Abstract
Circulating levels of adiponectin, an adipocyte-secreted protein associated with cardiovascular and metabolic risk, are highly heritable. To gain insights into the biology that regulates adiponectin levels, we performed an exome array meta-analysis of 265,780 genetic variants in 67,739 individuals of European, Hispanic, African American, and East Asian ancestry. We identified 20 loci associated with adiponectin, including 11 that had been reported previously (p < 2 × 10−7). Comparison of exome array variants to regional linkage disequilibrium (LD) patterns and prior genome-wide association study (GWAS) results detected candidate variants (r2 > .60) spanning as much as 900 kb. To identify potential genes and mechanisms through which the previously unreported association signals act to affect adiponectin levels, we assessed cross-trait associations, expression quantitative trait loci in subcutaneous adipose, and biological pathways of nearby genes. Eight of the nine loci were also associated (p < 1 × 10−4) with at least one obesity or lipid trait. Candidate genes include PRKAR2A, PTH1R, and HDAC9, which have been suggested to play roles in adipocyte differentiation or bone marrow adipose tissue. Taken together, these findings provide further insights into the processes that influence circulating adiponectin levels.
Keywords: adiponectin, genetics, genome-wide association study, obesity, cardio metabolic traits, lipids, exome
Introduction
Adiponectin is an adipose tissue-derived hormone that affects energy homeostasis and may link adiposity with the risk of type 2 diabetes (T2D), hyperinsulinemia, and metabolic syndrome.1, 2 Higher serum concentrations of adiponectin are associated with protection against inflammation and lower risk for obesity, cardiovascular disease, and T2D.1, 3, 4 Genetic overlap has been observed between adiponectin levels and metabolic syndrome, as well as the metabolic syndrome-related traits of high-density lipoprotein (HDL) cholesterol and plasma insulin.5, 6 Mendelian randomization (MR) studies have suggested a causal role for adiponectin in metabolic syndrome and insulin sensitivity, but not coronary artery disease, insulin resistance, or T2D.7, 8, 9, 10 Experimental manipulations in mice have demonstrated that acute depletion of adiponectin results in systemic insulin resistance and poor lipid homeostasis, leading to hyperlipidemia.11, 12, 13 Thus, understanding the pathophysiology of adiponectin levels may lead to therapeutic targets for the prevention and treatment of cardiovascular disease.2, 14, 15
Circulating levels of adiponectin are under substantial genetic influence. Twin and family studies have estimated that 30%–70% of the variability in adiponectin levels can be explained by genetic variation.16, 17, 18 The largest adiponectin genome-wide association study (GWAS) meta-analysis performed to date tested associations between ∼2.4 million variants, imputed to the HapMap reference panel, in up to 35,355 individuals primarily of European ancestry (83%) and identified 12 loci, including ADIPOQ (MIM: 605441), which encodes the adiponectin protein, and CDH13 (MIM: 601364), which encodes cadherin-13, a receptor that binds to circulating adiponectin.14 A subsequent adiponectin GWAS meta-analysis in up to 18,079 East Asians, using HapMap-imputed variants, identified one additional locus near WDR11-FGFR2 (MIM: 606417, 176943).19 These common genetic variants accounted for only 5% of the variance in adiponectin levels.14, 19, 20 Further investigation into the genetic basis for adiponectin levels and the associated loci would provide further insight into the genetic architecture and biological processes influencing adiponectin.
To discover and characterize candidate loci influencing circulating levels of adiponectin in order to further understand its role in insulin sensitivity and related traits, we combined exome array association results of up to 67,739 individuals from four ancestries (60,465 European, 3,271 African, 2,568 East Asian, and 1,435 Hispanics) to conduct the largest genetic analysis of adiponectin levels to date. Exome-based association studies, containing mostly nonsynonymous variants (n = 213,082, 80.2% of all variants in the analysis), may potentially provide results with more direct biological interpretability. However, nonsynonymous variant associations do not constitute sufficient evidence to implicate the target gene(s) underlying an association signal.21 Therefore, we used extent of linkage disequilibrium (LD), gene expression-variant association data, and pathway analyses to suggest genes and mechanisms through which the previously unreported variants may act to affect adiponectin levels.
Material and Methods
Study Design and Participants
Our meta-analysis consisted of 25 studies (28 datasets) comprising up to 67,739 adult individuals (≥18 years) of the following ancestries: (1) European (n ≤ 60,465), (2) East Asian (n ≤ 2,568), (3) African American (n ≤ 3,271), and (4) Hispanic (n ≤ 1,435). All participating institutions and coordinating centers approved this project, and informed consent was obtained from all study participants. In our primary discovery analyses, we combined data of all ancestries for both sex-specific and sex-combined analyses (Figure S1). Study-specific design, sample quality control, and descriptive statistics are provided in Tables S1–S3.
Genotype Calling
Each of the 25 contributing studies followed a standardized protocol and performed genotype calling of variants on the Illumina HumanExome Beadchip using the designated manufacturer’s software. Study-specific quality control measures of the genotyped variants were implemented before association analyses (Table S2). We excluded variants with a call rate < 98%, Hardy Weinberg equilibrium p < 1 × 10−6, or large allele frequency deviations from the reference populations (>0.60 for all ancestry analyses and >0.30 for ancestry-specific population analyses).
Statistical Analyses
We created residuals of adiponectin levels (in μg/mL) after adjustment for age, body mass index (BMI), and principal components (PCs, derived from GWAS data, variants with minor allele frequency [MAF] > 1% on the exome array, or ancestry-informative markers on the exome array), as well as any study-specific covariates, using a linear regression model. For studies with unrelated individuals, residuals were calculated separately by sex; for family-based studies, sex was included as a covariate in the model. Additionally, residuals for case-control studies were calculated in case subjects and control subjects separately. Finally, residuals were subjected to inverse normal transformation to obtain distributions with a mean of 0 and a standard deviation of 1, yielding approximately normally distributed trait values for downstream analyses. In additional analyses performed without adjustment for BMI and adjusted for fat percentage instead of BMI, results were similar (Figures S2–S5, Table S4) with one additional locus detected in models unadjusted for BMI (rs900399 in LEKR1 [MIM: 613536]); other signals became less significant.
Each study performed single-variant association analyses between inverse normal-transformed adiponectin levels (adjusted for age, sex, BMI, and other study-specific covariates) and exome array variants, including up to 28,868 common (MAF ≥ 5%) and 236,912 low-frequency (1% ≤ MAF < 5%) or rare (MAF < 1%) variants, using either RAREMETALWORKER or RVTEST (Table S2).22, 23 All analyses were performed in sex-combined and sex-specific groups, accounting for potential cryptic relatedness (kinship matrix), in a linear mixed model.
We applied a centralized quality control procedure to individual cohort association summary statistics using EasyQC24 to: (1) assess adiponectin transformation, (2) compare allele frequency alignment to the 1000 Genomes Project phase 1 reference panel to detect strand errors, and (3) examine quantile-quantile plots per study to detect population stratification, cryptic relatedness, and genotype biases.
Summary statistics were meta-analyzed using fixed-effects inverse-variance analyses in an all-ancestry, sex-combined, additive model, and variant associations that reached p < 2 × 10−7 were considered array-wide significant (Bonferroni corrected for multiple testing; 0.05/265,780 variants).21, 25, 26 Meta-analyses were carried out using RAREMETAL27 by two analysts at two sites in parallel. For all significant variants identified in the meta-analyses, we tested for differences between women-specific and men-specific beta estimates using EasyStrata.28 Each variant that reached psexhet < 0.05/(number of variants tested) was considered significant. In secondary analyses, we also performed analyses with adiponectin levels unadjusted for BMI and with adiponectin levels adjusted for fat percentage instead of BMI, as well as analyses stratified by sex (Figures S2–S8).
For gene-based analyses, we applied the sequence kernel association test (SKAT)29 and variable threshold (VT)30 gene-based methods in RAREMETAL using two different sets of criteria (broad and strict).26, 31 These two sets of criteria were used to select variants with a MAF < 5% within each ancestry group based on coding variant annotation from five prediction algorithms (PolyPhen2, HumDiv and HumVar, LRT, MutationTaster, and SIFT). The broad gene-based tests comprised of nonsense, stop-loss, splice site, and missense variants annotated as damaging by at least one algorithm mentioned above. The strict gene-based tests included only nonsense, stop-loss, splice site, and missense variants annotated as damaging by all five algorithms. Statistical significance for gene-based tests was set at a Bonferroni-corrected threshold of p < 2.5 × 10−6 based on approximately 20,000 genes.32 As secondary analyses were nested and/or highly correlated with the primary analysis, we chose the same stringent Bonferroni-corrected significance threshold for all analyses.
We also used the RAREMETAL R package to identify independent association signals across all ancestries and European (sex-combined and sex-specific) meta-analysis results. In 1 Mb windows centered on each lead (most significantly associated) variant, we performed sequential rounds conditioning on the lead variant to identify additional signals at p < 2 × 10−7.
Assessment for Bias
To address possible collider bias33, 34 in our significant association results, we used the summary statistics from an exome-chip study of BMI, a GWAS of body fat percentage, and our adiponectin results from models unadjusted for BMI or fat percentage.26 Our principal aim was to exclude the possibility that some of the discovered adiponectin-SNP associations are entirely driven by the association of the SNPs and obesity traits correlated with adiponectin levels. To this end, BMI and fat percentage-adjusted associations were corrected for potential bias due to phenotypic correlation between adiponectin levels and each covariate as well as possible association between each variant and covariate. Strength and significance of association with adiponectin unadjusted, adjusted for BMI, or adjusted for fat percentage were compared with each other, and those of covariates and corrected statistics. Corrected effect sizes were estimated using the following equation:33
where ρBMI x adiponectin = −0.31 and ρfat percent x adiponectin = −0.27.35
Associations with Cardiometabolic Traits
We evaluated each of the nine previously unreported loci that we detect here for association in prior adiponectin GWAS meta-analyses from ADIPOGen14 and AGEN19 and with other cardiometabolic traits and diseases. For these traits, we examined existing genome- and exome-wide meta-analysis results from consortia including GIANT (BMI26 and waist-to-hip-ratio adjusted for BMI36), GLGC (total cholesterol, HDL, LDL, and triglycerides),25 T2D,21 and MAGIC (HbA1c, fasting insulin, fasting glucose, 2-hour glucose).37, 38, 39, 40 We also examined the UK Biobank for associations with cardiometabolic traits and diseases. In addition, PhenoScanner was used to perform a PheWAS on all available traits, plasma proteins, and metabolites. We report proteins and metabolites when the lead exome variant and the variant most strongly associated with the protein or metabolite within 1 Mb exhibit high pairwise LD (r2 > 0.80). We did not identify any plasma proteins that met our criteria.
