Abstract
Atomoxetine is a non-stimulant medication used to treat attention-deficit/hyperactivity disorder. CYP2D6 polymorphisms influence the metabolism of atomoxetine thereby affecting drug efficacy and safety. We summarize evidence from the published literature supporting these associations and provide therapeutic recommendations for atomoxetine based on CYP2D6 genotype (updates at www.cpicpgx.org).
Keywords: atomoxetine, ADHD, CYP2D6, CPIC, pharmacogenetics, pharmacogenomics
INTRODUCTION
The purpose of this guideline is to provide information to allow the interpretation of clinical CYP2D6 genotype tests so that the results can be used to guide the use of atomoxetine. Detailed guidelines for prescribing of atomoxetine as well as analyses of cost effectiveness are beyond the scope of this document. The Clinical Pharmacogenetic Implementation Consortium (CPIC®) guidelines are periodically updated at https://cpicpgx.org/guidelines/ and http://www.pharmgkb.org.
FOCUSED LITERATURE REVIEW
A systematic literature review focused on CYP2D6 genotype and atomoxetine use was conducted (details in Supplement).
GENE: CYP2D6
CYP2D6 is highly polymorphic with over 100 known allelic variants and subvariants identified (www.PharmVar.org; CYP2D6 Allele Definition Table (1, 2)). CYP2D6 alleles have been extensively studied in multiple geographically, racially, and ethnically diverse groups and significant differences in allele frequencies have been observed (CYP2D6 Allele Frequency Table (1, 2)). The most commonly reported alleles are categorized into functional groups as follows: normal function (e.g., CYP2D6*1, *2 and *35), decreased function (e.g., CYP2D6*9, *10, *17, *29 and *41), and no function (e.g., CYP2D6*3-*6) (3, 4). Because CYP2D6 is subject to deletions and gene duplications or multiplications, many clinical laboratories also report copy number variations. CYP2D6*5 represents a gene deletion (no function allele) whereas gene duplications and multiplications are denoted by “xN” (e.g., CYP2D6*1xN with xN representing the number of CYP2D6 gene copies). Alleles carrying two or more normal function gene copies are categorized as alleles with increased function.
The combination of alleles is used to determine a patient’s diplotype. Each functional group is assigned an activity value ranging from 0 to 1 (e.g., 0 for no, 0.5 for decreased and 1.0 for normal function) (4). If an allele contains multiple copies of a functional gene, the value is multiplied by the number of copies present. Thus, the CYP2D6 activity score is the sum of the values assigned to each allele, which typically range from 0 to 3.0 but may exceed 3.0 in rare cases (4).
The CYP2D6 activity score can be translated into a standardized phenotype classification system (CYP2D6 Allele Definition Table (1, 2)): patients with an activity score of 0 are poor metabolizers (PMs), those with a score of 0.5 are considered intermediate metabolizers (IMs), those with a score of 1.0 −2.0 represent normal metabolizers (NMs) and patients with a score >2 are classified as ultrarapid metabolizers (UMs). However, diplotypes with an activity score of 1.0 give rise to less activity towards certain drugs including tamoxifen and atomoxetine compared to those with an AS of 1. 5 or 2.0; therefore, patients with an activity score of 1.0 may be classified as IMs by some reference laboratories. Thus, for this guideline, an activity score of 1.0 is classified as a CYP2D6 NM or IM (Table 1). This is in contrast to the classification used in some previous guidelines (5, 6) but similar to the recently published guideline on CYP2D6 and tamoxifen (7). Note that genotypes with an activity score of 1 are classified as NMs in the CYP2D6 Genotype to Phenotype Table (1) and CPIC will update the CPIC website and this table if needed. Efforts to standardize CYP2D6 genotype to phenotype translation system are ongoing. Currently, a CYP2D6 Genotype-to-Phenotype Working Group is also reviewing the classification of the decreased function CYP2D6*10 allele (discussions regarding the accuracy of CYP2D6*10 classification using a value of 0.5 for activity score calculation have been ongoing for years (7)). This allele appears to convey a reduction in activity across many substrates, which led to a special recommendation for CYP2D6*10-containing diplotypes for tamoxifen and for atomoxetine (Table 2).
TABLE 1.
ASSIGNMENT OF LIKELY CYP2D6 PHENOTYPES BASED ON DIPLOTYPES
| Likely phenotype b | Activity Score |
Genotypesa | Examples of CYP2D6 diplotypes |
|---|---|---|---|
| CYP2D6 ultrarapid metabolizer | >2 | An individual carrying duplications of functional alleles | *1/*1xN, *1/*2xN, *2/*2xNc |
| CYP2D6 normal metabolizer | 1.5 −2.0 | An individual carrying two normal function alleles or one normal function and one decreased function allele | *1/*1, *1/*2, *1/*9, *1/*41, *2/*2 |
| CYP2D6 normal metabolizer or intermediate metabolizer (controversy remains)d | 1.0 | An individual carrying two decreased function alleles or one normal function and one no function allele. An activity score (AS) of 1.0 is associated with decreased atomoxetine metabolism compared to those with an AS of 1.5 or 2. |
*1/*4, *1/*5, *41/*41, *10/*10 |
| CYP2D6 intermediate metabolizer | 0.5 | An individual carrying one decreased function and one no function allele | *4/*10, *4/*41, *5/*9 |
| CYP2D6 poor metabolizer | 0 | An individual carrying only no functional alleles | *3/*4, *4/*4, *5/*5, *5/*6 |
Assignment of allele function and citations for allele function can be found https://www.pharmgkb.org/page/cyp2d6RefMaterials (CYP2D6 Allele Definition Table and CYP2D6 Allele Functionality Table (1, 2)). For a complete list of CYP2D6 diplotypes and resulting phenotypes, see the CYP2D6 Genotype to Phenotype Table (1, 2). Note that genotypes with an activity score of 1 are classified as NMs in the online CYP2D6 genotype to phenotype table.
