Key Points
Question
To what extent can inpatient violence risk assessment be performed by applying machine learning techniques to clinical notes in patients’ electronic health records?
Findings
In this prognostic study, machine learning was used to analyze clinical notes recorded in electronic health records of 2 independent psychiatric health care institutions in the Netherlands to predict inpatient violence. Internal predictive validity was measured using areas under the curve, which were 0.797 for site 1 and 0.764 for site 2; however, applying pretrained models to data from other sites resulted in significantly lower areas under the curve.
Meaning
The findings suggest that inpatient violence risk assessment can be performed automatically using already available clinical notes without sacrificing predictive validity compared with existing violence risk assessment methods.
This prognostic study develops and validates a multivariable prediction model for assessing inpatient violence risk, using machine learning techniques applied to clinical notes written in patients’ electronic health records.
Abstract
Importance
Inpatient violence remains a significant problem despite existing risk assessment methods. The lack of robustness and the high degree of effort needed to use current methods might be mitigated by using routinely registered clinical notes.
Objective
To develop and validate a multivariable prediction model for assessing inpatient violence risk based on machine learning techniques applied to clinical notes written in patients’ electronic health records.
Design, Setting, and Participants
This prognostic study used retrospective clinical notes registered in electronic health records during admission at 2 independent psychiatric health care institutions in the Netherlands. No exclusion criteria for individual patients were defined. At site 1, all adults admitted between January 2013 and August 2018 were included, and at site 2 all adults admitted to general psychiatric wards between June 2016 and August 2018 were included. Data were analyzed between September 2018 and February 2019.
Main Outcomes and Measures
Predictive validity and generalizability of prognostic models measured using area under the curve (AUC).
Results
Clinical notes recorded during a total of 3189 admissions of 2209 unique individuals at site 1 (mean [SD] age, 34.0 [16.6] years; 1536 [48.2%] male) and 3253 admissions of 1919 unique individuals at site 2 (mean [SD] age, 45.9 [16.6] years; 2097 [64.5%] male) were analyzed. Violent outcome was determined using the Staff Observation Aggression Scale–Revised. Nested cross-validation was used to train and evaluate models that assess violence risk during the first 4 weeks of admission based on clinical notes available after 24 hours. The predictive validity of models was measured at site 1 (AUC = 0.797; 95% CI, 0.771-0.822) and site 2 (AUC = 0.764; 95% CI, 0.732-0.797). The validation of pretrained models in the other site resulted in AUCs of 0.722 (95% CI, 0.690-0.753) at site 1 and 0.643 (95% CI, 0.610-0.675) at site 2; the difference in AUCs between the internally trained model and the model trained on other-site data was significant at site 1 (AUC difference = 0.075; 95% CI, 0.045-0.105; P < .001) and site 2 (AUC difference = 0.121; 95% CI, 0.085-0.156; P < .001).
Conclusions and Relevance
Internally validated predictions resulted in AUC values with good predictive validity, suggesting that automatic violence risk assessment using routinely registered clinical notes is possible. The validation of trained models using data from other sites corroborates previous findings that violence risk assessment generalizes modestly to different populations.
Introduction
Violence in psychiatric inpatient wards remains a significant problem. A study1 combining data from 35 sites worldwide shows 14% to 20% of patients commit at least 1 act of violence during inpatient treatment, and surveys2 consistently show most practitioners being affected by violence at some point during their career. Adverse effects on both patients’ and caregivers’ well-being, such as injury, low morale, and high absentee levels, are well known.3,4
As an important part of managing inpatient violence, structured violence risk assessment (VRA) instruments have been proposed on the basis of a combination of static and dynamic risk factors. Their predictive validity surpasses that of unstructured clinical judgment, and a reasonable adoption in practice has been achieved, with more than half of all risk assessments performed using an instrument.5 However, meta-analyses reveal that only a small subset of risk factors for violent behavior generalize to different populations,6,7 and VRA instruments are consequently limited by the robustness of the individual factors that compose them.8,9 In addition, the time needed to perform a structured assessment, ranging from minutes to hours, has been identified as an obstacle for daily practice. Although adopting a VRA instrument diminished the number of violent incidents in 1 randomized clinical trial,10 other research11,12 suggests that its benefits in practice are still moderate because of its limitations.
Developing a prognostic model based on textual data registered in patients’ electronic health records (EHRs) might offer a novel approach to improve VRA. The fact that these data are unstructured and originally designated for treatment presents methodologic challenges but also opportunities in combating selection bias and exploring new associations.13 Machine learning, a term that refers to a set of statistical techniques that learn from large and potentially noisy data sets, is eminently well suited for this kind of task. Prognostic models obtained using these techniques are automatically tailored to the relevant population and can be fitted in the care process without imposing additional administrative load, circumventing drawbacks of structured VRA instruments. Although many fields of medicine have seen convincing cases of algorithms aiding clinical decision making (eg, cardiology,14 dermatology,15 and oncology16), the field of psychiatry still seems only on the verge of transforming in this direction.17,18 In this prognostic study, we tested to what extent textual data from the EHR can be used to automatically assess violence risk by developing and validating multivariable prediction models based on routinely collected clinical notes from 2 independent psychiatric treatment centers in the Netherlands.
