Significance
The use of specific IL-2/anti–IL-2 complexes (IL-2 cplxs) has shown great potency in the prevention of allograft rejection in mice. While long-term tolerance could be achieved toward islet allografts, clinically relevant skin graft models have remained elusive so far. Using a new protocol, this study documents that IL-2 cplxs can greatly prolong the survival of skin allografts and prevent sensitization of the recipient. The data provide support for the importance of regulatory mechanisms and organ-specific approaches when targeting allograft rejection.
Keywords: transplantation, tolerance, regulatory T cells, interleukin-2
Abstract
Injection of Interleukin-2 (IL-2) complexed with a particular anti–IL-2 monoclonal antibody (mab) JES6-1 has been shown to selectively expand CD4+Foxp3+ T regulatory T cells (Tregs) in vivo. Although the potency of this approach with regard to transplantation has already been proven in an islet transplantation model, skin graft survival could not be prolonged. Since the latter is relevant to human allograft survival, we sought to improve the efficiency of IL-2 complex (cplx) treatment for skin allograft survival in a stringent murine skin graft model. Here, we show that combining low doses of IL-2 cplxs with rapamycin and blockade of the inflammatory cytokine IL-6 leads to long-term (>75 d) survival of major histocompatibility complex-different skin allografts without the need for immunosuppression. Allograft survival was critically dependent on CD25+FoxP3+ Tregs and was not accompanied by impaired responsiveness toward donor alloantigens in vitro after IL-2 cplx treatment was stopped. Furthermore, second donor-type skin grafts were rejected and provoked rejection of the primary graft, suggesting that operational tolerance is not systemic but restricted to the graft. These findings plus the lack of donor-specific antibody formation imply that prolonged graft survival was largely a reflection of immunological ignorance. The results may represent a potentially clinically translatable strategy for the development of protocols for tolerance induction.
CD4+CD25+FoxP3+ regulatory T cells (Tregs) are critical regulators of immune homeostasis and peripheral self-tolerance and act by suppressing the proliferation and cytokine production of effector T cells (Teffs) by various mechanisms which are still not fully elucidated (1). While Tregs are crucial for the prevention of autoimmune diseases and allergies, they also suppress antitumor and antiviral responses. During the last decade, Tregs have received a lot of attention for the quest of tolerance induction in the field of organ transplantation (2), stimulated by promising preclinical findings in mice (3–5) and nonhuman primate models (6, 7). Soon thereafter, the first clinical trials were started using adoptive Treg transfer for the prevention of graft-versus-host disease (GVHD) following hematopoietic stem cell transplantation (HSCT) (8). Ongoing clinical phase I/II trials using Treg therapy for prevention of rejection in solid organ transplantation are demonstrating significant potential for reduction of immunosuppressive therapy. However, despite its potential, clinical application of adoptive Treg transfer is challenged by the lack of unique Treg surface markers, the relatively small numbers of Tregs that can be obtained without extensive ex vivo expansion and debate concerning the best source of Tregs. Preparation of Tregs by ex vivo expansion is being investigated but this approach is expensive and its effectiveness still uncertain. In light of these problems, the alternative approach of boosting Treg cell numbers in situ is clearly attractive (9).
Tregs are dependent on exogenous IL-2 for their survival as FoxP3 expression not only confers regulatory activity but also prevents IL-2 synthesis by these cells (10). Tregs constitutively express the high affinity IL-2 receptor alpha (IL-2Rα or CD25) to form a trimeric receptor complex with strong binding affinity for IL-2. In contrast, resting CD8 T cells and natural killer (NK) cells only express low-affinity IL-2 receptor beta (IL-2Rβ, CD122) and the common gamma chain (γc, CD132), leading to weaker affinity for IL-2. Exogenous low-dose IL-2 administration leads to expansion of Tregs in mice (11) and has been used successfully in clinical trials for type 1 diabetes (T1D) and treatment of GVHD (12, 13). However, boosting Treg proliferation by IL-2 injections is fraught with several risks and hurdles (14). First, IL-2 is toxic at higher doses and can lead to vascular leak syndrome which causes severe pulmonary edema. Second, the half-life of injected IL-2 is very short, which requires frequent dosing. Third, and probably most importantly, there can be parallel expansion of other IL-2 responsive cells, including CD4+CD25+ effector T cells, CD8 T cells, and NK cells, which could cause accelerated graft rejection instead of tolerance induction. By using particular IL-2/anti–IL-2 mab complexes (IL-2 cplxs) for injection, these problems are largely avoided (15). Here, the capacity of anti–IL-2 mab JES6-1 complexed with IL-2 to selectively expand Tregs in vivo and cause permanent acceptance of fully mismatched islet allografts has already been demonstrated in mice (16). Nevertheless, this approach failed to enhance survival of skin allografts (17). Other workers showed that mTOR blockade with rapamycin acts synergistically in a low-dose IL-2 model and significantly prolonged skin graft survival; however, daily injections were required and all grafts were eventually rejected by day 60 (18). In a major histocompatibility complex (MHC) mismatched F1 → parent model, IL-2 cplxs were shown to synergize with CD40L blockade by hindering CD8 alloreactivity (19). Importantly, little or no protective effect was observed with low-dose IL-2 or IL-2 cplxs alone in the BALB/c to B6 strain combination, a stringent model for clinical transplantation.
In this study, we attempted to achieve long-term survival of MHC-disparate skin allografts by modifying the existing protocol for Treg expansion in vivo. Since previous studies used only brief (3 d) treatment with IL-2 cplx, it was important to examine the effects of prolonged IL-2 cplx treatment. We also examined whether graft survival could be improved by IL-6 blockade (20, 21). In this respect, histologic rejection of allografts is known to be associated with IL-6 expression, both in experimental models (22) and patients (23, 24), and injection of anti–IL-6 mab can delay graft rejection (25). We show here that a combination of these treatments led to extended graft survival. Thus, increasing the treatment with IL-2/JES6-1 complexes to 30 d plus addition of rapamycin and a short course of IL-6 mab injections caused skin allografts to be accepted for prolonged periods [mean survival time (MST) 76 d] in the absence of immunosuppression.
Materials and Methods
Mice.
Female C57BL/6 (B6, H-2b) BALB/c (H-2d), and C3H (H-2k) mice were obtained from the Australian BioResources Pty, Ltd (ABR) facility in Moss Vale, NSW or Charles River Laboratories (Sulzfeld, Germany). B10.D2 (H-2d, minor antigen matched to B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the Medical University of Vienna. Mice were used at the age of 6–8 wk of age with an average weight of 18–20 g and housed under conventional barrier protection in ventilated filter cages (up to 5 mice). Animals were handled in accordance with protocols approved by the Garvan Institute of Medical Research and St Vincent’s Hospital Animal Experimentation and Ethics Committee, which comply with the Australian code of practice for the care and use of animals for scientific purposes. Experiments performed at the Medical University of Vienna were covered by the ethics votum of the Austrian Federal Ministry of Science, Research and Economy GZ:BMWFW-66.009/0028-WF/v/3b/2015 (according to “Tierversuchsgesetz” 2012, BGB. I Nr. 114/2012).
Preparation and Administration of IL-2/Anti–IL-2 Mab Complexes.
