Abstract
The voltage-gated sodium channel subunit β4 (SCN4B) regulates neuronal activity by modulating channel gating and has been implicated in ethanol consumption in rodent models and human alcoholics. However, the functional role for Scn4b in ethanol-mediated behaviors is unknown. We determined if genetic global knockout or targeted knockdown of Scn4b in the central nucleus of the amygdala (CeA) altered ethanol drinking or related behaviors. We used four different ethanol consumption procedures (continuous and intermittent two-bottle choice, drinking-in-the dark, and chronic intermittent ethanol vapor) and found that male and female Scn4b knockout mice did not differ from their wild-type littermates in ethanol consumption in any of the tests. Knockdown of Scn4b mRNA in the CeA also did not alter two-bottle choice ethanol drinking. However, Scn4b knockout mice demonstrated longer duration of the loss of righting reflex induced by ethanol, gaboxadol, pentobarbital, and ketamine. Knockout mice showed slower recovery to basal levels of handling-induced convulsions after ethanol injection, which is consistent with the increased sedative effects observed in these mice. However, Scn4b knockout mice did not differ in the severity of acute ethanol withdrawal. Acoustic startle responses, ethanol-induced hypothermia, and clearance of blood ethanol also did not differ between the genotypes. There were also no functional differences in the membrane properties or excitability of CeA neurons from Scn4b knockout and wild-type mice. Although we found no evidence that Scn4b regulates ethanol consumption in mice, it was involved in the acute hypnotic effects of ethanol and other sedatives.
Keywords: sodium channel subunit Scn4b, alcohol, knockout mice, central amygdala neurons, two-bottle choice ethanol drinking, drinking-in-the dark, chronic intermittent ethanol vapor, loss of righting reflex, sedatives, acute withdrawal
INTRODUCTION
Voltage-gated sodium channels are essential for controlling the rapid rise of membrane potential following depolarization during an action potential. Sodium channels are formed by the association of the pore-forming α subunit with one or more β subunits (Catterall, 2000). A single α subunit forms the functional core of the channel (Barchi, 1988), while each of the β subunits modulates channel gating and kinetics in distinct ways and may even function independently of α subunits (Brackenbury and Isom, 2011). The β4 subunit (Yu et al., 2003), encoded by the Scn4b gene, slows inactivation by allowing channels to evoke a resurgent current upon repolarization, thus promoting excitability (Aman et al., 2009; Bant and Raman, 2010; Grieco et al., 2005). It also regulates cell adhesion, migration, and extension of neurites (Miyazaki et al., 2007; Oyama et al., 2006). Recent work using Scn4b knockout (KO) mice revealed a shift in action potential threshold and dramatic impairment in the induction of spike-timing-dependent long-term depression in medium spiny neurons of the nucleus accumbens (Ji et al., 2017).
Gene expression profiling in brain has been an important tool to identify the potential molecular targets and biological pathways that are altered by ethanol and other drugs of abuse. In whole brain, expression of Scn4b was upregulated in ethanol-naive mice from different lines with a genetic predisposition for high ethanol consumption and was suggested to be a quantitative trait gene at chromosome 9 locus for ethanol preference in mice (Mulligan et al., 2006; Tabakoff et al., 2008). However, Scn4b expression was downregulated in several brain regions in ethanol-naive HDID-1 mice and P rats and in ethanol-treated HDID-1 and C57BL/6J mice (Suppl. Table 1). A GeneNetwork analysis of different datasets showed that Scn4b expression correlated with ethanol-related phenotypes (e.g., ethanol consumption, ethanol-induced hypothermia, and ethanol-induced motor ataxia) in BXD recombinant inbred mouse lines (Suppl. Table 2). One variant of SCN4B was also identified within a coregulated gene network that correlated with lifetime consumption of alcohol in human alcoholics (Farris et al., 2015).
Although there is evidence that Scn4b may be associated with ethanol behaviors, the contribution of this subunit has not been functionally validated. Here, we determined if genetic deletion or knockdown of Scn4b in male and female mice altered the behavioral effects of ethanol. Our comprehensive study was carried out in multiple laboratories using global KO mice and mice with targeted knockdown of Scn4b in the central nucleus of the amygdala (CeA) and examined different models of ethanol drinking and other behavioral and electrophysiological responses. Although the mutant mice did not differ from their control mice in ethanol consumption, Scn4b KO mice were more sensitive to the acute hypnotic effects of ethanol and other sedatives.
MATERIALS AND METHODS
Animals
As described previously (Ji et al., 2017), gene targeting and embryonic stem cell technologies were used to create mice in which Scn4b Exon 2 was flanked by loxP sites. Floxed mice (Strain 129 X C57BL/6J) were mated to a Cre global deleter transgenic mouse line (C57BL/6J) to generate Scn4b KO mice that did not express functional protein. Breeding pairs of cre recombined heterozygous KO mice on a C57BL/6J N2 background produced all wild-type (WT) and homozygous KO animals that were studied. These mice were shipped to The University of Texas at Austin for ethanol drinking and other behavioral tests and to The Scripps Research Institute for ethanol drinking and electrophysiological studies (described in the following sections). Mice (2–3 months old) were allowed to adapt to the testing room for one week before behavioral experiments began. Male C57BL/6J mice (~ 5 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, MA) and were housed at the Portland VA Medical Center. These mice were ~ 10 weeks of age when they were used to study the effect of Scn4b knockdown in the CeA on two-bottle choice (2BC) ethanol drinking.
Mice were group-housed 4–5 per cage (except during ethanol drinking tests), and food and water were available ad libitum (except during the drinking-in-the-dark test in which mice only had access to ethanol for either 2 or 4 h). All mice were naïve and used once for a single test. The University of Texas at Austin and Portland VA Medical Center vivaria were maintained on a 12-h light/dark cycle with lights on at 7:00 a.m., and The Scripps Research Institute vivarium was maintained on a 12-h light/dark cycle with lights off at 8:00 a.m. The temperature and humidity of the rooms were kept constant.
Animal care procedures at each institution were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and adequate measures were taken to minimize pain or discomfort. Experiments were approved by the Institutional Animal Care and Use Committees at The University of Texas at Austin, Oregon Health & Science University and Portland Veterans Affairs facility, and The Scripps Research Institute. Loss of righting reflex (LORR), hypothermia, acute ethanol withdrawal, startle reflex, ethanol clearance, continuous and intermittent 2BC ethanol drinking, and drinking-in-the-dark (DID) experiments in WT and Scn4b KO mice were performed at The University of Texas at Austin. DID, chronic intermittent ethanol (CIE) exposure, and electrophysiology experiments in WT and Scn4b KO mice were performed at The Scripps Research Institute. Scn4b knockdown in the CeA and 2BC drinking experiments in C57BL/6J mice were performed at Oregon Health & Science University.
Loss of Righting Reflex
Sensitivity to the sedative effects of ethanol (3.6 g/kg, i.p.) or other sedatives was measured by the duration of the LORR. Each drug was tested in naïve mice (n = 6–10 per genotype per sex). When mice became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 30 s. Sleep time was defined as the time from being placed in the supine position until the righting reflex was regained. All injectable ethanol (Aaper Alcohol and Chemical, Shelbyville, KY) solutions were prepared in 0.9% saline (20%, v/v) and injected i.p. Gaboxadol (Sigma-Aldrich, St. Louis, MO; 55 mg/kg, i.p.), ketamine (Sigma-Aldrich; 175 mg/kg, i.p.), and pentobarbital (Sigma-Aldrich; 50 mg/kg, i.p.) were dissolved in 0.9% saline and injected at 0.01 ml/g of body weight.
Hypothermia
Mice (n = 5–7 per genotype per sex) were placed into ventilated hypothermia chambers to acclimate for 60 min. Mice were then removed to record baseline temperatures with a glycerol-lubricated probe (1.2 mm ball × 2cm length; Sensortek Thermalert TH-8) that was inserted into the rectum for 5 s. Immediately following baseline recording, each mouse was injected with ethanol (3.6 g/kg, i.p.) and placed back in its chamber. Rectal temperatures were monitored over 5 h with measurements taken every 30 min following ethanol injection. Data from male and female mice were analyzed separately.
Acute Withdrawal from Ethanol
Mice (n = 9–10 per genotype per sex) were scored for handling-induced convulsion (HIC) severity 30 min before and immediately before ethanol injection, and these pre-drug baseline scores were averaged. A dose of 4 g/kg of ethanol in saline was injected i.p., and the HIC score was measured every hour until the HIC level reached baseline. Acute withdrawal was measured as the area under the curve, above the pre-drug level (Crabbe et al., 1991). To evoke HIC, the mouse was picked up by the tail and, if necessary, gently rotated 180°. The HIC was scored as follows: 5, tonic-clonic convulsion when lifted; 4, tonic convulsion when lifted; 3, tonic-clonic convulsion after a gentle spin; 2, no convulsion when lifted, but tonic convulsion elicited by a gentle spin; 1, facial grimace only after a gentle spin; 0, no convulsion or grimace after a gentle spin.
