Abstract
Introduction
The physiologic importance of fast CO2/HCO3- interconversion in various tissues requires the presence of carbonic anhydrase (CA, EC 4.2.1.1). Fourteen CA isozymes are present in humans, all of them being used as biomarkers.
Area covered
A great number of patents and articles were focused on the use of CA isozymes as biomarkers for various diseases and syndromes in the recent years, in an ascending trend over the last decade. The review highlights the most important studies related with each isozyme and covers the most recent patent literature.
Expert opinion
The CAs biomarker research area expanded significantly in recent years, shifting from the predominant use of CA IX and CA XII in cancer diagnostic, staging and prognosis towards a wider use of CA isozymes as disease biomarkers. CA isozymes are currently used either alone, in tandem with other CA isozymes and/or in combination with other proteins for the detection, staging and prognosis of a huge repertoire of human dysfunctions and diseases, ranging from mild transformation of the normal tissues to extreme shifts in tissue organization and function. The techniques used for their detection/quantitation and the state-of-the art in each clinical application are presented through relevant clinical examples and corresponding statistical data.
Keywords: carbonic anhydrases, biomarker, physiology, pathology
1. Introduction
Carbonic anhydrase (CA, E. C. 4. 2. 1. 1) is a zinc metalloprotein that catalyzes the reversible hydration of carbon dioxide to produce a bicarbonate ion and a proton (CO2 + H2O → HCO3- + H+). The enzyme is ubiquitously spread with the vegetal and animal kingdoms, a fact that is quite natural considering that carbon dioxide is the end product of aerobic metabolism in living organisms. It catalyzes the reaction to rates that reach diffusion-limit, thus serving the metabolic needs of various organisms, from the simplest to the most complex ones [1, 2].
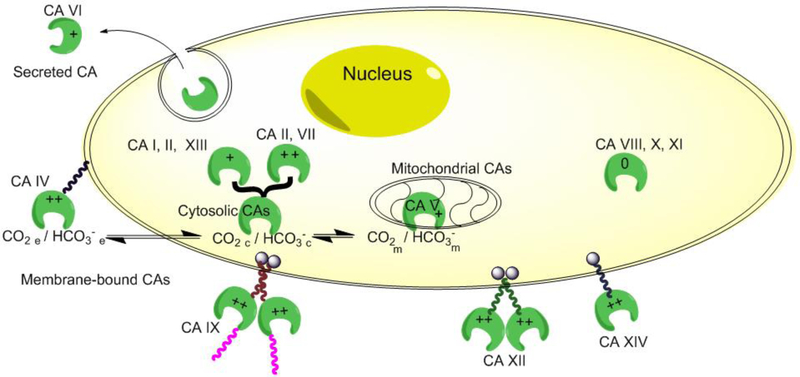
Five separate gene families encode these enzymes, namely α-CAs, β-CAs, γ-CAs, δ-CAs and η-CAs [1, 3–5]. The α-CAs are the only group found in mammals, with 14 different CA isozymes being described to date in humans (hCAs). They evolved to catalyze CO2/HCO3- interconversion in different macro- and micro-environments of the human body and consequently are involved in physiologic and pathologic processes such as respiration and CO2 excretion, pH homeostasis, secretion of electrolytes, gluconeogenesis, lipogenesis, and ureagenesis, bone resorption and calcification, and also in tumorigenicity. In terms of subcellular localization, one can distinguish cytosolic isozymes (CA I, CA II, CA III, CA VII, CA XIII), mitochondrial isozymes (CA VA and CA VB), membrane-bound isozymes (CA IV, CA IX, CA XII, CA XIV), and a secreted isozyme (CA VI). Besides these functional isozymes, there are three acatalytic representatives (CA VIII, CA X, CA XI) that do not contain Zn and whose function and role in the organism is not completely understood (Figure 1 and Table 1) [1, 2, 6].
Figure 1.
Cartoon depicting the 14 human CA isozymes, their relative catalytic activity and subcellular localization. For a more detailed overview, see Table 1.
Table 1.
The 14 human CA isozymes, their kinetic properties, main structural features and normal cell/tissue/organ distribution. See also Figure 1.
| CA isozyme, cellular localization | Main structural features | Kinetic properties kcat (s−1) | Normal organ/tissue location | Physiologic function |
|---|---|---|---|---|
| CA I (Cytosol) | Catalytic domain | 2 × 105 | Red blood cells, neutrophils, corneal epithelium, lens, ciliary body epithelium of the eye, sweat glands, adipose tissue, myoepithelial cells of the epithelium of the large intestine, zona glomerulosa of the adrenal glands | Involved in equilibration of CO2/HCO3− pools maintains the pH in blood, other body fluids, facilitates the CO2 transport from tissues to lungs |
| CA II (Cytosol) | Catalytic domain | 1.4 × 106 | oligodendrocytes and epithelium of the choroid plexus of the brain, ciliary body, lens, Muller cells of retina of the eyes, acinar cells of the salivary glands, type II epithelial cells of the lung, perivenous hepatocytes of the liver, proximal tubule, distal tubule and intercalated cells of the cortical collecting ducts of the kidneys, endothelial cells, erythrocytes, platelets, neutrophils, gastric parietal cells, epithelial cells of the duodenum, intestine and colon, pancreatic ducts cells, uterine endometrial cells, epithelial cells of seminal vesicle and ductus deferens, spermatozoa, zona glomerulosa cells of the adrenal glands, bone osteoclasts | Contribute to equilibration of dissolved CO2/HCO3− pools in blood and maintain the pH homeostasis in blood and in cytoplasm of cells |
| CA III (Cytosol) | Catalytic domain | 1 × 104 | skeletal muscles, adipocytes, uterus, prostate, lungs, kidneys, colon, and testis, red blood cells | Equilibrates dissolved CO2/HCO3− pools in and maintain the pH homeostasis in cytoplasm of muscle cells, confers resistance to oxidative stress of natural sources or induced via consumption of alcohol and various drugs |
| CA IV (External membrane-bound) | Catalytic domain linked to a phosphatidyl inositol glycan (GPI) membrane anchor | 1.1 × 106 | apical plasma membrane of certain capillary beds (pulmonary microvasculature, cortical capillaries, choriocapillaries of the eye, microcapillaries of skeletal and cardiac muscle), apical plasma membrane of the colon GI epithelial cells and of certain segments of the nephron, specific epithelial cells of the human reproductive tract | Involved in the equilibration of CO2/HCO3− pools in the extracellular space, where the concentration of Cl− ions is much higher than in the cytosol |
| CA VA (Mitochondria) | Catalytic domain | 2.9 × 105 | liver | Involved in gluconeogenesis, ureagenesis, lipogenesis, supply HCO3− to pyruvate carboxylase within gluconeogenesis and lipogenesis pathways, to carbamoylphosphate synthetase in ureagenesis pathway, to propionyl-CoA carboxylase and to 3-methylcrotonyl-CoA carboxylase within the branched chain amino acids catabolism |
| CA VB (Mitochondria) | Catalytic domain | 9.5 × 105 | skeletal and heart muscles, kidneys, pancreas, GI tract, brain and spinal cord | |
| CA VI (Secreted in saliva and milk) | Catalytic domain | 3.4 × 105 | secreted by salivary and mammary glands | In saliva, it neutralizes the acid generated from ingested food and also from decomposition of food by bacteria living in the oral cavity, thus protecting the upper alimentary canal from acidity |
| CA VII (Cytosol) | Catalytic domain | 9.5 × 105 | mainly in the CNS, also encountered in liver, skeletal muscles, stomach, duodenum, colon | Equilibrates dissolved CO2/HCO3− pools in and maintain the pH homeostasis in cytoplasm of CNS cells and in other tissues |
| CA VIII (Cytosol) | Catalytic domain (non-functional) | N/A | Purkinje cells of cerebellum, neural cell bodies and some astrocytes, liver, lungs, heart, gut, thymus, kidneys | Influences inositol triphosphate (ITP) binding to its receptor ITPR1 on the endoplasmatic reticulum, thus modulating calcium signaling inside the cells |
| CA IX (External membrane-bound) | Dimer consisting of proteoglycan, catalytic domain, transmembrane domain, cytoplasmic domain | 3.8 × 105 | epithelium of stomach, bile duct, gallbladder duct, pancreatic duct, rapidly-proliferating normal cells of small intestine, and (low expression) in the CNS | Involved in the equilibration of CO2/HCO3− pools in the extracellular space, plays a significant role in cell survival under hypoxic conditions, over-expressed in hypoxic tumors |
| CA X (Cytosol) | Catalytic domain (non-functional) | N/A | myelin sheath in the brain | Plays an important role in myelin sheath organization in normal brain development |
| CA XI (Cytosol) | N/A | CNS: choroid plexus and pia arachnoid areas, within neural body, neurites, and astrocytes, but not within oligodendroglia, Spinal cord, GI tract (stomach, small intestine, colon), pancreas, liver, kidneys, skeletal muscles, ovaries, lymph nodes, adrenal gland, thyroid, salivary glands | Unknown | |
| CA XII (External membrane-bound) | Dimer consisting of catalytic domain, transmembrane domain, cytoplasmic domain | 4.2 × 105 | colon, rectum, esophagus, pancreas, kidneys, prostate, brain, endometrium, ovaries, testis, sweat glands of skin, breast epithelium and non-pigmented ciliary epithelial cells of the eye | Involved in the equilibration of CO2/HCO3− pools in the extracellular space, enhanced expression in certain tumors |
| CA XIII (Cytosol) | Catalytic domain | 1.5 × 105 | colon, small intestine, testis, uterine cervix, endometrial glands and the thymus | Contribute to equilibration of dissolved CO2/HCO3− pools and helps maintain the pH homeostasis in cytoplasm of different cells, regulates the HCO3− ion concentration and pH homeostasis in the cervical and endometrial mucus thus maintaining the mobility of sperms and ensuring normal fertilization process |
| CA XIV (External membrane-bound) | Monomer consisting of catalytic domain, transmembrane domain, cytoplasmic domain | 3.1 × 105 | highly expressed in the kidney, brain, retina and the heart, also abundant expression in the skeletal muscle, liver, and lungs | Involved in the equilibration of CO2/HCO3− pools in the extracellular space, interacts with bicarbonate transporters, involved in acid–base balance in muscles and in other tissues in response to chronic hypoxia, in hyperactivity of the heart, and in pH regulation in the retina |
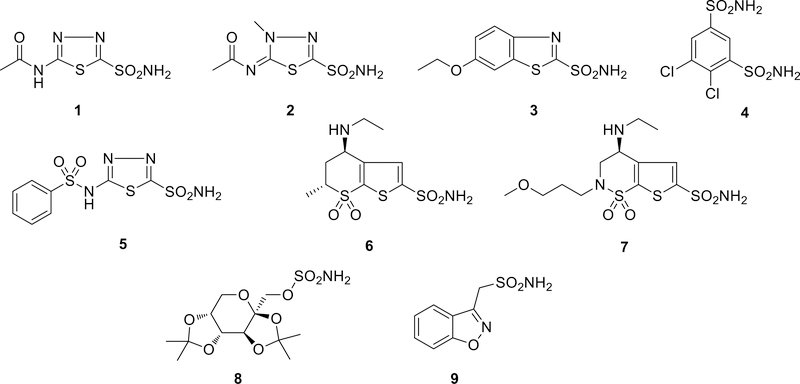
The fourteen human isozymes have a high structural homology, with key amino acid differences within their active site making them to vary quite widely in their kinetic parameters (Table 1) and also in their susceptibility to different inhibitors. Two main classes of inhibitors are currently known, the metal-complexing anions and the sulfonamides and their isosteres sulfamates and sulfamides [1, 7]. The second class was extensively used in human therapy, with sulfonamide inhibitors such as acetazolamide 1, methazolamide 2, ethoxzolamide 3, dichlorphenamide 4, benzolamide 5, dorzolamide 6 and brinzolamide 7 being used as diuretics, and in the management of edema, mountain sickness, epilepsy, and glaucoma (Figure 2) [1, 6, 8–10]. Anti-epileptic drugs topiramate 8 and zonisamide 9 are also potent CA inhibitors (CAIs). Other promising CA inhibitors are currently under advanced clinical stages in the treatment of hypoxic tumors [1, 6, 9–13] and were also used recently in the selective delivery of toxic drugs to tumors [14, 15]. Slower isozymes can be activated with CA activators, with potential applications in memory and learning enhancement [1, 16–23] The boundary between CA activation and inhibition was also revealed recently [24].
Figure 2.
CA inhibitors in clinical use, administered either systemically (compounds 1-5, 8, 9) or topically (antiglaucoma compounds 6 and 7, administered directly into the eye). One may notice that all compounds contain a primary sulfonamide/sulfamate group that binds the Zn (II) ion in the active site of CAs.
As mentioned above, CA isozymes are involved in many organs, where they are involved in different physiologic and pathologic processes and are biochemically coupled with other enzymes in various pathways. Alteration of these normal pathways in various dysfunctions or diseases trigger CA isozyme up/down-regulation from the gene level, which constitutes the main premise for using CAs as disease markers. Different diseases can also change the normal distribution of CA isozymes within the human body, leading to their appearance in large amounts in locations where they do not normally reside and/or decrease of their level in normal locations. These premises constitute the base for using CA isozymes as biomarkers for various human dysfunctions and diseases, which will be subsequently detailed in the following section.
2. Carbonic anhydrases as biomarkers for human diseases
2. 1. Carbonic anhydrase I as a disease biomarker
CA I is ubiquitously spread across body tissues. It is also present in significant amount in red blood cells (RBCs), together with CA II. Thus, CA I is five to six times more abundant than CA II in human erythrocytes, although it has only about 15% of the CA II activity. Even though CA I is the most abundant non-hemoglobin protein in RBCs, no hematologic abnormalities result from its absence. Both CA I and CA II contribute to equilibration of dissolved CO2/HCO3- pools in blood, maintain the pH blood homeostasis, and facilitate the CO2 transport from tissues to lungs [25]. The amount of free CA I protein in blood is normally very low in normal subjects but it can significantly increase in certain pathological conditions. CA I is also found in neutrophils, in the corneal epithelium, lens, ciliary body epithelium of the eye, in sweat glands, in the adipose tissue, in the myoepithelial cells of the epithelium of the large intestine, and in zona glomerulosa of the adrenal glands (Table 2) [25].
Table 2.
Distribution of isozyme CA I in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Red blood cells, neutrophils Eye (corneal epithelium, lens, ciliary body epithelium of the eye) Sweat glands, Adipose tissue, Large intestine (myoepithelial cells) Adrenal glands (zona glomerulosa) |
Immunostaining, WB | [25] | |
| Diseased | Blood/serum | |||
| • Breast cancer | MS (MRM) | [26] | ||
| • Non-small cell lung cancer | MALDI-MS, ELISA | [27] | ||
| 2DICAL-MS | [28] | |||
| • Prostate cancer | [29] | |||
| • Sepsis | Leucine-rich-alpha-2-glycoprotein | SELDI-TOF-MS, WB, ELISA | [30] | |
| • Schistosomiasis | [31] | |||
| • Diabetic nephropathy (with several other biomarkers) | Many other (complex set) | |||
In this context, Bio-Medieng Co. and Seul National University R&DB Foundation patented a method for early diagnostic of breast cancer relying on selective detection and quantification of a multiple biomarker set. The biomarker set included two apolipoproteins, fibronectin, neural cell adhesion molecule L1-like protein and CA I. The method relies on detection of two or more biomarkers in patient blood samples via multiple reaction monitoring (MRM) through triple quadrupole mass spectrometry. The biomarkers were detected directly via MRM or after being bound by specific antibodies raised against them, on biochips developed for this purpose. The authors claimed that the method avoids traditional biopsy and has a high diagnostic accuracy and sensitivity. Interestingly, CA I biomarker displayed the highest cancer/normal selectivity ratio of 1.6 (P < 0.001) among all biomarkers within this set [26].
Similarly, Wang et al. discovered via a combination of two-dimensional electrophoresis and matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) that CA I isozyme was significantly elevated in the sera of stage I non-small cell lung cancer (NSCLC) patients (3.18 ± 1.27 ng/mL, n = 22, measured via ELISA) as compared to cohorts of patients having benign tumors (2.21 ± 0.71 ng/mL, n = 18) and a normal control group (2.04 ± 0.63 ng/mL, n = 18) (P = 0.001) and concluded that CA I might serve as a novel biomarker for early detection of NSCLC [27].
