Abstract
Tweet:
Compared with non-Hispanic whites, Hispanics under 12 years of age with multiple positive autoantibodies had lower risk of progression to type 1 diabetes, but obesity/overweight increased their risk more than non-Hispanic whites.#ethnicdisparities
HYPOTHESIS:
We hypothesized that progression of islet autoimmunity and type 1 diabetes mellitus differ among races/ethnicities in at-risk individuals.
METHODS:
In this study, we analysed the data from The Type 1 Diabetes TrialNet Pathway to Prevention Study. We studied 4,873 non-diabetic, autoantibody-positive relatives of individuals with type 1 diabetes followed prospectively (11% Hispanics, 80.9% Non-Hispanic Whites, 2.9% Non-Hispanic Blacks and 5.2% Non-Hispanic Others). Primary outcomes were time from single confirmed to multiple autoantibody positivity, and time from multiple autoantibody positivity to type 1 diabetes mellitus diagnosis.
RESULTS:
Conversion from single to multiple autoantibody positivity was less common in Hispanics than Non-Hispanic Whites (HR=0.66, 95%CI=0.46–0.96, p=0.028) adjusting for autoantibody type, age, sex, Diabetes Prevention Trial Type 1 Risk Score and HLA DR3-DQ2/DR4-DQ8 genotype. In participants screened as multiple autoantibody-positive (n=2,834), time to type 1 diabetes did not differ by race/ethnicity overall (p=0.91). In children <12 years old at multiple autoantibody-positivity determination, being overweight/obese had differential effects by ethnicity; while overweight/obesity increased type 1 diabetes risk by 36% in Non-Hispanic Whites (HR=1.36, 95%CI=1.04–1.77, p=0.024), the risk was nearly quadrupled in Hispanics (HR=3.8, 95%CI=1.6–9.1, p=0.0026). We did not observe this interaction in participants ≥12 years at autoantibody-positivity determination, although this group size was limited. No significant differential risks were observed between Non-Hispanic Blacks and Non-Hispanic Whites.
CONCLUSIONS/INTERPRETATION:
The risk and rate of progression of islet autoimmunity were lower in Hispanic compared to Non-Hispanic White relatives, while significant differences in the development of type 1 diabetes mellitus were limited to children <12 years old and modified by BMI.
Keywords: diabetes in childhood, genetics of type 1 diabetes, prediction and prevention of type 1 diabetes, weight regulation and obesity
INTRODUCTION:
Type 1 diabetes mellitus is a chronic, autoimmune condition characterized by β-cell destruction that leads to insulin deficiency. Studies of the natural history and pathogenesis of type 1 diabetes have shown that it is a disease continuum with variable progression along well-defined stages: presymptomatic β-cell autoimmunity with normoglycaemia, presymptomatic β-cell autoimmunity with dysglycaemia, and symptomatic β-cell autoimmunity with dysglycaemia [1]. A wealth of data has been generated on genetic, immunological and metabolic risk factors that enable us to predict type 1 diabetes risk and design studies to intervene early in the autoimmune process, before the onset of symptoms. In genetically susceptible children with positivity for multiple autoantibodies, the 10-year risk of developing type 1 diabetes is 70% with lifetime risk reaching 100% [2]. A predictive score, i.e. the Diabetes Prevention Trial – Type 1 Risk Score (DPTRS), has been proposed to estimate the type 1 diabetes risk in at-risk individuals [3].
The growing public health impact of studies examining racial/ethnic differences is underscored by recent data demonstrating that the increase in type 1 diabetes incidence disproportionally affects racial and ethnic minorities [4]. However, much of the knowledge on type 1 diabetes pathogenesis stems from studies primarily conducted in the Non-Hispanic White (NHW) population, and its generalizability to other races/ethnicities has not been established. The incidence of type 1 diabetes in children varies by race/ethnicity; for instance, the SEARCH study reported 27, 19 and 14.8 new cases per 100,000 youth per year in 2012, respectively, in NHW, Non-Hispanic Blacks (NHB) and Hispanics [4]. While a limited number of studies showed that there are significant racial/ethnic differences in genetic, immunologic, metabolic, and clinical characteristics [5–13], the risk and rate of progression of islet autoimmunity and type 1 diabetes development among different racial/ethnic groups have not been identified. A full understanding of these and associated factors may inform the design of future prediction models and prevention trials and eventually patient care.
The Type 1 Diabetes TrialNet is a NIH-funded international consortium of clinical research centres aiming to prevent or delay type 1 diabetes. Relatives of individuals with type 1 diabetes are offered screening for the presence of islet autoantibodies and, if positive, enrolment in the Pathway to Prevention (PTP) Study, and if eligible, prevention studies [14]. An increasing number of participants of minority racial/ethnic background, especially Hispanic ethnicity, provided us with a unique opportunity to compare the natural course prior to development of type 1 diabetes in Hispanic and NHW individuals.
We hypothesized that islet autoimmunity and type 1 diabetes progression significantly differ among races/ethnicities in at-risk individuals. This study aimed to compare the rates and risk factors of progression of islet autoimmunity and type 1 diabetes development among races/ethnicities in at-risk individuals.
METHODS:
Design and Settings:
We analysed the data from the Type 1 Diabetes TrialNet PTP Study. The TrialNet PTP Study screens relatives of individuals with type 1 diabetes to identify participants for monitoring and/or prevention studies. One to 45-year-old first degree relatives and one to 20-year-old second degree relatives of individuals with type 1 diabetes were eligible for PTP screening; of note, due to rescreen guidelines and allowable timeframes, PTP participants could have been identified as autoantibody positive after 45 years of age. Eligible relatives were first tested for the presence of islet autoantibodies, including glutamic acid decarboxylase 65 (GAD65) autoantibody, islet antigen 2 (IA-2) autoantibody and micro-insulin autoantibody (mIAA) followed by islet cell autoantibody (ICA) if ≥1 positive autoantibody(ies) on initial screening test [15]. Additionally, zinc transporter 8 (ZnT8) autoantibody measurement was also performed consistently starting in 2012 in participants with ≥1 positive autoantibody(ies) on initial screening test [16]. Participants who were negative for all tested autoantibodies were eligible for yearly rescreening until 18 years of age. Participants with single autoantibody positivity confirmed on a consecutive visit within one year were defined as single confirmed autoantibody positive, whereas those with two or more positive autoantibodies at any screening or follow-up were defined as multiple autoantibody positive. Single confirmed and multiple autoantibody-positive participants were offered enrolment in “Monitoring” and, if eligible and interested, prevention studies. Baseline risk assessments included OGTT, HbA1c and HLA typing. Participants with multiple positive autoantibodies were monitored semi-annually through the PTP; single confirmed positive participants were monitored semi-annually until 2012, after which they were monitored annually. This monitoring includes OGTT, HbA1c and autoantibody tests. Details of the screening and follow-up processes have previously been described [15, 16]. All participants and/or their parents provided written informed consent and assent, as appropriate, approved by local Institutional Review Boards.
