Abstract
Gibberellins (GA) are key positive regulators of seed germination. Although the GA effects on seed germination have been studied in a number of species, little is known about the transcriptional reprogramming modulated by GA during this phase in species other than Arabidopsis thaliana. Here we report the transcriptome analysis of soybean embryonic axes during germination in the presence of paclobutrazol (PBZ), a GA biosynthesis inhibitor. We found a number of differentially expressed cell wall metabolism genes, supporting their roles in cell expansion during germination. Several genes involved in the biosynthesis and signaling of other phytohormones were also modulated, indicating an intensive hormonal crosstalk at the embryonic axis. We have also found 26 photosynthesis genes that are up-regulated by PBZ at 24 hours after imbibition (HAI) and down-regulated at 36 HAI, which led us to suggest that this is part of a strategy to implement an autotrophic growth program in the absence of GA-driven mobilization of reserves. Finally, 30 transcription factors (mostly from the MYB, bHLH, and bZIP families) were down-regulated by PBZ and are likely downstream GA targets that will drive transcriptional changes during germination.
Subject terms: Plant sciences, Computational biology and bioinformatics
Introduction
Gibberellins (GAs) constitute a large family of diterpenoid compounds that are ubiquitous in higher plants. Some GAs regulate processes such as seed germination, root, and stem elongation, leaf expansion, flower, and fruit development1,2. Seed germination typically starts with imbibition and ends with testa rupture, followed by emergence of the embryonic axis3. During this relatively short period, metabolic activity resumes, mitochondria and DNA damaged during desiccation are repaired, stored mRNAs are translated or degraded, and new transcriptional programs are activated. This complex series of interconnected events is fueled by the mobilization of stored reserves and gradually shifts towards photosynthesis and autotrophic growth4–6.
Over the past decades, seminal studies unequivocally demonstrated the role of GA in promoting seed germination7, in particular because GA-deficient mutants (e.g. ga1-3 and ga2-1) often require exogenous GA to germinate8,9. Further, the inhibition of radicle emergence in the presence of GA biosynthesis inhibitors (e.g. uniconazole and paclobutrazol, PBZ) indicates that GA is essential for seed germination10–12. PBZ is a plant growth retardant that blocks GA biosynthesis by inhibiting kaurene oxidase13. Other key GA biosynthesis enzymes are GA20- and GA3-oxidases (GA20ox and GA3ox, respectively), whereas GA2-oxidases (GA2ox) inactivate GA. During late germination, GA is synthesized at the radicle, hypocotyls, and micropylar endosperm14. GA is recognized by soluble receptors of the GIBBERELLIN INSENSITIVE DWARF1 (GID1) family15, which comprises the subfamilies GID1ac and GID1b in eudicots. Although very similar at the primary sequence level, different lines of evidence indicate that these subfamilies are functionally divergent2,16,17. The GA-GID1 complex promotes the degradation of DELLA transcriptional repressors via the 26S proteasome pathway18. Further, enhanced germination has been reported in loss-of-function DELLA-mutants19. GA is also notorious for its antagonistic interactions with ABA, a well-known seed germination inhibitor. In addition, GA has also been proposed to positively interact with brassinosteroids (BRs) and ethylene, which are ABA antagonists during seed germination19–21.
During seed germination, GA enhances embryo growth by promoting cell elongation and weakening of the surrounding tissues14,19. Several genes regulated by GA or DELLA have been identified during Arabidopsis seed germination, seedling and floral development14,22–24. In addition, various genes related to hormone pathways and cell wall metabolism were modulated by GA14,22. Despite the valuable information accumulated on the biochemical details of GA signaling and interactions with other hormones, little is known about the transcriptional programs driven by GA in germinating seeds of species other than A. thaliana. To date, only one report investigated the transcriptome of embryonic axes during soybean (Glycine max) germination25. Although this study showed a conspicuous activation of GA biosynthesis genes, it does not allow one to distinguish GA-driven transcriptional alterations. In the present work, we report the transcriptome of soybean embryonic axes during seed germination in the presence of the GA biosynthesis inhibitor PBZ, aiming to uncover the genes that are regulated by GA. We show that PBZ: 1) up-regulates several photosynthesis genes; 2) modulates the expression of numerous genes involved in the biosynthesis, signaling, and transport of other hormones, suggesting an intensive hormonal cross-talk during germination; 3) modulates the expression of several genes encoding cell wall modifying enzymes, supporting their roles in embryo cell expansion during germination and; 4) represses several transcription factors (TFs) in a time-specific fashion, indicating that these TFs might drive the transcriptional reprogramming mediated by GA during germination.
Results and Discussion
Transcriptome sequencing and functional analysis of differentially expressed genes
We conducted an initial assay to investigate the effects of PBZ on soybean seed germination. A pilot experiment was used to define the PBZ concentration used here (not shown). As expected, PBZ administration reduced radicle length, fresh weight and dry weight, resulting in a delay in germination (Supplementary Figure S1). Embryonic axes at 12, 24, and 36 hours after imbibition (HAI) were carefully separated from the cotyledons and submitted to RNA extraction, library preparation, and sequencing on an Illumina HiSeq. 2500 instrument (see methods for details). A total of 18 libraries (three biological replicates, with or without PBZ) were sequenced, resulting in a total of 14 to 67 million reads per sample (Supplementary Table S1). High-quality reads were mapped to the soybean reference genome (Wm82.a2.v1) and used for downstream analysis. Overall, 97.2% of the reads mapped to the reference genome (Supplementary Table S1). In general, we found good correspondence (Supplementary Figure S2) and high pair-wise correlations (0.95 to 0.99) (Supplementary Table S2) between the biological replicates. Genes with RPKM (Reads Per Kilobase per Million mapped reads) greater than or equal to 1 were considered expressed. In total, 29,204, 29,467, 31,065, 30,887, 32,636, and 32,466 genes were found to be expressed in 12C (control), 12P (PBZ), 24C, 24P, 36C, and 36P, respectively (Fig. 1A). Approximately 62.43% of the soybean protein-coding genes (34,990 genes) were expressed in at least at one time point (Supplementary Table S3), which is comparable to a previously published soybean germination transcriptome25.
Figure 1.
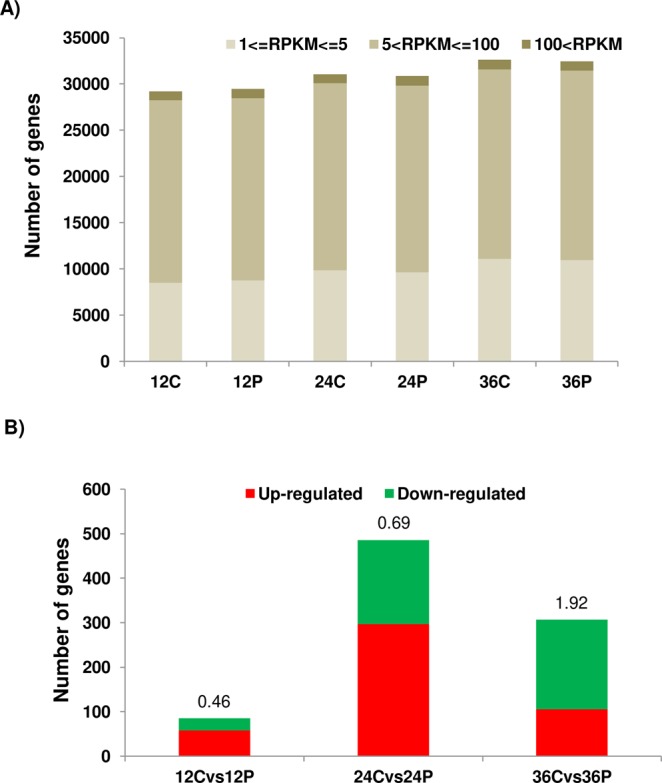
Gene expression profiling during seed germination. (A) Number of expressed genes (RPKM ≥1) and their estimated expression levels in each sample. (B) Number of DEGs at 12, 24 and 36 HAI. Numbers above the vertical bars stand for the ratio between down- and up-regulated genes. In the x-axes labels, C and P stand for control and PBZ, respectively.
