Abstract
Mitochondrial dysfunction is now recognized as a contributing factor to the early pathology of multiple human conditions including neurodegenerative diseases. Mitochondria are signaling organelles with a multitude of functions ranging from energy production to a regulation of cellular metabolism, energy homeostasis, stress response, and cell fate. The success of these complex processes critically depends on the fidelity of mitochondrial dynamics that include the ability of mitochondria to change shape and location in the cell, which is essential for the maintenance of proper function and quality control, particularly in polarized cells such as neurons. This review highlights several aspects of alterations in mitochondrial dynamics in Alzheimer’s disease, which may contribute to the etiology of this debilitating condition. We also discuss therapeutic strategies to improve mitochondrial dynamics and function that may provide an alternative approach to failed amyloid-directed interventions.
Keywords: Mitochondria, Alzheimer’s disease, fission, fusion, axonal trafficking, mitophagy, mitochondria-targeted therapeutics
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder characterized by a progressive decline in cognitive function where age is the greatest risk factor (1). Hallmarks of the disease include the accumulation of amyloid-β (Aβ) peptides and aggregation of hyperphosphorylated Tau protein (pTau), which contribute to increased synaptic dysfunction and neuroinflammation before the onset of clinical symptoms and neuronal loss (2). However, consistent failure of clinical trials focused on strategies to reduce Aβ formation or increase its clearance questions its role in AD etiology (3). Alternative mechanisms are well documented in early AD and include impaired glucose metabolism, mitochondrial dysfunction and altered energy homoeostasis (4). Decreased glucose utilization detected using fluorodeoxyglucose positron emission tomography (FDG-PET) imaging in patients with mild cognitive impairment (MCI), a prodromal stage of AD, suggests that abnormal energy homeostasis in the brain might be the underlying disease mechanism. The brain is the most energy-consuming organ in the body. Energy required to support axonal growth and synaptic activity is generated primarily in mitochondria in the form of adenosine triphosphate (ATP). Mitochondria can produce approximately 30 molecules of ATP per one molecule of glucose in a chain of enzymatic reactions known as oxidative phosphorylation (OXPHOS) where approximately 2 ATP molecules are generated in the cytoplasm during glycolysis (5) (Fig. 1A). Therefore, reduced glucose availability could devastate cells with high energy demands such as neurons. Recent clinical and basic science data demonstrate that molecular mechanisms of AD and diabetes significantly overlap where altered insulin growth factor (IGF) signaling results in the activation of a cascade of pathological events leading to insulin resistance, inflammation, activation of glycogen synthase kinase 3β (GSK3β), increased production of Aβ and pTau, mitochondrial dysfunction and oxidative damage (6). Once initiated, the pathological chain of events exacerbates with time, creating a vicious cycle where the role of mitochondria becomes increasingly important based on the abilities to either mitigate or contribute to disease progression (7, 8).
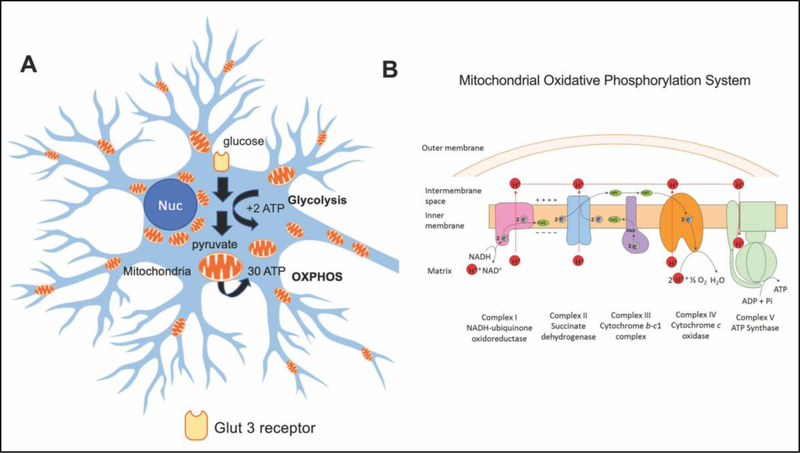
Figure 1: Generation of adenosine triphosphate (ATP) via glycolysis or oxidative phosphorylation (OXPHOS) pathways in neurons.
(A) Glucose uptake via Glut 3 receptors provides the substrate for a series of glycolytic reactions to generate two molecules of ATP in the cytoplasm. Conversion of glucose to pyruvate is necessary for production of ATP via OXPHOS in mitochondria that results in ~30–36 ATP molecules per one molecule of glucose. (B) The OXPHOS machinery is located at the mitochondrial inner membrane and consists of five complexes where complexes I – IV are involved in electron transfer and proton export to maintain protein gradient that is utilized by complex V to generate ATP to support neuronal function and synaptic activity.
Mitochondria are unique organelles that are the only ones apart from the nucleus that have their own DNA (mtDNA). They also are very dynamic. Within their life cycle, new mitochondria are produced by a process known as biogenesis, and old organelles are removed by an autophagic process known as mitophagy. Mitochondria respond to environmental changes and energy demands of the cell by fusing together to form an interconnected reticulum, and by dividing to either increase mitochondrial number or to segregate portions of the organelle for degradation via mitophagy (9–11). Neurons have a unique cellular architecture with multiple specialized compartments including the cell body, dendrites, synaptic terminals and axons that can reach a meter in length. To support neuronal activities, mitochondria must be delivered from the cell body where biogenesis occurs, to synaptic sites and to distal parts of axons via anterograde transport (12). Mitochondrial autophagic degradation takes place in the cell body requiring damaged organelles to return via retrograde trafficking (12). These transport mechanisms are tightly interconnected with mitochondrial shape and size that are regulated by fission and fusion, which together constitute mitochondrial dynamics. Consequently, it is no wonder that disturbances in the fidelity of these mechanisms can potentiate neuronal dysfunction. In the past two decades, perturbation of these dynamic mitochondrial systems has emerged as a central pathophysiological phenomenon in a broad range of human diseases including AD (13–15). In this review, we will discuss current progress in the understanding of the mechanisms of altered mitochondrial dynamics that is essential to the development of effective therapeutic strategies (16).
Mitochondrial fission and fusion in AD
Mitochondria have two membranes with distinct functions, the outer mitochondrial membrane (OMM) and the inner one (IMM) (Fig. 1B). The IMM forms multiple folds referred to as cristae that harbor components of OXPHOS. The OMM functions as a gateway to facilitate transport of proteins and other materials into the mitochondrion. Both membranes work in concert. In case of mitochondria, the saying “structure is function” is exceptionally true. Mitochondrial size and shape are very important for proper function. Morphological changes are regulated and balanced by a set of proteins collectively referred to as fission and fusion proteins. Fusion proteins include dynamin GTPase regulators Optic Atrophy 1 (OPA1), Mitofusin 1 (MFN1), Mitofusin 2 (MFN2) and fission proteins that consist of dynamin-like GTPase regulator Dynamin related protein 1 (DRP1) and a number of receptor/adaptor proteins. OPA1 protein is localized to the mitochondrial intermembrane space (IMS) by its transmembrane domain where it acts to insulate mitochondrial cristae while MFN1 and MFN2 are anchored on the OMM. Here, the MFNs can facilitate fusion of opposing mitochondrial membranes in a homotypic or heterotypic manner (17). Both MFN1 and MFN2 structurally and functionally complement OPA1 (18), and together these dynamins orchestrate a precise mechanical chain of events, which culminates in the coordinated fusion of the mitochondrial double membrane (19, 20). Furthermore, OPA1 is regulated by a set of proteases that can cleave it into eight different isoforms by alternative splicing of exons 4, 4b and 5b (21). Initially upon import into the mitochondrion, OPA1 is processed by matrix metalloproteases, which cleave a mitochondrial targeting sequence, followed by mitochondrial ATPase proteases associated with diverse functions (AAA+) to alter OPA1 splicing in response to changes in cellular metabolism and increased stress (22–25). This splicing is responsible for creating both the long (l-OPA1) and short (s-OPA1) isoforms of OPA1. These proteases include ATPase Family Gene 3 Like Matrix AAA Peptidase Subunit 2 (AFG3L2), Paraplegin, Presenilin Associated Rhomboid Like (PARL), Yeast Mitochondrial AAA Metalloprotease Like 1 ATPase (YME1L) and Overlapping with the M-AAA Protease 1 Homolog (OMA1) (26).
