Abstract
Testosterone is an anabolic steroid and the principal sex hormone in males. Maintaining adequate levels of testosterone throughout the life span of male is very desirable, especially it is now well-known that low levels of testosterone is associated with various aging diseases/disorders. Therefore, still, so many research studies have focused on enhancing serum levels of testosterone in males. Here, we intended to systematically discuss and present the impact of honey on serum levels of testosterone in males. This was conducted by searching PubMed, Scopus, and Embase electronic databases for research articles from May 1993 through April 2019 using the keywords “honey” and “honeybee” versus “testosterone”. Moreover, references from relevant published articles were also reviewed and cited to frame an integral discussion, conclusion, and future research needs. In conclusion, the collective evidence, which is mainly based on in vivo system studies, reveals that oral administration of honey increases serum testosterone level in males. Mechanistically, honey may increase serum level of testosterone by increasing the production of luteinizing hormone, enhancing the viability of Leydig cells, reducing oxidative damage in Leydig cells, enhancing StAR gene expression, and inhibiting aromatase activity in the testes. However, further research studies on humans, mainly clinical trials, in this specific research approach are still needed to confirm the effect of honey on testosterone.
Keywords: Honey, Testosterone, Luteinizing hormone, Antioxidants, Oxidative stress, Leydig cells, Food science
1. Introduction
Testosterone is an anabolic steroid considered as the foremost sex hormone in males [1, 2]. Chemically, testosterone belongs to the androstane class, which is a 19-carbon tetracyclic hydrocarbon structure [3]. It contains a hydroxyl and keto groups at the seventeen and the three positions, respectively.
Since 1935, where the name “testosterone” was firstly coined, as per Scopus and PubMed data bases, to date, there are more than one hundred thousand research studies on testosterone and its biological perspectives; more than one-third of these studies were published in the last decade. One important factor behind such increased research intention is the impact of testosterone on humans' health, particularly males. Studies have shown that testosterone is not only a key contributor/controller of male reproduction and maturation of external genital features [4], but also it is associated with well-being and general health of males [5]. Low serum levels of testosterone were found to be associated with wide-range of aging disorders/diseases such as diabetes [6, 7], Alzheimer [8, 9], atherosclerosis [10, 11], cancer [12], osteoporosis [13, 14], and infertility [15, 16]. Other disorders of deficient levels of testosterone include depression [17, 18], anxiety [17, 19], and fatigue [20].
Aging in males is accompanied by a reduction in free levels of testosterone [21]. Therefore, collectively, based on the above evidence, numerous research studies have intended to present the role of specific types of foods or herbs on serum testosterone level. One such example in this specific research context was to explore the effectiveness of honeybee on testosterone, especially various honeybee products are used in folk medicine to treat numerous diseases/disorders [22, 23, 24].
In general, regardless of its variety, honey is a natural product and an ancient part of humans' food [25]. It composes mainly form sugars such as fructose (∼38%), glucose (∼32%), maltose (∼7.1%), sucrose (∼1.3%), maltodextrin and other sugars (∼1.5%), in addition to other constituents such as amino acids, enzymes (e.g., invertase [26], catalase [27], α-and β-glucosidase [28], acid phosphatase, glucose oxidase [29], and diastase [30]), vitamins (e.g., pyridoxine, thiamine, riboflavin, niacin, and pantothenic acid), minerals (iron, calcium, copper, magnesium, manganese, zinc, and phosphorus), carotenoids, and aromatic substances [31, 32]. Moreover, honey is rich with natural antioxidants such flavonoids and phenolic acids, which exerts a wide range of biological properties [31]. Furthermore, the color and the flavor of honey is due to presence of various organic acids such as gluconic acid, Levulinic acid, formic acid, citric acid, lactic acid, aspartic acid, fumaric acid, butyric acid, malic acid, succinic acid, and others [31, 33]. However, it is worth mentioning that the exact composition of honey depends on its floral source as well as the environmental and seasonal factors [34].
Here, we intended to systematically discuss and summarize the impact of honey on serum levels of testosterone. This was conducted by searching Scopus, PubMed, and Embase electronic databases for research articles (published only in English language) from May 1993 through April 2019 using the keywords “honey” and “honeybee” versus “testosterone”. Moreover, references from relevant published articles were also discussed and cited to accomplish an integral review and conclusion.
Main text
2.1. Effect of honey on serum testosterone
Table 1 demonstrates the direct research studies conducted on honey and its reported effects on testosterone. As shown in the table, the target of these studies was serum testosterone; only one study was targeted urinary testosterone (marked by UT). The studies were conducted on different honey varieties from different countries (India, Malaysia, Turkey, Iran, Nigeria, and Italy). Up to the present time, the majority of these studies were animal studies, in particular, in rodent studies; only two studies were conducted on human males.
Table 1.
