Abstract
Sleep optimizes waking behavior, however, waking experience may also influence sleep. We used the fruit fly Drosophila melanogaster to investigate the relationship between visual experience and sleep in wild-type and mutant flies. We found that the classical visual mutant, optomotor-blind (omb), which has undeveloped horizontal system/vertical system (HS/VS) motion-processing cells and are defective in motion and visual salience perception, showed dramatically reduced and less consolidated sleep compared to wild-type flies. In contrast, optogenetic activation of the HS/VS motion-processing neurons in wild-type flies led to an increase in sleep following the activation, suggesting an increase in sleep pressure. Surprisingly, exposing wild-type flies to repetitive motion stimuli for extended periods did not increase sleep pressure. However, we observed that exposing flies to more complex image sequences from a movie led to more consolidated sleep, particularly when images were randomly shuffled through time. Our results suggest that specific forms of visual experience that involve motion circuits and complex, nonrepetitive imagery, drive sleep need in Drosophila.
Keywords: sleep, visual behaviour, Drosophila, optogenetics
Statement of Significance.
How is sleep affected by experiences we have during wakefulness? In this study we found that flies that cannot detect motion, and also have attention-like defects, have a reduced need for sleep. In contrast, activating neurons involved in detecting motion led to an increased need for sleep. Interestingly, showing flies repetitive motion stimuli had no effect on their sleep, whereas showing flies complex and surprising image sequences led to more consolidated sleep. This suggests that visual attention-like processes, which are usually required when there is more visual clutter or an element of surprise, may drive the need for sleep.
Introduction
Sleep is universal, yet it is still a matter of debate why animals need it and how sleep is regulated in different brains. Sleep need varies among species, depends on age and environment, and differs greatly among individuals of the same species [1]. The reasons for variation in sleep need are unclear, but may relate to distinct sleep functions that are homeostatically regulated. Sleep is crucial for learning, attention, and memory formation [2, 3], and these processes appear to be conserved among animals, as sleep maintains attention processes even in the fruit fly, Drosophila melanogaster [4]. Sleep is a homeostatic process, which adjusts to experiences during wakefulness by driving plasticity processes during sleep [5–8]. The degree to which an animal interacts with its environment when it is awake may thus affect the quantity and quality of its sleep, and understanding this relationship may help elucidate sleep functions.
How is sleep affected by waking experience? Studies in humans and rodents have shown that extended wakefulness increases sleep pressure, measurable in the slow-wave activity characteristic of non-rapid eye movement sleep [8, 9]. The quality of the waking experience is also important, with exploratory behavior, exposure to novel objects or training in motor tasks in rodents increasing slow-wave sleep [9–11]. In humans, slow-wave activity increases in specific brain regions following working memory, visual perception, or visuomotor tasks, and in some cases is correlated with improved performance [12–15]. Likewise, rapid eye movement sleep is also affected by recent events experienced during wakefulness, most obviously in the content of our dreams. Thus, in vertebrate species it seems that different sleep stages can be altered by the quality of waking experience.
Sleep research on the fruit fly, Drosophila, has also shed light on how waking experience affects sleep need. Depriving flies of sleep causes them to subsequently sleep more and deeper [16–18]. Again, the quality of the experience also matters: flies sleep more after increased social interaction [19, 20], or following a “richer” waking experience with social, mechanical, and visual cues [21]. In other cases, sleep can be suppressed by environmental factors that compete with the need for sleep, including starvation [22] and sexual arousal [23, 24]. However, there is still little known about how a fly’s visual perception of its environment influences its sleep architecture. One way this can be addressed in Drosophila is by examining sleep in flies exposed to different visual stimuli, and in mutant flies with defective vision.
Visual perception relies on interpretation of color, contrast, and motion [25]. One of the best-studied visual mutants in Drosophila is optomotor-blind (omb): these mutants cannot perceive motion due to failed development of motion-sensitive neurons [26, 27]. Interestingly, though omb mutants fail to see wide-field motion stimuli they still respond to small moving objects by orienting toward them (“object tracking” or “fixation”) [27, 28]. Despite extensive investigation into the visual behavior of omb mutants, whether they have sleep defects that relate to their altered visual perception is unknown.
Here, we investigated the relationship between sleep and visual behavior. We first characterized sleep and visual behavior in omb flies, which lack motion-detection pathways. Next, we examined sleep in flies that had motion circuits chronically activated. Finally, we looked at whether distinct types of visual stimuli affect sleep, by exposing wild-type flies to simple and complex visual stimuli during the day, and examining sleep following visual exposure.
Materials and Methods
Animals
Flies were cultured at 25°C, 50%–60% humidity on standard medium, also used in sleep experiments (agar, yeast, sugar, water, nipagen, propionic acid) under a 12 hour light to 12 hour dark cycle. Canton-S (CS) flies were used as the control wild-type strain. The ombH31 mutant (kindly provided by Martin Heisenberg, University of Würzburg, Germany) was outcrossed five times into the CS background. Each outcross was performed by (1) crossing ombH31/ombH31 virgin females to CS males followed by (2) crossing the resulting ombH31/+ female and ombH31/y male progeny, followed by (3) selecting the ombH31/ombH31 (1 × outcrossed) progeny. ombH31/ombH31 progeny from each outcross were selected based on phenotyping flies for optomotor responses to 16 Hz gratings, with ombH31/ombH31 progeny completely lacking an optomotor response compared to their ombH31/+ siblings who showed a strong optomotor response (see Figure 2F, right panel). The deficiency mutations, df(1)rb5 and df(1)rb13B, were kindly provided by Gert Pflugfelder (Johannes Gutenberg Universität Mainz, Germany). The R27B03-Gal4 (#49211) and 3A-Gal4 (#51629) lines were obtained from Bloomington Stock Centre, Indiana. The UAS-Chrimson (P[20xUAS-IVS-CsChrimson.mVenus]attp18) strain was a gift from Vivek Jayaraman, Janelia Farm Research Campus, United States. Gal4/+ and UAS-Chrimson/+ strains were produced by crossing the Gal4 and UAS-Chrimson strains to w1118.
