Abstract
OBJECTIVE
To identify novel modifiable risk factors of gestational diabetes mellitus (GDM) by examining the association between prepregnancy habitual folate intake and GDM risk.
RESEARCH DESIGN AND METHODS
The study included 14,553 women in the Nurses’ Health Study II who reported at least one singleton pregnancy between the 1991 and 2001 questionnaires. Prepregnancy intakes of total folate, supplemental folate, and food folate were assessed using a food frequency questionnaire administered every 4 years. Incident GDM was ascertained from a self-reported physician diagnosis. Relative risks (RRs) of GDM were estimated using log-binomial models, with adjustment for demographic, lifestyle, and dietary factors.
RESULTS
Over the study follow-up, 824 incident GDM cases were reported among 20,199 pregnancies. Women with adequate total folate intake (≥400 μg/day) had an RR of GDM of 0.83 (95% CI 0.72, 0,95, P = 0.007) compared with women with inadequate intake (<400 μg/day). This association was entirely driven by supplemental folate intake. The RRs of GDM for 1–399, 400–599, and ≥600 μg/day of supplemental folate intake were 0.83, 0.77, and 0.70, respectively, compared with no supplemental folate intake (Ptrend = 0.002). The association between supplemental folate intake and GDM risk largely persisted after additional adjustment for intake of multivitamins and other micronutrients, as well as among women who likely planned for the pregnancy.
CONCLUSIONS
Higher habitual intakes of supplemental folate before pregnancy were significantly associated with lower GDM risk. If confirmed, these findings indicate that prepregnancy folic acid supplementation could offer a novel and low-cost avenue to reduce GDM risk.
Gestational diabetes mellitus (GDM) is a common complication in pregnancy, estimated to affect 5–9% of pregnancies in the U.S. in 2009–2010 (1). It is associated not only with adverse perinatal outcomes but also with long-term cardiometabolic risk in mothers and their offspring (2). Identifying potentially modifiable factors that contribute to the prevention of GDM may improve the health and well-being of both women and their children.
Folate is a B vitamin that occurs naturally in foods such as vegetables, fruits, and beans. Its synthetic form, folic acid, is commonly used in dietary supplements and fortified foods. Folate serves as a cofactor in one-carbon metabolism essential for nucleotide synthesis and methylation. Low folate intake leads to increased homocysteine levels, which is a risk factor for cardiovascular disease and stroke (3). Emerging evidence suggests that low folate intake and high homocysteine levels may play a role in metabolic disturbances, including insulin resistance (4), dyslipidemia (4–6), and liver damage (4,7), through compromised methylation capacity and oxidative stress, whereas folic acid supplementation may improve these metabolic parameters (6,8–10). These metabolic disturbances have been implicated in the pathogenesis of GDM. However, it is unclear whether higher folate intake is associated with lower GDM risk. Epidemiologic studies addressing this question are sparse, and findings are inconsistent (11–20). Importantly, most of the studies had a cross-sectional design and a small sample size (11–16,18), and none considered potential confounding from other aspects of diet. Our study examined prepregnancy habitual intake of folate in relation to the risk of GDM in a large prospective cohort followed over 10 years accounting for potential confounding from other aspects of diet. In a subgroup of the study sample, we also explored whether the association was modified by a common single nucleotide polymorphism, methylenetetrahydrofolate reductase (MTHFR) C677T, which leads to lower folate and higher homocysteine levels (21).
Research Design and Methods
Study Population
The Nurses’ Health Study II (NHS II) enrolled 116,678 female nurses aged 25–44 years in 1989 and followed them using biennial questionnaires, where women reported their lifestyle and disease outcomes. Response rates for each questionnaire cycle were >90%. The current study included women who reported at least one singleton pregnancy lasting >6 months. Only pregnancies that occurred after the return of the first dietary assessment in 1991 were included in the current analyses. GDM was last ascertained on the 2001 questionnaire, when most participants had passed their reproductive age. Pregnancies were excluded if the woman reported a prior GDM diagnosis, as they might consequently modify their diet and lifestyle in subsequent pregnancies. Pregnancies were also excluded if the woman had prior diagnosis of type 2 diabetes, cardiovascular disease, or cancer other than skin cancer or if she had not returned any food frequency questionnaire (FFQ) before the pregnancy, had >70 FFQ items missing, or reported improbable total energy intake (<600 or >3,500 kcal/day). A total of 20,199 pregnancies among 14,553 women were included in this study. This study was approved by the institutional review board of Partners Health Care, Boston, Massachusetts.
Dietary Assessment
Women responded to a semiquantitative FFQ (22) in 1991 and every 4 years thereafter, where they reported the frequencies in which they consumed a specific portion of each of the 131 food or food group items during the past year. They also reported their use of dietary supplements, including the brand, dose, and frequency of use. We obtained the nutrient contents of each item from a nutrient database derived from the U.S. Department of Agriculture with additional information from manufacturers (23). For dietary information collected after 1998, we used an updated database, which reflects the mandatory folic acid fortification of cereal and grain products. Intake of each nutrient, including folate, was estimated as the sum of the intake from foods and supplements. Nutrient intakes from food were adjusted for total energy intake using the nutrient residual method (24). This study evaluated folate intake from supplements and food, together (i.e., total folate) and separately, as the exposures of interest. Folate intake estimated by the FFQ has shown moderate to high correlation in previous studies with an estimate from prospectively collected diet records (r = 0.71) (25), red blood cell folate levels (r = 0.51) (26), and plasma folate levels (r = 0.63) (27). The Alternate Healthy Eating Index 2010 (AHEI-2010) was derived and used as a measure of overall dietary quality (28).
Outcome Assessment
Women reported incident GDM diagnosis on the biennial questionnaire up to 2001. GDM diagnosis was attributed to the first pregnancy when more than one eligible pregnancy was reported during the questionnaire period. An earlier study among a subsample of NHS II participants reported that 94% of self-reported GDM diagnosis was confirmed by medical record review, while among women who had a pregnancy uncomplicated by GDM, 83% reported a glucose loading test, and 100% reported frequent urine screening in pregnancy, consistent with a high degree of GDM surveillance (29). The National Diabetes Data Group criteria (30) for diagnosing GDM were widely adopted during the study follow-up period, between 1991 and 2001.
