Abstract
Rationale, aims and objectives
Biologics are substantially more expensive than their conventional counterparts but it is unclear whether extra costs deliver better health outcomes. We compare clinical and economic outcomes between teriparatide (monthly cost ~1120) and bisphosphonates (monthly costs ~14) among postmenopausal women with osteoporosis.
Methods
From a 5% random sample of Medicare beneficiaries, we selected women newly diagnosed with osteoporosis between January 1, 2007 and December 31, 2011 and who initiated teriparatide or bisphosphonates after the diagnosis. We followed them until they switched osteoporosis treatment, died, or December 31, 2011. Clinical outcomes included hip fracture, vertebral fracture, fracture of radius, ulna or carpal bones, other upper limb fractures, other lower limb fractures and any fracture. Economic outcomes included medical costs, pharmacy costs, and total costs associated with osteoporosis. Using conventional propensity score, high-dimensional propensity score and instrumental variable analysis, we constructed Cox Proportional Hazards models to evaluate the risk of fracture and two-part models to compare costs.
Results
Teriparatide users had higher risk of fracture and higher costs, compared to similar bisphosphonates users. The hazard ratios of fracture for teriparatide relative to bisphosphonates ranged from 1.37 to 2.12, depending on methods. There was no difference in the risk of hip fracture between treatment groups. Total annual costs related to osteoporosis were between ~2733 and ~3352 higher for teriparatide users.
Conclusions
The biological agent, teriparatide, is more expensive yet less effective than conventional treatment, bisphosphonates.
Keywords: Biopharmaceuticals, Comparative Effectiveness, Osteoporosis, Teriparatide, Bisphosphonates, Bone Fractures
INTRODUCTION
Biologic therapies have become promising alternative treatments for many conditions including cancer, inflammatory diseases, and osteoporosis. Unlike conventional pharmaceuticals, which are chemically synthesized small molecules, biologics are manufactured in living systems and are large, complex molecules. Biologics are very expensive, often costing ~50,000 to ~100,000 per year.(1) Despite their high costs, the use of biologics has increased at a rate twice as fast as conventional drugs since 2000. (2, 3)
However, the effectiveness of some biologics compared to conventional treatments remains unclear in some cases. One example is osteoporosis. Two biologics are available to treat osteoporosis: teriparatide and denosumab; they were approved by the Food and Drug Administration in November 2002 and June 2010, respectively. Common agents in conventional treatment for postmenopausal osteoporosis are bisphosphonates. (4) The Medicare gross cost of a monthly treatment with teriparatide is ~1120, in comparison to ~14 for a generic formulation of alendronate, the most commonly prescribed bisphosphonate.
Two randomized trials compared clinical outcomes of alendronate and teriparatide in treating postmenopausal osteoporosis, and found that the incidence of non-vertebral fractures(5) and back pain(6) was significantly lower among teriparatide users. However, a cost-effectiveness analysis based on clinical trials data concluded that teriparatide was more expensive and less effective than alendronate.(7) Evidence gained from clinical trials data may not be generalizable to patients in the real-world practice. For example, previous studies suggest that only between 3 and 21% of osteoporosis patients meet the eligibility criteria of clinical trials to compare osteoporosis drugs.(8) Thus, it is important to assess the effectiveness of osteoporosis treatments using large existing data that reflect the real-world clinical practice. This, unfortunately, does not exist in the current literature for the case of teriparatide and bisphosphonates.
In this paper, we used 2006–2011 Medicare pharmacy and medical claims data for a 5% random sample of Medicare beneficiaries to compare incidence of fracture and costs between teriparatide and bisphosphonates among postmenopausal women newly diagnosed with osteoporosis. [We did not study denosumab because it was approved in June 2010 and in our data there were only 8 patients satisfying our selection criteria].