We compared the transcriptional profiles of PBZ-treated seeds at each time point with their respective controls and found a total of 85, 486, and 307 differentially expressed genes (DEGs) at 12, 24, and 36 HAI, respectively (Supplementary Table S4). Because PBZ is a GA antagonist, PBZ down- and up-regulated genes (i.e. PBZ-down and PBZ-up, respectively) are likely those induced and repressed by GA. The absolute number of genes down-regulated by PBZ and their ratios to up-regulated genes increased along germination (Fig. 1B; Supplementary Table S4). In DEG counts, 24 HAI was the most notable time point (with 297 and 189 up- and down-regulated genes, respectively; Fig. 1B). On the other hand, 12 HAI had the lowest number of DEGs (58 and 27 up- and down-regulated genes, respectively), indicating that GA transcriptional programs are mostly activated between 12 and 24 HAI and decrease afterwards, when most seeds had completed germination (Supplementary Figure S1). We tested the expression of 15 DEGs by real-time qPCR (RT-qPCR). Primer pairs for three of these genes failed to amplify a specific single PCR product and were not evaluated. All the remaining 12 genes had their transcripts successfully amplified and were confirmed as differentially expressed (Supplementary Figure S3). Thus, our RT-qPCR data support the findings obtained by the large-scale transcriptome comparisons.
Notably, we found 63 genes that are significantly up-regulated at 24 HAI and down-regulated at 36 HAI by PBZ (Supplementary Table S5, Supplementary Figure S4). About 41% (26 out of 63) of these genes are related with photosynthesis and their up-regulation by PBZ at 24 HAI followed by a down-regulation at 36 HAI might be a strategy to anticipate the transition to autotrophic growth in the absence of energetic resources resulting from proper GA signaling. This gene set encodes chloroplast ATP synthase subunits, RuBisCO, chloroplast ribosomal proteins, DNA-directed RNA polymerase subunit beta (rpoC1), YCF3, and several photosystem I and II subunits. Decrease in the expression of plastidial RNA polymerases (i.e. rpoB and rpoC1) caused aberrant chloroplast development and diminished photoautotrophic growth in A. thaliana26. Chloroplast YCF3 encodes a thylakoid protein that is essential for photosystem I complex biogenesis in tobacco27 and Chlamydomonas reinhardtii28. The regulation of photosynthesis genes by GA has also been recently demonstrated in rice seedlings under submergence29. Interestingly, all these 26 genes are nuclear encoded copies of genes that are located in the soybean chloroplast (Reference Sequence: NC_007942). Most of these copies seem to be functional, as they encode proteins with high sequence coverage (68 to 100%) and similarity (78 to 100%) with their plastidial counterparts (Supplementary Table S6). In the same way, 17 out of 63 genes encode proteins similar to those encoded by mitochondrial genes (Reference sequence: JX463295) (Supplementary Table S6). Collectively, these genes might integrate a system to reduce the dependence on cotyledonary reserves and optimize ATP production. Out of these 43 genes with organellar copies, 41 have been assigned to soybean reference chromosomes, suggesting that they are not annotated as nuclear genes because of contamination with organelle DNA fragments.
Gene Ontology and KEGG pathway enrichment analysis
Aiming to unravel major trends in the DEG lists, we conducted Gene Ontology (GO) and KEGG pathway enrichment analyses. There was no enrichment of GO terms or KEGG pathways at 12 HAI. In up-regulated genes at 24 HAI, we found a total of 19 enriched GO terms, including terms related with photosynthesis and translation (Supplementary Table S7). Three of the GO terms enriched in the genes up-regulated at 24 HAI were also found enriched in the genes down-regulated at 36 HAI, namely “generation of precursor metabolites and energy”, “photosynthesis”, and “thylakoid” (Supplementary Table S7), providing further support to the results discussed in the previous section.
KEGG pathway enrichment analysis revealed that ‘plant hormone signal transduction’ was enriched in down-regulated genes at 24 HAI and 36 HAI, supporting the regulation of other hormonal pathways by GA, and possibly their cross-talk, during germination (Fig. 2, Supplementary Table S8). These genes are involved in BR, auxin, jasmonic acid, ABA, and cytokinin signaling or biosynthesis. Given their indispensable roles in regulating seed germination, genes related with hormone signaling and biosynthesis are discussed in more detail in the next section. In down-regulated genes at 36 HAI, a number of genes encoding chaperones resulted in the enrichment of the pathway ‘protein processing in endoplasmic reticulum’. Phenylpropanoid biosynthesis genes were enriched in PBZ-down (7 genes) and PBZ-up genes (6 genes) at 24 HAI and 36 HAI, respectively. These genes include β-glucosidases, peroxidases, and spermidine hydroxycinnamoyl transferases that might be involved in cell wall modification or oxidative stress response (Supplementary Table S8). ‘Biosynthesis of secondary metabolites’ genes were enriched in PBZ-down (15 genes) at 24 HAI and, both in PBZ-up (23 genes) and PBZ-down genes (15 genes) at 36 HAI. Most of these PBZ-up genes encode UDP-glycosyltransferases, cytochrome P450 proteins, and brassinosteroid-6-oxidases, whereas PBZ-down genes encode 3-ketoacyl-CoA synthases, 1-amino-cyclopropane-1-carboxylate synthases (ACS), and peroxidases (Supplementary Tables S8). ‘Glutathione metabolism’, ‘RNA polymerase’, ‘purine metabolism’, ‘nucleotide excision repair’, ‘pyrimidine metabolism’, and spliceosome pathways were only enriched in up-regulated genes at 24 HAI (Supplementary Tables S8). All ‘glutathione metabolism’ DEGs encode glutathione-S-transferases (GSTs) and their up-regulation is related to an increased antioxidant capacity30. Increased antioxidant capacity and DNA repair mechanisms at 24 HAI in response to PBZ might be part of a tolerance mechanism to cope with the germination delay, which is in line with a recent study that proposed a link between DNA repair and antioxidant activity in Medicago truncatula seed germination and seedling establishment31.
Figure 2.