IL-2/anti–IL-2 mab complexes were prepared as previously described (15, 16). Briefly, recombinant mouse IL-2 (PeproTech) was mixed with anti–IL-2 mab (clone JES6-1; purified from hybridomas) in a ratio of 1:5 (16) and incubated at 37 °C for 30 min. Mice were injected intraperitoneally (i.p.) in a final volume of 100–200 µL.
Tolerance Protocol.
Groups of age-matched B6 recipients received 3 d of IL-2 cplx induction, followed by injections starting on day 1 thrice a week until day 30 (LD 0.5 µg IL-2/2.5 µg JES6-1, i.p.). Rapamycin (LC Laboratories) was administered as induction on day −1, d 0, followed by injections thrice a week from day 1 until day 29 (1 mg/kg i.p.). Antiinflammatory treatment consisted of short-term administration of anti–IL-6 mab (clone MP5-20F3, BioXcell) at days −1/1/4/6 (300 µg, i.v.).
Flow Cytometric Analysis and Antibodies.
Antibodies against mouse CD3 (17A2), CD8 (53-6.7), CD44 (IM7), CD122 (5H4), NK1.1 (PK136), PD-1 (CD279, RMP1-12), CD16/CD32 (Fc-block), 7AAD (viability staining solution; purchased from BioLegend), CD11c (HL3), CD80 (B7-1, 16–10A1), CD86 (B7-2, GL1; purchased from BD Biosciences), CD4 (RM4-5), CD25 (PC61 and 7D4), CTLA4 (CD152, UC10-4B9), CD45.2 (104), Ki67 (SolA15), CD62L (MEL-14), FoxP3 (FJK-16s), and fixable viability dye (Fixable Viability Dye eFluor 780 or 450 from eBioscience) and appropriate isotype controls were used. Cell suspensions were incubated for 10 min with Fc-block, then stained for surface markers for 30 min. Intracellular Foxp3 and Ki67 staining was performed by using eBioscience Fixation/Permeabilization kit according to the manufacturer’s instructions. Red blood cell lysis buffer (Sigma Aldrich) was used for removal of red blood cells in peripheral blood mononuclear cells (PBMCs) and spleen cells. Flow cytometric analysis was performed with BD FACS CantoII and FlowJo software.
Anti-Donor Antibodies (Flow Cytometric Crossmatch).
Recipient serum harvested ∼2 wk postgraft rejection (or at indicated timepoints) was heat inactivated and incubated with recipient-type and donor-type thymocytes (which are low in Fc-receptors, thus reducing background staining). Binding of serum IgG Abs to thymocytes was analyzed by flow cytometry using FITC-conjugated rat anti-mouse IgG1 and IgG2a/2b (BioLegend, San Diego).
Mixed Lymphocyte Reaction and Proliferation Assay.
One-way mixed lymphocyte reactions (MLRs) were performed as described in detail previously (26). Briefly, 4 × 105 unseparated responder cells [splenocytes or lymph node (LN) cells from naïve B6 or skin graft recipients] were incubated in triplicates with 4 × 105 irradiated (30 Gray) stimulator cells (splenocytes) of either B6 (recipient) or BALB/c (donor) origin or with medium only. After 96 h of incubation, allo-specific proliferation among nonirradiated (negative for Fixable Viability Dye eFluor 780) responder cells was assessed by measuring the expression of Ki67 in CD4 and CD8 T cells. For polyclonal proliferation assays, responder splenocytes (naïve B6 or skin graft recipients) were stimulated with plate-bound anti-CD3/anti-CD28 (145-2C11, BioLegend). Proliferation was assessed by Ki67 expression among CD4 and CD8 responder T cells.
To measure early donor-specific hyporesponsiveness, 4 × 105 lymph node cells from naïve B6 or skin graft recipients were labeled with Violet Proliferation Dye 450 (VPD) (BD Biosciences) and cultured with 4 × 105 irradiated (30 Gray) stimulator cells (splenocytes) of either B6 (recipient), BALB/c (donor) or C3H (third party) origin. In indicated experiments, responder cells were depleted of CD25+ cells by magnetic bead cell sorting (MACS CD25 microbead kit, Miltenyi Biotec). Proliferation after 96 h was assessed by cell division dependent dye dilution via flow cytometry. The stimulation index (SI) was calculated as follows: SI = proliferation of responder cells in wells with allogeneic stimulator cells added/proliferation of the same responders in wells containing syngeneic stimulator cells (proliferation against “self”).
Skin Grafting.
Full thickness tail skin from BALB/c (donor) or B10.D2 (donor MHC without minor mismatches) mice was grafted on the side of the chest, secured with bandaids for 6 d and visually inspected thereafter at short intervals. Grafts were considered to be rejected when less than 10% remained viable. Mice were anesthetized either with isoflurane inhalation anesthesia or with ketamine (Ketalar, 100 mg/kg) and xylazin (Rompun, 5 mg/kg). Postoperative analgesia consisted of buprenorphin (Buprenovet, day 0; 0.01–0.05 mg/kg/d i.p.), followed by piritramid (Dipidolor, 15 mg in 250 mL 0.4% glucose water) in drinking water ad libitum for 1 wk.
Isolation of Graft-Infiltrating Lymphocytes.
Skin grafts were minced and incubated for 1 h at 37 °C in RPMI containing 2 mg/mL Collagenase D (Sigma). Digestion was stopped with RPMI containing 1% FCS and filtered through a 100-µm cell strainer.
Statistics.
Data were statistically analyzed with GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) using a 2-tailed t test with unequal variances to compare differences between groups. Multiple measurements at different timepoints were compared using a 2-way ANOVA. Skin allograft survival was calculated according to the Kaplan–Meier product limit method and compared between groups using the log-rank test. A P value of less than 0.05 was considered to be statistically significant.
Results
IL-2/Anti–IL-2 Mab Complexes Synergize with IL-6 Blockade to Expand Tregs In Vivo and Prolong Skin Allograft Survival.
