Highlights
-
•
Cardiac amyloidosis has high associated morbidity and, until recently, limited treatment options.
-
•
This review discusses the mechanism and clinical trial performance of multiple emerging therapies.
-
•
Additional studies should identify optimal treatment paradigms and biomarker strategies for cardiac response to therapy.
Key Words: cardiac amyloidosis, clinical trials, therapeutics
Abbreviations and Acronyms: AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; ASCT, autologous stem cell transplantation; CA, cardiac amyloidosis; GLS, global longitudinal strain; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; MMP, matrix metalloproteinase; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; SAP, serum amyloid P
Summary
Cardiac amyloidosis is a restrictive cardiomyopathy that results from the deposition of misfolded light chain or transthyretin proteins, most commonly, in cardiac tissue. Traditionally, treatment options for light chain (AL) and transthyretin (ATTR) amyloidosis have been limited. However, there are now multiple novel therapeutics in development and several therapeutics recently approved that promise to revolutionize clinical management of AL and ATTR. Most of these agents disrupt specific stages of amyloidogenesis such as light chain or transthyretin protein production, formation of amyloidogenic intermediates, or amyloid fibril aggregation. Others aim to remove existing amyloid tissue deposits using monoclonal antibody technology. Although these advances represent an important step forward in the care of cardiac amyloidosis patients, additional studies are needed to define the optimal treatment paradigms for AL and ATTR and to validate clinical, imaging, or serum biomarker strategies that may confirm a cardiac response to therapy.
Central Illustration
Cardiac amyloidosis is a restrictive cardiomyopathy that results from the deposition of amyloid fibrils in cardiac tissue, causing progressive heart failure (HF) with median survival of 2 to 4 years 1, 2. Two types of amyloid protein cause more than 95% of cardiac amyloidosis: immunoglobulin light chain, which causes light chain amyloidosis (AL), and transthyretin, which causes hereditary or wild-type (previously called senile systemic amyloidosis) transthyretin amyloidosis (ATTR).
Although hereditary ATTR (3) and AL (4) are uncommon conditions, wild-type ATTR is likely underdiagnosed particularly among patients with HF with preserved ejection fraction (EF) and calcific aortic stenosis 5, 6, 7, 8, 9. Despite growing clinical recognition of the disease, availability of reliable noninvasive diagnostic techniques (10), and significant associated morbidity and mortality, pharmacologic agents have yet to receive regulatory approval specifically for AL or ATTR with cardiac involvement.
This review highlights the new and emerging therapies for AL and ATTR with emphasis on their mechanistic effects on the amyloid disease-producing process and performance in early clinical trials. It is apparent that additional studies are needed to define the optimal treatment paradigms and to measure therapeutic cardiac response in light chain (AL-CA) and transthyretin (ATTR-CA) cardiac amyloidosis.
Light Chain Amyloidosis
Background
AL results from the extracellular deposition of amyloid fibrils composed of monoclonal immunoglobulin light chains, which are produced by an underlying clonal plasma cell proliferative disorder such as multiple myeloma (MM) (Central Illustration, panel A). Ten percent to 15% of patients with MM develop AL; however, the majority of AL patients have <10% plasma cells in the bone marrow (11). Monoclonal gammopathy of undetermined significance (MGUS) is a known precursor of both MM and AL, and progresses to AL in 1.0% of cases with a relative risk of 8.8 as compared to the general population (12). The light chain amyloid fibrils in AL derive mostly from N-terminal amino acid residues of light chain immunoglobulin variable regions, and arise more commonly from λ light chains than κ (11).
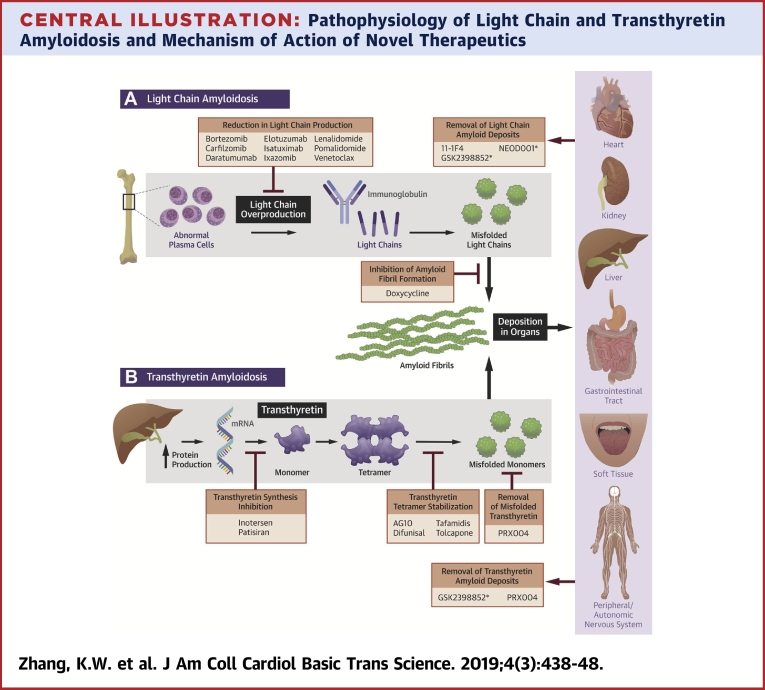
Central Illustration.
Pathophysiology of Light Chain and Transthyretin Amyloidosis and Mechanism of Action of Novel Therapeutics
(A) Abnormal plasma cells overproduce κ or λ light chains, which misfold and aggregate into amyloid fibrils. The light chain amyloid fibrils deposit in multiple end-organs causing organ dysfunction. Carfilzomib, daratumumab, elotuzumab, isatuximab, ixazomib, pomalidomide, and venetoclax reduce light chain production; doxycycline inhibits formation of light chain amyloid fibrils; 11-1F4, GSK2398852, and NEOD001 remove light chain amyloid deposits from tissue. (B) Transthyretin is synthesized in hepatocytes as a homotetramer, which dissociates into alternatively folded monomers that self-assemble to form insoluble amyloid fibrils. The transthyretin amyloid fibrils deposit in multiple end-organs causing organ dysfunction. Patisiran and inotersen inhibit transthyretin synthesis by targeting transthyretin mRNA; AG10, diflunisal, tafamidis, and tolcapone stabilize the transthyretin tetramer; PRX004 targets misfolded transthyretin and removes tissue amyloid deposits; GSK2398852 removes amyloid deposits from tissue. *Drug development has been suspended or terminated.