Sleep
Female virgins were collected and grown at 25°C in groups of approximately 20–30 flies per vial prior to experiment. When flies had reached 3–5 days of age, they were collected under CO2 anesthesia (at least 20 hours prior to experiment) and placed into individual glass tubes (Trikinetics, Waltham, MA) with food at one end and cotton wool at the other. Locomotion was measured using the Drosophila ARousal Tracking (DART) system [29]. Sleep was defined as a period of inactivity lasting 5 minutes or longer, as this has been shown to correspond with increased arousal thresholds [16, 17, 30]. For examining the effects of visual stimuli on sleep, flies were first allowed to adapt in the DART setup for more than 24 hours, with a constant blue stimulus (8 am–8 pm). Following adaptation, visual stimuli (e.g. movie scene or moving gratings) were presented to flies from 8 am to 8 pm on successive days, with the order of the visual stimuli scrambled across different experiments. For optogenetic experiments, flies were grown on 0.2 μM of all-trans-retinal (ATR) (or control food, without ATR) during the experiment and at least 1 day prior. Flies were exposed to low-intensity white light from 8 am to 8 pm throughout the experiment, with additional red light illumination during Chrimson activation using Red-Orange LEDs (Luxeon Rebel, 617 nm, 700 mA, Phillips LXM2-PH021-0070) as previously [31]. Flies were illuminated with 9.5 µW/mm2 from four light-emitting diode (LED) arrays. All Chrimson activation experiments were performed twice (and confirmed to have consistent effects on sleep) and data from the two experiments were pooled.
Visual behavior
We used a modified version of Buridan’s paradigm [32] as described previously [4, 33]. Briefly flies with clipped wings walked freely on a platform surrounded by a water-filled moat (preventing escape). All experiments lasted 3 minutes, and during optomotor experiments the direction of the grating (clockwise or anticlockwise) was switched after 1.5 minutes. Visual stimuli were presented on the walls of the arena, which consisted of 6 LED panels of green (520 nm) and blue (468 nm) LEDs that formed a hexagon. Open-source tracking software was used to record the position of the fly using a camera above the arena [34].
Visual stimuli
LED panels consisted of 1024 individual LED units (32 rows by 32 columns), controlled by LED Studio software (Shenzen Sinorad, Medical Electronics, Shenzen, China), with a 200 Hz refresh rate not visible to the flies. During sleep experiments, visual stimuli were presented on panels of blue LEDs (~500 lux, 468 nm), placed on both sides of the flies, slanted approximately 30° inward such that they were above the flies. For simple visual experiments, the control condition consisted of homogenous blue light, and motion stimuli were presented as vertically moving gratings (moving up on one side and down on the other) of dark and blue stripes 9° in width moving at a temporal speed of 3 Hz (54° per second) or 16 Hz (288° per second). These stimuli were created in Vision Egg software [35], written in Python programming language. For experiments exposing flies to movies, a 90 second action sequence from the movie Terminator 2: Judgement Day was compressed into 32 × 32 pixels in blue scale, and was presented continuously on repeat at a frame rate of 16 Hz (from 8 am to 8 pm). Movies were presented as a spatially shuffled version in which all pixel positions were randomized, a temporally shuffled version in which the temporal sequence of movie frames was randomized, or as a normal movie sequence. Movie sequences were compressed, converted into blue scale, and shuffled using Mathematica programming language.
Data analyses
Visual behavior was analyzed with CeTran (3.4) software [34], and with custom-made scripts in R programming language, as previously [4, 33]. Briefly stripe deviation was calculated as the smallest angle between the fly’s trajectory and either of the vertical objects [34], whereas optomotor responses represented the angular velocity of the fly (turning angle/second) moving in the grating direction. DART software was used to analyze sleep, and statistical analyses were performed using Prism, R, and MATLAB software. Lilliefors tests confirmed normal distribution of the data. t test, Mann–Whitney U-test, One-way analysis of variance (with Tukey’s multiple comparisons) or Kruskal–Wallis multiple comparisons were used to detect significant differences between groups.
Results
omb mutants sleep much less than wild-type flies
As in humans and other animals, visual perception in Drosophila involves detection of color, motion, and changes in luminance [25]. It is still unclear which of these visual-processing pathways might contribute to experience-dependent changes in sleep. We hypothesized that motion processing would be particularly important for several reasons: (1) motion is a highly salient feature of vision that invariably drives strong responses to turn in the same direction of motion (optomotor response), (2) motion detection engages much of the fly brain, and therefore may require increased homeostatic functions of sleep, (3) sleep-dependent changes in dendritic structure in Drosophila have been shown to occur in the horizontal system (HS) and vertical system (VS) motion-processing neurons [21].
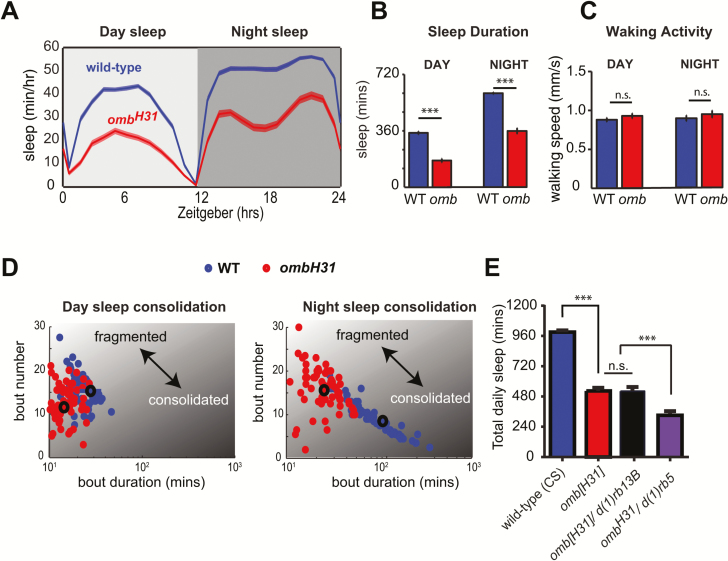
If motion detection contributes to sleep need, blocking it should result in reduced sleep. We turned to a classical visual response mutant, ombH31, which lacks HS and VS neurons and correspondingly has impaired motion responses [26, 27]. We first outcrossed the ombH31 mutation to our wild-type (CS) background, which was used as the control strain (see Materials and Methods section), and then examined sleep in these mutants. Interestingly, we found that ombH31 mutants slept much less than wild-type flies (Figure 1, A and B). However, walking speed during wakefulness was not affected in ombH31 mutants, suggesting that they did not simply have locomotor defects (Figure 1C). We next examined sleep bout number and sleep bout duration, with fewer and longer sleep bouts indicating more consolidated sleep, whereas more frequent and shorter sleep bouts indicated more fragmented sleep (Figure 1D). Interestingly, this analysis revealed that omb mutants have dramatically different sleep patterns compared to wild-type flies, with more fragmented sleep particularly at night (Figure 1D, right panel). Notably, where wild-type flies showed a strong correlation between sleep bout number and sleep bout duration at night (r = −0.8593, p < 0.0001), this effect was absent in ombH31 mutants (r = 0.0125, p = 0.9194). Together, these results show that ombH31 mutants have reduced sleep and altered sleep architecture, raising the possibility that sleep need may depend in part on visual information processing in motion circuits of the fly eye.
Figure 1.