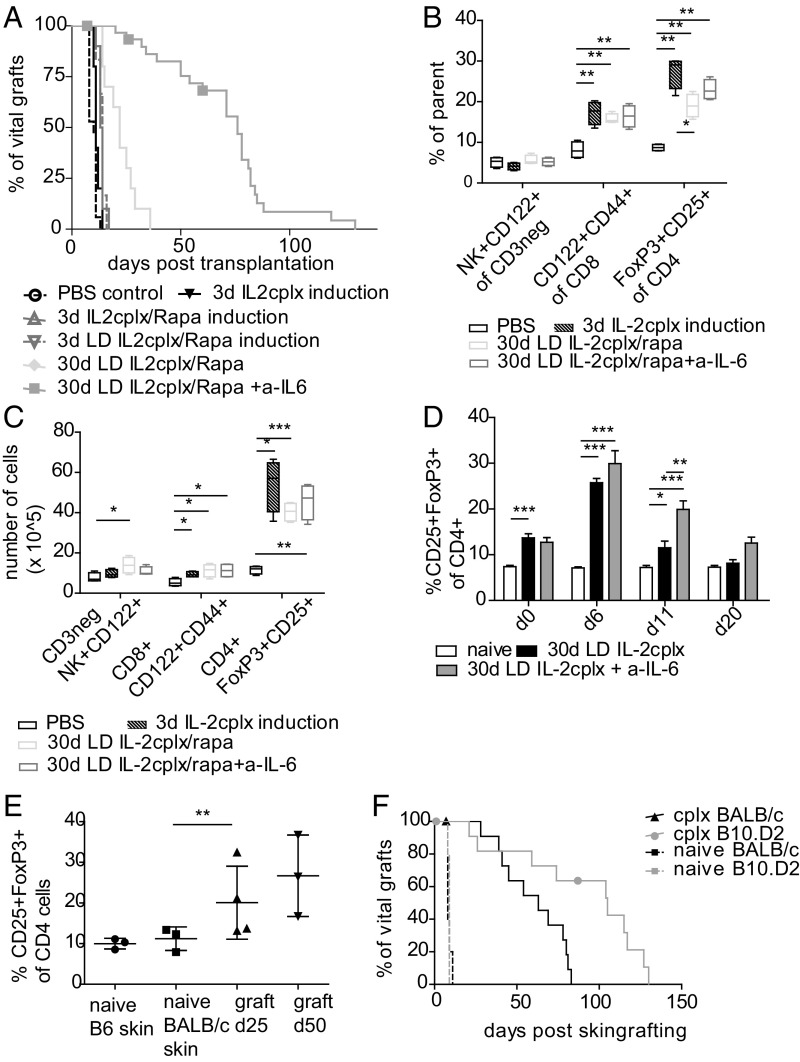
We initially investigated the potency of IL-2 cplx to prolong skin graft survival based on a protocol that induced long-term survival of islet allografts (16). Groups of B6 mice received fully mismatched skin grafts from BALB/c donors after 3 d of daily treatment with IL-2 cplxs (1 µg IL-2/5 µg JES6-1 per injection). However, this treatment failed to cause a prolongation of MST of the grafts, i.e., MST of 11 d vs. MST of 10 d in PBS-treated controls [P = not significant (n.s.)] (Fig. 1A). Accordingly, to improve graft MST, we added rapamycin (IL-2 cplx/rapa), lowered the dose of IL-2–0.5 µg per injection (LD IL-2; complexed with 2.5 µg JES6-1), and extended the length of treatment to 30 d (30 d LD IL-2 cplx/rapa); in addition, based on previous findings (21, 27, 28), we added a short-course (4 doses on days −1/1/4/6) of antiinflammatory treatment using anti–IL-6 mab [30 d LD IL-2 cplx/rapa + anti–IL-6 (a–IL-6)]. For all of these regimens, no treatment was given after 30 d.
Fig. 1.
Combined treatment with low dose IL-2 cplxs, rapamycin, and anti–IL-6 potentiates Treg function and prolongs skin allograft survival. Groups of mice were treated with IL-2 cplx-based tolerance regimens and grafted with fully mismatched BALB/c skin. Groups of mice received PBS (control; n = 17 [5]; MST = 10 d), IL-2 cplxs only (cplx; n = 5 [1]; MST = 11 d), IL-2 cplx combined with rapamycin (cplx/rapa; n = 10; MST = 13.5 d; low-dose (LD) cplx/rapa; n = 6 [1]; MST = 14 d) or prolonged treatment with cplx and rapamycin with (30 d LD cplx/rapa + a–IL-6 n = 31 [5]; MST = 76 d) and without anti–IL-6 (30 d LD cplx/rapa n = 10; MST = 22). (A) Survival curves of cumulative data of several independent experiments are shown (indicated in brackets), log-rank test. (B and C) Changes of leukocyte subsets were analyzed in the spleen on day 6 (d6) (*P < 0.05, **P < 0.005, ***P < 0.0005 2-tailed t test with unequal variances) and (D) in peripheral blood over time at indicated timepoints (two-way ANOVA *P < 0.05, **P < 0.01, ***P < 0.001). Mean percentages (B and D) and total cell numbers (C) are shown. Error bars indicate SD. (E) Mice were treated with IL-2 cplx-based tolerance protocol were grafted with 2 BALB/c grafts on contralateral sides of the back. Macroscopically intact grafts were harvested for analysis on d25 and d50. Graft-infiltrating T cells subsets were analyzed for percentage of CD25+FoxP3+ (Tregs; gated on CD45+CD4+). Mean percentages are shown, error bars indicate SD, groups were compared using a 2-tailed t test with unequal variances (**P < 0.01). (F) Mice were grafted with BALB/c (full mismatch) or B10.D2 (MHC mismatch, with minimal minor H antigen difference) skin, and groups were treated with IL-2 cplx-based tolerance regime. Naïve recipients uniformly rejected allografts within 10 d (BALB/c graft MST = 8 d; B10.D2 graft MST = 9 d; n = 5 per group), whereas graft survival was prolonged after IL-2 cplx-based treatment in all groups (BALB/c graft MST = 61.5 d; B10.D2 graft MST = 105 d, P = 0.0075 vs. BALB/c; n = 12 per group). Data were pooled from 2 independent experiments.
Individually, these treatments caused little or no increase in MST. However, the combination of these treatments was highly effective, resulting in an MST of 76 d (P < 0.0001) with some mice accepting grafts for >100 d without macroscopic signs of rejection; for IL-2/rapa, extending the injections to 70 d did not lead to a further increase in MST (SI Appendix, Fig. S1). To the best of our knowledge, this long-term skin allograft acceptance in the absence of immunosuppressive treatment or hematopoietic stem cell transplantation is demonstrable in the stringent BALB/c → B6 strain combination (29). Since treatment with a–IL-6 alone or in combination with rapamycin failed to prolong skin graft survival (MST = 15), IL-2 cplx treatment was crucial for the operational tolerant state (SI Appendix, Fig. S2).
Treatment with IL-2 cplxs has been shown to preferentially expand Tregs in vivo in peripheral blood as well as lymphoid organs without concomitant analogous proliferation of NK and CD8 cells (16, 30). When examined in spleen at day 6, all of the above treatments caused a marked increase in percentage and total numbers of Tregs but only a small increase in memory (CD122+CD44+) CD8 T cells and no increase in NK (CD3+NK+CD122+) cells (Fig. 1 B and C). At day 6, neither rapamycin nor anti–IL-6 mab, alone or in combination, potentiated Treg expansion by IL-2 cplx. Surprisingly, when cell numbers were examined in peripheral blood of mice given repeated IL-2 cplx injections (0.5 µg IL-2/2.5 µg JES6-1 per injection; starting at day −3), Treg numbers initially increased significantly until day 6 but then fell progressively and almost reached background levels by day 20 (Fig. 1D); this decline was less marked with a combination of IL-2 cplxs and anti–IL-6 mab. A similar decline in Treg numbers was also seen under ongoing low-dose IL-2 therapy (18), so we hypothesized that Tregs might home to other organs and the graft, respectively. To examine T cell infiltration in the grafts, IL-2 cplx–treated B6 mice bearing 2 BALB/c grafts on contralateral flanks had their grafts removed on days 25 and 50 posttransplant followed by enzymatic digestion to prepare cells for flow cytometric phenotyping. With regard to graft infiltrating cells, we found enrichment in CD4+CD25+FoxP3+ Tregs at 25 and 50 d postgrafting relative to naïve skin (Fig. 1E). Hence, the capacity of the cplx protocol to prolong graft survival may in part reflect intragraft regulation by graft-infiltrating Tregs.