The exact mechanism of AL pathogenesis and the resultant pathophysiological changes that lead from MGUS or MM to AL are currently unknown. The monoclonal plasma cells in AL share many genetic abnormalities with the plasma cells in MM and MGUS, namely, gain of chromosomes 7, 9, 11, 15, and 18, loss of chromosome 18 (13), translocation of the immunoglobulin heavy chain gene at 14q32 14, 15, and deletion of the long arm of chromosome 13 [del(13q)] (16). However, functional gene expression analyses show a unique molecular profile for AL as compared with MM including higher expression of CD27 SDF-1 (17). AL clonal plasma cells also show a number of deregulated genes and pathways involving protein processing and folding.
Current standard-of-care for newly diagnosed AL is based on antimyeloma therapy and consists of autologous stem cell transplantation (ASCT) for eligible patients or bortezomib-based chemotherapy for high-risk patients who are transplant-ineligible. Five-year survival with upfront bortezomib-based regimens lags behind that with upfront ASCT (55% versus 84%) 18, 19, although the 2 strategies have not been compared in a clinical trial setting and the transplant-ineligible population is generally sicker with more comorbidities. Additionally, up to 45% of patients have progression of cardiac disease despite complete hematologic response with initial therapy (20), possibly related to obstructive intramural coronary amyloidosis, subendocardial ischemia, and/or interstitial fibrosis 21, 22. There remains a critical need for novel therapeutic agents that improve cardiac response to AL therapy.
Proteasome inhibitors
Protein degradation is an essential cellular function carried out by the proteasome. Proteasome inhibition causes cell cycle arrest and cellular apoptosis, with proliferating malignant cells showing higher susceptibility than normal cells (23). Bortezomib is a boronic acid dipeptide that inhibits the 20S proteasome by binding to the catalytic β subunits lining the inner ring of the 20S core particle in a reversible manner 23, 24.
Ixazomib is a second-generation boronic acid dipeptide proteasome inhibitor that binds reversibly to the 20S proteasome. Because of a shorter proteasome dissociation half-life, ixazomib has significantly larger volume of distribution and greater tumor proteasome inhibition than bortezomib in vivo, allowing for higher doses of administration and greater plasma exposure (24). Compared to lenalidomide and dexamethasone alone, addition of ixazomib reduced disease progression or death in patients with relapsed/refractory MM with a hazard ratio (HR) of 0.74 (25). No excess of adverse events was seen with ixazomib. A phase I/II trial of ixazomib in 27 patients with relapsed/refractory AL showed encouraging hematologic (52%) and organ (56%) response rates with only 3 patients experiencing dose-limiting toxicity (NCT01318902) (26). A phase III trial of ixazomib in the AL population is ongoing (NCT01659658) (Table 1).
Table 1.
Emerging Therapeutics for Light Chain and Transthyretin Amyloidosis
| Mechanism of Action | Drug Name | Route | Frequency | FDA Approved? | FDA Approved For Amyloid? | Latest Amyloidosis Clinical Trial Phase | NCT Clinical Trial Number |
|---|---|---|---|---|---|---|---|
| Light chain amyloidosis | |||||||
| Reduction in light chain production | |||||||
| Proteasome Inhibitor | Carfilzomib | IV | Twice weekly for 3 weeks per 28-day cycle | Yes | No | I/II | NCT01789242 |
| Ixazomib | PO | Once weekly for 3 weeks per 28-day cycle | Yes | No | III | NCT01659658 | |
| Immunomodulator | Pomalidomide | PO | Once daily for 3 weeks per 28-day cycle | Yes | No | I/II (terminated) | NCT01807286 |
| Anti-CD38 mAb | Daratumumab | SQ | Once weekly (then once) per 28-day cycle | Yes | No | III | NCT03201965 |
| Isatuximab | IV | Weekly (then biweekly) per 28-day cycle | No | No | II | NCT03499808 | |
| Anti-SLAMF7 | Elotuzumab | IV | Weekly (then biweekly) per 28-day cycle | Yes | No | II | NCT03252600 |
| BCL2 Inhibitor | Venetoclax | PO | Daily | Yes | No | I | NCT03000660 |
| Inhibition of amyloid fibril formation | |||||||
| Doxycycline | PO | Twice daily | Yes | No | II/III | NCT02207556, NCT03474458, NCT03401372 | |
| Clearance of amyloid deposits | |||||||
| 11-1F4 | IV | Once weekly | No | No | I | NCT02245867 | |
| NEOD001 | IV | Once every 28 days | No | No | III (terminated) | NCT02312206 | |
| Transthyretin amyloidosis | |||||||
| Transthyretin tetramer stabilizer | |||||||
| AG10 | PO | Twice daily | No | No | II | NCT03458130 | |
| Diflunisal | PO | Twice daily | Yes | No | III | NCT00294671 | |
| Tafamidis | PO | Daily | No | No | III | NCT01994889 | |
| Tolcapone | PO | Daily | Yes | No | II | NCT02191826 | |
| Transthyretin synthesis inhibitor | |||||||
| Inotersen | SQ | Weekly | Yes | Yes | III | NCT01737398 | |
| Patisiran | IV | Once every 3 weeks | Yes | Yes | III | NCT01960348 | |
| Removal of amyloid deposits | |||||||
| PRX004 | IV | Once every 28 days | No | No | I | NCT03336580 | |
| Light chain and transthyretin amyloidosis | |||||||
| Removal of amyloid deposits | |||||||
| GSK2398852 | IV | Once per 11-day cycle | No | No | II (suspended) | NCT03044353 | |
FDA = U.S. Food and Drug Administration; IV = intravenous; mAb = monoclonal antibody; NCT = National Clinical Trial; PO = oral; SQ = subcutaneous.
Carfilzomib is a modified epoxyketone that binds the 20S proteasome irreversibly and with higher selectivity than bortezomib. As compared with lenalidomide and dexamethasone alone, the addition of carfilzomib in relapsed MM improved 24-month survival by 21% and also led to significant improvement in hematologic response rate (27). Cardiovascular toxicities (hypertension, cardiac failure, and ischemic heart disease) were among the major adverse effects. In a phase I/II trial of carfilzomib monotherapy in 28 patients with previously treated AL, 63% showed hematologic response although pulmonary, renal, and cardiac toxicities were common (28) (NCT01789242). Because of advanced cardiac disease in many AL patients, the role of carfilzomib in standard AL treatment paradigms remains to be determined.
Immunomodulatory agents
The immunomodulatory agents (IMiDs) include thalidomide and its analogues, and have therapeutic efficacy in a wide range of malignancies. Recent studies have shown that the IMiDs exert antimyeloma activity by activating the E3 ubiquitin ligase activity of the protein cereblon. Binding of the IMiDs to cereblon results in the rapid ubiquitination and degradation of Ikaros (IKZF1) and Aiolos (IKZF3), 2 key transcriptional regulators of B and T cell development (29). Lenalidomide is a second-generation synthetic thalidomide that has been in use for AL. In 69 patients with AL treated with lenalidomide and dexamethasone, 16% of patients achieved a complete response (CR) in an intent-to-treat analysis (NCT00091260) (30). The median time to achieve a CR was 6 months with 60% of CRs considered durable.