Annotation and Colocalization of Association Signals with eQTL Data
We examined whether any of the lead exome-wide associated variants were associated with expression (FDR < 1% for METSIM for rigor; FDR < 5% for GTEx as available) of nearby transcripts located within 1 Mb in subcutaneous or visceral adipose tissue (eQTLs).41, 42 Briefly, gene expression was measured in subcutaneous adipose tissue in 770 Finnish male participants from the Metabolic Syndrome in Men Study (METSIM) using the Affymetrix U219 microarray. From GTEx v7, we used gene expression data from 385 subcutaneous and 315 visceral adipose samples. Variant-trait and eQTL signals may colocalize when the lead exome variant and the variant most strongly associated with the expression level of the corresponding transcript (eSNP) exhibit high pairwise LD (r2 > 0.80). For such signals identified in the METSIM eQTL data, we also further assessed colocalization using reciprocal conditional analyses to test the association between the lead exome variant and transcript level when the eSNP was also included in the model, and vice versa; we reported exome-wide and eQTL signals as colocalized if the association for the eSNP was not significant (FDR > 1%) when conditioned on the adiponectin-associated exome variant.
Gene Set Enrichment Analysis
We performed a gene set enrichment analysis based on the European summary statistics of variants from the adiponectin adjusted for BMI association results using EC-DEPICT. EC-DEPICT, designed for use in exome array analyses,26, 43 assesses enrichment for a group of 14,462 gene sets (including canonical pathways, protein-protein interaction networks, and mouse phenotypes) that have been “reconstituted” using large-scale co-expression data. Briefly, enrichment p values were calculated using 2,000 permutations of 378,141 European samples from the UK Biobank, where only variants present on both the ExomeChip and UK Biobank arrays were included for analysis.26, 44 The input data included genes directly containing the most significant nonsynonymous coding variant per locus, where loci were defined as ±1 Mb from the index variant. We performed EC-DEPICT analyses using variants with an association of p < 5 × 10−4 across (1) all variants and (2) variants with MAF < 5%. We considered a false discovery rate of less than 0.05 to be statistically significant.
We also performed a pathway analysis by applying PASCAL to the European exome-wide summary statistics from the adiponectin (adjusted for BMI) association results.45 The method derives gene-based scores and subsequently tests for over-representation of high gene scores in pre-defined biological pathways. For the gene-based scores we performed both MAX and SUM estimations: the former being more powerful when the association is driven by a single variant in/near the focal gene, while the latter is preferred in case of allelic heterogeneity. We used both standard pathway libraries and dichotomized (Z-score > 3) reconstituted gene sets from DEPICT. A reference dataset from UK10K (TwinsUK46 and ALSPAC47 studies, n = 3,781) was used for LD estimation. Unlike EC-DEPICT, p value thresholds were not used to select input variants from the exome-wide data. Variants with MAF < 1% in the reference dataset were included in the analysis. To separate the contribution of coding (nonsynonymous and synonymous) variants, we performed PASCAL analyses across (1) all variants and (2) only coding variants. p < 5 × 10−5 was considered statistically significant after Bonferroni correction for multiple testing with 1,000 independent pathways.
Results
We conducted meta-analyses of single-variant association summary statistics, including data from up to 67,739 individuals from 25 studies (Tables S1–S3) across four ethnic groups; 89% of individuals had European ancestry. Each study performed single-variant association analyses adiponectin adjusted for age, sex, and BMI and exome array variants, and summary statistics from each dataset were meta-analyzed using fixed-effects analyses in an all-ancestry, sex-combined, additive model.21, 25, 26 In secondary analyses, we also examined adiponectin levels unadjusted for BMI, adiponectin levels adjusted for fat percentage instead of BMI, and models stratified by sex (Figures S2–S8). Participants were broadly representative of adults (age 18–95 years) of both sexes (51.2% male) with a wide distribution of BMI and adiponectin levels (Table S3).
Association Results
The primary sex-combined analysis of adiponectin levels adjusted for BMI identified variants (p < 2.0 × 10−7) at 11 known loci (in/near LYPLAL1 [MIM: 616548], IRS1 [MIM: 147545], STAB1 [MIM: 608560], ADIPOQ, ARL15 [MIM: unknown]/FST [MIM: 136470], PDE3A [MIM: 123805], CLIP1 [MIM: 179838], DNAH10 [MIM: 605884], CMIP [MIM: 610112], CDH13, and PEPD [MIM: 613230]) and 8 loci (in/near KIF9 [MIM: 607910], DALRD3 [MIM: unknown], FAM13A [MIM: 613299], SLC39A8 [MIM: 608732], SNX13 [MIM: 606589], GIMAP7 [MIM: 616961], RIC8B [MIM: 609147], and SLC38A8 [MIM: 615585]) (Tables 1 and S5; Figures 1, 2, S7, and S9). Lead variants at each locus were primarily common (MAF ≥ 5%), with the exception of one low-frequency variant at SLC39A8 (rs13107325; MAF = 4.7%) and one rare variant at SLC38A8 (rs145119400; MAF = 0.5%). An all-ancestry, women-only analysis identified an additional locus (rs1199354, OPLAH [MIM: 614243], MAF = 10.1%) that also exhibited significant sex heterogeneity (p = 2.5 × 10−5; Table 1; Figure S10). Low to moderate levels of heterogeneity were detected among the lead variants at each locus (Table S5). We further tested the association of adiponectin levels with aggregated variants within each gene by performing gene-based association tests; however, no additional loci were detected (Table S6).
Table 1.
Adiponectin Loci in Sex-Combined and Sex-Specific Meta-analyses (Additive, adjBMI)
| Variant | Chr | Position | Nearest Gene | Annotation | EA | EAF |
Sex-Combined |
Women |
Men |
Sex Diff. |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | p | n | Effect | p | n | Effect | p | n | pa | |||||||
| Loci Achieving Exome-wide Significance | ||||||||||||||||
| rs2276853 | 3 | 47,282,303 | KIF9 | missense (p.Arg638Gly) | A | 0.594 | −0.033 | 3.35E−08 | 65,521 | −0.039 | 1.98E−06 | 31,788 | −0.029 | 4.18E−04 | 33,733 | 4.17E−01 |
| rs3087866 | 3 | 49,054,692 | DALRD3 | missense (p.Gln299Arg) | C | 0.786 | −0.040 | 2.14E−08 | 65,521 | −0.034 | 5.88E−04 | 31,788 | −0.046 | 3.67E−06 | 33,733 | 3.86E−01 |
| rs13133548 | 4 | 89,740,128 | FAM13A | intronic | A | 0.489 | −0.039 | 5.91E−12 | 65,521 | −0.033 | 3.24E−05 | 31,788 | −0.041 | 1.98E−07 | 33,733 | 4.61E−01 |
| rs13107325 | 4 | 103,188,709 | SLC39A8 | missense (p.Ala391Thr) | T | 0.047 | 0.072 | 1.05E−07 | 59,606 | 0.078 | 1.13E−05 | 31,788 | 0.050 | 1.25E−02 | 33,733 | 2.98E−01 |
| rs10282707 | 7 | 17,911,038 | SNX13 | intronic | T | 0.383 | −0.040 | 1.96E−10 | 56,826 | −0.024 | 1.07E−02 | 24,421 | −0.053 | 3.44E−10 | 32,405 | 2.00E−02 |
| rs3735080 | 7 | 150,217,309 | GIMAP7 | missense (p.Arg83Cys) | T | 0.245 | −0.049 | 7.70E−13 | 65,521 | −0.036 | 2.72E−04 | 31,788 | −0.057 | 6.22E−10 | 33,733 | 1.05E−01 |
| rs11993554 | 8 | 145,111,529 | OPLAH | synonymous (p.Phe612Phe) | G | 0.101 | 0.030 | 3.75E−03 | 61,431 | 0.076 | 9.76E−08 | 29,427 | −0.011 | 4.66E−01 | 32,004 | 2.52E−05 |
| rs10861661 | 12 | 107,174,646 | RIC8B | intronic | C | 0.229 | −0.040 | 1.75E−09 | 59,489 | −0.035 | 4.74E−04 | 27,455 | −0.046 | 3.17E−06 | 32,034 | 4.42E−01 |
| rs145119400 | 16 | 84,075,593 | SLC38A8 | missense (p.Pro57Leu) | A | 0.005 | −0.300 | 8.95E−13 | 59,107 | −0.306 | 1.78E−03 | 28,412 | −0.296 | 1.80E−10 | 30,695 | 9.23E−01 |
| Previously Reported Loci Achieving Exome-wide Significance | ||||||||||||||||
| rs2791552 | 1 | 219,652,033 | LYPLAL1 | intergenic | C | 0.676 | 0.676 | 1.75E−14 | 55,387 | −0.055 | 8.97E−09 | 26,391 | −0.054 | 7.16E−09 | 28,996 | 9.68E−01 |
| rs2943641 | 2 | 227,093,745 | IRS1 | intergenic | C | 0.646 | 0.646 | 1.76E−21 | 65,521 | −0.048 | 2.21E−08 | 31,788 | −0.069 | 2.56E−16 | 33,733 | 7.30E−02 |
| rs13303 | 3 | 52,558,008 | STAB1 | missense (p.Met2506Thr) | C | 0.569 | 0.569 | 2.69E−21 | 61,431 | 0.061 | 8.28E−13 | 29,427 | 0.060 | 4.74E−12 | 32,004 | 9.53E−01 |
| rs17366568 | 3 | 186,570,453 | ADIPOQ | ncRNA/exonic | A | 0.118 | 0.118 | 1.43E−145 | 51,153 | −0.216 | 1.61E−64 | 31,788 | −0.239 | 5.86E−89 | 25,849 | 1.97E−01 |
| rs4311394 | 5 | 53,300,662 | ARL15 | intronic | G | 0.257 | 0.257 | 1.68E−10 | 59,931 | −0.046 | 1.54E−06 | 28,914 | −0.044 | 3.84E−06 | 31,017 | 9.13E−01 |
| rs7134375 | 12 | 20,473,758 | PDE3A | intergenic | A | 0.410 | 0.410 | 3.69E−20 | 65,521 | 0.054 | 2.88E−11 | 31,788 | 0.053 | 5.36E−11 | 33,733 | 9.14E−01 |
| rs11057405 | 12 | 122,781,897 | CLIP1 | intronic | A | 0.086 | 0.086 | 2.90E−14 | 65,521 | −0.092 | 1.74E−10 | 31,788 | −0.068 | 1.24E−06 | 33,733 | 2.29E−01 |
| rs11057353 | 12 | 124,265,687 | DNAH10 | missense (p.Ser167Pro) | C | 0.645 | 0.645 | 2.74E−19 | 65,521 | 0.061 | 3.47E−13 | 31,788 | 0.047 | 2.19E−08 | 33,733 | 2.17E−01 |
| rs2925979 | 16 | 81,534,790 | CMIP | intronic | C | 0.693 | 0.693 | 1.38E−37 | 61,801 | 0.093 | 6.72E−26 | 29,767 | 0.072 | 1.91E−16 | 32,034 | 8.80E−02 |
| rs3865188 | 16 | 82,650,717 | CDH13 | intergenic | T | 0.472 | 0.472 | 1.14E−08 | 59,420 | −0.042 | 7.28E−07 | 28,616 | −0.027 | 1.11E−03 | 30,804 | 2.27E−01 |
| rs4805885 | 19 | 33,906,123 | PEPD | intronic | C | 0.603 | 0.603 | 2.85E−25 | 61,431 | 0.076 | 4.63E−19 | 29,427 | 0.052 | 5.77E−28 | 32,004 | 5.20E−02 |
Loci achieving exome-wide significance (p < 2E−07) in sex-combined and/or sex-specific all-ancestry meta-analyses. Beta effect size from an additive model corresponding to the effect allele for inverse-normal transformed adiponectin levels adjusted for age, BMI, and other study-specific covariates. The smallest p value for each variant is shown in bold. Physical positions based on hg19. Abbreviation: Chr, chromosome; EA, effect allele; EAF, effect allele frequency; n, sample size.
