Abstract
In humans, early life exposure to inorganic arsenic is associated with adverse health effects. Inorganic arsenic in utero or in early postnatal life also produces adverse health effects in offspring of pregnant mice that consumed drinking water containing low part per billion levels of inorganic arsenic. Because aggregate exposure of pregnant mice to inorganic arsenic from both drinking water and food has not been fully evaluated in experimental studies, quantifying arsenic exposure of the developing mouse is problematic. Here, we determined levels of total arsenic and arsenic species in natural ingredient rodent diets that are composed of many plant and animal-derived foodstuffs and in a purified ingredient rodent diet that is composed of a more restricted mixture of foodstuffs. In natural ingredient diets, total arsenic levels ranged from ~60 to ~400 parts per billion, and in the purified ingredient diet, total arsenic level was 13 parts per billion. Inorganic arsenic was the predominant arsenic species in trifluoroacetic acid extracts of each diet. Various exposure scenarios were evaluated using information on inorganic arsenic levels in diet and drinking water and on daily food and water consumption of pregnant mice. In a scenario in which pregnant mice consumed drinking water with 10 parts per billion of inorganic arsenic and a natural ingredient diet containing 89 parts per billion of inorganic arsenic, drinking water contributed only ~20% of inorganic arsenic intake. Quantitation of arsenic species in diets used in studies in which drinking water is the nominal source of arsenic exposure provides more accurate dosimetry and improves understanding of dose–response relations. Use of purified ingredient diets will minimize the discrepancy between the target dosage level and the actual dosage level attained in utero exposure studies designed to evaluate effects of low level exposure to inorganic arsenic.
Graphical Abstract
INTRODUCTION
Epidemiological studies have identified inorganic arsenic (iAs) as a developmental toxicant and carcinogen. That is, exposure to iAs during early life can produce adverse health effects that may be manifested soon after exposure or later in life.1,2 Evidence of developmental toxicity and carcinogenicity of iAs in humans includes increased risk of cancer in later life,3,4 diminished intrauterine growth and low birth weight,5–9 increased susceptibility to infections in early life,10,11 altered development of immune function,12 lower growth rates in early childhood,13 and altered maturation of lung function.14,15 In addition, neurodevelopmental and behavioral effects have been associated with early life iAs exposure.16–21 Notably, these epidemiological studies have examined outcomes in populations that used water supplies that typically contained iAs in the range of 10–1000 parts per billion (ppb, μg As L–1). Scenarios for early life exposure to iAs are likely complex for individuals who manifest signs and symptoms attributable to iAs exposure and may include both in utero exposure to iAs and its methylated metabolites (derived from maternal or fetal metabolism) and postnatal exposure through ingestion and metabolism of iAs.
Studies demonstrating a range of adverse health effects of iAs exposure in developing humans have prompted studies of the developmental toxicity and carcinogenicity of iAs in animal models. The exemplar of such studies was evaluation of iAs as a transplacental carcinogen in the mouse. The adult offspring of mice that were exposed to 42.5 or 85 ppm (ppm, mg As L–1) of iAs (as arsenite) in drinking water between gestational days 8 and 18 have increased tumor incidence in liver, adrenal cortex, ovary, and lung, indicating that a limited interval of exposure to iAs in utero was sufficient to increase cancer risk in later life.22 Studies of effects of exposure restricted to the in utero period have been complemented by whole life exposure studies in which exposure of the offspring of iAs-exposed mice continued postnatally. A comparison of tumor production in mice exposed only in utero or by whole life treatment found that, although both exposure regimens produced tumors, the sites and yields of tumors differed.23 Whole life exposure studies also found that continuous pre- and postnatal exposure to lower drinking water concentrations of arsenite (6–24 ppm of iAs) produced tumors in male and female mice.24 The tumorigenicity of iAs in a continuous exposure scenario has been evaluated at much lower drinking water concentrations.25 Here, female mice were exposed to 50–5000 ppb of iAs (as arsenite) in drinking water before and during gestation. After delivery, nursing dams and offspring were maintained on drinking water containing arsenite at the same level as received during pregnancy. At weaning, offspring of treated females continued to receive drinking water with arsenite at the same levels used during in utero and preweaning exposure. Whole life exposure to arsenite increased tumor occurrence in mice at 104 weeks of age. Significantly increased tumor numbers were found in lungs of male mice in the 50 and 500 ppb groups but not in the 5000 ppb group. In female mice, lung adenoma yield was significantly increased in the 50 ppb group. Non-monotonic tumor response rates and concerns about appropriate controls for background tumor incidence have prompted discussion of these results.26–28 However, these studies strongly suggest that early life exposure to iAs at relatively low levels can have adverse health effects.
Other studies illustrate adverse noncancer health effects associated with early life exposure to iAs in mice. Adult offspring of female mice that received 50 ppb iAs (as arsenate) throughout gestation and through weaning at 23 days of age displayed neurobehavioral changes that may be linked to arsenic-induced epigenetic changes in glucocorticoid receptor regulation.29–32 In male offspring of mice that received drinking water containing 100 ppb iAs (as arsenite) from gestational day 5 to parturition, early life exposure to iAs altered metabolic status and exacerbated changes in hepatocellular structure associated with consumption of a high fat and carbohydrate diet.33 Offspring of mice exposed to drinking water containing 100 ppb iAs (as arsenite) from gestational day 8 until parturition exhibited reversible changes in lung structure and function, changes in expression of genes involved in mucociliary function and innate immunity, and diminished resistance to influenza A infection in early life.34–36 Exposure of pregnant mice to either 10 ppb or 42.5 ppm of iAs in drinking water from gestational day 10 until parturition resulted in an earlier age of vaginal opening and higher body weight in female offspring and in glucose intolerance, increased body weight, and increased body fat in all offspring.37 Compared with untreated females, fertility in females exposed to 42.5 ppm iAs was reduced; however, exposure to 10 ppb iAs did not affect fertility. Diminished postnatal growth has been reported in mice produced by dams that were maintained on drinking water containing 10 ppb of arsenite throughout pregnancy.38
Adverse health effects in offspring of mice exposed to relatively low (ppb) levels of iAs in drinking water during pregnancy suggest that this model may be appropriate for developmental toxicity studies. To date, the dosimetric measure for these studies has been the concentration of iAs in the drinking water supplied to pregnant mice; however, drinking water is not the only source of iAs in these studies.Food is also a source of iAs that has not been systemically evaluated in most studies using the in utero model. Consideration of the relative contribution of drinking water and the food supply is required to determine aggregate exposure to iAs in in utero exposure scenarios.