A summary of the research studies conducted on honey and its reported effects on serum and urinary levels of testosterone.
| Honey variety (source) | Dose | Duration | Population | Effect on testosterone | Ref. |
|---|---|---|---|---|---|
| Honey (India) | 2 mL day−1 | 30 days | Male bonnet monkeys | (±) ST | [78] |
| Honey (Aboca, Italy) | 20 g day−1 | 21 days | Human males | (±) UT | [79] |
| Tualang honey (Malaysia) | 0.2 g kg−1 | 2 weeks | Ovariectomized female rats | (+) ST | [80] |
| Honey (Iran) | 1 g kg−1day−1 | 40 days | Diabetes-induced male rats | (+) ST | [41] |
| Honey (Iran) | 1 g kg−1day−1 | 40 days | Male rats | (+) ST | [41] |
| Tualang honey (Malaysia) | 20 g (Control: 250 g of Tribulus terrestris) | 12 weeks | Oligospermic males | (±) ST | [81] |
| Honey (Nigeria) | 100 mg kg−1 of body weight | 35 days | Wistar rats | (±) ST | [40] |
| Honey (Nigeria) + nicotine | 100 mg kg−1 of body weight +1 mg kg−1 body weight | 35 days | Wistar rats | (+) ST | [40] |
| Mad honey (Turkey) | 80 mg kg−1 | 30 days | Male rats | (+) ST | [82] |
| Persian honey (Iran) | 5% of diet | During ischemia +50 days reperfusion | Male rats with ischemia/reperfusion induced testicular injury | (+) ST | [42] |
| Honey (Nigerian) | 10 mL kg −1 day−1 | 4 weeks | Sucrose-fed male rats | (+) ST | [83] |
| Persian honey (Iran) | 5% of diet | During ischemia +50 days reperfusion | Chemotherapy (Busulfan)-treated male rats | (+) ST | [84] |
| Persian honey (Iran) | 5% of diet | During ischemia +50 days reperfusion | Male rats with ischemia/reperfusion induced testicular injury | (+) ST | [84] |
| Tualang honey (Malaysia) | 1.2 g kg−1 day−1 | 13 weeks | Adult male rats exposed to cigarette smoke | (+) ST | [56] |
(+) Increase; (–) Decrease; (±) No effect; ST: serum testosterone; UT: urinary testosterone.
As illustrated in the Table 1, all summarized studies that show the effect of honey on testosterone were conducted on male population. Only one in-vivo study was conducted on ovariectomized female rats. The honey doses that are utilized in the in-vivo system studies were vary from ∼80 mg to 14.5 g kg−1 of body weight per day. These doses, in humans, for an adult male of 60 kg weight, equals to approximately 13 mg to 2.35 g kg−1 of body weight [35]. And, the duration of supplementation in these studies were ranged from ∼14 days to ∼50 days. While, in both human studies, the dose used was 20 g per day over approximately 3–12 weeks duration.
Collectively, as a result, the main stream of this specific research context reveals that oral administration of honey increases serum testosterone level in males. As summarized in the table, the effect of honey on testosterone is ranging from “positive” (+) to “no change” (±); there is no any “negative” (-) influence of honey administration on testosterone level. However, still, there is lack of human studies in this specific research approach.
2.2. Mechanistic studies
2.2.1. Effect of honey on luteinizing hormone
Luteinizing hormone, a glycoprotein also named lutropin, is released from the anterior pituitary in response to the pulses by gonadotropin-releasing hormone [36, 37]. uteinizing hormone acts on Leydig cells, polyhedral epithelioid cells located adjacent to the seminiferous tubules, in the testes [36]. Luteinizing hormone regulates the expression of 17β-hydroxysteroid dehydrogenase, an enzyme catalyzes the conversion of androstenedione [38], a steroid hormone also termed androst-4-ene-3,17-dione, to testosterone, which induces certain genes in testicular Sertoli cells and enhances the differentiation of spermatogonia [5, 39]. Therefore, mechanistically, serum testosterone level is directly influenced by the amount of luteinizing hormone produced.
In this particular discussion, the effect of honey on luteinizing hormone has been revealed in a number of studies; for example, the study conducted by Kolawole et al. have shown that nicotine-treated Wistar rats orally administered honey at 100 mg kg−1 of body weight, for 35 days, had higher serum level of luteinizing hormone compared with the control [40]. In addition, honey supplemented at 1 g kg−1 day−1, for 40 days, was found to upregulate luteinizing hormone in both normal and diabetes-induced male rats [41]. Moreover, male rats with ischemia/reperfusion induced testicular injury fed honey at 5% of the diet had higher serum levels of luteinizing hormone compared with the control [42]. According to this evidence, the positive effect of honey on serum testosterone level is modulated by increased production of luteinizing hormone.
2.2.2. Effect of honey on testicular tissue
A study conducted by Mohamed et al. (2011) showed that honey at 1.2 g kg−1 day−1, for 13 weeks, increases the number of Leydig cells, seminiferous tubules diameter, and epithelial heights of the testes in male rats with cigarette smoke-induced testicular damage [43]. In addition, assessed by electron micrograph, honey was found to maintain viable and normal structure (e.g., normal cellular organelles and nucleus) of Leydig cells in the testes of male marmosets [44]. Moreover, bee products mixture (80 mg honey, 60 mg pollen, 20 mg larvae, 20 mg royal jelly, and 2 mL propolis) twice a day (30 and 60 mg) protected against pentylenetetrazole-induced testicular damage (particularly Leydig cells and seminiferous tubules) in male Wistar rat [45]. Accordingly, it can be suggested that honey administration is beneficial, at least, to maintain normal testicular tissue, which afterwards positively influence the production of testosterone.