Test for sex difference; values significant at the table-wide Bonferroni threshold of 0.05/20 = 2.5E−03
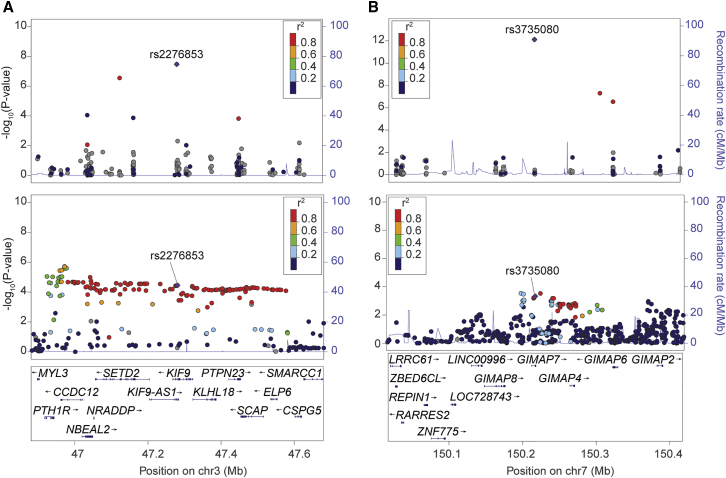
Figure 1.
Loci in Adiponectin Exome-wide Meta-analysis Located within Broader Trait-Associated Regions
KIF9 locus (A) and GIMAP7 locus (B). Each point represents a variant in the meta-analysis, plotted with hg19 genomic position on the x axis and p value (on a −log10 scale) on the y axis. The upper plots show the current exome-wide meta-analysis, and the lower plots show the genome-wide ADIPOGen consortium meta-analysis.14 In each plot, the lead variant identified in the exome array meta-analysis is represented in purple, and the color of all other variants indicate the LD with the lead variant in European ancestry haplotypes from the 1000 Genomes Phase 3 reference panel. In both examples, the lead variant from the exome-wide analysis may not be the best representative of the adiponectin-associated signal.
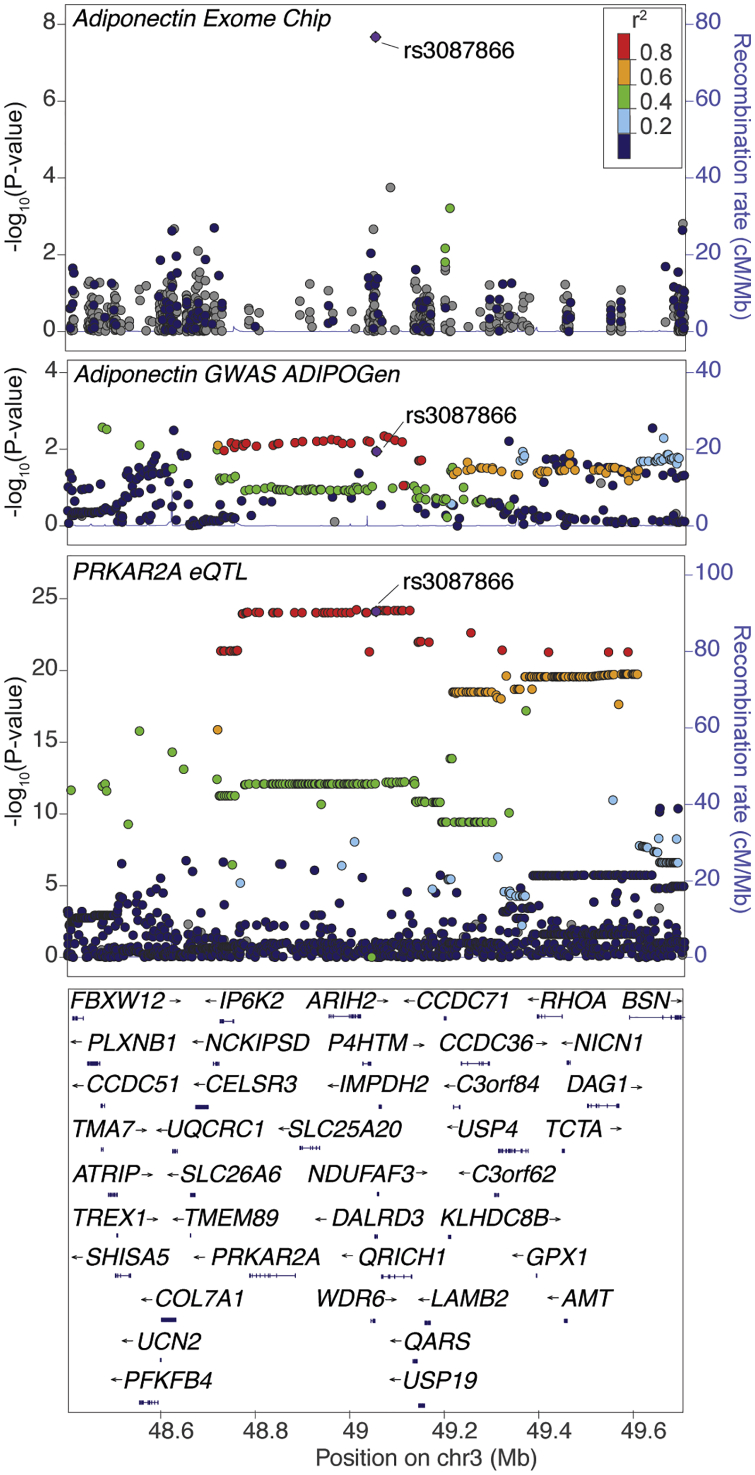
Figure 2.
Subcutaneous Adipose eQTL for PRKAR2A Colocalizes with the DALRD3 Adiponectin Exome-wide Locus
rs3087866 (purple diamond) shows the strongest association with adiponectin levels in the exome-wide meta-analysis (top plot). In the ADIPOGen genome-wide adiponectin meta-analysis (middle plot),14 rs3087866 and 85 proxy variants (r2 > 0.80; 1000Gp3) are nominally associated with adiponectin levels. The same variants exhibit the strongest association with expression of PRKAR2A in subcutaneous adipose tissue (lower plot).41 Colocalized eQTL signals are also observed for AMT and NICN1 (see Figure S12). Each point represents a variant, plotted with hg19 genomic position on the x axis and p value (on a −log10 scale) on the y axis. The color of all other variants indicates the LD with the lead variant in European ancestry haplotypes from the 1000 Genomes Phase 3 reference panel.
To investigate the potential for collider bias in the adiponectin analysis resulting from adjusting for a correlated covariate, BMI, we investigated the behavior of variants associated with adiponectin adjusted for BMI in analyses of adiponectin without adjustment for BMI and analyses of BMI alone, estimating the effect of bias. Nearly all of the adiponectin-associated variants identified showed a genuine effect on adiponectin levels, and any bias caused by adjusting for BMI was minimal. Only one locus (SLC39A8; rs13107325) exhibited potential pleiotropy and/or a collider bias effect due to BMI adjustment (Table S7). It is possible that applying rank-based inverse normal transformation to the residuals as opposed to the adiponectin values could have reintroduced inflation from the covariates and identified false positive results.48 However, examination of cohort-specific QQ plots, comparison of models unadjusted and adjusted for BMI, and the results from the collider bias analysis suggest any inflation is unlikely.
To identify additional association signals at the 20 significant loci, we implemented conditional analyses and identified 3 additional, already established loci that harbored multiple distinct variants associated with adiponectin: ADIPOQ (9 signals; r2 = 0.00–0.19), DNAH10-CCDC92 (2 signals; r2 = 0.07; MIM: 614848), and SLC38A8 (2 signals; r2 = 0.00) (Table S8; Figure S11). These results are consistent with previous reports of multiple signals at these loci for adiponectin and/or other cardiometabolic traits.49, 50 While the exome array-based analysis does not fully characterize the number or identity of the signals due to the relatively low density of analyzed variants, these findings support the presence of multiple adiponectin association signals at these loci.
Other Trait Associations
To further characterize the lead variants, we examined their associations with a range of cardiometabolic traits, including anthropometric, blood pressure, glycemic, and lipid traits and T2D (Tables 2, S9, and S10). Of the nine loci, six have previously shown a genome-wide significant association (p < 5 × 10−8) with at least one additional cardiometabolic trait: WHRadjBMI (four loci), HDL cholesterol (three loci), fat free mass index (two loci), body fat percentage (two loci), hip circumference (two loci), diastolic blood pressure (two loci), systolic blood pressure (one locus), hypertension (one locus), and BMI (one locus). The adiponectin signal associated with the largest number of other traits is SLC39A8, which also exhibited suggestive pleiotropy in the collider bias analysis (Table S7). That lead variant (p.Ala391Thr; rs13107325) has previously been shown to associate with BMI, blood pressure traits, HDL, other obesity-related traits, as well as Crohn disease and schizophrenia.51 An additional PheWAS was performed in all available traits, plasma proteins, and metabolites (Tables S11 and S12). The additional trait associations may suggest pleiotropic or mediating effects at these loci.
Table 2.
Biological Candidate Genes at Previously Unreported Adiponectin-Associated Loci
| Lead Variant | EAF | Nearest Gene | n Variants r2 ≥ 0.80 (Distance kb) | n Variants r2 ≥ 0.60 (Distance kb) | Function/Nonsynonymous Variants (r2 > 0.8) | Adipose eQTL Gene (p < 5 × 10−8) | Other Trait Associations (p < 1 × 10−4) | Literature Candidate |
|---|---|---|---|---|---|---|---|---|
| rs2276853 | 0.59 | KIF9 | 330 (597 kb) | 353 (597 kb) | KIF9 (p.Arg638Gly)∗; SETD2 (p.Pro1918His); SCAP (p.Val543Ile); NBEAL2 (p.Arg511Gly); PTPN23 (p.Ala818Thr) | – | WHRadjBMI, adiponectin, T2D | PTH1R |
| rs3087866 | 0.79 | DALRD3 | 73 (819 kb) | 210 (833 kb) | DALRD3 (p.Gln299Arg)∗ | AMT, NICN1, PRKAR2A, QRICH1 | FFMI, WHRadjBMI, Adiponectin, Menarche, T2D | PRKAR2A |
| rs13133548 | 0.49 | FAM13A | 41 (35 kb) | 54 (35 kb) | – | FAM13A | BF%, HC, HDL, WHRadjBMI, Adiponectin, BMI, T2D, TG | FAM13A |
| rs13107325 | 0.05 | SLC39A8 | 3 (51 kb) | 4 (196 kb) | SLC39A8 (p.Ala391Thr)∗ | – | BMI, BF%, DBP, FFMI, HC, HDL, HTN, SBP, WC, WHRadjBMI, Adiponectin, TG | SLC39A8 |
| rs10282707 | 0.38 | SNX13 | 9 (104 kb) | 16 (130 kb) | – | – | HDL, TC | HDAC9 |
| rs3735080 | 0.24 | GIMAP7 | 278 (119 kb) | 294 (146 kb) | GIMAP7 (p.Arg83Cys)∗; GIMAP6 (p.Gly241Ser) | – | DBP, FFMI, HTN, SBP | REPIN1; RARRES2 |
| rs11993554 | 0.10 | OPLAH | 37 (57 kb) | 122 (107 kb) | OPLAH (p.Phe612Phe∗, p.Arg31Gln, p.Val340Ile, p.Ser284Arg); EPPK1 (p.Arg936Trp) | – | WHRadjBMI | MAF1 |
| rs10861661 | 0.23 | RIC8B | 14 (134 kb) | 16 (134 kb) | – | – | WHRadjBMI, HDL, TG, HC | CRY1 |
| rs145119400 | 0.005 | SLC38A8 | 2 (257 kb) | 2 (257 kb) | SLC38A8 (p.Pro57Leu)∗ | – | – | CDH13; MBTPS1 |
Biological candidate genes at each of the loci previously not shown to be association with adiponectin. Number of variants in moderate to strong linkage disequilibrium and the distance spanned obtained using data from 1000 Genomes Phase 3 and build hg19 (LDLink). Nonsynonymous variants noted with an asterisk (∗) are the lead variants identified at each locus. eQTLs were identified in METSIM subcutaneous adipose and/or GTEx subcutaneous or visceral adipose tissues (see Table S15). Other trait associations were identified from available summary statistics from the ADIPOGen, DIAGRAM, GIANT, and GLGC consortia, along with the UK Biobank (see Tables S9–S11). Abbreviations: BMI, body mass index; BF%, body fat percentage; DBP, diastolic blood pressure; FFMI, fat free mass index; HC, hip circumference; HDL, high density lipoprotein cholesterol; HTN, hypertension; Menarche, age at menarche; SBP, systolic blood pressure; WC, waist circumference; WHRadjBMI, waist-hip-ratio adjusted for BMI.
