Abstract
Background
We applied an in vitro model to evaluate the efficacy of a heparinized 40% ethanol-based lock solution in a wide variety of clinical isolates causing C-RBSI.
Methods
A total of 100 clinical strains were collected retrospectively from the blood of patients with C-RBSI. The reduction in biomass and metabolic activity of biofilms was measured using the crystal violet (CV) assay and XTT assay, respectively. Regrowth inhibition (RI) was measured within 24 hours and 72 hours of ethanol lock therapy. Percentage reduction of ≥ 85% in RI was considered to be successful.
Results
Ethanol lock was more effective in reducing metabolic activity than in reducing biomass (83% vs. 50%, respectively). Percentages of RI diminished as regrowth was prolonged (57% for 24 hours and 17% for 72 hours of regrowth). No statistically significant intraspecies differences were found in biofilm reduction or in RI (p>0.05).
Conclusions
The use of heparinized 40% ethanol lock solution for 72 hours significantly reduced biomass and metabolic activity in clinical isolates from patients with C-RBSI. However, as biofilm has an important regrowth rate, 40% ethanol solution was not able to fully eradicate biofilm in vitro.
Introduction
Catheter-related bloodstream infection (C-RBSI) is one of the most problematic nosocomial infections, with high rates of morbidity and mortality, high associated costs, and prolonged hospital stays in patients with long-term catheters that cannot be removed [1, 2]. The main causative agents of C-RBSI are gram-positive cocci, gram-negative bacilli, and yeasts, owing to their capacity to form robust biofilms on the catheter surface [3, 4].
When C-RBSI is suspected, guidelines recommend catheter withdrawal [5]. However, patients with long-term catheters and no alternative central access have to maintain their catheter in place [6]. In these cases, the main approach is to use conservative treatments based on the application of intravenous antibiotics combined with antibiotic lock therapy [1]. In a recent study published by Freire et al., ALT was successful in 75.9% of cancer patients, with an improvement in patient outcome [7]. However, increasing antimicrobial resistance rates are making antibiotic lock therapy less eligible; therefore, many other lock solutions are being tested [6, 8, 9].
Ethanol has high anti-biofilm activity. It is also easy to use and cost-effective, with no reports of associated resistance with promising results in clinical trials showing catheter salvage rates of between 71% and 100% [10–13]. Ethanol lock has been used widely for prophylactic proposes in C-RBSI [11, 12, 14, 15]. However the use of ethanol lock solutions for C-RBSI treatment is scarce [16]. Moreover, these solutions still have some controversies regarding dose, time of treatment, combination with anticoagulants and adverse effects [17, 18]. Hence, this study demonstrated that in an in vitro model ethanol at a relatively low concentration, such as 40% can be combined with heparin and can be effective in controlling C-RBSI. However, this study represents the first step in this kind of research as it. This solution must be evaluated in an in vivo model such as a murine model to analyse its efficacy and safety in vivo before being applied in clinical trials, which would be the last step of evaluation. Thus, our study opens new ways for ethanol lock solution research.
In a previous study by our group, a solution of 40% ethanol combined with 60 international units of heparin proved highly active against bacterial and fungal biofilms in ATCC strains [19]. However, the behavioral characteristics of ATCC strains differ from those of clinical strains [20]. Therefore, we applied an in vitro model to test the efficacy of a heparinized ethanol-based lock solution in a wide variety of clinical strains isolated from patients with C-RBSI. Hence, our study is the first to describe the efficacy of 40% ethanol-heparin lock solution in a large sample of clinical strains.
Materials and methods
Strains
A total of 100 clinical strains were collected retrospectively from the blood of patients with C-RBSI. Their distribution was as follows: 20 Staphylococcus aureus (10 methicillin-susceptible S. aureus and 10 methicillin-resistant S. aureus), 20 coagulase-negative staphylococci (CoNS) (10 S. epidermidis), 20 Escherichia coli, 20 Enterococci spp. (10 E. faecium and 10 E. faecalis), and 20 Candida albicans.
Biofilm formation
Biofilms were grown as described in our previous study [19]. Briefly, a loopful of fresh culture was inoculated in 20 ml of medium and incubated at 30°C with shaking overnight. After 3 cycles of centrifugation-resuspension with PBS, inocula were adjusted to 0.5 or 0.35 McFarland for bacteria and yeast, respectively. One hundred microliters of each suspension were placed in a polypropylene 96-well microtiter plate and incubated at 37°C for 24 hours. Fresh medium (Sigma-Aldrich, Spain) was used as a negative control. Plates (Francisco Soria, Spain) were washed 3 times with PBS. All strains were tested in triplicate.
