Abstract
Parkinson’s disease (PD) is a progressive aging disorder that affects millions worldwide, thus, disease-modifying-therapies are urgently needed. PD pathology includes α-synuclein (aSyn) accumulation as synucleinopathy. Loss of GM1 gangliosides occurs in PD brain, which is modeled in GM2 synthase transgenic mice. GM2+/− mice have low, not absent GM1 and develop age-onset motor deficits, making them an excellent PD drug testing model. FTY720 (fingolimod) reduces synucleinopathy in A53T aSyn mice and motor dysfunction in 6-OHDA and rotenone PD models, but no one has tested FTY720 in mice that develop age-onset PD-like motor problems. We confirmed that GM2+/−mice had equivalent rotarod, hindlimb reflexes, and adhesive removal functions at 9 mo. From 11 mo, GM2+/− mice received oral FTY720 or vehicle 3x/week to 16 mo. As bladder problems occur in PD, we also assessed GM2+/− bladder function. This allowed us to demonstrate improved motor and bladder function in GM2+/− mice treated with FTY720. By immunoblot, FTY720 reduced levels of proNGF, a biomarker of bladder dysfunction. In humans with PD, arm swing becomes abnormal, and brachial plexus modulates arm swing. Ultrastructure of brachial plexus in wild type and GM2 transgenic mice confirmed abnormal myelination and axons in GM2 transgenics. FTY720 treated GM2+/− brachial plexus sustained myelin associated protein levels and reduced aggregated aSyn and PSer129 aSyn levels. FTY720 increases brain derived neurotrophic factor (BDNF) and we noted increased BDNF in GM2+/− brachial plexus and cerebellum, which contribute to rotarod performance. These findings provide further support for testing low dose FTY720 in patients with PD.
Keywords: Brachial plexus synucleinopathy, Fingolimod, Improved bladder function, Low dose drug, Motor improvement, Myelination biomarker increases, Neuroprotection, Parkinson’s disease
INTRODUCTION
Parkinson’s disease (PD) is an age-onset neurodegenerative disorder that affects ~ 1% the population over 50 and ~4% over 85 yr of age (Bekris et al. 2010). Males are 1.5 times more likely than females to be diagnosed with PD (Wooten et al. 2004). Early onset PD (21–49 years old) and juvenile onset PD (before 21 years old) do occur, but most PD is age-related and considered to be sporadic (Bonifati 2012), with familial PD accounting for only ~ 5% of all cases.
Most PD brains at autopsy exhibit a loss of midbrain substantia nigra pars compacta (SNc) dopaminergic neurons, which is apparent as a loss of neuromelanin-pigment. SNc supplies dopaminergic input to striatum that helps control normal body movement (Turner & Desmurget 2010) in association with cerebellar function (Bostan & Strick 2010; Bostan et al. 2010). The dying back of SNc axons that innervate striatum is part of the progressive pathology that subsequently leads to PD motor symptoms (Alexander 2004; Cheng et al. 2010).
Surviving midbrain SNc neurons often contain Lewy Bodies, inclusions with abundant aSyn, a presynaptic protein that broadly contributes to dopamine regulation (Perez et al. 2002; Benskey et al. 2016; Porras & Perez 2014; Mor & Ischiropoulos 2018; Perez & Hastings 2004; Peng et al. 2005; Tehranian et al. 2006). Phosphorylation of aSyn PSer129 not only affects normal aSyn function but also increases its aggregation potential (Lou et al. 2010; Oueslati 2016). aSyn aggregation in Lewy bodies/neurites is common in both sporadic and familial PD. Furthermore, though under-appreciated, it is known that cerebellum is affected in patients with PD (Wu & Hallett 2013) in part by interconnecting circuitry with the striatum (Bostan & Strick 2010; Bostan et al. 2010). Moreover, PSer129 aSyn has been found in adrenal gland, retina, salivary gland, enteric nervous system, and pancreas of PD patients; confirming extensive synucleinopathy in PD (Stoddard 1994; Bencsik et al. 2014; Antunes et al. 2016; Martinez-Valbuena et al. 2018; Beach et al. 2014; Ortuno-Lizaran et al. 2018; Fumimura et al. 2007; Beach et al. 2010; Jain 2011), further establishing that PD is more than just a brain disorder. Thus, protective therapies that reduce synucleinopathy are urgently needed.
Among key biological molecules that regulate optimal neuronal function are gangliosides, sialic acid-bearing glycosphingolipids that are highly expressed in the brain (Schnaar 2010). The most abundant mature gangliosides are GM1 (mono, with one sialic acid), GD1a/GD1b (di, with two sialic acids), and GT1b (tri, with three sialic acids) (Palmano et al. 2015; Sturgill et al. 2012; Posse de Chaves & Sipione 2010). GD1a and GT1b deficiencies contribute to neurodegeneration (Ohmi et al. 2014) and GM1 levels are significantly lower, but not entirely absent in SNc of PD brain (Wu et al. 2012a; Hadaczek et al. 2015). Gangliosides are also ligands that enhance myelin stability (Vyas & Schnaar 2001) and recent data from our lab confirmed bladder innervation myelination abnormalities in GM2−/− KO mice entirely lacking GM2/GD2 Synthase, the enzyme for mature ganglioside synthesis (Gil-Tommee et al. 2019). GM2−/− KO mice have only immature GM3 gangliosides a species of gangliosides that reportedly are elevated in sera of PD patients (Chan et al. 2017). GM1 has been shown to reduce aSyn aggregation in vitro (Martinez et al. 2007), suggesting that normal GM1 levels act to keep aSyn soluble. Prior data also confirm that ganglioside decreases in PD can be alleviated by treating with GM1 or with the GM1-analogue LIGA-20, both of which reduce PD motor symptoms (Schneider et al. 2010; Schneider et al. 1998). These data imply that mature GM1 gangliosides contribute significantly to brain function and that mice with reduced GM1 levels and progressive motor dysfunction make an excellent PD model (Wu et al. 2012a; Hadaczek et al. 2015) supporting their use for testing candidate PD therapies.
In addition to gangliosides, myelin associated proteins contribute to neuron function by supportive effects. For example, myelin basic protein (MBP) enhances neuronal signaling while mutations or absence of MBP cause impaired motor function in vivo (Nave 2010; Harauz & Boggs 2013). Similarly, the oligodendrocyte-specific-protein 2’,3’-cyclic nucleotide-3’-phosphodiesterase (CNP), contributes to axonal integrity (Lappe-Siefke et al. 2003). Moreover, oligodendrocytes and other glial cells produce neurotrophic factors that are protective of brain neurons, and GM1 itself functions as a trophic factor (Mocchetti 2005; Mocchetti & Bachis 2004).
PD has no cure and existing treatments can only modify symptoms and do not slow disease progression. Levodopa, a dopamine precursor that crosses the blood brain barrier then is converted to dopamine, acts to reduce PD rigidity and bradykinesia. Another PD treatment, deep brain stimulation (DBS), is more often used in those who respond poorly to PD medications. However, DBS surgery is invasive so it is not a first line PD therapy. Even so, a recent report showed less tremor progression in patients after DBS during a relatively early stage of the disease (Hacker et al. 2018). PD patients also can benefit from exercise/physical therapy programs, which improve muscle tone, balance, and quality of life in PD patients (Oertel 2017).