See the CYP2D6 Frequency Table for race-specific allele and phenotype frequencies or see Gaedigk et al (1, 2, 36).
Where xN represents the number of CYP2D6 gene copies. For individuals with CYP2D6 duplications or multiplications, see supplemental data for additional information on how to translate diplotypes into phenotypes.
Patients with an activity score of 1.0 may be classified as intermediate metabolizers by some reference laboratories. A group of CYP2D6 experts are currently working to standardize the CYP2D6 genotype to phenotype translation system. CPIC will update the CPIC website accordingly (CYP2D6 Genotype to Phenotype Table (1, 37)).
TABLE 2.
DOSING RECOMMENDATIONS FOR ATOMOXETINE BASED ON CYP2D6 GENOTYPE FOR CHILDREN
| Phenotype | Activity score |
Implication | Therapeutic Recommendation | Classification of Recommendationa |
|---|---|---|---|---|
| CYP2D6 ultrarapid metabolizer | >2 | Based on very limited data available for CYP2D6 ultrarapid metabolizers taking atomoxetine, it is unlikely ultrarapid metabolizers would achieve adequate serum concentrations for the intended effect at standard dosing. | Initiate with a dose of 0.5 mg/kg/day and increase to 1.2 mg/kg/day after 3 days. If no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/ml.b,c | Moderate |
| CYP2D6 normal metabolizer | 1.5–2.0 | Normal metabolizers of atomoxetine have a lower likelihood of response as compared to poor metabolizers. This is associated with increased discontinuation due to lack of efficacy as compared to poor metabolizers. | Initiate with a dose of 0.5 mg/kg and increase to 1.2 mg/kg/day after 3 days. If no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/ml.b,c | Moderate |
| CYP2D6 normal metabolizer or intermediate metabolizer (controversy remains)d |
1.0 (no *10 allele present) |
Possibly higher atomoxetine concentrations as compared to normal metabolizers but questionable clinical significance. Normal metabolizers with AS of 1 may be at an increased risk of increased discontinuation as compared to poor metabolizers. | Initiate with a dose of 0.5 mg/kg and increase to 1.2 mg/kg/day after 3 days If no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/ml.b,c | Moderate |
| CYP2D6 normal metabolizer or intermediate metabolizer (controversy remains)d |
1.0 (*10 present) |
Decreased metabolism of atomoxetine and higher atomoxetine concentrations as compared to normal metabolizers. Individuals with activity score of 1.0 with CYP2D6*10 may be at an increased risk of increased discontinuation as compared to poor metabolizers. | Initiate with a dose of 0.5 mg/kg/day and if no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a plasma concentration 2–4 h after dosing. If response is inadequate and concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Moderate |
| CYP2D6 intermediate metabolizer |
0.5 | Decreased metabolism of atomoxetine and higher atomoxetine concentrations as compared to normal metabolizers. Intermediate metabolizers may be at an increased risk of discontinuation as compared to poor metabolizers. | Initiate with a dose of 0.5 mg/kg/day and if no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a plasma concentration 2–4 h after dosing. If response is inadequate and concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Moderate |
| CYP2D6 poor metabolizer |
0 | Significantly decreased metabolism of atomoxetine may result in higher concentrations as compared to non-poor metabolizers. This may increase the occurrence of side effects, but also a greater improvement of ADHD symptoms as compared to non-poor metabolizers in those who tolerate treatment. Poor metabolizer status is associated with lower final dose requirements as compared to non-poor metabolizers. | Initiate with a dose of 0.5 mg/kg/day and if no clinical response and in the absence of adverse events after 2 weeks, consider obtaining a plasma concentration 4 h after dosing. If response is inadequate and concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Strong |
Rating scheme described in the Supplement.
Therapeutic range of 200 to 1000 ng/ml has been proposed (27).
Limited data are available regarding the relationship between atomoxetine plasma concentrations and clinical response. Available information suggests that clinical response is greater in PMs compared to non-PMs and may be related to the higher plasma concentrations 1 to 1.5 hours after dosing in PMs compared to non-PMs administered a similar dose. Furthermore, modest improvement in response, defined as reduction in ADHD-RS, is observed at peak concentrations greater than 400 ng/ml.
CPIC has general classified patients with activity score of 1 as “normal metabolizer.” However, in the case of atomoxetine, prescribing recommendations for those with an AS of 1.0 are allele-dependent, based on the presence of the CYP2D6*10 allele.