Conversely, mitochondrial fission is coordinated by DRP1 and a complement of receptor proteins such as Mitochondrial Fission 1 protein (Fis1), Mitochondrial fission factor (Mff), endophilin-B1, mitochondrial protein 18 (MTP18), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51, respectively) (27, 28). Once recruited to the OMM, DRP1 can oligomerize into a ring-like structure at positions marked by the endoplasmic reticulum (ER) and the actin cytoskeleton. This ring can then pinch the OMM to induce mitochondrial fission upon influx of ER calcium into mitochondria (29). This mechanism can be modulated by alternative post-translational modifications of DRP1 including phosphorylation, SUMOylation and O-GlcNAcylation (27). Counter-intuitively, OPA1 performs a dual role in both mitochondrial fission and fusion. Under conditions of cellular stress, proteases YME1L and OMA1 cleave OPA1 to produce and accumulate s-OPA1. s-OPA1 has been shown to induce fission of the IMM and facilitate mitochondrial fragmentation (30).
Mitochondrial shape ranges from small round structures to elongated tubular networks (Fig. 2). Mitochondrial size appears to directly relate to their function, distribution, quality and interaction with other cellular compartments and organelles. Very rapid shape shifting (within minutes) occurs in response to stress or metabolic changes in order to maintain cellular homoeostasis. For example, under conditions of glucose deprivation or serum starvation, there is an increase in mitochondrial fragmentation that has been proposed to provide mitochondria with increased surface area for accessibility of metabolic substrates to carrier proteins (32, 33). This could be beneficial in case of a high fat diet (HFD) enriched with saturated fatty acids where fragmentation was associated with increased mitochondrial intake of dietary fats (32, 34). Intriguingly, a different type of a HFD consisting of polyunsaturated fatty acids elicited a distinctive response where increased mitochondrial fusion correlated with augmented fatty acid utilization and reduced weight gain (32, 34). Similarly, mitochondrial fusion was found in cases of nitrogen source depletion (lack of glutamine or amino acids) that can be further exaggerated with a combination of nitrogen source and glucose deficiency in cases of more extreme starvation (32). Benefits of mitochondrial hyper-fusion include a protection against cellular apoptosis (35), increased complementation of mitochondrial components to maintain function, and even an increase in ATP production (36–40). Moreover, fission/fusion machinery is linked to mitochondrial singling via reactive oxygen (ROS) or nitrogen (RNS) species. Excessive ROS and RNS production is a well-defined characteristic of AD that may lead to several harmful effects including DNA, protein and lipid damage (41). However, under non-pathological, physiological levels (mitohormetic levels), ROS/RNS act as signaling molecules that can regulate several pathways associated with protective mitochondrial function and metabolism including mitochondrial dynamics and cristae remodeling (42). This occurs through either direct modification of fission/fusion proteins or by modifying reduction/oxidation sensors both internal and external to the mitochondrion, to alter downstream pathway signaling (43–45). Taken together, these observations suggest the existence of dynamic cellular signaling pathways, which are highly sensitive to the diversity of intra- and extracellular changes including concentration of nutrients and metabolites that can fine-tune mitochondrial dynamics to provide the appropriate response to these metabolic cues.
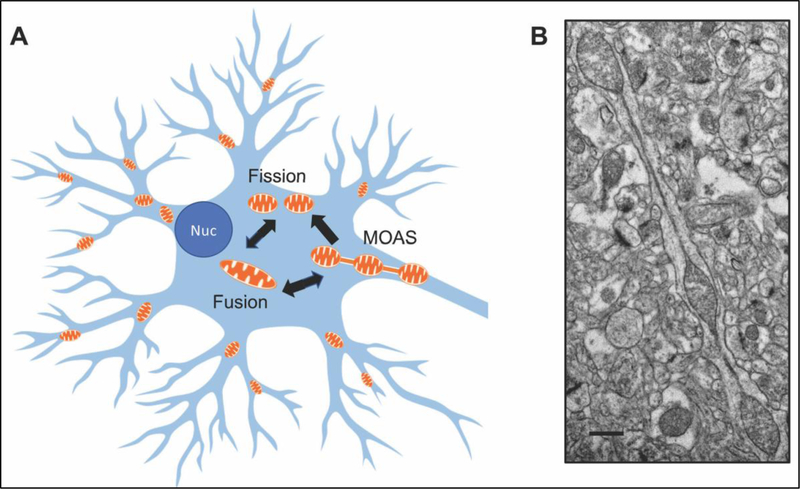
Figure 2: Mitochondrial fission/fusion dynamics in neurons.
(A) Mitochondria in neurons vary in shape and size constantly undergoing fission and fusion in response to metabolic demands. Intermediate morphological phenotype, mitochondria-on-a-string (MOAS), may represent a response to energetic stress that promotes mitochondrial stability and function. In AD, increased levels of MOAS and mitochondrial fragmentation were observed supporting high level of energetic stress. (B) Example of MOAS in a neuropil in brain tissue of a 3xTgAD female mouse 12 months of age. Micrograph was generated as part of the study described in (31). Scale bar, 0.5 μM.
Given the essential role of mitochondrial dynamics, any alterations in the fidelity of fission/fusion machinery could have devastating effect on mitochondrial function, energy and redox homeostasis. Indeed, altered mitochondrial dynamics are well documented in AD patients and model organisms with a bias towards increased mitochondrial fragmentation (46). This increased fission becomes even more pronounced with a pathological increase in levels of Aβ and pTau, and their interaction with mitochondrial fission regulators in disease progression (47–50). Investigations using various cell lines that overexpress mutant human amyloid precursor protein (APP) revealed increased mitochondrial fragmentation and perinuclear localization indicating a collapse of the mitochondrial network, which could be due to elevated levels of total DRP1 and/or changes in DRP1 post -translational modifications such as an increase in S-nitrosylation and a decrease in pS636, a known phosphorylation signal for inhibiting DRP1 mitochondrial translocation (51). Imbalanced dynamics may also occur with disturbances of fusion regulators, although this is not always the case (52–54). Interestingly, the analysis of fission/fusion dynamics conducted in AD patient primary fibroblasts demonstrated that similar to other cell models, the mitochondrial network had lost its integrity (55, 56). However, in contrast to neuronal models, these peripheral cells had reduced levels of DRP1 where mitochondria length was not different from control cells under basal conditions. Only when the mitochondrial OXPHOS in these cells was challenged with the ionophore uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (CCCP) and the rate of mitochondrial morphological recovery was monitored, was an underlying bias towards mitochondrial fission revealed (56). These discrepancies could be explained by several factors including the cell and tissue origin, where bioenergetics may include a dependence on OXPHOS vs. glycolysis. In addition, in some in vitro experiments, concentrations of Aβ used to mimic the acute stress response were outside of the physiological range, raising caution in the data interpretation in respect to in vivo disease mechanisms. Nevertheless, in postmortem AD human brain tissue, increased expression of DRP1 and FIS1 and decreased expression of MFN1, MFN2, OPA1 and TOM40 (a channel-forming subunit of a translocase of the mitochondrial outer membrane that is essential for protein transport into mitochondria) were identified in frontal cortex at early (Braak stages I and II), definite (Braak stages III and IV) and severe (Braak stages V and VI) stages of AD leading to mitochondrial fragmentation (57). However, the examination of mitochondrial morphology using brain tissue from multiple mouse models of AD produced inconsistent results, where in some cases, mitochondrial fragmentation associated with the elevated levels of DRP1 and FIS1 and reduced levels of OPA1, MFN1 and MFN2 was confirmed but in the others, elongated mitochondria associated with inhibited activity of DRP1 were found (31, 40, 57).
To further investigate mitochondrial morphology in respect to AD development, we analyzed hippocampal and cortical brain tissue from AD patients and four mouse models of AD using three dimensional electron microscopy (3D EM) (31). This study revealed the presence of a novel phenotype that we termed mitochondria-on-a-string (MOAS, Fig. 2B) (31). MOAS represent a very long mitochondrion where bulbous parts of the organelle are connected with a double membrane approximately 40 – 60 nm in diameter and ~5 μm in length (aka nanotunnels). These structures were found in the brain of AD patients, mice with tauopathy, aging wild type mice and non-human primates. They were also found in the brain of young wild type mice a few minutes after the induction of acute hypoxia (31, 58, 59). This extremely prevalent and dynamic formation of MOAS was attributed to calcium flux and bioenergetic stress, where fission arrest may promote the residual functioning of mitochondria under stress conditions making them resistant to mitophagy (31, 60–62). The presence of MOAS vs. fragmented mitochondria identified in AD emphasizes the complexity of mitochondrial dynamics and the need for further research using advanced techniques and models to better understand the role of mitochondrial fission and fusion at different stages of the disease.