Methods
In this study, we used data extracted from EHRs of 2 independent psychiatric treatment centers in the Netherlands. Data sources were not connected to each other or to sources outside the separate hospitals. We used deidentified data sets by deidentifying clinical notes using the Deindentification Method for Dutch Medical Text (DEDUCE) method.19 Demographic variables were limited to sex, year of birth, and Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnosis. The study was reviewed and approved by the University Medical Center Utrecht ethical committee. The committee assessed that obtaining informed consent retroactively was not necessary because of the retrospective nature of the study, the number of participants, the fact that no extra data were obtained, and the use of deidentified data. This report follows the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline20 and Reporting Guidance for Violence Risk Assessment Predictive Validity Studies (RAGEE).21
Cohort Definition
Site 1, used for internal method validation, was the psychiatry department of the academic medical center in Utrecht, the Netherlands. It delivers both secondary and tertiary care in 4 closed short-term treatment wards, including an acute ward and wards that focus on treatment of patients with psychotic disorders, mood disorders, and developmental disorders. A new admission was registered both when a new patient was admitted and when a patient was transferred between psychiatric wards. We allowed an absence of 2 weeks at most during admission, such as for discharge and readmission or temporary admission in a nonpsychiatric department; longer absences were registered as a new admission. Admissions in the developmental disorder ward were excluded according to patient age and the nature of violence. All admissions in other wards that started between January 2013 and August 2018 were included in the data set. We defined no exclusion criteria according to diagnosis, comorbidity, or other psychopathological conditions to maximize the translational value of predictive models. The resulting data set consisted of 3201 admissions of 2211 unique patients.
Site 2, used for external method validation, was a general psychiatric hospital that delivers secondary care, with an additional focus on addiction care. It consists of 47 treatment wards in the area of Rotterdam, the Netherlands. To match the original data set, admissions to 2 forensic psychiatric wards, 25 long-term care wards, and 9 wards that exclusively offer addiction care were not included in the study. All admissions in the 11 retained wards that started between June 2016 and August 2018 were included in the data set. Other conditions were kept equal. The resulting data set consisted of 3277 admissions of 1937 unique patients. Details explaining how data sets from both sites were extracted from EHR systems and how data quality was secured are shown in eAppendix 1 in the Supplement. We did not merge data sets but used the data set from site 1 for developing a machine learning approach, then used the data set from site 2 for externally validating this approach, and finally exchanged trained models between the sites.
Data Selection
Clinical notes that were written by psychiatrists and nurses were directly extracted from patients’ EHRs. We hypothesized that free text contains information that cannot easily be captured in structured form (eg, behavioral cues or social interactions) yet is relevant for VRA. Notes that were written in the 4 weeks before admission up to the first 24 hours of admission were included in the data sets. Admissions with fewer than 100 words registered after 24 hours (12 admissions in site 1 and 24 admissions in site 2) were excluded from the data set.
Outcome Variable
Reports of violent incidents were used to determine the outcome for each admission. In both sites mandatory reporting of all violent incidents takes place, including patient-staff and patient-patient violence. On the incident form, staff members who were involved in the incident were required to fill in structured information, a textual description of the incident, and incident severity as measured by the Staff Observation Aggression Scale–Revised.22 Our definition of a violent incident included all threatening and violent behavior of a verbal or physical nature directed at another person but excluded self-harm and inappropriate behavior, such as substance use, sexual intimidation, or vandalism. A positive outcome was defined as the presence of at least 1 incident in the first 4 weeks of admission, excluding the first 24 hours. No distinction in incident severity was made.
Exploratory Analysis
To examine the potential predictive power hidden in clinical notes, we extracted the 1000 most frequent terms in the clinical notes, including bigrams, as binary variables. We then assessed the strength of each term’s association with the outcome using a χ2 test and computed Matthews correlation coefficients to obtain the direction of the association. We selected the top 10% of predictors on the basis of their χ2 scores in 1000 repeated samples with replacement, computing the fraction of times a term was included among the top predictors as a measure of within–data set generalizability of predictors.
Machine Learning Models
We used a machine learning approach to perform VRA. Machine learning algorithms are able to detect patterns, if present, in historical data, and a prediction of the future course of treatment can be made on the basis of those patterns. Such an approach applied to textual data must comprise 2 steps: transforming clinical notes into a suitable numerical representation and subsequently feeding these numerical representations into a classification model.
To transform the clinical notes into a numerical form, we used the novel paragraph2vec algorithm,23 which learns an accurate numerical representation from a large corpus of text in an unsupervised way (ie, unrelated to outcome). This algorithm, founded in deep learning theory, is capable of using not only verbatim words in a text to determine a representation but also word order and the context of words such as negations. In previous work,24 we have shown the added value of this technique over a traditional bag-of-words approach when applied to VRA. The model was trained using a large internal set of clinical notes (ie, not only notes relevant for assessment), with model settings based on available literature without optimization (eAppendix 2 and eTable 1 in the Supplement).