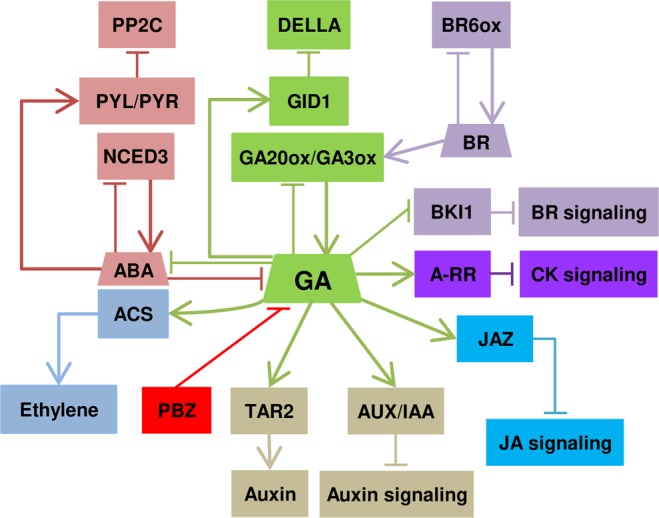
Schematic model of hormonal crosstalk with gibberellin during G. max seed germination. The model was derived from a careful literature curation based on differentially expressed genes discussed along the manuscript. Positive interactions are indicated by arrows and T bars indicate repression. Abbreviations: Pyrabactin Resistance (PYR); PYR-like (PYL); Protein Phosphatase 2 C (PP2C); Nine-cis-epoxycarotenoid dioxygenase 3 (NCED3); Abscisic acid (ABA); Aminocyclopropane-1-carboxylic acid synthase (ACS); Paclobutrazol (PBZ); Gibberellin (GA); GIBBERELLIN INSENSITIVE DWARF 1 (GID1); GA 20-oxidase (GA20ox); GA 3-oxidase (GA3ox); Tryptophan aminotransferases 2 (TAR2); Auxin/Indole-3-Acetic Acid(AUX/IAA); Brassinosteroid (BR); BR 6-oxidase (BR6ox); BRI1 kinase inhibitor (BKI1); Type-A response regulator (A-RR); JASMONATE ZIM DOMAIN (JAZ); Jasmonic acid (JA).
Feedback regulation and cross-talk with other hormones
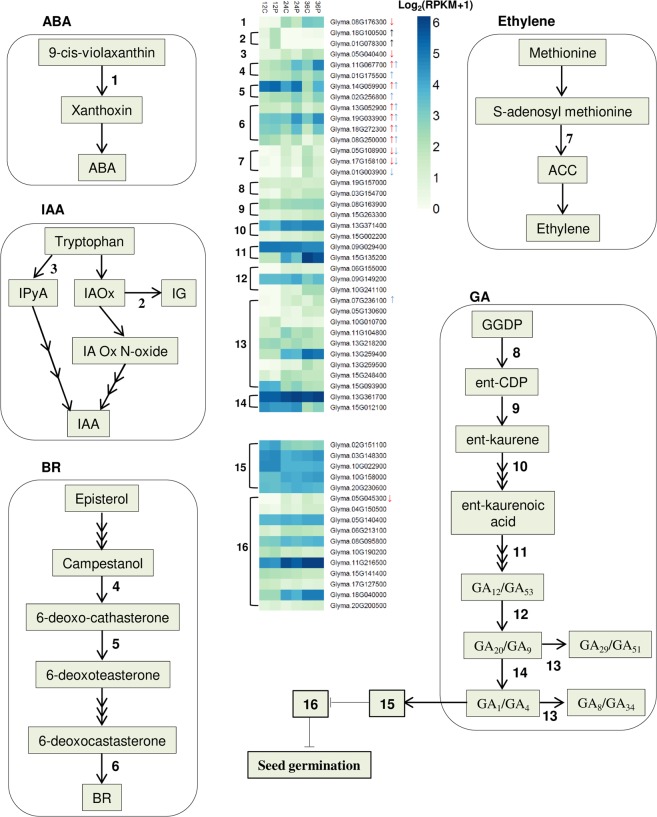
GA biosynthesis can be divided into early (CPS, KS, KO, and KAO) and late (e.g. GA20ox and GA3ox) stages1. While early GA biosynthesis genes are generally not affected by GA32, a negative GA-mediated feedback mechanism involving the down-regulation of late GA biosynthesis genes and up-regulation of the GA-deactivating GA2ox has been proposed as a system to keep balanced GA levels1. Although not included by our statistical thresholds, we found GA3ox and GA20ox genes with greater expression in the presence of PBZ at 24 HAI and 36 HAI (Fig. 3, Supplementary Table S9), which might indicate a compensating mechanism in response to PBZ. Long- and short-distance GA movement are also critical for developmental processes such as seed germination33. Recently, some transporters from the NPF and SWEET families were shown to transport GA in planta34,35. Curiously, NPF3 transports GA and ABA in A. thaliana35. We found two NPF3 genes strongly up-regulated by PBZ at 36 HAI, which is in accordance with the GA-mediated repression of NPF3 expression35. The spatiotemporal expression pattern of NPF3 has been proposed as a key aspect of its functionality35. In line with this, recent elegant works in A. thaliana showed that GA gradients correlate with cell length in dark-grown hypocotyls36,37. We hypothesize that this might be the case in soybean embryonic axes, particularly in the context of the recently described radicle-derived growth pattern in germinating soybean embryos38.
Figure 3.
Hormone biosynthesis pathways. Some GA deactivation and signaling genes discussed are also included. Up- and down-regulated genes are shown with up and down arrows. Black, red and blue arrows represent differential expression at 12, 24, and 36 HAI, respectively. Genes without arrows are expressed in at least one condition, although not classified as differentially expressed by our statistical thresholds. Genes are numbered as follows: 1) nine-cis-epoxycarotenoid dioxygenase 3 (NCED3); 2) SUR2; 3) tryptophan aminotransferase related 2 (TAR2); 4) DWARF4 (DWF4); 5) DWARF3 (DWF3); 6) brassinosteroid-6-oxidase 2 (BR6ox2); 7) 1-amino-cyclopropane-1-carboxylate synthase (ACS); 8) ent-copalyl diphosphate synthase (CPS); 9) ent-kaurene synthase (KS); 10) ent-kaurene oxidase (KO); 11) ent-kaurenoic acid oxidase (KAO); 12) GA 20-oxidase (GA20ox); 13) GA 2-oxidase (GA2ox); 14) GA 3-oxidase (GA3ox); 15) GIBBERELLIN INSENSITIVE DWARF1 (GID1) [Glyma.02G151100 (GID1b1), Glyma.10G022900 (GID1b2), Glyma.03G148300 (GID1b3), Glyma.10G158000 (GID1c1), and Glyma.20G230600 (GID1c2); 16) DELLA. Abbreviations: Abscisic Acid (ABA); indole-3-pyruvic acid (IPyA); Indol-3-acetaldoxime (IAOx); Indol-3-acetaldoxime N-oxide (IA Ox N-oxide); indole glucosinolates (IG); Indole-3-acetic acid (IAA); Brassinosteroid (BR); 1-aminocyclopropane-1-carboxylic acid (ACC); geranyl geranyl diphosphate (GGDP); ent-copalyl diphosphate (ent-CDP).
In addition to biosynthesis and transport, we have also investigated GA signaling genes. We found 11 DELLA genes (one PBZ-down at 24 HAI) and all 5 GID1s17 expressed in at least one time point (Fig. 3, Supplementary Table S9). Almost all DELLAs showed greater expression in the absence of PBZ (Fig. 3, Supplementary Table S9). The expression levels of GID1b1, GID1b2, and GID1b3 were greater in PBZ than in controls (except GID1b1 and GID1b3 at 24 HAI), supporting that GID1b is particularly important under low GA concentrations, as previously hypothesized by us and others2,17. Collectively, our results support that the low GA production resulting from PBZ administration activates an intricate system involving GA biosynthesis, signaling, and transport genes, probably to minimize the effects of impaired GA production to allow germination to occur.
Other phytohormones
ABA is the most notorious GA antagonist for its inhibitory effect on seed germination1,19. The regulatory step in ABA biosynthesis is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED), which is transcriptionally regulated by positive and negative feedback loops in different species39,40. The ABA receptor (PYL) inhibits the protein phosphatase 2 C (PP2C) in the presence of ABA41. We found one NCED3 (Glyma.08G176300) and two PP2Cs as PBZ-down and one PYL5 as PBZ-up (Figs 3, 4, Supplementary Table S9). In addition, two ABA transporters, ABCG40 (up-regulated, Glyma.19G169400) and NRT1.2 (down-regulated, Glyma.08G296000) were also differentially expressed upon PBZ treatment (Supplementary Table S9). Collectively, these results show that GA modulates different genes involved in ABA biosynthesis, signaling and transport, which might directly interfere with GA:ABA gradients along germinating soybean embryonic axes. This GA:ABA dynamics might be involved in the differential cell expansion patterns observed in germinating soybean embryos38.
