Abstract
Study Objectives
Determine abnormalities in levels of iron-management proteins in neuronal origin-enriched extracellular vesicles (nEVs) in restless legs syndrome (RLS).
Methods
We used immunoprecipitation for neuronal marker L1CAM to isolate nEVs from the serum of 20 participants with RLS from a study including magnetic resonance imaging (MRI) determinations of iron deposition in the substantia nigra and hematologic parameters and 28 age- and sex-matched Controls.
Results
RLS compared with Control participants showed higher levels of nEV total ferritin but similar levels of transferrin receptor and ferroportin. Western blot analysis showed that heavy- but not light-chain ferritin was increased in nEVs of RLS compared with Control participants. In RLS but not Control participants, nEV total ferritin was positively correlated with systemic iron parameters; the two groups also differed in the relation of nEV total ferritin to MRI measures of iron deposition in substantia nigra.
Conclusions
Given the neuronal origin and diversity of EV cargo, nEVs provide an important platform for exploring the underlying pathophysiology and possible biomarkers of RLS.
Keywords: restless legs syndrome, exosomes, extracellular vesicles, iron, ferritin
Statement of Significance
This is the first study using serum extracellular vesicles to assess in vivo pathogenic processes known to be involved in restless legs syndrome (RLS). The study is based on a well-characterized cohort and findings were associated with multimodal assessments including magnetic resonance imaging and hematologic parameters. The results are novel and provide further evidence for the role of impaired iron homeostasis in RLS pathogenesis. This study can potentially motivate a line of investigation to develop blood-based biomarkers for RLS.
Introduction
Restless legs syndrome (RLS) is a sensorimotor disorder of unknown etiology characterized by a compulsive urge to move the legs to relieve uncomfortable sensations [1]. Multiple studies have implicated the presence of altered brain iron homeostasis in RLS [2], including findings of lower cerebrospinal fluid (CSF) ferritin and higher CSF transferrin [3], and lower brain iron concentration by neuroimaging [4–6]. Brain autopsy studies also demonstrated changes in iron-management proteins in the substantia nigra of RLS cases consistent with regional brain iron insufficiency [7]. Importantly, RLS and Control participants in these studies had similarly normal body iron stores as determined by serum iron-related measurements. A dissociation between systemic and brain iron homeostasis is indicated by studies employing quantitative trait loci analysis of BXD recombinant inbred strains of mice, derived from C57BL/6J and DBA/2J progenitors, that was used to quantify variation in brain iron concentration in response to iron-restricted diet; this analysis revealed high variability in brain iron concentration that was dissociable form iron intake and attributable to genomic polymorphisms [8, 9]. The dissociation between iron levels in the brain and periphery implies that existing measures of systemic iron metabolism are inadequate as biomarkers of RLS and motivates the search for biomarkers that may reflect brain iron metabolism.
Extracellular vesicles (EVs) are membranous nanoparticles that carry variable cargo of lipids, RNA, DNA, and proteins representative of their cellular origin, including neurons [10, 11]. EVs share transmembrane proteins with their cell of origin that can be used as an antigen for immunocapture. Neuronal origin-enriched extracellular vesicles (nEVs) derived from blood may provide an easily accessible and biological diverse material for in vivo assessment of brain-based pathophysiology in RLS. In our lab, we use a two-step isolation technique to, first, isolate a heterogeneous population of total extracellular vesicles and, second, enrich for neuronal origin using immunoprecipitation against neural cell adhesion molecule L1CAM [12]. So far, this technique has been used to identify biomarkers for neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease [13–18]. If nEVs are of potential value in assessing the underlying neural mechanisms in RLS, it would be expected that, at a minimum, there should be some differences in the primary iron-regulatory proteins found between RLS and Controls.
For this study, we isolated nEVs from serum obtained from RLS cases and Controls participants to determine differences in the levels of the primary iron-management proteins. In addition, we examined correlations between the individual iron-management proteins and either serum indices of peripheral iron status or brain indices of iron status in the substantia nigra as determined by magnetic resonance imaging (MRI). Although much of the secondary analysis was exploratory, few correlations were hypothesized a priori, including a correlation between nEV ferritin and serum ferritin (similar to the correlation between serum and CSF ferritin [19]) and correlations between any of the nEV iron-management proteins and MRI-determined iron in the substantia nigra.
Methods
Participants and clinical methods
This study is based on serum samples from 20 RLS and 20 age and sex-matched Control participants in an investigational study of RLS approved by the Johns Hopkins Institutional Review Board; all participants provided written informed consent. The study involved transcranial magnetic stimulation studies, and therefore participants were excluded if they did not have a strong hand preference or had significant activities involving hand movements, e.g. musicians or artists. Participants with apnea–hypopnea index > 15/hr on a screening polysomnogram or with either a diagnosis or history of any significant neurological, psychiatric, medical, or sleep disorder (e.g. peripheral neuropathy, seizure disorder, dementia, congestive heart failure, psychotic disorder, and substance abuse), other than RLS, were excluded from the study. RLS was diagnosed by both a validated structured clinical interview modified to exclude mimics (HCDI/HTDI) [20] and a clinical evaluation by a neurologist with RLS expertise. RLS patients were tapered off all RLS medication and were free of RLS medications for at least 5 nights before periodic leg movements in sleep (PLM) were measured for 5 nights prior to the sleep lab evaluation using the PAM-RL (Phillips Respironics) [21, 22]. People with RLS were rejected, if on the average of the 5 nights or the screening polysomnogram they did not have PLMS/hr > 15, and Controls were rejected, if they had PLMS/hr > 15. PLMS were scored based on the 2006 World Association of Sleep Medicine standards [23] adjusted for the PAM-RL.