Startle Reflex
Acoustic startle responses were measured using SR-LAB test stations and software (San Diego Instruments, San Diego, CA) as described previously (Findlay et al., 2003). Test sessions began by placing the mouse in the plexiglass holding cylinder for a 5-min acclimation period. Over the next 8 min, mice (n = 6 per genotype per sex) were presented with each of seven trial types across five discrete blocks of trials for a total of 35 trials. The inter-trial interval was 10–20 s. One trial measured the response to no stimulus (baseline movement). The other six trials measured the responses to a startle stimulus, consisting of a 40-ms sound burst of 90, 95, 100, 105, 110, or 115 dB. Startle amplitude was measured every millisecond over a 65-ms period beginning at the onset of the startle stimulus. The trials were presented in pseudorandom order such that each type was presented once within a block of six trials. The maximum startle amplitude (Vmax) over this sampling period was taken as the dependent variable. A background noise level of 70 dB was maintained over the duration of the test session. Due to effects of body weight on startle responses, data from male and female mice were analyzed separately.
Ethanol Clearance
Mice (n = 4–5 per genotype per sex) were injected with a single dose of ethanol (4 g/kg, i.p.), and blood samples were taken from the retro-orbital sinus 30, 60, 120, 180, and 240 min after injection. Samples (~20 μl) were collected into capillary tubes and centrifuged for 6 min at 3100g in a Haematospin 1400 centrifuge (Analox Instruments, Lunenburg, MA). Plasma samples were stored at −20°C until blood ethanol concentrations (BECs) were determined in 5-μl aliquots using an AM1 Alcohol Analyzer (Analox Instruments). The machine was calibrated every 15 samples using an industry standard, and BECs were determined using commercially available reagents according to the manufacturer’s instructions. Samples were averaged from duplicate runs and expressed as mg/dl.
Continuous Two-Bottle Choice Ethanol Drinking
The 24-h 2BC protocol, in which mice had access to water and increasing concentrations of ethanol (3–18%, v/v), was carried out as described (Blednov et al., 2017). Each concentration of ethanol was offered for four days. Bottles were weighed daily and their positions changed daily to control for side preferences. Food was available ad libitum, and mice (n = 15–17 per genotype per sex) were weighed every four days. The amount of ethanol and water consumed (g/kg) was measured after 24-h continuous access sessions. Because female C57BL/6J mice typically consume more ethanol than males, data from male and female mice were analyzed separately for this and the intermittent 2BC procedure.
Intermittent Two-Bottle Choice Ethanol Drinking
Intermittent access to ethanol induces higher voluntary consumption in rats (Simms et al., 2008) and mice (Rosenwasser et al., 2013). We used this method to measure ethanol consumption in mice (n = 22–24 per genotype per sex) with access to a bottle of 15% (v/v) ethanol and a bottle of water during 24-h drinking sessions offered every-other-day. The placement of the ethanol bottle was alternated with each drinking session to control for side preferences. Food was available ad libitum, and mice were weighed every four days. The amount of ethanol and water consumed (g/kg) was measured every-other-day after 24-h access. Each point in the graphs (e.g., days 2, 4, 6, 8, 10, 12) represents the average of two drinking days with different bottle positions.
Drinking-in-the-Dark
A limited access, one-bottle DID test, which produces pharmacologically relevant levels of ethanol in the blood (Rhodes et al., 2005) was performed at The Scripps Research Institute. Beginning 3 h after lights off, the water bottle was replaced with a bottle containing a 20% (v/v) ethanol solution. The ethanol bottle remained in place for 2 h (day 1–3) and for 4 h (day 4). Other than these short periods of ethanol drinking, male (n = 13–18) and female (n = 8–11) mice had unlimited access to water. The amount of ethanol consumed was calculated as g/kg during 2-h or 4-h sessions. Blood ethanol samples were taken after the 4-h drinking session on day 4.
Chronic Intermittent Ethanol-Two-Bottle Choice
The chronic intermittent ethanol (CIE)-2BC procedure has been successfully used in rats (Vendruscolo and Roberts, 2014) and C57BL/6J mice (Becker and Lopez, 2004; Finn et al., 2007; Griffin et al., 2009) to model the motivational aspects of ethanol dependence and excessive ethanol drinking associated with the addicted state. For the first 15 days of testing (5 days per week for 3 weeks), 30 min before the lights went off (7:30 a.m.), mice were individually housed for 2 h with access to two drinking tubes, one containing 15% (v/v) ethanol and the other containing water. Mice then were divided by sex and genotype, based on equal ethanol and water consumption, into two balanced treatment groups that were exposed to intermittent ethanol vapor or air in identical chambers (n = 10–14 mice per sex per genotype per group). Chambers consisted of standard plastic, mouse-sized shoebox cages containing up to four mice per chamber (La Jolla Alcohol Research Inc., La Jolla, CA). Ethanol vapor was created by pumping 95% ethanol into a 2L Erlenmeyer vacuum flask kept at 50°C on a warming tray. Air was blown over the bottom of the flask at a rate of 11 L/min. Concentrations of ethanol vapor were adjusted by varying the rate at which the ethanol was pumped into the flask which, in turn, was based on the BECs of the mice (target of 175–225 mg/dl). The CIE group was injected with 1.75 g/kg ethanol and 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor, Sigma-Aldrich) and placed in the chambers to receive vapor for 16 h (with 8 h off). Following the fourth day of exposure, mice were allowed 72 h of undisturbed time in their home cages. The mice were then given five days of access to two bottles containing 15% ethanol or water for 2 h. The four days of ethanol vapor or air exposure and five days of 2BC testing were repeated for a total of three cycles. The control (CTL) group was injected with 68.1 mg/kg pyrazole in 0.9% saline and placed in chambers delivering air for the same periods as the ethanol vapor group and received 2BC testing at the same time as the vapor group. Following each 16 h bout of ethanol vapor exposure, mice were removed and on the 3rd day of each cycle, tail blood was sampled for BECs. Blood was collected in capillary tubes and emptied into 1.5 ml centrifuge tubes containing evaporated heparin and kept on ice. Samples were centrifuged and serum was decanted into fresh 1.5 ml centrifuge tubes. The serum was injected into an oxygen-rate alcohol analyzer (Analox Instruments) for BEC determination.
Electrophysiology
Adult male Scn4b KO mice (n = 6; average weight was 40.2 ± 1.6 g and average age was 32.5 ± 3.7 weeks) and their WT littermates (n = 4; average weight was 35.8 ± 2.7 g and average age was 30.0 ± 4.5 weeks) were used for electrophysiology. To prepare brain slices, mice were anesthetized with 3–5% isoflurane and brains were isolated in ice-cold artificial cerebrospinal fluid, containing the following in mM: NaCl 126, KCl 3, MgSO4 2, NaH2PO4 1, NaHCO3 25, and CaCl2 2 (300 mOsmol; bubbled with 95% O2–5% CO2). We used a Vibratome VT1000S (Leica Microsystems, Buffalo Grove, IL) to cut 300-μm coronal slices containing the CeA. The slices were then allowed to recover at 37°C for 30 min followed by incubation at room temperature for an additional 30 min. The patch pipettes were filled with an internal solution containing the following in mM: K-gluconate 100, KCl 30, MgSO4 1, CaCl2 1, EGTA 10, Na HEPES 10, and K2ATP 2 (pH 7.4 and 290 mOsmol). Recordings were conducted in the whole-cell current-clamp configuration using a Multiclamp 700B amplifier and pClamp 10.2 software (Molecular Devices, Sunnyvale, CA) for data acquisition. The recordings were low-pass filtered at 2–5 kHz and sampled at 10–40 kHz using an Axon Digidata 1440A interface. We recorded the current-voltage relationship (IV) by holding CeA neurons (n = 20–31) at −70 mV and performing a set of 19 hyperpolarizing and depolarizing steps (duration 600 ms) with injected currents ranging from −120 to 60 pA (with 10 pA/step). We analyzed voltage responses of CeA neurons under current-step stimulation and calculated various physiological parameters using the software NeuroExpress developed by Dr. A. Szucs.
Scn4b Knockdown in CeA
Male C57BL/6J mice were prepped for aseptic surgical procedures and anesthetized with isoflurane and oxygen. Over a period of 1 min, mice were given 100 nl bilateral intracranial microinjections into the CeA as described previously (Lasek and Azouaou, 2010). The injector was left in place for a short period of time and removed slowly to prevent tracing. Mice were then given 30 mg/ml injections of ketorolac tromethamine and allowed to recover on a warming pad in a quiet space.