Of importance, reference laboratories providing clinical CYP2D6 genotyping may use varying methods to assign phenotypes. Therefore, it is advisable to note a patient’s CYP2D6 diplotype and to calculate its activity score before making therapeutic decisions about atomoxetine therapy. See the CYP2D6 Diplotype to Phenotype Table for a comprehensive translation of diplotype to phenotype (1, 2).
Genetic Test Interpretation
Clinical laboratories rarely sequence through the CYP2D6 gene or interrogate every known variant position. Instead, they typically test for variants that are used to determine common allele haplotypes using the star-allele (*) nomenclature system. Allele definitions are maintained by the Pharmacogene Variation Consortium (www.PharmVar.org). The CYP2D6 Allele Definition Table and CYP2D6 Allele Functionality Table and tables found on the PharmGKB website contain a list of CYP2D6 alleles (1, 2), the specific combination of variants that can be used to determine the allele, functional status, and frequency across major ethnic populations as reported in the literature.
Genetic test results are reported as diplotypes, or the combination of the maternal and paternal alleles (e.g. CYP2D6*1/*2). Phenotypes are assigned based on the reported CYP2D6 diplotype, as summarized in Table 1 and in the CYP2D6 Dipl Table.
The limitations of genetic testing as described here include: (1) rare variants are often not detected; (2) known star alleles (*) which are not tested by a specific lab will not be reported, and instead, the patient will be reported as a *1, and 3) most tests are not designed to detect unknown or de novo variants. Supplemental Material (Genetic Test Interpretation Section) contains additional information regarding CYP2D6 genetic test interpretation and phenotype assignment.
Available Genetic Test Options
See Supplemental Material and the Genetic Testing Registry (GTR®) (www.ncbi.nlm.nih.gov/gtr/) for more information on commercially available clinical testing options.
Incidental findings
Currently, there are no diseases or conditions which have been consistently linked to variation in the CYP2D6 gene independent of drug metabolism and response.
Other considerations
CYP2D6 is the primary enzyme responsible for the metabolism of many other commonly-used medications. It is important to note that variation in CYP2D6 may have implications for other therapies that are beyond the scope of this guideline. CPIC guidelines exist for other drugs metabolized by CYP2D6 (3, 6–9).
DRUGS: ATOMOXETINE
Background
Based on a parent-reported survey in 2016, an estimated 6.1 million children (9 %) between the ages of 2–17 years received a diagnosis of attention-deficit/hyperactivity disorder (ADHD) (10). Although not a first-line agent for the treatment of ADHD, atomoxetine was the first non-stimulant medication approved in the United States to treat ADHD in 2002. Originally developed in the 1980s to treat adult depression, but only approved for the treatment of ADHD, atomoxetine is a selective norepinephrine reuptake inhibitor (11). A surge in atomoxetine prescriptions occurred following its approval, peaking in 2004 when it was the third most commonly prescribed medication for ADHD after methylphenidate and amphetamine/dextroamphetamine; however, following 2004 prescriptions gradually decreased to around 2 million per year in 2010 (12). Unlike stimulants, atomoxetine has a delayed onset to clinical effect and typically takes 2–4 weeks for full impact on symptoms to be observed (13).
Atomoxetine is an active parent compound and is metabolized by CYP2D6 to an active metabolite, 4-OH-atomoxetine; however, this metabolite is rapidly glucuronidated to the inactive 4-OH-atomoxetine-O-glucuronide and the unconjugated metabolite circulates at concentrations approximately 100-fold lower than the parent compound (14, 15). To a lesser extent, atomoxetine is also metabolized by CYP2C19 to N-desmethylatomoxetine, which is subsequently metabolized via CYP2D6 to N-desmethyl-4-hydroxyatomoxetine (Figure 1).
Figure 1. Atomoxetine Pathway, Pharmacokinetics.
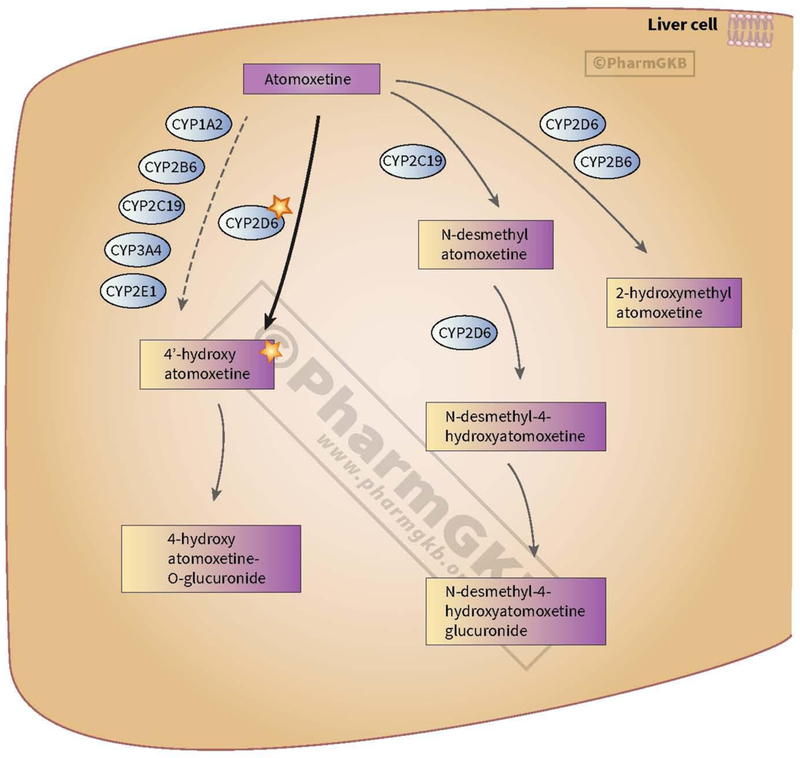
Data observed from Reference (38), M. Whirl-Carrillo, E.M. McDonagh, J. M. Hebert, L. Gong, K. Sangkuhl, C.F. Thorn, R.B. Altman and T.E. Klein. “Pharmacogenomics Knowledge for Personalized Medicine” Clinical Pharmacology & Therapeutics (2012) 92(4): 414–417.