Participants / Inclusion and Exclusion Criteria:
Between 2004 and July 31, 2017, 182,145 relatives were screened in the TrialNet PTP study at 21 Clinical Centres and approximately 100 collaborating Clinical Sites in US, Canada, UK, Finland, Italy, Germany, Australia and New Zealand. A total of 5,703 autoantibody positive participants who had at least one follow-up visit were identified in the TrialNet PTP – Monitoring Cohort. Exclusion criteria included fasting blood glucose <2.8 mmol/L or ≥7 mmol/L, 2-hour OGTT blood glucose ≥11.1 mmol/L, type 1 diabetes at first monitoring visit, missing fasting or 2-hour OGTT blood glucose data, and for the current analysis, unknown or missing ethnicity data. Individuals with glucose <2.8 mmol/L were excluded because of potential for data quality issues and those with fasting glucose ≥7 mmol/L and 2-hour OGTT glucose ≥11.1 mmol/L were excluded because of suspected type 1 diabetes.
Race and Ethnicity Categorization:
Race and ethnicity categorizations were based on self-report and on the standard NIH classifications and definitions for race and ethnicity [17]. Individuals who listed more than one race were categorized as multiracial. We evaluated individuals based on these NIH-defined groups, and also on composite race/ethnicity groups. Specifically, participants were assigned to one of the four racial/ethnic groups: Hispanics, Non-Hispanic Whites (NHWs), Non-Hispanic Blacks and Non-Hispanic Other Races. Non-Hispanic multiracial participants were included in the “Non-Hispanic Other Races” category.
Anthropometric Measures and Laboratory Analysis:
Body Mass Index (BMI):
BMI was calculated using data from the first monitoring visit. BMI percentiles (BMI%iles) were calculated for all participants ≥2 years old; BMI%iles were calculated for adults over 20 years old by imputing 20 as their age to be able to evaluate BMI%iles as a continuous measure across all participants. Classification as overweight was defined as a BMI ≥85th but below 95th percentile, and obesity was defined as a BMI ≥95th percentile adjusting for age and sex according to Centers for Disease Control and Prevention criteria. Because of very limited number of underweight participants, all participants with BMI%ile <85th percentile are considered lean weight.
HLA typing:
HLA genotyping was performed at TrialNet HLA Laboratory at the Barbara Davis Center, which receives whole blood from clinical sites and extracts DNA using the AutoGen QuickGene-610 instrument. In this analysis, participants were classified by the presence or absence of the highest risk genotype, i.e. DR3-DQ2 (DRB1*0301 – DQA1*0501 – DQB1*0201) and DR4–DQ8 (DQA1*0301 – DQB1*0302 with DRB1*0401, *0402 or *0405). Further information on HLA typing are provided in Electronic Supplemental Methods.
Autoantibody Assays:
GAD65, IA-2, mIAA, and ZnT8 autoantibodies were measured by radioimmunoassay in the TrialNet Core Laboratory at the Barbara Davis Center for Childhood Diabetes in Denver, Colorado. During the 2015 Islet Autoantibody Standardization Program Workshop, sensitivities and specificities were 52 and 100% respectively for mIAA, 82 and 99% respectively for GAD65 autoantibody, 72 and 100% respectively for IA-2 autoantibody, and 70 and 97% respectively for ZnT8 autoantibody [18]. ICA positivity was tested by indirect immunofluorescence in the Diagnostic Referral Laboratories at the University of Florida. An ICA value greater than 5 Juvenile Diabetes Foundation units was considered positive.
OGTT:
Participants’ glycaemic status was tested with an OGTT (oral glucose dose 1.75 g/kg, maximum 75 grams) after an overnight fast. C-peptide (nmol/L) and glucose (mmol/L) measurements were performed in the fasting state followed by 30, 60, 90, and 120 minutes later. Trapezoid method was used to calculate area under the curve (AUC) for C-peptide.
Diagnosis of Diabetes:
Diabetes was diagnosed according to TrialNet Natural History Study of the Development of Type 1 Diabetes Protocol (TrialNet Protocol TN01); fasting plasma glucose ≥7.0 mmol/L, 2-hour plasma glucose during an OGTT ≥11.1 mmol/L, a random plasma glucose ≥11.1 mmol/L with symptoms of hyperglycaemia or presence of unequivocal hyperglycaemia including acute metabolic decompensation (diabetic ketoacidosis). The first 3 criteria were required to be met on two occasions with a strong preference that at least one of the two testing occasions includes an OGTT. HbA1c level ≥48 mmol/mol from a laboratory that is using a The National Glycohemoglobin Standardization Program certified assay standardized to The Diabetes Control and Complications Trial was also accepted as a confirmatory criterion.
DPTRS:
A metabolic risk score was calculated for each individual based on a model including log-BMI, age, log-fasting C-peptide, and post-challenge glucose and C-peptide sums from 2-hour OGTT at baseline assessment [3]. This score was used to compare races/ethnicities both as continuous variable and dichotomized variable (<6.5 and ≥6.5). For the purpose of this analysis, we used <6.5 and ≥6.5 to define low and high DPTRS, respectively, based on the previously published differential diabetes risk [19].