For the microinjections, we used lentivirus vectors expressing either a shRNA targeting Scn4b or a scrambled control shRNA and enhanced green fluorescent protein. The 19-nucleotide targeting sequences were: shScn4b-10, 5’-GCTGGGAACCGAGGCAATA; shScn4b-268, 5’-GACCCTAAGGTGAGAGTGA (Ji et al., 2017); and scrambled, 5’-GCGCTTAGCTGTAGGATTC (Lasek et al., 2007). Sequences were cloned into the vector backbone pLL3.7 and virus was produced as described previously (Lasek et al., 2007). Lentiviral plasmids expressing shScn4b-10 and shScn4b-268 reduced expression of a co-transfected Scn4b cDNA in mouse Neuro-2a cells by ~80% compared with the plasmid expressing the control shRNA (one-way ANOVA F2, 6 = 105, p < 0.0001, n = 3 per group; Suppl. Fig. 1), and shScn4b-268 was shown to reduce expression of Scn4b transcripts by 25% in infected nucleus accumbens (Ji et al., 2017).
Two-Bottle Choice Ethanol Drinking after Scn4b Knockdown
Thirty days post-surgery, mice (n = 10–12 per genotype) underwent a 2BC drinking protocol, where mice were acclimated to the taste and effects of ethanol by progressively increasing the concentration of ethanol (0%, 5%, 10% and 20% v/v) every four days (Metten et al., 2014). After four weeks, another identical 2BC drinking procedure was performed. Mice were individually housed and given one week of habituation, with continuous access to food and tap water, accessible from two 25-ml graduated glass cylinders fitted with rubber stoppers and stainless steel sipper tubes. Every other day, the position of the ethanol bottle was alternated to control for side preferences.
Statistical Analysis
Data are reported as the mean ± S.E.M. Prism (GraphPad Software, Inc., La Jolla, CA) and Statview Software (SAS Institute Inc., Cary, NC) were used to perform ANOVAs, Student’s t-tests, and Bonferroni, Dunnett’s, or Fisher’s PLSD post-hoc tests.
RESULTS
Behavioral and Other In Vivo Tests in Scn4b KO Mice
Loss of Righting Reflex
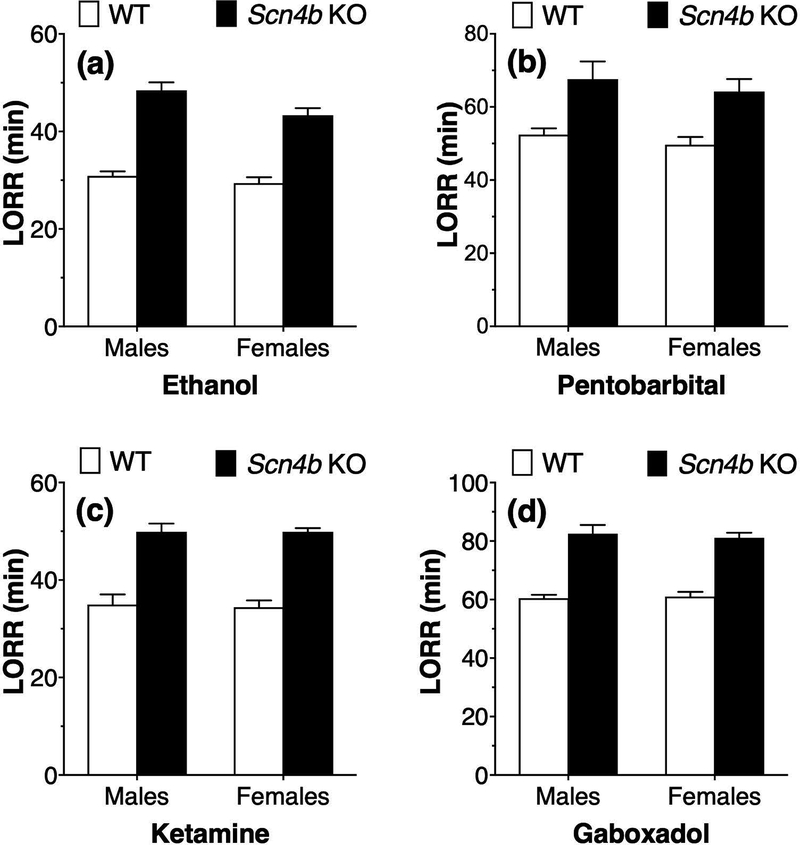
Compared with WT mice, male and female Scn4b KO mice showed longer duration of LORR after administration of ethanol (3.6 g/kg; Fig. 1a), pentobarbital (50 mg/kg; Fig. 1b), ketamine (175 mg/kg; Fig. 1c), or gaboxadol (55 mg/kg; Fig. 1d). For ethanol-treated mice, there was a significant effect of genotype (F1,23 = 126.7, p < 0.0001) and sex (F1,23 = 5.5, p < 0.05) but no genotype x sex interaction (F1,23 = 1.67, p = 0.21). For pentobarbital-treated mice, there was a significant genotype effect (F1,28 = 20.2, p < 0.0001) but no effect of sex (F1,28 = 0.87, p = 0.36) and no interaction (F1,28 = 0.01, p = 0.92). For ketamine-treated mice, there was a significant genotype effect (F1,26 = 105.6, p < 0.0001) but no effect of sex (F1,26 = 0.04, p = 0.85) and no interaction (F1,26 = 0.03, p = 0.86). For gaboxadol-treated mice, there was also a significant genotype effect (F1,31 = 118.1, p < 0.0001) but no effect of sex (F1,31 = 0.05, p = 0.83) and no genotype x sex interaction (F1,31 = 0.24, p = 0.63).
Figure 1. Prolonged duration of loss of righting reflex by ethanol and other sedatives in Scn4b KO mice.
Effect of i.p. injections of (a) ethanol (3.6 g/kg, n = 6–8 per genotype per sex), (b) pentobarbital (50 mg/kg, n = 6–10 per genotype per sex), (c) ketamine (175 mg/kg, n = 6–9 per genotype per sex), or (d) gaboxadol (55 mg/kg, n = 8–9 per genotype per sex) on the duration of loss of righting reflex (LORR) in male and female WT and Scn4b KO mice.
Hypothermia
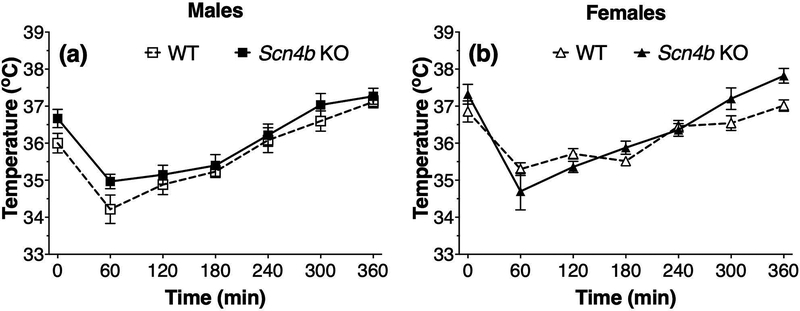
We measured the acute hypothermic response to ethanol (3.6 g/kg, injected at time 0) in male and female mice and found that the maximum reduction in body temperature occurred 60 min after injection followed by a gradual recovery period over the next 5 h (effect of time: F6,60 = 31.2, p < 0.0001 for males and F6,60 = 37.9, p < 0.0001 for females; Fig. 2). There was no significant genotype effect in male (F1,10 = 3.1, p = 0.11) or female mice (F1,10 = 1.3, p = 0.29). There was no significant genotype x time interaction in males (F6,60 = 0.55, p = 0.77), but this interaction was significant in females (F6,60 = 3.3, p < 0.01).
Figure 2. No main effect of genotype in ethanol-induced hypothermia in Scn4b KO and WT mice.
Effect of ethanol (3.6 g/kg, i.p.) injected at time 0 on body temperature in male (a, n = 6 per genotype) and female (b, n = 5–7 per genotype) WT and Scn4b KO mice.