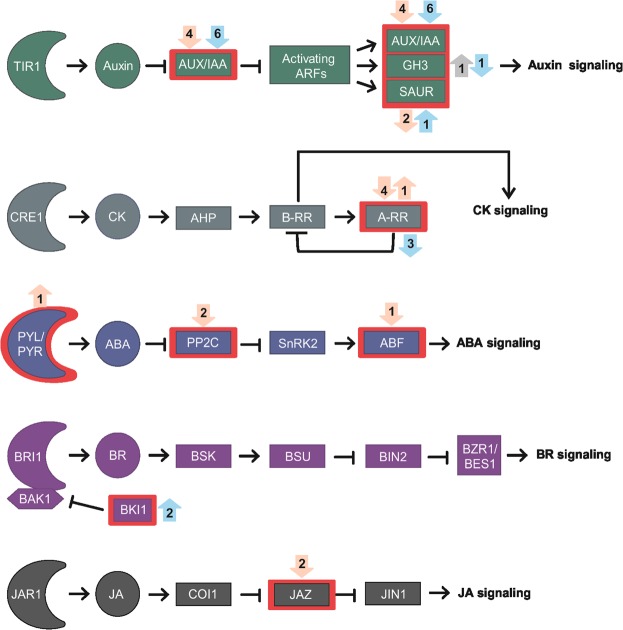
Figure 4.
Hormone signal transduction. Rectangles with red lines represent gene families with at least one DEG. Up and down arrows represent PBZ up- and down-regulated genes. Numbers of DEGs are shown in circles adjacent to the red rectangles. Grey, light orange, and light blue arrows represent DEGs at 12, 24, and 36 HAI, respectively. Abbreviations: transport inhibitor response 1 (TIR1); Auxin/Indole-3-Acetic Acid (Aux/IAA); auxin-responsive Gretchen Hagen3 (GH3); small auxin upregulated RNA (SAUR); CYTOKININ RESPONSE 1 (CRE 1); Cytokinin (CK); His-containing phosphotransfer protein (AHP); Type-B response regulator (B-RR); Type-A response regulator (A-RR); Pyrabactin Resistance (PYR); PYR-like (PYL); Abscisic acid (ABA); Protein Phosphatase 2C (PP2C); Sucrose non-fermenting 1-related protein kinases subfamily 2 (SnRK2s); Abscisic acid responsive element-binding factor (ABF); Brassinosteroid-insensitive 1 (BRI1); BRI1-associated receptor kinase 1 (BAK1); Brassinosteroid (BR); BRI1 kinase inhibitor (BKI1); Brassinosteroid signaling kinases (BSK); BRI1-suppressor (BSU); brassinosteroid-insensitive 2 (BIN2); Brassinazole-resistant 1 (BZR1); BRI1-ethyl methanesulfonate-suppressor 1 (BES1); JASMONATE RESISTANT 1 (JAR1); Jasmonic acid (JA); Coronatine Insensitive 1 (COI1); JASMONATE ZIM DOMAIN (JAZ); JASMONATE INSENSITIVE 1 (JIN1).
GA and ethylene positively interact with each other, promoting seed germination in several species42. Multiple lines of evidence, including PBZ administration, support the positive regulation of ethylene biosynthesis and signaling by GA14,43–46. Further, several ethylene biosynthesis genes are expressed in soybean embryonic axes during germination25. Accordingly, we found three PBZ-down 1-amino-cyclopropane-1-carboxylate synthase (ACS) genes (Fig. 3, Supplementary Table S9). ACS catalyzes the first committed and rate-limiting step in ethylene biosynthesis47. Our results suggest that up-regulation of ACS by GA is likely a key part of the synergy between GA and ethylene during soybean germination.
Several studies have shown that auxin inhibits or delays seed germination in wheat48, Arabidopsis49, and soybean50. On the other hand, exogenous GA4 up-regulated auxin biosynthesis and carrier genes in germinating Arabidopsis seeds14, supporting a complex GA-auxin cross-talk during soybean germination. There are multiple tryptophan-dependent IAA biosynthesis pathways in plants51. The tryptophan aminotransferases TAR1 and TAR2 convert trp to indole-3-pyruvate (IPA), which is converted to indole acetic acid (IAA) by the YUCCA flavin monooxygenase52. Further, superroot2 (SUR2) encodes the cytochrome P450 monooxygenase CYP83B1, involved in glucosinolate biosynthesis and auxin homeostasis53,54. We found two PBZ-up SUR2 at 12 HAI and one PBZ-down TAR2 at 24 HAI, indicating that GA promotes IAA production at these time points. We also found one auxin transporter (PIN, PBZ-up) and eleven auxin-responsive genes, including seven PBZ-down Auxin/Indole-3-Acetic Acid (Aux/IAA) repressors, small auxin upregulated RNA (SAUR), and the auxin-responsive Gretchen Hagen3 (GH3) family were differentially expressed at least at one of the time-point (Figs 3, 4, Supplementary Table S9). Although apparently conflicting with the promotion of IAA biosynthesis at 12 and 24 HAI, the down-regulation of several AUX/IAA genes by PBZ at 24 HAI and 36 HAI suggests that GA represses auxin signaling during late germination. Accordingly, three AUX/IAA genes have been recently demonstrated to promote hypocotyl elongation in A. thaliana55.
BRs typically induce seed germination and BR biosynthesis genes (DET2, DWF4, DWF3, BR6ox1, and ROT3) are up-regulated when endogenous BR concentrations are reduced56. Interestingly, six and eight BR biosynthesis genes were PBZ-up at 24 and 36 HAI, respectively (Fig. 3, Supplementary Table S9). BR promotes GA biosynthesis by regulating GA20ox1 and GA3ox1 expression in A. thaliana57. Further, GA partially rescued hypocotyl elongation defects resulting from BR deficiency57. Our group has proposed that BR signaling regulates cell expansion during soybean germination25. Taken together, the up-regulation of BR biosynthesis by PBZ might be involved in the activation of late GA biosynthesis genes to counter PBZ effects on GA production. This hypothesis also explains the observation that PBZ delays germination without a clear effect on germination rates (Supplementary Figure S1E). Finally, since BR also promotes GA biosynthesis in rice58, the emergence of this regulatory module probably predates the diversification of monocotyledonous and dicotyledonous species.
Antagonistic interactions between GA and cytokinin (CK) have been reported in different plants59–61. Type-A response regulators negatively regulate CK signaling by competing with type-B response regulators for phosphoryl transfer from the upstream Arabidopsis Hpt proteins or by interacting with other pathway components62. We found four and three PBZ-down type-A response regulators at 24 HAI and 36 HAI, respectively (Figs 2, 4, Supplementary Table S9). Since CK biosynthesis genes were not differentially expressed, our results indicate GA antagonizes CK by the up-regulation of negative CK signaling regulators during soybean germination.