The numerical representations of text were subsequently fed into a support vector machine with a radial kernel,25 a model that has previously been shown as appropriate for text classification.26 It works by first mapping data points to a higher-dimensional space and then inferring a decision boundary that maintains a maximum margin to these data points. New data points are subsequently classified according to the side of the boundary on which they lie.
Statistical Analysis
Model training and estimation of model predictive validity were done in a nested cross-validation setup, ensuring that admissions used for learning models were never used to simultaneously determine predictive validity. Different admissions of the same patient were additionally never split over different folds to ensure that predictions were not influenced by information from future admissions of the same patient. The final area under the curve (AUC) was computed by averaging the AUCs of the 5 outer cross-validation folds, while CIs and SEs were established using the method of DeLong et al.27 Additionally, performance metrics, such as sensitivity, specificity, and relative risk, were computed by pooling predictions over folds.28 The experimental setup is detailed further in eAppendix 3 in the Supplement. After finalizing the results in site 1, an external validation of the machine learning approach was performed in site 2 by training a new model with equal experimental setup. To further elucidate model performance, we investigated predictive validity for early-violence vs late-violence and short-admission vs long-admission subgroups. Finally, trained models were exchanged between sites to test their generalizability.
For the tokens discovered in exploratory analysis, the association with the outcome was determined using a χ2 test with a Holm-Bonferroni correction to control the familywise error rate. Differences in AUCs between various internal and external validations were tested for significance using the method of DeLong et al27 and Robin et al.29 We used a paired test when comparing 2 models on the same data set (ie, when comparing the cross-validated assessment and assessment using a pretrained model) to account for correlation between the 2 AUCs. In all other cases we used an unpaired test. All statistical significances in this study were assessed using 2-sided tests, and P < .01 was considered significant. The code for machine learning and statistical analysis was developed in Python software version 3.6 (Python Software Foundation) and is publicly available (eAppendix 4 in the Supplement).
Qualitative Evaluation
After finalizing the method and results in both sites, a qualitative evaluation was conducted in a focus group with participants, including practitioners, data analysts, and patient representatives from both sites. Participants discussed the method as presented by a researcher (V.M.) and interpreted the results. The participants’ attitude toward the method was positive, and its translation between sites was deemed appropriate. No changes were introduced to the study as a result of the focus group.
Results
Data Sets
The final data sets (Table 1) consisted of 3189 admissions from 2209 unique patients in site 1 and 3253 admissions from 1919 unique patients in site 2. Populations differed in age (mean [SD] age, 34.0 [16.6] and 45.9 [16.6] years, respectively), sex (1536 [48.2%] and 2097 [64.5%] men, respectively), and distribution of diagnoses. In both sites, the most commonly occurring diagnosis was schizophrenia or other psychotic disorders, followed by mood disorders and personality disorders in site 1 and substance-related disorders and bipolar disorders in site 2. Similar median (interquartile range [IQR]) lengths of stay (16.0 [6.0-41.0] and 15.0 [5.0-40.5] days), median (IQR) length of clinical notes (2091 [1541-2981] and 1961 [1160-3060] words), and admissions with a violent incidence (290 [9.1%] and 247 [7.7%]) were registered in both sites.
Table 1. Descriptive Statistics of the Data Sets Obtained From the 2 Sites.
| Characteristic | No. (%) | |
|---|---|---|
| Site 1 | Site 2 | |
| Demographic characteristics | ||
| Age, mean (SD), y | 34.0 (16.6) | 45.9 (16.6) |
| Men | 1536 (48.2) | 2097 (64.5) |
| Data set | ||
| Admissions, No. | 3189 | 3253 |
| Unique patients, No. | 2209 | 1919 |
| Length of stay, median (IQR), d | 16.0 (6.0-41.0) | 15.0 (5.0-40.5) |
| No. of words in notes, median (IQR) | 2091 (1541-2981) | 1961 (1160-3060) |
| Admissions with violent incidents | 290 (9.1) | 247 (7.7) |
| Incidents | ||
| During admission, No. | 962 | 652 |
| During first 4 wk | 658 (68.4) | 318 (48.8) |
| During first 24 h | 90 (9.4) | 42 (6.4) |
| Staff Observation Aggression Scale–Revised score, median (IQR) [range] | 12.0 (8.0-16.0) [2-21] | 11.0 (7.0-14.0) [2-19] |
| Diagnostic and Statistical Manual of Mental Disorders diagnosisa | ||
| Anxiety disorder | 92 (2.9) | 63 (1.9) |
| Bipolar disorder | 65 (2.0) | 170 (5.2) |
| Delirium, dementia, amnesia, and other cognitive disorders | 20 (0.6) | 109 (3.4) |
| Depressive disorder | 106 (3.3) | 150 (4.6) |
| Developmental disorder | 180 (5.6) | 29 (0.9) |
| Eating disorder | 57 (1.8) | 10 (0.3) |
| Mood disorder | 580 (18.2) | 10 (0.3) |
| Personality disorder | 214 (6.7) | 116 (3.6) |
| Substance-related disorder | 99 (3.1) | 373 (11.5) |
| Schizophrenia or other psychotic disorder | 860 (27.0) | 685 (21.1) |
| None within 12 wk | 795 (24.9) | 1392 (42.8) |
| Other | 121 (3.8) | 146 (4.5) |
Abbreviation: IQR, interquartile range.