Image reproduced and is licensed under CC BY-SA 4.0 from PharmGKB.
The recommended initial daily dose of atomoxetine is 0.5 mg/kg in children and adolescents up to 70 kg with a target and maximum dose of 1.2 mg/kg/day and 1.4 mg/kg/day, respectively. In children and adolescents over 70 kg and adults, the initial daily dose of atomoxetine is 40 mg/day, with a target and maximum daily dose of 80 mg/day and 100 mg/day, respectively (according to the product labeling). In patients taking a CYP2D6 inhibitor or those who are known to be CYP2D6 PMs, the product labeling currently recommends starting therapy at the usual daily dose and increasing to the recommended target doses if the drug is well tolerated and symptoms fail to improve after four weeks.
In registry clinical trials for the efficacy and safety of atomoxetine, separation from placebo was, on average, observed after 1–2 weeks of treatment (13). Incremental increases in response may occur for up to 24 weeks or longer. Pharmacokinetic studies highlight that doses producing atomoxetine peak concentrations greater than 200 ng/ml 1–4 hours after dosing may increase the likelihood of response (16). Common dose-related side effects from atomoxetine occurring more frequently in CYP2D6 PMs compared to non-PMs in children or adults include dry mouth, blurred vision, sleep disturbances, decreased weight or appetite, constipation, depression, tremor, feeling jittery, excoriation, dry eye or conjunctivitis, syncope, urinary retention, sexual dysfunction, hyperhidrosis, peripheral coldness, and elevated blood pressure (16).
Linking genetic variability to variability in drug-related phenotypes
Atomoxetine pharmacokinetics.
A strong association exists between CYP2D6 genotype and atomoxetine pharmacokinetic variability (see Table S2). Atomoxetine is considered a CYP2D6 “sensitive substrate” by the U.S. Food and Drug Administration (FDA) for evaluating drug-drug interactions. As such, CYP2D6 genetic variation has a profound effect on atomoxetine pharmacokinetics (17). The range of values observed for atomoxetine exposure, most often reported as either the area under the drug plasma concentration-time curve (AUC) or the maximum concentration (Cmax), is substantial. Atomoxetine exposure (AUC) is, on average, 10-fold higher in CYP2D6 PMs compared to non-PMs (14–16, 18, 19). However, the comparison of group mean values obscures the full range of exposures that may be present in a population. For example, a CYP2D6 genotype-stratified single-dose pharmacokinetic study (n=23 children) observed a 30-fold range in AUC when dosed using the FDA-recommended initial dose of 0.5 mg/kg. Decreasing atomoxetine exposure was associated with genotype, i.e. increasing CYP2D6 activity scores (15). It should be noted that in early in vivo drug development studies, the depth of genotyping only differentiated between CYP2D6 PMs and non-PMs. Subsequent studies that performed more comprehensive genotyping suggest that CYP2D6 IMs (e.g., a CYP2D6 activity score of 0.5) have pharmacokinetic profiles that differ from both PMs and NMs (15).
Ex vivo studies evaluating metabolic capacity in human liver microsomes that have been genotyped for CYP2D6 provide evidence that increased exposure is due to reduced metabolic capacity in both IMs and PMs (11, 20, 21). The most studied decreased function CYP2D6 allele in the context of atomoxetine is the CYP2D6*10 variant. Individuals with two CYP2D6*10 alleles had higher atomoxetine exposure (5-fold higher peak concentration) when compared to individuals with at least one normal function allele (22–24). Individuals heterozygous for *10 and one fully functional CYP2D6 allele had higher atomoxetine exposure compared to individuals carrying two fully functioning alleles.
Atomoxetine response/toxicity.
The likelihood of favorable treatment response and side effects are both reported to be higher in CYP2D6 PMs compared to non-PMs, which is likely due to increased exposure to parent drug in the PMs. The extent of improvement in ADHD symptoms, i.e. mean change in ADHD symptom rating scale scores, was greater in PMs compared to non-PMs, while CYP2D6 non-PMs were also more likely to discontinue atomoxetine therapy due to inefficacy as compared to CYP2D6 PMs (16). Current evidence is limited to comparisons between CYP2D6 PMs and non-PMs; thus, there is no evidence correlating efficacy and/or drug discontinuation with other CYP2D6 phenotype classes. Higher exposures to atomoxetine may also partially explain a greater percentage of side effects in CYP2D6 PMs, such as increases in heart rate and diastolic blood pressure, when compared to non-PMs (16, 19, 25). However, in a retrospective study that evaluated atomoxetine response in participants enrolled from six atomoxetine randomized controlled trials (n=618), beneficial response was noted in 47% of patients, 13% of patients were determined to have minimal response, and 40% had no response (26). These data suggest at least two distinct atomoxetine response groups within the observed population. Additionally, it can also be inferred that CYP2D6 genotype alone does not account for the atomoxetine response distribution described in the above investigation, given that CYP2D6 PMs frequency is ~5–10% of the population (Frequency table (2)).