Surprisingly, little work is done to demonstrate the direct connection between altered mitochondrial dynamics and bioenergetics in AD (15, 52, 63). Mitochondrial fission and fusion are proposed to be involved in the maintenance and assembly of mitochondrial ETC complexes suggesting that any alterations in mitochondrial dynamics could affect energy production (64). Most of the studies linked altered mitochondrial dynamics to morphological alterations and cellular distribution. Fusion-deficient mitochondria are larger in diameter, which could preclude their entrance into dendrites and axons with narrow diameter affecting synaptic function. An excessive fission might impact energy production by affecting cristae integrity and the assembly of the OXPHOS complexes (65). However, the definitive demonstration of the effect of altered fission/fusion machinery on the integrity and function of the enzymes of the OXPHOS and TCA cycle remains to be done.
Mitochondrial axonal transport and autophagy in AD
Mitochondria are transported within neurons from (anterograde transport) and to (retrograde transport) the cell body via the mechanism known as axonal transport (Fig. 3) (66). Mitochondrial motility in neurons is essential for providing ATP to the sites of synapses, to promote axonal growth, for calcium buffering, and for ensuring mitochondrial repair and degradation (67). Mitochondrial trafficking in neurons can be facilitated along microtubule tracks or actin filaments based on the cellular compartment. The structure and polarity of microtubules within axons and dendrites are different, with approximately 90% of microtubules oriented with their positive end away from the cell body in axons. In dendrites, microtubules have mixed orientation and density at the proximal end to the cell body with polarity and organization becoming more reminiscent of axons at the distal sites (68). To facilitate axonal transport, adaptor proteins such as syntabulin, mitochondrial Rho small GTPase (MIRO) and Milton are associated with motor proteins of the kinesin-1 and kinesin-3 family to transport mitochondria towards the (+) end of microtubules in the anterograde direction (69). The protein complexes consisting of dynein and dynactin proteins direct mitochondria to the (−) end of microtubules facilitating retrograde transport (67, 69). Thus, the kinesin motors typically transport mitochondria in the anterograde direction in axons while both kinesin and dynein can perform bidirectional movement of mitochondria in dendrites (Fig. 3). It is also possible for mitochondria to move along actin filaments in dendritic spines, growth cones and synaptic boutons for short-range redistribution using myosin motors (70–72). The integrity of microtubules is essential for mitochondrial motility in neurons where hyperphosphorylation of microtubule binding protein Tau (pTau) observed in AD and tauopathies, negatively impact mitochondrial transport. Moreover, proteins such as MFN2 that play essential roles in mitochondrial fission and fusion, have been shown to participate in mitochondrial transport interacting with murine MIRO proteins where mutations or deletions in MFN2 lead to a longer pausing and slowing of axonal transport in both directions (73). Furthermore, emerging evidence suggests that there is a tightly orchestrated interconnection between environmental changes and mitochondrial biogenesis, fission, fusion, transport and degradation where details of molecular mechanisms are not well understood and require further investigation (66, 73). Indeed, the essential connection between mitochondrial transport and synaptic activity has been established when a loss of function of syntabulin, an adaptor protein that mediates presynaptic mitochondrial motility, resulted in reduced synaptic plasticity (74, 75). Cellular signaling cues such as Ca2+, ROS, oxygen level, nutrients and ATP act to regulate these motor/adaptor proteins and determine mitochondrial movement and position. Both MIRO and Milton can be modified to encourage mitochondrial stalling by Ca2+ binding to MIRO near synaptic termini (76) and O-GlcNAc transferase (OGT) adding a GlcNAc sugar to serine and threonine residues of Milton (77). Oxygen deprivation has been found to recruit hypoxia upregulated mitochondrial movement regulator (HUMMR) to the kinesin/MIRO/Milton complex to promote anterograde transport (78). Furthermore, mitochondrial motility is also regulated by levels of ATP and ADP with greater velocity in regions with higher ATP levels while in the areas with low ATP, such as synaptic terminals, mitochondria dock (79). This signaling crosstalk aids in the partitioning and compartmentalization of mitochondria within different neuronal compartments (67). Although MIRO and Milton have been identified as mammalian adaptors responsible for the transport of mitochondria by kinesin, additional motor and adaptor proteins participate in, the mechanisms of axonal trafficking ensuring proper mitochondrial distribution in the cell (80–82).
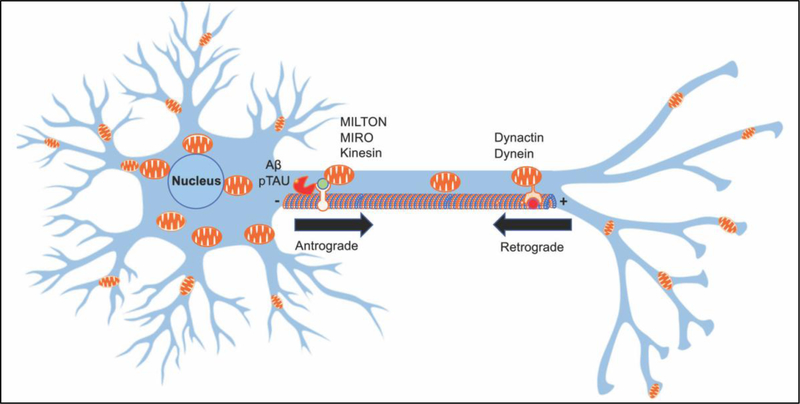
Figure 3: Mitochondrial transport in neurons.
Neurons have distinct cellular compartments comprised of the cell body, axons, dendrites, dendritic spines, axonal growth cones and synaptic boutons. Mitochondria are transported from the cell body to distal parts of neurons or to the sites of high energy demand along microtubule tracks using kinesin (anterograde direction) or dynein (retrograde direction) motor protein complexes. In AD, increased accumulation of Aβ and hyperphosphorylated Tau leads to abnormal trafficking in both directions compromising energy support for synaptic function.
In AD, an impairment of mitochondrial axonal transport precedes the accumulation of toxic protein aggregates and is linked to disturbed axonal integrity and synaptic function (40, 46, 83, 84). While the precise molecular mechanisms underlying mitochondrial transport inhibition in AD remain to be elucidated, a disturbance in mitochondrial motility is tightly linked with unbalanced fission/ fusion regulators, increased levels of both Aβ and pTau, and oxidative stress (46, 84). One of the first investigations in this area was conducted by Reddy’s group in primary neurons from the Tg2576 mice expressing mutant human APP protein (46). In this study, the authors found significantly decreased anterograde mitochondrial movement, increased mitochondrial fission and decreased fusion, abnormal mitochondrial and synaptic proteins, and defective mitochondrial function in primary neurons from Tg2576 mice compared to wild-type neurons (46). Furthermore, application of mitochondria-targeted antioxidant peptide SS31 (6’-dimethyltyrosine-Lys-Phe-NH2, will be discussed below) restored mitochondrial transport and synaptic viability, and decreased the percentage of defective mitochondria (46). We later reported similar findings in neurons from mice expressing another familial AD mutation in PS1 gene and in neurons form double transgenic APP/PS1 mice (40). In multiple studies, treatment of primary neuronal cultures with Aβ-derived diffusible ligands has been shown to reduce mitochondrial motility and distribution in axons (85–89). Detrimental effect of Aβ on mitochondrial motility was further confirmed in experiments using Drosophila and murine animal models of AD where the accumulation of oligomeric Aβ resulted in a depletion of synaptic mitochondria (84). Additional investigations of the relationship between different Aβ species and mitochondrial transport conducted in our laboratory indicated that toxic Aβ peptides with a higher propensity to aggregate have a larger impact on mitochondrial motility where extracellular fibrils were having the greatest impact possibly facilitating trafficking abnormalities via interacting with the neuronal plasma membrane (89). Interestingly, trafficking inhibition in these experiments did not affect mitochondrial ATP production and bioenergetics parameters indicating a differential onset of mitochondrial dysfunction in disease progression where alterations in mitochondrial motility may precede the loss of mitochondrial ability to generate energy. Apart from Aβ, overexpression and/or hyperphosphorylation of Tau has also been shown to disrupt both the distribution and localization of mitochondria in cellular and murine models of AD (90). Investigation into the molecular mechanism of transport inhibition has identified several key proteins, including Aβ and pTau, which participate in a feedback loop of enhanced biochemical instability. Glycogen synthase kinase 3 (GSK3), a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues, has been shown to mediate the impairment of mitochondrial transport in AD through phosphorylation and deactivation of mitochondrial transport motor proteins and through increased phosphorylation of Tau at AT8 sites (Ser199, Ser202, Thr205) (91). These Tau modifications not only promoted the development of filamentous pTau aggregates but also lead to increased microtubule instability that contributes to the inhibition of transport. Moreover, expression of mutant Tau (K369I) has also been reported to trap the kinesin motor protein Jip1 in the soma causing the impairment of axonal transport (92). Interestingly, the RNAi-mediated knockdown of MIRO has been shown to lead to an increase in pTau (Ser262) (93) demonstrating the interconnection between mitochondrial transport and the development of toxic protein aggregates in AD. These trafficking dysfunctions coupled with the pTau and Aβ-mediated downregulation of OXPHOS complex activity can reduce synaptic function and promote cognitive decline (94, 95).