Statistical Methods:
Descriptive analyses were used to summarize characteristics across all participants as well as within single autoantibody-confirmed and multiple autoantibody positive cohorts. Characteristics were compared between the race/ethnicity composite groups using Kruskal-Wallis tests for continuous variables, and chi-square tests for categorical/dichotomized factors, where Fisher exact tests were used as appropriate with small numbers in subset groups. At-risk individuals who enrolled in prevention trials were censored at the time of entry in the trial. Primary outcomes for these analyses were time to multiple positive autoantibodies in single confirmed autoantibody positive participants and time to type 1 diabetes diagnosis in participants with multiple positive autoantibodies. Time to multiple positive autoantibodies was defined as the time from single confirmed autoantibody positive determination to the time when ≥2 positive autoantibodies were identified. Time to progression to type 1 diabetes diagnosis was defined as the time from participants being identified as having multiple positive autoantibodies to the time when they were diagnosed with type 1 diabetes. Those who had not progressed at their last follow up visit were censored at that time point. The two analysis cohorts were not mutually exclusive, as those single confirmed autoantibody positive participants who subsequently converted to multiple positive autoantibodies were included in the multiple autoantibody positive cohort upon that time. Kaplan-Meier methods were used to estimate the proportion of participants who have not had an event (e.g., development of type 1 diabetes) by a certain time. Estimated event rates were also calculated using cumulative incidence analyses. Univariate and multivariable Cox regression models were used to evaluate prognostic utility of the various markers and factors in relation to time to progression to multiple positive autoantibodies or type 1 diabetes diagnosis for the single confirmed and multiple positive autoantibody cohorts, respectively. Cumulative incidence of events of interest were graphed and also adjusted for identified factors of interest using methodology of Therneau et al, and Nieto et al [20, 21]. Multivariable models utilized a hybrid variable selection approach based on backward selection and all subsets regression approaches. Age at autoantibody determination was evaluated as a continuous measure; however, an optimal cut-point for age was identified using recursive partitioning analyses (rpart package in R), which uses a tree-based method and iteratively evaluates all possible cut-points of age that best differentiated participants’ prognosis in relation to, for instance, time to progression to type 1 diabetes. [22, 23]. Statistical significance was determined at p<0.05. All analyses were performed using the statistical program R version 3.4.1 for Windows.
RESULTS:
A total of 4,873 TrialNet PTP participants, comprising 11% Hispanics, 80.9% NHWs, 2.9% NHBs and 5.2% Non-Hispanic Other Races, were followed prospectively. At screening, 2,039 participants (42%) were single confirmed autoantibody positive while 2,834 (58%) were positive for multiple autoantibodies. Median follow-up for single to multiple autoantibody conversion was 1.9 years (IQR: 0.7 to 4.2 years) and for progression to type 1 diabetes was 1.0 years (IQR: 0.4 to 2.9 years) in event-free participants for the respective outcomes. A total of 363/2,039 (18%) participants progressed from single to multiple autoantibody positivity. Across all 4,873 participants, 591 (12%) progressed to type 1 diabetes during follow-up (65 single confirmed and 526 multiple autoantibody positive) (Figure 1). The estimated cumulative incidence of type 1 diabetes rate at 5-years was 39% (95%CI=36%−42%) in the multiple autoantibody positive cohort and 26.4% (95%CI=24%−29%) across all autoantibody positive participants.
Figure 1:
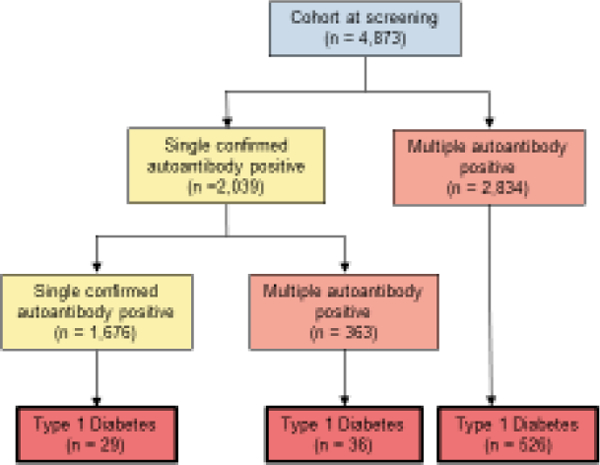
Flow-diagram of islet autoimmunity progression and type 1 diabetes development in TrialNet PTP participants. (*T1D: Type 1 Diabetes Mellitus, PTP: Pathway to Prevention)
We observed differences in the distributions of baseline characteristics between the race/ethnicity groups (Table 1). The pairwise comparisons of baseline characteristics between NHBs versus NHWs, and Non-Hispanic Other Races versus NHWs were not included, because among these respective groups, there were no significant differences in primary outcomes i.e. time to multiple positive autoantibodies in single confirmed autoantibody positive participants and time to type 1 diabetes in participants with multiple positive autoantibodies.
Table 1:
Baseline characteristics at screening by race and ethnicity groups (n=4,873). (*NHWs: Non-Hispanic Whites, NHBs: Non-Hispanic Blacks)
| Hispanics (n=537) |
NHWs (n=3940) |
NHBs (n=143) |
Non-Hispanic Other Races (n=253) |
p-value (Overall) |
p-value (Hispanic vs NHWs) |
|
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| median | 12.4 | 12.0 | 11.2 | 11.7 | 0.51 | 0.19 |
| range | 1.3 to 48.7 | 1.06 to 51.8 | 2.6 to 45.7 | 1.7 to 46 | ||