Acute Withdrawal from Ethanol
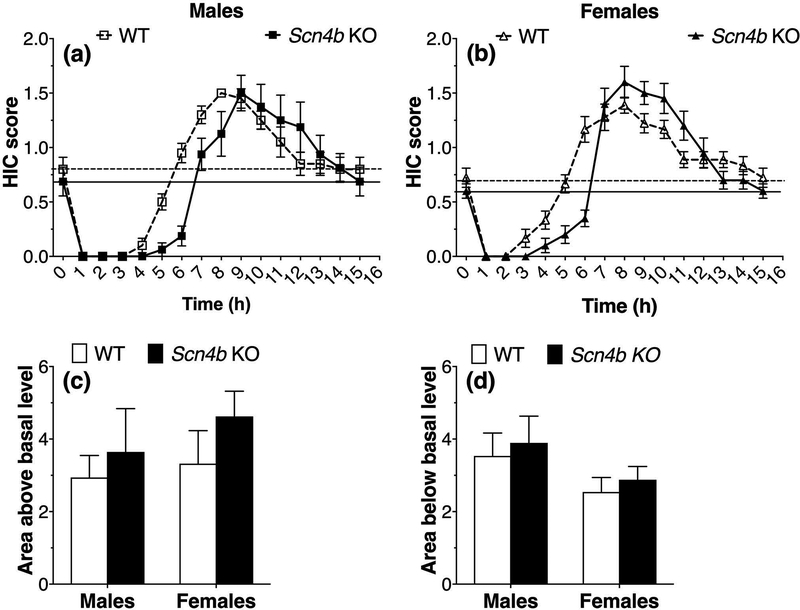
A single dose of ethanol (4 g/kg, given at time 0) suppressed basal HICs in mice for about 4 h followed by increased HICs (effect of time: F15,240 = 58.2, p < 0.0001 for males and F15,255 = 81.9, p < 0.0001 for females; Fig. 3a,b). The recovery to basal levels was slower in Scn4b KO mice of both sexes, but the area below basal did not differ from WT as discussed below. The slower recovery after 4 g/kg ethanol is consistent with the prolonged sedative effects in Scn4b KO mice after 3.6 g/kg ethanol injection shown in Fig. 1. Acute withdrawal from ethanol is represented by HIC scores above the basal level. Although there was no main effect of genotype in males (F1,16 = .93, p = 0.35) or females (F1,17 = .72, p = 0.41), there was a significant genotype x time interaction (F15,240 = 4.2, p < 0.0001 for males and F15,255 = 7.3, p < 0.0001 for females; Fig. 3a,b). No group differences were found for areas above or below the basal HIC level (Fig. 3c,d). Two-way ANOVA of the area above the curve showed no effect of genotype (F1,33 = 1.4, p = 0.24), sex (F1,33 = .65, p = 0.43), or genotype x sex interaction (F1,33 = .13, p = 0.73). Two-way ANOVA of the area below the curve also showed no effect of genotype (F1,33 = .41, p = 0.53), sex (F1,33 = 3.4, p = 0.07), or genotype x sex interaction (F1,33 = 0.0004, p = 0.98).
Figure 3. Scn4b KO mice show slower recovery to basal handling-induced convulsions after ethanol injection but do not differ in acute withdrawal from ethanol.
Effect of ethanol (4 g/kg, i.p.) injected at time 0 on handling-induced convulsions (HIC) in male (a, n = 9–10 per genotype) and female (b, n = 9–10 per genotype) WT and Scn4b KO mice. Area of the HIC curve above the basal level (c) in male and female mice. Area below the basal level (d) in male and female mice.
Startle Response
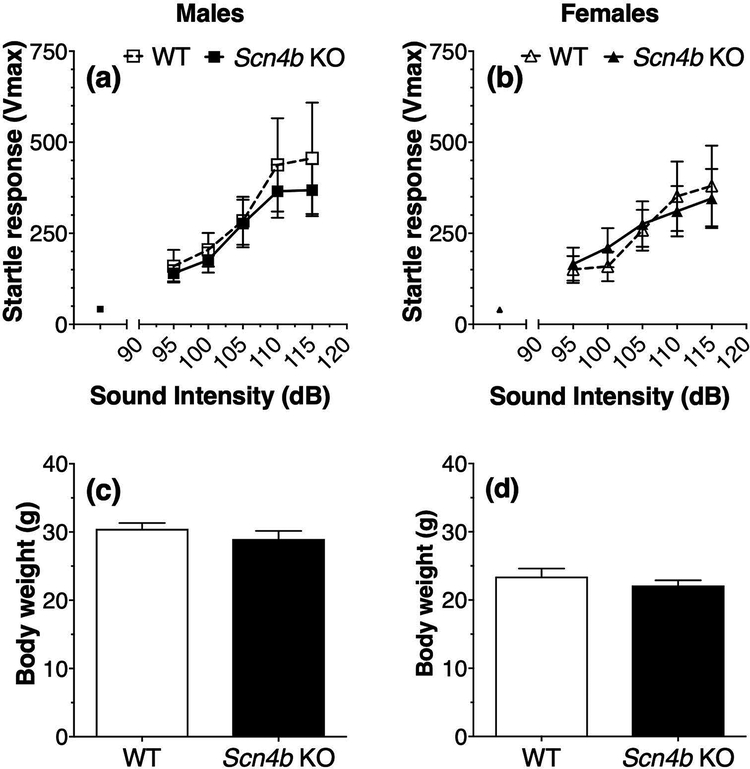
There were no differences in basal (white noise) responses in WT and Scn4b KO male mice (Vmax or startle magnitude: 42.02 + 5.5 and 40.2 + 4.8, respectively) or in WT and KO female mice (40.3 + 3.9 and 38.3 + 4.5, respectively) (Fig. 4a,b). Startle responses depended on the sound intensity (F4,40 = 12.4, p < 0.0001 for males and F4,40 = 13.4, p < 0.0001 for females), but there was no effect of genotype in males (F1,10 = 0.20, p = 0.66) or females (F1,10 = 0.0003, p = 0.99) and no interaction of sound intensity and genotype in males (F4,40 = 0.27, p = 0.90) or females (F4,40 = 0.64, p = 0.64) (Fig. 4a,b). There were no differences in body weight between male WT and KO mice (Student’s t-test; t(10) = 1.0, p = 0.33; Fig. 4c) or female WT and KO mice (Student’s t-test; t(10) = 0.93, p = 0.38; Fig. 4d).
Figure 4. No genotype-dependent differences in startle responses and body weight in Scn4b KO and WT mice.
Basal and sound-induced increases in startle responses in male (a, n = 6 per genotype) and female (b, n = 6 per genotype) WT and Scn4b KO mice. Body weight in the same male (c) and female (d) Scn4b KO and WT mice.
Ethanol Clearance
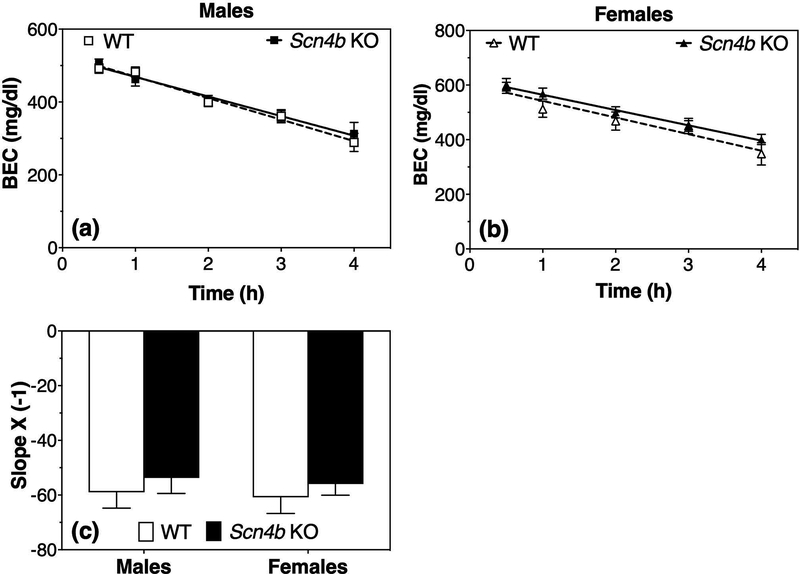
Fig. 5 shows the clearance of ethanol from blood in male (Fig. 5a) and female (Fig. 5b) mice. In males, there was a significant effect of time (F4,32 = 58.8, p < 0.0001) but no effect of genotype (F1,8 = 0.08, p = 0.78) or genotype x time interaction (F4,32 = 0.61 interaction, p = 0.66). In females, there was a significant effect of time (F4,28 = 163.5, p < 0.0001) and time x genotype interaction (F4,28 = 4.6, p < 0.01) but no genotype effect (F1,7 = 0.68, p = 0.44). Post-hoc analysis showed no significant differences between WT and KO mice at any time point. The slopes of the regression lines are shown in Fig. 5c; two-way ANOVA of these data showed no significant effects of genotype (F1,15 = 0.86, p = 0.37), sex (F1,15 = 0.14, p = 0.71), or genotype x sex interaction (F1,15 = 0.001, p = 0.97). These studies were carried out at The University of Texas at Austin, and similar results showing no genotype effect were replicated using female mice at The Scripps Research Institute (A. J. Roberts, data not shown).
Figure 5. No genotype-dependent differences in ethanol clearance in Scn4b KO and WT mice.