FTY720/fingolimod is an approved oral treatment for relapsing/remitting multiple sclerosis (MS) (Sanford 2014; Brinkmann 2009; Brinkmann et al. 2010). The drug is protective by increasing BDNF expression (Vargas-Medrano et al. 2014; Vidal-Martinez et al. 2016; Deogracias et al. 2012) and also by improving myelination in MS patients as well as in MS models (Gurevich et al. 2018; Yazdi et al. 2015).
In association with PD, BDNF has been shown to be a major neurotrophin for SNc dopaminergic neurons (Dluzen et al. 1999), thus using a drug that can stimulate BDNF expression has considerable potential as a PD therapeutic. In addition to its role in PD pathology, we discovered that aSyn normally interacts with and modulates protein phosphatase 2A (PP2A) enhancing its activity (Peng et al. 2005; Lou et al. 2010; Farrell et al. 2014; Wang et al. 2009). We also found that in PD brain, PP2A activity is reduced in tissue with robust aSyn aggregation (Wu et al. 2012b). This suggests that a loss of aSyn function toward PP2A as aSyn accumulates in Lewy bodies/neurites may require enhancement. We and others have shown that FTY720 also stimulates PP2A activity (Vargas-Medrano et al. 2014; Lek et al. 2017; Perrotti & Neviani 2008). Here we show the protective benefits of low dose FTY720 in parkinsonian GM2+/− mice. Our findings suggest that FTY720 has the potential to counteract PD pathology, reverse dysfunction and slow disease progression. As the drug is already Food and Drug Administration (FDA) approved, it could be repurposed in short order for PD clinical trials.
EXPERIMENTAL PROCEDURES
Mice and Genotyping
A heterozygous (+/−) breeding pair of GM2/GD2 synthase/B4galnt1 mice (Sheikh et al. 1999) was a gift from Drs. Ledeen and Wu of Rutgers New Jersey Medical School and used to produce most mice for this study. Experiments were conducted on protocols approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee (IACUC #11025) in accordance with AALAC and National Institutes of Health guidelines. Mice were housed under 12-hr light/dark cycles with access to food and water ad libitum. Genotyping was performed using DNA purified from 3 mm tail snips with QIAamp DNA Mini Kit (QIAGEN.com). PCR primers were 5′-TAC CAG GCC AAC ACA GCA-3′ and 5′-CAG GTC CAG GGG CGT CTT-3′. PCR products stained with ethidium bromide were photographed under UV light to identify bands of 2.9 Kb and 2.5 Kb in GM2+/− mice as previously described (Wu et al. 2001).
FTY720 Preparation and Dosing
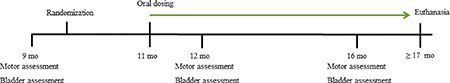
FTY720 was dissolved in dimethyl sulfoxide (DMSO) to create a 5 mg/mL stock solution that was stored at −20°C. Animal weights were obtained the day before dosing to calculate the amount of FTY720 needed for 0.5 mg/kg/mouse dosing. Beginning at 11 mo of age, mice received oral 0.5 mg/kg FTY720 or an equivalent amount of DMSO, as vehicle 3x/week. Doses were prepared and given in a blinded manner according to mouse ear punch patterns. For drug delivery, mice were held by the scruff of the neck, in a semi-supine position, with solutions delivered using a 10 μl pipette with the pipette tip gently placed in the mouth.
As previously confirmed by others, GM2+/− mice can begin showing motor deficits by 7 mo (Wu et al. 2012a), thus we set 9 mo as our baseline and randomly selected mice after confirming equivalent motor and bladder function. For behavioral tests, mice were placed in clean quiet rooms 15 min prior to evaluation in order to acclimate to the environment. Tests were performed between 1:00 – 3:00 PM on each test day for 9, 12 and 16 mo old GM2+/− mice (as shown in the time line below).
Behavioral Assessments
Rotarod. Balance and coordination were measured using rotarod (Colburn Instruments, PA) by experimenters blinded to the treatment condition for this and all behavioral tests. Mice were pre-trained in two independent sessions at a constant rate of 4 rpm and then at 8 rpm. On the day of testing, each mouse received an accelerating warm-up trial. Three trials were then performed with the rotarod set to accelerate from 4 – 40 rpm over 5 min time, with rotation increasing in increments of 0.12 rpm/sec. Between trials, mice were allowed a resting period of 5 min. Accelerating rotarod tests for each time point were done over two consecutive days. Adhesive removal sensorimotor tests. Adhesive removal allows determining motor response to a sensory stimulus (Fleming et al. 2004). Each mouse was restrained and a small adhesive sticker was placed on their forehead using forceps. The mouse was placed in a cage and a timer was set to record how long it took each mouse to remove the sticker, typically with their forepaws. The maximum amount of time each mouse was tested was 2 min (120 sec), and if a mouse failed to remove the sticker it scored 120. This testing was performed over 5 trials with mice having a short break between trials, over 2 consecutive days. Hindlimb reflex tests. To measure motor nerve function we evaluated hindlimb reflexes. Each mouse was suspended by the tail for 5 sec during which the position of the hindlimbs was scored as previously described (Chiavegatto et al. 2000). Data were collected over 3 trials performed on 2 different days, with short breaks between trials. Scores range from impaired to normal as follows: 0 = one or both hindlimbs paralyzed, 1 = hindlimbs and paws close to the body with clasping toes, 2 = loss of flexion of hindlimbs, 3 = hindlimbs extended < 90°angle, and 4 = hindlimbs extended > 90°angle. Urinary Void Spot Analysis. Food and water were removed during 1 hr tests performed on 5 different days, with each mouse placed individually into a clean cage (between 10:00–11:00 am) with cage bottom covered with filter paper (Bio–Rad, Hercules, CA, USA, cat# 1650962) as before (Gil-Tommee et al. 2019). Filters were collected, labeled, and allowed to dry. Void spots were illuminated with a UV light and counted as small (≤ 0.2 cm2) or large (> 0.2 cm2) by individuals blinded to treatments using established methods (Bjorling et al. 2015; Gil-Tommee et al. 2019; Hodges et al. 2008; Studeny et al. 2008; Yu et al. 2014; Birder et al. 2002; Hamill et al. 2012). Pooled data for each mouse at 12 and 16 mo time points provided the final values.
Euthanasia and Tissue Collection
Animals were euthanized by CO2 inhalation followed by decapitation. Tissues were rapidly dissected, placed in labeled tubes and stored at −80°C until analysis. We collected brachial plexus, brain, and urinary bladders at necropsy. For electron microscopy, tissue was handled as described below.
Transmission Electron Microscopy
Dissected brachial plexus was placed in 4% PFA overnight then rinsed and transferred to 10 mL pH 7.2 containing 2.5% glutaraldehyde-0.1 M imidazole buffer. Tissues were then immersed in 2 mL 2% osmium tetroxide in imidazole buffer for 4 hr and washed in distilled water followed by dehydration in a graded series of ethanol (50%, 80%, absolute). Sequentially, tissue sections were immersed in a mixture of 1:1 absolute ethanol and propylene oxide and then in propylene oxide in sealed vials on a rotating mixer for 15 min. Finally, tissue was infiltrated with a 1:1 mixture of propylene oxide and epoxy resin overnight in open vials then embedded with epoxy resin in molds at 60°C for 48 hr. Embedded tissue was first ‘thick’ sectioned at 0.25 micrometers with glass knives and stained with epoxy tissue stain (Electron Microscopy Sciences, Hatfield, PA). Selected areas were then thin sectioned at 70 nm with diamond knives and mounted on Formvar-Carbon coated grids and stained with 2% uranyl acetate and lead citrate solution. Digital images were collected with a mid-mount XR611 camera (AMT, Inc., Woburn, MA) in a model H-7650 electron microscope (Hitachi High-Technologies, Dallas, TX).
