Abstract
Background:
Sarcopenia is the most common complication of cirrhosis and adversely affects quality of life and outcomes before, during, and after liver transplantation. We studied predictors of sarcopenia and sarcopenic obesity in patients with cirrhosis undergoing liver transplant (LT) evaluation.
Methods:
A retrospective analysis of 207 adult cirrhotic patients that underwent LT from January 2008 to December 2013 was performed at our institution.
Results:
Two hundred seven patients were evaluated, 68% were male with a mean age of 54 ± 8 years. The most common etiology of cirrhosis was alcoholic liver disease (38.6%), followed by chronic hepatitis C (38.2%), nonalcoholic steatohepatitis (NASH) (21.7%), and hepatocellular carcinoma (HCC) (24.6%). The mean body mass index of the cohort was of 30.1 ± 5.7 kg/m2. Forty-eight percent of these patients were obese. Of the 207 patients, 88% had computed tomographic (CT) scans within 90 days before transplant; of these, 59% had sarcopenia found during LT evaluation. Of the patients with pretransplant sarcopenia, 59 had CT scan at 6 months posttransplant and 56 (95%) remained sarcopenic. Of the 56 patients who had sarcopenia at 6 months, 31 had available CT scans at 1 year, and 100% persisted with sarcopenia. These 31 subjects had a mean skeletal muscle index of 35 at 6 months and 36 at 1 year. SO was found in 41.7% of our patients.
On multivariable regression analysis, obesity and age were found to be independently associated with pretransplant sarcopenia after controlling for gender and alcohol liver disease diagnosis (P = 0.00001, odds ratio [OR] 0.22, and P = 0.008, OR 2.0, respectively). A multivariable logistic regression analysis found that NASH as cause of cirrhosis and model of end-stage liver disease score are independent predictors of sarcopenic obesity after controlling for age, gender, alcoholic liver disease diagnosis, and HCC (P = 0.014 and 0.038, respectively; 95% confidence interval, 1.44–25.26 and 1.00–1.15, respectively; OR 6.03, 1.08, respectively).
Conclusions:
Sarcopenia and sarcopenic obesity is seen in a significant number of patients with cirrhosis undergoing LT evaluation. Sarcopenia progresses after LT initially and does not recover at least within the first year after surgery. Obesity is an independent predictor of pretransplant sarcopenia and NASH was associated with 6-fold increased risk of having sarcopenic obesity in cirrhotic patients in our cohort.
Keywords: cirrhosis, liver transplantation, sarcopenia, obesity
Introduction
In normal aging, changes in body composition result in a shift toward decreased muscle mass and increased fat mass. The loss of muscle tissue that occurs with aging is called sarcopenia. Although this term is applied clinically to denote muscle wasting, it is often used to describe a set of cellular processes in the muscle such as denervation, mitochondrial dysfunction, and inflammation. Sarcopenia has been also used as an alternative term for decreased mobility and function, increased fatigue, and reduced energy needs.1 Sarcopenia can also be present as a result of chronic diseases and malignancy. Age-related changes in the body composition as well as the increased prevalence of obesity determine a combination of excess weight and reduced muscle mass or strength, recently defined as sarcopenic obesity (SO).1 Common inflammatory pathways have linked sarcopenia and obesity yet the interplay between these two entities is poorly understood.2 Roubenoff hypothesized that both sarcopenia and obesity are similar behaviorally and biologically.3 Sarcopenia and obesity are often thought as a prelude to frailty, known to adversely predict hospitalizations, morbidity, and mortality.4 Studies have shown that sarcopenia and visceral obesity in the elderly may synergistically increase their effect on physical disability, metabolic disorders, and cardiovascular diseases.5 It has been observed that the association of functional status impairment is stronger for SO than for either obesity or sarcopenia alone.1
