Abstract
Background
Osteogenesis imperfecta is caused by a genetic defect resulting in an abnormal type I collagen bone matrix which typically results in multiple fractures with little or no trauma. Bisphosphonates are used in an attempt to increase bone mineral density and reduce these fractures in people with osteogenesis imperfecta. This is an update of a previously published Cochrane Review.
Objectives
To assess the effectiveness and safety of bisphosphonates in increasing bone mineral density, reducing fractures and improving clinical function in people with osteogenesis imperfecta.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Inborn Errors of Metabolism Trials Register which comprises references identified from comprehensive electronic database searches, handsearches of journals and conference proceedings. We additionally searched PubMed and major conference proceedings.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Inborn Errors of Metabolism Register: 28 April 2016.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing bisphosphonates to placebo, no treatment, or comparator interventions in all types of osteogenesis imperfecta.
Data collection and analysis
Two authors independently extracted data and assessed the risk of bias of the included trials.
Main results
Fourteen trials (819 participants) were included. Overall, the trials were mainly at a low risk of bias, although selective reporting was an issue in several of the trials. Data for oral bisphosphonates versus placebo could not be aggregated; a statistically significant difference favouring oral bisphosphonates in fracture risk reduction and number of fractures was noted in two trials. No differences were reported in the remaining three trials which commented on fracture incidence. Five trials reported data for spine bone mineral density; all found statistically significant increased lumbar spine density z scores for at least one time point studied. For intravenous bisphosphonates versus placebo, aggregated data from two trials showed no statistically significant difference for the number of participants with at least one fracture, risk ratio 0.56 (95% confidence interval 0.30 to 1.06). In the remaining trial no statistically significant difference was noted in fracture incidence. For spine bone mineral density, no statistically significant difference was noted in the aggregated data from two trials, mean difference 9.96 (95% confidence interval ‐2.51 to 22.43). In the remaining trial a statistically significant difference in mean per cent change in spine bone mineral density z score favoured intravenous bisphosphonates at six and 12 months. Data describing growth, bone pain, and functional outcomes after oral or intravenous bisphosphonate therapy, or both, as compared to placebo were incomplete among all studies, but do not show consistent improvements in these outcomes. Two studies compared different doses of bisphosphonates. No differences were found between doses when bone mineral density, fractures, and height or length z score were assessed. One trial compared oral versus intravenous bisphosphonates and found no differences in primary outcomes. Two studies compared the intravenous bisphosphonates zoledronic acid and pamidronate. There were no significant differences in primary outcome. However, the studies were at odds as to the relative benefit of zoledronic acid over pamidronate for lumbosacral bone mineral density at 12 months.
Authors' conclusions
Bisphophonates are commonly prescribed to individuals with osteogenesis imperfecta. Current evidence, albeit limited, demonstrates oral or intravenous bisphosphonates increase bone mineral density in children and adults with this condition. These were not shown to be different in their ability to increase bone mineral density. It is unclear whether oral or intravenous bisphosphonate treatment consistently decreases fractures, though multiple studies report this independently and no studies report an increased fracture rate with treatment. The studies included here do not show bisphosphonates conclusively improve clinical status (reduce pain; improve growth and functional mobility) in people with osteogenesis imperfecta. Given their current widespread and expected continued use, the optimal method, duration of therapy and long‐term safety of bisphosphonate therapy require further investigation. In addition, attention should be given to long‐term fracture reduction and improvement in quality of life indicators.
Plain language summary
Bisphosphonate therapy for osteogenesis imperfecta
Review question
We reviewed the evidence about the effect and safety of bisphosphonates in increasing bone mineral density, reducing fractures and improving clinical function in people with osteogenesis imperfecta. This is an update of a previously published Cochrane Review.
Background
Osteogenesis imperfecta is also known as brittle bone disease. It is a genetic condition which can be passed on from a parent to child or occur in the child without any other family history. An affected person is at risk for frequent breaks of the long bones or collapse of the bones of the spine. There is no cure for osteogenesis imperfecta and treatment is mostly supportive.
Search date
The evidence is current to: 28 April 2016.
Study characteristics
This review looked at trials studying one of the groups of medications known as bisphosphonates which are more typically used to treat osteoporosis. They are used in osteogenesis imperfecta to try and reduce the number of bone fractures in affected individuals.
We included 14 trials, and most of these did not show a major reduction in fractures in affected individuals when treated with bisphosphonates.
Key results
Each trial independently showed significant improvements in bone mineral density after treatment with oral or intravenous bisphosphonates. Bone pain, growth and quality of life indicators were not reported in enough detail and the effects of this treatment need further investigation. The long‐term effectiveness and safety of bisphosphates, as well as dose and duration of therapy, require extended evaluation
Quality of the evidence
The majority of trials analysed were small and not powered to show a statistically significant difference in many outcome measures.
Background
A definition of terms is available at: www.cochrane.org/glossary.
Description of the condition
Osteogenesis imperfecta (OI) is an inherited, and most often autosomal dominant condition, caused by mutations in genes encoding type I collagen (Steiner 2013). Several recessive genetic defects have been identified as causing OI. This condition (sometimes called brittle bone disease) is characterized by bone fragility, and predisposition to fractures, in many cases with minimal or no trauma. Low bone mass is a common but not universal feature. In addition to multiple fractures, individuals with OI also commonly exhibit joint hypermobility, blue or grey‐blue scleral colour, dentinogenesis imperfecta (a genetic disorder of tooth development), and premature hearing loss (Cole 2002).
Type I collagen is the most abundant protein of bone and is also present in ligaments, tendons, dentin, sclera, and skin. Normal bone matrix is composed of 90% Type I collagen fibers and 10% non‐collagenous proteins. These collagen fibers are usually oriented in a preferential direction with hydroxyapatite [Ca10(PO4)6(OH)2] crystals located in the ground substance within these fibers. Hydroxyapatite crystals provide mechanical rigidity and strength to bone whereas collagen fibers provide resilience. Individuals with OI have less or poorer quality (or both) type‐I collagen than unaffected people, causing their bones to deform or fracture (or both). In 80% to 90% of people with OI, mutations in one of the two genes encoding type I collagen chains, COL1A1 and COL1A2, are found (Byers 1991).
The exact incidence of OI is unknown as milder forms may be unrecognized. Finnish data published in 2002 by Kuurila suggests six per 100,000 individuals are affected with the disorder (Kuurila 2002). Other studies suggest the incidence of severe OI may be as high as 1 in 25,000 live births (Byers 2000; Connor 1985; Orioli 1995). Parents with OI caused by collagen mutations have a dominant disorder and 50% risk of having an affected child with each pregnancy. The majority of children with OI have inherited the disorder from a parent. De novo mutations account for approximately 35% of children with OI (OIF 2008). There are a number of less common causes of OI which are inherited as a recessive disorder in which case the risk is 25%. In general, these recessive forms of OI are more severe.
Prior to the availability of molecular genetic analyses, four major phenotypic classifications of OI were identified based on Sillence criteria which includes inheritance mode, clinical presentation and radiographic findings (Sillence 1979). Further refinement of these classifications was made with molecular genetic analyses (Byers 1991; Byers 1992; Glorieux 2000; OIF 2008; Steiner 2013). However, recently, the classification has once again been reviewed as the clinical utility of multiple subtypes of OI based on molecular diagnoses was limited. A return to the more clinically useful classification scheme has been recommended as represented in Table 1 (Sillence 2012). The diagnosis is made by a combination of history including family history, clinical examination, and radiographic findings with genetic and/or biochemical testing available for diagnostic confirmation.
1. Classification of osteogenesis imperfecta.
| Syndrome names | # of subtypes | Subtypes and genetic basis | Previous type |
| Classic Non‐deforming OI with Blue Sclerae | 2 | Ia ‐ With normal teeth or Opalescent Dentine caused by COL1A1 Ib ‐ caused by COL1A2 |
I |
| Common variable OI with Normal Sclerae | 2 | IVa ‐ caused by COL1A1 IVb ‐ caused by COL1A2 |
IV |
| OI with calcification in interosseous membranes | 1 | V ‐ caused by 5'UTR mutation in IFITM5 | V |
| Progressively Deforming OI with normal sclerae | 10 | III ‐gene x III‐COL1A1 or COL1A2 inherited as autosomal dominant III ‐ CRTAP, P3H1/LEPRE1, PPIB, FKBP10, SERPINH1, SP7/OSX, SERPINF1 and BMP 1 |
III |
International Nomenclature Committee for Constitutional Disorders of the Skeleton, Australia.
In about 80% to 90% of individuals affected by OI, mutations in either of the genes encoding the pro‐α1 or pro‐α2 chains of type I collagen (COL1A1 or COL1A2) can be identified (Byers 2013). Of those without collagen gene mutations, a number will have mutations in proteins involved in post‐translational modification or transport of type 1 collagen. These mutations tend to result in the progressively deforming type of OI (formerly Type III) or lethal OI (formerly Type II) and are inherited in an autosomal recessive manner. Genes involved include the following; CRTAP, P3H1/LEPRE1, PPIB, FKBP10, SERPINH1, SP7/OSX, SERPINF1, BMP1 and WNT1 (Alanay 2010; Barnes 2006; Becker 2011; Cabral 2007; Cho 2012; Christiansen 2010; Lapunzina 2010; Martinez‐Glez 2012; Pyott 2013Van Dijk 2009). The subtype of OI associated with interosseous calcification and hypertrophic callus and inherited in an autosomal dominant manner was recently identified to be caused by a 5'UTR mutation of the IFITM5 gene (Semler 2012).
Description of the intervention
There is no cure for OI and therapy is largely supportive at present. Therapy is varied and individualized depending upon OI severity, degree of impairment and age of the individual. Orthopedic management is paramount; surgical intervention or bracing of lower limbs, or both, is often required. Physical and occupational therapy are mainstays of therapy. Pharmacologic agents including growth hormone, calcitonin, parathyroid hormone, sodium fluoride, and vitamins have been administered in attempts to reduce fractures and deformities in OI. Oral and intravenous (IV) bisphosphonates are currently the most promising pharmacologic therapy and are routinely used for OI, since clinical trials of these agents have consistently shown improvements in bone mineral density (BMD) in people with OI. A prior version of this review and other systematic reviews have since found consistent improvements of BMD in people with OI who are treated with various bisphosphonates (Castillo 2009). Some trials also show fracture risk reduction and growth enhancement.
How the intervention might work
Bisphosphonates act by inactivating osteoclasts, the cells that break down bone tissue, thereby inhibiting bone resorption (Fisher 1999). There are two different types of bisphosphonates, nitrogenous and non‐nitrogenous. Nitrogenous bisphosphonates disrupt osteoclast formation, survival and cytoskeletal dynamics. Non‐nitrogenous bisphosphonates initiate osteoclast apoptosis. The bisphosphonates vary in their efficacy and absorption when taken orally, making direct comparison challenging. An additional table lists the currently available bisphosphonates (Table 2).
2. Current bisphosphonates.
| Bisphosphonate | Mechanism of action | Route of administration |
| Alendronate* (Fosamax) | Nitrogenous | Oral |
| Clodronate (Bonefos) | Non‐nitrogenous | Oral, IV |
| Etidronate (Didronel) | Non‐nitrogenous | Oral |
| Ibandronate (Boniva) | Nitrogenous | Oral |
| Neridronate* | Nitrogenous | IV |
| Olpadronate* | Nitrogenous | Oral, IV |
| Pamidronate* (Aredia) | Nitrogenous | IV |
| Risendronate (Actonel) | Nitrogenous | Oral |
| Tiludronate (Skelid) | Non‐nitrogenous | Oral |
| Zolendronate (Zometa, Reclast) | Nitrogenous | IV |
Nitrogenous bisphosphonates disrupt osteoclast formation, survival and cytoskeletal dynamics. They contain nitrogen (a colourless tasteless odourless element that as a diatomic gas is relatively inert and constitutes 78% of the atmosphere and that is a constituent of organic compounds found in all living tissues).
Non‐nitrogenous bisphosphonates initiate osteoclast apoptosis.
IV: intravenous
Bisphosphonates are widely used in post‐menopausal women to treat osteoporosis where they have been shown to increase bone density, decrease bone turnover (Reid 2002) and reduce fractures (Black 1996). Although increases in BMD are not expected to alter the underlying defective Type I collagen in OI, it is anticipated that increased BMD might lead to decreased fracture rates analogous to bisphosphonate therapy in post‐menopausal women with osteoporosis (Reid 2002). Animal models give reason for optimism as increases in BMD in a mouse model of OI are accompanied by decreases in fracture rate (Camacho 2001). Still, caution is advised, since the biology of OI differs from osteoporosis and improving bone density without altering resiliency may not lead to desired functional improvements (Marini 2003). A report of bisphosphonate‐induced osteopetrosis validates these concerns (Whyte 2003).
Why it is important to do this review
There is currently no consensus on the effectiveness and safety of these agents in the treatment of OI. Optimal timing of treatment in both children and adults remains undefined, as does dose and duration of treatment. Additionally, it is not fully understood whether fracture healing or post‐surgical healing (or both) in people with OI will be impacted by bisphosphonate therapy. The optimal duration of bisphosphonate use is unclear even in post‐menopausal women (FDA 2011). Emerging data from clinical trials and observational studies support an association between bisphosphonate use and atypical subtrochanteric femur fractures. These observations prompted the FDA to issue a warning regarding this possible adverse event (Erviti 2013). One of the authors (RS) observed such a complication in a teenage patient treated with alendronate.
Children's natural proclivity towards increased BMD and growth, coupled with the tendency for decreased fractures with advancing age in children with OI, make data comparison between adults and children difficult. Large, multicentre, randomised, placebo‐controlled trials to better assess the specific effects of bisphosphonate therapy for OI are still indicated. The goals for use of pharmacologic agents in OI include increased bone density as measured by dual‐energy X‐ray absorptiometry (DEXA), decreased fracture incidence, lessening of deformity, reduced pain, and improved growth and mobility.
This is an update of a previously published Cochrane Review (Dwan 2014; Phillipi 2008).
Objectives
To assess the effectiveness and safety of bisphosphonates in increasing BMD, reducing fractures and improving clinical function in people with OI.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials, published or unpublished.
Types of participants
Children (defined as age 0 to 18 years) and adults with OI diagnosed using accepted diagnostic criteria, based on clinical or laboratory findings, or both. Individuals affected with all types of OI are included in this review.
Types of interventions
Bisphosphonates to improve BMD in OI compared to placebo, no treatment control group, or comparator interventions, such as sodium fluoride; testosterone; vitamin C; vitamin D; flavonoids; calcitonin; growth hormone; parathyroid hormone; and different formulations or treatment regimens of bisphosphonates.
Types of outcome measures
Primary outcomes
-
Fracture reduction
numbers of breaks
frequency of breaks (rates)
Change in BMD as assessed by DEXA
Secondary outcomes
Change in biochemical markers of bone and mineral metabolism (e.g. bone alkaline phosphatase measurements)
Growth (z scores; vertebral heights)
Bone pain (as assessed by self‐reported questionnaires of pain and analgesic use)
Quality of life (e.g. functional changes in mobility, strength, well‐being and completion of activities of daily living (ADLs))
Lung function (e.g. pulmonary function testing)
Outcome data were grouped at six months and then annually. If outcome data had been recorded at other time periods, consideration was given to examining these as well.
Search methods for identification of studies
Electronic searches
We searched the Cystic Fibrosis and Genetic Disorders Group's Inborn Errors of Metabolism Trials Register using the term: osteogenesis imperfecta.
The Inborn Errors of Metabolism Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE and the prospective handsearching of one journal ‐ Journal of Inherited Metabolic Disease. Unpublished work was identified by searching through the abstract books of the Society for the Study of Inborn Errors of Metabolism conference and the SHS Inborn Error Review Series. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
We have run searches of PubMed and Ovid MEDLINE from 1966 to June 2016. The search strategies are listed in the additional tables (Table 3; Table 4).
3. Search strategy ‐ PubMed 1996 to June 2016.
| MESH terms |
| 1. osteogenesis imperfecta (827) 2. randomised controlled trial or Randomized Controlled Trials (49443) 3. 1 and 2 (4) 4. from 4 keep (2) |
4. Search strategy ‐ Ovid MEDLINE 1996 to June 2016.
| Search terms |
| 1. osteogenesis imperfecta (163) 2. randomised controlled trial (11846) 3. Randomized Controlled Trials (11906) 4. Combine 1, 2, 3 (7) 5. From 7 keep 2 (2) |
Date of the most recent search of the Group's Cystic Fibrosis and Genetic Disorders Group Inborn Errors of Metabolism Trials Register: 28 April 2016.
Searching other resources
The major conference proceedings from the Osteogenesis Imperfecta Foundation national conference, 1995 to March 2005, and the American Society for Bone and Mineral Research annual meeting proceedings, 1996 to March 2005, were also hand searched in order to identify pertinent unpublished work. In addition, the authors contacted the pharmaceutical companies, who manufacture bisphosphonates (November 2004), for information on any relevant RCTs, but only one manufacturer responded. The data from the Novartis study concluded on 03 June 2008 are included in this review.