Optomotor-blind mutants have reduced and fragmented sleep. (A) Sleep across 24 hours in ombH31 and wild-type (CS) flies. (B) Total sleep duration during the day and the night. (C) Average walking speed during wakefulness. (D) Scatterplots depicting day sleep consolidation (left panel) and night sleep consolidation (right panel), where consolidated sleep is indicated by high bout duration and low bout number, whereas fragmented sleep is indicated by low bout duration and high bout number. Averaged data points for each condition are indicated by larger circles with black outline. (E) Total sleep averaged across 24 hours in wild type, ombH31, ombH31/d(1)rb13B, and ombH31/d(1)rb5 flies. n = 60 flies per group for all panels. ***p < 0.001, by t tests in (B and C) and one-way analysis of variance with Tukey’s multiple comparisons in (E). Error bars show the SEM.
Next, we confirmed that the sleep defects in omb mutants are indeed linked to defects in visual circuits. The ombH31 mutation is a genetic inversion that disrupts the optic lobe regulatory (OLR) regions upstream of the omb gene, preventing development of primarily the HS and VS neurons [27, 36] (Figure 2A). However, in addition to the HS/VS neuronal defects, ombH31 mutants also have reduced omb expression in inner optic chiasm giant glia cells that support the normal development of the inner-optic-chiasm (IOC), a region that connects the medulla, lobula, and lobula plate of the Drosophila visual system [37]. It has previously been shown that when the ombH31 mutation is placed in trans-heterozygosity with the deficiency mutations, df(1)rb5 and df(1)rb13B, which disrupt only part of the same OLR region, the IOC develops normally whereas the motion-processing cells still do not develop [36, 37]. We found that ombH31/deficiency heterozygotes had sleep defects comparable to ombH31 mutants (Figure 1E), suggesting that the reduced sleep phenotype observed in ombH31 mutants are likely due to defective HS/VS neurons, rather than other brain abnormalities.
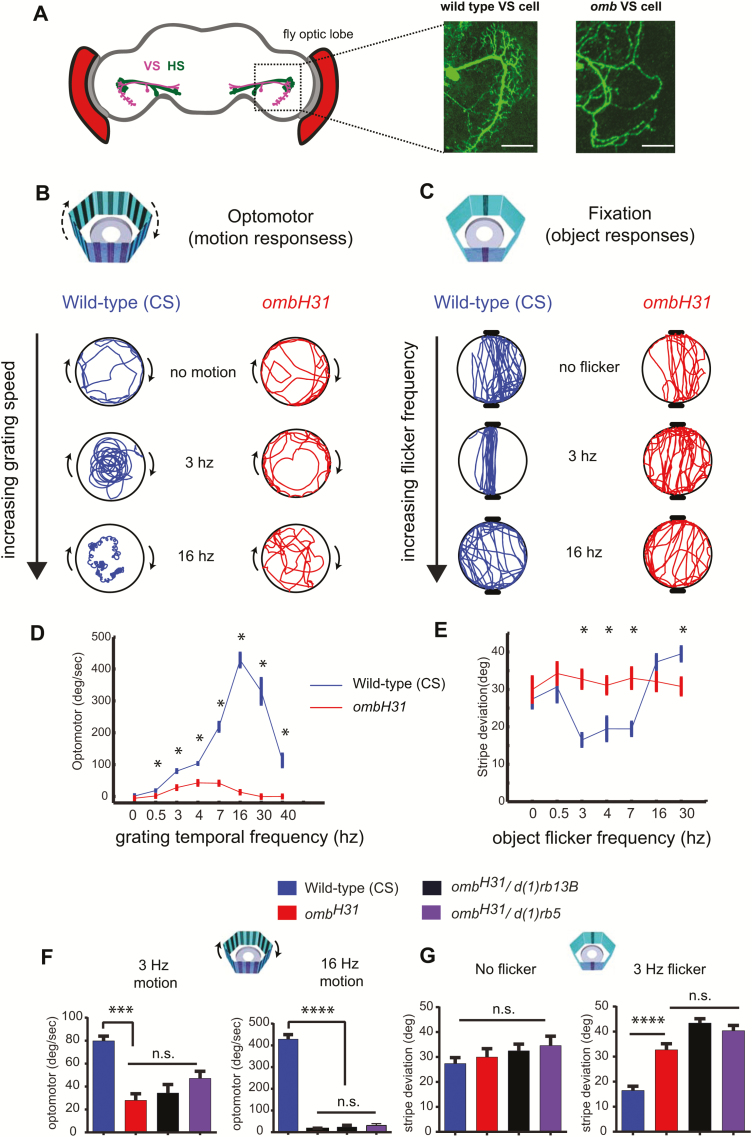
Figure 2.
Visual responses to motion and objects in wild-type and optomotor-blind flies are modulated differently by temporal frequency. (A) HS and VS neurons reside in the lobula plate of the fly optic lobe, and detect horizontal and vertical motion, respectively (left panel). Example images of a normal VS cell in a wild-type fly, and an undeveloped VS cell in an ombH31 mutant (right panel). Scale bar = 25 µm. (B and C) Example responses of wild-type and ombH31 flies to temporal frequencies of motion (B) and object flicker (C). (D and E) Quantification of optomotor responses (D) and object fixation behavior (E) in wild-type and ombH31 mutants. (F) Optomotor responses to 3 Hz (left panel) or 16 Hz (right panel) moving gratings in wild type, ombH31, ombH31/d(1)rb13B, and ombH31/d(1)rb5 flies. (G) Object fixation for stationary objects (left panel) or 3 Hz flickering objects (right panel) in wild type, ombH31, ombH31/d(1)rb13B, and ombH31/d(1)rb5 flies. *p < 0.05, t tests between wild-type and ombH31 mutant at each temporal frequency in (D and E) and ***p < 0.001, ****p < 0.0001, one-way analysis of variance with Tukey’s multiple comparisons in (F and G). n = 10 flies per condition in (D and E) and 10 flies per group in (F and G). Error bars show the SEM.
Visual phenotypes in omb flies
To better understand the visual defects of omb flies, we next performed a thorough characterization of visual behavior in omb and wild-type flies in a recently developed arena for freely walking flies [4, 32], based on Buridan’s paradigm [32]. In our visual arena, we examined optomotor responses (tendency to turn in the same direction as a moving grating), and fixation behavior (orientation and walking back and forth between two objects), and looked at changes in visual responses of wild-type and omb mutants as we altered grating speed and object flicker frequency (Figure 2, B and C). Optomotor responses were examined across a range of temporal frequencies, as it has previously been shown that flies have different behavioral and neural responses to different motion frequencies [38–41]. In our free-walking paradigm, wild-type flies responded robustly to a 3 Hz grating (Figure 2B, wild type, “3 Hz”), consistent with a previous study showing that optomotor responses in tethered walking flies were strongest at a temporal frequency of 3 Hz [38]. However, we found that unlike tethered flies, the peak in optomotor responses in freely walking flies was shifted to higher frequencies, with the strongest response evoked by a 16 Hz grating (Figure 2D, blue trace). As expected, ombH31 mutants had much lower responses in general, with weak responses to 3–7 Hz gratings, and responses close to 0 at the wild-type peak, 16 Hz (Figure 2, B and D, red traces).