Pomalidomide is a newer thalidomide analogue with more potent antimyeloma activity than lenalidomide. As with lenalidomide, it inhibits pro-inflammatory cytokine production and angiogenesis, induces apoptosis and cell cycle arrest, and activates T cells and natural killer cells (31). A phase I trial of pomalidomide and dexamethasone in 33 patients with previously treated AL showed hematologic response rate of 48% and organ response rate of 15% (32) (NCT01807286). Twenty-seven patients (82%) had cardiac involvement but only 4 showed cardiac response. This trial was terminated early during the phase I portion without continuation to phase II.
Trials using the IMiDs thalidomide, lenalidomide, and pomalidomide have repeatedly shown that patients with AL have a poor tolerance for these agents when used at the standard doses typically prescribed for MM, although it is unclear why this occurs (33). The precise role and optimal sequence of the IMiDs in the treatment of AL remains unclear.
Monoclonal antibodies
CD38 and SLAMF7 (also known as CD319 and CS1) are cell surface antigens specific to MM cells that are being evaluated as targets of monoclonal antibody (mAb) therapeutics in MM and AL.
CD38 is a type II transmembrane glycoprotein that associates with cell-surface receptors in lipid rafts, regulates cytoplasmic Ca2+ flux, and mediates signal transduction in lymphoid and myeloid cells (34). It is expressed at low level on normal lymphoid and myeloid cells and in some tissues of nonhematopoietic origin, but it is highly and uniformly expressed on plasma cells in patients with MM and AL 35, 36. Daratumumab is a human immunoglobulin (Ig) G1κ mAb that targets the extracellular domain of CD38 (37). In vitro, daratumumab kills MM cells by antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, and is highly efficacious in both early- and late-treatment settings in in vivo tumor models (37). Two large phase III trials showed improved progression-free survival (PFS) with daratumumab in combination with either lenalidomide and dexamethasone or bortezomib and dexamethasone in patients with relapsed/refractory MM 38, 39. A retrospective analysis of 25 patients with refractory AL who received daratumumab and dexamethasone showed overall hematologic response rate of 76% including CR in 36% (40). Therapy was well tolerated without any grade 3 or 4 reactions observed, even among the 72% of patients with cardiac involvement. A phase III trial of daratumumab, cyclophosphamide, bortezomib, and dexamethasone in patients with newly diagnosed AL is ongoing (NCT03201965).
Isatuximab is a chimeric IgG1 monoclonal antibody that targets CD38 as an allosteric antagonist (41). In vivo, isatuximab showed antitumor activity in multiple cancer models including MM, lymphoma, and leukemia. In a phase Ib trial of isatuximab, lenalidomide, and dexamethasone for relapsed/refractory multiple myeloma, the overall hematologic response rate was 56% and median PFS was 8.5 months, indicative of clinical benefit without significant toxicity (42). A phase II trial of isatuximab in patients with relapsed/refractory AL is ongoing (NCT03499808).
SLAMF7 is a member of the Signaling Lymphocyte Activation Molecule Family and is expressed at low level on natural killer cells, CD8+ T cells, B cells, and mature dendritic cells (43). Normal plasma cells and MM cells express high levels of SLAMF7 mRNA and protein (44), and targeting of SLAMF7 using mAbs led to antibody-dependent cellular cytotoxicity and reduction in myeloma activity in vivo. Although the mechanism of SLAMF7 upregulation in MM is unknown, small interfering RNA inhibition of SLAMF7 expression inhibited MM cell adhesion to bone marrow stromal cells (45). Elotuzumab is a humanized recombinant IgG1κ mAb that targets the extracellular region of SLAMF7 (44). In patients with relapsed/refractory MM, the addition of elotuzumab to lenalidomide and dexamethasone improved median PFS from 14.9 to 19.4 months, with overall response rate (ORR) of 79% (improved from 66%) (46). Elotuzumab gained U.S. Food and Drug Administration approval for relapsed/refractory MM in 2015, and is currently in a phase II trial for patients with relapsed AL (NCT03252600).
Small molecule inhibitors
Modulation of intrinsic apoptotic pathways offers an additional mechanism by which to treat AL amyloidosis. BCL-2 is a central regulator of programmed cell death that binds and sequesters pro-apoptotic proteins (47). Elevated expression of anti-apoptotic proteins is common in MM and associated with resistance to therapy (48). Venetoclax is a small molecule that binds with high affinity to the binding pocket on BCL-2 specific for the BH3 region of intrinsic pro-apoptotic proteins (47). In vitro, venetoclax inhibits interaction of BCL-2 with pro-apoptotic proteins within the mitochondria, and induces apoptosis in lymphoma and leukemia cells as well as tumor regression in in vivo solid tumor models (47). In human myeloma cell lines and primary MM samples, sensitivity to venetoclax was highest in the presence of the (11;14) translocation, which correlated with higher ratios of BCL2 and MCL1 mRNA (49). t(11;14) is seen in 15% to 20% of patients with MM, and may be a marker of intermediate-risk disease (50).
A phase I trial of venetoclax monotherapy in 66 patients with relapsed/refractory MM showed an ORR of 21%; among patients with t(11;14), the ORR was doubled at 40% (51). Similarly, time to progression of disease was 6.6 months in patients with t(11;14) and 1.9 months in those without t(11;14). There were no adverse cardiac events, and changes in cardiac parameters with therapy were not evaluated. A separate phase Ib trial of venetoclax in combination with bortezomib and dexamethasone showed better ORR at 67%, with time to progression of 9.7 months (52). Again, patients with higher BCL2 expression had a higher ORR (94%) as compared to patients with low BCL2 expression (59%). A phase I trial of venetoclax and dexamethasone in patients with relapsed/refractory AL is ongoing (NCT03000660).
