Abstract
Microbial infection is an important cause of acute-on-chronic liver failure (ACLF), which is a syndrome that results in multiple organ dysfunction or failure and is accompanied by an increased short-term risk of mortality. Early detection and treatment of microbial infection can effectively reduce the mortality of patients with ACLF. However, antimicrobial resistance has recently increased due to the increased use of antimicrobial agents. Therefore, it is important to choose appropriate antibiotics and antifungal agents for early prevention or treatment of patients with microbial infection and ACLF to reduce the occurrence of drug resistance and to reduce patient mortality. This review summarizes the current status in the understanding of the epidemiology, pathogenesis, early diagnosis, treatment, and strategies for prevention of microbial infection in patients with ACLF.
MeSH Keywords: Antibiotics, Antineoplastic; Bacterial Infections; Inflammation; Liver Failure, Acute
Background
Acute-on-chronic liver failure (ACLF) is the term used for the syndrome of severe damage to the liver resulting in impaired liver functions, including liver synthesis, detoxification, excretion, and biotransformation, that has high short-term mortality [1–3]. The main clinical manifestations of ACLF are coagulopathy, jaundice, hepatic encephalopathy, and ascites. Among the many causes, bacterial infection is the most common trigger for ACLF in up to 33% of cases [4]. Once ACLF occurs, the condition can rapidly deteriorate, leading to multiple organ dysfunction or failure, and a high risk of mortality of 33% and 51% at 28 and 90 days, respectively [5]. This review aims to summarize the current understanding of microbial infection associated with ACLF, in terms of the epidemiology, pathogenesis, early diagnosis and treatment, and strategies for prevention.
Epidemiology of Acute-on-Chronic Liver Failure (ACLF)
The syndrome of ACLF has an infectious and non-infectious cause. The most important infectious agents are bacteria. The common types of bacterial infections result in bacteremia, spontaneous bacterial peritonitis, urinary tract infection, pneumonia, and soft tissue infection [6]. Urinary tract infections (28.5%) and spontaneous bacterial peritonitis (22.5%) are the most common infections [7].
The main source of early infection in patients with cirrhosis of the liver is from intestinal bacterial flora. Gram-negative bacteria and Enterococcus are the most common pathogens. The common pathogen that causes spontaneous bacterial peritonitis is Escherichia coli, and Klebsiella pneumoniae is a common cause of pneumonia [8–10]. However, due to the increasing clinical use of invasive procedures and the inappropriate use of antibiotics, the epidemiological pattern of microbial infection is changing. The infection rate for Gram-positive bacteria (mainly Staphylococcus) is increasing, as is the development of antimicrobial resistance, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) in patients with cirrhosis [11]. Bajaj et al. showed that the prevalence of Gram-positive bacterial infection (32.9%) was significantly greater than Gram-negative bacterial infection (26.8%) in ACLF in patients with liver cirrhosis hospitalized in 18 hepatology referral centers in the United States and Canada [7]. In 2018, Mucke et al. showed that the infection rate from Gram-positive bacteria was 52.1% in patients with liver cirrhosis [12]. Inappropriate use of antibiotics and changes in the clinical environment have increased the chances of infection with multidrug-resistant (MDR) bacteria and pan-drug resistant (PDR) bacteria and fungi, which have become the main causes of the failure of antibiotic treatment [9,13,14].
Definition and Diagnosis of ACLF
Due to the varied etiology and differences in geographical and ethnic cause and prevalence, there are no unified clinical guidelines for the diagnosis and treatment of ACLF. Two important definitions in clinical practice are from the Asian Pacific Association for the Study of the Liver (APASL) [15], and the European Association for the Study of the Liver (EASL) [4]. Table 1 summarizes the diagnostic criteria used from the perspective of the predisposition, infection/inflammation, response, organ failure (PIRO) concept. The diagnostic criteria of the EASL guidelines are mainly for Europe and North America, and alcoholic liver disease (ALD) is the main cause of ACLF. In these geographical areas, hepatitis B virus (HBV) infection is rare. The diagnostic criteria from the APASL are mainly for the Asia-Pacific region and Africa, where the main infectious cause of ACLF is HBV. Also, the APASL guidelines do not include the same prognostic scoring as the EASL guidelines. In 2017, Wu et al. aimed to clarify the clinical and pathological characteristics of patients with hepatitis B virus-associated ACLF (HBV-ACLF) in the Asia-Pacific region using the findings from a prospective study and proposed new diagnostic criteria and a prognostic scoring system, the Chronic Liver Failure Consortium criteria [16]. The predictive accuracy of the criteria proposed by Wu et al. at 28 days and 90 days was higher than that of other scoring systems [16]. However, as there are still no uniform and precise diagnostic criteria and prognostic scoring systems for ACLF, prospective, large-scale, multicenter, controlled clinical studies are required to provide the evidence required to develop future guidelines.
Table 1.
Diagnostic criteria used from the perspective of the predisposition, infection/inflammation, response, organ failure (PIRO) concept for acute-on-chronic liver failure (ACLF).
| Asian Pacific Association for the Study of the Liver (APASL) [15] | European Association for the Study of the Liver (EASL) [4] | |
|---|---|---|
| Predisposition | Chronic liver with/without cirrhosis | Acute decompensation of cirrhosis |
| Injury | Intrahepatic (main: HBV reaction) | Intrahepatic (main: active alcoholism) Extrahepatic (main: bacterial infections) Unknown reasons |
| Response | TB ≥5 mg/dl and INR ≥1.5 complications with 4 week of ascites and/or HE High 28-day mortality |
One or more organ failure High 28-day mortality |
| Organ failure | Liver: TB ≥5 mg/dl and INR ≥1.5 | Liver: TB ≥12mg/dl Kidney: creatinine ≥2mg/dl Coagulation: INR ≥2.5 or PLT ≤20000/mm3 Circulation: MAP ≤70 mmHg Respiration: PaO2/FiO2 ≤200 or SpO2/FiO2 ≤214 Cerebral: grade III or IV HE |
HBV – hepatitis B virus; INR – International Sensitivity Index; TB – total bilirubin; PLT – platelets; MAP – mean arterial pressure; PaO2 – partial pressure of arterial oxygen; FiO2 – fraction of inspired oxygen; SpO2 – pulse oximetric saturation; HE – hepatic encephalopathy.