Following PLM evaluation, all people with RLS were free of RLS medications for at least 11 days when RLS severity was assessed using IRLS and blood samples were collected. All participants had fasted for 12 hr prior to collection of their early-morning blood samples used in this study. Following the blood draw, RLS and Control participants underwent 7T-MRI scanning to acquire MR phase data for quantitative susceptibility mapping (QSM) based on measurement of tissue magnetic susceptibility, known to be proportional to regional tissue iron content as reported previously [24]. Basic demographics, blood biomarkers of systemic iron metabolism, and hematologic parameters for patient and Control participants, as well as clinical measures for participants with RLS used in this study are presented in Table 1.
Table 1.
Baseline demographics and clinical parameters
| RLS | JH Controls | NIA Controls | P value (RLS vs. Controls) | |
|---|---|---|---|---|
| Number of participants | 20 | 18 | 10 | – |
| International Restless Legs Scale (RLS severity) | 24.7 ±6.9 | – | – | – |
| Age (years) | 58 ± 2.1 | 57 ± 2.1 | 60 ± 2.3 | .534 |
| Sex | 13F/7M | 12F/6M | 5F/5M | .768 |
| Serum ferritin (ng/mL) | 43.2 ± 6 | 38.9 ± 6 | 63.8 ± 18 | .396 |
| Serum iron (μg/dL) | 87.3 ± 7.5 | 95.6 ± 7.5 | – | .439 |
| Total Iron Binding Capacity (TIBC) (μg/dL) | 43.2 ± 6 | 38.9 ± 6 | – | .543 |
| % saturation | 87.3±2.3 | 95.6 ± 2.3 | – | .444 |
| Hemoglobin (g/dL) | 13.6 ± .24 | 13.5 ± .24 | – | .618 |
| RDW (d/dL) | 13.39 ± .13 | 13.4 ± .13 | – | .814 |
| MCHC (g/dL) | 33.6 ± .19 | 33.8 ± .19 | – | .316 |
Variables except for sex are presented as mean ± standard deviation. P values for differences were calculated using unpaired t-test for continuous variables and Fisher’s exact test for sex (M = males; F = females).
We noticed that JH Controls had relatively low-serum ferritin levels for what could be expected for their age, although no different from levels of participants with RLS. To increase the number of Controls with participants with serum ferritin levels more typical for their age, an additional cohort of 10 Control participants were selected from the National Institute on Aging (NIA) biobank of approved studies to boost the number of Control participants. These Controls were age- and sex-matched to people with RLS, did not have a diagnosis or history of any significant neurological, psychiatric, medical, or sleep disorder, had a normal neurologic exam, and were not taking any neuroactive medications. Their samples were collected in the early morning after 12 hr of fasting. NIA Controls did not have MRI and detailed hematology measures, but did have serum ferritin data.
MRI acquisition and processing
MRI scanning was done using a 7T Achieva scanner (Philips Healthcare, Best, The Neatherlands) with a 32-channel head coil (Novamedical). Morning (08:00–10:00 am) scans were used since RLS symptoms are reduced in the morning, thereby reducing movement problems. Foam pad and straps were used to restrict head movements. The QSM-based magnetic susceptibility calculations were obtained from MR phase measurements using a 3D single-echo gradient-echo (GRE) sequence and 0.8 mm isotropic resolution as described previously [24]. QSM images were later segmented automatically with coregistration to a Hopkins Atlas and visual adjustment as needed by a neuroradiologist to derive the substantia nigra region of interest, which was further subdivided into its pars compacta and pars reticularis, among other regions of interest [24]. The central CSF region in lateral ventricles excluding choroid plexus regions which had obvious hypointensity in the QSM image, was used as the reference region to determine the final values of tissue magnetic susceptibility in different brain regions. Because the tissue magnetic susceptibility is known to be proportional to regional tissue iron content in gray matter [24], we consider these measures as MRI surrogates for regional iron.
Blood sample processing
Preanalytical factors for blood collection and storage comply with guidelines for EV biomarker analysis [25]. Blood samples from Johns Hopkins and NIA were collected in SST tubes and were centrifuged immediately to isolate serum, which was aliquoted into 0.5 mL aliquots and frozen at −80°C. All samples were randomly labeled using 5-digit codes that had no relation to clinical grouping. RLS and Control samples were then merged using 2 × 2 matrix randomization. All investigators involved in nEV isolation and analysis (S.C., S.G., and D.K.) were blinded to the sample identities during nEV isolation, protein quantifications, and statistical analyses. Two of the Control samples did not have sufficient serum for adequate nEV extraction and therefore the Johns Hopkins cohort included 20 participants with moderate-to-severe RLS and 18 Controls. The 10 additional samples from NIA Control participants were processed similarly.
nEV isolation and characterization
The methodology used to isolate EVs enriched for neuronal origin from peripheral blood has been implemented in multiple [16–18, 26, 27, 28] prior studies and has been described in detail in a methodology paper [12]. We defrosted samples once immediately prior to processing. Total EVs were precipitated with a particle precipitation solution, Exoquick (System Biosciences, Inc., Mountainview, CA). To isolate nEVs resuspended total EVs were incubated for 1 hr at 4°C with 4 μg of mouse anti-human CD171 biotinylated antibody (i.e. antibody against neural cell adhesion molecule L1CAM; clone 5G3, Thermo Scientific, Inc.) and, subsequently, 15 μL of Pierce Streptavidin Plus UltraLink Resin (Thermo Scientific, Inc.) and 25 μL 3% BSA, for 4 hr at 4°C. After centrifugation, 0.1M glycine-HCl was added to pellet containing nEVs to detach them from beads, the pH of the nEV-containing supernatant was neutralized, followed by addition of detergent solution MPER and inhibitors followed by two freeze-thaw cycles to lyse nEVs. Intact nEVs diluted 1:100 to achieve a desired range of 3–15 × 108/mL were used for nanoparticle tracking analysis (NTA) with Nanosight NS500 (Malvern, Amesbury, United Kingdom) to calculate average nEV concentration and diameter.