| missing | 0 | 0 | 1 | 0 | ||
| Sex, n (%) | ||||||
| female | 303 (57%) | 2001 (51%) | 84 (59%) | 144 (57%) | 0.006 | 0.02 |
| male | 230 (43%) | 1934 (49%) | 59 (41%) | 107 (43%) | ||
| missing | 4 | 5 | 0 | 2 | ||
| Autoantibody status at screening, n (%) | ||||||
| Single confirmed (+) | 280 (52%) | 1585 (40%) | 48 (34%) | 126 (50%) | <0.00001 | <0.00001 |
| Multiple (+) | 257 (48%) | 2355 (60%) | 95 (66%) | 127 (50%) | ||
| 1 | 280 (52%) | 1585 (40%) | 48 (34%) | 126 (50%) | <0.00001 | <0.00001 |
| 2 | 113 (21%) | 1003 (26%) | 46 (36%) | 68 (27%) | ||
| 3 | 63 (12%) | 606 (15%) | 23 (12%) | 25 (10%) | ||
| 4 | 45 (8%) | 462 (12%) | 13 (10%) | 21 (8%) | ||
| 5 | 32 (6%) | 236 (6%) | 11 (7%) | 9 (4%) | ||
| Missing at least one autoantibody titre | 4 | 48 | 2 | 4 | ||
| DPTRS | ||||||
| median | 5.85 | 6.18 | 6.13 | 6.04 | <0.0001 | <0.00001 |
| range | 0.78 to 10.1 | 0.49 to 10.1 | 3.5 to 9.1 | 1.3 to 9.76 | ||
| <6.5, n (%) | 383 (75%) | 2289 (61%) | 89 (67%) | 155 (67%) | <0.0001 | <0.00001 |
| >= 6.5, n (%) | 126 (25%) | 1460 (39%) | 43 (33%) | 77 (33%) | ||
| missing | 28 | 191 | 11 | 21 | ||
| BMI percentile | ||||||
| Median | 81.1 | 67.8 | 76.4 | 69.0 | <0.0001 | <0.0001 |
| Range | 0 to 99.8 | 0 to 100 | 2.0 to 99.6 | 0 to 99.2 | ||
| Normal/Lean | 286 (55%) | 2682 (70%) | 75 (56%) | 164 (67%) | <0.0001 | <0.0001 |
| Overweight | 98 (19%) | 612 (16%) | 24 (18%) | 49 (20%) | ||
| Obese | 139 (26%) | 534 (14%) | 36 (27%) | 32 (13%) | ||
| missing | 14 | 112 | 8 | 8 | ||
| Subgroup 1: Single confirmed autoantibody positives (n=2039) | ||||||
| n=280 | n=1585 | n=48 | n=126 | |||
| Antibody type, n (%) | ||||||
| GAD65 | 195 (69.5%) | 1118 (70.5%) | 38 (79%) | 89 (70.5%) | 0.12 | 0.21 |
| IA-2 | 7 (2.5%) | 76 (5%) | 1 (2%) | 1 (1%) | ||
| mIAA | 78 (28%) | 391 (24.5%) | 9 (19%) | 36 (28.5%) | ||
| HLA DR3-DQ2/DR4-DQ8, n (%) | ||||||
| Both DR3-DQ2 and DR4-DQ8 present | 19 (7%) | 202 (13%) | 0 | 11 (10%) | 0.001 | 0.009 |
| Not both DR3-DQ2 and DR4-DQ8 present | 252 (93%) | 1306 (87%) | 46 (100%) | 104 (90%) | ||
| DR3-DQ2 and/or DR4-DQ8 present | 152 (56%) | 1142 (76%) | 29 (63%) | 80 (70%) | <0.0001 | <0.0001 |
| Neither present | 119 (44%) | 366 (24%) | 17 (37%) | 35 (30%) | ||
| missing | 9 | 77 | 2 | 11 | ||
| Fasting C-peptide (nmol/L) | ||||||
| median | 0.59 | 0.53 | 0.62 | 0.52 | 0.015 | 0.0015 |
| range | 0.13 to 1.93 | 0.02 to 2.5 | 0.17 to 1.27 | 0.06 to 3.38 | ||
| missing | 0 | 3 | 0 | 0 | ||
| Mean C-peptide AUC (nmol/L) | ||||||
| median | 2.19 | 1.98 | 1.9 | 2.0 | 0.0007 | <0.0001 |
| range | 0.31 to 8.23 | 0.28 to 8.27 | 0.72 to 3.91 | 0.4 to 8.77 | ||
| missing | 8 | 42 | 2 | 9 | ||
| BMI percentile | ||||||
| Median | 82.9 | 71.0 | 89.1 | 66.5 | <0.0001 | <0.0001 |
| Range | 0.45 to 99.8 | 0 to 100 | 2.8 to 99.6 | 0 to 99.1 | ||
| Normal/Lean | 139 (51%) | 1036 (67%) | 20 (44.5%) | 85 (70%) | <0.0001 | <0.0001 |
| Overweight | 52 (19%) | 253 (16.5%) | 11(24.5%) | 23 (19%) | ||
| Obese | 81 (30%) | 254 (16.5%) | 14 (31%) | 14 (11%) | ||
| missing | 8 | 42 | 3 | 4 | ||
| Subgroup 2: Multiple autoantibody positives (n=2834) | ||||||
| n=257 | n=2355 | n=95 | n=127 | |||
| HLA DR3-DQ2/DR4-DQ8, n (%) | ||||||
| Both DR3-DQ2 and DR4-DQ8 present | 58 (23%) | 532 (24%) | 11 (12%) | 21 (18%) | 0.03 | 0.99 |
| Not both DR3-DQ2 and DR4-DQ8 present | 194 (77%) | 1688 (76%) | 80 (88%) | 97 (82%) | ||
| DR3-DQ2 and/or DR4-DQ8 present | 183 (73%) | 1867 (84%) | 67 (74%) | 93 (79%) | <0.0001 | <0.0001 |
| Neither present | 69 (27%) | 353 (16%) | 24 (26%) | 25 (21%) | ||
| Missing | 5 | 135 | 4 | 9 | ||
| Fasting C-peptide (nmol/L) | ||||||
| median | 0.49 | 0.45 | 0.41 | 0.44 | 0.046 | 0.01 |
| range | 0.11 to 1.71 | 0.05 to 2.48 | 0.08 to 1.24 | 0.10 to 1.61 | ||
| missing | 0 | 5 | 0 | 0 | ||
| Mean C-peptide AUC (nmol/L) | ||||||
| median | 1.75 | 1.621 | 1.72 | 1.63 | 0.012 | 0.003 |
| range | 0.36 to 7.49 | 0.16 to 7.89 | 0.48 to 3.53 | 0.68 to 4.71 | ||
| missing | 10 | 52 | 2 | 6 | ||
| BMI percentile | ||||||
| Median | 79.3 | 65.9 | 73.5 | 72.1 | 0.0001 | 0.0006 |
| Range | 0 to 99.7 | 0 to 100 | 1.98 to 99.6 | 0.6 to 99.2 | ||
| Normal/Lean | 147 (59%) | 1646 (72%) | 55 (61%) | 79 (64%) | <0.0001 | <0.0001 |
| Overweight | 36 (18%) | 359 (16%) | 13 (14%) | 26 (21%) | ||
| Obese | 58 (23%) | 280 (12%) | 22 (24%) | 18 (15%) | ||
| missing | 6 | 70 | 5 | 4 | ||
In progression from single confirmed to multiple positive autoantibodies, we found that Hispanic ethnicity, age at screening, sex, DPTRS, and HLA DR3-DQ2/DR4-DQ8 were significant factors affecting time to progression (ESM Table 1). We found that Hispanic ethnicity was significantly associated with a protective effect for conversion to multiple positive autoantibodies (HR=0.66, 95%CI=0.46–0.96; p=0.028, ESM Table 1) ) after adjustment for positive autoantibody type, age, sex, DPTRS, obesity and HLA DR3-DQ2/DR4-DQ8 genotype (Figure 2). This lower likelihood in Hispanic participants to progress to multiple positive autoantibody compared to NHWs was also observed in participants who did not have HLA DR3-DQ2/DR4-DQ8, even after adjustment for potential confounders (HR=0.63, 95%CI=0.42–0.94, p=0.024).