The most well studied pharmacokinetic parameter for atomoxetine relates plasma drug concentrations that approximate the Cmax of the parent compound to reduction of ADHD symptoms (16). Given this evidence, the therapeutic recommendation for each CYP2D6 phenotype class also includes guidance for plasma drug concentration testing, as a means to estimate atomoxetine exposure (i.e. exposure check). These target reference values are from the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology, and are meant to guide the clinician in the event that patient response to atomoxetine is inadequate (27). Included with these guidelines is a plasma concentration (henceforth described as an ‘exposure check’) performed post drug administration which is included to rule out inadequate systemic exposure as a cause of non-response in individuals with a CYP2D6 activity score of 1 or more. This CPIC guideline also includes a grading system to evaluate the strength of evidence between various CYP2D6 genotype-predicted phenotypes and the corresponding therapeutic recommendation. Data from both in vitro and in vivo studies (Table S1), as well as consensus recommendations were used in formulating the guidance in Table 2.
Therapeutic Recommendations
Tables 2 and 3 summarize the therapeutic recommendations for atomoxetine based on CYP2D6 phenotype in children and adults, respectively. Although not routinely ordered, patients may benefit from a single time point atomoxetine exposure check to guide therapy. Exposure check concentrations between 200 – 1000 ng/mL are generally considered to be “therapeutic” (16, 28), however for individuals with comorbidities a higher exposure target may be warranted, as was done in a study evaluating children with both ADHD and Oppositional Defiant Disorder (29). We propose that the plasma concentration exposure check be used with an individual’s CYP2D6 genotype to help clinicians guide dose selection and titration as discussed below. Based on pharmacokinetic knowledge that CYP2D6 metabolism phenotypes influence atomoxetine peak concentration and half-life, Tables 2 and 3 propose that prescribers consider measuring peak concentrations 1 to 2 hours after dosing in known CYP2D6 UMs, NMs and IMs with high activity (activity score 1.0 without a CYP2D6*10 allele), 2 to 4 hours after dosing in CYP2D6 IMs with low activity (activity score 0.5) and in individuals with AS of 1 when the CYP2D6*10 allele is present, and 4 hours after dosing in PMs.
TABLE 3.
DOSING RECOMMENDATIONS FOR ATOMOXETINE BASED ON CYP2D6 GENOTYPE FOR ADULTS
| Phenotype | Activity score |
Implication | Therapeutic Recommendation | Classification of Recommendationa |
|---|---|---|---|---|
| CYP2D6 ultrarapid metabolizer | >2 | Based on very limited data available for CYP2D6 ultrarapid metabolizers taking atomoxetine, it is unlikely ultrarapid metabolizers would achieve adequate serum concentrations for the intended effect at standard dosing. | Initiate with a dose of 40 mg/day and increase to 80 mg/day after 3 days. If no clinical response and in the absence of adverse events after 2 weeks, consider increasing dose to 100 mg/day. If no clinical response observed after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/mlb,c Dosages greater than 100 mg/day may be needed to achieve target concentrations.d | Moderate |
| CYP2D6 normal metabolizer | 1.5–2.0 | Normal metabolizers of atomoxetine have a lower likelihood of response as compared to poor metabolizers. This is associated with increased discontinuation due to lack of efficacy as compared to poor metabolizers. | Initiate with a dose of 40 mg/day and increase to 80 mg/day after 3 days. If no clinical response and in the absence of adverse events after 2 weeks, consider increasing dose to 100 mg/day. If no clinical response observed after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/ml.b,c Dosages greater than 100 mg/day may be needed to achieve target concentrations.d | Moderate |
| CYP2D6 Normal metabolizer or Intermediate metabolizer (controversy remains)e |
1.0 (*10 allele not present) |
Possibly higher atomoxetine concentrations as compared to normal metabolizers but questionable clinical significance. Normal metabolizers may be at an increased risk of increased discontinuation as compared to poor metabolizers. | Initiate with a dose of 40 mg/day and increase to 80 mg/day after 3 days. If no clinical response and in the absence of adverse events after 2 weeks, consider increasing dose to 100 mg/day. If no clinical response observed after 2 weeks, consider obtaining a peak plasma concentration (1 to 2 hours after dose administered). If <200 ng/ml, consider a proportional increase in dose to approach 400 ng/ml.b,c Dosages greater than 100 mg/day may be needed to achieve target concentrations.d | Moderate |
| CYP2D6 Normal metabolizer or Intermediate metabolizer (controversy remains)e |
1.0 (*10 allele present) |
Decreased metabolism of atomoxetine higher atomoxetine concentrations as compared to normal metabolizers. Individuals with activity score of 1.0 with CYP2D6*10 may be at an increased risk of increased discontinuation as compared to poor metabolizers. | Initiate with a dose of 40 mg/day and if no clinical response and in the absence of adverse events after 2 weeks increase dose to 80 mg/day. If response is inadequate after 2 weeks consider obtaining a plasma concentration 2–4 h after dosing. If concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Moderate |
| CYP2D6 intermediate metabolizer |
0.5 | Decreased metabolism of atomoxetine higher atomoxetine concentrations as compared to normal metabolizers. Intermediate metabolizers may be at an increased risk of discontinuation as compared to poor metabolizers. | Initiate with a dose of 40 mg/day and if no clinical response and in the absence of adverse events after 2 weeks increase dose to 80 mg/day. If response is inadequate after 2 weeks consider obtaining a plasma concentration 2–4 h after dosing. If concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Moderate |
| CYP2D6 poor metabolizer | 0 | Significantly decreased metabolism of atomoxetine may result in higher | Initiate with a dose of 40 mg/day and if no clinical response and in the absence of adverse events after 2 weeks increase dose to 80 mg/day. If response is inadequate after 2 weeks consider obtaining a plasma concentration 2–4 h after dosing. If concentration is <200 ng/ml, consider a proportional dose increase to achieve a concentration to approach 400 ng/ml.b,c If unacceptable side effects are present at any time, consider a reduction in dose. | Moderate |
Rating scheme described in the Supplement.