Quantification of nEV proteins
The three primary iron-management proteins assessed in this study were total ferritin, ferroportin, and transferrin receptor (TfR). The TfR functions as the primary cellular iron importer, whereas ferroportin functions as the primary cellular iron exporter [29]. Total ferritin, as determined by ELISA, is a combination of light-chain ferritin, which is the primary cellular iron storage protein and heavy-chain ferritin, which acts both as an iron storage and transport protein [29]. Proteins in the nEV lysate were quantified using Enzyme-Linked Immunosorbent Assays (ELISA), i.e. TfR (Cat. #ab217790; Abcam, Cambridge, MA), total ferritin (Cat. #ab200018; Abcam, Cambridge, MA), and ferroportin (Cat. #LS-F12550-1; LifeSpan Bioscience Inc, Seattle, WA). All assays were conducted in duplicate and the average coefficients of variance (CVs) were 4.6% (TfR), 6.2% (total ferritin), and 6.5% (ferroportin). All samples were diluted 1:1 with diluents provided by the manufacturers, and the concentration was corrected for this dilution. We calculated the standard curve separately for each plate using standards provided by the manufacturers. The lowest limit of quantification (LLOQ; defined as the concentration of the standard with (1) signal above the mean of the blank plus 2.5 SD of the blank, (2) CV among duplicates <20%, and (3) recovery >80% and <120%) was calculated for each assay and plate and the highest LLoQ was used as the global LLOQ for subsequent analyses. The LLoQ was 0.100 ng/mL for Transferrin Receptor, 0.334 ng/mL for total ferritin, and 0.135 ng/mL for ferroportin. For TfR all samples were above the LLoQ and within the linear range of the standard curve; therefore, all values were included in the analysis. For total ferritin, 11 samples were below LLoQ. If values for these samples were above the limit of detection (LOD = 0.02 ng/mL) and had CV < 15%, were assigned the LLoQ value (5 RLS, 5 Control samples), but if CV > 15% or below the LOD, they were excluded from the analysis (1 Control sample). For ferroportin, one sample (later determined to be RLS) was below the LLoQ, but above the LOD (.05 ng/mL) and had a CV < 15%; therefore, it was assigned the LLoQ value. In addition, plate to plate variability was assessed using nEVs from two Control participants outside the cohort as internal standards; between-plate CVs were under 10% for all assays. These criteria resulted in valid data from 20 RLS and 18 Control samples for TfR and ferroportin and from 20 RLS and 28 Control samples for ferritin.
The total ferritin ELISA showed that one group had on average higher nEV total ferritin levels than the other, although there was overlap between the two groups. Next, we sought to determine if the difference in nEV total ferritin was a result of difference in heavy, light, or both forms of ferritin through Western blot (WB) analyses. During WB experiments, NIA Investigators were still blinded to Groups identity (RLS vs. Controls). In a first experiment, we sought to maximize our ability to detect differences for the various types of ferritin by comparing a participant with extreme high and low values of total ferritin. Therefore, we selected three participants with the highest nEV total ferritin from the high-total ferritin group and three participants with the lowest nEV total ferritin from the low-total ferritin group. In a second experiment, we sought to confirm that group differences for different ferritin types were not due to selection of extreme cases. Therefore, we selected RLS and Control participants with a wide range of nEV total ferritin values: 1 RLS and 1 Control participant with total ferritin of 0.14 ng/mL (low range), 1 RLS and 1 Control participant with total ferritin of 1.06 ng/mL (mid-range), and 1 RLS and 1 Control participant with total ferritin of 2.84 ng/mL (high-range). Results from both types of approaches were similar. Separate blots were conducted for ferritin light chain (Cat# ab69090, MW: 19kDa; Abcam, Cambridge, MA) and ferritin heavy chain (Cat# sc-135667, MW: 21kDa; Santa Cruz Biotechnology, Dallas, TX), and Alix was used as a loading control for nEV concentration (Cat# sc-99010, MW: 95kDa; Santa Cruz Biotechnology, Dallas, TX). The blots were developed using LiCor QuickWestern Kit (Cat# P/N 926–68100; LiCor Biosciences, Lincoln, NE) and read using the Odyssey CLx (LiCor Biosciences, Lincoln, NE).
Statistical analyses
Analyses were performed using SPSS version 25. To assess group differences, we performed mixed model analyses of nEV biomarkers as dependent variables, with Group (A and B, later identified as RLS and Control) as fixed factor, and nEV concentration included as covariate to normalize for differential nEV yield, as previously done [30]. (nEV concentration values were significantly skewed and values were log-transformed to assure normal distribution; for all other nEV biomarkers, values were normally distributed and raw values were used in the analysis.) For significant effects of Group, t-tests of least square means (estimated marginal means) were used to determine the direction and significance of pairwise differences. (Bar graphs of nEV biomarkers depict estimated marginal means and standard errors of the mean.) In exploratory models in which age and sex were also included, these demographic variables had no significant effects and did not contribute to model fit; therefore, they were not included in the basic model. Also, follow-up exploratory models included biomarkers measured in the soluble phase of serum (e.g. for nEV ferritin, follow-up exploratory analysis included serum ferritin as covariate) to determine whether differences in nEV biomarkers may be attributed to differences in the soluble phase of serum. Finally, Spearman’s correlations (computed for both groups combined and then separately for RLS and Control participants) were used to explore possible relationships between nEV biomarkers and nEV concentration, blood parameters, and magnetic susceptibilities, proportional to iron deposition within ROIs.