Figure 2:
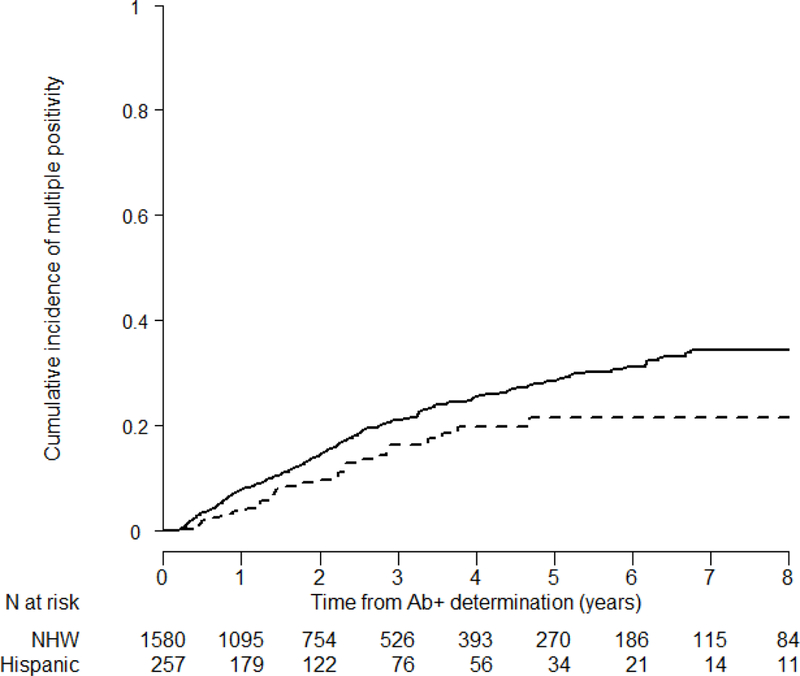
Cumulative incidence of multiple autoantibody positivity in Hispanics vs NHWs TrialNet PTP participants with single confirmed autoantibody positivity at screening (p=0.01). Curves are adjusted for autoantibody type, age, sex, DPTRS, and HLA DR3-DQ2/DR4-DQ8 status. We used the following lines for each group: the solid line for NHWs, and the dashed line for Hispanics. (*PTP: Pathway to Prevention, NHWs: Non-Hispanic Whites, Ab+: autoantibody positive)
Among multiple autoantibody positive participants at screening, cumulative incidence of and time to type 1 diabetes did not differ significantly by race/ethnicity in the overall cohort, or between Hispanic vs. NHW participants after adjusting for age, sex, DPTRS, number of autoantibodies, HLA DR3-DQ2/DR4-DQ8 genotype, and BMI (ESM Table 2). Cut-point analyses identified 12 years for age in relation to time to progression to type 1 diabetes. In the overall cohort, there was a significant three-way interaction between age, being overweight/obese vs. not, and Hispanic vs. NHW (p=0.006, ESM Table 2). Stratified analyses showed that in children <12 years old, ethnicity (Hispanic vs. NHW) was a significant effect modifier on the effects of overweight/obesity on cumulative incidence and rate of progression to type 1 diabetes (p=0.025). In children <12 years old at the time of multiple autoantibody determination, although it was not significantly different, lean Hispanics appeared to have a lower rate of progression to type 1 diabetes (HR=0.65, 95% CI=0.36–1.17, p=0.15; Figure 3) than lean NHWs after adjusting for sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8. However, in this age group, overweight/obesity increased likelihood and rate of progression to type 1 diabetes by 36% in NHWs (HR=1.36, 95%CI=1.04–1.77, p=0.024, ESM Table 3); while the likelihood and rate of progression to type 1 diabetes was nearly quadrupled in Hispanics (HR=3.8, 95%CI=1.6–9.1; p=0.0026, ESM Table 3) even after adjusting for sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8 (Figure 3). In participants ≥12 years old at multiple autoantibody determination, there were no significant differences among Hispanic vs NHWs. However, we also observed a significant interaction between the BMI%ile as a continuous measure and Hispanic ethnicity (p=0.012). Although BMI%ile was not a significant factor in NHW participants ≥12 years old (HR=0.997, 95%CI=0.991–1.004; p=0.38), we observed a significant effect of BMI%ile (HR=0.96, 95%CI=0.94–0.99; p=0.007) in Hispanic participants ≥12 years old even with the much more limited number of participants in that group. However, with only 13 events in this restricted cohort, we consider this an interesting and hypothesis-generating observation that warrants further investigation.
Figure 3:
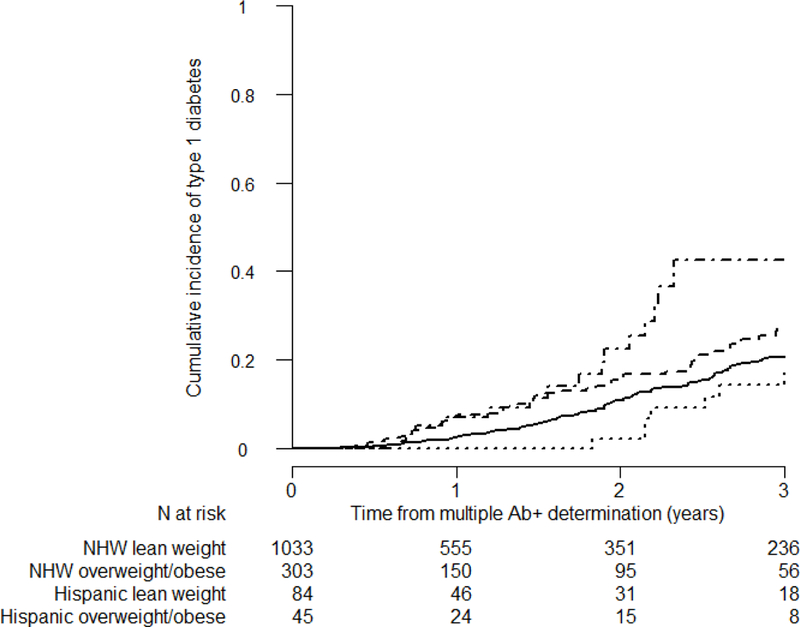
Cumulative incidence of type 1 diabetes mellitus in TrialNet PTP participants <12 years old with multiple positive autoantibodies, by ethnicity (i.e. Hispanic vs NHW) and BMI groups. Curves are adjusted for sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8 status. We used the following lines for each group: the solid line for lean NHWs, the dashed line for overweight/obese NHWs, the dotted line for lean Hispanics, and the dash-dotted line for overweight/obese Hispanics. (*PTP: Pathway to Prevention, NHWs: Non-Hispanic Whites, Ab+: autoantibody positive)
To further assess the role and effect modification by Hispanic ethnicity on progression to type 1 diabetes, we also evaluated this outcome in the overall cohort of all autoantibody positive participants (n=4,873) and found similar findings while adjusting for the number of autoantibodies and other potential confounders (Figure 4, ESM Table 4, and Table 5). No significant differential risks were observed between NHBs and NHWs.