We next characterized object fixation in wild-type and omb mutants. We used visual flicker to add salience to the otherwise static objects, as a fly’s attention can be drawn toward an object that is moved or oscillated [27, 42, 43]. Previous experiments suggested that omb flies may be unable to fixate on objects [26, 44, 45], although these defects were debated and somewhat dependent on the type of behavioral paradigm used [27]. We found that fixation performance depended on the flicker frequency, much like optomotor performance depended on the speed of the moving grating (Figure 2, C and E). Wild-type flies appeared to be most responsive to objects flickering between 3–7 Hz, compared to lower or higher frequencies (Figure 2C, blue traces, and Figure 2E, blue traces showing that stripe deviation is lower, indicating better fixation, between 3–7 Hz). Interestingly, ombH31 mutants responded like wild-type flies to non-flickering objects but failed to display stronger responses to 3-7Hz flickering objects (Figure 2, C and E, red traces), suggesting that motion-detection circuits may be required to derive salience information from flicker. This suggests that HS/VS motion-processing circuits also convey information about visual salience in the frequency domain for visual objects.
We next confirmed the ombH31 visual defects of omb mutants by placing the ombH31 mutation in trans-heterozygosity with the deficiency mutations, df(1)rb5 and df(1)rb13B, in which as mentioned earlier, the IOC develops normally whereas the motion-processing cells still do not develop [36, 37]. We found that ombH31/deficiency heterozygotes were still defective in detecting motion (Figure 2F), and that they could still fixate normally on stationary objects while being impaired for detecting flicker salience effects (Figure 2G), confirming that these visual phenotypes are likely related to having defective HS/VS motion-processing cells.
Optogenetic activation of motion-processing neurons increases sleep
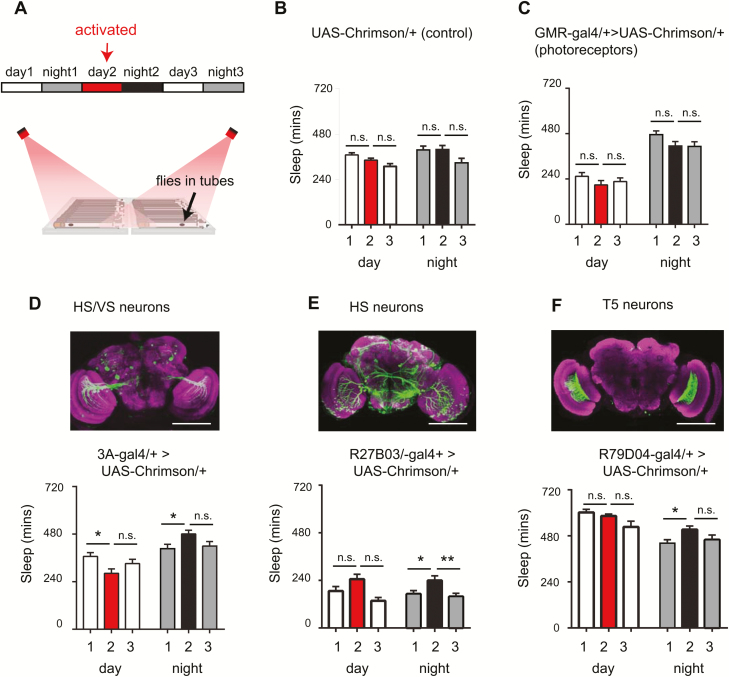
Considering that omb mutants have disrupted HS/VS neurons and sleep less than wild-type flies, we wondered whether the activity of these neurons might affect sleep. To address this question, we expressed a red-light-activated channelrhodopsin, Chrimson [31], in a Gal4 circuit which expresses in HS and VS neurons, 3A-Gal4 [46], and another Gal4 circuit that expresses in HS neurons [47]. This allowed us to transiently activate HS/VS neurons in wild-type flies during the day, and record sleep before, during and after activation (Figure 3A). Importantly, sleep was not altered in a UAS-Chrimson/+ control (Figure 3B), indicating there was no effect of red light on sleep during the red-light exposure or afterward. Furthermore, sleep was not altered when we activated a more general and peripheral component of the visual pathway—the photoreceptors (Figure 3C). Interestingly, however, activation of HS and VS cells led to an increase in sleep at nighttime, following activation compared to the previous night (Figure 3, D and E: “night 1” vs “night 2”). This effect on nighttime sleep did not seem to depend on how much sleep flies had during the day (during activation), because 3A/+>UAS-Chrimson/+ flies showed reduced sleep during red-light activation, and R27B03/+>UAS-Chrimson/+ flies showed no difference in sleep. Furthermore, nighttime sleep was not increased in NO-ATR controls, suggesting red light alone did not affect nighttime sleep (Supplementary Figures S1, A–E). Overall, our results suggest that optogenetic activation of HS and VS cells led to an increase in nighttime sleep.
Figure 3.
Activation of motion circuits increases nighttime sleep. (A) Flies expressing a red-light-activated channelrhodopsin (Chrimson) were placed in the recording set up and sleep was analyzed under baseline conditions on day 1 (normal white light from 8 am to 8 pm, followed by 12 hours darkness at night), an activated condition on day 2 (normal white light + 12 hours red light illumination from 8 am to 8 pm, followed by 12 hours darkness at night), and recovery conditions (same as baseline). (B and C) Total sleep duration was unchanged in UAS-Chrimson/+ (genetic control) or in GMR-Gal4/+>UAS-Chrimson/+ (flies expressing Chrimson in photoreceptors). (D–F) Upper panel: whole-mount brain immunostaining of three motion circuits: 3A-Gal4>UAS-GFP (HS and VS neurons), R27B03-Gal4>UAS-GFP (HS cells), and R79D04/+>UAS-GFP (T5 neurons). Brains were immunostained with anti-GFP (green) and anti-Bruchpilot (BRP, nc82, magenta). Scale bar = 100 µm. Lower panel: total daytime and nighttime sleep for flies expressing Chrimson in the aforementioned circuits, under baseline, activated and recovery conditions. n =31 flies in (B), 33 flies in (C), 33 flies in (D), 30 flies in (E), and 47 flies in (F). Error bars indicate the SEM. *p < 0.05, **p < 0.01, one-way analysis of variance with Tukey’s multiple comparisons.