Data availability statement
Anonymized data will be shared by request from any qualified investigator.
Results
Differences in iron-management proteins between RLS and Control participants
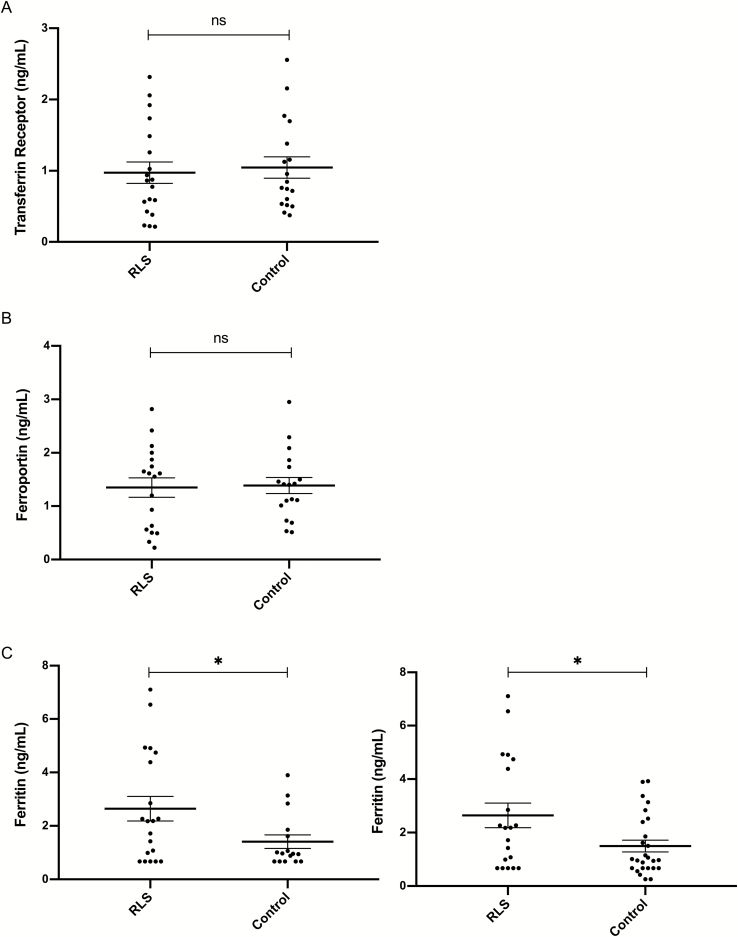
nEV TfR: There was no significant effect of Group (Figure 1A), with participants with RLS showing similar levels [1099 ± 847 pg/mL (mean ± SD)] compared with Controls (1046 ± 632 pg/mL), whereas nEV concentration was positively associated with nEV TfR values (F = 11.696, df = 35, p = 0.002). In follow-up analysis, the effect of the nEV concentration * Group interaction term was nonsignificant suggesting that the association between nEV TfR and nEV concentration did not differ between the two groups.
Figure 1.
Iron management proteins in L1CAM + nEVs. Graphs depict values (mean ± SD) of Transferrin Receptor (TfR) (A), Ferroportin (B), and Ferritin (C) in 20 RLS and 18 Control participants—except for 17 and 28 Control participants for Ferritin (C) (ns = nonsignificant). In (C) * depicts p = 0.032 in the first graph (including RLS and Control participants from JH) and p = 0.017 in the second graph (including additional Control participants from the NIA); values below the LLoQ with CV < 15% were assigned the LLoQ value. Group differences remained significant, even if we analyzed raw signal or did not set concentration values to the LLoQ.
nEV ferroportin: There was no significant effects of Group (Figure 1B) or nEV concentration, with participants with RLS showing similar levels (2.26 ± 3.2 ng/mL) compared with Controls (1.38 ± 0.6 ng/mL).
nEV total ferritin (ELISA): There was a significant effect of Group (F = 5.015, df = 34, p = 0.032), with participants with RLS showing higher levels (2.58 ± 2.1 ng/mL) compared with Controls (1.31 ± 1.1 ng/mL; p = 0.023), whereas the effect of nEV concentration was nonsignificant. In exploratory models including age and sex, Group significance increased further (F = 8.368, df = 32, p = 0.007) and sex was also highly significant (F = 14.476, df = 32, p = 0.001), with women having much lower nEV ferritin levels (1.31 ± 1 ng/mL) than men (3.13 ± 2.37 ng/mL). In an exploratory model including serum ferritin as a covariate (to rule out that group differences in nEV ferritin were attributable to serum ferritin), Group differences remained significant (F = 5.028, df = 33, p = 0.032).
In an analysis involving 20 RLS and 28 Control participants (with the addition of 10 Control participants from NIA studies), total ferritin in nEVs remained significantly higher in RLS (2.58 ± 2.1 ng/mL) compared with Control participants (1.36 ± 1.17 ng/mL) both without covarying serum ferritin (F = 6.162, df = 45, p = 0.017, Figure 1C) and with covarying serum ferritin (F = 10.023, df = 44, p = 0.003). Similarly, in an analysis involving 20 participants with RLS and 10 Control participants from NIA studies, total ferritin in nEVs remained significantly higher in RLS (2.58 ± 2.1 ng/mL) compared with Control participants (1.64 ± 1.34 ng/mL; F = 6.948, df = 26, p = 0.014).
