Abstract
Early life exposure to stress has been suggested to be a crucial factor for the development of the brain and its functions. It is well documented that childhood stress is a risk factor for sleep problems in adulthood. Piper betle L. leaf extract (PB) has been used in several traditional medicines to cure various ailments. Recently, PB has been proved to have antidepressant activity. The literature suggests that antidepressants affect the synthesis and release of melatonin through several mechanisms. Thus, this study investigated the potential role of PB for the treatment of sleep disruption after early life stress exposure. Firstly, dexamethasone (DEX) (2 and 20 mg/l for 24 h) was administered to zebrafish larvae on the 4th day post-fertilization (dpf) to induce early life stress. The effects of stress on behaviour during adulthood, melatonin level and stress-related gene expression (nfkb) in the brain were then studied. Next, the possible role of PB (10 and 30 mg/Kg) was studied by measuring its effect on behaviour and by quantifying the expression levels of several melatonin-related (MT1, MT2, aanat1, aanat2) and stress-related (nfkb) genes by qPCR. DEX-treated zebrafish exhibited anxious behaviour, along with a lower level of melatonin and a higher mRNA expression of nfkb. After treatment with PB, a similar effect on behaviour and gene expression levels as the melatonin treatment group (10 mg/kg; positive control) was seen in adult zebrafish. These molecular confirmations of the observed behavioural effects of the PB indicate a possible role in the treatment of early life stress-induced sleep disruption.
Keywords: Piper betel L., sleep disruption, stress, glucocorticoid, melatonin, zebrafish
Introduction
Stress is a complex condition with negative emotional and biological effects. Stress can be detrimental to human health regardless of whether it occurs during the prenatal period, infancy, childhood, adolescence, adulthood or in old age. Stress has an impact on the brain structures involved in cognition and mental health (1). Early-life stress causes critical developmental complications and impacts brain functions in both animals and humans (2–4). Early life stress also increases the anxiety level of fish (4) and alters social behaviour in rodents (2). Similarly in humans, exposure to stress in early life increases the risk of developing mental health problems such as hyperactivity (3) and sleep problems (5). While stress is percieved to have negative consequences, this may not always be true in the case of acute stress (6). In contrast to chronic stress, acute stress results from a single exposure to a stressor on a scale of min to hours (6). The effects of chronic stress are not as clear as it paradoxically can have both positive and negative effects in the case of cognition (7).
We know that sleep is an important physiological and behavioral state of human homeostasis. The negative impact of disturbed sleep on cognitive functions, mood and overall health are very much evident (8). Melatonin has a significant role in sleep initiation and maintenance in humans. Its administration promotes sleep in fish (9), birds (10) diurnal primates (11,12) and in humans (13). The effects of reduced melatonin levels on sleep are still under investigation. Some studies have suggested that circulating melatonin levels are significantly lower in aged individuals as compared to young healthy adults, which affects their sleep (14–16). Similarly, Zhdanova et al (17) has shown that an age-dependent decline in nighttime brain melatonin levels reduces the sleep time in zebrafish. Haimov et al (16), has also proven a significant correlation between a low level of melatonin production and insomnia. Based on other studies, stress also negatively affects melatonin synthesis (18,19), which further modulates a variety of physiological processes such as sleep regulation.
AANAT is the first enzyme in the conversion of serotonin to melatonin and its activity plays an important role in melatonin production. Roseboom et al (20), has observed that after the onset of darkness, aanat mRNA levels increases, followed shortly by increases in AA-NAT protein levels and hence enzyme activity. Literature suggests that there are two aanats which are expressed (aanat1 in the retina and aanat2 in the pineal organ) in teleost fish. Retina aanat1 mRNA peaks in the late afternoon and pineal annat2 mRNA peaks six h later (21).
Glucocorticoids have been identified as the main corticosteroids that protect an organism against stress (22), but excessive exogenous glucocorticoids can induce low birth weight in infants, hypertension in adulthood as well as increased anxiety-related behaviour in rats (23,24) and in zebrafish (4). In literature, it is also documented that glucocorticoids have an inhibitory effect on melatonin synthesis (25,26). Literature also suggests that Nfkb is potential biomarker for oxidative stress (27) and is found in almost all animal cell types. It is also involved in cellular responses to stimuli such as stress, cytokines and free radicals (27). This is significant because melatonin also prevents oxidative stress (28) and inhibits nfkb expression (29).
Piper betle L
(PB) is one of the most popular plants in the Asiatic region and ranks second to tea and coffee in terms of daily consumption (30). PB leaf extract and its purified compounds are well-known ethnomedicines and are associated with a number of potential therapeutic efficacies. It has been reported to have antimicrobial, antibacterial, antioxidant, anticancer, antidiabetic and anti-inflammatory activities (31). In recent studies, PB has been shown to exhibit analgesic and antidepressant activities as well (32,33).
Although most behavioural research in the past was done with rodent models, zebrafish are an upcoming animal model due to their prolific breeding, which results in a large number of offspring for experimental purposes in a relatively short duration (34). Other advantages of zebrafish as compared to rodents in terms of an animal model are their longer lifespan and robust phenotypes, as they display apparent and easily quantifiable behavioural endpoints (35). The variety of behavioural and molecular tools available for use with zebrafish makes them outstanding models for helping to investigate neuromolecular mechanisms. Zebrafish can provide a comparatively quick indication of possible functional efficacy. In addition, this diurnal vertebrate has several fundamental similarities to humans (36). All these characteristics make zebrafish a better animal model for behavioural research. Due to their cost effectiveness, reliability and scalability, this study opted for zebrafish as an animal model over rodents. In the context of this experiment, zebrafish are also emerging as a promising model organism for experimental studies of sleep and its related disorders as its pineal gland and retina contains circadian oscillators that drive the rhythms for synthesizing melatonin (37) and regulating sleep in a similar fashion as in humans (38).
Thus, the aim of the present study was to evaluate the effect of early life stress induced via the glucocorticoid dexamethasone (DEX), on melatonin synthesis and adult zebrafish behaviour. This study also aims to assess the potential role of PB for the treatment of stress induced sleep disruption.