Data collection and analysis
Selection of studies
Two authors (CP and RS, or CP and DB) read the papers identified by the review search strategy for relevancy and then assessed the trials for inclusion in the review based on the criteria outlined above. They identified important comparisons within each class (e.g. oral versus IV bisphosphonates). If disagreement arose on the suitability of a trial for inclusion in the review or its quality, they reached a consensus by discussion.
Data extraction and management
Two authors (CP and KD) independently extracted data using a structured form including date of publication, participant characteristics (especially demographics and type of OI), setting, detailed nature of intervention and control or, comparator, detailed nature of outcomes (i.e. bone density by DEXA, fractures, linear growth, bone turnover markers, bone pain and functional assessments).
Assessment of risk of bias in included studies
Previous versions of this review assessed the methodological quality of included studies based on the methods described by Jüni (Jüni 2001).
This version of the review has used the Cochrane risk of bias assessment as described in the Cochrane Hadbook for Systematic Reviews of Interventions 5.1 (Higgins 2011a). Two authors (CP and KD) evaluated the included studies independently for the domains listed below as 'low risk', 'high risk' or 'unclear risk' of bias and resolved any disagreement by discussion.
Random sequence generation
Concealment of allocation
Blinding of participants, personnel, outcome assessment
Incomplete outcome data
Selective reporting
Other potential sources of bias
Measures of treatment effect
For binary outcome measures (fracture reduction) the authors collected data on the number of participants for each outcome event and allocated treatment group. The authors calculated a pooled estimate of the treatment effect for each outcome across trials using risk ratios (RR) and 95% confidence intervals (CIs) where appropriate.
For continuous outcomes (change in BMD, change in biochemical markers of bone and mineral metabolism, growth, bone pain, quality of life, lung function) the authors recorded either the mean change from baseline for each group or mean post‐treatment or intervention values and standard deviation (SD) or standard error (SE) for each group. The authors calculated a pooled estimate of treatment effect by calculating the mean difference (MD) and 95% CIs.
Unit of analysis issues
The authors included one cross‐over trial in the review and treated the data from this trial as parallel data; i.e. as if the results from the two periods were independent (Seikaly 2005), Elbourne reports that using this approach is conservative, due to the fact that it ignores the within‐patient correlation (Elbourne 2002).
For count data (number of fractures) the authors calculated the relative rate from the information given in the published papers from three trials using Poisson regression (Bishop 2010; Sakkers 2004; Senthilnathan 2008).
Dealing with missing data
When the original papers presented data in a graph form, the authors sometimes estimated values for use in the review (Chevrel 2006). They calculated SDs when investigators reported SEs of the mean data (Seikaly 2005). The investigators of the Adami and Gatti trials kindly provided unpublished data on BMD (Adami 2003; Gatti 2005).
Assessment of heterogeneity
The authors will assess heterogeneity by visual inspection of the forest plots, Chi² test and I² statistic. The I² statistic describes the percentage of total variation across trials due to heterogeneity rather than chance (Higgins 2003). The values of I² lie between 0% and 100%, and a simplified categorization of heterogeneity that the authors plan to use is of low (I² value of 25%), moderate (I² value of 50%), and high (I² value of 75%) (Higgins 2003).
Assessment of reporting biases
In future reports, the authors plan to create a funnel plot in order to assess publication bias. If the authors observe asymmetry, they will investigate other reasons for this. Heterogeneity and selective outcome reporting would be possible sources for this bias.
Data synthesis
In this review the authors have analysed data using a fixed‐effects model. However, if in future updates, if they include a sufficient number of trials and find significant heterogeneity, they plan to use a random‐effects model of statistical analysis and investigate possible causes of heterogeneity further (see below).
The authors used meta‐analytic methods such as the inverse variance method for continuous outcomes and the Mantel‐Haenszel method for categorical outcomes.
Meta‐analysis of the available data was limited due to the different agents used (oral versus IV bisphosphonates), different outcome measures, different populations (adults versus children), different reporting indices (z score versus t score versus total BMD), and variable inclusion of a placebo or control group.
Subgroup analysis and investigation of heterogeneity
Where the authors find heterogeneity, and if they have sufficient trials included in a meta‐analysis (i.e. four or more), they will investigate the possible causes further. Proposed subgroup analyses are by age (adults versus child); type of OI; and severity of disease (mild or severe).
Sensitivity analysis
When the authors include sufficient trials, they will perform a sensitivity analysis based on the overall risk of bias of the trials, including and excluding quasi‐randomized trials.
Results
Description of studies
Please also refer to 'Table 5' for further details.
5. Study comparison: outcome data reported by individual studies.
| Study ID | Biochemical markers | BMD | Fracture incidence | Growth | Bone pain | Quality of life |
| Adami 2003 | Decrease in bone‐specific alkaline phosphatase 20%, decrease 25% in serum sCTx, decrease 20% in urinary free‐deoxypyridinoline in IV Neridronate group vs placebo. | Increase spine BMD 3.0 +/‐ 4.6%, hip 4.3 +/‐ 3.9% vs no significant change placebo. | 14% decrease in rate of fracture. | Not addressed. | Not addressed. | Not addressed. |
| Barros 2012 | Not addressed. | At the end of the follow‐up period, both groups showed a decrease in the fracture rate (P = 0.025 and P = 0.048, respectively) but no information was given comparing the groups. | There was no significant change in height in both groups throughout the treatment | Not addressed. | Not addressed. | |
| Bishop 2010 | Decrease in bone‐specific alkaline phosphatase 1%, decrease 21% serum NTx over 2‐year trial period (grouped data). | Lumbar spine BMD increased significantly only in the 2 mg/kg/wk group | Overall reduction in non‐vertebral fracture incidence in all groups during trial as compared to two years prior. Dose groups did not differ. | No difference between groups in height z scores. | No difference between groups in pain scores. | No difference between groups in grip strength or self reported function. |
| Bishop 2013 | The mean values for serum 25‐hydroxyvitamin D and intact parathyroid hormone were within normal ranges, and the changes from baseline were small at all time‐points for both treatment groups | Lumbar spine BMD increased significantly | significant decrease in risk of recurrent clinical fracture | No difference between groups in height z scores. | no difference in pain scales between the groups and the data was not shown | Not addressed. |
| Chevrel 2006 | Decrease in bone resorption markers (collagen peptides, osteocalcin) at one year. Alkaline phosphatase unchanged. | Increase spine BMD. Increase femur BMD. Effects seen primarily in first year of therapy. | No difference in vertebral or peripheral fracture rate. Not adequately powered. | Not addressed. | No difference in pain except an increase with alendronate at 36 month time point. | Not addressed. |
| DiMeglio 2006 | Decrease in alkaline phosphatase and bone alkaline phosphatase; decrease in urine NTX/Cr. | Increase BMD, BMC and area z scores in both oral and IV therapy. | Decreased fracture incidence with time when both groups are combined. | Increase height and length combined group z scores compared to normal children. | Not addressed. | Not addressed. |
| Gatti 2005 | Significant decrease in alkaline phosphatase from baseline. Groups did not differ. | Increase spine BMD in first 12 months, then groups were no longer different. BMD continued to be different from baseline. Initial increase (first 12 months) in height/projected lumbar spine area, followed by no change at 12 ‐ 26 months. | Relative risk reduction 0.36% (CI, 0.15 ‐ 0.87; P < 0.02). | Initial increase (first 12 months) in height/projected lumbar spine area, followed by no change at 12 ‐ 26 months. | Not addressed. | Not addressed. |
| Letocha 2005 | No significant change from baseline as measured at each infusion time. | Increase spine BMD in treatment group as compared to control group. Increases were seen in the first 12 months but no further increases were noted with extended therapy. | Decreased upper extremity fractures in the first year of treatment with no further decrease in the second year. No change in fracture incidence of lower extremity long bone fracture in the first or second year. | Growth rates were unchanged. | No difference in self‐reported pain scores. | No difference in muscle strength or gross motor abilities. |
| Zoledronic Acid 2008 | The secondary endpoints of relative change from baseline in biomarkers of bone turnover (serum ƒÀ‐CTx, P1NP and BALP, in participants greater than or equal to 3 years of age) all had statistically significant greater reductions in the zoledronic acid group compared to the pamidronate group at both 6 and 12 months | The primary analysis of the percentage change in LS BMD at month 12 relative to baseline in the ITT (LOCF) population demonstrated that zoledronic acid was not only non‐inferior, but superior, to pamidronate with an 8% greater increase in LS BMD and both 95% confidence intervals above zero | No difference between groups in fractures. | No difference between groups in mean supine length, mean vertebral spine length and height. | No statistically significant differences were identified between treatment groups | Not addressed. |
| Rauch 2009 | Decrease collagen type‐1 N‐telopeptide in treatment group compared to placebo. | Increase spine BMD in treatment group compared to placebo. | No detectable difference in new fractures. | No difference in height z scores. | No difference in bone pain. | No difference in grip force. |
| Sakkers 2004 | No significant change between groups. | Increase spine z score 1.67 SD vs no significant change placebo. | 31% decrease in rate of fracture. | No significant change. | Not addressed. | No significant difference in mobility / ambulation; muscle strength or self care. |
| Seikaly 2005 | Decrease in urinary NTX/Cr. No change in serum markers or other urinary markers of bone turnover. | Increase in BMD z score 0.89 with alendronate compared to ‐0.12 with placebo. | Non‐significant trend toward decreased fractures. | Increased height z scores (0.41 vs 0.11) when alendronate is compared to placebo. | Decreased pain scores and decreased use of analgesia. | Improved well being scores. Increase in self care. No change in mobility. |
| Senthilnathan 2008 | Decrease in bone specific alkaline phosphatase and serum NTx in both groups. | Increase in LSB mass for both groups. Increased LSBMD in 12/mg/kg/yr group after adjustment. | No differences in crush fractured vertebrae between groups. Improved crush fractures in all but one infant. | No difference in length z scores between groups. | Not addressed. | Not addressed. |
| Ward 2010 | Decrease in uNTx (62%) compared to placebo (32%). | Increase in LSBMD compared to placebo. | No difference in fractures. | No difference in height z scores. | No difference in bone pain. | No difference in mobility. |
IV: intravenous NTX/Cr: N‐linked telopeptides/creatinine sCTx: serum cross‐laps vs: versus
Results of the search
Twenty‐one trials were identified and reviewed, of which 14 (including 819 participants) RCTs met the inclusion criteria (Adami 2003; Barros 2012; Bishop 2010; Bishop 2013; Chevrel 2006; DiMeglio 2006; Gatti 2005; Letocha 2005; Rauch 2009; Sakkers 2004; Seikaly 2005; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008).
Included studies
Six trials compared an oral bisphosphonate to placebo (Bishop 2013; Chevrel 2006; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010), while three trials compared an IV bisphosphonate to placebo (Adami 2003; Gatti 2005; Letocha 2005). One trial compared different doses of oral bisphosphonates (Bishop 2010) and one trial compared different doses of IV bisphosphonates (Senthilnathan 2008). One trial compared oral to IV bisphosphonates (DiMeglio 2006). Two trials compared different IV bisphosphonates (Barros 2012; Zoledronic Acid 2008).
Twelve trials enrolled 709 children, one of which included participants up to 19 years of age (Ward 2010), which we included as children (Barros 2012; Bishop 2010; Bishop 2013; DiMeglio 2006; Gatti 2005; Letocha 2005; Sakkers 2004; Rauch 2009; Seikaly 2005; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008) and two trials enrolled 110 adults (Adami 2003; Chevrel 2006).
Excluded studies
Eight trials were excluded from the review (Antoniazzi 1996; Antoniazzi 2006; Antoniazzi 2010; DiMeglio 2004; Gerber 1998; Granda 1977; Orwoll 2014; Ward 2005). Four trials were not RCTs (Antoniazzi 1996; Antoniazzi 2006; DiMeglio 2004; Ward 2005). A further trial did not evaluate bisphosphonates but rather long‐leg braces (Gerber 1998). One trial studied pyrophosphate levels in OI disease severity rather than improvement in bone density or fracture reduction (Granda 1977), and a further trial investigated response to growth hormone rather than bisphosphonates (Antoniazzi 2010). The eighth trial investigated teriparatide, a parathyroid hormone analogue and not a bisphosphonates (Orwoll 2014).
Risk of bias in included studies
The risk of bias has been assessed for this update and information is included below and in Characteristics of included studies tables.
Allocation
Generation of the allocation sequence
Six trials were described as randomised, by computer‐generated random numbers and were deemed to have a low risk of bias (Bishop 2010; Chevrel 2006; DiMeglio 2006; Letocha 2005; Sakkers 2004; Seikaly 2005). One further trial used an interactive voice response system and was deemed to have a low risk of bias (Bishop 2013).
Six trials were described as randomised, although no information on the randomised procedures used was given and the trials are therefore at an unclear risk of bias (Adami 2003; Barros 2012; Rauch 2009; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008). Similarly, the Gatti trial was described as a RCT but the method was not fully described and participants were assigned according to OI type to either an active or control group (unclear risk of bias) (Gatti 2005).
Concealment of allocation
For eight trials, the method of allocation concealment was not stated and therefore were at an unclear risk of bias (Adami 2003; Barros 2012; Gatti 2005; Letocha 2005; Rauch 2009; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008).
For the Sakkers trial, generation of the randomisation sequence was done independently of the researchers by an outside group, therefore concealment was deemed at low risk of bias (Sakkers 2004). Similarly, for the Seikaly trial allocation was concealed by a pharmacist at the institution and was found to be at a low risk of bias (Seikaly 2005). For the Cheverel trial, allocation was concealed by giving the randomised list to the researchers who then assigned each new trial participant the subsequent number on the list (low risk of bias) (Chevrel 2006). For the DiMeglio trial, allocation concealment was ensured by a clinic nurse who assigned treatment (low risk of bias) (DiMeglio 2006). Two trials used allocation by a remote telephone system randomisation and was at a low risk of bias (Bishop 2010; Bishop 2013).
Blinding
Six trials were described as double blinded and therefore are at a low risk of bias (Bishop 2010; Bishop 2013; Chevrel 2006; Sakkers 2004; Seikaly 2005; Ward 2010).
The Adami trial stated that "prevalent vertebral fractures were identified and graded blindly by a semi quantitative scale", so was therefore at a low risk of bias for outcome assessors (Adami 2003). The Letocha trial was described as unblinded but the investigators were stated as blinded to vertebral area/compression (Letocha 2005) so outcome assessors were at a low risk of bias. The Gatti trial was not described as blinded and is therefore at an unclear risk of bias (Gatti 2005).
Two trials were open label and therefore at a high risk of bias (Barros 2012; DiMeglio 2006). One trial was open label but the outcome assessors were blind to treatment allocation therefore this trial was deemed at a low risk of bias (Zoledronic Acid 2008). One trial had carers, participants and outcome assessors blinded and was therefore at low risk of bias (Senthilnathan 2008). Details of blinding were not stated in one trial and was therefore at an unclear risk of bias (Rauch 2009).
Incomplete outcome data
For the Sakkers trial, an intention‐to‐treat analysis was undertaken. Two participants (one placebo and one treatment) withdrew from the trial but were accounted for in the final analysis and is at low risk of bias (Sakkers 2004). It was also reported that intention‐to‐treat analyses were performed in the Cheverel trial, and dropouts were described, therefore the trial is at a low risk of bias (Chevrel 2006).
In the Adami trial, per protocol analyses were performed; although intention‐to‐treat analyses were planned, they were not applied as all participants completed the treatment follow up, indicating a low risk of bias (Adami 2003). Similarly, in the Gatti trial, intention‐to‐treat analyses were planned but not applied as all participants completed the treatment follow up (Gatti 2005).
In the Letocha trial a per protocol and repeated‐measures model was used but its is not clear if all participants completed the trial, so is therefore at an unclear risk of bias (Letocha 2005).
For the Seikaly trial it is unclear whether intention‐to‐treat analyses were performed introducing potential attrition bias, therefore this trial is at an unclear risk of bias (Seikaly 2005).
The type of analysis performed is not stated for the DiMeglio trial, although changes in group assignment were made when participants had difficulty tolerating the assigned regimen (DiMeglio 2006). Loss to follow up for each group is reported therefore the trial is at low risk of bias.
One trial did not provide any information on the reasons that participants dropped out of the trial and they did not state if an intention‐to‐treat analysis was used (Barros 2012). This trial was therefore deemed at an unclear risk of bias.
For six trials all dropouts were reported indicating a low risk of bias (Bishop 2010; Bishop 2013; Rauch 2009; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008).