Sarcopenia is the most common complication of cirrhosis and adversely affects response to stress, quality of life, and outcomes before, during, and after liver transplantation.6–11 While the traditional clinical profile of a patient with progressive worsening of liver disease is loss of muscle and adipose tissue mass, the clinical profile of patients with cirrhosis is changing with the rapid increase in the number patients with nonalcoholic fatty liver disease that occurs in the setting of obesity.6 Overweight and obesity are now endemic in Western countries. Nonalcoholic steatohepatitis (NASH) is one of the most common forms of chronic liver disease in developed countries. NASH and sarcopenia seem to have similar pathophysiological backgrounds, such as chronic inflammation, vitamin D deficiency, oxidative stress, and decreased physical activity.12–16 Patients with cirrhosis may develop simultaneous loss of skeletal muscle and gain of adipose tissue, culminating in this condition of SO. The pathogenesis of SO and myosteatosis and its relationship with NASH in cirrhotic patients is not completely elucidated, but it seems to be the result of a complex interaction involving dysregulation of fatty acid oxidation and ketogenesis, gluconeogenesis from amino acids, glycogenolysis, and selective utilization of aromatic amino acids in the liver and branched chain amino acids in the skeletal muscle. The presence of sarcopenia, SO, and myosteatosis has been associated with worse survival in patients with cirrhosis compared with patients with cirrhosis and no muscular abnormalities.17 Numerous indirect methods have been used to quantify body composition in patients with cirrhosis; however, most of these methods lack either availability and/or reproducibility. Cross-sectional imaging studies, including computed tomographic (CT) scan, or magnetic resonance imaging are the gold standard tools to quantify skeletal muscle mass and constitute a good resource for objective and detailed nutritional/metabolic assessment of cirrhotic patients and identification of sarcopenia.8,18,19 We aim to study predictors of sarcopenia and SO in patients with cirrhosis undergoing liver transplant (LT) evaluation.
Methods
In this retrospective study, data from 207 adult cirrhotic patients who underwent LT from January 2008 to December 2013 at the University of Kentucky transplant center were collected.
Clinical and laboratory assessment.
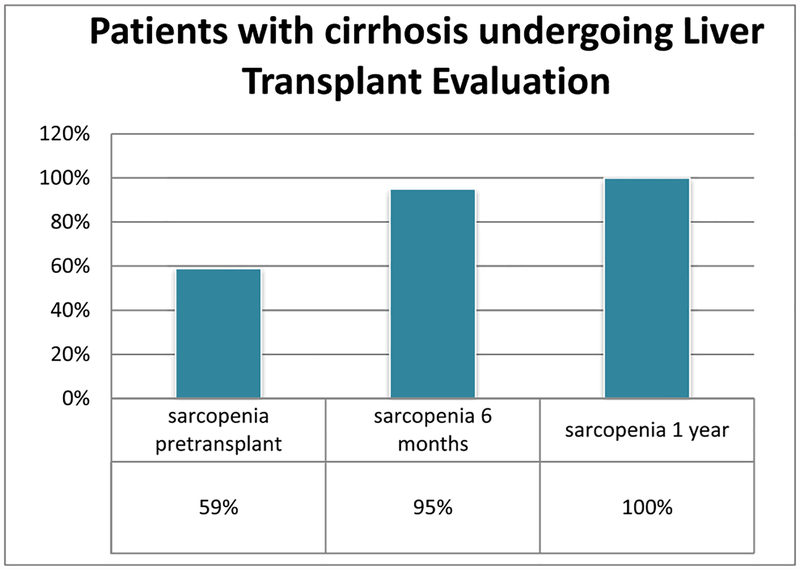
Clinical data included sex, age, weight, height, body mass index (BMI), cirrhosis etiology, presence of ascites. Hospitalization and transplant procedure details such as hospitalization length of stay, intensive care unit (ICU) length of stay, readmission, duration of intubation, transfusions, OR time, and cold ischemic time were also recollected (Fig. 1)
Figure 1.
Sarcopenia progression.
Laboratory data included alanine aminotransferase, aspartate aminotransferase, total bilirubin, total protein, albumin, complete blood count, sodium, creatinine, international normalized ratio (INR), and laboratory model for end-stage liver disease (MELD) score was also calculated. Patients with multiorgan transplants were excluded from the analysis.
Diagnosis of sarcopenia based on the skeletal muscle mass on CT imaging.