Materials and methods
Preparation of the plant extract
PB dried leaves were obtained from the herbal farm, Sungai Buloh, Malaysia. Following the extraction protocol used by Ganguly et al (39), the dried leaves were first grounded to powder form in a hand crusher. The powdered leaves (300 gm) were extracted twice with absolute ethanol (900 ml each time) and filtered through Whatman no. 1 filter paper. The alcoholic extract was subsequently dried in a rotary evaporator to obtain a semi solid extract with an approximate yield of 5%. Then extract was stored in −20°C until needed. The PB extract was dissolved in 1% of Dimethyl sulfoxide (DMSO) before being used. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was employed to identify the chemical constituents in the alcoholic extract of PB. PB was found to have a qualitative composition of eugenol, allylpyrocatechol, caryophyllene and camphor.
Luciferase reporter gene assay
A luciferase reporter gene assay was performed to determine the affinity of PB for the melatonin receptors MT1 and MT2. This method measures G protein signalling through changes in the levels of the secondary messenger cAMP. In case of the melatonin receptor assay, stimulation of Gi-coupled melatonin receptors decreases the expression of intracellular cAMP, leading to a decrease in the transcription level of the reporter gene luciferase and hence the degree of bioluminescence produced. For this experiment, the method described by Chen et al (40) was used, with some minor modifications for Luciferase Reporter Gene Assay. Briefly, human embryonic kidney (HEK) (41) cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C and in an atmosphere of 5% CO2. On the second day after checking the confluency of the cells, the transfection was done using a suspension of plasmid (MT1 or MT2), Luciferase reporter (CRE), OPTI-MEM (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h, the media and cell morphology was checked and then treated with either 0.25 mM of the positive controls (MT1 receptor assay positive control: 6-chloromelatonin; MT2 receptor assay positive control: 2-iodomelatonin) or different PB extract doses (10, 30, 50 µg/ml). Forskolin (1:1,000) was also added as a stimulator of adenylylcyclase, to raise the cAMP level so that the inhibition of cAMP is easier to detect. After 6 h of treatment, the dual Luciferase assay was performed as per the manufacturer's (Promega Corporation, Madison, WI, USA) instructions. For the data analysis, the luciferase activity of each sample was normalized to the renilla luciferase activity and the relative luciferase activity was calculated with the help of the formula:
Animal breeding
Adult zebrafish (Danio rerio; 3–4 months-old) of heterogeneous wild-type stock (standard short-fin phenotype) were obtained from a local commercial supplier (Akuarium Batu Karang Laut Sdn Bhd, Selangor, Malaysia). All fish were housed in the animal facility department of Monash University, Malaysia under standard conditions. They were maintained under 14 h Light (200 lux): 10 h Dark (5 lux) regime [light onset: 8 am (ZT; Zeitgeber Time=0); light offset: 10 pm (ZT=14)] and fed twice daily to ensure a constant source of nourishment. They were kept under the above conditions for two weeks to acclimatize the zebrafish before breeding them. In the present study, 10 adult zebrafish (5 females and 5 males) were used as breeders to procure eggs. We have used the standard breeding method as described by Khor et al (4).
Dexamethasone (DEX) exposure for early life stress
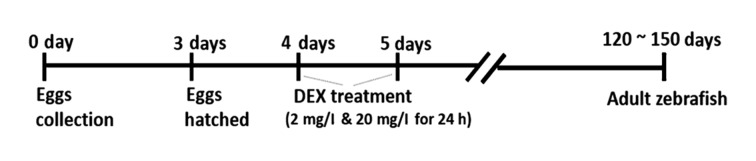
To create an early life stress model (Fig. 1), zebrafish larvae were treated with dexamethasone 21-phosphate salt (DEX; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) (2 and 20 mg/l) and with tap water (control group) at 4 dpf (days post fertilization) for 24 h. All the larvae were randomly divided into groups. The doses used for DEX treatment (2, 20 mg/l) were chosen as per the doses established by Khor et al (4) in their model of zebrafish anxiety like behaviour. Although the paper also tested the effect of 200 mg/l of DEX, this dose resulted in very apprent changes in the swimming pattern of the zebrafish. After the treatment, the larval zebrafish were maintained until the adult stage (120~150 dpf; weight range of 0.7–0.9 grams) under the standard conditions previously mentioned (14:10 LD) till subjected to experimentation. Only adult male zebrafish were used in the study. All experimental procedures implemented in this study follow the guidelines on the care and the use of animals, with ethical approval by the Monash Animal Research Platform (MARP) Animal Ethics Committee, Monash University, Australia (MARP/2014/133).
Figure 1.
Early life stress induced sleep disorder model.
Grouping and treatment
A total of 200 zebrafish were used in the present study. All zebrafish were randomly divided into groups using a physical randomisation method. In the first part of the study, all adult zebrafish which were earlier exposed to DEX treatment; divided into three groups (n=10 per group) consisting of treatments with DEX (2, 20 mg/l) and water (control) to explore the effect of early life stress on behaviour (locomotor activity and % of time spent in the upper zone of the tank), the level of melatonin and nfkb expression in adult zebrafish. Secondly, the fish were divided into eight groups (n=8–10 per group) consisting of early life exposure to DEX (2 and 20 mg/l) and treatment with either PB 10 mg/Kg, PB 30 mg/Kg, melatonin 10 mg/kg or vehicle (DMSO), to determine the effect of PB on behavior, melatonin receptor and synthesis as well as stress related gene expression in adult zebrafish. Intraperitoneal injections were used for the PB, melatonin and vehicle (DMSO) treatment, using a standard method according to Kundap et al (42). The melatonin dose was chosen based on the work of Cuesta et al (43) whom determined that this dose produced the highest positive effects when injected into seabream (teleost fish).
The experimenter was unaware of the identity of the control and treatment groups while processing the samples for analysis.
