Highlights
-
•
Cardiac fibroblasts become activated following injury and participate in repair and remodeling of the heart.
-
•
The authors discuss the phenotypic alterations and role of fibroblasts in infarcted and failing hearts.
-
•
In failing hearts, fibroblasts may deposit ECM proteins, increasing myocardial stiffness, but may also exert protective and reparative actions.
-
•
Future studies will focus on characterization of the phenotypic heterogeneity of cardiac fibroblasts that may explain their functional diversity.
Key Words: cytokines, extracellular matrix, fibroblast, infarction, remodeling
Abbreviations and Acronyms: AT1, angiotensin type 1; ECM, extracellular matrix; FAK, focal adhesion kinase; FGF, fibroblast growth factor; IL, interleukin; lncRNA, long noncoding ribonucleic acid; MAPK, mitogen-activated protein kinase; miRNA, micro–ribonucleic acid; MRTF, myocardin-related transcription factor; PDGF, platelet-derived growth factor; RNA, ribonucleic acid; ROCK, Rho-associated coiled-coil containing kinase; ROS, reactive oxygen species; SMA, smooth muscle actin; TGF, transforming growth factor; TRP, transient receptor potential
Summary
Expansion and activation of fibroblasts following cardiac injury is important for repair but may also contribute to fibrosis, remodeling, and dysfunction. The authors discuss the dynamic alterations of fibroblasts in failing and remodeling myocardium. Emerging concepts suggest that fibroblasts are not unidimensional cells that act exclusively by secreting extracellular matrix proteins, thus promoting fibrosis and diastolic dysfunction. In addition to their involvement in extracellular matrix expansion, activated fibroblasts may also exert protective actions, preserving the cardiac extracellular matrix, transducing survival signals to cardiomyocytes, and regulating inflammation and angiogenesis. The functional diversity of cardiac fibroblasts may reflect their phenotypic heterogeneity.
Central Illustration
Most myocardial conditions are associated with “fibrosis,” expansion of the cardiac interstitium, due to deposition of extracellular matrix (ECM) proteins 1, 2, 3. In human patients with a wide variety of cardiac diseases, the extent of fibrotic changes is a strong predictor of adverse outcome. In patients with heart failure with reduced ejection fraction, and in those with heart failure with preserved ejection fraction, prominent fibrotic remodeling is associated with higher mortality, increased hospitalization rates, and an increased incidence of adverse cardiac events 4, 5, 6, 7. Moreover, in subjects with diabetes, expansion of the myocardial interstitial space is associated with mortality and with heart failure hospitalizations (8). The association between fibrosis and poor prognosis may reflect the adverse functional consequences of ECM deposition on systolic and diastolic function or the proarrhythmic effects of fibrotic myocardial lesions. However, because the adult mammalian heart lacks significant endogenous regenerative capacity, cardiac fibrosis may also reflect activation of reparative mechanisms in response to primary cardiomyocyte injury. To what extent fibrotic cardiac remodeling represents a primary myocardial disease that mediates dysfunction and causes adverse outcome remains unknown.
Fibroblasts are the main effector cells of cardiac fibrosis. The adult mammalian heart contains abundant fibroblasts that expand following injury and can produce large amounts of ECM proteins. Animal model studies have identified cardiac fibroblasts both as critical reparative cells that maintain the structural integrity of the infarcted ventricle and as cellular effectors of heart failure that deposit stiff ECM in the interstitium, reducing myocardial compliance. The functional heterogeneity of fibroblast populations, their remarkable phenotypic plasticity, and the limited information on the characteristics and properties of fibroblasts in human myocardial diseases have hampered dissection of reparative and maladaptive fibroblast actions. In this review we describe the role of fibroblasts in failing and remodeling hearts. We discuss the dynamic alterations of fibroblasts in injury and repair of the infarcted heart and their role in remodeling and dysfunction of the ventricle in conditions associated with chronic heart failure.
Fibroblasts in Cardiac Homeostasis
Fibroblasts are defined and identified on the basis of functional and morphological criteria as cells of mesenchymal origin that lack a basement membrane and are involved in the formation and maintenance of connective tissues by producing a wide range of ECM proteins (9). Although several fibroblast markers have been proposed (Table 1), their specificity is limited. Moreover, considering that resident fibroblast populations in many tissues are heterogeneous (10) and undergo dynamic phenotypic changes following injury, identification of reliable markers that label all fibroblast subsets is a major challenge. Thus, characterization of fibroblasts typically requires the combined use of fibroblast-related markers (including ECM proteins that reflect their matrix-synthetic function) and exclusion criteria reflecting the absence of expression of endothelial, hematopoietic cell and vascular mural cell–specific proteins.
Table 1.
Sensitivity and Specificity of Markers Used to Identify Cardiac Fibroblasts
| Marker | Sensitivity | Specificity |
|---|---|---|
| Vimentin | Labels all fibroblasts 180, 181. | Also expressed by other cells of mesenchymal origin (endothelial cells [182], vascular smooth muscle cells [183], etc.). |
| α-SMA | Expressed by activated myofibroblasts in fibrotic hearts 22, 41, 138. Not expressed by quiescent fibroblasts (137). | Also expressed by vascular mural cells. |
| Col1α1 | Synthesis of structural collagens is a hallmark of fibroblasts in normal and remodeling hearts 42, 141. | Although synthesis of structural collagens by cells other than fibroblasts has been reported, expression of Col1α1 in cardiac endothelial cells, immune cells, vascular smooth muscle cells, and pericytes is negligible when compared to fibroblasts (141). Because of labeling of the surrounding matrix, antibodies to collagens may be suboptimal for fibroblast identification. Col1α1-GFP reporter mice represent a robust tool for identification of fibroblasts in many organs, including the heart (42). |
| Periostin | Expressed by fibroblasts in neonatal hearts but not by fibroblasts in normal adult hearts (184). Highly expressed in activated cardiac fibroblasts after injury 185, 186. | May also be expressed by subsets of vascular smooth muscle cells (187). |
| Fibronectin ED-A | Highly expressed by activated myofibroblasts (188). | Deposited in the matrix (189). May also colocalize with macrophages, endothelial cells, and other cell types 190, 191. |
| PDGFRα | Highly expressed in cardiac fibroblasts in normal (41) and pressure-overloaded myocardium (141). | Although vascular smooth muscle cells have been reported to express PDGFRα, especially under conditions of stress (192), PDGFRα-GFP reporter lines seem to predominantly identify cells with fibroblast-like characteristics (193). |
| DDR2 | High expression in cardiac fibroblasts in normal adult hearts (194), colocalizing with vimentin and col1α1 (195). May also be expressed in various subpopulations of infarct fibroblasts and myofibroblasts (196). | DDR2 expression has been reported in activated endothelial cells (197) and in stretched vascular smooth muscle cells (198). It is unclear whether this affects the specificity of DDR2 for fibroblasts in injured and remodeling hearts. |
| Antigen recognized by MEFSK4 | The MEFSK4 antibody labels through flow cytometry almost all PDGFRα+, Col1α1+ cardiac fibroblasts (14). No antibodies are available for immunohistochemistry. | MEFSK4 has been reported to label a small subpopulation of pericytes (14). |
| Cluster of differentiation 90 (Thy1) | Identifies a subpopulation of fibroblasts in the normal and remodeling myocardium 14, 141, 199, 200. | Also expressed by immune cells, lymphatic endothelial cells, and pericytes (201). |
| Sca1 | Identifies a subpopulation (∼60%) of PDGFRα+, Col1α1+ fibroblasts in the murine heart (14). | Lacks specificity. In Sca1-GFP reporter mice, Sca1 expression colocalized with endothelial and pericyte markers (202). |
| Tcf21 | Labels the majority of fibroblast-like cells in normal myocardium (19). In infarcted and pressure-overloaded hearts, accumulation of Tcf21+ fibroblast-like cells is noted; however, according to a single report, Tcf21 may not label activated α-SMA+ myofibroblasts (203). | Relatively specific for fibroblast populations. Not expressed by immune cells (CD45+) (203), endothelial cells, and vascular smooth muscle cells (19). |
| FSP1 | No expression in fibroblasts in the normal adult myocardium. In the infarcted and pressure-overloaded heart, there is a marked expansion of FSP1+ cells. The majority of these cells cannot be identified as α-SMA+ myofibroblasts 141, 184. | Lacks specificity. The majority of FSP1+ cells in injured and remodeling hearts are endothelial cells, macrophages, and vascular smooth muscle cells 184, 204. |
| FAP | Not expressed in normal cardiac fibroblasts (174). Labels many activated fibroblasts in infarcted rat hearts and in human myocardial samples from patients with post-infarction heart failure (205). | Specific for activated fibroblasts. However, in human failing hearts, FAP expression has been reported in small populations of inflammatory cells and endothelial cells (205). |
Col1α1 = collagen 1α1; DDR2 = discoidin domain receptor 2; FAP = fibroblast-activation protein; FSP1 = fibroblast-specific protein 1; GFP = green fluorescent protein; PDGFRα = platelet-derived growth factor α; Sca1 = stem cell antigen–1; SMA = smooth muscle actin; Tcf21 = transcription factor 21.
The myocardium contains a large population of resident fibroblasts enmeshed within the ECM network 11, 12. For many years, fibroblasts were considered the most abundant noncardiomyocytes in the adult mammalian myocardium. A study using flow cytometry in adult mice identified 27% of myocardial cells as discoidin domain–containing receptor 2–positive fibroblasts and only 7% of the cells as CD31+ endothelial cells (13), a finding quite surprising considering the high vascular content of the mammalian heart. In contrast, a more recent study using a combination of fibroblast reporter mouse models and cell-specific antibodies suggested that cardiac fibroblasts represent <20% of noncardiomyocytes and are greatly outnumbered by endothelial cells (which represent more than 60% of noncardiomyocytes) (14). Differences in the strategies used for cardiac cell isolation, and variability in the sensitivity and specificity of the methodological approaches used for cellular identification, may account, at least in part, for conflicting results in various investigations. Moreover, the relative numbers of various interstitial cell populations in the myocardium are also dependent on the age, sex, and species of the subjects studied. It should be emphasized that most of our knowledge on the characteristics of cardiac fibroblasts is based on studies in rodents, and relatively little is known regarding the density, phenotype, and distribution of fibroblasts in normal human hearts.