Nondietary Covariates
Women reported their frequencies engaging in common recreational activities in 1991, 1997, and 2001, from which their total physical activity was estimated. They reported their race and family history of diabetes in 1989. They also reported their height, weight, parity, smoking status, oral contraceptive use, use of ovulation induction medication, and concurrent infertility (i.e., having tried to become pregnant for >1 year without success since the last questionnaire cycle) in each biennial questionnaire, and the values from the most recent questionnaire cycle were used in the analysis. A validation study among a subsample of NHS I participants found a high correlation between self-reported weight and weight measured by a technician (r = 0.97) (31). Women’s BMI was calculated as weight in kilograms divided by the square of height in meters. Missing values in covariates were coded as a separate category.
Statistical Analysis
Participant characteristics at baseline in 1991 were presented by categories of folate intake in 1991. Differences across the categories were compared using ANOVA test for continuous variables and χ2 test for categorical variables.
Cumulative average amounts of nutrient and total energy intakes and physical activities before pregnancy were derived to reduce measurement error and to represent long-term diet (32). Relative risks (RRs) and 95% CIs of incident GDM in relation to categories of folate intake (i.e., quartiles and adequacy [<400 μg/day and ≥400 μg/day] of total folate intake, categories of supplemental folate intake [0, 1‒399, 400‒599, and 600‒2000 μg/day], and quartiles of food folate intake) were estimated using log-binomial models with generalized estimating equations and robust variance estimates. Log-Poisson models were used in instances when log-binomial models did not converge, which provides comparable estimates with wider CIs. Linear trends of GDM risk across categories of folate intake were examined by fitting the models using the median intake of each category of folate intake as a continuous variable. In a series of models, RRs and linear trends were estimated with adjustment for potential confounders, which were selected a priori, including age (months), race (white, African American, Hispanic, Asian, others), parity (0, 1, 2, ≥3), family history of diabetes (yes, no), prepregnancy BMI (<21.0, 21.0‒22.9, 23.0‒24.9, 25.0‒26.9, 27.0‒28.9, 29.0‒30.9, 31.0‒32.9, 33.0‒34.9, ≥35.0, and missing), cigarette smoking (never, past, current), alcohol use (0, 0.1‒5.0, 5.1‒9.9, ≥10 g/day), and quartiles of physical activity, total energy intake, glycemic load, and intakes of saturated fat, total fiber, and heme iron. In addition, restricted cubic spline models were used to flexibly model potential nonlinear relations between continuous folate intake and GDM risk.
To evaluate whether intakes of specific micronutrients or multivitamin use confounded the associations between folate intake and GDM risk, we performed the analyses with additional adjustments for other micronutrients (i.e., quartiles of vitamins B1, B2, B3, B5, B6, B12, A, C, and D, calcium, and magnesium) and multivitamin intake (times/week). To evaluate the possibility that women who used folate supplements may have a lower GDM risk due to a higher likelihood of pregnancy planning, we restricted the analyses to women who were likely planning for pregnancy: those who were currently married and not using oral contraceptives (n = 14,594), who concurrently used ovulation induction medications (n = 2,159), and who reported concurrent infertility (n = 1,984). To evaluate the extent to which our findings were robust to unmeasured confounders, we calculated the E-values (33) for the estimated associations between folate intake and GDM risk. E-value reflects the magnitude of associations an unmeasured confounder must have with both the exposure and the outcome to fully explain the observed association between the exposure and the outcome. For a protective exposure, the E-value can be estimated as follows (33):
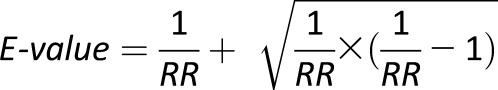 |
To examine potential effect modification by major risk factors of GDM, we stratified the analyses by age (<35 vs. ≥35 years), parity (nulliparous vs. parous), family history of diabetes (yes vs. no), and prepregnancy BMI (<25, 25‒29, ≥30 kg/m2). To test the extent to which the findings were sensitive to model specifications, we used the updated prepregnancy dietary intakes from the most recent dietary assessment instead of the cumulative average intakes. We also adjusted for AHEI dietary quality score instead of individual nutrients.
Last, we conducted an exploratory analysis to examine the association between folate intake and GDM risk by MTHFR C677T genotypes (CC/CT vs. TT) among women genotyped for studies of GDM and case-control studies of other conditions (34). This analysis is restricted to white women, as only 3.7% (n = 38) of this subsample was nonwhite. A total of 1,313 pregnancies reported by 999 women during follow-up were included in this analysis. Compared with women without genotyping information, those with genotyping information on average were slightly older and more likely to have a family history of diabetes. Other demographic and lifestyle characteristics measured in the study were similar between the two groups. Odds ratios (ORs) and 95% CIs were estimated using logistic regression models with generalized estimating equations and robust variance estimates. All analyses in subsets of the study sample were adjusted for the same set of covariates as was used in the analyses in the full sample but used fewer categories to ensure model convergence.
Results
During the study follow-up, 824 incident GDM cases were reported among the 20,199 pregnancies. At baseline in 1991, women who had higher folate intake from any source (i.e., food folate and supplemental folate) were more likely to be white, parous, and physically active and to have overall higher dietary quality and to use multivitamins, vitamin B-complex, and folic acid; they were less likely to be a current smoker and consumed less alcohol (Table 1).
Table 1.