According to the criteria of Bajaj et al. [17], ACLF infection can be divided into spontaneous bacteremia, spontaneous bacterial peritonitis, urinary tract infection, pneumonia, cellulitis, bacterial enteritis, and fungal infection [18]. These specific diagnostic criteria are shown in Table 2.
Table 2.
Common types of infection and diagnostic crtiteria in patients with acute-on-chronic liver failure (ACLF).
| Type of infection | Diagnostic criteria |
|---|---|
| Spontaneous bacteremia | Positive blood cultures without a source of infection |
| Spontaneous bacterial peritonitis | Ascitic fluid polymorphonuclear cells >250/ml |
| Urinary tract infection | Urine white blood cell >15/high power field with either positive urine Gram stain or culture |
| Pneumonia | New pulmonary infiltrate in the presence of: (1) at least one respiratory symptom (cough, sputum production, dyspnea, pleuritic pain); (2) at least one finding on auscultation (rales or crepitation) or one sign of infection (core body temperature >38°C or <36°C, shivering or leucocyte count >10,000/mm3 or <4,000/mm3) in the absence of antibiotics |
| Cellulitis | Fever with cellulitis |
| Bacterial enteritis | Diarrhea or dysentery with a positive stool culture for Salmonella, Shigella, Yersinia, Campylobacter, or pathogenic E. coli |
| Fungal infection | Do not consider host factors and clinical manifestations Pathology identifies invasive fungal infection Isolation of pathogenic fungi from infected or non-infected areas |
Impaired Function of the Intestinal Barrier in Microbial Infection
There are four kinds of cells in the intestinal epithelium that include columnar intestinal cells, goblet cells, secretory cells, and Paneth cells. The intestinal epithelial tight junction protein is an important structural component of the intestine [19] and maintains the integrity of the intestinal mucosal epithelial barrier [20]. In patients with cirrhosis, the structure of tight junction proteins changes, and bacteria, toxins, and inflammatory mediators in the intestinal lumen can leak into the surrounding tissues [21,22]. The migration of microorganisms, or their pathogen-associated molecular patterns (PAMPs), from the intestinal lumen to the extra-intestinal tissue is known as bacterial translocation. The translocation of bacteria is closely related to the occurrence and development of infection [23]. In non-cirrhotic chronic liver disease, the intestinal barrier function of the patient can also be impaired [24]. Bacterial translocation and endotoxemia can impair the contractility of mesenteric vessels and increase portal hypertension in patients with liver cirrhosis, and cirrhosis and portal hypertension affect the microbiota and increase bacterial translocation [25,26]. It is currently believed that the intestinal barrier, microbiota, liver, and immune system maintain balance through complex interactions [27,28]. When the balance between these organs and systems is disturbed, intestinal permeability can increase, although the exact mechanism is not clear [27,28]. Bacterial translocation and bacterial products pass through the intestinal barrier and are commonly associated with cirrhosis [29].
Paneth cells are located at the base of the intestinal crypt and play an important role in innate immunity in the intestine but also participate in acquired immune and inflammatory responses [30]. Teltschik et al. have shown that Paneth cells are key effectors of host immune responses to intestinal pathogens in animal models of cirrhosis [31]. One of the distinguishing features is that Paneth cells contain cytoplasmic endocrine granules that contain antimicrobial peptides, including lysozyme, secretory phospholipase A2, cryptdin-related sequence peptide, angiogenic factors, and defensins [32,33]. During bacterial infection, changes in lysozyme cause changes in lymphocyte trafficking, cell autophagy, via an autophagy-based alternative secretion pathway [34]. Also, cryptdin-related sequence peptide can be combined with and reduces the immune stimulating activity of lipopolysaccharide (LPS) [35]. When Paneth cells and intestinal crypts are damaged, they induce the expression of tumor necrosis factor-α (TNF-α), which is involved in inflammation and in the regeneration of crypt epithelial cells [36–39]. TNF-α can downregulate the expression of the occludin promoter, inhibit its dephosphorylation, and lead to increased permeability of the intestinal mucosa, and the number, morphology, and location of Paneth cells can vary depending on the nature of the infection [36–39].
Microbial Infection as a Trigger that Changes the Pro-Inflammatory and Anti-Inflammatory Systems
There are pro-inflammatory and anti-inflammatory systems in the human body [40]. Pro-inflammatory factors are involved in cellular immune responses, and anti-inflammatory factors are involved in immune regulation. The two systems are in equilibrium to maintain normal immunity. Once a pro-inflammatory factor is produced, it not only activates the immune response, but can also promote the production of other pro-inflammatory factors, resulting in amplification effects, or an immune cascade phenomenon [41]. A persistent inflammatory response can constitute a second immunological attack that causes the inflammatory response to expand leading to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) [42,43].
Cytokines can be classified into pro-inflammatory cytokines (IFNγ, IL-1β, TNFα, IL-6, IL-8, IL-2, and IL-17A) and anti-inflammatory cytokines (IL-10, IL-1Ra, and TGFβ) [44–46]. Proinflammatory cytokines play a major role in the induction of systemic inflammation and the development of MODS. However, anti-inflammatory cytokines help to regulate and eliminate acute inflammation. Recently, Brinkhoff et al. showed that, in an experimental model, the pro-inflammatory cytokines IFNγ, TNFα, IL-2, and IL-17A begin to decrease at 3 hours after LPS injection, and IL-10 levels remained stable [47]. When systemic IL-10 levels returned to baseline, the ability of T-cells to produce IFNγ or IL-17A was normalized after 24 hours [47]. The ability of anti-inflammatory regulatory T-cells (Tregs) to produce IL-10 remained stable and did not change during endotoxemia [47].