Selective reporting
Eight trials were classified as having a low risk of bias as outcomes stated in the methods sections were reported (Adami 2003; Bishop 2013; Chevrel 2006; Gatti 2005; Seikaly 2005; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008). However, we had no access to protocols for this judgement, although trial registry information was available for one trial (Zoledronic Acid 2008). Five trials were judged as having high risk of bias as outcomes were only reported as non‐significant and no further data were reported (Bishop 2010; DiMeglio 2006; Letocha 2005; Rauch 2009; Sakkers 2004). One trial was classed as having an unclear risk of bias as decreased fracture rate was not fully reported (Barros 2012).
Other potential sources of bias
Some trials employed retrospective methods to assess fracture rates and may be subject to recall bias (Adami 2003; Bishop 2010; DiMeglio 2006; Gatti 2005; Letocha 2005). The natural proclivity towards reduced fractures and growth with age makes comparison difficult if adjusted scoring such as z scores are not used by researchers.
Effects of interventions
As mentioned above, data were sometimes estimated when presented in graph form (Chevrel 2006). Standard deviations were calculated when SEs of the mean data were reported by the trial investigators (Seikaly 2005). For the Adami and Gatti trials, the intervention groups each received treatment for 24 and 36 months respectively, and the control groups also began active therapy at 12 months for the remainder of each trial (Adami 2003; Gatti 2005). Data are reported in this review at the 6 and 12 month time‐points for both trials where there is a comparison between intervention versus control (Adami 2003; Gatti 2005).
One cross‐over trial was included in the review, data from this trial were treated as parallel data (Seikaly 2005). For count data (number of fractures), the relative rate was calculated from the information given in the published papers or received from authors from five trials using Poisson regression (Adami 2003; Bishop 2010; Gatti 2005; Sakkers 2004; Senthilnathan 2008).
Oral bisphosphonates compared to placebo or no treatment control group
Six trials were included in this comparison (Bishop 2013; Chevrel 2006; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010).
Primary outcomes
1. Fracture incidence
Each of the six trials reported on this outcome (Bishop 2013; Chevrel 2006; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010). The Sakkers trial reported a 31% reduction in relative risk for fracture after treatment with oral olpadronate, and when analysed in the review this produced a hazard ratio of 0.69 (95% CI 0.52 to 0.91) (Analysis 1.1 ‐ as reported in the paper) and a statistically significantly decreased fracture number, relative rate (RR) 0.40 (95% CI 0.24 to 0.69) (Analysis 1.2) (Sakkers 2004). The Bishop trial also reported risk of recurrent clinical fracture and when analysed in the review this produced a HR of 0.58 (95% CI: 0.37 to 0.92) (P = 0.0416) at 12 months, which was statistically significant in favour of oral risendronate (Analysis 1.1). The Bishop trial also reported statistically significant differences in clinical non‐vertebral (29 out of 92 versus 24 out of 49, P = 0.0446) and long‐bone fractures (18 out of 94 versus 17/ out of 49) and time to first fracture, HR 0.53 (95% CI 0.31 to 0.92) (P = 0.0337) (Bishop 2013).
1.1. Analysis.
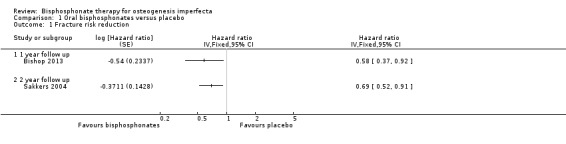
Comparison 1 Oral bisphosphonates versus placebo, Outcome 1 Fracture risk reduction.
1.2. Analysis.
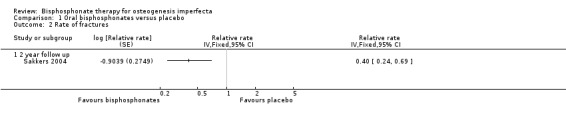
Comparison 1 Oral bisphosphonates versus placebo, Outcome 2 Rate of fractures.
Seikaly noted "a tendency to decrease the frequency of bone fractures" with alendronate versus placebo that did not reach significance (Seikaly 2005). Data cannot be entered into the meta‐analysis for this cross‐over trial as the total number of fractures (and not number of participants with one fracture or more) were reported across treatment groups. Three trials (Chevrel 2006; Rauch 2009; Ward 2010) also showed no statistically significant difference in the number of people with at least one fracture with bisphosphonates compared to placebo, two trials at 24 months, RR 1.05 (95% CI 0.82 to 1.35) (Rauch 2009; Ward 2010) and one at 36 months, RR 0.97 (95% CI 0.48 to 1.95) (Chevrel 2006) (Analysis 1.3). However, Chevrel was not adequately powered to detect differences in fracture rate (Chevrel 2006). Rauch reported 11 fractures from seven of the 13 participants (0 to 2 per participant) in the risedronate group and 11 fractures from six of the 13 participants (0 to 4 per participant) in the placebo group (Rauch 2009).
1.3. Analysis.
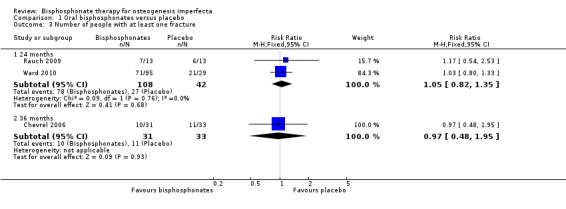
Comparison 1 Oral bisphosphonates versus placebo, Outcome 3 Number of people with at least one fracture.
2. Change in BMD as assessed by DEXA
a. Mean per cent change in spine BMD
Each of the six trials reported on this outcome (Bishop 2013; Chevrel 2006; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010). For the Sakker trial, data are not available in an appropriate form to be entered into the meta‐analyses (Sakkers 2004). Data for this trial were presented as within‐group changes (not presented in this review) showing statistically significant improvements in the bisphosphonates group and statistically non‐significant changes in the placebo group (Sakkers 2004). One trial found a statistically significantly increase in lumbar spine density z scores at six months, in favour of oral bisphosphonates, MD 0.39 (95% CI 0.28, 0.50) (Analysis 1.4) (Bishop 2013). Two trials found a statistically significantly increased lumbar spine density z scores at 12 months, in favour of oral bisphosphonates, MD 0.51 (95%CI 0.35 to 0.68) (Analysis 1.4) (Bishop 2013; Seikaly 2005). Two trials reported a significant change in lumbar spine area BMD z score at 24 months, MD 0.99 (95% CI 0.70, 1.28) in favour of oral bisphosphonates (Analysis 1.4) (Rauch 2009; Ward 2010).
1.4. Analysis.
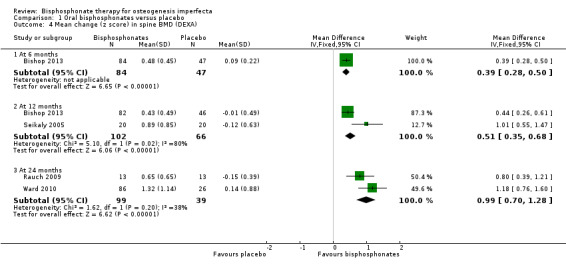
Comparison 1 Oral bisphosphonates versus placebo, Outcome 4 Mean change (z score) in spine BMD (DEXA).
Chevrel reported a significant increase in lumbar spine BMD throughout the three years of the trial (Chevrel 2006). It was reported that the increase was much greater after the first 12 months, although this continued, without reaching a plateau to the end of treatment at 36 months; for lumbar spine at 12 months, MD 7.00 (95% CI 3.87 to 10.13) and at 36 months, MD 9.40 (95% CI 5.44 to 13.36) (Chevrel 2006) (Analysis 1.5). Three studies reported a significant difference at two years, MD 17.31 (95% CI 5.01 to 29.62) although there was a considerable amount of heterogeneity (I² = 77%) (Chevrel 2006; Rauch 2009; Ward 2010) (Analysis 1.5).
1.5. Analysis.
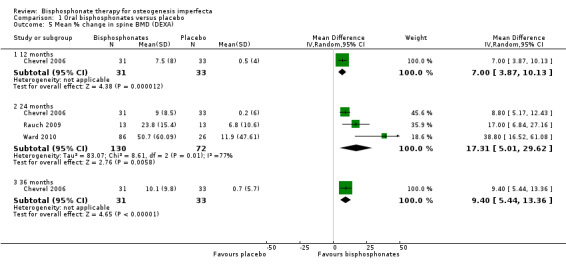
Comparison 1 Oral bisphosphonates versus placebo, Outcome 5 Mean % change in spine BMD (DEXA).
b. Mean per cent change in total femur BMD
One trial reported on this outcome (Chevrel 2006). The mean per cent change in total femur BMD were reported by Chevrel at 36 months, the increase in the alendronate group was statistically significantly greater than that in the placebo group; total femur BMD, MD 3.00 (95% CI 2.73 to 3.27) (Chevrel 2006) (Analysis 1.6).
1.6. Analysis.
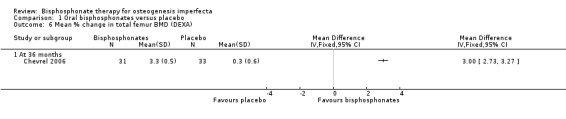
Comparison 1 Oral bisphosphonates versus placebo, Outcome 6 Mean % change in total femur BMD (DEXA).
Secondary Outcomes
1. Change in biochemical markers of bone and mineral metabolism and bone histology
The varied markers chosen for study in the included trials we reviewed prohibited direct comparison, but a narrative description of the findings are presented.
Each of the six trials reported on this outcome (Chevrel 2006; Bishop 2013; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010). Sakkers reported (in narrative format only) no statistically significant change in urine or serum markers between the olpadronate and placebo groups (Sakkers 2004). Seikaly also found no change in serum or urinary markers of bone turnover between the alendronate and placebo groups. However, since data for this cross‐over trial were presented separately by treatment arm for each of the four arms of the trial, we were unable to analyse this as if it were a parallel trial as planned (See: Unit of analysis issues) (Seikaly 2005). A decrease in some bone resorption markers (collagen peptides, osteocalcin) with alendronate administration was noted by Chevrel while alkaline phosphatase levels were unchanged (Chevrel 2006). In the Ward trial, no difference was found in serum alkaline phosphatase, but there was a difference in uNTx to creatinine ratio (Ward 2010). Rauch reported a difference in risedronate and placebo for serum NTX %, but no difference for alkaline phosphatase % and urine NTX/Cr % (Rauch 2009). The Bishop trial reported normal and unchanged values for serum 25‐hydroxyvitamin D and intact parathyroid hormone at all time‐points for both treatment groups (Bishop 2013). They additionally report statistically significant changes in both urine NTx/Cr and bone‐specific alkaline phosphatase at the six‐ and 12‐month time periods (Bishop 2013).
2. Growth
Five trials reported on this outcome (Bishop 2013; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010). Sakkers narratively reported no differences in seated height or radiographic assessments of lumbar vertebral height between olpadronate and placebo at 24‐months follow up (Sakkers 2004). Bishop and Seikaly found a statistically significant increase in height growth z scores in response to 12 months of alendronate therapy, MD 0.24 (95% CI 0.04 to 0.43) (Bishop 2013; Seikaly 2005), but Rauch and Ward did not find a statistically significant difference at 24 months, MD 0.08 (95% CI ‐0.19 to 0.35) (Rauch 2009; Ward 2010) (Analysis 1.7). This outcome was not addressed in the remaining trial (Chevrel 2006).
1.7. Analysis.
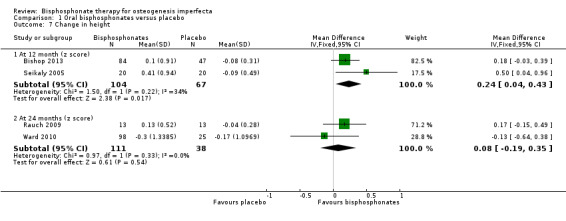
Comparison 1 Oral bisphosphonates versus placebo, Outcome 7 Change in height.
3. Bone pain
Five trials reported on this outcome (Bishop 2013; Chevrel 2006; Rauch 2009; Seikaly 2005; Ward 2010). For the alendronate group, statistically significant decreases in pain scores and analgesic use at 12 months were reported by Seikaly, MD ‐3.63 (95% CI ‐5.17 to ‐2.09) and MD ‐2.00 (95% CI ‐3.57 to ‐0.43), respectively (Analysis 1.8; Analysis 1.9) (Seikaly 2005). Here, the interaction of treatment and order were not statistically significant, indicating that differences found between alendronate and placebo were not explained by order of administration. At 24 months Ward reported a non‐significant difference (in favour of the alendronate group), MD ‐0.73 (95% CI ‐2.64, 1.18) (Ward 2010). The number of participants with bone pain was also reported as 37% (38 out of 102) in the alendronate group and 57% (17 out of 30) in the placebo group. Chevrel narratively reported that the pain score was similar in both groups from 0 to 30 months and reported end of trial data that showed an increase at 36 months with alendronate, MD 1.30 (95% CI 0.14 to 2.46) (Analysis 1.8) (Chevrel 2006). Rauch reported that the number of participants suffering from bone pain at the end of the trial was 31% (four out of 13) in both the risedronate group and the placebo group and the difference was not statistically significant (Rauch 2009). Bishop did not identify a difference in pain scales between the groups and the data was not shown. However, they do report pain as an adverse events reported by at least 10% of participants in either group (pain in 14 out of 94 versus five out of 49 and pain in the arms and back in 20 out of 94 versus 14 out of 49) (Bishop 2013).
1.8. Analysis.
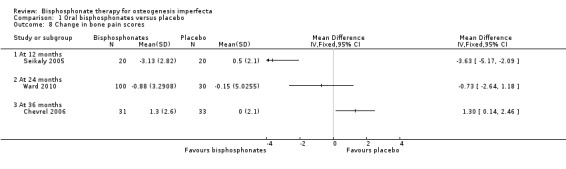
Comparison 1 Oral bisphosphonates versus placebo, Outcome 8 Change in bone pain scores.
1.9. Analysis.
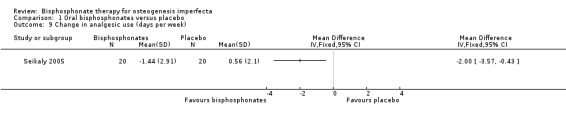
Comparison 1 Oral bisphosphonates versus placebo, Outcome 9 Change in analgesic use (days per week).
Bone pain was not evaluated by Sakkers (Sakkers 2004).
4. Quality of life
Four of the included trials evaluated at least one quality of life outcome (Chevrel 2006; Sakkers 2004; Seikaly 2005; Ward 2010). Seikaly reported a statistically significant increase in well‐being as assessed by scored participant recall, MD 3.19 (95% CI 2.25 to 4.13) (Seikaly 2005) (Analysis 1.10); improved self‐care skills or ADLs (assessed by Pediatric Evaluation of Disability Inventory (PEDI), a validated measurement tool) with alendronate versus placebo, MD 3.58 (95% CI 1.06 to 6.10) (Analysis 1.11); but no improvements in mobility as assessed by WeeFIM (a validated measurement tool for transfers, locomotion, access to stairs), MD 0.79 (95% CI ‐3.31 to 4.89) (Analysis 1.12). In contrast, Sakkers compared olpadronate to placebo and narratively reported that there were no changes in functional outcomes as assessed by PEDI, nor did the authors find changes in grip or hip flexor strength. Mobility as assessed by another validated scale (Bleck) was also not improved when compared to placebo controls (Sakkers 2004). These functional outcomes were not addressed in other included studies (Chevrel 2006). Chevrel was the only trial to assess hearing and did not find any difference (as assessed by Rinne testing %) with alendronate administration, MD ‐0.10 (95% CI ‐2.88 to 2.68) (Chevrel 2006) (Analysis 1.13).
1.10. Analysis.
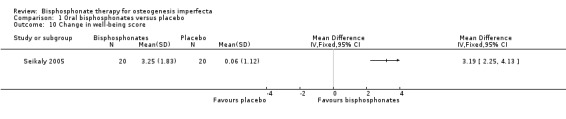
Comparison 1 Oral bisphosphonates versus placebo, Outcome 10 Change in well‐being score.
1.11. Analysis.
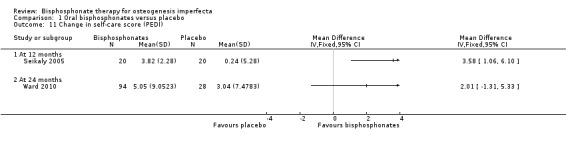
Comparison 1 Oral bisphosphonates versus placebo, Outcome 11 Change in self‐care score (PEDI).
1.12. Analysis.
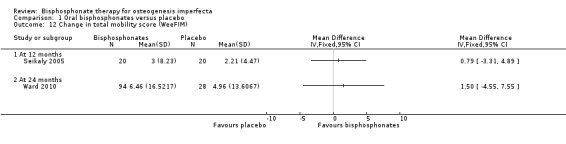
Comparison 1 Oral bisphosphonates versus placebo, Outcome 12 Change in total mobility score (WeeFIM).