Figure 4:
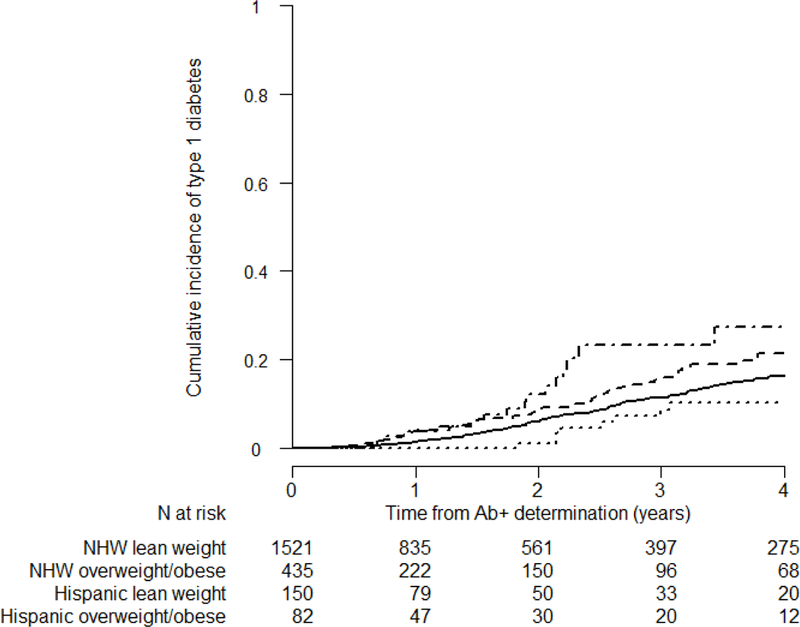
Cumulative incidence of type 1 diabetes mellitus in TrialNet PTP participants <12 years old in all at-risk cohort (i.e., both single confirmed and multiple autoantibody positive cohort), by ethnicity (i.e. Hispanic vs NHW) and BMI groups. Curves are adjusted for sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8 status. We used the following lines for each group: the solid line for lean NHWs, the dashed line for overweight/obese NHWs, the dotted line for lean Hispanics, and the dash-dotted line for overweight/obese Hispanics. (*PTP: Pathway to Prevention, NHWs: Non-Hispanic Whites, Ab+: autoantibody positive)
DISCUSSION:
In our study of the role of race and ethnicity in relation to the progression of islet autoimmunity and type 1 diabetes development in at-risk individuals from the TrialNet PTP cohort, we found that conversion from single to multiple autoantibody positivity was less common in Hispanics than NHWs after adjustment for autoantibody type, age, sex, DPTRS, obesity, and HLA DR3-DQ2/DR4-DQ8. Although time from multiple autoantibody positivity to type 1 diabetes did not differ by race/ethnicity in the overall cohort, we found that Hispanic ethnicity as well as age (specifically <12 or ≥12 years) were significant effect modifiers on the influence of BMI%ile on rates of progression to type 1 diabetes. In participants <12 years old at multiple autoantibody determination, being overweight or obese was a significant factor for progression to type 1 diabetes in both Hispanic and NHW participants; however, this risk was much more pronounced in Hispanic than in the NHW participants (HRs of 3.8 vs. 1.36, respectively) even adjusting for sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8. There was also an indication that Hispanic ethnicity could also modify the effects of BMI%ile in participants ≥12 years old at autoantibody determination.
Single to multiple autoantibody conversion is a crucial step in islet autoimmunity progression and type 1 diabetes risk [1]. After adjustment for confounding factors, we observed that progression to multiple autoantibody positivity was less common in Hispanics compared to NHWs. The slower progression in Hispanics suggests lower frequency of predisposing characteristics beyond those that we adjusted for, for example additional type 1 diabetes-linked HLA haplotypes or non-HLA genetic factors, or environmental factors. Further research is warranted to understand the basis of this ethnic difference and its impact on type 1 diabetes prediction models as well as type 1 diabetes prevention efforts. Finally, the lower relative ratio of type 1 diabetes development in single confirmed autoantibody positive versus multiple autoantibody positive participants in our cohort is similar to previous reports [1] and underscores the role of multiple islet autoantibody positivity in progression to type 1 diabetes [2].
Although there was no difference in time to type 1 diabetes in multiple autoantibody positive cohort among the races/ethnicities overall, Hispanics appeared to have lower likelihood of progressing to type 1 diabetes than NHWs among lean children <12 years after adjustment of sex, number of autoantibodies, DPTRS, and HLA DR3-DQ2/DR4-DQ8. Differences in additional type 1 diabetes-associated HLA haplotypes and/or non-HLA genetic factors, as well as epigenetic or environmental factors may explain the lower relative risk and rate of progression in this group. The significant role of Hispanic ethnicity on modifying the effects of BMI in this age group was best illustrated through the greater detrimental effect of elevated BMI in Hispanics than in NHWs in children <12 years old. This phenomenon may be related to the limitation of BMI in estimating percent body fat and adiposity, which was shown previously to disproportionally affect US Hispanics [24]. Accordingly, BMI has been shown to increase the risk of cardiovascular disease in Hispanics more than non-Hispanics [25]. Higher adiposity and consequently higher insulin resistance for a given BMI%ile in Hispanics might have contributed to this disproportional detrimental effect.
Similarly, the lack of clear ethnic differences in progression in individuals ≥12 years old could be due to lower statistical power because of a smaller sample size or fewer cases of progression in individuals >12 years old, as expected since it is known that older age is associated with slower progression from multiple autoantibody positivity to type 1 diabetes [2, 26, 27]. The ethnic/racial differences in progression to type 1 diabetes in older individuals may be identified in larger cohorts with a longer observation period in future studies. To the contrary, others reported that high risk autoantibody profiles (presence of IA2 and/or ZnT8) and genetic factors (HLA class I and non-HLA genes), but not age, are independent risk factors in progression from multiple autoantibody positivity to type 1 diabetes [28–31].