Blood ethanol concentration (BEC) measured after ethanol (4 g/kg, i.p.) administration in male (a, n = 5 per genotype) and female (b, n = 4–5 per genotype) WT and Scn4b KO mice. (c) Slopes of the regression lines in male and female mice.
Ethanol Drinking Tests in Scn4b KO mice
Continuous Two-Bottle Choice
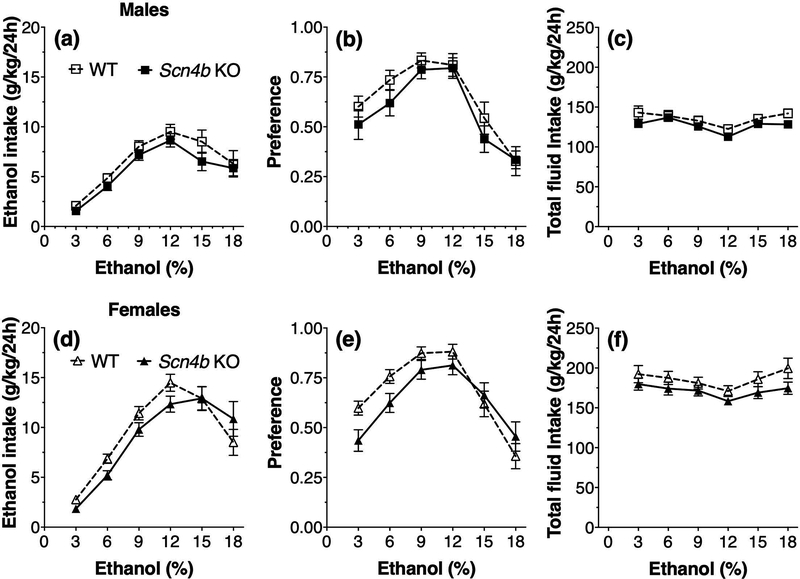
In the 2BC procedure using increasing concentrations of ethanol (3–18%, v/v) in male mice (Fig. 6a), there was a significant effect of ethanol concentration (F5,145 = 36.9, p < 0.0001) but no genotype effect (F1,29 = 1.6, p = 0.21) or interaction between genotype and ethanol concentration (F5,145 = 0.42, p = 0.83) on ethanol consumption. For ethanol preference (Fig. 6b), there was a significant effect of ethanol concentration (F5,145 = 36.3, p < 0.0001) but no genotype (F1,29 = 0.96, p = 0.34) or genotype x ethanol interaction effects (F5,145 = 0.65, p = 0.66). For total fluid intake in male mice (Fig. 6c), there was also an effect of ethanol concentration (F5,145 = 14.8, p < 0.0001) but no genotype (F1,29 = 1.7, p = 0.20) or interaction effects (F5,145 = 1.4, p = 0.22). In the 2BC test in female mice (Fig. 6d), there was a significant effect of ethanol concentration (F5,160 = 64.5, p < 0.0001) and interaction between genotype and ethanol concentration (F5,160 = 2.4, p < 0.05) for amount of ethanol consumed but no main effect of genotype (F1,32 = 0.56, p = 0.46). For ethanol preference (Fig. 6e), there was also a significant effect of ethanol concentration (F5,160 = 44.3, p < 0.0001) and interaction between genotype and ethanol concentration (F5,160 = 3.8, p < 0.01) but no main effect of genotype (F1,32 = 0.85, p = 0.36). Although female KO mice drank slightly less than WT mice, post-hoc analysis using the Bonferroni multiple comparison test did not reveal significant genotype differences at any ethanol concentration. For total fluid intake in female mice (Fig. 6f), there was a significant effect of ethanol concentration (F5,160 = 9.7, p < 0.0001) but no effect of genotype (F1,32 = 1.9, p = 0.18) and no interaction between genotype and ethanol concentration (F5,160 = 1.1, p = 0.38).
Figure 6. No main effect of genotype in ethanol consumption and preference in the continuous two-bottle choice test in Scn4b KO and WT mice.
Ethanol intake (a), preference for ethanol (b), and total fluid intake (c) in male WT and Scn4b KO mice (n = 15–16 per genotype). Ethanol intake (d), preference for ethanol (e), and total fluid intake (f) in female WT and Scn4b KO mice (n = 17 per genotype). The amount of ethanol and water consumed (g/kg) was measured after 24-h continuous access sessions. Each ethanol concentration (3–18%) was offered for four days and bottle positions were alternated daily.
Intermittent Two-Bottle Choice
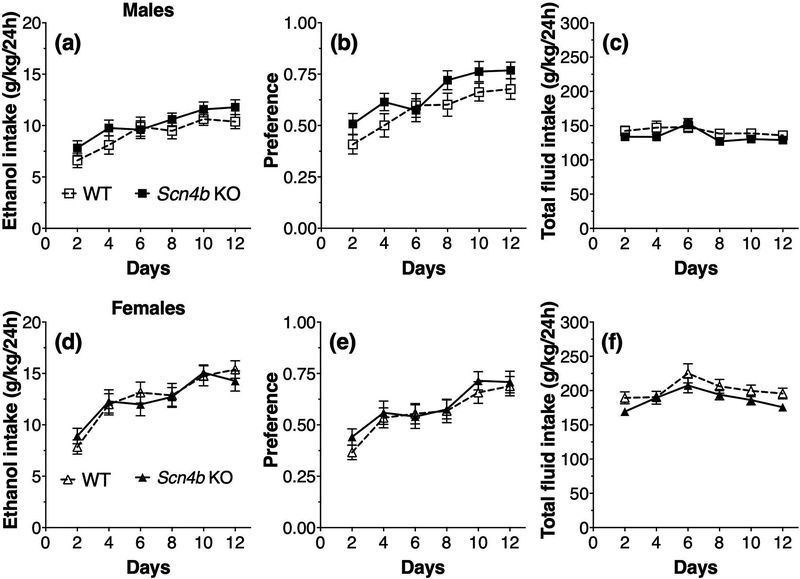
During intermittent (every-other-day) drinking in male mice (Fig. 7a), there was a significant effect of days of treatment (F5,215 = 17.8, p < 0.0001) on ethanol (15% v/v) consumption but no effect of genotype (F1,43 = 1.4, p = 0.25) or interaction between genotype and days of treatment (F5,215 = 0.96, p = 0.44). For ethanol preference (Fig. 7b), there was a significant effect of days of treatment (F5,215 = 21.8, p < 0.0001) but no effect of genotype (F1,43 = 2.0, p = 0.16) or interaction between genotype and treatment days (F5,215 = 1.5, p = 0.19). For total fluid intake in male mice (Fig. 7c), there was a significant treatment effect (F5,215 = 5.8, p < 0.0001) but no genotype (F1,43 = 0.99, p = 0.32) or interaction effects (F5,215 = 1.3, p = 0.27). In female mice, there was a significant effect of days of treatment (F5,220 = 24.9, p < 0.0001) on every-other-day ethanol (15% v/v) consumption but no effect of genotype (F1,44 = 0.01, p = 0.91) or interaction between genotype and days of treatment (F5,220 = 0.84, p = 0.53) (Fig. 7d). For ethanol preference (Fig. 7e), there was a significant effect of days of treatment (F5,220 = 18.4, p < 0.0001) but no effect of genotype (F1,44 = 0.29, p = 0.59) or interaction between genotype and treatment days (F5,220 = 0.42, p = 0.83). For total fluid intake in female mice (Fig. 7e), there was a significant treatment effect (F5,220 = 7.8, p < 0.0001) but no genotype (F1,44 = 2.5, p = 0.12) or interaction effects (F5,220 = 0.60, p = 0.70).
Figure 7. No genotype-dependent differences in ethanol consumption and preference in the intermittent two-bottle choice test in Scn4b KO and WT mice.
Ethanol intake (a), preference for ethanol (b), and total fluid intake (c) in male WT and Scn4b KO mice (n = 22–23 per genotype). Ethanol intake (d), preference for ethanol (e), and total fluid intake (f) in female WT and Scn4b KO mice (n = 22–24 per genotype). The amount of ethanol (15%) and water consumed (g/kg) was measured every-other-day after 24-h access. Each point represents two days of drinking with alternating bottle positions.