Age-standardized characteristics of study population (n = 14,553) according to categories of folate intake in 1991 in NHS II
| Total folate* |
Supplemental folate |
Food folate |
||||
|---|---|---|---|---|---|---|
| Q1 |
Q4 |
None |
≥600 μg/day |
Q1 |
Q4 |
|
| No. of participants | 3,600 | 3,413 | 6,791 | 1,161 | 3,418 | 3,601 |
| Folate intake, μg/day, median (range) | 246 (81‒294) | 873 (698‒2,770) | 0 | 900 (600‒2,000) | 189 (46‒212) | 342 (302‒1,030) |
| Age, years | 31.8 ± 3.2 | 31.9 ± 3.3 | 31.9 ± 3.3 | 31.8 ± 3.2 | 31.3 ± 3.1 | 32.4 ± 3.3 |
| White, % | 90.8 | 94.1 | 92.4 | 93.7 | 91.0 | 93.6 |
| Family history of diabetes, % | 11.7 | 10.8 | 11.3 | 9.9 | 11.2 | 11.0 |
| Nulliparous, % | 35.2 | 32.5 | 36.6 | 15.1 | 32.0 | 43.9 |
| Current smoking, % | 12.5 | 6.9 | 10.6 | 5.4 | 11.3 | 9.2 |
| Alcohol, g/day | 3.2 ± 5.6 | 2.5 ± 4.4 | 3.2 ± 5.2 | 2.1 ± 4.2 | 2.6 ± 5.1 | 3.4 ± 5.1 |
| BMI, kg/m2 | 23.6 ± 4.8 | 23.1 ± 4.0 | 23.4 ± 4.4 | 23.5 ± 4.3 | 23.6 ± 4.8 | 22.9 ± 3.9 |
| Physical activity, MET h/week | 19.0 ± 26.2 | 26.7 ± 33.5 | 22.6 ± 29.2 | 24.4 ± 34.1 | 17.8 ± 24.3 | 30.9 ± 35.5 |
| AHEI score | 39.2 ± 9.3 | 45.9 ± 10.4 | 43.0 ± 10.4 | 43.5 ± 10.7 | 37.3 ± 8.9 | 49.8 ± 10.0 |
| Total calories, kcal/day | 1,753 ± 554 | 1,762 ± 536 | 1,780 ± 545 | 2,029 ± 556 | 1,801 ± 570 | 1,804 ± 552 |
| Carbohydrate, %E | 48.1 ± 7.5 | 52.0 ± 7.1 | 49.9 ± 7.4 | 52.0 ± 6.6 | 49.1 ± 7.9 | 53.0 ± 7.2 |
| Glycemic load† | 120 ± 22 | 126 ± 21 | 122 ± 21 | 125 ± 19 | 123 ± 24 | 127 ± 21 |
| Glycemic index† | 54.8 ± 3.4 | 53.6 ± 3.2 | 54.2 ± 3.3 | 53.7 ± 3.0 | 55.4 ± 3.3 | 52.8 ± 3.1 |
| Protein, %E | 18.8 ± 3.4 | 19.6 ± 3.4 | 19.2 ± 3.4 | 19.5 ± 3.2 | 18.3 ± 3.5 | 19.6 ± 3.5 |
| Total fat, %E | 33.4 ± 5.5 | 29.5 ± 5.3 | 31.5 ± 5.6 | 29.9 ± 5.2 | 33.2 ± 5.8 | 28.3 ± 5.1 |
| Saturated fat, %E | 12.1 ± 2.4 | 10.7 ± 2.3 | 11.3 ± 2.4 | 11.0 ± 2.2 | 12.2 ± 2.6 | 10.0 ± 2.2 |
| Monounsaturated fat, %E | 12.8 ± 2.3 | 11.0 ± 2.3 | 11.9 ± 2.4 | 11.2 ± 2.2 | 12.7 ± 2.5 | 10.5 ± 2.2 |
| Polyunsaturated fat, %E | 5.6 ± 1.3 | 5.3 ± 1.3 | 5.5 ± 1.3 | 5.2 ± 1.4 | 5.5 ± 1.4 | 5.4 ± 1.3 |
| Trans fat, %E | 1.9 ± 0.7 | 1.4 ± 0.5 | 1.6 ± 0.6 | 1.5 ± 0.5 | 1.9 ± 0.7 | 1.3 ± 0.5 |
| Cholesterol, mg/day† | 245 ± 62 | 233 ± 67 | 239 ± 65 | 236 ± 64 | 236 ± 61 | 232 ± 74 |
| Total fiber, g/day† | 15.1 ± 3.6 | 19.4 ± 6.2 | 17.8 ± 5.3 | 18.4 ± 5.3 | 14.5 ± 4.4 | 22.0 ± 6.0 |
| Heme iron, mg/day† | 1.2 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.4 |
| Vitamin A, μg/day† | 798 ± 570 | 2,795 ± 1,423 | 1,043 ± 739 | 2,799 ± 1,531 | 1,310 ± 1,046 | 2,008 ± 1,381 |
| Vitamin C, mg/day† | 136 ± 173 | 382 ± 382 | 166 ± 189 | 349 ± 320 | 176 ± 235 | 334 ± 326 |
| Vitamin E, mg/day† | 11.3 ± 33.1 | 33.4 ± 61.7 | 12.7 ± 36.3 | 30.7 ± 60.1 | 15.6 ± 33.9 | 25.9 ± 57.5 |
| Total folate (B9), μg/day† | 240 ± 40 | 966 ± 315 | 313 ± 106 | 1215 ± 301 | 415 ± 308 | 624 ± 315 |
| Food folate, μg/day† | 216 ± 40 | 288 ± 79 | 260 ± 71 | 271 ± 69 | 186 ± 25 | 358 ± 59 |
| Supplemental folate, μg/day | 3.4 ± 16 | 570 ± 316 | 0 ± 0 | 914 ± 198 | 168 ± 274 | 209 ± 289 |
| Thiamin (B1), mg/day† | 1.7 ± 2.0 | 5.5 ± 10.0 | 1.9 ± 2.2 | 5.4 ± 10.0 | 2.8 ± 5.8 | 3.8 ± 7.1 |
| Riboflavin (B2), mg/day† | 2.0 ± 1.9 | 6.3 ± 10.0 | 2.2 ± 2.1 | 6.3 ± 9.9 | 3.2 ± 4.7 | 4.3 ± 7.1 |
| Niacin (B3), mg/day† | 23.6 ± 12.8 | 50.9 ± 26.6 | 25.6 ± 13.8 | 49.5 ± 26.9 | 31.6 ± 19.5 | 38.4 ± 25.2 |
| Pantothenic acid (B5), mg/day† | 5.0 ± 4.0 | 15.7 ± 15.0 | 5.6 ± 4.4 | 13.3 ± 17.6 | 8.3 ± 13.3 | 11.4 ± 12.7 |
| Vitamin B6, mg/day† | 3.4 ± 13.4 | 12.1 ± 25.4 | 3.7 ± 13.8 | 14.9 ± 26.4 | 5.2 ± 14.9 | 8.6 ± 23.7 |
| Cobalamin (B12), μg/day† | 5.9 ± 3.7 | 16.1 ± 16.8 | 6.7 ± 4.9 | 18.0 ± 23.4 | 8.5 ± 7.0 | 11.2 ± 14.2 |
| Multivitamin, % | 7.9 | 90.8 | 2.7 | 95.4 | 42.9 | 52.3 |
| Frequency of multivitamin intake, days/week | 0.2 ± 0.9 | 6.4 ± 2.5 | 0.1 ± 0.9 | 7.3 ± 1.9 | 2.3 ± 3.2 | 2.9 ± 3.4 |
| Vitamin B-complex, % | 1.3 | 5.7 | 1.5 | 4.7 | 2.3 | 5.4 |
| Folic acid, % | 0.0 | 2.1 | 0.0 | 4.6 | 0.3 | 0.9 |
Values are means ± SD for continuous variables and percentages for categorical variables. All values except age are standardized to the age distribution of the study population. Comparisons of all characteristics across quartiles of folate intake are significant, except for family history of diabetes. Q, quartile; %E, % of energy.