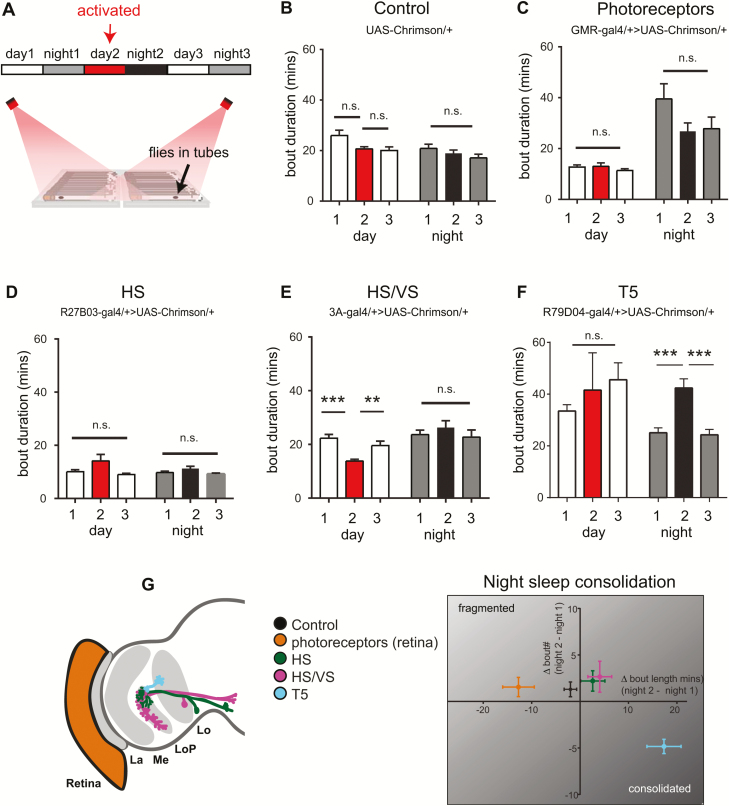
We next asked whether neurons upstream of the HS and VS neurons in the visual motion pathway may affect sleep need. To probe this question, we focused on T5 columnar neurons of the lobula, which respond to dark moving edges, and signal to the dendrites of the HS and VS cells [48–50]. We optogenetically activated T5 neurons and analyzed sleep as previously. Interestingly, we found that similar to activation of HS and VS cells, T5 cell activation also increased sleep specifically at nighttime, following activation (Figure 3F). To further analyze the effects of optogenetic activation of these visual circuits, we examined sleep consolidation by measuring sleep bout duration (Figure 4, A–F) and sleep bout number (Supplementary Figure S2) across the night and the day. No differences in day or night sleep bout duration were seen in UAS-Chrimson/+ controls, or with activation of photoreceptors (GMR-Gal4/+>UAS-Chrimson/+), or HS neurons (R27B03-Gal4/+>UAS-Chrimson/+) (Figure 4, B–D). Activation of HS and VS cells together (3A-Gal4/+>UAS-Chrimson) led to a small but significant reduction in day time sleep bout duration (Figure 4E, red compared to white bars), while having no effect on nighttime sleep. The most striking effect occurred with activation of T5 neurons, leading to more consolidated sleep at nighttime following red-light activation, indicated by increased sleep bout duration (Figure 4F, black bar vs gray bars) and significantly fewer bouts (Supplementary Figure S2E, gray bars vs black bar). Figure 4G shows a summary of night sleep consolidation for all strains tested, in which it is clear that T5 activation led to more consolidated sleep (indicated by a positive change in bout length, i.e. longer bouts, and a negative change in bout number, i.e. fewer bouts, when the night following activation (night 2) was compared to baseline conditions (night 1). Changes in sleep architecture following T5 activation were not due to locomotion impairments, as walking speed of the flies remained the same (walking speed [mm/s]: baseline: 28.9 ± 1.1, post-red: 26.8 ± 0.97, recovery:26.91 ± 1.7; no significant differences between post-red vs baseline or recovery, Kruskal–Wallis multiple comparisons). Furthermore, there were no differences in sleep consolidation observed in no-ATR control flies (Supplementary Figure S3). We noticed that sleep varied considerably in strains with different genetic backgrounds, which can be seen in the different levels of baseline sleep when comparing Gal4>Chrimson to Gal4/+ and UAS-Chrimson/+ controls (Supplementary Figure S4), and as such we always looked at effects of activation within each strain, rather than between different genotypes. Overall, our results suggest that activation of HS, VS, and T5 neurons in the motion-detection pathway increase total sleep (Figure 3), but only activation of T5 neurons leads to more consolidated sleep (Figure 4).
Figure 4.
Activation of T5 motion-detection neurons consolidates nighttime sleep. (A) Flies expressing a red-light-activated channelrhodopsin (Chrimson) were placed in the recording set up and sleep was analyzed under baseline conditions on day 1 (normal white light from 8 am to 8 pm, followed by 12 hours darkness at night), an activated condition on day 2 (normal white light + 12 hours red light illumination from 8 am to 8 pm, followed by 12 hours darkness at night), and recovery conditions (same as baseline). Analyses in this figure are from the same dataset as in Figure 3. (B–F) Sleep bout duration during the day and night across all three conditions for the UAS-Chrimson/+ control (B) and red-light-activated optic lobe circuits (C–F). (G) Summary graph showing sleep consolidation for the aforementioned strains, viewed as a change in sleep bout number/duration following red-light activation (night 2), normalized to baseline sleep conditions (night 1). Activation of T5 neurons (cyan) led to consolidation of nighttime sleep as observed by an increase in sleep bout duration and decrease in sleep bout number. n = 31 flies in (B), 33 flies in (C), 33 flies in (D), 30 flies in (E), and 47 flies in (F). Error bars indicate the SEM. **p < 0.01, ***p < 0.001, one-way analysis of variance with Tukey’s multiple comparisons.