One-Bottle Drinking-in-the-Dark
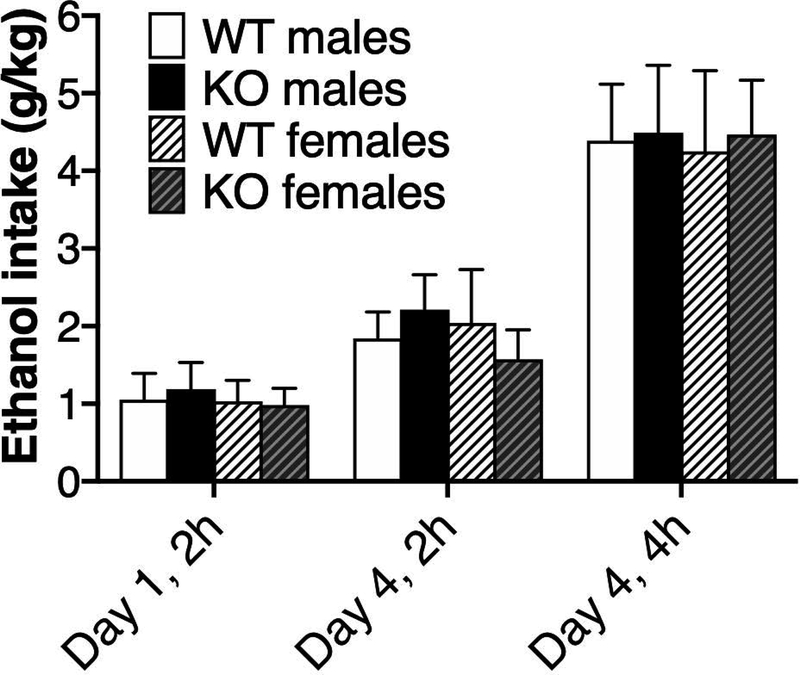
The one-bottle DID procedure capitalizes on the circadian rhythm in drinking and uses discrete ethanol exposure times to increase levels of consumption. There were no significant effects of genotype, sex, or genotype x sex on ethanol (20% v/v) drinking (Fig. 8). For the effect of genotype, the respective ANOVA results for day 1 (2 h), day 4 (2 h), and day 4 (4 h) were F1,60 = 0.03, p = 0.87; F1,60 = 0.06, p = 0.81; and F1,60 = 0.22, p = 0.64. Respective ANOVA results for the effect of sex were F1,60 = 0.15, p = 0.70; F1,60 = 0.16, p = 0.69; and F1,60 = 0.32, p = 0.58. Respective ANOVA results for genotype x sex interaction were F1,60 = 0.10, p = 0.76; F1,60 = 1.3, p = 0.27; and F1,60 = 0.34, p = 0.56. BECs (mg/dl) on day 4 were 82.5 ± 16.1 and 76.0 ± 19.5 for male WT and KO mice, respectively. In females, BECs were 60.6 ± 18.6 and 51.1 ± 11.6 in WT and KO mice, respectively. There were no significant effects of genotype (F1,60 = 1.0, p = 0.32), sex (F1,60 = 1.3, p = 0.27), or genotype x sex (F1,60 = 0.32, p = 0.58) on BECs. These studies were carried out at The Scripps Research Institute, and similar DID results showing no effect of genotype in male and female mice (n = 9–10 per genotype per sex) were replicated at The University of Texas at Austin (Y. A. Blednov, data not shown).
Figure 8. No genotype-dependent differences in ethanol consumption in the drinking-in-the-dark test in Scn4b KO and WT mice.
Ethanol intake was measured in male (n = 13–18 per genotype) and female (n = 8–11 per genotype) WT and Scn4b KO mice using one bottle of ethanol (20% v/v) for 2-h drinking sessions (days 1–3) and 4-h on day 4.
Chronic Intermittent Ethanol-Two-Bottle Choice
In the CIE model, increased ethanol consumption is typically observed after 2BC limited-access periods are cycled with chronic passive exposure to ethanol vapor. Figure 9 shows 2BC ethanol intake under baseline and CIE conditions in male and female WT and Scn4b KO mice. Respective BEC averages for the three ethanol cycles (CIE1, CIE2, and CIE3) were 140.51, 174.57, and 176.79 mg/dl for male WT mice; 130.79, 176.47, and 194.96 mg/dl for male KO mice; 138.64, 163.06, and 179.98 mg/dl for female WT mice; and 146.58, 162.65, and 192.38 mg/dl for female KO mice. We used 4-way repeated measures ANOVA with cycle as the within-subject factor (Baseline, CIE1, CIE2, CIE3) and the three between-subject factors as genotype, group (CTL vs CIE), and sex. There was no significant effect of genotype (F1,88 = 0.004, p = 0.95); however, there was an overall sex difference in ethanol intake (F1,88 = 22.9, p < 0.0001), with females drinking more than males. There was also an effect of group x cycle (F3,264 = 11.2, p < 0.0001). Post-hoc analyses using Fisher’s PLSD tests showed that WT and KO males and females drank more ethanol following CIE3 compared with their baseline drinking levels (p < 0.01). There were no significant changes in drinking in any of the CTL groups. Thus, CIE vapor increased ethanol intake and BECs, but there were no significant differences between WT and Scn4b KO mice (Fig. 9).
Figure 9. No genotype-dependent differences in ethanol consumption in the chronic intermittent ethanol-two-bottle choice test in Scn4b KO and WT mice.
Ethanol intake was measured in male (n = 10–14 per genotype per group) and female (n = 12–14 per genotype per group) WT and Scn4b KO mice following air vapor control (CTL) and chronic intermittent ethanol (CIE) vapor conditions.
Electrophysiology in CeA Neurons
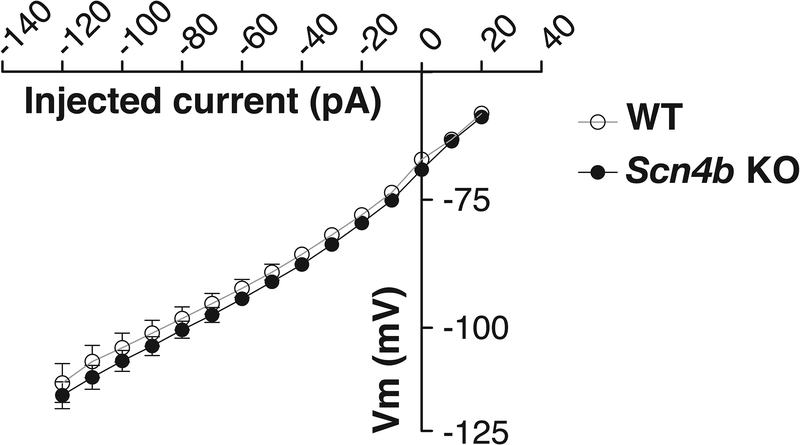
In addition to our in vivo studies, we further characterized the effects of Scn4b deletion at the cellular level. We used a current-voltage (I-V) relationship protocol to examine the intrinsic membrane properties and excitability of CeA neurons in Scn4b KO vs. WT mice. Based on the firing properties of CeA neurons, the neurons can be divided into three cell types: low-threshold bursting (LTB), late responding (LR), and regular spiking (RS) neurons (Herman et al., 2013). The data from all CeA neurons were combined because in our data set none of the CeA cell types in Scn4b KO and WT mice showed significant alterations in the tested parameters, and the distribution of the individual cell types was comparable (WT: 25% LTB, 30% LR, and 45% RS; Scn4b KO: 22% LTB, 26% LR, and 51% RS). There were no significant differences in the resting membrane potentials of CeA neurons between the genotypes (WT: −54.65 ± 1.8 mV vs. KO: −51.65 ± 1.7 mV; t-test, p > 0.05). There were no significant genotype differences (t-test; p > 0.05) in the membrane properties, excitability (Table 1), and current-voltage relationship (Fig. 10) of the CeA neurons. Also, we did not observe significant differences in the properties associated with action potentials in the CeA neurons between WT and Scn4b KO mice (Table 2).
Table 1. Physiological properties of CeA neurons from Scn4b KO and WT mice.
The membrane time constant was obtained by monoexponential fit of the initial part of the voltage responses under negative current steps. The voltage sag and afterdepolarization (ADP) values were plotted against the level of the injected current, and the corresponding relationships were fitted with linear functions. The slope of the linear fit is proportional with the magnitude of the voltage sag and ADP associated with the action of the intrinsic h-current.
| Mice (number of cells) | Resistance (MOhm) | Capacitance (pF) | Membrane Time Const. (ms) | Voltage Sag Slope (mV/pA) | ADP Slope (mV/pA) | Rheobase (pA) | Number of Spikes at Max Intensity | Spike Threshold (mV) |
|---|---|---|---|---|---|---|---|---|
| WT (n = 20) |
502.9 ± 31.3 | 83.7 ± 6.9 | 30.4 ± 1.9 | −0.08 ± 0.01 | −0.10 ± 0.01 | 27.9 ± 2.8 | 6.0 ± 0.7 | −41.6 ± 1.0 |
|
Scn4b KO (n = 31) |
520.2 ± 26.7 | 81.6 ± 5.2 | 30.6 ± 1.3 | −0.08 ± 0.01 | −0.10 ± 0.01 | 27.8 ± 2.2 | 6.1 ± 0.6 | −43.1 ± 0.7 |
Figure 10. No genotype-dependent differences in the whole-cell current-voltage (I-V) relationship of CeA neurons from Scn4b KO and WT mice.