*Includes food folate and folic acid from supplements and fortified food.
†Energy adjusted using residual method.
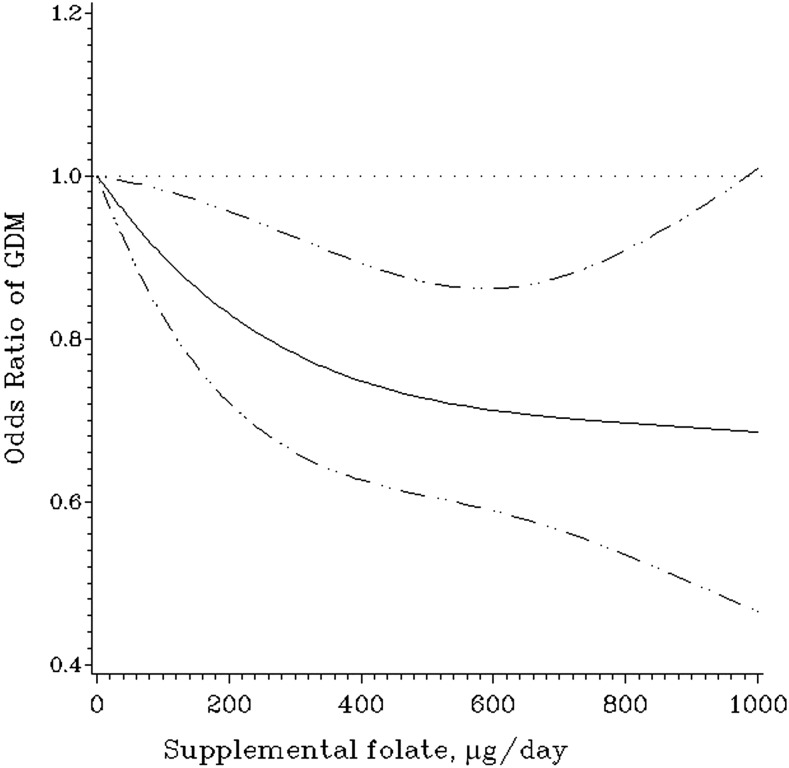
Total folate intake was inversely associated with GDM risk (Table 2). After adjustment for age, race, parity, family history of diabetes, prepregnancy BMI, cigarette smoking, alcohol use, physical activity, and intakes of total energy, saturated fat, glycemic load, total fiber, and heme iron, the RRs of GDM across increasing quartiles of total folate intake were 1.00 (reference), 1.01 (95% CI 0.84, 1.21), 0.81 (0.67, 0.99), and 0.81 (0.66, 0.98), respectively (Ptrend = 0.009). Each 100 µg/day increase in total folate intake was associated with an RR of 0.96 (0.94, 0.99) for GDM. Adequate total folate intake (≥400 µg/day) was associated with an RR of 0.83 (0.72, 0.95) for GDM (P = 0.007) compared with inadequate intake (<400 µg/day). The association between total folate intake and GDM risk was entirely driven by supplemental folate (Table 2). The RRs of GDM by supplemental folate intake were 0.83 (0.71, 0.98) for 1‒399 µg/day, 0.77 (0.64, 0.93) for 400‒599 µg/day, and 0.70 (0.52, 0.94) for ≥600 µg/day compared with no supplemental folate intake (Ptrend = 0.002). Each 100 µg/day increase in supplemental folate intake was associated with an RR of 0.95 (0.92, 0.98) for GDM. The restricted cubic spline model demonstrated linear (P = 0.21 for curvature) associations between supplemental folate intake and GDM risk (Fig. 1). Food folate intake was not associated with GDM risk (Ptrend = 0.66).
Table 2.