What is the function of resident fibroblasts in normal mammalian hearts? In the developing myocardium, cardiac fibroblasts have been suggested to regulate cardiomyocyte proliferation through a fibronectin/β1-integrin–mediated pathway (15). In adult hearts, normal cardiac function may require interactions between cardiomyocytes and the surrounding ECM. Cardiac fibroblasts, enmeshed into the endomysium and perimysium, may play an important role in regulation of the synthesis and turnover of ECM components, thus preserving the structural integrity of the ventricle 16, 17, 18. Mice with global germline loss of transcription factor 21, which is essential for cardiac fibroblast development, had greatly decreased collagen levels in the cardiac interstitium and exhibited dysmorphic hearts that lacked a distinct apex (19). Although these findings are consistent with an important role of fibroblasts in cardiac development, the consequences of fibroblast depletion on cardiac homeostasis in adult mice have not been investigated.
In addition to their critical role in the formation of the cardiac ECM network, fibroblasts may also contribute to cellular communication in the cardiac syncytium. Given their strategic location in the interstitium, cardiac fibroblasts have been suggested to facilitate communication between myocardial layers (20). Cardiac fibroblasts express high levels of connexins (connexin-40, connexin-43, and connexin-45) and establish functional gap junctional channels with neighboring cardiomyocytes, modulating their electrophysiological properties (21). Thus, fibroblasts may act as electric couplers of myocytes from different regions that would normally be isolated by connective tissue, contributing to the synchronization of the contraction.
Phenotypic Changes and Role of Cardiac Fibroblasts in the Infarcted Myocardium
Fibroblasts exhibit remarkable phenotypic plasticity and undergo dramatic alterations in their gene expression profile and functional properties in response to mechanical stress or to stimulation with soluble mediators. In vitro, cardiac fibroblasts cultured in the low-tension environment of a collagen-based pad have dendritic morphology, synthesize low levels of collagen, and have negligible expression of myofibroblast markers, such as α-smooth muscle actin (SMA) (22). In contrast, when cultured in plates, fibroblasts undergo conversion to myofibroblasts, exhibiting activation of mechanosensitive signaling pathways that trigger incorporation of α-SMA into stress fibers and induce synthesis of ECM proteins. In vivo, cardiac fibroblasts respond to changes in their microenvironment by acquiring a wide range of phenotypic profiles, thus serving as inflammatory, matrix-synthetic, or proangiogenic cells depending on the context (Central Illustration).
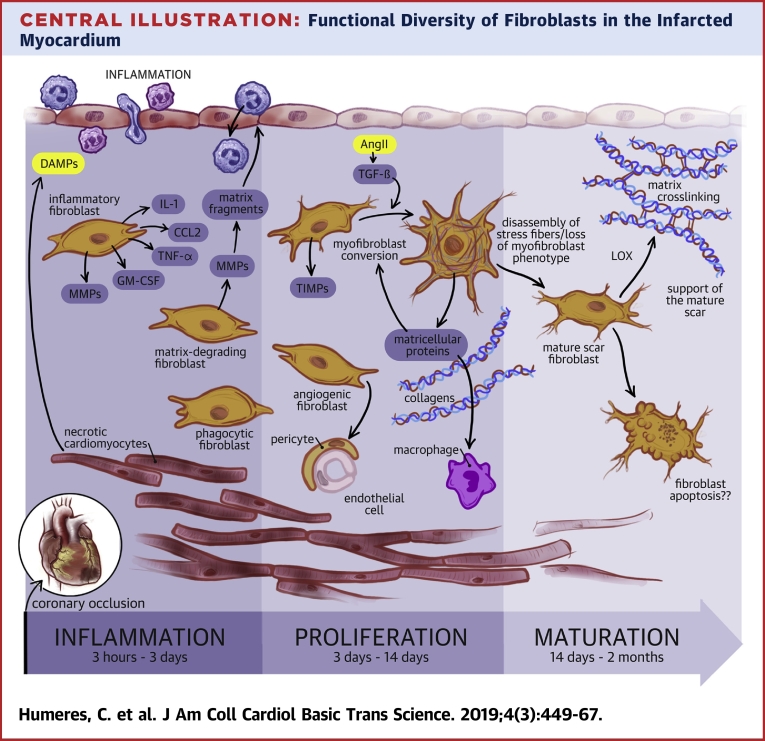
Central Illustration.
Functional Diversity of Fibroblasts in the Infarcted Myocardium
In the dynamic environment of the infarcted heart, cardiac fibroblasts expand, undergo phenotypic changes, and are implicated in a wide range of functions. Coronary occlusion causes death of cardiomyocytes in the area of injury. During the inflammatory phase of infarct healing, Damage-Associated Molecular Patterns (DAMPs) released by dying cells activate a pro-inflammatory phenotype in cardiac fibroblasts that secrete cytokines (such as IL-1, TNF-α, and GM-CSF), and chemokines (such as CCL2) contributing to recruitment and activation of leukocytes. Cytokine-stimulated fibroblasts also secrete matrix metalloproteinases (MMPs), promoting extracellular matrix degradation and release of pro-inflammatory matrix fragments. Some studies have suggested that infarct fibroblasts may also function as phagocytic cells; however, considering the abundance of macrophages in the healing infarct the relative contribution of “phagocytic fibroblasts” remains unclear. Clearance of the infarcted heart from dead cells stimulates anti-inflammatory signals, leading to suppression of inflammation and transition to the proliferative phase of infarct healing. Fibroblasts expand, predominantly through recruitment of resident populations and undergo myofibroblast conversion, incorporating α-SMA into cytoskeletal stress fibers. Activated myofibroblasts are the main matrix-synthetic cells in the infarcted heart and produce both structural extracellular matrix proteins and matricellular macromolecules. In addition to their contribution in matrix production, fibroblast populations may also contribute to regulation of the angiogenic response and may regulate macrophage phenotype. During scar maturation fibroblasts exhibit disassembly of α-SMA-decorated stress fibers, and may produce matrix-crosslinking enzymes such as lysyl-oxidases (LOX). Reduction of fibroblast numbers in mature scars has been suggested to involve activation of apoptosis. The molecular basis for the phenotypic transitions of cardiac fibroblasts in the phases of infarct healing remains poorly understood. The functional diversity of fibroblasts in the infarcted heart may reflect sequential activation of distinct fibroblast subpopulations, or may result from coordinated responses of the fibroblasts to the dynamic changes in their microenvironment.
In myocardial infarction, sudden occlusion of a coronary artery results in the death of up to 1 billion cardiomyocytes, triggering an intense inflammatory reaction (23). Because the massive loss of cardiomyocytes overwhelms the extremely limited regenerative potential of the adult mammalian heart, the infarcted myocardium heals through formation of a scar. Thus, repair of the infarcted heart is dependent on a well-orchestrated cellular response, composed of 3 distinct but overlapping phases. During the inflammatory phase, innate immune activation in response to release of damage-associated molecular patterns by dying cardiomyocytes and degraded ECM triggers cytokine and chemokine induction and recruits leukocytes that clear the infarct from necrotic and apoptotic cells and remove matrix debris (24). Macrophages phagocytosing apoptotic cells undergo transition to an anti-inflammatory phenotype, mediating suppression of inflammation and activation of a reparative program that orchestrates the proliferative phase of cardiac repair, characterized by expansion of myofibroblasts and vascular cells. The maturation phase follows and is associated with quiescence of fibroblasts, recruitment of mural cells by infarct neovessels, and formation of a crosslinked collagenous scar (25). During the 3 phases of infarct healing, cardiac fibroblasts undergo rapid phenotypic transitions from quiescence to a pro-inflammatory and matrix-degrading phenotype to a matrix-synthetic myofibroblast phenotype, only to revert to quiescence as the scar matures. Emerging evidence suggests that fibroblasts do not simply follow the changes in their microenvironment but serve as critical regulators of the cellular events in every phase of cardiac repair (26).
The fibroblasts in the inflammatory phase of infarct healing
Fibroblasts are capable of producing large amounts of proinflammatory cytokines and chemokines in response to stimulation with reactive oxygen species (ROS), Toll-like receptor ligands, or interleukin (IL)-1β 27, 28, 29. During the early post-ischemic phase, interstitial fibroblasts may sense damage-associated molecular patterns released by dying cardiomyocytes, activating a proinflammatory program (Figure 1). Considering that several other cell types, including cardiomyocytes, endothelial cells, immune cells, and vascular mural cells, can also secrete proinflammatory mediators (30), the relative role of resident cardiac fibroblasts as inflammatory cells remains unclear. In vivo studies have suggested that infarct fibroblasts may exhibit activation of the NRLP3 inflammasome 31, 32, thus serving as an important source of active IL-1β, a critical proinflammatory cytokine in the infarcted myocardium (33). A recent study suggested that fibroblasts may stimulate leukocyte recruitment in the infarcted myocardium by secreting large amounts of granulocyte/macrophage colony-stimulating factor (34). To what extent proinflammatory fibroblasts also contribute other chemokines or cytokines to the infarct environment remains unknown. Cytokine-activated proinflammatory fibroblasts also secrete proteases that play an important role in clearance of the infarct from matrix debris (35). Associative data have suggested that in addition to their role as proinflammatory and matrix-degrading cells, fibroblasts may protect cardiomyocytes from ischemic injury (36). The molecular signals responsible for the prosurvival actions are unclear.
Figure 1.
Fibroblasts in the Inflammatory Phase of Infarct Healing
During the inflammatory phase of infarct healing, cardiac fibroblasts secrete proinflammatory mediators and matrix-degrading proteases. Damage-associated molecular patterns (DAMPs) released by necrotic cells and matrix fragments activate Toll-like receptor signaling in cardiac fibroblasts. Proinflammatory cytokines (such as interleukin [IL]–1β and tumor necrosis factor [TNF]–α) released by endothelial cells, immune cells, and cardiomyocytes and activation of reactive oxygen species (ROS) accentuate fibroblast inflammatory activity. IL-1/IL-1RI signaling has been suggested to reduce α-smooth muscle actin (α-SMA) expression, preventing myofibroblast conversion. Cytokines and chemokines (such as IL-1β, TNF-α, IL-6, and granulocyte/macrophage colony-stimulating factor [GM-CSF]) secreted by activated fibroblasts may contribute to the recruitment of leukocytes, whereas protease release may promote matrix degradation. Considering that several other cell types are capable of secreting inflammatory mediators, the relative contribution of fibroblasts is unclear. The cartoon was designed using Servier Medical Art (https://smart.servier.com). DNA = deoxyribonucleic acid; HMGB1 = high-mobility group protein B1; MMP = matrix metalloproteinase; TNFR = tumor necrosis factor receptor.
