Abstract
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are the most recently approved class of diabetes drugs. Unlike other agents, SGLT2 inhibitors act on the kidney to promote urinary glucose excretion. SGLT2 inhibitors provide multiple benefits, including decreased HbA1c, body weight, and blood pressure. These drugs have received special attention because they decrease the risk of major adverse cardiovascular events and slow progression of diabetic kidney disease (1–3). Balanced against these impressive benefits, the U.S. Food and Drug Administration–approved prescribing information describes a long list of side effects: genitourinary infections, ketoacidosis, bone fractures, amputations, acute kidney injury, perineal necrotizing fasciitis, and hyperkalemia. This review provides a physiological perspective to understanding the multiple actions of these drugs complemented by a clinical perspective toward balancing benefits and risks.
Physiology of the SGLT Family
SGLT1 and SGLT2
Twelve members of the SLC5A gene family have been identified. Sodium–glucose cotransporter 1 (SGLT1) (encoded by the SLC5A1 gene), first identified in epithelial cells of the intestine, illustrates how these cotransporters function (4). SGLT1 in the apical membrane mediates cotransport of glucose and Na+ into epithelial cells (Fig. 1). Because Na+ is actively extruded by Na/K-ATPase, the extracellular Na+ concentration substantially exceeds the intracellular concentration. Movement of Na+ down its electrochemical gradient provides the energy required for active transport of glucose. Subsequently, members of the SLC2A gene family (e.g., GLUT2) facilitate exit of glucose from the cell by diffusion (4).
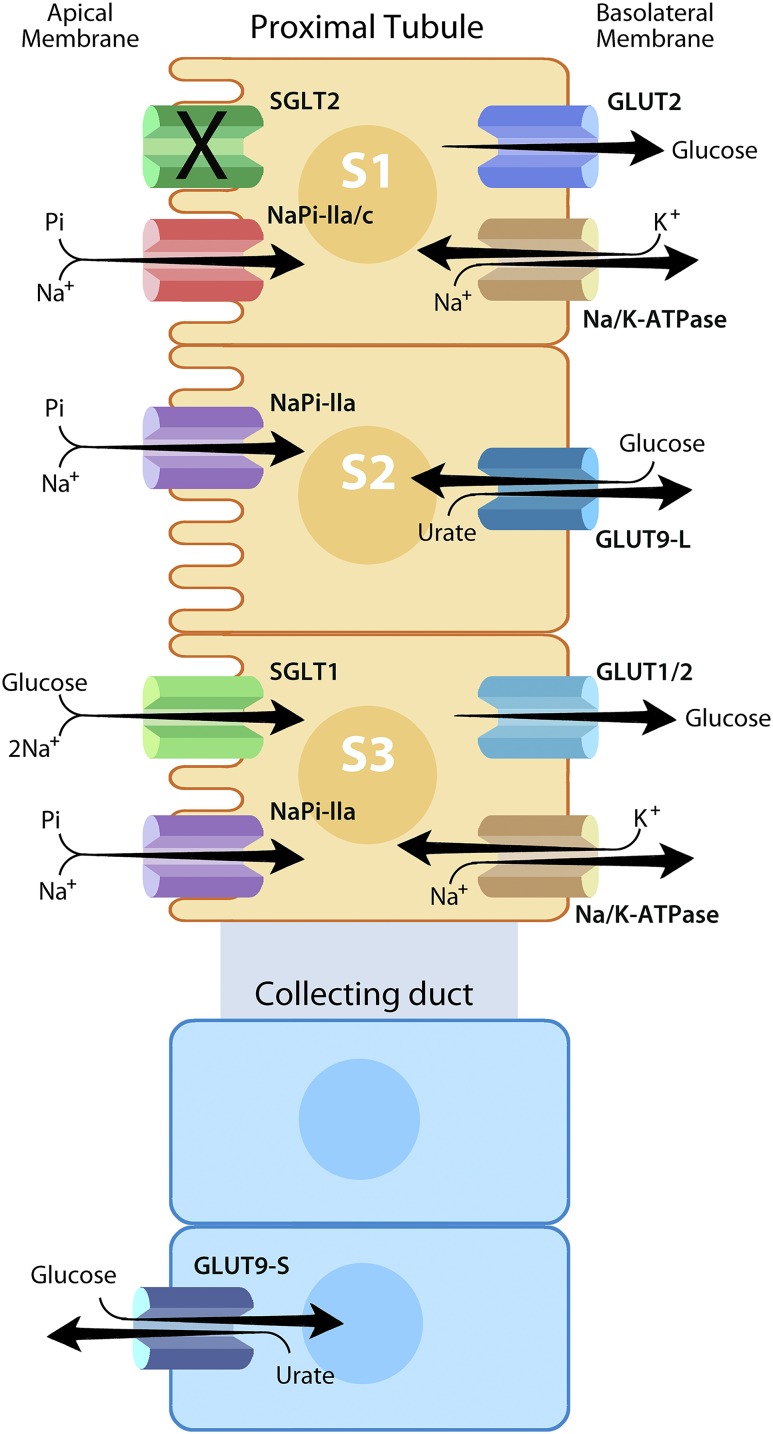
Figure 1.
Role of selected solute transporters related to tubular reabsorption of glucose. SGLT2 (encoded by the SLC5A2 gene) is a high-capacity, low-affinity SGLT located in the S1 segment of the renal proximal tubule. Under physiological conditions, SGLT2 mediates reabsorption of >90% of the filtered glucose load. SGLT1 (encoded by the SLC5A1 gene) is a low-capacity, high-affinity SGLT located in the S3 segment of the renal proximal tubule, which mediates near-complete reabsorption of the glucose that escapes reabsorption by SGLT2. SGLT family transporters are located on the apical membrane of renal tubular epithelial cells and mediate active transport of glucose into epithelial cells. GLUT2 and/or GLUT1 (encoded by SLC2A2 and SLC2A1, respectively) are located on the basolateral membrane and mediate passive diffusion of glucose out of renal tubular epithelial cells. We have indicated stoichiometries for SGLT family transporters: one Na+ ion for SGLT2 and two Na+ ions per glucose molecule for SGLT1. However, the figure does not indicate stoichiometries for other transporters. Na/K-ATPases are also located on the basolateral membrane and mediate active transport of Na+ out of cells, thereby establishing an electrochemical gradient for Na+ that provides the energy required to drive active transport of glucose. SGLT2 inhibitors block cotransport of glucose and Na+ in the S1 segment of the proximal tubule. Two sodium phosphate cotransporters (NaPi-IIa and NaPi-IIc encoded by SLC34A1 and SLC34A3, respectively) compete with SGLT2 and SGLT1 for energy stored in the Na+ gradient to drive active transport. Thus, when SGLT2 is inhibited, this indirectly promotes reabsorption of phosphate. Furthermore, GLUT9 (encoded by SLC2A9) is an antiporter that catalyzes the exchange of glucose for uric acid. A short isoform located on the apical membrane in the collecting duct may mediate uric acid secretion in exchange for glucose reabsorption driven by SGLT2 inhibitor–induced glucosuria (23). A long isoform located on basolateral membranes in the proximal tubule S2 segment may mediate uric acid reabsorption. Inhibition of SGLT2-mediated glucose reabsorption might decrease extracellular glucose concentrations near the basolateral membrane, thereby partially inhibiting uric acid reabsorption. Illustration by T. Phelps, used with permission from the Department of Art as Applied to Medicine, Johns Hopkins University.