In a related clinical study, Takakura and collaborators discovered that CA I can serve as a potential biomarker for detection of prostate cancer (PC). The motivation of their study was that there is currently a diagnostic gray zone of PC via measurement of serum prostate-specific antigen (PSA) levels. In this diagnostic uncertain zone, characterized by PSA values ranging from 4 to 10 ng/mL, biopsies reveal no evidence of cancer in 75% of these subjects, raising the need of addressing additional biomarkers. After analyzing enriched plasma proteins from 25 prostate cancer patients and 15 healthy controls using a label-free quantitative shotgun proteomics platform called 2DICAL (2-dimensional image converted analysis of liquid chromatography and mass spectrometry) the authors identified CA I by tandem MS. Independent immunological assays revealed that plasma CA I levels in 54 prostate cancer patients were significantly higher than those in 60 healthy controls, with a P = 0.022. Therefore it was concluded that CA I can potentially serve as a valuable plasma biomarker and that the combination of PSA and that CA I may enhance the accuracy of early diagnosis of prostate cancer in patients with gray-zone PSA level [28].
Besides cancer-related applications, National University Corporation Chiba University patented a method for diagnosing sepsis by measuring CA I or leucine-rich alpha-2-glycoprotein in a blood sample. The patients were diagnosed with sepsis if the concentration of CA 1 or leucine-rich alpha-2-glycoprotein was significantly higher than in healthy persons or non-septic kidney injury patients [29]. In another study, Kardoush et al. sought to identify novel biomarkers of human Schistosoma mansoni infection by studying serum proteins in a mouse model of schistosomiasis, followed by confirmation in chronically infected patients. Acute (6 weeks) and chronic (12 weeks) sera from S. mansoni–infected C57Bl/6 mice, as well as sera from chronically infected patients, were assessed using two proteomic platforms: surface-enhanced, laser desorption and ionization, time-of-flight mass spectrometry (SELDI-TOF MS) and Velos Orbitrap mass spectrometry. Several candidate biomarkers were further evaluated by Western blot and/or enzyme-linked immunosorbent assay (ELISA). CA I was identified among the most promising biomarkers for schistosomiasis in both mouse and human samples. Interestingly, CA I was identified as a negative biomarker, with a progressive reduction of serum CA I levels over the 12-week infection period confirmed in both species by both Western blot (murine and human: both P < 0.001) and by ELISA (human: P < 0.01) [30].
Bio-Rad and CNRS France proposed a method for the in vitro detection of an increased risk of diabetic nephropathy in a subject suffering from diabetes and being normoalbuminuric. The method involved the detection of at least two proteins selected from a biomarker set that included heparan sulfate proteoglycan core protein (or fragments thereof), CA I, prothrombin (or fragments thereof), tetranectin, CD59 glycoprotein, plasma serine protease inhibitor, mannan-binding lectin serine protease 2 (or isoforms thereof), antithrombin-III, alpha-1-antitrypsin, collagen alpha-1(I) chain, alpha-enolase, histone H2B type 1-O, glutaminyl-peptide cyclotransferase, protein AMBP and zinc-alpha-2-glycoprotein. A kit that facilitates biomarker detection was also patented (Table 2) [31].
2. 2. Carbonic anhydrase II as a disease biomarker
CA II is the most active CA isozyme, having a turnover rate for CO2 hydration approaching diffusion limit (Kcat = 1.4 × 106 s−1, Table 1), and has the widest distribution in the body, being expressed in the cytosol of cells from virtually every tissue or organ [1]. It is found in large amounts in oligodendrocytes and epithelium of the choroid plexus in the brain, in the ciliary body, lens, Muller cells of retina of the eyes, in acinar cells of the salivary glands, in the type II epithelial cells of the lung, in the perivenous hepatocytes of the liver, in the proximal tubule, distal tubule and intercalated cells of the cortical collecting ducts of the kidneys. It is also found in endothelial cells, erythrocytes, platelets, neutrophils, in gastric parietal cells, in the epithelial cells of the duodenum, intestine and colon, in pancreatic ducts cells, uterine endometrial cells, epithelial cells of seminal vesicle and ductus deferens, spermatozoa, in zona glomerulosa cells of the adrenal glands and in bone osteoclasts (Table 3) [25]. The impact of this CA isozyme in the human body is best exemplified by CA II deficiency syndrome, a human autosomal recessive disorder characterized by osteopetrosis, renal tubular acidosis, and cerebral calcification. Subjects suffering from this disorder are characterized by developmental delay, short stature, cognitive defects, and bone fragility [25].
Table 3.
Distribution of isozyme CA II in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Endothelial cells, red blood cells, platelets, neutrophils | Immunostaining, WB | [25] | |
| Brain (oligodendrocytes and epithelium of the choroid plexus) | ||||
| Eye (ciliary body, lens, Muller cells of retina) | ||||
| Salivary glands (acinar cells) | ||||
| Lung (type II epithelial cells) | ||||
| GI tract (gastric parietal cells, epithelial cells of the duodenum, small intestine and colon, pancreatic duct cells) | ||||
| Liver (perivenous hepatocytes) | ||||
| Kidneys (proximal tubule, distal tubule, intercalating cells of the cortical collecting ducts) | ||||
| Reproductive tract (endometrial cells of the uterus, epithelial cells of seminal vesicle and ductus deferens, spermatozoa) | ||||
| Adrenal glands (zona glomerulosa cells) | ||||
| Bones (bone osteoclasts) | ||||
| Diseased | Blood/plasma | |||
| • Hemolysis; also hemoglobinopathies, disseminated intravascular coagulation, malaria, pulmonary hypertension, anemia | CA I | Stopped-flow Assay | [32] | |
| • Atherosclerosis | Immunostaining | [33] | ||
| • Gastrointestinal stromal tumors | Immunostaining WB | [34] | ||
Researchers from Case Western Reserve University patented an assay to detect hemolysis within a blood sample by quantifying carbonic anhydrases I and II. As mentioned above, CA I and CA II are found in large amounts in erythrocytes. When hemolysis occurs, both proteins are released in the bloodstream. However, the circulation time of the free proteins is short (t½ of about 2h) due to their removal by reticuloendothelial system after binding by a transferrin-like protein. The assay exploits the catalytic properties of CA towards the CO2 hydration reaction. It relies on mixing a human sample including red blood cells (RBCs) and/or RBC lysate with an out-of-equilibrium CO2/HCO3− solution (0.5% CO2/22 mM HCO3−, at physiologic pH = 7.2. This solution is typically made in a stopped flow spectrometer cell from two dissimilar CO2/HCO3− solutions, one containing 0% CO2/0% HCO3−, pH 7.03 and another one containing 1% CO2/44 mM HCO3−, pH 8.41, which are mixed rapidly in the measuring cell of the instrument. The resulted out-of-equilibrium solution spontaneously equilibrates and its pH raises to 7.5, being monitored by the fluorescent dye pyranine. Since the equilibration speed depends on the amount of CA present, this method allows quantification of CA in the sample, which is proportional with the extent of RBC hemolysis in vivo. It is claimed that the assay, due to its precise nature, can be utilized to assess RBC hemolysis in patients on drugs that would predispose them to hemolysis, as well as patients suffering from other conditions such as hemoglobinopathies, disseminated intravascular coagulation, malaria, pulmonary hypertension, anemia. The assay might be also useful for assessing RBC status/fragility prior to blood transfusions and CA release following lysis of a wide range of cells and tissues [32].
Clofent-Sanchez and collaborators disclosed the phage display selection and characterization of human single-chain scFv antibodies that target the vascular endothelial cell surface proteins and the sub-endothelial molecular repertoire of an atherosclerosis mouse model. In one example, CA II was identified as the target for a scFv that stained an area rich in macrophage- and smooth muscle cell-derived foam cells under endothelium and a deeper area rich in necrotic cells adjacent to the internal elastic lamina in advanced lesions. Therefore, it is claimed that anti-CA II antibodies have the potential to be utilized for imaging vulnerable plaques in atherosclerosis [33].
Parkilla and collaborators have recently revealed the use of CA II as a biomarker for gastrointestinal stromal tumors (GISTs). These tumors constitute the most common mesenchymal tumors of the gastrointestinal tract, which are usually generated from expression of mutant KIT or PDGFRA receptor tyrosine kinase oncogenes. As a consequence, most GISTs display strong expression of KIT that allow their reliable diagnosis. However, a subset of GISTs that lacks KIT expression was evidenced and in order to distinguish them from other sarcomas/leiomyosarcomas new biomarkers are needed. After evaluating CA II expression in 175 GISTs (two cohorts including 152 and 23 cases) via Western blotting, authors observed that CA II is highly expressed in GIST cell lines, with 95% of GISTs being positive for the protein. Interestingly, the CA II expression in GISTs did not correlate with KIT or PDGFRA mutated proteins expressed by these tumors. Biomarker selectivity was good, with immunoreactivity for CA II being either absent or low in other mesenchymal tumor categories analyzed. High CA II expression was associated with a better disease-specific survival rate compared to low or no expression of the protein (Mantel–Cox test, P < 0.0001), which pleads for use of CA II as a useful biomarker in diagnosis of this tumor type among mesenchymal tumors (Table 3) [34].
2. 3. Carbonic anhydrase III as a disease biomarker
CA III is a low activity isozyme, with a Kcat of about 100 smaller than that of CA II (Table 1) [1]. This is due to particularities of its active site, which also make this isozyme much more resistant to inhibition with sulfonamides as compared with CA II and other (fast) isozymes. It can be found in large amounts in skeletal muscles (up to 8 % of the soluble protein), in adipocytes, and, in low amounts, in uterus, red cells, prostate, lung, kidney, colon, and testis (Table 4) [25, 35].
Table 4.
Distribution of isozyme CA III in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Skeletal muscles | Immunostaining, WB | [25, 35] | |
| Adipocytes | ||||
| Red blood cells (small amount) | ||||
| Lungs, kidneys, colon, uterus, testis (small amounts) | ||||
| Diseased | Liver tissue | |||
| • downregulation in liver injury due to alcoholism | CPS-1, isoaspartate (upregulated) | WB | [36, 37] | |
| Blood/serum | Myoglobin | Radioimmunoassay | [38, 39, 40] | |
| • acute myocardial infarction | H-FABP | Biochip array | [41] | |
| • acute coronary syndrome | WB, ELISA | [42] | ||
| • vasculitis | WB, MALDI-TOF | [43] | ||
| Synovial membrane | ||||
| • rheumatoid arthritis | ||||
The distinctive nature of CA III relative to other CAs is the presence of two reactive sulfhydryl groups, which can form a disulfide linkage. Through these S-containing groups, CA III can contribute to resistance to oxidative stress, of either natural sources or induced via consumption of alcohol, various drugs, etc. Prolonged ethanol feeding was shown to affect pathways that catalyze the re-methylation of homocysteine to form methionine in the liver methionine metabolic pathway, lowering hepatocyte S-adenosylmethionine (SAM) levels [44]. With alcohol consumption being a major healthcare problem, Kharbanda et al. recently examined the effects of prolonged ethanol consumption on liver enzymes, in search for biomarkers to characterize and assess liver injury. Within this study, male Wistar rats were exposed to ethanol for 4 weeks, after which liver protein were analyzed using one dimensional and two-dimensional polyacrylamide gel electrophoresis (1D- and 2D-PAGE) techniques. For quantitation of CA III during exposure to ethanol, western blotting using anti-CA III antibodies was utilized. The authors observed a downregulation of CA III due to ethanol-induced biochemical stress [36]. Following this study, the same team suggested the use of CA III, carbamoyl phosphate synthase-1 (CPS-1), and isoaspartate as biomarkers of liver injury. Alcohol intake induces isoaspartate protein damage by inhibiting protein isoaspartyl methyltransferase (PIMT), an enzyme that triggers repair of isoasparate-containing proteins. In their study, the liver proteome of ethanol-fed rats was compared with the hepatic proteome of PIMT- KO mice. The rats were exposed to ethanol for either 4 weeks, or for 8 weeks. Quantification of isoaspartate-related protein damage was performed by extracting the liver and methylating with 3H-SAM using exogenous PIMT to radiolabel the isoaspartate, followed by resolving by 1D PAGE and visualization via western blotting. It was observed that nearly 98% of CA III was downregulated in the ethanol-exposed rats after 4 weeks (P = 0.009), while CPS-1 increased by 20% and 64%. The levels of CPS-1 increased with alcohol consumption, suggesting that elevated isoaspartate and CPS-1 levels and reduced CA-III levels can be associated with hepatocellular injury caused by alcoholism (Table 4) [37].
On the other hand, the particular biodistribution of CA III isozyme, its high abundance in skeletal muscles and its absence from myocardial muscle, propelled CA III as a serum marker for skeletal muscle damage and also for acute myocardial infarction (AMI), in tandem with myoglobin. In a seminal study, Vaanen et al. [38] measured serum concentrations of myoglobin (S-Myo) and CA III by specific radioimmunoassay in 26 patients with acute myocardial infarction, 14 patients with neuromuscular diseases, and six healthy subjects, before and after physical exercise. Authors have found that S-Myo was increased in infarct patients, whereas S-CA III was not altered. On the contrary,in patients with neuromuscular diseases and in healthy subjects after physical exercise, both S-Myo and S-CA III were significantly increased. Thus, the presence of both myoglobin and CA III in blood (low myoglobin/CA III ratio) indicated skeletal muscle damage, while presence of only myoglobin (or high myoglobin/CA III ratio) indicated potential AMI [38, 39, 45]. It was further proven that within the first critical 6 hours of chest pain, a key symptom of an MI, myoglobin/carbonic anhydrase III ratio is a more sensitive parameter for detection of early acute myocardial infarction than creatine kinase (CK), creatine kinase myocardial band protein (CK-MB) or myoglobin alone (P < 0.001). Myoglobin remains, though, one of the earliest cardiac markers that elevates in serum after AMI [39]. Following these studies, the ratio between serum myoglobin and carbonic anhydrase III was used to evaluate the success of thrombolytic treatments following an MI, as well as screening patients in need of rescue angioplasty post thrombolysis [40].
Building on these studies, Sawicki and collaborators investigated the use of CA III in the diagnosis of acute coronary syndrome (ACS). This is frequently a challenging task, with biomarkers playing a key role in the evaluation, immediate risk stratification and implementation of appropriate therapy in the management of patients with suspected ACS. Cardiac troponins remain the best established biomarkers in ACS for both diagnosis and risk assessment due to their high sensitivity and specificity for detecting myocardial necrosis. Unfortunately, troponin release is delayed for several hours after the onset of ischemic injury and suffer from interference by other than ACS conditions involving myocardial damage. Using a biochip array technology, authors attempted to identify potential biomarkers of ACS shortly after the symptom onset, in a study group consisting of 42 patients suspected for ACS. Although CA III failed to act as biomarker in predicting ACS conditions, the authors identified other proteins such as H-FABP that displayed a very good efficacy in early detection of ACS (90.5%), especially when used in conjunction with troponins detection. This study highlights the importance of disease biology/biochemistry and its intrinsic link with the disease biomarkers [41].
Robert-Pachot and collaborators attempted to identify new autoantibodies with potential utility for the diagnosis of rheumatoid arthritis (RA) via immunoblotting on synovial membrane proteins. Using MALDI-TOF mass spectrometry and 2D electrophoresis, the team identified CA III as the target protein recognized by autoantibodies in RA sera. The sensitivity of these new autoantibodies for RA, using the immune-enzymatic technique, was found to be about 17%. Specificity was found to be very high when compared to non-autoimmune diseases (100%), with CA III being essentially absent from the synovial membrane of non-RA patients. However, the specificity was found to be weak (67%) when comparing the levels of the CA III autoantibodies in other autoimmune diseases, particularly systemic lupus erythematosus (SLE) [43]. Saito et al. expanded the use of CA III autoantibodies quantification to the diagnostic of vasculitis - clinical syndromes characterized by blood vessel wall inflammation that lead to tissue or end organ damage. Thus, the Japanese research team tested the prevalence of anti-CA III antibodies in diverse vasculitis such as polyarteritis nodosa (PAN), microscopic polyangiitis (MPA), Wegener’s granulomatosis (WG) and Takayasu’s arteritis (TA), using immunological assays such as ELISA and the Western Blotting technique. A significantly higher prevalence of anti-CA III antibodies was found in MPA patients versus healthy subjects (MPA, 1½3 (47.8%); healthy controls, 2/32 (6.3%); P < 0.001). Further, MPA patients positive for anti-CAIII antibodies had higher Birmingham vasculitis activity scores (BVAS - a typical disease assessment in systemic vasculitis) as compared to anti-CAIII antibody-negative patients. A commonly used diagnostic test for MPA patients is myeloperoxidase – anti‐nuclear cytoplasmic antibody (MPO‐ANCA) test, which is quite limited, with up to 40% of patients diagnosed with MPA that are negative in ANCA test. In this context, one should mention that a weak and not significant negative correlation was observed between anti-CAIII antibody levels and MPO-ANCA levels, suggesting the possibility of utilizing anti-CAIII antibodies for diagnosis of MPA in MPO-ANCA negative patients. Importantly, no significant differences were found in anti-CAIII autoantibody levels between MPA and the other primary vasculitis (Table 4) [42].
