Abstract
The blood–brain barrier (BBB) is a highly complex and dynamic structure, mainly composed of brain microvascular endothelial cells, pericytes, astrocytes and the basement membrane (BM). The vast majority of BBB research focuses on its cellular constituents. Its non-cellular component, the BM, on the other hand, is largely understudied due to its intrinsic complexity and the lack of research tools. In this review, we focus on the role of the BM in BBB integrity. We first briefly introduce the biochemical composition and structure of the BM. Next, the biological functions of major components of the BM in BBB formation and maintenance are discussed. Our goal is to provide a concise overview on how the BM contributes to BBB integrity.
Keywords: basement membrane, blood-brain barrier, laminin, collagen Iv
Introduction
The blood–brain barrier (BBB) is a highly complex and dynamic structure, mainly composed of brain microvascular endothelial cells (BMECs), pericytes, astrocytes and a non-cellular component—the basement membrane (BM).1–3 By tightly regulating what enters the brain, the BBB functions to maintain the homeostasis of the central nervous system.4–6 Consistent with this important role, BBB disruption has been found in a variety of neurological disorders.2 7 8 The vast majority of BBB research, however, focuses on its cellular constituents, including BMECs, pericytes and astrocytes. It has been shown that BMECs contribute to BBB’s barrier property via forming tight junctions at the intercellular space9 and limiting transcellular transport (transcytosis).10 11 Pericytes, mural cells that cover capillaries in the vasculature, play important roles in the formation, maturation and maintenance of the BBB.12 Astrocytes, by interacting with pericytes and BMECs through their endfeet,13 participate in BBB maintenance and ion/water transport.14 15 The readers are referred to the following references for more information on the functions of these cells in BBB integrity.16–21
Unlike the cellular constituents of the BBB, the BM is largely understudied probably due to its intrinsic complexity. Recent studies suggest that the BM also contributes substantially to vascular barrier function.22–25 In this review, we summarise recent findings on the function of the BM in BBB integrity. First, we briefly introduce the biochemical composition and structure of the BM. Next, we discuss the function of each major component of the BM in BBB formation and maintenance.
Basement membrane
The BM is a unique form of the extracellular matrix (ECM) found predominantly underneath endothelial and epithelial cells. It exerts many important functions, including structural support, cell anchoring and signalling transduction.26–28 In the brain, two types of BM are found: an endothelial BM and a parenchymal BM (figure 1), which are separated by pericytes.29–31 Under physiological conditions, the two BM layers are indistinguishable and look like one in areas without pericytes (figure 1). Structurally, the BM is a highly organised protein sheet with a thickness of 50–100 nm.32–34 Biochemically, the BM consists of four major ECM proteins: collagen IV, laminin, nidogen and perlecan. These ECM proteins are synthesised predominantly by BMECs, pericytes and astrocytes at the BBB. The functional significance of each BM component in BBB integrity is discussed below.
Figure 1.
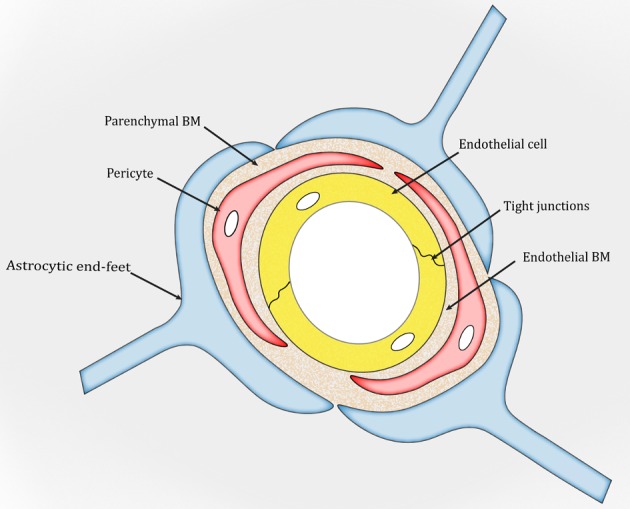
Schematic illustration of the blood–brain barrier. BM, basement membrane.