The kidney makes use of similar mechanisms to mediate glucose reabsorption. SGLT2 is located in the S1 segment of the proximal tubule where it mediates reabsorption of ∼90% of glucose filtered at the glomerulus (5) (Fig. 1). SGLT1 is located downstream in the S3 segment where it mediates reabsorption of glucose that escapes SGLT2 (5). SGLT1 and SGLT2 have differentiated functional characteristics matched with their distinct physiological roles. SGLT2 is expressed at high levels, providing sufficient capacity to reabsorb most of the filtered glucose. SGLT1 is expressed at lower levels but has higher affinity for glucose (Km ∼0.5 mmol/L for SGLT1 vs. ∼5 mmol/L for SGLT2), enabling SGLT1 to function efficiently at low glucose concentrations (4). Furthermore, the two transporters have different stoichiometries. While SGLT2 transports one ion of Na+ per molecule of glucose, SGLT1 transports two Na+ ions per molecule of glucose (4). The larger number of Na+ ions provides more energy, thereby enabling SGLT1 to transport glucose up a steeper chemical gradient. This combination of a low Km plus two–Na+ ion stoichiometry enables SGLT1 to drive glucose concentrations to near-zero levels in the urine. Although SGLT2 is located primarily in the renal proximal tubule, SGLT1 is located in a number of epithelial membranes, including small intestine, bile duct, pancreatic duct, and salivary glands (4).
Other SLC5A Family Members
SGLT4 and SGLT5 (encoded by SLC5A9 and SLC5A10, respectively) cotransport Na+ together with either glucose or other hexoses (e.g., mannose, fructose) (4). Both SGLT4 and SGLT5 are expressed in the proximal tubule and may also contribute to renal tubular glucose reabsorption. During evolution, SGLT3 lost the ability to transport glucose as a result of substitution of glutamate for glutamine 457 (6). Nevertheless, SGLT3 retains capabilities to bind glucose and transport Na+. Because glucose concentrations regulate the rate at which SGLT3 transports Na+, SGLT3 is hypothesized to function as a glucose sensor rather than as an SGLT. Indeed, SGLT3 may function physiologically as a portal glucose sensor (7). While phlorizin has been reported to inhibit many SGLT family members, marketed selective SGLT2 inhibitors are less well characterized with respect to inhibition of SGLT3, SGLT4, or SGLT5.
Drug Discovery
Target Identification and Target Validation
Pharmacologic Validation
Phlorizin is a natural product derived from the root bark of apple trees as first reported in 1835 (8,9). It induces glucosuria by inhibiting renal tubular glucose reabsorption. Parenteral phlorizin was reported to decrease plasma glucose levels in animal models of diabetes, thereby alleviating glucose toxicity, increasing insulin sensitivity, and enhancing insulin secretion (10–12). These observations first suggested that an SGLT inhibitor might be an effective, glucose-lowering treatment for diabetes. Phlorizin is degraded by intestinal lactase-phlorizin hydrolase, and the glucosuric effect of a single enteral dose wanes over several hours, necessitating multiple large oral doses to sustain maximal glucosuria (13). Phlorizin thus seemed impractical for pharmaceutical use.
Human Genetic Validation
Biallelic loss-of-function mutations in the SGLT2 gene (SLC5A2) were identified as the cause of familial renal glucosuria (14,15). These patients excreted ∼30–200 g/1.73 m2/day of glucose in their urine (corresponding to ∼120–800 kcal/1.73 m2/day) (4). Although patients with familial renal glucosuria are normoglycemic, this degree of glucosuria was hypothesized to be sufficient to decrease HbA1c in patients with diabetes. Furthermore, patients with familial renal glucosuria appeared to be healthy, suggesting that a selective SGLT2 inhibitor might be safe. In contrast, patients with biallelic loss-of-function mutations in the SGLT1 gene (SLC5A1) exhibit a syndrome characterized by glucose-galactose malabsorption (4). Taken together, these observations inspired pharmaceutical companies to initiate programs to discover selective SGLT2 inhibitors to treat diabetes.
Medicinal Chemistry
Phlorizin provided the chemical starting point for medicinal chemistry programs. The phlorizin scaffold was modified to achieve desired selectivity for inhibition of SGLT2, oral bioavailability, and sufficiently long half-lives to drive clinical efficacy and to generate intellectual property to protect marketing exclusivity. Washburn and colleagues (16) extended the half-life by removing oxygen from the glycosidic linkage connecting the sugar and aglycone moieties, a modification that is incorporated in all currently approved SGLT2 inhibitors. Although all approved SGLT2 inhibitors are relatively selective for SGLT2 versus SGLT1, the degree of selectivity varies among members of the class. Sotagliflozin is described as a dual SGLT1-SGLT2 inhibitor, but even sotagliflozin is ∼20-fold more potent as an inhibitor of SGLT2 (IC50 1.8 nmol/L) than of SGLT1 (IC50 36 nmol/L) (17).
Pharmacology
Renal Effects
Direct Effects: Glucosuria and Natriuresis
These drugs inhibit proximal tubular reabsorption of both glucose and Na+. For healthy volunteers with normal renal function and weighing ∼70 kg, canagliflozin (300 mg) induces excretion of ∼50 g/day of glucose (18). However, there is considerable interindividual variation, ranging from ∼30 to ∼90 g/day (∼25–65% of the filtered glucose load). SGLT2 inhibition triggers compensatory mechanisms that increase glucose flux through SGLT1 (and possibly other glucose transporters located downstream in the renal tubule). Consequently, net glucose excretion is less than would be predicted from complete SGLT2 inhibition. Because patients with diabetes have higher levels of plasma glucose, they experience quantitatively greater degrees of glucosuria when treated with SGLT2 inhibitors. Conversely, smaller quantities of glucose are excreted in patients with impaired renal function because of lower filtered glucose load.
SGLT2 inhibitors induce natriuresis by inhibiting transport of one Na+ ion for every molecule of glucose that is not absorbed. As with other diuretics, SGLT2 inhibitor–induced natriuresis is transient, owing to compensatory mechanisms that increase Na reabsorption at other nephron sites. Increased activity of the renin-angiotensin-aldosterone system contributes to this process (19,20). Thus, negative Na+ balance is eventually terminated, and a new steady state is established at a reduced level of total body Na+. It is uncertain whether SGLT2 inhibitors create sustained reductions in plasma volume in patients with diabetes. Two studies directly measured changes in plasma volume in response to SGLT2 inhibitors. One demonstrated a decrease in plasma volume 1 week after initiation of canagliflozin, which was not sustained at 12 weeks (21). The other reported a decreased plasma volume after 12 weeks of dapagliflozin (22). In the dapagliflozin study, the median reduction in plasma volume was offset by a comparable increase in red blood cell mass (22).
Indirect Effects: Increased Serum Phosphorus and Decreased Serum Uric Acid
SGLT2 inhibitors also alter renal tubular function indirectly. Inhibition of SGLT2-mediated glucose reabsorption in the proximal tubule increases delivery of glucose to downstream segments of the nephron. GLUT9 functions as a glucose-uric acid antiporter (Fig. 1). The short isoform of GLUT9 is expressed on the apical side of collecting duct epithelial cells (23) where it mediates both 1) reabsorption of glucose from the tubular lumen and 2) secretion of uric acid into tubular fluid. Consequently, delivery of glucose to the collecting duct drives increased secretion of uric acid into the lumen, which mediates the observed decrease in serum uric acid (24).
In 1944, Pitts and Alexander (25) reported that phlorizin promotes tubular phosphate reabsorption. They postulated that glucose transporters and phosphate transporters compete for “an element common to the two mechanisms.” We now know that both glucose and phosphate are reabsorbed by Na+-dependent cotransporters: SGLT1 and SGLT2 for glucose and NaPi-IIa and NaPi-IIc for phosphate (Fig. 1). The electrochemical gradient for Na+ represents the common element for which these Na+-dependent cotransporters compete. When SGLT2 is inhibited, this crosstalk mechanism may indirectly increase proximal tubular phosphate reabsorption. The increase in tubular reabsorption of phosphate mediates the SGLT2 inhibitor–induced increase in serum phosphorus (18).