2. 4. Carbonic anhydrase IV as a disease biomarker
CA IV is a membrane-bound CA, associated to membranes via a glycosylphosphatidylinositol anchor. It is a fast isozyme (Kcat = 1.1 × 106 s−1), similarly to CA II [1, 46–48]. CA IV was found to be more resistant to inhibition by halide ions than CA II, being adapted to catalyze the CO2/HCO3- interconversion in the extracellular space, where the concentration of Cl- ions is much higher than in the cytosol. The isozyme is expressed on the apical plasma membrane of certain capillary beds, GI epithelial cells and of certain segments of the nephron. It is also expressed on specific epithelial cells of the human reproductive tract, on the plasma face of the pulmonary microvasculature, cortical capillaries, choriocapillaries of the eye, microcapillaries of skeletal and cardiac muscle, and on the microvasculature and apical plasma membrane of the colon (Table 5) [25].
Table 5.
Distribution of isozyme CA IV in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Capillary beds (apical face): • pulmonary vasculature, • cortical capillaries, • choriocapillaries of the eye, • skeletal and cardiac muscles microcapillaries |
Immunostaining, WB | [25, 47, 48] | |
| Epithelial cells of • GI tract • Reproductive tract |
||||
| Diseased | Blood/Serum | |||
| • acute myocardial infarction | MMP-9, PGLYRP1, MANSC1, QPCT, IRAK3, VNN3 | RT-PCR, DNA chip, WB, ELISA, radioimmunoassay, rocket immunoelectrophoresis | [49] | |
| • autoimmune pancreatitis | gamma-globulin and IgG | WB | [50] | |
| • apendicitis | many other (complex set) | Illumina BeadChip Arrays | [51] | |
Since CA IV is an extracellular isozyme, it can be selectively targeted by membrane-impermeant inhibitors, either charged [52–55] or polymeric [56, 57]. Some of these membrane-impermeant inhibitors were used for the detection of CA IV and other membrane-bound isozymes in vitro, ex vivo and in vivo [1, 9, 58].
Researchers from Catholic University Industry Academic Cooperation Foundation of South Korea patented biomarkers and a kit for early diagnosis of acute myocardial infarction. The biomarker comprises a protein expressed within 4 h after the acute myocardial infarction, selected form a set comprising CA IV, matrix metalloproteinase-9 (MMP-9), peptidoglycan recognition protein 1 (PGLYRP1), MANSC domain-containing protein 1 (MANSC1), glutaminyl-peptide cyclotransferase (QPCT), interleukin 1 receptor associated kinase 3 (IRAK3) and Vanin 3 (VNN3). Detection of the biomarker was done either at mRNA or at protein level in a blood sample, via RT-PCR, DNA chip and by western blot, ELISA, radioimmunoassay analysis and/or diffusion, rocket immunoelectrophoresis [49].
Nishimori et al. assessed the potential of antibodies to CA IV in diagnosis of autoimmune pancreatitis (AIP). The authors detected serum antibodies to CA IV in patients with idiopathic chronic pancreatitis, including AIP patients, via western blot technique. Moreover, it was found that the presence of serum antibodies against CA IV was significantly correlated with serum gamma-globulin and IgG levels in AIP patients, suggesting that CA IV can constitute a target antigen that is commonly expressed in epithelial cells of specific tissues involved in AIP and AIP-related diseases [50]. CA IV was also included as a potential biomarker for appendicitis within a relatively large pool of proteins, in a recent application from The George Washington University Corporation [51].
2. 5. Carbonic anhydrases VA and VB as disease biomarkers
Carbonic anhydrases VA and VB are two mitochondrial isozymes with medium-high activity (Kcat VA = 2.9 × 105 s−1, Kcat VB = 9.5 × 105 s−1), which are important for gluconeogenesis and ureagenesis - two metabolic pathways that depend in part on mitochondrial enzymes. They supply HCO3- to pyruvate carboxylase within gluconeogenesis and lipogenesis pathways, to carbamoylphosphate synthetase in ureagenesis pathway, to propionyl-CoA carboxylase and to 3-methylcrotonyl-CoA carboxylase within the branched chain amino acids catabolism. CA VA is found mainly in the liver, while CA VB is found in skeletal and heart muscles, kidneys, pancreas, GI tract, brain and spinal cord (Table 6) [1, 59, 60, 61, 62, 63, 64, 65]. The important role played by these isozymes in the body was recently evidenced in the CA V-deficiency syndrome, characterized by lethargy, hyperlactatemia, and hyperammonemia of unexplained origin during the neonatal period and early childhood [66]. Following administration of carglumic acid, Van Karnebeek and collaborators successfully resolved hyperammonemia in three children suffering from this syndrome and suggested that diagnostic molecular analysis of CA VA should be considered in newborns and other persons displaying this biochemical imbalance. Moreover, the authors indicated that CA VA deficiency should be added to the list of treatable inborn errors of metabolism potentially causing intellectual disability [66].
Table 6.
Distribution of isozyme CA VA and VB in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | CA VA • liver |
[1, 59, 60, 61, 62, 63] | ||
| CA VB • skeletal and heart muscles, • kidneys, • pancreas, • GI tract, • brain and spinal cord |
||||
| Diseased | peripheral blood mononuclear cells (PMBC) – CA VB • pancreatic cancer • pancreatic cancer/ chronic pancreatitis |
Complex gene set Complex gene set, CA19–9 |
whole genome cDNA microarray technique whole genome cDNA microarray technique | [68] [69] |
On the other hand, due to their involvement in the anabolic processes presented above, CA VA and VB constitute major targets for anti-obesity therapy, which includes treatment with primary sulfonamide drugs with potent CA inhibitory activity topiramate 8 and zonisamide 9 (Figure 2), and their combinations with other agents [67].
Bain and collaborators identified CA VB gene and CA VB as potential biomarker for the diagnostic of pancreatic cancer (PC) via transcriptional profiling of peripheral blood mononuclear cells (PMBC). Thus, the authors analyzed PBMC samples from 26 PC patients and 33 matched healthy controls using whole genome cDNA microarray technique, which allowed them to identify a set of genes significantly different between PC cases and healthy controls. Within this gene set, 65 genes displayed at least a 1.5 fold change in expression versus normal expression profiles. Further unsupervised hierarchical clustering analysis identified CA VB within an eight-gene predictor set that could distinguish PC patients from healthy controls with an accuracy of 79% in a blinded subset of samples from treatment naive patients, with a sensitivity of 83% and a specificity of 75% (Table 6) [68]. In a subsequent study, the same team validated their initial findings and refined the gene set predictor (still containing the CA VB gene) on a larger cohort of 177 patients, including 95 PC patients, 35 patients with chronic pancreatitis (CP) and 47 healthy subjects, using the same techniques, with the goal of differentiating resectable PC from CP. Multivariate models for PBMC gene expression were applied in order to assess if any combination of the proteins from the gene set was diagnostically superior to the main PC biomarker approved, CA19–9, which lacks selectivity and specificity. It was found that addition of four PBMC gene subset expression level (including CA VB) to CA19–9 significantly improved CA19–9’s diagnostic abilities when comparing resectable PC to CP patients (p = 0.023), thus offering a new tool for early PC diagnosis (Table 6) [69].
2. 6. Carbonic anhydrase VI as a disease biomarker
CA VI is a medium-fast isozyme (Kcat = 3.4 × 105 s−1), which is the only secreted CA isozyme in human subjects, being found in saliva and milk. In saliva, it neutralizes the acid generated from ingested food and also from decomposition of food by bacteria living in the oral cavity, thus protecting the upper alimentary canal from acidity (Table 7) [1, 25, 70].
Table 7.
Distribution of isozyme CA VI in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Saliva | [1, 25, 70] | ||
| Human milk | ||||
| Diseased | Saliva | |||
| • oral cavities | α-amylase | ELISA, zymography | [71] | |
| • enamel erosion | mucin 5B, statherine | [72] | ||
| Saliva, blood/serum | ||||
| • Sjögren’s Disease • secondary Sjogren’s syndrome |
salivary gland protein 1, parotid secretory protein | ELISA, WB | [73, 74] | |
In this context, Borghi et al. revealed a direct relationship between α-amylase and CA VI from saliva, the visible bacterial biofilm, and early childhood caries. This longitudinal study found that CA VI activity was significantly higher in saliva of children with caries (P ≤ 0.05), and that α-amylase activity was significantly higher in saliva of caries‐free children (P < 0.0001) (Table 7) [71]. In the same framework, Colgate-Palmolive Company patented a kit diagnosing the amplified predisposition to enamel erosion in a mammal based on two methods, both measuring and quantifying the concentrations of proteins critical to proper enamel formation. The first method measures concentrations of biomarkers mucin 5B, carbonic anhydrase VI, and statherine in a saliva sample from the oral cavity using specific antibodies against these targets, and compares the concentrations of these proteins to control samples. Based on the results generated with this kit, concentrations lower than 250 mg/ml mucin 5B, < 35 mg/ml of CA 6, or > 15 mg/ml of statherin indicate susceptibility to enamel erosion. The second method requires a sample of acquired enamel pellicle (AEP) from a patient and measures concentrations of total protein, statherin, and calcium concentrations in the AEP. The determined concentrations of these proteins are compared to control values generated from normal patients. Since AEP provides a protective layer on enamel, it is claimed that a reduction in the AEP concentration of [statherin] < 30 ng or of [Ca2+] < 0.001 mol/mm2 were indicative of erosion susceptibility. The authors claimed the need for this kit in identifying highly susceptible patients for proper treatment, along with further evaluation of dental erosion (Table 7) [72].
The Research Foundations of State University of New York patented a kit to identify patients with early Sjögren’s Disease (SD) - an autoimmune disease characterized by dryness of the mouth and eyes. SD can occur alone (primary SD) or can be associated with another underlying autoimmune disease (secondary Sjogren’s syndrome (SS)) such as rheumatoid arthritis, systemic sclerosis (scleroderma), systemic lupus erythematosus, or polymyositis. Primary SS presents the greatest diagnostic challenge, since the symptoms are vague and non-specific, and because primary SD can trigger a wide range of systemic and extraglandular ocular complications. As the disease progresses, the lacrimal glands vital for tear production become irreversibly destroyed, leading to the symptoms observed clinically. Primary SD was associated with an increased risk of cerebrovascular events, myocardial infarction, predisposition to hypertension and hypertriglyceridemia and various forms of lymphoma compared to the general population [73, 74]. The proposed method involves the detection of antibodies against salivary gland protein 1 (SP-1), parotid secretory protein (PSP), carbonic anhydrase VI, in saliva, blood, or serum samples, via antigen-antibody reaction quantified by immunohistochemical techniques such as Western blotting and ELISA. Individuals were identified as having Sjögren’s disease if above-mentioned antibodies or a combination of them were present in the biological sample prelevated. Furthermore, the kit, containing purified proteins salivary gland protein 1 (SP-1), parotid secretory protein (PSP) and carbonic anhydrase VI (CA VI), could be utilized to validate the absence of Sjögren’s Disease. Thus, absence of PSP and SP-1 antibodies, as well as low levels of CA 6 antibodies were considered indicative that the patient does not have Sjögren’s Disease. The value of the kit is deemed important due to its ability for early diagnosis of this disease (Table 7) [74].
2. 7. Carbonic anhydrase VII as a disease biomarker
CA VII is a fast isozyme (Kcat = 9.5 × 105 s−1), found in large amounts in the CNS, but also encountered in colon, liver, skeletal muscles, duodenum, stomach [1]. In the CNS CA VII is found in the neurons of hippocampus, together with CA II. However, CA VII is not found in glial cells, which contain just CA II. Using a novel CA VII (Car7) knockout (KO) mouse model, as well as a CA II (Car2) KO, and a CA II/VII double KO mouse models, Ruusuvuori et al. have shown that mature hippocampal pyramidal neurons are endowed with these two cytosolic isoforms that enhance bicarbonate-driven GABAergic excitation during intense GABAA-receptor activation. It appears that CA VII is predominantly expressed by neurons starting around postnatal day 10 (P10), while CA II is expressed in neurons at P20. The authors also found that CA VII mRNA is expressed in the human cerebral cortex at a very early age, that points towards CA VII being a key molecule in age-dependent neuronal pH regulation (Table 8) [75, 76]. Earlier studies from the same group have shown that CA VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal neurons in response to high-frequency stimulation [77], making CA VII an important modulator of long-term synaptic plasticity, with direct implications in memory and learning processes [17, 20, 21].
Table 8.
Distribution of isozyme CA VII in normal and pathologic tissues
On the other hand, CA VII was recently suggested as a genetic marker for detecting colorectal cancer, within a larger group of biomarkers, by the National Defense Medical Center Taiwan. Thus, Drs. Chu and Chang from the above-mentioned institution revealed an increased expression of CA VII in colorectal cancer cells vs normal counterparts and suggested the use of CA VII levels as a predictor of malignancy propensity of sampled tissues (Table 8) [78].
2. 8. Carbonic anhydrases VIII, X, XI as disease biomarkers
CAs VIII, X, XI are CA isozymes devoid of catalytic activity due to absence of one or more histidine residues needed to coordinate the Zn2+ ion in their active site. These inactive isozymes are known as CA-related proteins (CA-RPs) and their physiologic role is relatively poorly understood.
CA VIII is the most structurally-studied protein among the three CA-RPs, known to be present in the Purkinje cells of cerebellum, in the neural cell bodies and some astrocytes, and in other human tissues such as the liver, lung, heart, gut, thymus, and kidney (Table 9) [6, 79, 80, 81]. Picaud et al. revealed that the core domain of hCA VIII adopts the classical architecture of the mammalian CA enzymes, with a 10-stranded central β-sheet surrounded by several short α-helices and β-strands, resembling closely the cytosolic isozymes CA II and CA XIII [82]. It was proved that CA-RP VIII influences inositol triphosphate (ITP) binding to its receptor ITPR1 on the endoplasmic reticulum, thus modulating calcium signaling inside the cells [79, 83]. Reverse-transcription polymerase chain reaction (RT-PCR) analysis has also revealed the presence of CA VIII mRNA in developing human lungs and, to a lesser extent, in normal lungs, where it is expressed in the pulmonary epithelium of developing lungs, and also into the bronchial ciliated cells of adult lungs [84]. A mutations in CA-RP VIII gene (S100P) that significantly reduces the protein stability [79] has been associated with ataxia, mild mental retardation and a predisposition for quadrupedal gait in humans and with lifelong gait disorder in mice. This suggests an important role for CA-RP VIII in the normal brain [79, 85, 86]. CA-RP VIII has been recently identified as an autoantigen involved in the pathogenesis of melanoma-associated paraneoplastic cerebellar degeneration (PCD), a disease characterized by the selective damage of the Purkinje cells of the cerebellum, believed to be immune-mediated (Table 9) [87].
Table 9.
Distribution of isozyme CA-RP VIII in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Brain (Purkinje cells of cerebellum, neural cell bodies, astrocytes) Lungs, Heart, Liver, gut, Thymus, Kidneys |
[6, 79, 80, 81, 84] | ||
| Diseased | Blood/Serum | |||
| • melanoma-associated paraneoplastic cerebellar degeneration | WB | [87] | ||
| • Non-small cell lung cancer (squamous cell carcinomas, adenocarcinomas, and adenosquamous cell carcinomas) | Northern-blot analysis, RT-PCR, immunostaining | [84] | ||
| • Colon cancer | RT-PCR, immunocytochemical analysis | [88] | ||
Importantly, CA-RP VIII was found to be strongly expressed in many lung cancers, including squamous cell carcinomas, adenocarcinomas, and adenosquamous cell carcinomas. The protein was abundantly expressed in cancer cells at the front of tumor progression, suggesting an important role in non-small cell lung carcinomas progression [84]. CA-RP VIII was also significantly over-expressed, at both mRNA and protein levels, in certain colon cancers, where it potentiates the proliferative and invasive abilities, both in vitro and in vivo. Small interfering RNA (siRNA)-mediated knockdown of CA-RP VIII translated into significant inhibition of cell proliferation and colony formation in a HCT116 colon cancer cell line expressing CA-RP VIII (Table 9) [88].