Collagen IV
Collagen IV, the most abundant component of the BM, is a trimeric protein containing three α-chains. Currently, six collagen IV α-chains (COL4A1–6) have been identified.35–37 Unlike COL4A3–6, which are more spatially and temporally restricted, COL4A1 and COL4A2 are present in almost all BMs and are highly conserved across species.38 It has been shown that ablation of COL4A1/2 results in abnormal BM structure and embryonic lethality at E10.5–E11.5, although BM formation during early development is unaffected,39 suggesting that collagen IV is required for the maintenance but not formation of the BM. In addition, mice with splice mutation lacking exon 41 of COL4A1 in both alleles die during embryogenesis, whereas those with such mutation in one allele show cerebrovascular defects, including porencephaly and intracerebral haemorrhage.40–42 To examine the relative contribution of each cell type at the BBB, exon 41 of COL4A1 was ablated in BMECs, pericytes and astrocytes, respectively. Although loss of exon 41 of COL4A1 in astrocytes caused very mild intracerebral haemorrhage, such mutation in BMECs or pericytes resulted in fully penetrant intracerebral haemorrhage and incompletely penetrant porencephaly.42 These results suggest that loss of exon 41 of COL4A1 in both BMECs and pericytes contributes to cerebrovascular defects. Consistent with these reports, various missense mutations in COL4A1/2 lead to brain malformation and intracerebral haemorrhage with different severity.43–45 Together, these results suggest a crucial role of collagen IV in vascular integrity. The major findings in these studies have been summarised in table 1.
Table 1.
Loss-of-function studies on major BM components
| Genes | Knockout/mutation | Cre promoter | Knockout phenotype | References |
| Collagen IV | ||||
| Collagen 4A1/2 | Global knockout | – | BM structural deficiencies and embryonic lethality (E10.5–E11.5) | 39 |
| Collagen 4A1 | Lacking exon 41 in both alleles | – | Embryonic lethality | 45 |
| Collagen 4A1 | Lacking exon 41 in one allele | – | Intracerebral haemorrhage and porencephaly | 40 41 |
| Collagen 4A1 | Conditional knockout | Rosa26-CreER, Tie2-Cre, Pdgfrb-Cre, Gfap-Cre | Intracerebral haemorrhage and porencephaly with different severity | 42 |
| Collagen 4A1/2 | Missense mutations | – | Vascular defects and brain malformations | 43 44 |
| Collagen 4A2 | Missense mutations | – | Intracerebral haemorrhage | 45 |
| Laminin | ||||
| Laminin α2 | Global knockout | – | BBB disruption | 58 |
| Laminin α4 | Global knockout | – | Haemorrhage during embryonic/neonatal stage | 68 |
| Laminin α5 | Global knockout | – | Embryonic lethality (~E17) and defects in neural tube closure and neural crest cell migration | 52–54 |
| Conditional knockout | Tie2-Cre (endothelium) |
No gross CNS abnormalities under homeostatic conditions | 70 71 | |
| Laminin β1 | Global knockout | – | Embryonic lethality (E5.5–E6.5) | 55 |
| Laminin γ1 | Global knockout | – | Embryonic lethality (E5.5–E6.5) | 55–57 |
| Conditional knockout | Nestin-Cre (neural progenitors) | BBB breakdown and intracerebral haemorrhage | 1 87 | |
| Conditional knockout | Pdgrfb-Cre (mural cells) | Hydrocephalus and BBB breakdown | 51 | |
| Nidogen | ||||
| Nidogen-1 | Global knockout | – | Mild BM alteration in brain capillaries | 72–74 |
| Nidogen-2 | Global knockout | – | No effect on BM formation | 75 |
| Nidogen-1 and nidogen-2 | Global knockout | – | BM defect and perinatal lethality | 77–79 |
| Perlecan | ||||
| Perlecan | Global knockout | – | Embryonic lethality (E10–E12) | 84–86 |
BBB, blood–brain barrier; BM, basement membrane; CNS, central nervous system.