The role of fibroblasts in the proliferative phase of infarct healing
The potential role of infarct fibroblasts in phagocytosis and suppression of inflammation
Activation of the post-infarction inflammatory reaction is followed by rapid suppression of proinflammatory gene synthesis and subsequent resolution of the leukocytic infiltrate, marking the transition to the proliferative phase of infarct healing. Phagocytosis of apoptotic cells plays a key role in downmodulation of inflammation, stimulating release of anti-inflammatory signals, such as IL-10 and transforming growth factor (TGF)-β. To what extent fibroblasts participate in repression and resolution of post-infarction inflammation remains unknown. A recent study suggested that activated fibroblasts may serve as phagocytes, engulfing apoptotic cells from the infarct zone (37). Considering the abundance of phagocytic macrophages in the healing infarct (38), the relative contribution of fibroblasts in clearance of dead cells is unclear. Whether any phagocytic actions of fibroblasts are accompanied by secretion of IL-10 or TGF-β and by acquisition of an anti-inflammatory phenotype has not been investigated.
Expansion of activated fibroblasts in the infarcted myocardium
Expansion of cardiac fibroblasts and acquisition of a matrix-synthetic myofibroblast phenotype are prominent features of the proliferative phase of infarct healing. In addition to the abundant resident cardiac fibroblasts that can respond to activating signals, several other cell types have been proposed as important cellular sources for the expanding infarct myofibroblast population. Endothelial cells can undergo endothelial-to-mesenchymal transition in response to growth factor stimulation, acquiring a matrix-synthetic phenotype. Hematopoietic fibroblast progenitors can also contribute to the expansion of activated fibroblasts in injury sites. Pericytes and vascular smooth muscle cells can undergo fibroblast conversion, contributing to fibrotic responses. Over the past 10 years, studies combining bone marrow transplantation experiments, parabiosis, and lineage tracing strategies have attempted to explore the cellular origin of fibroblasts in the infarcted heart (Table 2). Although earlier investigations had suggested important contributions of endothelial cells and hematopoietic progenitors to the infarct myofibroblast population 39, 40, recent studies using lineage-tracing approaches with several different Cre drivers demonstrated that resident cardiac fibroblasts are the main source for activated myofibroblasts in the infarcted heart, with much smaller contributions of endothelial and hematopoietic cells 41, 42. It should be emphasized that the studies investigating the origin of infarct myofibroblasts have several limitations that may explain, at least in part, conflicting findings (43). First, the use of nonspecific fibroblast markers or Cre drivers with questionable specificity may limit the reliability of the findings. For example, some of the studies suggesting major contributions of endothelial cells to the myofibroblast population were based on nonspecific Cre drivers (such as the Tie1-Cre line) (44). Identification of fibroblasts represents another major challenge due to the absence of specific markers (Table 1). Thus, in many cases, conclusions regarding conversion of other lineages into fibroblasts are based on immunofluorescence data showing expression of nonspecific markers, such as fibroblast-specific protein–1 or α-SMA 40, 44.
Table 2.
Cellular Origin of Fibroblasts in Myocardial Infarction
| Reference # | Main Conclusions of the Study | Strategies Used to Study the Cellular Origin of Infarct Fibroblasts | Markers Used for Fibroblast Identification |
|---|---|---|---|
| (41) | Activated fibroblasts in infarcted and remodeling hearts are derived from Tcf21+ tissue-resident fibroblasts. Endothelial cells, myeloid cells, and smooth muscle cells do not significantly contribute to the activated fibroblast population. | Lineage-tracing analysis using Cre drivers to study the fate of resident cardiac fibroblasts (Tcf21MCM), activated myofibroblasts (PostnMCM), myeloid cells (LysMCre), endothelial cells (Cdh5Cre), and vascular smooth muscle cells (Myh11CreERT2). | Vimentin, PDGFRα, α-SMA, FSP1 |
| (137) | Resident Tcf21+ cardiac fibroblasts become activated and proliferative within 2–4 days after nonreperfused infarction, then undergo myofibroblast conversion, secreting large amounts of ECM proteins. Finally in mature scars, fibroblasts show reduced expression of α-SMA and express tendon genes. | The fate of fibroblasts was studied using 3 different lineage-tracing models: Tcf21MCM/+ (resident cardiac fibroblasts), Postn-MCM (activated fibroblasts), and Acta2-CreERT2 (activated myofibroblasts). | Vimentin, α-SMA |
| (206) | Epicardial-derived resident mesenchymal cells, not bone marrow cells, are the main source of fibroblasts in the infarcted heart. | WT1Cre mice were used for permanent genetic tracing of epicardium-derived cells. Mice reconstituted with RFP+ bone marrow cells were used to study bone marrow origin. | Collagen I, FSP1, DDR2, CD90, α-SMA |
| (207) | Following nonreperfused infarction, subsets of epicardium-derived cells differentiate into fibroblasts and smooth muscle cells. | Lineage tracing of epicardium derived cells by using inducible WT1CreERT2 mice. | FSP1, procollagen I, collagen III, fibronectin, α-SMA |
| (42) | The vast majority of activated collagen-producing fibroblasts (∼96%) in nonreperfused infarcts are derived from epicardial cells. Hematopoietic, bone marrow lineages, and endothelial cells do not significantly contribute to the fibroblast population. | Lineage-tracing models to label epicardial cells (Wt1-Cre), endothelial cells (Tie2-Cre), hematopoietic cells (Vav-Cre). Transplantation with RFP+ bone marrow to study bone marrow origin. | Breeding with collagen 1α1-GFP reporter mice, α-SMA |
| (39) | Post-infarction, 35%–40% of α-SMA+ mesenchymal cells are derived from endothelial cells, possibly through endothelial-to-mesenchymal transition. | The endothelial cell-specific endothelial-SCLCreERT mouse line was used to trace endothelial cells. | α-SMA expression, Snail, FSP1, vimentin and collagen I mRNA expression |
| (208) | 24% of myofibroblasts in nonreperfused myocardial infarcts originate from bone marrow cells. | Transplantation with EGFP-tagged bone marrow, or bone marrow from proCol1α2 gene-driven luciferase or β-Gal reporter mice. | αSMA staining, β galactosidase activity in pro-Col1α2-driven chimeric mice |
| (40) | 25% of vimentin+ fibroblasts and 57% of α-SMA+ myofibroblasts in nonreperfused infarcts are derived from bone marrow cells. | Transplantation with bone marrow from EGFP reporter mice to document bone marrow origin. | α-SMA, vimentin |
| (209) | Blood-derived cells contributed to the myofibroblast population. Treatment with G-CSF enhances recruitment of bone marrow–derived myofibroblasts. | Transplantation of GFP+ bone marrow. | Vimentin, α-SMA |
| (210) | Gli-1+ perivascular cells contribute to the myofibroblast population in the infarcted myocardium (approximately 60% of activated myofibroblasts are derived from Gli1+ cells). | Lineage tracing using Gli1CreERT2 mice. | Collagen I, PDGFRα, α-SMA |
ECM = extracellular matrix; EGFP = enhanced green fluorescent protein; G-CSF = granulocyte-colony stimulating factor; mRNA = messenger ribonucleic acid; other abbreviations as in Table 1.
Second, the timing of reperfusion may have dramatic effects on the fate of resident myocardial cells and on recruitment of blood-derived progenitors, thus altering the relative contribution of various cell types to the expansion and activation of fibroblasts. Early reperfusion results in accentuated and accelerated leukocyte influx and could also augment infiltration of the infarct zone with bone marrow–derived fibroblast progenitors. Prolonged coronary occlusion, in contrast, may cause ischemic death of large numbers of interstitial and vascular cells in the infarct zone, thus reducing their relative contribution to myofibroblast expansion.
Third, considering that all lineage-tracing studies were performed in mouse models, there is practically no information on the origin of myofibroblasts in human myocardial infarction.
Myofibroblast migration in the border zone of the infarct
In the healing infarct, fibroblasts undergo conversion to myofibroblasts, expressing contractile proteins, such as α-SMA and the embryonic isoform of smooth muscle myosin heavy chain, synthesizing periostin, and secreting large amounts of ECM proteins 22, 45. In animal models of myocardial infarction, myofibroblasts are localized predominantly in the border zone, forming well-organized arrays (46). Fibroblast migration to the infarct border zone may be mediated by growth factors, such as TGF-β and fibroblast growth factors (FGFs) 47, 48, and by proinflammatory cytokines, such as IL-1β, tumor necrosis factor–α, and cardiotrophin-1 27, 49. It has also been suggested that chemokines, such as monocyte chemoattractant protein–1/C-C motif chemokine ligand 2, may promote the migration of bone marrow–derived fibroblast progenitors in injured tissues. Considering the robust evidence documenting no significant contribution of hematopoietic cells on infarct fibroblast populations (42), the potential significance of this mechanism is unclear. C-C motif chemokine ligand 2 may contribute to fibrosis through recruitment and activation of fibrogenic monocytes and macrophages 50, 51 rather than through recruitment of circulating fibroblast progenitors or modulation of fibroblast function. A recently published investigation identified a subpopulation of atypical monocytes with a critical contribution in bleomycin-induced pulmonary fibrosis (52). Whether fibrogenic monocyte subsets with distinct phenotypic profiles are recruited in remodeling hearts has not been investigated. Other members of the chemokine family, such as the CXC chemokine interferon-γ–inducible protein–10/CXCL10, may inhibit fibroblast migration, serving as an endogenous inhibitory signal that restrains the fibrotic response following injury 53, 54.
Fibroblast migration is dependent on the continuous formation and disruption of adhesive interactions between fibroblast surface proteins and the surrounding cardiac ECM. Migration involves well-orchestrated activation of integrins on cardiac fibroblast cytoplasmic membrane (55), linked with the production of proteases that degrade the matrix (56) and expression of specialized matrix proteins that locally activate or transduce growth factor–mediated signals (57).
The effects of neurohumoral pathways on activation of infarct myofibroblasts
After migrating to the infarct border zone, fibroblasts acquire a proliferative matrix-synthetic phenotype through the local induction of fibrogenic mediators (Figure 2). Neurohumoral pathways are critically implicated in regulation of fibroblast function following myocardial infarction. Potent activation of the renin-angiotensin-aldosterone system in infarcted hearts (58) stimulates myofibroblast conversion, proliferation, and ECM protein synthesis both through direct actions, and via induction of TGF-β 59, 60. The fibrogenic actions of angiotensin II are mediated predominantly through engagement of the angiotensin type 1 (AT1) receptor 61, 62, 63, 64. In contrast, the AT2 receptor may exert inhibitory functions, suppressing fibroblast proliferation and ECM synthesis (65), and has been suggested to restrain profibrotic signaling (66). Although extensive in vivo evidence supports the profibrotic actions of AT1 signaling in experimental models of myocardial infarction (67), to what extent the prosurvival effects of angiotensin-converting enzyme inhibition and AT1 blockade in patients with acute myocardial infarction are mediated through attenuation of angiotensin-induced fibrosis remains unknown.