2.2.3. Antioxidant activity of honey: effect on serum testosterone
The antioxidant activity of honey has been revealed in various occasions [46, 47, 48]. Above and beyond, it was suggested to be a useful marker for determining the variety of honey (i.e., botanical origin of honey) [34]. In fact, the antioxidant activity of honey is attributable to the presence of various phenolic compounds (flavonoids and non-flavonoids (mainly phenolic acids)) such as caffeic acid, vanillic acid, þ-coumaric acid, syringic acid, ferulic acid, quercetin, myricetin, kaempferol, pinobanksin, chrysin, pinocembrin, ellagic acid, 3-hydroxybenzoic acid, galangin, 4-hydroxybenzoic acid, chlorogenic acid, rosmarinic acid, hesperetin, gallic acid, and others [31, 49].
In general, accumulation of free radicals, in particular reactive oxygen species [50, 51], in live cells over antioxidants leads to formation of oxidative stress [52]. It is well-known that the state of oxidative stress diminishes the function of the cell [53, 54], which may be attributable to increased levels of oxidative injury to the main functional biomolecules (e.g., proteins) [55]. In particular, such oxidative stress state, when occurs in Leydig cells in the testes, may reduce testosterone synthesis [6], and hence the level of serum testosterone. It was reported that oxidative stress reduces both enzymatic and non-enzymatic antioxidant reservoir in Leydig cells [41]. Accordingly, given that honey is very rich with antioxidants, administration of honey may enhance the performance of Leydig cells, and hence the production of testosterone, mainly by enhancing the antioxidant defense mechanism.
Indeed, for example, it has been shown that adult male Sprague-Dawley rats exposed (whole body exposure) three times daily to cigarette smoke for 13 weeks had higher testicular levels of lipid peroxidation (high thiobarbituric acid reactive substances) and oxidative stress (lower total antioxidant capacity, reduced superoxide dismutase activity, and reduced catalase activity) [43], and honey supplementation at 1.2 g−1 kg−1 day−1 during the same period of time significantly reduced the testicular lipid peroxidation (low thiobarbituric acid reactive substances) and oxidative stress (higher levels of total antioxidant capacity and restored levels of superoxide dismutase and catalase activities) [43]. Directly, adult male rats exposed to cigarette smoke supplemented, for 13 weeks, with Tualang honey at 1.2 g−1 kg−1 day−1 had higher level of serum testosterone compared with the control [56]. In addition, another in-vivo system study conducted by Nasrolahi et al. concluded that administration of honey at 1.0 g kg−1 day−1, for 40 days, inhibits diabetes-induced oxidative damage in testicular tissues and increases serum level of testosterone.
2.2.4. Effect of bioactive molecule chrysin on aromatase (estrogen synthase) enzyme
Chrysin (5,7-dihydroxyflavone) is a flavonoid presents at high levels in propolis and honey [57]. It has a wide range of pharmacological properties, mainly antioxidant [58], anticancer [59], and anti-inflammatory [60]. It has been shown that chrysin, orally supplemented at 50 mg kg−1 day−1, for 2 months, significantly increased the testicular level of glutathione as well as the activity of the antioxidant enzymes (catalase, glutathione peroxidase, and superoxide dismutase) [61]. Chrysin has been recognized as a potent inhibitor of the enzyme aromatase, which is an enzyme catalyzes the conversion of testosterone to estradiol [62, 63]. Such biological properties has let chrysin, especially when used at high doses, to be used as a testosterone-boosting agent [61, 64, 65, 66]. Accordingly, administration of honey, as a rich source of chrysin, may enhance, albeit partially, the production of testosterone.
Several researches have demonstrated the effect of chrysin on the upregulation of the StAR gene [65, 67]. Chrysin was found to stimulate testicular steroidogenesis by enhancing the expression of the StAR gene, which is encoding for steroidogenic acute regulatory protein (StAR protein) [65]. StAR protein plays an important role in the steroidogenesis process by facilitating the transport of cholesterol from the outer mitochondrial membrane to the internal mitochondrial membrane [65]. Thus, cholesterol cleavage occurs into pregnenolone in Leydig cells, which increases the synthesis of testosterone.
2.2.5. Effect of certain bioactive molecules in honey on testosterone production
Quercetin, a potent antioxidant flavonoid, was found to be present in honey. The effect of quercetin on testosterone has been explored in various occasions. For example, quercetin supplemented for 15 days at approximately 50 mg kg−1day−1 was found to enhance serum level of testosterone in male rats with arsenic-induced reproductive toxicity [68]. In addition, quercetin supplemented at approximately 10 mg−1 kg−1 for 3 weeks significantly enhanced testosterone level in male rats with atrazine-induced reproductive damage [69]. Moreover, male rats with di-(2-ethylhexyl) phthalate-induced reproductive damage administered quercetin at 0.09 g−1 kg−1 day−1, for 15 days, had higher serum levels of testosterone compared with the control [70]. Further, quercetin at 20 mg−1 kg−1, for 14 days, restored the plasma levels of testosterone in male rats with sulphasalazine-induced reproductive toxicity [71]. Furthermore, quercetin at 20 mg−1 kg−1, for 4 weeks, attenuated testosterone depletion in male Wistar rats induced by cadmium compared with the control [72]. This evidence underscores the role of quercetin, and hence, albeit partially, the role of honey, in enhancing testosterone production, especially in chemical-induced reproductive toxicity conditions.