Laminin
Laminin is a T-shaped or cruciform-shaped trimeric protein composed of α, β and γ chains. So far, five α, four β and three γ chains have been identified.46 47 Various combinations of these subunits generate a large number of laminin isoforms. Although BMECs, pericytes and astrocytes all make laminin at the BBB, they synthesise different laminin isoforms. For example, BMECs generate laminin-α4β1γ1 (-411) and laminin-511,29 48 astrocytes predominately make laminin-211,29 49 whereas pericytes mainly synthesise laminins containing α4, α5 and γ1.50 51 Due to this cell-specific expression pattern, laminin shows differential distribution between endothelial and parenchymal BMs.30 Specifically, astrocyte-derived laminin-211 is predominantly found in parenchymal BM, whereas endothelial cell–derived laminin-411 and laminin-511 are mainly located in endothelial BM.
To investigate laminin’s function in BBB integrity, a variety of laminin loss-of-function mutants have been generated. Global knockout of most laminin subunits, including α5,52–54 β155 or γ1,55–57 leads to embryonic lethality, preventing investigation of their functions in BBB integrity. To overcome this limitation and enable investigation of laminin’s function in a cell-specific manner, we generated a series of conditional knockout lines targeting the laminin γ1 chain, a common subunit found in almost all laminin isoforms at the BBB. In a previous study, we showed that loss of astrocyte-derived laminin (laminin-211) led to age-dependent BBB breakdown and intracerebral haemorrhage.1 Consistent with our finding, laminin α2 null mutants displayed postnatal BBB disruption.58 These results suggest an indispensable role of astrocytic laminin in BBB maintenance.
In addition, we also generated transgenic mice with laminin deficiency in vascular smooth muscle cells (vSMCs, termed SKO hereafter)51 59 and mural cells (vSMCs and pericytes, termed PKO hereafter).51 60 In a mixed genetic background, the PKO mice demonstrated BBB breakdown and hydrocephalus, and usually died within 4 months.51 None of these deficits were observed in SKO mice,51 suggesting that it is the loss of pericyte-derived rather than vSMC-derived laminin that causes these changes. Given that hydrocephalus itself can cause BBB compromise, it remains unclear whether BBB disruption in PKO mice is due to loss of pericytic laminin or secondary to hydrocephalus. Based on that hydrocephalus is highly genetic background dependent,61–67 we hypothesise that we can eliminate or reduce hydrocephalus by crossing the PKO mice into different backgrounds. We are currently testing this hypothesis in our laboratory.
Unlike laminin α5 global knockout mice, laminin α4 null mutants are viable.68 They show compromised vascular integrity and haemorrhage at perinatal stage but not in adulthood.68 Since laminin α5 expression in the vasculature starts after birth,48 69 it is believed that loss of laminin α4 is compensated by laminin α5, which rescues the haemorrhagic phenotype in adulthood. Recently, mice with laminin α5 deficiency in endothelial cells were generated.70 71 These mutants fail to display any obvious defects under homeostatic conditions,70 71 again suggesting potential compensation between laminin α4 and α5. Due to this mutual compensation, the role of endothelial laminin in BBB integrity remains largely unknown. These loss-of-function studies are summarised in table 1.
Nidogen
Nidogen, also known as entactin, functions to stabilise the collagen IV and laminin networks. Two nidogen isoforms (nidogen-1 and nidogen-2) have been identified in mammals.72 Interestingly, mice deficient for nidogen-1 or nidogen-2 are grossly normal, except that a mild alteration in brain capillary BM is observed in nidogen-1 mutants.72–74 In addition, although nidogen-1 expression is unchanged in nidogen-2 null mice,75 redistribution and upregulation of nidogen-2 have been observed in nidogen-1 null mice.76 These results indicate the existence of compensatory mechanism between nidogen-1 and nidogen-2. Consistent with this speculation, deletion of both nidogen-1 and nidogen-2 leads to severe BM defects and perinatal lethality.77–79 It remains unclear how nidogens contribute to BBB integrity.