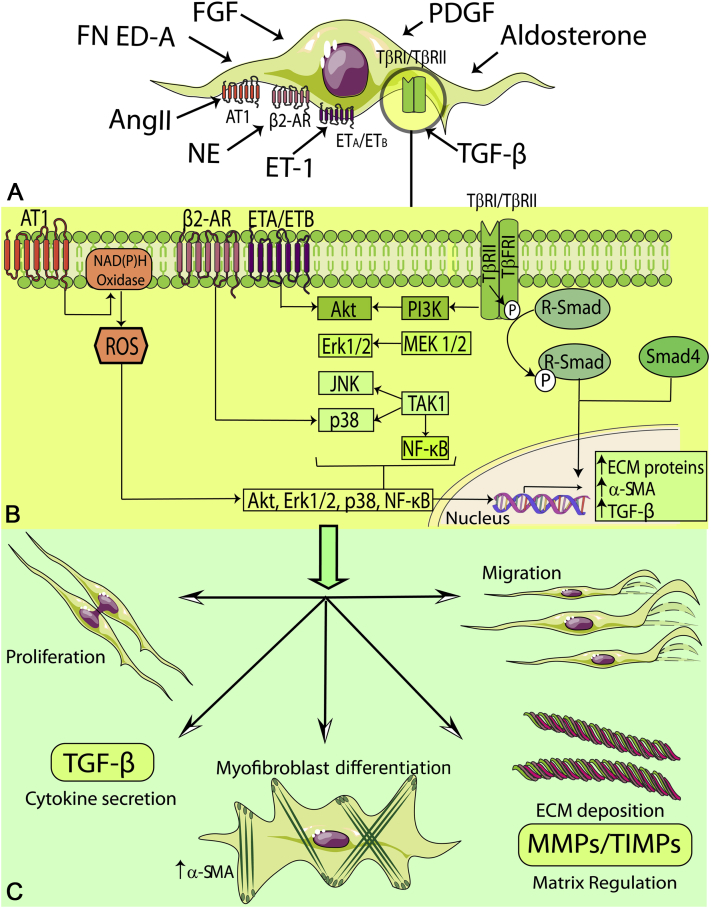
Figure 2.
Fibroblasts in the Proliferative Phase of Infarct Healing
During the proliferative phase of infarct healing, fibrogenic growth factors and neurohumoral mediators trigger myofibroblast conversion and stimulate fibroblast proliferation, migration, and activation. A wide range of fibrogenic mediators, induced during the proliferative phase of cardiac repair, are implicated in myofibroblast activation. Neurohumoral mediators, such as angiotensin II (AngII), aldosterone, and norepinephrine (NE), growth factors (transforming growth factor [TGF]-βs, fibroblast growth factors [FGFs], platelet-derived growth factors [PDGFs]), and specialized matrix proteins, such as ED-A fibronectin and matricellular proteins cooperate to activate intracellular signaling pathways that promote myofibroblast conversion and proliferation and modulate expression of extracellular matrix (ECM) proteins and of genes associated with matrix metabolism. The cartoon was designed using Servier Medical Art (https://smart.servier.com). AR = adrenergic receptor; ET = endothelin; MMP = matrix metalloproteinase; NF = nuclear factor; ROS = reactive oxygen species; SMA = smooth muscle actin; TIMP = tissue inhibitor of metalloproteinase.
Both animal model studies and investigations in human patients suggest that aldosterone contributes to myocardial fibrosis (68). Mineralocorticoid receptor inhibition attenuated fibrosis in experimental models of nonreperfused myocardial infarction (69) and reduced levels of biomarkers reflecting collagen synthesis in patients with acute myocardial infarction (70). The cellular basis for these effects remains unclear. Aldosterone-mediated signaling has been suggested to modulate the phenotype of all cells involved in cardiac repair, driving macrophages toward a fibrogenic phenotype (71), activating T cells (72), inducing cardiomyocyte-derived fibrogenic signals (73), and directly stimulating fibroblast proliferation and collagen synthesis 74, 75.
The adrenergic system is also prominently activated following myocardial infarction. Stimulation of β2-adrenergic receptor signaling directly stimulates proliferation of cardiac fibroblasts through effects that may involve p38 mitogen-activated protein kinase (MAPK) signaling 76, 77, 78. Chronic pharmacological stimulation or transgenic overexpression of β-adrenergic receptor causes myocardial fibrosis (79); whether fibrotic remodeling is due to direct activation of fibroblasts or reflects reparative fibrosis in response to cardiomyocyte death remains unknown. Activation of G protein-coupled receptor kinase 2 in cardiac fibroblasts may transduce, at least in part, the fibrogenic actions of β-adrenergic receptors in the infarcted myocardium 80, 81.
The role of TGF-βs in fibroblast activation
The fibrogenic growth factor TGF-β is a central mediator in myofibroblast conversion following myocardial infarction. All 3 TGF-β isoforms are markedly up-regulated in the infarcted heart; TGF-β1 and TGF-β2 are induced earlier, whereas TGF-β3 exhibits a late peak and a prolonged time course of expression (82). Whether TGF-β isoforms play distinct roles following infarction remains unknown. Most myocardial cell types are capable of secreting TGF-β as an inactive complex bound to the latency-associated peptide (forming the small latent complex), and latent TGF-β-binding protein (forming the large latent complex). Several mediators, including ROS, cell surface integrins, proteases, and matricellular proteins (such as thrombospondin-1), have been implicated in generation of active TGF-β in the healing infarct 83, 84, 85, 86, 87. The active TGF-β dimer binds and sequentially transphosphorylates type II and type I TGF-β receptors, activating downstream canonical signaling pathways through receptor-activated Smad proteins (R-Smads-Smad2/3) and Smad-independent pathways (88). Both Smad-dependent and non-Smad pathways have been implicated in α-SMA and ECM protein up-regulation, triggering myofibroblast conversion and activation in healing myocardial infarction (89). In the infarcted heart, activation of Smad3-dependent signaling in cardiac fibroblasts plays a crucial role in formation of well-organized fibroblast arrays in the infarct border zone by inducing integrin expression (90).
Endothelin-1
The endothelium-derived peptide endothelin-1 is a potent vasoconstrictor but has also been reported to exert fibroblast-activating effects. Endothelin-1 secreted by TGF-β- or angiotensin II–stimulated endothelial cells may stimulate fibroblast proliferation, myofibroblast conversion, and ECM synthesis through activation of the endothelin-A receptor and downstream Rac/PI3K/Akt signaling pathways (91). In vivo, cardiac-specific endothelin-1 overexpression caused myocardial fibrosis associated with biventricular systolic and diastolic dysfunction (92), whereas endothelin antagonism attenuated adverse fibrotic remodeling following myocardial infarction (93).
The role of FGFs and platelet-derived growth factors in the activation of infarct fibroblasts
FGF2 may stimulate a proliferative phenotype in infarct fibroblasts through activation of p38 MAPK and protein kinase Cδ signaling pathways (94). In vivo, FGF2-knockout mice had reduced proliferation of infarct fibroblasts, associated with decreased ECM synthesis. These defects resulted in impaired scar formation and infarct expansion. In contrast, FGF2 overexpression increased fibroblast proliferation and accentuated ECM deposition (95). PDGFs and PDGF receptors (PDGFRs) are also overexpressed in the infarcted myocardium and may play role in regulation of fibroblast function (96). Activation of PDGFRα signaling may promote fibroblast activation; in contrast, PDGFRβ actions are important for maturation of the infarct vasculature. In vitro, PDGF-AA potently stimulates cardiac fibroblast proliferation and ECM protein synthesis (97). In vivo, PDGFRα and PDGFRβ neutralization reduced collagen deposition in reperfused myocardial infarcts (96); however, PDGFRβ inhibition also prevented the recruitment of mural cells by infarct neovessels, perturbing maturation of the infarct vasculature (96).
The role of specialized ECM proteins in the regulation of infarct fibroblast phenotype
Tissue injury is associated with induction of specialized matricellular proteins that do not play a primary structural role but regulate cellular responses by transducing or modulating signaling cascades. Fibroblasts are important cellular targets of specialized ECM proteins. The ED-A domain of fibronectin plays an important role in conversion of fibroblasts into myofibroblasts 98, 99, 100. Moreover, several matricellular macromolecules, including thrombospondins (87), osteopontin (101), tenascin-C (102), secreted protein acidic and rich in cysteine (103), periostin (104), and osteoglycin (105), have been implicated in activation of myofibroblasts in healing infarcts. Nonfibrillar collagens, such as collagen VI, are also involved in the activation of a myofibroblast phenotype following myocardial infarction (106). Most specialized matrix proteins act by binding to fibroblast surface molecules, such as integrins and syndecans, or by modulating activity of growth factors and proteases.
Intracellular molecular pathways involved in fibroblast activation
In the healing infarct, induction of fibrogenic stimuli, such as damage-associated molecular patterns, cytokines and growth factors, neurohumoral mediators, and matricellular proteins cooperate to stimulate intracellular cascades involved in myofibroblast conversion, migration, proliferation, and induction of a matrix-synthetic transcriptional program (107). Experimental studies have identified several essential intracellular pathways that contribute to fibroblast activation.
The ROS system acts as a common effector of many fibrogenic signals (108). Angiotensin II activates downstream ROS-sensitive kinases (109) and stimulates collagen synthesis through ROS generation (110). Aldosterone-induced fibroblast activation is mediated, at least in part, through oxidative stress (111). Moreover, extensive evidence suggests that ROS mediate the fibrogenic actions of TGF-β and critically regulate matrix metabolism by modulating synthesis and activity of proteases involved in ECM degradation (112).
Ca2+ oscillations have also been implicated in the regulation of fibroblast phenotype and function (113). Angiotensin II or TGF-β may induce fibrogenic actions, at least in part through activation of members of the transient receptor potential (TRP) family of cationic channels. In cardiac fibroblasts, the calcium channel TRPC6 has been implicated in myofibroblast conversion by activating a calcineurin–nuclear factor of activated T cells cascade 114, 115.