Extent of LD Surrounding Exome Array Signals
The identification of nonsynonymous variant associations has the potential to pinpoint functional genes. However, evidence of association is not sufficient to conclude that these variants or genes have causal effects on trait levels. To assess the potential role of variants at previously unreported adiponectin loci, we assessed the LD with neighboring variants using 1000 Genomes Phase 3 for the European population to estimate the extent of the locus and to identify additional candidate variants (r2 > 0.80) (Figures 1, 2, and S9; Table 2). Variants at four loci (nearest genes KIF9, DALRD3, GIMAP7, and OPLAH) are each part of wider LD blocks containing between 37 and 330 variants that span a distance of 35 to 819 kb including multiple genes; three of these loci include nonsynonymous variants in more than one gene (Figures 1 and 2; Table 2). For example, at the KIF9 locus (Figure 1, Table 2), the lead variant, rs2276853, encodes KIF9 p.Arg638Gly. However, 330 additional variants spanning nearly 600 kb are in high LD with the lead variant (r2 > 0.80), including nonsynonymous variants in four additional genes (SETD2 [MIM: 612778], SCAP [MIM: 601510], NBEAL2 [MIM: 614169], PTPN23 [MIM: 606584]). The wide LD regions motivated further investigation of possible mechanisms and gene(s) through which candidate variant(s) act to affect adiponectin levels.
Most of the nonsynonymous variants identified in the meta-analysis have low or inconsistent evidence of functional consequences in algorithms, suggesting that other variants may have functional effects (Table S13). Because the exome array captures only a limited set of variants and the extent of LD surrounding each of locus can be vast, we further interrogated each locus to determine whether the lead variant is the best representative of the association signal. To do so, we examined the association results of the exome array loci within the ADIPOGen Consortium genome-wide meta-analysis of adiponectin levels (n = 35,355; HapMap imputed).14 At six loci (KIF9, GIMAP7, DALRD3, RIC8B, SNX13, and FAM13A), a variant other than the exome array lead variant showed a more significant association with adiponectin (Figures 1 and 2, Table 2). For example, at the KIF9 locus, genome-wide association results showed variants in moderate LD (0.60 < r2 < 0.80) with the lead exome array variant that had stronger association with adiponectin levels.14 These variants are located near PTH1R (MIM: 168468), and reduced PTH1R leads to increased adipocyte differentiation and higher adiponectin levels (Table S14),52 suggesting PTH1R as a strong candidate gene at this locus. A seventh locus, SLC38A8, could not be compared to the genome-wide association data because neither the lead variant nor either of the two variants in strong LD (r2 > 0.80) with it were evaluated by ADIPOGen.14 These genome-wide analyses suggest that the genes harboring the lead exome array variants may not be the best candidate genes and that the association signals may act via other variants and possibly other nearby genes instead.
Comparison with eQTL Signals
To aid in the identification of candidate genes at the nine previously unreported association signals, we examined whether any of the variants associated with adiponectin are colocalized (see Material and Methods) with expression levels of nearby transcripts in subcutaneous or visceral adipose tissue. For this analysis, we compared the pattern of variant association with adiponectin levels to the pattern of variant association in the expression quantitative trait locus (eQTL) analysis in GTEx42 and the METSIM study (Table S15).41 Two loci showed evidence of colocalized associations. The adiponectin-associated variant rs13133548 is in high LD (r2 = 0.95) with rs72614904, the variant most strongly associated with expression level of FAM13A (β = 0.190, p = 8.10 × 10−9); alleles associated with lower adiponectin are associated with higher FAM13A expression level. Consistent with these associations, FAM13A has been shown to control adipocyte lipolysis (Table S14).53 The next adiponectin-associated variant rs3087866 is in high LD (r2 = 0.99) with rs6446200, the variant most strongly associated with expression levels of PRKAR2A (MIM: 176910) (β = 0.652, p = 5.79 × 10−25; Figure 2), AMT (r2 = 0.92, β = −0.364, p = 1.14 × 10−8), and NICN1 (MIM: 611516) (r2 = 0.90, β = 0.408, p = 1.69 × 10−11) (Figure S12). At this signal, alleles associated with lower adiponectin are associated with higher PRKAR2A and NICN1 expression level, but lower AMT (MIM: 238310) expression level. The strongest eQTL association signal is with PRKAR2A, which encodes a subunit of cAMP-dependent protein kinase, an essential regulator of lipid and glucose metabolism that plays a role in energy homeostasis.54 Although the exome array variants may not be the best representative variant for these association signals, the LD between the exome array variants and the lead eQTL variants is strong, suggesting that FAM13A, PRKAR2A, AMT, and NICN1 are plausible candidate genes for regulatory effects at these loci.
Candidate Pathways
To further characterize the biological processes and candidate pathways enriched within the identified adiponectin-associated loci, we performed gene set enrichment analyses using PASCAL and EC-DEPICT.26, 43, 45 The PASCAL analyses assuming single association signals from each locus (MAX score) showed significant (p < 5 × 10−5) evidence for enrichment in gene sets for the adipocyte differentiation-related ATXN1 protein-protein interaction sub-network55 (Tables S16A, S16B, and S17), and the PASCAL analyses that considered multiple association signals from the same loci (SUM score) provided additional evidence for enrichment in gene sets for abnormal white adipose tissue physiology, decreased gonadal fat pad weight, and the adipogenesis-related NR3C1 protein-protein interaction sub-network (Tables S16C, S16D, and S17).56 None of the gene sets from EC-DEPICT achieved statistical significance (FDR < 0.05), possibly due to sample size and the number of loci detected (especially for the analysis restricted to low-frequency and rare variants [MAF < 5%]) (Tables S18A and S18B). The enriched processes obtained with these approaches suggest that the adiponectin association signals may alter adipose tissue- and obesity-related pathways.
Discussion
This exome-array based meta-analysis of 265,780 variants in up to 67,739 individuals from four different ancestries identified 30 distinct signals in 20 loci associated with circulating adiponectin levels, including nine loci previously not reported to be associated with adiponectin. Two of the independent signals are located in regions that exhibit extensive LD, and comparison of lead exome array variants to the patterns of association in the previously performed, smaller ADIPOGen GWAS dataset14 suggests the possible contribution of noncoding variants acting on more distant candidate genes. eQTL colocalizations and evaluation of gene functions helped detect potential genes and mechanisms through which the variants may act to affect adiponectin levels. Candidate genes include FAM13A, SLC39A8, PRKAR2A, PTH1R, and HDAC9. Eight of the association signals have been found to be associated with other obesity and lipid traits; the evidence of association with adiponectin levels also guides interpretation of genes and mechanisms for these traits.
Once an association with a nonsynonymous variant is detected, it is simple to hypothesize a causal connection between the variant, the gene in which it is located, and the examined phenotype. We demonstrate that this assumption is often inaccurate, particularly with regards to common nonsynonymous variants identified through an exome array analysis. For example, the common (MAF = 0.41), adiponectin-associated variant, rs2276853, encodes missense variant p.Arg638Gly in KIF9, kinesin family member 9, a regulator of spindle dynamics localized to the nuclear envelope that is essential for mitosis.57 Examination of the surrounding wide LD region using GWAS data from the previous ADIPOGen study14 showed that moderately correlated variants 300 kb upstream near PTH1R, parathyroid hormone receptor 1, exhibited a more significant association with adiponectin levels. Reduced levels of PTH1R lead to increased adipocyte differentiation into mature adipocytes responsible for adiponectin secretion,52 suggesting that the variant alleles may act to reduce PTH1R expression, leading to higher adipocyte differentiation and higher adiponectin levels. PTH1R has also been studied extensively in bone marrow, which has been shown to secrete higher levels of adiponectin than white adipose tissue,58 suggesting that the effect of this locus on adiponectin levels might be mediated by secretion of adiponectin from the bone marrow.
Another example of a nonsynonymous variant representing a potential false lead for causality with adiponectin is rs3087866 (p.Gln299Arg; MAF = 0.21) located within an exon of DALRD3, a locus that has also been associated with central adiposity.36 While the exact function is unknown, DALRD3 encodes a protein with an anticodon “DALR” binding domain, similar to that of tRNA synthetases. At this locus, however, the strongest colocalized eQTL is for PRKAR2A, which encodes a regulatory subunit of protein kinase A. Prkar2a knockout mice weigh less than wild-type littermates and are resistant to diet-induced obesity, glucose intolerance, and fatty liver.59 At the human adiponectin-associated locus, the rs3087866-C allele was associated with decreased adiponectin levels and decreased adipose PRKAR2A expression. This observation is consistent with the smaller adipose tissue depots observed in knockout mice59 and supports the possibility that PRKAR2A contributes to the associations with both adiponectin and central adiposity. The other colocalized eQTLs for AMT and NICN1 at this locus were weaker and less clearly relevant to adipose biology.
Exome arrays contain variants with sparse coverage, and trait-associated variants can be located hundreds of kilobases from their target genes. Here, rs10282707 (MAF = 0.38), previously associated with HDL cholesterol,25 is located within an intron of SNX13, sorting nexin 13. This gene belongs to the sorting nexin family, a G protein signaling regulator family of molecules that are involved in intracellular trafficking and regulate G alpha subunits of heterotrimeric G proteins. Located 200 kb downstream of SNX13, HDAC9 (MIM: 606543) encodes histone deacetylase 9, which alters chromosome structure and affects transcription factor access to DNA. HDAC9 has been shown to be a negative regulator of adipocyte differentiation.60 Higher levels of Hdac9 prevented adipocyte differentiation, and knockout of Hdac9 in mice protected from adipose tissue dysfunction and systemic metabolic disease during high fat feeding.61 In humans, the allele associated with higher adiponectin and lower HDL cholesterol levels may act by reducing HDAC9 expression or function. The GWAS data helped guide interpretation of candidate genes at this locus.