Gene Expression
Total mRNA extracted from cerebellum used a miRNeasy mini kit (Qiagen, catalog no. 217004) and RNase-free DNase kit (Qiagen, catalog no. 79254) according to the manufacturer. RNA concentration and purity were assessed using NanoDrop 2000 spectrophotometry (Thermo Scientific). RNA quality was assessed by measuring 28S/18S band ratios in RNA “bleach” gels exactly as described (Aranda et al. 2012; Segura-Ulate et al. 2017). Reverse transcription of mRNAs was performed using the High Capacity RNA-to-cDNA kit (Applied Biosystems, catalog no. 4387406) as per manufacturer instructions. Amplification was measured by real-time quantitative PCR (qPCR) with Taqman probes in a RealPlex Mastercycler 2 (Eppendorf Inc., Westbury, NY). Relative expression of mRNAs was measured using Taqman probe assays (Life Technologies, Inc.) for BDNF (catalog no. Mm04230607_s1), glial cell line derived neurotrophic factor (GDNF) (Mm00599849_m1), nerve growth factor (NGF) (Mm00443039_m1), and ciliary neurotrophic factor (CNTF) (Mm00446373_m1) with GAPDH (catalog no. Mm99999915_g1) and eukaryotic 18S ribosomal RNA (catalog no. Hs99999901_s1) as internal expression controls. Molecular assays were evaluated in 2–3 independent experiments in duplicate or triplicate.
Protein Isolation and Immunoblots
Soluble and insoluble protein was isolated as before (Waxman & Giasson 2008; Gil-Tommee et al. 2019; Wu et al. 2012b; Vidal-Martinez et al. 2016). Standard immunoblot tissue was homogenized in 6 volumes (w/v) of ice-cold buffer containing 1 mM AEBSF, 5 μM aprotinin and 1 mM benzamidine using a Bullet Blender (Next Advance, Inc., Averill Park, NY, USA). Samples were sonicated and spun at 14,000 × g for 10 min at 4°C to remove particulates. Protein concentrations were determined by bicinchoninic acid assay (Thermo-Fisher, USA). Gels, loaded with 10 – 50 μg protein per sample were transferred to nitrocellulose, blocked in buffer containing 5% nonfat milk, and incubated in primary antibodies overnight at 4°C. Antibodies included aSyn (C20, sc-7011-R, Santa Cruz Biotechnology, Santa Cruz, CA, USA), aSyn PSer129 (11A5, gift of Dr. J. Anderson of Elan Pharmaceuticals), β-actin (4970S, Cells Signaling, Danvers, MA, USA), CNP (D83E10, Cell Signaling, Danvers, MA, USA), MBP (ab40390, Abcam), proNGF (H-20, sc-548, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and BDNF (ab203573, Abcam). Secondary antibodies were IRDye 800CW (green) or 680RD (red) to generate signals that were quantified in Image Studio (v2) by LiCor Odyssey (Lincoln, NE, USA). Biochemical and molecular evaluations were from multiple independent experiments.
Statistics
Student t tests were performed using Prism 6 Software (GraphPad Inc., San Diego, CA, USA). Group effects (GM2+/− Vehicle vs FTY720), time effects, and group × time interactions were evaluated by parametric repeated measures ANOVA (RM-ANOVA) with post hoc Bonferroni tests using SPSS (v20, IBM, New York, NY). Expression of trophic factor mRNAs were calculated by comparative Ct method (2−ΔΔCt) using the Relative Expression Software Tool (REST) (Pfaffl et al. 2002) (http://www.REST.de.com). Data represent mean ± standard error (SEM), except for mRNA whisker box plots created with REST 2009, demonstrating median (dotted line inside the box), interquartile ranges 1 and 3 (upper and lower edges of the box), and maximum and minimum expression values (top and bottom whiskers). Significance was set to p < 0.05 for all assessments.
RESULTS
FTY720 improves movement and sensorimotor function in aging GM2+/− parkinsonian mice
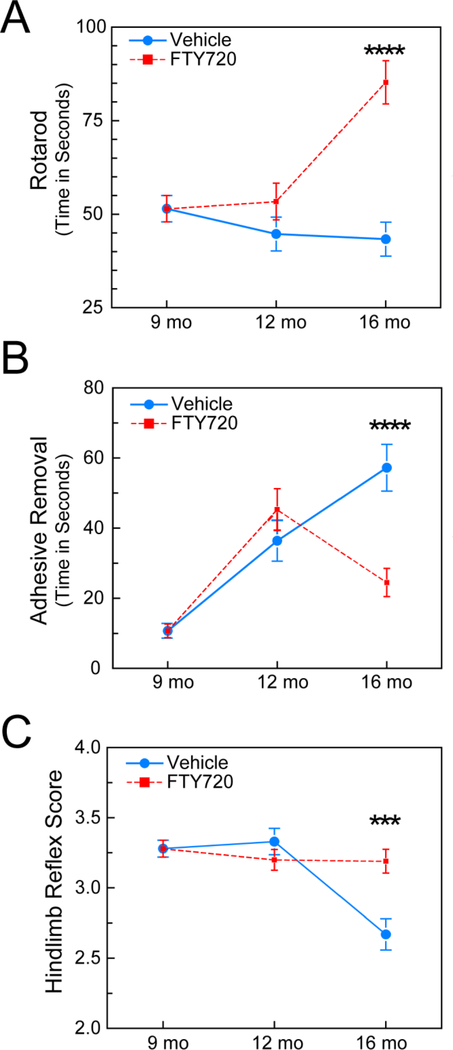
Rotarod measures balance and coordination as well as physical endurance. At 9 mo, in baseline tests, all GM2+/− mice performed similarly. Dosing stared at 11 mo, and at 12 mo, after 4 weeks of FTY720 or vehicle, GM2+/− mice treated with FTY720 showed no decline in performance while vehicle treated littermates showed a trend toward a decline in performance. However, by 16 mo, after 5 mo of FTY720 or vehicle, the FTY720 treated GM2+/− mice performed significantly better than their vehicle treated littermates, and even better than their own performance at 9 and 12 mo (Fig. 1A).
Figure 1. GM2+/− movement is improved or sustained in response to FTY720.
All motor tasks were evaluated at baseline (9 mo) and again at 12 mo and 16 mo for GM2+/− mice treated with vehicle or FTY720. (A) FTY720 treated GM2+/− mice significantly improved their time on the rotarod at 16 mo, while vehicle treated littermates rotarod performance declined. (B) Adhesive removal timing is better when riming is shorter. GM2+/− mice treated with FTY720 performed significantly better than vehicle treated GM2+/− littermates at 16 mo. (C) The hindlimb reflexes of GM2+/− mice treated with FTY720 were sustained at relatively normal levels, while vehicle treated GM2+/− mice developed poorer hindlimb reflexes by 16 mo. RM-ANOVA, ***, p < .001; ****, p < .0001.