MAPKs exhibit a broad range of functions in many different cellular responses, including cell proliferation, survival, migration, and differentiation. Both in vitro and in vivo studies suggest an important role for MAPK signaling pathways in fibroblast activation. Fibroblast-specific loss-of-function approaches suggested that activation of p38α MAPK, the major isoform expressed in cardiac fibroblasts (116), promotes myofibroblast conversion following infarction through activation of the transcription factor serum response factor and the signaling effector calcineurin 78, 117. The serum response factor/myocardin-related transcription factor (MRTF) axis plays a dominant role in regulation of α-SMA transcription and subsequent myofibroblast conversion 118, 119. In vivo, mice with global loss of MRTF-A had attenuated fibrosis following myocardial infarction (120). Whether these observations reflect abrogation of MRTF-dependent effects on fibroblasts remains unclear, considering that MRTF-A may also modulate cardiomyocyte and vascular cell phenotype and function 121, 122.
Noncoding ribonucleic acids in regulation of infarct fibroblasts
A growing body of evidence demonstrates that noncoding ribonucleic acids (RNAs), including small noncoding microRNA (miRNAs) and long noncoding RNA (lncRNA), may be implicated in the regulation of fibroblast activity in the infarcted heart 123, 124. MiRNAs may act by modulating several profibrotic target pathways, including the TGF-β/Smad system, angiotensin II/MAPK signaling, the RhoA/Rho-associated coiled-coil containing kinase (ROCK) cascade, the MRTF/serum response factor axis, and the cationic channels regulating calcium responses (125). Several miRNAs, such as miR-29 and miR-101, function as negative regulators of cardiac fibroblasts; repression of these miRNAs by fibrogenic stimuli, such as TGF-β, may activate a fibrogenic program in response to infarction 126, 127. Members of the miR-15 family have also been suggested to exert antifibrotic actions by inhibiting the TGF-β pathway (128). In contrast to other antifibrotic miRNAs, miR-15 is up-regulated following cardiac injury and may play a role in restraining the fibrotic response.
Other miRNAs may function as activators of the fibrogenic cascade, promoting myofibroblast conversion and activation in the infarcted heart. MiR-21 is markedly induced in infarct fibroblasts (129) and may exert fibrogenic actions by stimulating MAPK activation in cardiac fibroblasts (130) or by targeting the TGF-β cascade (131). In addition to its effects on the fibrotic response, fibroblast-derived miR-21, packaged into exosomes, may exert paracrine effects on cardiomyocyte hypertrophy and immune cell activation (132).
Evidence on the role on lncRNAs in fibroblast activation following infarction is limited (133). Wisp2 superenhancer–associated RNA, a cardiac fibroblast–enriched lncRNA, has been implicated in fibroblast proliferation, activation, and survival following myocardial infarction (134). The species specificity of lncRNAs (only 15% of mouse lncRNAs are expressed in humans and vice versa) is a major limiting factor in the use of animal models to understand their role in human diseases (135).
Fibroblasts in scar maturation
In healing infarcts, secretion of structural ECM proteins by activated myofibroblasts is followed by induction of matrix crosslinking enzymes that contribute to scar maturation. As the scar matures, the density of activated myofibroblasts is dramatically reduced (45). Depletion of myofibroblasts from the mature scar may reflect apoptosis of fibroblasts (136) or loss of α-SMA expression and acquisition of a distinct fibroblast phenotype, characterized by high expression of tendon genes (137). The molecular signals responsible for apoptosis, or deactivation of scar myofibroblasts remain unknown.
Chronic activation of fibroblasts in the remodeling noninfarcted myocardium
As the infarcted heart heals, the surviving myocardium exhibits chronic remodeling, associated with cardiomyocyte hypertrophy and interstitial fibrotic changes. Increased wall stress in noninfarcted myocardial segments may activate interstitial fibroblasts, promoting a matrix-synthetic phenotype and contributing to segmental dysfunction. Although chronic fibrotic changes have been reported in remodeling noninfarcted segments, and some studies have suggested persistence of myofibroblasts for many years in patients surviving an acute infarction (138), the relative contribution of chronic fibroblast activation in the pathogenesis of post-infarction heart failure remains unknown.
The Fibroblasts in the Pressure-Overloaded Myocardium
Increased afterload is a common pathophysiologic companion of many cardiac pathologic conditions, including hypertensive heart disease and aortic stenosis. A pressure load imposes mechanical stress on all myocardial cells and triggers a series of molecular events leading to hypertrophic and fibrotic ventricular remodeling and ultimately heart failure (139). Expansion of resident cardiac fibroblast populations is a prominent characteristic of cardiac pressure overload 140, 141 and is associated with activation of a matrix-synthetic program and subsequent deposition of collagens in interstitial and perivascular areas. Neurohumoral activation has been critically implicated in pressure overload–induced cardiac fibrosis. Angiotensin II–mediated AT1 activation mediates interstitial fibrosis in models of left ventricular pressure overload (142), through direct actions and via induction of inflammatory cytokines and growth factors. Although fibrogenic actions of pro-inflammatory cytokines, such as tumor necrosis factor–α and IL-6, have been reported in pressure-overload models, whether these effects involve direct modulation of fibroblast phenotype or reflect indirect actions on macrophages or cardiomyocytes remains unknown 143, 144. Moreover, TGF-β-driven activation of Smad-dependent signaling has been implicated in fibroblast activation in the pressure-overloaded heart (145).
Considering that mechanical stress is the primary insult in the pressure-overloaded myocardium, activation of mechanosensitive signaling pathways in cardiac fibroblasts may be the critical initial event involved in the pathogenesis of interstitial fibrosis. The focal adhesion-integrin complex is a primary mechanosensor in fibroblasts and transduces molecular signals that promote ECM gene transcription and myofibroblast conversion (146). Focal adhesion kinase (FAK) is a critical molecular link between mechanical stress and fibroblast activation. In vitro, FAK activation has been demonstrated to mediate mechanosensitive or growth factor–induced myofibroblast conversion 147, 148, 149. In vivo, FAK knockdown attenuated fibrotic changes in a model of cardiac pressure overload (150). However, considering the broad effects of FAK activation on cardiomyocytes and vascular and interstitial cells, the cellular basis for these effects is unclear. Evidence documenting the role of fibroblast-specific FAK activation in cardiac fibrosis is lacking.
Mechanosensitive ion channels have also been implicated in pressure overload–induced fibroblast activation (146). TRPC3 and TRPV4 have been implicated in myofibroblast conversion in response to mechanical stress or to growth factor stimulation 151, 152. A recent study demonstrated a critical role for fibroblast-specific activation of the TWIK-related potassium channel in the activation of a fibrogenic response in the pressure-overloaded myocardium (153).
Mechanosensitive or neurohumoral activation of the small GTP-binding protein RhoA may also play an important role in fibroblast proliferation and activation following pressure overload, signaling through the ROCKs, ROCK1 and ROCK2 154, 155. In an experimental model of cardiac pressure overload, pharmacological inhibition of the RhoA-ROCK pathway attenuated fibrosis (156). Fibroblast-specific ROCK2 signaling has been suggested to mediate angiotensin II–mediated fibrosis, through induction of FGF2 and of the matricellular protein CCN2 (157). In addition to the direct fibrogenic actions of mechanosensitive signaling pathways, pressure overload may activate fibroblasts indirectly, through mechanical stress–induced actions on cardiomyocytes, T lymphocytes, or macrophages 158, 159.
It should be emphasized that the contribution of fibroblasts in the pressure-overloaded myocardium is not limited to synthesis of ECM proteins and subsequent increase in ventricular stiffness. Activated fibroblasts may function as potent modulators of cardiomyocyte prosurvival and hypertrophic responses by secreting growth factors or through the release of miRNA-containing exosomes 160, 161. Recent work suggested that TGF-β/Smad–dependent matrix-preserving actions of activated myofibroblasts prevent the generation of proinflammatory ECM fragments and play a critical role in protection of the pressure-overloaded myocardium from inflammation and systolic dysfunction (162). Thus, activated fibroblasts in the pressure-overloaded heart are not unidimensional cells that mediate fibrosis and dysfunction but may also exert protective actions preventing myocardial injury (Figure 3). Whether the diverse actions of fibroblasts in the remodeling myocardium are mediated through distinct fibroblast subpopulations remains unknown.
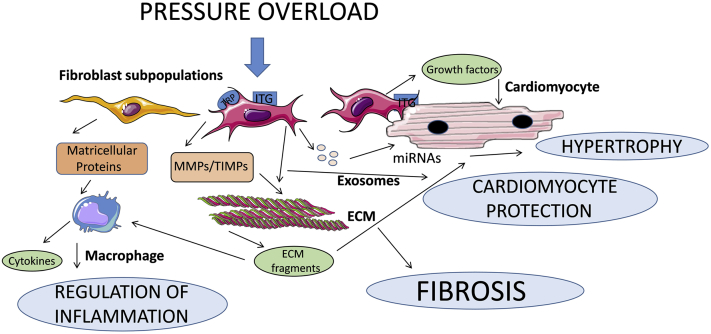
Figure 3.
The Phenotypic Heterogeneity of Cardiac Fibroblast Populations May Explain Their Functional Diversity in Injured and Remodeling Hearts
In the pressure-overloaded myocardium, mechanical stress activates mechanosensitive signaling pathways in cardiac fibroblasts that may involve integrins (ITGs) and stress-activated ion channels (such as transient receptor potential [TRP] channels). Traditional views consider the fibroblasts as matrix-producing cells that secrete large amounts of fibrillar and nonfibrillar collagens, increasing extracellular matrix (ECM) deposition and promoting fibrosis and diastolic dysfunction. However, recent evidence challenges this unidimensional view of fibroblasts, suggesting that they may also play protective roles, by preserving the ECM, thus preventing generation of proinflammatory matrix fragments and by transducing prosurvival cascades in cardiomyocytes. Secretion of matricellular proteins that bind to the structural components of the ECM and modulate signaling responses and release of micro–ribonucleic acid (miRNA)–containing exosomes that may modulate cardiomyocyte responses represent major additional mechanisms implicated in fibroblast actions. The diverse effects of fibroblasts in vivo may reflect their phenotypic heterogeneity, as different fibroblast subsets may exert distinct actions. MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase.
Fibroblasts in the Volume-Overloaded Heart
Conditions associated with volume overload, such as severe aortic or mitral valve regurgitation, are associated with marked ventricular dilation and progressive systolic dysfunction. Studies in experimental models of chordal rupture–induced mitral regurgitation in the dog 163, 164 and of aortocaval fistula in the rat 165, 166 suggest that volume overload causes unique interstitial perturbations that may contribute to adverse remodeling. In contrast to the marked increase in collagen deposition noted in pressure-overloaded hearts, the volume-overloaded myocardium exhibits a marked loss of interstitial collagen associated with increased MMP expression 163, 164, reduced collagen synthesis (167), and accentuated collagen degradation 166, 168. The matrix-degrading phenotype of interstitial cells in volume-overloaded hearts has been attributed to release of cardiomyocyte-derived TNF-α (165) or to downmodulation of TGF-β signaling (163). Whether these changes reflect perturbations of fibroblast phenotype and function in response to volume overload-induced stretch remains unknown.