With available CT, the L3 vertebral level was identified, and SliceOmatic 5. 0 software (Tomovision, Montreal, Canada) was used to measure the cross-sectional area of surrounding muscles (psoas, paraspinals, transversus abdominis, rectus abdominis, and internal and external obliques). The muscle cross-sectional area at L3–L4 has been correlated with whole body muscle mass in patients with and without cancer and has been validated against dual X-ray absorptiometry. The specific areas of measurement were identified and muscle area were analyzed by two expert abdominal radiologists using tissue-specific Hounsfield unit thresholds. Patients without preoperative CT scan were excluded from the analysis.
The L3 skeletal muscle area was normalized to stature by the division of the muscle area by the height squared. Sarcopenia is defined as a reduction in the quantity of muscle mass > 2 standard deviations below normal.
Sarcopenia cutoffs for the lumbar skeletal muscle index (SMI) were based on a CT-based sarcopenia study of patients with cancer; the same cutoffs that were also used by Montano-Loza et al. in patients with cirrhosis. Sarcopenia was considered present when the L3 muscle area was ≤52.4 cm2/m2 in males and ≤ 38.5 cm2/m2 in females. Patients who had sarcopenia before LT with follow-up CT scans at 6 and 12 months after liver transplantation were analyzed to study the progression of sarcopenia. Obesity was defined as BMI > 30, obesity class I as BMI 30–35, obesity class II as 35–40 and obesity class III as BMI ≥ 40 as described by the World Health Organization. SO was defined as obesity class I, II, or II in the setting of sarcopenia (Fig. 2).
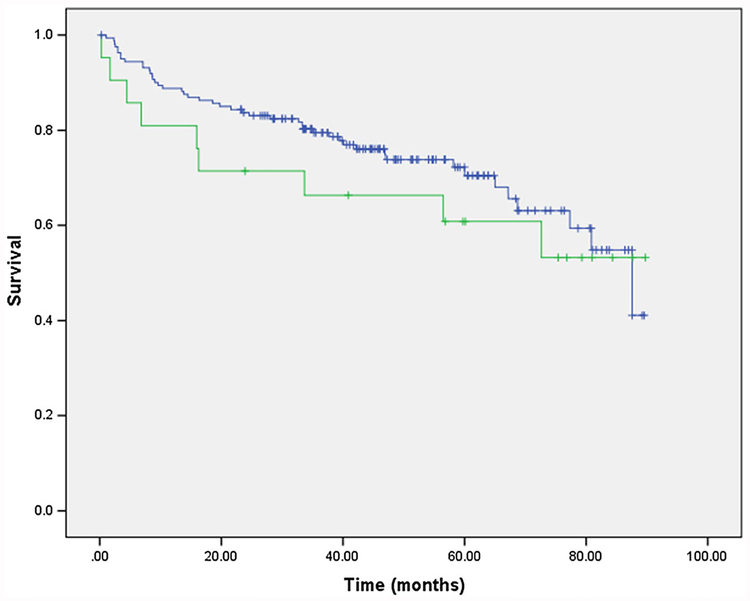
Figure 2.
Kaplan–Meier survival curve in patients with and without pretransplant SO.
Statistical analysis.
Continuous variables are presented as mean ± SD and compared using Student t test. Chi-square test was used for categorical variables. A multivariable logistic regression analysis was performed to find predictors of pretransplant and posttransplant sarcopenia. Overall survival (OS) of patients with and without pre-LT sarcopenia and with sarcopenia at 6 and 12 months was calculated using Kaplan–Meier and comparisons between groups were conducted by using log-rank test. Statistical significance was set at P < 0.05. Data were analyzed using IBM SPSS software, version 22.
Results
Demographic and clinical characteristics.
Of these 207 patients, 68% were male with a mean age of 54 ± 8 years. The most common etiology of cirrhosis was alcoholic liver disease (ALD) (38.6%), followed by chronic hepatitis C (38.2%), NASH (21.7%), and hepatocellular carcinoma (HCC) (24.6%). The mean MELD score at transplant was 21.8 ± 8. Other clinical and laboratory data are summarized in Table 1. The mean cold ischemic time was 360.41 ± 101.22 minutes. The mean operative time was 7.49 ± 1.96 h with a mean hospital and ICU length of 17.31 ± 25 and 7.31 ± 15 days, respectively (Table 2).