We also evaluated sensorimotor function by timing the ability to remove an adhesive sticker placed on the forehead of GM2+/− mice. As can be appreciated in Figure 1B, at 9 mo, all GM2+/− mice removed the sticker within approximately 10 sec. At 12 mo, all GM2+/− mice took much longer to remove the sticker. By 16 mo, after 5 mo treatment with FTY720 or vehicle, the FTY720 GM2+/− mice had improved timing (~ 25 seconds) while vehicle treated GM2+/− performance continued to worsen (~57 seconds) (Fig. 1B).
As a test of motor nerve function, we also measured hindlimb reflexes of GM2+/− mice. At 9 mo all GM2+/− mice had scores that were within a relatively normal range (3.25). At 12 mo, after 4 weeks of FTY720 or vehicle, all GM2+/− mice still had similar scores regardless of treatment (3.25). However, by 16 mo, after 5 mo of FTY720 or vehicle, the FTY720 treated GM2+/− mice had hindlimb reflexes similar to their 9 and 12 mo scores, while vehicle treated littermates had scores consistent with a significant decline (~2.6). These findings demonstrate that FTY720 sustained hindlimb reflexes (Fig. 1C), while vehicle treated GM2+/− mice showed parkinsonian progression akin to what occurs over time in PD.
Bladder function is also improved by FTY720 in GM2+/− mice
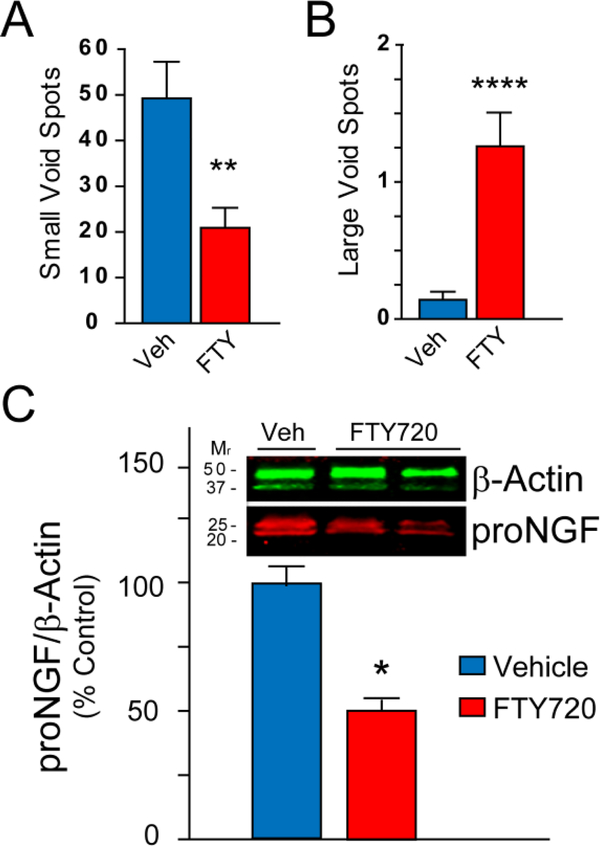
Abnormal bladder function is common in patients with PD (Araki et al. 2000), and we confirmed bladder dysfunction in the related GM2−/− KO parkinsonian model (Gil-Tommee et al. 2019). Those findings prompted us to measure bladder function in GM2+/− mice treated with vehicle or FTY720. Mice with bladder hyperreflexia produce more small spots and fewer large spots as measured using a urinary void spot test (described in Experimental Procedures). We noted that aging GM2+/− mice treated with vehicle had significantly more small spots (Fig. 2A) and significantly fewer large spots (Fig. 2B) than littermate GM2+/− mice treated with FTY720. This suggested that bladder function was significantly improved by FTY720. As an additional measure of bladder health we biochemically evaluated proNGF protein levels in bladders of vehicle and FTY720 treated GM2+/− mice, as proNGF is known to increase along with bladder dysfunction (Ryu et al. 2018). Evaluation of bladder proNGF by immunoblot revealed that FTY720 significantly reduced bladder proNGF, as can be appreciated in Figure 2C (p < 0.05).
Figure 2. FTY720 improves voiding function and reduces proNGF levels in GM2+/− bladder.
The void spot test is a measurement tool in which fewer small spots and more large spots indicate normal bladder function. Levels of proNGF increase with bladder dysfunction. (A) FTY720 treated GM2+/− mice have significantly fewer void spots at 16 mo. (B) FTY720 treated GM2+/− mice have significantly more large void spots at 16 mo. (C) Representative immunoblots from vehicle and FTY720 treated GM2+/− bladder, confirm reduced proNGF levels in GM2+/− mice treated with FTY720. Student’s t test, *, p < .05, **, p < 0.01; ****, p < .0001.
FTY720 reduces pathological aSyn and increases BDNF and myelin associated protein levels in brachial plexus of aging GM2+/− mice
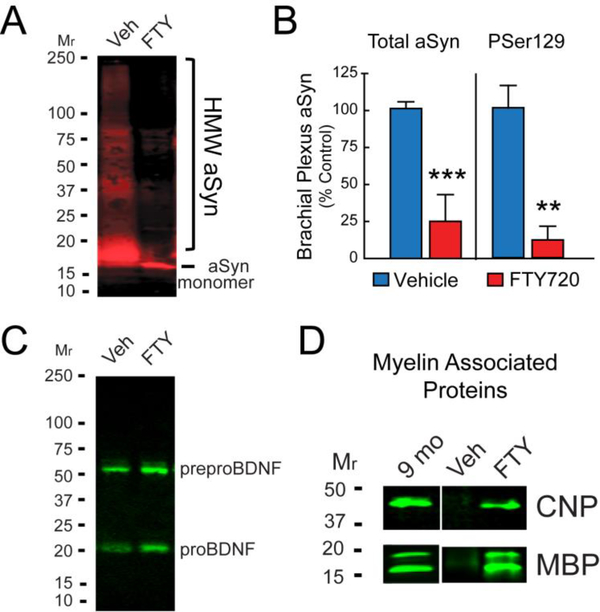
Optic and sciatic nerves of GM2−/− KO mice have abnormal myelination/degeneration (Sheikh et al. 1999) and aSyn has been shown to increase in aging GM2−/− KO and GM2+/− parkinsonian mouse brain (Wu et al. 2012a; Wu et al. 2011). However, no one has evaluated the brachial plexus of GM2+/− mice for synucleinopathy or protection in response to a therapeutic. We chose to do so because brachial plexus is the main innervation of the forelimb that is associated with rotarod and adhesive removal tests, and also contributes to arm swing in humans (Mirelman et al. 2016). We collected brachial plexus, brain, and urinary bladders at necropsy. We assessed aSyn aggregation in GM2+/− brachial plexus by immunoblot and saw abundant aggregated aSyn in vehicle treated GM2+/− mice (Fig. 3A, lane 1). In contrast, brachial plexus of FTY720 treated GM2+/− mice had monomeric aSyn with no high molecular weight bands (Fig. 3A, lane 2). These findings suggest that FTY720 has potent anti-synucleinopathy properties, as previously seen in A53T aSyn mice treated with FTY720 (Vidal-Martinez et al. 2016). Evaluation of multiple brachial plexus samples by dot blot allowed us to quantify total aSyn levels, which was reduced in GM2+/− mice by FTY720 (Fig. 3B, left; Vehicle = 1.01 ± 0.02; FTY720 = 0.25 ± 0.08; p < .0001). As Lewy body aggregates are enriched in PSer129 aSyn we also quantified PSer129 aSyn on dot blots and saw that FTY720 significantly reduced aSyn PSer129 levels in GM2+/− brachial plexus (Fig. 3B, right side; Vehicle = 1.01 ± 0.22; FTY720 = 0.25 ± 0.08; p < .001). We then measured brachial plexus BDNF by immunoblot, and found that FTY720 increased proBDNF and preproBDNF levels in GM2+/− brachial plexus compared to vehicle treated littermates (Fig. 3C). We also measured markers of myelin and axonal integrity, MBP and CNP on brachial plexus by immunoblot. It is known that MBP and CNP proteins decrease in association with aging and/or pathology (Ansari & Loch 1975; Xie et al. 2013; Kuhlmann et al. 2008; Wang et al. 2004). After equal protein loading, we compared MBP and CNP levels in 9 mo GM2+/− brachial plexus as a positive control, to MBP and CNP protein in brachial plexus of vehicle treated GM2+/− mice, which was nearly absent (Fig. 3D). In contrast, FTY720 treated GM2+/− brachial plexus had CNP and MBP levels similar to those seen at 9 mo (Fig. 3D), a time when GM2+/− motor function was relatively normal (Fig. 1A – 1C).