Percentage relative to the total number of admissions.
Machine Learning Models
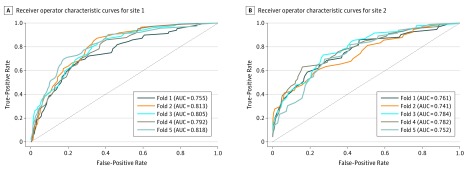
Several performance metrics of predictive validity, both for in-site validation using nested cross-validation and for other-site validation of pretrained models, were computed (Table 2). Optimal hyperparameters are shown in eTable 2 in the Supplement. An optimal AUC of 0.797 (95% CI, 0.771 to 0.822) was achieved for the internal validation of the method in site 1, while the optimal AUC for the external validation of the method in site 2 was 0.764 (95% CI, 0.732 to 0.797) (Figure). The difference in internal cross-validation AUCs between the 2 sites was not significant (AUC difference = 0.032; 95% CI, −0.009 to 0.074; P = .12). Specificity (ie, prediction in the negative class) of models was higher (0.935 to 0.947) than sensitivity (ie, prediction in the positive class; 0.334 to 0.336). The relative risk of violent outcome for patients with predicted high risk vs low risk was 5.121 (95% CI, 4.109-6.330) in site 1 and 6.297 (95% CI, 4.956-7.922) in site 2.
Table 2. Predictive Validity of Prognostic Models in Both Sites and Both Internally and Externally Trained.
| Evaluation | Internal Cross-validation | External Model | ||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 1 | Site 2 | |
| Model evaluated in site | 1 | 2 | 1 | 2 |
| Model trained in site | 1 | 2 | 2 | 1 |
| AUC (95% CI) [SE] | 0.797 (0.771-0.822) [0.013] | 0.764 (0.732-0.797) [0.017] | 0.722 (0.690-0.753) [0.016] | 0.643 (0.610-0.675) [0.017] |
| Admissions, No. | 3189 | 3253 | 3189 | 3253 |
| Negative, No. (%) | ||||
| True | 2711 (85.0) | 2847 (87.5) | 2682 (84.1) | 2793 (85.9) |
| False | 193 (6.1) | 164 (5.0) | 218 (6.8) | 214 (6.6) |
| Positive, No. (%) | ||||
| True | 97 (3.0) | 83 (2.6) | 72 (2.3) | 33 (1.0) |
| False | 188 (5.9) | 159 (4.9) | 217 (6.8) | 213 (6.5) |
| Specificity (95% CI) | 0.935 (0.930-0.940) | 0.947 (0.943-0.951) | 0.925 (0.921-0.930) | 0.929 (0.926-0.933) |
| Sensitivity (95% CI) | 0.334 (0.287-0.383) | 0.336 (0.285-0.389) | 0.248 (0.205-0.296) | 0.134 (0.097-0.179) |
| Relative risk (95% CI) | 5.121 (4.109-6.330) | 6.297 (4.956-7.922) | 3.314 (2.581-4.214) | 1.885 (1.305-2.673) |
Abbreviation: AUC, area under the curve.
Figure. Receiver Operator Characteristic Curves for Internal Cross-validations.
Receiver operator characteristic curves are shown for each fold, according to internal cross-validation in site 1 (A) and site 2 (B). Dashed diagonal lines denote an area under the curve (AUC) of 0.5, ie, predictive validity equivalent to chance. AUC indicates area under the curve.
The validation of pretrained models in the other site resulted in AUCs of 0.722 (95% CI, 0.690-0.753) in site 1 and 0.643 (95% CI, 0.610-0.675) in site 2. The difference in AUCs between the internally trained model and the model trained on other-site data was significant both in site 1 (AUC difference = 0.075; 95% CI, 0.045-0.105; P < .001) and site 2 (AUC difference = 0.121; 95% CI, 0.085-0.156; P < .001). Although specificity was still similar, both sensitivity and relative risk were lower compared with in-site validations.
We examined model performance in assessing early vs late violence and violence during short vs long admissions. In both internal and external validations and in both sites, predictive validity was higher for early violence than for late violence as well as higher for short admissions than for long admissions. However, the difference was never significant. For example, for the internal validation in site 1, the difference in AUCs for assessing early violence vs late violence was 0.046 (95% CI, −0.003 to 0.094; P = .06), and the difference in AUCs for assessing violence during short admissions vs long admissions was 0.012 (95% CI, −0.041 to 0.066; P = .65). Full subgroup analysis is included in eTable 3 and eTable 4 in the Supplement.