CA X was the second CARP reported, after CA VIII [81, 89]. Sequence analysis of cDNA libraries showed significant homology (25–57%) to other CA isozymes and revealed the lack of two histidine residues required for Zn-binding and catalytic activity similarly to another inactive CA isozyme, CA-RP XI [81]. Using monoclonal antibodies against human CA-RP X, it was found that the protein it is expressed in the cytoplasm of the myelin sheath (Table 10) [81]. Moreover, it was also found that a decreased CA-RP X level correlated well with the demyelinization of axons in the human brain in acute disseminated encephalomyelitis, correlation supported by almost complete disappearance of CA-RP X protein in a mouse model of incomplete myelinization [81, 90]. These findings are supporting an important role played by CA-RP X in myelin sheath organization in normal brain development [80, 81]. Additionally, comparative analysis of the CA-RP X expression pattern from more than 17000 microarrays, revealed protein upregulation in several cancers (Table 10) [80, 91].
Table 10.
Distribution of isozyme CA-RP X in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | CNS • cytoplasm of the myelin sheath cells |
immunostaining | [81, 89] | |
| Diseased | CNS (cytoplasm of the myelin sheath - downregulation) • acute disseminated encephalomyelitis |
Immunostaining | [81, 90] | |
| Tumors (various types) | Many other (large set) | Affymetrix gene expression array | [80, 91] | |
CA XI is the last CARP, similar in structure with CA-RP X. CA-RP XI was found to be expressed in CNS, in the choroid plexus and pia arachnoid areas, within neural body, neurites, and astrocytes, but not within oligodendroglia [81]. RT-PCR-driven expression analysis, together with northern blot analysis, revealed the presence of CA XI mRNA in large amounts in the brain, followed by expression in the spinal cord, pancreas, liver, kidney, thyroid salivary gland, skeletal muscles, ovaries, small intestine, colon, stomach, lymph nodes and adrenal gland, at substantial lower levels (Table 11) [80]. Increases expression of CA-RP XI in these tissues can be correlated with risk of cancer. Thus, an elevated expression of CA-RP XI was found in 91 % of gastrointestinal stromal tumors (GISTs) specimens analyzed immunohistochemically, with an elevated expression of the protein found at the periphery of GISTs. Interestingly, CA-RP VIII was also found over-expressed in the same specimens, although less frequently – only 59% of all cases. The correlation between expression patterns of CARP XI and GIST was also confirmed in vitro, in the GIST cell line GIST-T1. Through RT-PCR, Southern blot, and immunocytochemistry, it was confirmed that ectopic expression of CA-RP XI in GIST-T1 cells induced cell proliferation and invasion (Table 11) [92]. Similarly to CA-RP X, upregulation of CA-RP XI mRNA was linked to several cancers and pathological conditions via microarrays screening (Table 11) [80, 91].
Table 11.
Distribution of isozyme CA-RP XI in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Brain • choroid plexus and pia arachnoid areas, • neural body, neurites, and astrocytes Spinal cord, GI tract (stomach, small intestine, colon) Pancreas, Liver, Kidneys, Skeletal muscles, Ovaries, Lymph nodes, Adrenal gland, thyroid, salivary glands |
[80, 81] | ||
| Diseased | Gastrointestinal stromal tumors | CA-RP VIII | Immunostaining, RT-PCR, Southern blot, immunocytochemistry | [92] |
| Other tumors (various types) | Many other (large set) | Affymetrix gene expression array | [80, 91] | |
| Gliomas, astrocytomas, oligodendroglial tumors | CA II, CA IX, CA XII | Immunostaining | [93] | |
Karjalainen et al. [93] recently assessed the expression of CA-RPs VIII and CA-RPs XI in human astrocytomas or oligodendroglial tumors and analyzed their association to different clinicopathological features. The authors also attempted to correlate the expression of these CA-RPs with the expression of other CA isozyme traditionally associated with tumors, namely the cytosolic CA II, and the membrane-bound CA IX and CA XII. An analysis of the two CA-RPs in 405 gliomas of different grades via immunostaining revealed that CA-RP VIII was expressed in 13% of the astrocytomas and in 9% of the oligodendrogliomas, while CA-RP XI was expressed in 7% of the astrocytic and in 1% of the oligodendroglial tumor specimens. The benign tumors such as pilocytic astrocytomas did not express CARPs at all. Another finding was that the presence of these CARPs was associated with a more benign behavior in grade II-IV astrocytomas (both CA-RP VIII and XI), and in oligodendrogliomas and oligoastrocytomas (CA-RP VIII only). No correlation was found between CA-RP VIII expression and the expression of other CAs and between the expression of CA-RP XI and CA VII, CA IX, and CA XII. Interestingly, the expression of CA-RP XI was found to be positively correlated with the cytoplasmic CA II in astrocytic tumors (P = 0.037). It was concluded that CA-RPs do play a role in tumorigenesis of diffusively infiltrating gliomas through an unknown mechanism that requires further investigations (Table 11) [93].
2. 9. Carbonic anhydrases IX as a disease biomarker
Carbonic anhydrase IX (CA IX) is a medium-fast (Kcat = 3.8 × 105 s−1), membrane-bound, isozyme of carbonic anhydrase. It is a transmembrane isozyme, possessing an N-terminal proteoglycan domain, the catalytic domain, a single-pass transmembrane region, and an intracellular tail [1, 94, 95]. Mass spectrometry experiments showed that CA IX protein is dimeric, possessing an intermolecular disulfide bond between two similar Cys residue located on separate CA catalytic domains [96]. CA IX normal expression is limited to the epithelium of stomach, bile duct, gallbladder duct, pancreatic duct, rapidly-proliferating normal cells of small intestine, and, to a lower extent, to the CNS, where it can be found mainly in the ventricular-lining cells and in the choroid plexus (Table 12) [94, 97, 98]. However, CA IX is strongly upregulated in many hypoxic tumors, where it plays a significant role in tumor cell survival, invasiveness and metastasis [94]. Fast-growing tumors are becoming quickly hypoxic, triggering the stabilization of HIF-1 in cytoplasm, followed by relocation to the nucleus, where it triggers the expression of a plethora of genes encoding proteins involved in cell survival and proliferation under hypoxic conditions, including CA IX [1, 94]. Hypoxic tumor cells rely on glycolysis as the main source of energy production, and produce large amounts of acidic byproducts pyruvate and lactate. CA IX plays a defensive role towards pH regulation inside the tumor cell, moving protons from cytoplasm to the external milieu via reversible HCO3- dehydration/CO2 hydration, in tandem with cytosolic CA isozymes such as CA II [1, 94, 99–102]. Giving its central role in promoting tumor cell survival, CA IX expression is upregulated in many types of cancers including breast, kidney, colon, ovarian, head-and-neck, pancreatic and lung cancer [1, 94, 98, 103]. This expression pattern renders CA IX a potential biomarker and a compelling therapeutic target for the detection and treatment of hypoxic solid tumors (Table 12) [1, 6, 9, 10, 11, 14, 15, 56, 100, 102, 104–113].
Table 12.
Distribution of isozyme CA IX in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | GI tract • epithelium of stomach, bile duct, gallbladder duct, pancreatic duct, • rapidly-proliferating normal cells of small intestine |
Immunostaining | [94, 96, 97, 98] | |
| CNS • ventricular-lining cells • choroid plexus |
Immunostaining | [94, 96, 97, 98] | ||
| Diseased | Tumors • renal cell carcinoma, colon, lung, breast, ovarian, head-and-neck, pancreatic cancer, transitional cell carcinoma of the urinary tract |
Imaging/detection via CA IX antibodies and CA IX inhibitors, Immunostaining, WB | [1, 6, 9, 11, 100, 102, 110, 111, 112, 113] [114, 115, 116, 117, 118, 119] |
|
| Blood • renal cell carcinoma • non-small cell lung cancer |
ELISA ELISA |
[120] [117] |
||
| Urine • transitional cell carcinoma of the urinary tract |
WB | [121] | ||
In this context, Hulick et al. investigated the relationship between the CA IX blood levels and the progression of renal cell carcinoma (RCC). They utilized an anti-CA IX antibody (M75)-based enzyme-linked immunosorbent assay to determine CA IX levels in blood obtained before and after nephrectomy for clinically localized disease in patients with clear cell RCC, papillary and chromophobe RCC, oncocytoma, or benign kidney lesions. Comparison with CA IX levels in blood drawn from normal control individuals revealed a significant reduction in the blood levels of CA IX (P < 0.006), after nephrectomy for localized disease, in the majority of patients with clear cell RCC (57%). On the other hand, blood samples from patients with non-clear cell RCC, a benign disease, or those having undergone de-bulking nephrectomy for metastatic disease did not show a decrease in CA IX levels after nephrectomy. The follow-up measurements of CA IX levels in a small group of patients indicated that rising CA IX levels directly correlate with disease progression (Table 12) [120].
In another study, Genega et al. investigated the level of expression of CA IX in primary and metastatic renal neoplasms and correlated this expression to the grade and type of the tumor. They evaluated the CA IX expression in 366 cancer cases, among which 317 cases were primary and 42 cases were metastatic tumors. The cases were divided based on tumor type as follows: 308 renal cell carcinomas (186 clear cell, 52 papillary, 35 chromophobe, 20 unclassified, 15 Xp11.2 translocation), 26 oncocytomas, 2 metanephric adenomas, 1 urothelial carcinoma, 1 mixed epithelial and stromal tumor, 1 angiomyolipoma, 21 unknown and 6 with more than one tumor type. CA IX immunostaining was carried out using the mouse monoclonal antibody MN-75 [122] on one representative section of tumor from each case. Samples were scored based on the staining intensity of the cytoplasmic membrane and the percentage of positive cells such that samples in which > 85% of tumor cells stained for CA IX were considered as high CA IX expressing tumors, whereas those in which ≤ 85% of tumor cells stained for CA IX were considered as low CA IX expressing tumors. Their results showed a correlation between high CA IX expression and tumor type (clear cell versus non-clear cell). Moreover, a statistically significant association between the expression and grade in primary clear cell carcinomas was found, thus supporting the evidence that CA IX could be used as an immunohistochemical marker to reliably distinguish between different tumor types and if its expression is correlated to RCC grade (Table 12) [114].
In a more comprehensive study, Luong-Player et al. examined the level of expression of CA IX in different type of malignancies as well as in normal tissues. The study included 1,551 cases encompassing 1,160 malignant tumors, 69 benign neoplasms, and 322 normal tissues. Immunohistochemical staining of CA IX was performed using a mouse monoclonal antibody, clone MRQ-54. Cytoplasmic membrane staining was regarded as a positive result. The staining intensity was graded as weak or strong. The distribution of staining was recorded as negative (< 5% of tumor cells stained), 1+ (5%−25%), 2+ (26%−50%), 3+ (51%−75%), or 4+ (> 75%). The results revealed the overexpression of CA IX in clear renal cell carcinoma (CRCC) with 90% of low-grade CRCCs and 86% of high-grade CRCCs cleared showing CA IX overexpression. On the other hand, all chromophobe renal cell carcinoma (ChRCC) and renal oncocytomas were CA IX negative. Moreover, 90 % of the intrahepatic cholangiocarcinoma (ICC) cases showed a significant level of CA IX overexpression, while only 15 % of the hepatocellular carcinoma (HCC) cases were CA IX positive. All carcinomas of the breast, thyroid, or prostate as well as normal renal tubules except one case showed no staining and were reported as CA IX negative. These findings validated CA IX as a potential diagnostic biomarker in differentiating CRCC from ChRCC and oncocytoma. The authors also provided evidence that CA IX can be used to distinguish low-grade CRCC from normal renal tubules, which can be challenging in small biopsy specimens or fine-needle aspirations. Moreover, these findings rendered CA IX as an adjunctive immunohistochemical tool in separating cholangiocarcinomas from HCC and identify the role of CA IX as an important marker in separating CRCCs from other lesions with clear cell features, such as clear cell carcinoma of the ovary and uterus (Table 12) [115].
Besides serving as a diagnostic biomarker for renal carcinomas, CA IX is also considered to be a potential diagnostic epitope for colorectal cancer. In this context, Korkeila et al. evaluated the level of CA IX expression in tumor samples obtained from 166 rectal cancer patients treated by preoperative radio- or chemo-radiotherapy or surgery only. Operative samples harvested from non-irradiated patients served as controls. The level of CA IX expression was evaluated using a rabbit polyclonal antibody for CA IX by three different strategies - either positive or negative, proportion of positivity and staining intensity. The results of immunohistochemical analysis were also validated by western blotting analysis. Results revealed that CA IX staining was positive in 44%, weak in 15% and moderate or strong in 29% of the operative samples. The proportion of CA IX positive staining and staining intensity were directly correlated. Moreover, the staining intensity of CA IX in the operative samples was shown to be significantly dependent on the treatment categories. The long-course radiotherapy group samples were CA IX positive with moderate/strong staining intensity. On the other hand, the chemo-radiotherapy group samples were mostly CA IX negative, implying the synergistic effect of chemotherapy in enhancing the treatment outcome. This reflects the prognostic significance of CA IX in rectal cancer, suggesting that strong staining intensity of CA IX is an adverse prognostic factor in rectal cancer (Table 12) [116].
Furthermore, CA IX has proven to be a predictive prognostic tool for lung adenocarcinoma as shown by Nakao and collaborators [123]. In this study, the level of CA IX expression was evaluated immunohistochemically in cancer associated fibroblasts (CAF) and cancer cells in 158 resected cases of lung adenocarcinoma. CA IX staining was done using a rabbit polyclonal antibody for CA IX and the samples were considered positive for CA IX if cell membrane and cytoplasmic staining was present in > 10 % of the CAFs and > 20 % of the cancer cells. The results revealed that CA IX expression by cancer cells was observed in 25.3% of the cases, while its expression by stromal spindle cells that were morphologically identified as fibroblasts was observed in 24.7% of the cases. Moreover, CA IX expression by both cancer cells and fibroblasts was observed in 11.4% of the total cases. Interestingly, all cases that were positive for CA IX expression by cancer cells or fibroblasts were cases of invasive carcinoma (Table 12) [123].
In the same context of using CA IX as a biomarker for lung cancer, Ilie et al. investigated the expression of CA IX in tumor tissue and/or plasma of patients with non-small cell lung cancer (NSCLC). They generated tissue microarrays of 555 NSCLC tissue samples for quantification of CA IX expression. The plasma level of CA IX was determined by ELISA in 209 of these NSCLC patients and in 58 healthy individuals. The CA IX tissue immunostaining and plasma levels were correlated with clinicopathological factors and patient outcome. High tissue expression of CAIX was correlated with shorter overall survival (OS) (P = 0.05) and disease-specific survival (DSS) of patients (P = 0.002). It is found that the plasma level of CA IX was significantly higher in patients with NSCLC than in healthy individuals (P<0.001) and associated with shorter OS (P < 0.001) and DSS (P < 0.001), mostly in early stage I+II NSCLC. Besides, high CA IX tissue expression (P = 0.002) was associated with poor prognosis in patients with resectable NSCLC. In addition, a high plasma level of CA IX was an independent variable predicting poor OS (P < 0.001) in patients with NSCLC. The authors concluded that high expression of CA IX in tumor tissue is a predictor of poor survival, and a high plasma level of CA IX is an independent prognostic biomarker in patients with NSCLC, particularly in early-stage I+II carcinomas (Table 12) [117].