Complex visual sequences consolidate sleep
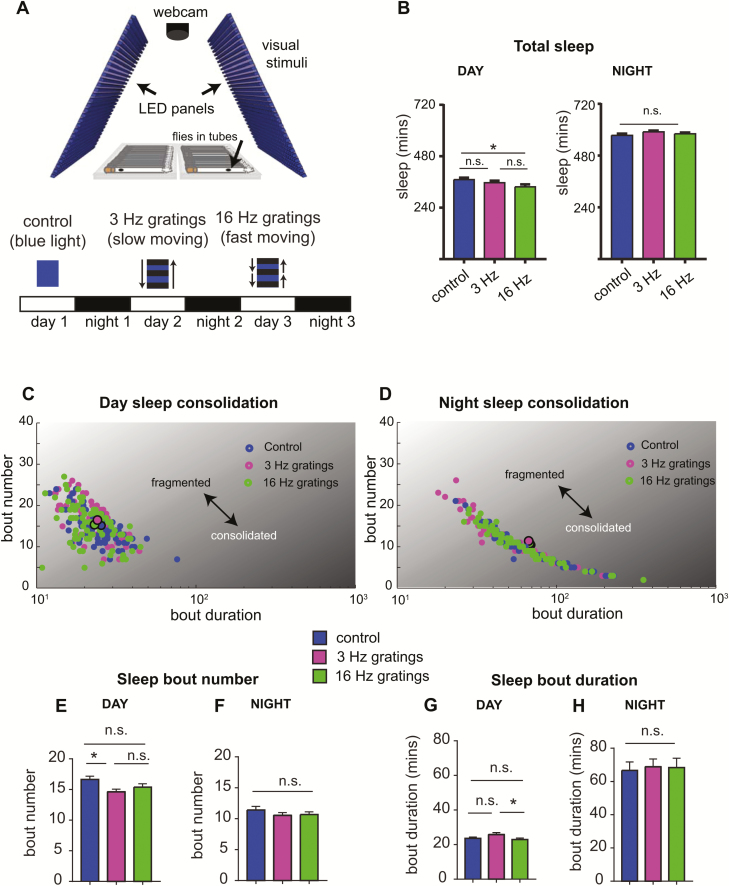
As activating motion-detection circuits increased sleep, we hypothesized that exposing wild-type flies to visual motion (which should also excite these cells) would similarly increase sleep. We therefore tested how flies responded to two different motion speeds: a slow 3 Hz grating (which is known to elicit strong behavioral and neural responses [38–41] and a fast 16 Hz grating (which evoked the strongest optomotor responses in our previous experiments). Flies were exposed to these two types of motion stimuli in addition to a control stimulus of constant blue light (Figure 5A), and stimulus order was rearranged across three independent experiments. Surprisingly, we found that these simple motion stimuli had little effect on sleep—although flies slept significantly less during the 16 Hz motion compared to control flies (Figure 5B, left panel), there were no changes in total sleep following visual stimulation (at night) (Figure 5B, right panel). A closer examination of sleep consolidation revealed a small but significant reduction of day bout number during exposure to both types of motion stimuli (day time sleep, Figure 5E), whereas bout duration was not different from controls (Figure 5G). No differences were observed following exposure to the different stimuli (Figure 5, G and H). Overall, these results suggest that exposure to simple and repetitive visual motion stimuli, whether fast or slow, have little influence on sleep need.
Figure 5.
Simple visual stimuli have little effect on sleep consolidation in CS flies. (A) Visual stimuli were presented on LED panels while sleep was recorded with a webcam to measure fly locomotion in tubes. Three different visual stimuli were presented to flies: control stimulus (constant blue light), 3 Hz (slow moving) gratings, and 16 Hz (fast moving) gratings. The order of the movies was shuffled across three independent experiments. Visual stimuli were presented during the day (8 am–8 pm) across three consecutive days, and sleep was analyzed across the day and night. (B) Total sleep duration for flies exposed to the different visual stimuli during the day (left panel) and following visual stimulation, at night (right panel). (C and D) Scatterplots showing sleep consolidation for wild-type flies during the day (C) and night (D). Averaged data points for each condition are indicated by larger circles with black outline. (E–H) Quantification of sleep bout number for the day (E) and night (F), as well as sleep bout duration for the day (G) and night (H). n > 70 flies, three experiments. *p < 0.05, one-way analysis of variance with Tukey’s multiple comparisons. Error bars show the SEM.
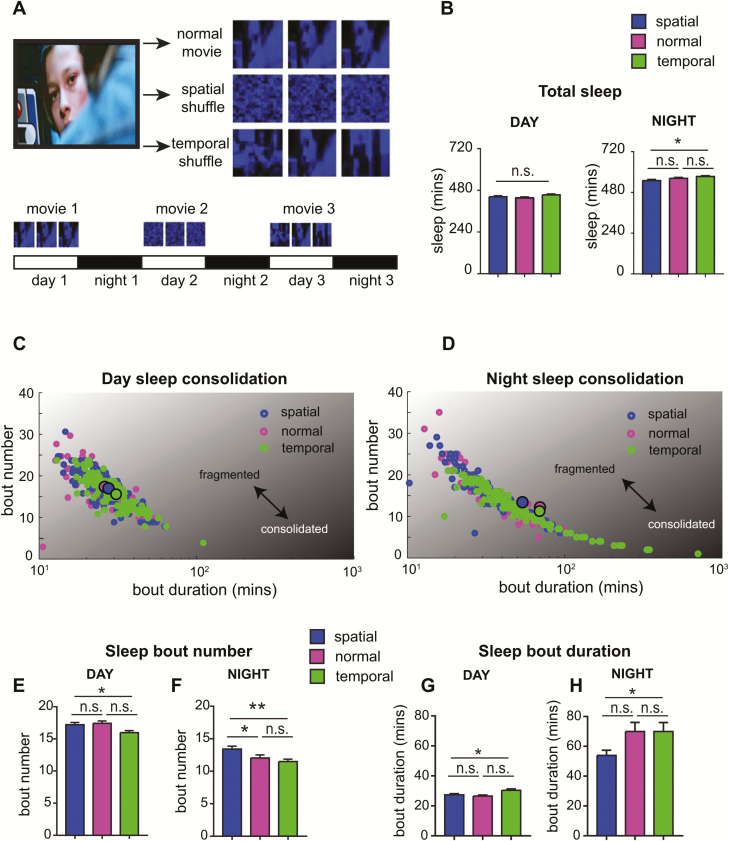
We then hypothesized that more complex visual stimuli might affect sleep. To test this hypothesis, we exposed flies to action scenes from a classic action film, which contain a variety of shapes and movement. We used three different movie types (1) the normal movie sequence (2) a spatially shuffled movie (in which pixels are shuffled such that all object forms and motion are lost), and (3) a temporally shuffled movie (in which movie frames are shuffled such that images remain intact but all temporal information is lost) (Figure 6A). Flies watched each movie during the day, while spontaneous sleep was recorded during the day and night (Figure 6A; three movie orders were tested to address any possible sequence effects). Interestingly, we found that although there were no differences in total sleep duration during the movies (Figure 6B, left panel), flies that had seen the temporally shuffled movies appeared to sleep more at night compared to flies that had seen spatially shuffled movies (Figure 6B, right panel). Sleep also appeared to be more consolidated during the day and the night for flies that watched the temporally shuffled movies (Figure 6, C and D) compared to the spatially shuffled movies, as indicated by a reduction in sleep bout number (Figure 6, E and F) and an increase in sleep bout duration (Figure 6, G and H). An increase in sleep consolidation was also apparent for flies that watched normal movie sequences, although only for nighttime sleep, which revealed significantly fewer sleep bouts (Figure 6F), and a trend (nonsignificant) for longer sleep bouts (Figure 6H). Overall this suggests that the imagery of these movie scenes, which comprises objects and motion (which is lost in the spatial shuffle), can drive sleep need in flies.