A palatable and nutritionally adequate food supply that meets the nutritional requirements of the mouse is a requirement for use of mice in biomedical research.39,40 For most experimental studies, mice receive so-called natural ingredient diets that are composed of ingredients derived from grains, seeds, and other plant- and animal-derived foodstuffs which contain iAs at ppb levels.41–43 Use of a natural ingredient diet in studies that evaluate effects of in utero exposure to iAs may be an unquantified source of exposure. Alternatives to natural diets are so-called purified ingredient diets that are compounded from readily available processed ingredients (e.g., sugar, starch, vegetable oil, casein, vitamin and mineral mixes). The use of purified diets has been identified as a strategy that reduces variation in composition that is inherent in natural ingredient diets.44 For studies designed to examine health outcomes of offspring of pregnant mice that receive ppb levels of iAs in drinking water, use of a purified ingredient diet with a low iAs concentration could reduce exposure to arsenic. Reducing dietary iAs levels permits better control over dosing regimens, ensuring that the amount and form of arsenic used to create the in utero exposure scenario are known and quantifiable.
In the work reported here, we have characterized arsenical species present in some natural ingredient diets and a purified ingredient diet that could be used in studies of the effects of in utero exposure to iAs. Based on estimated intake of food and water by pregnant mice during gestation, we have estimated aggregate intake of iAs apportioned between arsenic derived from drinking water (the dose) and from food (the diet). This analysis shows that the relative contribution of these two sources depends on the rodent diet used and suggests that accurately estimating the dose requires attention to both food supply and the dosing medium, drinking water, as sources of iAs.
METHODS
Reagents and Standards.
Water (18.2 MΩ cm) provided from a Milli-Q Academic water purification system from Millipore GmbH (Vienna, Austria) was used throughout this study. Nitric acid ROTIPURAN 68%, p.a., further purified in a MLS duoPUR sub-boiling unit (MLS GmbH, Leutkirch, Germany), pyridine ROTIPURAN ≥99.5%, p.a., formic acid ROTIPURAN ≥99.5%, p.a., aqueous ammonia solution ROTIPURAN, ≥25%, p.a., trifluoroacetic acid (TFA) ≥99.9%, and hydrogen peroxide 30% w/w were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Malonic acid (>99%) was obtained from Sigma-Aldrich (Vienna).
For total arsenic measurements by ICPMS, a single element standard of arsenic (1000 ± 3 mg As L–1 in 2% nitric acid) was used in conjunction with single element standards of germanium (1000 ± 2 mg L–1 in 2% HNO3; 0.5% HF), indium (1000 ± 2 mg L–1 in 2% HNO3), and tellurium (1000 ± 2 mg L–1 in 2% HNO3), which served as internal standards; all single element standards were obtained from Carl Roth GmbH & Co. KG (Karlsruhe, Germany).
For the determination of arsenic species by HPLC/ICPMS, standard solutions were prepared for arsenate (As(V)) from Na2HAsO4∙7H2O, >98% from Merck (Darmstadt, Germany), and dimethylarsinate (DMAs), prepared from sodium dimethylarsinate (>98%) purchased from Fluka Chemie (Buchs, Switzerland). Methylarsonate (MA) was prepared in-house from As2O3 and CH3I (Meyer reaction). In addition, the following standards were prepared from previously synthesized in-house arsenic compounds (purity >99% by NMR and HPLC/mass spectrometry): arsenobetaine (AB), trimethylarsine oxide (TMAO), arsenocholine (AC), and tetrame-thylarsonium ion (TETRA). Finally, standards for the four arsenosugars commonly found in marine algae (so-called glycerol-, phosphate-, sulfonate-, and sulfate sugars) were prepared from previously isolated compounds or from synthesis.45,46 Structures of arsenic species of potential interest in this study are shown in Supplemental Figure S1.
Rodent Diets.
Eleven natural ingredient rodent diets in pelleted form (diets 1–11) and one purified ingredient rodent diet in powdered form (diet 12) were obtained from vendors in the United States. The purified ingredient diet evaluated here was AIN-93G rodent diet; this diet meets nutritional requirements of mice during periods of rapid growth and during pregnancy and lactation.44 All diets were stored at –20 °C before processing for analysis.
Sample Preparation.
Samples (8–10 pellets) of diets 1–11 were ground in a Retsch ZM 200 centrifugal mill (Retsch GmbH, Haan, Germany) to a particle size of <0.25 mm. Powdered diet samples were stored in 50 mL polypropylene tubes (Greiner, Bio-one, Frickenhausen, Germany) at 4 °C.
Total Arsenic Analysis.
For determination of total arsenic content of diets, samples were analyzed in triplicate. Each replicate used about 250 mg (weighed with a precision of 0.1 mg) of a powdered diet sample that was weighed directly into a 12 mL quartz tube. After addition of 2 mL of nitric acid and 2 mL of internal standard (100 μg L–1 Ge, In, Te in 1% nitric acid), samples were transferred to a Teflon rack of the Ultraclave microwave system and covered with Teflon caps. Microwave digestions were performed with an Ultraclave III (MLS GmbH, Leutkirch, Germany) at an argon pressure of 4 × 106 Pa, while samples were heated to 250 °C for 30 min. After cooling to room temperature, digested samples were transferred to 15 mL polypropylene tubes (Greiner, Bio-one, Frickenhausen, Germany) and diluted with water to 10 mL.
Arsenic concentrations of digestion solutions were determined using an Agilent 7900ce ICPMS (Agilent Technologies, Waldbronn, Germany) equipped with a concentric Micro Mist nebulizer and a Scott double pass spray chamber. Helium was used as the collision cell gas to remove polyatomic interferences, and 74Ge, 115In, and 126Te were used as internal standards. The ICPMS response for As was enhanced by addition of 1% CO2 in argon as optional gas.47 Quantification was performed by external calibration with a calibration curve in the range of 0.01–50 μg As L–1. LOD was 0.002 μg As L–1 in solution equivalent to ca. 0.1 μg As kg–1 of rodent diet. For matrix matching, standards were prepared in 20% nitric acid with an internal standard providing 20 μg L–1 each of Ge, In, and Te. For analytical quality control, standard reference material ERM-BC211 (rice flour) with a certified arsenic content of 260 ± 13 μg As kg–1 was used; our obtained value was 265 ± 9 μg As kg–1 (n = 8).
Sample Extraction.
Extractions of powdered diet samples were performed in triplicate using water or TFA as extractant.
Water Extraction.
About 200 mg of each powdered sample was accurately weighed into a 50 mL polypropylene tube (Greiner, Bio-one, Frickenhausen, Germany), and 10 mL of water was added. Samples were extracted by shaking at room temperature for 60 min in a GFL-1083 shaking water bath (Gesellschaft fur Labortechnik, Burkwedel, Germany). Extracted samples were centrifuged for 15 min at 4700 rcf (relative centrifugal force) in a Rotina 420R centrifuge (Andreas Hettich GmbH & Co.KG, Tuttlingen, Germany). Supernates were collected and filtered through a PTFE syringe filter (0.2 μm; Bruckner Analysentechnik, Linz, Austria). Filtrates were analyzed for arsenic species using HPLC-ICPMS.