In another study, Kim et al. analyzed the level of CA IX expression associated with pancreatic neuroendocrine neoplasms (PanNETS). The level of expression was investigated immunohistochemically in 164 well-differentiated PanNETs and 23 incidentally identified pancreatic neuroendocrine microadenomas, using a primary antibody directed against CA IX. Moderate to strong staining of CA IX in > 10% of tumor cells was considered as positive. Results revealed that CA IX expression was observed in normal islets, while neuroendocrine microadenomas and small (< 1 cm) PanNETs showed loss of CA IX expression. CA IX expression was observed in 38 (23%) and 36 (22%) of PanNETs, respectively. CA IX expression was associated with larger size (p = 0.001), higher grade (p < 0.001), higher pT category (p < 0.001), lymph node (p = 0.003) and distant (p = 0.047) metastases, higher AJCC stage (p < 0.001), and lymphovascular (p < 0.001) and perineural (p = 0.002) invasion. Moreover, PanNET patients with CA IX expression had a shorter recurrence-free survival (5-year survival rate 47%) than those without CA IX expression (76%) by univariate (p = 0.001) but not multivariate analysis. These results suggested that CA IX expression was found mainly in normal islets, while neuroendocrine microadenomas and small PanNETs showed loss of CA IX expression. CA IX was found to be expressed in larger PanNETs, associated with aggressive clinicopathologic factors and poor recurrence-free survival by univariate but not multivariate analysis. The value of CA IX expression as prognostic marker for PanNET patients needs further studies (Table 12) [118].
Furthermore, CA IX expression was associated with transitional cell carcinoma (TCC) of the urinary tract. Hyrsl et al. studied the expression level of cell-associated CA IX in histological sections of the transitional cell carcinoma (TCC) of the urinary tract and of the soluble form of CA IX (s-CAIX) shed by the tumor into the serum and urine of TCC patients. A number of 23 patients with TCC or squamous cell carcinoma (SCC) and sixteen healthy persons as controls were enrolled in this study. Soluble s-CAIX were detected in urine samples using Western blots, as a double band at 50 and 54 kDa. In most cases, the presence of s-CA IX in the urine correlated with CA IX expression in the tumor. On the other hand, s-CA IX did not exceed the normal level in the serum of TCC patients. s-CAIX was found to be presented in the urine of patients with TCC of the urinary bladder and renal pelvis, allowing the detection of tumors in approximately 70% of the patients. In conclusion, the authors suggested that a simple, rapid and sensitive test, monitoring s-CA IX levels in urine can be developed for the early detection of relapse in patients following transurethral tumor resection (Table 12) [121].
Chu et al. analyzed the expression of CA IX in patients with invasive breast ductal carcinoma and attempted to correlate the expression level of this CA isozyme with histological grade, lymphatic metastasis, TNM stage and patient prognosis. Invasive breast ductal carcinoma is characterized by a heterogeneously hypoxic environment and therefore it is not surprising that the expression of CA IX was detected by non-biotin immunohistochemical method in 29 (29.0%) of 100 invasive breast ductal carcinomas investigated. The CA IX expression was found to be significantly correlated to lymph node metastasis (P = 0.015), TNM stage (P = 0.018) and overall survival rate (P = 0.0001) or disease-free survival rate (P = 0.0001), but not to age (P = 0.375), tumor size (P = 0.288) and histological grade (P = 0.526), thus proving CA IX as an independent prognostic factor (P = 0.002). It was also concluded that the hypoxic microenvironment in invasive breast ductal carcinoma might be associated with aggressive tumor phenotype of this cancer type (Table 12) [119].
2. 10. Carbonic anhydrases XII as a disease biomarker
CA XII is another medium-fast (Kcat = 4.2 × 105 s−1), membrane-bound isozyme, similar in general structure with CA IX. It contains the N-terminal CA domain, an α-helical transmembrane region, and a short intracytoplasmic tail, as does CA IX, but it does not have a proteoglycan domain. Similarly with CA IX, it forms a dimer with the two active sites oriented towards the extracellular milieu. The extracellular catalytic domain contains two asparagine residues that can be glycosylated, while the transmembrane domain contains the GXXXG and GXXXS motifs, which are involved in the dimerization of the protein polypeptide. The cytoplasmic domain at the C-terminus contains two potential sites for phosphorylation in the 29 amino acid sequence [1, 124–128].
CA XII is overexpressed in many cancers, including renal, breast, non-small cell lung cancer, etc [126, 127]. In fact, the protein was discovered in a cDNA library from a human renal cell carcinoma (RCC) using the serological expression screening method [124] and was also cloned from the same cells using the RNA differential display technology [127]. In RCC, CA XII expression has been found mostly in clear cell carcinomas and oncocytomas. In clear cell carcinomas, the level of CA XII expression correlates with the histological grade of the tumor [126, 129, 130]. Both CA IX and CA XII are overexpressed under hypoxic conditions [131], but the level of expression depends on the tissue type. In contrast to CA IX, CA XII is highly expressed in many normal tissues including colon (but not small intestine), kidney, prostate, endometrium, rectum, esophagus, brain, pancreas, ovary, testis, sweat glands of skin, breast epithelium and non-pigmented ciliary epithelial cells of the eye (Table 13) [126, 127, 132]. Importantly, the expression patterns of CA IX and CA XII are different and they overlap only marginally [132].
Table 13.
Distribution of isozyme CA XII in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | GI tract | Immunostaining | [126, 127, 132] | |
| • esophagus | ||||
| • colon (but not small intestine), | ||||
| • rectum | ||||
| Pancreas, | ||||
| Kidneys, | ||||
| Prostate, | ||||
| Brain, | ||||
| Endometrium, | ||||
| Ovaries, testis, | ||||
| Sweat glands of skin, | ||||
| Breast epithelium, non-pigmented ciliary epithelial cells of the eye | ||||
| Diseased | Tumors | CA IX (in some) | cDNA libraries screening, PCR, | [124, 126, 127, 131, 133] |
| • kidney (renal cell carcinoma) | Northern blotting | [126, 129, 130] | ||
| • breast cancer | [134] | |||
| • lung cancer | Immunostaining | [135] | ||
| • cervical cancer | Immunostaining, tissue microarrays | [136] [137] |
||
| • ovarian cancer | CA IX | Immunostaining | ||
| Immunostaining | [138] | |||
| • colorectal cancer | CA IX | [139, 140] | ||
| CA IX | Immunostaining | [141, 142] | ||
| • brain cancers | CA IX, CA II | Immunostaining | ||
| Immunostaining, WB, RT-PCR | [143] | |||
| • esophageal squamous cell carcinoma | Immunostaining | [144, 145, 146, 147] | ||
| Blood (mutated version) | ||||
| • Cystic fibrosis-like diseases | Genotyping, gene sequencing, RT-PCR, | [148, 149] | ||
| • Pancreatitis | [126, 148, 150] | |||
| • Sjogren’s syndrome | Large set of biomarkers considered | Immunostaining | [151, 152] | |
| Nucleus pulposus of vertebrae | ||||
| • Chronic back pain | CA IV, CA IX | cDNA microarray analysis, RT-PCR, immunostaining, flow cytometry | [153] | |
| Eyes (non-pigmented ciliary epithelial cells) • Glaucoma |
Immunostaining, Northern blotting | |||
As mentioned above, CA XII expression has been detected in many other tumors, besides RCC. Dinona (Seoul, KR) patented an antibody recognizing and binding to CA XII-expressing tumors for diagnostic purposes. The antibody binds a non-catalytic region, located at an N terminus of CA XII and can also be used in the alleviation, prophylaxis, therapy or CA XII-positive solid tumors [133].
In breast cancer CA XII expression is controlled by the estrogen receptor (ER) and is associated with positive ER alpha receptor status [126, 134, 154]. Immunohistochemistry assessment of CA XII expression in a series of 103 cases of invasive breast cancer determined that CA XII was present in 75% of tested patients and was associated with lower grade (P = 0.001), positive estrogen receptor status (P < 0.001), and negative epidermal growth factor receptor status (P < 0.001). CA XII expression was associated with an absence of necrosis (P < 0.001), and CA XII positive tumors were associated with a lower relapse rate (P = 0.04) and a better overall survival for the patient (P = 0.01). It was concluded that CA XII is a biomarker that is associated with a better prognosis in an unselected series of invasive breast carcinoma patients (Table 13) [134].
In another relevant study, 555 tumors from non-small cell lung cancer (NSCLC) patients were immunostained for CA XII using tissue microarrays (TMA) and the results were correlated with clinicopathological parameters and outcome of patients. CA XII was found overexpressed in 19 % of the patients and the expression of this biomarker was associated with tumors of lower grade (P = 0.015) and histological type (P < 0.001), being significantly higher in squamous cell carcinoma. High CAXII expression was correlated with better overall patient survival and better outcome of patients with resectable NSCLC (Table 13) [135].
CA XII expression was also correlated with survival outcome in well differentiated (WD), moderately differentiated (MD) or poorly differentiated (PD) cases of cervical cancers. Immunohistochemical detection of the CA XII biomarker in cervical cancer tissues from 183 patients undergoing radiotherapy revealed that CA XII expression was highly associated with the histologic grade of cervical cancer, with a fourfold reduction in CA XII gene expression in PD tumors. Lack of CA XII expression was associated with PD histology, with an odds ratio of 3.9 (P = 0.01). The authors concluded that CA XII may be used as a novel prognostic marker in combination with histologic grade of the tumors and that this combined category system may be applicable as an adjunct prognostic indicator of survival in patients with uterine cervical cancer treated with radiotherapy [136]. A comparison of CA IX versus CA XII prognostic power in primary cervical cancer tissues from 73 patients who underwent laparoscopic lymph nodule staging and two patients with clinical staging, done at the National Cancer Center, Korea, revealed that expression of CA IX, but not CA XII, in tumors is associated with the presence of lymph node metastases and poorer prognosis (Table 13) [137].
In a similar study, Hynninen and collaborators investigated CA IX and XII expression in patients with ovarian tumors via immunostaining and attempted to correlate the results with histopathological and clinical parameters [138]. The authors found that most cases of borderline mucinous cystadenomas, mucinous cystadenocarcinomas and serous cystadenocarcinomas were moderately or strongly positive for CA IX. The CA IX staining was most prominent in hypoxic regions of malignant tumours, Expression of CA XII was detected in all tumor categories, with a mean staining intensity weaker than for CA IX in all groups except for clear cell carcinomas. It was concluded that the wide expression of both CA IX and CA XII in ovarian tumours suggests that these isozymes could represent potential targets in ovarian cancer therapy, with CA IX being a superior histopathological marker protein for hypoxia in malignant ovarian tumors (Table 13) [138].
In colorectal tumors, CA XII expression was found to be very distinct from the normal tissues. The extent of positive staining of CA XII was found to increase with a high grade of dysplasia, especially in the deep parts of the adenomatous mucosa. Adenomas with severe dysplasia and carcinomas showed an equal, diffusely spread staining pattern for CA XII that seemed to be associated with the malignant behavior [139]. Therefore, it was concluded that CA XII, in addition to CA IX [140] might be useful in the histopathological diagnosis of colorectal tumors (Table 13) [139].
CA XII was found to be overexpressed, frequently together with CA IX, in several types of brain tumors, including gliomas, hemangioblastomas, and meningiomas. Statistical analysis of a total of 112 tumor samples collected from surgical specimens versus normal brain tissue specimens revealed that CA IX and CA XII expression differed significantly from normal brain in all but the grade I gliomas (P < 0.05), being over-expressed in all of the tumor groups except the grade I astrocytoma samples. Importantly, a significant correlation was observed between both CA IX and CA XII expression and increasing grade of malignancy in primary brain tumors (CA IX vs. tumor grade: correlation coefficient = 0.464, P < 0.01; CA XII vs. tumor grade: correlation coefficient = 0.409, P < 0.01) [141]. CA XII expression has also been detected in diffuse astrocytomas. An alternatively spliced variant of this isoform was identified in these tumors, which is 11 amino acids shorter than the normal CA XII protein. Both variants are expressed in the normal brain at very low levels. The study material consisted of 370 diffusively infiltrating astrocytic gliomas (grades II, III, and IV) and the overall survival was known for 287 patients (39 grade II, 30 grade III, and 218 grade IV). Interestingly, the shorter isoform of CA XII appeared to be the predominant form in the brain tumors examined, and its expression was found to be an independent prognostic factor in diffuse astrocytoma, linked with survival. Out of the 370 diffusely infiltrating astrocytomas analyzed, 363 cases (98%) showed immunoreactions for CA XII. CA XII expression was found to be correlated with poorer patient prognosis in univariate (p = 0.010, log-rank test) and multivariate survival analyses (p = 0.039, Cox analysis). Moreover, it was also found that simultaneous expression of CA II, CA IX, and CA XII predicted an extremely poor prognosis for the corresponding patients. Multivariate analysis further revealed that simultaneous expression of both CA XII and CA IX was also a valuable independent prognostic factor (Table 13) [142].
Ochi et al. have recently demonstrated that CA XII is an independent prognostic factor in advanced esophageal squamous cell carcinoma (ESCC) [143]. Immunohistochemical analysis performed on 70 primary tumor samples obtained from ESCC patients who underwent esophagectomy, revealed CA XII presence in the cell membranes of carcinoma cells. The expression of CA XII was related to the pT category but had no prognostic impact overall. However, when authors examined the expression of CA XII according to the pT category, they found that within pT2–3 ESCC, the 3-year survival rate of patients with the high grade expression of CA XII (29.1 %) was significantly lower than that of patients with the low grade expression of CA XII (70.3 %). A multivariate analysis conducted on these specimens has demonstrated that the expression of CA XII was one of the most important independent prognostic factors following radical esophagectomy in pT2–3 ESCC, suggesting that the expression of CA XII may be a valuable prognostic factor for patients with advanced ESCC (Table 13) [143].
Due to the important role played by CA XII normal physiological functions, the isozyme can act as a biomarker in several other dysfunctions and diseases besides cancer. For example, CA XII is involved in bicarbonate/chloride exchange and water movement in/out of cells and tissues [126, 147, 155] This involvement starts at the embryonic stage [126]. CA XII was shown to activate the ductal Cl-/HCO3- exchanger AE2, and to be associated with Na+/HCO3- cotransporter kNBC1, thus playing a key role in epithelial cell electrolyte homeostasis [155, 156]. Mutations in the CA XII gene are known to cause cystic fibrosis (CF)-like diseases [144, 145, 146, 147], pancreatitis [148, 149] and Sjogren’s syndrome [126, 148, 150] (Table 13). Along these lines, Muhammad et al. identified a novel autosomal recessive form of isolated salt wasting in sweat, which lead to severe infantile hyponatremic dehydration in three individuals from a small Bedouin clan. The subjects presented with failure to thrive (FTT) in infancy or in young age, hyponatremic dehydration, and hyperkalemia with isolated sweat salt wasting. Through genotyping and gene sequencing, the authors identified that this syndrome is associated with a Glu143Lys mutation in CA XII structure [146]. The same CA XII enzyme mutant cased similar symptoms in several consanguineous Israeli Bedouin kindred [145]. Interestingly, in this study Feldshtein and collaborators showed that the catalytic activity of p.Glu143Lys CA XII was around 70% of the wild-type enzyme, for the physiologic CO2 hydration reaction and was efficiently inhibited by acetazolamide (KI of 10 nM). It was also revealed that the p.Glu143Lys CA XII mutant was inhibited in the submicromolar range by chloride, bromide, or iodide (KIs of 0.37–0.73 mM), unlike the wild-type enzyme, which is not inhibited by these anions (KIs for these anions in the range of 73–215 mM) [145]. Since the reduction in activity of CA XII is not dramatic, the authors speculated that phenotype limited to the sweat glands, without affecting the normal activity of GI tract of kidneys [145]. In a related study, Lee et al. described variants in CA12 gene encoding carbonic anhydrase XII in two pedigrees exhibiting CF-like phenotypes with elevated sweat chloride levels, FTT, and lung disease [147]. The authors have shown that although the subjects contained normal CFTR variants, aberrant CA XII transcripts bearing either a p.His121Gln or the previously identified p.Glu143Lys protein mutants displayed completely diminished enzymatic activity at physiologic concentrations of sodium chloride that caused CF-like features in sweat gland and lungs [126, 147] (Table 13).