Two other electrolyte abnormalities have been reported in association with SGLT2 inhibitors: 1) small increases in serum magnesium concentration, possibly mediated by augmented Mg2+ reabsorption (26), and 2) increases in serum potassium, which are exacerbated by chronic kidney disease, potassium-sparing diuretics, or renin-angiotensin-aldosterone inhibitors (27). The mechanisms of these drug-induced changes in electrolytes have not been fully elucidated. However, the combination of increased glucagon and decreased insulin levels has been suggested to increase plasma potassium levels possibly by redistribution of potassium from intracellular to extracellular fluid (28).
Extrarenal Effects
Pancreatic α-Cells
SGLT2 inhibitors have been reported to increase plasma glucagon levels in clinical studies (29,30). Two publications suggested that dapagliflozin acts directly on pancreatic islets to promote glucagon secretion (31,32), but α-cells may also use other mechanisms to sense the loss of glucose in the urine.
Biliary Ducts and Sweat Glands
SGLT1 is reported to be expressed in cholangiocytes (33) where it mediates reabsorption of glucose from bile. When biliary glucose reabsorption was inhibited with phlorizin, bile volume increased. Although a truly selective SGLT2 inhibitor would not be predicted to affect bile ducts, less selective compounds (e.g., sotagliflozin) might inhibit SGLT1-mediated biliary glucose reabsorption. Similarly, evidence has been presented in the patent literature that phlorizin inhibits glucose reabsorption in the sweat gland, thereby increasing the volume of sweat (34). Although neither SGLT1 nor SGLT2 was detected (35), both SGLT3 and SGLT4 were reported to be expressed in sweat glands.
Pharmacokinetics
All SGLT2 inhibitors share similar chemical structures and exhibit similar pharmacokinetics (Table 1). Although it is outside the scope of this review to discuss the pharmacokinetics of individual drugs in depth, we will summarize several key points. SGLT2 inhibitors achieve maximal concentrations 1–1.5 h after a single oral dose, and terminal half-lives are in the range of 8–16 h (Table 1). Canagliflozin is extensively metabolized in the liver to two inactive metabolites, M5 and M7 (36). Less than 1% of the dose is excreted unchanged in the urine. Two uridine 5′-diphospho-glucuronosyltransferases (UGTs), UGT2B4 and UGT1A9, catalyze the formation of M5 and M7, respectively. Genetic variants in UGT1A9 and UGT2B4 have modest effects on canagliflozin pharmacokinetics (37).
Table 1.
Selected aspects of pharmacokinetics and drug metabolism
| Canagliflozin | Empagliflozin | Dapagliflozin | Ertugliflozin | |
|---|---|---|---|---|
| Half-life (hours) | 10.6–13.1 | 12.4 | 8–12.9 | 16.6 |
| Dosing adjustment in patients with impaired renal function | eGFR 45–60 mL/min/1.73 m2, use 100 mg; eGFR <45 mL/min/1.73 m2, do not initiate; contraindicated eGFR <30 mL/min/1.73 m2 | eGFR <45 mL/min/1.73 m2, do not initiate; contraindicated in severe renal impairment/dialysis | eGFR <60 mL/min/1.73 m2, do not initiate; contraindicated eGFR <30 mL/min/1.73 m2 | eGFR <60 mL/min/1.73 m2, do not initiate; contraindicated eGFR <30 mL/min/1.73 m2 |
| Metabolism: UGT isoforms | UGT1A9, UGT2B4 | UGT2B7, UGT1A3, UGT1A8, UGT1A9 | UGT1A9 | UGT1A9, UGT2B7 |
| Metabolism: other pathways | CYP3A4 (∼7%) | Pgp substrate |
eGFR, estimated glomerular filtration rate; Pgp, permeability glycoprotein.
Clinical Efficacy
Metabolic Effects
Decreased HbA1c
Drug-induced glucosuria provides the primary mechanism whereby SGLT2 inhibitors decrease HbA1c. Four SGLT2 inhibitors are approved for use in the U.S. (Table 2). When added to background therapy with metformin, these drugs decreased HbA1c by 0.4–0.8% (4–9 mmol/mol), starting from a baseline HbA1c of 7.90–8.2% (63–66 mmol/mol) (38–41). In a head-to-head study comparing pharmacodynamic responses to canagliflozin (300 mg) versus dapagliflozin (10 mg) (42), responses to both drugs were similar during the first 4 h. Nevertheless, although the dose of canagliflozin provides maximal inhibition of SGLT2 during the entire 24 h after administration of the drug, dapagliflozin provides only submaximal SGLT2 inhibition during hours 12–24. This interpretation is consistent with observations that canagliflozin (300 mg) decreases mean HbA1c by ∼0.77% (∼9 mmol/mol), whereas dapagliflozin (10 mg) decreases mean HbA1c by ∼0.5% (∼6 mmol/mol) (Table 2).
Table 2.
SGLT2 inhibitors: clinical efficacy and safety
| Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | |
|---|---|---|---|---|
| Baseline HbA1c | 7.94–7.96% | 7.9–8.2% | 7.9% | 8.1–8.2% |
| Baseline HbA1c (mmol/mol) | 63 | 63–66 | 63 | 65–66 |
| Δ HbA1c (low dose) | −0.62% (100 mg) | −0.4% (5 mg) | −0.6% (10 mg) | −0.5% (5 mg) |
| Δ HbA1c (low dose) (mmol/mol) | −7 (100 mg) | −4 (5 mg) | −7 (10 mg) | −6 (5 mg) |
| Δ HbA1c (high dose) | −0.77% (300 mg) | −0.5% (10 mg) | −0.6% (25 mg) | −0.7% (15 mg) |
| Δ HbA1c (high dose) (mmol/mol) | −9 (300 mg) | −6 (10 mg) | −7 (25 mg) | −8 (15 mg) |
| Δ Weight (low dose) | −2.5% (100 mg) | −2.2 kg (5 mg) | −2.0% (10 mg) | −1.8 kg (5 mg) |
| Δ Weight (high dose) | −2.9% (300 mg) | −2.0 kg (10 mg) | −2.5% (25 mg) | −1.7 kg (15 mg) |
| Δ Systolic BP (low dose) | −3.7 mmHg (100 mg) | −4.5 mmHg (5 mg) | −4.1 mmHg (10 mg) | −3.3 mmHg (5 mg) |
| Δ Systolic BP (high dose) | −5.4 mmHg (300 mg) | −5.3 mmHg (10 mg) | −4.8 mmHg (25 mg) | −3.8 mmHg (15 mg) |
| CV outcome trial* | CANVAS | DECLARE-TIMI 58 | EMPA-REG OUTCOME | |
| MACE risk reduction | 0.86 (0.75–0.97) | 0.93 (0.84–1.03) | 0.86 (0.74–0.99) | |
| Heart failure (hospitalization) | 0.67 (0.52–0.87) | 0.73 (0.61–0.88) | 0.65 (0.50–0.85) | |
| Death (CV) | 0.87 (0.72–1.06) | 0.98 (0.82–1.17) | 0.62 (0.49–0.77) | |
| Death (any cause) | 0.87 (0.74–1.01) | 0.93(0.82–1.03) | 0.68 (0.57–0.82) | |
| Nonfatal MI (except silent MI) | 0.85 (0.69–1.05) | 0.89(0.77–1.01) | 0.87 (0.70–1.09) | |
| Nonfatal stroke | 0.90 (0.71–1.15) | 1.01 (0.84–1.21) | 1.24 (0.92–1.67) | |
| Progression of kidney disease | 0.73 (0.67–0.79) | 0.53 (0.43–0.66) | 0.61 (0.53–0.70) |
Four SGLT2 inhibitors have been approved by the U.S. FDA for the treatment of type 2 diabetes. Data have been taken from the FDA-approved prescribing information for each drug and/or from the cardiovascular (CV) outcome trials (1–3,53). The prescribing information expresses HbA1c as a percent (NGSP units). HbA1c levels in International Federation of Clinical Chemistry (IFFC) units (mmol/mol) were generated using the conversion tool at http://www.ngsp.org/convert1.asp, which uses the following equation for the conversion: NGSP = (0.09148 × IFCC) + 2.152. Accordingly, it is not straightforward to accomplish the conversion for mean Δ HbA1c. For purposes of the article, we have estimated Δ HbA1c (mmol/mol) by assuming that the baseline HbA1c was ∼8.0% (64 mmol/mol) and calculating the change in HbA1c if that baseline HbA1c were decreased by reported change in HbA1c. For example, if the prescribing information reports that Δ HbA1c = −0.6%, this would correspond to an HbA1c of 7.4% (relative to a baseline HbA1c of 8.0%). The website converts an HbA1c of 7.4% to 57 mmol/mol. Thus, we have subtracted 64 mmol/mol from 57 mmol/mol, thereby converting a value of Δ HbA1c = −0.6% to Δ HbA1c = −7 mmol/mol. This should be viewed as an approximation. It would be necessary to convert data on individual patients before averaging to accomplish an exact unit conversion. BP, blood pressure; MI, myocardial infarction.