Caffeic acid, another phenolic compound present in honey, was found to enhance serum testosterone. For example, it was shown that male albino Wistar rats orally supplemented with caffeic acid at 50 mg kg−1 had higher serum levels of testosterone and luteinizing hormone compared with the control [73]. In addition, caffeic acid phenethyl ester, given intraperitonially at 10 μmol kg−1, for 30 days, enhanced serum testosterone level in male rats with cadmium-induced reproductive toxicity [74].
Also, ellagic acid, rosmarinic acid, p-coumaric acid, and ferulic acid are phenolic compounds present in honey and were found, whether directly or indirectly, to have positive effects on serum testosterone. Ellagic acid and rosmarinic acid at 10 mg kg−1day−1 and 75 mg kg−1day−1, respectively, for 2 weeks, were found to attenuate doxorubicin-induced testicular damage in male Sprague-Dawley rats [75]. p-coumaric acid at 50, 100, and 200 mg kg−1, for 4 weeks, significantly increased testosterone in male rats with ethanol-induced reproductive toxicity [76]. Administration of ferulic acid at 50 mg kg−1, for 8 weeks, restored serum testosterone levels in male rats with lead-induced testicular damage [77]. Collectively, it is evident that honey is rich with potent biomolecules that have a positive effect on testosterone production.
3. Conclusion
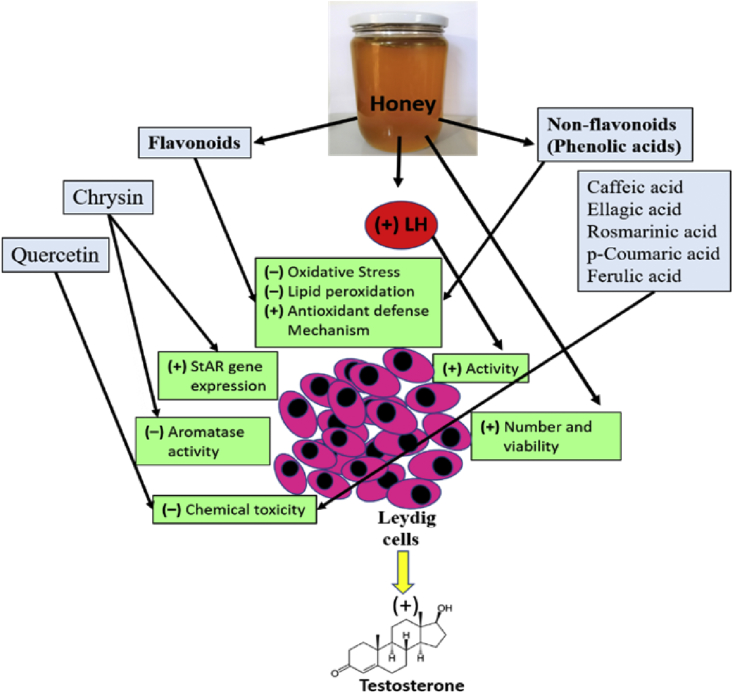
In conclusion, collectively, the main stream of this specific research approach reveals that oral administration of honey enhances serum testosterone level in males. The mechanisms by which honey increases serum testosterone may be by enhancing the production of luteinizing hormone, enhancing the viability of Leydig cells, reducing testicular oxidative injury, enhancing StAR gene expression, and inhibiting aromatase activity in the testes (Fig. 1). In addition, honey has been found to contain various bioactive compounds (e.g., phenolic acids) that may improve testosterone production. However, still, there is lack in the number of human studies in this research context. Therefore, conducting clinical trials that reveal the mechanisms of honey on serum testosterone level will be of great importance.
Fig. 1.