TFA Extraction.
About 200 mg of each powdered sample was accurately weighed into a 50 mL polypropylene tube, 10 mL of 0.02 M aqueous TFA was added, and samples were extracted at 95 °C for 60 min in a GFL-1083 shaking water bath. After cooling to room temperature, extracted samples were centrifuged for 15 min at 4700 rcf in a Rotina 420R centrifuge. Supernates were collected and filtered through a PTFE syringe filter (0.2 μm; Bruckner Analysentechnik, Linz, Austria). Filtrates were analyzed for arsenic species using HPLC-ICPMS.
Determination of Inorganic Arsenic and Organic Arsenic Species.
HPLC–ICPMS measurements were performed with an Agilent 1100 series HPLC (Agilent Technologies, Waldbronn, Germany). PEEK (polyetheretherketone) tubing (Upchurch Scientific, Oak Harbor, WA, USA) was used to connect the HPLC column to the ICPMS.
Chromatographic separation of anionic species was performed using a PRP-X100 column (150 × 4.6 mm, 5 μm particle size, Hamilton Company, Reno, Nevada, USA) with a mobile phase of 5 mmol L–1 malonic acid adjusted to pH 5.6 with aqueous ammonia at a flow rate of 1 mL min–1. Column temperature was 40 °C, and the injected sample volume was 20 μL. Quantification was done by external calibration against standard arsenic species based on peak areas (calibration from 0.05–20 μg As L–1). iAs (sum of arsenite and arsenate) was determined by HPLC following oxidation with H2O2. Detection limits for anionic arsenic species in solution, based on a S/N ratio of 3, were 0.05–0.07 μg As L–1 which, depending on arsenic species, corresponded to about 2–3 μg As kg–1 of rodent diet (see Table 1). Spiking experiments were performed with HPLC-ICPMS to confirm the identity of analytes in samples. Additionally, presence of the sulfonate arsenosugar, AB, and TMAO was confirmed by molecular mass spectrometry (see below).
Table 1.
Concentrations of Total Arsenic and of Aqueous Trifluoroacetic Acid-Extractable Arsenicals in Natural Ingredient Rodent Diets (1–11) or a Purified Ingredient Rodent Diet (12)
| TFA extractable As species (μg As/kg)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| diet | total As (μg/kg)a | EEc (%) | iAsd | DMAs | TMAO | AB | SO3-sugar | glycerol sugar | sum of quantified speciese in μg As/kg (% total As) |
| 1 | 128 ± 5 | 70 | 71 | 3.0 | <4 | <2 | 2.8 | 2.1 | 79 (62) |
| 2 | 130 ± 13 | 63 | 79 | 2.5 | <4 | <2 | <2 | <2 | 82 (63) |
| 3 | 91.0 ± 2.0 | 75 | 63 | <2 | <4 | <2 | <2 | <2 | 63 (69) |
| 4 | 95.5 ± 3.1 | 73 | 62 | <2 | <4 | <2 | <2 | <2 | 62 (65) |
| 5 | 62.2 ± 2.3 | 69 | 38 | <2 | <4 | <2 | <2 | <2 | 38 (61) |
| 6 | 363 ± 7 | 52 | 79 | 23 | 10 | 50 | <2 | <2 | 162 (45) |
| 7 | 100 ± 2 | 77 | 69 | <2 | <4 | <2 | <2 | <2 | 69 (69) |
| 8 | 79.4 ± 2.3 | 58 | 42 | <2 | <4 | <2 | <2 | <2 | 42 (53) |
| 9 | 405 ± 19 | 52 | 89 | 25 | 10 | 56 | <2 | <2 | 180 (44) |
| 10 | 361 ± 6 | 64 | 35 | 19 | 14 | 140 | <2 | <2 | 208 (58) |
| 11 | 138 ± 3 | 66 | 87 | <2 | <4 | 2.9 | <2 | <2 | 90 (65) |
| 12 | 12.8 ± 0.7 | 79 | 10 | <2 | <4 | <2 | <2 | <2 | 10 (78) |
Total arsenic concentration in homogenized sample of rodent diet.
Concentration of arsenicals in aqueous TFA extracts of rodent diets.
Extraction efficiency (EE) as percentage of total arsenic present in a homogenized diet sample that is present in the aqueous TFA extract of that diet.
Sum of iAsIII and iAsV.
Upper: Sum of concentrations of arsenic species quantified in aqueous TFA extract. Lower: Sum of concentrations of arsenic species quantified in aqueous TFA extract as a percentage of total arsenic concentration in homogenized sample of rodent diet.
Chromatographic separation of cationic species was performed with an Ionospher C5 column (100 × 3.0 mm, 5 μm particle size, Agilent Technologies, Germany) with a mobile phase of 10 mmol L–1 pyridine buffer adjusted to pH 2.8 with formic acid at a flow rate of 1 mL min–1. Column temperature was 30 °C, and the injected sample volume was 20 μL. Quantification was done by external calibration against standard arsenic species based on peak areas (calibration from 0.05–20 μg As L–1). Detection limits for cationic arsenic species in solution, based on a S/N ratio of 3, were 0.05 to 0.10 μg As L–1 which, depending on arsenic species, corresponded to about 2–4 μg As kg–1 of rodent diet (see Table 1). For all HPLC-ICPMS analyses, an optional gas (1% CO2 in argon) was introduced through a T-piece connecting the spray chamber and the torch to enhance the arsenic response. In addition to the signal at m/z 75 (75As, 40Ar35Cl), the signal at m/z 77 (40Ar37Cl) was monitored to detect possible chloride interference on m/z 75. Quality control for chromatographic separations used the standard reference material ERM-BC211 with certified contents of DMAs (119 ± 13 μg As kg–1) and iAs (124 ± 11 μg As kg–1); obtained values were 131 ± 3 μg As kg–1 for DMAs and 116 ± 4 μg As kg–1 for iAs (n = 3).