Endotoxemia Induces the Inhibition of Pro-Inflammatory T-Helper Cells
Endotoxemia may be considered to be a contributing factor to the immunological paralysis caused by sepsis [47]. The levels of pro-inflammatory cytokines in patients with cirrhosis are higher than in healthy people [48] and are positively correlated with the degree of liver damage [49]. When the infection remains uncontrolled, the release of immunosuppressive factors increases, and is associated with monocyte deactivation, decreased T-cell reactivity, and cell apoptosis can lead to loss of immune cells [50–52]. Severe inflammation can lead to shock and organ failure, while prolonged low immune conditions can lead to immunosuppression due to immune paralysis and inability to clear infections (Figures 1, 2) [53].
Figure 1.
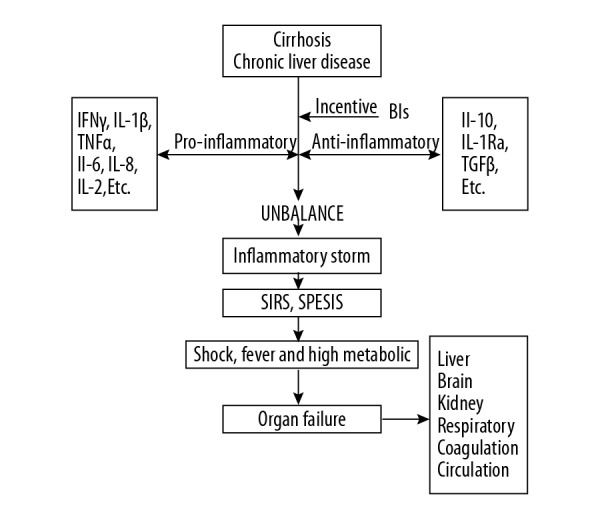
The role of bacterial infections in acute-on-chronic liver failure (ACLF). Bacterial infection plays an important role in the transition of patients from cirrhosis or chronic liver disease to acute-on-chronic liver failure (ACLF). Infection is also the most frequent precipitant of ACLF via the systemic inflammatory response. The development of multiorgan failure in ACLF characterized by significant alterations in systemic and hepatic hemodynamics and worsening liver function.
Figure 2.
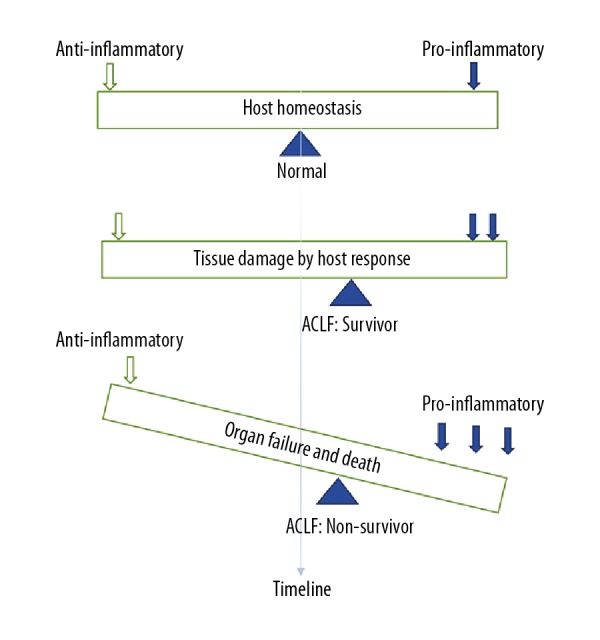
Schematic diagram of the balance between anti-inflammatory and pro-inflammatory factors in acute-on-chronic liver failure (ACLF). Effective control of infection requires a balanced response between the pro-inflammatory and anti-inflammatory mechanisms of the host. In patients with acute-on-chronic liver failure (ACLF), the promotion of pro-inflammatory factors can result in tissue damage, and the equilibrium point will shift, when the pro-inflammatory factors increased further, organ failure and death may occur.
The Relationship between Invasive Fungal Disease and ACLF
Acute injury and persistent hepatocyte damage in ACLF patients lead to a significant reduction in the efficiency of preventing and eliminating pathogens [54,55]. Bajaj et al. reported that the incidence of invasive fungal disease is 5% in patients with cirrhosis (43% in ACLF) and the mortality rate is much higher than patients without invasive fungal disease [56,57]. Other reports indicate that the incidence of fungal infections in patients with cirrhosis ranges from 1–66% [58,59]. Several studies have shown that risk factors for secondary fungal infections in patients with ACLF include intensive care unit admission, age, diabetes, use of adrenal glucocorticoids, and hospital admission [60,61]. Candida sp. and Aspergillus sp. are common pathogens, and common sites for infection include the urinary and respiratory tracts [62]. Invasive fungal disease can be a cause of ACLF, although the mechanism remains unclear. With the exacerbation of ACLF, the occurrence of immune paralysis can lead to invasive fungal disease. Also, invasive fungal disease can increase the cytokine response and aggravate organ failure. Early diagnosis and treatment are key to reducing patient mortality. Nipun et al. noted that the 1,3-β-D-glucan (BDG) and the galactomannan index (GMI) were effective biomarkers for the diagnosis of invasive fungal disease in patients with ACLF [63].
Early Diagnosis and Biomarkers of Bacterial Infection
Early diagnosis of microbial infection is a critical step in the management of patients with ACLF patients [64]. Some patients who are asymptomatic or lack specific clinical symptoms are easily missed. Bacterial culture is the gold standard method for diagnosing bacterial infections, but due to the low culture rate and the amount of time required, diagnosis may be delayed. Early diagnosis and timely initiation of appropriate antibiotic therapy are important to improve the prognosis of patients with bacterial infections, and biomarkers of infection may aid early diagnosis. Procalcitonin and C-reactive protein (CRP) are two acute-phase serum proteins that are commonly used as early markers of infection [65]. Stimulated by endotoxin released by some bacterial infections and inflammatory mediators, such as IL-1β, TNF and IL-6, almost all tissues can produce procalcitonin. At a critical value of 0.1 μg/L, procalcitonin is a very sensitive marker that can exclude infection. A procalcitonin test with a cutoff value of 0.25 μg/L is most helpful in the diagnosis of bacteremia in patients with community-acquired pneumonia (CAP) and urinary tract infection (UTI) [66]. Zhang et al. [67] reported that a CRP >12.15 mg/L was a reliable indicator of bacterial infection in patients with ACLF. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) was 0.948, the sensitivity was 96.6%, and the specificity was 83.3% for the CRP level. Compared with traditional culture methods, the sensitivity of polymerase chain reaction (PCR) (16S PCR) in the detection of bacterial DNA in ascites was 100%, and the specificity was 91.5% [68]. The reverse blot hybridization assay (REBA) used in the REBA Sepsis-ID test has been shown to be a fast and reliable test for the identification of Gram-positive and Gram-negative bacteria, fungi, and antibiotic resistance genes for MRSA and VRE [69,70].
