Abstract
Summary
Results from hundreds of genome-wide association studies (GWAS) are now freely available and offer a catalogue of the association between phenotypes across medicine with variants in the genome. With the aim of using this data to better understand therapeutic mechanisms, we have developed Drug Targetor, a web interface that allows the generation and exploration of drug–target networks of hundreds of phenotypes using GWAS data. Drug Targetor networks consist of drug and target nodes ordered by genetic association and connected by drug–target or drug–gene relationship. We show that Drug Targetor can help prioritize drugs, targets and drug–target interactions for a specific phenotype based on genetic evidence.
Availability and implementation
Drug Targetor v1.21 is a web application freely available online at drugtargetor.com and under MIT licence. The source code can be found at https://github.com/hagax8/drugtargetor.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
The number of genome-wide association studies (GWAS) is growing. Consortia are unravelling associations between genetic variants and traits with ever increasing sample sizes. Available GWAS cover many areas of medicine, behaviour and biology. In addition, initiatives such as the Genotype-Tissue Expression (GTEx) project (GTEx Consortium, 2013) investigate the tissue-dependent variation in gene expression levels and identify associations with expression quantitative trait loci (eQTLs), and methods such as S-PrediXcan (Barbeira et al., 2018) allow prediction of tissue-specific expression levels using GWAS summary statistics. These advances can help map GWAS associations to protein targets in a tissue-specific manner. Maggiora and Gokhale (2017) have shown that bipartite drug–target networks can be useful to assess polypharmacology (drugs binding several targets) or polyspecificity (targets interacting with dissimilar drugs), by providing a simple bipartite representation of drug–target interactions. In this paper, we present Drug Targetor (drugtargetor.com), a web interface that allows users to browse over 500 GWAS to identify drugs and targets of interest using bipartite drug–gene networks. These networks are phenotype-dependent: drugs and genes are ordered using GWAS-derived genetic scores. Drug Targetor uses several data layers: drug–target interactions (how a drug binds a target), drug–gene interactions (how a drug influences gene expression), genetic scores to order drugs and genes by association with a phenotype and predicted gene expression levels derived from GWAS and eQTLs.
2 Materials and methods
2.1 Bipartite drug–target networks
Drug Targetor v1.21 builds phenotype-dependent bipartite drug–gene networks using HTML 5 canvas and JavaScript (Supplementary Fig. S1). A bipartite network consists in two sets of disjoint and independent sets of nodes A and B. Edges connect nodes in A to nodes in B. In Drug Targetor networks, A = drugs and B = genes. Drugs and genes are connected by type of drug–gene or drug–target interaction (Table 1). Genes and drugs are ordered by genetic scores derived from GWAS. The web platform allows to choose the phenotype and tissue of interest, the drug ensemble to be used and the type of drug–target or drug–gene interaction. A total of 530 phenotypes are available in the interface as of October 2018. The drugs are subdivided into categories defined by the Anatomical Therapeutic Chemical Classification System (ATC, https://www.whocc.no/atc_ddd_index). Drug Targetor uses its own database of genetic scores for drugs and genes; users can choose to visualize the network for the top drugs, or a network corresponding to a specific ATC drug class.
Table 1.
Graphical elements of Drug Targetor networks
| Graphical element | Description |
|---|---|
| Blue connector | Agonist, positive modulator |
| Orange connector | Antagonist, negative modulator |
| Brown connector | Partial agonist |
| Purple connector | Modulator |
| Black connector | Interaction but unknown mechanism of action |
| Red connector | Decreases gene expression |
| Green connector | Increases gene expression |
| Grey connector | Unknown effect on gene expression |
| Left-hand table (drug table) | Drugs ordered by genetic score for a given phenotype |
| Right-hand table (gene table) | Genes ordered by genetic score for a given phenotype |
| Red/green cells (gene table) | Negative/positive tissue-dependent association result |
2.2 Phenotype-dependent drug and gene scores
Drugs and genes are ordered by GWAS-derived scores, which can be used as filters in the network construction. The drug score is the −log10(P-value) of the drug/phenotype association test, computed using MAGMA pathway analysis (de Leeuw et al., 2015) after mapping each drug to its interacting genes. Gene scores, on the other hand, are a combination of two gene-wise tests (cf. Supplementary Text S3 for details): MAGMA gene-wise association test and S-PrediXcan (Barbeira et al., 2018) tissue–gene association test, which uses eQTL data (Supplementary Text S4). The gene scores range from 1 to 7; genes with the highest score (7) are significant in both S-PrediXcan and MAGMA. Drug Targetor reports all S-PrediXcan z-scores; positive or negative z-scores correspond to up- or down-regulation in the tissue of interest.