Figure 3. FTY720 reduces synucleinopathy, increases BDNF protein, and sustains MBP and CNP at younger levels in aging GM2+/− brachial plexus.
(A) aSyn immunoblot from representative vehicle (Veh) treated GM2+/− mice shows abundant high molecular weight (HMW) aSyn, which is eliminated in brachial plexus of age matched FTY720 treated GM2+/− mice. (B) Quantification of total aSyn and pathological PSer129 aSyn from multiple brachial plexus samples confirms a significant reduction in synucleinopathy in FTY720 treated GM2+/− mice compared to vehicle treated GM2+/− mice. (C) Reprobing the immunoblot shown in A for BDNF, confirms FTY720 increased preproBDNF and proBDNF levels in brachial plexus, as compared to vehicle treated GM2+/− mice. (D) Myelin associated proteins MBP and CNP are abundant in brachial plexus of untreated 9 mo mouse, absent in 17 mo vehicle treated GM2+/mouse, and sustained in 17 mo GM2+/− mouse treated with FTY720. Student’s t test, **, p < 0.01; ***, p < .001.
Cerebellar BDNF mRNA is also increased in response to FTY720 in GM2+/− mice
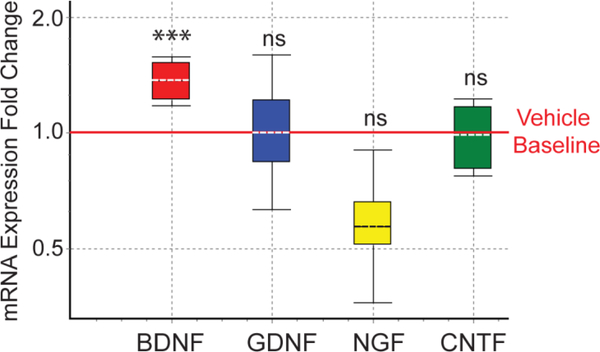
As FTY720 improved movement of GM2+/− mice (Fig. 1A – 1C) we measured neurotrophic factor expression in cerebellum, a brain region that contributes significantly to balance and coordination. Similar to our earlier findings in A53T aSyn transgenic mice treated with FTY720 (Vidal-Martinez et al. 2016), we saw significantly increased BDNF mRNA levels as measured using qPCR. However, GDNF, NGF, and CNTF levels were not changed after oral FTY720 in GM2+/− mice (Fig. 4). This BDNF increase in cerebellum parallels the BDNF increases noted in the brachial plexus of GM2+/− mice (Fig. 3C).
Figure 4. FTY720 increases BDNF mRNA in GM2+/− cerebellum.
Cerebellar qPCR from vehicle treated and FTY720 treated littermates confirm a significant increase in BDNF but not in GDNF, NGF, or CNTF neurotrophic factors. ***, p < .001, ns, not significant.
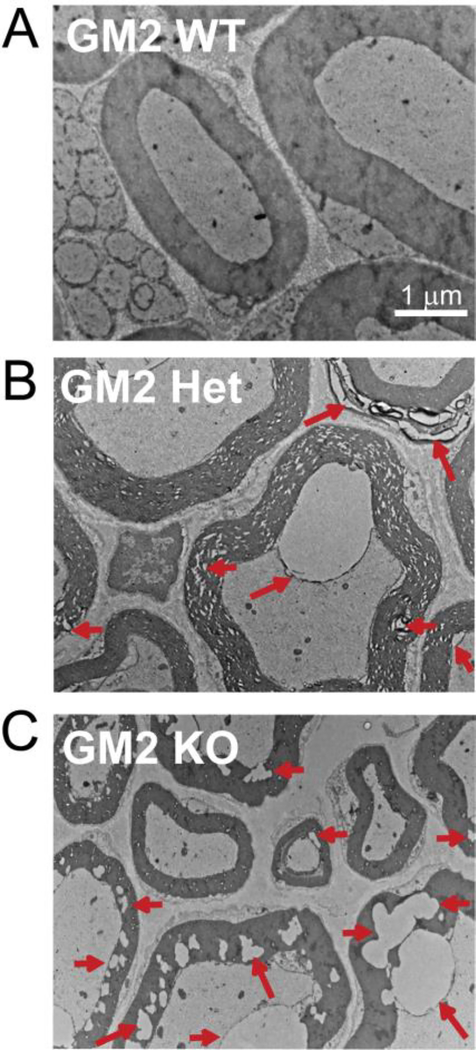
Abnormal brachial plexus myelin and axons in untreated GM2 transgenic mice
We previously demonstrated atypical myelination and axons of GM2−/− KO bladder innervation by transmission electron microscopy (TEM) (Gil-Tommee et al. 2019). As demonstrated above, vehicle treated GM2+/− mice showed a loss of MBP and CNP proteins in brachial plexus (Fig. 3D). Unfortunately, brachial plexus from treated mice was frozen and thus could not be evaluated by TEM. However, no one has ever assessed the brachial plexus of any GM2 mice by TEM. Thus, we obtained GM2 mice from Dr. Mariko Saito (Nathan Kline Institute, Orangeburg, NY) and in collaboration with Dr. Peter Cooke (New Mexico State University, Las Cruces, NM) performed brachial plexus TEM (Fig. 5). This demonstrated that brachial plexus axons and myelin sheaths in WT GM2 mouse are normal (Fig. 5A), in GM2+/− mice are abnormal (Fig. 5B), and even more abnormal in GM2−/− KO mice (Fig. 5C). Although we did not evaluate FTY720 treated GM2+/− brachial plexus by TEM, it is tempting to speculate that FTY720 may have improved its ultrastructure as we did see BDNF, MBP, and CNP levels increase in the nerve.
Figure 5. Brachial plexus myelination and axonal abnormalities in GM2 transgenic mice.
(A) Myelin sheaths are uniform and surround normal axons in the brachial plexus of a wild type (WT) GM2 mouse. (B) Arrows point to areas of abnormal myelin sheaths as well as to atypical axonal changes in the brachial plexus of a GM2+/− mouse. (C) Arrows point to areas of abnormal myelin sheaths and axonal changes in the brachial plexus of a GM2 synthase KO mouse. Scale bar = 1 μm.