Using DNA-based enzymes, or DNAzymes, bacteria can be detected down to one colony forming unit (CFU) after four hours of sample culture [71]. To visualize the bacterial concentration, Li et al. [72] expanded the enzyme strand to hybridize with urease-labeled DNA and immobilized the DNAzymes on magnetic beads. Each urease can be converted approximately ×1014 times, enabling another layer of signal amplification and allowing for the selective detection of approximately ×103 E. coli cells, in the absence of cell culture [72]. The use of an acoustic impedance matching buffer enables the separation of bacteria from high concentrations of blood cells [73], which can recover 99.7% of bacteria while removing more than 99.9% of blood cells. At high whole blood concentrations, such as 20%, it is possible to recover 90% of the bacteria while removing more than 99% of the blood cells [74]. One milliliter of an undiluted whole blood equivalent is processed in 12.5 minutes [74]. Also, the bacterial recovery rate is 90%, and the blood cell removal rate is greater than 99%, and the enriched bacteria can then be subjected to acoustic enrichment and PCR detection [74]. Although effective monitoring and high-sensitivity pathogen identification procedures are constantly being updated, the cost of expensive testing currently limits their widespread use. Therefore, new technologies for early diagnosis are still urgently needed and must be developed, updated, and generally used in clinical practice.
Treatment of Bacterial Infections in Patients with ACLF
Early detection of bacterial infections and timely use of reasonable antimicrobial agents are essential for the treatment of patients with ACLF. It has been estimated that in patients with ACLF and infection, mortality is increased by approximately 3.3% per hour of delay in treatment [75]. In 2014, Jalan et al. published a position statement on the treatment of infection in patients with cirrhosis, including community-acquired and nosocomial bacterial infections [76]. Treatment of community-acquired infections is recommended to include third-generation cephalosporins or amoxicillin/clavulanic acid, which are preferred for the treatment of spontaneous bacterial peritonitis, spontaneous bacterial empyema, spontaneous bacteremia, and pneumonia [76]. Simple urinary tract infections can be treated with quinolones or cotrimoxazole, and complex urinary tract infections (accompanied by sepsis) can be treated with third-generation cephalosporins or amoxicillin and clavulanic acid [76]. A strategy for treating pneumonia is to use quinolones alone [76]. Cellulitis can be treated with amoxicillin and clavulanic acid or a third-generation cephalosporin plus oxacillin [76]. Also, the addition of glycopeptides can be used when there is a high prevalence of MRSA or VSE, with third-generation cephalosporins remaining the main choice for treatment of community-acquired infections [76]. The treatment of hospital-acquired infections in hospitals with a low prevalence of MDR bacteria, is piperacillin/tazobactam or carbapenems for the treatment of spontaneous bacterial peritonitis, spontaneous bacterial empyema, and spontaneous bacteremia; the preferred treatment for pneumonia is piperacillin/tazobactam or carbapenems plus respiratory quinolones active against Pseudomonas; nitrofurantoin or fosfomycin can be selected for a simple urinary tract infection, and piperacillin/tazobactam or carbapenem can be selected for complex urinary tract infections (accompanied by sepsis); cellulitis can be treated with carbapenems or ceftazidime plus oxacillin [76]. Vancomycin or daptomycin are recommended for use in hospitals with a high prevalence of MRSA and VSE, and the use of linezolid is recommended for use in hospitals with a high prevalence of VRE (Table 3) [76].
Table 3.
Recommended empirical antibiotic treatment, according to Jalan et al. [72].
| Type of infection | Community-acquired infection | Nosocomial infection |
|---|---|---|
| Spontaneous bacteremia, SBP, SBE |
|
|
| Urinary tract infection | Simple:
|
Simple:
|
| Pneumonia |
|
|
| Cellulitis |
|
|
| Bacterial enteritis |
|
SBP – spontaneous bacterial peritonitis; SBE – spontaneous bacterial empyema; MRSA – methicillin-resistant Staphylococcus aureus; VSE – vancomycin-sensitive enterococci; VRE – vancomycin-resistant Enterococcus.
In hospitals with a high prevalence of MRSA and vancomycin-susceptible Enterococcus.
In hospitals with a high prevalence of vancomycin-resistant Enterococcus.
In a prospective study, Piano et al. [77] noted that ceftazidime and meropenem plus daptomycin showed significant differences in the treatment outcomes of spontaneous bacterial peritonitis in hospital-acquired infections. The response rate of broad-spectrum treatment increased from 25% to 86.7%, and the survival rate also increased significantly, with 94% responders and 50% non-responders [77]. Merli et al. [78] compared standard and broad-spectrum treatment for patients with cirrhosis, and the results showed that the failure rate of broad-spectrum treatment was lower than that of standard treatment (18% vs. 51%; P=0.001). Based on the above findings, we propose a combined approach to the treatment of patients with ACLF and hospital-acquired infections and recommend the use of broad-spectrum antibiotics, such as piperacillin plus combactam, or carbapenem plus glycopeptides. Also, we recommend adjusting the use of antibiotics in a timely manner, within 48 hours, according to the clinical condition of the patients and the microbial culture results (Figure 3).
Figure 3.