METHODS
Data source and study population
We obtained 2006–2011 pharmacy and medical data for a 5% random sample of Medicare beneficiaries from the Centers for Medicare and Medicaid Services (CMS). First, we selected women newly diagnosed with osteoporosis between January 1, 2007 and December 31, 2011 by using CMS Chronic Condition Warehouse indicator that traced the first diagnosis date back to January 1, 1999. The diagnosis of osteoporosis was defined as having one inpatient or two outpatient claims with International Classification of Diseases, Ninth Revision (ICD-9) codes 733.00, 733.01, 733.02, 733.03 or 733.09.(9) Second, we identified those individuals whose first treatment for osteoporosis was either a bisphosphonate (including alendronate, risedronate, ibandronate, and zoledronic acid) or teriparatide (N=42,536). Because we have 2006 Part D data for each individual, we had at least one year prior data to ensure they did not fill any prescription for an osteoporosis drug before (osteoporosis drugs are listed in Supplemental Methods in the Supplemental Material). Third, to constrain to postmenopausal women, we further restricted study participants to be 50 years or older at the time of initiating bisphosphonates or teriparatide. We followed each individual from the index date, defined as 180 days after drug initiation, until they switched their osteoporosis treatment, died, or end of the study period (December 31, 2011). We set the index date as 180 days after drug initiation for two reasons: (1) to allow treatments to achieve therapeutic effects;(10, 11) and (2) to make sure our clinical outcomes did not capture follow-up visits of fractures that happened before drug initiation.(11, 12) We did not consider discontinuation of treatment as a censoring event because of the residual effects of the treatments after discontinuation.(13, 14) Our final sample included 39,493 bisphosphonates users and 550 teriparatide users.
Outcomes
We compared clinical and economic outcomes between treatment groups. Clinical outcomes included hip fracture, vertebral fracture, fracture of radius, ulna or carpal bones, other upper limb fractures, other lower limb fractures and any fracture. We collected medical claims for these types of fracture since the index date (ICD-9 codes listed in Supplemental Table 1 in the Supplemental Material). We defined time to any fracture as days between the index date (180 days after drug initiation date) and the date of the first fracture. We also analyzed time to hip fracture, time to vertebral fracture, time to fracture of radius, ulna or carpal bones, time to other upper limb fractures and time to other lower limb fractures.
Economic outcomes included medical, pharmaceutical, and total costs associated with osteoporosis. Monthly medical costs were calculated as the monthly average of the total payment amount for all medical claims with primary or secondary ICD-9 codes for osteoporosis or fracture (ICD-9 codes listed in Supplemental Table 1 in the Supplemental Material) during follow-up period. Monthly pharmaceutical costs were calculated as the monthly average of the gross cost of prescriptions for either bisphosphonates or teriparatide during follow-up period. Total monthly costs were calculated as the sum of monthly medical and pharmaceutical costs.
Covariates
We adjusted for demographic variables and clinical characteristics. Demographic variables included age, race and Medicaid eligibility. Clinical characteristics included history of hip fracture, history of vertebral fracture, history of fracture of radius, ulna or carpal bones, history of other upper limb fractures, history other lower limb fractures, number of other CMS priority comorbidities (list in Table 1), and time between diagnosis and drug initiation. To identify history of fractures, we collected all medical claims for each type of fracture (ICD-9 codes listed in Supplemental Table 1 in the Supplemental Appendix) between January 1, 2006 (first data available) and date of drug initiation. History of fracture was defined as having at least one claim for the respective type of fracture before drug initiation.
Table 1.
Baseline Patient Characteristics Before Propensity Score Adjustments, by Treatment Group.
| Variable | Bisphosphonates (N=39493) |
Teriparatide (N=550) |
P-value |
|---|---|---|---|
| Age (%) | 0.089 | ||
| <65 | 8.78 | 11.45 | |
| 65–74 | 47.54 | 46.00 | |
| ≥75 | 43.68 | 42.55 | |
| Race (%) | <0.001 | ||
| White | 77.30 | 78.00 | |
| Black | 6.17 | 2.91 | |
| Asian | 5.60 | 2.73 | |
| Hispanic | 9.44 | 14.73 | |
| Native American | 0.48 | 0.91 | |
| Other | 1.03 | 0.70 | |
| Medicaid eligibility (%) | 36.16 | 45.82 | <0.001 |
| History of hip fracture (%) | 4.66 | 11.80 | <0.001 |
| History of vertebral fracture (%) | 5.50 | 19.10 | <0.001 |
| History of fracture of radius, ulna or carpal bones (%) | 3.20 | 5.27 | 0.006 |
| History of other upper limb fractures (%) | 3.24 | 5.27 | 0.008 |
| History of other lower limb fractures (%) | 6.46 | 13.27 | <0.001 |
| History of CMS priority cancera (%) | 10.61 | 7.09 | 0.008 |
| No. of CMS priority comorbiditiesb (%) | <0.001 | ||
| 0–2 | 46.11 | 35.45 | |
| 3–5 | 36.96 | 39.27 | |
| ≥6 | 16.93 | 25.27 | |
| Time between diagnosis and drug initiation | <0.001 | ||
| ≤2 months | 51.44 | 34.36 | |
NOTES:
Abbreviations: CMS= Centers for Medicare and Medicaid Services.