RRs (95% CI) of GDM according to prepregnancy folate intake
| GDM/pregnancy (n) | Model 1* | Model 2† | Model 3‡ | |
|---|---|---|---|---|
| Total folate, μg/day§ | ||||
| Q1 (81‒293) | 231/4,626 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Q2 (294‒422) | 220/4,875 | 0.88 (0.73, 1.05) | 0.97 (0.81, 1.16) | 1.01 (0.84, 1.21) |
| Q3 (423‒697) | 200/5,639 | 0.67 (0.56, 0.81) | 0.77 (0.64, 0.93) | 0.81 (0.67, 0.99) |
| Q4 (698‒2,770) | 173/5,059 | 0.65 (0.54, 0.79) | 0.75 (0.62, 0.91) | 0.81 (0.66, 0.98) |
| Ptrend | <0.001 | 0.001 | 0.009 | |
| Per 100 μg/day increase | 0.94 (0.91, 0.96) | 0.96 (0.93, 0.98) | 0.96 (0.94, 0.99) | |
| Total folate, μg/day | ||||
| Inadequate (<400) | 401/11,348 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Adequate (≥400) | 423/8,851 | 0.71 (0.62, 0.81) | 0.79 (0.69, 0.90) | 0.83 (0.72, 0.95) |
| P | <0.001 | <0.001 | 0.007 | |
| Supplemental folate, μg/day | ||||
| 0 | 405/8,650 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| 1‒399 | 230/5,831 | 0.78 (0.67, 0.92) | 0.83 (0.70, 0.97) | 0.83 (0.71, 0.98) |
| 400‒599 | 141/4,064 | 0.72 (0.59, 0.86) | 0.75 (0.62, 0.91) | 0.77 (0.64, 0.93) |
| ≥600 | 48/1,654 | 0.60 (0.45, 0.80) | 0.69 (0.51, 0.92) | 0.70 (0.52, 0.94) |
| Ptrend | <0.001 | <0.001 | 0.002 | |
| Per 100 μg/day increase | 0.94 (0.91, 0.96) | 0.95 (0.92, 0.98) | 0.95 (0.92, 0.98) | |
| Food folate, μg/day | ||||
| Q1 (46‒212) | 210/4,695 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
| Q2 (213‒254) | 226/5,335 | 0.92 (0.76, 1.11) | 1.02 (0.85, 1.23) | 1.05 (0.87, 1.27) |
| Q3 (255‒302) | 205/5,194 | 0.84 (0.70, 1.02) | 0.99 (0.82, 1.19) | 1.05 (0.86, 1.29) |
| Q4 (303‒1,030) | 183/4,975 | 0.77 (0.63, 0.94) | 0.91 (0.74, 1.11) | 1.06 (0.84, 1.33) |
| Ptrend | 0.006 | 0.28 | 0.66 | |
| Per 100 μg/day increase | 0.88 (0.79, 0.99) | 0.96 (0.87, 1.07) | 1.06 (0.94, 1.20) |
Q, quartile.
*Adjusted for age (months).
†Model 1 adjustment plus adjustment for race (white, African American, Hispanic, Asian, others), parity (0, 1, 2, ≥3), family history of diabetes (yes, no), physical activity (quartiles), prepregnancy BMI (<21.0, 21.0‒22.9, 23.0‒24.9, 25.0‒26.9, 27.0‒28.9, 29.0‒30.9, 31.0‒32.9, 33.0‒34.9, ≥35.0 kg/m2), cigarette smoking (never, past, current), and alcohol use (0, 0.1‒5.0, 5.1‒9.9, ≥10 g/day).
‡Model 2 adjustments plus adjustment for quartiles of dietary intakes of total energy, glycemic load, saturated fat, total fiber, and heme iron.
§Includes food folate and folic acid from supplements and fortified food.
Figure 1.
OR and 95% CI of GDM according to supplemental folate intake. The model was estimated using restricted cubic spline logistic regression models with three knots, taking account of age (months), race (white, African American, Hispanic, Asian, others), parity (0, 1, 2, ≥3), family history of diabetes (yes, no), physical activity (quartiles), prepregnancy BMI (<21.0, 21.0‒22.9, 23.0‒24.9, 25.0‒26.9, 27.0‒28.9, 29.0‒30.9, 31.0‒32.9, 33.0‒34.9, ≥35.0 kg/m2), cigarette smoking (never, past, current), alcohol use (0, 0.1‒5.0, 5.1‒9.9, ≥10 g/day), and quartiles of dietary intakes of total energy, glycemic load, saturated fat, total fiber, and heme iron.
In the multivariate models with additional adjustment for other micronutrients, the association between supplemental folate intake and GDM risk remained significant and generally unchanged (Supplementary Table 1). These findings suggest that the association between supplemental folate intake and GDM risk was not due to other micronutrients tested. In the multivariate model with additional adjustment for multivitamin intake, the association between supplemental folate intake and GDM risk largely persisted; the RRs of GDM by supplemental folate intake were 0.81 (95% CI 0.66, 1.00) for 1‒399 µg/day, 0.73 (0.52, 1.02) for 400‒599 µg/day, and 0.65 (0.42, 1.01) for ≥600 µg/day compared with no supplemental folate intake, although the linear trend became nonsignificant (Ptrend = 0.10) (Supplementary Table 1), whereas weekly frequency of multivitamin use was not associated with GDM risk (OR 1.01 [95% CI 0.96, 1.06], P = 0.69). This finding suggests that the major part of the association between supplemental folate intake and GDM risk was not explained by multivitamin intake. When the analysis was restricted to women who were likely planning for pregnancy—those who were married and not using oral contraceptives, who concurrently used ovulation induction medications, and who reported concurrent infertility—the association between supplemental folate and GDM risk largely remained at the same magnitude, although it was no longer significant among the latter two groups (Supplementary Fig. 1), where the sample sizes were much smaller (concurrent ovulation induction medications, n = 2,159; concurrent infertility, n = 1,984). These findings suggest that pregnancy planning was unlikely to account for the association between supplemental folate and GDM risk. The E-value for the association between the highest quartile of supplemental folate intake and GDM risk was 2.21, suggesting that a potential confounder must have relatively strong associations with both supplemental folate intake and GDM risk in order to fully explain the association between supplemental folate intake and GDM risk.