1.13. Analysis.
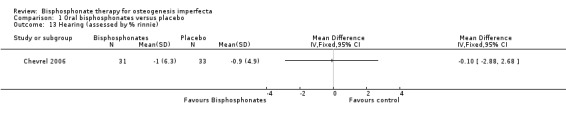
Comparison 1 Oral bisphosphonates versus placebo, Outcome 13 Hearing (assessed by % rinnie).
The Ward trial reported no change from baseline in self‐care, mobility and grip force at 24 months; self‐care, MD 2.01 (95% CI ‐1.31 to 5.33) (Analysis 1.11); and mobility, MD 1.50 (95% CI ‐4.55, 7.55) (Ward 2010) (Analysis 1.12).
Two trials did not report on quality of life (Bishop 2013; Rauch 2009).
5. Lung function
None of the included trials reported on this outcome (Bishop 2013; Chevrel 2006; Rauch 2009; Sakkers 2004; Seikaly 2005; Ward 2010).
IV Bisphosphonates compared to placebo or no treatment control group
Three trials were included in this comparison (Adami 2003; Gatti 2005; Letocha 2005).
Primary outcomes
1. Fracture incidence
Each of the included trials reported on this outcome (Adami 2003; Gatti 2005; Letocha 2005). For the Adami and Gatti trials, data on the number of participants with at least one fracture were obtained from the primary investigators. There was no statistically significant difference between the treatment and control groups, RR 0.56 (95% CI 0.30 to 1.06) (Adami 2003; Gatti 2005) (Analysis 2.1). Data were also obtained on the total number of fractures amongst participants for the treatment and control groups and these were one out of 31 and two out of 15 (respectively) for the Adami trial and 13 out of 42 and 18 out of 22 for the Gatti trial (Adami 2003; Gatti 2005). For the Letocha trial the incidence of fractures of the lower and upper extremities at 12 months did not change statistically significantly between the pamidronate and placebo groups from baseline, MD ‐0.11 (95% CI ‐0.96 to 0.74) (Analysis 2.2;); MD ‐0.22 (95% CI ‐0.67 to 0.23), respectively (Letocha 2005) (Analysis 2.3).
2.1. Analysis.
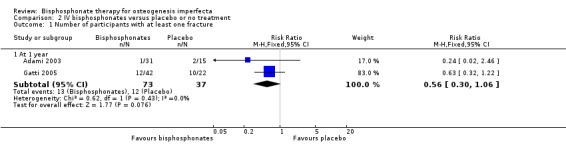
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 1 Number of participants with at least one fracture.
2.2. Analysis.
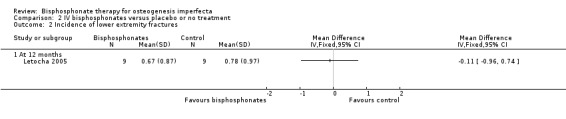
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 2 Incidence of lower extremity fractures.
2.3. Analysis.
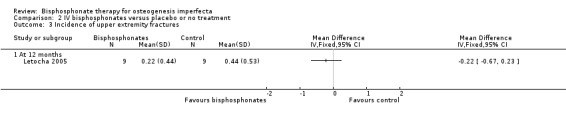
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 3 Incidence of upper extremity fractures.
2. Change in BMD as assessed by DEXA
a. Mean per cent change in spine BMD
Each of the included trials reported on this outcome (Adami 2003; Gatti 2005; Letocha 2005). Adami reported BMD at six month intervals from six to 24 months. In this trial, the intervention group received neridronate for 24 months and the control group began therapy at 12 months which continued for 12 months. We therefore report data here for the six‐ and 12‐month time points to compare intervention versus no treatment (Adami 2003). When summary statistics from two studies were calculated, there was no statistically significant differences between treatment and control groups in spine BMD at six months, MD 9.96 (95% CI ‐2.51 to 22.43) (I² = 89%) and at 12 months, MD 14.68 (95% CI ‐6.08 to 35.45) (I² = 95%) (Analysis 2.4) (Adami 2003; Gatti 2005). However, it should be noted that the I² values are very large, representing a considerable amount of heterogeneity, I² = 89% and 95% respectively, although there are only two studies. Letocha investigated the mean per cent change in spine BMD z score and found statistically significant increases with IV pamidronate at six months, MD 21.59 (95% CI 5.79 to 37.39) and 12 months, MD 25.60 (95% CI 11.48 to 39.72) (Letocha 2005) (Analysis 2.5).
2.4. Analysis.
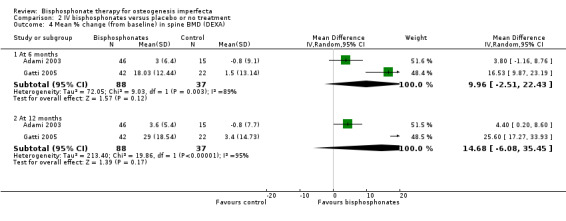
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 4 Mean % change (from baseline) in spine BMD (DEXA).
2.5. Analysis.
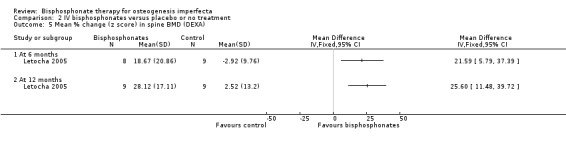
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 5 Mean % change (z score) in spine BMD (DEXA).
b. Mean per cent change in hip BMD
Two of the included trials reported on this outcome (Adami 2003; Gatti 2005). Adami and Gatti reported data on total hip BMD at six and 12 months. No statistically significant differences were noted when hip BMD data from these trials were combined, MD 6.16 (95% CI ‐3.57 to 15.90) and MD 11.27 (95% CI ‐3.69 to 26.22), respectively (Adami 2003; Gatti 2005) (Analysis 2.6).
2.6. Analysis.
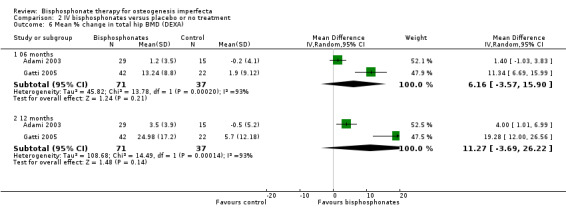
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 6 Mean % change in total hip BMD (DEXA).
We note for both mean per cent change in spine and hip BMD for the Adami and Gatti trials that there are large differences in the SDs reported for each of these two trials and whilst clinically it is appropriate for these trials to be combined, we plan to investigate this heterogeneity further once more trials are included (Adami 2003; Gatti 2005).
Secondary Outcomes
1. Change in biochemical markers of bone and mineral metabolism and bone histology
Two trials reported on this outcome (Adami 2003; Gatti 2005). Information was provided narratively within the text for each trial. Adami reported a decrease in: bone specific alkaline phosphatase (BSAP); serum C‐telopeptide (sCTx); and urinary free‐deoxy pyridinoline (ufDPD) in the neridronate group (within group data not presented) (Adami 2003). In the Gatti trial, statistically significant decreases in alkaline phosphatase were found with IV administration of neridronate in children (Gatti 2005).
2. Growth
One trial reported on this outcome (Letocha 2005). There were no statistically significant improvements in growth rate at 12 months, MD 1.07 (95% CI ‐2.24 to 4.38) (Letocha 2005) (Analysis 2.7). Growth was not measured in the remaining trials (Adami 2003; Gatti 2005).
2.7. Analysis.
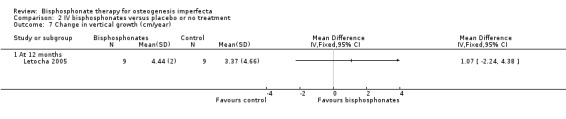
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 7 Change in vertical growth (cm/year).
3. Bone pain
One trial reported on this outcome (Letocha 2005). No changes in self‐reported bone pain on a self‐evaluation four‐point scale were found by Letocha, MD ‐0.11 (95% CI ‐0.83 to 0.61) (Letocha 2005) (Analysis 2.8). Bone pain was not addressed by the remaining two trials using IV bisphosphonates (Adami 2003; Gatti 2005).
2.8. Analysis.
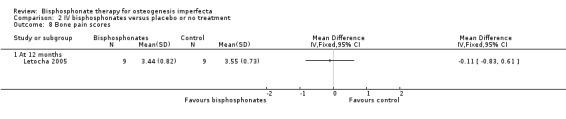
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 8 Bone pain scores.
4. Quality of life
One trial reported on this outcome (Letocha 2005). Letocha investigated muscle strength and gross motor function (using BAMF (Brief Assessment of Motor Function), a 10‐point gross motor assessment tool) (Letocha 2005). No differences in muscle strength or functional mobility were noted between the IV pamidronate and control groups during treatment, MD ‐3.18 (95% CI ‐18.97 to 12.61) (Analysis 2.9); MD ‐0.80 (95% CI ‐2.42 to 0.82), respectively (Letocha 2005) (Analysis 2.10). Outcomes reflecting quality of life were not evaluated or reported in the remaining IV bisphosphonates trials (Adami 2003; Gatti 2005).
2.9. Analysis.
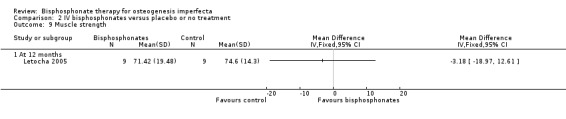
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 9 Muscle strength.
2.10. Analysis.
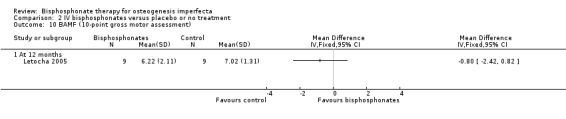
Comparison 2 IV bisphosphonates versus placebo or no treatment, Outcome 10 BAMF (10‐point gross motor assessment).
5. Lung function
None of the included trials reported on this outcome (Adami 2003; Gatti 2005; Letocha 2005).
Oral versus IV bisphosphonates
One trial was included in this comparison (DiMeglio 2006).
Primary outcomes
1. Fracture incidence
DiMeglio reported annualised fracture rates and found no difference between oral alendronate and IV pamidronate treatment groups, MD 0.50 (95% CI ‐0.64 to 1.64) (DiMeglio 2006) (Analysis 3.1).
3.1. Analysis.
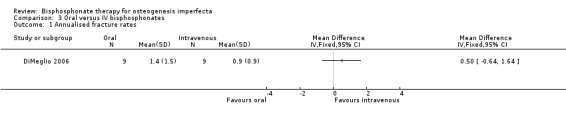
Comparison 3 Oral versus IV bisphosphonates, Outcome 1 Annualised fracture rates.
2. Change in BMD as assessed by DEXA
DiMeglio did not find a difference in BMD when they compared oral to IV therapy at 12 months, MD 0.30 (95% CI ‐1.11 to 1.71) and 24 months, MD 0.20 (95% CI ‐1.32 to 1.72) (DiMeglio 2006) (Analysis 3.2).
3.2. Analysis.
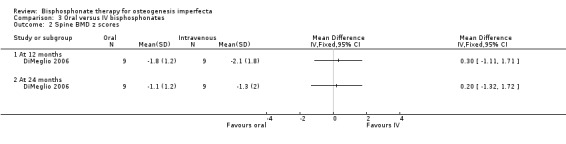
Comparison 3 Oral versus IV bisphosphonates, Outcome 2 Spine BMD z scores.
Secondary Outcomes
1. Change in biochemical markers of bone and mineral metabolism and bone histology
DiMeglio reported that there were no statistically significant differences in response between the two treatment groups at four, 12, or 24 months (DiMeglio 2006). Data were reported for the four‐ and 24‐month time‐periods for change in alkaline phosphonate (IU/L)) at four months, MD ‐12.00 (95% CI ‐86.09 to 62.09) and 12 months, MD ‐35.00 (95% CI ‐115.36 to 45.36) (Analysis 3.3); change in bone alkaline phosphonate (IU/L) at four months, MD 5.00 (95% CI ‐22.36 to 32.36) and 12 months, MD ‐12.00 (95% CI ‐39.78 to 15.78) (Analysis 3.4); and change in NTX/Cr (nMBCE/mM) at four months, MD ‐108.00 (95% CI ‐300.32 to 84.32) and 12 months, MD ‐111.00 (95% CI ‐269.38 to 47.38) (DiMeglio 2006) (Analysis 3.5).
3.3. Analysis.
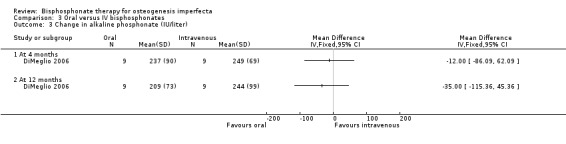
Comparison 3 Oral versus IV bisphosphonates, Outcome 3 Change in alkaline phosphonate (IU/liter).
3.4. Analysis.
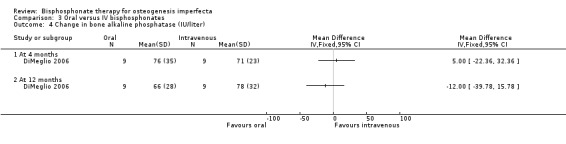
Comparison 3 Oral versus IV bisphosphonates, Outcome 4 Change in bone alkaline phosphatase (IU/liter).
3.5. Analysis.
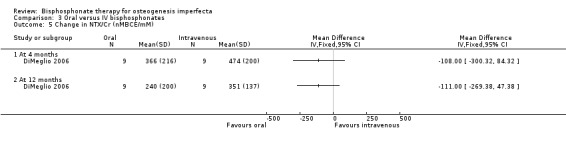
Comparison 3 Oral versus IV bisphosphonates, Outcome 5 Change in NTX/Cr (nMBCE/mM).
2. Growth
DiMeglio found no statistically significant difference in height compared to baseline in the oral or IV groups (within‐group data) (DiMeglio 2006).
3. Bone pain
DiMeglio did not investigate bone pain (DiMeglio 2006).
4. Quality of life
DiMeglio did not address quality of life indicators (DiMeglio 2006).
5. Lung function
DiMeglio did not investigate lung function (DiMeglio 2006).
Different doses of oral or IV bisphosphonates
Two trials were included in this comparison (Bishop 2010; Senthilnathan 2008). Bishop considered different doses of oral bisphosphonates and Senthilnathan considered different doses of IV bisphosphonates and were therefore not pooled in the analysis (Bishop 2010; Senthilnathan 2008).
Primary outcomes
1. Fracture incidence
Senthilnathan found no difference in the 6 mg/kg and 12 mg/kg dose in the number of crush‐fractured vertebrae with rate ratio 1.28 (95% CI 0.67 to 2.45) (Senthilnathan 2008) (Analysis 4.1).
4.1. Analysis.
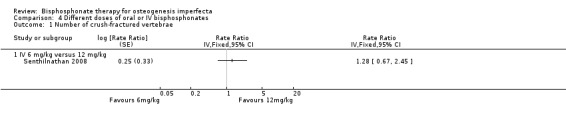
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 1 Number of crush‐fractured vertebrae.
Bishop found no difference in fracture reduction when comparing each of the three dose groups (0.2, 1, 2 mg/kg/week), RR 0.87 (95% CI 0.48 to 1.57), RR 0.78 (95% CI 0.44 to 1.37), RR 0.89 (95% CI 0.51 to 1.54), respectively (Bishop 2010) (Analysis 4.2).
4.2. Analysis.
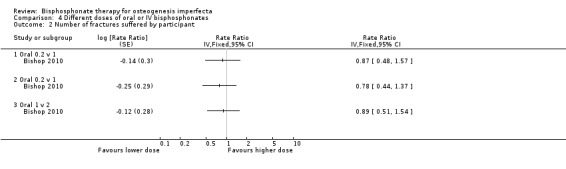
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 2 Number of fractures suffered by participant.
2. Change in BMD as assessed by DEXA
Senthilnathan found no difference in lumbar spine BMD at 12 months between the different doses, MD 45.00 (95% CI ‐30.15 to 120.15) (Senthilnathan 2008) (Analysis 4.3).
4.3. Analysis.
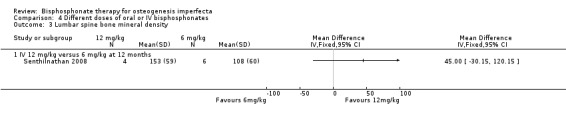
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 3 Lumbar spine bone mineral density.
Bishop found a statistically significant difference in lumbar spine z score BMD adjusting for the baseline value of each outcome and age when comparing 0.2 mg/kg versus 2 mg/kg, MD ‐1.18 (95% CI ‐1.97 to ‐0.39) (Bishop 2010) (Analysis 4.4). No difference was found at remaining two dose groups: 0.2 mg/kg/week versus 1 mg/kg/week, MD ‐0.50 (95% CI ‐1.29 to 0.29); 1 mg/kg/week versus 2 mg/kg/week, MD ‐0.68 (95% CI ‐1.46 to 0.10) (Bishop 2010) (Analysis 4.4).