Ethanol lock treatment
Each well was treated with 120 μl of 40% ethanol and 60 international units of heparin (heparin sodium, 5,000 IU/5 ml, Hospira Prod. Farm. y Hosp, S.L.) plus 120 μl of medium at 37°C for 72 hours. Positive controls were treated only with 120 μl of fresh medium. Ethanol solution and medium were replaced every 24 hours.
Biomass reduction assay
Reduction in biofilm biomass was evaluated using crystal violet (CV) dye. After ethanol treatment, plates were washed 3 times with PBS. After plates were completely dry, 200 μl of 99% methanol were added for 10 minutes at room temperature. Methanol was discarded, and 125 μl of 0.1% CV was added for 10 minutes at room temperature. Plates were washed with distilled water, and fixed CV was released using 125 μl of 30% acetic acid for 15 minutes. The volume was transferred to a new plate, and absorbance (550 nm) was measured in a spectrophotometer (Biochrom EZ Read 400, Mervilab, Spain). The percentage of reduction was calculated using Eq 1.
Metabolic activity reduction assay
Plates were washed 3 times with PBS, and metabolic activity was measured by adding 100 μl of a premixed solution of 10 ml of XTT (0.5 mg/ml) (Sigma-Aldrich, Spain) with 40 μl of menadione (1.72 mg/ml) (Sigma-Aldrich, Spain) protected from light. Plates were incubated at 37°C for 2 hours, and absorbance was measured at 492 nm in a spectrophotometer (Biochrom EZ Read 400, Mervilab, Spain). The percentage of reduction in metabolic activity was calculated using Eq 2.
Regrowth inhibition
After ethanol therapy was administered and wells were washed 3 times with PBS, 100 μl of fresh medium was added and incubated for 24 hours and 72 hours at 37°C. The medium was replaced every 24 hours. Absorbance was measured at 492 nm, and the percentage of regrowth inhibition (RI) for each incubation period was calculated using Eq 3.
Statistical analyses
Data were expressed as mean (SD) for percentage reduction in biomass, metabolic activity, and RI. We considered a percentage of ≥85% as successful RI according to clinical data on the success of antibiotic lock therapy [21].
Quantitative variables were compared using an ANOVA test and Games-Howell post hoc test. Qualitative variables were compared using the chi-square test. Statistical significance was set at p<0.05. All tests were performed using the statistical program SPSS 21.0 (IBM).
Results
Ethanol lock solution was successful in decreasing biomass and metabolic activity in 50% and 83% of cases, respectively. Table 1 describes the distribution of all bacteria and fungal species in which treatment was successful. Ethanol showed better anti-biofilm activity for XTT than for CV in all species.
Table 1. Distribution of strains in which 40% ethanol-60 IU heparin lock solution for 72 hours proved successful.
| Microorganism | Success rate*, N (%) | |
|---|---|---|
| CV | XTT | |
| S. aureus (N = 20) | 7 (35) | 8 (40) |
| CoNS (N = 20) | 5 (25) | 16 (80) |
| Enterococci sp. (N = 20) | 19 (95) | 19 (95) |
| E. coli (N = 20) | 19 (95) | 20 (100) |
| C. albicans (N = 20) | 0 (0) | 20 (100) |
| Total (N = 100) | 50 (50) | 83 (83) |
IU; international units; CV, crystal violet; XTT, 2,3-Bis-(2- methoxy 4-nitro-5-sulfophenyl)-2H-tetrazolium5-carboxanilide salt; CoNS, coagulase-negative staphylococci.
*Success rate was set as ≥85%.
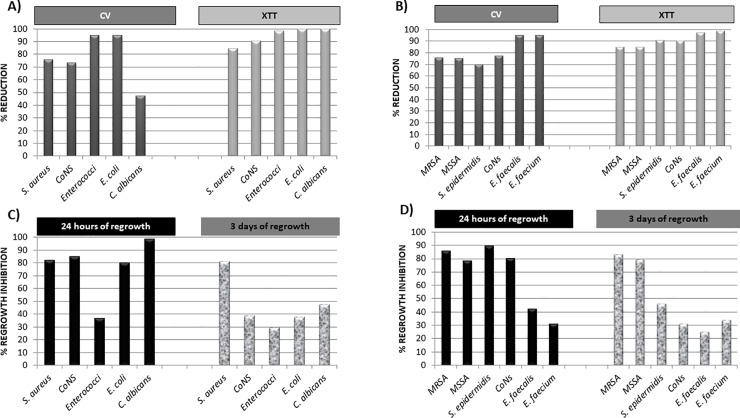
The overall percentages of reduction for CV and XTT assays are shown in Fig 1A. Percentages ranged between 47.5% (C. albicans) and 95.2% (Enterococci sp.) (p<0.001) for biomass reduction and 84.8% (S. aureus) and 100% (E. coli) (p<0.001) for metabolic activity reduction. No statistically significant intraspecies differences were found (p>0.05) (Fig 1B).