Behavior tests
All the behaviour tests were carried out with adult male zebrafish in the early h (9:00am-1:00pm) of the light phase, The zebrafish had their behaviour monitored in an experimental tank with the dimensions of 36×26×22 cm, filled with 10L of water. Lighting conditions were fixed (~400 lux) and the water temperature was at 28°C±1°C. A video tracking system (Sony Handycam PJ340) was used to record their behaviour. All data was analyzed by using SMART software (Panlab, Barcelona, Spain). For analysis, the experimental tank was divided into two equal halves (upper and lower zones). The sleep like behaviour parameters analysed in this study as adapted from Zhdanova et al (17), were locomotor activity-total distance travelled (cm) in the tank; time spent in upper zone of the tank (%) and quiescent state-inactive period (sec) in the given period of time (10 min). As per the criterion of Zhdanova et al (17), inactive period was defined as the number of sec in which the swimming speed of the fish is below 0.1 cm/sec. of Fish were injected intraperitoneally with their respective treatment doses of PB, melatonin or DMSO (as mentioned above) and after 30 min, they were subjected to behaviour monitoring.
Melatonin estimation
LCMS-MS was performed for the estimation of melatonin in adult zebrafish brains which were earlier exposed to DEX treatment. All zebrafish were divided into three groups consisting of treatments with DEX (2 mg/l, n=6; 20 mg/l, n=6) and control (water, n=6) as mentioned above. All brain samples were harvested at ZT 16 (after the 2 h of light offset). Melatonin is light sensitive, so as a precaution, all sampling was completed under very dim red light. Samples were harvested and homogenized in 400 µl of ice cold methanol with 0.1% formic acid. The homogenate was vortex mixed and centrifuged at 18,000 X g for 10 min at 4°C. The supernatant was used for further analysis by using the standard method of LCMS-MS (42).
Gene expression study
A new set of DEX-exposed fish groups were divided in a similar manner to the behavior study. They were injected intraperitoneally with their respective doses at ZT12 (2 h before lights offset) and all brain samples were harvested at ZT16 (after the 2 h of light offset). Because melatonin is light sensitive, all sampling was completed under the very dim red light as a precaution. All brain samples were collected in 200 µl of ice cold trizole (Invitrogen; Thermo Fisher Scientific, Inc.) and immediately stored in −80°C until needed.
RNA isolation and cDNA synthesis
Total mRNA was isolated by following the manufacturers' protocol. In brief, the brain tissue was properly homogenized in trizole and mixed with 40 µl of chloroform and centrifuged at 13,500 rpm (revolutions per minute) for 15 min at 4°C. The upper aqueous supernatant was transferred into new tubes and 100 µl of isopropanol was added, mixed and incubated for 10 min at room temperature and centrifuged for 10 min at 13,500 rpm at 4°C. Later, the supernatant was discarded and the pellets were subjected for rinsing with 200 µl of 75% ethanol. Then, the pellets were left for air drying for between 5 to 8 min. Finally, 15 µl of nuclease free water was added to each tube to dissolve the mRNA pellet. The concentration and purity of the isolated mRNA was measured using a NanoDrop Spectrophotometer. The mRNA samples were converted into cDNA using the Omniscript Reverse-transcription kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol, with a final volume of 20 µl.
Reverse transcription-quantitative-PCR (RT-qPCR)
Gene expression levels for melatonin receptor 1A (MT1), melatonin receptor 1B (MT2), arylalkylamine N-acetyltransferase 1 (aanat1), arylalkylamine N-acetyltransferase 1 (aanat2) and nuclear factor Kappa-light-chain-enhancer of activated B cells (nfkb) were quantified by real-time quantitative RT-qPCR (Step one; Applied Biosystems) using QuantiTect SYRB Green dye (Qiagen, Valencia, CA, USA). All the primer sets were provided by Qiagen (MT1:QT02113986; MT2:PPZ00432A; aanat1:QT02041431; aanat2:QT02183783; nfkb:QT02149980 and eef1a1b:QT02042684). The PCR mixture contained 1X SYBR-Green PCR master mix (Qiagen), 0.7 µM each forward and reverse primers and 1 µl of sample cDNA. The samples were incubated at 95°C for 2 min prior to thermal cycling (40 cycles of 95°C for 5 sec and 60°C for 15 sec). The relative expression values of the above genes was obtained by normalizing threshold cycle (Ct) values of genes of interest against Ct value of eef1a1b (housekeeping gene) [2^(Ct eef1a1b-Ct Gene of interest)] (42).
Statistical analysis
All obtained data was analyzed by one-way analysis of variance (one-way ANOVA) followed by Dunnett post-hoc test with the level of significance set as *P<0.05, **P<0.01, ***P<0.001 for the DEX-2 groups and #P<0.05, ##P<0.01, ###P<0.001 for the DEX-20 groups, using Graph Pad Prism v.5.0. The data is presented as mean ± standard error of the mean.
Results
Luciferase reporter gene assay
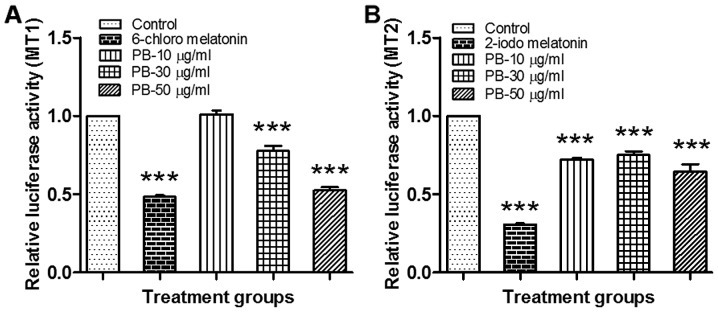
PB significantly decreased (P<0.001) the relative luminescence activity as compared to the vehicle control, which indicates that Piper betel extract exhibited a strong agonist activity towards Melatonin receptor 1A (Fig. 2A) and 1B (Fig. 2B).
Figure 2.