The associations of total, supplemental, and food folate with GDM risk were consistent across strata of age (<35 vs. ≥35 years), family history of diabetes (yes vs. no), and prepregnancy adiposity status (prepregnancy BMI <25, 25‒29, or ≥30 kg/m2). Although the association of total and supplemental folate intake with GDM risk appeared to be stronger among parous women than nulliparous women, the tests for multiplicative interaction were not statistically significant (data not shown). Using the updated prepregnancy dietary intakes from the most recent dietary assessment instead of the cumulative average intakes did not alter the results (Supplementary Table 2), and neither did adjusting for AHEI dietary quality score instead of individual nutrients (data not shown).
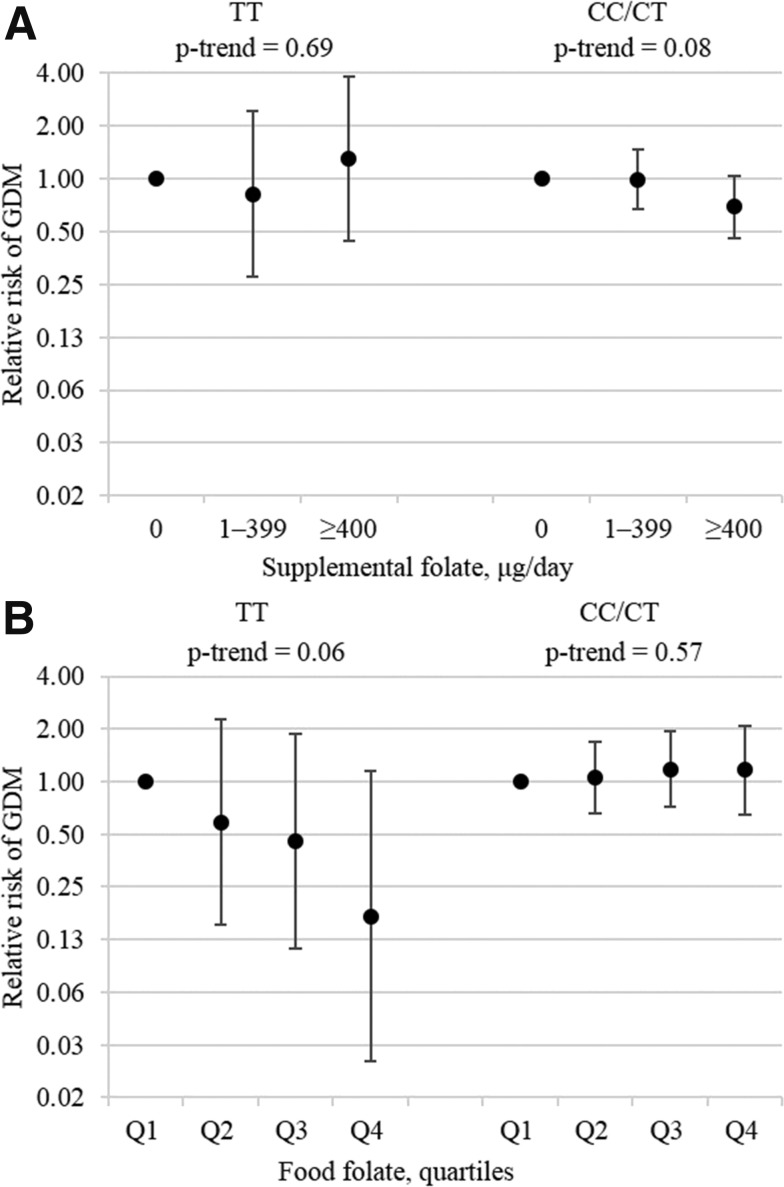
Among the subsample of 1,313 pregnancies reported by white women whose MTHFR C677T was genotyped, 1,136 (200 GDM cases) were in the CC/CT group and 177 (34 GDM cases) in the TT group. Total folate intake was not associated with GDM risk among women with either the TT genotype (Ptrend = 0.62) or the CC/CT genotype (Ptrend = 0.12). Supplemental folate intake was not associated with GDM risk among women with the TT genotype (Ptrend = 0.69); it was inversely associated with GDM risk among women with the CC/CT genotype, but the association was marginally short of significance (Ptrend = 0.08) (Fig. 2A). Food folate had a strong inverse association with GDM risk, which was close to significance among women with the TT genotype (Ptrend = 0.06), but it was not associated with GDM risk among women with the CC/CT genotype (Ptrend = 0.57) (Fig. 2B).
Figure 2.
RRs and 95% CI of GDM according to prepregnancy supplemental (A) and food folate (B) intake by MTHFR C677T genotypes among white women. The CC/CT and TT groups included 1,136 and 177 pregnancies, respectively. Models adjusted for age (months), nulliparity (yes, no), family history of diabetes (yes, no), physical activity (MET h/week), prepregnancy BMI (<25.0, 25.0‒29.9, and >30.0 kg/m2), current smoking (yes, no), alcohol use (yes, no), and dietary intakes of total energy (kcal/day), glycemic load (unit), saturated fat (% energy), total fiber (g/day), and heme iron (mg/day). Three categories of supplemental folate intake were used instead of four to avoid small numbers in some categories. Q, quartile.
Conclusions
In this large prospective cohort study, we found an inverse association between prepregnancy supplemental folate intake and GDM risk. Women who took ≥600 μg of supplemental folate per day before pregnancy had 30% lower GDM risk compared with those who did not take supplemental folate.
The Centers for Disease Control and Prevention and U.S. Preventive Services Task Force recommended that all women of childbearing age consume 400 µg of folic acid daily. In our study, adequate total folate intake (i.e., ≥400 μg/day) was significantly associated with lower risk of GDM. This association was entirely driven by folate from supplements; increasing supplemental folate intake was associated with decreasing risk of GDM even above the recommended intake of 400 µg/day. The maximum intake of supplemental folate in our study was 1,000 µg/day. In contrast, food folate was not associated with GDM risk. Folic acid, used in supplements, is more bioavailable than food folate (35); therefore, an equal quantity of folic acid would be expected to have a greater biological effect than food folate. In addition, folate intake from food was much lower than from supplements and thus may be insufficient to achieve a protective effect against GDM. Other studies have also reported supplemental folate to have stronger associations with relevant health outcomes than food folate (26,36). Importantly, the association between supplemental folate and GDM risk was not explained by multivitamin intake, other micronutrients, or pregnancy planning. Although unobserved confounding cannot be ruled out, the E-values estimated in our study suggest that a confounder must have relatively strong associations with both supplemental folate intakes and GDM risk to completely explain the observed association.