CMS priority types of cancer include breast cancer, colorectal cancer, endometrial cancer and lung cancer.
The number of other CMS priority comorbidities was calculated as the sum of previous history of acute myocardial infarction, atrial fibrillation, Alzheimer’s disease, related disorders or senile dementia, cataract, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, depression, diabetes, glaucoma, ischemic heart disease, history of stroke or transient ischemic attack, and rheumatoid arthritis or osteoarthritis.
Statistical analysis
We compared patient characteristics between bisphosphonates and teriparatide users using chi-square test or Fisher’s exact test. To evaluate balance of covariates between treatment groups, we calculated the standardized difference in covariate means.(15) Standardized differences whose absolute value is lower than 10% indicate balance between groups.(16)
To compare the hazard rates of fracture between groups, we constructed Cox Proportional Hazards models. Switch of osteoporosis treatment, death and end of the study were considered censoring events. These models were implemented with statistical software SAS 9.4 (Cary, NC).
To compare pharmacy, medical and total costs between groups, we constructed two-part models, because many individuals had zero costs. The first part was a logistic regression and the second part was a generalized linear model (GLM) with a gamma distribution and a log link function. Standard errors of the marginal effect of treatment were obtained by bootstrapping. These two-part models were constructed with a modification of the program by Deb et al. from the American Society of Health Economists Conference at Cornell,(17) using the statistical software Stata 13 (College Station, TX).
Methods for mitigating selection biases
The key issue when comparing two treatments using observational data is that individuals on one treatment group may not be comparable with individuals on the other group, which leads to selection bias.(18) We used propensity scores and instrumental variable analysis to mitigate this problem.
Propensity score analysis
We used two methods to calculate the probability of initiating teriparatide (propensity score), relative to bisphosphonates: conventional and high-dimensional propensity score. To incorporate propensity scores in our analysis, we performed matching and covariate adjustment.
To calculate the conventional propensity score, we conducted a logistic regression that predicted the probability of initiating teriparatide, controlling for all the covariates listed above. The logistic regression constructed to calculate the high-dimensional propensity score included two types of covariates: predefined covariates (same as in the conventional propensity score) and empirical covariates selected from the claims. The selection of the empirical covariates is explained in Supplemental Methods in the Supplemental Material.
We performed two methods using the scores: first, we simply included the calculated propensity score as an additional covariate in the models discussed above (Cox Proportional Hazard models for time to fracture and two-part models for costs); second, we used the nearest neighbor 1 to 5 matching approach with replacement to match treatment groups. Cox Proportional Hazard models and two-part models were constructed for matched samples.
Instrumental variable approach
We used the average copayment at the county level for one month supply of in the initial phase of Medicare Part D benefits as an instrument in our analysis. We assessed the validity of the instrument (Supplemental Methods in the Supplemental Material) and implemented it in our analysis following the two-stage residual inclusion method. In the first stage, we performed a logistic regression to predict the probability of initiating bisphosphonates or teriparatide, controlling for predefined covariates and the instrument. Then we calculated residuals as the difference between observed and predicted values of treatment. In the second stage, we constructed Cox Proportional Hazards models and two-part models including the residuals of the first-stage regression as a covariate.
RESULTS
Patient characteristics
The mean follow-up period was 27.9 months (Interquartile Range [IQR] = 17.2 to 40.2 months) for bisphosphonates group and 21.3 months (IQR = 10.3 to 30.2 months) for teriparatide group. Among bisphosphonates users, 66.4% initiated alendronate, 19.3 % risedronate, 14.2% ibandronate, and 0.04 % zoledronic acid.