To maintain functional mitochondria, cells utilize autophagy where damaged organelles are removed via lysosomal degradation (Fig. 4). For mitochondria-specific autophagic removal known as mitophagy, the collapsed mitochondrial membrane or the release of mitochondria-associated lipids serve as a signal to promote targeting mitochondria into autophagosomes (96). These autophagosomes then fuse with lysosomes for bulk degradation and component recycling, which primarily occur in the cell body although local autophagic processes in axons have also been identified (97). The triggers for autophagy vary including dysfunctional mitochondria, starvation and exercise. However, the most common reason includes the nutrient imbalance. A key sensor of energy homeostasis, AMP-activated protein kinase (AMPK), can activate or increase levels of mitophagy through a direct phosphorylation of unc-51 like activating kinase 1 (ULK1) under energetic stress (98). The most well described mitochondrial quality control mechanism includes the PINK1/PARKIN pathway although other mitophagy pathways have also been described (96). Loss of mitochondrial membrane potential typically stabilizes the protein PINK1 on the OMM, which then phosphorylates OMM proteins including MIRO, MFNs and ubiquitin (96). This leads to the translocation of the E3 ligase PARKIN that further ubiquitinates OMM proteins resulting in the recruitment of autophagic machinery. In neurons, the transport of dysfunctional mitochondria inside autophagic vacuoles (AV) from distal to sematodendritic regions is required for the majority of mitophagic degradation (99). To achieve this, autophagosomes fuse with late endosomes (LE) and form amphisomes together with dynein-snapin motors for transport of AVs back to the soma (100). However in AD, defective retrograde transport can contribute to disease pathogenesis with accumulation of amphisomes at axonal terminals due to Aβ mediated interruption of dynein-snapin coupling (101). Thus, the interconnection between multiple mechanisms of mitochondrial dysfunction in AD is complex where one (e.g., the impairment of axonal transport) could affect the other (e.g., mitophagy). Defective anterograde transport ensures that ‘young’ mitochondria from the soma are unable to fuse and share their components with other mitochondria in distal regions to promote their repair. Impaired retrograde transport affects the ability to remove dysfunctional mitochondria that may arise as a consequence of reduced integrity. Altogether, impairments in mitochondrial and neuronal transport mechanisms coupled with increased accumulation of AVs and reduced ability of lysosomes to fuse with autophagosomes promote the dysfunction of mitochondrial quality control in AD (102–104).
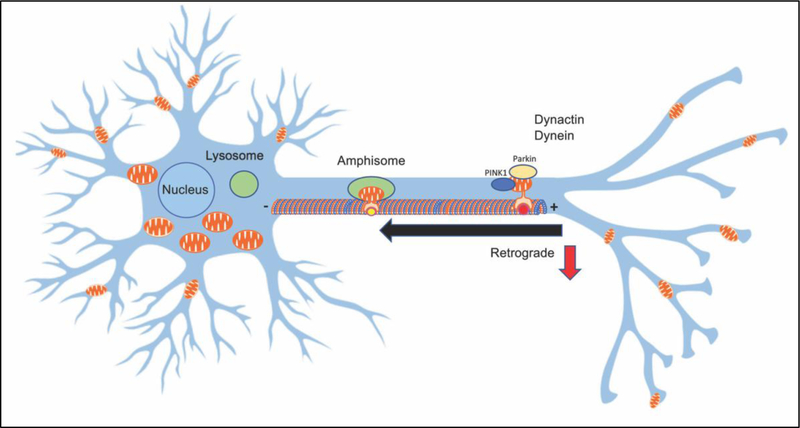
Figure 4: Mechanisms of mitochondrial quality control are interconnected with mechanisms of mitochondrial dynamics.
Dysfunctional mitochondria are transported in the retrograde direction from distal sites to the cell body for degradation and recycling by lysosomes via mechanism called mitophagy. The PINK1/ PARKIN proteins are involved in targeting mitochondria for mitophagy. Autophagosomes that are converted to amphisomes are transported by dynein-snapin motor complexes along microtubules to the cell body for lysosomal degradation. In AD, Aβ can interfere with the formation of these complexes leading to the accumulation of dysfunctional mitochondria and subsequent axonal swelling.
Therapeutic strategies to improve mitochondrial dynamics and function
Given the extent of mitochondria involvement in the development of AD, it is not surprising that they have become increasingly recognized as a therapeutic target. For in depth discussion of current state of therapeutic development in AD field we refer the reader to excellent reviews published recently (105–108). Below, we will summarize therapeutic strategies specifically related to mitochondria.
Approaches to improve mitochondria function are divided into two groups - pharmacological and non-pharmacological interventions. Among the latter, diet and exercise appear to directly improve multiple parameters of mitochondrial dynamics and function collectively referred to as mitochondrial fitness. A beneficial effect of caloric restriction and exercise on slowing the aging process and improvement of mitochondrial function has been shown in multiple model organisms and clinical trials. Extensive studies conducted in mutator mice harboring a defect in the proofreading-exonuclease activity of mitochondrial polymerase gamma (PolG) that results in the accumulation of mitochondrial DNA (mtDNA) mutations and premature aging demonstrated that the endurance exercise induced systemic mitochondrial biogenesis, prevented mtDNA depletion and mutations, increased mitochondrial oxidative capacity and respiratory chain assembly, restored mitochondrial morphology, and blunted pathological levels of apoptosis in multiple tissues of mtDNA mutator mice (109). Importantly, exercise not only improved mitochondrial function in peripheral organs but also completely blocked brain atrophy in these mice. Mutator mice display multiple pathologies that overlap with the observed in advanced aging and AD including deficits in the neurotransmitters acetylcholine, glutamate, aspartate and dopamine; a depletion in levels of nicotinamide adenine dinucleotide (NAD); increased poly(adenosine diphosphate-ribose) polymerase 1 (PARP1) activity in mouse cortex; deficits in carnitine metabolites and an upregulated antioxidant response; metabolic dysfunction and oxidative stress in the brain tissue; impaired rotarod performance and locomotor activity; and decreased protein levels of mitochondrial complexes I and IV subunits; decreased COX activity; and deficits in oxidative phosphorylation (110–113). While there are no reports indicating that mutator mice develop profound AD-like phenotype (possibly due to the relatively short life spanof these mice), crossing a mutator mouse with a mouse model of familial AD accelerated the disease phenotype supporting the hypothesis that mitochondrial dysfunction contributes to the pathogenesis of AD (114). These data highlight the relevance of the observations generated in mutator mice to the development and treatment of age-related neurodegenerative diseases.