HPLC-electrospray mass spectrometry was used to confirm the presence of the sulfonate arsenosugar in Diet 1 and of AB and TMAO in diet 10. For the sulfonate arsenosugar, HPLC separation was performed with a Dionex Ultimate 3000 series instrument (Thermo Fischer Scientific, Erlangen, Germany) on a PRP-X100 column (150 × 4.6 mm, 5 μm particle size) at 40 °C using 100 mM NH4CO3 pH 9.5 as the mobile phase at 1 mL min–1; injection volume was 20 μL. For AB and TMAO, HPLC separations were performed with IonoSpher 5C (100 × 3.0 mm; 5 μm); mobile phase, ammonium formate (20 mM incl. 3 % MeOH; pH 2.6); flow rate, 1.0 mL min–1; column temperature, 30 °C; injection volume, 10 μL. Compounds were measured with a high-resolution mass spectrometer (Q-Exactive Hybrid Quadrupole-Orbitrap MS from Thermo Fischer Scientific) at a resolution of 70,000 (fwhm) under electrospray ionization conditions in positive mode. The HPLC retention time and mass spectral data for the arsenicals in the rodent diet exactly matched well data obtained for the standards sulfonate arsenosugar (observed m/z 393.01868, Δm 2.1 ppm), AB (m/z 179.00478, Δm < 0.2 ppm), and TMAO (m/z 136.99432, Δm 0.8 ppm).
Statistical Methods.
Relations between biological variables (e.g., food consumption during pregnancy) or chemical components of diets were evaluated with linear regression analysis using SigmaPlot 13 (Systat, Inc., San Jose, CA). Appropriateness of use of linear regression analysis in the fitting of data was tested by ANOVA procedures that provided an F-statistic and an associated P value for these analyses.
RESULTS AND DISCUSSION
Arsenic in Natural Ingredient and Purified Ingredient Rodent Diets.
Table 1 lists total As concentrations in 12 rodent diets, including 11 natural ingredient diets and one purified ingredient diet. The lowest total As concentration (13 ng g–1) was found in the purified ingredient diet. Total As levels were much higher in natural ingredient diets, ranging between ~60 and ~400 ng g–1. Total As levels varied widely among natural ingredient diets: 3 diets contained ~360–405 ng As g–1) and 8 diets contained ~60–140 ng g–1. Differences in ingredients used in preparation of natural ingredient diets most likely account for this variation in total As levels. As discussed below, identification of arsenic species in diets and evaluation of product information sheets provided insights into sources for arsenic in natural ingredient diets. Notably, the current analysis did not examine possible temporal changes in levels of total or speciated arsenic in natural or purified ingredient diets. Changes in sources of foodstuffs used in diet composition could affect the contribution of diet to aggregate arsenic exposure.
Arsenic Species in Natural and Purified Ingredient Rodent Diets.
Before undertaking arsenic speciation analyses of rodent diets by HPLC-ICPMS, we considered the relative merits of water and aqueous TFA as extraction solvents. Aqueous TFA is a very effective extractant for the analysis of arsenic species in terrestrial samples, in which iAs predominates,48 but can degrade some organoarsenicals (e.g., arsenosugars) present in marine samples. As shown in Table 1 and Supporting Information Table S1, TFA extraction of rodent diets gave higher extraction efficiencies (52–79%) for total arsenic than did water extraction (24–53%). We did not observe TFA degradation of arsenic species except for the two natural ingredient diets (1, 7) that contained some arsenosugars. For example, the small amount of arsenosugars present in the water extract (~9 μg kg–1) of diet 1 was reduced ~3-fold in the TFA extract. Arsenic species data reported here are based on the TFA extraction procedure. Another important analytical consideration in speciation analysis is column recovery. In our analyses of rodent diets, column recovery was usually >85%. Taken together, these two analytical performance values indicate that the sum of arsenic species measured by our method accounted for ~45–78% of the total arsenic initially present in rodent diets.
For natural ingredient diets, iAs was the predominant arsenical species in the TFA-extractable fraction. The relation between diet composition and arsenic contents was examined using data from product information sheets. On a mass basis, dietary protein ranged from 14 to 25% and fat ranged from 4 to 9%. Notably, although there was a strong positive correlation (r2 = 0.60, P < 0.005) between dietary % protein and total As concentration, dietary % fat and total As concentration were not correlated. On a relative basis, corn and wheat products were the three most abundant ingredients in 6 of 11 diets and were the four most abundant ingredients in 5 of 11 diets. Barley, oats, and soybean meal were also components of some diets. Corn or wheat products may be important iAs sources in natural ingredient diets. Corn plants accumulate arsenic from soil, and arsenic in corn kernels is primarily inorganic.49 In wheat, highly extractable iAs is concentrated in the bran fraction of the grain.50,51 As noted above, there was no association between the level of fat in diet and total As level. For 9 of 11 diets, soybean oil was the sole fat source; two diets also included porcine fat. Because soybeans and soybean oil have been reported to contain only low levels of arsenic,52–55 soybean oil was unlikely to be a significant source of iAs for these diets.
Figure 1 shows relative levels of arsenic species present in TFA extracts of natural ingredient diets. In all cases, the predominant species was iAs. Di- and trimethylated arsenicals were detected in some diets. Three natural ingredient diets (6, 9, 10) with relatively high levels of DMAs, TMAO, and AB were unique because they contained fish meal. Fish meal has been reported to contain high levels of AB and can also contain TMAO.56,57 Arsenosugars were detected at low levels in two diets, although the dietary source of these compounds is unknown.
Figure 1.
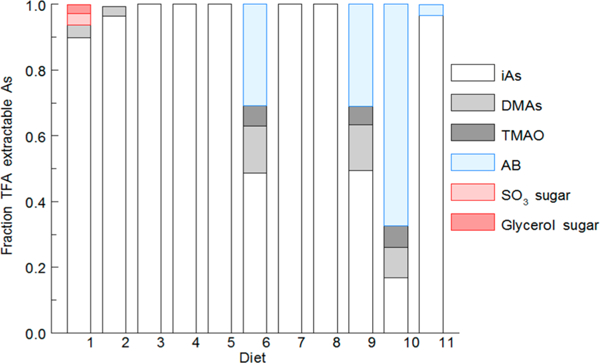
Relative levels of TFA-extractable arsenic species in natural ingredient rodent diets. iAs, sum of arsenite and arsenate; DMAs, dimethylarsinate acid; TMAO, trimethylarsine oxide; AB, arsenobetaine; SO3 sugar, sulfonate arsenosugar; glycerol sugar, glycerol arsenosugar.
In contrast to natural ingredient diets, the purified ingredient diet had a much lower total arsenic concentration that was mostly accounted for by iAs present in the TFA extract. Arsenic in the purified ingredient diet may be derived from cornstarch and casein, the two most abundant ingredients. As noted above, iAs can accumulate in corn, and low concentrations (<3 ng g–1) have been reported in cow’s milk, the source of casein.58 In addition, iAs may be a contaminant of phosphate salts, including potassium phosphate, a component of the mineral mix used in AIN-93G rodent diet.59
Estimating in Utero Exposure to Arsenic.
Data we obtained on levels of arsenicals in natural ingredient and purified ingredient rodent diets were used to estimate exposure to iAs in an exposure scenario in which pregnant mice were maintained with free access to a drinking water that had been modified to contain iAs at a desired dosing level and to a natural ingredient or a purified ingredient rodent diet. Thus, aggregate daily intake of iAs in these exposure scenarios would be the sum of iAs consumed from diet and drinking water.