Using the context of regional LD and existing adiponectin GWAS data from ADIPOGen14 is not sufficient for mapping of multiple association signals at a locus. For example, CDH13 encodes an extracellular cadherin-13 receptor that binds to adiponectin,62 and variants near CDH13 are well established to show very strong adiponectin association.14, 19 The strongest adiponectin-associated variant near CDH13 from the exome array analysis was rs3865188, located near the CDH13 promoter, although rs3865188 is in low LD (r2 = 0.16) with the lead variant in the genome-wide data, rs12051272.14 The exome array analysis also identified an adiponectin-associated variant (rs145119400, MAF = 0.005) nearly 1.5 Mb away within SLC38A8, and association analysis conditioning on rs145119400 identified yet another signal 260 kb upstream, within an exon of CDH13 (rs186440718, MAF = 0.003). Neither rs145119400 nor rs186440718 are available in the ADIPOGen consortium data, and rs145119400 is present in only one person in the 1000 Genomes Phase 3 reference panel. Thus, we were not able to determine the number of association signals, nor whether these three variants are all tagging the established CDH13 association signals, nor whether rs145119400 and SLC38A8 represent a distinct association signal and candidate gene for adiponectin biology. Future genome-wide association data in larger datasets with dense marker maps will provide valuable opportunities to perform more detailed genomic and fine-mapping analyses to identify potential candidate genes and pathways.
Identification of the gene(s) underlying genetic association signals is imperative to translate associated variants to biological processes. One method that has proven useful for the prediction of candidate functional genes is with the detection of colocalized eQTLs in relevant tissues.63, 64, 65 However, determination of colocalized GWASs and eQTL loci requires accurate identification of the variants most strongly associated with the phenotype and gene expression levels in both sets of data. With the sparse coverage of variants in the exome array, other untested variants in low to moderate LD with the lead exome variant may exhibit stronger trait associations, leading to false identification of a colocalized eQTL or, more likely, missing a colocalized eQTL signal. Larger and more diverse eQTL studies including both sexes and broader ancestry could help identify additional colocalized association signals. While exome array data can be valuable in identifying low-frequency or rare variants present on the array that are often poorly imputed from genotyping arrays, caution should be used in translating association results into candidate functional variants and genes.
Taken together, our results emphasize the value of using exome array analysis for identification of both coding and noncoding signals in discovery of candidate genes relevant for lipid and obesity biology. However, use of genome-wide association data to characterize exome array signals facilitated detection of the LD structure in the association signals, a few of which suggested that more strongly associated variants exist outside exons. As such, caution should be used in the interpretation of results from both exome array and exome-sequencing studies.66 Future GWASs in larger sample sizes will be valuable to validate these results and extend them to additional loci and genes that influence cardiometabolic traits and diseases.
Declaration of Interests
This work was conducted prior to M.E.G.’s current affiliation with the National Heart, Lung, and Blood Institute, and, as such, the views expressed in this article do not represent the views of the NHLBI, NIH, or other government entity. D.M.-K. is a part-time clinical research consultant for Metabolon, Inc. M.A.N.’s participation is supported by a consulting contract between Data Tecnica International and the National Institute on Aging, National Institutes of Health. V.S. has participated in a conference trip and received an honorarium sponsored by Novo Nordisk.
Acknowledgments
The authors thank all investigators, staff members, and study participants for their contributions to all the participating studies. Funding information for all participating cohorts are provided in the Supplemental Data.
J.B.-J. and T.H. were partially funded by the Novo Nordisk Foundation Center for Basic Metabolic Research, an independent Research Center at the University of Copenhagen. R.S.F. was supported by NHGRI F31 HG009850. T.M.F. was supported by the European Research Council (grant 323195:GLUCOSEGENES-FP7-IDEAS-ERC). H.M.H. was supported by NHLBI T32 HL007055 and T32 HL129982. T.K. was supported by the Novo Nordisk Foundation Center for Protein Research (grants NNF17OC0027594 and NNF14CC0001). T.O.K. was supported by the Danish Council for Independent Research (grant DFF – 6110-00183) and the Novo Nordisk Foundation (grant NNF17OC0026848). C.M.L. is supported by the Li Ka Shing Foundation, WT-SSI/John Fell funds, the NIHR Biomedical Research Centre, Oxford, Widenlife, and NIH (grant 5P50HD028138-27). M.I. McCarthy is a Wellcome Trust Senior Investigator (grant WT098381) and a National Institute of Health Research Senior Investigator, and the views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. J.B. Meigs is supported by NIH K24 DK080140. K.L.M. was supported by NIH R01DK072193 and R01DK093757. D.M.-K. is supported by Dutch Science Organization (ZonMW-VENI Grant 916.14.023). K.E.N. was supported by NIH R01DK089256, R01HD057194, U01HG007416, and R01DK101855 and AHA 13GRNT16490017. C.K.R. was supported by National Institutes of Health (grant 5T32GM67553). S.R. was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (grant 312062) and the Academy of Finland (grant 285380). V.S. was supported by the Finnish Foundation for Cardiovascular Research. C.N.S. was supported by the American Heart Association Postdoctoral Fellowships 15POST24470131 and 17POST33650016. J.G.W. is supported by U54GM115428 from the National Institute of General Medical Sciences. L.B.L.W. was supported by Wellcome Trust (WT083442AIA). H.Y. was funded by Diabetes UK RD Lawrence fellowship (grant 17/ 0005594). K.L.Y. was supported by KL2TR001109.
Published: June 6, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.05.002.
Contributor Information
Ruth J.F. Loos, Email: ruth.loos@mssm.edu.
Karen L. Mohlke, Email: mohlke@med.unc.edu.
Data Availability
Summary genetic association results can be found at http://www.thelooslab.com/#data. All other relevant data are in the paper and Supplemental Data.
Web Resources
Illumina HumanExome Beadchip Array, https://genome.sph.umich.edu/wiki/Exome_Chip_Design
OMIM, https://www.omim.org/
PhenoScanner, http://www.phenoscanner.medschl.cam.ac.uk
Supplemental Data
References
- 1.Azrad M., Gower B.A., Hunter G.R., Nagy T.R. Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine. 2013;43:586–592. doi: 10.1007/s12020-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Azrad, M., Gower, B.A., Hunter, G.R., and Nagy, T.R. (2013). Racial differences in adiponectin and leptin in healthy premenopausal women. Endocrine 43, 586-592. [DOI] [PMC free article] [PubMed]
- 2.Wang X., Bao W., Liu J., Ouyang Y.Y., Wang D., Rong S., Xiao X., Shan Z.L., Zhang Y., Yao P., Liu L.G. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, X., Bao, W., Liu, J., Ouyang, Y.Y., Wang, D., Rong, S., Xiao, X., Shan, Z.L., Zhang, Y., Yao, P., and Liu, L.G. (2013). Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36, 166-175. [DOI] [PMC free article] [PubMed]
- 3.Breitfeld J., Stumvoll M., Kovacs P. Genetics of adiponectin. Biochimie. 2012;94:2157–2163. doi: 10.1016/j.biochi.2012.03.004. [DOI] [PubMed] [Google Scholar]; Breitfeld, J., Stumvoll, M., and Kovacs, P. (2012). Genetics of adiponectin. Biochimie 94, 2157-2163. [DOI] [PubMed]
- 4.Goldstein B.J., Scalia R.G., Ma X.L. Protective vascular and myocardial effects of adiponectin. Nat. Clin. Pract. Cardiovasc. Med. 2009;6:27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]; Goldstein, B.J., Scalia, R.G., and Ma, X.L. (2009). Protective vascular and myocardial effects of adiponectin. Nat. Clin. Pract. Cardiovasc. Med. 6, 27-35. [DOI] [PMC free article] [PubMed]
- 5.Povel C.M., Boer J.M., Feskens E.J. Shared genetic variance between the features of the metabolic syndrome: heritability studies. Mol. Genet. Metab. 2011;104:666–669. doi: 10.1016/j.ymgme.2011.08.035. [DOI] [PubMed] [Google Scholar]; Povel, C.M., Boer, J.M., and Feskens, E.J. (2011). Shared genetic variance between the features of the metabolic syndrome: heritability studies. Mol. Genet. Metab. 104, 666-669. [DOI] [PubMed]
- 6.Henneman P., Aulchenko Y.S., Frants R.R., Zorkoltseva I.V., Zillikens M.C., Frolich M., Oostra B.A., van Dijk K.W., van Duijn C.M. Genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome-related traits. Diabetes Care. 2010;33:908–913. doi: 10.2337/dc09-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]; Henneman, P., Aulchenko, Y.S., Frants, R.R., Zorkoltseva, I.V., Zillikens, M.C., Frolich, M., Oostra, B.A., van Dijk, K.W., and van Duijn, C.M. (2010). Genetic architecture of plasma adiponectin overlaps with the genetics of metabolic syndrome-related traits. Diabetes Care 33, 908-913. [DOI] [PMC free article] [PubMed]
- 7.Au Yeung S.L., Schooling C.M. Adiponectin and coronary artery disease risk: A bi-directional Mendelian randomization study. Int. J. Cardiol. 2018;268:222–226. doi: 10.1016/j.ijcard.2018.03.132. [DOI] [PubMed] [Google Scholar]; Au Yeung, S.L., and Schooling, C.M. (2018). Adiponectin and coronary artery disease risk: A bi-directional Mendelian randomization study. Int. J. Cardiol. 268, 222-226. [DOI] [PubMed]
- 8.Yaghootkar H., Lamina C., Scott R.A., Dastani Z., Hivert M.F., Warren L.L., Stancáková A., Buxbaum S.G., Lyytikäinen L.P., Henneman P., GENESIS Consortium. RISC Consortium Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62:3589–3598. doi: 10.2337/db13-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yaghootkar, H., Lamina, C., Scott, R.A., Dastani, Z., Hivert, M.F., Warren, L.L., Stancakova, A., Buxbaum, S.G., Lyytikainen, L.P., Henneman, P., et al.; GENESIS Consortium; RISC Consortium (2013). Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes 62, 3589-3598. [DOI] [PMC free article] [PubMed]