Mechanisms of honey on testosterone level. LH, luteinizing hormone; (+), increase; (–), decrease.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Gagnidze K., Pfaff D.W. Sex on the brain. Cell. 2009;139:19–21. doi: 10.1016/j.cell.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Banihani S.A. Effect of paracetamol on semen quality. Andrologia. 2018;50 doi: 10.1111/and.12874. [DOI] [PubMed] [Google Scholar]
- 3.Karakas M., Schafer S., Appelbaum S., Ojeda F., Kuulasmaa K., Bruckmann B., Berisha F., Schulte-Steinberg B., Jousilahti P., Blankenberg S. Testosterone levels and type 2 diabetes-no correlation with age, differential predictive value in men and women. Biomolecules. 2018;8 doi: 10.3390/biom8030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNairn A.J., Chuang C.H., Bloom J.C., Wallace M.D., Schimenti J.C. Female-biased embryonic death from inflammation induced by genomic instability. Nature. 2019;567:105–108. doi: 10.1038/s41586-019-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banihani S.A. Testosterone in males as enhanced by onion (allium cepa l.) Biomolecules. 2019;9 doi: 10.3390/biom9020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banihani S.A. Ginger and testosterone. Biomolecules. 2018;8 doi: 10.3390/biom8040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Li X., Cai Z., Li H., Yang B. Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis. Aging Male. 2019:1–12. doi: 10.1080/13685538.2018.1557139. [DOI] [PubMed] [Google Scholar]
- 8.Asih P.R., Tegg M.L., Sohrabi H., Carruthers M., Gandy S.E., Saad F., Verdile G., Ittner L.M., Martins R.N. Multiple mechanisms linking type 2 diabetes and alzheimer's disease: testosterone as a modifier. J. Alzheimer's Dis. 2017;59:445–466. doi: 10.3233/JAD-161259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike C.J. Sex and the development of alzheimer's disease. J. Neurosci. Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kloner R.A., Carson C., 3rd, Dobs A., Kopecky S., Mohler E.R., 3rd. Testosterone and cardiovascular disease. J. Am. Coll. Cardiol. 2016;67:545–557. doi: 10.1016/j.jacc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Budoff M.J., Ellenberg S.S., Lewis C.E., Mohler E.R., 3rd, Wenger N.K., Bhasin S., Barrett-Connor E., Swerdloff R.S., Stephens-Shields A., Cauley J.A. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. J. Am. Med. Assoc. 2017;317:708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan A.L., Hu J.C., Morgentaler A., Mulhall J.P., Schulman C.C., Montorsi F. Testosterone therapy in men with prostate cancer. Eur. Urol. 2016;69:894–903. doi: 10.1016/j.eururo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamad N.V., Soelaiman I.N., Chin K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging. 2016;11:1317–1324. doi: 10.2147/CIA.S115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X., Li J., Zhang H., Wang H., Yin G., Miao D. Pyrroloquinoline quinone prevents testosterone deficiency-induced osteoporosis by stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption. Am J Transl Res. 2017;9:1230–1242. [PMC free article] [PubMed] [Google Scholar]
- 15.Banihani S.A. Effect of coenzyme q10 supplementation on testosterone. Biomolecules. 2018;8 doi: 10.3390/biom8040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Migdadi F., Banihani I., Banihani S.A. Clinico-hormonal correlation of oligospermic patients in the below sea level environment (Jordan valley) Neuroendocrinol. Lett. 2005;26:13–18. [PubMed] [Google Scholar]
- 17.McHenry J., Carrier N., Hull E., Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 2014;35:42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland W.E., Worthman C., Shanahan L., Costello E.J., Angold A. Early pubertal timing and testosterone associated with higher levels of adolescent depression in girls. J. Am. Acad. Child Adolesc. Psychiatry. 2019 doi: 10.1016/j.jaac.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder P.J., Bhasin S., Cunningham G.R., Matsumoto A.M., Stephens-Shields A.J., Cauley J.A., Gill T.M., Barrett-Connor E., Swerdloff R.S., Wang C. Effects of testosterone treatment in older men. N. Engl. J. Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petering R.C., Brooks N.A. Testosterone therapy: review of clinical applications. Am. Fam. Phys. 2017;96:441–449. [PubMed] [Google Scholar]
- 21.Vermeulen A., Goemaere S., Kaufman J.M. Testosterone, body composition and aging. J. Endocrinol. Investig. 1999;22:110–116. [PubMed] [Google Scholar]
- 22.Seres A., Ducza E., Gaspar R. Investigation of gestagenic effect of raw drone milk in rats. Acta Pharm. Hung. 2014;84:77–81. [PubMed] [Google Scholar]