Resultant MT1 and MT2 agonist acitivty of different PB concentrations. (A) Effect of different concentrations of PB (10, 30 and 50 µg/ml) and 6-chloro melatonin (positive control) on the luciferase activity in cells transfected with a luciferase reporter, plasmid (melatonin receptor MT1) and treated with vehicle. Data is presented as mean ± SEM and analyzed by one-way ANOVA in comparison with control (two independent experiments performed in quadruplicate). ***P<0.001 vs. Control. (B) Effect of different concentrations of PB (10, 30 and 50 µg/ml) and 2-iodo melatonin (positive control) on the luciferase activity in cells transfected with a luciferase reporter, plasmid (melatonin receptor MT2) and treated with vehicle. Data is presented as mean ± SEM and analyzed by one-way ANOVA in comparison with control (two independent experiments performed in quadruplicate). ***P<0.001 vs. Control. MT1, melatonin receptor type 1A; MT2, melatonin receptor type 1B; PB, Piper betle L. leaf extract; SEM, standard error of the mean; ANOVA, analaysis of variance.
Effect of early life DEX exposure in adult zebrafish on the behaviour and the level of melatonin and nfkb gene expression
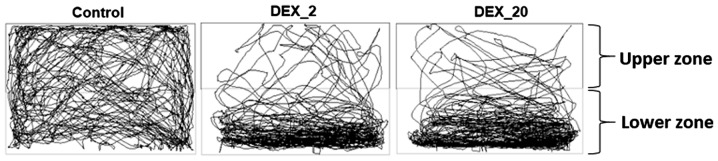
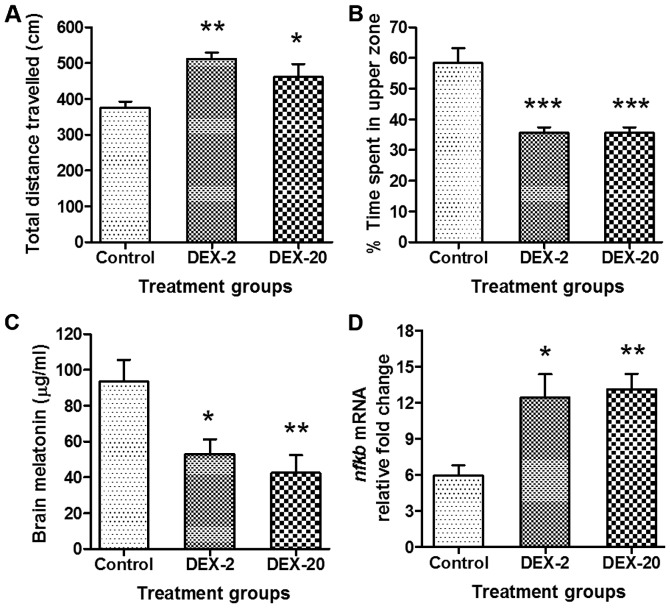
The groups of fish which were exposed to DEX (2 and 20 mg/l) in early life, show significantly increased (P<0.01 and P<0.05) total distance travelled in a given period of time (10 min) in comparison to control group of fish (Figs. 3 and 4A). Furthermore, the percentage of time spent in the upper zone was significantly decreased in DEX 2 mg/l (P<0.001) and 20 mg/l (P<0.001) treated fish in comparison to control group (Figs. 3 and 4B).
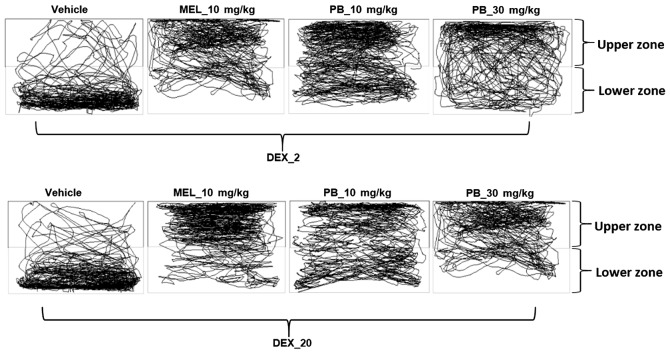
Figure 3.
Representative tracking images of zebrafish locomotor activity from each group obtained at the end of the analysis using SMART software. The tanks were divided into the upper zone and lower zone. Tracking patterns revealed DEX-treated fish had increased locomotor activity and less time spent in the upper zone of the tanks. DEX, dexamthasone.
Figure 4.
Effect of early life DEX treatment on the locomotion parameters, brain melatonin levels and nfkb mRNA expression level of adult zebrafish. (A) Effect of early life DEX treatment (2 and 20 mg/l) in adult zebrafish; total distance travelled, (B) % time spent in the upper zone of tank, (C) level of brain melatonin and (D) nfkb mRNA expression level. The data were analyzed by one way ANOVA followed by dunnett post-hoc test, and was represented as the mean ± SEM, n=8. *P<0.05; **P<0.01; ***P<0.001 vs. the Control Group. DEX, dexamthasone; nfkb, nuclear factor kappa-light-chain-enhancer of activated B cells; mRNA, messenger RNA; ANOVA, analaysis of variance; SEM, standard error of the mean.
In the case of melatonin estimation, fish which were exposed to DEX (2 and 20 mg/l) in early life show significantly reduced (P<0.05 and P<0.01) levels of melatonin in the adult zebrafish brain in comparison to control group (Fig. 4C).
The results also showed a significant elevation (P<0.05) and (P<0.01) in the expression level of nfkb in the DEX treated groups (2 and 20 mg/l) as compared to control group (Fig. 4D).