Doxycycline
Matrix metalloproteinases (MMPs) and their tissue inhibitors are key regulators of cardiac extracellular matrix homeostasis, which is disrupted by amyloid infiltration resulting in thickening and stiffening of the myocardium. Elevation in MMP and tissue inhibitor of matrix metalloproteinase serum levels and tissue expression have been associated with renal and cardiac damage in AL 53, 54, suggesting that inhibition of the MMP pathway may mitigate the cardiotoxic effects of light chain amyloid fibril deposition. Doxycycline is a semisynthetic tetracycline antibiotic that inhibits bacterial protein synthesis and, separately, also acts as an inhibitor of MMPs. Doxycycline inhibits the formation of light chain amyloid fibrils in vivo and ex vivo in a dose-dependent manner, and prevented light chain amyloid deposition in a mouse model of AL (55). In a retrospective cohort study of 103 patients with AL-CA, 24-month survival improved from 40% to 82% by administering doxycycline along with chemotherapy whereas cardiac response to therapy improved 3-fold to 60% (56). Multiple clinical trials of doxycycline in conjunction with plasma cell-directed therapy in patients with AL are ongoing (NCT02207556, NCT03474458, and NCT03401372).
Clearance of amyloid deposits
Mouse models of human AL amyloidomas showed spontaneous tumor regression in association with neutrophil infiltration and production of antibodies targeted to κ and λ light chain extracts (57), suggesting that exogenous administration of anti-amyloid antibodies may expedite clearance of amyloid deposits.
11-1F4 (CAEL-101) is a chimeric monoclonal IgG1 antibody that targets the VL fragment of human K Bence Jones protein, with stronger affinity for κ light chain than λ 57, 58. 11-1F4 interacts immunohistochemically with human AL deposits in hepatocytes, proximal renal tubules, and myocytes, and facilitates regression of AL amyloidomas in vivo (58). In a phase Ia/b dose-escalation study in 27 patients with relapsed/refractory AL, 62% of patients showed organ response at a median of 2 weeks after starting treatment (NCT02245867) (59). In patients with cardiac involvement, 11-1F4 led to a statistically significant improvement in global longitudinal strain (GLS) after 12 weeks of follow-up (60). A randomized phase II/III trial for newly diagnosed AL patients is planned, inclusive of a high-risk patient cohort with N-terminal prohormone of brain natriuretic peptide (NT-proBNP) > 8,500 ng/L. Additionally, a radiolabeled peptide comprising a 11-1F4 epitope and a pan-amyloid-reactive peptide is in development which may enable the use of 11-1F4 as a diagnostic imaging agent for AL and other forms of amyloidosis (61).
NEOD001 is a humanized mAb originally developed for secondary amyloidosis but found to target AL deposits with high affinity. It targets the C-terminal amino acid sequence of murine serum amyloid A protein, which is exposed during the process of AA amyloid deposition and is not accessible in the full-length serum amyloid A molecule (62). NEOD001 binds AL amyloid deposits in situ, induces phagocytic clearance of AL deposits, and expedites clearance of AL amyloidomas 63, 64. NEOD001 performed well in a phase I/II trial of patients with AL and persistent organ dysfunction after therapy (65). However, a phase II trial failed to meet its primary endpoint of cardiac response as measured by NT-proBNP (NCT02632786), and a subsequent phase III trial was discontinued prematurely for futility (NCT02312206) (66). Failure of these late-phase clinical trials may have stemmed from issues with study design, such as the use of biomarker-based endpoints in NCT02632786 and a composite clinical endpoint in NCT02312206 as opposed to best response analysis. Further development of NEOD001 for AL was halted in April 2018.
Transthyretin Amyloidosis
Background
Transthyretin is a 55,000-Dalton protein that is synthesized primarily in the liver, and serves as the primary serum transport protein for holo-retinol-binding protein and a minor transport protein for thyroxine. In plasma, <1% of transthyretin is thyroxine-bound due to higher binding affinity of thyroxine for thyroid binding globulin and albumin (67). Transthyretin is also synthesized in the choroid plexus and secreted into the cerebrospinal fluid (CSF), where it serves as the primary carrier of thyroxine (68).
The transthyretin protein contains 4 identical subunits rich in β-sheets that assemble into 2 αβ dimers. The αβ dimers contact one another at an extended β-sheet that forms 2 funnel-shaped hormone binding sites, where thyroxine binds with negative cooperativity 68, 69. Formation of transthyretin amyloid fibrils requires dissociation of the tetramer into alternatively folded monomers, which then self-assemble to form insoluble amyloid fibrils (Central Illustration, panel B) 70, 71. Binding of thyroxine stabilizes the native conformation of the transthyretin tetramer, preventing its dissociation into monomer units and subsequent aggregation into amyloid fibrils (72). In CSF, where transthyretin is highly thyroxine bound, transthyretin fibril formation is generally not observed (72).
The kinetics of tetramer dissociation also explain the predilection of mutant transthyretin for amyloid formation. L55P and V122I-mutant transthyretin dissociate rapidly to the monomeric amyloidogenic intermediate and aggregate to form amyloid fibrils at a correspondingly high rate, leading to nearly 100% disease penetrance with severe systemic disease (73). In comparison, the V30M mutant dissociates more slowly even than wild-type transthyretin and results in a milder disease phenotype (polyneuropathy) with penetrance as low as 2% 74, 75. The protective T119M variant exhibits extremely slow tetramer dissociation and fibril formation rates (73); compound heterozygotes expressing both T119M and V30M transthyretin exhibit either normal phenotype or only mild pathology of late onset (76).
Transthyretin tetramer stabilizers
Because of the disease-modifying effect of tetramer stabilization, transthyretin tetramer stabilizers have emerged as a novel class of therapeutics for ATTR. Nonsteroidal anti-inflammatory drugs such as diflunisal were among the first transthyretin tetramer stabilizers identified, binding with higher efficacy to the central hormone-binding funnel than thyroxine (77). Administration of diflunisal 250 mg twice daily in healthy subjects slowed transthyretin aggregation in vitro by 3-fold (78). In familial amyloid polyneuropathy, a phase III clinical trial of diflunisal 250 mg twice daily reduced the rate of progression of neurologic impairment and preserved quality of life over 2 years (NCT00294671) (79). Diflunisal was well tolerated in an open-label study of 13 patients with wild-type and hereditary ATTR-CA; hemoglobin, mean arterial pressure, and glomerular filtration rate remained stable over 0.9 years of follow-up without significant change in cardiac structure, function, or biomarkers (80). Larger randomized studies of diflunisal in the ATTR-CA population are needed.
Tafamidis is a small molecule ligand in the benzoxazole family that exhibits potent and selective transthyretin binding, and lacks nonsteroidal anti-inflammatory drugs activity. Tafamidis inhibits wild-type transthyretin amyloidogenesis in a dose-dependent manner, and also stabilizes the 2 most clinically significant amyloidogenic mutant homotetramers (V30M and V122I) with comparable potency and efficacy (81). In a phase III trial of 441 patients with wild-type and hereditary ATTR-CA, tafamidis led to a reduction in all-cause mortality (HR: 0.70, 95% confidence interval: 0.51 to 0.96) and cardiovascular-related hospitalization (HR: 0.68, 95% confidence interval: 0.56 to 0.81), with a number-needed-to-treat for the combined endpoint of all-cause mortality and cardiovascular-related hospitalization of 7.5 (82). It remains unknown whether tafamidis is effective in advanced amyloid disease, as patients with New York Heart Association (NYHA) functional class IV HF were excluded; a small study of patients with advanced familial amyloid polyneuropathy showed no significant clinical improvement with tafamidis (83).