Fig 1. Percentage reduction of biomass and metabolic activity and percentage of regrowth inhibition of biofilms after 72-hour treatment of 40% ethanol combined with 60 international units (IU) of heparin.
A) Overall percentage reduction in biomass and metabolic activity after 72-hour treatment with heparinized 40% ethanol lock solution. B) Percentage reduction in biomass and metabolic activity after 72-hour treatment with heparinized 40% ethanol lock solution according to species. C) Overall percentage of regrowth inhibition within 24 hours and 72 hours after 72-hour treatment with heparinized 40% ethanol lock solution. D) Percentage of regrowth inhibition within 24 hours and 72 hours after 72-hour treatment with heparinized 40% ethanol lock solution according to species. CV, crystal violet; XTT, 2,3-Bis-(2- methoxy 4-nitro-5-sulfophenyl)-2H-tetrazolium5-carboxanilide salt; CoNS, coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
As for RI after ethanol lock, rates decreased for all strains from 57% to 17% of success within 24 hours and 72 hours, respectively (Table 2). After 24 hours of regrowth, inhibition ranged from 98.5% (C. albicans) to 36.8% (Enterococci sp.) (p<0.001). In contrast, when regrowth was assessed within 72 hours, percentages of inhibition varied between 81.2% (S. aureus) and 29.4% (Enterococci sp.) (p<0.001). RI for S. aureus was ≥80% in both periods.
Table 2. Percentages of success in regrowth inhibition (RI) after 40% ethanol-60 IU heparin lock solution for 72 hours.
| Microorganism | Success rate* of RI, N (%) | |
|---|---|---|
| 24 hours | 72 hours | |
| S. aureus (N = 20) | 13 (65) | 10 (50) |
| CoNS (N = 20) | 12 (60) | 1 (5) |
| Enterococci sp. (N = 20) | 1 (5) | 0 (0) |
| E. coli (N = 20) | 11 (55) | 4 (20) |
| C. albicans (N = 20) | 20 (100) | 2 (10) |
| Total (N = 100) | 57 (57) | 17 (17) |
CoNS, coagulase-negative staphylococci.
*Success rate for RI was set as ≥85%.
Fig 1C shows the overall results for RI. No statistical intraspecies differences were found at 24 hours or at 72 hours after ethanol lock therapy (p>0.05) (Fig 1D).
Discussion
We found that 40% ethanol plus 60 IU of heparin was able to reduce metabolic activity by up to 85% in 5 of the most causative agents of C-RBSI after 72 hours of locking. However, these strains were able to regrow within 72 hours after ethanol therapy.
Although the frequency of C-RBSI has decreased in the last decade, this condition still represents a huge challenge in clinical settings, with high associated costs (€18,000€/episode), high mortality (up to 25%), and longer hospitalizations [22, 23]. Thus, research has focused on prophylaxis and treatment of C-RBSI in patients with no possibility of catheter replacement using different agents as lock therapy [24]. Antibiotics are the most common agent for lock therapy [25]. However, overuse of antibiotics is increasing the frequency of multidrug-resistant strains [6]. Ethanol has been proposed as an alternative to antibiotics in lock therapy [12, 13]. However, most clinical studies used 70% ethanol, which shows important adverse effects such as ethanol taste, nausea, dizziness, rupture of catheter lumen, or catheter occlusion [17]. In our previous study, we demonstrated that 40% ethanol for 72 hours was sufficient to reduce the metabolic activity of biofilm in ATCC strains [19]. Furthermore, this concentration of alcohol can be safely combined with heparin, which is required for locks of 24 hours or more [19]. In our study we demonstrated that this ethanol solution is also efficient in reducing biofilms of C-RBSI clinical isolates.