*For the CV outcome trials, data are hazard ratio (95% CI). Because the CV outcome study for ertugliflozin is still in progress, data are not yet available.
Although the primary pharmacology is mediated by the drug-induced increase in urinary glucose excretion, other mechanisms may also contribute to decreasing HbA1c. For example, SGLT2 inhibitors increase insulin sensitivity and enhance glucose-stimulated insulin secretion (29,30,43), probably mediated by alleviating glucotoxicity. In addition, SGLT2 inhibitors promote weight loss, which likely contributes to metabolic improvement.
Weight Loss
Glucosuria is associated with urinary loss of calories, which mediates a 2–3% weight loss over 6 months (Table 2). Ferrannini et al. (44) published a thorough study of the energetics of weight loss in patients with type 2 diabetes treated with empagliflozin (25 mg) for 90 weeks. During the course of the study, urinary energy loss averaged 206 kcal/day. If energy intake were kept constant, this magnitude of calorie loss would be predicted to induce a mean weight loss of 11.1 kg in women and 11.4 kg in men. However, the observed weight loss was considerably less: 3.2 kg in women and 3.1 kg in men. These data strongly suggest that homeostatic mechanisms triggered a compensatory increase in food intake. The discrepancy between observed and expected weight loss became increasingly apparent after 18 weeks of empagliflozin therapy when mean body weight reached a plateau presumably caused by increased food intake.
Compensatory Homeostatic Mechanisms
SGLT2 inhibitors do not cause fasting hypoglycemia in healthy volunteers probably because these drugs trigger increases in hepatic glucose production (29,30), which replaces some glucose excreted in the urine, thereby preventing hypoglycemia. SGLT2 inhibitors also trigger increases in plasma glucagon levels. It has been suggested that increased glucagon secretion is mediated by a direct effect of SGLT2 inhibitors on the pancreatic α-cell (31). In addition, SGLT2 inhibitors have been reported to decrease circulating insulin levels (29,30). This drug-induced decrease in insulin secretion would be predicted to decrease the paracrine effect of insulin to inhibit glucagon secretion.
In terms of energy balance, glucosuria is the metabolic equivalent of removing ∼50 g of carbohydrate from the diet, which triggers a shift to fatty substrate utilization (45) similar to what is observed with a ketogenic diet (46). Indeed, 4 weeks of empagliflozin (25 mg/day) has been shown to increase mean fasting β-hydroxybutyrate levels from 246 to 561 μmol/L in patients with type 2 diabetes (45). Furthermore, empagliflozin promoted lipid oxidation while inhibiting glucose oxidation and nonoxidative glucose disposal. Other SGLT2 inhibitors have also been demonstrated to increase circulating levels of ketone bodies (e.g., tofogliflozin) (47).
Cardiovascular Effects
Natriuresis and Decreased Blood Pressure
When canagliflozin (300 mg) was administered to healthy volunteers, 24-h urinary Na+ excretion increased by 1.25 g, a mean increase of 32% (18). As discussed above, natriuresis was transient, with a return to baseline within 5 days. As shown in a study in subjects with type 2 diabetes, levels of atrial natriuretic peptide and N-terminal pro-B-type natriuretic peptide decreased, and plasma renin activity increased (20). Drug-induced natriuresis likely contributes to a long-term reduction in blood pressure (3–5 mmHg systolic blood pressure) (Table 2). Other mechanisms have also been suggested. Despite a drug-induced decrease in blood pressure, it has been reported that SGLT2 inhibitors do not increase heart rate. For example, among patients with baseline heart rates >80 beats/min (bpm), luseogliflozin was associated with a 7.5-bpm decrease in heart rate compared with a 3.9-bpm decrease in placebo-treated patients (48). Similarly, dapagliflozin did not increase heart rates compared with placebo (49). Cherney et al. (50) reported that empagliflozin did not change circulating catecholamine levels in patients with type 1 diabetes. Furthermore, they observed that empagliflozin decreased arterial stiffness (50). In any case, Kubota et al. (51) published a description of the rationale and study design for a clinical trial to study the effect of empagliflozin on cardiac sympathetic activity in patients with acute myocardial infarction, and Tanaka et al. (52) published the rationale and study design for a clinical trial to study the effect of empagliflozin on endothelial function.
Cardioprotection
As required by the U.S. Food and Drug Administration (FDA), cardiovascular outcome trials have been initiated for all SGLT2 inhibitors approved in the U.S. The primary outcomes were related to cardiovascular safety, but prespecified hierarchical statistical analyses were conducted to test the hypothesis that there might be cardiovascular benefit. Tests for superiority were conducted only if the noninferiority analysis confirmed cardiovascular safety. Both the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) and the Canagliflozin Cardiovascular Assessment Study (CANVAS) demonstrated reductions of the risk of major adverse cardiovascular events (MACE), with hazard ratios of 0.86 for both empagliflozin (1) and canagliflozin (3). Data on secondary outcomes differed in some respects (e.g., differences in the risks of individual components of the primary composite MACE outcome cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke). These differences in secondary outcomes may reflect statistical variation rather than true differences. The Dapagliflozin Effect on Cardiovascular Events trial (DECLARE-TIMI 58) demonstrated that dapagliflozin achieved a statistically significant decrease in a composite end point of cardiovascular death or hospitalization for heart failure but a statistically insignificant trend toward fewer MACE (53). In the cardiovascular outcome trials for empagliflozin, canagliflozin, and dapagliflozin, SGLT2 inhibitor–treated patients experienced decreased risks of hospitalization for heart failure (Table 2). It is likely that the natriuretic action of these drugs contributes importantly to the heart failure benefit. All three studies exhibited a trend toward a 10–15% decrease in the risk of nonfatal myocardial infarction but did not achieve statistical significance in any of the individual studies. None of the studies demonstrated benefit with respect to the risk of nonfatal stroke (Table 2), with point estimates of hazard ratios ranging from 1.24 (empagliflozin) to 0.90 (canagliflozin). Whereas empagliflozin demonstrated a statistically significant decrease in the risk of cardiovascular death (hazard ratio 0.62), neither canagliflozin nor dapagliflozin was associated with a statistically significant decrease in the risk of cardiovascular death (Table 2). It is unknown whether the three drugs are truly differentiated with respect to cardiovascular benefit. It remains possible that random variation or differences in study design or patient population may contribute to the observed differences. For example, whereas >99% of patients in EMPA-REG OUTCOME had established cardiovascular disease, this was true for only 65.6% in CANVAS and only 40.6% of patients in DECLARE-TIMI 58.
EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58 were all designed as randomized placebo-controlled trials. Compared with placebo-treated patients, drug-treated patients had lower HbA1c and lower blood pressure. HbA1c was ∼0.6% lower at 3–6 months, but the difference decreased to ∼0.2% by the end of the trials. Mean systolic blood pressure was ∼4 mmHg lower, which may have contributed to the decrease in cardiovascular risk. It would be of interest to conduct a treat-to-target study design in which patients were randomized to an SGLT2 inhibitor–based regimen or a non-SGLT2 inhibitor–based regimen (e.g., a dipeptidyl peptidase 4 [DPP-4] inhibitor) plus a generic antihypertensive and treated to equivalent blood pressure and glycemic control to evaluate differences in cardiovascular risk. When DPP-4 inhibitors become generic, a combination of a generic DPP-4 inhibitor plus a generic antihypertensive would likely be substantially less expensive than a proprietary SGLT2 inhibitor. Accordingly, this type of head-to-head comparison has the potential to provide great value by decreasing the cost of pharmacotherapy. The long-term randomized clinical trials of SGLT2 inhibitors have been sponsored by pharmaceutical companies and have been designed as placebo-controlled trials (1,3). There would be great value in conducting randomized controlled comparative effectiveness studies. Unfortunately, the National Institutes of Health–sponsored Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) (54) does not include an SGLT2 inhibitor among the four drugs being evaluated as second-line therapy for combination with metformin.
It is instructive to compare these data with the Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD-BP) clinical trial and Systolic Blood Pressure Intervention Trial (SPRINT) (55). In the SPRINT study of patients without diabetes, intensive blood pressure control was associated with a hazard ratio of 0.75 (95% CI 0.64–0.89) for MACE. This represents a greater risk reduction than the hazard ratio of 0.86 observed in EMPA-REG OUTCOME (1) and CANVAS (3), but the magnitude of blood pressure reduction was also greater in SPRINT. Similarly, intensive blood pressure control was associated with a hazard ratio of 0.77 (95% CI 0.63–0.95) for MACE in the standard glycemia arm of the ACCORD-BP study. The standard glycemia arm of ACCORD-BP had a similar mean HbA1c (∼7.5% [58 mmol/mL]) as the SGLT2 inhibitor arms of EMPA-REG OUTCOME and CANVAS. In contrast, the placebo-treated arm of EMPA-REG OUTCOME and CANVAS had substantially higher mean levels of HbA1c (8.0–8.2% [64–66 mmol/mol]).
Exploratory analyses have evaluated correlations of various parameters with the observed decrease in the risk of cardiovascular death in empagliflozin-treated patients (56). Although the authors acknowledged that EMPA-REG OUTCOME was not designed to determine mechanisms, they applied statistical mediation analysis to generate hypotheses about possible causality. This approach identified several possible mediators (with percent mediation in parentheses): hematocrit (51.8%), albumin (25.5%), uric acid (24.6%), and fasting plasma glucose (16.1%). Although these hypotheses are thought provoking, a number of questions arise. For example, it is not clear whether exploratory statistical analysis can dissect the relative contributions of closely related physiological mechanisms, such as decreased blood pressure and increased hematocrit and albumin (all of which are triggered at least in part by drug-induced natriuresis). Furthermore, their mediation analysis placed great weight on how each parameter evolved over time. In the case of HbA1c and fasting plasma glucose, different conclusions were reached depending on how data were analyzed. Glycemic parameters seemed more important in an analysis that assessed chronic effects, whereas glycemia seemed less important in an analysis that focused on levels of HbA1c and fasting plasma glucose closer to the time of death. In the Epidemiology of Diabetes Interventions and Complications (EDIC) study, patients from the Diabetes Control and Complications Trial (DCCT) were followed up 11 years after the end of DCCT. While mean HbA1c levels were essentially identical in both arms (intensive vs. conventional treatment) at the time of the analysis, patients receiving intensive treatment a decade earlier experienced a 57% decrease in the risk of MACE (57). A subsequent analysis published on the 30th anniversary of the DCCT confirmed the cardiovascular benefit with longer follow-up (58). These data strongly suggest that prior glycemic control may be more important than the level of glycemic control at the time an adverse cardiovascular event actually occurs. Whether the metabolic memory effect is mediated directly by glycemic control or indirectly (e.g., by postponement of renal disease), these observations suggest that chronic glycemic control (rather than glycemic control at the time of the adverse event) may have been an important predictor of cardiovascular death in EMPA-REG OUTCOME (56).
SGLT2 inhibitor–induced natriuresis with plasma volume contraction could contribute to an acute increase in hematocrit as a result of hemoconcentration. In response to an SGLT2 inhibitor, however, hematocrit continues to increase gradually and attains maximum values only after 16 weeks of treatment (22). This gradual increase in hematocrit is unlikely due to plasma volume contraction because SGLT2 inhibitor–induced natriuresis is transient, with a new steady state being established within a few days. Rather, the increased hematocrit may be attributed to stimulation of renal erythropoietin secretion by SGLT2 inhibitors, leading to new red blood cell formation. Dapagliflozin has induced an early rise in serum erythropoietin accompanied by reticulocytosis and an increase in measured red cell mass (22,59). Empagliflozin has triggered a similar increase in serum erythropoietin (59).
Renoprotection
Empagliflozin, canagliflozin, and dapagliflozin have been reported to slow the progression of diabetic kidney disease (hazard ratio ∼0.70) (2,3,53). As discussed above for cardiovascular outcome trials, renal outcome data were obtained from placebo-controlled trials in which SGLT2 inhibitor–treated patients had a significantly lower mean HbA1c and blood pressure. While these two risk factors probably contributed to the observed renoprotection, it is not clear that the magnitude of decreases in HbA1c and blood pressure were sufficient to account for the magnitude of renoprotection. Other SGLT2 inhibitor–specific mechanisms have also been proposed to contribute to renal benefit. For example, hyperglycemia increases the quantity of glucose filtered at the glomerulus, which in turn drives upregulation of proximal tubular SGLTs, increasing renal proximal tubular reabsorption of both Na+ and glucose. This would decrease delivery of Na+ to the macula densa, thereby promoting hyperfiltration through the mechanism of tubuloglomerular feedback (19,60). Hyperfiltration is believed to be an important causal element in the pathogenesis of diabetic kidney disease. When empagliflozin was administered to patients with type 1 diabetes, the drug restored normal glomerular filtration rates in patients with hyperfiltration (19). Several other mechanisms have also been proposed to contribute to renoprotection, including decreased levels of serum uric acid, increased hematocrit, and elevated ketone body levels (2,3,61,62).
Safety and Side Effects
SGLT2 inhibitors are characterized by an attractive efficacy profile and a long list of adverse drug reactions. Although the net clinical benefit is positive for most patients, some side effects are serious, even potentially life threatening. Most of these effects were not recognized when the drugs were first approved but became apparent after approval. Most of these reactions are mechanism based and likely to be class effects. Nevertheless, individual SGLT2 inhibitors may vary with respect to the magnitude of the safety concern. For example, the approved dose of canagliflozin is at the top of the dose-response curve, whereas the approved dose of dapagliflozin has submaximal efficacy. Selection of a lower dose may represent a trade-off in which submaximal efficacy is the price for enhanced safety.
Genitourinary and Perineal Infections
SGLT2 inhibitors increase glucose concentrations in urine, which creates a rich culture medium for bacteria, thereby increasing the risk of urinary tract infections (Table 3). Glucosuria indirectly increases glucose concentrations on genital skin, which increases the risk of external genital infections, especially in women (Table 3). While many SGLT2 inhibitor–induced genitourinary infections are minor and readily treated, infections can be recurrent and may exert a substantial adverse effect on quality of life. Indeed, mycotic genital infections are reported to affect as many as 5–10% of women with type 2 diabetes receiving SGLT2 inhibitors. Furthermore, the FDA has warned that some patients experience serious urosepsis or potentially life-threatening necrotizing fasciitis of the perineum (Fournier gangrene). Fortunately, these serious infections are rare.
Table 3.