- 9.Mente A., Meyre D., Lanktree M.B., Heydarpour M., Davis A.D., Miller R., Gerstein H., Hegele R.A., Yusuf S., Anand S.S., SHARE Investigators. SHARE-AP Investigators Causal relationship between adiponectin and metabolic traits: a Mendelian randomization study in a multiethnic population. PLoS ONE. 2013;8:e66808. doi: 10.1371/journal.pone.0066808. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mente, A., Meyre, D., Lanktree, M.B., Heydarpour, M., Davis, A.D., Miller, R., Gerstein, H., Hegele, R.A., Yusuf, S., and Anand, S.S.; SHARE Investigators; SHARE-AP Investigators (2013). Causal relationship between adiponectin and metabolic traits: a Mendelian randomization study in a multiethnic population. PLoS ONE 8, e66808. [DOI] [PMC free article] [PubMed]
- 10.Gao H., Fall T., van Dam R.M., Flyvbjerg A., Zethelius B., Ingelsson E., Hägg S. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes. 2013;62:1338–1344. doi: 10.2337/db12-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gao, H., Fall, T., van Dam, R.M., Flyvbjerg, A., Zethelius, B., Ingelsson, E., and Hagg, S. (2013). Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 62, 1338-1344. [DOI] [PMC free article] [PubMed]
- 11.Xia J.Y., Sun K., Hepler C., Ghaben A.L., Gupta R.K., An Y.A., Holland W.L., Morley T.S., Adams A.C., Gordillo R. Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia. 2018;61:932–941. doi: 10.1007/s00125-017-4516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xia, J.Y., Sun, K., Hepler, C., Ghaben, A.L., Gupta, R.K., An, Y.A., Holland, W.L., Morley, T.S., Adams, A.C., Gordillo, R., et al. (2018). Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia 61, 932-941. [DOI] [PMC free article] [PubMed]
- 12.Holland W.L., Miller R.A., Wang Z.V., Sun K., Barth B.M., Bui H.H., Davis K.E., Bikman B.T., Halberg N., Rutkowski J.M. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]; Holland, W.L., Miller, R.A., Wang, Z.V., Sun, K., Barth, B.M., Bui, H.H., Davis, K.E., Bikman, B.T., Halberg, N., Rutkowski, J.M., et al. (2011). Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55-63. [DOI] [PMC free article] [PubMed]
- 13.Sattar N., Nelson S.M. Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J. Clin. Endocrinol. Metab. 2008;93:3299–3301. doi: 10.1210/jc.2008-1435. [DOI] [PubMed] [Google Scholar]; Sattar, N., and Nelson, S.M. (2008). Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J. Clin. Endocrinol. Metab. 93, 3299-3301. [DOI] [PubMed]
- 14.Dastani Z., Hivert M.F., Timpson N., Perry J.R., Yuan X., Scott R.A., Henneman P., Heid I.M., Kizer J.R., Lyytikäinen L.P., DIAGRAM+ Consortium. MAGIC Consortium. GLGC Investigators. MuTHER Consortium. DIAGRAM Consortium. GIANT Consortium. Global B Pgen Consortium. Procardis Consortium. MAGIC investigators. GLGC Consortium Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dastani, Z., Hivert, M.F., Timpson, N., Perry, J.R., Yuan, X., Scott, R.A., Henneman, P., Heid, I.M., Kizer, J.R., Lyytikainen, L.P., et al.; DIAGRAM+ Consortium; MAGIC Consortium; GLGC Investigators; MuTHER Consortium; DIAGRAM Consortium; GIANT Consortium; Global B Pgen Consortium; Procardis Consortium; MAGIC investigators; GLGC Consortium (2012). Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 8, e1002607. [DOI] [PMC free article] [PubMed]
- 15.Nawrocki A.R., Rajala M.W., Tomas E., Pajvani U.B., Saha A.K., Trumbauer M.E., Pang Z., Chen A.S., Ruderman N.B., Chen H. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]; Nawrocki, A.R., Rajala, M.W., Tomas, E., Pajvani, U.B., Saha, A.K., Trumbauer, M.E., Pang, Z., Chen, A.S., Ruderman, N.B., Chen, H., et al. (2006). Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 281, 2654-2660. [DOI] [PubMed]
- 16.Chuang L.M., Chiu Y.F., Sheu W.H., Hung Y.J., Ho L.T., Grove J., Rodriguez B., Quertermous T., Chen Y.D., Hsiung C.A., Tai T.Y., Stanford Asia-Pacific Program of Hypertension and Insulin Resistance Study Group Biethnic comparisons of autosomal genomic scan for loci linked to plasma adiponectin in populations of Chinese and Japanese origin. J. Clin. Endocrinol. Metab. 2004;89:5772–5778. doi: 10.1210/jc.2004-0640. [DOI] [PubMed] [Google Scholar]; Chuang, L.M., Chiu, Y.F., Sheu, W.H., Hung, Y.J., Ho, L.T., Grove, J., Rodriguez, B., Quertermous, T., Chen, Y.D., Hsiung, C.A., and Tai, T.Y.; Stanford Asia-Pacific Program of Hypertension and Insulin Resistance Study Group (2004). Biethnic comparisons of autosomal genomic scan for loci linked to plasma adiponectin in populations of Chinese and Japanese origin. J. Clin. Endocrinol. Metab. 89, 5772-5778. [DOI] [PubMed]
- 17.Comuzzie A.G., Funahashi T., Sonnenberg G., Martin L.J., Jacob H.J., Black A.E., Maas D., Takahashi M., Kihara S., Tanaka S. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2001;86:4321–4325. doi: 10.1210/jcem.86.9.7878. [DOI] [PubMed] [Google Scholar]; Comuzzie, A.G., Funahashi, T., Sonnenberg, G., Martin, L.J., Jacob, H.J., Black, A.E., Maas, D., Takahashi, M., Kihara, S., Tanaka, S., et al. (2001). The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J. Clin. Endocrinol. Metab. 86, 4321-4325. [DOI] [PubMed]
- 18.Lindsay R.S., Funahashi T., Krakoff J., Matsuzawa Y., Tanaka S., Kobes S., Bennett P.H., Tataranni P.A., Knowler W.C., Hanson R.L. Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes. 2003;52:2419–2425. doi: 10.2337/diabetes.52.9.2419. [DOI] [PubMed] [Google Scholar]; Lindsay, R.S., Funahashi, T., Krakoff, J., Matsuzawa, Y., Tanaka, S., Kobes, S., Bennett, P.H., Tataranni, P.A., Knowler, W.C., and Hanson, R.L. (2003). Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes 52, 2419-2425. [DOI] [PubMed]
- 19.Wu Y., Gao H., Li H., Tabara Y., Nakatochi M., Chiu Y.F., Park E.J., Wen W., Adair L.S., Borja J.B. A meta-analysis of genome-wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11-FGFR2. Hum. Mol. Genet. 2014;23:1108–1119. doi: 10.1093/hmg/ddt488. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, Y., Gao, H., Li, H., Tabara, Y., Nakatochi, M., Chiu, Y.F., Park, E.J., Wen, W., Adair, L.S., Borja, J.B., et al. (2014). A meta-analysis of genome-wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11-FGFR2. Hum. Mol. Genet. 23, 1108-1119. [DOI] [PMC free article] [PubMed]
- 20.Richards J.B., Waterworth D., O’Rahilly S., Hivert M.F., Loos R.J., Perry J.R., Tanaka T., Timpson N.J., Semple R.K., Soranzo N., GIANT Consortium A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]; Richards, J.B., Waterworth, D., O’Rahilly, S., Hivert, M.F., Loos, R.J., Perry, J.R., Tanaka, T., Timpson, N.J., Semple, R.K., Soranzo, N., et al.; GIANT Consortium (2009). A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 5, e1000768. [DOI] [PMC free article] [PubMed]
- 21.Mahajan A., Wessel J., Willems S.M., Zhao W., Robertson N.R., Chu A.Y., Gan W., Kitajima H., Taliun D., Rayner N.W., ExomeBP Consortium. MAGIC Consortium. GIANT Consortium Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 2018;50:559–571. doi: 10.1038/s41588-018-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mahajan, A., Wessel, J., Willems, S.M., Zhao, W., Robertson, N.R., Chu, A.Y., Gan, W., Kitajima, H., Taliun, D., Rayner, N.W., et al.; ExomeBP Consortium; MAGIC Consortium; GIANT Consortium (2018). Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50, 559-571. [DOI] [PMC free article] [PubMed]
- 22.Feng S., Liu D., Zhan X., Wing M.K., Abecasis G.R. RAREMETAL: fast and powerful meta-analysis for rare variants. Bioinformatics. 2014;30:2828–2829. doi: 10.1093/bioinformatics/btu367. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feng, S., Liu, D., Zhan, X., Wing, M.K., and Abecasis, G.R. (2014). RAREMETAL: fast and powerful meta-analysis for rare variants. Bioinformatics 30, 2828-2829. [DOI] [PMC free article] [PubMed]
- 23.Zhan X., Hu Y., Li B., Abecasis G.R., Liu D.J. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016;32:1423–1426. doi: 10.1093/bioinformatics/btw079. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhan, X., Hu, Y., Li, B., Abecasis, G.R., and Liu, D.J. (2016). RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics 32, 1423-1426. [DOI] [PMC free article] [PubMed]
- 24.Winkler T.W., Day F.R., Croteau-Chonka D.C., Wood A.R., Locke A.E., Mägi R., Ferreira T., Fall T., Graff M., Justice A.E., Genetic Investigation of Anthropometric Traits (GIANT) Consortium Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 2014;9:1192–1212. doi: 10.1038/nprot.2014.071. [DOI] [PMC free article] [PubMed] [Google Scholar]; Winkler, T.W., Day, F.R., Croteau-Chonka, D.C., Wood, A.R., Locke, A.E., Magi, R., Ferreira, T., Fall, T., Graff, M., Justice, A.E., et al.; Genetic Investigation of Anthropometric Traits (GIANT) Consortium (2014). Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 9, 1192-1212. [DOI] [PMC free article] [PubMed]
- 25.Liu D.J., Peloso G.M., Yu H., Butterworth A.S., Wang X., Mahajan A., Saleheen D., Emdin C., Alam D., Alves A.C., Charge Diabetes Working Group. EPIC-InterAct Consortium. EPIC-CVD Consortium. GOLD Consortium. VA Million Veteran Program Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017;49:1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, D.J., Peloso, G.M., Yu, H., Butterworth, A.S., Wang, X., Mahajan, A., Saleheen, D., Emdin, C., Alam, D., Alves, A.C., et al.; Charge Diabetes Working Group; EPIC-InterAct Consortium; EPIC-CVD Consortium; GOLD Consortium; VA Million Veteran Program (2017). Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 49, 1758-1766. [DOI] [PMC free article] [PubMed]
- 26.Turcot V., Lu Y., Highland H.M., Schurmann C., Justice A.E., Fine R.S., Bradfield J.P., Esko T., Giri A., Graff M., CHD Exome+ Consortium. EPIC-CVD Consortium. ExomeBP Consortium. Global Lipids Genetic Consortium. GoT2D Genes Consortium. EPIC InterAct Consortium. INTERVAL Study. ReproGen Consortium. T2D-Genes Consortium. MAGIC Investigators. Understanding Society Scientific Group Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 2018;50:26–41. doi: 10.1038/s41588-017-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Turcot, V., Lu, Y., Highland, H.M., Schurmann, C., Justice, A.E., Fine, R.S., Bradfield, J.P., Esko, T., Giri, A., Graff, M., et al.; CHD Exome+ Consortium; EPIC-CVD Consortium; ExomeBP Consortium; Global Lipids Genetic Consortium; GoT2D Genes Consortium; EPIC InterAct Consortium; INTERVAL Study; ReproGen Consortium; T2D-Genes Consortium; MAGIC Investigators; Understanding Society Scientific Group (2018). Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50, 26-41. [DOI] [PMC free article] [PubMed]