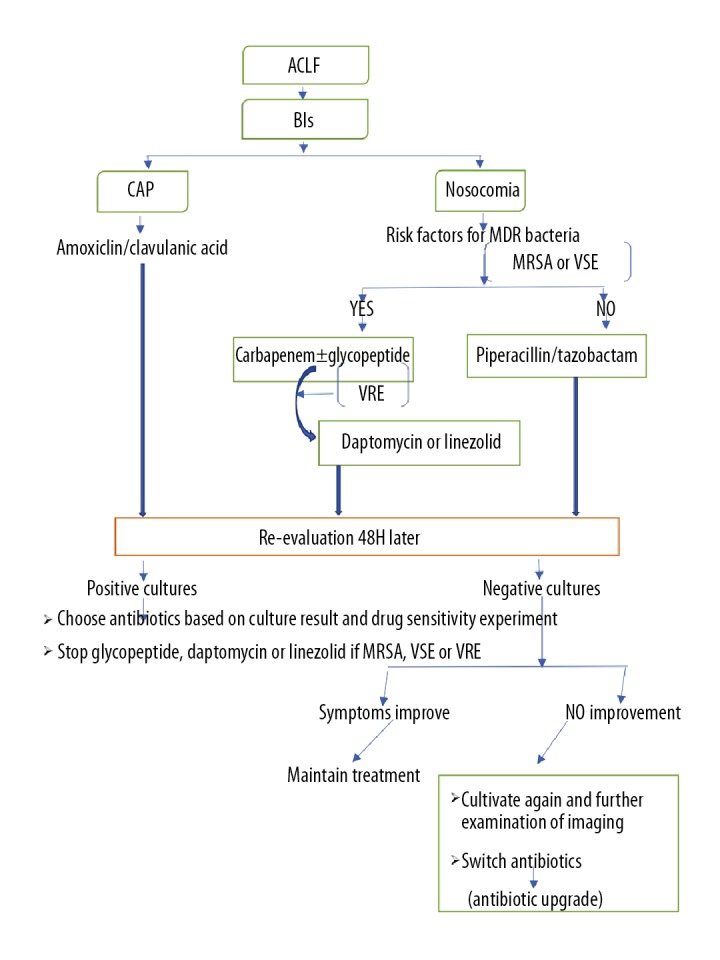
The approach to treatment for patients with acute-on-chronic liver failure (ACLF) with bacterial infections. Empirical anti-infective treatments should be based on the severity of infection, the risk factors for multidrug resistant (MDR) bacteria and epidemiological characteristics. BIs – bacterial infections; CAP – community-acquired infection; MDR – multidrug resistant; MRSA – methicillin-resistant Staphylococcus aureus; VSE – vancomycin-sensitive Enterococci; VRE – vancomycin-resistant Enterococci.
The Advantages and Disadvantages of Antibiotic Prophylaxis in Patients with ACLF
Adequate antibiotic prophylaxis can effectively reduce the incidence of bacterial infection and the risk of death in patients with cirrhosis [79]. Several studies have now identified three clinical conditions where antibiotics should be used prophylactically to prevent infection in patients with cirrhosis, and include the presence of low-protein ascites (<15 g/L), a previous history of spontaneous bacterial peritonitis, and the patient with ongoing bleeding [76,80,81]. These three conditions and the recommended prophylactic antibiotics are summarized in Table 4 [76,80,81].
Table 4.
Clinical conditions where antibiotics should be used prophylactically to prevent infection in patients with acute-on-chronic liver failure (ACLF) and the recommended prophylactic antibiotics.
| Type of prophylaxis | Condition | Antibiotic and dose | Duration |
|---|---|---|---|
| Primary prophylaxis | Low-protein ascites (<15g/L) and advanced cirrhosis |
|
Until LT Death |
| Secondary prophylaxis | Previous spontaneous bacterial peritonitis |
|
Until LT Death |
| Gastrointestinal bleeding | Patient currently bleeding |
|
7 days |
LT – liver transplantation; PO – by mouth; BID – once every 12 hrs; IV – intravenous. Child-Pugh score ≥9 points with serum bilirubin ≥3 mg/dL and/or impaired renal function (serum creatinine ≥1.2 mg/dL, blood urea nitrogen ≥25 mg/dL, or serum sodium ≥130 mEq/L).
In patients with advanced cirrhosis (at least two of the following: ascites, jaundice, hepatic encephalopathy and malnutrition) or ACLF.
However, the most serious problem associated with the use of prophylactic antibiotics is the development of multidrug resistance. The prevalence of MDR bacterial infections in patients with cirrhosis has been prohibitively high [64], and these high rates of resistance are associated with recent and frequently used antibiotics [82–84]. In common infections, recent antibiotic exposure and healthcare-acquired infections are predictors of empirical antibiotic resistance [84]. Therefore, the risks and benefits of prophylactic use of antibiotics require further evaluation. Rifaximin can non-selectively eliminate intestinal microbes and has a direct impact on bacterial translocation capacity [85]. Rifaximin can also reduce the incidence of hepatic encephalopathy [86]. However, rifaximin has a higher susceptibility to the development of bacterial MDR than norfloxacin [87]. Therefore, rifaximin can be used as an alternative to norfloxacin for the prevention of MDR bacterial infections. However, further randomized controlled trials are needed to confirm the true role of rifaximin in the prevention of infection in patients with cirrhosis.
Treatment of Secondary Invasive Fungal Infection in ACLF
In 2016, Nadim et al. reviewed the management of critically ill patients with cirrhosis and made recommendations for antifungal prevention and treatment, in patients with cirrhosis in three clinical situations [88]. Antifungal prophylaxis was recommended for patients in the intensive care unit who had received antibiotics for 48 h, whose clinical condition had not improved, and when the prevalence of fungal infection was more than 5%, when there is a risk of progression to invasive fungal disease, and when fungus was detected in the sputum of patients with endotracheal intubation [88]. Fever can be an indication for empirical antifungal therapy, even without microbiological or imaging evidence for fungal infection [88]. Early treatment with antifungal drugs may reduce mortality due to invasive fungal disease in patients with ACLF, even if there are no clinical signs of fungal infection. For patients with candidaemia, echinocandins (caspofungin: 70 mg/d initially, followed by 50 mg/d) are preferred, and fluconazole may be selected for the tapered treatment [89]. Cases of catheter-related candidemia should have the catheter removed in a timely manner, but in patients with asymptomatic candiduria, treatment may not be recommended [90]. In patients with invasive aspergillosis, voriconazole is recommended as the main antifungal agent [91]. In patients with ACLF, hepatocytes are severely damaged, the storage capacity of the liver is significantly reduced, and damage to renal function damage is more likely to occur. Therefore, amphotericin B and its liposomes should be avoided when selecting antifungals in patients with ACLF. Because of the potential hepatotoxicity of voriconazole, blood concentrations can be monitored if necessary and drug doses adjusted.