SGLT2 inhibitors: safety issues
| Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | |
|---|---|---|---|---|
| Urinary tract infections (low dose)* | 2.1 (100 mg) | 2.0 (5 mg) | 1.7 (10 mg) | 0.1 (5 mg) |
| Urinary tract infections (high dose)* | 0.6 (300 mg) | 0.6 (10 mg) | 0 (25 mg) | 0.2 (15 mg) |
| Genital infections (female) (low dose)* | 7.8 (100 mg) | 6.9 (5 mg) | 3.9 (10 mg) | 6.1 (5 mg) |
| Genital infections (female) (high dose)* | 8.8 (300 mg) | 5.4 (10 mg) | 4.9 (25 mg) | 9.2 (15 mg) |
| Genital infections (male) (low dose)* | 3.5 (100 mg) | 2.5 (5 mg) | 2.7 (10 mg) | 3.3 (5 mg) |
| Genital infections (male) (high dose)* | 3.1 (300 mg) | 2.4 (10 mg) | 1.2 (25 mg) | 3.8 (15 mg) |
| Δ LDL cholesterol (mg/dL) (low dose) | +4.5 (100 mg) | +2.3 (10 mg) | +2.6 (5 mg) | |
| Δ LDL cholesterol (mg/dL) (high dose) | +8.0 (300 mg) | +3.9 (10 mg) | +4.2 (25 mg) | +5.4 (15 mg) |
| Amputations (placebo) | 1.5 (placebo) | 0.1 (placebo) | ||
| Amputations (low dose) | 3.5 (100 mg) | 0.2 (5 mg) | ||
| Amputations (high dose) | 3.1 (300 mg) | 0.5 (15 mg) | ||
| Bone fractures (canagliflozin) | 4.0 (CANVAS) | |||
| Bone fractures (placebo) | 2.6 (CANVAS) |
Data are %, unless otherwise indicated. Selected data on various adverse effects are summarized for the four FDA-approved SGLT2 inhibitors and have been taken from the FDA-approved prescribing information for each drug. In some cases, the prescribing information provides data separately for placebo- and drug-treated patients. In those cases, we have calculated placebo-subtracted data by subtracting the data on placebo-treated patients from data on drug-treated patients. Puckrin et al. (84) conducted a meta-analysis of 86 randomized clinical trials. They reported a 3.37-fold (95% CI 2.89–3.93) increase in the risk of genital infections relative to placebo and a 3.89-fold (95% CI 3.14–4.82) increase relative to active comparators. They did not observe an increased risk of urinary tract infections: risk relative to placebo 1.03 (95% CI 0.96–1.11) and risk relative to active comparator 1.08 (0.93–1.25).
*Calculated by subtracting incidence in placebo-treated patients from incidence in drug-treated patients.
Exaggerated Pharmacology
Glucose Lowering
SGLT2 inhibitors do not ordinarily cause hypoglycemia in healthy volunteers and are unlikely to induce hypoglycemia when administered as monotherapy or in combination with non–hypoglycemia-inducing drugs such as metformin, DPP-4 inhibitors, thiazolidinediones, or glucagon-like peptide 1 receptor agonists. Nevertheless, there are pharmacodynamic interactions with hypoglycemia-inducing drugs. By decreasing baseline glucose levels, SGLT2 inhibitors increase the risk of hypoglycemia as a result of either insulin or sulfonylureas. The prescribing information recommends that physicians should consider a lower dose of insulin or insulin secretagogue to reduce the risk of hypoglycemia when combined with an SGLT2 inhibitor.
Ketoacidosis
SGLT2 inhibitors increase ketone body levels in healthy volunteers and patients with type 2 diabetes (45,47). In a small subpopulation of patients with type 2 diabetes, ketone body levels increase to the point where they cause ketoacidosis (63,64). Several factors have been suggested to increase the risk of SGLT2 inhibitor–induced ketoacidosis: anti-GAD65 autoantibodies, recent surgery, recent alcohol intake, and markedly decreased food intake (63,64). Some cases of SGLT2 inhibitor–associated ketoacidosis occur in the absence of marked hyperglycemia (e.g., <250 mg/dL) (63). This form of euglycemic ketoacidosis sometimes presents diagnostic challenges, leading to delay in initiation of appropriate therapy.
The risk of ketoacidosis is markedly increased in patients with type 1 diabetes receiving off-label treatment with an SGLT2 inhibitor, especially those whose insulin dose was decreased to mitigate the risk of hypoglycemia. At least eight clinical trials have been published evaluating the efficacy and safety of SGLT2 inhibitors in combination with insulin to treat patients with type 1 diabetes (65–72). Among the 4,076 SGLT2 inhibitor–treated patients in these eight clinical trials, 3.5% developed ketoacidosis, corresponding to a 5.8-fold increase in risk relative to the 0.6% risk among the 2,362 placebo-treated patients. The U.S. FDA estimated that there would be one additional case of ketoacidosis for every 26 patients receiving sotagliflozin. For every 100,000 patients with type 1 diabetes receiving SGLT2 inhibitors as adjunctive therapy, these data suggest there might be ∼4,000 additional cases of ketoacidosis. If one assumes a case fatality rate of 0.4% for diabetic ketoacidosis, this suggests that SGLT2 inhibitors might cause ∼16 additional deaths. Risks would likely be even greater if these drugs become commonly used in a real-world setting compared with clinical trials with highly selected patients and highly experienced clinicians. This dramatic increase in ketoacidosis is associated with relatively modest glycemic efficacy. For example, in the inTandem1 and inTandem2 (Efficacy, Safety, and Tolerability of Sotagliflozin as Adjunct Therapy in Adult Patients With Type 1 Diabetes Mellitus Who Have Inadequate Glycemic Control With Insulin Therapy) trials, the maximal decrease in HbA1c occurred after ∼8 weeks of sotagliflozin therapy. At 24 weeks, the placebo-subtracted decrease in HbA1c was in the range of 0.35–0.41% (3–4 mmol/mol); by 52 weeks, glycemic efficacy had waned to a placebo-subtracted decrease in HbA1c in the range of 0.21–0.32% (2–3 mmol/mol) (66,71). None of the eight clinical trials presented data to confirm whether glycemic efficacy is sustained beyond 52 weeks. When health authorities considered SGLT2 inhibitors to treat type 1 diabetes, they considered whether incremental improvement in glycemic control outweighs the increased risk of these potentially life-threatening side effects. In any case, in February 2019, the European Committee for Medicinal Products for Human Use recommended indications for the use of both dapagliflozin and sotagliflozin as adjunctive therapies for type 1 diabetes. On 22 March 2019, the U.S. FDA announced its decision not to approve sotagliflozin as adjunctive therapy for type 1 diabetes.
Natriuretic Effects
SGLT2 inhibitors promote natriuresis and plasma volume contraction, which are associated with decreased blood pressure. This mechanism likely contributes to several side effects listed in the prescribing information: hypotension, acute kidney injury, and impairment in renal function. These risks are greater in patients with preexisting renal impairment and the elderly as well as in patients receiving diuretics, ACE inhibitors, or angiotensin receptor blockers.
Amputations
The prescribing information for canagliflozin and ertugliflozin warns of an increased risk of lower-limb amputations, especially toes and the forefoot (3). While the mechanism of this risk has not been established, it is noteworthy that thiazide diuretics were associated with a sixfold increase in the risk of lower-limb amputations in patients with diabetes compared with ACE inhibitor–treated patients (73). This suggests that the increased risk of amputations may be mediated by volume contraction, which might decrease perfusion pressure to a foot in which blood flow is already compromised by atherosclerotic peripheral artery disease. This pathophysiology may be exacerbated by increased whole-blood viscosity as a result of increased concentration of erythrocytes in the case of SGLT2 inhibitors. Similarly, thiazide diuretics have been reported to increase blood viscosity (74,75). Increases in whole-blood viscosity may reduce blood flow in the dermal microvasculature, particularly where cutaneous blood flow may already be reduced by peripheral neuropathy (76,77). The prescribing information for canagliflozin recommends that the drug be discontinued if a patient develops a foot ulcer or a foot infection.