DISCUSSION
The World Health Organization lists neurological disorders as a great risk factor facing human health due to both aging and the exponential rise in the world’s population (https://www.who.int/news-room/fact-sheets/detail/ageing-and-health). As the main risk factor for PD is aging, its incidence is approaching epidemic levels. This awareness has prompted a concerted effort by the research community to identify early PD biomarkers and to develop protective therapies that can slow or halt PD progression. With this in mind, we began preclinically evaluating the anti-parkinsonian potential of the FDA approved MS drug, FTY720. Importantly, FTY720 has a long half-life (Kovarik et al. 2004; Meno-Tetang et al. 2006), allowing us to give the drug just 3 times/week, which was well-tolerated and protective. Our findings in this parkinsonian mouse model, GM2+/− mice, along with data from other parkinsonian models support FTY720 repurposing for PD (Ren et al. 2017; Zhao et al. 2017; Vidal-Martinez et al. 2016).
Movement abnormalities are typically the main symptom at PD diagnosis. However, few parkinsonian animal models replicate the age-onset movement problems seen in PD (Dawson et al. 2010; Farrell et al. 2014; Chesselet & Richter 2011; Potashkin et al. 2010). Fortunately, GM2+/− mice develop such motor deficits over time (Wu et al. 2012a; Wu et al. 2011), making it an excellent model for testing prolonged drug treatment. As it is also important to test drugs after symptoms have manifested, we aged our GM2+/− mice then treated them with FTY720 or vehicle from 11 – 16 mo. This allowed us to show that 5 mo of oral FTY720 significantly improved movement as measured by rotarod, adhesive removal and hindlimb reflex tests (Fig. 1). Bladder muscle can also be affected in PD, as previously shown in GM2−/− KO mice, which develop age-onset bladder dysfunction (Gil-Tommee et al. 2019). We measured void patterns of GM2+/− mice and saw that mice treated with FTY720 had more normal void patterns compared to vehicle treated littermates with hyperreflexia (Fig. 2A, 2B). In GM2−/− KO bladder, we found abundant proNGF, a biomarker of bladder dysfunction (Ryu et al. 2018; Gil-Tommee et al. 2019). We therefore measured bladder proNGF by immunoblot in FTY720 and vehicle treated mice, revealing significantly lower levels of proNGF in FTY720 treated GM2+/− mice (Fig. 2C).
Another common motor problem in PD is decreased arm swing, which may serve as a PD diagnostic marker (Mirelman et al. 2016). Brachial plexus modulates arm swing in humans (Souza et al. 2016; Pontzer et al. 2009; Collins et al. 2009), thus we evaluated brachial plexus of aging GM2+/− mice. By immunoblot we saw abundant Lewy-like aSyn aggregation in vehicle treated brachial plexus that was reversed by FTY720 (Fig. 3A). We also found abundant pathological PSer129 aSyn in the brachial plexus of vehicle treated mice that was reversed by FTY720 (Fig. 3B). As myelination problems can affect movement, we measured levels of key myelin associated proteins in GM2+/− brachial plexus, which revealed that FTY720 maintained MBP and CNP at young levels compared to a loss of MBP and CNP in vehicle treated GM2+/− brachial plexus by immunoblot (Fig. 3D).
FTY720 improves function at least in part by increasing BDNF expression in vivo and in vitro (Deogracias et al. 2012; Doi et al. 2013; Smith et al. 2015; Vidal-Martinez et al. 2016; Ren et al. 2017; Segura-Ulate et al. 2017). As brachial plexus showed changes in myelin associated proteins we also measured brachial plexus BDNF on immunoblots. This revealed increased levels of proBDNF and preproBDNF proteins in GM2+/− mice treated with FT720 (Fig. 3C). As cerebellum is a brain region that regulates balance and coordination, we also measured expression of BDNF and other trophic factors in GM2+/− cerebellum by qPCR. While a significant increase in BDNF mRNA was seen in cerebellum of FTY720 treated mice, the levels of GDNF, NGF, and CNTF were not increased after 3x/week FTY720 (Fig. 4). Remarkably, cerebellum also contributes to urinary function (Chou et al. 2013; Sugiyama et al. 2009).
We and others have shown abnormal myelination in nerves in GM2−/− KO mice (Gil-Tommee et al. 2019; Sheikh et al. 1999; Chiavegatto et al. 2000), but no one has assessed any nerves in GM2+/− mice. We saw increased levels of MBP and CNP myelin associated proteins in GM2 +/− brachial plexus in response to FTY720 (Fig. 3D). And though we could not perform TEM from aging studies with FTY720, we obtained GM2 mice in which to assess brachial plexus ultrastructure by TEM. This allowed us to show that WT brachial plexus had normal myelin surrounding healthy axons (Gil-Tommee et al. 2019), while GM2+/− and GM2−/− KO myelination and axons were abnormal (Fig. 5). These findings paralleling findings in brachial plexus for aSyn, MBP, and CNP (Fig. 3), which likely contributed to improved function of GM2+/− mice treated with FTY720.
In summary, low dose oral FTY720 given 3x/week was well-tolerated and increased BDNF expression in parkinsonian GM2+/− mice. FTY720 also reduced pathological Lewy-like aSyn aggregation in brachial plexus. Regarding urinary bladder, FTY720 improved voiding and reduced levels of the bladder dysfunction marker, proNGF. Taken together these preclinical findings in GM2+/− parkinsonian mice provide compelling support for testing FTY720 in patients with PD in hopes of slowing or delaying PD progression.
HIGHLIGHTS.