Pregnancy in mice is a physiological challenge that leads to increased food and water consumption, changes in physical activity, and altered thermoregulation.60–63 These adaptations support rapid fetal growth and development and prepare pregnant mice for the demands of parturition and lactation. Data on daily food and water consumption by pregnant C57BL/6J mice were used to develop linear regression equations to describe intake of food and water by mice during pregnancy (Figure 2).64,65 These daily estimates of food and water intake by pregnant mice were used to calculate intake of iAs from food and water in exposure scenarios which are described below. Although nonlinear curve fitting procedures might improve the agreement between data and equation, use of simple linear equations to describe these data was sufficient to represent the trend for increased food and water intake during pregnancy. Indeed, given substantial differences in food and water intake among inbred mice strains,66,67 it is likely a more sophisticated analysis of intakes during pregnancy would be developed for each mouse strain under evaluation.
Figure 2.
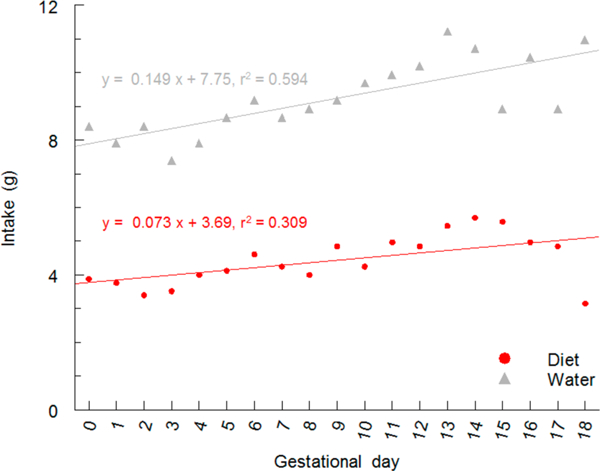
Intake of food and water by pregnant mice during gestation; data shown for food and water intake by pregnant C57BL/6 mice. For water intake, linear regression analysis evaluated by ANOVA yields a F-statistic of 24.8, P < 0.001). For food intake, linear regression analysis evaluated by ANOVA yields a F-statistic of 7.6, P < 0.014).
Estimated daily intake of iAs from diet and drinking water was examined for two exposure scenarios. For these calculations, levels of TFA-extractable iAs in diets were used to calculate daily intake of iAs. For a variety of foods, TFA has been shown to be an effective extractant.68 However, the relation between extractability by TFA of iAs in some foods and bioavailability is not well understood. For example, differences in the TFA-extractability of As in several vegetables do not appear to predict bioavailability of As in these foods as determined in juvenile swine.69 Here, daily intake of iAs from diet and drinking water was estimated in mice that consumed a natural ingredient rodent diet containing 89 ng of iAs g–1 (diet 9 in Table 1) or a purified ingredient rodent diet containing 10 ng of iAs g–1 (diet 12 in Table 1). For these exposure scenarios, pregnant mice had free access to the specified diet and to drinking water that contained 50 ng of iAs g–1. Figure 3 shows estimated daily intakes from diet or drinking water for these scenarios and temporal changes in the ratio of the amounts of iAs ingested from drinking water and from diet. In the scenario using the natural ingredient diet, intake of arsenic from drinking water and from diet was similar throughout ingestion, with ratios ranging from ~1.3 to 1.1. Use of a purified ingredient rodent diet markedly reduced the contribution of diet to daily iAs intake, resulting in water/diet ratios ranging from ~11.2 to 9.1.
Figure 3.
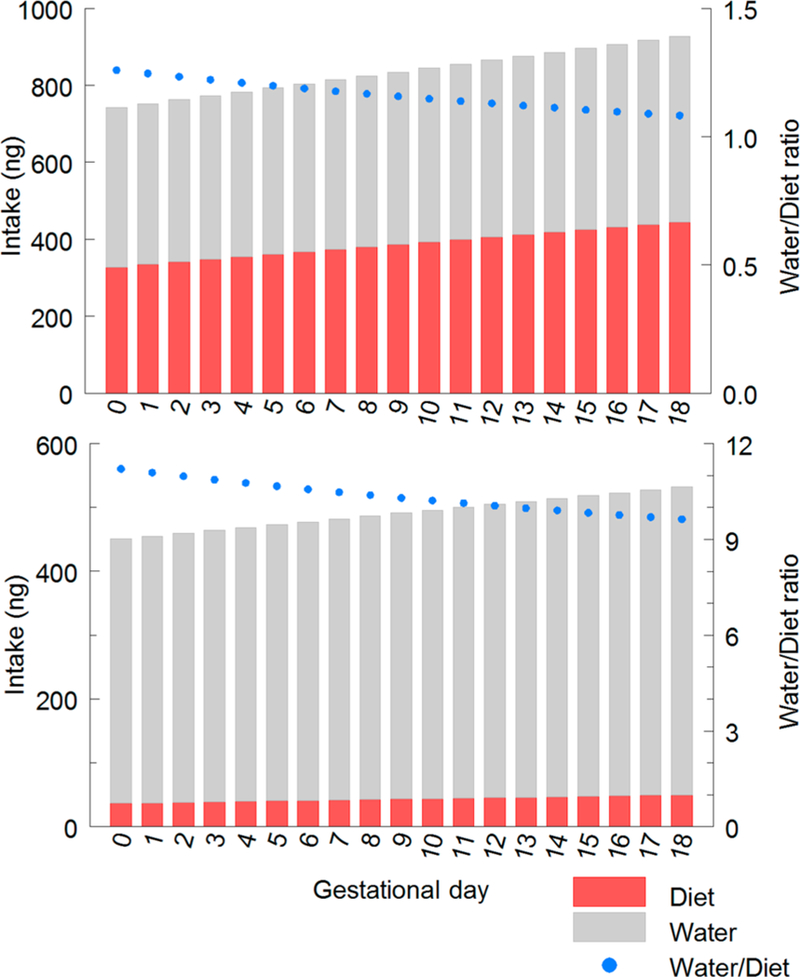
Estimated daily intake of inorganic arsenic from diet and drinking water by pregnant mice between day 0 and 18 of gestation. Upper: Pregnant mice with free access to a natural ingredient rodent diet containing 89 ng of TFA-extractable iAs per g (diet 9 in Table 1) and drinking water containing 50 ng of As per g. Lower: Pregnant mice with free access to a purified ingredient rodent diet containing 10 ng of TFA-extractable iAs per g (diet 12 in Table 1) and drinking water containing 50 ng of As per g. For each exposure scenario, calculated intake of inorganic arsenic based on estimated daily food and water intake shown in Figure 2. The ratio of inorganic arsenic ingested from drinking water and from diet is shown for both exposure scenarios.