- 23.Badolato M., Carullo G., Cione E., Aiello F., Caroleo M.C. From the hive: honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017;142:290–299. doi: 10.1016/j.ejmech.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 24.Samarghandian S., Farkhondeh T., Samini F. Honey and health: a review of recent clinical research. Pharmacogn. Res. 2017;9:121–127. doi: 10.4103/0974-8490.204647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zubair R., Aziz N. As smooth as honey--the historical use of honey as topical medication. JAMA Dermatol. 2015;151:1102. doi: 10.1001/jamadermatol.2015.1764. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez M.P., Huidobro J.F., Mato I., Muniategui S., Sancho M.T. Evolution of invertase activity in honey over two years. J. Agric. Food Chem. 2001;49:416–422. doi: 10.1021/jf0003350. [DOI] [PubMed] [Google Scholar]
- 27.Huidobro J.F., Sanchez M.P., Muniategui S., Sancho M.T. Precise method for the measurement of catalase activity in honey. J. AOAC Int. 2005;88:800–804. [PubMed] [Google Scholar]
- 28.Kubota M., Tsuji M., Nishimoto M., Wongchawalit J., Okuyama M., Mori H., Matsui H., Surarit R., Svasti J., Kimura A. Localization of alpha-glucosidases i, ii, and iii in organs of european honeybees, apis mellifera l., and the origin of alpha-glucosidase in honey. Biosci. Biotechnol. Biochem. 2004;68:2346–2352. doi: 10.1271/bbb.68.2346. [DOI] [PubMed] [Google Scholar]
- 29.Majtan J., Bohova J., Prochazka E., Klaudiny J. Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food. 2014;17:290–293. doi: 10.1089/jmf.2012.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sak-Bosnar M., Sakac N. Direct potentiometric determination of diastase activity in honey. Food Chem. 2012;135:827–831. doi: 10.1016/j.foodchem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C., Fett R. Honey. Chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Ciulu M., Solinas S., Floris I., Panzanelli A., Pilo M.I., Piu P.C., Spano N., Sanna G. Rp-hplc determination of water-soluble vitamins in honey. Talanta. 2011;83:924–929. doi: 10.1016/j.talanta.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 33.Mato I., Huidobro J.F., Simal-Lozano J., Sancho M.T. Significance of nonaromatic organic acids in honey. J. Food Prot. 2003;66:2371–2376. doi: 10.4315/0362-028x-66.12.2371. [DOI] [PubMed] [Google Scholar]
- 34.Dzugan M., Tomczyk M., Sowa P., Grabek-Lejko D. Antioxidant activity as biomarker of honey variety. Molecules. 2018;23 doi: 10.3390/molecules23082069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 36.Zirkin B.R., Papadopoulos V. Leydig cells: formation, function, and regulation. Biol. Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatiades G.A., Kaiser U.B. Gonadotropin regulation by pulsatile gnrh: signaling and gene expression. Mol. Cell. Endocrinol. 2018;463:131–141. doi: 10.1016/j.mce.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaya D.S., Augstine J., Menon V.P. Protective effect of testosterone against alcohol and paracetamol induced hepatotoxicity in rats. Indian J. Exp. Biol. 1995;33:194–200. [PubMed] [Google Scholar]
- 39.Wang Y., Chen F., Ye L., Zirkin B., Chen H. Steroidogenesis in leydig cells: effects of aging and environmental factors. Reproduction. 2017;154:R111–R122. doi: 10.1530/REP-17-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolawole T.A., Oyeyemi W.A., Adigwe C., Leko B., Udeh C., Dapper D.V. Honey attenuates the detrimental effects of nicotine on testicular functions in nicotine treated wistar rats. Niger. J. Physiol. Sci. 2015;30:11–16. [PubMed] [Google Scholar]
- 41.Nasrolahi O., Khaneshi F., Rahmani F., Razi M. Honey and metformin ameliorated diabetes-induced damages in testes of rat; correlation with hormonal changes. Iran J. Reprod. Med. 2013;11:1013–1020. [PMC free article] [PubMed] [Google Scholar]
- 42.Gholami M., Abbaszadeh A., Khanipour Khayat Z., Anbari K., Baharvand P., Gharravi A.M. Honey improves spermatogenesis and hormone secretion in testicular ischaemia-reperfusion-induced injury in rats. Andrologia. 2018;50 doi: 10.1111/and.12804. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed M., Sulaiman S.A., Jaafar H., Sirajudeen K.N. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. Int. J. Mol. Sci. 2011;12:5508–5521. doi: 10.3390/ijms12095508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijaykumar T., Singh D., Vanage G.R., Dhumal R.V., Dighe V.D. Bisphenol a-induced ultrastructural changes in the testes of common marmoset. Indian J. Med. Res. 2017;146:126–137. doi: 10.4103/ijmr.IJMR_927_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarraga-Galindo N., Vergara-Aragon P., Rosales-Melendez S., Ibarra-Guerrero P., Dominguez-Marrufo L.E., Oviedo-Garcia R.E., Hernandez-Ramirez H., Hernandez-Tellez B., Lopez-Martinez I.E., Sanchez-Cervantes I. Effects of bee products on pentylenetetrazole-induced seizures in the rat. Proc. West. Pharmacol. Soc. 2011;54:33–40. [PubMed] [Google Scholar]
- 46.Gyergyak K., Boros B., Marton K., Felinger A., Papp N., Farkas A. Bioactive constituents and antioxidant activity of some carpathian basin honeys. Nat. Prod. Commun. 2016;11:245–250. [PubMed] [Google Scholar]