In the canonical Jasmonic Acid (JA) signaling pathway, the receptor CORONATINE INSENSITIVE 1 (COI1) interacts with JA and promotes the proteasomal degradation of JASMONATE ZIM-domain (JAZ) repressors63. JAZ represses the transcription of JA-responsive genes through interaction with the MYC2 TF and other regulatory proteins63,64. JA and GA perform antagonistic roles in regulating hypocotyl elongation via physical interactions between JAZ and DELLA repressors. In summary, JA-mediated JAZ degradation releases DELLA to repress GA signaling (and hypocotyl elongation), whereas GA-mediated DELLA degradation releases JAZ to inhibit JA responses64,65. We found two PBZ-down JAZ genes at 24 HAI (Supplementary Table S9), indicating that GA represses JA signaling during germination. Interestingly, JAZ up-regulation might constitute an additional layer of JA repression, as GA-promoted DELLA degradation would release more JAZ proteins to repress JA signaling.
Gibberellins regulate cell wall remodeling enzymes
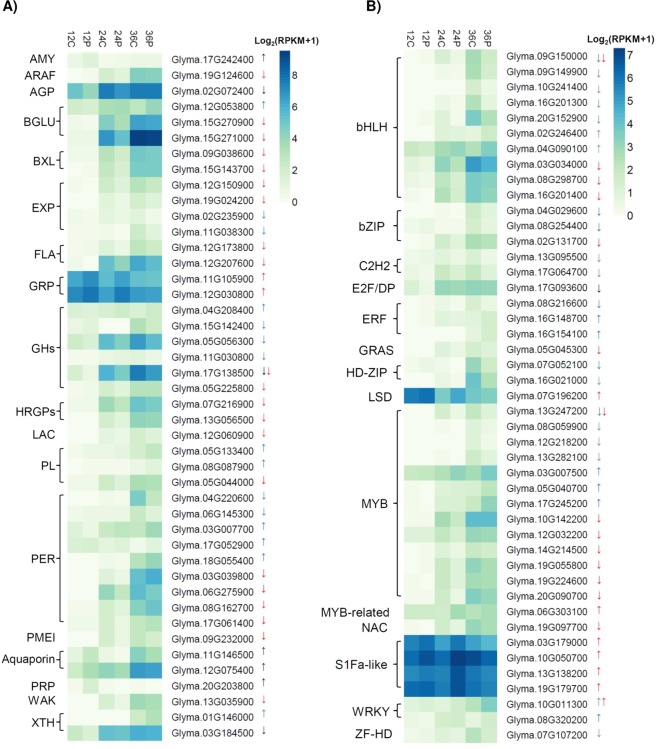
Several genes encoding cell elongation and cell wall remodeling enzymes, such as xyloglucan endotransglycosylase/hydrolases (XTH), pectin methylesterases (PME), expansins, pectin lyases, aquaporins, and others are induced by GA in Arabidopsis and tomato seed germination14,22,66–68. We found a number of these cell wall remodeling genes as differentially expressed (Fig. 5A, Supplementary Table S10). Peroxidases and glycosyl hydrolases (GHs) also play active roles in cell wall loosening69,70. Accordingly, nine and eight peroxidases and GHs were differentially expressed, respectively. Genes involved in pectin metabolism were also modulated by PBZ (Fig. 5A), suggesting that this process is also under GA regulation during germination. We also found other cell wall related DEGs, such as arabinogalactan-proteins, fasciclin-like AGPs, hydroxyproline (Hyp)-rich glycoproteins, and proline- or glycine-rich proteins, which play important roles in cell proliferation71–73 and expansion74. Several of those genes are also GA-responsive in cucumber, maize, and barley75–77. Importantly, 30 out of 44 cell wall DEGs were PBZ-down, supporting that the notorious effect of GA in promoting cell elongation.
Figure 5.
Genes encoding differentially expressed cell-wall remodeling enzymes (A) and transcription factors (B). Up- and down-regulated genes are shown with up and down arrows. Black, red, and blue arrows represent DEGs at 12, 24, and 36 HAI, respectively. Abbreviations: alpha amylase-like (AMY); alpha-L-arabinofuranosidase (ARAF); arabinogalactan protein (AGP); beta glucosidase (BGLU); beta-xylosidase (BXL); expansin (EXP); FASCICLIN-like arabinogalactan-protein (FLA); glycine-rich protein (GRP); Glycosyl hydrolase family protein (GH); hydroxyproline-rich glycoprotein family protein (HRGP); laccase (LAC); Pectin lyase-like superfamily protein (PL); Peroxidase superfamily protein (PER); pectin methylesterase inhibitor superfamily protein (PMEI); proline-rich protein (PRP); wall associated kinase (WAK); xyloglucan endotransglucosylase/hydrolase (XTH); basic helix-loop-helix (bHLH); Basic Leucine Zipper (bZIP); C2H2 zinc finger (C2H2); Ethylene response factor (ERF); GRAS (gibberellin insensitive (GAI), Repressor of ga1-3 (RGA), SCARECROW-LIKE 3 (SCR) gene family; Homeodomain-leucine zipper (HD-ZIP); LESION SIMULATING DISEASE (LSD); Myelobastosis (MYB); Zinc finger Homeodomain (ZF-HD); No apical meristem (NAM), ATAF, and CUC (cup-shaped cotyledon) (NAC) family.
Transcription factor genes modulated by paclobutrazol are likely drivers of GA-mediated transcriptional reprogramming
Because seed germination is mainly regulated by the embryonic axis, we have specifically investigated the differential expression of TFs in this tissue, as they might be major drivers of the GA transcriptional programs. A total of 45 TFs were differentially expressed upon PBZ treatment. Strikingly, one, 18, and 23 TFs were differentially expressed exclusively at 12, 24, and 36 HAI, respectively (Fig. 5B, Supplementary Table S11). This pattern indicates that differentially expressed TFs play specific roles at different germination times. Further, most of the differentially expressed TFs (66.7%) were down-regulated by PBZ and likely comprise regulators that are downstream of GA (Fig. 5B, Supplementary Table S11). The TF families with the greatest number of down-regulated members were MYB (myelobastosis; 10 down), bHLH (basic helix-loop-helix; 8 down), and bZIP (basic leucine zipper domain; 3 down), which is in line with previous studies in soybean25 and A. thaliana22 showing that MYB and bHLH are among the mostly activated TF families during germination. Interestingly, five and six of the PBZ-down MYB and bHLH genes, respectively, were also differentially expressed in a time-dependent manner during soybean germination25, further supporting that GA coordinates the transcription of specific TFs at different time points. Conversely, S1Fa-like (4 up) and WRKY (3 up) were the families that were most represented among PBZ-up TFs (Fig. 5B, Supplementary Table S11). S1Fa-like is a poorly-studied TF family that has been associated with photomorphogenesis78. Remarkably, all four soybean S1Fa-like TFs were strongly up-regulated by PBZ at 24 HAI, indicating that they might be part of the regulatory system to activate photosynthetic growth in response to low GA concentrations, as discussed above. Photomorphogenesis is regulated by a complex pathway involving GA and light in A. thaliana seedlings79,80. Nevertheless, no PIF or HY5 genes, which encode important regulators of photomorphogenesis, were modulated by PBZ.
Comparison with A. thaliana GA-responsive genes
Ogawa et al. identified a total of 230 and 127 up- and down-regulated genes during germination of A. thaliana ga1-3 seeds upon GA treatment14. Other study, also in A. thaliana, reported DEGs in imbibed seeds and developing flowers of wild type, ga1-3, and a quintuple DELLA null mutant (ga1 rga gai rgl1 rgl2)22. This latter study identified 541 and 571 up- and down-regulated GA-responsive genes in imbibed seeds. It is important to mention that Ogawa et al. used a microarray platform representing ~8,200 genes, while Cao et al. used one covering ~23,000 genes. This difference is likely an important factor accounting for the differences in DEG numbers between these studies. Overall, these studies have an overlap of 109 GA-up genes and 90 GA-down genes. Importantly, a significant fraction of these genes are also regulated by DELLA22.