AG10 is a synthetic small molecule transthyretin ligand whose basic protein structure was identified by high-throughput screen, and subsequently modified with a carboxylic acid group on the 2-fluorophenyl ring to optimize binding energetics to transthyretin (84). AG10 binds to wild-type transthyretin with higher affinity than tafamidis or diflunisal; furthermore, AG10 stabilizes V122I mutant transthyretin tetramers more effectively than tafamidis and is also more effective in preventing V122I amyloid fibril formation (84). Both tafamidis and AG10 protect cardiomyocytes from the proteotoxic effects of amyloidogenic V122I transthyretin in vitro. AG10 is currently in a phase II clinical trial for hereditary and wild-type ATTR-CA (NCT03458130); a phase III trial in ATTR-CA is projected to open in early 2019.
Tolcapone is a catechol-O-methyltransferase inhibitor originally approved as an adjunct to levodopa and carbidopa for the treatment of Parkinson’s disease. It binds to the thyroxine binding pocket at the transthyretin dimer-dimer interface with higher affinity than tafamidis, and is a stronger aggregation inhibitor than tafamidis (85). Similar to tafamidis, tolcapone inhibits aggregation of the V122I-transthyretin variant although with less efficacy than wild-type transthyretin. When incubated with human plasma, tolcapone prevents transthyretin tetramer dissociation for wild-type and V30M transthyretin with greater stabilizing activity than tafamidis. The unique ability of tolcapone to cross the blood-brain barrier indicates that it may treat transthyretin leptomeningeal amyloidosis, noting that the majority of transthyretin in the CSF is thyroxine bound. A phase II trial of tolcapone in familial amyloid polyneuropathy is ongoing (NCT02191826).
Transthyretin synthesis inhibitors
Patisiran is a second-generation, double-stranded small interfering RNA that targets the 3’ untranslated region of the transthyretin gene, which is conserved among wild-type transthyretin and all reported transthyretin mutations. Patisiran is modified with 2’-O-methyl ribonucleosides for improved stability and formulated in a lipid nanoparticle, which enables passage through the fenestrated vascular endothelium of the liver and targeted organ delivery (86). Endosomal endocytosis of the lipid nanoparticle delivers patisiran to the cytoplasm, where it triggers endogenous cellular pathways for controlling gene expression through RNA interference. Specifically, binding of patisiran to the RNA-induced silencing complex triggers separation of the 2 RNA strands and allows for binding of the antisense strand to transthyretin mRNA. The double-stranded RNA substrate is then cleaved and targeted for degradation, leading to a reduction in transthyretin protein levels (87). In animal models, patisiran reduced transthyretin deposition and facilitated regression of existing transthyretin deposits, with the extent of deposit regression correlating with the level of RNA interference–mediated knockdown (88).
In a phase III trial of patients with hereditary ATTR with polyneuropathy, patisiran significantly improved neuropathy and quality of life after 18 months of therapy in the overall cohort and in the subgroup with cardiac involvement (56%; NYHA functional class III/IV were excluded) (86). Within the cardiac subpopulation, patisiran led to a statistically significant reduction in NT-proBNP level, left ventricular (LV) wall thickness, and reduced worsening in GLS, suggestive of cardiac benefit (89). Further study of patisiran in the ATTR-CA population with the use of clinically relevant cardiac endpoints is needed.
Inotersen is a second-generation antisense oligonucleotide that targets the 3’ untranslated region of transthyretin mRNA. Its single-stranded synthetic oligomer contains 20 nucleotides with a modified phosphorothioate backbone as well as 2’-O-methoxyethyl modified ribonucleotides at each terminus, which confer increased hybridization affinity to target RNA, increased resistance to nuclease degradation, and reduced immunostimulatory activity (90). Systemically administered inotersen distributes at high levels to the liver, where it gains access to the intracellular space via endosome activity and then moves to the nucleus by passive diffusion and active transport due to its phosphorothioate backbone 91, 92. Once bound to the target RNA through Watson-Crick base-pairing, inotersen forms an RNA-DNA hybrid that triggers target mRNA degradation by RNase H via a central region of 10 2’-deoxynucleotide residues that is recognized by the enzyme (93).
In a phase III trial of patients with hereditary ATTR with polyneuropathy, inotersen significantly improved neuropathy and quality of life after 16 months of therapy in the overall cohort and in the subgroup with cardiomyopathy (68%; NYHA functional class III/IV were excluded) (94). Within the cardiomyopathy subgroup, there was no significant change in LVEF, GLS, LV wall thickness, LV mass, or lateral E/E’ ratio after 16 months of inotersen therapy, although baseline LVEF and GLS were relatively preserved at 64% and −14%, respectively. Thrombocytopenia and, rarely, glomerulonephritis are important safety concerns and should be monitored on a regular basis. The safety of inotersen in patients with a prior liver transplant for hereditary ATTR is not established. As with patisiran, further study of inotersen in the ATTR-CA population is needed.
Clearance of amyloid deposits
GSK2398852 is an anti-serum amyloid P component (SAP) antibody whose epitope is a glycoprotein in the pentraxin family, a group of highly evolutionarily conserved proteins that activate the innate immune response upon binding to microbial ligands, apoptotic cells, and amyloid fibrils 95, 96. The pentraxins have also been found to promote amyloid deposition and disease progression in vivo (97). In a mouse model of systemic amyloidosis, depletion of circulating SAP followed by administration of anti-SAP monoclonal IgG1 antibody led to infiltration of macrophages and other monocytic inflammatory cells into tissue amyloid deposits in a complement-dependent fashion (98). The inflammatory process was characterized by high-phagocyte endocytotic activity and engulfment of amyloid deposits by multinucleated giant cells, culminating in complete clearance of amyloid tissue deposits and restoration of normal tissue architecture.
In a phase I trial, 16 subjects with systemic amyloidosis and significant amyloid infiltration of the liver and spleen received GSK2398852 after depletion of circulating SAP without excess toxicity (99). Patients with cardiac involvement were excluded from this study, and there were no significant changes in extracellular volume of the heart, troponin T, or NT-proBNP. However, a phase II trial of GSK2398852 in patients with biopsy- or bone scintigraphy–proven ATTR-CA was suspended prematurely due to an adverse risk/benefit profile (NCT03044353).