Determination of group differences in nEV heavy- and light-chain ferritin
Based on the finding of group differences for nEV total ferritin, we conducted two WB experiments to determine whether the differences result from differences in heavy-chain, light-chain, or both forms of ferritin.
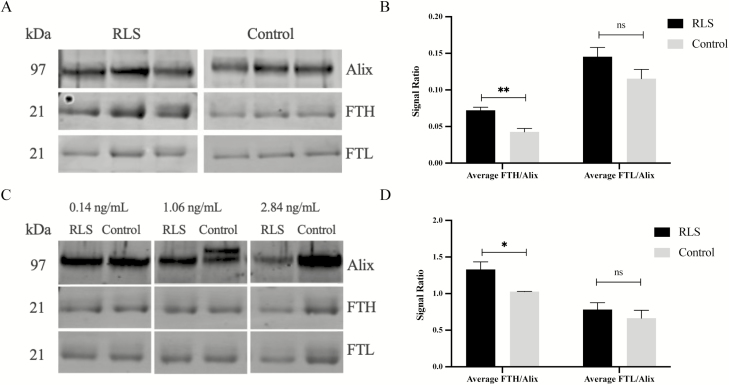
First, in an experiment aiming to maximize possible differences, we selected three participants with the highest nEV total ferritin from the RLS group and three participants with the lowest nEV total ferritin from the Control group, based on the total ferritin ELISA results. In this experiment, participants with RLS had higher levels of heavy-chain ferritin/Alix compared with Controls (p = 0.009), but similar levels of light-chain ferritin/Alix compared with Controls (p = 0.17; Figure 2, A and B).
Figure 2.
Altered Ferritin profile in L1CAM + nEVs in RLS. (A, B) People with RLS with the highest ferritin concentration and Control participants with the lowest ferritin concentrations were selected. Western blot (WB) image shows heavy-chain ferritin (FTH), light-chain ferritin (FTL), and Alix, a common nEV marker used for normalization. Normalized FTH was higher (** depicts p = 0.009) in people with RLS compared with Controls; there were no differences in FTL (ns refers to p = 0.17). (C, D) People with RLS and Controls with equivalent ferritin concentrations were selected. Normalized FTH was again higher (* depicts p = 0.04) in people with RLS compared with Controls; again there were no differences for FTL (ns refers to p = 0.45). Graphs depict mean ± SEM.
Second, we selected RLS and Control participants with a wide range of nEV total ferritin values: 1 RLS and 1 Control participant with total ferritin of 0.14 ng/mL (low range), 1 RLS and 1 Control participant with total ferritin of 1.06 ng/mL (mid-range), and 1 RLS and 1 Control participant with total ferritin of 2.84 ng/mL (high range). Similar to the first WB, participants with RLS had higher levels of heavy-chain ferritin/Alix compared with Controls (p = 0.04), but similar levels of light-chain ferritin/Alix compared with Controls (p = 0.45; Figure 2, C and D).
Intercorrelations between EV biomarkers and correlations with hematologic and systemic iron parameters
In both groups combined, nEV TfR was positively correlated with nEV concentration (ρ = 0.537, p = 0.001), negatively correlated with nEV ferroportin (ρ = −0.545, p < 0.001), showed a trend for positive correlation with nEV ferritin (ρ = 0.293, p = 0.074), and was not correlated with any serum biomarkers of iron metabolism or hematologic parameters (Table 2). In Control participants, the positive correlation between nEV TfR and nEV concentration was particularly strong (ρ = 0.765, p < 0.001), but there was only a weak nonsignificant trend in participants with RLS (ρ = 0.384, p = 0.095). Therefore, in participants with RLS, nEV TfR seems to be dissociated from their circulating concentration, although this correlational evidence is hardly definitive.
Table 2.