Although A. thaliana and soybean are distantly related and their seeds are remarkably different, we investigated the conservation of the DEGs identified in A. thaliana described above with the ones reported here using BLASTP (minimum query coverage and similarity of 50%). We found 178 and 124 differentially expressed soybean orthologs for 122 and 84 A. thaliana GA-up and GA-down genes, respectively. These soybean gene sets were named GA-up-orthologs and GA-down-orthologs, respectively. Curiously, a significant part (47.19% and 55.66% of the GA-up-orthologs and GA-down-orthologs, respectively) of these genes are modulated in opposite directions in the two species (Supplementary Table S12). Nevertheless, most of the genes related with cell-wall modification, GSTs, auxin responsive genes (AUX/IAA and SAUR), oxidoreductases (aldo-ketoreductases), and transferases are modulated in same directions in soybean and A. thaliana, whereas genes modulated in opposite directions between the species encode HSPs, cytochrome p450, serine carboxypeptides, late embryogenesis proteins and flavonol synthase/flavanone 3-hydroxylase (Supplementary Table S12). Proportionally and in absolute numbers, 24 HAI is the stage with the most conserved DEG profile between A. thaliana and soybean. Further, 351 out of the 468 soybean DEGs without a DEG ortholog in A. thaliana do have orthologs in the A. thaliana genome, indicating that several orthologous genes are differentially regulated in the two species. Finally, in addition to the evolutionary distance, there are also important technical aspects that require consideration. The A. thaliana studies used microarrays to investigate modulated genes in ga1-3 mutants either upon treatment with exogenous GA14 or in contrast with wild type seeds during germination22. Here we analyzed an RNA-Seq transcriptome of embryonic axes of germinating soybean seeds treated with PBZ. Both experimental designs have limitations; even the A. thaliana ga1-3 dry seeds have bioactive GA from the GA treatment used to rescue parental fertility of mutant plants14. In addition, administration of exogenous GA may have unintended effects due to locations and concentrations different from those found under natural conditions. On the other hand, while allowing the investigation with more natural GA concentrations and locations, chemical inhibition of GA biosynthesis probably does not shutdown GA signaling completely. Further, it is not unreasonable to expect that the inhibitor effects might be overcome after some time, for example by an increase in the levels of GA biosynthesis enzymes. A more detailed picture of the interspecies conservation of GA-driven transcriptional programs will be clearer when more species are studied using state-of-the-art RNA-Seq technologies.
Material and Methods
Plant material and growth conditions
G. max seeds (BRS-284, from EMBRAPA, Brazil) were used in this study. Seeds were surface sterilized with 70% ethanol for 1 minute and with commercial bleach (1% v/v) for 3 minutes, followed by three washes with sterile distilled water (30 seconds per wash). Seeds were germinated in 15 cm Petri dishes with 2 g of sterile cotton in two conditions: in the presence of 30 ml of sterile water (control) or sterile water with 200 µM paclobutrazol (Sigma Aldrich), a concentration that resulted in significant reductions in axis length, fresh and dry mass (Supplementary Figure S1). These reductions are in line with the classical GA phenotype of diminished GA signaling. Seeds were allowed to germinate in an incubation chamber at 28 °C and 12/12 h photoperiod (dark/light). We used three plates per sample, with 20 seeds per plate. Embryonic axes from dry seeds were also collected. For total RNA extraction, seeds were harvested at 12, 24, and 36 HAI in control and PBZ treated conditions. Embryonic axes were separated from cotyledons and immediately placed in RNAlaterTM (Qiagen) until RNA extraction. RNA was extracted from harvested embryonic axes using RNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions. Three independent biological replicates of each condition were used.
RNA purification, sequencing, and analysis
RNA-Seq libraries were prepared using the TruSeq RNA Sample Preparation Kit v2 and submitted to 1 × 100 bp single-end sequencing on a HiSeq 2500 instrument at LaCTAD (UNICAMP, Campinas, Brazil). Read quality was assessed by FastQC (v0.11.5; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Residual ribosomal RNA (rRNA) contamination was removed by aligning reads against the G. max rRNA sequences (GenBank: AH001765.2, AH001766.2) using BLASTN and novoalign (V3.06.05; http://www.novocraft.com) with default parameters. After removing rRNA reads, FastQC package was run again and showed that no further filtering steps were required. The G. max cv. Williams 82 reference genome81 was indexed using novoindex (hash length 13 and step size 1). Reads were aligned to the reference genome version 2 (Wm82.a2.v1) using novoalign (F ILM1.8 -o SAM -s 2). Only those reads uniquely mapped to the reference genome were subsequently analyzed. Gene expression levels were calculated with cufflinks (v2.1.1;–library-type fr-firststrand) with bias correction (-b option)82,83 and normalized by reads per kilobase of transcript per million mapped reads (RPKM). Genes with RPKM greater than or equal to one were considered expressed. Pairwise-correlations between biological replicates were computed using the cor function of R stats package (http://www.r-project.org/). The differential expression between Control vs PBZ at 12 HAI, 24 HAI, and 36 HAI were determined by cuffdiff (v2.2.1;–library-type fr-firststrand -b)82. Genes with at least two-fold difference in expression and q-value ≤ 0.05 were considered differentially expressed. Enrichment of Gene Ontology (GO) term was performed using agriGO (v2.0) with hypergeometric test, corrected by the Hochberg FDR method (FDR ≤0.05)84. Redundant GO terms were removed with REViGO85 using small similarity (0.05), the Uniprot database, and SinRel as the semantic similarity parameters. KOBAS 3.086 was used to assess the enrichment of DEGs in KEGG pathways (Fisher’s exact test, P < 0.05). Expressed genes (i.e. RPKM ≥1) were used as the background set for GO and KEGG enrichment analyses. G. max TFs were obtained from the Plant Transcription Factor Database (PlantTFDB 4.0)87. Heatmaps were created using the aheatmap function in the NMF package using R88. The datasets generated in this study have been deposited in the NCBI Gene Expression Omnibus database, under the accession number GSE112872.
Transcriptome validation of gene expression by Real-time qPCR
Three independent RNA biological replicates were selected for Real-Time qPCR analysis based on RPKM values obtained in the transcriptome analysis. RNA was isolated with RNeasy Plant Mini Kit (Qiagen). RNA quality was assessed by nanodrop and agarose gel electrophoresis. RNA was tested for genomic DNA contamination via conventional PCR. Whenever necessary, traces of genomic DNA were removed by Turbo DNAse treatment (Ambion). First strand complementary DNA (cDNA) was synthesized from 2 μg of RNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). The cDNA was used as template for real-time qRT-PCR analysis using SYBR green based detection in a StepOnePlus PCR System. Melting curves were examined to ensure single products. Results were quantified using the “delta-delta Ct” method and normalized to the constitutive gene Glyma.03G064800 (Bic-C2) transcript levels, as previously described89. Amplification with a second constitutive gene (Glyma.02G273700, F-box protein 2) gave similar results (not shown). Primer sequences and product sizes are provided at the Supplementary Table S13. One-tailed paired t-test for each gene was performed by comparison of the two different conditions (control x PBZ treated) using the GraphPad Prism 7.00 software.
Supplementary information
Supplementary tables S1, S2, S3, S5, S6, S7, S8, S10, S11, S12, S13
Acknowledgements
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ; grants E-26/010.002019/2014 and E-26/102.259/2013), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES; Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We thank Dr. Lupis Ribeiro for helping with the real-time qPCR experiments and the Life Sciences Core Facility (LaCTAD) of State University of Campinas (UNICAMP) for library preparation and RNA sequencing. The funding agencies had no role in the design of the study and collection, analysis, and interpretation of data and in writing.