PRX004 is a humanized IgG1 mAb that targets an epitope exposed on abnormal transthyretin protein. In preclinical models, PRX004 neutralized soluble cytotoxic forms of transthyretin, prevented amyloid fibril formation, and promoted clearance of organ amyloid deposits via phagocytosis (unpublished data, courtesy of Prothena Corporation, May 2018). A phase I dose escalation trial is currently ongoing (NCT03336580).
Future Directions
With the emergence of multiple novel therapeutics for AL and ATTR, there is an urgent need to define optimal drug combinations and sequences of drug delivery to reduce morbidity and mortality amongst patients with AL-CA and ATTR-CA. This is particularly important in the treatment of AL-CA, although large randomized clinical trials in this population are challenging due to low disease prevalence and poor performance status. Although initial therapy for AL with a bortezomib-based regimen versus ASCT is generally accepted, it remains unclear which regimens are optimal for relapsed/refractory disease (100) and how the novel AL therapeutics will fit into the treatment paradigm. It is likely that a multipronged treatment approach will be optimal for both AL-CA and ATTR-CA, and carefully executed clinical trials are needed to determine the optimal approach.
The impact of each novel therapeutic on cardiac biomarkers also requires clarification. NT-proBNP is an important cardiac biomarker that correlates inversely with prognosis in AL-CA and ATTR-CA 2, 20. A paradoxical increase in NT-proBNP has been described in AL patients on lenalidomide despite hematologic response and absence of cardiac symptoms, possibly reflecting IMiD-induced fluid retention as opposed to direct cardiotoxicity 101, 102. Additionally, a significant increase in NT-proBNP has been described in patients with advanced AL-CA on bortezomib, although it is unclear whether this represents drug toxicity or worsening of pre-existing cardiac disease (103). The utility of alternate cardiac biomarkers, such as troponin T, for assessment of drug-induced cardiotoxicity should be explored.
Finally, validated biomarkers that track with cardiac response to therapy are needed to rigorously assess the cardiac efficacy of emerging therapeutics in AL and ATTR. Challenges associated with NT-proBNP have been discussed, and also include optimal timing of NT-proBNP assessment (104). Whereas serial cardiac magnetic resonance imaging studies may be useful in clinical trials, this strategy is unlikely to be feasible in clinical practice. GLS by echocardiography has shown value for prognosis and diagnosis in CA, but its utility for tracking cardiac response to therapy has yet to be explored 105, 106. Six-minute walk distance was better on tafamidis as compared with placebo in clinical trials and correlated with cardiac response to chemotherapy in AL 82, 107, and could be implemented in future clinical trials and clinical practice as a marker of cardiac response to therapy.
Conclusions
Cardiac amyloidosis is a restrictive cardiomyopathy with high morbidity and mortality, and is increasingly recognized as an underdiagnosed condition. Although no therapies have been approved for AL- or ATTR-CA, a number of therapeutics targeted at neoplastic plasma cells, transthyretin protein production, and amyloid fibril formation are currently in clinical trials. Additionally, mAbs aiming to remove existing amyloid deposits may improve outcomes for patients with more advanced cardiac disease. Future efforts should define the optimal treatment paradigms for cardiac response in AL-CA and ATTR-CA, and identify effective clinical, imaging, and serum biomarker strategies that reflect cardiac response to therapy.
Footnotes
Dr. Stockerl-Goldstein is on the advisory board for Celgene; has stock equity in Abbott and AbbVie; and has received grants from Millenium Pharmaceuticals, Janssen Pharmaceuticals, BioLineRx Ltd., Pfizer Inc., and GlaxoSmithKline. Dr. Lenihan has received personal fees from Pfizer, Prothena, Akcea, and Takeda. Dr. Zhang has reported that she has no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and U.S. Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Cibeira M.T., Sanchorawala V., Seldin D.C. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–4352. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2017;44:1–8. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins P.N., Ando Y., Dispenzeri A., Gonzalez-Duarte A., Adams D., Suhr O.B. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47:625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer M.S., Elliott P., Comenzo R., Semigran M., Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135:1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed S.F., Mirzoyev S.A., Edwards W.D. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-López E., Gallego-Delgado M., Guzzo-Merello G. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 7.Scully P.R., Treibel T.A., Fontana M. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71:463–464. doi: 10.1016/j.jacc.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treibel T.A., Fontana M., Gilbertson J.A. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016:1–11. doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 9.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 11.Muller A.M.S., Geibel A., Neumann H.P.H. Primary (AL) amyloidosis in plasma cell disorders. Oncologist. 2006;11:824–830. doi: 10.1634/theoncologist.11-7-824. [DOI] [PubMed] [Google Scholar]
- 12.Kyle R.A., Larson D.R., Therneau T.M. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca R., Ahmann G.J., Jalal S.M. Chromosomal abnormalities in systemic amyloidosis. Br J Haematol. 1998;103:704–710. doi: 10.1046/j.1365-2141.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 14.Avet-Loiseau H., Daviet A., Saunier S., Bataille R. Chromosome 13 abnormalities in multiple myeloma are mostly monosomy 13. Br J Haematol. 2000;111:1116–1117. doi: 10.1046/j.1365-2141.2000.02488.x. [DOI] [PubMed] [Google Scholar]
- 15.Perfetti V., Coluccia A.M.L., Intini D. Translocation t(4;14)(p16.3;q32) is a recurrent genetic lesion in primary amyloidosis. Am J Pathol. 2001;158:1599–1603. doi: 10.1016/S0002-9440(10)64115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison C.J., Mazzullo H., Ross F.M. Translocations of 14q32 and deletions of 13q14 are common chromosomal abnormalities in systemic amyloidosis. Br J Haematol. 2002;117:427–435. doi: 10.1046/j.1365-2141.2002.03438.x. [DOI] [PubMed] [Google Scholar]
- 17.Abraham R.S., Ballman K.V., Dispenzieri A. Functional gene expression analysis of clonal plasma cells identifies a unique molecular profile for light chain amyloidosis. Blood. 2005;105:794. doi: 10.1182/blood-2004-04-1424. [DOI] [PubMed] [Google Scholar]
- 18.Palladini G., Sachchithanantham S., Milani P. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 19.Sidiqi M.H., Aljama M.A., Buadi F.K. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36:1323–1329. doi: 10.1200/JCO.2017.76.9554. [DOI] [PubMed] [Google Scholar]
- 20.Palladini G., Dispenzieri A., Gertz M.A. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 21.Hashimura H., Ishibashi-Ueda H., Yonemoto Y. Late gadolinium enhancement in cardiac amyloidosis: attributable both to interstitial amyloid deposition and subendocardial fibrosis caused by ischemia. Heart Vessels. 2016;31:990–995. doi: 10.1007/s00380-015-0658-0. [DOI] [PubMed] [Google Scholar]