In humans, beneficial effect of physical exercise is now well accepted as a strategy not only to promote health but also to manage patients with neurodegenerative disorders including AD (115). Beneficial effect of multifaceted interventions (diet, exercise, cognitive training and vascular monitoring) in at-risk elderly people was tested in multiple clinical trials (FINGER, MAPT and PreDIVA). The results of the trial conducted in the Finish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) showed that a multidomain lifestyle intervention can benefit cognition in elderly people with an elevated risk of dementia (116). This approach is now implemented world-wide through global initiatives with the ultimate goal to harmonize studies on dementia prevention, generate high-quality scientific evidence and promote its implementation (117). Additional studies confirmed multiple benefits of exercise in AD patients including improved brain blood flow, increased hippocampal volume, improved neurogenesis and cognitive function, decreased neuropsychiatric symptoms, and a slower disease progression (118). Moreover, exercise has been shown to have fewer side effects compared to pharmacological interventions. Grounded on a population-based perspective, the Alzheimer’s Association has identified regular physical exercise as one of the strategies to reduce the risk of cognitive decline and the development of dementia (119). However, the exact mechanism of the protective effect of exercise on the brain remains incompletely understood. The proposed pathways mainly converge on mitochondria where improved biogenesis and turnover ensure reduced production of ROS and improved bioenergetics (115). It is also important to note that various rigor and exercise strategies (aerobic vs. strength training) may differentially impact health outcomes in individuals based on age, sex and the disease stage granting further development of public health recommendations (120).
Similar to exercise, beneficial effect of caloric restriction on lifespan and healthspan is well documented in model organisms and humans (121). In healthy adults, 24 months of continuous 15 – 25% reduction in calorie intake resulted in improved quality of life and a significant decrease in levels of oxidative stress markers (122). Additional benefits of caloric restriction in healthy humans include reduced inflammation, decreased levels of circulating tumor necrosis factor-α and cardiometabolic risk factors, improved insulin signaling and immune defense against infections (123). Major mechanisms of caloric restriction include activation of autophagy/mitophagy via AMPK-dependent inhibition of mechanistic target of rapamycin (mTOR) pathway and activation of sirtuins that are essential modulators of cellular stress resistance, aging and cell death. In respect to mitochondria, caloric restriction has been shown to increase mitochondrial biogenesis and turnover leading to a lesser accumulation of dysfunctional organelles, improved mitochondrial dynamics, morphology and function, and decreased mitochondrial permeability improving calcium retention capacity ultimately leading to a protection against excitotoxicity, a major mechanism involved in neurodegeneration (124). Despite mounting evidence in support of caloric restriction to promote health in AD patients, implementation of this strategy in elderly population has proven difficult. Based on the understanding of key mechanisms, multiple caloric restriction mimetics have been recently developed or re-purposed and tested in clinical trials (123).
In addition to caloric restriction, ketogenic diet has been shown beneficial in slowing down the development of cognitive dysfunction in patients with MCI and AD. The ketogenic diet was developed at the Mayo Clinic in 1920 by Dr. Wilder as a strategy to treat epilepsy (125). This diet consists of high fat content, low levels of carbohydrates, and normal protein content, and mimics fasting where energy production in mitochondria is switched to the ketone body utilization (126). The neuroprotective effect of ketogenic diet in AD is attributed to the ability of ketone bodies to provide more efficient source of energy fuel for mitochondria under conditions where glucose uptake is altered. In addition, ketone bodies have been shown to improve mitochondrial respiration, decrease ROS production, improve antioxidant defense, and inhibit mitochondrial permeability transition ultimately protecting neuronal function (127). Indeed, multiple clinical trials conducted in MCI and AD patients suggest that this intervention has positive effect on cognitive function (128–131). However, the long-term implementation of ketogenic diet to patients is difficult and could be associated with adverse side effects (126).
A promising group of compounds have produced a beneficial effect by directly targeting mitochondria. Counter intuitively, these compounds partially inhibit complexes of the OXPHOS machinery, inducing a mild stress response reminiscent to mechanisms involved in caloric restriction and exercise. In particular, we found that modulation of mitochondrial complex I activity with small molecules attenuates the development of cognitive symptoms in multiple mouse models of familial AD when treatment was administered chronically at early stages of AD development (132). Beneficial mechanisms included activation of AMPK, subsequent deactivation of GSK3β, reduction of Aβ and pTau levels, restoration of axonal trafficking, enhanced levels of BDNF and synaptic proteins, augmented bioenergetics, increased ability of neurons to sustain oxidative stress, and restored cognitive function and behavior. Furthermore, broad application of partial complex I inhibitor metformin, an FDA – approved drug to treat Type II Diabetes Mellitus (T2DM), supports the feasibility of such approach in humans. Metformin is a natural product derived from the plant Galega officinalis and has a strong safety record of being used in herbal medicine since medieval times (133). The molecular targets and the mode of action of metformin are incompletely understood. However, it inhibits energy transduction by selectively suppressing efficient coupling of redox and proton transfer domains of complex I, where subsequent AMPK activation appears to be largely associated with a major long-term, clinically relevant effect on enhancing hepatic insulin sensitivity (133–135). Biological, clinical and epidemiological data suggest that T2DM increases risk of mild cognitive impairment, vascular dementia and AD. Clinical trials have found that application of antidiabetic drugs including metformin protected against cognitive decline in MCI and AD patients improving executive functioning, learning, memory and attention (136–138). These antidiabetic drugs positively affect mitochondrial and synaptic function, reduce neuroinflammation, and improve brain metabolism (139). Interestingly, recent systematic review reported that metformin reduced mortality and diseases of aging (cardiovascular disease and cancer) in patients who did not have diabetes demonstrating that effect of metformin on healthspan is independent of its antidiabetic properties (140). Thus, complex I inhibitors including metformin appear to mimic mechanisms involved in caloric restriction and exercise and could slow the aging process, improve memory, and reduce oxidative stress (141–146). Resveratrol is another compound that implements its beneficial effects on health and longevity via reducing the activity of the OXPHOS complexes I, III and V (147–149). Resveratrol activates sirtuins, a family of NAD+-dependent deacetylases that are central to the body’s response to diet and exercise. Resveratrol also stimulates key signaling pathways including the antioxidant defenses, reduction of inflammation via inhibiting NF-κB, AMPK activation leading to improved mitochondrial function and biogenesis through SIRT1 (sirtuin 1)/AMPK/PGC1α pathway and expression of vitagenes, which prevent the deleterious effects triggered by oxidative stress (150–152). Vitagenes are involved in preserving cellular homeostasis during stressful conditions and include, among others, heat shock proteins (Hsp) Hsp32, Hsp70, antioxidant genes such as thioredoxin, and a family of sirtuin proteins (153). Results of studies conducted in in vitro and in vivo models of AD provided evidence that resveratrol normalizes cholinergic neurotransmission, brain-derived neurotrophic factor expression, reduces oxidative stress, promotes β-amyloid peptides clearance and anti-amyloidogenic cleavage of APP, and reduces neuronal apoptosis (154). However, the utilization of resveratrol in humans has been challenging where limited bioavailability, pronounced side effects, and inconsistent results were reported in clinical trials (155).