4.4. Analysis.
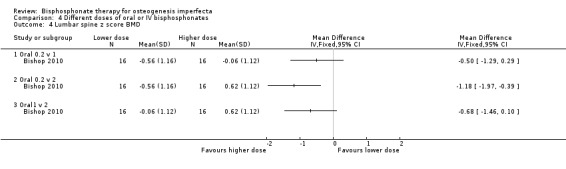
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 4 Lumbar spine z score BMD.
Secondary Outcomes
1. Change in biochemical markers of bone and mineral metabolism and bone histology
In the Senthilnatahn trial, the mean (SD) serum bone specific ALP declined from 235 (49) IU/l at baseline to 170 (46) IU/l at 12 months, remaining well above the upper limit of the adult normal range (48 IU/l). NTx fell from 2089 (1612) to 551 (120) nmol BCE/mmol creatinine over the same period. In percentage terms, ALP fell by 28% (17%) and NTx by 74% (27%) (Senthilnathan 2008).
In the Bishop trial, bone specific alkaline phosphatase activity declined by 1% from 81 ± 28 to 80 ± 39 IU/l (maximum decline for any child was 63%) and NTx by 21% from 2575 ± 1618 to 2044 ± 1031 nmol/mmol BCE (maximum decline for any child 70%) over the two‐year period of the trial (Bishop 2010).
2. Growth
Senthilnathan reported no difference in final length z score between the two dose groups, MD 2.10 (95% CI ‐0.50 to 4.70) (one trial) (Analysis 4.5) (Senthilnathan 2008). Bishop also found no difference in height z score for the three dose groups (0.2, 1, 2 mg/kg/week) at two years, (MD ‐0.56 (95% CI ‐2.46 to 1.34) (one trial), MD ‐1.15 (95% CI ‐2.87 to 0.57) (one trial), MD ‐0.59 (95% CI ‐2.34 to 1.16,) (one trial), respectively (Analysis 4.6) (Bishop 2010).
4.5. Analysis.
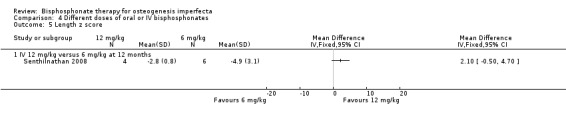
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 5 Length z score.
4.6. Analysis.
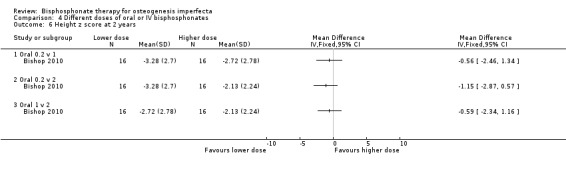
Comparison 4 Different doses of oral or IV bisphosphonates, Outcome 6 Height z score at 2 years.
3. Bone pain
Bishop found no difference between the groups in pain scores but no data was presented (Bishop 2010). Senthilnathan did not measure bone pain (Senthilnathan 2008).
4. Quality of life
Bishop reported that there were no statistically significant differences in PEDI and Gross Motor Function Measure scores between the groups. Mean (SD) grip strength in the right and left hands respectively was 33.6 (23.9) lb and 31.8 (21.6) lb at trial initiation and 38.4 (20.9) lb and 39.3 (21.8) lb at trial end. Neither difference was statistically significant and data are not presented separately for each treatment group (Bishop 2010).
Senthilnathan did not measure quality of life outcomes (Senthilnathan 2008).
5. Lung function
Neither of the included trials reported on this outcome (Bishop 2010; Senthilnathan 2008).
IV versus IV bisphosphonates
Two trials were included in this comparison which compared zoledronic acid to pamidronate efficacy in OI (Barros 2012; Zoledronic Acid 2008).
Primary outcomes
1. Fracture incidence
The zoledronic acid trial reported that there was no difference in the number of participants with fractures (32 (43%) versus 31 (41%)) (Zoledronic Acid 2008). The trial investigators also reported no difference in the change in number of fractures per participant from baseline, MD ‐0.41 (95% CI ‐1.45 to 0.63) (Analysis 5.1).
5.1. Analysis.
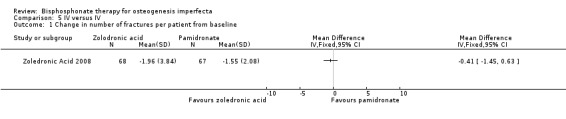
Comparison 5 IV versus IV, Outcome 1 Change in number of fractures per patient from baseline.
The Barros trial reported that at the end of the follow‐up period, both groups showed a decrease in the fracture rate (P = 0.025 and P = 0.048, respectively). No further data were reported (Barros 2012).
2. Change in BMD as assessed by DEXA
The zoledronic trial reported a statistically significant change in lumbar spine BMD, MD 8.06 (95% CI 0.48 to 15.64) favouring zoledronic acid (Zoledronic Acid 2008) (Analysis 5.2).
5.2. Analysis.
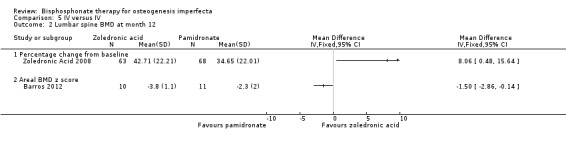
Comparison 5 IV versus IV, Outcome 2 Lumbar spine BMD at month 12.
The Barros trial appears to favour pamidronate in the analysis, MD ‐1.50 (95% CI ‐2.86 to ‐0.14) (Analysis 5.2), however, the trial report indicates that compared with the pamidronate group, the zoledronic acid group had a significant increase in lumbar spine z score at one year (P = 0.053) (Barros 2012).
Secondary Outcomes
1. Change in biochemical markers of bone and mineral metabolism and bone histology
The zoledronic trial reported there was a statistically significant reduction in CTx , P1NP and BSALP in the zoledronic group compared to the pamidronate group at both six and 12 months (Zoledronic Acid 2008).
There was no significant difference in osteocalcin (OC) or serum CTx between the groups. Data on other markers are reported in 'Table 2' of the trial report (Barros 2012).
2. Growth
The increases in both supine length and vertebral spine length were numerically greater in the pamidronate group compared to the zoledronic acid group, but the results were not statistically significant: MD ‐0.50 (95% CI ‐2.62 to 1.62) (Analysis 5.3); MD ‐0.80 (95% CI ‐2.39 to 0.79), respectively (Analysis 5.4). Height was not adjusted for age so it was not possible to tell from the data if there was an improvement in growth (z score) in those on bisphosphonates (Zoledronic Acid 2008).
5.3. Analysis.
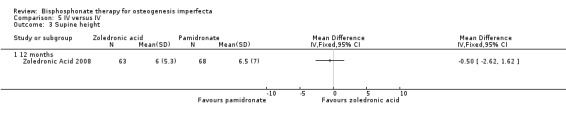
Comparison 5 IV versus IV, Outcome 3 Supine height.
5.4. Analysis.
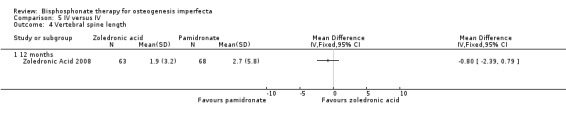
Comparison 5 IV versus IV, Outcome 4 Vertebral spine length.
In the Barros trial, there was no significant change in height in both groups throughout the treatment (pamidronate 111.7 (30.3) versus zoledronic acid 97.0 (16)). It is not clear what the number of participants were in each group so this data could not be included in the forest plot (Barros 2012).
3. Bone pain
In one trial, bone pain was assessed using the Wong‐Baker FACES at baseline, 6 and 12 months and did not identify a clear increase in bone pain compared to baseline nor did it identify a clear difference between treatment groups (Zoledronic Acid 2008). Most participants had no pain or minimal pain at baseline and at the end of the trial. Data are fully reported but were not summarised. Pain was also reported as an adverse event that occurred in at least 10% of participants in either treatment group (safety population, zoledronic acid 17.6% versus pamidronate 5.1%).
The Barros trial did not report on this outcome (Barros 2012).
4. Quality of life
Neither of the included trials reported on this outcome (Barros 2012; Zoledronic Acid 2008).
5. Lung function
Neither of the included trials reported on this outcome (Barros 2012; Zoledronic Acid 2008).
Discussion
Summary of main results
Fourteen trials were included in this review; 12 of these enrolled children (Barros 2012; Bishop 2010; Bishop 2013; DiMeglio 2006; Gatti 2005; Letocha 2005; Rauch 2009; Sakkers 2004; Seikaly 2005; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008) and two enrolled adults (Adami 2003; Chevrel 2006). To varying degrees, these studies investigated changes in BMD, fracture rate, markers of bone turnover, growth, pain and quality of life with bisphosphonate therapy. For a summary of outcomes reported, see 'Additional tables' (Table 5).
Overall completeness and applicability of evidence
All trials assessing BMD independently reported statistically significant increases after treatment with either oral or IV bisphosphonate and sometimes at separate sites (spine, hip, femur). However, it is difficult to compare these trials directly as different populations were included (adults versus children; for accurate comparisons children cannot be compared to adults due to high bone turnover during childhood and adolescence and open epiphyses). Additionally, different reporting indices were used (z score versus t score versus total BMD). As previously mentioned, the expected growth and BMD increases in children and adolescents with OI, coupled with their tendency for decreased fractures, make data comparison challenging. Statistically significant gains in spine and hip BMD were not seen with IV bisphosphonate administration at six and 12 months with combined summary statistics, indicating the need for continued rigorous study (Adami 2003; Gatti 2005). Interestingly, multiple trials reported the largest gains in BMD in the first year of therapy (Adami 2003; Bishop 2013; Chevrel 2006; Gatti 2005; Letocha 2005; Rauch 2009; Ward 2010) and gains in BMD were independent of administration of therapy in a placebo‐controlled cross‐over trial (Seikaly 2005). Two trials showed greater increases with higher dosing (Bishop 2010; Senthilnathan 2008); one of which showed a statistically significant increase in BMD only at the higher dosing schedule (Bishop 2010). These data possibly argue for the study and consideration of short‐course bisphosphonate therapy. When oral and IV bisphosphonates were directly compared, both statistically significantly increased spine BMD z scores at 12 and 24 months, but there were no differences in BMD between groups when route of administration was considered (DiMeglio 2006) (Analysis 3.2).
The combined data analyses supporting a change in fracture incidence after bisphosphonate therapy are less straightforward. Adami reported a 14% reduction of fractures after adults were treated with IV neridronate (Adami 2003). Sakkers found a 31% reduction in the relative risk of fracture of long bones after treatment with oral olpadronate in children affected with OI (Sakkers 2004). Relative risk of fracture was also reduced by 0.36% in another trial (Gatti 2005). Seikaly noted a non‐significant trend toward decreased fractures (Seikaly 2005). DiMeglio found no differences in fracture incidence with oral versus IV bisphosphonate administration and noted a decreased fracture incidence with time, but only when oral and IV groups were combined (DiMeglio 2006). Letocha found decreased upper extremity but not lower extremity fracture rates in the first year of therapy but no further increases were noted when therapy was extended (Letocha 2005). Bishop reported an overall tendency to reduced fracture incidence when compared to the fracture incidence over the prior two years. They noted no statistically significant difference between the dose of oral bisphosphonates (Bishop 2010). A larger trial by Bishop reported statistically significant difference in fracture incidence between children treated with risedronate and the placebo control group; 31% versus 49% respectively. However, during the following two years of open‐label study, the same groups reported fracture incidence of 53% and 65% respectively (Bishop 2013). Chevrel found no difference in vertebral or peripheral fracture rates but was not adequately powered to detect a difference (Chevrel 2006). A further three trials did not identify a statistically significant difference in fracture incidence, although the Senthilnathan trial did make note of an improvement in infant spinal crush fracture (Rauch 2009; Senthilnathan 2008; Ward 2010). It should be noted that none of the 14 included trials reported increased fractures with bisphosphonate treatment. When we further analysed the number of participants with at least one fracture, we found no difference in fractures in those treated with IV neridronate compared to the control population (Adami 2003; Gatti 2005) (Analysis 2.1). These trials employed retrospective fracture recall as a method for comparison, leading to potential recall bias. The number of participants randomised versus those lost to follow up was also unclear in one trial (Adami 2003). Consideration of reported heterogeneous results, in addition to the aforementioned natural tendency toward reduction in fractures with age highlight the importance of continued prospective, placebo‐controlled evaluation of bisphosphonates and fracture incidence in children. This is possible given widespread bisphosphonate use.
All 14 trials measured serum and urine markers (or both) of either bone formation or resorption (or both). The trials did not focus on the same biochemical markers, but each applied best practice with respect to sample collection and remained consistent to the marker chosen to represent bone turnover. Twelve trials reported decreases in serum (Adami 2003; Barros 2012; Bishop 2010; Chevrel 2006; DiMeglio 2006; Gatti 2005; Rauch 2009; Senthilnathan 2008; Zoledronic Acid 2008) or urine type I collagen by products (Adami 2003; Bishop 2013; DiMeglio 2006; Seikaly 2005; Ward 2010) whereas two trials reported no statistical difference in biochemical markers of bone turnover between treatment and control groups or from baseline (Letocha 2005; Sakkers 2004). The clinical significance and utilization of these biochemical markers of bone turnover are not universally utilized, however, the assumption is they act as a proxy for efficacy of therapy. These markers are not specific to process but indicate a change in bone homeostasis and are not reliable indicators of acute change. The varied markers chosen for study in the trials we reviewed prohibited direct comparison, but more systematic study of these markers could assist investigators in assessing response to individual bisphosphonates and their dosing, as well as participant compliance or concordance, or both, with therapy.
Bisphosphonate therapy was not conclusively shown to impact vertical growth. Ten trials included in this review commented on growth. Seikaly reported increased height z scores with oral bisphosphonates (Seikaly 2005). Growth as assessed by height and length combined z scores compared to normal children was increased in another trial when children receiving oral and IV therapies were combined (DiMeglio 2006). Letocha found growth rates were unchanged by bisphosphonate therapy (Letocha 2005). Seated height and vertebral height were also not different in the treatment versus placebo group of another trial (Sakkers 2004). Two trials compared different bisphosphonates to one another and did not show a significant difference in vertical growth rates between the therapies under investigation, they however did not compare data to normalized standards and thus no commentary can be made on their impact on vertical growth (Barros 2012; Zoledronic Acid 2008). No difference in z scores were noted in the remaining five trials (Bishop 2010; Bishop 2013; Rauch 2009; Senthilnathan 2008; Ward 2010). It is not within the scope of this review to distinguish between bisphosphonate responsiveness in different OI types, however it is interesting to note that milder forms of OI showed more improvements in growth and BMD in at least one trial (DiMeglio 2006).
It is important to determine whether bisphosphonate administration translates into functional changes. Bone pain and quality of life were addressed in several trials. Decreased pain scores and decreased analgesic use were reported in one trial (Seikaly 2005). The remaining trials which included pain and other quality of life measures found no difference in self‐reported pain scores (Bishop 2010; Bishop 2013; Chevrel 2006; Letocha 2005; Rauch 2009; Ward 2010; Zoledronic Acid 2008). The exception within these data was of increased pain with bisphosphonates at 36 months only (Chevrel 2006). When the authors re‐evaluated this difference, they found it statistically significant in the intention‐to‐treat analysis, but not in the per protocol analysis. Seikaly found improved well‐being scores and increases in self‐care abilities but no change in mobility (Seikaly 2005). However, Sakkers found no changes in self‐care abilities, nor did the authors find changes in strength or mobility when compared to placebo controls (Sakkers 2004). Additionally, no differences in gross motor abilities or other quality of life measures were found in several trials (Bishop 2010; Bishop 2013 ; Letocha 2005; Rauch 2009; Ward 2010). Chevrel was the only trial to assess hearing and found no change in Rinne testing with bisphosphonate therapy (Chevrel 2006). Taken together, the data presented here do not support consistent improvements in these quality of life indicators with bisphosphonate administration. Nevertheless, clinicians caring for people affected by OI have the strong anecdotal impression bisphosphonate treatment results in reduction in pain and subsequent increased mobility and functionality. These secondary outcomes were assessed by a few trials in this review, but represent an important need in future research. One may argue fracture rates may not be the only appropriate primary outcome of interest if a population with fragile bones were to have increased mobility as a result of treatment. It is difficult to quantify general well‐being, perhaps a more granular analysis needs to be developed looking at participation in daily events with less emphasis on pain.
Trials including IV and oral bisphosphonate use were included in this review. Mean bisphosphonate oral bioavailability (alendronate) in children is comparable to that found in adult trials, with approximately 50% localizing to bone (Ward 2005). However, individual oral bioavailability of alendronate varies as much as ten‐fold and could contribute to variable responsiveness (Ward 2005). It was noted that there was a large individual variance in bioavaliability of zolendronate in children, but there was no apparent effect of gender, body weight or creatinine clearance in participants with normal renal function (Zoledronic Acid 2008). We did not separately assess different bisphosphonate preparations although we have distinguished between oral and IV therapy. Different preparations and pharmacogenetic variability potentially underlie heterogeneous responses in individual participants. Additionally, although we made no attempt to distinguish between OI type and response to bisphosphonate therapy, it is entirely plausible different OI types will respond to therapy differently as one trial suggests (DiMeglio 2004).