Non-Antimicrobial Treatment Strategies
Non-antibiotic strategies include four categories, which are summarized in Figure 4. First, the intestinal flora can be regulated with the use of probiotics, which are living bacteria that can replace or increase beneficial bacteria in the digestive tract and help improve intestinal function [92]. Prebiotics are indigestible food ingredients that promote the growth of beneficial bacteria, such as fermentable fibers. Synbiotics are a combination of probiotics and prebiotics. However, the addition of probiotics to norfloxacin has not been shown to reduce the incidence of peritonitis or death [93]. A fecal microbiota transplant is an emerging approach for the treatment of imbalance of the gastrointestinal flora [94].
Figure 4.
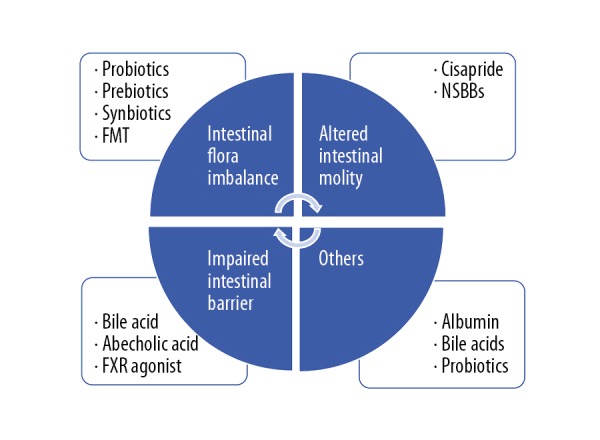
Summary of the four categories of non-antibiotic treatment for patients with acute-on-chronic liver failure (ACLF) with bacterial infections. The mechanisms are shown in the circle and the treatment strategies are shown in the box. FMT – fecal microbiota transplant; NSBBs – non-selective beta-blockers; FXR – non-steroidal farnesoid X receptor.
A second approach is to increase gastrointestinal motility, and the combination of norfloxacin and cisapride has been shown to significantly reduce the incidence of peritonitis in patients with cirrhosis [95]. However, cisapride extended the Q-T interval in some patients, which limits its use [95]. Non-selective beta-blockers have been shown to increase intestinal peristalsis and improve bacterial translocation in patients with ACLF [96]. Also, the use of non-selective beta-blockers and endoscopic band ligation can effectively reduce bleeding caused by esophageal varices in patients with cirrhosis and portal hypertension [96]. However, non-selective beta-blockers and abecholic acid have been shown to have a potential impact on blood pressure and heart rate, and the advantages and disadvantages of using non-selective beta-blockers in patients with ACLF require further study.
The third non-antimicrobial treatment strategy is to protect the function of the intestinal barrier, as bile acids affect the antibacterial activity of the intestinal mucosa directly or indirectly by regulating the expression of several host genes [97,98]. Non-steroidal farnesoid X receptor (FXR) agonists are nuclear receptors that regulate the metabolism of bile acids, lipids, and carbohydrates [99]. Abecholic acid is a potent semi-synthetic bile acid and FXR agonist that reduces intestinal inflammation and bacterial translocation [100]. The role of FXR in infection prevention deserves further study.
As a fourth treatment approach, albumin can reduce the risk of infection in patients with alcoholic cirrhosis at physiological concentration levels, but the specific mechanism remains unclear [101]. In patients with chronic end-stage liver disease, decreased intraluminal concentrations of bile acids can promote bacterial overgrowth [98]. Probiotics can promote mucosal barrier function and regulate intestinal flora, inhibiting the growth of pathogenic microorganisms [102].
Conclusions and Perspective
Microbial infection from bacterial and fungal organisms are a common trigger for the occurrence and development of ACLF, and early diagnosis and treatment are essential to improve the prognosis for these patients. The proliferation of multidrug-resistant bacteria has complicated the choices for the treatment of microbial infections in patients with ACLF. New methods are needed to identify the strains of organisms that cause infection and their antibiotic susceptibility. In the future, new antimicrobial agents will be required to treat infections due to drug-resistant organisms, and non-antibiotic approaches should be identified to prevent infection in patients with ACLF.
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China, grant number: 81670567
Conflict of interest
None.