This is the first study to examine folate intake from both food and supplements in relation to GDM risk with adjustment for other major dietary factors. We are only aware of two existing studies examining folic acid supplementation before or during pregnancy in relation to GDM risk (19,20); the results were contradictory. The first study was conducted in a hospital in China including 166 women with GDM. It found that women who received folic acid supplementation intervention had a lower risk of developing GDM (19). However, the study excluded women who smoked cigarettes, drank alcohol, had chronic diseases, or took prescription medications during the perinatal period from the intervention group but not from the control group. Thus, it is unclear whether the lower risk of GDM was due to folic acid supplementation or different exclusion criteria between the two groups. The second was a population-based cohort study in one city in China including 249 women with GDM. It found that women who took folic acid supplements had a higher risk of GDM compared with women who never took vitamin supplements (20). However, it is difficult to interpret the findings, as details of the study methods and results were not reported. Several studies have examined serum or red blood cell folate levels before or during pregnancy in relation to GDM risk (11–18). Most of them reported a null association (11–14,17). However, two of these studies excluded women who took folic acid supplements (11,12). In addition, most of these studies were based on relatively small sample sizes (≤50 women with GDM) (11,12,14,15,17). Thus, they were likely underpowered to detect a moderate association. Two of the studies found a positive folate-GDM association conditional on vitamin B12 deficiency (15,16), whereas vitamin B12 deficiency is expected to be rare in our sample given that only 1% of our sample had vitamin B12 intakes below the recommended daily allowance. Overall, a comparison of the results between this and the previous studies should take into consideration differences in timing (long-term before pregnancy vs. immediately before or during pregnancy) and measurement (folate intake vs. serum or red blood cell folate or folic acid supplementation) of the exposure, as well as background levels of exposure to folate and other nutrients across different populations.
In the exploratory analysis, we found suggestive evidence that lower food folate intake was associated with an increased risk of GDM among women with the MTHFR 677TT genotype but not among those with the CC/CT genotypes. MTHFR is responsible for converting folate to the 5-methyltetrahydrofolate form that is critical for methylation reactions. The MTHFR 677T variant renders the enzyme thermoliable, and individuals with the homozygote variant (TT) have substantially lower folate levels and higher homocysteine levels compared with those with the CC genotype with the same amount of folate intake (21). Thus, individuals with the TT genotype may be more susceptible to lower food folate intake. In contrast, lower food folate intake may not affect GDM risk among women with the common C variant, which was consistent with the findings in the overall population. However, supplemental folate was not associated with GDM risk among women with the TT genotype. The lack of association between supplemental folate and GDM risk among women with TT genotype could be due to the small sample size in this exploratory analysis.
Several mechanisms may explain the inverse association between folate intake and adverse metabolic outcomes including GDM. First, inadequate folate intake leads to compromised methylation capacity and impaired phosphatidylcholine synthesis, which is essential for very low-density lipoprotein assembly and homeostasis (5,6). Consistent with this mechanism, inadequate folate intake has been shown to lead to liver steatosis in mice (37). Second, folic acid supplementation may upregulate AMPK among mice fed with a high-fat diet, improving insulin resistance and hepatic inflammation (8,9). Lastly, folic acid supplementation—directly (10) or via lowering blood homocysteine levels (7)—may protect against oxidative stress in mice, which is known to contribute to endothelial dysfunction and insulin resistance (38); among human populations, homocysteine levels have also been positively associated with GDM risk (39).
This study has several strengths. It was based on a large, prospectively followed cohort of women, which conferred adequate statistical power to detect modest associations and enabled us to establish a temporal relationship between the exposure and the outcome. We also had comprehensive assessments of dietary intake, which allowed the examination of food folate and supplemental folate separately and enabled us to adjust for confounding by other dietary factors; the repeated dietary assessment also likely reduced measurement error and potentially reflected long-term diet (32).
Several potential limitations merit discussion. First, measurement error in self-reported dietary intake was likely. However, folate intake measured from the FFQ previously showed good correlation with prospectively collected diet records (r = 0.71) (25), red blood cell folate (r = 0.51) (26), and plasma folate levels (r = 0.63) (27). In addition, due to the prospective design, such measurement error is not likely to vary with respect to GDM status. Second, as in all observational studies, residual confounding cannot be ruled out. However, we adjusted for a comprehensive list of risk factors for GDM, including physical activity and other dietary factors. We also conducted sensitivity analyses to examine potential confounding from other micronutrients, multivitamin use, and pregnancy planning. Significant associations of folate intake from supplements with GDM persisted in these sensitivity analyses. Lastly, our exploratory analysis stratified by MTHFR C677T genotype was conducted in a small subsample, conferring limited statistical power. Future studies with larger sample sizes are needed to further examine whether MTHFR polymorphisms may modify the association between folate intake and GDM risk.
In the present large prospective cohort study, we found higher prepregnancy habitual folate intake from supplements to be significantly associated with lower risk of GDM. In addition, doses of folate supplement greater than current recommendation were associated with an even lower GDM risk. Given that pregnancy concerns both women and their fetus and that GDM is only one such relevant outcome, future data both from animal models and human epidemiologic studies are warranted to determine the appropriate supplementation dose. GDM is a substantial burden on the health of mothers and children, and folic acid supplementation has been widely recommended among all women of reproductive age to prevent birth defects. Moreover, data from one recent intervention study demonstrated that promoting folic acid supplementation can be achieved through a community-based prepregnancy care program (40). If confirmed, our findings indicate that prepregnancy folic acid supplementation could offer a feasible, novel, and low-cost avenue to reduce the risk of GDM.