Exploratory Analysis
Of the 1000 most frequent terms from clinical notes, the top 20 terms by generalizability within a data set were selected (Table 3).30 Several terms, such as aggressive, angry, verbal, threatening, and irritated, can directly be associated with violence, whereas other terms, such as reacts, walks, and speaks, describe behavioral cues that may indirectly be associated with violence. The terms aggressive and walked and their synonyms are seen in both sites. Other terms do not directly co-occur in both sites but have a counterpart with a similar meaning (eg, colleague vs staff and door vs office). All terms generalize well within the data set, being chosen among the top 10% in repeated sampling at least 95% of the time. In site 1, the terms aggressive, reacts, and offered generalize best within the data set, whereas in site 2 the terms verbal, threatening, and aggression compose the top 3. The 47 terms in site 1 and 21 terms in site 2 with highest χ2 scores were significantly associated with the outcome after applying a Holm-Bonferroni correction. Matthews correlation coefficients ranged from −0.14 to 0.17, showing weak correlations. Most terms had a positive correlation with violent outcome, except status voluntary and dejected in site 1, which were negatively correlated with violent outcome (status voluntary: Matthews correlation coefficient, −0.12; 95% CI, −0.14 to −0.09; P < .001; dejected: Matthew correlation coefficient, −0.14; 95% CI, −0.17 to −0.11; P < .001).
Table 3. Results of Exploratory Analysis.
| Ranka | Site 1 | Site 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Term (English Translation)b | Ratio | MCC (95% CI)c | P Valued | Term (English Translation)b | Ratio | MCC (95% CI)c | P Valued | |
| 1 | Agressief (aggressive) | 1.00 | 0.17 (0.13 to 0.21) | <.001 | Verbaal (verbal) | 1.00 | 0.14 (0.10 to 0.18) | <.001 |
| 2 | Reageert (reacts) | 1.00 | 0.15 (0.11 to 0.19) | <.001 | Dreigend (threatening) | 1.00 | 0.13 (0.08 to 0.16) | <.001 |
| 3 | Aangeboden (offered) | 1.00 | 0.14 (0.11 to 0.18) | <.001 | Agressie (aggression) | 1.00 | 0.15 (0.11 to 0.17) | <.001 |
| 4 | Boos (angry) | 1.00 | 0.16 (0.12 to 0.19) | <.001 | Hierop ([up]on this) | 1.00 | 0.13 (0.09 to 0.16) | <.001 |
| 5 | Deur (door) | 1.00 | 0.14 (0.10 to 0.18) | <.001 | Kantoor (office) | 1.00 | 0.12 (0.08 to 0.16) | <.001 |
| 6 | Loopt (walks) | 1.00 | 0.15 (0.11 to 0.18) | <.001 | Personeel (staff) | 1.00 | 0.12 (0.07 to 0.16) | <.001 |
| 7 | Ibs (arrest) | 1.00 | 0.14 (0.10 to 0.17) | <.001 | Aangesproken (spoke to) | 1.00 | 0.11 (0.08 to 0.15) | <.001 |
| 8 | Aanbieden (offer) | 1.00 | 0.12 (0.08 to 0.15) | <.001 | Agressief (aggressive) | 0.99 | 0.11 (0.08 to 0.15) | <.001 |
| 9 | Noodmedicatie (emergency medication) | 0.99 | 0.14 (0.10 to 0.17) | <.001 | Gevaar agressie (danger aggression) | 0.99 | 0.11 (0.07 to 0.15) | <.001 |
| 10 | Liep (walked) | 0.99 | 0.12 (0.08 to 0.16) | <.001 | Agitatie (agitation) | 0.99 | 0.11 (0.07 to 0.14) | <.001 |
| 11 | Agressie (aggression) | 0.99 | 0.13 (0.09 to 0.18) | <.001 | Geirriteerd (irritated) | 0.99 | 0.10 (0.06 to 0.14) | .001 |
| 12 | Vraagt (asks) | 0.99 | 0.13 (0.10 to 0.17) | <.001 | Separeer (seclusion room) | 0.99 | 0.10 (0.06 to 0.15) | <.001 |
| 13 | Status vrijwillig (status voluntary) | 0.99 | −0.12 (−0.14 to −0.09) | <.001 | Loopt (walks) | 0.99 | 0.11 (0.08 to 0.14) | .02 |
| 14 | Psychotisch (psychotic) | 0.98 | 0.12 (0.09 to 0.16) | <.001 | Grond (ground) | 0.98 | 0.10 (0.06 to 0.14) | <.001 |
| 15 | Collega (colleague) | 0.98 | 0.11 (0.07 to 0.15) | <.001 | Aanvang (commencement) | 0.98 | 0.11 (0.08 to 0.14) | .01 |
| 16 | Spreekt (speaks) | 0.97 | 0.12 (0.08 to 0.15) | <.001 | Mede (also) | 0.98 | 0.10 (0.07 to 0.14) | .001 |
| 17 | Gehouden (obliged) | 0.97 | 0.11 (0.07 to 0.15) | <.001 | Dhr wilde (Mr wanted) | 0.98 | 0.10 (0.06 to 0.14) | .001 |
| 18 | Beoordelen (judge), verb | 0.96 | 0.11 (0.07 to 0.15) | <.001 | Liep (walked) | 0.98 | 0.10 (0.06 to 0.14) | .006 |
| 19 | Momenten (moments) | 0.96 | 0.12 (0.08 to 0.15) | <.001 | Geagiteerd (agitated) | 0.96 | 0.10 (0.06 to 0.14) | .01 |
| 20 | Somber (dejected) | 0.95 | −0.14 (−0.17 to −0.11) | <.001 | cvd (not available) | 0.96 | 0.10 (0.06 to 0.14) | .004 |
Abbreviation: MCC, Matthews correlation coefficient.