Perlecan
Perlecan, also known as heparan sulfate proteoglycan 2 (HSPG2), is an extremely large protein present in most BMs.80 It has various domains (I–V) and motifs, which enable them to interact with a large number of molecules,81–83 such as ECM proteins and heparin-binding growth factors. Loss-of-function studies demonstrated that perlecan-deficient mice died at E10–E12. In addition, many complex phenotypes in multiple tissues/organs were found in these mutants, although BM formation was not affected.84–86 These results suggest that perlecan is dispensable for BM formation but required for embryogenesis. Due to this early embryonic lethality, the function of perlecan in BBB integrity remains unknown.
Concluding remarks
The BBB plays essential roles in brain homeostasis under physiological conditions and disease pathogenesis/progression under pathological conditions. Recent studies strongly suggest that the BM also actively participates in BBB regulation. However, how exactly the BM regulates BBB integrity at the molecular and cellular levels is largely unknown due to its intrinsic complexity and the lack of research tools. With the advancement in genetics and biochemistry, we are starting to answer this important question. This knowledge will widen/deepen our understanding of BBB regulation and promote the development of innovative therapies for neurological disorders with BBB disruption.
Footnotes
Contributors: LX and AN did the literature search. All authors wrote the manuscript. YY edited the manuscript.
Funding: This work was partially supported by the American Heart Association Scientist Development Grant (16SDG29320001).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Patient consent for publication: Not required.
References
- 1. Yao Y, Chen ZL, Norris EH, et al. . Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 2014;5:3413 10.1038/ncomms4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 2008;57:178–201. 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 3. He Y, Yao Y, Tsirka SE, et al. . Cell-culture models of the blood–brain barrier. Stroke 2014;45:2514–26. 10.1161/STROKEAHA.114.005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persidsky Y, Ramirez SH, Haorah J, et al. . Blood–brain barrier : structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006;1:223–36. 10.1007/s11481-006-9025-3 [DOI] [PubMed] [Google Scholar]
- 5. Abbott NJ, Patabendige AA, Dolman DE, et al. . Structure and function of the blood–brain barrier. Neurobiol Dis 2010;37:13–25. 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 6. Campos-Bedolla P, Walter FR, Veszelka S, et al. . Role of the blood–brain barrier in the nutrition of the central nervous system. Arch Med Res 2014;45:610–38. 10.1016/j.arcmed.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 7. Bell RD, Winkler EA, Sagare AP, et al. . Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010;68:409–27. 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montagne A, Barnes SR, Sweeney MD, et al. . Blood–brain barrier breakdown in the aging human hippocampus. Neuron 2015;85:296–302. 10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kniesel U, Wolburg H. Tight junctions of the blood–brain barrier. Cell Mol Neurobiol 2000;20:57–76. 10.1023/A:1006995910836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004;84:869–901. 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- 11. Fenstermacher J, Gross P, Sposito N, et al. . Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci 1988;529:21–30. 10.1111/j.1749-6632.1988.tb51416.x [DOI] [PubMed] [Google Scholar]
- 12. Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011;21:193–215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 13. Wong AD, Ye M, Levy AF, et al. . The blood–brain barrier: an engineering perspective. Front Neuroeng 2013;6:7 10.3389/fneng.2013.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010;119:7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montgomery DL. Astrocytes: form, functions, and roles in disease. Vet Pathol 1994;31:145–67. 10.1177/030098589403100201 [DOI] [PubMed] [Google Scholar]
- 16. Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013;36:437–49. 10.1007/s10545-013-9608-0 [DOI] [PubMed] [Google Scholar]