Although specific data were not extracted, bisphosphonate therapy administered for one to three years appears to be safe and well‐tolerated in the adults and children treated here. Adverse effects of bisphosphonates are few and minor in this population (gastrointestinal complaints ‐ often comparable to placebo in the trials we reviewed, fever, headache, small decreases in lymphocyte counts) and the drugs are generally well‐tolerated (Bishop 2013; DiMeglio 2006; Letocha 2005; Rauch 2009; Ward 2005; Senthilnathan 2008; Ward 2010; Zoledronic Acid 2008). Flu‐like symptoms described as "acute phase reactions" were common with IV administration of bisphosphonates, particularly with the first infusion (DiMeglio 2006; Letocha 2005; Zoledronic Acid 2008) but rarely contributed to trial withdrawal. Intravenous access was difficult in one trial not included in this review where four of nine children less than three years required central line placement for IV bisphosphonate administration (DiMeglio 2004). Intravenous access was otherwise not reported to be problematic in children or adults. Clinical and laboratory evidence of hypocalcemia was reported in the zoledronic acid clinical trial which identified 22% of the participants treated with zolderonic acid and 9% of participants treated with pamidronate to have hypocalcemia (Zoledronic Acid 2008). However, clinical or laboratory evidence of hypocalcemia was not found (Bishop 2013; Seikaly 2005; Ward 2005) nor was evidence of hepatic or renal dysfunction or changes in nephrocalcinosis as measured by ultrasound in those treated with bisphosphonates (Barros 2012; Sakkers 2004; Seikaly 2005). Emerging data from clinical trials and observational studies do, however, support a concern for bisphosphonate use and atypical subtrochanteric femur fractures (Erviti 2013). The optimal method, dose, initiation and duration of therapy remain unclear. However, IV neridronate has been administered to infants with OI as young as neonates with reported improved growth and lowered fracture incidence (Antoniazzi 2006). A more extensive safety review of bisphosphonate use in children with low BMD and fragility fractures in juvenile idiopathic arthritis has been recently published (Thornton 2006). A rare, but serious side effect of bisphonates is osteonecrosis of the jaw. This presumably occurs from over‐suppression of bone turnover and occurs more commonly in elderly people with cancer taking primarily IV bisphosphonates (Woo 2006). To our knowledge, osteonecrosis of the jaw has not been reported in children or adults with OI.
We identified a recent abstract outlining an RCT evaluating subcutaneous teriparatide in adults (www.ashg.org/2013meeting/abstracts/fulltext/f130121209.htm). These data show statistically significant changes in BMD as well as significant changes in markers of bone metabolism. No comment was made of fracture incidence but as this data has not yet been published in a peer‐review journal, further analysis and discussion of this data was not undertaken. The use of parathyroid analogues is further supported by another recent publication where individuals who continued to fracture after bisphosphonate therapy were treated with teriparatide and showed a positive response in biomarkers (Gatti 2013). To date, the focus of pharmacological treatment for OI has been on bisphosphonates. Byers reviewed pharmacological agents used prior to 1991 (Byers 1992).
Quality of the evidence
Nineteen studies were identified and reviewed, 14 of which were randomised control trials and met the inclusion criteria. These 14 trials represent a total of 819 participants. The majority of trials analysed were small and not powered to show a statistically significant difference in many outcome measures. The primary and secondary outcome measures were reasonably evenly represented within these trials, methodologies varied and direct comparison of data was at times not possible. Meta‐analysis of the available data was limited due to the different agents used (oral versus IV bisphosphonates), different outcome measures, different populations (adults versus children), different reporting indices (z score versus t score versus total BMD), and variable inclusion of a placebo or control group. Sufficient commonality existed amongst the stated primary and secondary outcomes to enable reliable data comparison to internal validation of conclusions drawn.
Potential biases in the review process
This version of the review has used the Cochrane risk of bias assessment as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011b).
As stated above, the meta‐analysis was limited due to the variability across the studies and potential biases were acknowledged during this analysis and subsequent data extrapolation.
Agreements and disagreements with other studies or reviews
One other relevant review has been published (Castillo 2009). The authors reviewed seven studies and concluded that the studies favoured an overall improvement in BMD but that fracture reduction and growth improvement were not universally supported. They additionally concluded that further studies were required to evaluate the broader impact of therapy, including, but not limited to, deformity, daily function and quality of life.
Authors' conclusions
Implications for practice.
The results from the included trials provide evidence for statistically significant improvement in BMD in individuals affected with OI when treated with either oral or IV bisphosphonates. It remains to be seen whether this increase in BMD is a surrogate marker for fracture reduction and clinical functional improvement. At this time, comparative data remains inconclusive though several trials reported here individually show improvements in fracture incidence. Additionally, the long‐term safety of bisphosphonates in OI, particularly when used in children, and the risk of bisphosphonates to cause atypical femur fractures, impair perioperative or fracture bone healing (or both), as well as increased bone density if unmonitored, have not been sufficiently evaluated. The duration of use once BMD is improved requires thoughtful study. Effects of bisphosphonates on growth, bone deformity, mobility, and pain have been reviewed but absolute benefits remain inconclusive,
Implications for research.
Despite widespread use, a number of questions concerning bisphosphonate therapy in children and adults with OI remain unanswered. In particular whether increases in BMD convincingly translate into fracture reduction and functional improvement.
Additionally, the following remain unclear.
Are bisphosphonates equally safe and effective in children and adults? What are the long‐term effects of osteoclast inhibitors like bisphosphonates on the immature growing skeleton? Will they be licensed for use in children (www.fda.gov/cder/foi/esum/2003/20560se1‐038BPCA.PDF)?
Are there differences in the safety or efficacy of IV versus oral bisphosphonates? Are there differences in safety and efficacy of individual bisphosphonates in OI? Does the optimal method, dosage, length of therapy as well as optimal therapeutic window also warrant investigation?
Will the efficacy of bisphosphonates in increasing BMD be confirmed in larger studies, and with more heterogeneous participants? Does the efficacy of bisphosphonates differ by OI clinical type or mutation type? Are bisphosphonates useful in OI types which do not increase bone turnover?
Do bisphosphonates, when used in people with OI, delay or impair fracture healing, or peri‐operative bone healing?
Well‐designed, adequately‐powered, placebo‐controlled RCTs assessing the longitudinal effects of bisphosphonates on BMD, fracture reduction and healing, and changes in quality of life indicators such as function and pain should be studied in both children and adults with OI. These trials should be prospective, longitudinal, double‐blinded, using comparable assessments of change including z scores for BMD and vertical growth, as well as validated assessments measuring pain and quality of life outcomes. Quality of life outcomes should be broadened to include other factors important to people with OI, such as hearing and dentition and biochemical markers of bone turnover should be carefully assessed to determine if they are an adequate proxy for dose efficacy and subject concordance with therapy. Spontaneous versus non‐spontaneous fractures should be investigated in these trials as well as bone healing after fractures and operative intervention with bisphosphonates.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2016 | New citation required but conclusions have not changed | For this update, only minor changes to the review have been made throughout the text. |
| 17 October 2016 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group's Inborn errors of Metabolism Trials Register identified two references, of which one was disregarded as not relevant and one was an additional reference to an already excluded study (Orwoll 2014). |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 28 July 2014 | Amended | Contact details updated. |
| 17 July 2014 | New search has been performed | A search of the Group's Inborn Errors of Metabolism Register identified six new trials eligible for inclusion in the review (Barros 2012; Bishop 2010; Bishop 2013; Rauch 2009; Senthilnathan 2008; Zoledronic Acid 2008). In addition to this, one already included trial, previously published in abstract form only (Glorieux 2004), has now been published in full and further information has been included in the review (Ward 2010). |
| 17 July 2014 | New citation required but conclusions have not changed | The review update six new trials. Two trials evaluated oral bisphosphonates versus placebo (Bishop 2013; Rauch 2009); one trial evaluated different doses of oral bisphosphonates (Bishop 2010); one trial evaluated different doses of intravenous infused bisphosphonates (Senthilnathan 2008); and two trials compared different intravenous bisphosphonates (Barros 2012; Zoledronic Acid 2008). No major changes to the conclusions were made as compared to the previous published version (Phillipi 2008). |
| 28 May 2008 | Amended | Converted to new review format. |
Acknowledgements
Janet Reeder (JR) was the lead author on the original published protocol and her valuable contributions to this review are gratefully acknowledged.
Tracey Remmington (TR) was a co‐author on a previous version of the review (Phillipi 2008).
The authors acknowledge the Osteogenesis Imperfecta Foundation for providing useful background information and for their encouragement. We also thank the investigators of specific trials (Adami 2003 and Gatti 2005) for providing unpublished data.
Data and analyses
Comparison 1. Oral bisphosphonates versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fracture risk reduction | 2 | Hazard ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 1 year follow up | 1 | Hazard ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 year follow up | 1 | Hazard ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Rate of fractures | 1 | Relative rate (Fixed, 95% CI) | Totals not selected | |
| 2.1 2 year follow up | 1 | Relative rate (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of people with at least one fracture | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.35] |
| 3.2 36 months | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.48, 1.95] |
| 4 Mean change (z score) in spine BMD (DEXA) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [0.28, 0.50] |
| 4.2 At 12 months | 2 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.35, 0.68] |
| 4.3 At 24 months | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [0.70, 1.28] |
| 5 Mean % change in spine BMD (DEXA) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 12 months | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 7.0 [3.87, 10.13] |
| 5.2 24 months | 3 | 202 | Mean Difference (IV, Random, 95% CI) | 17.31 [5.01, 29.62] |
| 5.3 36 months | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 9.4 [5.44, 13.36] |
| 6 Mean % change in total femur BMD (DEXA) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in height | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 12 month (z score) | 2 | 171 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.04, 0.43] |
| 7.2 At 24 months (z score) | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.19, 0.35] |
| 8 Change in bone pain scores | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 At 36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Change in analgesic use (days per week) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Change in well‐being score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Change in self‐care score (PEDI) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Change in total mobility score (WeeFIM) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Hearing (assessed by % rinnie) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. IV bisphosphonates versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with at least one fracture | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1 year | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.06] |
| 2 Incidence of lower extremity fractures | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Incidence of upper extremity fractures | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean % change (from baseline) in spine BMD (DEXA) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 9.96 [‐2.51, 22.43] |
| 4.2 At 12 months | 2 | 125 | Mean Difference (IV, Random, 95% CI) | 14.68 [‐6.08, 35.45] |
| 5 Mean % change (z score) in spine BMD (DEXA) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Mean % change in total hip BMD (DEXA) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 06 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 6.16 [‐3.57, 15.90] |
| 6.2 12 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | 11.27 [‐3.69, 26.22] |
| 7 Change in vertical growth (cm/year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Bone pain scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Muscle strength | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 BAMF (10‐point gross motor assessment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Oral versus IV bisphosphonates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Annualised fracture rates | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Spine BMD z scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in alkaline phosphonate (IU/liter) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in bone alkaline phosphatase (IU/liter) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in NTX/Cr (nMBCE/mM) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Different doses of oral or IV bisphosphonates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of crush‐fractured vertebrae | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 IV 6 mg/kg versus 12 mg/kg | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of fractures suffered by participant | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Oral 0.2 v 1 | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Oral 0.2 v 1 | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Oral 1 v 2 | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Lumbar spine bone mineral density | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 IV 12 mg/kg versus 6 mg/kg at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lumbar spine z score BMD | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Oral 0.2 v 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Oral 0.2 v 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Oral1 v 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Length z score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 IV 12 mg/kg versus 6 mg/kg at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Height z score at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Oral 0.2 v 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Oral 0.2 v 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Oral 1 v 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. IV versus IV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in number of fractures per patient from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Lumbar spine BMD at month 12 | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Percentage change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Areal BMD z score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Supine height | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Vertebral spine length | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adami 2003.
| Methods | Single centre, double‐blinded, placebo‐controlled RCT. Parallel for 1 year then the control group crossed over for an additional year. |
|
| Participants | 46 adults (23 male) were randomised. Mean age (range): 34.9 years (21 to 50 years). Any type of OI. | |
| Interventions | IV neridronate versus no treatment.
Dosage: 100 mg diluted in 250 ml of saline solution infused intravenously in 30 min every 3 months.
Trial period: 24 months.
Groups: 31 participants in the neridronate group versus 15 in the control group.
After 12 months the control group also received intervention (neridronate). All participants seen at 3‐month intervals, but full clinical evaluation including bone densitometry measurements by DXA and fasting serum and urinary (second morning voiding) biochemistry was obtained per‐protocol every 6 months. Radiographs of the spine (anteroposterior and lateral views) were obtained at baseline and after 12 and 24 months. |
|
| Outcomes | Fractures, spine BMD, hip BMD, BSAP, sCTX , ufDPD. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised according to type of OI. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Prevalent vertebral fractures were identified and graded blindly by a semi quantitative scale. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 of 78 consented participants completed the trial. Per protocol analyses were performed; although intention‐to‐treat analyses were planned they were not applied as all participants completed the treatment follow up – interim analysis. |
| Selective reporting (reporting bias) | Low risk | Change in biochemical markers of bone and mineral metabolism is partially reported as a graph. Other outcomes mentioned are reported. |
| Other bias | Low risk | None. |
Barros 2012.
| Methods | Parallel, open‐label RCT. | |
| Participants | Children and adolescents with a diagnosis of OI (types I, III and IV) with a history of at least one minimal trauma fracture in the past year and no prior exposure to bisphosphonates. Age: 1 ‐ 15 years. 17 males and 6 females. Exclusion criteria not stated. |
|
| Interventions | PAM: < 3 years of age (1 mg/kg/day over 2 consecutive days every 3 months); > 3 years of age (1 mg/kg/day over 2 consecutive days every 4 months). ZOL: < 3 years of age (0.025 mg/kg/day over 2 consecutive days every 3 months); > 3 years of age (0.050 mg/kg/day over 2 consecutive days every 4 months). Treatment period: 1 year. |
|
| Outcomes | Height Weight Biochemistry BMD (lumbar spine and total body) BMC |
|
| Notes | Baseline characteristics differ between the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated as randomised. Baseline characteristics appear to differ between the groups 12 pieces of paper marked with treatment 1 and 12 pieces of paper marked with treatment 2 were put in a closed box. As the participants were seen and agreed to participate, one paper was drawn from the box to define which treatment the participant would receive, because of this, the age could not be paired. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given. |
| Selective reporting (reporting bias) | Unclear risk | Decreased fracture rate not reported completely, but no indication if this is due to the results. |
| Other bias | Low risk | None. |
Bishop 2010.
| Methods | Multicentre, parallel, double‐blinded RCT dose‐ranging trial of risedronate. | |
| Participants | 53 children randomised and 48 received treatment 0.2 mg/kg/wk = 17 1 mg/kg/wk = 18 2 mg/kg/wk = 18 Mean age 0.2 mg/kg/wk = 10.8 (3.8 ‐ 17) years 1 mg/kg/wk = 10.8 (5.2 ‐ 16.5) years 2 mg/kg/wk = 11 (5.8 ‐ 17) years Sex of participants (M/F) 0.2 mg/kg/wk = 6/11 1 mg/kg/wk = 8/10 2 mg/kg/wk = 4/14 OI type (I, III, IV) 0.2 mg/kg/wk = 0/4/13 1 mg/kg/wk = 0/2/16 2 mg/kg/wk = 1/1/16 |
|
| Interventions | Children were randomised to receive 0.2 mg, 1 mg or 2 mg/kg/week risedronate for 2 years, dose prescribed to the nearest 5 mg. Oral doses administered were multiples of the same tablet of sizes 2.5, 5, 15, 30 or 35 mg; for instance a child weighing 50 kg randomised to 2 mg/kg/week would receive 3 x 35 mg i.e. 105 mg. | |
| Outcomes | Fracture reduction as assessed by skeletal survey Change in BMD Change in biochemical markers of bone/mineral metabolism Growth Bone pain/functional outcome Pediatric evaluation of disability inventory (PEDI) Gros motor function measure (GMFM) Grip strength at trial initiation vs 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was by multiple permuted blocks, stratified according to age (up to 10.99 years, or above) allocated by a remote telephone system. |
| Allocation concealment (selection bias) | Low risk | Allocation was by a remote telephone system. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Children, their families and medical staff were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants withdrew during the 2‐year period of the trial and were lost to follow up. 1 child did not like the taste of the tablets, 1 developed an inflammatory bowel condition thought not to be related to the medication, 3 withdrew without giving a reason. The 5 children were distributed between groups 1/2/2. Consort diagram shown. |
| Selective reporting (reporting bias) | High risk | Bishop states that there was no difference between the groups in pain scores but no data was presented. For quality of life, data was not presented separately by treatment group. |
| Other bias | Low risk | None |
Bishop 2013.
| Methods | Parallel, double‐blind RCT. | |
| Participants | Children and adolescents with a diagnosis of OI (types not stated) with a history of at least 1 non‐traumatic/low‐impact fracture and BMD z score of ‐1.0 or less for total body or LS OR a real BMD z score of ‐2.0 or less regardless of fracture history. Those considered ineligible weighed less than 10kg, had cancer or untreated rickets, had low vitamin D or prior treatments which might have affected the results or disease severe enough they would have normally been given treatment anyway. 4 ‐ 15 years 71 females 72 males |
|
| Interventions | 94 risedronate 49 placebo (10 kg ‐ 30 kg) 2.5 mg daily (> 30 kg) 5 mg daily |
|
| Outcomes | Primary: % change from baseline lumbar spine BMD Secondary: % change from baseline in total body BMD at Month 12 % change from baseline in lumbar spine BMC at month 12 % change from baseline in total body BMC at month 12 Lumbar spine z score ‐ % change from baseline to month 12 Total body z score ‐ % change from baseline to month 12 % change from baseline in lumbar spine bone area at month 12 % change from baseline in total body bone area month 12 New morphometric vertebral fracture at month 12 Categorization by number of new morphometric vertebral fracture at month 12 Probability of fracture in 12 months Number of clinical fractures, month 12 Serum BAP ‐ % change from baseline to month 12 Urine NTX/Cr ‐ % change from baseline at month 12 Wong‐Baker FACES Pain Rating Scale ‐ change from baseline to month 12 Bone Age (years), change from baseline to month 12 Annualized growth velocity ‐ change from baseline to month 12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized in a 2:1 ratio by a telephone based interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Telephone based interactive voice response system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial treatment was masked from participants, investigators, and trial centre personnel during the first year. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants discontinued in the intervention arm and reasons were provided. An ITT analysis was undertaken. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported. |
| Other bias | Low risk | None |
Chevrel 2006.
| Methods | Single‐centre, double‐blinded, placebo‐controlled RCT. Parallel trial. |
|
| Participants | 64 adults (39 males) were randomised. Age range: > 20 years (treatment: mean (SD) 37 (12); placebo mean (SD) 36 (12). All types of OI. | |
| Interventions | Oral alendronate vs placebo.