Our data provide insight on the impact of race/ethnicity in type 1 diabetes progression and may be valuable for the design of predictive models and prevention trials. Future studies aimed at identifying factors (e.g. genetic, epigenetic, environmental, etc.) contributing to slower progression in Hispanics will advance our understanding of the natural history of type 1 diabetes and may have a significant impact on the prevention of type 1 diabetes. Such analyses may also allow for the determination of categorical diabetes subtypes with important therapeutic implications.
We observed that NHWs were more likely to have multiple positive autoantibodies compared to Hispanics, similar to a previous study conducted in individuals with newly diagnosed type 1 diabetes [32]. The higher incidence of islet autoimmunity observed in NHWs could be due to the different distribution of type 1 diabetes-associated HLA haplotypes/genotypes, with increased frequency of susceptibility [6] and decreased frequency of protective types [33] compared to Hispanics.
Counselling families on type 1 diabetes risk in family members is an integral part of modern diabetes care [34]. If confirmed, our findings highlight that the differential effect of race/ethnicity may need to be taken into consideration when counselling at-risk family members. Seroconversion from single confirmed to multiple positive autoantibodies (i.e., pre-symptomatic phase of type 1 diabetes) was less common in Hispanics. Hispanic families with at-risk children younger than 12 years old may also be informed that overweight/obesity increases their risk for progression to type 1 diabetes more significantly than NHW children. This knowledge may encourage Hispanic families to make healthy lifestyle changes. Furthermore, this finding has important public health implications due to the higher prevalence of overweight/obesity [35] and greater annual rate of increase in type 1 diabetes incidence in Hispanics compared to NHWs [4].
Limitations of the study are lack of adjustment for other HLA [36, 37] and non-HLA loci [37, 38] that are known to be associated with type 1 diabetes development despite adjustment for the presence of the highest risk genotype, the observational nature of the study allowing to identify associations without implications on causality, and the smaller number of participants in minority groups other than Hispanics, which limited our ability to delineate racial/ethnic differences in those groups. Longer follow up of the participants will increase the precision of the estimates of progression. Because of the design of the TrialNet PTP study, the duration of positivity of single or multiple autoantibodies prior to screening is not known. However, the racial differences in the proportion of multiple autoantibody positive individuals at screening (60% in NHWs vs 48% in Hispanics, Table 1) reflects the differential rates of early progression. Thus, the differences between Hispanics and NHWs in progression from single to multiple autoantibody positivity could have been more robust if participants were followed since birth in a different study design. Our study focused on racial/ethnic differences in progression of islet autoantibodies (from single to multiple) and development of type 1 diabetes; analyses of differences in the first appearance of autoantibodies will require cohorts that follow individuals from birth. Additionally, caution should be taken in applying our data to the determination of type 1 diabetes risk in the general population, as our study participants were relatives of individuals with type 1 diabetes, and thus at increased risk for the development of type 1 diabetes. The major strengths of this study were the relatively large sample size, including a significant number of Hispanics, and the availability of comprehensive type 1 diabetes predictive data (e.g., HLA DR3-DQ2/DR4-DQ8, DPTRS, etc.). These characteristics enabled us to compare the races/ethnicities for outcome measures while adjusting for confounding factors.
In conclusion, progression of islet autoimmunity, from single to multiple positive autoantibodies, was less common in Hispanics compared to NHWs, while differences in progression from multiple autoantibodies to type 1 diabetes were limited to children <12 years old and modified by BMI. Further research is warranted to investigate factors playing a role in the racial/ethnic heterogeneity of type 1 diabetes pathogenesis. Better insight into these factors will allow for adequate counselling of at-risk individuals and for the development of prediction models and design of prevention trials.
Supplementary Material
Research in Context:
What is already known about this subject?
Type 1 diabetes mellitus is a disease continuum with variable progression along well-defined stages.
A wealth of data has been generated on genetic, immunological and metabolic risk factors that enable us to predict type 1 diabetes risk.
A limited number of studies showed that there are significant racial/ethnic differences in genetic, immunologic, metabolic, and clinical characteristics.
There are also racial/ethnic differences in incidence and prevalence of type 1 diabetes.
What is the key question?
Do progression of islet autoimmunity and type 1 diabetes differ among races/ethnicities in at-risk individuals?
What are the new findings?
Conversion from single to multiple autoantibody positivity was less common in Hispanics than Non-Hispanic Whites after adjusting for confounding factors.
Being overweight or obese corresponded with significantly increased rates of type 1 diabetes in participants who were less than 12 years old at autoantibody presentation. These increased rates in relation to overweight/obesity were more pronounced in Hispanic participants (nearly four-fold increase) than in non-Hispanic White participants (increase by only 36%).
How might this impact on clinical practice in the foreseeable future?
It provides important findings that can be used in identification of different type 1 diabetes phenotypes.
It impacts the prediction of progression of islet autoimmunity and development of type 1 diabetes based on race/ethnicity.
ACKNOWLEDGEMENTS:
The datasets generated during and/or analyzed during the current study are available in the NIDDK Central Repository.
Funding: The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Abbreviations:
- BMI%iles
BMI percentiles
- DPTRS
Diabetes Prevention Trial Type 1 Risk Score
- GAD65
glutamic acid decarboxylase 65
- IA-2
islet antigen 2
- ICA
islet cell autoantibody
- mIAA
micro-insulin autoantibody
- NHW
Non-Hispanic White
- NHB
Non-Hispanic Black
- PTP
Pathway to Prevention
- ZnT8
zinc transporter 8
Footnotes
Duality of Interests: The authors declare that there is no duality of interest associated with this manuscript.
Prior Presentation: The part of this study was presented as oral presentation at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, June 9-13, 2017.