Effect of PB on adult zebrafish behaviour which were earlier exposed to DEX treatment
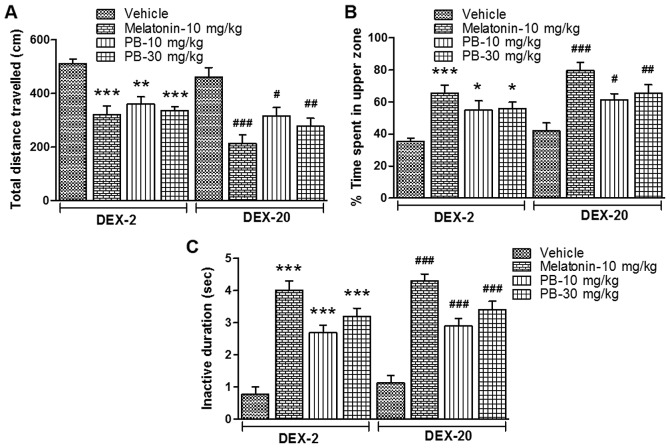
After the treatment of melatonin (positive control; 10 mg/kg) and PB (10 and 30 mg/kg) on the groups of fish which were earlier exposed to DEX (2 and 20 mg/l), they showed significantly reduced [(DEX-2; P<0.001, P<0.01 and P<0.001) (DEX-20; P<0.001, P<0.05 and P<0.01)] locomotor activity in given period of time (10 min) (Figs. 5 and 6A) and their time spent in the upper zone of the tank significantly increased [(DEX-2; P<0.001, P<0.05 and P<0.05) (DEX-20; P<0.001, P<0.05 and P<0.01)] (Figs. 5 and 6B). Furthermore their quiescent state (inactive duration) also significantly increased [(DEX-2; P<0.001, P<0.001 and P<0.001) (DEX-20; P<0.001, P<0.001 and P<0.001)] in comparison to their respective vehicle treated group (Fig. 6C).
Figure 5.
Representative tracking images of zebrafish locomotor activity from each group obtained at the end of the analysis using SMART software. The tanks were divided into the upper zone and lower zone. Tracking patterns revealed DEX-treated fish had increased locomotor activity and less time spent in the upper zone of the tanks. After the treatment with PB (10 and 30 mg/kg) they exhibtied similar swimming patterns as the melatonin (10 mg/kg)-treated group (positive control). DEX, dexamthasone; PB, Piper betle L. leaf extract.
Figure 6.
Effect of melatonin and PB on zebrafish sleep-like behaviour. Effect of melatonin (10 mg/kg; positive control) and PB (10 and 30 mg/Kg) on early life DEX treated (2 and 20 mg/ml) adult zebrafish on sleep-like behaviour, which is characterized by (A) total distance travelled, (B) % time spent in the upper zone of the tank (place preference) and (C) inactive duration (quiescent phase). The data were analyzed by one way ANOVA followed by dunnett post-hoc test, and was represented as the mean ± SEM, n=8. *P<0.05; **P<0.01; ***P<0.001 vs. the DEX-2 Vehicle Group. #P<0.05; ##P<0.01; ###P<0.001 vs. the DEX-20 vehicle group. PB, Piper betle L. leaf extract; DEX, dexamthasone; ANOVA, analaysis of variance; SEM, standard error of the mean.
Effect of PB on gene expression of adult zebrafish which were earlier exposed to DEX treatment
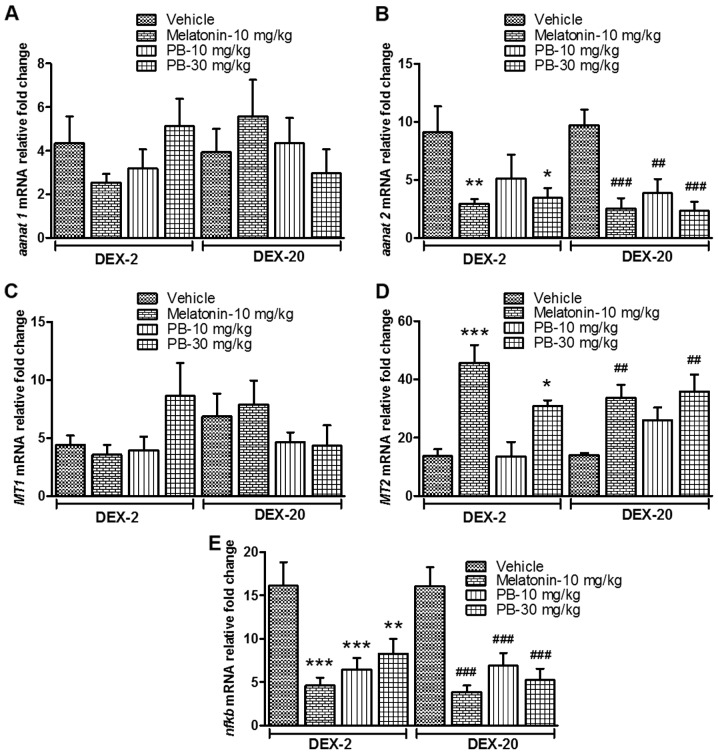
After the treatment of melatonin (positive control; 10 mg/kg) and PB (10 and 30 mg/Kg) on the groups of fish which were earlier exposed to DEX (2 and 20 mg/l), they show significantly reduced mRNA expression level of aanat2 [(DEX-2; P<0.01 and P<0.05) (DEX-20; P<0.001, P<0.01 and P<0.001)] (Fig. 7B) and nfkb [(DEX-2; P<0.001, P<0.001 and P<0.01) (DEX-20; P<0.001, P<0.001 and P<0.001)] (Fig. 7E). In the case of MT2, the mRNA expression level significantly increased [(DEX-2; P<0.001 and P<0.05) (DEX-20; P<0.01 and P<0.01)] in comparison to their respective vehicle treated group (Fig. 7D), but there was no significant change in aanat1 and MT1 gene expression.
Figure 7.
Effect of PB on sleep-related genes in early life DEX treated adult zebrafish. Effect of PB (10 and 30 mg/kg) on early life DEX treated (2 and 20 mg/ml) adult zebrafish on sleep related gene; (A) aanat1 mRNA expression level, (B) aanat2 mRNA expression level, (C) MT1 mRNA expression level, (D) MT2 mRNA expression level and (E) stress related gene nfkb mRNA expression level. The data were analyzed by one way ANOVA followed by dunnett post-hoc test, and was represented as the mean ± SEM, n=8. **P<0.01; ***P<0.001 vs. the DEX-2 Vehicle Group. ##P<0.01; ###P<0.001 vs. the DEX-20 Vehicle Group. PB, Piper betle L. leaf extract; DEX, dexamthasone; aanat1, arylalkylamine N-acetyltransferase 1; aanat2, arylalkylamine N-acetyltransferase 2; MT1, melatonin receptor type 1A; mRNA, messenger RNA; MT2, melatonin receptor type 1B; ANOVA, snalaysis of variance; SEM, standard error of the mean.