Fibroblast Activation in the Aging and Diabetic Heart
Aging, diabetes, obesity, and metabolic dysfunction are associated with progressive interstitial and perivascular fibrosis that may contribute to the pathogenesis of heart failure with preserved ejection fraction 5, 169, 170, 171, 172, 173. In contrast to the rapid accumulation of α-SMA-expressing myofibroblasts in models of acute cardiac injury, diabetes and aging do not typically trigger myofibroblast conversion but may cause induction of a matrix-preserving program in cardiac interstitial cells (174). The cellular events and molecular mechanisms mediating fibrosis in senescent and diabetic hearts remain poorly understood. In aging hearts, fibroblast activation may involve the cooperation of several distinct pathways, including age-associated induction of the ROS system, activation of neurohumoral mediators, and stimulation of cytokine and TGF-β-mediated responses (175). In diabetic subjects, in contrast, hyperglycemia may result in accumulation of advanced glycation end-products that crosslink the cardiac ECM, while directly activating fibroblasts by triggering receptor for advanced glycation end-product–mediated signals (176) and accentuating age-associated changes. Diabetes-associated induction of matricellular proteins, such as thrombospondin-1, may also promote fibrosis by activating growth factor–dependent signaling in cardiac fibroblasts (177). Fibroblast activation in diabetic hearts may also reflect microvascular inflammation or resident macrophage stimulation and subsequent secretion of fibrogenic signals 5, 178. It should be emphasized that although aging is associated with increased basal interstitial and perivascular collagen deposition, senescent animals exhibit perturbed fibroblast responses following injury, associated with blunted activation of growth factor signaling pathways (179).
Conclusions and Future Directions
Expansion and activation of resident fibroblast populations play an important role in repair and remodeling of the injured heart and are implicated in the pathogenesis of systolic and diastolic dysfunction in chronic heart failure. Emerging evidence suggests that activated fibroblasts are not unidimensional matrix-producing cells but exhibit a wide range of phenotypes and may regulate inflammatory, hypertrophic, and prosurvival responses. Several important questions remain to be answered. Does the diversity of functional effects of cardiac fibroblasts in the remodeling heart reflect actions of distinct fibroblast subpopulations? If so, what are the phenotypic characteristics, origin, and fate of these fibroblast subsets? Does the functional pluralism of fibroblasts reflect their high responsiveness to changes in their microenvironment? Which molecular signals and environmental cues drive the dramatic phenotypic changes of cardiac fibroblasts in remodeling hearts? Do cell biological processes documented in mouse models recapitulate the phenotypic changes of fibroblasts in human hearts? Answering these questions is critical in order to design novel therapeutic approaches for patients with heart failure.
Footnotes
Dr. Frangogiannis’s laboratory is supported by National Institutes of Health grants R01 HL76246 and R01 HL85440 and by U.S. Department of Defense grants PR151134 and PR151029. Dr. Humeres is supported by American Heart Association post-doctoral award 19POST34450144. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and U.S. Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Frangogiannis N.G. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Berk B.C., Fujiwara K., Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki T., Fukumoto Y., Sugimura K. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. Comparison between preserved and reduced ejection fraction heart failure. Circ J. 2011;75:2605–2613. doi: 10.1253/circj.cj-11-0568. [DOI] [PubMed] [Google Scholar]
- 5.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 6.Kato S., Saito N., Kirigaya H. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2015;191:314–319. doi: 10.1016/j.ijcard.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Roy C., Slimani A., de Meester C. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2018;20:55. doi: 10.1186/s12968-018-0477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong T.C., Piehler K.M., Kang I.A. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–664. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivey M.J., Tallquist M.D. Defining the cardiac fibroblast. Circ J. 2016;80:2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phipps R.P., Borrello M.A., Blieden T.M. Fibroblast heterogeneity in the periodontium and other tissues. J Periodont res. 1997;32:159–165. doi: 10.1111/j.1600-0765.1997.tb01398.x. [DOI] [PubMed] [Google Scholar]
- 11.Souders C.A., Bowers S.L., Baudino T.A. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(Suppl II):17–26. [PubMed] [Google Scholar]
- 13.Banerjee I., Fuseler J.W., Price R.L., Borg T.K., Baudino T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 14.Pinto A.R., Ilinykh A., Ivey M.J. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M., Tsuchihashi T., Ivey K.N. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eghbali M., Blumenfeld O.O., Seifter S. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 17.Brown R.D., Ambler S.K., Mitchell M.D., Long C.S. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 18.Spinale F.G. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 19.Acharya A., Baek S.T., Huang G. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber K.T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 21.Kohl P., Camelliti P., Burton F.L., Smith G.L. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005;38:45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 22.Shinde A.V., Humeres C., Frangogiannis N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 2017;1863:298–309. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frangogiannis N.G. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 24.Shinde A.V., Frangogiannis N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Frangogiannis N.G. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell M.D., Laird R.E., Brown R.D., Long C.S. IL-1beta stimulates rat cardiac fibroblast migration via MAP kinase pathways. Am J Physiol Heart Circ Physiol. 2007;292:H1139–H1147. doi: 10.1152/ajpheart.00881.2005. [DOI] [PubMed] [Google Scholar]
- 28.Richter K., Kietzmann T. Reactive oxygen species and fibrosis: further evidence of a significant liaison. Cell Tissue Res. 2016;365:591–605. doi: 10.1007/s00441-016-2445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boza P., Ayala P., Vivar R. Expression and function of toll-like receptor 4 and inflammasomes in cardiac fibroblasts and myofibroblasts: IL-1beta synthesis, secretion, and degradation. Mol Immunol. 2016;74:96–105. doi: 10.1016/j.molimm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Frangogiannis N.G., Lindsey M.L., Michael L.H. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi M., Takahashi M., Hata T. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 32.Sandanger O., Ranheim T., Vinge L.E. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 33.Bujak M., Dobaczewski M., Chatila K. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anzai A., Choi J.L., He S. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxena A., Chen W., Su Y. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrial M., Da Silva C.C., Pillot B. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2014;68:56–65. doi: 10.1016/j.yjmcc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Nakaya M., Watari K., Tajima M. Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest. 2017;127:383–401. doi: 10.1172/JCI83822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBerge M., Yeap X.Y., Dehn S. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res. 2017;121:930–940. doi: 10.1161/CIRCRESAHA.117.311327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aisagbonhi O., Rai M., Ryzhov S., Atria N., Feoktistov I., Hatzopoulos A.K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mollmann H., Nef H.M., Kostin S. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Kanisicak O., Khalil H., Ivey M.J. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore-Morris T., Cattaneo P., Guimaraes-Camboa N. Infarct fibroblasts do not derive from bone marrow lineages. Circ Res. 2018;122:583–590. doi: 10.1161/CIRCRESAHA.117.311490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alex L., Frangogiannis N.G. The cellular origin of activated fibroblasts in the infarcted and remodeling myocardium. Circ Res. 2018;122:540–542. doi: 10.1161/CIRCRESAHA.118.312654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeisberg E.M., Tarnavski O., Zeisberg M. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 45.Frangogiannis N.G., Michael L.H., Entman M.L. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 46.Blankesteijn W.M., Essers-Janssen Y.P., Verluyten M.J., Daemen M.J., Smits J.F. A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart. Nat Med. 1997;3:541–544. doi: 10.1038/nm0597-541. [DOI] [PubMed] [Google Scholar]
- 47.Detillieux K.A., Sheikh F., Kardami E., Cattini P.A. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 48.Stawowy P., Margeta C., Kallisch H. Regulation of matrix metalloproteinase MT1-MMP/MMP-2 in cardiac fibroblasts by TGF-beta1 involves furin-convertase. Cardiovasc Res. 2004;63:87–97. doi: 10.1016/j.cardiores.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Freed D.H., Chilton L., Li Y. Role of myosin light chain kinase in cardiotrophin-1-induced cardiac myofibroblast cell migration. Am J Physiol Heart Circ Physiol. 2011;301:H514–H522. doi: 10.1152/ajpheart.01041.2010. [DOI] [PubMed] [Google Scholar]
- 50.Dewald O., Zymek P., Winkelmann K. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 51.Frangogiannis N.G., Dewald O., Xia Y. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 52.Satoh T., Nakagawa K., Sugihara F. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 53.Bujak M., Dobaczewski M., Gonzalez-Quesada C. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saxena A., Bujak M., Frunza O. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103:217–227. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manso A.M., Kang S.M., Ross R.S. Integrins, focal adhesions, and cardiac fibroblasts. J Investig Med. 2009;57:856–860. doi: 10.231/JIM.0b013e3181c5e61f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghersi G., Dong H., Goldstein L.A. Regulation of fibroblast migration on collagenous matrix by a cell surface peptidase complex. J Biol Chem. 2002;277:29231–29241. doi: 10.1074/jbc.M202770200. [DOI] [PubMed] [Google Scholar]
- 57.Murphy-Ullrich J.E. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2012;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 59.Kagami S., Border W.A., Miller D.E., Noble N.A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell S.E., Katwa L.C. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 61.Schorb W., Booz G.W., Dostal D.E., Conrad K.M., Chang K.C., Baker K.M. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 62.Sadoshima J., Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 63.Crabos M., Roth M., Hahn A.W., Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thibault G., Lacombe M.J., Schnapp L.M., Lacasse A., Bouzeghrane F., Lapalme G. Upregulation of alpha(8)beta(1)-integrin in cardiac fibroblast by angiotensin II and transforming growth factor-beta1. Am J Physiol Cell Physiol. 2001;281:C1457–C1467. doi: 10.1152/ajpcell.2001.281.5.C1457. [DOI] [PubMed] [Google Scholar]
- 65.Ohkubo N., Matsubara H., Nozawa Y. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.cir.96.11.3954. [DOI] [PubMed] [Google Scholar]