- 17. Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol 2015;7:a020412 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005;57:173–85. 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- 19. Stamatovic SM, Johnson AM, Keep RF, et al. . Junctional proteins of the blood–brain barrier: new insights into function and dysfunction. Tissue Barriers 2016;4:e1154641 10.1080/21688370.2016.1154641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss N, Miller F, Cazaubon S, et al. . The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 2009;1788:842–57. 10.1016/j.bbamem.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 21. Zhao Z, Nelson AR, Betsholtz C, et al. . Establishment and dysfunction of the blood–brain barrier. Cell 2015;163:1064–78. 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohashi KL, Tung DK, Wilson J, et al. . Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation 1996;3:199–210. 10.3109/10739689609148289 [DOI] [PubMed] [Google Scholar]
- 23. Yadav R, Larbi KY, Young RE, et al. . Migration of leukocytes through the vessel wall and beyond. Thromb Haemost 2003;90:598–606. 10.1160/TH03-04-0220 [DOI] [PubMed] [Google Scholar]
- 24. Hoshi O, Ushiki T. Neutrophil extravasation in rat mesenteric venules induced by the chemotactic peptide N-formyl-methionyl-luecylphenylalanine (fMLP), with special attention to a barrier function of the vascular basal lamina for neutrophil migration. Arch Histol Cytol 2004;67:107–14. 10.1679/aohc.67.107 [DOI] [PubMed] [Google Scholar]
- 25. Bixel MG, Petri B, Khandoga AG, et al. . A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood 2007;109:5327–36. 10.1182/blood-2006-08-043109 [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011;209:139–51. 10.1530/JOE-10-0377 [DOI] [PubMed] [Google Scholar]
- 27. Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood–brain barrier formation and stroke. Dev Neurobiol 2011;71:1018–39. 10.1002/dneu.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–9. 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sixt M, Engelhardt B, Pausch F, et al. . Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 2001;153:933–46. 10.1083/jcb.153.5.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hallmann R, Horn N, Selg M, et al. . Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev 2005;85:979–1000. 10.1152/physrev.00014.2004 [DOI] [PubMed] [Google Scholar]
- 31. Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 2008;67:1113–21. 10.1097/NEN.0b013e31818f9ca8 [DOI] [PubMed] [Google Scholar]
- 32. Ruben GC, Yurchenco PD. High resolution platinum-carbon replication of freeze-dried basement membrane. Microsc Res Tech 1994;28:13–28. 10.1002/jemt.1070280104 [DOI] [PubMed] [Google Scholar]
- 33. Vracko R, Benditt EP. Capillary basal lamina thickening. Its relationship to endothelial cell death and replacement. J Cell Biol 1970;47:281–5. 10.1083/jcb.47.1.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 2011;3:a004911 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hudson BG, Reeders ST, Tryggvason K, et al. . Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993;268:26033–6. [PubMed] [Google Scholar]
- 36. Filie JD, Burbelo PD, Kozak CA. Genetic mapping of the alpha 1 and alpha 2 (IV) collagen genes to mouse chromosome 8. Mamm Genome 1995;6:487–87. 10.1007/BF00360662 [DOI] [PubMed] [Google Scholar]
- 37. Sado Y, Kagawa M, Naito I, et al. . Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J Biochem 1998;123:767–76. 10.1093/oxfordjournals.jbchem.a022003 [DOI] [PubMed] [Google Scholar]
- 38. Sado Y, Kagawa M, Kishiro Y, et al. . Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol 1995;104:267–75. 10.1007/BF01464322 [DOI] [PubMed] [Google Scholar]
- 39. Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, et al. . Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004;131:1619–28. 10.1242/dev.01037 [DOI] [PubMed] [Google Scholar]
- 40. Gould DB, Phalan FC, van Mil SE, et al. . Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med 2006;354:1489–96. 10.1056/NEJMoa053727 [DOI] [PubMed] [Google Scholar]
- 41. Gould DB, Phalan FC, Breedveld GJ, et al. . Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 2005;308:1167–71. 10.1126/science.1109418 [DOI] [PubMed] [Google Scholar]