Therapeutic range of 200 to 1000 ng/ml has been proposed (27).
Limited data are available regarding the relationship between atomoxetine plasma concentrations and clinical response. Available information suggests that clinical response is greater in PMs compared to non-PMs and may be related to the higher plasma concentrations 1 to 1.5 hours after dosing in PMs compared to non-PMs administered a similar dose. Furthermore, modest improvement in response, defined as reduction in ADHD-RS, is observed at peak concentrations greater than 400 ng/ml.
Doses above 120 mg/day have not been evaluated.
CPIC has general classified patients with activity score of 1 as “normal metabolizer.” However, in the case of atomoxetine, prescribing recommendations for those with an AS of 1.0 are allele-dependent, based on the presence of the CYP2D6*10 allele.
Very limited data exist for CYP2D6 UMs taking atomoxetine, but it is unlikely these individuals would achieve adequate serum concentrations with standard atomoxetine dosing (30). As discussed above, CYP2D6 non-PMs have a lower likelihood of treatment response as compared to CYP2D6 PMs (16). Thus, for CYP2D6 UMs and NMs, recommendations are to initiate standard atomoxetine dosing (see Table 2 and 3 for pediatric and adult dosing, respectively) and if no clinical response is observed after two weeks, consider obtaining a peak plasma concentration one to two hours after dose administration. If the peak concentration is less than 200 ng/ml, consider increasing the dose proportionally to approach 400 ng/ml (16). It is important to note that doses above 120 mg have not been extensively evaluated, although they may be necessary to achieve target concentrations in some patients. While CYP2D6 NMs with activity scores of 1 (without the presence of the CYP2D6* 1 0 allele) have higher atomoxetine plasma concentrations compared to NMs with an AS of 2, the clinical significance of this difference is unclear. Thus, CYP2D6 NMs with an AS of 1 (without the presence of the CYP2D6*10 allele) should be treated similarly to CYP2D6 NMs with AS of 2.
CYP2D6 PMs, IMs, and NMs with an AS of 1 in the presence of the CYP2D6*10 allele have significantly decreased metabolism of atomoxetine, which may increase the risk of side effects (16, 19, 25). However, these individuals may also have greater improvement of ADHD symptoms and lower dose requirements as compared to non-PMs. Therefore, the recommendation for these phenotype groups are to initiate with a standard starting dose (see Table 2 and 3 for pediatric and adult dosing, respectively) and if there is an inadequate trajectory of symptom improvement after 2 weeks (in the absence of side effects), consider obtaining a plasma concentration two-four hours after dosing. If response is inadequate and side effects are not present, consider adjusting the dose proportionally to approach 400 ng/ml.
Recommendations for Incidental Findings
Not applicable
Other considerations
In addition to CYP2D6, variation in atomoxetine response has also been examined with its pharmacodynamic target, the norepinephrine transporter, SLC6A2. Ramoz et. al. describes significant associations between 20 SNPs within SLC6A2 and responders as compared to non-responders of atomoxetine (31). If other studies replicated this finding, future guidelines may consider incorporating this result in its recommendations.
Individuals taking atomoxetine along with a strong CYP2D6 inhibitor (e.g. bupropion, fluoxetine, paroxetine) may experience higher than expected concentrations based on their CYP2D6 genotype through a process known as phenoconversion. This has been described for paroxetine and fluoxetine in non-PM metabolizers taking atomoxetine (32–34). For the duration of the phenoconversion (for fluoxetine it may last up to 2–3 months after fluoxetine discontinuation in average patients and longer in some individuals (35)) the individual phenotypically resembles a CYP2D6 PM regardless of genotype.
Implementation resources for this guideline.