Discussion
Melatonin receptors (MT1 and MT2) are valuable molecular targets for the drug discovery of sleep related problems. In preliminary in-vitro studies, PB significantly decreased the relative luminescence activity as compared the vehicle control, with 50 µg/ml of PB showing a level of activity that is similar to that of the MT1 positive control (6-chloro melatonin). This indicates that PB can act as a full agonist against MT1 at a concentration of 50 µg/ml as it shows similar efficacy to the melatonin analogue 6-chloro melatonin. In contrast, PB is only a partial agonist of MT2 as it produced a sub-maximal response as compared to the MT2 positive control (2-iodomelatonin), at all tested concentrations. This could be due to the slight difference between the MT1 and MT2 receptors, in which the MT2 receptor possesses an additional hydrophobic pocket at the region which corresponds to the melatonin N1-C2 binding region (44). This suggests that the PB constituents which bind to the MT1 and MT2 receptors, do not possess a lipophilic side chain at the melatonin N1 or C2 topological equivalent. While we did not perform a quantitive LCMS-MS analysis of our extract, any active constituents are likely to have a similar structure to that of melatonin and thus future research could focus on the isolation of the active constituents for further testing and development. Future studies could also utilise MT1 (Fabomotizole) and MT2 (Luzindole) antagonists as another confirmatory test.
Melatonin is the principal hormone involved in multiple physiological processes, including the sleep-wake cycle. It is functionally associated with sleep only in diurnally active species (45). For this reason, we have chosen melatonin as a positive control in our experiments with the diurnal zebrafish. Zebrafish larvae exposed to DEX (2 and 20 mg/l) show a decrease in the level of melatonin production at night time in the adult zebrafish brain. This clearly indicates the interference of the glucocorticoid DEX in the formation of melatonin. A number of studies have shown that melatonin synthesis is influenced by glucocorticoids and is negatively affected by stress in numerous animals (19,25,46,47). These findings suggest that neonatal exposure of DEX could affect the neuronal and hormonal signaling pathways related to melatonin synthesis.
In addition to this, our data shows increased zebrafish locomotor activity and decreased time spent in the upper zone of the tank, which is characteristic of anxiety like behaviour as seen in previous zebrafish studies (4). Some other studies have also demonstrated that the DEX can disrupt the glucocorticoid receptor signaling pathway and alter the neuroendocrine system, which impacts behaviour such as hyper activity in adulthood (3,23), that can interfere with sleep behaviour. Furthermore, nfkb mRNA expression is significantly higher in DEX treated fish in comparison to normal fish, which suggests that early life stress has an impact on the expression of stress-activated proteins, which can further influence normal sleep regulation as sleep is a resting state with anti-oxidative properties (48).
Nowadays, sleep disorders are becoming a general societal problem in all age groups of people. Studies suggest that sleep problems have a negative impact on health and cognitive performance (49). Stress is the most powerful contributor to poor sleep and exposure to stress during childhood has been suggested to be a crucial factor for the development of sleep disorders in adulthood (5). Currently, many substances are available for the treatment of sleep disorders, but they have some sort of side effects such as daytime sleepiness (50). The interest of researchers in medicinal plants as a natural source of many active components has noticeably increased in the past 20 years as drugs against various pharmacological targets are developed. Nowadays, natural products are in great demand due to their prevalent biological properties and for the discovery of many types of effective bioactive compounds in natural products (33). In the present study, PB has enhanced sleep like behaviour (decrease in total distance travelled, increased time spent in the upper zone and in a quiescent state), which is similar to melatonin treated group which we have used as a positive control in the study. Additionally, PB was found to significantly suppress nfkb mRNA expression in early life stressed fish, similar to that seen in the melatonin treated group. This suggets that PB has similar anti-oxidative properties and potentially it can help to reduce oxidative stress induced by early life stress.
In the present study, we did not find any changes in aanat1 mRNA expression. This is interesting as in zebrafish, aanat1 is thought to be expressed exclusively in the retina and is naturally lower at night when we collected our brain samples, as it peaks in the late afternoon (51). In the case of aanat2, mRNA expression was suppressed after the treatment of PB and melatonin in DEX treated fish. We speculate that increased levels of melatonin initiated a negative feedback mechanism to suppress aanat2 expression and downregulate the further production of melatonin as it has reached peak levels. This is because rhythmic transcription of aanat ensures melatonin production is turned on only after the onset of darkness (52). For a clear mechanism of action of aanat2 in zebrafish brain, further research should be carried out.
Moreover, it is well established that the effect of melatonin on sleep is mediated by melatonin receptors (9). In the present study, PB significantly increased the MT2 mRNA expression in the DEX treated fish, which is similar to melatonin treated group which we have used as a positive control. Increased expression of MT2 mRNA suggests that it facilitates the expression of melatonin to maintain an optimum level throughout the dark h to expedite physiological activities. Pandi-Perumal et al (53) has suggested that melatonin exerts its physiological actions by interacting with melatonin receptors. On the other hand, there was no significant change in MT1 mRNA expression. Further investigations are necessary to examine whether MT1 exhibits different expression patterns in the zebrafish brain which might explain the discrepancies observed between both types of receptors. Alternatively, the results could be explained by the high level of exogenous melatonin in the positive control group. This would trigger the same negative feedback mechanisms that result in a reduced level of annat2 and hence melatonin production as previously discussed. In addition, melatonin undergoes biexponential decay in blood plasma, with a first distribution half-life of 2 min and a second metabolic half-life of 20 min due to melatonin catabolism by the liver (54). It is possible that these correction mechanisms results in an overshoot, causing melatonin levels to fall below their normal concentration at ZT16 when the zebrafish were removed for gene expression analysis, four h after treatment. As the level of melatonin falls below normal, MT2 expression is upregulated to compensate for this, but not MT1 as melatonin has a higher affinity for MT1 as compared to MT2 (55). The PB extract at a dose of 30 mg/kg also shows a similar effect on the melatonin related genes, lending credence to the idea that it can be used to improve sleep disruption as a result of a deficiency in melatonin.