Supplementary Material
Article Information
Funding. The NHS II was funded by National Institutes of Health research grants DK112940, CA50385, P30 DK46200, UM1 CA176726, and R01 CA67262. M.L., G.B., J.L.M., and C.Z. were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract nos. HHSN275201000020C, HHSN275201500003C, HHSN275201300026I, and HHSN275201100002I). A.J.G. was supported by a National Institute of Environmental Health Sciences Career Development Grant (K99ES026648).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.L., S.L., and C.Z. contributed to the study design. J.E.C. and F.B.H. were involved in data collection. M.L. and S.L. analyzed data. M.L. drafted the manuscript. A.J.G. conducted technical review. C.Z. obtained funding. M.L., S.L., J.E.C., A.J.G., S.H.L., S.N.H., X.W., M.D., G.B., A.A.B., S.F.O., J.L.M., F.B.H., and C.Z. contributed to the interpretation of the results and revision of the manuscript for important intellectual content and approved the final version of the manuscript. M.L. and C.Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 30th Society for Pediatric and Perinatal Epidemiologic Research Annual Meeting, Seattle, WA, 18–20 June 2017.
Footnotes
M.L. and S.L. contributed equally as first authors.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2198/-/DC1.
S.H.L. is currently affiliated with the Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA.
References
- 1.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373:1789–1797 [DOI] [PubMed] [Google Scholar]
- 3.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002;325:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pravenec M, Kozich V, Krijt J, et al. . Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am J Hypertens 2013;26:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett 2009;583:1215–1225 [DOI] [PubMed] [Google Scholar]
- 6.da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors 2014;40:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matté C, Stefanello FM, Mackedanz V, et al. . Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci 2009;27:337–344 [DOI] [PubMed] [Google Scholar]
- 8.Sid V, Wu N, Sarna LK, Siow YL, House JD, O K. Folic acid supplementation during high-fat diet feeding restores AMPK activation via an AMP-LKB1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 2015;309:R1215–R1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buettner R, Bettermann I, Hechtl C, et al. Dietary folic acid activates AMPK and improves insulin resistance and hepatic inflammation in dietary rodent models of the metabolic syndrome. Horm Metab Res 2010;42:769–774 [DOI] [PubMed]
- 10.Sarna LK, Wu N, Wang P, Hwang SY, Siow YL, O K. Folic acid supplementation attenuates high fat diet induced hepatic oxidative stress via regulation of NADPH oxidase. Can J Physiol Pharmacol 2012;90:155–165 [DOI] [PubMed] [Google Scholar]
- 11.Guven MA, Kilinc M, Batukan C, Ekerbicer HC, Aksu T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch Gynecol Obstet 2006;274:333–337 [DOI] [PubMed] [Google Scholar]
- 12.Seghieri G, Breschi MC, Anichini R, et al. . Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metabolism 2003;52:720–723 [DOI] [PubMed] [Google Scholar]
- 13.Sukumar N, Venkataraman H, Wilson S, et al. Vitamin B12 status among pregnant women in the UK and its association with obesity and gestational diabetes. Nutrients 2016;8:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarim E, Bagis T, Kilicdag E, et al. . Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand 2004;83:543–547 [DOI] [PubMed] [Google Scholar]
- 15.Krishnaveni GV, Hill JC, Veena SR, et al. . Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009;52:2350–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr 2018;37:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell SK, Lynch J, Esterman A, McDermott R. Pre-pregnancy predictors of diabetes in pregnancy among Aboriginal and Torres Strait Islander women in North Queensland, Australia. Matern Child Health J 2012;16:1284–1292 [DOI] [PubMed] [Google Scholar]
- 18.Berglund SK, García-Valdés L, Torres-Espinola FJ, et al.; PREOBE team . Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: an observational cohort study (PREOBE). BMC Public Health 2016;16:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Jiang J, Xu M, et al. . Individualized supplementation of folic acid according to polymorphisms of methylenetetrahydrofolate reductase (MTHFR), methionine synthase reductase (MTRR) reduced pregnant complications. Gynecol Obstet Invest 2015;79:107–112 [DOI] [PubMed] [Google Scholar]
- 20.Zhu B, Ge X, Huang K, et al. . Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care 2016;39:e36–e37 [DOI] [PubMed] [Google Scholar]
- 21.Crider KS, Zhu JH, Hao L, et al. . MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr 2011;93:1365–1372 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, et al. . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 25 [Internet], 2012. Nutrient Data Laboratory Home Page, available from https://www.ars.usda.gov/ba/bhnrc/ndl. Accessed 1 October 2012
- 24.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27 [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126; discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Stampfer MJ, Colditz GA, et al. . Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med 1998;129:517–524 [DOI] [PubMed] [Google Scholar]
- 27.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993;57:182–189 [DOI] [PubMed] [Google Scholar]
- 28.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon CG, Willett WC, Carey VJ, et al. . A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997;278:1078–1083 [PubMed] [Google Scholar]
- 30.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473 [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Stampfer MJ, Rimm E, et al. . Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540 [DOI] [PubMed] [Google Scholar]
- 33.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274 [DOI] [PubMed] [Google Scholar]
- 34.Ding M, Chavarro J, Olsen S, et al. . Genetic variants of gestational diabetes mellitus: a study of 112 SNPs among 8722 women in two independent populations. Diabetologia 2018;61:1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc 2004;63:529–536 [DOI] [PubMed] [Google Scholar]
- 36.Gaskins AJ, Rich-Edwards JW, Hauser R, et al. . Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet Gynecol 2014;124:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen KE, Wu Q, Wang X, Deng L, Caudill MA, Rozen R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr 2010;140:1736–1741 [DOI] [PubMed] [Google Scholar]
- 38.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev 2006;22:423–436 [DOI] [PubMed] [Google Scholar]
- 39.Gong T, Wang J, Yang M, et al. . Serum homocysteine level and gestational diabetes mellitus: a meta-analysis. J Diabetes Investig 2016;7:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto JM, Hughes DJF, Evans ML, et al. . Community-based pre-pregnancy care programme improves pregnancy preparation in women with pregestational diabetes. Diabetologia 2018;61:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.