Among other innovative pharmacological strategies designed to improve mitochondrial function are antioxidant therapies that mitigate local ROS production in mitochondria compared to the reduction of global levels of ROS. These compounds include coenzyme Q10, idebenone, creatine, MitoQ, MitoVitE, MitoTEMPOL, latrepirdine, methyleneblue, triterpenoids, a series of Szeto-Schiller (SS) peptides, curcumin, Ginkgo biloba, and omega-3 polyunsaturated fatty acids (107). These mitochondria-targeted compounds have been extensively evaluated in multiple laboratories using various in vivo and in vitro models of AD. Multiple benefits of these compounds include improved bioenergetics, reduced oxidative stress, improved mitochondrial dynamics and trafficking. For example, a peptide, 6’-dimethyltyrosine-Lys-Phe-NH2 (SS31) binds to cardiolipin, a lipid that is localized specifically to the mitochondrial membranes, and promotes the efficiency of mitochondrial OXPHOS machinery. SS31 has been shown efficacious in protecting against Aβ-induced oxidative stress, synaptic loss, mitochondrial dysfunction, and abnormal calcium homeostasis in vitro and in vivo (156, 157). Some of these compounds have demonstrated promising results in clinical trials (158, 159). However, in many cases the precise mechanism of pharmacological intervention remains uncertain. For example, application of quinazolinone, a mitochondria division inhibitor 1 (Mdivi-1), originally described as a selective inhibitor of mitochondrial fission protein Drp1, induced neuroprotection in in vitro and in vivo models of AD, Parkinson’s disease (PD), traumatic brain injury and other diseases via enhancing mitochondrial fusion, increasing mitochondrial biogenesis and levels of synaptic proteins (160–163). More recently, Mdivi-1 was shown to act as a reversible mitochondrial complex I inhibitor that reduces ROS production suggesting that positive changes in mitochondrial function and reduced fission may not be related to the proposed direct effect of Mdivi-1 on Drp1 GTPase activity (164). Nevertheless, recent extensive studies conducted in neuronal N2a cells support the notion that Mdivi-1 is a Drp1 inhibitor that reduces mitochondrial fragmentation (165). Furthermore, application of Mdivi-1 appears to significantly improve energy production under conditions where complexes, I, II and IV of the ETC are impaired (165). It remains to be determined whether Mdivi-1 acts via multiple mechanisms including partial inhibition of complex I. Since Mdivi-1 has been considered for clinical trials, additional investigations into its molecular targets might be necessary to ensure safety and efficacy of the application in humans (163). Similarly, implementation of antioxidant therapies has met with mixed success. While common antioxidants, such as vitamins E and C, did not produce consistent results in clinical trials, novel approaches include the design of molecules that could specifically target mitochondria to reduce ROS generated by a dysfunctional organelles (166). Oral administration of MitoQ, a ubiquinone linked to a lipophilic triphenylphosphonium cation, produced encouraging results reducing oxidative stress in multiple animal models advancing MitoQ into clinical trials (167). However, future work is required to unequivocally demonstrate the feasibility of mitochondria-targeted antioxidants as a therapeutic strategy for AD. An additional approach recently developed by Anavex was shown beneficial in clinical phase 2a trial for AD where ANAVEX 2–73, a sigma 1 receptor agonist, improved cognitive performance and functional measure in patients. A chaperone protein, sigma 1 is activated in response to acute and chronic cellular stressors and modulates multiple mechanisms involved in neurodegeneration such as glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function (168). Most recent data suggest that application of ANAVEX 2–73 may mediate beneficial effect also via activation of autophagy (169). Similarly, activation of mitophagy has been shown as an essential mechanism of neuroprotection in multiple animal models of AD granting further development of mitophagy activators for clinical application (170)
In conclusion, multiple promising therapeutic strategies aiming to improve cellular energetics and mitochondrial dynamics and function are under the development for the prevention and treatment of neurodegenerative diseases. These strategies are exceptionally important as they also promote healthy aging. Since the greatest risk factor for most of neurodegenerative diseases is age, the development or implementation of health enhancing strategies could delay the onset of debilitating age-related conditions including AD.
Highlights.
Mitochondrial dynamics, trafficking and turnover are essential for neuronal function
Mitochondrial dynamics and function are affected early in Alzheimer’s Disease
Therapeutic strategies to support mitochondrial dynamics could be beneficial for AD
Acknowledgments
This work was supported by the National Institutes of Health NIA RF1AG55549, NINDS R01NS107265, and RO1AG062135 grants (all to ET).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- 1.Guerreiro R, Bras J. The age factor in Alzheimer’s disease. Genome Med. 2015;7:106. doi: 10.1186/s13073-015-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Strooper B, Karran E. The Cellular Phase of Alzheimer’s Disease. Cell. 2016;164(4):603–15. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 3.Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin Investig Drugs. 2017;26(6):735–9. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J Alzheimers Dis. 2018;62(3):1403–16. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich PR. The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans. 2003;31(Pt 6):1095–105. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 6.Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1078–89. doi: 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842(8):1219–31. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1105–21. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbe K, Murley A, Nunnari J. Determinants and functions of mitochondrial behavior. Annu Rev Cell Dev Biol. 2014;30:357–91. doi: 10.1146/annurev-cellbio-101011-155756. [DOI] [PubMed] [Google Scholar]
- 10.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–46. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–87. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11(1):11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 14.Trotta AP, Chipuk JE. Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci. 2017;74(11):1999–2017. doi: 10.1007/s00018-016-2451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Perry G, Smith MA, Wang X. Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2013;33 Suppl 1:S253–62. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20 Suppl 2:S591–607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117(Pt 26):6535–46. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 18.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177–89. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134(3):333–44. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 21.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–55. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523(1–3):171–6. [DOI] [PubMed] [Google Scholar]
- 23.Satoh M, Hamamoto T, Seo N, Kagawa Y, Endo H. Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem Biophys Res Commun. 2003;300(2):482–93. [DOI] [PubMed] [Google Scholar]
- 24.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126(1):163–75. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–77. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consolato F, Maltecca F, Tulli S, Sambri I, Casari G. m-AAA and i-AAA complexes coordinate to regulate OMA1, the stress-activated supervisor of mitochondrial dynamics. J Cell Sci. 2018;131(7). doi: 10.1242/jcs.213546. [DOI] [PubMed] [Google Scholar]
- 27.Hu C, Huang Y, Li L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int J Mol Sci. 2017;18(1). doi: 10.3390/ijms18010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166(7):1027–39. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffen J, Koehler CM. ER-mitochondria contacts: Actin dynamics at the ER control mitochondrial fission via calcium release. J Cell Biol. 2018;217(1):15–7. doi: 10.1083/jcb.201711075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204(6):919–29. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K, Sesaki H, Poon WW, Gylys KH, Patterson ER, Parisi JE, Diaz Brinton R, Salisbury JL, Trushina E. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci Rep. 2016;6:18725. doi: 10.1038/srep18725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putti R, Sica R, Migliaccio V, Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol. 2015;6:109. doi: 10.3389/fphys.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lionetti L, Mollica MP, Donizzetti I, Gifuni G, Sica R, Pignalosa A, Cavaliere G, Gaita M, De Filippo C, Zorzano A, Putti R. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One. 2014;9(3):e92753. doi: 10.1371/journal.pone.0092753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Long Q, Liu J, Tang H, Li Y, Bao F, Qin D, Pei D, Liu X. Mitochondrial fusion provides an ‘initial metabolic complementation’ controlled by mtDNA. Cell Mol Life Sci. 2015;72(13):2585–98. doi: 10.1007/s00018-015-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westermann B Bioenergetic role of mitochondrial fusion and fission. Biochimica et biophysica acta. 2012;1817(10):1833–8. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 38.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19(4):630–41. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, Siddiqui A, Tamura Y, Sesaki H, Wengenack TM, Dzeja PP, Poduslo JF. Defects in Mitochondrial Dynamics and Metabolomic Signatures of Evolving Energetic Stress in Mouse Models of Familial Alzheimer’s Disease. PLoS One. 2012;7(2). doi: ARTN e32737 DOI 10.1371/journal.pone.0032737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid Med Cell Longev. 2016;2016:8590578. doi: 10.1155/2016/8590578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cid-Castro C, Hernandez-Espinosa DR, Moran J. ROS as Regulators of Mitochondrial Dynamics in Neurons. Cell Mol Neurobiol. 2018;38(5):995–1007. doi: 10.1007/s10571-018-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014;2:123–39. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015;22(2):207–18. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Human Molecular Genetics. 2011;20(23):4515–29. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Human Molecular Genetics. 