- 22.Dubrey S., Falk R.H. Amyloidosis and the heart. Br J Cardiol. 1995;2:193–199. [Google Scholar]
- 23.Rajkumar S.V., Richardson P.G., Hideshima T., Anderson K.C. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Kupperman E., Lee E.C., Cao Y. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 25.Moreau P., Masszi T., Grzasko N. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 26.Sanchorawala V., Palladini G., Kukreti V. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–606. doi: 10.1182/blood-2017-03-771220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart A.K., Rajkumar S.V., Dimopoulos M.A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 28.Cohen A.D., Landau H., Scott E.C. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. 2016;128:645. [Google Scholar]
- 29.Stewart A.K. How thalidomide works against cancer. Science. 2014;343:256–257. doi: 10.1126/science.1249543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchorawala V., Finn K.T., Fennessey S. Durable hematologic complete responses can be achieved with lenalidomide in AL amyloidosis. Blood. 2010;116:1990–1991. doi: 10.1182/blood-2010-07-295485. [DOI] [PubMed] [Google Scholar]
- 31.Gay F., Mina R., Troia R., Bringhen S. Pharmacokinetic evaluation of pomalidomide for the treatment of myeloma. Expert Opin Drug Metab Toxicol. 2013;9:1517–1527. doi: 10.1517/17425255.2013.827169. [DOI] [PubMed] [Google Scholar]
- 32.Dispenzieri A., Buadi F., Laumann K. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dispenzieri A., Lacy M.Q., Zeldenrust S.R. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–470. doi: 10.1182/blood-2006-07-032987. [DOI] [PubMed] [Google Scholar]
- 34.Deaglio S., Vaisitti T., Billington R. CD38/CD19 : a lipid raft — dependent signaling complex in human B cells. Blood. 2007;109:5390–5398. doi: 10.1182/blood-2006-12-061812. [DOI] [PubMed] [Google Scholar]
- 35.Lin P., Owens R., Tricot G., Wilson C.S. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 36.Lisenko K., Schönland S.O., Jauch A. Flow cytometry-based characterization of underlying clonal B and plasma cells in patients with light chain amyloidosis. Cancer Med. 2016;5:1464–1472. doi: 10.1002/cam4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Weers M., Tai Y.-T., van der Veer M.S. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 38.Dimopoulos M.A., Oriol A., Nahi H. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo A., Chanan-Khan A., Weisel K. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman G.P., Schrier S.L., Lafayette R.A., Arai S., Witteles R.M., Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900–902. doi: 10.1182/blood-2017-01-763599. [DOI] [PubMed] [Google Scholar]
- 41.Deckert J., Wetzel M.C., Bartle L.M. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20:4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 42.Martin T., Baz R., Benson D.M. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294–3303. doi: 10.1182/blood-2016-09-740787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malaer J.D., Mathew P.A. CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma. Am J Cancer Res. 2017;7:1637–1641. [PMC free article] [PubMed] [Google Scholar]
- 44.Hsi E.D., Steinle R., Balasa B. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai Y.-T., Dillon M., Song W. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces aritibody-dependent cellular cytotoxicity in the bone marrow mitieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lonial S., Dimopoulos M., Palumbo A. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 47.Oltersdorf T., Elmore S.W., Shoemaker A.R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 48.Bodet L., Gomez-Bougie P., Touzeau C. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118:3901–3910. doi: 10.1182/blood-2010-11-317438. [DOI] [PubMed] [Google Scholar]
- 49.Touzeau C., Ryan J., Guerriero J. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016;30:761–764. doi: 10.1038/leu.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman G.P., Gertz M.A., Dispenzieri A. Impact of cytogenetic classification on outcomes following early high-dose therapy in multiple myeloma. Leukemia. 2016;30:633–639. doi: 10.1038/leu.2015.287. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S., Kaufman J.L., Gasparetto C. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401–2409. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 52.Moreau P., Chanan-Khan A., Roberts A.W. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130:2392–2400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 53.Keeling J., Herrera G.A. Matrix metalloproteinases and mesangial remodeling in light chain-related glomerular damage. Kidney Int. 2005;68:1590–1603. doi: 10.1111/j.1523-1755.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 54.Biolo A., Ramamurthy S., Connors L.H. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail. 2008;1:249–257. doi: 10.1161/CIRCHEARTFAILURE.108.788687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward J.E., Ren R., Toraldo G. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118:6610–6617. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wechalekar A.D., Whelan C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017;7:89–91. doi: 10.1038/bcj.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hrncic R., Wall J., Wolfenbarger D.A. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol. 2000;157:1239–1246. doi: 10.1016/S0002-9440(10)64639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solomon A., Weiss D.T., Wall J.S. Therapeutic potential of chimeric amyloid-reactive monoclonal antibody 11-1F4. Clin Cancer Res. 2003;9 3831s LP–8s. [PubMed] [Google Scholar]
- 59.Edwards C.V., Gould J., Langer A.L. Final analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with relapsed or refractory AL amyloidosis. Blood. 2017;130(suppl 1) 509 LP-509. [Google Scholar]
- 60.Shames S., Jeff G., Maurer M.S., Lentzsch S. Cardiac response to chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with AL amyloidosis with global longitudinal strain: results from the phase 1b trial. J Am Soc Echocardiogr. 2018;31:B14. [Google Scholar]
- 61.Stuckey A., Williams A., Richey T. Preliminary pharmacokinetic study of a bispecific peptide for pretargeting immunotherapy of amyloidosis using PreClinical SPECT/CT. J Nucl Med. 2018;59(suppl 1):1834. [Google Scholar]
- 62.Wall J.S., Kennel S.J., Richey T. Generation and characterization of anti-AA amyloid-specific monoclonal antibodies. Front Immunol. 2011;2:1–11. doi: 10.3389/fimmu.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renz M., Torres R., Dolan P.J. 2A4 binds soluble and insoluble light chain aggregates from AL amyloidosis patients and promotes clearance of amyloid deposits by phagocytosis. Amyloid. 2016;23:168–177. doi: 10.1080/13506129.2016.1205974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wall J.S., Kennel S.J., Williams A. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gertz M.A., Landau H., Comenzo R.L. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varga C., Lentzsch S., Comenzo R.L. Beyond NEOD001 for systemic light-chain amyloidosis. Blood. 2018;132:1992–1993. doi: 10.1182/blood-2018-07-865857. [DOI] [PubMed] [Google Scholar]