Taken together, the results of the present study demonstrated that early life exposure of glucocorticoid (DEX) has a negative impact on the development of the brain and produces long-term effects that persist till adulthood. This is chacracterised by anxious behaviour, a decrease in melatonin production and increased nfkb mRNA expression. Our results also suggest that PB could be effective against such conditions and it is a promising candidate for the improvement of sleep disruption related to early life stress.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DEX
dexamethasone
- PB
Piper betle L. leaves extract
- ZT
Zeitgeber time
- MT1
melatonin receptor type 1A
- MT2
melatonin receptor type 1B
- aanat1
arylalkylamine N-acetyltransferase 1
- aanat2
arylalkylamine N-acetyltransferase 2
- nfkb
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- eef1a1b
eukaryotic translation elongation factor 1 alpha 1b
Funding
The present study was supported by Fundamental Research Grant Scheme (FRGS), Ministry of Education Malaysia (MOE) (grant no. FRGS/1/2014/SG03/MUSM/03/2).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YK performed all the experiments and was responsible for the writing of the manuscript in its entirety. BC, MFS and IO were involved in conceptualizing and proofreading. All authors gave their final approval for the submission of the manuscript.
Ethics approval and consent to participate
All experimental procedures implemented in this study follow the guidelines on the care and the use of animals, with ethical approval by the Monash Animal Research Platform (MARP) Animal Ethics Committee, Monash University, Australia (MARP/2014/133).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 2.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1111/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60:364–371. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Khor YM, Soga T, Parhar IS. Caffeine neuroprotects against dexamethasone-induced anxiety-like behaviour in the Zebrafish (Danio rerio) Gen Comp Endocrinol. 2013;181:310–315. doi: 10.1016/j.ygcen.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield EA, Lee C, Friedman EL, Springer KW. Childhood abuse as a risk factor for sleep problems in adulthood: Evidence from a US national study. Ann Behav Med. 2011;42:245–256. doi: 10.1007/s12160-011-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn N, Hermans EJ, Fernández G. Cognitive benefit and cost of acute stress is differentially modulated by individual brain state. Soc Cogn Affect Neurosci. 2017;12:1179–1187. doi: 10.1093/scan/nsx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S29–S34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/S0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 10.Mintz EM, Phillips NH, Berger RJ. Daytime melatonin infusions induce sleep in pigeons without altering subsequent amounts of nocturnal sleep. Neurosci Lett. 1998;258:61–64. doi: 10.1016/S0304-3940(98)00849-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhdanova IV, Cantor ML, Leclair OU, Kartashov AI, Wurtman RJ. Behavioral effects of melatonin treatment in non-human primates. Sleep Res Online. 1998;1:114–118. [PubMed] [Google Scholar]
- 12.Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol Behav. 2002;75:523–529. doi: 10.1016/S0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 13.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature and performance. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi H, Kato KI, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982;55:27–29. doi: 10.1210/jcem-55-1-27. [DOI] [PubMed] [Google Scholar]
- 15.Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. Human melatonin production decreases with age. J Pineal Res. 1986;3:379–388. doi: 10.1111/j.1600-079X.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 16.Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, Herer P, Tzischinsky O, Lavie P. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhdanova I, Yu L, Lopez-Patino M, Shang E, Kishi S, Guelin E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res Bull. 2008;75:433–441. doi: 10.1016/j.brainresbull.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido Y, Aluru N, McGuire A, Park YJ, Vijayan MM, Takemura A. Effect of cortisol on melatonin production by the pineal organ of tilapia, Oreochromis mossambicus. Comp Biochem Physiol A Mol Integr Physiol. 2010;155:84–90. doi: 10.1016/j.cbpa.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Larson ET, Winberg S, Mayer I, Lepage O, Summers CH, Øverli Ø. Social stress affects circulating melatonin levels in rainbow trout. Gen Comp Endocrinol. 2004;136:322–327. doi: 10.1016/j.ygcen.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Roseboom PH, Coon SL, Baler R, McCune SK, Weller JL, Klein DC. Melatonin synthesis: Analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 21.Coon SL, Bégay V, Deurloo D, Falcón J, Klein DC. Two arylalkylamine N-acetyltransferase genes mediate melatonin synthesis in fish. J Biol Chem. 1999;274:9076–9082. doi: 10.1074/jbc.274.13.9076. [DOI] [PubMed] [Google Scholar]
- 22.Munck A, Náray-Fejes-Tóth A. Glucocorticoids and stress: Permissive and suppressive actions. Ann N Y Acad Sci. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- 23.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Tongjaroenbuangam W, Ruksee N, Mahanam T, Govitrapong P. Melatonin attenuates dexamethasone-induced spatial memory impairment and dexamethasone-induced reduction of synaptic protein expressions in the mouse brain. Neurochem Int. 2013;63:482–491. doi: 10.1016/j.neuint.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Benyassi A, Schwartz C, Ducouret B, Falcón J. Glucocorticoid receptors and serotonin N-acetyltransferase activity in the fish pineal organ. Neuroreport. 2001;12:889–892. doi: 10.1097/00001756-200104170-00004. [DOI] [PubMed] [Google Scholar]