CeA neurons (n = 20 and 31 cells from WT and Scn4b KO mice, respectively) were held at −70 mV, and hyperpolarizing and depolarizing currents were injected using 10 pA increments (−120 to 60 pA).
Table 2. Action potential properties of CeA neurons from Scn4b KO and WT mice.
Spike after-hyperpolarization after the first spike is calculated relative to the spike threshold. Maximal positive slope represents a maximal voltage slope during the spike upshot phase (mediated primarily by sodium channels). Maximal negative slope represents a maximal voltage slope during the spike falling phase (mediated primarily by potassium channels).
| Mice (number of cells) | Amplitude(mV) | Spike Rise Time (ms) | Spike Decay Time (ms) | Half-Width (ms) | Spike After-hyperpolari-zation (mV) | Maximal Positive Slope (mV/ms) | Phase of Maximal Positive Slope | Maximal Negative Slope (mV/ms) | Phase of Maximal Negative Slope | Pos/Neg Slope Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| WT (n = 20) |
86.4 ± 1.8 | 1.2 ± 0.05 | 3.0 ± 0.2 | 1.8 ± 0.1 | 12.5 ± 0.9 | 156.5 ± 10.8 | 0.6 ± 0.01 | −51.4 ± 4.7 | 0.73 ± 0.01 | 3.3 ± 0.2 |
|
Scn4b KO (n = 31) |
86.9 ± 1.7 | 1.1 ± 0.03 | 2.6 ± 0.2 | 1.6 ± 0.1 | 11.3 ± 0.9 | 172.2 ± 7.5 | 0.59 ± 0.01 | −56.9 ± 2.9 | 0.73 ± 0.01 | 3.2 ± 0.1 |
Two-Bottle Choice Ethanol Drinking after Scn4b Knockdown in CeA
Male mice received bilateral microinjections into the CeA of lentivirus expressing shScn4b-10, shScn4b-268, or a control shRNA. Thirty days following the intracranial microinjections, mice underwent two rounds of 2BC drinking using increasing ethanol concentrations (0%, 5%, 10%, and 20% v/v). There was a 4-week off period between the drinking tests. There were no differences in ethanol intake between the two procedures, and data from both drinking procedures were combined. Ethanol (20% v/v) intake and preference were not altered in mice expressing shScn4b-10 or shScn4b-268 compared with the control group treated with control shRNA (Table 3). There was also no effect of CeA knockdown on 5% or 10% ethanol consumption (data not shown).
Table 3. No effect of Scn4b knockdown in the CeA on ethanol intake and preference.
Thirty days following microinjections of shScn4b-10, shScn4b-10, or a control shRNA into the CeA, mice underwent two 2BC drinking procedures using increasing ethanol concentrations of 0%, 5%, 10%, and 20% (v/v). There was a 4-week waiting period between the procedures. Data from both drinking procedures were combined (n = 10–12 mice per genotype).
| Group | Ethanol (20%) Intake | Preference |
|---|---|---|
| shScn4b-268 | 13.5 ± 1.9 g/kg/24h | 0.43 ± 0.04 |
| shScn4b-10 | 14.6 ± 1.0 g/kg/24h | 0.47 ± 0.03 |
| Control | 14.8 ± 1.9 g/kg/24h | 0.47 ± 0.05 |
DISCUSSION
Our comprehensive study was carried out in multiple laboratories using both male and female mice, different methods to inhibit Scn4b expression, and different tests of acute, chronic, and dependence-driven consumption. We have provided corroborating evidence that Scn4b is not a critical gene for regulation of ethanol drinking. We also validated the DID and blood ethanol clearance results in two laboratories, further increasing the robustness of our approach and conforming to the National Institutes of Health mandate for increased rigor and reproducibility in research.
Although Scn4b was not a critical gene for ethanol consumption, it was involved in the acute hypnotic actions of ethanol and other sedatives. The slower recovery to basal HIC levels after ethanol injection is also consistent with the increased sedative effects in Scn4b KO mice. The sedative/hypnotic actions were not specific to ethanol, as gaboxadol, ketamine, and pentobarbital all increased sleep time in Scn4b KO mice. Gaboxadol and pentobarbital target GABAA receptors and ketamine blocks NMDA receptors, although pentobarbital (Bachmann et al., 2002; Wartenberg et al., 2001) and ketamine (Flood and Krasowski, 2000) may also act on other ion channels. Thus, Scn4b may indirectly alter the function of several inhibitory and excitatory synaptic processes, including those mediated by GABAA- and NMDA-receptor operated channels. Sodium channel β (and α) subunits are highly expressed in excitable cells and have significant effects on neuronal excitability in vivo (Brackenbury and Isom, 2011). Longer drug-induced sedation in Scn4b KO mice is consistent with the prediction that global deletion of Scn4b would decrease neuronal excitability, thus exacerbating effects of sedative/hypnotic drugs. This is also consistent with the finding that Scn4b KO mice showed less stimulation in response to amphetamine, perhaps due to decreased excitability of core nucleus accumbens medium spiny neurons (Ji et al., 2017). However, our electrophysiology results showed that Scn4b deletion does not alter the cellular physiology of CeA neurons. While the expression of Scn4b is high in the nucleus accumbens, expression in the CeA is very low (Allen Brain Atlas, http://mouse.brain-map.org/experiment/show/73636111). Based on the low expression and our negative electrophysiological results, we propose that Scn4b does not play a role in the regulation of the membrane properties and excitability of CeA neurons. Furthermore, when we evaluated basal HICs and acoustic startle responses as behavioral indices of CNS excitability, KO mice did not differ from their WT littermates in these tests. It’s possible that Scn4b-mediated changes in excitability may be specific for brain regions and neuronal subpopulations that exhibit higher expression of the subunit (e.g., nucleus accumbens neurons), and that this could impact the behavioral effects of certain drugs of abuse, such as observed for amphetamine (Ji et al., 2017).
Previous whole brain transcriptome studies showed upregulated expression of Scn4b in mice with a genetic predisposition for high ethanol consumption (Mulligan et al., 2006; Tabakoff et al., 2008). Although expression was upregulated in ethanol-naive inbred mouse strains, it was downregulated in the selectively bred HDID mice and P rats (Suppl. Table 1); thus the expression patterns were not consistent across the different genetic models. Many other individual genes were also altered in these studies and differential changes in expression do not suggest which genes contribute to drinking risk or which are associated with other genetic perturbations or cumulative molecular effects. Although Scn4b expression correlated with ethanol drinking, ataxia, and hypothermia (Suppl. Table 2), our current findings show that genetic manipulation of Scn4b failed to regulate ethanol intake or hypothermia; however, our LORR results provide some support for the correlation with ataxia. Overall, expression-based analyses have been limited in their ability to predict single genetic determinants of complex drinking behaviors in brain. Considering that Scn4b is enriched in medium spiny dopamine neurons, changes in its expression may reflect activity or plasticity of those neurons. Thus, a change in gene expression could indicate the importance of particular cells rather than the importance of the specific gene. While there is one known isoform of Scn4b in rodents, there are three coding isoforms of SCN4B in humans. Network analysis revealed that only the shortest variant was part of a coregulated network that correlated with lifetime alcohol consumption, but it was not differentially expressed between human alcoholic and control brains (Farris et al., 2015). It is not known if this variant might function differently than the other sodium channel isoforms. Also, this gene may only be a minor player within an important network, with other genes in the network driving the behavior. We must further consider that the microarray and RNA-seq studies that suggested Scn4b as a gene of interest represent a mere snapshot of varied cell types at single time points. Because of the plasticity of individual brain cells and differing patterns of gene expression as a function of time, pinpointing the expression changes most relevant for drug behaviors will require greater temporal and cellular resolution. The discrepancy between many genomic and functional validation studies underscores the need for studying transcriptome plasticity across species, brain regions, cell types, and time periods of the addiction cycle to view the dynamic cellular changes that drive excessive ethanol consumption.
A potential limitation of our work in KO mice is that deletion of Scn4b throughout development may lead to upregulation of other β subunits, particularly Scn2b, which is expressed in some of the same brain regions as Scn4b (although at a neuronal level, Scn2b and Scn4b localization was complimentary rather than overlapping) (Yu et al., 2003). However, β2 protein levels marginally decreased in spinal cord and cerebellum in Scn4b KO mice while β1 levels were slightly upregulated in these regions (Miyazaki et al., 2014). Expression of β1 and β2 did not change in cortex and striatum, and β3 expression was not altered in any of the regions studied. Thus, only β1 was slightly upregulated in a region-dependent manner, and in situ hybridization of β1–3 subunits revealed no compensatory localization changes in Scn4b KO mice (Miyazaki et al., 2014). Another consideration is that our knockdown experiments only examined the CeA, and it is possible that knockdown in striatal or other brain regions could reduce ethanol consumption. However, this possibility is mitigated by the lack of effect of the global KO in four different drinking tests. The drinking tests we used are associated with intoxicating blood ethanol levels and model different drinking patterns in humans.