CA XII is also present in nuclear pulposus (NP) cells of the inter-vertebral disks. Aging-dependent degeneration of nucleus pulposus of the intervertebral discs causes spinal cord stiffness and back pain. These degenerative processes were recently correlated with CA XII expression level in NP cells [151]. Thus, Powell et al. conducted a cDNA microarray analysis of 16 human samples from 6 donors. From 552 genes found up-regulated in NP cells, 90 contained transmembrane domains, and 28 were quantified by RT-PCR. The most intense CA XII labeling was observed in the NP of discs from young subjects and from degenerative tissue, suggesting a possible implication of CA XII as a biomarker for chronic back pain [151]. However, the usefulness as CA XII as a biomarker for inter-vertebral disk degeneration and back pain was recently challenged in the study of Richardson et al. that used gene expression analysis (N=60) and immunohistochemistry (N=56) to validate the previously identified NP marker genes in adult human NP cells from a range of ages and degenerate states. The study confirmed the expression of NP markers FoxF1, Pax-1, keratin-8/18, CA XII, and the expression of notochordal (NC) cell markers brachyury, galectin-3 and CD24 in cells of the NP irrespective of age or degeneration. Immunohistochemical data, combined with flow cytometry (N = 5), identified a small number of CA XII+Gal3+T+CD24+ cells, which suggests the possible presence of a sub-population of cells with an NC-like phenotype in adult NP tissue. This finding supported the authors hypothesis that at least a sub-population of adult human NP cells are notochordally derived and retain notochordal marker expression, further complicating the biomarker landscape. Overall, it was concluded that the NP contains a heterogeneous population of cells, which may possess varied phenotypic and functional profiles and that further investigations are needed to improve our understanding of IVD homeostasis and repair (Table 13) [152].
CA XII was also found to be over-expressed in glaucoma. Liao et al. investigated the expression and tissue distribution of CA genes CA9 (encoding CA IX) and CA12 (encoding CA XII) in fetal, neonatal, and adult human eyes with and without glaucoma (Table 13) [153]. Thus, CA IX and CA XII expression was assessed by immunostaining in 16 normal and 10 glaucomatous eyes, and in cultured non-pigmented ciliary epithelial cells (NPE), together with quantification of CA4, CA9, and CA12 mRNA via northern blot hybridization in cultured NPE cell from normal and glaucoma donors. CA XII isozymes was found to be localized primarily to the NPE with an expression prominent during embryonic eye development but decreasing significantly in adults. In contrast, the CA IX expression in the NPE was found to be very low. The epithelium of cornea and lens expressed both enzymes at low levels during development and in adult eye, and no expression was detected in the retina. Importantly, only CA XII isozyme was found to be expressed in higher levels in the NPE from glaucoma eyes as compared with normal eyes. A similar pattern was found in cultured NPE cell lines, with a five-fold increase in the CA12 mRNA level in the NPE cells from a glaucoma patient as compared with the normal subjects. No expression of the CA4 gene encoding CA IV isozyme was detected on these northern blots, pointing to a central role played by CA XII, together with CA IX, in the ciliary cells, in aqueous humor production and in glaucoma, contrary to previous beliefs centered on CA IV activity. Therefore CA XII can act as a predictive biomarker for glaucoma and may prompt the development of new topical CA inhibitors with selectivity for this isozyme, following the success of other (non-selective) CA inhibitors for management of glaucoma [1, 157–159].
2. 11. Carbonic anhydrases XIII as a disease biomarker
Carbonic anhydrase XIII is recognized as the most recently characterized isozyme of CA in humans [160]. It is a monomeric compact globular protein, which demonstrates a moderate catalytic activity with Kcat = 1.5 × 105 s−1 at 25°C and pH = 7.5 rendering it the second least active form among other cytosolic isozymes of CA, after CA III [1, 160, 161]. The CA XIII expression has been reported in the cytosol of cells of different body organs including the colon, small intestine, testis, uterine cervix, some endometrial glands and the thymus [162]. Owing to its abundance in the reproductive system organs, CA XIII is known to play a crucial role in regulating the HCO3- ion concentration and pH homeostasis in the cervical and endometrial mucus, in maintaining the mobility of spermatozoids and ensuring normal fertilization process (Table 14) [161].
Table 14.
Distribution of isozyme CA XIII in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | GI tract | immunostaining | [1, 160, 161] [162] | |
| • Colon, small intestine, | ||||
| Testis, uterine cervix, endometrial glands | ||||
| Thymus | ||||
| Diseased | Colorectal tumors | CA I, CA II, | Immunostaining | [163] |
| Serum | ||||
| • Sjogren’s syndrome | CA I, CA II, CA VI, CA VII |
ELISA determination of antibodies against target proteins | [164] | |
No clinical significance has been reported for the level of expression of CA XIII in humans. However, the downregulation of the level of expression of CA XIII has been reported in patients diagnosed with colorectal cancer [163]. In a study performed by Kummola et al. the expression of CA XIII was analyzed in both neoplastic and normal tissue specimens collected from the same patients. CA XIII-specific antibodies and an immunohistochemical staining method were used in this study. For comparison, the tissue sections were also immunostained for other cytosolic isozymes, CA I and II. It was observed that the expression of CA XIII as well as CA I and II are down-regulated in tumor cells compared to the normal tissue, with lowest signal being detected in carcinoma samples. The authors suggested that the down-regulation of CA XIII and cytosolic CA I, CA II in colorectal cancer may result from the reduced levels of a common transcription factor or loss of closely linked CA I, CA II and CA XIII alleles on chromosome 8 (Table 14) [163].
In another relevant study, Pertovaara et al. examined carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren’s syndrome [164]. It has been known that anti-carbonic anhydrase II (anti-CA II) antibodies have been related to renal manifestations of primary Sjogren’s syndrome, but not all primary Sjogren’s syndrome patients with renal tubular acidosis displayed anti-CA II antibodies. In order to clarify the role of different CA isozymes in this disease and whether their antibodies are associated with renal manifestations of primary Sjogren’s syndrome, the authors analyzed anti-CA XIII antibodies, together with anti-CA I, II, VI, VII antibodies. Using ELISA, tests were performed in 74 primary Sjogren’s syndrome patients fully characterized nephrologically and, as controls, in 56 subjects with sicca symptoms, but without displaying primary Sjogren’s syndrome. The levels of anti-CA I, II, VI and VII antibodies were found to be significantly higher in patients with primary Sjogren’s syndrome as compared with subjects with sicca symptoms but not in the primary Sjogren’s syndrome. The anti-CA antibodies could not be associated with the presence of complete or incomplete renal tubular acidosis or proteinuria or urinary α₁m excretion in patients with primary Sjogren’s syndrome. However, the study revealed that levels of anti-CA II, VI and XIII antibodies correlated significantly with renal acidification capacity and urinary pH, and inversely with serum sodium concentrations in patients with primary Sjogren’s syndrome (Table 14) [164].
2. 12. Carbonic anhydrases XIV as a disease biomarker
Carbonic anhydrase XIV is another membrane-bound isozyme of CA with an extracellular catalytic domain, a single transmembrane helix, and a short intracellular polypeptide segment [165]. CA XIV demonstrates a moderate catalytic activity with Kcat = 3.1 × 105 [1, 101]. Studies reported the role of CA XIV in interacting with bicarbonate transporters and its involvement in acid–base balance in muscles and erythrocytes in response to chronic hypoxia, hyperactivity of the heart, and pH regulation in the retina [101]. It is highly expressed in the kidney, retina and the heart. It also shows abundant expression in the skeletal muscle, liver, brain, and lung [166].
A study by Kaunisto et al [167] demonstrated the key role of CA XIV in urine acidification. The level of CA XIV mRNA and protein expression was determined by western blot technique in extracts of cortex and medulla of mouse kidney. Moreover, the pattern of expression of CA XIV in the nephron of both rat and mouse kidney was determined by immunofluorescence using polyclonal antibodies against mouse CA XIV. Results revealed an abundant expression of CA XIV in apical plasma membranes of the S1 and S2 segments of proximal tubules, and weaker expression in the basolateral membranes. Also, strong staining was seen in the initial portion of the thin descending limb of Henle, overall confirming a significant role of CA XIV in urinary acidification (Table 15) [167].
Table 15.
Distribution of isozyme CA XIV in normal and pathologic tissues
| Status | Biodistribution | Other biomarkers co-assessed | Method of assessment | References |
|---|---|---|---|---|
| Normal | Kidneys Brain (on neurons, not on glial cells) Retina (on glial cells, not on neurons, retinal pigment epithelium, Muller cells and astrocytes) Skeletal muscles Lungs Liver |
Immunostaining | [1, 101, 166, 168, 169][170] | |
| Diseases | Tumors • Gliomas, • Melanomas, • Liver cancer • Uterine cancer |
Immunostaining | [171, 172] |
Nagelhus et al. analyzed the subcellular distribution of CA XIV in retina by high-resolution immunogold cytochemistry and revealed that the distribution pattern of the protein in cells of retina is completely opposite from the distribution pattern in brain cells. Thus, in retina CA XIV was found in glial cells but not in neurons, whereas in brain it was found on neurons but not on glial cells. CA XIV was also found strongly expressed on retinal pigment epithelium (RPE). The authors suggested that the enrichment of CA XIV on specific membrane domains of glial cells and RPE suggests specialization of this isozyme in buffering pH and volume in the surrounding extracellular spaces of retinal neurons, in CO2 removal from neural retina and in the modulation of photoreceptor function [168]. Ogilvie et al. further investigated the physiologic role of CA XIV in retina in conjunction with CA IV, which is expressed in the choriocapillaris overlying the retina, by characterizing the physiological and morphological phenotype of the CA XIV-null, CA IV-null, and CA IV/CA XIV-double-null mouse retinas [169]. Through the use of flash electroretinograms, performed at 2, 7, and 10 months of age, the authors showed that the rod/cone a-wave, b-wave, and cone b-wave were significantly reduced (26–45%) in the CA XIV-null mice compared with wild-type littermates. No differences in retinal morphology were observed between wild-type and CA XIV-null mice and Müller cells and rod bipolar cells had a normal appearance in all animals. It was concluded that CA XIV is the isozyme mainly responsible for producing a normal retinal light response. While retinas of CA IV-null mice showed no functional or morphological differences compared with normal littermates, CA IV/CA XIV double mutant animals showed a greater deficit in light response than the CA XIV-null retina ones, pointing to a minor contribution of CA IV, besides CA XIV to normal retinal physiology and homeostasis [169].
Parkkila et al. analyzed the expression of CA XIV on neurons and axons in mouse and human brain using antibodies against mouse and human [170]. Immunostaining revealed the presence of CA XIV on neuronal membranes and axons in both mouse and human brain. The highest expression was seen on large neuronal bodies and axons in the anterolateral part of pons and medulla oblongata. The hippocampus, corpus callosum, cerebellar white matter and peduncles, pyramidal tract, and choroid plexus tested positive for CA XIV presence, revealing an important role of this isozyme in modulating excitatory synaptic transmission in brain (Table 15) [170].
Besides its expression in normal tissues, CA XIV was shown to be significantly overexpressed in different types of cancer including gliomas, melanomas, liver- and uterine cancer (Table 15) [171, 172].
3. Conclusion
A great number of patents and articles were focused on the use of CA isozymes as biomarkers for various diseases and syndromes in the recent years, in an ascending trend over the last decade. All CA isozymes were used as biomarkers, including the inactive CA-related proteins, thus confirming the usefulness of CA and its isozymes in early diagnosis and prognosis of different pathophysiologies linked with imbalances and diseases.
4. Expert opinion
The physiologic importance of CO2/HCO3- interconversion in various tissues requires the presence of carbonic anhydrase, which helps reach the thermodynamic equilibrium between the two species almost instantaneously. Fourteen CA isozymes evolved in humans, with eleven of them being able to catalyze the same reaction in different microenvironments. Three CA-related proteins, although catalytically inactive, seem to be expressed and retained in their corresponding microenvironments too. Overall, all CA isozymes play important roles in biochemical homeostasis and dynamics of humans and higher vertebrates. Their role may change in time as a result of genetic and epigenetic (re)programming due to normal aging and acquired/developed diseases. The intrinsic expression and dynamic of CA isozymes at the level of different tissues/organs can therefore be used to early detect, confirm and assess the progress/stage of different diseases and even predict patient prognosis.
The field matured in recent years, shifting from the predominant use of CA IX and CA XII in cancer diagnostic, staging and prognosis towards a wider use of CA isozymes as disease biomarkers. As shown above, essentially all CA isozymes were proved to be useful as biomarkers, either alone, in tandem with other CA isozymes and/or in combination with other proteins for the detection, staging and prognosis of a huge repertoire of human dysfunctions and diseases, ranging from mild transformation of the normal tissues (light imbalances and dysfunctions, early disease states) to extreme shifts in tissue organization and function (autoimmune diseases, cancer).
As a consequence, specific antibodies are currently available for the selective detection of all fourteen CA isozymes. They can be either standard, or truncated (e.g. scFv ones), developed classically or through phage display technologies using cell cultures or specific tissues expressing the desired protein target. Antibodies can be developed against different areas of the core catalytic region of CA isozymes or can be designed to recognize structural regions unique only to one isozyme (e.g. proteoglycan region of CA IX). These antibodies constitute primary tools for the detection and quantitation of CA isozymes in different human and animal samples, ranging from blood, urine, saliva, to biopsy specimens and different tissue samples. Standard techniques such as immunostaining, ELISA, western blotting, allow quantitation of CA isozymes in normal and diseased tissues, often in tandem with protein separation techniques such as gel electrophoresis (1D/2D). Interestingly, stopped-flow techniques, normally used for quantification of kinetic parameters of CA isozymes, were also adapted for quantification of these protein targets in different samples. Quantitation of expression of CA isozymes is often performed also at the mRNA level, using RT-PCR and related techniques. State-of-the-art mass spectrometry techniques, including simple and triple quadrupole MS, Orbitrap MS, MALDI, etc are continuously being developed and refined as detection and quantitation tools for CA isozymes in simple or very complex matrices. The excellent sensitivity of the MS techniques permits the reliable quantitation of the level of different CA isozymes in normal and disease tissues within a very broad concentration range, thus allowing improved correlations between the level of CA biomarker and disease stage/progression. Moreover, the MS techniques can simultaneous detect and quantitate multiple protein targets. This advantage is frequently used to follow different proteins across disease progression, either different CA isozymes (e.g. CA II/CA IX/CA XII in various pre-malignant or malignant tissues) or CA isozymes in tandem with other relevant proteins (multiple examples), irrespective of the sense in which the level of proteins is expressed within disease progression (enhanced/reduced expression). Once the set of reliable biomarkers is identified, it can be clustered and used to design a specific chip dedicated to the disease to be screened/tracked. Often the set of biomarkers and their specific dynamic in different locations (tissues/blood/urine) can be tailored to discriminate specific stages within the same disease, or to discriminate between different diseases sharing the same epitopes. It can be also used for the evaluation, immediate risk stratification and implementation of appropriate therapy in the management of patients suffering from a particular disease.
All the above-mentioned biological, biochemical and biophysical tools and techniques that allow the efficient tracking and quantitation of various CA isozymes within the human body and their use as disease biomarkers also contribute to a better understanding of the biochemical and physiological role of CA isozymes in normal physiology and pathology, ensuring a constant progress of the CA research field.
Article highlights.
Isoforms of CA evolved to perform the efficient equilibration of CO2/HCO3- in different environments. The normal biodistribution of all 14 CA isozymes is presented in detail, together with kinetic parameters, biochemical and physiologic details relevant for their activity.
All CA isozymes are currently used as biomarkers, either alone, in combination with other CA isozymes, or as part of more complex biomarker sets.
For each CA isozyme we are revealing significant biodistribution differences that appear between normal and diseased states, which constitute the base for their use as biomarkers. The use of CA biomarkers expanded dramatically in recent years from cancer diagnostic, staging and prognosis to a wide repertoire of human dysfunctions and diseases.
Relevant statistical data acquired in human subjects or in animal models of human diseases were compiled and presented for each CA isozyme from patent and literature data whenever possible, revealing the ability of each protein to discern between related pathologies or stages of a given disease. It allows one to evaluate the intrinsic quality of each CA biomarker and the limitations of its use. The correlation between the CA biomarker level and patient prognosis was also presented for a plethora of diseases in which CAs are involved, with statistical details.
All methods currently available to detect/quantify CA isozymes were compiled from the newest patents and from relevant publications available for each isozyme. Besides traditional antibody-based detection/quantitation methods and RT-PCR, one can observe the recent widespread use of mass spectrometry-based techniques and chip-based screening technologies, including their state-of-the-art versions, presented for each CA biomarker/disease case.