- 27.Liu D.J., Peloso G.M., Zhan X., Holmen O.L., Zawistowski M., Feng S., Nikpay M., Auer P.L., Goel A., Zhang H. Meta-analysis of gene-level tests for rare variant association. Nat. Genet. 2014;46:200–204. doi: 10.1038/ng.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, D.J., Peloso, G.M., Zhan, X., Holmen, O.L., Zawistowski, M., Feng, S., Nikpay, M., Auer, P.L., Goel, A., Zhang, H., et al. (2014). Meta-analysis of gene-level tests for rare variant association. Nat. Genet. 46, 200-204. [DOI] [PMC free article] [PubMed]
- 28.Winkler T.W., Kutalik Z., Gorski M., Lottaz C., Kronenberg F., Heid I.M. EasyStrata: evaluation and visualization of stratified genome-wide association meta-analysis data. Bioinformatics. 2015;31:259–261. doi: 10.1093/bioinformatics/btu621. [DOI] [PMC free article] [PubMed] [Google Scholar]; Winkler, T.W., Kutalik, Z., Gorski, M., Lottaz, C., Kronenberg, F., and Heid, I.M. (2015). EasyStrata: evaluation and visualization of stratified genome-wide association meta-analysis data. Bioinformatics 31, 259-261. [DOI] [PMC free article] [PubMed]
- 29.Wu M.C., Lee S., Cai T., Li Y., Boehnke M., Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, M.C., Lee, S., Cai, T., Li, Y., Boehnke, M., and Lin, X. (2011). Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 89, 82-93. [DOI] [PMC free article] [PubMed]
- 30.Price A.L., Kryukov G.V., de Bakker P.I., Purcell S.M., Staples J., Wei L.J., Sunyaev S.R. Pooled association tests for rare variants in exon-resequencing studies. Am. J. Hum. Genet. 2010;86:832–838. doi: 10.1016/j.ajhg.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Price, A.L., Kryukov, G.V., de Bakker, P.I., Purcell, S.M., Staples, J., Wei, L.J., and Sunyaev, S.R. (2010). Pooled association tests for rare variants in exon-resequencing studies. Am. J. Hum. Genet. 86, 832-838. [DOI] [PMC free article] [PubMed]
- 31.Purcell S.M., Moran J.L., Fromer M., Ruderfer D., Solovieff N., Roussos P., O’Dushlaine C., Chambert K., Bergen S.E., Kähler A. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]; Purcell, S.M., Moran, J.L., Fromer, M., Ruderfer, D., Solovieff, N., Roussos, P., O’Dushlaine, C., Chambert, K., Bergen, S.E., Kahler, A., et al. (2014). A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, 185-190. [DOI] [PMC free article] [PubMed]
- 32.Kiezun A., Garimella K., Do R., Stitziel N.O., Neale B.M., McLaren P.J., Gupta N., Sklar P., Sullivan P.F., Moran J.L. Exome sequencing and the genetic basis of complex traits. Nat. Genet. 2012;44:623–630. doi: 10.1038/ng.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kiezun, A., Garimella, K., Do, R., Stitziel, N.O., Neale, B.M., McLaren, P.J., Gupta, N., Sklar, P., Sullivan, P.F., Moran, J.L., et al. (2012). Exome sequencing and the genetic basis of complex traits. Nat. Genet. 44, 623-630. [DOI] [PMC free article] [PubMed]
- 33.Aschard H., Vilhjálmsson B.J., Joshi A.D., Price A.L., Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am. J. Hum. Genet. 2015;96:329–339. doi: 10.1016/j.ajhg.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aschard, H., Vilhjalmsson, B.J., Joshi, A.D., Price, A.L., and Kraft, P. (2015). Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am. J. Hum. Genet. 96, 329-339. [DOI] [PMC free article] [PubMed]
- 34.Day F.R., Loh P.R., Scott R.A., Ong K.K., Perry J.R. A Robust Example of Collider Bias in a Genetic Association Study. Am. J. Hum. Genet. 2016;98:392–393. doi: 10.1016/j.ajhg.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Day, F.R., Loh, P.R., Scott, R.A., Ong, K.K., and Perry, J.R. (2016). A Robust Example of Collider Bias in a Genetic Association Study. Am. J. Hum. Genet. 98, 392-393. [DOI] [PMC free article] [PubMed]
- 35.Willer C.J., Speliotes E.K., Loos R.J., Li S., Lindgren C.M., Heid I.M., Berndt S.I., Elliott A.L., Jackson A.U., Lamina C., Wellcome Trust Case Control Consortium. Genetic Investigation of ANthropometric Traits Consortium Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]; Willer, C.J., Speliotes, E.K., Loos, R.J., Li, S., Lindgren, C.M., Heid, I.M., Berndt, S.I., Elliott, A.L., Jackson, A.U., Lamina, C., et al.; Wellcome Trust Case Control Consortium; Genetic Investigation of ANthropometric Traits Consortium (2009). Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25-34. [DOI] [PMC free article] [PubMed]
- 36.Justice A.E., Karaderi T., Highland H.M., Young K.L., Graff M., Lu Y., Turcot V., Auer P.L., Fine R.S., Guo X., CHD Exome+ Consortium. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. EPIC-CVD Consortium. ExomeBP Consortium. Global Lipids Genetic Consortium. GoT2D Genes Consortium. InterAct. ReproGen Consortium. T2D-Genes Consortium. MAGIC Investigators Protein-coding variants implicate novel genes related to lipid homeostasis contributing to body-fat distribution. Nat. Genet. 2019;51:452–469. doi: 10.1038/s41588-018-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Justice, A.E., Karaderi, T., Highland, H.M., Young, K.L., Graff, M., Lu, Y., Turcot, V., Auer, P.L., Fine, R.S., Guo, X., et al.; CHD Exome+ Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; EPIC-CVD Consortium; ExomeBP Consortium; Global Lipids Genetic Consortium; GoT2D Genes Consortium; InterAct; ReproGen Consortium; T2D-Genes Consortium; MAGIC Investigators (2019). Protein-coding variants implicate novel genes related to lipid homeostasis contributing to body-fat distribution. Nat. Genet. 51, 452-469. [DOI] [PMC free article] [PubMed]
- 37.Wheeler E., Leong A., Liu C.T., Hivert M.F., Strawbridge R.J., Podmore C., Li M., Yao J., Sim X., Hong J., EPIC-CVD Consortium. EPIC-InterAct Consortium. Lifelines Cohort Study Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS Med. 2017;14:e1002383. doi: 10.1371/journal.pmed.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wheeler, E., Leong, A., Liu, C.T., Hivert, M.F., Strawbridge, R.J., Podmore, C., Li, M., Yao, J., Sim, X., Hong, J., et al.; EPIC-CVD Consortium; EPIC-InterAct Consortium; Lifelines Cohort Study (2017). Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS Med. 14, e1002383. [DOI] [PMC free article] [PubMed]
- 38.Manning A.K., Hivert M.F., Scott R.A., Grimsby J.L., Bouatia-Naji N., Chen H., Rybin D., Liu C.T., Bielak L.F., Prokopenko I., DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Multiple Tissue Human Expression Resource (MUTHER) Consortium A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 2012;44:659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manning, A.K., Hivert, M.F., Scott, R.A., Grimsby, J.L., Bouatia-Naji, N., Chen, H., Rybin, D., Liu, C.T., Bielak, L.F., Prokopenko, I., et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Multiple Tissue Human Expression Resource (MUTHER) Consortium (2012). A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 44, 659-669. [DOI] [PMC free article] [PubMed]
- 39.Scott R.A., Lagou V., Welch R.P., Wheeler E., Montasser M.E., Luan J., Mägi R., Strawbridge R.J., Rehnberg E., Gustafsson S., DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scott, R.A., Lagou, V., Welch, R.P., Wheeler, E., Montasser, M.E., Luan, J., Magi, R., Strawbridge, R.J., Rehnberg, E., Gustafsson, S., et al.; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium (2012). Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44, 991-1005. [DOI] [PMC free article] [PubMed]
- 40.Scott R.A., Scott L.J., Mägi R., Marullo L., Gaulton K.J., Kaakinen M., Pervjakova N., Pers T.H., Johnson A.D., Eicher J.D., DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888–2902. doi: 10.2337/db16-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]; Scott, R.A., Scott, L.J., Magi, R., Marullo, L., Gaulton, K.J., Kaakinen, M., Pervjakova, N., Pers, T.H., Johnson, A.D., Eicher, J.D., et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium (2017). An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 66, 2888-2902. [DOI] [PMC free article] [PubMed]
- 41.Civelek M., Wu Y., Pan C., Raulerson C.K., Ko A., He A., Tilford C., Saleem N.K., Stančáková A., Scott L.J. Genetic regulation of adipose gene expression and cardio-metabolic traits. Am. J. Hum. Genet. 2017;100:428–443. doi: 10.1016/j.ajhg.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; Civelek, M., Wu, Y., Pan, C., Raulerson, C.K., Ko, A., He, A., Tilford, C., Saleem, N.K., Stančakova, A., Scott, L.J., et al. (2017). Genetic regulation of adipose gene expression and cardio-metabolic traits. Am. J. Hum. Genet. 100, 428-443. [DOI] [PMC free article] [PubMed]
- 42.GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]; GTEx Consortium (2013). The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580-585. [DOI] [PMC free article] [PubMed]
- 43.Pers T.H., Karjalainen J.M., Chan Y., Westra H.J., Wood A.R., Yang J., Lui J.C., Vedantam S., Gustafsson S., Esko T., Genetic Investigation of ANthropometric Traits (GIANT) Consortium Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pers, T.H., Karjalainen, J.M., Chan, Y., Westra, H.J., Wood, A.R., Yang, J., Lui, J.C., Vedantam, S., Gustafsson, S., Esko, T., et al.; Genetic Investigation of ANthropometric Traits (GIANT) Consortium (2015). Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 6, 5890. [DOI] [PMC free article] [PubMed]
- 44.Marouli E., Graff M., Medina-Gomez C., Lo K.S., Wood A.R., Kjaer T.R., Fine R.S., Lu Y., Schurmann C., Highland H.M., EPIC-InterAct Consortium. CHD Exome+ Consortium. ExomeBP Consortium. T2D-Genes Consortium. GoT2D Genes Consortium. Global Lipids Genetics Consortium. ReproGen Consortium. MAGIC Investigators Rare and low-frequency coding variants alter human adult height. Nature. 2017;542:186–190. doi: 10.1038/nature21039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marouli, E., Graff, M., Medina-Gomez, C., Lo, K.S., Wood, A.R., Kjaer, T.R., Fine, R.S., Lu, Y., Schurmann, C., Highland, H.M., et al.; EPIC-InterAct Consortium; CHD Exome+ Consortium; ExomeBP Consortium; T2D-Genes Consortium; GoT2D Genes Consortium; Global Lipids Genetics Consortium; ReproGen Consortium; MAGIC Investigators (2017). Rare and low-frequency coding variants alter human adult height. Nature 542, 186-190. [DOI] [PMC free article] [PubMed]
- 45.Lamparter D., Marbach D., Rueedi R., Kutalik Z., Bergmann S. Fast and Rigorous Computation of Gene and Pathway Scores from SNP-Based Summary Statistics. PLoS Comput. Biol. 2016;12:e1004714. doi: 10.1371/journal.pcbi.1004714. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lamparter, D., Marbach, D., Rueedi, R., Kutalik, Z., and Bergmann, S. (2016). Fast and Rigorous Computation of Gene and Pathway Scores from SNP-Based Summary Statistics. PLoS Comput. Biol. 12, e1004714. [DOI] [PMC free article] [PubMed]