We previously used global KO or targeted genetic knockdown to test seven different neuroimmune genes that were nominated by gene expression studies and found that ethanol consumption was reduced in all of the mutants (Blednov et al., 2012; Truitt et al., 2016). These studies provided evidence that gene expression profiling can identify molecular determinants of ethanol drinking and that neuroimmune/inflammatory pathways are relevant targets. Bhandari and colleagues also successfully used gene expression data to identify an ethanol-sensitive gene and then validate that the candidate gene alters ethanol-related behaviors in mutant models (Bhandari et al., 2012). Many single genetic deletions in mice have been associated with decreased ethanol consumption (Mayfield et al., 2016), but most were based on the known function of the gene from behavioral traits. A critical and more difficult question is how to use genomic information to predict single genes that are important for ethanol consumption or other traits. The present work shows that despite the transcriptome-based evidence, experimental validation did not support a strong role for Scn4b in many of the behavioral actions of ethanol. A better alternative to the single gene approach may be to integrate expression signatures in different brain areas, species, and stages of alcohol addiction with computational, systems pharmacology approaches to identify drug targets for the altered gene networks (Ferguson et al., 2018).
Supplementary Material
Supplemental Figure 1. Knockdown of Scn4b by shRNA in Neuro-2a cells. Cells were co-transfected with lentiviral plasmids expressing an shRNA to Scn4b (shScn4b-10 or shScn4b-268) or a scrambled control shRNA (Control) and a plasmid expressing the cDNA for mouse Scn4b using lipofectamine 2000. RNA was isolated from cells 48 h after transfection, cDNA prepared using a reverse transcription kit, and expression of Scn4b transcript levels measured by quantitative real-time PCR. ****p < 0.0001 by Dunnett’s multiple comparisons post-hoc test when compared with control.
Acknowledgements
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (AA013520/INIA Project awarded to YAB and RAH; AA025479/INIA Core to RAH; AA006399 to RAH;AA010422 and AA020889/INIA Project awarded to GEH; AA020893/INIA Project to AJR; AA016654 to AWL; AA013498/INIA Project to MR; U24 AA013517/INIA Project to MB; AA013484 to RJH). The authors thank Elizabeth Osterndorff-Kahanek (Waggoner Center for Alcohol and Addiction Research, The University of Texas at Austin, USA) for data analysis and preparation of Suppl. Tables 1 and 2, and Dr. A. Szucs (Dept. Physiology and Neurobiology, Eötvös Lóránd University, Hungary; and BioCircuits Institute, University of California San Diego, USA) for the NeuroExpress software. The authors have no conflicts of interest to declare.
REFERENCES
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM (2009) Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci. 29, 2027–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A, Mueller S, Kopp K, Brueggemann A, Suessbrich H, Gerlach U, Busch AE (2002) Inhibition of cardiac potassium currents by pentobarbital. Naunyn Schmiedebergs Arch Pharmacol. 365, 29–37. [DOI] [PubMed] [Google Scholar]
- Bant JS & Raman IM (2010) Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc Natl Acad Sci U S A. 107, 12357–12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi RL (1988) Probing the molecular structure of the voltage-dependent sodium channel. Annu Rev Neurosci. 11, 455–495. [DOI] [PubMed] [Google Scholar]
- Becker HC & Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 28, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Hill JS, Farris SP, Costin B, Martin I, Chan CL, Alaimo JT, Bettinger JC, Davies AG, Miles MF, Grotewiel M (2012) Chloride intracellular channels modulate acute ethanol behaviors in Drosophila, Caenorhabditis elegans and mice. Genes Brain Behav. 11, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, Ruiz CI, Cullins MA, Da Costa A, Osterndorff-Kahanek EA, Homanics GE, Harris RA (2017) Mutation of the inhibitory ethanol site in GABAA rho1 receptors promotes tolerance to ethanol-induced motor incoordination. Neuropharmacology. 123, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA (2012) Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 17, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL (2011) Na channel beta subunits: Overachievers of the ion channel family. Front Pharmacol. 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 26, 13–25. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK (1991) Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 550, 1–6. [DOI] [PubMed] [Google Scholar]
- Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD (2015) Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry. 20, 1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson LB, Harris RA, Mayfield RD (2018) From gene networks to drugs: systems pharmacology approaches for AUD. Psychopharmacology (Berl). 235, 1635–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GS, Phelan R, Roberts MT, Homanics GE, Bergeson SE, Lopreato GF, Mihic SJ, Blednov YA, Harris RA (2003) Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. J Neurosci. 23, 8051–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ (2007) Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41). Alcohol Clin Exp Res. 31, 939–949. [DOI] [PubMed] [Google Scholar]
- Flood P & Krasowski MD (2000) Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology. 92, 1418–1425. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM (2005) Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 45, 233–244. [DOI] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC (2009) Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl). 201, 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013) Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 33, 3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Saha S, Gao G, Lasek AW, Homanics GE, Guildford M, Tapper AR, Martin GE (2017) The sodium channel beta4 auxiliary subunit selectively controls long-term depression in core nucleus accumbens medium spiny neurons. Front Cell Neurosci. 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek AW & Azouaou N (2010) Virus-delivered RNA interference in mouse brain to study addiction-related behaviors. Methods Mol Biol. 602, 283–298. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Janak PH, He L, Whistler JL, Heberlein U (2007) Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 6, 728–735. [DOI] [PubMed] [Google Scholar]
- Mayfield J, Arends MA, Harris RA, Blednov YA (2016) Genes and alcohol consumption: Studies with mutant mice. Int Rev Neurobiol. 126, 293–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Iancu OD, Spence SE, Walter NA, Oberbeck D, Harrington CA, Colville A, McWeeney S, Phillips TJ, Buck KJ, Crabbe JC, Belknap JK, Hitzemann RJ (2014) Dual-trait selection for ethanol consumption and withdrawal: genetic and transcriptional network effects. Alcohol Clin Exp Res. 38, 2915–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Oyama F, Inoue R, Aosaki T, Abe T, Kiyonari H, Kino Y, Kurosawa M, Shimizu J, Ogiwara I, Yamakawa K, Koshimizu Y, Fujiyama F, Kaneko T, Shimizu H, Nagatomo K, Yamada K, Shimogori T, Hattori N, Miura M, Nukina N (2014) Singular localization of sodium channel beta4 subunit in unmyelinated fibres and its role in the striatum. Nat Commun. 5, 5525. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Oyama F, Wong HK, Kaneko K, Sakurai T, Tamaoka A, Nukina N (2007) BACE1 modulates filopodia-like protrusions induced by sodium channel beta4 subunit. Biochem Biophys Res Commun. 361, 43–48. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE (2006) Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 103, 6368–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama F, Miyazaki H, Sakamoto N, Becquet C, Machida Y, Kaneko K, Uchikawa C, Suzuki T, Kurosawa M, Ikeda T, Tamaoka A, Sakurai T, Nukina N (2006) Sodium channel beta4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington’s disease transgenic mice. J Neurochem. 98, 18–529. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S (2013) Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol. 18, 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 32, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K, Hu W, Bhave SV, Finn DA, Grahame NJ, Hoffman PL (2008) The genomic determinants of alcohol preference in mice. Mamm Genome. 19, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt JM, Blednov YA, Benavidez JM, Black M, Ponomareva O, Law J, Merriman M, Horani S, Jameson K, Lasek AW, Harris RA, Mayfield RD (2016) Inhibition of IKKbeta reduces ethanol consumption in C57BL/6J mice. eNeuro. 3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF & Roberts AJ (2014) Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 48, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenberg HC, Wartenberg JP, Urban BW (2001) Human cardiac sodium channels are affected by pentobarbital. Eur J Anaesthesiol. 18, 306–313. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R (2003) Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 23, 7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Knockdown of Scn4b by shRNA in Neuro-2a cells. Cells were co-transfected with lentiviral plasmids expressing an shRNA to Scn4b (shScn4b-10 or shScn4b-268) or a scrambled control shRNA (Control) and a plasmid expressing the cDNA for mouse Scn4b using lipofectamine 2000. RNA was isolated from cells 48 h after transfection, cDNA prepared using a reverse transcription kit, and expression of Scn4b transcript levels measured by quantitative real-time PCR. ****p < 0.0001 by Dunnett’s multiple comparisons post-hoc test when compared with control.