The above results show that modifying our current IL-2 cplx protocol for tolerance induction markedly extended skin allograft survival in a stringent MHC-incompatible strain combination without immunosuppression. In the BALB/c → B6 combination used above, the donor and host displayed both MHC and minor H antigen differences. In the MHC-different, minor H near-identical B10.D2 → B6 combination, graft survival after IL-2 cplx treatment was significantly prolonged to an MST of 105 d, relative to MST of 63 d for BALB/c grafts in the experiments shown (Fig. 1F); without IL-2 cplx treatment, all grafts were rejected rapidly at 8–9 d. In the experiments discussed below, we used the clinically relevant fully mismatched BALB/c → B6 combination.
Role of Tregs in Graft Survival.
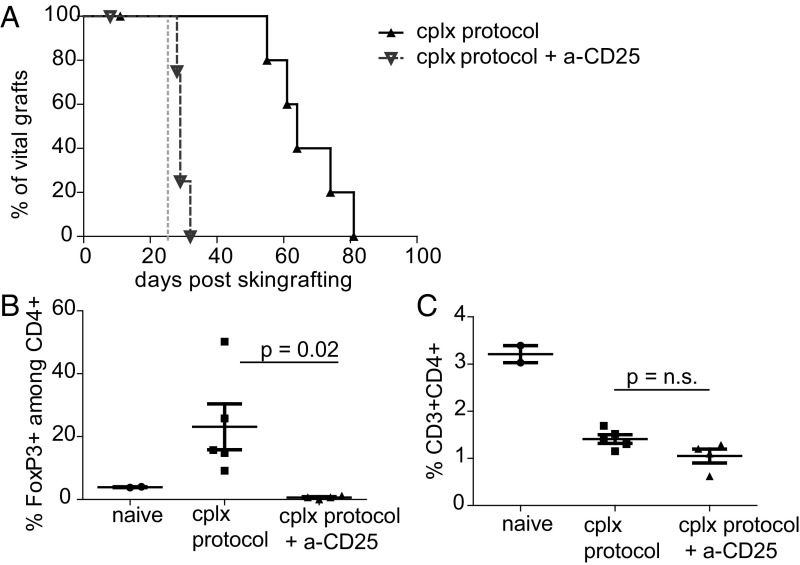
Using the above protocol combining IL-2 cplx, rapamycin and anti–IL-6 (referred to hereafter as IL-2 cplx protocol), we investigated the mechanisms responsible for graft survival. Notably, Treg depletion by anti-CD25 mab injection rapidly abrogated graft acceptance in all mice, suggesting that Tregs actively prevent rejection in these mice (Fig. 2A). All mice that received Treg depleting antibodies on day 25 after grafting rejected their graft within 5 d of starting the injections, whereas in non–Treg-depleted mice, skin allografts survived for 4–8 wk in the absence of immunosuppressive treatment. Flow cytometric analysis in PBMCs indicated that FoxP3+ Tregs were efficiently depleted by addition of CD25-specific mab (Fig. 2B), whereas percentages of overall CD4 T cells did not change significantly, in line with most conventional CD4 cells being CD25 negative (Fig. 2C). Levels of total CD4 cells in peripheral blood of both groups treated with IL-2 cplxs were significantly lower than in naïve mice due to ongoing treatment with rapamycin (31). Collectively, these data indicate that operational tolerance in the grafted mice is actively maintained by Tregs.
Fig. 2.
Depletion of Tregs during IL-2 cplx treatment leads to rapid graft rejection. (A) Mice were treated with IL-2 cplx-based tolerance protocol (cplx protocol) and grafted with BALB/c skin (n = 6; MST = 64 d); some mice were treated with anti-CD25 mab (day 25, indicated with dashed gray line) to deplete Tregs (cplx protocol + a-CD25: n = 5; MST = 29 d, P = 0.0029). (B and C) Peripheral blood was analyzed on day 30 to measure frequency of (B) FoxP3+ Tregs among CD4+ subset and (C) total CD4 T cells among leukocytes (naïve n = 2, cplx protocol n = 5, cplx protocol + a-CD25 n = 4; 2-tailed t test with unequal variances).
IL-2 Cplx-Based Tolerance Protocols Impair Development of Anti-Donor Antibodies.
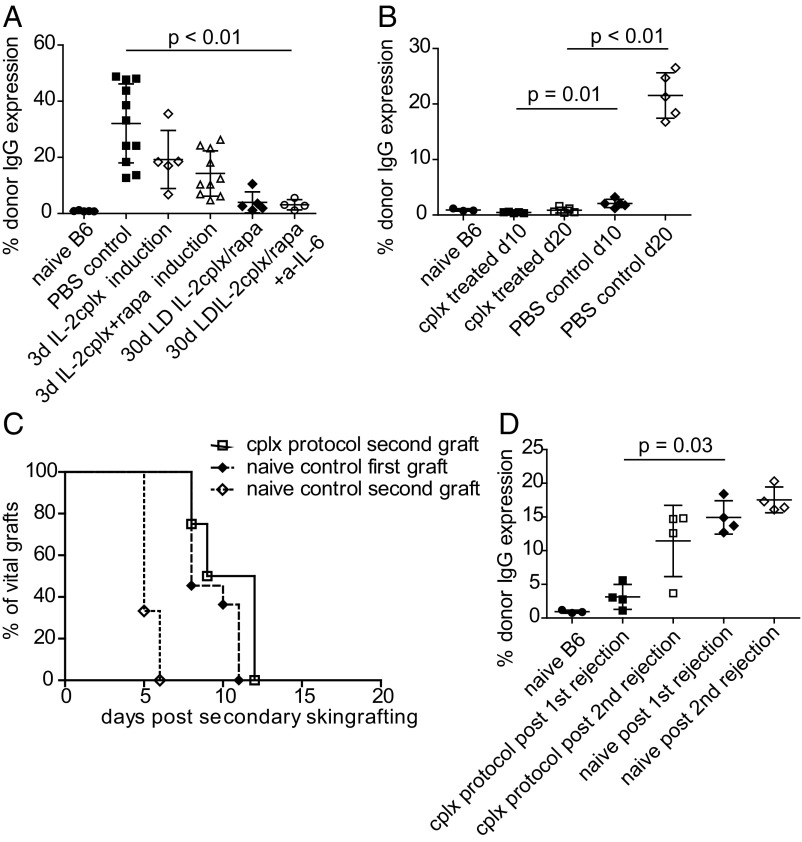
Examining the serum of the skin-grafted mice revealed that IL-2 cplxs markedly impaired development of donor-specific IgG antibodies (Fig. 3A). In particular, mice that received the optimal combined tolerogenic treatment were almost devoid of anti-donor antibodies after eventual graft rejection. Likewise, in contrast to untreated (PBS) controls, donor-specific antibodies in the tolerized mice were also undetectable at early stages (10 d and 20 d) after grafting (Fig. 3B); antibodies to control recipient IgG were absent in all groups (SI Appendix, Fig. S3). Importantly, optimal IL-2 cplx protocol ablated humoral immunity to the graft.
Fig. 3.