Intercorrelations between EV biomarkers and correlations with hematologic and systemic iron parameters
| Both groups combined | RLS participants | Control participants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | nEV Concentration (E10 particles/mL) | nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | nEV Concentration (E10 particles/mL) | nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | nEV Concentration (E10 particles/mL) | |||
| Spearman’s ρ | nEV TfR (pg/mL) | Correlation Coefficient | 1.000 | −.545 ** | .293 | .537 ** | 1.000 | −.535 * | .411 | .384 | 1.000 | −.624 ** | .222 | .765 ** |
| Sig. (2−tailed) | . | .000 | .074 | .001 | . | .015 | .072 | .095 | . | .006 | .376 | .000 | ||
| nEV Ferroportin (pg/mL) | Correlation Coefficient | −−.545** | 1.000 | −.247 | −.568 ** | −.535 * | 1.000 | −.215 | −.487 * | −.624 ** | 1.000 | −.300 | −.721 ** | |
| Sig. (2−tailed) | .000 | . | .135 | .000 | .015 | . | .363 | .030 | .006 | . | .226 | .001 | ||
| nEV Ferritin (ng/mL) | Correlation Coefficient | .293 | −.247 | 1.000 | .116 | .411 | −.215 | 1.000 | .071 | .222 | −.300 | 1.000 | .348 | |
| Sig. (2−tailed) | .074 | .135 | . | .488 | .072 | .363 | . | .767 | .376 | .226 | . | .157 | ||
| nEV Concentration (E10 particles/mL) | Correlation Coefficient | .537 ** | −.568 ** | .116 | 1.000 | .384 | −.487 * | .071 | 1.000 | .765 ** | −.721 ** | .348 | 1.000 | |
| Sig. (2−tailed) | .001 | .000 | .488 | . | .095 | .030 | .767 | . | .000 | .001 | .157 | . | ||
| Ferritin (mg/mL) | Correlation Coefficient | .231 | −.236 | (.607 ** ) .580 ** | .041 | .435 | −.202 | .782 ** | .051 | −.112 | −.268 | (.479 *) .298 | .129 | |
| Sig. (2−tailed) | .163 | .149 | .000 | .807 | .056 | .394 | .000 | .830 | .657 | .267 | (.011) .229 | .610 | ||
| Iron (μg/dL) | Correlation Coefficient | .129 | −.185 | .326 * | .148 | .173 | −.350 | .525 * | .314 | .056 | .215 | .172 | −.079 | |
| Sig. (2−tailed) | .442 | .259 | .046 | .376 | .466 | .130 | .018 | .177 | .826 | .378 | .496 | .756 | ||
| %Saturation | Correlation Coefficient | .189 | −.176 | .343 * | .188 | .302 | −.391 | .508 * | .365 | .034 | .230 | .224 | −.020 | |
| Sig. (2-tailed) | .255 | .284 | .035 | .259 | .196 | .088 | .022 | .113 | .893 | .343 | .372 | .938 | ||
| Hemoglobin (g/dL) | Correlation Coefficient | .111 | −.103 | .431 ** | −.018 | .224 | −.085 | .567 ** | −.083 | −.043 | .018 | .258 | .075 | |
| Sig. (2-tailed) | .508 | .532 | .007 | .914 | .342 | .723 | .009 | .729 | .864 | .943 | .301 | .766 | ||
| RDW (d/dL) | Correlation Coefficient | .006 | .162 | −.169 | −.165 | .021 | .255 | −.405 | −.057 | −.054 | .099 | .055 | −.331 | |
| Sig. (2-tailed) | .972 | .324 | .311 | .322 | .930 | .278 | .077 | .811 | .831 | .687 | .828 | .179 | ||
| MCHC (g/dL) | Correlation Coefficient | −.110 | −.171 | −.103 | .163 | −.074 | −.200 | −.062 | .162 | −.128 | −.175 | .106 | .256 | |
| Sig. (2-tailed) | .511 | .298 | .540 | .328 | .756 | .397 | .795 | .494 | .614 | .473 | .676 | .304 | ||
Spearman’s ρ and 2-tailed significance are listed for both groups combined and for separate groups. Significant correlations (p < 0.05) are in bold font; trends for significant correlations (0.1 < p < 0.05) are in bold and italic font (* depicts p < 0.05; ** depicts p < 0.01). Parentheses indicate correlations including the NIA cohort of additional Controls.
In both groups combined, nEV ferroportin was negatively correlated with nEV concentration (ρ = 0.568, p < 0.001), negatively correlated with nEV TfR (ρ = −0.545, p < 0.001; Table 2), and was not correlated with nEV ferritin, serum biomarkers of iron metabolism, or hematologic parameters.
In both groups combined, nEV total ferritin was not correlated with nEV concentration, nEV TfR, or nEV ferroportin, but was positively correlated with serum ferritin (ρ = 0.58, p < 0.001 in RLS and Control participants from JH; ρ = 0.607, p < 0.001 including Control participants from the NIA), %saturation (ρ = 0.343, p = 0.035), iron (ρ = 0.326, p = 0.046), and hemoglobin (ρ = 0.431, p = 0.007). In participants with RLS, nEV total ferritin showed a trend for positive correlation with nEV TfR (ρ = 0.411, p = 0.072) and was positively correlated with serum ferritin (ρ = 0.782, p < 0.001), %saturation (ρ = 0.508, p = 0.022), iron (ρ = 0.525, p = 0.018), and hemoglobin (ρ = 0.567, p = 0.009). In Control participants, nEV total ferritin was correlated with serum ferritin (ρ = 0.479, p = 0.011 including Control participants from the NIA), but not with nEV concentration, nEV TfR, nEV ferroportin, or other serum biomarkers of iron metabolism or hematologic parameters. Overall, the pattern that emerges is that, in participants with RLS, nEV ferritin is closely related to parameters of systemic iron metabolism, but there is no such relationship in Control participants.
Correlations between EV biomarkers and magnetic susceptibility in the substantia nigra
nEV TfR and nEV ferroportin showed no correlations with regional magnetic susceptibility in the substantia nigra (Table 3). In both groups combined, nEV total ferritin showed a trend for positive correlation with magnetic susceptibility in the substantia nigra pars compacta only (ρ = 0.34, p = 0.053). In participants with RLS, nEV total ferritin had no correlations with regional magnetic susceptibility in the substantia nigra. In Controls, nEV total ferritin showed a trend for positive correlation with magnetic susceptibility in the substantia nigra (ρ = 0.482, p = 0.058), which reached significance for its pars reticularis (ρ = 0.524, p = 0.037). Therefore, in Controls, nEV total ferritin was significantly related to magnetic susceptibility/iron deposition in substantia nigra, but this relationship was not present in RLS.
Table 3.