2012;21(11):2538–47. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manczak M, Sesaki H, Kageyama Y, Reddy PH. Dynamin-related protein 1 heterozygote knockout mice do not have synaptic and mitochondrial deficiencies. Biochimica et biophysica acta. 2012;1822(6):862–74. doi: 10.1016/j.bbadis.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manczak M, Kandimalla R, Fry D, Sesaki H, Reddy PH. Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum Mol Genet. 2016;25(23):5148–66. doi: 10.1093/hmg/ddw330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kandimalla R, Manczak M, Fry D, Suneetha Y, Sesaki H, Reddy PH. Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum Mol Genet. 2016;25(22):4881–97. doi: 10.1093/hmg/ddw312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandimalla R, Reddy PH. Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim Biophys Acta. 2016;1862(4):814–28. doi: 10.1016/j.bbadis.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva DF, Selfridge JE, Lu J, E L, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet. 2013;22(19):3931–46. doi: 10.1093/hmg/ddt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105(49):19318–23. doi: 0804871105 [pii] 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. Journal of Alzheimer’s disease: JAD. 2010;20 Suppl 2:S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. The American journal of pathology. 2008;173(2):470–82. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Maestro P, Gargini R, Garcia E, Perry G, Avila J, Garcia-Escudero V. Slower Dynamics and Aged Mitochondria in Sporadic Alzheimer’s Disease. Oxid Med Cell Longev. 2017;2017:9302761. doi: 10.1155/2017/9302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Human Molecular Genetics. 2011;20(13):2495–509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morozov YM, Datta D, Paspalas CD, Arnsten AF. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol Aging. 2017;51:9–18. doi: 10.1016/j.neurobiolaging.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyumentsev MA, Stefanova NA, Kiseleva EV, Kolosova NG. Mitochondria with Morphology Characteristic for Alzheimer’s Disease Patients Are Found in the Brain of OXYS Rats. Biochemistry (Mosc). 2018;83(9):1083–8. doi: 10.1134/S0006297918090109. [DOI] [PubMed] [Google Scholar]
- 60.Vincent AE, Turnbull DM, Eisner V, Hajnoczky G, Picard M. Mitochondrial Nanotunnels. Trends Cell Biol. 2017;27(11):787–99. doi: 10.1016/j.tcb.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincent AE, White K, Davey T, Philips J, Ogden RT, Lawless C, Warren C, Hall MG, Ng YS, Falkous G, Holden T, Deehan D, Taylor RW, Turnbull DM, Picard M. Quantitative 3D Mapping of the Human Skeletal Muscle Mitochondrial Network. Cell Rep. 2019;27(1):321. doi: 10.1016/j.celrep.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavorato M, Iyer VR, Dewight W, Cupo RR, Debattisti V, Gomez L, De la Fuente S, Zhao YT, Valdivia HH, Hajnoczky G, Franzini-Armstrong C. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc Natl Acad Sci U S A. 2017;114(5):E849–E58. doi: 10.1073/pnas.1617788113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez MJ, Ponce DP, Osorio-Fuentealba C, Behrens MI, Quintanilla RA. Mitochondrial Bioenergetics Is Altered in Fibroblasts from Patients with Sporadic Alzheimer’s Disease. Front Neurosci. 2017;11:553. doi: 10.3389/fnins.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 2011;108(31):12920–4. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, Ellisman MH, Taylor SS. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem. 2011;286(4):2918–32. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125(Pt 9):2095–104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334(1):35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Namba T, Funahashi Y, Nakamuta S, Xu C, Takano T, Kaibuchi K. Extracellular and Intracellular Signaling for Neuronal Polarity. Physiol Rev. 2015;95(3):995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 69.Course MM, Wang X. Transporting mitochondria in neurons. F1000Res. 2016;5. doi: 10.12688/f1000research.7864.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, Panaretos NS, Slater SQ, Rengarajan M, Feliu M, Cheney RE. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol. 2009;19(23):2008–13. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shneyer BI, Usaj M, Henn A. Myo19 is an outer mitochondrial membrane motor and effector of starvation-induced filopodia. J Cell Sci. 2016;129(3):543–56. doi: 10.1242/jcs.175349. [DOI] [PubMed] [Google Scholar]
- 72.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30(26):8984–92. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(12):4232–40. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma H, Cai Q, Lu W, Sheng ZH, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J Neurosci. 2009;29(41):13019–29. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends in cell biology. 2010;20(2):102–12. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Hajnoczky G, Booth D, Csordas G, Debattisti V, Golenar T, Naghdi S, Niknejad N, Paillard M, Seifert EL, Weaver D. Reliance of ER-mitochondrial calcium signaling on mitochondrial EF-hand Ca2+ binding proteins: Miros, MICUs, LETM1 and solute carriers. Curr Opin Cell Biol. 2014;29:133–41. doi: 10.1016/j.ceb.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Lim S, Hoffman D, Aspenstrom P, Federoff HJ, Rempe DA. HUMMR, a hypoxia- and HIF-1alpha-inducible protein, alters mitochondrial distribution and transport. J Cell Biol. 2009;185(6):1065–81. doi: 10.1083/jcb.200811033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mironov SL. ADP regulates movements of mitochondria in neurons. Biophys J. 2007;92(8):2944–52. doi: 10.1529/biophysj.106.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reck-Peterson SL, Redwine WB, Vale RD, Carter AP. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol. 2018;19(6):382–98. doi: 10.1038/s41580-018-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lopez-Domenech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 2018;37(3):321–36. doi: 10.15252/embj.201696380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melkov A, Abdu U. Regulation of long-distance transport of mitochondria along microtubules. Cell Mol Life Sci. 2018;75(2):163–76. doi: 10.1007/s00018-017-2590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307(5713):1282–8. [DOI] [PubMed] [Google Scholar]
- 84.Cai Q, Tammineni P. Mitochondrial Aspects of Synaptic Dysfunction in Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1087–103. doi: 10.3233/JAD-160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-Derived Diffusible Ligands Cause Impaired Axonal Transport of Mitochondria in Neurons. Neurodegener Dis. 2010;7(1–3):56–9. doi: 000283484 [pii] 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26(41):10480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107(43):18670–5. doi: 1006586107 [pii] 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: science.1194653 [pii] 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L, Trushin S, Christensen TA, Tripathi U, Hong C, Geroux RE, Howell KG, Poduslo JF, Trushina E. Differential effect of amyloid beta peptides on mitochondrial axonal trafficking depends on their state of aggregation and binding to the plasma membrane. Neurobiol Dis. 2018;114:1–16. doi: 10.1016/j.nbd.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng Y, Bai F. The Association of Tau With Mitochondrial Dysfunction in Alzheimer’s Disease. Front Neurosci. 2018;12:163. doi: 10.3389/fnins.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shahpasand K, Uemura I, Saito T, Asano T, Hata K, Shibata K, Toyoshima Y, Hasegawa M, Hisanaga S. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J Neurosci. 2012;32(7):2430–41. doi: 10.1523/JNEUROSCI.5927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ittner LM, Ke YD, Gotz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. 2009;284(31):20909–16. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iijima-Ando K, Sekiya M, Maruko-Otake A, Ohtake Y, Suzuki E, Lu B, Iijima KM. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer’s disease-related tau phosphorylation via PAR-1. PLoS Genet. 2012;8(8):e1002918. doi: 10.1371/journal.pgen.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–62. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756–71. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu CT. Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol Dis. 2019;122:23–34. doi: 10.1016/j.nbd.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206(5):655–70. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu SB, Pekkurnaz G. Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J Mol Biol. 2018;430(21):3922–41. doi: 10.1016/j.jmb.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol. 2012;22(6):545–52. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes use the ride-on service for retrograde transport toward the soma. Autophagy. 2015;11(8):1434–6. doi: 10.1080/15548627.2015.1062203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tammineni P, Ye X, Feng T, Aikal D, Cai Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. Elife. 2017;6. doi: 10.7554/eLife.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 103.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–91. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- 104.Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. The Journal of cell biology. 2005;171(1):87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fish PV, Steadman D, Bayle ED, Whiting P. New approaches for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2019;29(2):125–33. doi: 10.1016/j.bmcl.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 106.Mullane K, Williams M. Alzheimer’s disease (AD) therapeutics - 2: Beyond amyloid - Re-defining AD and its causality to discover effective therapeutics. Biochemical pharmacology. 2018;158:376–401. doi: 10.1016/j.bcp.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 107.Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 108.Oliver DMA, Reddy PH. Small molecules as therapeutic drugs for Alzheimer’s disease. Mol Cell Neurosci. 2019;96:47–62. doi: 10.1016/j.mcn.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108(10):4135–40. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5(7):e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuke S, Kametani M, Yamada K, Kasahara T, Kubota-Sakashita M, Kujoth GC, Prolla TA, Hitoshi S, Kato T. Heterozygous Polg mutation causes motor dysfunction due to mtDNA deletions. Ann Clin Transl Neurol. 