Dosage: 10 mg.
Trial period: 36 months. Participants seen at baseline and during the 3 years of the trial: every year in Lyon with intermediary visits at months 6, 18, and 30 by their local physician. BMD of the lumbar spine and of both hips (total femur) was measured at baseline and at 12, 24, and 36 months. Radiographs of the spine (anteroposterior and lateral views) were obtained at baseline and 36 months. Overall pain score was evaluated at baseline and every 6 months during 3 years with a visual analog scale score (0 – 10). Each participant underwent audiometry and impedancometry at baseline and at 36 months. |
|
| Outcomes | Dietary calcium intake, spine BMD, hip BMD, spine radiographs, fractures, pain score, audiometry, impedancometry, serum and urine samples for biochemical markers of bone turnover, transiliac bone biopsies. | |
| Notes | Allocation intervention ‐ adequate. Computer‐generated list. Not adequately powered for fracture outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list was drawn up by the pharmacy department |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by giving the randomised list to the researchers who then assigned each new trial subject the subsequent number on the list |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded (trial personnel and participants) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analyses were performed. BMD follow up was complete for 62 randomised participants, including the 3 in the alendronate group and 1 in the placebo group who withdrew from the trial for personal reasons without drug related adverse effects. |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported. |
| Other bias | Unclear risk | Not adequately powered for fracture outcome. |
DiMeglio 2006.
| Methods | Single centre, unblinded RCT. Parallel trial. |
|
| Participants | 18 children (7 males) were randomised. Mean age: 8.7 years. Oral arm mean: 9 years (range 3.8 ‐ 12.7 years); IV arm mean: 8.4 years (3 ‐ 13.7 years). OI types I, III, IV included. | |
| Interventions | IV pamidronate disodium 1 mg/kg/day every 4 months compared to oral alendronate 1 mg/kg daily.
Pamidronate diluted in 250 or 500 ml of normal saline given by slow infusion over 4 hours. Alendronate was given as 1 or 2 10 mg tablets, rounded to the nearest 10 mg dose for weight.
Trial period: 24 months. BMD, BMC, and area at the spine (L2–L4) and total body were measured every 4 months by DXA. |
|
| Outcomes | BMC, BMD, posteroanterior radiographs of left hand and wrist, radiographs of suspected fractures, blood and urine samples. | |
| Notes | Allocation of intervention ‐ adequate. Computer random number generator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician created a randomisation schedule with a computer random number generator using a block‐randomisation scheme. Participants were stratified according to clinical severity of OI, pubertal stage, and bone age. |
| Allocation concealment (selection bias) | Low risk | Random number sequences were concealed until the principal investigator assigned a group according to the stratification scheme. A nurse then consulted the randomisation schedule and determined whether the participant would receive oral or IV treatment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The type of analysis performed is not stated for the DiMeglio trial, although changes in group assignment were made when participants had difficulty tolerating the assigned regimen. One child on oral alendronate was lost to follow up after 8 months and is therefore not included in this analysis. |
| Selective reporting (reporting bias) | High risk | Height was partially reported as graphs with groups combined as there was no statistically significant difference between groups. Bone pain was also not reported although not stated as measured. |
| Other bias | Low risk | None. |
Gatti 2005.
| Methods | Single centre, unblinded, RCT. No placebo control. Parallel trial. |
|
| Participants | 64 children 6 ‐ 11 years were assigned to neridronate for 3 years (n = 44) or received no treatment for one year then received neridronate for two years (n = 22). All OI types included. | |
| Interventions | Neridronate (2 mg/kg every 3 months) compared to no treatment.
Trial period: 36 months. After 12 months, the control group also received treatment (neridronate). All participants were seen at 3‐monthly intervals, but full clinical evaluation, including bone densitometry measurements by DXA and fasting serum and urinary (second morning voiding) biochemistry, was obtained per‐protocol every 6 months before the infusion of neridronate. |
|
| Outcomes | BMD and height/projected area of spine and total hip, fractures, markers of bone turnover. | |
| Notes | Allocation of intervention ‐ unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned according to OI type to either an active or control group with a ratio 2:1. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses were planned but not applied as all participants completed the treatment follow‐up. |
| Selective reporting (reporting bias) | Low risk | Graphs only presented for change in biochemical markers of bone and mineral metabolism. |
| Other bias | Low risk | None. |
Letocha 2005.
| Methods | Single centre, non blinded, RCT. No placebo control. Parallel trial. |
|
| Participants | 18 children were randomised.
Age range: 4 ‐ 13 years. OI type III and IV included. |
|
| Interventions | IV pamidronate versus control.
Dosage: 10 mg/m2/day for 3 days every 3 months.
Trial period: 12 months initially, then 7 participants in the treatment group were given an additional 6 ‐ 21 months of IV pamidronate.
Note: 4 children in each group were receiving recombinant growth hormone (rGH) (0.06 mg/kg/day for 6 days/week). All participants were seen quarterly at the National Institutes of Health Clinical Center. Serum markers of bone formation and growth parameters were measured at each visit. Antero‐posterior (AP) and lateral radiographs of the spine and lower extremity long bones and DXA at vertebrae L1–L4 were obtained at baseline and every 6 months. QCT scans of the spine were performed at the National Institutes of Health Clinical Center at 0 and 12 months. |
|
| Outcomes | L1 ‐ L4 DXA, spine QCT, spine radiographs, musculoskeletal and functional testing. | |
| Notes | Allocation of intervention ‐ adequate. "Randomly‐generated numbers". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Trial was stated as non‐blind but during measurements, the investigator was blinded to participant identifiers, the order of the films, and whether they came from the treatment or control groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A per protocol and repeated‐measures model was used. It is not clear if all randomised participants completed the trial. |
| Selective reporting (reporting bias) | High risk | Reported no significant change in biochemical markers of bone and mineral metabolism. |
| Other bias | Low risk | None. |
Rauch 2009.
| Methods | Single centre, placebo‐controlled RCT. Parallel trial. |
|
| Participants | Risedronate: 13 Placebo: 13 Mean age (SD): 11.9 (4) years placebo, 11.7 (3.6) years risedronate 15 male, 11 female OI types I |
|
| Interventions | Risedronate (or placebo) 15 mg once per week if < 40 kg 30 mg once per week if > 40 kg |
|
| Outcomes | Every 3 months for bone pain, grip force, blood and urine tests and anthropometric measurements Every 12 months for bone age, radiographs of spine, BMD by DXA Baseline and 24 months for radiographs of long bones (or whenever a fracture sustained) Bone biopsy at 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised in equal number to receive either risedronate or placebo. Both risedronate and placebo pills were provided by the manufacturer." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Risedronate: 2 withdrew (lack of effect, refused to take pills) Placebo: 1 withdrew (lack of effect). |
| Selective reporting (reporting bias) | High risk | Bone age stated as measured but not reported and grip strength was reported as not significant. Authors have been contacted for data. |
| Other bias | Low risk | None. |
Sakkers 2004.
| Methods | Single centre, double‐blinded, placebo‐controlled RCT. Parallel trial. |
|
| Participants | 34 children (16 male) were randomised. Age range: 3 ‐ 18 years. OI types I, III and IV. | |
| Interventions | Oral olpadronate versus placebo. Dosage: 10 mg/m2 daily. Trial period: 24 months. | |
| Outcomes | Fractures, spinal BMC, spinal BMD, calcaneal BMC, calcaneal BMD, muscle strength, self care, mobility, height (body and seated), arm span, head circumference, body weight, heights of each lumbar vertebral body, urinary analysis, blood samples (but only 9 from each group).
Adverse effects reported. BMC and BMD of the lumbar spine (L1 – L4) and of the os calcis were measured before randomisation, after 1 year, and at the end of the trial. Domains of functional outcome were measured at the beginning of the trial and every 6 months, and anthropometric and radiographic variables were measured every 12 months. All laboratory assessments were done at baseline and at 3 months, 12 months, and 24 months. |
|
| Notes | Allocation of intervention by computer‐generated randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Researchers were not aware of treatment allocation. Generation of the randomisation sequence was done independently of the researchers by an outside group. For each participant the Julius Centre was contacted by telephone for treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded (outcome assessors blind). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was undertaken. 2 participants (1 placebo and 1 treatment) withdrew from the trial but were accounted for in the final analysis. Consort diagram presented. |
| Selective reporting (reporting bias) | High risk | Reported no significant change in biochemical markers of bone and mineral metabolism and narratively reported no differences in seated height or radiographic assessments of lumbar vertebral height between olpadronate and placebo at 24 months follow up. |
| Other bias | Low risk | None. |
Seikaly 2005.
| Methods | Single centre, double‐blinded, placebo‐controlled RCT. Cross‐over trial. |
|
| Participants | 20 children (11 males) were randomised. 17 completed. Age range: 3 ‐ 15 years. Mean (SD) 9.8 (1.06) years; median 10.53 years. OI types: I, III, IV. | |
| Interventions | Oral alendronate versus placebo.
Dosage: 5 mg/d (participants who weighed < 30 kg); or 10 mg/d (participants who weighed > 30 kg).
Trial period: 24 months (12 months each trial period). Participants were evaluated at baseline, every 3 months thereafter (except when otherwise indicated), and at the conclusion of the trial. |
|
| Outcomes | Physical evaluation, food records, blood and urine analysis, stool guaiac, DEXA, renal ultrasound, skeletal survey. | |
| Notes | Allocation of intervention ‐ adequate. Computer‐generated randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation by pharmacist. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by a pharmacist at the institution. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether intention‐to‐treat analyses were performed introducing potential attrition bias. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. |
| Other bias | Low risk | None. |
Senthilnathan 2008.
| Methods | Single‐centre, parallel, double blinded, RCT. | |
| Participants | 12 randomised, 6 in each group. Number who received treatment and were analysed Low dose = 6 High dose = 4 Infants with OI who had long bone fractures with consequent deformity at birth or within the first 3 months of life, and/or multiple crush fractured vertebrae 7 males and 5 females under one year of age. |
|
| Interventions | 6 or 12 mg/kg/year of pamidronate by IV infusion. | |
| Outcomes | Change in BMD as assessed by DEXA, Change in biochemical markers of bone and mineral metabolism (e.g., bone alkaline phosphatase measurements), growth. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Carers and participants blinded and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One infant died from pneumonia 3 weeks after receiving the first infusion and one infant was withdrawn due to non attendance for scheduled infusions. Both were in the 12 mg/kg group (high dose). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. |
| Other bias | Unclear risk | Problems with accrual. |
Ward 2010.
| Methods | Multicentre, parallel, double‐blinded placebo‐controlled RCT. | |
| Participants | 139 randomised 4 ‐ 19 years (ALN 11.0 (3.6) years; placebo 11.1 (4.0) years 78 males 61 females Children and adolescents with a diagnosis of OI types III or IV or a diagnosis of OR type I associated with 1 or more of the following: chronic pain; more than 3 fractures (including vertebrae) per year with minimal trauma for the previous 2 years; or limb deformity requiring surgery. |
|
| Interventions | ALN or placebo for 24 months (< 40 kg) 5 mg daily (> 40 kg) 10 mg daily Placebo = 30 ALN = 109 Analysed: ALN = 83; placebo = 26 |
|
| Outcomes | Fracture reduction Change in BMD as assessed by DEXA Change in biochemical markers of bone and mineral metabolism Growth Bone pain PEDI score Grip strength |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized in a 3:1 ratio and stratified according to their weight at baseline. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind. outcome assessors blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total = 30, ALN = 26, Placebo = 4. Reasons given per group in figure 1 – consort diagram. Modified ITT. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. |
| Other bias | Low risk | None. |
Zoledronic Acid 2008.
| Methods | Randomized (Phase II), open‐label, active control, parallel assignment, safety/efficacy trial. 9 clinic sites: UCLA (Los Angeles, USA); Alfred I. DuPont Hospital for Children (Delaware, USA); Intermountain Orthopedics (Idaho, USA); St Jude Children's Research Hospital (Illinois, USA); Children's Hospital (Nebraska, USA); Children's Hospital Medical Center (Ohio, USA); Oregon Health & Science University (Portland, USA); Vanderbilt University Medical Center (Tennessee, USA); Texas Chilren's Hospital (Texas, USA). |
|
| Participants | Children 1 to 17 years of age N = 155 randomised but baseline data collected on 150. Mean (SD) age: zoledronic acid = 8.6 (4.25); pamidronate = 8.5 (4.20). Zoledronic acid: female n = 36, male n = 38. Pamidronate: female n = 31, male n = 45. Inclusion criteria (included, but not limited to): • Children age 1 to 17 • OI type III or IV or • OI type I with ≥ 3 minimal trauma fractures (including vertebral fractures) in the 2 previous years or with a history of limb deformity requiring surgery. • Serum 25 hydroxy vitamin D ≥ 15 ng/mL Exclusion criteria (included but not limited to) • Renal disease: defined as a serum creatinine value above the upper limit of normal (age and sex‐matched) or pathologic proteinuria (any participant with a urine protein to creatinine ratio of > 0.3 is to be excluded) or history of prior clinically significant renal disease (e.g. nephritis or nephrotic syndrome). One repeat assessment of the urine protein to creatinine was allowed. The assessment was made within 2 weeks of the first assessment and the sample was a urine collection of a first morning void, after an overnight fast. • Any disease or abnormality that would prevent accurate BMD measurements of the lumbar spine (i.e. intra‐abdominal calcification that may prohibit accurate data collection/interpretation; severe scoliosis, kyphosis, or metal implants, etc.) • Any clinically significant clinical laboratory abnormalities at screening • History or evidence of an intestinal malabsorption syndrome |
|
| Interventions | Zoledronic acid participants ≥ 1 to < 3 years of age were to be administered 0.025 mg/kg as a 30 to 45‐minute IV infusion every 3 months for one year. Participants ≥3 years to ≤ 17 years of age were to be administered 0.05 mg/kg as a 30‐minute IV infusion every 3 months for one year. Pamidronate participants were to be administered 4‐hour IV infusions of the trial drug. Participants ≥1 year to < 2 years of age were to be administered 0.5 mg/kg/day on each of three consecutive days every 2 months for one year. Participants ≥2 years to < 3 years of age were to be administered 0.75 mg/kg/day on each of three consecutive days every 3 months for one year. Participants ≥ 3 years to ≤ 17 years of age were to be administered 1 mg/kg/day on each of three consecutive days every 3 months for one year. ZA = 74; P = 76. |
|
| Outcomes | Primary outcome measure: change in lumbar spine BMD at month 12 relative to baseline. Secondary outcome measure: change in z score of the lumbar spine at month 12 relative to baseline. |
|
| Notes | Multicenter trial. USA. Financial assistance: Novartis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Because of the difference in dosing between the active controlled infusions it was not possible to blind the participants or treating physicians to the trial drugs. However, the radiologists responsible for assessing the primary endpoints (e.g. change from baseline in LS BMD at 12 months) were blinded to treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 155 were randomised and the intent‐to‐treat population included 63 participants in the zoledronic acid group and 68 participants in the pamidronate group. Participants who did not have a baseline measurement or were lost to follow up without any post‐baseline measurement were excluded from the analyses. Missing values were imputed using the last post‐baseline observation carried forward (LOCF). Reasons for participants who dropped out were described. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Data analysed and presented in different ways. Graphs presented for some outcomes. |
| Other bias | Low risk | None. |
ALN: alendronate BMC: bone mineral content BMD: bone mineral density BSAP: bone‐specific alkaline phosphatase DEXA, DXA: dual‐energy x‐ray absorptiometry IV: intravenous NTx: N‐linked telopeptides OI: osteogenesis imperfecta PAM: pamidronate QCT:quantitative computer‐assisted tomography RCT: randomised controlled trial sCTX: serum cross‐laps SD: standard deviation ufDPD: urinary free‐deoxy pyridinoline vs: versus ZOL: zoledronic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antoniazzi 1996 | Not a randomised controlled trial. |
| Antoniazzi 2006 | Not a randomised controlled trial. |
| Antoniazzi 2010 | Investigated response to growth hormone rather than bisphosphonates. |
| DiMeglio 2004 | Not a randomised controlled trial. |
| Gerber 1998 | Not a bisphosphonate therapy ‐ leg braces. |
| Granda 1977 | Evaluated pyrophosphate levels in OI disease severity not improved in bone density or fracture reduction. |
| Orwoll 2014 | Participants were randomised to teriparatide 20 mcg sub‐q or placebo and this is not a bisphosphonate. |
| Ward 2005 | Not a randomised controlled trial. An open‐label, oral bioavailability study. Does not address the outcomes of interest. |
mcg: microgram OI: osteogenesis imperfecta sub‐q: subcutaneously
Differences between protocol and review
Title change from 'Pharmacologic therapy for osteogenesis imperfecta' to more accurately refect the content of the review.