Author Contributions
Conceptualization: T.M.V., A.E.A.O.; Formal analysis: R.K.G., E.A.G.O., A.E.A.O., T.M.V.; Funding acquisition: T.M.V. and A.E.A.O.; Investigation: R.K.G., E.A.G.O., B.C.R., R.N.d.F., A.E.A.O., T.M.V.; Methodology: R.K.G., E.A.G.O., A.E.A.O., B.C.R., R.N.d.F., T.M.V.; Project administration: T.M.V.; Resources: R.N.d.F., T.M.V., A.E.A.O.; Software: R.K.G.; Supervision: T.M.V.; Visualization: R.K.G., E.A.G.O., B.C.R.; Writing – original draft: T.M.V., R.K.G.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rajesh K. Gazara and Eduardo A. G. de Oliveira contributed equally.
Change history
10/8/2019
The HTML version of this paper was updated shortly after publication, following a technical error that resulted in the incorrect labelling of the Supplementary Information files. This has now been corrected in the HTML; the PDF version of the paper was correct from the time of publication.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45898-2.
References
- 1.Olszewski N, Sun T-P, Gubler F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell. 2002;14(Suppl):S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanimoto, E. & Hirano, K. In Plant Roots: The Hidden Half (eds Amram Eshel & Tom Beeckman) Ch. 13, 13-11–13-14 (CRC Press 2013).
- 3.Bewley JD. Seed Germination and Dormancy. The Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley, J. D., Bradford, K. J., Hilhorst, H. W. M. & Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy. (Springer New York 2013).
- 5.Rajjou L, et al. Seed Germination and Vigor. Annual Review of Plant Biology. 2012;63:507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- 6.Weitbrecht K, Müller K, Leubner-Metzger G. First off the mark: Early seed germination. Journal of Experimental Botany. 2011;62:3289–3309. doi: 10.1093/jxb/err030. [DOI] [PubMed] [Google Scholar]
- 7.Urbanova, T. & Leubner-Metzger, G. In Annual Plant Reviews, Volume 49 Ch. 9, 253–284 (2016).
- 8.Groot SPC, Bruinsma J, Karssen CM. The role of endogenous gibberellin in seed and fruit development of tomato: Studies with a gibberellin-deficient mutant. Physiologia Plantarum. 1987;71:184–190. [Google Scholar]
- 9.Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. Theoretical and Applied Genetics. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. The Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karssen CM, Zaorski S, Kepczynski J, Groot SPC. Key Role for Endogenous Gibberellins in the Control of Seed Germination. Ann. Bot. 1989;63:71–80. [Google Scholar]
- 12.Nambara E, Akazawa T, McCourt P. Effects of the gibberellin biosynthetic inhibitor uniconazol on mutants of Arabidopsis. Plant Physiology. 1991;97:736–738. doi: 10.1104/pp.97.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedden P, Graebe JE. Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. Journal of Plant Growth Regulation. 1985;10:111–122. [Google Scholar]
- 14.Ogawa M, et al. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueguchi-Tanaka M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 16.Voegele A, Linkies A, Muller K, Leubner-Metzger G. Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. Journal of Experimental Botany. 2011;62:5131–5147. doi: 10.1093/jxb/err214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazara RK, Moharana KC, Bellieny-Rabelo D, Venancio TM. Expansion and diversification of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) family in land plants. Plant Molecular Biology. 2018;97:435–449. doi: 10.1007/s11103-018-0750-9. [DOI] [PubMed] [Google Scholar]
- 18.Murase K, Hirano Y, Sun T-p, Hakoshima T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature. 2008;456:459–463. doi: 10.1038/nature07519. [DOI] [PubMed] [Google Scholar]
- 19.Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- 20.Holdsworth MJ, Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytologist. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 21.Linkies A, et al. Ethylene Interacts with Abscisic Acid to Regulate Endosperm Rupture during Germination: A Comparative Approach Using Lepidium sativum and Arabidopsis thaliana. The Plant Cell. 2009;21:3803–3822. doi: 10.1105/tpc.109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao D, Cheng H, Wu W, Soo HM, Peng J. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiology. 2006;142:509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemhauser JL, Hong F, Chory J. Different Plant Hormones Regulate Similar Processes through Largely Nonoverlapping Transcriptional Responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Zentella R, et al. Global Analysis of DELLA Direct Targets in Early Gibberellin Signaling in Arabidopsis. The Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellieny-Rabelo D, et al. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Scientific Reports. 2016;6:36009. doi: 10.1038/srep36009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hricova A. The SCABRA3 Nuclear Gene Encodes the Plastid RpoTp RNA Polymerase, Which Is Required for Chloroplast Biogenesis and Mesophyll Cell Proliferation in Arabidopsis. Plant Physiology. 2006;141:942–956. doi: 10.1104/pp.106.080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruf S, Kössel H, Bock R. Targeted inactivation of a tobacco intron-containing open reading frame reveals a novel chloroplast-encoded photosystem I-related gene. The Journal of cell biology. 1997;139:95–102. doi: 10.1083/jcb.139.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau E. The chloroplast ycf3 and ycf4 open reading frames of Chlamydomonas reinhardtii are required for the accumulation of the photosystem I complex. The EMBO Journal. 1997;16:6095–6104. doi: 10.1093/emboj/16.20.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang J, et al. Transcriptomic Analysis of Gibberellin- and Paclobutrazol-Treated Rice Seedlings under Submergence. International Journal of Molecular Sciences. 2017;18:2225. doi: 10.3390/ijms18102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roxas VP, Smith RK, Allen ER, Allen RD. Overexpression of glutathione S-transferase/glutathioneperoxidase enhances the growth of transgenic tobacco seedlings during stress. Nature Biotechnology. 1997;15:988–991. doi: 10.1038/nbt1097-988. [DOI] [PubMed] [Google Scholar]
- 31.Pagano A, Araujo SS, Macovei A, Leonetti P, Balestrazzi A. The Seed Repair Response during Germination: Disclosing Correlations between DNA Repair, Antioxidant Response, and Chromatin Remodeling in Medicago truncatula. Front Plant Sci. 2017;8:1972. doi: 10.3389/fpls.2017.01972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helliwell CA, et al. Cloning of the Arabidopsis ent-kaurene oxidase gene GA3. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9019–9024. doi: 10.1073/pnas.95.15.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binenbaum J, Weinstain R, Shani E. Gibberellin Localization and Transport in Plants. Trends in Plant Science. 2018;23:410–421. doi: 10.1016/j.tplants.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Kanno Y, et al. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nature Communications. 2016;7:13245. doi: 10.1038/ncomms13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tal I, et al. The Arabidopsis NPF3 protein is a GA transporter. Nature. Communications. 2016;7:11486. doi: 10.1038/ncomms11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizza A, Walia A, Lanquar V, Frommer WB, Jones AM. In vivo gibberellin gradients visualized in rapidly elongating tissues. Nat Plants. 2017;3:803–813. doi: 10.1038/s41477-017-0021-9. [DOI] [PubMed] [Google Scholar]
- 37.Rizza A, Jones AM. The makings of a gradient: spatiotemporal distribution of gibberellins in plant development. Curr Opin Plant Biol. 2018;47:9–15. doi: 10.1016/j.pbi.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza NM, Topham AT, Bassel GW. Quantitative analysis of the 3D cell shape changes driving soybean germination. Journal of Experimental Botany. 2017;68:1531–1537. doi: 10.1093/jxb/erx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espasandin FD, Maiale SJ, Calzadilla P, Ruiz OA, Sansberro PA. Transcriptional regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) gene by putrescine accumulation positively modulates ABA synthesis and drought tolerance in Lotus tenuis plants. Plant Physiology and Biochemistry. 2014;76:29–35. doi: 10.1016/j.plaphy.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, et al. Negative feedback regulation of ABA biosynthesis in peanut (Arachis hypogaea): a transcription factor complex inhibits AhNCED1 expression during water stress. Scientific Reports. 2016;6:37943. doi: 10.1038/srep37943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umezawa T, et al. Type 2 C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proceedings of the National Academy of Sciences. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H. Ethylene, a key factor in the regulation of seed dormancy. Front Plant Sci. 2014;5:539. doi: 10.3389/fpls.2014.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D. Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. Journal of Plant Growth Regulation. 2004;23:44–53. [Google Scholar]