Our results are consistent with those of other in vitro studies. Öncü et al. reported the anti-biofilm activity of 40% ethanol in Candida species [26]. Using 1 cm of silicon catheter, the authors demonstrated that 40% ethanol was able to inhibit growth of clinical C. albicans isolates in Sabouraud dextrose agar within 30 minutes [26]. Using the XTT assay, Peters et al., [27] reported complete inhibition of metabolic activity with 40% ethanol for 4 hours in C. albicans and S. aureus prototype strains. Moreover, Chaudhury et al. and Qu et al., did not recover any colonies of S. aureus or CoNS after 1 hour of 40% ethanol lock therapy [28, 29]. In the case of gram-negative bacilli, Chamber et al., showed bactericidal effects when 30%-90% ethanol was applied to mature biofilms for 8 hours [30].
While very impressive in terms of reduced metabolic activity, our results show that regrowth inhibition was low in almost all the tested species within 24 hours and 72 hours after ethanol lock therapy. Regrowth of almost all the strains did not achieve a 100% reduction, except for E. coli, which showed a 95.1% reduction in biomass, suggesting that some metabolically active cells were still able to resuscitate after the addition of fresh medium. This phenomenon, known as viable but non-culturable (VBNC) cells, remains markedly challenging for clinicians and accounts for the high rates of treatment failure [31]. We hypothesize that this kind of cell could be present in all tested strains, as the factors inducing this cellular state include environmental stress and changes in pH, both of which are associated with exposure to ethanol [32–34]. Moreover, percentages of reduction in biomass and metabolic activity differed substantially, thus supporting the conversion of active cells to VBNC cells. Although the CV and XTT assays are complementary for biofilm characterization [35], CV can dye viable cells that cannot be detected using XTT, as they are metabolically inactive. Furthermore, Parasuraman et al. have demonstrated in a new study that confocal microscopy could be very useful in evaluating the cell viability which would be very useful for detecting VBNC cells and hence, the real efficacy of ethanol lock solution [36]. The authors also demonstrated the usefulness of nanoparticles for inhibiting biofilm formation and extracellular matrix production [36]. The use of ethanol encapsulated in nanoparticles could improve ethanol penetration in the inner layers of biofilms and thus, eradicating the biofilm and completely inhibit cell regrowth.
To the best of our knowledge, this is the first in vitro study to describe the anti-biofilm activity of ethanol in such a large collection of clinical C-RBSI strains, with the novelty of adding heparin in the lock solution. Furthermore, given that ethanol lock therapy has not previously been reported to be efficacious for Enterococci sp., our findings provide new insight into the treatment of C-RBSI.
Our study is subject to limitations. First, the in vitro model does not mimic clinical conditions and is subject to high biological variations even between wells [37]. However, this system is ideal for screening purposes and for testing different antibiotics and disinfectants in a large numbers of strains simultaneously [37]. Second, we did not count colony-forming units, although our results are similar to those obtained by other authors [26, 28, 29]. Third, we only studied the efficacy of ethanol lock therapy in monomicrobial biofilms and it would be necessary to assess this efficacy in polymicrobial biofilms due to the clinical relevance that they represent.
In conclusion, our results provide a good preliminary insight into the effect of 40% ethanol-60 IU heparin as lock solution for C-RBSI. Although future studies are needed to improve ethanol penetration and its action in VBNC cells, this lock solution could be an alternative to conservative antibiotic lock therapy solutions for maintaining catheters until they can be replaced.
Supporting information
(SPS)
(XLSX)
(SAV)
(ZIP)
Acknowledgments
We thank Thomas O'Boyle for his help in the preparation of the manuscript and Dr. Escribano, Dr. Guinea and our colleagues, especially María Ángeles Bordallo-Cardona, for providing us with the C. albicans clinical strains.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
M. Guembe is supported by the Miguel Servet Program (ISCIIIMICINN, MS13/00268) from the Health Research Fund (FIS) of the Carlos III Health Institute (ISCIII), Madrid, Spain. The study has been partially financed by the European Regional Development Fund (FEDER) “A way of making Europe”.