REFERENCES:
- [1].Insel RA, Dunne JL, Atkinson MA, et al. (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care 38: 1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ziegler AG, Rewers M, Simell O, et al. (2013) Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama 309: 2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sosenko JM, Krischer JP, Palmer JP, et al. (2008) A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes care 31: 528–533 [DOI] [PubMed] [Google Scholar]
- [4].Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. (2017) Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. The New England journal of medicine 376: 1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barinas-Mitchell E, Pietropaolo S, Zhang YJ, et al. (2004) Islet cell autoimmunity in a triethnic adult population of the Third National Health and Nutrition Examination Survey. Diabetes 53: 1293–1302 [DOI] [PubMed] [Google Scholar]
- [6].Black MH, Lawrence JM, Pihoker C, et al. (2013) HLA-associated phenotypes in youth with autoimmune diabetes. Pediatric diabetes 14: 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gay EC, Hamman RF, Carosone-Link PJ, et al. (1989) Colorado IDDM Registry: lower incidence of IDDM in Hispanics. Comparison of disease characteristics and care patterns in biethnic population. Diabetes care 12: 701–708 [DOI] [PubMed] [Google Scholar]
- [8].Kostraba JN, Cruickshanks KJ, Neville TG, et al. (1992) Clinical characteristics of IDDM in Hispanics and non-Hispanic whites. Little evidence of heterogeneity by ethnicity. Diabetes care 15: 1303–1309 [DOI] [PubMed] [Google Scholar]
- [9].Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ (2003) Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes care 26: 2876–2882 [DOI] [PubMed] [Google Scholar]
- [10].Morahan G (2012) Insights into type 1 diabetes provided by genetic analyses. Current opinion in endocrinology, diabetes, and obesity 19: 263–270 [DOI] [PubMed] [Google Scholar]
- [11].Tosur M, Redondo MJ (2017) Heterogeneity of Type 1 Diabetes: The Effect of Ethnicity. Current diabetes reviews [DOI] [PubMed] [Google Scholar]
- [12].Gandhi K, Tosur M, Schaub R, Haymond MW, Redondo MJ (2017) Racial and ethnic differences among children with new-onset autoimmune Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association 34: 1435–1439 [DOI] [PubMed] [Google Scholar]
- [13].Redondo MJ, Libman I, Cheng P, et al. (2018) Racial/Ethnic Minority Youth With Recent-Onset Type 1 Diabetes Have Poor Prognostic Factors. Diabetes care [DOI] [PubMed] [Google Scholar]
- [14].Skyler JS, Greenbaum CJ, Lachin JM, et al. (2008) Type 1 Diabetes TrialNet--an international collaborative clinical trials network. Annals of the New York Academy of Sciences 1150: 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. (2009) The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatric diabetes 10: 97–104 [DOI] [PubMed] [Google Scholar]
- [16].Meah FA, DiMeglio LA, Greenbaum CJ, et al. (2016) The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 59: 1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].NIH (2015) Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. NOT-OD-15–089 [Google Scholar]
- [18].Fouts A, Pyle L, Yu L, et al. (2016) Do Electrochemiluminescence Assays Improve Prediction of Time to Type 1 Diabetes in Autoantibody-Positive TrialNet Subjects? Diabetes care 39: 1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sosenko JM, Skyler JS, Mahon J, et al. (2011) Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes care 34: 1785–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Therneau TMCC, Atkinson EJ (2015) Adjusted Survival Curves [Google Scholar]
- [21].Nieto FJ, Coresh J (1996) Adjusting survival curves for confounders: a review and a new method. American journal of epidemiology 143: 1059–1068 [DOI] [PubMed] [Google Scholar]
- [22].Xu P, Krischer JP, Type 1 Diabetes TrialNet Study G (2016) Prognostic Classification Factors Associated With Development of Multiple Autoantibodies, Dysglycemia, and Type 1 Diabetes-A Recursive Partitioning Analysis. Diabetes care 39: 1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Redondo MJ, Geyer S, Steck AK, et al. (2018) TCF7L2 Genetic Variants Contribute to Phenotypic Heterogeneity of Type 1 Diabetes. Diabetes care 41: 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wong WW, Strizich G, Heo M, et al. (2016) Relationship between body fat and BMI in a US hispanic population-based cohort study: Results from HCHS/SOL. Obesity 24: 1561–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jayawardene WP, Lohrmann D, Dickinson S, Talagala S, Torabi M (2017) Clinical measures of obesity and cumulative cardiometabolic risk in adolescents. Clinical obesity 7: 11–21 [DOI] [PubMed] [Google Scholar]
- [26].Steck AK, Dong F, Waugh K, et al. (2016) Predictors of slow progression to diabetes in children with multiple islet autoantibodies. Journal of autoimmunity 72: 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krischer JP, Liu X, Lernmark A, et al. (2017) The Influence of Type 1 Diabetes Genetic Susceptibility Regions, Age, Sex, and Family History on the Progression From Multiple Autoantibodies to Type 1 Diabetes: A TEDDY Study Report. Diabetes 66: 3122–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gorus FK, Balti EV, Messaaoui A, et al. (2017) Twenty-Year Progression Rate to Clinical Onset According to Autoantibody Profile, Age, and HLA-DQ Genotype in a Registry-Based Group of Children and Adults With a First-Degree Relative With Type 1 Diabetes. Diabetes care 40: 1065–1072 [DOI] [PubMed] [Google Scholar]
- [29].Balke EM, Balti EV, Van der Auwera B, et al. (2018) Accelerated Progression to Type 1 Diabetes in the Presence of HLA-A*24 and -B*18 Is Restricted to Multiple Islet Autoantibody-Positive Individuals With Distinct HLA-DQ and Autoantibody Risk Profiles. Diabetes care 41: 1076–1083 [DOI] [PubMed] [Google Scholar]
- [30].Achenbach P, Hummel M, Thumer L, Boerschmann H, Hofelmann D, Ziegler AG (2013) Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia 56: 1615–1622 [DOI] [PubMed] [Google Scholar]
- [31].Lipponen K, Gombos Z, Kiviniemi M, et al. (2010) Effect of HLA class I and class II alleles on progression from autoantibody positivity to overt type 1 diabetes in children with risk-associated class II genotypes. Diabetes 59: 3253–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aviles-Santa L, Maclaren N, Raskin P (2004) The relationship between immune-mediated Type 1 diabetes mellitus and ethnicity. J Diabetes Complications 18: 1–9 [DOI] [PubMed] [Google Scholar]
- [33].Erlich HA, Zeidler A, Chang J, et al. (1993) HLA class II alleles and susceptibility and resistance to insulin dependent diabetes mellitus in Mexican-American families. Nature genetics 3: 358–364 [DOI] [PubMed] [Google Scholar]
- [34].Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook A (2014) Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes care 37: 2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ogden CL, Carroll MD, Fryar CD, Flegal KM (2015) Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief: 1–8 [PubMed] [Google Scholar]
- [36].Ilonen J, Kiviniemi M, Lempainen J, et al. (2016) Genetic susceptibility to type 1 diabetes in childhood - estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatric diabetes 17 Suppl 22: 8–16 [DOI] [PubMed] [Google Scholar]
- [37].Redondo MJ, Steck AK, Pugliese A (2017) Genetics of type 1 diabetes. Pediatric diabetes [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Steck AK, Xu P, Geyer S, et al. (2017) Can Non-HLA Single Nucleotide Polymorphisms Help Stratify Risk in TrialNet Relatives at Risk for Type 1 Diabetes? The Journal of clinical endocrinology and metabolism 102: 2873–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