- 26.Meneses-Santos D, Buonfiglio DDC, Peliciari-Garcia RA, Ramos-Lobo AM, Souza DDN, Carpinelli AR, Carvalho CRO, Sertie RAL, Andreotti S, Lima FB, et al. Chronic treatment with dexamethasone alters clock gene expression and melatonin synthesis in rat pineal gland at night. Nat Sci Sleep. 2018;10:203–215. doi: 10.2147/NSS.S158602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Berg R, Haenen G, Van den Berg H, Bast A. Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br J Nutr. 2001;86(Suppl 1):S121–S127. doi: 10.1079/BJN2001340. [DOI] [PubMed] [Google Scholar]
- 28.Baydas G, Ozer M, Yasar A, Koz S, Tuzcu M. Melatonin prevents oxidative stress and inhibits reactive gliosis induced by hyperhomocysteinemia in rats. Biochemistry (Mosc) 2006;71(Suppl 1):S91–S95. doi: 10.1134/S0006297906130153. [DOI] [PubMed] [Google Scholar]
- 29.Gilad E, Wong HR, Zingarelli B, Virág L, O'Connor M, Salzman AL, Szabó C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NFkappaB activation. FASEB J. 1998;12:685–693. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 30.Kumar N, Misra P, Dube A, Bhattacharya S, Dikshit M, Ranade S. Piper betle Linn. a maligned Pan-Asiatic plant with an array of pharmacological activities and prospects for drug discovery. Cur Sci. 2010;99:922–932. [Google Scholar]
- 31.Dwivedi V, Tripathi S. Review study on potential activity of Piper betle. J Pharmacogn Phytochem. 2014;3:93–98. [Google Scholar]
- 32.Alam B, Akter F, Parvin N, Pia RS, Akter S, Chowdhury J, Sifath-E-Jahan K, Haque E. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna J Phytomed. 2013;3:112–125. [PMC free article] [PubMed] [Google Scholar]
- 33.Swati B, Madhusudhanan N. Antidepressant activity of ethanolic extract of piper betle leaves in mice. Cur Res Neuroscience. 2012;2:11–16. doi: 10.3923/crn.2012.11.16. [DOI] [Google Scholar]
- 34.Hoo JY, Kumari Y, Shaikh MF, Hue SM, Goh BH. Zebrafish: a versatile animal model for fertility research. BioMed Res Int. 2016;2016:9732780. doi: 10.1155/2016/9732780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: Filling the gap between in vitro and rodent models for drug development. Rev Neurosci. 2011;22:75–84. doi: 10.1515/rns.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idda ML, Bertolucci C, Vallone D, Gothilf Y, Sánchez-Vázquez FJ, Foulkes NS. Chapter 3-Circadian clocks: Lessons from fish. Prog Brain Res. 2012;199:41–57. doi: 10.1016/B978-0-444-59427-3.00003-4. [DOI] [PubMed] [Google Scholar]
- 37.Kazimi N, Cahill GM. Development of a circadian melatonin rhythm in embryonic zebrafish. Brain Res Dev Brain Res. 1999;117:47–52. doi: 10.1016/S0165-3806(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 38.Zhdanova IV. Sleep and its regulation in zebrafish. Rev Neurosci. 2011;22:27–36. doi: 10.1515/rns.2011.005. [DOI] [PubMed] [Google Scholar]
- 39.Ganguly S, Mula S, Chattopadhyay S, Chatterjee M. An ethanol extract of Piper betle Linn. mediates its anti-inflammatory activity via down-regulation of nitric oxide. J Pharm Pharmacol. 2007;59:711–718. doi: 10.1211/jpp.59.5.0012. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Xu Z, Wu D, Li J, Song C, Lu W, Huang J. Luciferase reporter gene assay on human 5-HT receptor: Which response element should be chosen? Sci Rep. 2015;5:8060. doi: 10.1038/srep08060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham FL, Smiley J, Russell W, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 42.Kundap UP, Kumari Y, Othman I, Shaikh M. Zebrafish as a model for epilepsy-induced cognitive dysfunction: A pharmacological, biochemical and behavioral approach. Front Pharmacol. 2017;8:515. doi: 10.3389/fphar.2017.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuesta A, Cerezuela R, Esteban MA, Meseguer J. In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream. J Pineal Res. 2008;45:70–78. doi: 10.1111/j.1600-079X.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 44.Zlotos DP. Recent progress in the development of agonists and antagonists for melatonin receptors. Curr Med Chem. 2012;19:3532–3549. doi: 10.2174/092986712801323153. [DOI] [PubMed] [Google Scholar]
- 45.Pandi-Perumal SR, Srinivasan V, Spence DW, Cardinali DP. Role of the melatonin system in the control of sleep: Therapeutic implications. CNS Drugs. 2007;21:995–1018. doi: 10.2165/00023210-200721120-00004. [DOI] [PubMed] [Google Scholar]
- 46.Zawilska JB, Sadowska M. Prolonged treatment with glucocorticoid dexamethasone suppresses melatonin production by the chick pineal gland and retina. Pol J Pharmacol. 2002;54:61–66. [PubMed] [Google Scholar]
- 47.López-Patiño MA, Gesto M, Conde-Sieira M, Soengas JL, Míguez JM. Stress inhibition of melatonin synthesis in the pineal organ of rainbow trout (Oncorhynchus mykiss) is mediated by cortisol. J Exp Biol. 2014;217:1407–1416. doi: 10.1242/jeb.087916. [DOI] [PubMed] [Google Scholar]
- 48.Villafuerte G, Miguel-Puga A, Rodríguez EM, Machado S, Manjarrez E, Arias-Carrión O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid Med Cell Longev. 2015;2015:234952. doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 50.Pagel J, Parnes BL. Medications for the treatment of sleep disorders: An overview. Prim Care Companion J Clin Psychiatry. 2001;3:118–125. doi: 10.4088/PCC.v03n0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Appelbaum L, Vallone D, Anzulovich A, Ziv L, Tom M, Foulkes N, Gothilf Y. Zebrafish arylalkylamine-N-acetyltransferase genes-targets for regulation of the circadian clock. J Mol Endocrinol. 2006;36:337–347. doi: 10.1677/jme.1.01893. [DOI] [PubMed] [Google Scholar]
- 52.Klein DC. Arylalkylamine N-acetyltransferase: ‘The Timezyme’. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 53.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A. A review of melatonin, its receptors and drugs. Eurasian J Med. 2016;48:135–141. doi: 10.5152/eurasianjmed.2015.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.