Figure 6.
Complex image sequences drive sleep consolidation. (A) Visual scenes from the movie “Terminator” were presented to flies in three different formats: normal movie, spatial shuffle (pixel positions were randomly shuffled), and temporal shuffle (movie frames were randomly shuffled through time). The order of the movies was alternated across three independent experiments. (B) Total sleep duration for day time sleep (during the movies, left panel) and nighttime sleep (following movies, right panel). (C and D) Scatterplots depicting day time sleep consolidation (C) and nighttime sleep consolidation (D), where consolidated sleep is indicated by high bout duration and low bout number, whereas fragmented sleep is indicated by low bout duration and high bout number. Averaged data points for each condition are indicated by larger circles with black outline. (E–H) Sleep bout number during the day (E) and night (F) and sleep bout duration during the day (G) and night (H) for the flies that observed the different movies. *p < 0.05, **p < 0.01, one-way analysis of variance with Tukey’s multiple comparisons. n = 150 flies, three experiments.
Discussion
Sleep is often defined by an animal’s loss of awareness, or its disconnection from waking experience. Yet it is clear that waking experience and sleep are closely linked. Our study supports this idea, showing that sleep need can be modulated by visual experience, through activation of specific visual circuits or by exposure to complex visual stimuli.
Our observations that disrupting motion detection (in an omb mutant) decreases sleep, whereas activating motion-detection circuits increases sleep, suggests that visual circuits can drive sleep need. Sleep in omb mutants was decreased by approximately 50%, comparable to some of the shortest sleeping mutants derived from behavioral screens, such as Shaker [51] and sleepless [52]. Why should a visual mutant, omb, sleep less? We know from studies in insects and mammals that richer waking experiences, particularly those that require visual perception, attention and learning, drive the need for sleep [9, 11–15, 18, 19, 21]. This could relate to synaptic homeostasis: the more an animal experiences, pays attention, and learns during wake, the more sleep-dependent plasticity will be required to consolidate or rescale synaptic connections [3, 8, 53]. It is therefore possible that omb mutants require less sleep because they have a more limited visual experience, particularly if it affects their ability to pay attention. Our finding that unlike wild-type flies, omb did not fixate more strongly on flickering objects (within a particular frequency range) compared to stationary objects, suggests that they have impaired visual salience processing. Thus, the reduced sleep in omb mutants may not only be related to motion-processing defects, but may also be linked to other visual impairments. Indeed, although activating HS and VS neurons could increase subsequent sleep, suggesting that motion detection can drive sleep need, the effect was subtle compared to the dramatically reduced sleep in omb mutants. Our observation that activation of the T5 neurons also increased sleep, and additionally caused an increase in the consolidation of sleep (reducing bout number and increasing bout duration) suggests that other visual pathways aside from HS and VS neurons can influence sleep architecture. Future studies should probe whether some types of visual neurons can consolidate sleep more than others, or whether the effect depends on cumulative activity of many visual circuits. Furthermore, a key question is how sensory neurons in the visual system signal the need for sleep to central brain circuits, such as the fan-shaped body, ellipsoid body, and upstream tubercular-bulbar (TuBu) neurons, where sleep pressure signals are thought to be generated and detected [54–58].
Our finding that temporally shuffled movie sequences lead to more consolidated sleep, compared to spatially shuffled scenes, suggests that object-related information and unpredictability may be a key factor that drives sleep need. Although the temporally shuffled movie sequences retain object information (such as people or buildings), the spatially shuffled versions do not. Thus, the temporally shuffled sequences could provide an element of surprise, as there is no predictable movement of objects in these scenes. This element of surprise may engage a fly’s attention, leading to an overall increase in visual processing that drives the need to sleep. Consistent with this idea, more simple and repetitive stimuli (the 3 Hz and 16 Hz gratings) had no effect on sleep consolidation compared to a control stimulus (static blue light), possibly because they do not contain any novelty. Indeed, in a previous study it was shown that visual novelty increases selective 20–30 Hz activity in the fly brain, and that this attention-like response is attenuated over repeated exposure to the same stimuli [59]. Interestingly, a recent study in mice found that slow-wave activity is increased following extended wakefulness through exposure to novel objects, which increases cortical firing, but using a more artificial method of increasing cortical firing through optogenetic activation did not have the same effect [9]. This suggests that exposure to novelty, and attention toward visual objects may be an important factor in driving sleep need.
Our observed effects of visual experience on sleep are admittedly subtle compared to the known effects of social interactions on sleep [18, 19]. Again, this may be related to attention. Social interactions engage various senses to drive adaptive behaviors such as courting or fighting. These behaviors, by yielding real outcomes for the flies (as opposed to passively watching movies but having no control over them), are probably much more attention grabbing. In line with this, we have previously shown that when a fly is in control of its visual surroundings, and thus likely to be paying more attention to them, there is increased temporal coordination between brain regions, compared to when it views the same visual scene passively [43]. This suggests that the brain operates differently when a fly is actively or passively viewing a scene, with increased temporal coordination likely leading to an increased need for synaptic tuning during sleep [3]. One prediction from these findings is that closed-loop control of the visual world (as might happen for any animal actively engaged in its visual environment) should more strongly increase sleep need than passive viewing. Another point to consider is that social interactions such as courtship and fighting are associated with valence (e.g. a mate is seen as “good” and a competitor as “bad”). It is therefore possible that situations that involve reward or punishment signals in the brain may also be a key driver of sleep need. Future work in Drosophila should further explore whether visual circuits that evoke valence (such as the detection of mates or predators) may be involved in driving sleep need, and how these processes in turn might be regulated by sleep.
Funding
Funding for this research was provided by the National Institutes of Health, the Australian Research Council, and the National Health and Medical Research Council.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
We thank Richard Faville and Ben Kottler for assistance and development of DART Software, David Kirszenblat for assistance with programming visual stimuli, and Deniz Ertekin and Aoife Larkin for comments on the manuscript. We thank Martin Heisenberg, Gert Pflugfelder, Vivek Jayaraman, and the Bloomington Stock Centre for flies.