Treatment with IL-2 cplxs prevents alloantibody production humoral response and impairs memory cell differentiation. (A) Sera of mice were collected ∼2 wk post skin-graft rejection and analyzed for the presence of donor graft-specific IgG (naïve B6 n = 5; PBS control n = 11; 3 d cplx = 5; 3 d cplx/rapa n = 10; 30 d LD cplx/rapa n = 5; 30 d LD cplx/rapa + a–IL-6 n = 4). (B) Sera of naïve, cplx-treated or PBS-treated control mice were analyzed for donor-specific IgG early after skin grafting (day 10, day 20; n = 3–5 per group). (C) Rejection of a second BALB/c graft applied without additional treatment ∼2 wk after rejection of the first graft (naïve first: n = 11, MST = 8 d; naïve second n = 4, MST = 5 d, P < 0.0001 vs. naïve first; cplx second: n = 4, MST = 10.5 d, P = 0.0058 vs. naïve second; P = 0.1383 vs. naïve first; log-rank test). (D) Sera of mice were analyzed for the presence of anti-donor IgG after the first and second rejection (n = 2–4 per group). Groups were compared with a 2-tailed t test with unequal variances.
Effects of Applying a Second Skin Allograft after Primary Graft Rejection.
To seek further information on how the IL-2 cplx protocol prolonged graft survival, we placed another donor-type skin graft on the opposite flank after the mice rejected their primary graft (>100 d). Surprisingly, these second grafts were rejected at the same rate (MST = 10.5) as primary grafts on normal mice (MST of 8 d); in marked contrast, sensitized PBS controls rejected a secondary donor graft in accelerated fashion (MST of 5 d, P > 0.0001 against naïve; P = 0.0058 vs. cplx-treated sensitized; Fig. 3C). Moreover, after rejection of the second graft, anti-donor antibody production was comparable to the level seen in naïve mice after primary graft rejection (Fig. 3D). These data, plus the lack of donor-specific antibodies (see above), suggest that the active regulatory mechanisms that delayed rejection of the first graft largely prevented initial sensitization and eventually restored the immune system to a naïve level.
Effect of Applying a Second Skin Graft before Primary Graft Rejection.
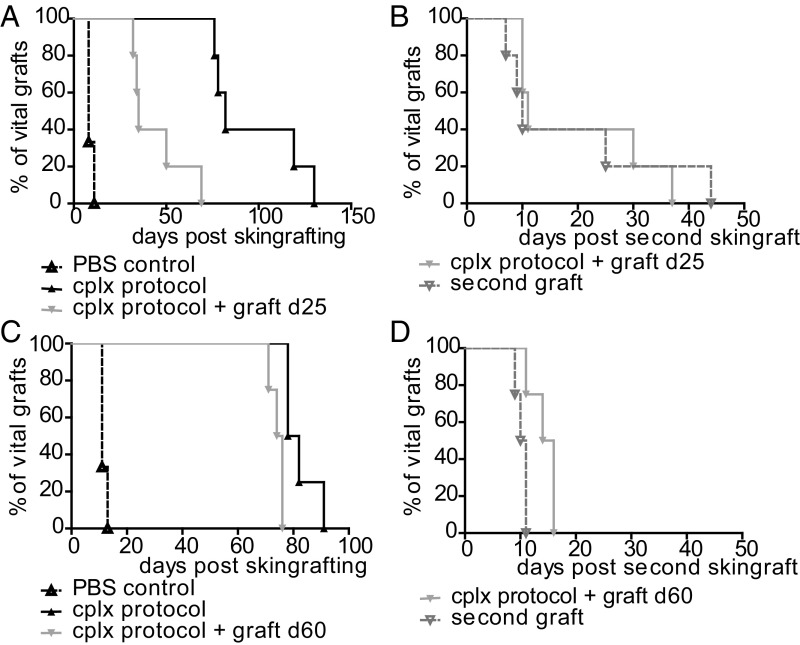
In an additional set of experiments, we challenged mice with a second donor type graft before rejection of the primary graft. Here, the second graft was applied at 25 d or 60 d after initial grafting. At 25 d postgrafting (still under IL-2 cplx and rapamycin treatment), placing a second BALB/c graft on the contralateral flank caused accelerated rejection of the first graft (MST of 35 d) relative to mice that did not receive the second graft (MST of 85 d) (Fig. 4A). In this situation, both grafts were rejected at about the same time after applying the second graft (Fig. 4B). Interestingly, rejection of both grafts was rapid for some mice but slow for others, implying varying degrees of an operational tolerant state, presumably reflecting variable persistence of Treg function in the treated mice.
Fig. 4.
Challenge with a second donor-type graft breaks tolerance. Groups of mice received BALB/c skin grafts under tolerogeneic IL-2 cplx protocol. Operationally tolerant mice (graft macroscopically intact) were grafted with a second graft of BALB/c strain (A and B) at day 25 (d25) (PBS control n = 3, cplx protocol n = 5, cplx protocol + graft d25 n = 5; P = 0.0018 ± second graft) or (C and D) at d60 (PBS control n = 3, cplx protocol n = 4, second graft: cplx protocol + graft d60 n = 4; P = 0.0091 ± second graft) after primary skin grafting. Overall survival of the primary grafts with or without additional antigen challenge on (A) d25 or (C) d60 and survival of the grafts after placing a second donor-type graft transplanted on (B) d25 and (D) d60 (second graft d25 MST = 10 d; second graft d60 MST = 10.5 d; P = n.s. vs. PBS control) are shown. Log-rank test was used for comparison of survival curves.
When the mice received a second graft at 60 d (well after ceasing treatment at 30 d), the second graft caused prompt accelerated rejection of the first graft (MST of 75 d), relative to controls that received only the first graft (MST of 80 d) (Fig. 4C). Rejection of the second graft occurred rapidly, i.e., at 10.5 d, as for primary rejection by untreated control mice (Fig. 4D, compare with Fig. 1A). Hence, in contrast to 25 d, there was no apparent tolerance when the second graft was applied at 60 d.
Operational Tolerance Induced by IL-2 Cplx-Based Protocols Is Not Systemic.