Correlations between EV biomarkers and magnetic susceptibility within the substantia nigra
| Both groups combined | RLS participants | Control participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | nEV TfR (pg/mL) | nEV Ferroportin (pg/mL) | nEV Ferritin (ng/mL) | |||
| Spearman’s ρ | Substantia nigra susceptibility (ppm) | Correlation Coefficient | .103 | −.115 | .242 | .282 | −.108 | .118 | −.253 | .088 | .482 |
| Sig. (2-tailed) | .570 | .517 | .175 | .273 | .680 | .653 | .345 | .736 | .058 | ||
| Substantia nigra pars compacta susceptibility (ppm) | Correlation Coefficient | .161 | −.007 | .340 | .414 | −.120 | .228 | −.256 | .167 | .353 | |
| Sig. (2-tailed) | .369 | .966 | .053 | .098 | .646 | .379 | .339 | .523 | .180 | ||
| Substantia nigra pars reticularis susceptibility (ppm) | Correlation Coefficient | .040 | −.135 | .229 | .189 | −.088 | .125 | −.344 | .017 | .524 * | |
| Sig. (2-tailed) | .823 | .448 | .201 | .468 | .736 | .633 | .192 | .948 | .037 | ||
Spearman’s ρ and 2-tailed significance are listed for both groups combined and for separate groups. Significant correlations (p < 0.05) are in bold font; trends for significant correlations (0.1 < p < 0.05) are in bold and italic font (* depicts p < 0.05; ** depicts p < 0.01).
Discussion
RLS has been firmly associated with altered brain iron metabolism [2]. However, our ability to study altered brain iron metabolism in RLS patients in vivo is, in part, hampered by the investigational tools available. CSF samples are not easily obtainable and MRI measures of brain iron relate only to regional ferritin-bound iron, thereby limiting their scope of investigation to a narrow aspect of iron management abnormalities in RLS. The presence of iron-management proteins in EVs [12, 31] raised the possibility of probing neuronal iron management using circulating nEVs. This study confirmed the presence of the key iron-management proteins in nEVs and demonstrated higher heavy-chain ferritin in RLS compared with Control participants. This finding supports our primary hypothesis indicating that nEV may be of value as investigational tools for RLS.
In the last 18 years, there have been a multitude of studies that have utilized various techniques to determine the role of brain iron metabolism in RLS. The current consensus is that idiopathic RLS is associated with a relative brain iron insufficiency [2]. The current study, however, found that nEV total ferritin was increased, not decreased in RLS. One interpretation of this finding is that increased nEV ferritin in RLS reflects increased neuronal export of ferritin that may contribute to decreased brain ferritin. Ferritin is known to be secreted through secretory lysosomes and the MVB-exosome pathway [32] and was previously found in human urinary exosomes [33, 34]. The two WB experiments generated the highly consistent finding that, while nEVs contain both heavy- and light-chain ferritin, only heavy-chain ferritin accounts for the higher levels of total ferritin in nEVs from RLS compared with Control participants. Of note, heavy-chain ferritin was also specifically decreased in RLS compared with Controls in the substantia nigra and choroid plexus of autopsy cases, without corresponding differences in light-chain ferritin [7, 35, 36]. Thus, the specific increase in nEV heavy-chain ferritin in RLS could contribute to the reduction of heavy-chain ferritin in the brain.
From previous studies of nEVs in Alzheimer’s disease, we were accustomed to expect the direction of differences to match that of autopsy studies (e.g. insulin receptor substrate-1 [IRS-1] [28] or lysosomal enzymes [17]) and interpreted this to mean that the content of nEVs matches the neuronal intracellular content. In line with the interpretation of nEV cargo, in Controls, nEV ferritin was significantly associated with magnetic susceptibility/iron deposition in the substantia nigra; in essence, brain regional iron deposits were associated with the levels of the main iron storage protein. This expectation was refuted in people with RLS who showed no associations between magnetic susceptibility in the substantia nigra and nEV ferritin or other regulatory proteins, suggesting a dissociation between nEV cargo and intraneuronal content, at least for this region. Moreover, in the present study, the direction of the abnormality in nEVs was the opposite of what was previously observed on brain autopsy studies [7, 35, 36]. One plausible explanation for this surprising finding is that, in RLS, excessive levels of heavy ferritin-bound iron may be loaded into nEVs leading to the intraneuronal iron depletion observed in autopsy studies of RLS. Intracellularly, iron (and other metals) is associated with proteins implicated in neurodegenerative disease pathogenesis (such as prion protein, amyloid precursor protein, and a-synuclein), which are known to be secreted in EVs [31]. Therefore, excessive iron efflux may occur from neurons that need to excrete potentially hazardous protein cargo leading to intraneuronal iron depletion. This hypothesis, however, requires further testing in studies examining the neurodegenerative protein cargo in nEVs from patients with varying degrees of RLS severity. We also found that, in RLS, nEV ferritin did not correlate with brain iron deposition in SN by MRI, further suggesting dysregulated iron export via nEVs independent of neuronal iron stores. A hint for an alternative explanation is provided by the fact that, in RLS but not Control participants, nEV total ferritin and serum ferritin and hemoglobin were positively correlated. A similar positive correlation was previously seen between serum and CSF ferritin levels [37]. This suggests that, in RLS, brain iron regulation may lose its independence from systemic iron regulation and brain iron levels may more closely reflect peripheral stores.
TfR is the primary importer of iron into cells and its expression is regulated by intracellular iron concentration [38]. In reticulocytes, TfR has been shown to be secreted with exosomes and is known to interact with the ESCRT accessory protein Alix during its sorting into MVBs [39]. Sorting TfR into endosomes is also a mechanism of trafficking iron into the cytoplasm [40]. In this study, we showed the presence of TfR in nEVs and its association with nEV concentration, especially, in Control participants. These observations suggest that the production of nEVs may be normally regulated by TfR to help maintain cellular iron homeostasis and that this regulation may be disrupted in RLS. Testing these mechanistic hypotheses is beyond the scope of the present study and requires studies in cellular and animal models in which iron availability, various sources of neuronal stress, and the cellular machinery for EV production and release are simultaneously manipulated.