There were significant differences in patient characteristics between treatment groups (Table 1). Bisphosphonates users were more likely to be African Americans (6.2% vs. 2.9%) and Asians (5.6% vs. 2.7%) than teriparatide users. The proportion of Medicare beneficiaries also eligible for Medicaid coverage was higher among teriparatide group (p-value<0.001). In addition, teriparatide users had a higher prevalence of history of fracture and a higher number of concomitant comorbidities.
After conventional propensity score matching, treatment groups were similar for all covariates analyzed (Table 2 and Supplemental Table 2 in the Supplemental Material). However, treatment groups matched on the high-dimensional propensity score were not balanced on the prevalence of history of vertebral fracture (p-value=0.021, standardized difference= −11.5%).
Table 2.
Comparison of Baseline Patient Characteristics of the Matched Cohorts.
| After Conventional PS Matching | After HDPS Matching | |||||
|---|---|---|---|---|---|---|
| Variable | Bisphosphonates (N=2747) |
Teriparatide (N=550) |
P- value |
Bisphosphonates (N=1695) |
Teriparatide (N=467) |
P-value |
| Age (%) | 0.903 | 0.813 | ||||
| <65 | 11.14 | 11.45 | 9.52 | 10.49 | ||
| 65–74 | 45.29 | 46.00 | 47.12 | 46.47 | ||
| ≥75 | 43.57 | 42.55 | 43.36 | 43.04 | ||
| Race (%) | 0.290 | 0.992 | ||||
| White | 78.41 | 78.00 | 77.81 | 77.52 | ||
| Black | 2.95 | 2.91 | 3.41 | 3.21 | ||
| Asian | 2.58 | 2.73 | 3.21 | 3.00 | ||
| Hispanic | 14.56 | 14.73 | 13.84 | 14.78 | ||
| Native American | 1.27 | 0.91 | 0.97 | 0.86 | ||
| Other | 0.22 | 0.72 | 1.38 | 1.07 | ||
| Medicaid eligibility (%) | 47.47 | 45.82 | 0.479 | 42.80 | 45.40 | 0.309 |
| History of hip fracture (%) | 11.36 | 11.82 | 0.757 | 9.62 | 10.71 | 0.478 |
| History of vertebral fracture (%) | 17.29 | 19.09 | 0.312 | 13.79 | 17.99 | 0.021 |
| History of fracture of radius, ulna or | 5.27 | |||||
| carpal bones (%) | 4.84 | 0.669 | 4.78 | 5.14 | 0.748 | |
| History of other upper limb fractures | 5.82 | 5.27 | ||||
| (%) | 0.611 | 4.48 | 5.14 | 0.540 | ||
| History of other lower limb fractures | 13.27 | |||||
| (%) | 13.47 | 0.902 | 13.74 | 14.13 | 0.825 | |
| History of CMS priority cancer (%) | 6.48 | 7.09 | 0.598 | 7.18 | 7.49 | 0.811 |
| No. of CMS priority comorbidities (%) | 0.691 | 0.409 | ||||
| 0–2 | 35.13 | 35.45 | 38.83 | 35.76 | ||
| 3–5 | 37.90 | 39.27 | 38.12 | 38.97 | ||
| ≥6 | 26.97 | 25.27 | 23.05 | 25.27 | ||
| Time between diagnosis and drug | ||||||
| initiation | 0.552 | 0.293 | ||||
| ≤2 months | 33.05 | 34.36 | 38.17 | 35.55 | ||
NOTES:
Abbreviations: PS= Propensity Score, HDPS=High-Dimensional Propensity Score, CMS= Centers for Medicare and Medicaid Services.
CMS priority types of cancer include breast cancer, colorectal cancer, endometrial cancer and lung cancer.
The number of other CMS priority comorbidities was calculated as the sum of previous history of acute myocardial infarction, atrial fibrillation, Alzheimer’s disease, related disorders or senile dementia, cataract, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, depression, diabetes, glaucoma, ischemic heart disease, history of stroke or transient ischemic attack, and rheumatoid arthritis or osteoarthritis.