References
- 1.Banares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: The RELIEF trial. Hepatology. 2013;57(3):1153–62. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Gao S, Duan Z, Hu KQ. Overview on acute-on-chronic liver failure. Front Medicine. 2016;10(1):1–17. doi: 10.1007/s11684-016-0439-x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Pandey G, Bansal D, et al. NMR-based urinary profiling of lactulose/mannitol ratio used to assess the altered intestinal permeability in acute on chronic liver failure (ACLF) patients. Magn Reson Chem. 2017;55(4):289–96. doi: 10.1002/mrc.4525. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Gustot T, Fernandez J, Garcia E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–52. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 6.Bunchorntavakul C, Chavalitdhamrong D. Bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. World J Hepatol. 2012;4(5):158–68. doi: 10.4254/wjh.v4.i5.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj JS, O’Leary JG, Reddy KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60(1):250–56. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thulstrup AM, Sorensen HT, Schonheyder HC, et al. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31(6):1357–61. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 9.Bartoletti M, Giannella M, Caraceni P, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61(1):51–58. doi: 10.1016/j.jhep.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7(3):309–19. doi: 10.1080/21505594.2016.1141162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez J, Bert F, Nicolas-Chanoine MH. The challenges of multi-drug-resistance in hepatology. J Hepatol. 2016;65(5):1043–54. doi: 10.1016/j.jhep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Mucke MM, Rumyantseva T, Mucke VT, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38(4):645–53. doi: 10.1111/liv.13568. [DOI] [PubMed] [Google Scholar]
- 13.Bartoletti M, Giannella M, Lewis R, et al. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. 2018;24(5):546.e541–48. doi: 10.1016/j.cmi.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Park H, Jang KJ, Jang W, et al. Appropriate empirical antibiotic use and 30-d mortality in cirrhotic patients with bacteremia. World J Gastroenterol. 2015;21(12):3587–92. doi: 10.3748/wjg.v21.i12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8(4):453–71. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67(12):2181–91. doi: 10.1136/gutjnl-2017-314641. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: The North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56(6):2328–35. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: A pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42(4):439–46. doi: 10.1111/j.1365-2362.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 20.Bellot P, Frances R, Such J. Pathological bacterial translocation in cirrhosis: Pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33(1):31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 21.Ahluwalia V, Wade JB, Heuman DM, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. Metab Brain Dis. 2014;29(4):1017–25. doi: 10.1007/s11011-014-9507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SC, Wang W, Ren WY, et al. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol. 2003;9(3):534–38. doi: 10.3748/wjg.v9.i3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardo A, Bartoli R, Lorenzo-Zuniga V, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31(4):858–63. doi: 10.1053/he.2000.5746. [DOI] [PubMed] [Google Scholar]
- 24.Liang FF, Wang J, Li L, et al. [Chronic liver disease increases with damage to intestinal barrier function]. Zhonghua Gan Zang Bing Za Zhi. 2018;26(8):612–17. doi: 10.3760/cma.j.issn.1007-3418.2018.08.010. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 25.Tazi KA, Moreau R, Herve P, et al. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: Role of Akt signaling. Gastroenterology. 2005;129(1):303–14. doi: 10.1053/j.gastro.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Wiest R, Das S, Cadelina G, et al. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104(9):1223–33. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quigley EM, Monsour HP. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):262–69. doi: 10.1055/s-0035-1562946. [DOI] [PubMed] [Google Scholar]
- 28.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–75. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thalheimer U, De Iorio F, Capra F, et al. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: A pilot study. Eur J Gastroenterol Hepatol. 2010;22(10):1228–34. doi: 10.1097/MEG.0b013e32833b4b03. [DOI] [PubMed] [Google Scholar]
- 30.Ouellette AJ, Selsted ME. Paneth cell defensins: Endogenous peptide components of intestinal host defense. FASEB J. 1996;10(11):1280–89. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 31.Teltschik Z, Wiest R, Beisner J, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55(4):1154–63. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 32.Clevers HC, Bevins CL. Paneth cells: Maestros of the small intestinal crypts. Ann Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 33.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–85. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 34.Bel S, Pendse M, Wang Y, et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science. 2017;357(6355):1047–52. doi: 10.1126/science.aal4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson ML, Karlsson-Sjoberg JM, Putsep KL. CRS-peptides: Unique defense peptides of mouse Paneth cells. Mucosal Immunol. 2012;5(4):367–76. doi: 10.1038/mi.2012.22. [DOI] [PubMed] [Google Scholar]
- 36.Adolph TE, Mayr L, Grabherr F, Tilg H. Paneth cells and their antimicrobials in intestinal immunity. Curr Pharm Des. 2018;24(10):1121–29. doi: 10.2174/1381612824666180327161947. [DOI] [PubMed] [Google Scholar]
- 37.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 38.Holly MK, Smith JG. Paneth cells during viral infection and pathogenesis. Viruses. 2018;10(5) doi: 10.3390/v10050225. pii: E225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez Rodriguez NR, Eloi MD, Huynh A, et al. Expansion of Paneth cell population in response to enteric Salmonella enterica serovar typhimurium infection. Infect Immun. 2012;80(1):266–75. doi: 10.1128/IAI.05638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinsky MR. Dysregulation of the immune response in severe sepsis. Am J Med Sci. 2004;328(4):220–29. doi: 10.1097/00000441-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72(9):3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–48. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 43.Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9(4):401–10. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 44.Adib-Conquy M, Cavaillon JM. [Host inflammatory and anti-inflammatory response during sepsis]. Pathol Biol (Paris) 2012;60(5):306–13. doi: 10.1016/j.patbio.2012.03.011. [in French] [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–70. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 46.Wiewel MA, Harmon MB, van Vught LA, et al. Risk factors, host response and outcome of hypothermic sepsis. Crit Care. 2016;20(1):328. doi: 10.1186/s13054-016-1510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkhoff A, Sieberichs A, Engler H, et al. Pro-inflammatory Th1 and Th17 cells are suppressed during human experimental endotoxemia whereas anti-inflammatory IL-10 producing T-cells are unaffected. Front Immunol. 2018;9:1133. doi: 10.3389/fimmu.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byl B, Roucloux I, Crusiaux A, et al. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104(5):1492–97. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- 49.Claria J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64(4):1249–64. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 50.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 51.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scandinavian J Infect Dis. 2003;35(9):585–92. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 52.Oberholzer TG, Grobler SR, Rossouw RJ, et al. [Microleakage of four different amalgam binding systems]. SADJ. 2001;56(2):64–70. [in Afrikaans] [PubMed] [Google Scholar]