- 42. Jeanne M, Jorgensen J, Gould DB. Molecular and genetic analyses of collagen type IV mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation 2015;131:1555–65. 10.1161/CIRCULATIONAHA.114.013395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Favor J, Gloeckner CJ, Janik D, et al. . Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics 2007;175:725–36. 10.1534/genetics.106.064733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuo DS, Labelle-Dumais C, Mao M, et al. . Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by Col4a1 and Col4a2 mutations. Hum Mol Genet 2014;23:1709–22. 10.1093/hmg/ddt560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeanne M, Labelle-Dumais C, Jorgensen J, et al. . COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet 2012;90:91–101. 10.1016/j.ajhg.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Durbeej M. Laminins. Cell Tissue Res 2010;339:259–68. 10.1007/s00441-009-0838-2 [DOI] [PubMed] [Google Scholar]
- 47. Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci 2017;74:1095–115. 10.1007/s00018-016-2381-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorokin LM, Pausch F, Frieser M, et al. . Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol 1997;189:285–300. 10.1006/dbio.1997.8668 [DOI] [PubMed] [Google Scholar]
- 49. Jucker M, Tian M, Norton DD, et al. . Laminin alpha 2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience 1996;71:1153–61. 10.1016/0306-4522(95)00496-3 [DOI] [PubMed] [Google Scholar]
- 50. Stratman AN, Malotte KM, Mahan RD, et al. . Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009;114:5091–101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gautam J, Zhang X, Yao Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep 2016;6:36450 10.1038/srep36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nguyen NM, Miner JH, Pierce RA, et al. . Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol 2002;246:231–44. 10.1006/dbio.2002.0658 [DOI] [PubMed] [Google Scholar]
- 53. Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol 1998;143:1713–23. 10.1083/jcb.143.6.1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coles EG, Gammill LS, Miner JH, et al. . Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Dev Biol 2006;289:218–28. 10.1016/j.ydbio.2005.10.031 [DOI] [PubMed] [Google Scholar]
- 55. Miner JH, Li C, Mudd JL, et al. . Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 2004;131:2247–56. 10.1242/dev.01112 [DOI] [PubMed] [Google Scholar]
- 56. Smyth N, Vatansever HS, Meyer M, et al. . The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci 1998;857:283–6. 10.1111/j.1749-6632.1998.tb10133.x [DOI] [PubMed] [Google Scholar]
- 57. Smyth N, Vatansever HS, Murray P, et al. . Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 1999;144:151–60. 10.1083/jcb.144.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Menezes MJ, McClenahan FK, Leiton CV, et al. . The extracellular matrix protein laminin α2 regulates the maturation and function of the blood–brain barrier. J Neurosci 2014;34:15260–80. 10.1523/JNEUROSCI.3678-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yao Y, Norris EH, Strickland S. The cellular origin of laminin determines its role in blood pressure regulation. Cell Mol Life Sci 2015;72:999–1008. 10.1007/s00018-014-1732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao Y, Norris EH, Mason CE, et al. . Laminin regulates PDGFRβ(+) cell stemness and muscle development. Nat Commun 2016;7:11415 10.1038/ncomms11415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ding H, Wu X, Boström H, et al. . A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet 2004;36:1111–6. 10.1038/ng1415 [DOI] [PubMed] [Google Scholar]
- 62. Fredriksson L, Li H, Fieber C, et al. . Tissue plasminogen activator is a potent activator of PDGF-CC. Embo J 2004;23:3793–802. 10.1038/sj.emboj.7600397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baribault H, Penner J, Iozzo RV, et al. . Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 1994;8:2964–73. 10.1101/gad.8.24.2964 [DOI] [PubMed] [Google Scholar]
- 64. Threadgill DW, Dlugosz AA, Hansen LA, et al. . Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 1995;269:230–4. 10.1126/science.7618084 [DOI] [PubMed] [Google Scholar]
- 65. Bonyadi M, Rusholme SA, Cousins FM, et al. . Mapping of a major genetic modifier of embryonic lethality in TGF beta 1 knockout mice. Nat Genet 1997;15:207–11. 10.1038/ng0297-207 [DOI] [PubMed] [Google Scholar]