The top 20 terms with highest within–data set generalizability (ratio) are included.
The Van Dale Dutch–English Dictionary, 3rd edition,30 was used for translations.
Matthews correlation coefficient is computed to assess the direction of association between the term and outcome.
P values derived from χ2 test, and a Holm-Bonferroni correction was applied to obtain corrected P values.
Discussion
To our knowledge, this is the first time that readily available clinical notes from patients’ EHRs were used to assess inpatient violence risk. We applied machine learning techniques to retrospective textual data, to train a model that differentiates patients who show violent behavior during the first 4 weeks of admission from patients who do not. As far as we know, no study has performed VRA using clinical text, and no study has tested automatic VRA in multiple sites. The AUCs of internally cross-validated predictions (0.797 and 0.764) from this study lie in the range that can be seen as acceptable for application in practice. Although in-site validation of models obtained good results, other-site validation of pretrained models resulted in significantly lower predictive validity, corroborating previous findings that VRA generalizes modestly over different populations. This strengthens the case for using locally developed and/or trained models and methods for VRA. Our choice to balance between false-positive and false-negative findings for reporting outcomes resulted in higher predictive validity in the low-risk class (eg, sensitivity) than in the high-risk class (eg, specificity), which is largely in line with existing VRA research. To our knowledge, no assessment method has shown both high sensitivity and high specificity, characterizing the difficulty of performing VRA and the need for further improvements.
Violence risk assessment is a research topic that has been thoroughly described, and the predictive validity of many existing methods, such as VRA checklists and unstructured clinical judgment, has been reported in literature. Although our study, based on other data sets, does not allow making strong claims about whether machine learning improves predictive validity,31 we note that our internally validated predictive validities of AUC = 0.797 and AUC = 0.764 lie in the same range of existing methods while overcoming some of their drawbacks. For example, a study by Fazel et al32 assessed median (IQR) predictive performance of the 4 most commonly used VRA instruments over 30 different studies (AUC = 0.72 [0.68-0.78]), while another study by Teo et al33 assessed the level of accuracy of psychiatric residents (AUC = 0.52) and trained psychiatrists (AUC = 0.70). A study by Suchting et al34 performed automatic VRA based on roughly 300 structured variables with comparable performance to our approach (AUC = 0.78).
The terms obtained in exploratory analysis, before application of modeling techniques, demonstrate a potential new type of risk factor that should be taken into account. Violence risk assessment instruments are often based on a combination of static factors (eg, previous violent behavior or employment status) and dynamic factors (eg, hostility or disorder symptoms). The terms we extracted from text are mostly dynamic and pertain to behavioral cues (eg, angry or walked) and social interactions (eg, reacts or offered), which may be more difficult to capture in a structured instrument but appear to provide important additional information.
A major strength of our research is the translational value that is obtained by using clinical notes from the EHR. Clinical text is already recorded as part of treatment by most psychiatric health care institutions, implying that our machine learning approach can be widely used to support violence management in daily practice. Second, applying a flexible machine learning approach allows method customization to local requirements and furthermore reveals the predictive validity for the relevant population, which is of particular importance given the lack of robustness and generalizability of existing models and methods. Finally, much attention has been devoted to the actuarial vs clinical debate,35 pertaining to the question of whether actuarial VRA instruments or VRA instruments based on clinical judgment are superior. Our approach essentially combines both approaches by using clinical judgment captured in clinical notes as input for an actuarial tool. This allows leveraging of health care professionals’ clinical experience while establishing a reasonably objective judgment through subsequent statistical modeling.
Limitations
This study has limitations. One limitation is that the data obtained from EHRs were originally designated for treatment rather than research. This introduces some noise to our data set, in clinical notes and in violence incident reports, for example, in reporting discrepancies among different wards. This source of measurement uncertainty cannot be quantified, warranting some caution when interpreting our results. Furthermore, we predominantly used AUC, a measure of discrimination, to measure the predictive validity of our models. This measure is known to have some limitations, such as an inability to account for prevalence.36 We used a black box modeling approach combining the paragraph2vec and support vector machine algorithms to assess violence risk, inhibiting a straightforward substantiation of probability of violent behavior. Although the terms obtained in exploratory analysis together with the subgroup analysis of predictive validity have elucidated the problem context to some extent, they do not directly explain model behavior. How such explanations can reliably be obtained, both at the patient level and the model level, is still a topic of ongoing research in computer science.37 An exploration of model explainability is included in eAppendix 5 and the eFigure in the Supplement.