Contributions of diet and drinking water to cumulative intake of iAs by pregnant mice through gestational day 18 were calculated using two natural ingredient rodent diets (diets 5 and 9) and purified rodent diet (diet 12). For each diet, exposure scenarios included ingestion of drinking water that contained 10, 50, or 100 ng of iAs g–1. Figure 4 shows absolute contributions of diet and drinking water to iAs intake for these combinations of arsenic-containing diets and drinking water supplies. The ratio of iAs intake from drinking water and from diet varied markedly among the combinations examined here. For the worst case exposure scenario (lowest iAs level in drinking water and highest iAs level in diet), iAs ingested from drinking water would account for only ~20% of the cumulative dose. In contrast, for exposure scenarios combining the purified ingredient diet with the lowest level of iAs in drinking water, iAs ingested from water would account for about 65% of the cumulative exposure. In scenarios combining the two higher water dosage levels and the purified ingredient diet, more than 90% of iAs exposure would be derived from drinking water.
Figure 4.
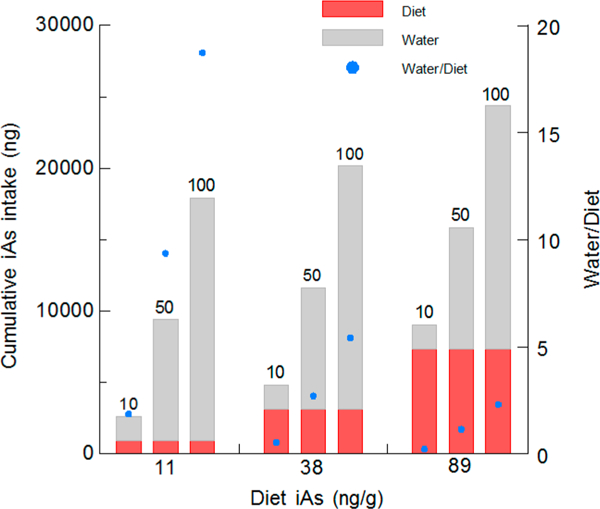
Cumulative intake of inorganic arsenic by pregnant mice from diet and drinking water. From gestational 0–18, pregnant mice have free access to natural ingredient diets (diet 5 or 9 from Table 1) or a purified ingredient rodent diet (diet 12 from Table 1) and drinking water that contains 10, 50, or 100 ng of inorganic arsenic per g. The purified ingredient diet contains 10 ng of inorganic arsenic per g. The two natural ingredient rodent diets contain 38 and 89 ng of inorganic arsenic per g. For each exposure scenario, the ratio of arsenic ingested from drinking water and from diet is shown.
Because a brief period of exposure of a pregnant mouse to low levels of iAs in drinking water can produce an array of adverse effects in offspring, it is important to understand the dose–response relation between arsenic exposure and adverse effect. Understanding dose–response relations for iAs requires quantitation of aggregate exposure to this agent from all sources. The amendment of drinking water with known concentrations of iAs is a relatively easy experimental manipulation, and quantitation of iAs in water is a relatively simple analytical procedure. In contrast, quantitation of arsenic species present in food requires efficient extraction of these species from a complex matrix and an analytical approach that can quantify a diverse array of arsenic-containing species. Although there are limited data on the bioavailability of inorganic or methylated arsenic species in the mouse or juvenile swine, there is little information on the bioavailability of iAs or other arsenicals present in complex food matrices.70,71 Given these factors, the most expedient strategy to reduce the confounding influence of diet in iAs exposure is use of a purified ingredient diet instead of a natural ingredient diet. Minimizing the contribution of diet to aggregate iAs exposure will improve our understanding of dose–response relations underlying the actions of iAs as a developmental toxicant or carcinogen.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Georg Raber and the NAWI Graz Central Lab (Environmental Metabolomics) for accurate mass measurements.
Funding
This work was supported in part by NIH grant R01ES022697 to M.S.
ABBREVIATIONS
- iAs
inorganic arsenic
- ppb
parts per billion
- ppm
parts per million
- TFA
trifluoroacetic acid
- As(V)
arsenate
- DMAs
dimethylarsinate
- MA
methylarsonate
- AB
arsenobetaine
- TMAO
trimethylarsine oxide
- AC
arsenocholine
- TETRA
tetramethylarsonium ion
- rcf
relative centrifugal force
- PEEK
polyetheretherketone
- fwhm
full width at half-maximum
Footnotes
Preparation of this document has been funded by the U.S. Environmental Protection Agency. This document has been subjected to review by the National Health and Environmental Effects Research Laboratory (NHEERL) and approved for publication. Approval does not signify that the contents reflect the views of the Agency nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemres-tox.7b00309.
Structures of relevant arsenicals and information on water-extractable arsenicals in mouse diets (PDF)
REFERENCES
- (1).U.S. Environmental Protection Agency. (1996) Guidelines for Reproductive Toxicity Risk Assessment. EPA/630/R-96/009 (October 1996) (www.epa.gov/sites/production/files/2014-11/documents/guidelines_repro_toxicity.pdf).