Funding
This paper was funded by the National Institutes of Health (R03EB026189) and the Temple University School of Pharmacy Drug Discovery Program. A Shabana and UK Mondal would like to thank TU Graduate School for a Dissertation Completion Grant.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discovery. 2008;7:168–181.** Comprehensive review summarizing the therapeutic applications of carbonic anhydrases
- 2.Supuran CT. Structure and function of carbonic anhydrases. Biochem J. 2016. July 15;473(14):2023–32. [DOI] [PubMed] [Google Scholar]
- 3.Supuran CT, Capasso C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites. 2017. November 11;7(4).* Comprehensive review summarizing the bacterial carbonic anhydrases
- 4.Zolfaghari Emameh R, Barker HR, Syrjanen L, et al. Identification and inhibition of carbonic anhydrases from nematodes. J Enzyme Inhib Med Chem. 2016;31(sup4):176–184. [DOI] [PubMed] [Google Scholar]
- 5.Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets. 2015;19(12):1689–704.* Important review summarizing structure and applications of bacterial, fungal and protozoan carbonic anhydrases
- 6.Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev. 2012. August 8;112(8):4421–68.**Comprehensive review on the structure and function of carbonic anhydrases, drug design
- 7.Ilies MA, Banciu MD. Non-sulfonamide carbonic anhydrase inhibitors In: Supuran CT, Scozzafava A, Conway J, editors. Carbonic Anhydrase: Its Inhibitors and Activators. Boca Raton: CRC Press; 2004. p. 207–239. [Google Scholar]
- 8.Krishnamurthy VM, Kaufman GK, Urbach AR, et al. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. Chem Rev. 2008. March;108(3):946–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akocak S, Ilies MA. Next-generation primary sulfonamide carbonic anhydrase inhibitors In: Supuran CT, Capasso C, editors. Targeting carbonic anhydrases. London: Future Science; 2014. p. 35–51. [Google Scholar]
- 10.Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017. January;12(1):61–88. [DOI] [PubMed] [Google Scholar]
- 11.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discovery. 2011. September 16;10(10):767–77.*Comprehensive review dealing with involvement of carbonic anhydrases in tumor pathophisiology
- 12.Supuran CT, Scozzafava A, Jurca BC, et al. Carbonic Anhydrase Inhibitors. Part 49. Synthesis of Substituted- Ureido and Thioureido Derivatives of Aromatic/ Heterocyclic Sulfonamides with Increased Affinities for Isozyme I. Eur J Med Chem. 1998;33:83–93. [Google Scholar]
- 13.Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011. March 24;54(6):1896–902. [DOI] [PubMed] [Google Scholar]
- 14.Krall N, Pretto F, Decurtins W, et al. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl. 2014. April 14;53(16):4231–5. [DOI] [PubMed] [Google Scholar]
- 15.Shabana AM, Mondal UK, Alam MR, et al. pH-Sensitive Multiligand Gold Nanoplatform Targeting Carbonic Anhydrase IX Enhances the Delivery of Doxorubicin to Hypoxic Tumor Spheroids and Overcomes the Hypoxia-Induced Chemoresistance. ACS Appl Mater Interfaces. 2018. May 30;10(21):17792–17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supuran CT. Carbonic anhydrase activators. Future Med Chem. 2018. March 1;10(5):561–573.* Arecent review on carbonic anhydrase activators
- 17.Sun MK, Alkon DL. Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci. 2002. February;23(2):83–9. [DOI] [PubMed] [Google Scholar]
- 18.Dave K, Scozzafava A, Vullo D, et al. Pyridinium derivatives of histamine are potent activators of cytosolic carbonic anhydrase isoforms I, II and VII. Org Biomol Chem. 2011. April 21;9(8):2790–800. [DOI] [PubMed] [Google Scholar]
- 19.Draghici B, Vullo D, Akocak S, et al. Ethylene bis-imidazoles are highly potent and selective activators for isozymes VA and VII of carbonic anhydrase, with a potential nootropic effect. Chem Commun (Camb). 2014. June 7;50(45):5980–3. [DOI] [PubMed] [Google Scholar]
- 20.Canto de Souza L, Provensi G, Vullo D, et al. Carbonic anhydrase activation enhances object recognition memory in mice through phosphorylation of the extracellular signal-regulated kinase in the cortex and the hippocampus. Neuropharmacology. 2017. May 15;118:148–156. [DOI] [PubMed] [Google Scholar]
- 21.Sanku RKK, John JS, Salkovitz M, et al. Potential learning and memory disruptors and enhancers in a simple, 1-day operant task in mice. Behav Pharmacol. 2018. March 21. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt A, Mondal UK, Supuran CT, et al. Crystal Structure of Carbonic Anhydrase II in Complex with an Activating Ligand: Implications in Neuronal Function. Mol Neurobiol. 2018. September;55(9):7431–7437. [DOI] [PubMed] [Google Scholar]
- 23.Ilies M, Banciu MD, Ilies MA, et al. Carbonic anhydrase activators: design of high affinity isozymes I, II, and IV activators, incorporating tri-/tetrasubstituted-pyridinium-azole moieties. J Med Chem. 2002. January 17;45(2):504–10. [DOI] [PubMed] [Google Scholar]
- 24.Dave K, Ilies MA, Scozzafava A, et al. An inhibitor-like binding mode of a carbonic anhydrase activator within the active site of isoform II. Bioorg Med Chem Lett. 2011. May 1;21(9):2764–8. [DOI] [PubMed] [Google Scholar]
- 25.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Shin HS, Kang UB, et al. , Multiple biomarker set for breast cancer diagnosis, method of detecting the same, and diagnosis kit for breast cancer using antibody against the same. US20150024960 2015.
- 27.Wang DB, Lu XK, Zhang X, et al. Carbonic anhydrase 1 is a promising biomarker for early detection of non-small cell lung cancer. Tumour Biol. 2016. January;37(1):553–9. [DOI] [PubMed] [Google Scholar]
- 28.Takakura M, Yokomizo A, Tanaka Y, et al. Carbonic anhydrase I as a new plasma biomarker for prostate cancer. ISRN Oncol. 2012;2012:768190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taka-Aki N, Tomoaki H, Diagnostic Agent For Sepsis WO2018088455. 2018.
- 30.Kardoush MI, Ward BJ, Ndao M. Serum Carbonic Anhydrase 1 is a Biomarker for Diagnosis of Human Schistosoma mansoni Infection. Am J Trop Med Hyg. 2017. April;96(4):842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granier C, Molina F, Salvetat N, et al. Early prediction markers of diabetic nephropathy. EP2963422 2016.
- 32.Geyer RR, Zhao P, Parker MD, et al. Assay and method for quantitating carbonic anhydrase activity and assessing red blood hemolysis. US20160355867 2016. [DOI] [PMC free article] [PubMed]
- 33.Clofent-Sanchez G, Deramchia K, Jacobin AJ, et al. Antibodies for molecular imaging of vulnerable plaques in atherosclerosis. WO2011IB03011 2013.
- 34.Parkkila S, Lasota J, Fletcher JA, et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod Pathol. 2010. May;23(5):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter N, Jeffery S, Shiels A, et al. Characterization of human carbonic anhydrase III from skeletal muscle. Biochem Genet. 1979. October;17(9–10):837–54. [DOI] [PubMed] [Google Scholar]
- 36.Kharbanda KK, Vigneswara V, McVicker BL, et al. Proteomics reveal a concerted upregulation of methionine metabolic pathway enzymes, and downregulation of carbonic anhydrase-III, in betaine supplemented ethanol-fed rats. Biochem Biophys Res Commun. 2009. April 17;381(4):523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter WG, Vigneswara V, Newlaczyl A, et al. Isoaspartate, carbamoyl phosphate synthase-1, and carbonic anhydrase-III as biomarkers of liver injury. Biochem Biophys Res Commun. 2015. March 13;458(3):626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaananen HK, Syrjala H, Rahkila P, et al. Serum carbonic anhydrase III and myoglobin concentrations in acute myocardial infarction. Clin Chem. 1990. April;36(4):635–8. [PubMed] [Google Scholar]
- 39.Vuori J, Syrjala H, Vaananen HK. Myoglobin/carbonic anhydrase III ratio: highly specific and sensitive early indicator for myocardial damage in acute myocardial infarction. Clin Chem. 1996. January;42(1):107–9. [PubMed] [Google Scholar]
- 40.Vuotikka P, Uusimaa P, Niemela M, et al. Serum myoglobin/carbonic anhydrase III ratio as a marker of reperfusion after myocardial infarction. Int J Cardiol. 2003. October;91(2–3):137–44. [DOI] [PubMed] [Google Scholar]
- 41.Sawicki M, Sypniewska G, Krintus M, et al. Multi-Marker Approach with the Use of Biochip Cardiac Array Technology for Early Diagnosis in Patients with Acute Coronary Syndromes. EJIFCC. 2008. December;19(3):160–71. [PMC free article] [PubMed] [Google Scholar]
- 42.Saito R, Watanabe H, Asano T, et al. Anti-carbonic anhydrase III autoantibodies in vasculitis syndrome. Int J Rheum Dis. 2013. June;16(3):339–46. [DOI] [PubMed] [Google Scholar]
- 43.Robert-Pachot M, Desbos A, Moreira A, et al. Carbonic anhydrase III: a new target for autoantibodies in autoimmune diseases. Autoimmunity. 2007. July;40(5):380–9. [DOI] [PubMed] [Google Scholar]
- 44.Barak AJ, Beckenhauer HC, Tuma DJ, et al. Effects of prolonged ethanol feeding on methionine metabolism in rat liver. Biochem Cell Biol. 1987. March;65(3):230–3. [DOI] [PubMed] [Google Scholar]
- 45.Kemp M, Donovan J, Higham H, et al. Biochemical markers of myocardial injury. Br J Anaesth. 2004. July;93(1):63–73. [DOI] [PubMed] [Google Scholar]
- 46.Stams T, Nair SK, Okuyama T, et al. Crystal structure of the secretory form of membrane-associated human carbonic anhydrase IV at 2.8-A resolution. Proc Natl Acad Sci U S A. 1996. November 26;93(24):13589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990. May 25;265(15):8795–801. [PubMed] [Google Scholar]
- 48.Wistrand PJ, Knuuttila KG. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int. 1989. March;35(3):851–9. [DOI] [PubMed] [Google Scholar]
- 49.Nam SU, Noh JH, Kim BJ, et al. A Marker for Early Diagnosis of Acute Myocardial Infarction. KR2013141044 2013.
- 50.Nishimori I, Miyaji E, Morimoto K, et al. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut. 2005. February;54(2):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chawla LS, Mccaffrey TA, Mcpherson P, et al. Blood biomarkers for appendicitis and diagnostics methods using biomarkers. WO2016065202A1 2016.
- 52.Supuran CT, Ilies MA, Scozzafava A. Carbonic anhydrase inhibitors - Part 29: Interaction of isozymes I, II and IV with benzolamide-like derivatives. European Journal of Medicinal Chemistry. 1998;33:739–751. [Google Scholar]
- 53.Supuran CT, Scozzafava A, Ilies MA, et al. Carbonic anhydrase inhibitors - Part 53 - Synthesis of substituted-pyridinium derivatives of aromatic sulfonamides: The first non-polymeric membrane-impermeable inhibitors with selectivity for isozyme IV. European Journal of Medicinal Chemistry. 1998. Jul-Aug;33(7–8):577–594. [Google Scholar]
- 54.Supuran CT, Scozzafava A, Ilies MA, et al. Carbonic anhydrase inhibitors: synthesis of sulfonamides incorporating 2,4,6-trisubstituted-pyridinium-ethylcarboxamido moieties possessing membrane-impermeability and in vivo selectivity for the membrane-bound (CA IV) versus the cytosolic (CA I and CA II) isozymes. J Enzyme Inhib. 2000;15(4):381–401. [DOI] [PubMed] [Google Scholar]
- 55.Scozzafava A, Briganti F, Ilies MA, et al. Carbonic anhydrase inhibitors: synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus cytosolic isozymes. J Med Chem. 2000. January 27;43(2):292–300. [DOI] [PubMed] [Google Scholar]
- 56.Akocak S, Alam MR, Shabana AM, et al. PEGylated Bis-Sulfonamide Carbonic Anhydrase Inhibitors Can Efficiently Control the Growth of Several Carbonic Anhydrase IX-Expressing Carcinomas. Journal of medicinal chemistry. 2016. May 26;59(10):5077–88. [DOI] [PubMed] [Google Scholar]
- 57.Maren TH, Conroy CW, Wynns GC, et al. Renal and cerebrospinal fluid formation pharmacology of a high molecular weight carbonic anhydrase inhibitor. J Pharmacol Exp Ther. 1997. January;280(1):98–104. [PubMed] [Google Scholar]
- 58.Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opinion on Therapeutic Patents. 2000;10(5):575–600. [Google Scholar]
- 59.Nagao Y, Platero JS, Waheed A, et al. Human mitochondrial carbonic anhydrase: cDNA cloning, expression, subcellular localization, and mapping to chromosome 16. Proc Natl Acad Sci U S A. 1993. August 15;90(16):7623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagao Y, Batanian JR, Clemente MF, et al. Genomic organization of the human gene (CA5) and pseudogene for mitochondrial carbonic anhydrase V and their localization to chromosomes 16q and 16p. Genomics. 1995. August 10;28(3):477–84. [DOI] [PubMed] [Google Scholar]
- 61.Dodgson SJ, Forster RE 2nd, Storey BT, et al. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980. September;77(9):5562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujikawa-Adachi K, Nishimori I, Taguchi T, et al. Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J Biol Chem. 1999. July 23;274(30):21228–33. [DOI] [PubMed] [Google Scholar]
- 63.Shah GN, Hewett-Emmett D, Grubb JH, et al. Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci U S A. 2000. February 15;97(4):1677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah GN, Rubbelke TS, Hendin J, et al. Targeted mutagenesis of mitochondrial carbonic anhydrases VA and VB implicates both enzymes in ammonia detoxification and glucose metabolism. Proc Natl Acad Sci U S A. 2013. April 30;110(18):7423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghandour MS, Parkkila AK, Parkkila S, et al. Mitochondrial carbonic anhydrase in the nervous system: expression in neuronal and glial cells. J Neurochem. 2000. November;75(5):2212–20. [DOI] [PubMed] [Google Scholar]
- 66.van Karnebeek CD, Sly WS, Ross CJ, et al. Mitochondrial carbonic anhydrase VA deficiency resulting from CA5A alterations presents with hyperammonemia in early childhood. Am J Hum Genet. 2014. March 6;94(3):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat. 2013;23(6):725–35. [DOI] [PubMed] [Google Scholar]
- 68.Baine MJ, Chakraborty S, Smith LM, et al. Transcriptional profiling of peripheral blood mononuclear cells in pancreatic cancer patients identifies novel genes with potential diagnostic utility. PLoS One. 2011. February 10;6(2):e17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baine MJ, Menning M, Smith LM, et al. Differential gene expression analysis of peripheral blood mononuclear cells reveals novel test for early detection of pancreatic cancer. Cancer Biomark. 2011;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parkkila S, Parkkila AK, Vierjoki T, et al. Competitive time-resolved immunofluorometric assay for quantifying carbonic anhydrase VI in saliva. Clin Chem. 1993. October;39(10):2154–7. [PubMed] [Google Scholar]
- 71.Borghi GN, Rodrigues LP, Lopes LM, et al. Relationship among alpha amylase and carbonic anhydrase VI in saliva, visible biofilm, and early childhood caries: a longitudinal study. Int J Paediatr Dent. 2017. May;27(3):174–182. [DOI] [PubMed] [Google Scholar]
- 72.Zaidel L, Miller S, Carpentier G, et al. Diagnostic methods. WO2013095367 2013.
- 73.Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjogren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ambrus JL, Shen JL Method of diagnosing Sjogren’s disease. EP20110735215 2011.
- 75.Ruusuvuori E, Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell Biochem. 2014;75:271–90. [DOI] [PubMed] [Google Scholar]
- 76.Ruusuvuori E, Huebner AK, Kirilkin I, et al. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013. August 14;32(16):2275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruusuvuori E, Li H, Huttu K, et al. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci. 2004. March 17;24(11):2699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu C-M, Chang Y Genetic marker for detecting colorectal cancer and method using the same. TW20130113645 2013.