References
- 1.Chaves F, Garnacho-Montero J, Del Pozo JL, Bouza E, Capdevila JA, de Cueto M, et al. Executive summary: Diagnosis and Treatment of Catheter-Related Bloodstream Infection: Clinical Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC) and the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC). Enferm Infecc Microbiol Clin. 2018;36(2):112–9. 10.1016/j.eimc.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 2.Dibb MJ, Abraham A, Chadwick PR, Shaffer JL, Teubner A, Carlson GL, et al. Central Venous Catheter Salvage in Home Parenteral Nutrition Catheter-Related Bloodstream Infections: Long-Term Safety and Efficacy Data. JPEN J Parenter Enteral Nutr. 2016;40(5):699–704. 10.1177/0148607114549999 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Creixems M, Munoz P, Martin-Rabadan P, Cercenado E, Guembe M, Bouza E. Evolution and aetiological shift of catheter-related bloodstream infection in a whole institution: the microbiology department may act as a watchtower. Clin Microbiol Infect. 2013;19(9):845–51. 10.1111/1469-0691.12050 [DOI] [PubMed] [Google Scholar]
- 4.Gominet M, Compain F, Beloin C, Lebeaux D. Central venous catheters and biofilms: where do we stand in 2017? Apmis. 2017;125(4):365–75. 10.1111/apm.12665 [DOI] [PubMed] [Google Scholar]
- 5.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassallo M, Dunais B, Roger PM. Antimicrobial lock therapy in central-line associated bloodstream infections: a systematic review. Infection. 2015;43(4):389–98. 10.1007/s15010-015-0738-1 [DOI] [PubMed] [Google Scholar]
- 7.Freire MP, Pierrotti LC, Zerati AE, Benites L, da Motta-Leal Filho JM, Ibrahim KY, et al. Role of Lock Therapy for Long-Term Catheter-Related Infections by Multidrug-Resistant Bacteria. Antimicrob Agents Chemother. 2018;62(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaftari AM, Hachem R, Szvalb A, Taremi M, Granwehr B, Viola GM, et al. A Novel Nonantibiotic Nitroglycerin-Based Catheter Lock Solution for Prevention of Intraluminal Central Venous Catheter Infections in Cancer Patients. Antimicrob Agents Chemother. 2017;61(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, Mehta P, Chen C, Daines DA, Mermel LA, Chen HL, et al. Antimicrobial Efficacy and Safety of a Novel Gas Plasma-Activated Catheter Lock Solution. Antimicrob Agents Chemother. 2018;62(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broom J, Woods M, Allworth A, McCarthy J, Faoagali J, Macdonald S, et al. Ethanol lock therapy to treat tunnelled central venous catheter-associated blood stream infections: results from a prospective trial. Scand J Infect Dis. 2008;40(5):399–406. 10.1080/00365540701756953 [DOI] [PubMed] [Google Scholar]
- 11.Metcalf SC, Chambers ST, Pithie AD. Use of ethanol locks to prevent recurrent central line sepsis. J Infect. 2004;49(1):20–2. 10.1016/j.jinf.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 12.Tan M, Lau J, Guglielmo BJ. Ethanol locks in the prevention and treatment of catheter-related bloodstream infections. Ann Pharmacother. 2014;48(5):607–15. 10.1177/1060028014524049 [DOI] [PubMed] [Google Scholar]
- 13.Rajpurkar M, McGrath E, Joyce J, Boldt-MacDonald K, Chitlur M, Lusher J. Therapeutic and prophylactic ethanol lock therapy in patients with bleeding disorders. Haemophilia. 2014;20(1):52–7. 10.1111/hae.12241 [DOI] [PubMed] [Google Scholar]
- 14.Broom JK, Krishnasamy R, Hawley CM, Playford EG, Johnson DW. A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients—the HEALTHY-CATH trial. BMC Nephrol. 2012;13:146 10.1186/1471-2369-13-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worth LJ, Slavin MA, Heath S, Szer J, Grigg AP. Ethanol versus heparin locks for the prevention of central venous catheter-associated bloodstream infections: a randomized trial in adult haematology patients with Hickman devices. J Hosp Infect. 2014;88(1):48–51. 10.1016/j.jhin.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Blackwood RA, Issa M, Klein K, Mody R, Willers M, Teitelbaum D. Ethanol Lock Therapy for the Treatment of Intravenous Catheter Infections That Have Failed Standard Treatment. J Pediatric Infect Dis Soc. 2017;6(1):94–7. 10.1093/jpids/piv060 [DOI] [PubMed] [Google Scholar]
- 17.Mermel LA, Alang N. Adverse effects associated with ethanol catheter lock solutions: a systematic review. J Antimicrob Chemother. 2014;69(10):2611–9. 10.1093/jac/dku182 [DOI] [PubMed] [Google Scholar]