Before an automatic VRA approach can be used in practice, some important challenges need to be addressed. Our results point out that both high sensitivity and high specificity are unlikely to be achieved simultaneously. Further research is needed to point out the desired balance between false-positives and false-negatives, and hence, whether our prognostic models are most useful to identify patients at high or at low risk of violence. Additionally, what level of substantiation is necessary before automatic VRA can be used in practice also remains an open question, which should be addressed in discussion with professionals in the field.
Conclusions
In the near future, we envision that further advancements toward a data-driven psychiatric practice will be made and that EHR data will become an even more valuable asset in supporting important decisions in the clinical process. Machine learning approaches have been able to contribute substantially in other fields of medicine, and our study provides evidence that such progress is possible in mental health care as well. Although some crucial challenges need to be addressed before adoption is possible, this study highlights the potential value of EHR data, and clinical notes in particular, for decision support. Such support systems may in the future be widely applied in daily practice, contributing to more effective and efficient psychiatric treatment.
eAppendix 1. Data Set Formation
eAppendix 2. Paragraph2vec Model Training
eAppendix 3. Cross-validation Procedure
eAppendix 4. Code and Data Availability
eAppendix 5. Model Explainability
eFigure. Two Samples of Local Explanations of Models
eTable 1. Chosen Paragraph2vec Model Settings
eTable 2. Optimal Hyperparameters and Optimal AUC Based on the Inner Cross-validation Loop
eTable 3. Subgroup Analysis of Model Performance: Early vs Late Violence
eTable 4. Subgroup Analysis of Model Performance: Short vs Long Admissions
eReferences
References
- 1.Iozzino L, Ferrari C, Large M, Nielssen O, de Girolamo G. Prevalence and risk factors of violence by psychiatric acute inpatients: a systematic review and meta-analysis. PLoS One. 2015;10(6):. doi: 10.1371/journal.pone.0128536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Leeuwen ME, Harte JM. Violence against mental health care professionals: prevalence, nature and consequences. J Forensic Psychiatry Psychol. 2017;28(5):581-. doi: 10.1080/14789949.2015.1012533 [DOI] [Google Scholar]
- 3.Inoue M, Tsukano K, Muraoka M, Kaneko F, Okamura H. Psychological impact of verbal abuse and violence by patients on nurses working in psychiatric departments. Psychiatry Clin Neurosci. 2006;60(1):29-36. doi: 10.1111/j.1440-1819.2006.01457.x [DOI] [PubMed] [Google Scholar]
- 4.Nijman H, Bowers L, Oud N, Jansen G. Psychiatric nurses’ experiences with inpatient aggression. Aggress Behav. 2005;31(3):217-227. doi: 10.1002/ab.20038 [DOI] [Google Scholar]
- 5.Singh JP, Desmarais SL, Hurducas C, et al. International perspectives on the practical application of violence risk assessment: a global survey of 44 countries. Int J Forensic Ment Health. 2014;13(3):193-206. doi: 10.1080/14999013.2014.922141 [DOI] [Google Scholar]
- 6.Dack C, Ross J, Papadopoulos C, Stewart D, Bowers L. A review and meta-analysis of the patient factors associated with psychiatric in-patient aggression. Acta Psychiatr Scand. 2013;127(4):255-268. doi: 10.1111/acps.12053 [DOI] [PubMed] [Google Scholar]
- 7.Steinert T. Prediction of inpatient violence. Acta Psychiatr Scand Suppl. 2002;106(412):133-141. doi: 10.1034/j.1600-0447.106.s412.29.x [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Wong SCP, Coid J. The efficacy of violence prediction: a meta-analytic comparison of nine risk assessment tools. Psychol Bull. 2010;136(5):740-767. doi: 10.1037/a0020473 [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Grann M, Fazel S. A comparative study of violence risk assessment tools: a systematic review and metaregression analysis of 68 studies involving 25,980 participants. Clin Psychol Rev. 2011;31(3):499-513. doi: 10.1016/j.cpr.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 10.Abderhalden C, Needham I, Dassen T, Halfens R, Haug HJ, Fischer JE. Structured risk assessment and violence in acute psychiatric wards: randomised controlled trial. Br J Psychiatry. 2008;193(1):44-50. doi: 10.1192/bjp.bp.107.045534 [DOI] [PubMed] [Google Scholar]
- 11.Viljoen JL, Cochrane DM, Jonnson MR. Do risk assessment tools help manage and reduce risk of violence and reoffending? a systematic review. Law Hum Behav. 2018;42(3):181-214. doi: 10.1037/lhb0000280 [DOI] [PubMed] [Google Scholar]
- 12.Wand T. Investigating the evidence for the effectiveness of risk assessment in mental health care. Issues Ment Health Nurs. 2012;33(1):2-7. doi: 10.3109/01612840.2011.616984 [DOI] [PubMed] [Google Scholar]