2.3 Drug–target and drug–gene connections
Different types of interactions can be selected in Drug Targetor and are used to connect drugs and targets: drug–gene interactions (how a drug influences gene expression), and drug–target interactions (how a drug interacts with a protein target). Drug–target interactions are further divided into drug mechanism of action (antagonist, agonist, modulator), and data measuring binding of a compound to a target (Supplementary Text S1). Different colours are attributed to the connections depending on interaction type (cf. Table 1). The different data sources and their references are reported in Supplementary Table S1.
3 Example
We present an example of a Drug Targetor network based on a type 2 diabetes GWAS (Scott et al., 2017) in Figure 1.
Fig. 1.
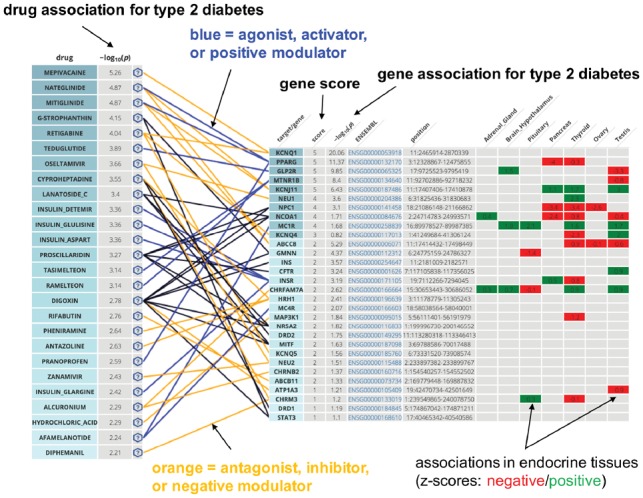
Drug Targetor network representing top drugs and their targets with drug and gene scores derived from a type 2 diabetes genome-wide association study by Scott et al. (2017). Drug and gene scores were computed using MAGMA. Tissue-wise gene associations (z-scores) in the gene table were computed using S-PrediXcan
Drugs with highest score are represented with their top targets (for gene filtering options, cf. Supplementary Text S5). Top-ranked drugs include already known diabetes drugs agonists of the glitazone receptor (PPARG gene), but also melatonin receptor 1B agonists. A recent study showed an improvement of sleep quality in type 2 diabetes patients with insomnia treated with ramelteon (Tsunoda et al., 2016); evidence also points towards a link between melatonin and glucose homeostasis (Lardone et al., 2014). Drug Targetor suggestions are supported by literature, indicating that such networks could be suggestive of repurposing opportunities.
Funding
H.G. and G.B. acknowledge funding from the US National Institute of Mental Health [PGC3: U01 MH109528]. We gratefully acknowledge capital and computing equipment funding from the Maudsley Charity [grant reference 980]; and Guy’s and St Thomas’s Charity [grant reference STR130505].
Conflict of Interest: G.B. reports consultancy and speaker fees from Eli Lilly and Illumina and grant funding from Eli Lilly. H.A.G. and C.H. report no conflict of interest.
Supplementary Material
References
- Barbeira A.N. et al. (2018) Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun., 9, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw C.A. et al. (2015) MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol., 11, e1004219.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium (2013) The Genotype-Tissue expression (GTEx) project. Nat. Genet., 45, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardone P.J. et al. (2014) Melatonin and glucose metabolism: clinical relevance. Curr. Pharm. Des., 20, 4841–4853. [DOI] [PubMed] [Google Scholar]
- Maggiora G., Gokhale V. (2017) A simple mathematical approach to the analysis of polypharmacology and polyspecificity data. F1000Res., 6, 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R.A. et al. (2017) An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes, 66, 2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda T. et al. (2016) The effects of ramelteon on glucose metabolism and sleep quality in type 2 diabetic patients with insomnia: a pilot prospective randomized controlled trial. J. Clin. Med. Res., 8, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