- (2).National Research Council (2000) Scientific frontiers in developmental toxicology and risk assessment. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- (3).Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, Perez L, Cortes S, Balmes JR, Liaw J, and Smith AH (2014) Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am. J. Epidemiol. 180, 1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, Duran V, Cuevas S, Garcia J, Meza R, Valdes R, Valdes G, Benitez H, VanderLinde V, Villagra V, Cantor KP, Moore LE, Perez SG, Steinmaus S, and Smith AH (2014) Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Cancer Epidemiol., Biomarkers Prev. 23, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Davis MA, Higgins J, Li Z, Gilbert-Diamond D, Baker ER, Das A, and Karagas MR (2015) Preliminary analysis of in utero low-level arsenic exposure and fetal growth using biometric measurements extracted from fetal ultrasound reports. Environ. Health 14, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bloom MS, Neamtiu IA, Surdu S, Pop C, Anastasiu D, Appleton AA, Fitzgerald EF, and Gurzau ES (2016) Low level arsenic contaminated water consumption and birth outcomes in Romania-An exploratory study. Reprod. Toxicol. 59, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Henn BC, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, Coull BA, Bellinger DC, and Wright RO (2016) Prenatal arsenic exposure and birth outcomes among a population residing near a mining-related Superfund site. Environ. Health Perspect 124, 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, and Karagas MR (2016) Relation between in utero arsenic exposure and birth outcomes in a cohort of mothers and their newborns from New Hampshire. Environ. Health Perspect 124, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, Quamruzzaman Q, Rahman M, and Christiani DC (2016) Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort. Epidemiology 27, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Farzan SF, Korrick S, Li Z, Enelow R, Gandolfi AJ, Madan J, Nadeau K, and Karagas MR (2013) In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ. Res. 126, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, Baker E, and Karagas MR (2016) Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a U.S. cohort. Environ. Health Perspect 124, 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nadeau KC, Li Z, Farzan S, Koestler D, Robbins D, Fei DL, Malipatlolla M, Maecker H, Enelow R, Korrick S, and Karagas MR (2014) In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin. Immunol. 155, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Saha KK, Engstrom A, Hamadani JD, Tofail F, Rasmussen KM, and Vahter M (2012) Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environ. Health Perspect 120, 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Olivas-Calderon E, Recio-Vega R, Gandolfi AJ, Lantz RC, Gonzalez-Cortes T, Gonzalez-De Alba C, Froines JR, and Espinosa-Fematt JA (2015) Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol. Appl. Pharmacol. 287, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, and Gonzalez-De Alba C (2015) In utero and early childhood exposure to arsenic decreases lung function in children. J. Appl. Toxicol. 35, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan K, Kline J, Ahsan H, and Graziano JH (2011) Arsenic exposure and motor function among children in Bangladesh. Environ. Health Perspect 119, 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, Ronquillo D, and Stoltzfus RJ (2011) Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environ. Res. 111, 670–676. [DOI] [PubMed] [Google Scholar]
- (18).Tyler CR, and Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr. Environ. Health Rep 1, 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rahman M, Sohel N, Hore SK, Yunus M, Bhuiya A, and Streatfield PK (2015) Prenatal arsenic exposure and drowning among children in Bangladesh. Global Health Action 8, 28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wasserman GA, Liu X, Loiacono NJ, Kline J, Factor-Litvak P, van Geen A, Mey JL, Levy D, Abramson R, Schwartz A, and Graziano JH (2014) A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ. Health 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Kline J, Siddique AB, Shahriar H, Uddin MN, van Geen A, Mey JL, Balac O, and Graziano JH (2016) Child intelligence and reductions in water arsenic and manganese: A two-year follow-up study in Bangladesh. Environ. Health Perspect 124, 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Waalkes MP, Liu J, and Diwan BA (2007) Transplacental arsenic carcinogenesis in mice. Toxicol. Appl. Pharmacol. 222, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ahlborn GJ, Nelson GM, Grindstaff RD, Waalkes MP, Diwan BA, Allen JW, Kitchin KT, Preston RJ, Hernandez-Zavala A, Adair B, Thomas DJ, and Delker DA (2009) Impact of life stage and duration of exposure on arsenic-induced proliferative lesions and neoplasia in C3H mice. Toxicology 262, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tokar EJ, Diwan BA, Ward JM, Delker DA, and Waalkes MP (2011) Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol. Sci. 119, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Waalkes MP, Qu W, Tokar EJ, Kissling GE, and Dixon D (2014) Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses. Arch. Toxicol. 88, 1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Cohen SM, Arnold LL, Klaunig JE, and Goodman JI (2014) Re: Waalkes et al. : Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses, Arch. Toxicol. 2014. Arch. Toxicol. 88, 2061–2062. [DOI] [PubMed] [Google Scholar]
- (27).Cohen SM, Arnold LL, Klaunig JE, and Goodman JI (2015) Response to the Waalkes et al. , Letter to the editor concerning our “letter to the editor, Re: Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human relevant doses, Waalkes et al., Arch Toxicol, 2014”. Arch. Toxicol. 89, 2167–2168. [DOI] [PubMed] [Google Scholar]
- (28).Waalkes MP, Qu W, Tokar EJ, Kissling GE, and Dixon D (2014) Response to letter to the editor by Cohen et al. (2014) “Re: Waalkes et al.: Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses, Arch Toxicol, 2014”. Arch. Toxicol. 88, 2063–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Martinez EJ, Kolb BL, Bell A, Savage DD, and Allan AM (2008) Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. NeuroToxicology 29, 647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Goggin SL, Labrecque MT, and Allan AM (2012) Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neuro-Toxicology 33, 1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Allan AM, Hafez AK, Labrecque MT, Solomon ER, Shaikh MN, Zheng X, and Ali A (2015) Sex-Dependent effects of developmental arsenic exposure on methylation capacity and methylation regulation of the glucocorticoid receptor system in the embryonic mouse brain. Toxicol. Rep 2, 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tyler CR, Hafez AK, Solomon ER, and Allan AM (2015) Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain. Toxicol. Appl. Pharmacol. 288, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ditzel EJ, Li H, Foy CE, Perrera AB, Parker P, Renquist BJ, Cherrington NJ, and Camenisch TD (2016) Altered Hepatic Transport by Fetal Arsenite Exposure in Diet-Induced Fatty Liver Disease. J. Biochem. Mol. Toxicol. 30, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ramsey KA, Bosco A, McKenna KL, Carter KW, Elliot JG, Berry LJ, Sly PD, Larcombe AN, and Zosky GR (2013) In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environ. Health Perspect 121, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ramsey KA, Foong RE, Sly PD, Larcombe AN, and Zosky GR (2013) Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ. Health Perspect 121, 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ramsey KA, Larcombe AN, Sly PD, and Zosky GR (2013) In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacol. Toxicol. 14, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE, and Yao HH (2016) Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ. Health Perspect 124, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kozul-Horvath CD, Zandbergen F, Jackson BP, Enelow RI, and Hamilton JW (2012) Effects of low-dose drinking water arsenic on mouse fetal and postnatal growth and development. PLoS One 7, e38249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).National Research Council (1995) Nutrient Requirements of Laboratory Animals, 4th revised ed., The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- (40).National Research Council (1996) Guide for the Care and Use of Laboratory Animals The National Academies Press, Washington, DC. [Google Scholar]