- 13.Menger V, Spruit M, Hagoort K, Scheepers F. Transitioning to a data driven mental health practice: collaborative expert sessions for knowledge and hypothesis finding. Comput Math Methods Med. 2016;2016:9089321. doi: 10.1155/2016/9089321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944. doi: 10.1371/journal.pone.0174944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118. doi: 10.1038/nature21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J. 2014;13:8-17. doi: 10.1016/j.csbj.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of ‘precision psychiatry.’ BMC Med. 2017;15(1):80. doi: 10.1186/s12916-017-0849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh AM, Stewart R, John A, et al. ; MQ Data Science Group . Data science for mental health: a UK perspective on a global challenge. Lancet Psychiatry. 2016;3(10):993-998. doi: 10.1016/S2215-0366(16)30089-X [DOI] [PubMed] [Google Scholar]
- 19.Menger V, Scheepers F, van Wijk LM, Spruit M. DEDUCE: a pattern matching method for automatic de-identification of Dutch medical text. Telemat Inform. 2018;35(4):727-736. doi: 10.1016/j.tele.2017.08.002 [DOI] [Google Scholar]
- 20.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. doi: 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 21.Singh JP, Yang S, Mulvey EP; RAGEE Group . Reporting guidance for violence risk assessment predictive validity studies: the RAGEE Statement. Law Hum Behav. 2015;39(1):15-22. doi: 10.1037/lhb0000090 [DOI] [PubMed] [Google Scholar]
- 22.Nijman HLI, Muris P, Merckelbach HLGJ, et al. The staff observation aggression scale-revised (SOAS-R). Aggress Behav. 1999;25(3):197-209. doi: [DOI] [Google Scholar]
- 23.Le QV, Mikolov T Distributed representations of sentences and documents. https://arxiv.org/abs/1405.4053. Accessed May 31, 2019.
- 24.Menger V, Scheepers F, Spruit M. Comparing deep learning and classical machine learning approaches for predicting inpatient violence incidents from clinical text. Appl Sci. 2018;8(6):981. doi: 10.3390/app8060981 [DOI] [Google Scholar]
- 25.Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20(3):273-297. doi: 10.1007/BF00994018 [DOI] [Google Scholar]
- 26.Joachims T. Text categorization with support vector machines: learning with many relevant features. In: Nédellec C, Rouveirol C, eds. Machine Learning: ECML-98. Lecture Notes in Computer Science (Lecture Notes in Artificial Intelligence) Berlin, Germany: Springer; 1998;1398:137-142. doi: 10.1007/BFb0026683 [DOI] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 28.Forman G, Scholz M. Apples-to-apples in cross-validation studies: pitfalls in classifier performance measurement. SIGKDD Explor. 2010;12(1):49-57. doi: 10.1145/1882471.1882479 [DOI] [Google Scholar]
- 29.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannay M. Van Dale Dutch-English Dictionary. 3rd edition Utrecht, Netherlands: Van Dale Lexicografie, 1997. [Google Scholar]
- 31.Kattan MW. Factors affecting the accuracy of prediction models limit the comparison of rival prediction models when applied to separate data sets. Eur Urol. 2011;59(4):566-567. doi: 10.1016/j.eururo.2010.11.039 [DOI] [PubMed] [Google Scholar]
- 32.Fazel S, Singh JP, Doll H, Grann M. Use of risk assessment instruments to predict violence and antisocial behaviour in 73 samples involving 24 827 people: systematic review and meta-analysis. BMJ. 2012;345:e4692. doi: 10.1136/bmj.e4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo AR, Holley SR, Leary M, McNiel DE. The relationship between level of training and accuracy of violence risk assessment. Psychiatr Serv. 2012;63(11):1089-1094. doi: 10.1176/appi.ps.201200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchting R, Green CE, Glazier SM, Lane SD. A data science approach to predicting patient aggressive events in a psychiatric hospital. Psychiatry Res. 2018;268:217-222. doi: 10.1016/j.psychres.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 35.Monahan J, Skeem JL. The evolution of violence risk assessment. CNS Spectr. 2014;19(5):419-424. doi: 10.1017/S1092852914000145 [DOI] [PubMed] [Google Scholar]
- 36.Halligan S, Altman DG, Mallett S. Disadvantages of using the area under the receiver operating characteristic curve to assess imaging tests: a discussion and proposal for an alternative approach. Eur Radiol. 2015;25(4):932-939. doi: 10.1007/s00330-014-3487-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller T. Explanation in artificial intelligence: insights from the social sciences. Artif Intell. 2019;267:1-38. doi: 10.1016/j.artint.2018.07.007 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Data Set Formation
eAppendix 2. Paragraph2vec Model Training
eAppendix 3. Cross-validation Procedure
eAppendix 4. Code and Data Availability
eAppendix 5. Model Explainability
eFigure. Two Samples of Local Explanations of Models
eTable 1. Chosen Paragraph2vec Model Settings
eTable 2. Optimal Hyperparameters and Optimal AUC Based on the Inner Cross-validation Loop
eTable 3. Subgroup Analysis of Model Performance: Early vs Late Violence
eTable 4. Subgroup Analysis of Model Performance: Short vs Long Admissions
eReferences