Bone Fracture
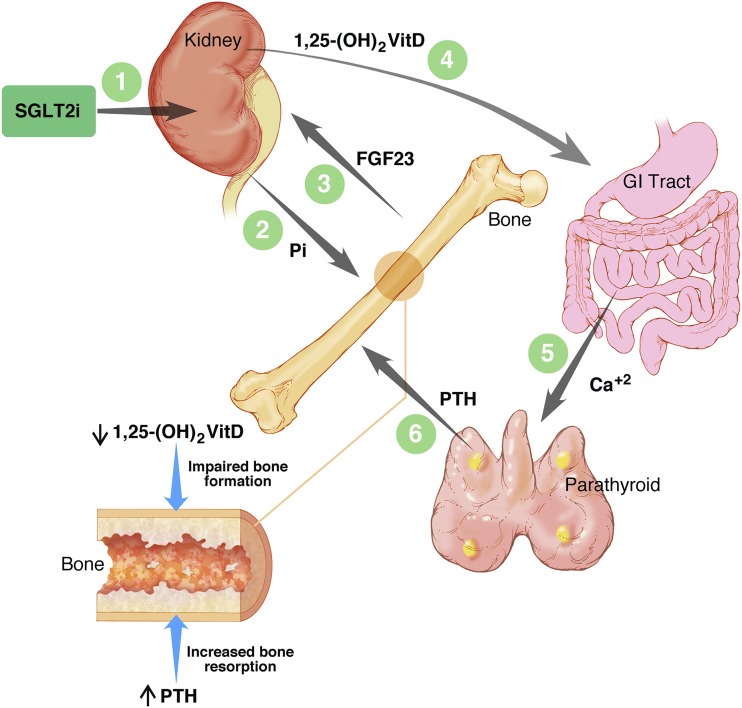
Canagliflozin has been reported to accelerate loss of bone mineral density and increase the risk of fracture (78). While dapagliflozin increased the risk of fracture in patients with impaired renal function (79), adverse effects on bone health have not been reported for dapagliflozin in patients with normal renal function or with either empagliflozin or ertugliflozin. The prescribing information for canagliflozin warns of increased fracture risk, but this is not presently treated as a class effect for all SGLT2 inhibitors. Several factors have been proposed as possible mediators of the increased fracture risk (78): increased risk of falls as a result of postural hypotension, drug-induced weight loss, and decreased estradiol levels in women. We have proposed an alternative hypothesis (18) (Fig. 2). As discussed above, canagliflozin triggers an increase in serum phosphorus by promoting renal tubular reabsorption of phosphate (18). This in turn triggers an increase in plasma fibroblast growth factor 23 (FGF23), a decrease in 1,25-dihydroxyvitamin D, and an increase in parathyroid hormone (PTH) levels. These endocrine changes would be predicted to impair bone formation and increase bone resorption (Fig. 2). Because there is considerable interindividual variation in the magnitude of these effects, we proposed that patients with the most marked changes in 1,25-dihydroxyvitamin D and PTH levels might be at greatest risk for bone loss and fracture (18).
Figure 2.
Impact of canagliflozin on bone health: pharmacodynamic effects on hormones regulating bone and mineral metabolism. SGLT2 inhibitors promote tubular reabsorption of phosphate, thereby increasing serum phosphorus levels. In pharmacodynamic studies of canagliflozin in healthy volunteers (18), the increase in serum phosphorus was followed promptly by an increase in plasma FGF23, which in turn triggered a decrease in plasma levels of 1,25-dihydroxyvitamin D [1,25-(OH)2VitD]. The decrease in 1,25-(OH)2VitD levels was followed by an increase in plasma levels of parathyroid hormone (PTH) presumably triggered by decreased gastrointestinal absorption of calcium. The increased levels of serum phosphorus and FGF23 returned toward baseline levels in this 5-day study conducted in healthy volunteers. However, in studies of canagliflozin in patients with type 2 diabetes, the increase in serum phosphorus (Pi) was sustained for at least 26 weeks (80). On the lower left side of the diagram, similar to the pathophysiology of bone disease associated with chronic kidney disease, the decrease in 1,25-(OH)2VitD and increase in PTH (18,81,82) may contribute to mediating the adverse effect of canagliflozin on bone health (78,83). Illustration by T. Phelps, used with permission from the Department of Art as Applied to Medicine, Johns Hopkins University.
Concluding Remarks
Research on SGLT2 inhibitors began almost 200 years ago with the discovery of phlorizin. DeFronzo and colleagues (10–12) suggested that drug-induced glycosuria offers an innovative therapeutic approach to the treatment of diabetes. Subsequently, mutations in SLC5A2 (the gene encoding SGLT2) were identified as the cause of familial renal glucosuria (14). Over the past two decades, pharmaceutical industry research translated these scientific insights into selective SGLT2 inhibitors, drugs that are used by >1 million patients with type 2 diabetes. These drugs provide many benefits: improved glycemic control, weight loss, and decreased blood pressure. Compared with placebo, SGLT2 inhibitors have been demonstrated to decrease the risk of MACE and slow the progression of diabetic kidney disease. While most patients derive net clinical benefit, health authorities have identified a number of serious adverse drug reactions, some of them potentially life threatening. Challenges remain to place this class of drugs into the context of precision medicine to define criteria enabling physicians to prescribe SGLT2 inhibitors to patients likely to derive the greatest benefit and least likely to experience serious harm. This class of drugs represents a triumph for the biomedical research enterprise, incorporating important contributions from both academia and industry to translate scientific insights into innovative therapies to benefit people struggling with diabetes.
Article Information
Funding. The authors acknowledge grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01-DK-118942 to A.L.B. and S.I.T. and 5R21-DK-105401 to S.I.T).