Incidence of fracture
Table 3 shows the unadjusted incidence of fracture by treatment group. Among teriparatide users, 23.1% had a fracture during the study period, in comparison to 14.5% of bisphosphonates users (p-value<0.001). The unadjusted incidence of hip fracture was not different between treatment groups; however, the incidence of vertebral fracture was higher among teriparatide users than bisphosphonates users: 7.8% vs. 3.6% (p-value<0.001).
Table 3.
Unadjusted Incidence of Fracture and Monthly Costs, by Treatment Group.
| Bisphosphonates | Teriparatide | P-Value | |
|---|---|---|---|
| Clinical outcomes - no. patients (%) | |||
| Any fracture | 5727 (14.5) | 127 (23.09) | <0.001 |
| Hip fracture | 1596 (4.04) | 31 (5.64) | 0.059 |
| Vertebral fracture | 1421 (3.60) | 43 (7.82) | <0.001 |
| Fracture of radius, ulna or carpal bones | 963 (2.44) | 24 (4.36) | 0.004 |
| Other upper limb fractures | 1132 (2.87) | 21 (3.82) | 0.185 |
| Other lower limb fractures | 2074 (5.25) | 35 (6.36) | 0.246 |
| Monthly osteoporosis-related costs - (~) | |||
| Medical costs | 12. 21 | 30. 25 | <0.001 |
| Pharmacy costs | 19.24 | 318.00 | <0.001 |
| Total costs | 31.45 | 348.25 | <0.001 |
NOTES:
The unadjusted incidence of fracture was obtained as the percentage of study participants in each group experiencing the clinical outcome during follow-up period. Monthly medical costs were calculated as the monthly average of the total payment amount for all medical claims with primary or secondary ICD-9 codes for osteoporosis or fracture during follow-up period. Monthly pharmaceutical costs were calculated as the monthly average of the gross cost of prescriptions for either bisphosphonates or teriparatide during follow-up period. Total monthly costs were calculated as the sum of monthly medical and pharmaceutical costs. The estimates for the average medical costs are only ~25.14 and ~42.66 because 42% of our study participants had zero monthly medical costs during all follow-up period. These estimates are consistent with previous studies.(19)
Figure 1 shows the hazard ratios for fractures for teriparatide relative to bisphosphonates, estimated by six methods. All methods associated teriparatide with higher risk of any fracture. The point estimates for the hazard ratio of any fracture for teriparatide relative to bisphosphonates ranged from 1.37 for high-dimensional propensity score matching to 2.12 for instrumental variable analysis. None of the methods found differences in the risk of hip fracture, other upper limb fracture and other lower limb fracture between treatment groups. However, all methods except high-dimensional propensity score matching found a higher risk of vertebral fracture among teriparatide users. In addition, teriparatide was associated with a higher risk of fracture of radius, ulna or carpal bones by all methods except for approaches based on high-dimensional propensity score.
Figure 1.
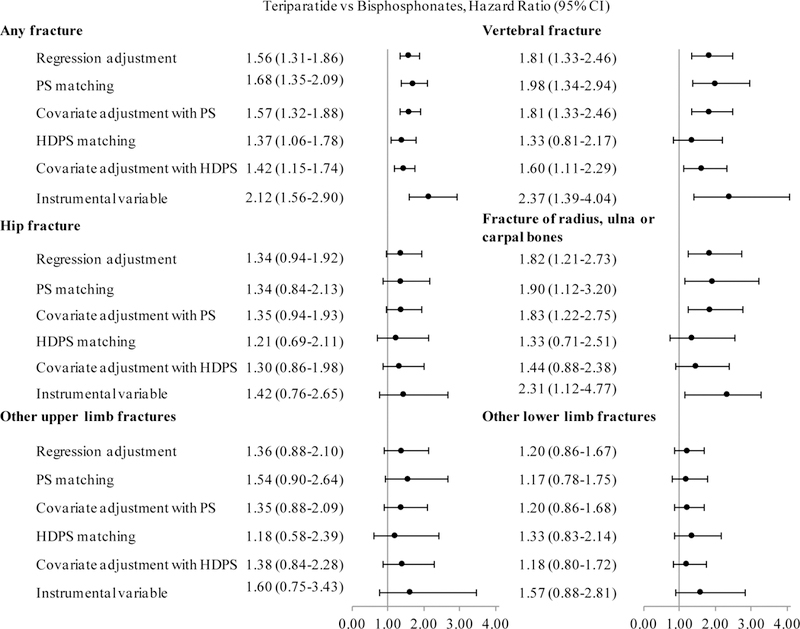
Hazard Ratios for Teriparatide Relative to Bisphosphonates, by Type of Fracture and Method.