Contributions of authors
Review 2008
Robert Steiner (RS) conceived the review. RS and JR wrote the protocol. CP, RS and TR all contributed to the original review.
Review 2014
Kerry Dwan (KD), CP and RS independently examined and evaluated all relevant studies and drafted the text, with input from TR and Donald Basel (DB). CP,TR and KD input the data into the review. CP and DB revised the background and discussion from the original written by JR and RS, and RS edited these.
Review 2016
KD, CP, RD and DB all contributed to the this minor update.
DB acts as guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
All authors: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adami 2003 {published and unpublished data}
- Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, et al. Intravenous neridronate in adults with osteogenesis imperfecta. Journal of Bone and Mineral Research 2003;18(1):126‐30. [DOI] [PubMed] [Google Scholar]
- Gatti D, Viapiana O, Lippolis I, Braga V, Prizzi R, Rossini M, et al. Intravenous bisphosphonate therapy increases radial width in adults with osteogenesis imperfecta. Journal of Bone and Mineral Research 2005;20(8):1323‐6. [DOI] [PubMed] [Google Scholar]
Barros 2012 {published data only}
- Barros ER, Saraiva GL, Oliveira TP, Lazaretti‐Castro M. Safety and efficacy of a 1‐year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2012;25(5‐6):485‐91. [DOI] [PubMed] [Google Scholar]
Bishop 2010 {published data only}
- Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, et al. A randomised controlled dose‐ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. Journal of Bone and Mineral Research 2010;25(1):32‐40. [DOI] [PubMed] [Google Scholar]
Bishop 2013 {published data only}
- Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta:a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;382(9902):1424‐32. [DOI] [PubMed] [Google Scholar]
Chevrel 2006 {published data only}
- Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina‐Granade G, Duboeuf F, et al. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3‐year randomized placebo‐controlled trial. Journal of Bone and Mineral Research 2006;21(2):300‐6. [DOI] [PubMed] [Google Scholar]
DiMeglio 2006 {published data only}
- DiMeglio LA, Peacock M. Two‐year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. Journal of Bone and Mineral Research 2006;21(1):132‐40. [DOI] [PubMed] [Google Scholar]
- Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2005;18(1):43‐53. [DOI] [PubMed] [Google Scholar]
Gatti 2005 {published and unpublished data}
- Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, et al. Intravenous neridronate in children with osteogenesis imperfecta: A randomized controlled study. Journal of Bone and Mineral Research 2005;20(5):758‐63. [DOI] [PubMed] [Google Scholar]
Letocha 2005 {published data only}
- Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short‐term functional improvement. Journal of Bone and Mineral Research 2005;20(6):977‐86. [DOI] [PubMed] [Google Scholar]
Rauch 2009 {published data only}
- Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. Journal of Bone and Mineral Research 2009;24(7):1282–9. [DOI] [PubMed] [Google Scholar]
Sakkers 2004 {published data only}
- Sakkers R, Kok D, Engelbert R, Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2‐year randomised placebo‐controlled study. Lancet 2004;363(9419):1427‐31. [] [DOI] [PubMed] [Google Scholar]
Seikaly 2005 {published data only}
- Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. Journal of Pediatric Orthopaedics 2005;25(6):786‐91. [DOI] [PubMed] [Google Scholar]
Senthilnathan 2008 {published data only}
- Senthilnathan S, Walker E, Bishop NJ. Two doses of pamidronate in infants with osteogenesis imperfecta. Archives of Disease in Childhood 2008;93(5):398‐400. [DOI] [PubMed] [Google Scholar]
Ward 2010 {published data only}
- Glorieux FH, Rauch F, Ward LM, Smith P, Verbruggen N, Heydon N, et al. Alendronate in the treatment of pediatric osteogenesis imperfecta [abstract]. Journal of Bone and Mineral Research 2004;20 Suppl:S12. [] [Google Scholar]
- Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. Journal of Clinical Endocrinology and Metabolism 2011;96(2):355‐64. [DOI] [PubMed] [Google Scholar]
Zoledronic Acid 2008 {unpublished data only}
- Food, Drug Administration (FDA). Center for Drug Evaluation and Research. Statistical review and evaluation ‐ clinical studies: Zometa (zoledronic acid) intravenous injection (i.v.) 4 mg lyophilized powder. Treatment of children with osteogenesis imperfecta (OI). http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm072901.pdf (accessed 07 April 2014).
- NCT00063479. Bisphosphonate treatment of osteogenesis imperfecta. http://clinicaltrials.gov/show/NCT00063479 (accessed 08 March 2013).
References to studies excluded from this review
Antoniazzi 1996 {published data only}
- Antoniazzi F, Bertoldo F, Mottes M, Valli M, Sirpresi S, Zamboni G, et al. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. Journal of Pediatrics 1996;129(3):432‐9. [DOI] [PubMed] [Google Scholar]
Antoniazzi 2006 {published data only}
- Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. Journal of Pediatrics 2006;149(2):174‐9. [DOI] [PubMed] [Google Scholar]
Antoniazzi 2010 {published data only}
- Antoniazzi F, Monti E, Venturi G, Franceschi R, Doro F, Gatti D, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. European Journal of Endocrinology 2010;163(3):479‐87. [DOI] [PubMed] [Google Scholar]
DiMeglio 2004 {published data only}
- DiMeglio LA, Ford L, McClintock C, Peacock M. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone 2004;35(5):1038‐45. [DOI] [PubMed] [Google Scholar]
Gerber 1998 {published data only}
- Gerber LH, Binder H, Berry R, Siegel KL, Kim H, Weintrob J, et al. Effect of withdrawal of bracing in matched pairs of children with osteogenesis imperfecta. Archives of Physical Medicine and Rehabilitation 1998;79(1):46‐51. [DOI] [PubMed] [Google Scholar]
Granda 1977 {published data only}
- Granda JL, Falva KA, Bullough PG. Phyrophosphate levels and magnesium oxide therapy in osteogenesis imperfecta. Clinical Orthopaedics and Related Research 1977;Jul‐Aug(216):228‐31. [PubMed] [Google Scholar]
Orwoll 2014 {unpublished data only}
- NCT00131469. Study of Teriparatide (FORTEO) to Treat Adults With Osteogenesis Imperfecta (OI). http://clinicaltrials.gov/show/NCT00131469 (accessed 08 March 2013). []
- Nagamani S, Shapiro J, Veith S, Wang Y, Lapidus J, Reeder JL, et al. Teriparatide, the first anabolic agent for treatment of osteogenesis imperfecta improves bone mineral density at the hip and spine: a randomized, blinded, placebo‐controlled trial [abstract]. Proceedings of the 63rd Annual Meeting of the American Society of Human Genetics; 2013 Oct 22‐26; Boston. Boston: American Society of Human Genetics, 2013:27.
- Orwoll ES, Shapiro J, Veith S, Wang Y, Lapidus J, Vanek C, et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. Journal of Clinical Investigation 2014;124(2):491‐8. [CENTRAL: 977911; CRS: 5500050000000133; EMBASE: 2014112169; PUBMED: 24463451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2005 {published data only}
- Ward LM, Denker AE, Porras A, Shugarts S, Kline W, Travers R, et al. Single‐dose pharmacokinetics and tolerability of alendronate 35‐ and 70‐milligram tablets in children and adolescents with osteogenesis imperfecta type I. Journal of Clinical Endocrinology and Metabolism 2005;90(7):4051‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Alanay 2010
- Alanay Y, Avaygan H, Camacho N, Utine GE, Boduroglu K, Aktas D, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal‐recessive osteogenesis imperfecta. American Journal of Human Genetics 2010;86(4):551‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2006
- Barnes AM, Chang W, Morello R, Cabral WA, Weis MA, Eyre DR, et al. Deficiency of cartilage‐associated protein in recessive lethal osteogenesis imperfecta. New England Journal of Medicine 2006;355(26):2757‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2011
- Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal‐recessive osteogenesis imperfecta. American Journal of Human Genetics 2011;88(3):362‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1996
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348(9041):1535‐41. [DOI] [PubMed] [Google Scholar]
Byers 1991
- Byers PH, Wallis GA, Willing MC. Osteogenesis imperfecta: translation of mutation to phenotype. Journal of Medical Genetics 1991;28(7):433‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byers 1992
- Byers PH, Steiner RD. Osteogenesis imperfecta. Annual Review of Medicine 1992;43:269‐82. [DOI] [PubMed] [Google Scholar]
Byers 2000
- Byers PH. Osteogenesis imperfecta: perspectives and opportunities. Current Opinion in Pediatrics 2000;12(6):603‐9. [DOI] [PubMed] [Google Scholar]
Byers 2013
- Byers 2013 [pers comm]. OI [personal communication]. Email to: D Basel 2013.
Cabral 2007
- Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, et al. Prolyl 3‐hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nature Genetics 2007;39(3):359‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Camacho 2001
- Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, et al. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcified Tissue International 2001;69(2):94‐101. [DOI] [PubMed] [Google Scholar]
Castillo 2009
- Castillo H, Samson‐Fang L, American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Developmental Medicine and Child Neurology 2009;51(1):17‐29. [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho TJ, Lee KE, Lee SK, Song SJ, Kim KJ, Jeon D, et al. A Single Recurrent Mutation in the 5'‐UTR of IFITM5 Causes Osteogenesis Imperfecta Type V. American Journal of Human Genetics 2012;91(2):343‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christiansen 2010
- Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, Alrasheed S, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. American Journal of Human Genetics 2010;86(3):389‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2002
- Cole WG. Advances in Osteogenesis Imperfecta. Clinical Orthopaedics and Related Research 2002;401:6‐16. [DOI] [PubMed] [Google Scholar]
Connor 1985
- Connor JM, Connor RA, Sweet EM, Gibson AA, Patrick WJ, McNay MB, et al. Lethal neonatal chondrodysplasias in the West of Scotland 1970‐1983 with a description of a thanatophoric, dysplasialike, autosomal recessive disorder, Glasgow variant. American Journal of Medical Genetics 1985;22(2):243‐53. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Erviti 2013
- Erviti J, Alonso A, Oliva B, Gorricho J, López A, Timoner J, et al. Oral bisphosphonates are associated with increased risk of subtrochanteric and diaphyseal fractures in elderly women: a nested case–control study. http://bmjopen.bmj.com/content/3/1/e002091 (accessed 06 February 2013). [DOI] [PMC free article] [PubMed]
FDA 2011
- Food, Drug Administration (FDA). Background document for meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf (accessed 07 March 2013).
Fisher 1999
- Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro [abstract]. Proceedings of the National Academy of Sciences of the United States of America 1999;96:133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gatti 2013
- Gatti D, Rossini M, Viapiana O, Povino MR, Liuzza S, Fracassi E, et al. Teriparatide treatment in adult patients with osteogenesis imperfecta type I. Calcified Tissue International 2013;93(5):448‐52. [DOI] [PubMed] [Google Scholar]
Glorieux 2000
- Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. Journal of Bone and Mineral Research 2000;15(9):1650‐8. [DOI] [PubMed] [Google Scholar]
Glorieux 2004
- Glorieux FH, Rauch F, Ward LM, Smith P, Verbruggen N, Heyden N, et al. Alendronate in the treatment of pediatric osteogenesis imperfecta [abstract]. Journal of Bone and Mineral Research 2004;20(Suppl):1043. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jüni 2001
- Jüni T, Altman, DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuurila 2002
- Kuurila K, Kaitila I, Johansson R, Grenman R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. The Annals of Otology, Rhinology, and Laryngology 2002;111(10):939‐46. [DOI] [PubMed] [Google Scholar]
Lapunzina 2010
- Lapunzina P, Aglan M, Temtamy S, Caparros‐Martin JA, Valencia M, Leton R, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. American Journal of Human Genetics 2010;87(1):110‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marini 2003
- Marini JC. Do bisphosphonates make children's bones better or brittle?. New England Journal of Medicine 2003;349(5):423‐6. [DOI] [PubMed] [Google Scholar]
Martinez‐Glez 2012
- Martínez‐Glez V, Valencia M, Caparrós‐Martín JA, Aglan M, Temtamy S, Tenorio J, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Human Mutation 2012;33(2):343‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
OIF 2008
- Osteogenesis Imperfecta Foundation. Facts about Osteogenesis Imperfecta. www.oif.org (accessed August 2008).
Orioli 1995
- Orioli IM, Castilla EE, Scarano G, Mastroiacovo P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. American Journal of Medical Genetics 1995;59(2):209‐17. [DOI] [PubMed] [Google Scholar]
Pyott 2013
- Pyott SM, Tran TT, Leistritz DF, Pepin MG, Mendelsohn NJ, Temme RT, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. American Journal of Human Genetics 2013;92(4):590‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2002
- Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. New England Journal of Medicine 2002;346(9):653‐61. [DOI] [PubMed] [Google Scholar]
Semler 2012
- Semler O, Garbes L, Keupp K, Swan D, Zimmermann K, Becker J, et al. A Mutation in the 5'‐UTR of IFITM5 Creates an In‐Frame Start Codon and Causes Autosomal‐Dominant Osteogenesis Imperfecta Type V with Hyperplastic Callus. American Journal of Human Genetics 2012;91(2):349‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sillence 1979
- Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. Journal of Medical Genetics 1979;16(2):101‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sillence 2012
- Sillence D. Osteogenesis Imperfecta. International committee for constitutional disorders of the skeleton. Sydney, 2012.
Steiner 2013
- Steiner RD, Adsit J, Basel D. Gene Reviews; COL1A1/2‐Related Osteogenesis Imperfecta. http://www.ncbi.nlm.nih.gov/books/NBK1295/ (Initial Posting: January 28, 2005; Last Update: February 14, 2013).
Thornton 2006
- Thornton J, Ashcroft DM, Mughal MZ, Elliott RA, O'Neill TW, Symmons D. Systematic review of effectiveness of bisphosphonates in treatment of low bone mineral density and fragility fractures in juvenile idiopathic arthritis. Archives of Disease in Childhood 2006;91(9):753‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dijk 2009
- Dijk FS, Nesbitt IM, Zwikstra EH, Nikkels PGJ, Piersma SR, Fratantoni SA, et al. PPIB mutations cause severe osteogenesis imperfecta. American Journal of Human Genetics 2009;85(4):521‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whyte 2003
- Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate‐induced osteopetrosis. New England Journal of Medicine 2003;349(5):457‐63. [DOI] [PubMed] [Google Scholar]
Woo 2006
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine 2006;144(10):753‐61. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dwan 2014
- Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD005088.pub3] [DOI] [PubMed] [Google Scholar]
Phillipi 2008
- Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005088.pub2] [DOI] [PubMed] [Google Scholar]