Duality of Interest. A.L.B. receives partial research support provided to the University of Maryland School of Medicine by Regeneron Pharmaceuticals. B.R.L. was previously employed by Bristol-Myers Squibb (2006–2012), Janssen Research and Development (2013–2014), and Pfizer (2014–2017) and owns stock in Bristol-Myers Squibb, Merck, Pfizer, and Eli Lilly. S.I.T. discloses previous employment at Bristol-Myers Squibb (2000–2013), consultancy for Ionis Pharmaceuticals, research support provided to the University of Maryland School of Medicine by Regeneron Pharmaceuticals, and ownership of stock in Celgene. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 2.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 4.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017;26:27–38 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi L, Díez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One 2010;5:e10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaere F, Duchampt A, Mounien L, et al. . The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab 2012;2:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev 2005;21:31–38 [DOI] [PubMed] [Google Scholar]
- 9.Jörgens V. The roots of SGLT inhibition: Laurent-Guillaume de Koninck, Jean Servais Stas and Freiherr Josef von Mering. Acta Diabetol 2019;56:29–31 [DOI] [PubMed] [Google Scholar]
- 10.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest 1991;87:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldring W, Welsh C. The effects on renal activity of the oral administration of phlorizin in man. J Clin Invest 1934;13:749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santer R, Kinner M, Lassen CL, et al. . Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003;14:2873–2882 [DOI] [PubMed] [Google Scholar]
- 15.Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol 2010;5:133–141 [DOI] [PubMed] [Google Scholar]
- 16.Meng W, Ellsworth BA, Nirschl AA, et al. . Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2008;51:1145–1149 [DOI] [PubMed] [Google Scholar]
- 17.Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res 2015;12:101–110 [DOI] [PubMed] [Google Scholar]
- 18.Blau JE, Bauman V, Conway EM, et al. . Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 2018;3:e99123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherney DZ, Perkins BA, Soleymanlou N, et al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Takano K, Iijima H, et al. . Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther 2017;34:436–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sha S, Polidori D, Heise T, et al. . Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014;16:1087–1095 [DOI] [PubMed] [Google Scholar]
- 22.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Takahashi M, Yan K, Sakurai H. Expression of SLC2A9 isoforms in the kidney and their localization in polarized epithelial cells. PLoS One 2014;9:e84996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chino Y, Samukawa Y, Sakai S, et al. . SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014;35:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitts RF, Alexander RS. The renal reabsorptive mechanism for inorganic phosphate in normal and acidotic dogs. Am J Physiol 1944;142:648–662 [Google Scholar]
- 26.Tang H, Zhang X, Zhang J, et al. . Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia 2016;59:2546–2551 [DOI] [PubMed] [Google Scholar]
- 27.Weir MR, Kline I, Xie J, Edwards R, Usiskin K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 2014;30:1759–1768 [DOI] [PubMed] [Google Scholar]
- 28.Massara F, Martelli S, Cagliero E, Camanni F, Molinatti GM. Influence of glucagon on plasma levels of potassium in man. Diabetologia 1980;19:414–417 [DOI] [PubMed] [Google Scholar]
- 29.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonner C, Kerr-Conte J, Gmyr V, et al. . Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 32.Pedersen MG, Ahlstedt I, El Hachmane MF, Göpel SO. Dapagliflozin stimulates glucagon secretion at high glucose: experiments and mathematical simulations of human A-cells. Sci Rep 2016;6:31214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazaridis KN, Pham L, Vroman B, de Groen PC, LaRusso NF. Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am J Physiol 1997;272:G1168–G1174 [DOI] [PubMed] [Google Scholar]
- 34.Sembrowich WL, Anderson CR, Kennedy WR, inventors. Method and apparatus for non-invasively monitoring plasma glucose levels. U.S. patent 5,036,861A. 6 August 1991
- 35.Ono E, Murota H, Mori Y, et al. . Sweat glucose and GLUT2 expression in atopic dermatitis: implication for clinical manifestation and treatment. PLoS One 2018;13:e0195960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamidi RN, Cuyckens F, Chen J, et al. . Metabolism and excretion of canagliflozin in mice, rats, dogs, and humans. Drug Metab Dispos 2014;42:903–916 [DOI] [PubMed] [Google Scholar]
- 37.Francke S, Mamidi RN, Solanki B, et al. . In vitro metabolism of canagliflozin in human liver, kidney, intestine microsomes, and recombinant uridine diphosphate glucuronosyltransferases (UGT) and the effect of genetic variability of UGT enzymes on the pharmacokinetics of canagliflozin in humans. J Clin Pharmacol 2015;55:1061–1072 [DOI] [PubMed] [Google Scholar]
- 38.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 39.Lavalle-González FJ, Januszewicz A, Davidson J, et al. . Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Häring HU, Merker L, Seewaldt-Becker E, et al.; EMPA-REG MET Trial Investigators . Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014;37:1650–1659 [DOI] [PubMed] [Google Scholar]
- 41.Dagogo-Jack S, Liu J, Eldor R, et al. . Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab 2018;20:530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha S, Polidori D, Farrell K, et al. . Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab 2015;17:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merovci A, Mari A, Solis-Herrera C, et al. . Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab 2015;100:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrannini E, Baldi S, Frascerra S, et al. . Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195 [DOI] [PubMed] [Google Scholar]
- 46.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22 [DOI] [PubMed] [Google Scholar]
- 47.Kaku K, Watada H, Iwamoto Y, et al.; Tofogliflozin 003 Study Group . Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 2014;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: subanalysis of placebo-controlled, double-blind clinical trials. J Diabetes Investig 2018;9:638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöström CD, Johansson P, Ptaszynska A, List J, Johnsson E. Dapagliflozin lowers blood pressure in hypertensive and non-hypertensive patients with type 2 diabetes. Diab Vasc Dis Res 2015;12:352–358 [DOI] [PubMed] [Google Scholar]
- 50.Cherney DZ, Perkins BA, Soleymanlou N, et al. . The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubota Y, Yamamoto T, Tara S, et al. . Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: rationale. Diabetes Ther 2018;9:2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka A, Shimabukuro M, Okada Y, et al.; EMBLEM Trial Investigators . Rationale and design of a multicenter placebo-controlled double-blind randomized trial to evaluate the effect of empagliflozin on endothelial function: the EMBLEM trial. Cardiovasc Diabetol 2017;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 54.Nathan DM, Buse JB, Kahn SE, et al.; GRADE Study Research Group . Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beddhu S, Chertow GM, Greene T, et al. . Effects of intensive systolic blood pressure lowering on cardiovascular events and mortality in patients with type 2 diabetes mellitus on standard glycemic control and in those without diabetes mellitus: reconciling results from ACCORD BP and SPRINT. J Am Heart Assoc 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inzucchi SE, Zinman B, Fitchett D, et al. . How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]
- 57.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathan DM, Bayless M, Cleary P, et al.; DCCT/EDIC Research Group . Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes 2013;62:3976–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 2016;8:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanton RC. Sodium glucose transport 2 (SGLT2) inhibition decreases glomerular hyperfiltration: is there a role for SGLT2 inhibitors in diabetic kidney disease? Circulation 2014;129:542–544 [DOI] [PubMed] [Google Scholar]
- 61.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115–1122 [DOI] [PubMed] [Google Scholar]
- 62.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 2017;13:11–26 [DOI] [PubMed] [Google Scholar]
- 63.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015;100:2849–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 66.Buse JB, Garg SK, Rosenstock J, et al. . Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathieu C, Dandona P, Gillard P, et al.; DEPICT-2 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 68.Garg SK, Strumph P. Effects of sotagliflozin added to insulin in type 1 diabetes. N Engl J Med 2018;378:967–968 [DOI] [PubMed] [Google Scholar]
- 69.Dandona P, Mathieu C, Phillip M, et al.; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:864–876 [DOI] [PubMed] [Google Scholar]
- 70.Dandona P, Mathieu C, Phillip M, et al.; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 2018;41:2552–2559 [DOI] [PubMed] [Google Scholar]
- 71.Danne T, Cariou B, Banks P, et al. . HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 72.Rosenstock J, Marquard J, Laffel LM, et al. . Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 73.Erkens JA, Klungel OH, Stolk RP, Spoelstra JA, Grobbee DE, Leufkens HG. Antihypertensive drug therapy and the risk of lower extremity amputations in pharmacologically treated type 2 diabetes patients. Pharmacoepidemiol Drug Saf 2004;13:139–146 [DOI] [PubMed] [Google Scholar]
- 74.Khder Y, Bray des Boscs L, el Ghawi R, et al. . Calcium antagonists and thiazide diuretics have opposite effects on blood rheology and radial artery compliance in arterial hypertension: a randomized double-blind study. Fundam Clin Pharmacol 1998;12:457–462 [DOI] [PubMed] [Google Scholar]
- 75.Stoltz JF, Zannad F, Kdher Y, et al. . Influence of a calcium antagonist on blood rheology and arterial compliance in hypertension: comparison with a thiazide diuretic. Clin Hemorheol Microcirc 1999;21:201–208 [PubMed] [Google Scholar]
- 76.Cho YI, Mooney MP, Cho DJ. Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol 2008;2:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des 2005;11:2301–2309 [DOI] [PubMed] [Google Scholar]
- 78.Watts NB, Bilezikian JP, Usiskin K, et al. . Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon H. Canagliflozin: clinical efficacy and safety. Endocrinology and Metabolic Drugs Advisory Committee Meeting [Internet], 2013. Available from https://wayback.archive-it.org/7993/20170405220021/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM336234.pdf. Accessed on 8 July 2015
- 81.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015;3:8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol 2018;14:473–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bilezikian JP, Watts NB, Usiskin K, et al. . Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 2016;101:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol 2018;55:503–514 [DOI] [PubMed] [Google Scholar]