- (41).Arslan B, Djamgoz MBA, and Akun E (2016) Arsenic: A review on exposure pathways, accumulation, mobility and transmission into the human food chain. Rev. Environ. Contam. Toxicol. 243, 27–51. [DOI] [PubMed] [Google Scholar]
- (42).Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, Rohl C, Schupp T, Wollin KM, and Hengstler JG (2015) High exposure to inorganic arsenic by food: the need for risk reduction. Arch. Toxicol. 89, 2219–2227. [DOI] [PubMed] [Google Scholar]
- (43).European Food Safety Authority (2014) Dietary exposure to inorganic arsenic in the European population. EFSA Journal 12, 3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Reeves PG, Nielsen FH, and Fahey GC Jr, (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123, 1939–1951. [DOI] [PubMed] [Google Scholar]
- (45).Francesconi KA, Edmonds JS, Stick RV, Skelton BW, and White AH (1991) Arsenic-containing ribosides from the brown alga Sargassum lacerifolium: X-ray molecular structure of 2-amino-3-[5′-deoxy-5′-(dimethylarsinoyl)ribosyloxy]-propane-1-sulphonic acid. J. Chem. Soc., Perkin Trans. 1, 2707–2716. [Google Scholar]
- (46).Traar P, Rumpler A, Madl T, Saischek G, and Francesconi KA (2009) Synthesis of Naturally-Occurring Arsenic-Containing Carbohydrates. Aust. J. Chem. 62, 538–545. [Google Scholar]
- (47).Kuehnelt D, Juresa D, Kienzl N, and Francesconi KA (2006) Marked individual variability in the levels of trimethylselenonium ion in human urine determined by HPLC/ICPMS and HPLC/vapor generation/ICPMS. Anal. Bioanal. Chem. 386, 2207–2212. [DOI] [PubMed] [Google Scholar]
- (48).Raber G, Stock N, Hanel P, Murko M, Navratilova J, and Francesconi KA (2012) An improved HPLC-ICPMS method for determining inorganic arsenic in food: Application to rice, wheat and tuna fish. Food Chem. 134, 524–532. [Google Scholar]
- (49).Rosas-Castor JM, Guzman-Mar JL, Hernandez-Ramirez A, Garza-Gonzalez MT, and Hinojosa-Reyes L (2014) Arsenic accumulation in maize crop (Zea mays): a review. Sci. Total Environ. 488–489, 176–187. [DOI] [PubMed] [Google Scholar]
- (50).Cubadda F, Ciardullo S, D’Amato M, Raggi A, Aureli F, and Carcea M (2010) Arsenic contamination of the environment-food chain: a survey on wheat as a test plant to investigate phytoavailable arsenic in Italian agricultural soils and as a source of inorganic arsenic in the diet. J. Agric. Food Chem. 58, 10176–10183. [DOI] [PubMed] [Google Scholar]
- (51).Zhao FJ, Stroud JL, Eagling T, Dunham SJ, McGrath SP, and Shewry PR (2010) Accumulation, distribution, and speciation of arsenic in wheat grain. Environ. Sci. Technol. 44, 5464–5468. [DOI] [PubMed] [Google Scholar]
- (52).Bradicich R, Foster NE, Hons FE, Jeffus MT, and Kennner CT (1969) Residues in food and feed. Arsenic in cottonseed products and various commodities. Pest. Monit. J. 3, 139–141. [PubMed] [Google Scholar]
- (53).Wauchope RD (1978) Selenium and arsenic levels in soybeans from different production regions of the United States. J. Agric. Food Chem. 26, 226–288. [DOI] [PubMed] [Google Scholar]
- (54).Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, and Menzel DB (1999) A market basket survey of inorganic arsenic in food. Food Chem. Toxicol. 37, 839–846. [DOI] [PubMed] [Google Scholar]
- (55).Svarc-Gajic JV, Suturovic ZJ, Marjanovic NJ, and Kravic SZ (2005) Determination of As(III) and As(V) in oilseeds by chronopotentiometric stripping analysis: development of a method. Mol. Nutr. Food Res. 49, 337–342. [DOI] [PubMed] [Google Scholar]
- (56).Petursdottir AHE (2010) Determination of toxic and non-toxic arsenic species in Icelandic fish meal. Master’s Thesis, Faculty of Physical Sciences, School of Engineering and Natural Sciences, University of Iceland, Reykjavik, http://skemman.is/stream/get/1946/6357/18152/1/MasterThesis-final.pdf. [Google Scholar]
- (57).Petursdottir AHE, Jorundsdottir HO, and Gunnlaugsdottir H (2010) Food safety and added value of Icelandic fishmeal. Determination of toxic and nontoxic arsenic species in fish meal. Skýrsla Matís 45–10 Desember 2010 ISSN 1670–7192, http://www.matis.is/media/matis/utgafa/45-10-Arsenic-in-Icelandic-fishmeakl.pdf.
- (58).Lynch HN, Greenberg GI, Pollock MC, and Lewis AS (2014) A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. Sci. Total Environ. 496, 299–313. [DOI] [PubMed] [Google Scholar]
- (59).Schrodter K, Bettermann G, Staffel T, Wahl F, Klein T, and Hofmann T (2012) Phosphoric Acid and Phosphatesin Ulmanns Encyclopedia of Industrial Chemistry, Vol 26, pp 679–724, Wiley-VCH Verlag, Weinheim. [Google Scholar]
- (60).Speakman JR (2008) The physiological costs of reproduction in small mammals. Philos. Trans. R Soc. Lond. Philos. Trans. R. Soc.j B 363, 375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Makarova EN, Kochubei ED, and Bazhan NM (2010) Regulation of food consumption during pregnancy and lactation in mice. Neurosci. Behav. Physiol. 40, 263–267. [DOI] [PubMed] [Google Scholar]
- (62).Gamo Y, Troup C, Mitchell SE, Hambly C, Vaanholt LM, and Speakman JR (2013) Limits to sustained energy intake. XX. Body temperatures and physical activity of female mice during lactation. J. Exp. Biol. 216, 3751–3761. [DOI] [PubMed] [Google Scholar]
- (63).Finlay JB, Liu X, Ermel RW, and Adamson TW (2015) Maternal Weight Gain as Predictor of Litter Size in Swiss Webster, C57BL/6J, and BALB/cJ mice. J. Am. Assoc. Lab. Anim. Sci. 54, 694–699. [PMC free article] [PubMed] [Google Scholar]
- (64).Fieldwick DM (2013) Body Weight Regulation During Pregnancy in the mouse. Thesis, Bachelor of Medical Science with Honors, University of Otago, New Zealand (http://hdl.handle.net/1052¾057). [Google Scholar]
- (65).Ladyman SR, Fieldwick DM, and Grattan DR (2012) Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction 144, 83–90. [DOI] [PubMed] [Google Scholar]
- (66).Kutscher CL (1974) Strain differences in drinking in inbred mice during ad libitum feeding and food deprivation. Physiol. Behav. 13, 63–70. [DOI] [PubMed] [Google Scholar]
- (67).Bachmanov AA, Reed DR, Beauchamp GK, and Tordoff MG (2002) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Raber G, Stock N, Hanel P, Murko M, Navratilova J, and Francesconi KA (2012) An improved HPLC-ICPMS method for determining inorganic arsenic in food: Application to rice, wheat and tuna fish. Food Chem. 134, 524–532. [Google Scholar]
- (69).Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, and Naidu R (2008) Application of an in vivo swine model for the determination of arsenic bioavailability in contaminated vegetables. Chemosphere 71, 1963–1969. [DOI] [PubMed] [Google Scholar]
- (70).Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, and Naidu R (2006) In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ. Health Perspect 114, 1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Bradham KD, Scheckel KG, Nelson CM, Seales PE, Lee GE, Hughes MF, Miller BW, Yeow A, Gilmore T, Serda SM, Harper S, and Thomas DJ (2011) Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environ. Health Perspect 119, 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