- 66. George EL, Georges-Labouesse EN, Patel-King RS, et al. . Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993;119:1079–91. [DOI] [PubMed] [Google Scholar]
- 67. Heiman-Patterson TD, Sher RB, Blankenhorn EA, et al. . Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: a window of opportunity in the search for genetic modifiers. Amyotroph Lateral Scler 2011;12:79–86. 10.3109/17482968.2010.550626 [DOI] [PubMed] [Google Scholar]
- 68. Thyboll J, Kortesmaa J, Cao R, et al. . Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol 2002;22:1194–202. 10.1128/MCB.22.4.1194-1202.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Patton BL, Miner JH, Chiu AY, et al. . Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol 1997;139:1507–21. 10.1083/jcb.139.6.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song J, Lokmic Z, Lammermann T, et al. . Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival. Proc Natl Acad Sci USA 2013;110:E2915–E2924. 10.1073/pnas.1218131110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Song J, Zhang X, Buscher K, et al. . Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep 2017;18:1256–69. 10.1016/j.celrep.2016.12.092 [DOI] [PubMed] [Google Scholar]
- 72. Kang SH, Kramer JM. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol Biol Cell 2000;11:3911–23. 10.1091/mbc.11.11.3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dong L, Chen Y, Lewis M, et al. . Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest 2002;82:1617–30. 10.1097/01.LAB.0000042240.52093.0F [DOI] [PubMed] [Google Scholar]
- 74. Murshed M, Smyth N, Miosge N, et al. . The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 2000;20:7007–12. 10.1128/MCB.20.18.7007-7012.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schymeinsky J, Nedbal S, Miosge N, et al. . Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol 2002;22:6820–30. 10.1128/MCB.22.19.6820-6830.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol 2002;21:611–21. 10.1016/S0945-053X(02)00070-7 [DOI] [PubMed] [Google Scholar]
- 77. Bader BL, Smyth N, Nedbal S, et al. . Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol 2005;25:6846–56. 10.1128/MCB.25.15.6846-6856.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Böse K, Nischt R, Page A, et al. . Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J Biol Chem 2006;281:39620–9. 10.1074/jbc.M607886200 [DOI] [PubMed] [Google Scholar]
- 79. Mokkapati S, Baranowsky A, Mirancea N, et al. . Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J Invest Dermatol 2008;128:2259–67. 10.1038/jid.2008.65 [DOI] [PubMed] [Google Scholar]
- 80. Knox SM, Whitelock JM. Perlecan: how does one molecule do so many things? Cell Mol Life Sci 2006;63:2435–45. 10.1007/s00018-006-6162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Costell M, Sasaki T, Mann K, et al. . Structural characterization of recombinant domain II of the basement membrane proteoglycan perlecan. FEBS Lett 1996;396(2-3):127–31. 10.1016/0014-5793(96)01082-4 [DOI] [PubMed] [Google Scholar]
- 82. Dolan M, Horchar T, Rigatti B, et al. . Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J Biol Chem 1997;272:4316–22. 10.1074/jbc.272.7.4316 [DOI] [PubMed] [Google Scholar]
- 83. Hopf M, Göhring W, Mann K, et al. . Mapping of binding sites for nidogens, fibulin-2, fibronectin and heparin to different IG modules of perlecan. J Mol Biol 2001;311:529–41. 10.1006/jmbi.2001.4878 [DOI] [PubMed] [Google Scholar]
- 84. Arikawa-Hirasawa E, Watanabe H, Takami H, et al. . Perlecan is essential for cartilage and cephalic development. Nat Genet 1999;23:354–8. 10.1038/15537 [DOI] [PubMed] [Google Scholar]
- 85. Costell M, Gustafsson E, Aszódi A, et al. . Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 1999;147:1109–22. 10.1083/jcb.147.5.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rossi M, Morita H, Sormunen R, et al. . Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. Embo J 2003;22:236–45. 10.1093/emboj/cdg019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen ZL, Yao Y, Norris EH, et al. . Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol 2013;202:381–95. 10.1083/jcb.201212032 [DOI] [PMC free article] [PubMed] [Google Scholar]