- 47.Ahmad N.S., Abdul Aziz A., Kong K.W., Hamid M.S.A., Cheong J.P.G., Hamzah S.H. Dose-response effect of tualang honey on postprandial antioxidant activity and oxidative stress in female athletes: a pilot study. J. Altern. Complement. Med. 2017;23:989–995. doi: 10.1089/acm.2017.0129. [DOI] [PubMed] [Google Scholar]
- 48.Kus P.M., Jerkovic I., Tuberoso C.I., Marijanovic Z., Congiu F. Cornflower (centaurea cyanus l.) honey quality parameters: chromatographic fingerprints, chemical biomarkers, antioxidant capacity and others. Food Chem. 2014;142:12–18. doi: 10.1016/j.foodchem.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Suarez J.M., Giampieri F., Gonzalez-Paramas A.M., Damiani E., Astolfi P., Martinez-Sanchez G., Bompadre S., Quiles J.L., Santos-Buelga C., Battino M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012;50:1508–1516. doi: 10.1016/j.fct.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 50.Hani S.B., Bayachou M. Salvia fruticosa reduces intrinsic cellular and h2o2-induced DNA oxidation in hek 293 cells; assessment using flow cytometry. Asian Pac. J. Trop. Biomed. 2014;4:399–403. doi: 10.12980/APJTB.4.2014C1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banihani S.A. Histamine-2 receptor antagonists and semen quality. Basic Clin. Pharmacol. Toxicol. 2016;118:9–13. doi: 10.1111/bcpt.12446. [DOI] [PubMed] [Google Scholar]
- 52.Banihani S.A. Vitamin b12 and semen quality. Biomolecules. 2017;7 doi: 10.3390/biom7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banihani S.A. Radish (raphanus sativus) and diabetes. Nutrients. 2017;9 doi: 10.3390/nu9091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alzoubi K.H., Hasan Z.A., Khabour O.F., Mayyas F.A., Al Yacoub O.N., Banihani S.A., Azab M.A., Alrabadi N. The effect of high-fat diet on seizure threshold in rats: role of oxidative stress. Physiol. Behav. 2018;196:1–7. doi: 10.1016/j.physbeh.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 56.Mahaneem Mohamed, Sulaiman Siti Amrah, Jaafar Hasnan, Sirajudeen Kuttulebbai Nainamohamed Salam, Ismail Zul Izhar Mohd, Islam M.N. Effect of honey on testicular functions in rats exposed to cigarette smoke. J. ApiProduct ApiMed. Sci. 2011;3:12–17. [Google Scholar]
- 57.Wang X., Morris M.E. Effects of the flavonoid chrysin on nitrofurantoin pharmacokinetics in rats: potential involvement of abcg2. Drug Metab. Dispos. 2007;35:268–274. doi: 10.1124/dmd.106.011684. [DOI] [PubMed] [Google Scholar]
- 58.Lapidot T., Walker M.D., Kanner J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J. Agric. Food Chem. 2002;50:7220–7225. doi: 10.1021/jf020615a. [DOI] [PubMed] [Google Scholar]
- 59.Patel R.V., Mistry B., Syed R., Rathi A.K., Lee Y.J., Sung J.S., Shinf H.S., Keum Y.S. Chrysin-piperazine conjugates as antioxidant and anticancer agents. Eur. J. Pharm. Sci. 2016;88:166–177. doi: 10.1016/j.ejps.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Hwang S.H., Kim H.Y., Zuo G., Wang Z., Lee J.Y., Lim S.S. Anti-glycation, carbonyl trapping and anti-inflammatory activities of chrysin derivatives. Molecules. 2018;23 doi: 10.3390/molecules23071752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciftci O., Ozdemir I., Aydin M., Beytur A. Beneficial effects of chrysin on the reproductive system of adult male rats. Andrologia. 2012;44:181–186. doi: 10.1111/j.1439-0272.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 62.Jeong H.J., Shin Y.G., Kim I.H., Pezzuto J.M. Inhibition of aromatase activity by flavonoids. Arch Pharm. Res. (Seoul) 1999;22:309–312. doi: 10.1007/BF02976369. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira G.A., Ferraz E.R., Souza A.O., Lourenco R.A., Oliveira D.P., Dorta D.J. Evaluation of the mutagenic activity of chrysin, a flavonoid inhibitor of the aromatization process. J. Toxicol. Environ. Health. 2012;75:1000–1011. doi: 10.1080/15287394.2012.696517. [DOI] [PubMed] [Google Scholar]
- 64.Brown G.A., Vukovich M.D., Martini E.R., Kohut M.L., Franke W.D., Jackson D.A., King D.S. Endocrine and lipid responses to chronic androstenediol-herbal supplementation in 30 to 58 year old men. J. Am. Coll. Nutr. 2001;20:520–528. doi: 10.1080/07315724.2001.10719061. [DOI] [PubMed] [Google Scholar]
- 65.Jana K., Yin X., Schiffer R.B., Chen J.J., Pandey A.K., Stocco D.M., Grammas P., Wang X. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse leydig cells. J. Endocrinol. 2008;197:315–323. doi: 10.1677/JOE-07-0282. [DOI] [PubMed] [Google Scholar]
- 66.Dhawan K., Kumar S., Sharma A. Beneficial effects of chrysin and benzoflavone on virility in 2-year-old male rats. J. Med. Food. 2002;5:43–48. doi: 10.1089/109662002753723214. [DOI] [PubMed] [Google Scholar]