- 44.Calvo AP, Nicolas C, Nicolas G, Rodriguez D. Evidence of a cross-talk regulation of a GA 20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus sylvatica seeds. Physiologia Plantarum. 2004;120:623–630. doi: 10.1111/j.0031-9317.2004.0270.x. [DOI] [PubMed] [Google Scholar]
- 45.Hua J, et al. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. The Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 47.Yang SF, Hoffman NE. Ethylene Biosynthesis and its Regulation in Higher Plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1984;35:155–189. [Google Scholar]
- 48.Ramaih S, Guedira M, Paulsen GM. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Functional Plant Biology. 2003;30:939. doi: 10.1071/FP03113. [DOI] [PubMed] [Google Scholar]
- 49.Park J, et al. Integration of Auxin and Salt Signals by the NAC Transcription Factor NTM2 during Seed Germination in Arabidopsis. Plant Physiology. 2011;156:537–549. doi: 10.1104/pp.111.177071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuai H, et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Scientific Reports. 2017;7:12620. doi: 10.1038/s41598-017-13093-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashiguchi K, et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Y. Auxin Biosynthesis. The Arabidopsis Book. 2014;12:e0173. doi: 10.1199/tab.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlier I, et al. The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc Natl Acad Sci USA. 2000;97:14819–14824. doi: 10.1073/pnas.260502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. The Plant Cell. 2001;13:101–111. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed JW, et al. Three Auxin Response Factors Promote Hypocotyl Elongation. Plant Physiology. 2018;178:864–875. doi: 10.1104/pp.18.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka K, et al. Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiology. 2005;138:1117–1125. doi: 10.1104/pp.104.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unterholzner SJ, et al. Brassinosteroids Are Master Regulators of Gibberellin Biosynthesis in Arabidopsis. The Plant Cell. 2015;27:2261–2272. doi: 10.1105/tpc.15.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tong H, et al. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. The Plant Cell. 2014;26:4376–4393. doi: 10.1105/tpc.114.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleishon S, Shani E, Ori N, Weiss D. Negative reciprocal interactions between gibberellin and cytokinin in tomato. New Phytologist. 2011;190:609–617. doi: 10.1111/j.1469-8137.2010.03616.x. [DOI] [PubMed] [Google Scholar]
- 60.Fonouni-Farde C, et al. DELLA1-Mediated Gibberellin Signaling Regulates Cytokinin-Dependent Symbiotic Nodulation. Plant Physiology. 2017;175:1795–1806. doi: 10.1104/pp.17.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenboim-Wainberg Y. Cross Talk between Gibberellin and Cytokinin: The Arabidopsis GA Response Inhibitor SPINDLY Plays a Positive Role in Cytokinin Signaling. The Plant Cell. 2005;17:92–102. doi: 10.1105/tpc.104.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.To JPC, et al. Cytokinin Regulates Type-A Arabidopsis Response Regulator Activity and Protein Stability via Two-Component Phosphorelay. The Plant Cell. 2007;19:3901–3014. doi: 10.1105/tpc.107.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song S, Qi T, Wasternack C, Xie D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr Opin Plant Biol. 2014;21:112–119. doi: 10.1016/j.pbi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Yang DL, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci USA. 2012;109:E1192–1200. doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen F. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. Journal of Experimental Botany. 2002;53:215–223. doi: 10.1093/jexbot/53.367.215. [DOI] [PubMed] [Google Scholar]
- 67.Park J, et al. Gibberellin Signaling Requires Chromatin Remodeler PICKLE to Promote Vegetative Growth and Phase Transitions. Plant Physiology. 2017;173:1463–1474. doi: 10.1104/pp.16.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen F, Bradford KJ. Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiology. 2000;124:1265–1274. doi: 10.1104/pp.124.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minic Z, Jouanin L. Plant glycoside hydrolases involved in cell wall polysaccharide degradation. Plant Physiology and Biochemistry. 2006;44:435–449. doi: 10.1016/j.plaphy.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Liszkay A, Kenk B, Schopfer P. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta. 2003;217:658–667. doi: 10.1007/s00425-003-1028-1. [DOI] [PubMed] [Google Scholar]
- 71.Langan KJ, Nothnagel EA. Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma. 1997;196:87–98. [Google Scholar]
- 72.Serpe MD, Nothnagel EA. Effects of Yariv phenylglycosides on Rosa cell suspensions: Evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta. 1994;193:542–550. [Google Scholar]
- 73.Ringli C, Keller B, Ryser U. Glycine-rich proteins as structural components of plant cell walls. Cellular and molecular life sciences: CMLS. 2001;58:1430–1441. doi: 10.1007/PL00000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willats WG, Knox JP. A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of beta-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. The Plant journal: for cell and molecular biology. 1996;9:919–925. doi: 10.1046/j.1365-313x.1996.9060919.x. [DOI] [PubMed] [Google Scholar]
- 75.Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I. CsAGP1, a Gibberellin-Responsive Gene from Cucumber Hypocotyls, Encodes a Classical Arabinogalactan Protein and Is Involved in Stem Elongation. Plant Physiology. 2003;131:1450–1459. doi: 10.1104/pp.015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki Y, Kitagawa M, Knox JP, Yamaguchi I. A role for arabinogalactan proteins in gibberellin-induced alpha-amylase production in barley aleurone cells. The Plant journal: for cell and molecular biology. 2002;29:733–741. doi: 10.1046/j.1365-313x.2002.01259.x. [DOI] [PubMed] [Google Scholar]
- 77.Liu W-Y, et al. Anatomical and transcriptional dynamics of maize embryonic leaves during seed germination. Proceedings of the National Academy of Sciences. 2013;110:3979–3984. doi: 10.1073/pnas.1301009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou DX, Bisanz-Seyer C, Mache R. Molecular cloning of a small DNA binding protein with specificity for a tissue-specific negative element within the rps1 promoter. Nucleic acids research. 1995;23:1165–1169. doi: 10.1093/nar/23.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alabadí D, et al. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. The Plant Journal. 2007;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 80.Alabadı D, Gil J, Bla ´zquez MA, García-Martínez JL. Gibberellins Repress Photomorphogenesis in Darkness. Plant Physiology. 2004;134:1050–1057. doi: 10.1104/pp.103.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmutz J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 82.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian T, et al. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Research. 2017;45:W122–W129. doi: 10.1093/nar/gkx382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PloS one. 2011;7:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J, Mao X, Cai T, Luo J, Wei L. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Research. 2006;34:W720–W724. doi: 10.1093/nar/gkl167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin J, et al. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Research. 2017;45:D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yim AK, et al. Using RNA-Seq Data to Evaluate Reference Genes SuiTable for Gene Expression Studies in Soybean. PloS one. 2015;10:e0136343. doi: 10.1371/journal.pone.0136343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables S1, S2, S3, S5, S6, S7, S8, S10, S11, S12, S13