- 46.Moayyeri A., Hammond C.J., Valdes A.M., Spector T.D. Cohort Profile: TwinsUK and healthy ageing twin study. Int. J. Epidemiol. 2013;42:76–85. doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]; Moayyeri, A., Hammond, C.J., Valdes, A.M., and Spector, T.D. (2013). Cohort Profile: TwinsUK and healthy ageing twin study. Int. J. Epidemiol. 42, 76-85. [DOI] [PMC free article] [PubMed]
- 47.Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Molloy L., Ness A., Ring S., Davey Smith G. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Boyd, A., Golding, J., Macleod, J., Lawlor, D.A., Fraser, A., Henderson, J., Molloy, L., Ness, A., Ring, S., and Davey Smith, G. (2013). Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 42, 111-127. [DOI] [PMC free article] [PubMed]
- 48.Pain O., Dudbridge F., Ronald A. Are your covariates under control? How normalization can re-introduce covariate effects. Eur. J. Hum. Genet. 2018;26:1194–1201. doi: 10.1038/s41431-018-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pain, O., Dudbridge, F., and Ronald, A. (2018). Are your covariates under control? How normalization can re-introduce covariate effects. Eur. J. Hum. Genet. 26, 1194-1201. [DOI] [PMC free article] [PubMed]
- 49.Hivert M.F., Manning A.K., McAteer J.B., Florez J.C., Dupuis J., Fox C.S., O’Donnell C.J., Cupples L.A., Meigs J.B. Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes. 2008;57:3353–3359. doi: 10.2337/db08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hivert, M.F., Manning, A.K., McAteer, J.B., Florez, J.C., Dupuis, J., Fox, C.S., O’Donnell, C.J., Cupples, L.A., and Meigs, J.B. (2008). Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes 57, 3353-3359. [DOI] [PMC free article] [PubMed]
- 50.Jee S.H., Sull J.W., Lee J.E., Shin C., Park J., Kimm H., Cho E.Y., Shin E.S., Yun J.E., Park J.W. Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 2010;87:545–552. doi: 10.1016/j.ajhg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jee, S.H., Sull, J.W., Lee, J.E., Shin, C., Park, J., Kimm, H., Cho, E.Y., Shin, E.S., Yun, J.E., Park, J.W., et al. (2010). Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 87, 545-552. [DOI] [PMC free article] [PubMed]
- 51.Pickrell J.K., Berisa T., Liu J.Z., Ségurel L., Tung J.Y., Hinds D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016;48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pickrell, J.K., Berisa, T., Liu, J.Z., Segurel, L., Tung, J.Y., and Hinds, D.A. (2016). Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709-717. [DOI] [PMC free article] [PubMed]
- 52.Fan Y., Hanai J.I., Le P.T., Bi R., Maridas D., DeMambro V., Figueroa C.A., Kir S., Zhou X., Mannstadt M. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;25:661–672. doi: 10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fan, Y., Hanai, J.I., Le, P.T., Bi, R., Maridas, D., DeMambro, V., Figueroa, C.A., Kir, S., Zhou, X., Mannstadt, M., et al. (2017). Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 25, 661-672. [DOI] [PMC free article] [PubMed]
- 53.Lundbäck V., Kulyte A., Strawbridge R.J., Ryden M., Arner P., Marcus C., Dahlman I. FAM13A and POM121C are candidate genes for fasting insulin: functional follow-up analysis of a genome-wide association study. Diabetologia. 2018;61:1112–1123. doi: 10.1007/s00125-018-4572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lundback, V., Kulyte, A., Strawbridge, R.J., Ryden, M., Arner, P., Marcus, C., and Dahlman, I. (2018). FAM13A and POM121C are candidate genes for fasting insulin: functional follow-up analysis of a genome-wide association study. Diabetologia 61, 1112-1123. [DOI] [PMC free article] [PubMed]
- 54.London E., Nesterova M., Stratakis C.A. Acute vs chronic exposure to high fat diet leads to distinct regulation of PKA. J. Mol. Endocrinol. 2017;59:1–12. doi: 10.1530/JME-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]; London, E., Nesterova, M., and Stratakis, C.A. (2017). Acute vs chronic exposure to high fat diet leads to distinct regulation of PKA. J. Mol. Endocrinol. 59, 1-12. [DOI] [PMC free article] [PubMed]
- 55.Chu A.Y., Deng X., Fisher V.A., Drong A., Zhang Y., Feitosa M.F., Liu C.T., Weeks O., Choh A.C., Duan Q. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 2017;49:125–130. doi: 10.1038/ng.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chu, A.Y., Deng, X., Fisher, V.A., Drong, A., Zhang, Y., Feitosa, M.F., Liu, C.T., Weeks, O., Choh, A.C., Duan, Q., et al. (2017). Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 49, 125-130. [DOI] [PMC free article] [PubMed]
- 56.Lee M.J., Fried S.K. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 2014;38:1228–1233. doi: 10.1038/ijo.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, M.J., and Fried, S.K. (2014). The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 38, 1228-1233. [DOI] [PMC free article] [PubMed]
- 57.Tikhonenko I., Magidson V., Gräf R., Khodjakov A., Koonce M.P. A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell. Mol. Life Sci. 2013;70:1285–1296. doi: 10.1007/s00018-012-1205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tikhonenko, I., Magidson, V., Graf, R., Khodjakov, A., and Koonce, M.P. (2013). A kinesin-mediated mechanism that couples centrosomes to nuclei. Cell. Mol. Life Sci. 70, 1285-1296. [DOI] [PMC free article] [PubMed]
- 58.Cawthorn W.P., Scheller E.L., Learman B.S., Parlee S.D., Simon B.R., Mori H., Ning X., Bree A.J., Schell B., Broome D.T. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cawthorn, W.P., Scheller, E.L., Learman, B.S., Parlee, S.D., Simon, B.R., Mori, H., Ning, X., Bree, A.J., Schell, B., Broome, D.T., et al. (2014). Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20, 368-375. [DOI] [PMC free article] [PubMed]
- 59.London E., Nesterova M., Sinaii N., Szarek E., Chanturiya T., Mastroyannis S.A., Gavrilova O., Stratakis C.A. Differentially regulated protein kinase A (PKA) activity in adipose tissue and liver is associated with resistance to diet-induced obesity and glucose intolerance in mice that lack PKA regulatory subunit type IIα. Endocrinology. 2014;155:3397–3408. doi: 10.1210/en.2014-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]; London, E., Nesterova, M., Sinaii, N., Szarek, E., Chanturiya, T., Mastroyannis, S.A., Gavrilova, O., and Stratakis, C.A. (2014). Differentially regulated protein kinase A (PKA) activity in adipose tissue and liver is associated with resistance to diet-induced obesity and glucose intolerance in mice that lack PKA regulatory subunit type IIα. Endocrinology 155, 3397-3408. [DOI] [PMC free article] [PubMed]
- 60.Chatterjee T.K., Idelman G., Blanco V., Blomkalns A.L., Piegore M.G., Jr., Weintraub D.S., Kumar S., Rajsheker S., Manka D., Rudich S.M. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J. Biol. Chem. 2011;286:27836–27847. doi: 10.1074/jbc.M111.262964. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chatterjee, T.K., Idelman, G., Blanco, V., Blomkalns, A.L., Piegore, M.G., Jr., Weintraub, D.S., Kumar, S., Rajsheker, S., Manka, D., Rudich, S.M., et al. (2011). Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J. Biol. Chem. 286, 27836-27847. [DOI] [PMC free article] [PubMed]
- 61.Chatterjee T.K., Basford J.E., Knoll E., Tong W.S., Blanco V., Blomkalns A.L., Rudich S., Lentsch A.B., Hui D.Y., Weintraub N.L. HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes. 2014;63:176–187. doi: 10.2337/db13-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chatterjee, T.K., Basford, J.E., Knoll, E., Tong, W.S., Blanco, V., Blomkalns, A.L., Rudich, S., Lentsch, A.B., Hui, D.Y., and Weintraub, N.L. (2014). HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes 63, 176-187. [DOI] [PMC free article] [PubMed]
- 62.Williams A.S., Kasahara D.I., Verbout N.G., Fedulov A.V., Zhu M., Si H., Wurmbrand A.P., Hug C., Ranscht B., Shore S.A. Role of the adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PLoS ONE. 2012;7:e41088. doi: 10.1371/journal.pone.0041088. [DOI] [PMC free article] [PubMed] [Google Scholar]; Williams, A.S., Kasahara, D.I., Verbout, N.G., Fedulov, A.V., Zhu, M., Si, H., Wurmbrand, A.P., Hug, C., Ranscht, B., and Shore, S.A. (2012). Role of the adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PLoS ONE 7, e41088. [DOI] [PMC free article] [PubMed]
- 63.Conde L., Bracci P.M., Richardson R., Montgomery S.B., Skibola C.F. Integrating GWAS and expression data for functional characterization of disease-associated SNPs: an application to follicular lymphoma. Am. J. Hum. Genet. 2013;92:126–130. doi: 10.1016/j.ajhg.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Conde, L., Bracci, P.M., Richardson, R., Montgomery, S.B., and Skibola, C.F. (2013). Integrating GWAS and expression data for functional characterization of disease-associated SNPs: an application to follicular lymphoma. Am. J. Hum. Genet. 92, 126-130. [DOI] [PMC free article] [PubMed]
- 64.Albert F.W., Kruglyak L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 2015;16:197–212. doi: 10.1038/nrg3891. [DOI] [PubMed] [Google Scholar]; Albert, F.W., and Kruglyak, L. (2015). The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 16, 197-212. [DOI] [PubMed]
- 65.Brænne I., Civelek M., Vilne B., Di Narzo A., Johnson A.D., Zhao Y., Reiz B., Codoni V., Webb T.R., Foroughi Asl H., Leducq Consortium CAD Genomics‡ Prediction of Causal Candidate Genes in Coronary Artery Disease Loci. Arterioscler. Thromb. Vasc. Biol. 2015;35:2207–2217. doi: 10.1161/ATVBAHA.115.306108. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brænne, I., Civelek, M., Vilne, B., Di Narzo, A., Johnson, A.D., Zhao, Y., Reiz, B., Codoni, V., Webb, T.R., Foroughi Asl, H., et al.; Leducq Consortium CAD Genomics‡ (2015). Prediction of Causal Candidate Genes in Coronary Artery Disease Loci. Arterioscler. Thromb. Vasc. Biol. 35, 2207-2217. [DOI] [PMC free article] [PubMed]
- 66.Huyghe J.R., Jackson A.U., Fogarty M.P., Buchkovich M.L., Stančáková A., Stringham H.M., Sim X., Yang L., Fuchsberger C., Cederberg H. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat. Genet. 2013;45:197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huyghe, J.R., Jackson, A.U., Fogarty, M.P., Buchkovich, M.L., Stančakova, A., Stringham, H.M., Sim, X., Yang, L., Fuchsberger, C., Cederberg, H., et al. (2013). Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat. Genet. 45, 197-201. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary genetic association results can be found at http://www.thelooslab.com/#data. All other relevant data are in the paper and Supplemental Data.