NOTES:
Abbreviations: PS= Propensity Score, HDPS=High-Dimensional Propensity Score.
Hazard ratios estimated by Cox Proportional Hazards models. All models controlled for age, race, Medicaid eligibility, history of hip fracture, history of vertebral fracture, history of fracture of radius, ulna or carpal bones, history of other upper limb fracture, history of other lower limb fracture, history of CMS priority cancer, number of other CMS priority conditions, and time between osteoporosis diagnosis and treatment initiation. The models constructed using covariate adjustment also included the respective propensity score as a covariate. The models constructed with instrumental variable analysis also included the residuals of the first-stage logistic regression that predicted the probability of initiating teriparatide vs. bisphosphonates on the basis of the instrument and predefined covariates.
Difference in costs
Before adjusting for patient characteristics, teriparatide was associated with higher medical costs, pharmaceutical and total costs than bisphosphonates (Table 3). Table 4 shows the adjusted difference in monthly osteoporosis medical costs, pharmaceutical and total costs between treatment groups, by method used. All methods found a highly significant association between teriparatide use and higher costs. Covariate adjustment using conventional propensity score was the method that found the smallest differences in total costs between treatment groups; this method found that the total monthly costs associated with osteoporosis were ~228 higher for teriparatide users than bisphosphonates users (p-value<0.001).
Table 4.
Difference in Costs between Teriparatide and Bisphosphonates, by Types of Costs and Method.
| Difference Teriparatide - Bisphosphonates | ||||||
|---|---|---|---|---|---|---|
| Method | Medical costs |
P- value |
Pharmaceutical costs |
P- value |
Total costs |
P- value |
| Regression adjustment | 8.85 | <0.001 | 249.70 | <0.001 | 229.75 | <0.001 |
| Conventional PS matching | 9.20 | <0.001 | 262.26 | <0.001 | 249.21 | <0.001 |
| Covariate adjustment using conventional PS | 8.82 | <0.001 | 246.02 | <0.001 | 227.72 | <0.001 |
| HD-PS matching | 9.90 | <0.001 | 288.27 | <0.001 | 279.36 | <0.001 |
| Covariate adjustment using HD-PS | 8.22 | <0.001 | 246.00 | <0.001 | 228.54 | <0.001 |
| Instrumental variable | 13.07 | 0.01 | 269.47 | <0.001 | 237.74 | <0.001 |
NOTES:
Abbreviations: PS= Propensity Score, HDPS=High-Dimensional Propensity Score.
Monthly medical costs were calculated as the monthly average of the total payment amount for all medical claims with primary or secondary ICD-9 codes for osteoporosis or fracture during follow-up period. Monthly pharmaceutical costs were calculated as the monthly average of the gross cost of prescriptions for either bisphosphonates or teriparatide during follow-up period. Total monthly costs were calculated as the sum of monthly medical and pharmaceutical costs. Marginal effects of treatment were calculated using two-part models that controlled for age, race, Medicaid eligibility, history of hip fracture, history of vertebral fracture, history of fracture of radius, ulna or carpal bones, history of other upper limb fracture, history of other lower limb fracture, history of CMS priority cancer, number of other CMS priority conditions, and time between osteoporosis and treatment initiation. The models constructed using covariate adjustment also included the respective propensity score as a covariate. The models constructed with instrumental variable analysis also included the residuals of the first-stage logistic regression that predicted the probability of initiating teriparatide vs. bisphosphonates on the basis of the instrument and predefined covariates.