This study provides further support for utilizing nEVs to probe diverse brain pathologies including diseases without pathogenic protein accumulation, such as RLS. However, the technique has limitations including the presence of L1CAM in non-neural tissues resulting in a subpopulation of mixed origin. Moreover, there was substantial overlap of nEV ferritin levels between groups and the differences were not diagnostic. Moreover, the study was limited by its small number of participants with moderate to severe RLS (with only 8 out of 20 having late age-of-onset of RLS [>45 years] [41]), which prevents generalizability of the findings for other features of RLS, such as the distinction between early- and late-onset RLS. Given the small number of RLS participants, we did not have the statistical power to further subdivide them into subgroups by age-of-onset for RLS or by sex. These limitations should be addressed in future larger studies. However, this study provides tantalizing new evidence for the role of iron regulation in RLS pathogenesis and can inform intriguing new hypotheses. For a disease without existing blood biomarkers, these findings are novel and open the road for further investigation in larger cohorts to determine performance in diagnostic classification.
Funding
This study was supported in part by National Institutes of Health (NIH) grants R01NS075184 (Allen, Earley, and Li), R01NS101283 (Earley, Allen, Van Zijl, and Li), and P41 EB015909 (van Zijl and Li). This research was supported in part by the Intramural Research Program of the National Institute on Aging and NIH.
Conflict of interest statement. None declared.
References
- 1. Earley CJ. Clinical practice. Restless legs syndrome. N Engl J Med. 2003;348(21):2103–2109. [DOI] [PubMed] [Google Scholar]
- 2. Earley CJ, et al. Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis–Ekbom Disease). Sleep Med. 2014;15(11):1288–1301. [DOI] [PubMed] [Google Scholar]
- 3. Earley CJ, et al. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–1700. [DOI] [PubMed] [Google Scholar]
- 4. Allen RP, et al. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56(2):263–265. [DOI] [PubMed] [Google Scholar]
- 5. Earley CJ, et al. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7(5):458–461. [DOI] [PubMed] [Google Scholar]
- 6. Rizzo G, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Disord. 2013;28(13):1886–1890. [DOI] [PubMed] [Google Scholar]
- 7. Connor JR, et al. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134(Pt 4):959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jellen LC, et al. Systems genetics analysis of iron regulation in the brain. Biochimie. 2009;91(10):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jellen LC, et al. Systems genetic analysis of the effects of iron deficiency in mouse brain. Neurogenetics. 2012;13(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellingham SA, et al. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fauré J, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. [DOI] [PubMed] [Google Scholar]
- 12. Mustapic M, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winston CN, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst). 2016;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi M, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128(5):639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapogiannis D, et al. Pathogenic proteins in neurally-derived blood exosomes as diagnostic and prognostic biomarkers for Alzheimer’s disease. Ann Neurol. 2014;76:S92–S92. [Google Scholar]
- 16. Goetzl EJ, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30(12):4141–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goetzl EJ, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetzl EJ, et al. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol. 2015;2(7):769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Earley CJ, et al. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep. 2005;28(9):1069–1075. [DOI] [PubMed] [Google Scholar]
- 20. Hening WA, et al. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9(3):283–289. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi M, et al. The validity of the PAM-RL device for evaluating periodic limb movements in sleep and an investigation on night-to-night variability of periodic limb movements during sleep in patients with restless legs syndrome or periodic limb movement disorder using this system. Sleep Med. 2014;15(1):138–143. [DOI] [PubMed] [Google Scholar]
- 22. Gschliesser V, et al. PLM detection by actigraphy compared to polysomnography: a validation and comparison of two actigraphs. Sleep Med. 2009;10(3):306–311. [DOI] [PubMed] [Google Scholar]
- 23. Zucconi M, et al. ; International Restless Legs Syndrome Study Group (IRLSSG). The official world association of sleep medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006;7(2):175–183. [DOI] [PubMed] [Google Scholar]
- 24. Li X, et al. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Witwer KW, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiandaca MS, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11(6):600–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goetzl EJ, et al. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018;32(2):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapogiannis D, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29(2):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr. 2000;20:627–662. [DOI] [PubMed] [Google Scholar]
- 30. Eitan E, et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell. 2017;16(6):1430–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellingham SA, et al. The secret life of extracellular vesicles in metal homeostasis and neurodegeneration. Biol Cell. 2015;107(11):389–418. [DOI] [PubMed] [Google Scholar]
- 32. Truman-Rosentsvit M, et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. 2018;131(3):342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Principe S, et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics. 2013;13(10-11):1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dear JW, et al. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013;13(10-11):1572–1580. [DOI] [PubMed] [Google Scholar]
- 35. Connor JR, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304–309. [DOI] [PubMed] [Google Scholar]
- 36. Connor JR, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62(9):1563–1567. [DOI] [PubMed] [Google Scholar]
- 37. Mizuno S, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. [DOI] [PubMed] [Google Scholar]
- 38. Ponka P, et al. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31(10):1111–1137. [DOI] [PubMed] [Google Scholar]
- 39. Géminard C, et al. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5(3):181–193. [DOI] [PubMed] [Google Scholar]
- 40. Mayle KM, et al. The intracellular trafficking pathway of transferrin. Biochim Biophys Acta. 2012;1820(3):264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allen RP, et al. Family history study of the restless legs syndrome. Sleep Med. 2002;3 (Suppl):S3–S7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.