- 79.Aspatwar A, Tolvanen ME, Ortutay C, et al. Carbonic anhydrase related protein VIII and its role in neurodegeneration and cancer. Curr Pharm Des. 2010;16(29):3264–76. [DOI] [PubMed] [Google Scholar]
- 80.Aspatwar A, Tolvanen ME, Parkkila S. An update on carbonic anhydrase-related proteins VIII, X and XI. J Enzyme Inhib Med Chem. 2013. December;28(6):1129–42. [DOI] [PubMed] [Google Scholar]
- 81.Taniuchi K, Nishimori I, Takeuchi T, et al. Developmental expression of carbonic anhydrase-related proteins VIII, X, and XI in the human brain. Neuroscience. 2002;112(1):93–9. [DOI] [PubMed] [Google Scholar]
- 82.Picaud SS, Muniz JR, Kramm A, et al. Crystal structure of human carbonic anhydrase-related protein VIII reveals the basis for catalytic silencing. Proteins. 2009. August 1;76(2):507–11. [DOI] [PubMed] [Google Scholar]
- 83.Hirota J, Ando H, Hamada K, et al. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J. 2003. June 1;372(Pt 2):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akisawa Y, Nishimori I, Taniuchi K, et al. Expression of carbonic anhydrase-related protein CA-RP VIII in non-small cell lung cancer. Virchows Arch. 2003. January;442(1):66–70. [DOI] [PubMed] [Google Scholar]
- 85.Turkmen S, Guo G, Garshasbi M, et al. CA8 mutations cause a novel syndrome characterized by ataxia and mild mental retardation with predisposition to quadrupedal gait. PLoS Genet. 2009. May;5(5):e1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaya N, Aldhalaan H, Al-Younes B, et al. Phenotypical spectrum of cerebellar ataxia associated with a novel mutation in the CA8 gene, encoding carbonic anhydrase (CA) VIII. Am J Med Genet B Neuropsychiatr Genet. 2011. December;156B(7):826–34. [DOI] [PubMed] [Google Scholar]
- 87.Bataller L, Sabater L, Saiz A, et al. Carbonic anhydrase-related protein VIII: autoantigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2004. October;56(4):575–9. [DOI] [PubMed] [Google Scholar]
- 88.Nishikata M, Nishimori I, Taniuchi K, et al. Carbonic anhydrase-related protein VIII promotes colon cancer cell growth. Mol Carcinog. 2007. March;46(3):208–14. [DOI] [PubMed] [Google Scholar]
- 89.Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol. 1996. February;5(1):50–77. [DOI] [PubMed] [Google Scholar]
- 90.Mikoshiba K, Okano H, Miyawaki A, et al. Molecular genetic analyses of myelin deficiency and cerebellar ataxia. Prog Brain Res. 1995;105:23–41. [DOI] [PubMed] [Google Scholar]
- 91.Kilpinen S, Autio R, Ojala K, et al. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 2008;9(9):R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morimoto K, Nishimori I, Takeuchi T, et al. Overexpression of carbonic anhydrase-related protein XI promotes proliferation and invasion of gastrointestinal stromal tumors. Virchows Arch. 2005. July;447(1):66–73. [DOI] [PubMed] [Google Scholar]
- 93.Karjalainen SL, Haapasalo HK, Aspatwar A, et al. Carbonic anhydrase related protein expression in astrocytomas and oligodendroglial tumors. BMC Cancer. 2018. May 23;18(1):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015. April;31:52–64. [DOI] [PubMed] [Google Scholar]
- 95.Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci U S A. 2009. September 22;106(38):16233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hilvo M, Baranauskiene L, Salzano AM, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. Journal of Biological Chemistry. 2008. October 10;283(41):27799–809. [DOI] [PubMed] [Google Scholar]
- 97.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001. March;158(3):905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zavada J, Zavadova Z, Pastorek J, et al. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br J Cancer. 2000. June;82(11):1808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillies RJ, Gatenby RA. Metabolism and Its Sequelae in Cancer Evolution and Therapy. Cancer J. 2015. Mar-Apr;21(2):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sneddon D, Niemans R, Bauwens M, et al. Synthesis and in Vivo Biological Evaluation of (68)Ga-Labeled Carbonic Anhydrase IX Targeting Small Molecules for Positron Emission Tomography. J Med Chem. 2016. July 14;59(13):6431–43. [DOI] [PubMed] [Google Scholar]
- 101.Mboge MY, Mahon BP, McKenna R, et al. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites. 2018. February 28;8(1), pii: E19.*A recent review on the involvement of carbonic anhydrases in pH homeostasis in normal cells and in tumors
- 102.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013. December 17;4:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004. November 19;577(3):439–45. [DOI] [PubMed] [Google Scholar]
- 104.Ilies MA, Vullo D, Pastorek J, et al. Carbonic anhydrase inhibitors. Inhibition of tumor-associated isozyme IX by halogenosulfanilamide and halogenophenylaminobenzolamide derivatives. Journal of medicinal chemistry. 2003. May 22;46(11):2187–96. [DOI] [PubMed] [Google Scholar]
- 105.Kanfar N, Tanc M, Dumy P, et al. Effective Access to Multivalent Inhibitors of Carbonic Anhydrases Promoted by Peptide Bioconjugation. Chemistry. 2017. May 17;23(28):6788–6794. [DOI] [PubMed] [Google Scholar]
- 106.Lomelino C, McKenna R. Carbonic anhydrase inhibitors: a review on the progress of patent literature (2011–2016). Expert Opin Ther Pat. 2016. August;26(8):947–56. [DOI] [PubMed] [Google Scholar]
- 107.Supuran CT, Winum JY. Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med Chem. 2015;7(11):1407–14.* A comprehensive review on the use of CA inhibitors in treatment of different tumors
- 108.Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008–2018). Expert Opin Ther Pat. 2018. August 9:1–12.* A recent patent review on the use of CA inhibitors in cancer treatment
- 109.Benej M, Pastorekova S, Pastorek J. Carbonic anhydrase IX: regulation and role in cancer. Subcell Biochem. 2014;75:199–219. [DOI] [PubMed] [Google Scholar]
- 110.Yang X, Minn I, Rowe SP, et al. Imaging of carbonic anhydrase IX with an 111In-labeled dual-motif inhibitor. Oncotarget. 2015. October 20;6(32):33733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Groves K, Bao B, Zhang J, et al. Synthesis and evaluation of near-infrared fluorescent sulfonamide derivatives for imaging of hypoxia-induced carbonic anhydrase IX expression in tumors. Bioorg Med Chem Lett. 2012. January 1;22(1):653–7. [DOI] [PubMed] [Google Scholar]
- 112.Krall N, Pretto F, Mattarella M, et al. A 99mTc-Labeled Ligand of Carbonic Anhydrase IX Selectively Targets Renal Cell Carcinoma In Vivo. J Nucl Med. 2016. June;57(6):943–9. [DOI] [PubMed] [Google Scholar]
- 113.Minn I, Koo SM, Lee HS, et al. [64Cu]XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget. 2016. August 30;7(35):56471–56479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Genega EM, Ghebremichael M, Najarian R, et al. Carbonic anhydrase IX expression in renal neoplasms: correlation with tumor type and grade. Am J Clin Pathol. 2010. December;134(6):873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luong-Player A, Liu H, Wang HL, et al. Immunohistochemical reevaluation of carbonic anhydrase IX (CA IX) expression in tumors and normal tissues. Am J Clin Pathol. 2014. February;141(2):219–25. [DOI] [PubMed] [Google Scholar]
- 116.Korkeila E, Talvinen K, Jaakkola PM, et al. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer. 2009. March 24;100(6):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ilie M, Mazure NM, Hofman V, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer. 2010. May 25;102(11):1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim JY, Lee SH, An S, et al. Carbonic anhydrase 9 expression in well-differentiated pancreatic neuroendocrine neoplasms might be associated with aggressive behavior and poor survival. Virchows Arch. 2018. May;472(5):739–748. [DOI] [PubMed] [Google Scholar]
- 119.Chu X, Zhao P, Lv Y, et al. Expression of carbonic anhydrase-9 correlates with metastasis and prognosis of Chinese patients with invasive breast ductal carcinoma. Int J Clin Exp Pathol. 2016;9(2):1446–1452. [Google Scholar]
- 120.Hulick P, Zimmer M, Margulis V, et al. Blood Levels of Carbonic Anhydrase 9 Correlate with Clear Cell Renal Cell Carcinoma Activity. Clinical Proteomics. 2009. March 01;5(1):37–45. [Google Scholar]
- 121.Hyrsl L, Zavada J, Zavadova Z, et al. Soluble form of carbonic anhydrase IX (CAIX) in transitional cell carcinoma of urinary tract. Neoplasma. 2009;56(4):298–302. [DOI] [PubMed] [Google Scholar]
- 122.Pastorekova S, Zavadova Z, Kostal M, et al. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992. April;187(2):620–6. [DOI] [PubMed] [Google Scholar]
- 123.Nakao M, Ishii G, Nagai K, et al. Prognostic significance of carbonic anhydrase IX expression by cancer-associated fibroblasts in lung adenocarcinoma. Cancer. 2009. June 15;115(12):2732–43. [DOI] [PubMed] [Google Scholar]
- 124.Tureci O, Sahin U, Vollmar E, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A. 1998. June 23;95(13):7608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Whittington DA, Waheed A, Ulmasov B, et al. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci U S A. 2001. August 14;98(17):9545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waheed A, Sly WS. Carbonic anhydrase XII functions in health and disease. Gene. 2017. August 5;623:33–40.* A comprehensive review on CA XII involvement in physiologic and pathologic processes
- 127.Ivanov SV, Kuzmin I, Wei M-H, et al. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proceedings of the National Academy of Sciences. 1998;95(21):12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ulmasov B, Waheed A, Shah GN, et al. Purification and kinetic analysis of recombinant CA XII, a membrane carbonic anhydrase overexpressed in certain cancers. Proc Natl Acad Sci U S A. 2000. December 19;97(26):14212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parkkila S, Parkkila AK, Saarnio J, et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000. December;48(12):1601–8. [DOI] [PubMed] [Google Scholar]
- 130.Parkkila S, Rajaniemi H, Parkkila A-K, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proceedings of the National Academy of Sciences. 2000;97(5):2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wykoff CC, Beasley NJP, Watson PH, et al. Hypoxia-inducible Expression of Tumor-associated Carbonic Anhydrases. Cancer Research. 2000;60(24):7075–7083. [PubMed] [Google Scholar]
- 132.Ondriskova E, Debreova M, Pastorekova S. Tumor-Associated Carbonic Anhydrases IX and XII 2015. In: Carbonic Anhydrases as Biocatalysts - From Theory to Medical and Industrial Applications. Amsterdam: Elsevier; pp. 169–205.* A comprehensive recent review on the tumor-associated CA IX and CA XII
- 133.Moon YR, Ji GY Antibody binding to carbonic anhydrase and use thereof. US201615344764 2016. [Google Scholar]
- 134.Watson PH, Chia SK, Wykoff CC, et al. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. British Journal Of Cancer. 2003;88:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ilie MI, Hofman V, Ortholan C, et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. 2011. April 1;128(7):1614–23. [DOI] [PubMed] [Google Scholar]
- 136.Yoo CW, Nam BH, Kim JY, et al. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat Oncol. 2010. November 1;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee S, Shin HJ, Han IO, et al. Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer Sci. 2007. March;98(3):329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hynninen P, Vaskivuo L, Saarnio J, et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology. 2006. December;49(6):594–602. [DOI] [PubMed] [Google Scholar]
- 139.Kivela A, Parkkila S, Saarnio J, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000. February;156(2):577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saarnio J, Parkkila S, Parkkila AK, et al. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998. July;153(1):279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Proescholdt MA, Mayer C, Kubitza M, et al. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005. October;7(4):465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haapasalo J, Hilvo M, Nordfors K, et al. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol. 2008. April;10(2):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ochi F, Shiozaki A, Ichikawa D, et al. Carbonic Anhydrase XII as an Independent Prognostic Factor in Advanced Esophageal Squamous Cell Carcinoma. J Cancer. 2015;6(10):922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Quinton PM. Role of epithelial HCO3(−) transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010. December;299(6):C1222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feldshtein M, Elkrinawi S, Yerushalmi B, et al. Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII. Am J Hum Genet. 2010. November 12;87(5):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muhammad E, Leventhal N, Parvari G, et al. Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12. Hum Genet. 2011. April;129(4):397–405. [DOI] [PubMed] [Google Scholar]
- 147.Lee M, Vecchio-Pagan B, Sharma N, et al. Loss of carbonic anhydrase XII function in individuals with elevated sweat chloride concentration and pulmonary airway disease. Hum Mol Genet. 2016. May 15;25(10):1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee MG, Ohana E, Park HW, et al. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012. January;92(1):39–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maleth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014. June;55(6):337–45. [DOI] [PubMed] [Google Scholar]
- 150.Almstahl A, Wikstrom M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol. 2003. May;48(5):337–44. [DOI] [PubMed] [Google Scholar]
- 151.Power KA, Grad S, Rutges JP, et al. Identification of cell surface-specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011. December;63(12):3876–86. [DOI] [PubMed] [Google Scholar]
- 152.Richardson SM, Ludwinski FE, Gnanalingham KK, et al. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep. 2017. May 4;7(1):1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liao SY, Ivanov S, Ivanova A, et al. Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma. J Med Genet. 2003. April;40(4):257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gruvberger S, Ringnér M, Chen Y, et al. Estrogen Receptor Status in Breast Cancer Is Associated with Remarkably Distinct Gene Expression Patterns. Cancer Research. 2001;61(16):5979–5984. [PubMed] [Google Scholar]
- 155.Hong JH, Muhammad E, Zheng C, et al. Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3(−) secretion revealed by disease causing human mutation. J Physiol. 2015. December 15;593(24):5299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007. January;71(2):103–15. [DOI] [PubMed] [Google Scholar]
- 157.Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem. 2014;75:349–59. [DOI] [PubMed] [Google Scholar]
- 158.Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agents. Curr Top Med Chem. 2007;7(9):849–54. [DOI] [PubMed] [Google Scholar]
- 159.Baldwin JJ, Ponticello GS, Anderson PS, et al. Thienothiopyran-2-sulfonamides: novel topically active carbonic anhydrase inhibitors for the treatment of glaucoma. Journal of medicinal chemistry. 1989. 1989/12/01;32(12):2510–2513. [DOI] [PubMed] [Google Scholar]
- 160.Lehtonen J, Shen B, Vihinen M, et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem. 2004. January 23;279(4):2719–27. [DOI] [PubMed] [Google Scholar]
- 161.Di Fiore A, Monti SM, Hilvo M, et al. Crystal structure of human carbonic anhydrase XIII and its complex with the inhibitor acetazolamide. Proteins. 2009. January;74(1):164–75. [DOI] [PubMed] [Google Scholar]
- 162.Hilvo M, Innocenti A, Monti SM, et al. Recent advances in research on the most novel carbonic anhydrases, CA XIII and XV. Curr Pharm Des. 2008;14(7):672–8. [DOI] [PubMed] [Google Scholar]
- 163.Kummola L, Hamalainen JM, Kivela J, et al. Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer. 2005. April 18;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pertovaara M, Bootorabi F, Kuuslahti M, et al. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren’s syndrome. Rheumatology (Oxford). 2011. August;50(8):1453–7. [DOI] [PubMed] [Google Scholar]
- 165.Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem. 2007. July 1;15(13):4336–50. [DOI] [PubMed] [Google Scholar]
- 166.Mori K, Ogawa Y, Ebihara K, et al. Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem. 1999. May 28;274(22):15701–5. [DOI] [PubMed] [Google Scholar]
- 167.Kaunisto K, Parkkila S, Rajaniemi H, et al. Carbonic anhydrase XIV: luminal expression suggests key role in renal acidification. Kidney Int. 2002. June;61(6):2111–8. [DOI] [PubMed] [Google Scholar]
- 168.Nagelhus EA, Mathiisen TM, Bateman AC, et al. Carbonic anhydrase XIV is enriched in specific membrane domains of retinal pigment epithelium, Muller cells, and astrocytes. Proc Natl Acad Sci U S A. 2005. May 31;102(22):8030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ogilvie JM, Ohlemiller KK, Shah GN, et al. Carbonic anhydrase XIV deficiency produces a functional defect in the retinal light response. Proc Natl Acad Sci U S A. 2007. May 15;104(20):8514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Parkkila S, Parkkila AK, Rajaniemi H, et al. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci U S A. 2001. February 13;98(4):1918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013. April 2;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012. May;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]