- 66.Kurisu S., Ozono R., Oshima T. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.hyp.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 67.Schieffer B., Wirger A., Meybrunn M. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 68.Lijnen P., Petrov V. Induction of cardiac fibrosis by aldosterone. J Mol Cell Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 69.van den Borne S.W., Isobe S., Zandbergen H.R. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. J Am Coll Cardiol Img. 2009;2:187–198. doi: 10.1016/j.jcmg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi M., Tsutamoto T., Wada A. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559–2565. doi: 10.1161/01.CIR.0000068340.96506.0F. [DOI] [PubMed] [Google Scholar]
- 71.Rickard A.J., Morgan J., Tesch G., Funder J.W., Fuller P.J., Young M.J. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 72.Li C., Sun X.N., Zeng M.R. Mineralocorticoid receptor deficiency in T cells attenuates pressure overload-induced cardiac hypertrophy and dysfunction through modulating T-cell activation. Hypertension. 2017;70:137–147. doi: 10.1161/HYPERTENSIONAHA.117.09070. [DOI] [PubMed] [Google Scholar]
- 73.Rickard A.J., Morgan J., Bienvenu L.A. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension. 2012;60:1443–1450. doi: 10.1161/HYPERTENSIONAHA.112.203158. [DOI] [PubMed] [Google Scholar]
- 74.Neumann S., Huse K., Semrau R. Aldosterone and D-glucose stimulate the proliferation of human cardiac myofibroblasts in vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- 75.Brilla C.G., Zhou G., Matsubara L., Weber K.T. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 76.Kim J., Eckhart A.D., Eguchi S., Koch W.J. Beta-adrenergic receptor-mediated DNA synthesis in cardiac fibroblasts is dependent on transactivation of the epidermal growth factor receptor and subsequent activation of extracellular signal-regulated kinases. J Biol Chem. 2002;277:32116–32123. doi: 10.1074/jbc.M204895200. [DOI] [PubMed] [Google Scholar]
- 77.Turner N.A., Porter K.E., Smith W.H., White H.L., Ball S.G., Balmforth A.J. Chronic beta2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003;57:784–792. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 78.Molkentin J.D., Bugg D., Ghearing N. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation. 2017;136:549–561. doi: 10.1161/CIRCULATIONAHA.116.026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen M.N., Kiriazis H., Ruggiero D. Spontaneous ventricular tachyarrhythmias in beta2-adrenoceptor transgenic mice in relation to cardiac interstitial fibrosis. Am J Physiol Heart Circ Physiol. 2015;309:H946–H957. doi: 10.1152/ajpheart.00405.2015. [DOI] [PubMed] [Google Scholar]
- 80.Woodall M.C., Woodall B.P., Gao E., Yuan A., Koch W.J. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ Res. 2016;119:1116–1127. doi: 10.1161/CIRCRESAHA.116.309538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Travers J.G., Kamal F.A., Valiente-Alandi I. Pharmacological and activated fibroblast targeting of gbetagamma-GRK2 after myocardial ischemia attenuates heart failure progression. J Am Coll Cardiol. 2017;70:958–971. doi: 10.1016/j.jacc.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dewald O., Ren G., Duerr G.D. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu R.M., Desai L.P. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishimura S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 86.Rifkin D.B., Mazzieri R., Munger J.S., Noguera I., Sung J. Proteolytic control of growth factor availability. APMIS. 1999;107:80–85. doi: 10.1111/j.1699-0463.1999.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 87.Frangogiannis N.G., Ren G., Dewald O. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 88.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 89.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 90.Kong P., Shinde A.V., Su Y. Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation. 2018;137:707–724. doi: 10.1161/CIRCULATIONAHA.117.029622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi-Wen X., Chen Y., Denton C.P. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller E.E., Momen A., Masse S. Electrical remodelling precedes heart failure in an endothelin-1-induced model of cardiomyopathy. Cardiovasc Res. 2011;89:623–633. doi: 10.1093/cvr/cvq351. [DOI] [PubMed] [Google Scholar]
- 93.Miyauchi T., Fujimori A., Maeda S. Chronic administration of an endothelin-A receptor antagonist improves exercise capacity in rats with myocardial infarction-induced congestive heart failure. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S64–S67. doi: 10.1097/01.fjc.0000166212.04674.8b. [DOI] [PubMed] [Google Scholar]
- 94.House S.L., Branch K., Newman G., Doetschman T., Schultz J.l.J. Cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2 is mediated by the MAPK cascade. Am J Physiol Heart Circ Physiol. 2005;289:H2167–H2175. doi: 10.1152/ajpheart.00392.2005. [DOI] [PubMed] [Google Scholar]
- 95.Virag J.A., Rolle M.L., Reece J., Hardouin S., Feigl E.O., Murry C.E. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zymek P., Bujak M., Chatila K. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 97.Simm A., Nestler M., Hoppe V. Mitogenic effect of PDGF-AA on cardiac fibroblasts. Basic Res Cardiol. 1998;93(Suppl 3):40–43. doi: 10.1007/s003950050209. [DOI] [PubMed] [Google Scholar]
- 98.Dugina V., Fontao L., Chaponnier C., Vasiliev J., Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 99.Kohan M., Muro A.F., White E.S., Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24:4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arslan F., Smeets M.B., Riem Vis P.W. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 101.Lenga Y., Koh A., Perera A.S., McCulloch C.A., Sodek J., Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 102.Tamaoki M., Imanaka-Yoshida K., Yokoyama K. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schellings M.W., Vanhoutte D., Swinnen M. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oka T., Xu J., Kaiser R.A. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Aelst L.N., Voss S., Carai P. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res. 2015;116:425–436. doi: 10.1161/CIRCRESAHA.116.304599. [DOI] [PubMed] [Google Scholar]
- 106.Naugle J.E., Olson E.R., Zhang X. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 107.Roche P.L., Filomeno K.L., Bagchi R.A., Czubryt M.P. Intracellular signaling of cardiac fibroblasts. Compr Physiol. 2015;5:721–760. doi: 10.1002/cphy.c140044. [DOI] [PubMed] [Google Scholar]
- 108.Cheng T.H., Cheng P.Y., Shih N.L., Chen I.B., Wang D.L., Chen J.J. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003;42:1845–1854. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 109.Ohtsu H., Frank G.D., Utsunomiya H., Eguchi S. Redox-dependent protein kinase regulation by angiotensin II: mechanistic insights and its pathophysiology. Antioxid Redox Signal. 2005;7:1315–1326. doi: 10.1089/ars.2005.7.1315. [DOI] [PubMed] [Google Scholar]
- 110.Lijnen P., Papparella I., Petrov V., Semplicini A., Fagard R. Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species. J Hypertens. 2006;24:757–766. doi: 10.1097/01.hjh.0000217860.04994.54. [DOI] [PubMed] [Google Scholar]
- 111.Iglarz M., Touyz R.M., Viel E.C., Amiri F., Schiffrin E.L. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction with the renin-angiotensin system. Am J Hypertens. 2004;17:597–603. [PubMed] [Google Scholar]
- 112.Siwik D.A., Pagano P.J., Colucci W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 113.Mukherjee S., Ayaub E.A., Murphy J. Disruption of calcium signaling in fibroblasts and attenuation of bleomycin-induced fibrosis by nifedipine. Am J Respir Cell Mol Biol. 2015;53:450–458. doi: 10.1165/rcmb.2015-0009OC. [DOI] [PubMed] [Google Scholar]
- 114.Nishida M., Onohara N., Sato Y. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 115.Davis J., Burr A.R., Davis G.F., Birnbaumer L., Molkentin J.D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sinfield J.K., Das A., O’Regan D.J., Ball S.G., Porter K.E., Turner N.A. p38 MAPK alpha mediates cytokine-induced IL-6 and MMP-3 expression in human cardiac fibroblasts. Biochem Biophys Res Commun. 2013;430:419–424. doi: 10.1016/j.bbrc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bageghni S.A., Hemmings K.E., Zava N. Cardiac fibroblast-specific p38alpha MAP kinase promotes cardiac hypertrophy via a putative paracrine interleukin-6 signaling mechanism. FASEB J. 2018;32:4941–4954. doi: 10.1096/fj.201701455RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomasek J.J., McRae J., Owens G.K., Haaksma C.J. Regulation of alpha-smooth muscle actin expression in granulation tissue myofibroblasts is dependent on the intronic CArG element and the transforming growth factor-beta1 control element. Am J Pathol. 2005;166:1343–1351. doi: 10.1016/s0002-9440(10)62353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lighthouse J.K., Small E.M. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52–60. doi: 10.1016/j.yjmcc.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Small E.M., Thatcher J.E., Sutherland L.B. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weng X., Yu L., Liang P. Endothelial MRTF-A mediates angiotensin II induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;80:23–33. doi: 10.1016/j.yjmcc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 122.Trembley M.A., Quijada P., Agullo-Pascual E. Mechanosensitive gene regulation by myocardin-related transcription factors is required for cardiomyocyte integrity in load-induced ventricular hypertrophy. Circulation. 2018;138:1864–1878. doi: 10.1161/CIRCULATIONAHA.117.031788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Creemers E.E., van Rooij E. Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ Res. 2016;118:108–118. doi: 10.1161/CIRCRESAHA.115.305242. [DOI] [PubMed] [Google Scholar]
- 124.Thum T. Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol. 2014;11:655–663. doi: 10.1038/nrcardio.2014.125. [DOI] [PubMed] [Google Scholar]
- 125.Piccoli M.T., Bar C., Thum T. Non-coding RNAs as modulators of the cardiac fibroblast phenotype. J Mol Cell Cardiol. 2016;92:75–81. doi: 10.1016/j.yjmcc.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 126.van Rooij E., Sutherland L.B., Thatcher J.E. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan Z., Sun X., Shan H. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-beta1 pathway. Circulation. 2012;126:840–850. doi: 10.1161/CIRCULATIONAHA.112.094524. [DOI] [PubMed] [Google Scholar]
- 128.Tijsen A.J., van der Made I., van den Hoogenhof M.M. The microRNA-15 family inhibits the TGFbeta-pathway in the heart. Cardiovasc Res. 2014;104:61–71. doi: 10.1093/cvr/cvu184. [DOI] [PubMed] [Google Scholar]
- 129.Roy S., Khanna S., Hussain S.R. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thum T., Gross C., Fiedler J. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 131.Yuan J., Chen H., Ge D. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem. 2017;42:2207–2219. doi: 10.1159/000479995. [DOI] [PubMed] [Google Scholar]
- 132.Bang C., Batkai S., Dangwal S. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang X., Zhang F. Long noncoding RNA: a new contributor and potential therapeutic target in fibrosis. Epigenomics. 2017;9:1233–1241. doi: 10.2217/epi-2017-0020. [DOI] [PubMed] [Google Scholar]
- 134.Micheletti R., Plaisance I., Abraham B.J. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017;9:eaai9118. doi: 10.1126/scitranslmed.aai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paralkar V.R., Mishra T., Luan J. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood. 2014;123:1927–1937. doi: 10.1182/blood-2013-12-544494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hayakawa K., Takemura G., Kanoh M. Inhibition of granulation tissue cell apoptosis during the subacute stage of myocardial infarction improves cardiac remodeling and dysfunction at the chronic stage. Circulation. 2003;108:104–109. doi: 10.1161/01.CIR.0000074225.62168.68. [DOI] [PubMed] [Google Scholar]