GM2+/− mice, with reduced GM1 levels, develop age-onset parkinsonian symptoms
Reduced GM1 levels increase GM2+/− synucleinopathy and loss of myelin markers
Low dose FTY720 reduces synucleinopathy in the GM2+/− nervous system
Low dose FTY720 increases BDNF and myelin markers in GM2+/− mice
Movement and bladder function are improved by low dose FTY720 in GM2+/− mice
ACKNOWLEDGEMENTS
We are grateful to Drs. Ledeen, Wu, and Saito for GM2 mice and to Dr. Peter Cooke for TEM. We also thank members of the LARC facility at TTUHSC El Paso for excellent animal care. We are also grateful for support from the: National Institutes of Health BUILDingSCHOLARS Program (to VDP/RGP), Fogarty International Center-U.S./Costa Rica Neuropsychiatric Genetics Research Training Program (NCOD-5D43TW008333) fellowship (to CGT/RGP); the Lizanell and Colbert Coldwell Foundation, El Paso Community Foundation, Perez Family Research and Ms. Ana Mae Doyle Gift Funds (to RGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander GE (2004) Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues in clinical neuroscience 6, 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari KA and Loch J (1975) Decreased myelin basic protein content of the aged human brain. Neurology 25, 1045–1050. [DOI] [PubMed] [Google Scholar]
- Antunes L, Frasquilho S, Ostaszewski M et al. (2016) Similar alpha-Synuclein staining in the colon mucosa in patients with Parkinson’s disease and controls. Movement disorders : official journal of the Movement Disorder Society 31, 1567–1570. [DOI] [PubMed] [Google Scholar]
- Araki I, Kitahara M, Oida T and Kuno S (2000) Voiding dysfunction and Parkinson’s disease: urodynamic abnormalities and urinary symptoms. The Journal of urology 164, 1640–1643. [PubMed] [Google Scholar]
- Aranda PS, LaJoie DM and Jorcyk CL (2012) Bleach gel: A simple agarose gel for analyzing RNA quality. Electrophoresis 33, 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI et al. (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta neuropathologica 119, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Carew J, Serrano G et al. (2014) Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neuroscience letters 571, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris LM, Mata IF and Zabetian CP (2010) The genetics of Parkinson disease. Journal of geriatric psychiatry and neurology 23, 228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsik A, Muselli L, Leboidre M, Lakhdar L and Baron T (2014) Early and persistent expression of phosphorylated alpha-synuclein in the enteric nervous system of A53T mutant human alpha-synuclein transgenic mice. Journal of neuropathology and experimental neurology 73, 1144–1151. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, Perez RG and Manfredsson FP (2016) The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson’s disease. J Neurochem 137, 331–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S et al. (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nature neuroscience 5, 856–860. [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML and Hill WG (2015) Evaluation of voiding assays in mice: impact of genetic strains and sex. American Journal of Physiology - Renal Physiology 308, F1369–F1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V (2012) Autosomal recessive parkinsonism. Parkinsonism & related disorders 18 Suppl 1, S4–6. [DOI] [PubMed] [Google Scholar]
- Bostan A and Strick P (2010) The Cerebellum and Basal Ganglia are Interconnected. Neuropsychol Rev 20, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP and Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A 107, 8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V (2009) FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. British journal of pharmacology 158, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S and Burtin P (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature reviews. Drug discovery 9, 883–897. [DOI] [PubMed] [Google Scholar]
- Chan RB, Perotte AJ, Zhou B et al. (2017) Elevated GM3 plasma concentration in idiopathic Parkinson’s disease: A lipidomic analysis. PLoS ONE 12, e0172348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-C, Ulane CM and Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Annals of neurology 67, 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF and Richter F (2011) Modelling of Parkinson’s disease in mice. Lancet neurology 10. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Sun J, Nelson RJ and Schnaar RL (2000) A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Experimental neurology 166, 227–234. [DOI] [PubMed] [Google Scholar]
- Chou YC, Jiang YH, Harnod T and Kuo HC (2013) Characteristics of neurogenic voiding dysfunction in cerebellar stroke: a cross-sectional, retrospective video urodynamic study. Cerebellum 12, 601–606. [DOI] [PubMed] [Google Scholar]
- Collins SH, Adamczyk PG and Kuo AD (2009) Dynamic arm swinging in human walking. Proceedings. Biological sciences / The Royal Society 276, 3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS and Dawson VL (2010) Genetic animal models of Parkinson’s disease. Neuron 66, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE and Barde YA (2012) Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci U S A 109, 14230–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Story GM, Xu K, Kucera J and Walro JM (1999) Alterations in nigrostriatal dopaminergic function within BDNF mutant mice. Experimental neurology 160, 500–507. [DOI] [PubMed] [Google Scholar]
- Doi Y, Takeuchi H, Horiuchi H et al. (2013) Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS One 8, e61988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell KF, Krishnamachari S, Villanueva E, Lou H, Alerte TN, Peet E, Drolet RE and Perez RG (2014) Non-motor parkinsonian pathology in aging A53T alpha-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J Neurochem 128, 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS and Chesselet MF (2004) Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 9434–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumimura Y, Ikemura M, Saito Y et al. (2007) Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. Journal of neuropathology and experimental neurology 66, 354–362. [DOI] [PubMed] [Google Scholar]
- Gil-Tommee C, Vidal-Martinez G, Annette Reyes C, Vargas-Medrano J, Herrera GV, Martin SM, Chaparro SA and Perez RG (2019) Parkinsonian GM2 synthase knockout mice lacking mature gangliosides develop urinary dysfunction and neurogenic bladder. Experimental neurology 311, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich M, Waknin R, Stone E and Achiron A (2018) Fingolimod-improved axonal and myelin integrity of white matter tracts associated with multiple sclerosis-related functional impairments. CNS neuroscience & therapeutics 24, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker ML, DeLong MR, Turchan M et al. (2018) Effects of deep brain stimulation on rest tremor progression in early stage Parkinson disease. Neurology 91, e463–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Wu G, Sharma N, Ciesielska A, Bankiewicz K, Davidow AL, Lu ZH, Forsayeth J and Ledeen RW (2015) GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Experimental neurology 263, 177–189. [DOI] [PubMed] [Google Scholar]
- Hamill RW, Tompkins JD, Girard BM, Kershen RT, Parsons RL and Vizzard MA (2012) Autonomic dysfunction and plasticity in micturition reflexes in human alpha-synuclein mice. Developmental neurobiology 72, 918–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G and Boggs JM (2013) Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J Neurochem 125, 334–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SJ, Zhou G, Deng FM et al. (2008) Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin II knockout mice. The Journal of urology 179, 2046–2051. [DOI] [PubMed] [Google Scholar]
- Jain S (2011) Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism & related disorders 17, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik JM, Schmouder R, Barilla D, Wang Y and Kraus G (2004) Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. British journal of clinical pharmacology 57, 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Lassmann H and Brück W (2008) Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta neuropathologica 115, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR and Nave KA (2003) Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature genetics 33, 366–374. [DOI] [PubMed] [Google Scholar]
- Lek S, Vargas-Medrano J, Villanueva E, Marcus B, Godfrey W and Perez RG (2017) Recombinant alpha- beta- and gamma-Synucleins Stimulate Protein Phosphatase 2A Catalytic Subunit Activity in Cell Free Assays. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Montoya SE, Alerte TN et al. (2010) Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem 285, 17648–17661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Valbuena I, Amat-Villegas I, Valenti-Azcarate R, Carmona-Abellan MDM, Marcilla I, Tunon MT and Luquin MR (2018) Interaction of amyloidogenic proteins in pancreatic beta cells from subjects with synucleinopathies. Acta neuropathologica 135, 877–886. [DOI] [PubMed] [Google Scholar]
- Martinez Z, Zhu M, Han S and Fink AL (2007) GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry 46, 1868–1877. [DOI] [PubMed] [Google Scholar]
- Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P and Jusko WJ (2006) Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(−4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug metabolism and disposition: the biological fate of chemicals 34, 1480–1487. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Bernad-Elazari H, Thaler A et al. (2016) Arm swing as a potential new prodromal marker of Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 31, 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I (2005) Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cellular and Molecular Life Sciences CMLS 62, 2283–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I and Bachis A (2004) Brain-derived neurotrophic factor activation of TrkB protects neurons from HIV-1/gp120-induced cell death. Crit Rev Neurobiol 16, 51–57. [DOI] [PubMed] [Google Scholar]
- Mor DE and Ischiropoulos H (2018) The Convergence of Dopamine and α-Synuclein: Implications for Parkinson’s Disease. Journal of Experimental Neuroscience 12, 1179069518761360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468, 244–252. [DOI] [PubMed] [Google Scholar]
- Oertel WH (2017) Recent advances in treating Parkinson’s disease. F1000Research 6, 260–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi Y, Ohkawa Y, Tajima O, Sugiura Y, Furukawa K and Furukawa K (2014) Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord. Journal of neuroinflammation 11, 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuno-Lizaran I, Beach TG, Serrano GE, Walker DG, Adler CH and Cuenca N (2018) Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Movement disorders : official journal of the Movement Disorder Society 33, 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oueslati A (2016) Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? Journal of Parkinson’s disease 6, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmano K, Rowan A, Guillermo R, Guan J and Mc Jarrow P (2015) The Role of Gangliosides in Neurodevelopment. Nutrients 7, 3891–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L and Perez RG (2005) Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci 118, 3523–3530. [DOI] [PubMed] [Google Scholar]
- Perez RG and Hastings TG (2004) Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J Neurochem 89, 1318–1324. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F and Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 3090–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti D and Neviani P (2008) Protein phosphatase 2A (PP2A), a drugable tumor suppressor in Ph1(+) leukemias. Cancer metastasis reviews 27, 159–168. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW and Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic acids research 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H, Holloway J. H. t., Raichlen DA and Lieberman DE (2009) Control and function of arm swing in human walking and running. The Journal of experimental biology 212, 523–534. [DOI] [PubMed] [Google Scholar]
- Porras JL and Perez RG (2014) Potential contribution of alpha-synuclein dysregulation to Parkinson’s disease pathology In: Nova Publishers - Alpha-Synuclein, (Kanowitz M. P. a. H. C. ed.), pp. 51–70. NOVA Science Publishers, NY. [Google Scholar]
- Posse de Chaves E and Sipione S (2010) Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS letters 584, 1748–1759. [DOI] [PubMed] [Google Scholar]
- Potashkin JA, Blume SR and Runkle NK (2010) Limitations of animal models of Parkinson’s disease. Parkinson’s disease 2011, 658083–658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Han M, Wei X, Guo Y, Shi H, Zhang X, Perez RG and Lou H (2017) FTY720 Attenuates 6-OHDA-Associated Dopaminergic Degeneration in Cellular and Mouse Parkinsonian Models. Neurochemical research 42, 686–696. [DOI] [PubMed] [Google Scholar]
- Ryu JC, Tooke K, Malley SE et al. (2018) Role of proNGF/p75 signaling in bladder dysfunction after spinal cord injury. The Journal of clinical investigation 128, 1772–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M (2014) Fingolimod: a review of its use in relapsing-remitting multiple sclerosis. Drugs 74, 1411–1433. [DOI] [PubMed] [Google Scholar]
- Schnaar RL (2010) Brain gangliosides in axon-myelin stability and axon regeneration. FEBS letters 584, 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Roeltgen DP, Mancall EL, Chapas-Crilly J, Rothblat DS and Tatarian GT (1998) Parkinson’s disease: improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology 50, 1630–1636. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Sendek S, Daskalakis C and Cambi F (2010) GM1 ganglioside in Parkinson’s disease: Results of a five year open study. Journal of the neurological sciences 292, 45–51. [DOI] [PubMed] [Google Scholar]
- Segura-Ulate I, Yang B, Vargas-Medrano J and Perez RG (2017) FTY720 (Fingolimod) reverses alpha-synuclein-induced downregulation of brain-derived neurotrophic factor mRNA in OLN-93 oligodendroglial cells. Neuropharmacology 117, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW and Schnaar RL (1999) Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci U S A 96, 7532–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Dubost V, Brinkmann V, Jivkov M, Huck C and Theil D (2015) Fingolimod Up-Regulates BDNF Expression within the CNS And Down-Regulates Tissue Pathology in a Pre-Clinical Model of Chronic Neuroinflammation (P1.159). Neurology 84. [Google Scholar]
- Souza L, Lemos T, Silva DC, de Oliveira JM, Guedes Corrêa JF, Tavares PL, Oliveira LA, Rodrigues EC and Vargas CD (2016) Balance Impairments after Brachial Plexus Injury as Assessed through Clinical and Posturographic Evaluation. Frontiers in human neuroscience 9, 715–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard SL (1994) The adrenal medulla and Parkinson’s disease. Reviews in the neurosciences 5, 293–307. [DOI] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S et al. (2008) Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. Journal of molecular neuroscience : MN 36, 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill ER, Aoki K, Lopez PH et al. (2012) Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 22, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Sakakibara R, Tsunoyama K, Takahashi O, Kishi M, Ogawa E, Terada H and Tomaru T (2009) Cerebellar Ataxia and Overactive Bladder after Encephalitis Affecting the Cerebellum. Case reports in neurology 1, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranian R, Montoya SE, Van Laar AD, Hastings TG and Perez RG (2006) Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem 99, 1188–1196. [DOI] [PubMed] [Google Scholar]
- Turner RS and Desmurget M (2010) Basal ganglia contributions to motor control: a vigorous tutor. Current opinion in neurobiology 20, 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Medrano J, Krishnamachari S, Villanueva E, Godfrey WH, Lou H, Chinnasamy R, Arterburn JB and Perez RG (2014) Novel FTY720-based compounds stimulate neurotrophin expression and phosphatase activity in dopaminergic cells. ACS Med Chem Lett 5, 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Martinez G, Vargas-Medrano J, Gil-Tommee C, Medina D, Garza NT, Yang B, Segura-Ulate I, Dominguez SJ and Perez RG (2016) FTY720/Fingolimod reduces synucleinopathy and improves gut motility in A53T Mice: Contributions of pro-brain-derived neurotrophic factor (Pro-BDNF) and mature BDNF. J Biol Chem 291, 20811–20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas AA and Schnaar RL (2001) Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration. Biochimie 83, 677–682. [DOI] [PubMed] [Google Scholar]
- Wang DS, Bennett DA, Mufson EJ, Mattila P, Cochran E and Dickson DW (2004) Contribution of changes in ubiquitin and myelin basic protein to age-related cognitive decline. Neuroscience research 48, 93–100. [DOI] [PubMed] [Google Scholar]
- Wang J, Lou H, Pedersen CJ, Smith AD and Perez RG (2009) 14–3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: localization of dopamine regulatory proteins to mitochondria. J Biol Chem 284, 14011–14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA and Giasson BI (2008) Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. Journal of neuropathology and experimental neurology 67, 402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK and Patrie J (2004) Are men at greater risk for Parkinson’s disease than women? Journal of Neurology, Neurosurgery & Psychiatry 75, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Kulkarni N, Amin R and Ledeen RW (2011) Mice lacking major brain gangliosides develop parkinsonism. Neurochemical research 36, 1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Kulkarni N and Ledeen RW (2012a) Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. Journal of neuroscience research 90, 1997–2008. [DOI] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu ZH and Ledeen RW (2001) Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc Natl Acad Sci U S A 98, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lou H, Alerte TN, Stachowski EK, Chen J, Singleton AB, Hamilton RL and Perez RG (2012b) Lewy-like aggregation of alpha-synuclein reduces protein phosphatase 2A activity in vitro and in vivo. Neuroscience 207, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T and Hallett M (2013) The cerebellum in Parkinson’s disease. Brain : a journal of neurology 136, 696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Zhang JC, Fu H and Chen J (2013) Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat CNS. International journal of molecular medicine 32, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Yazdi A, Baharvand H and Javan M (2015) Enhanced remyelination following lysolecithin-induced demyelination in mice under treatment with fingolimod (FTY720). Neuroscience 311, 34–44. [DOI] [PubMed] [Google Scholar]
- Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG and Zeidel ML (2014) Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. American Journal of Physiology - Renal Physiology 306, F1296–F1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Yang X, Yang L, Li M, Wood K, Liu Q and Zhu X (2017) Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 31, 172–179. [DOI] [PubMed] [Google Scholar]