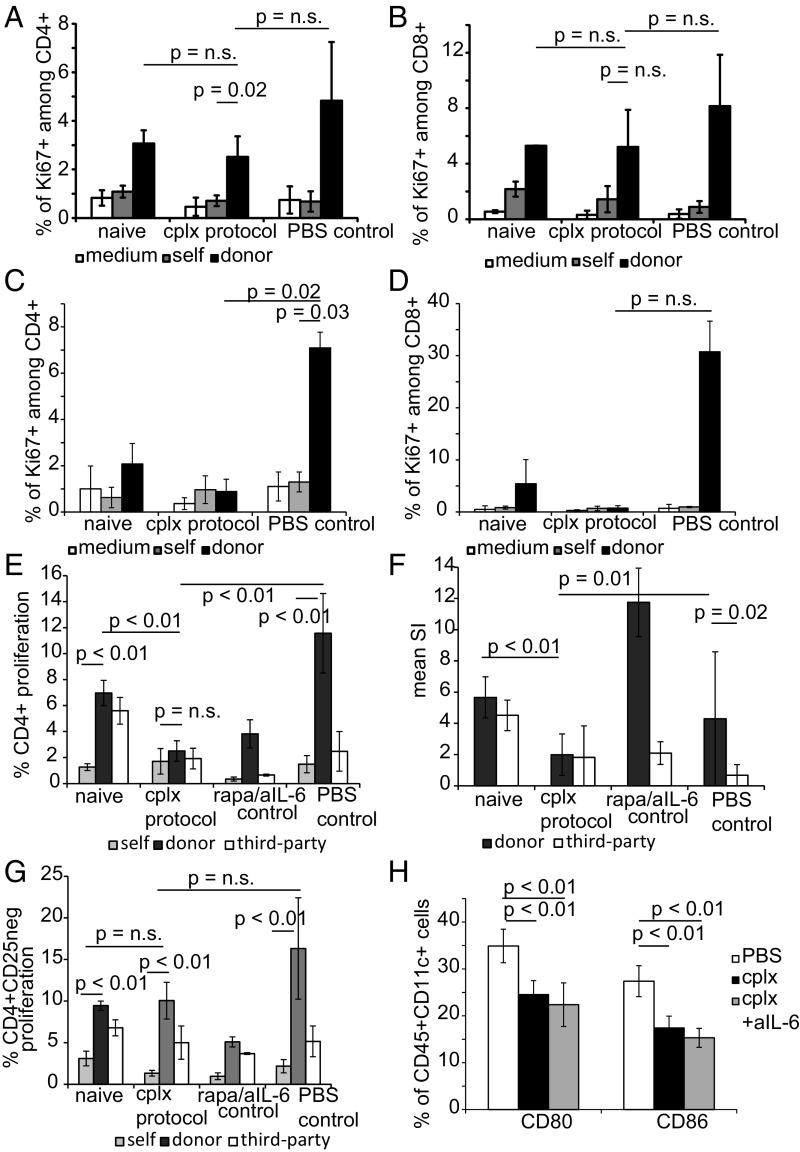
To directly investigate whether donor-specific sensitization or tolerance had occurred in the IL-2 cplx mice, we examined responsiveness to donor antigens (irradiated spleen cells) in vitro. When tested in MLRs at a stage when grafts were macroscopically intact (day 50), the host mice did not show a detectable alteration in donor-specific responsiveness in either CD4 or CD8 T cells, in comparison with naïve or PBS control T cells (Fig. 5 A and B); likewise, in vitro polyclonal proliferative responses of CD4 and CD8 T cells in response to CD3/CD28 ligation cells were not impaired (SI Appendix, Fig. S4). These findings indicated that, at day 50, responsiveness to graft alloantigens was much the same as for naïve mice, indicating neither tolerance nor sensitization. Responses at earlier timepoints were somewhat different. Thus, at day 20 responses to graft alloantigens were very low and no higher than with autologous antigen-presenting cells (APCs) (Fig. 5 C and D). A likely explanation for this finding is that the early elevation in Treg numbers induced by the IL-2 cplx protocol induced nonspecific suppression.
Fig. 5.
IL-2 cplx-based tolerance protocol does not induce systemic (donor) hyporesponsiveness. (A–D) Responsiveness to graft alloantigens was evaluated by in vitro T cell proliferation assays. LN cells were prepared from groups of B6 recipients of BALB/c skin grafts at day 50 (A and B) or day 20 (C and D) after skin transplantation; LN cells from normal B6 mice (naïve) and grafted mice not given IL-2 cplx (PBS control) were used as controls. The 4 × 105 unseparated LN cells were cultured alone (medium) or with 4 × 105 irradiated spleen cells from B6 (self) or BALB/c (donor) mice for 4 d and then assessed for Ki67 expression by flow cytometry. Mean expression of Ki67 expression in CD4 (A and C) and CD8 (B and D) T cells in triplicate cultures is shown (naïve = 2, cplx protocol = 4, PBS control = 3; 2-tailed t test with unequal variances for P values). (E–G) Responsiveness to graft versus third-party alloantigens. LN cells were prepared from B6 recipients of BALB/c skin grafts at day 14 after grafting and starting treatment with IL-2 cplx (cplx protocol) or a mixture of rapamycin and anti–IL-6 mab (rapa/IL-6 control), with untreated grafted mice (PBS control) as a control. LN cells were labeled with VPD and 4 × 105 cells were cultured with 4 × 105 irradiated spleen cells from B6 (self), BALB/c (donor), or C3H (third party) mice for 4d and then assessed for VPD dilution by flow cytometry. Responder cells were either unseparated (E and F) or depleted of CD25+ cells to remove Tregs (G) before culture. (E and G) Mean percentage of CD4 cells that expressed a low density of VPD (indicating proliferation) is shown for triplicate cultures. (F) The mean (SI ratio of responses to BALB/c and C3H relative to responses to B6 stimulators) of unseparated LN cells is shown. Results are pooled from 2 separate experiments and involve cells taken from multiple mice (naïve n = 4, cplx protocol n = 6, rapa/anti–IL-6 n = 2, PBS control n = 5). (H) B7 expression on host DCs. Expression of B7 molecules CD80 and CD86 was measured at day 14 in CD11+ splenocytes of PBS-treated (n = 4), cplx-treated (n = 7), and cplx/anti–IL-6 treated (n = 7) mice (data pooled from 2 independent experiments). Data are shown as mean percentage, error bars depict SD, P values were calculated using a 2-tailed t test with unequal variances.
To examine this possibility, we tested responsiveness to graft versus third-party alloantigens in a VPD dye dilution assay with unseparated CD4 cells and also CD25-depleted CD4 cells at ∼day 15 after grafting. With unseparated CD4 cells, responses to graft (BALB/c, H2d) and third-party (C3H, H2k) alloantigens were both low relative to naïve mice, consistent with nonspecific suppression by elevated Treg numbers (Fig. 5E). Such suppression was not seen with control mice that were skin grafted but treated only with rapa plus IL-6 mab or with PBS alone, i.e., situations where Tregs were not expanded. Here, elevated responses to graft antigens relative to third-party antigens occurred (Fig. 5E); this selective sensitization to graft antigens was seen clearly when responses to graft and third-party antigens were expressed as SIs (Fig. 5F). For mice treated with the complete IL-2 cplx protocol, the notable finding was that Treg removal from the responder CD4 cells before culture did unmask alloreactivity (Fig. 5G). Here, the significant finding was that responses of CD25−CD4+ cells to graft and third-party alloantigens closely resembled the responses of naïve CD4 cells, whereas cells from control PBS mice showed enhanced (although not statistically significant) responses to the graft antigens.
Collectively, these in vitro findings were consistent with the notion that long-term maintenance of allografts in mice treated with the IL-2 cplx protocol was largely a reflection of ignorance mediated by Tregs. In considering how Tregs limit responsiveness to alloantigens, other workers have reported that injecting mice with IL-2 cplx causes a significant reduction in levels of B7 (CD80, CD86) on dendritic cells (DCs) (32). We have confirmed this finding for CD11c+ spleen cells in mice treated with the IL-2 cplx protocol used here (Fig. 5H).
Discussion
Tregs are key players in the maintenance of self-tolerance as indicated by the lethal pathology that develops upon Treg depletion (33). For this reason, Treg therapy is considered a promising therapeutic tool for induction of tolerance, both for autoimmune diseases and organ transplantation. Although Treg therapy has received a lot of attention and several suppressive subpopulations of T cells have been described and characterized, there is no consensus on which particular Treg subset is best suited for therapeutic manipulation, nor is it clear under what conditions the cells should be expanded, either in vitro or in vivo (34). Moreover, although the function of Tregs has been demonstrated in various preclinical models (3, 16, 35–37), manipulating their numbers to induce efficient tolerance in vivo has proved difficult. In particular, to date no protocol utilizing Tregs to confer transplantation tolerance toward skin allografts has been successful in a stringent mouse model with an intact T cell repertoire.