The guideline supplement contains resources that can be used within electronic health records (EHRs) to assist clinicians in applying genetic information to patient care for the purpose of drug therapy optimization (see Resources to incorporate pharmacogenetics into an electronic health record with clinical decision support section in the supplement). Clinical implementation resources include cross-references for drug and gene names to widely-used terminologies and standardized nomenclature systems, workflow diagrams, a table that translates genotype test results into a predicted phenotype with genetic test interpretation, and example text for documentation in the EHR and point-of-care alerts.
POTENTIAL BENEFITS AND RISKS FOR THE PATIENT
The potential benefit of using an individual’s CYP2D6 genotype to guide atomoxetine dosing is that clinicians can be alerted to individuals who are more likely to fail treatment at standard dosing (e.g., NMs or UMs) or be at an increased risk of adverse effects (e.g., PMs). The FDA recommends atomoxetine doses up to 100 mg/day in adults or 1.2 mg/kg/day in children. This guideline proposes that CYP2D6 UMs (activity score >2 and 1–2% of US patients) and NMs (activity score 1–2 and 77–92% of US patients) may need higher than FDA recommended doses to achieve concentrations associated with clinical response, based on low atomoxetine peak concentrations (<200 ng/ml). It is estimated that up to 1/3 of US patients may be in this category of needing higher than recommended doses (30), but the exact prevalence needs to be established by future clinical studies. A potential risk of testing is the misinterpretation of genetic test results, as rare or novel variants are typically not interrogated. If an individual carries a rare variant, the actual phenotype may differ from the phenotype predicted by the genotypes included on a specific lab test. An individual’s intrinsic CYP2D6 activity may also be impacted by other factors including epigenetics, diet, comorbidities, or co-medications. Any of these factors, including the co-medication with a CYP2D6 inhibitor, would be reflected through atomoxetine TDM. Although CYP2D6 genotyping is usually reliable when performed in qualified laboratories, the possibility for error in genotyping, contamination, or mislabeling of the sample remains.
CAVEATS: APPROPRIATE USE AND/OR POTENTIAL MISUSE OF GENETIC TESTS
Rare CYP2D6 variants may not be included in the genotype testing used by some laboratories, and patients with rare variants may be assigned a “wild-type” (CYP2D6*1) genotype by default. Thus, there is a small risk that an assigned “wild-type” allele could potentially harbor a no or decreased function variant. Furthermore, it is important that the genetic testing platform includes testing for gene copy number to identify CYP2D6 ultrarapid metabolizers or gene deletions that may decrease CYP2D6 AS. Caution should be used regarding molecular diagnostics of CYP2D6 gene copy number variation since commercially available genotyping results may differ between diagnostic laboratories depending on assay design. Like all diagnostic tests, CYP2D6 genotype is one of multiple pieces of information that clinicians should consider when making their therapeutic choice for each patient. Furthermore, there are several other factors that cause potential uncertainty in the genotyping results and phenotype predictions. These are discussed in detail in the Supplemental Material online.
In this guideline, we propose that exposure be assessed to guide subsequent dosing decisions when the desired clinical response has not been achieved, but we also recognize that this recommendation has limitations: available exposure-response data from clinical trials are derived from concentrations drawn 60–90 min after dosing; although this measure may not be the best predictor of response, the relationship between other exposure measures, such as trough concentration at steady state, steady state AUC, unbound atomoxetine concentrations, or total active compounds (unbound ATX + unbound 4-OH aglycone), has not been evaluated.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the critical input of Dr. Mary V. Relling (St Jude Children’s Research Hospital) and the members of the Clinical Pharmacogenetics Implementation Consortium (CPIC).
FUNDING
This work was funded by the National Institutes of Health (NIH) for CPIC (R24GM115264; U24HG010135–01) and PharmGKB (R24GM61374), PharmVar (R24GM123930). Atomoxetine studies conducted by the authors (JTB, AG and JSL) were supported by R01HD058556.
Footnotes
DISCLAIMER
Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision-making, as well as to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variation among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health care provider to determine the best course of treatment for the patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be solely made by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to property related to any use of CPIC’s guidelines, or for any errors or omissions.
CPIC is a registered service mark of the U.S. Department of Health & Human Services (HHS).
CONFLICT OF INTEREST
THE AUTHORS DECLARED NO COMPETING INTERESTS FOR THIS WORK.
Supplementary Material
(2018–0834CR_Supplemental_Material.pdf)
Supplement to: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Atomoxetine Therapy
(2018–0834CR_Implementation_Tables.zip)
Implementation Tables
REFERENCES
- (1).PharmGKB. Gene Reference Materials for CYP2D6. <https://www.pharmgkb.org/page/cyp2d6RefMaterials>. Accessed September 16 2016.
- (2).CPIC. CPIC Guideline for Atomoxetine based on CYP2D6 genotype. <https://cpicpgx.org/guidelines/cpic-guideline-for-atomoxetine-based-on-cyp2d6-genotype>.