- 18.Perez-Granda MJ, Barrio JM, Munoz P, Hortal J, Rincon C, Rabadan PM, et al. Ethanol lock therapy (E-Lock) in the prevention of catheter-related bloodstream infections (CR-BSI) after major heart surgery (MHS): a randomized clinical trial. PLoS One. 2014;9(3):e91838 10.1371/journal.pone.0091838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso B, Pérez-Granda MJ, Rodríguez-Huerta A, Rodríguez C, Bouza E, Guembe M. The optimal ethanol lock therapy regimen for treatment of biofilm-associated catheter infections: Results from an in vitro study. J Hosp Infect. 2018. [DOI] [PubMed] [Google Scholar]
- 20.Alnuaimi AD, O'Brien-Simpson NM, Reynolds EC, McCullough MJ. Clinical isolates and laboratory reference Candida species and strains have varying abilities to form biofilms. FEMS Yeast Res. 2013;13(7):689–99. 10.1111/1567-1364.12068 [DOI] [PubMed] [Google Scholar]
- 21.Bookstaver PB, Gerrald KR, Moran RR. Clinical outcomes of antimicrobial lock solutions used in a treatment modality: a retrospective case series analysis. Clinical pharmacology: advances and applications. 2010;2:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. International Journal of Critical Illness and Injury Science. 2014;4(2):162–7. 10.4103/2229-5151.134184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riu M, Terradas R, Sala M, Comas M, Knobel H, Grau S, et al. [Costs associated with nosocomial bacteraemias in a University Hospital]. Enferm Infecc Microbiol Clin. 2012;30(3):137–42. 10.1016/j.eimc.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Salonen BR, Bonnes SL, Vallumsetla N, Varayil JE, Mundi MS, Hurt RT. A prospective double blind randomized controlled study on the use of ethanol locks in HPN patients. Clin Nutr. 2018;37(4):1181–5. 10.1016/j.clnu.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 25.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clinical infectious diseases. 2011;52(9):e162–e93. 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oncu S. Optimal dosage and dwell time of ethanol lock therapy on catheters infected with Candida species. Clin Nutr. 2014;33(2):360–2. 10.1016/j.clnu.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 27.Peters BM, Ward RM, Rane HS, Lee SA, Noverr MC. Efficacy of ethanol against Candida albicans and Staphylococcus aureus polymicrobial biofilms. Antimicrob Agents Chemother. 2013;57(1):74–82. 10.1128/AAC.01599-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhury A, Rangineni J, B V. Catheter lock technique: in vitro efficacy of ethanol for eradication of methicillin-resistant staphylococcal biofilm compared with other agents. FEMS Immunol Med Microbiol. 2012;65(2):305–8. 10.1111/j.1574-695X.2012.00950.x [DOI] [PubMed] [Google Scholar]
- 29.Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA. Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase-negative staphylococcal biofilms. J Med Microbiol. 2009;58(Pt 4):442–50. 10.1099/jmm.0.006387-0 [DOI] [PubMed] [Google Scholar]
- 30.Chambers ST, Peddie B, Pithie A. Ethanol disinfection of plastic-adherent micro-organisms. J Hosp Infect. 2006;63(2):193–6. 10.1016/j.jhin.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 31.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:258 10.3389/fmicb.2014.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lleo MM, Bonato B, Tafi MC, Signoretto C, Boaretti M, Canepari P. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol. 2001;91(6):1095–102. [DOI] [PubMed] [Google Scholar]
- 33.Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S. Current Perspectives on Viable but Non-Culturable (VBNC) Pathogenic Bacteria. Front Public Health. 2014;2:103 10.3389/fpubh.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zandri G, Pasquaroli S, Vignaroli C, Talevi S, Manso E, Donelli G, et al. Detection of viable but non-culturable staphylococci in biofilms from central venous catheters negative on standard microbiological assays. Clin Microbiol Infect. 2012;18(7):E259–61. 10.1111/j.1469-0691.2012.03893.x [DOI] [PubMed] [Google Scholar]
- 35.Alonso B, Lucio J, Perez-Granda MJ, Cruces R, Sanchez-Carrillo C, Bouza E, et al. Does biomass production correlate with metabolic activity in Staphylococcus aureus? J Microbiol Methods. 2016. [DOI] [PubMed] [Google Scholar]
- 36.Parasuraman P, Antony AP, B SLS, Sharan A, Siddhardha B, Kasinathan K, et al. Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling. 2019;35(1):89–103. 10.1080/08927014.2019.1570501 [DOI] [PubMed] [Google Scholar]
- 37.Coenye T, Nelis HJ. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods. 2010;83(2):89–105. 10.1016/j.mimet.2010.08.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SPS)
(XLSX)
(SAV)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.