DISCUSSION
Using several approaches to mitigate selection biases, we consistently found that teriparatide was associated with higher risk of fracture and higher costs than bisphosphonates. The hazard ratios for the risk of any fracture for teriparatide relative to bisphosphonates ranged from 1.37 to 2.12, depending on which method was used. The annual medical and drug costs related to osteoporosis were between ~2733 and ~3352 higher for teriparatide users, relative to bisphosphonates users.
Our results are consistent with the previous cost-effectiveness analysis that found that teriparatide was more costly and less effective than alendronate.(7) A randomized clinical trial compared the risk of fracture between teriparatide and alendronate in 146 postmenopausal women with osteoporosis and found that patients on teriparatide had lower rates of any fracture.(5) However, we found that after adjustment for patient characteristics, new patients on teriparatide had higher risk of any fracture, compared to similar patients on bisphosphonates. The differences in patient characteristics between the clinical trial and our study explain this discrepancy: first, patients with other severe or chronically disabling conditions than osteoporosis or with history of cancer were excluded from the trial. In contrast, 89% of our study participants had at least one CMS priority condition other than osteoporosis and 10% had history of cancer. Second, patients in our study were considerably older than in the clinical trial (mean age: 74.2 vs. 65.5 years). Third, only 2% of the subjects included in the clinical trial belonged to other racial groups than White or Hispanic, in comparison to 13% of our sample. Finally, our estimate for the average medical costs associated with osteoporosis is similar to results from a previous study that estimated the monthly medical costs of osteoporosis in €32.5, around ~45.(19)
Our study has three main limitations. First, we had a relative small group of teriparatide users, because we focused on newly diagnosed patients among whom the initiation of teriparatide was a rare event. However, we have a big enough sample size to estimate precise results and our results are robust for the six models we used. Second, like all studies using claims data, we did not have detailed clinical information, such as the results of laboratory tests. In particular, we did not observe levels of bone mineral density, which affect both treatment initiation and incidence of fractures. Unbalanced distribution of bone mineral density and of other unobserved confounding factors between treatment groups could have led to biased estimates. Therefore, more studies are necessary to confirm our results on the effectiveness of teriparatide as first-line treatment of osteoporosis, as compared to bisphosphonates. In particular, it would be informative to conduct similar comparative effectiveness studies using registry data or prospective observational data, which may contain more information on potential underlying confounders than claims data. Third, our study only included postmenopausal women newly diagnosed with osteoporosis and therefore, the generalizability of our results to treatment-experienced patients is certainly limited.
Nevertheless, our study has several important contributions. Currently, teriparatide is only recommended as first-line treatment of osteoporosis in women with severe osteoporosis who have a contraindication or are intolerant to bisphosphonates.(20, 21) We found that use of teriparatide among postmenopausal women newly diagnosed with osteoporosis is more expensive and less effective than bisphosphonates. Therefore, our results support the current recommendations not to use teriparatide as first-line treatment of post-menopausal osteoporosis except for those special cases. However, in our data, we observed that teriparatide was used as first-line therapy for some post-menopausal women. It is unlikely that our teriparatide group included patients with a contraindication or intolerant to bisphosphonates for two reasons: first, because of our sample selection, patients on teriparatide group did not use bisphosphonates before initiating teriparatide and therefore, it is unlikely that they were intolerant to bisphosphonates. Second, most contraindications for bisphosphonates are only for oral forms, and we also studied the use of intravenous formulations. Nevertheless, some teriparatide users may have been representative of patients with glucocorticoid-induced osteoporosis. In other cases, the first-line use of teriparatide may have been incentivized by the generous coverage for this drug under Medicare Part D. In our study sample, the median (mean) out-of-pocket cost was ~6 (~116) for a 30-day supply of teriparatide, which normally costs ~1120 per month. About 46% of teriparatide users in our sample also had Medicaid coverage so they paid little copayment.
In conclusion, we found that teriparatide was more costly yet less effective than bisphosphonates in the treatment of postmenopausal osteoporosis. Our study shows the importance of conducting comparative effectiveness studies between new biological agents and conventional treatments with real-world clinical data. This analysis could be extended to other therapeutic alternatives in the treatment of osteoporosis and in particular denosumab, a biopharmaceutical approved for the treatment of osteoporosis in 2010.