- 53.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385–96. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Wasmuth HE, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42(2):195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Bajaj JS, Rajender Reddy K, Tandon P, et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am J Gastroenterol. 2018;113(4):556–63. doi: 10.1038/ajg.2017.471. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870–80. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 58.Lahmer T, Brandl A, Rasch S, et al. Fungal peritonitis: underestimated disease in critically ill patients with liver cirrhosis and spontaneous peritonitis. PLoS One. 2016;11(7):e0158389. doi: 10.1371/journal.pone.0158389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Yang Q, Huang J, Li L. Risk factors for invasive pulmonary aspergillosis and hospital mortality in acute-on-chronic liver failure patients: A retrospective cohort study. Int J Med Sci. 2013;10(12):1625–31. doi: 10.7150/ijms.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassan EA, Abd El-Rehim AS, Hassany SM, et al. Fungal infection in patients with end-stage liver disease: Low frequency or low index of suspicion. Int J Infect Dis. 2014;23:69–74. doi: 10.1016/j.ijid.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Muskett H, Shahin J, Eyres G, et al. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit Care. 2011;15(6):R287. doi: 10.1186/cc10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin LN, Zhu Y, Che FB, et al. Invasive fungal infections secondary to acute-on-chronic liver failure: A retrospective study. Mycoses. 2013;56(4):429–33. doi: 10.1111/myc.12044. [DOI] [PubMed] [Google Scholar]
- 63.Verma N, Singh S, Taneja S, et al. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int. 2019;39(3):503–13. doi: 10.1111/liv.13981. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology. 2012;55(5):1551–61. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 65.Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: A systematic review and meta-analysis. Diagn Microbiol Infect Dis. 2014;80(1):72–78. doi: 10.1016/j.diagmicrobio.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 66.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Chen P, Gao H, et al. Bacterial infection and predictors of mortality in patients with autoimmune liver disease-associated acute-on-chronic liver failure. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/5108781. 5108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardick J, Won H, Jeng K, et al. Identification of bacterial pathogens in ascitic fluids from patients with suspected spontaneous bacterial peritonitis by use of broad-range PCR (16S PCR) coupled with high-resolution melt analysis. J Clin Microbiol. 2012;50(7):2428–32. doi: 10.1128/JCM.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi Y, Wang HY, Lee G, et al. PCR-reverse blot hybridization assay for screening and identification of pathogens in sepsis. J Clin Microbiol. 2013;51(5):1451–57. doi: 10.1128/JCM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HY, Kim S, Kim J, et al. Comparison of multiplex real-time PCR and PCR-reverse blot hybridization assay for the direct and rapid detection of bacteria and antibiotic resistance determinants in positive culture bottles. J Med Microbiol. 2016;65(9):962–74. doi: 10.1099/jmm.0.000319. [DOI] [PubMed] [Google Scholar]
- 71.Aguirre SD, Ali MM, Salena BJ, Li Y. A sensitive DNA enzyme-based fluorescent assay for bacterial detection. Biomolecules. 2013;3(3):563–77. doi: 10.3390/biom3030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tram K, Kanda P, Salena BJ, et al. Translating bacterial detection by DNAzymes into a litmus test. Angewandte Chemie (International ed in English) 2014;53(47):12799–802. doi: 10.1002/anie.201407021. [DOI] [PubMed] [Google Scholar]
- 73.Ohlsson P, Petersson K, Augustsson P, Laurell T. Acoustic impedance matched buffers enable separation of bacteria from blood cells at high cell concentrations. Sci Rep. 2018;8(1):9156. doi: 10.1038/s41598-018-25551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohlsson P, Evander M, Petersson K, et al. Integrated acoustic separation, enrichment, and microchip polymerase chain reaction detection of bacteria from blood for rapid sepsis diagnostics. Anal Chem. 2016;88(19):9403–11. doi: 10.1021/acs.analchem.6b00323. [DOI] [PubMed] [Google Scholar]
- 75.Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436–42. doi: 10.1038/ajg.2014.212. [DOI] [PubMed] [Google Scholar]
- 76.Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–24. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 77.Piano S, Fasolato S, Salinas F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63(4):1299–309. doi: 10.1002/hep.27941. [DOI] [PubMed] [Google Scholar]
- 78.Merli M, Lucidi C, Di Gregorio V, et al. An empirical broad-spectrum antibiotic therapy in health-care-associated infections improves survival in patients with cirrhosis: A randomized trial. Hepatology. 2016;63(5):1632–39. doi: 10.1002/hep.28332. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2016;63(6):2019–31. doi: 10.1002/hep.28330. [DOI] [PubMed] [Google Scholar]
- 80.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 81.Runyon BA. Management of adult patients with ascites due to cirrhosis: An update. Hepatology. 2009;49(6):2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35(1):140–48. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 83.Chaulk J, Carbonneau M, Qamar H, et al. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: A single-centre experience and summary of existing studies. Can J Gastroenterol Hepatol. 2014;28(2):83–88. doi: 10.1155/2014/429536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10(11):1291–98. doi: 10.1016/j.cgh.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123–27. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 87.Ramos JM, Vidal I, Bellot P, et al. Comparison of the in vitro susceptibility of rifaximin versus norfloxacin against multidrug resistant bacteria in a hospital setting. A proof-of-concept study for use in advanced cirrhosis. Gut. 2016;65(1):182–83. doi: 10.1136/gutjnl-2015-309421. [DOI] [PubMed] [Google Scholar]
- 88.Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64(3):717–35. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 89.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaushik A, Kest H. The role of antifungals in pediatric critical care invasive fungal infections. Crit Care Res Pract. 2018;2018 doi: 10.1155/2018/8469585. 8469585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patterson TF, Thompson GR, 3rd, Denning DW, et al. Executive summary: Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):433–42. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome, and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39(10):1113–25. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pande C, Kumar A, Sarin SK. Addition of probiotics to norfloxacin does not improve efficacy in the prevention of spontaneous bacterial peritonitis: A double-blind placebo-controlled randomized-controlled trial. Eur J Gastroenterol Hepatol. 2012;24(7):831–39. doi: 10.1097/MEG.0b013e3283537d61. [DOI] [PubMed] [Google Scholar]
- 94.Wiest R, Albillos A, Trauner M, et al. Corrigendum to “Targeting the gut-liver axis in liver disease” [J Hepatol 67 (2017) 1084–1103] J Hepatol. 2018;68(6):1336. doi: 10.1016/j.jhep.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Itha S, Sharma A, Bhatt G. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol. 2006;21(10):1634. doi: 10.1111/j.1440-1746.2006.04107.x. [DOI] [PubMed] [Google Scholar]
- 96.Mookerjee RP, Pavesi M, Thomsen KL, et al. Treatment with non-selective beta-blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64(3):574–82. doi: 10.1016/j.jhep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Akoda K, Van den Bossche P, Marcotty T, et al. Nutritional stress affects the tsetse fly’s immune gene expression. Med Vet Entomol. 2009;23(3):195–201. doi: 10.1111/j.1365-2915.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 98.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103(10):3920–25. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cariou B, Staels B. The expanding role of the bile acid receptor FXR in the small intestine. J Hepatol. 2006;44(6):1213–15. doi: 10.1016/j.jhep.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Verbeke L, Farre R, Verbinnen B, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185(2):409–19. doi: 10.1016/j.ajpath.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58(5):1836–46. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 102.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol. 2000;95(1 Suppl):S2–4. doi: 10.1016/s0002-9270(99)00806-0. [DOI] [PubMed] [Google Scholar]