2014;1(11):909–20. doi: 10.1002/acn3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai Y, Kiselak T, Clark J, Clore E, Zheng K, Cheng A, Kujoth GC, Prolla TA, Maratos-Flier E, Simon DK. Behavioral and metabolic characterization of heterozygous and homozygous POLG mutator mice. Mitochondrion. 2013;13(4):282–91. doi: 10.1016/j.mito.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clark-Matott J, Saleem A, Dai Y, Shurubor Y, Ma X, Safdar A, Beal MF, Tarnopolsky M, Simon DK. Metabolomic analysis of exercise effects in the POLG mitochondrial DNA mutator mouse brain. Neurobiol Aging. 2015;36(11):2972–83. doi: 10.1016/j.neurobiolaging.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kukreja L, Kujoth GC, Prolla TA, Van Leuven F, Vassar R. Increased mtDNA mutations with aging promotes amyloid accumulation and brain atrophy in the APP/Ld transgenic mouse model of Alzheimer’s disease. Mol Neurodegener. 2014;9:16. doi: 10.1186/1750-1326-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bernardo TC, Marques-Aleixo I, Beleza J, Oliveira PJ, Ascensao A, Magalhaes J. Physical Exercise and Brain Mitochondrial Fitness: The Possible Role Against Alzheimer’s Disease. Brain Pathol. 2016;26(5):648–63. doi: 10.1111/bpa.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 117.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–66. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 118.Cass SP. Alzheimer’s Disease and Exercise: A Literature Review. Curr Sports Med Rep. 2017;16(1):19–22. doi: 10.1249/JSR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 119.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718–26. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 120.Pedersen BK. Which type of exercise keeps you young? Curr Opin Clin Nutr Metab Care. 2019;22(2):167–73. doi: 10.1097/MCO.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 121.Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, Sabatini DM, de Cabo R, Fontana L. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12(4):645–51. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018;27(4):805–15 e4. doi: 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019;29(3):592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 124.Amigo I, Menezes-Filho SL, Luevano-Martinez LA, Chausse B, Kowaltowski AJ. Caloric restriction increases brain mitochondrial calcium retention capacity and protects against excitotoxicity. Aging Cell. 2017;16(1):73–81. doi: 10.1111/acel.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rogovik AL, Goldman RD. Ketogenic diet for treatment of epilepsy. Can Fam Physician. 2010;56(6):540–2. [PMC free article] [PubMed] [Google Scholar]
- 126.Wlodarek D Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients. 2019;11(1). doi: 10.3390/nu11010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action In: th, Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD) 2012. [PubMed] [Google Scholar]
- 128.Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–4. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 129.Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, Yoshida S, Ashida K, Nakamura K, Takahashi T, Kunugi H. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci Lett. 2019;690:232–6. doi: 10.1016/j.neulet.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 130.Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:28–36. doi: 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fortier M, Castellano CA, Croteau E, Langlois F, Bocti C, St-Pierre V, Vandenberghe C, Bernier M, Roy M, Descoteaux M, Whittingstall K, Lepage M, Turcotte EE, Fulop T, Cunnane SC. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15(5):625–34. doi: 10.1016/j.jalz.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 132.Zhang L, Zhang S, Maezawa I, Trushin S, Minhas P, Pinto M, Jin LW, Prasain K, Nguyen TD, Yamazaki Y, Kanekiyo T, Bu G, Gateno B, Chang KO, Nath KA, Nemutlu E, Dzeja P, Pang YP, Hua DH, Trushina E. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer’s Disease. EBioMedicine. 2015;2(4):294–305. doi: 10.1016/j.ebiom.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cameron AR, Logie L, Patel K, Erhardt S, Bacon S, Middleton P, Harthill J, Forteath C, Coats JT, Kerr C, Curry H, Stewart D, Sakamoto K, Repiscak P, Paterson MJ, Hassinen I, McDougall G, Rena G. Metformin selectively targets redox control of complex I energy transduction. Redox Biol. 2018;14:187–97. doi: 10.1016/j.redox.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016;24(4):521–2. doi: 10.1016/j.cmet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 136.Cao B, Rosenblat JD, Brietzke E, Park C, Lee Y, Musial N, Pan Z, Mansur RB, McIntyre RS. Comparative efficacy and acceptability of antidiabetic agents for Alzheimer’s disease and mild cognitive impairment: A systematic review and network meta-analysis. Diabetes Obes Metab. 2018;20(10):2467–71. doi: 10.1111/dom.13373. [DOI] [PubMed] [Google Scholar]
- 137.Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, Detre JA, Wolk DA, Arnold SE. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis Assoc Disord. 2017;31(2):107–13. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luchsinger JA, Perez T, Chang H, Mehta P, Steffener J, Pradabhan G, Ichise M, Manly J, Devanand DP, Bagiella E. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J Alzheimers Dis. 2016;51(2):501–14. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herath PM, Cherbuin N, Eramudugolla R, Anstey KJ. The Effect of Diabetes Medication on Cognitive Function: Evidence from the PATH Through Life Study. BioMed research international. 2016;2016:7208429. doi: 10.1155/2016/7208429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 141.Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119(5):652–65. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Handschin C Caloric restriction and exercise “mimetics”: Ready for prime time? Pharmacol Res. 2016;103:158–66. doi: 10.1016/j.phrs.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8–9):685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 144.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37(1):47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255–60. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2018;65(4):1225–36. doi: 10.3233/JAD-180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, Vessieres E, Ayer A, Henrion D, Lenaers G, Reynier P, Procaccio V. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS One. 2015;10(12):e0144290. doi: 10.1371/journal.pone.0144290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25(2–3):87–97. [PubMed] [Google Scholar]
- 149.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130(5):1115–23. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tellone E, Galtieri A, Russo A, Giardina B, Ficarra S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid Med Cell Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bastianetto S, Menard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852(6):1195–201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 152.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35(3):146–54. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trovato Salinaro A, Cornelius C, Koverech G, Koverech A, Scuto M, Lodato F, Fronte V, Muccilli V, Reibaldi M, Longo A, Uva MG, Calabrese V. Cellular stress response, redox status, and vitagenes in glaucoma: a systemic oxidant disorder linked to Alzheimer’s disease. Front Pharmacol. 2014;5:129. doi: 10.3389/fphar.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014;6:218. doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wahab A, Gao K, Jia C, Zhang F, Tian G, Murtaza G, Chen J. Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules. 2017;22(8). doi: 10.3390/molecules22081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochimica et biophysica acta. 2012;1822(5):639–49. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reddy PH, Manczak M, Kandimalla R. Mitochondria-targeted small molecule SS31: a potential candidate for the treatment of Alzheimer’s disease. Hum Mol Genet. 2017;26(8):1483–96. doi: 10.1093/hmg/ddx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar A, Singh A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front Pharmacol. 2015;6:206. doi: 10.3389/fphar.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feniouk BA, Skulachev VP. Cellular and molecular mechanisms of action of mitochondria-targeted antioxidants. Curr Aging Sci. 2016. [DOI] [PubMed] [Google Scholar]
- 160.Manczak M, Kandimalla R, Yin X, Reddy PH. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 2018. doi: 10.1093/hmg/ddy335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bido S, Soria FN, Fan RZ, Bezard E, Tieu K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-alpha-synuclein rat model of Parkinson’s disease. Sci Rep. 2017;7(1):7495. doi: 10.1038/s41598-017-07181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu Q, Xia SX, Li QQ, Gao Y, Shen X, Ma L, Zhang MY, Wang T, Li YS, Wang ZF, Luo CL, Tao LY. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res. 2016;1630:134–43. doi: 10.1016/j.brainres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 163.Smith G, Gallo G. To mdivi-1 or not to mdivi-1: Is that the question? Dev Neurobiol. 2017;77(11):1260–8. doi: 10.1002/dneu.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A, Yadava N, Ge SX, Francis TC, Kennedy NW, Picton LK, Kumar T, Uppuluri S, Miller AM, Itoh K, Karbowski M, Sesaki H, Hill RB, Polster BM. The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev Cell. 2017;40(6):583–94 e6. doi: 10.1016/j.devcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Manczak M, Kandimalla R, Yin X, Reddy PH. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 2019;28(2):177–99. doi: 10.1093/hmg/ddy335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans. 2016;44(5):1219–26. doi: 10.1042/BST20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71(6):1056–63. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lahmy V, Meunier J, Malmstrom S, Naert G, Givalois L, Kim SH, Villard V, Vamvakides A, Maurice T. Blockade of Tau hyperphosphorylation and Abeta(1)(−)(4)(2) generation by the aminotetrahydrofuran derivative ANAVEX2–73, a mixed muscarinic and sigma(1) receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38(9):1706–23. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Christ MG, Huesmann H, Nagel H, Kern A, Behl C. Sigma-1 Receptor Activation Induces Autophagy and Increases Proteostasis Capacity In Vitro and In Vivo. Cells. 2019;8(3). doi: 10.3390/cells8030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktaschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–12. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]