Supplementary Material
Acknowledgements
We acknowledge funding from Commonwealth Foundation, Agency for Healthcare Research and Quality (No. R01 HS018657), and Institute of Medicine (IOM-2000000523). Sponsors played no role in the study conduct, data analysis or report generation. In addition, the publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript. We would like to thank Dr. Seo Hyon Baik for his advice with the statistical analysis and Dr. Antonio Pinera Guirao for his clinical insights.
Footnotes
Conflicts of interest and financial disclosure: None.
References
- 1.Lee TH, Emanuel EJ. Tier 4 drugs and the fraying of the social compact. New England Journal of Medicine. 2008;359(4):333. [DOI] [PubMed] [Google Scholar]
- 2.Aitken M, Berndt ER, Cutler DM. Prescription drug spending trends in the United States: looking beyond the turning point. Health Affairs. 2009;28(1):w151–w60. [DOI] [PubMed] [Google Scholar]
- 3.Grabowski H, Cockburn I, Long G. The market for follow-on biologics: how will it evolve? Health Affairs. 2006;25(5):1291–301. [DOI] [PubMed] [Google Scholar]
- 4.Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clinic Proceedings. 2008;83(9):1032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. Journal of Clinical Endocrinology and Metabolism. 2002;87(10):4528–35. [DOI] [PubMed] [Google Scholar]
- 6.Miller PD, Shergy WJ, Body JJ, Chen P, Rohe ME, Krege JH. Longterm reduction of back pain risk in women with osteoporosis treated with teriparatide compared with alendronate. Journal of Rheumatology. 2005;32(8):1556–62. [PubMed] [Google Scholar]
- 7.Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Archives of Internal Medicine. 2006;166(11):1209–17. [DOI] [PubMed] [Google Scholar]
- 8.Dowd R, Recker RR, Heaney RP. Study subjects and ordinary patients. Osteoporosis International. 2000;11(6):533–6. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New Oral Anticoagulants and the Risk of Intracranial Hemorrhage: Traditional and Bayesian Meta-analysis and Mixed Treatment Comparison of Randomized Trials of New Oral Anticoagulants in Atrial Fibrillation. JAMA Neurology. 2013;28(10). [DOI] [PubMed] [Google Scholar]
- 10.Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. Journal of Managed Care Pharmacy. 2011;17(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riihimaki J, Sund R, Vehtari A. Analysing the length of care episode after hip fracture: a nonparametric and a parametric Bayesian approach. Health Care Management Science. 2010. June;13(2):170–81. [DOI] [PubMed] [Google Scholar]
- 12.Ireland A, Kelly P. Total length of stay, costs and outcomes at final discharge for admitted patients with hip fracture: linked episode data for Australian veterans and war widows. Internal Medicine. 2013;43(12):1280–6. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein JS, Arnold AL. Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. Journal of Clinical Endocrinology and Metabolism. 1999;84(4):1214–9. [DOI] [PubMed] [Google Scholar]
- 14.Peris P, Torra M, Olivares V, Reyes R, Monegal A, Martínez-Ferrer A, Guañabens N. Prolonged bisphosphonate release after treatment in women with osteoporosis. Relationship with bone turnover. Bone. 2011;49(4):706–9. [DOI] [PubMed] [Google Scholar]
- 15.d’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17(19):2265–81. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J Statistical power analysis for the behavioral sciences: Psychology Press; 1988. [Google Scholar]
- 17.Deb P, Manning W, Norton E. Minicourse: Modelling health care costs and counts. American Society of Health Economists Conference; Cornell; 2010. [Google Scholar]
- 18.Hernán MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]
- 19.Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. The direct and indirect costs of the chronic management of osteoporosis: a prospective follow-up of 3440 active subjects. Osteoporosis International. 2006;17(9):1346–52. [DOI] [PubMed] [Google Scholar]
- 20.Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(1):25–54. [DOI] [PubMed] [Google Scholar]
- 21.NICE technology appraisal guidance. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. 2008. [cited 2015 March 20]; Available from: https://www.nice.org.uk/guidance/ta161/chapter/1-guidance.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