- (3).Crews KR et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 95, 376–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ & Leeder JS The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83, 234–42 (2008). [DOI] [PubMed] [Google Scholar]
- (5).Crews KR et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther 91, 321–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hicks JK et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther 98, 127–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Goetz MP et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin Pharmacol Ther 103, 770–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hicks JK et al. Clinical Pharmacogenetics Implementation Consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther 102, 37–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bell GC et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin Pharmacol Ther 102, 213–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD & Blumberg SJ Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol 47, 199–212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Farid NA, Bergstrom RF, Ziege EA, Parli CJ & Lemberger L Single-dose and steady-state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol 25, 296–301 (1985). [DOI] [PubMed] [Google Scholar]
- (12).Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J & Murphy D Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 130, 23–31 (2012). [DOI] [PubMed] [Google Scholar]
- (13).Savill NC et al. The efficacy of atomoxetine for the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a comprehensive review of over a decade of clinical research. CNS Drugs 29, 131–51 (2015). [DOI] [PubMed] [Google Scholar]
- (14).Sauer JM et al. Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism. DrugMetab Dispos 31, 98–107 (2003). [DOI] [PubMed] [Google Scholar]
- (15).Brown JT, Abdel-Rahman SM, van Haandel L, Gaedigk A, Lin YS & Leeder JS Single dose, CYP2D6 genotype-stratified pharmacokinetic study of atomoxetine in children with ADHD. Clin Pharmacol Ther 99, 642–50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Michelson D, Read HA, Ruff DD, Witcher J, Zhang S & McCracken J CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry 46, 242–51 (2007). [DOI] [PubMed] [Google Scholar]
- (17).FDA. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. <https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm#table2-1> (2017).
- (18).Ring BJ, Gillespie JS, Eckstein JA & Wrighton SA Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos 30, 319–23 (2002). [DOI] [PubMed] [Google Scholar]
- (19).Trzepacz PT, Williams DW, Feldman PD, Wrishko RE, Witcher JW & Buitelaar JK CYP2D6 metabolizer status and atomoxetine dosing in children and adolescents with ADHD. Eur Neuropsychopharmacol 18, 79–86 (2008). [DOI] [PubMed] [Google Scholar]
- (20).Dinh JC, Pearce RE, Van Haandel L, Gaedigk A & Leeder JS Characterization of atomoxetine biotransformation and implications for development of PBPK models for dose individualization in children. Drug Metab Dispos 44, 1070–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Monroe WM Fifty years of change. Va Med 116, 281–2 (1989). [PubMed] [Google Scholar]
- (22).Byeon JY et al. Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38, 2083–91 (2015). [DOI] [PubMed] [Google Scholar]
- (23).Matsui A et al. Pharmacokinetics, safety, and tolerability of atomoxetine and effect of CYP2D6*10/*10 genotype in healthy Japanese men. J Clin Pharmacol 52, 388–403 (2012). [DOI] [PubMed] [Google Scholar]
- (24).Cui YM et al. Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele. Br J Clin Pharmacol 64, 445–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Fijal BA et al. CYP2D6 predicted metabolizer status and safety in adult patients with attention-deficit hyperactivity disorder participating in a large placebo-controlled atomoxetine maintenance of response clinical trial. J Clin Pharmacol 55, 1167–74 (2015). [DOI] [PubMed] [Google Scholar]
- (26).Newcorn JH, Sutton VK, Weiss MD & Sumner CR Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the Integrated Data Exploratory Analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry 48, 511–8 (2009). [DOI] [PubMed] [Google Scholar]
- (27).Schoretsanitis G et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians<sup/>. World J Biol Psychiatry 19, 162–74 (2018). [DOI] [PubMed] [Google Scholar]
- (28).Hiemke C et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51, el (2018). [DOI] [PubMed] [Google Scholar]
- (29).Hazell P et al. Relationship between atomoxetine plasma concentration, treatment response and tolerability in attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Atten Defic Hyperact Disord 1, 201–10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).de Leon J Translating pharmacogenetics to clinical practice: Do cytochrome P450 2D6 ultrarapid metabolizers need higher atomoxetine doses? J Am Acad Child Adolesc Psychiatry 54, 532–4 (2015). [DOI] [PubMed] [Google Scholar]
- (31).Ramoz N et al. A haplotype of the norepinephrine transporter (Net) gene Slc6a2 is associated with clinical response to atomoxetine in attention-deficit hyperactivity disorder (ADHD). Neuropsychopharmacology 34, 2135–42 (2009). [DOI] [PubMed] [Google Scholar]
- (32).Todor I et al. Evaluation of a potential metabolism-mediated drug-drug interaction between atomoxetine and bupropion in healthy volunteers. J Pharm Pharm Sci 19, 198–207 (2016). [DOI] [PubMed] [Google Scholar]
- (33).Paulzen M, Clement HW & Grunder G Enhancement of atomoxetine serum levels by co-administration of paroxetine. Int JNeuropsychopharmacol 11, 289–91 (2008). [DOI] [PubMed] [Google Scholar]
- (34).Kratochvil CJ et al. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry 44, 915–24 (2005). [DOI] [PubMed] [Google Scholar]
- (35).Spina E, Pisani F & de Leon J Clinically significant pharmacokinetic drug interactions of antiepileptic drugs with new antidepressants and new antipsychotics. Pharmacol Res 106, 72–86 (2016). [DOI] [PubMed] [Google Scholar]
- (36).Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T & Leeder JS Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).CPIC. CPIC Guideline for Tamoxifen based on CYP2D6 genotype. <https://cpicpgx.org/guidelines/cpic-guideline-for-tamoxifen-based-on-cyp2d6-genotype/>.
- (38).Whirl-Carrillo M et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92, 414–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