As mentioned earlier, short-term injection of IL-2/JES6-1 mab complexes to expand Tregs in vivo led to indefinite survival of islet allografts in most mice (14) and provided potent suppression in a number of other transplantation and autoimmune disease models (16, 17, 38–40). However, this treatment conspicuously failed to enhance survival of skin allografts. Other groups had partial success by combining IL-2 cplx treatment with T cell depletion and adoptive Treg transfer and reported prolonged survival of skin allografts with an MST of 36 d (30). In another study, a combination of short-term IL-2 cplx treament and CD8 T cell depletion caused only a mild prolongation of skin graft survival in the BALB/c → B6 combination, though prolonged survival (>40 d) was seen with just a single MHC II mismatch (bm12 → B6) strain combination (17). Interestingly, the combination with costimulation blocker CTLA4Ig was detrimental for graft survival in this setting (38). Others reported promising results with daily injections of low-dose IL-2 plus rapamycin and achieved enhanced survival of MHC-mismatched skin allografts with an MST of 24 d (18). Most importantly, in all of these studies, however, the enhanced survival of fully MHC-mismatched grafts was only moderate.
In the studies reported here, our primary aim was to achieve prolonged survival of fully MHC-incompatible skin allografts in the absence of continuous immunosuppression. Initial studies where IL-2 cplx injection was combined with other individual treatments led to only a mild prolongation of graft rejection in the strongly immunogenic BALB/c → B6 combination. However, prolonging the injection of IL-2 cplx to 30 d and including treatment with a mixture of rapamycin and short-term IL-6 blockade led to extended graft survival with some of the grafts remaining viable for up to 100 d and an overall MST of 76 d. Including IL-6 mab in the protocol was especially important. IL-6 is a major proinflammatory cytokine secreted by many cell types, including macrophages and T cells, and is known to be synthesized during infection and after trauma to promote immune responses and tissue repair (22). As mentioned earlier, IL-6 blockade was shown previously to delay allograft rejection, especially mediated by CD4 T cells (25). In our hands, however, IL-6 blockade+/− rapa had no effect in prolonging graft survival in the absence of IL-2, yet led to long-term survival in combination with IL-2. In this respect, it is of interest that IL-6 blockade accentuated Treg expansion by IL-2 cplx and thereby may function by prolonging Treg function. This notion is in line with reports that IL-6 blockade in mice can stabilize Treg function (41) and also led to an increase in Treg numbers (20), both for thymic-derived and peripherally induced Tregs, and thereby reduces the severity of GVHD (42). Whether IL-6 blockade has similar effects on human Tregs is still unclear (43), although recent data have shown that anti–IL-6R mab (tocilizumab) therapy is beneficial for renal transplantation (44).
With the optimal IL-2 cplx protocol, it is of interest that allograft MST was even longer with B10.D2 grafts (105 d) than with BALB/c grafts (76 d), implying that tolerance induction was less effective with combined minor H plus MHC disparity than with MHC disparity alone. The reason for this difference is unclear, although it may be relevant that in vivo allogeneic responses to MHC antigens involve both CD4 and CD8 T cells, whereas minor H responses are mediated largely by CD8 cells (45, 46). In this respect, future studies will be needed to establish whether or not the capacity of the IL-2 cplx protocol to enhance allograft survival applies equally to CD4 and CD8 T cells. As mentioned above, the capacity of IL-6 blockade to prolong graft survival largely affects CD4 T cells (25).
With regard to how the IL-2 cplx protocol enhances graft survival, the prolongation of MHC-incompatible allograft survival was clearly Treg dependent as indicated by the rapid onset of graft rejection following Treg removal by injection of CD25 mab. The simplest explanation for these data is that the expansion of Tregs induced by the optimal IL-2 cplx protocol largely prevented an immune response to the graft antigens, thereby leading to an operational state of ignorance to the graft. This notion fits with the failure to induce alloantibody production soon or late after grafting, and also with the rapid rejection of the primary graft after application of a second graft, the lack of evidence for any apparent change in reactivity to host antigens seen in vitro, and the finding that regrafting with donor skin after eventual rejection of the primary graft did not lead to accelerated rejection of the second graft. However, these findings do not exclude some degree of initial sensitization to the graft antigens; indeed, despite apparent normal responsiveness in vitro, the very rapid tempo of rejection (5 d) seen following CD25 mab injection at 2 wk after grafting supports this possibility. Hence, an immune response may have simmered but was kept inert by the presence of Tregs. Nevertheless, it is notable that, when tested in MLR, Treg-depleted CD4 cells prepared at 2 wk postgrafting showed little or no signs of sensitization to the graft donor. As a whole, the data suggest that eventual late rejection of the grafts reflected the late onset of a primary response: at this stage the mice showed no signs of either sensitization or tolerance to the graft antigens.
This scenario raises the issue of why the primary grafts were eventually rejected. Since Treg numbers declined progressively during and after cessation of IL-2 cplx treatment, we envisage that the onset of host reactivity to the graft correlated with a gradual diminution of Treg function and was mediated largely by naïve rather than sensitized T cells. In addition, the slow tempo of rejection may have reflected that; because of their prolonged survival, the grafts were largely depleted of donor APCs (so-called passenger leukocytes) and were thus relatively resistant to rejection when Treg function waned. Here, a key issue is whether the host can ever be totally ignorant of an organ transplant (leaving aside grafting in a sequestered site). Other workers addressed this question by placing skin allografts on immunoincompetent SCID mice followed months later by reconstitution of the mice with fetal liver cells taken from syngeneic normal mice. Thus, after reconstitution, would newly formed T cells arising in the host thymus be able to detect and reject the well-established healthy allografts? Perhaps surprisingly, the grafts were all rejected, though with a slow tempo of 47 d (47). This finding supports the view that, in the current model, the grafts were initially maintained by Treg-mediated ignorance: mild sensitization to graft antigens may have occurred but at a level too weak to cause rejection. This state of operational tolerance lapsed when Treg numbers waned, allowing the progressive onset of an immune response by host T cells. Whether this response was mediated entirely by T cells that persisted from the time of initial grafting or by newly formed T cells arising in the thymus after grafting, or both, is unclear. Experiments with adult thymectomized mice will be needed to resolve this question.
As for other forms of tolerance mediated by Tregs, the issue of how these cells regulate the immune response is still controversial (8, 48). In addition to release of inhibitory mediators such as IL-10 and TGFβ, Tregs are now known to function in part by reducing the levels of B7 (CD80 and CD86) costimulatory molecules on DCs by CTLA4-mediated transendocytosis (49–51), a process that can be accentuated by increasing Treg numbers. Thus, other workers reported that increasing Treg numbers by injection of the same IL-2/mab complexes used here caused a prominent decrease in the background level of B7 on DCs (32). This finding, which we have confirmed in this study, suggests that the prolonged survival of allografts reported here may be largely a reflection of Treg-mediated reduced expression of costimulatory molecules on host APCs, thus leading to a failure to activate graft-reactive T cells. This state of ignorance then wanes gradually when Treg numbers—and B7 density on DCs—return to normal, thereby allowing nontolerant naïve T cells to eventually see the graft antigens in immunogenic form and mount a primary response. As mentioned earlier, ignorance could be accentuated by persistence of graft-infiltrating Tregs.