- 67.Purkey H.E., Dorrell M.I., Kelly J.W. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors in blood plasma. Proc Natl Acad Sci U S A. 2001;98:5566–5571. doi: 10.1073/pnas.091431798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamilton J.A., Benson M.D. Transthyretin: a review from a structural perspective. Cell Mol Life Sci. 2001;58:1491–1521. doi: 10.1007/PL00000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klabunde T., Petrassi H.M., Oza V.B., Raman P., Kelly J.W., Sacchettini J.C. Rational design of potent human transthyretin amyloid disease inhibitors. Nat Struct Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 70.Colon W., Kelly J.W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 71.Lai Z., Colón W., Kelly J.W. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 72.Miroy G.J., Lai Z., Lashuel H.A., Peterson S.A., Strang C., Kelly J.W. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc Natl Acad Sci U S A. 1996;93:15051–15056. doi: 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammarstrom P., Jiang X., Hurshman A.R., Powers E.T., Kelly J.W. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002;99(suppl 4):16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coelho T. Familial amyloid polyneuropathy: new developments in genetics and treatment. Curr Opin Neurol. 1996;9:355–359. [PubMed] [Google Scholar]
- 75.Holmgren G., Costa P.M., Andersson C. Geographical distribution of TTR met30 carriers in northern Sweden: discrepancy between carrier frequency and prevalence rate. J Med Genet. 1994;31:351–354. doi: 10.1136/jmg.31.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hammarström P., Schneider F., Kelly J.W. Trans-suppression of misfolding in an amyloid disease. Science. 2001;293:2459–2462. doi: 10.1126/science.1062245. [DOI] [PubMed] [Google Scholar]
- 77.Peterson S.A., Klabunde T., Lashuel H.A., Purkey H., Sacchettini J.C., Kelly J.W. Inhibiting transthyretin conformational changes that lead to amyloid fibril formation. Proc Natl Acad Sci U S A. 1998;95:12956–12960. doi: 10.1073/pnas.95.22.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sekijima Y., Dendle M.A., Kelly J.W. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;1:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 79.Berk J.L., Suhr O.B., Obici L. Repurposing diflunisal for familial amyloid polyneuropathy. JAMA. 2013;310:2658. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castaño A., Helmke S., Alvarez J., Delisle S., Maurer M.S. Diflunisal for ATTR cardiac amyloidosis. Congest Hear Fail. 2012;18:315–319. doi: 10.1111/j.1751-7133.2012.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bulawa C.E., Connelly S., DeVit M. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 83.Lozeron P., Théaudin M., Mincheva Z., Ducot B., Lacroix C., Adams D. Effect on disability and safety of tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20:1539–1545. doi: 10.1111/ene.12225. [DOI] [PubMed] [Google Scholar]
- 84.Penchala S.C., Connelly S., Wang Y. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci U S A. 2013;110:9992–9997. doi: 10.1073/pnas.1300761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sant’Anna R., Gallego P., Robinson L.Z. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat Commun. 2016;7:10787. doi: 10.1038/ncomms10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams D., Gonzalez-Duarte A., O’Riordan W.D. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 87.Bumcrot D., Manoharan M., Koteliansky V., Sah D.W.Y. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butler J.S., Chan A., Costelha S. Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis. Amyloid. 2016;23:109–118. doi: 10.3109/13506129.2016.1160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon S.D., Adams D., Kristen A. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis: an analysis of the APOLLO study. Circulation. 2019;139:431–443. doi: 10.1161/CIRCULATIONAHA.118.035831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rinaldi C., Wood M.J.A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 91.Hartig R., Shoeman R.L., Janetzko A., Grub S., Traub P. Active nuclear import of single-stranded oligonucleotides and their complexes with non-karyophilic macromolecules. Biol Cell. 1998;90:407–426. [PubMed] [Google Scholar]
- 92.Lorenz P., Misteli T., Baker B.F., Bennett C.F., Spector D.L. Nucleocytoplasmic shuttling: a novel in vivo property of antisense phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 2000;28:582–592. doi: 10.1093/nar/28.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu H., Lima W.F., Zhang H., Fan A., Sun H., Crooke S.T. Determination of the role of the human RNase H1 in the pharmacology of DNA-like antisense drugs. J Biol Chem. 2004;279:17181–17189. doi: 10.1074/jbc.M311683200. [DOI] [PubMed] [Google Scholar]
- 94.Benson M.D., Waddington-Cruz M., Berk J.L. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wade W.F. Pattern Recognition Receptors and the Innate Immune Network. In: Tang W.-F., editor. Molecular Medical Microbiology (Second Edition) Vol. 1. Elsevier Ltd.; Philadelphia, Pennsylvania: 2015. pp. 449–474. [Google Scholar]
- 96.Pepys M.B., Dyck R.F., de Beer F.C., Skinner M., Cohen A.S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979;38:284–293. [PMC free article] [PubMed] [Google Scholar]
- 97.Botto M., Hawkins P.N., Bickerstaff M.C. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 98.Bodin K., Ellmerich S., Kahan M.C. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richards D.B., Cookson L.M., Berges A.C. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 100.Milani P., Gertz M.A., Merlini G., Dispenzieri A. Attitudes about when and how to treat patients with AL amyloidosis: an international survey. Amyloid. 2017;24:213–216. doi: 10.1080/13506129.2017.1370421. [DOI] [PubMed] [Google Scholar]
- 101.Dispenzieri A., Dingli D., Kumar S.K. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010;85:757–759. doi: 10.1002/ajh.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tapan U., Seldin D.C., Finn K.T. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. 2010;116:3021. doi: 10.1182/blood-2010-09-305136. [DOI] [PubMed] [Google Scholar]
- 103.Hussain A.S., Hari P., Brazauskas R. Changes in cardiac biomarkers with bortezomib treatment in patients with advanced cardiac amyloidosis. Am J Hematol. 2015;90:E212–E213. doi: 10.1002/ajh.24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wechalekar A., Foard D., Whelan C. Challenges of using NT-Probnp for response assessment in systemic AL amyloidosis — analysis of a prospective study. Blood. 2016;128:4511. [Google Scholar]
- 105.Barros-Gomes S., Williams B., Nhola L.F. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. J Am Coll Cardiol Img. 2017;10:398–407. doi: 10.1016/j.jcmg.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Phelan D., Collier P., Thavendiranathan P. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 107.Decker I., Goodman S.A., Phillips S.E., Lenihan D.J., Cornell R.F. The six-minute walk test is a valuable measure of functional change following chemotherapy for AL (light-chain) cardiac amyloidosis. Br J Haematol. 2017;177:481–483. doi: 10.1111/bjh.14585. [DOI] [PubMed] [Google Scholar]