- 137.Fu X., Khalil H., Kanisicak O. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Willems I.E., Havenith M.G., De Mey J.G., Daemen M.J. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 139.Frohlich E.D., Susic D. Pressure overload. Heart Fail Clin. 2012;8:21–32. doi: 10.1016/j.hfc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Ali S.R., Ranjbarvaziri S., Talkhabi M. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–635. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 141.Moore-Morris T., Guimaraes-Camboa N., Banerjee I. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dickstein K. The role of losartan in the management of patients with heart failure. Clin Ther. 2001;23:1456–1477. doi: 10.1016/s0149-2918(01)80120-x. [DOI] [PubMed] [Google Scholar]
- 143.Nakajima S., Roswit W.T., Look D.C., Holtzman M.J. A hierarchy for integrin expression and adhesiveness among T cell subsets that is linked to TCR gene usage and emphasizes V delta 1+ gamma delta T cell adherence and tissue retention. J Immunol. 1995;155:1117–1131. [PubMed] [Google Scholar]
- 144.Zhao L., Cheng G., Jin R. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ Res. 2016;118:1918–1929. doi: 10.1161/CIRCRESAHA.116.308688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Khalil H., Kanisicak O., Prasad V. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saucerman J.J., Tan P.M., Buchholz K.S., McCulloch A.D., Omens J.H. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol. 2019;16:361–378. doi: 10.1038/s41569-019-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leask A. Focal adhesion kinase: a key mediator of transforming growth factor beta signaling in fibroblasts. Adv Wound Care (New Rochelle) 2013;2:247–249. doi: 10.1089/wound.2012.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang P., Wang W., Wang X. Focal adhesion kinase mediates atrial fibrosis via the AKT/S6K signaling pathway in chronic atrial fibrillation patients with rheumatic mitral valve disease. Int J Cardiol. 2013;168:3200–3207. doi: 10.1016/j.ijcard.2013.04.113. [DOI] [PubMed] [Google Scholar]
- 149.Chan M.W., Arora P.D., Bozavikov P., McCulloch C.A. FAK, PIP5KIgamma and gelsolin cooperatively mediate force-induced expression of alpha-smooth muscle actin. J Cell Sci. 2009;122:2769–2781. doi: 10.1242/jcs.044008. [DOI] [PubMed] [Google Scholar]
- 150.Clemente C.F., Tornatore T.F., Theizen T.H. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- 151.Adapala R.K., Thoppil R.J., Luther D.J. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harada M., Luo X., Qi X.Y. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation. 2012;126:2051–2064. doi: 10.1161/CIRCULATIONAHA.112.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abraham D.M., Lee T.E., Watson L.J. The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. J Clin Invest. 2018;128:4843–4855. doi: 10.1172/JCI95945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Loirand G., Sauzeau V., Pacaud P. Small G proteins in the cardiovascular system: physiological and pathological aspects. Physiol Rev. 2013;93:1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- 155.Shimizu T., Liao J.K. Rho kinases and cardiac remodeling. Circ J. 2016;80:1491–1498. doi: 10.1253/circj.CJ-16-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phrommintikul A., Tran L., Kompa A. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H1804–H1814. doi: 10.1152/ajpheart.01078.2007. [DOI] [PubMed] [Google Scholar]
- 157.Shimizu T., Narang N., Chen P. Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction. JCI Insight. 2017;2:93187. doi: 10.1172/jci.insight.93187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nishida M., Sato Y., Uemura A. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27:3104–3115. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Laroumanie F., Douin-Echinard V., Pozzo J. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–2124. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 160.Xiang F.L., Fang M., Yutzey K.E. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun. 2017;8:712. doi: 10.1038/s41467-017-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lyu L., Wang H., Li B. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89:268–279. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Russo I., Cavalera M., Huang S. Protective effects of activated myofibroblasts in the pressure-overloaded myocardium are mediated through smad-dependent activation of a matrix-preserving program. Circ Res. 2019;124:1214–1227. doi: 10.1161/CIRCRESAHA.118.314438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zheng J., Chen Y., Pat B. Microarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009;119:2086–2095. doi: 10.1161/CIRCULATIONAHA.108.826230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nagatomo Y., Carabello B.A., Coker M.L. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000;278:H151–H161. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 165.Chen Y., Pat B., Zheng J. Tumor necrosis factor-alpha produced in cardiomyocytes mediates a predominant myocardial inflammatory response to stretch in early volume overload. J Mol Cell Cardiol. 2010;49:70–78. doi: 10.1016/j.yjmcc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chancey A.L., Brower G.L., Peterson J.T., Janicki J.S. Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation. 2002;105:1983–1988. doi: 10.1161/01.cir.0000014686.73212.da. [DOI] [PubMed] [Google Scholar]
- 167.Childers R.C., Sunyecz I., West T.A., Cismowski M.J., Lucchesi P.A., Gooch K.J. Role of the cytoskeleton in the development of a hypofibrotic cardiac fibroblast phenotype in volume overload heart failure. Am J Physiol Heart Circ Physiol. 2018;316:H596–H608. doi: 10.1152/ajpheart.00095.2018. [DOI] [PubMed] [Google Scholar]
- 168.Cox M.J., Hawkins U.A., Hoit B.D., Tyagi S.C. Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation. 2004;109:2123–2128. doi: 10.1161/01.CIR.0000127429.53391.78. [DOI] [PubMed] [Google Scholar]
- 169.Cavalera M., Wang J., Frangogiannis N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Russo I., Frangogiannis N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Biernacka A., Cavalera M., Wang J. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail. 2015;8:788–798. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sorop O., Heinonen I., van Kranenburg M. Multiple common co-morbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress and myocardial stiffening. Cardiovasc Res. 2018;114:954–964. doi: 10.1093/cvr/cvy038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen F., Chen D., Zhao X. Interleukin-6 deficiency facilitates myocardial dysfunction during high fat diet-induced obesity by promoting lipotoxicity and inflammation. Biochim Biophys Acta. 2017;1863:3128–3141. doi: 10.1016/j.bbadis.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 174.Alex L., Russo I., Holoborodko V., Frangogiannis N.G. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;315:H934–H949. doi: 10.1152/ajpheart.00238.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen W., Frangogiannis N.G. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao J., Randive R., Stewart J.A. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. 2014;5:860–867. doi: 10.4239/wjd.v5.i6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gonzalez-Quesada C., Cavalera M., Biernacka A. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Packer M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur J Heart Fail. 2018;20:873–878. doi: 10.1002/ejhf.1167. [DOI] [PubMed] [Google Scholar]
- 179.Bujak M., Kweon H.J., Chatila K., Li N., Taffet G., Frangogiannis N.G. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Camelliti P., Green C.R., LeGrice I., Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 181.Chang H.Y., Chi J.T., Dudoit S. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Franke W.W., Schmid E., Osborn M., Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979;81:570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gabbiani G., Schmid E., Winter S. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kong P., Christia P., Saxena A., Su Y., Frangogiannis N.G. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–H1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shimazaki M., Nakamura K., Kii I. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takeda N., Manabe I., Uchino Y. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Snider P., Hinton R.B., Moreno-Rodriguez R.A. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Santiago J.J., Dangerfield A.L., Rattan S.G. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 189.Dobaczewski M., Bujak M., Zymek P., Ren G., Entman M.L., Frangogiannis N.G. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 190.Doddapattar P., Gandhi C., Prakash P. Fibronectin splicing variants containing extra domain a promote atherosclerosis in mice through Toll-like receptor 4. Arterioscler Thromb Vasc Biol. 2015;35:2391–2400. doi: 10.1161/ATVBAHA.115.306474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chang M.L., Chen J.C., Alonso C.R., Kornblihtt A.R., Bissell D.M. Regulation of fibronectin splicing in sinusoidal endothelial cells from normal or injured liver. Proc Natl Acad Sci U S A. 2004;101:18093–18098. doi: 10.1073/pnas.0408439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hu Y., Bock G., Wick G., Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. FASEB J. 1998;12:1135–1142. doi: 10.1096/fasebj.12.12.1135. [DOI] [PubMed] [Google Scholar]
- 193.Chen L., Acciani T., Le Cras T., Lutzko C., Perl A.K. Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goldsmith E.C., Hoffman A., Morales M.O. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 195.Chang Y., Guo K., Li Q., Li C., Guo Z., Li H. Multiple directional differentiation difference of neonatal rat fibroblasts from six organs. Cell Physiol Biochem. 2016;39:157–171. doi: 10.1159/000445613. [DOI] [PubMed] [Google Scholar]
- 196.Squires C.E., Escobar G.P., Payne J.F. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 197.Zhang S., Bu X., Zhao H. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J Pathol. 2014;232:436–448. doi: 10.1002/path.4311. [DOI] [PubMed] [Google Scholar]
- 198.Shyu K.G., Chao Y.M., Wang B.W., Kuan P. Regulation of discoidin domain receptor 2 by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Hypertension. 2005;46:614–621. doi: 10.1161/01.HYP.0000175811.79863.e2. [DOI] [PubMed] [Google Scholar]
- 199.Furtado M.B., Costa M.W., Pranoto E.A. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res. 2014;114:1422–1434. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hudon-David F., Bouzeghrane F., Couture P., Thibault G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007;42:991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 201.Jurisic G., Iolyeva M., Proulx S.T., Halin C., Detmar M. Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res. 2010;316:2982–2992. doi: 10.1016/j.yexcr.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Uchida S., De Gaspari P., Kostin S. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Braitsch C.M., Kanisicak O., van Berlo J.H., Molkentin J.D., Yutzey K.E. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schneider M., Kostin S., Strøm C.C. S100A4 is upregulated in injured myocardium and promotes growth and survival of cardiac myocytes. Cardiovasc Res. 2007;75:40–50. doi: 10.1016/j.cardiores.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 205.Tillmanns J., Hoffmann D., Habbaba Y. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. doi: 10.1016/j.yjmcc.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 206.Ruiz-Villalba A., Simón A.M., Pogontke C. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–2066. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 207.Zhou B., Honor L.B., He H. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van Amerongen M.J., Bou-Gharios G., Popa E. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 209.Fujita J., Mori M., Kawada H. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750–2759. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- 210.Kramann R., Schneider R.K., DiRocco D.P. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]