The clinical application of the current data is still unclear. Since the skin grafts were eventually rejected, clinicians might be reluctant to use this treatment without accompanying immunosuppression. However, it should be emphasized that our studies are limited to skin grafts and, given past success with islet allografts (16), it will be important to use the IL-2 cplx protocol to examine survival of clinically relevant heart and kidney transplants. With modification to deplete NK cells, this approach might be used to promote engraftment of donor hematopoietic cells (30). Success here might be a useful aid for the long-term goal of inducing central tolerance for organ transplantation (52).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903165116/-/DCSupplemental.
References
- 1.Vignali D. A., Collison L. W., Workman C. J., How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q., Bluestone J. A., Regulatory T-cell therapy in transplantation: Moving to the clinic. Cold Spring Harb. Perspect. Med. 3, a015552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joffre O., et al. , Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat. Med. 14, 88–92 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang J. Y., et al. , Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 118, 3619–3628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadig S. N., et al. , In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat. Med. 16, 809–813 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashuda H., Shimizu A., Uchiyama M., Okumura K., Prolongation of renal allograft survival by anergic cells: Advantages and limitations. Clin. Transplant. 24 (suppl. 22), 6–10 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Ezzelarab M. B., et al. , Regulatory T cell infusion can enhance memory T cell and alloantibody responses in lymphodepleted nonhuman primate heart allograft recipients. Am. J. Transplant. 16, 1999–2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai K., Uchiyama M., Hester J., Wood K., Issa F., Regulatory T cells for tolerance. Hum. Immunol. 79, 294–303 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Vaikunthanathan T., Safinia N., Boardman D., Lechler R. I., Lombardi G., Regulatory T cells: Tolerance induction in solid organ transplantation. Clin. Exp. Immunol. 189, 197–210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot J. D., Rasmussen J. P., Gavin M. A., Rudensky A. Y., A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Grinberg-Bleyer Y., et al. , IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 207, 1871–1878 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosmaczewska A., Low-dose interleukin-2 therapy: A driver of an imbalance between immune tolerance and autoimmunity. Int. J. Mol. Sci. 15, 18574–18592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klatzmann D., Abbas A. K., The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Siegel J. P., Puri R. K., Interleukin-2 toxicity. J. Clin. Oncol. 9, 694–704 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Boyman O., Kovar M., Rubinstein M. P., Surh C. D., Sprent J., Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311, 1924–1927 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Webster K. E., et al. , In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 206, 751–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vokaer B., Charbonnier L. M., Lemaître P. H., Le Moine A., Impact of interleukin-2-expanded regulatory T cells in various allogeneic combinations on mouse skin graft survival. Transplant. Proc. 44, 2840–2844 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Pilon C. B., et al. , Administration of low doses of IL-2 combined to rapamycin promotes allogeneic skin graft survival in mice. Am. J. Transplant. 14, 2874–2882 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Govender L., Wyss J. C., Kumar R., Pascual M., Golshayan D., IL-2-mediated in vivo expansion of regulatory T cells combined with CD154-CD40 co-stimulation blockade but not CTLA-4 Ig prolongs allograft survival in naive and sensitized mice. Front. Immunol. 8, 421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granofszky N., et al. , Anti-interleukin-6 promotes allogeneic bone marrow engraftment and prolonged graft survival in an irradiation-free murine transplant model. Front. Immunol. 8, 821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei J., et al. , Administration of anti-interleukin-6 monoclonal antibody prolongs cardiac allograft survival. Transpl. Int. 23, 1271–1281 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Zhao X., et al. , Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. Am. J. Transplant. 12, 90–101 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Waiser J., et al. , Interleukin-6 expression after renal transplantation. Nephrol. Dial. Transplant. 12, 753–759 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Batal I., et al. , Glomerular inflammation correlates with endothelial injury and with IL-6 and IL-1β secretion in the peripheral blood. Transplantation 97, 1034–1042 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Booth A. J., et al. , IL-6 promotes cardiac graft rejection mediated by CD4+ cells. J. Immunol. 187, 5764–5771 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahr B., et al. , Regulatory T cells promote natural killer cell education in mixed chimeras. Am. J. Transplant. 17, 3049–3059 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Keely S., Foster P. S., Stop press: Eosinophils drafted to join the Th17 team. Immunity 43, 7–9 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Hock K., et al. , Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6. Am. J. Transplant. 14, 2011–2022 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Williams M. A., et al. , Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J. Immunol. 165, 6849–6857 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Mahr B., et al. , IL-2/α-IL-2 complex treatment cannot be substituted for the adoptive transfer of regulatory T cells to promote bone marrow engraftment. PLoS One 11, e0146245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevel N., Allenbach Y., Klatzmann D., Salomon B., Benveniste O., Beneficial role of rapamycin in experimental autoimmune myositis. PLoS One 8, e74450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corry D. B., Kheradmand F., Induction and regulation of the IgE response. Nature 402 (suppl.), B18–B23 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Lahl K., et al. , Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204, 57–63 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley J. L., June C. H., Blazar B. R., Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity 30, 656–665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilat N., et al. , Treg-therapy allows mixed chimerism and transplantation tolerance without cytoreductive conditioning. Am. J. Transplant. 10, 751–762 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilat N., et al. , T-regulatory cell treatment prevents chronic rejection of heart allografts in a murine mixed chimerism model. J. Heart Lung Transplant. 33, 429–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duran-Struuck R., et al. , Effect of ex vivo-expanded recipient regulatory T cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in cynomolgus macaques. Transplantation 101, 274–283 (2017). Correction in: Transplantation102, e252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charbonnier L. M., et al. , CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am. J. Transplant. 12, 2313–2321 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Xu H., et al. , Utility of IL-2 complexes in promoting the survival of murine orthotopic forelimb vascularized composite allografts. Transplantation 102, 70–78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan J. J., et al. , IL-2/anti-IL-2 complexes ameliorate lupus nephritis by expansion of CD4+CD25+Foxp3+ regulatory T cells. Kidney Int. 91, 603–615 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Hsieh W. C., Hsu T. S., Chang Y. J., Lai M. Z., IL-6 receptor blockade corrects defects of XIAP-deficient regulatory T cells. Nat. Commun. 9, 463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X., et al. , Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 114, 891–900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinewietfeld M., Hafler D. A., The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 25, 305–312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J., et al. , Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am. J. Transplant. 17, 2381–2389 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Sprent J., Schaefer M., Lo D., Korngold R., Properties of purified T cell subsets. II. In vivo responses to class I vs. class II H-2 differences. J. Exp. Med. 163, 998–1011 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korngold R., Sprent J., Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J. Exp. Med. 165, 1552–1564 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajan T. V., Shultz L. D., Greiner D. L., Lack of peripherally induced tolerance to established skin allografts in immunologically reconstituted scid mice. Dev. Immunol. 3, 45–50 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothstein D. M., Camirand G., New insights into the mechanisms of Treg function. Curr. Opin. Organ Transplant. 20, 376–384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou T. Z., et al. , A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J. Immunol. 194, 2148–2159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qureshi O. S., et al. , Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi J., et al. , Unregulated antigen-presenting cell activation by T cells breaks self tolerance. Proc. Natl. Acad. Sci. U.S.A. 116, 1007–1016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sachs D. H., Kawai T., Sykes M., Induction of tolerance through mixed chimerism. Cold Spring Harb. Perspect. Med. 4, a015529 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.