- 67.Cormier M., Ghouili F., Roumaud P., Bauer W., Touaibia M., Martin L.J. Influences of flavones on cell viability and camp-dependent steroidogenic gene regulation in ma-10 leydig cells. Cell Biol. Toxicol. 2018;34:23–38. doi: 10.1007/s10565-017-9395-8. [DOI] [PubMed] [Google Scholar]
- 68.Baltaci B.B., Uygur R., Caglar V., Aktas C., Aydin M., Ozen O.A. Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia. 2016;48:1202–1213. doi: 10.1111/and.12561. [DOI] [PubMed] [Google Scholar]
- 69.Abdel Aziz R.L., Abdel-Wahab A., Abo El-Ela F.I., Hassan N.E.Y., El-Nahass E.S., Ibrahim M.A., Khalil A.A.Y. Dose- dependent ameliorative effects of quercetin and l-carnitine against atrazine- induced reproductive toxicity in adult male albino rats. Biomed. Pharmacother. 2018;102:855–864. doi: 10.1016/j.biopha.2018.03.136. [DOI] [PubMed] [Google Scholar]
- 70.Abd-Ellah M.F., Aly H.A., Mokhlis H.A., Abdel-Aziz A.H. Quercetin attenuates di-(2-ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum. Exp. Toxicol. 2016;35:232–243. doi: 10.1177/0960327115580602. [DOI] [PubMed] [Google Scholar]
- 71.Osawe S.O., Farombi E.O. Quercetin and rutin ameliorates sulphasalazine-induced spermiotoxicity, alterations in reproductive hormones and steroidogenic enzyme imbalance in rats. Andrologia. 2018;50 doi: 10.1111/and.12981. [DOI] [PubMed] [Google Scholar]
- 72.Ujah G.A., Nna V.U., Agah M.I., Omue L.O., Leku C.B., Osim E.E. Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male wistar rats. Andrologia. 2018;50 doi: 10.1111/and.12866. [DOI] [PubMed] [Google Scholar]
- 73.Erboga M., Kanter M., Aktas C., Bozdemir Donmez Y., Fidanol Erboga Z., Aktas E., Gurel A. Anti-apoptotic and anti-oxidant effects of caffeic acid phenethyl ester on cadmium-induced testicular toxicity in rats. Biol. Trace Elem. Res. 2016;171:176–184. doi: 10.1007/s12011-015-0509-y. [DOI] [PubMed] [Google Scholar]
- 74.Akomolafe S.F., Akinyemi A.J., Oboh G., Oyeleye S.I., Ajayi O.B., Omonisi A.E., Owolabi F.L., Atoyebi D.A., Ige F.O., Atoki V.A. Co-administration of caffeine and caffeic acid alters some key enzymes linked with reproductive function in male rats. Andrologia. 2018;50 doi: 10.1111/and.12839. [DOI] [PubMed] [Google Scholar]
- 75.Georgy G.S., Maher O.W. Ellagic acid and rosmarinic acid attenuate doxorubicin-induced testicular injury in rats. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21937. [DOI] [PubMed] [Google Scholar]
- 76.Nishi K., Ramakrishnan S., Gunasekaran V.P., Parkash K., Ramakrishnan A., Vijayakumar N., Ganeshan M. Protective effects of pcoumaric acid on ethanol induced male reproductive toxicity. Life Sci. 2018;209:1–8. doi: 10.1016/j.lfs.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 77.Hasanein P., Fazeli F., Parviz M., Roghani M. Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia. 2018;50 doi: 10.1111/and.12798. [DOI] [PubMed] [Google Scholar]
- 78.Gill-Sharma M.K., D'Souza S., Parte P., Balasinor N., Choudhuri J., Majramkar D.D., Aleem M., Juneja H.S. Effect of oral tamoxifen on semen characteristics and serum hormone profile in male bonnet monkeys. Contraception. 2003;67:409–413. doi: 10.1016/s0010-7824(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 79.Gambelunghe C., Rossi R., Sommavilla M., Ferranti C., Rossi R., Ciculi C., Gizzi S., Micheletti A., Rufini S. Effects of chrysin on urinary testosterone levels in human males. J. Med. Food. 2003;6:387–390. doi: 10.1089/109662003772519967. [DOI] [PubMed] [Google Scholar]
- 80.Zaid S.S., Sulaiman S.A., Sirajudeen K.N., Othman N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats--animal model for menopause. BMC Complement Altern. Med. 2010;10:82. doi: 10.1186/1472-6882-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ismail S.B., Bakar M.B., Nik Hussain N.H., Norhayati M.N., Sulaiman S.A., Jaafar H., Draman S., Ramli R., Wan Yusoff W.Z. Comparison on the effects and safety of tualang honey and tribestan in sperm parameters, erectile function, and hormonal profiles among oligospermic males. Evid Based Complement Alternat Med. 2014;2014:126138. doi: 10.1155/2014/126138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatli O., Karaca Y., Turkmen S., Gulgen G.S., Sahin A., Eryigit U., Fazli O., Karaguzel E., Mentese A., Orem A. The effect of mad honey on testosterone levels of male rats. Bratisl. Lek. Listy. 2016;117:677–680. doi: 10.4149/BLL_2016_130. [DOI] [PubMed] [Google Scholar]
- 83.Oyelowo Oluwakemi Tinuolaoluwa, Adekunbi Daniel Abiodun, Dada K.A. Protective role of nigerian honey on sperm indices and testis in sucrose-fed rats. Bangladesh J. Med. Sci. 2014;13:180–189. [Google Scholar]
- 84.Gholami M., Abbaszadeh A., Baharvand P., Hasanvand A., Hasanvand A., Gharravi A.M. Protective effects of Persian honey, apis mellifera meda skorikov on side effects of chemotherapy and ischemia/reperfusion induced testicular injury. J. Complement. Integr. Med. 2018;15 doi: 10.1515/jcim-2016-0035. [DOI] [PubMed] [Google Scholar]