References
- 1. Campbell SS, et al. . Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8(3):269–300. [DOI] [PubMed] [Google Scholar]
- 2. Stickgold R, et al. . Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16(2):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirszenblat L, et al. . The yin and yang of sleep and attention. Trends Neurosci. 2015;38(12):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirszenblat L, et al. . Sleep regulates visual selective attention in Drosophila. J Exp Biol. 2018;221:1–34. doi: 10.1242/jeb.191429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diering GH, et al. . Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355(6324):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vivo L, et al. . Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, et al. . REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tononi G, et al. . Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez AV, et al. . Why does sleep slow-wave activity increase after extended wake? Assessing the effects of increased cortical firing during wake and sleep. J Neurosci. 2016;36(49):12436–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massimini M, et al. . Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A. 2007;104(20):8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanlon EC, et al. . Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32(6):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huber R, et al. . Local sleep and learning. Nature. 2004;430(6995):78–81. [DOI] [PubMed] [Google Scholar]
- 13. Pugin F, et al. . Local increase of sleep slow wave activity after three weeks of working memory training in children and adolescents. Sleep. 2015;38(4):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mascetti L, Muto V, Matarazzo L, et al. . The impact of visual perceptual learning on sleep and local slow-wave initiation. J Neurosci. 2013;33(8):3323–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landsness EC, et al. . Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32(10):1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaw PJ, et al. . Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. [DOI] [PubMed] [Google Scholar]
- 17. Huber R, et al. . Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27(4):628–639. [DOI] [PubMed] [Google Scholar]
- 18. van Alphen B, et al. . A dynamic deep sleep stage in Drosophila. J Neurosci. 2013;33(16):6917–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ganguly-Fitzgerald I, et al. . Waking experience affects sleep need in Drosophila. Science. 2006;313(5794):1775–1781. [DOI] [PubMed] [Google Scholar]
- 20. Donlea JM, et al. . Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324(5923):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bushey D, et al. . Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332(6037):1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keene AC, et al. . Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20(13):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen D, et al. . Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun. 2017;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckwith EJ, et al. . Regulation of sleep homeostasis by sexual arousal. Elife. 2017;6:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulk A, et al. . Vision in Drosophila: seeing the world through a model’s eyes. Annu Rev Entomol. 2013;13(50):313–332. [DOI] [PubMed] [Google Scholar]
- 26. Heisenberg M, et al. . Optomotor-blindH31—a Drosophila mutant of the lobula plate giant neurons. J Comp Physiol A. 1978;124(4):287–296. [Google Scholar]
- 27. Heisenberg M, et al. . Vision in Drosophila. Genetics of Microbehavior. Berlin, Germany; Berlin, Germany: Springer; 1984. [Google Scholar]
- 28. Bahl A, et al. . Object tracking in motion-blind flies. Nat Neurosci. 2013;16(6):730–738. [DOI] [PubMed] [Google Scholar]
- 29. Faville R, et al. . How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci Rep. 2015;5:8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hendricks JC, et al. . Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. [DOI] [PubMed] [Google Scholar]
- 31. Klapoetke NC, et al. . Independent optical excitation of distinct neural populations. Nat Methods. 2014;11(3):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Götz KG. Visual guidance in Drosophila. Basic Life Sci. 1980;16:391–407. [DOI] [PubMed] [Google Scholar]
- 33. Ferguson L, et al. . Transient dysregulation of dopamine signaling in a developing Drosophila arousal circuit permanently impairs behavioral responsiveness in adults. Front Psychiatry. 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colomb J, et al. . Open source tracking and analysis of adult Drosophila locomotion in Buridan’s paradigm with and without visual targets. PLoS One. 2012;7(8):e42247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Straw AD. Vision egg: an open-source library for realtime visual stimulus generation. Front Neuroinform. 2008;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hofmeyer K, et al. . Optomotor-blind expression in glial cells is required for correct axonal projection across the Drosophila inner optic chiasm. Dev Biol. 2008;315(1):28–41. [DOI] [PubMed] [Google Scholar]
- 37. Brunner A, et al. . Mutations in the proximal region of the optomotor-blind locus of Drosophila melanogaster reveal a gradient of neuroanatomical and behavioral phenotypes. J Neurogenet. 1992;8(1):43–55. [DOI] [PubMed] [Google Scholar]
- 38. Götz KG, Wenking H. Visual control of locomotion in the walking fruitfly Drosophila. J Comp Physiol. 1973;85(3):235–266. [Google Scholar]
- 39. Joesch M, et al. . Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr Biol. 2008;18(5):368–374. [DOI] [PubMed] [Google Scholar]
- 40. Chiappe ME, et al. . Walking modulates speed sensitivity in Drosophila motion vision. Curr Biol. 2010;20(16):1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schnell B, et al. . Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol. 2010;103(3):1646–1657. [DOI] [PubMed] [Google Scholar]
- 42. Sareen P, et al. . Attracting the attention of a fly. Proc Natl Acad Sci U S A. 2011;108(17):7230–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paulk AC, et al. . Closed-loop behavioral control increases coherence in the fly brain. J Neurosci. 2015;35(28):10304–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bülthoff H. Drosophila mutants disturbed in visual orientation. Biol Cybern. 1982;45(1):71–77. [Google Scholar]
- 45. Bülthoff H, Götz KG, Herre M. Recurrent inversion of visual orientation in the walking fly, Drosophila melanogaster. J Comp Physiol A. 1982;148(4):471–481. [Google Scholar]
- 46. Scott EK, et al. . Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J Comp Neurol. 2002;454(4):470–481. [DOI] [PubMed] [Google Scholar]
- 47. Pfeiffer BD, et al. . Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105(28):9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maisak MS, et al. . A directional tuning map of Drosophila elementary motion detectors. Nature. 2013;500(7461): 212–216. [DOI] [PubMed] [Google Scholar]
- 49. Borst A, et al. . Common circuit design in fly and mammalian motion vision. Nat Neurosci. 2015;18(8):1067–1076. [DOI] [PubMed] [Google Scholar]
- 50. Fisher YE, et al. . Orientation selectivity sharpens motion detection in Drosophila. Neuron. 2015;88(2):390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cirelli C, et al. . Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434(7037):1087–1092. [DOI] [PubMed] [Google Scholar]
- 52. Koh K, et al. . Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321(5887):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diekelmann S, et al. . The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 54. Liu S, et al. . Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell. 2016;165(6):1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donlea JM, et al. . Recurrent circuitry for balancing sleep need and sleep. Neuron. 2018;97(2):378–389.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo F, et al. . A circadian output circuit controls sleep-wake arousal threshold in Drosophila. Neuron. 2018; 100(3): 624–635. [DOI] [PubMed] [Google Scholar]
- 57. Kirszenblat L, et al. . Sleep in Drosophila. In: Hans Dringenberg, ed. . Handbook of Sleep Research. Vol. 30, 1st ed. Cambridge, MA; Academic Press; 2019 June 1. In Press. https://www.elsevier.com/books/handbook-of-sleep-research/dringenberg/978-0-12-813743-7 [Google Scholar]
- 58. Raccuglia D, et al. . Network-specific synchronization of electrical slow-wave oscillations regulates sleep in Drosophila. bioRxiv. 2019. doi: 10.1101/542498 [DOI] [PubMed] [Google Scholar]
- 59. van Swinderen B. Attention-like processes in Drosophila require short-term memory genes. Science. 2007;315(5818):1590–1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.