Table 1.
Demographic and clinical characteristics
| Parameter | n = 207 | Percentage or range |
|---|---|---|
| Age | 54 ± 8 | 22–72 |
| Gender (M:F) | 142:65 | 69:31 |
| BMI | 30.1 ± 5.7 | 17.4–47.9 |
| MELD score | 21 ± 8 | 6–46 |
| Height | 67 ± 4 | 64–72 |
| Weight | 198 ± 45 | 140–261 |
| Etiology of liver disease | 80 | 38.6 |
| ALD | 51 | 24.6 |
| HCC | 79 | 38.2 |
| HCV | 45 | 21.7 |
| NASH | ||
| Totalbilirubin | 6.3 ± 8 | 0.3–57.9 |
| Creatinine | 1.6 ± 1.4 | 0.33–7 |
| INR | 1.6 ± 0.47 | 1–4 |
| Obesity type 1 | 57 | 27.5 |
| Obesity type 2 | 46 | 22.2 |
| Obesity type 3 | 4 | 2 |
| pre-Lumbar index | 46.25 ± 10.56 | 23.61–74.69 |
| Lumbar index 6 months | 40.72 ± 10.46 | 15.83–68.57 |
| Lumbar index 1 year | 41.51 ± 9.74 | 21.35–68.65 |
| Ascities | 148 | 7.5 |
ALD, alcoholic liver disease; BMI, body mass index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, model for end stage liver disease; NASH, nonalcoholic steatohepatitis.
Table 2.
Resource utilization
| Parameter | n = 207 | Percentage or range |
|---|---|---|
| ICU length of stay | 7.31 ± 15 | 1–150 |
| Hospitallength of stay | 17.31 ± 25 | 0–259 |
| Readmission | 82 | 40 |
| Cold ischemic time | 360.4 ± 101 | 160–821 |
| OR time | 7.49 ± 1.96 | 3.9–17.2 |
| PCRBCS | 11.68 ± 10.9 | 0–72 |
ICU, intensive care unit; OR, odds ratio; PCRBCS.
Obesity, sarcopenia, and SO in cirrhosis.
The mean BMI of the cohort was of 30.1 ± 5.7 kg/m2. Obesity was found in 101 (48.8%) of the patients. Of the 207 patients, 182 patients (88%) had CT scans within 90 days before transplant, of these 108 (59%) had sarcopenia pre-LT defined by the cutoff values. Sarcopenia was more prevalent in men 82 (75%) than women 26 (24%) (Graphic 1). Of 108 patients with pretransplant sarcopenia, 38 (35.2%) had obesity and 70 (64.8%) were nonobese. Of 56 patients that had sarcopenia at 6 months, 23 (41.1%) were obese and 33 (58.9) nonobese. At 1 year of 31 patients that had sarcopenia, 9 (29%) were obese compared with 22 (71%) nonobese. In a sub-group analysis, older patients (50, or older) with sarcopenia were associated with significantly increased rates of 30-day readmissions (P = 0.05). On multivariable regression analysis, obesity and age were found to be independently associated with pretransplant sarcopenia after controlling for gender and alcohol liver disease diagnosis (P = 0.00001, odds ratio [OR] 0.22; and P = 0.008, OR 2.0, respectively). (Table 3)
Table 3.
Multiple logistic regression analyses of factors associated with sarcopenia
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | 0.94 | 0.90–0.99 | 0.008 |
| Gender | 0.49 | 0.23–1.02 | 0.058 |
| Obesity | 0.22 | 0.11–0.44 | < 0.001 |
| ALD | 1.98 | 0.95–4.10 | 0.067 |
ALD, alcoholic liver disease; CI, confidence interval.
Failure to reverse sarcopenia after successful liver transplantation.
Of the patients with pretransplant sarcopenia, 59 had CT scan at 6 months posttransplant and 56 (95%) remain sarcopenic. Of the 56 patients that had sarcopenia at 6 months, 31 had available CT scans at 1 year, and sarcopenia persisted in 100%. These 31 subjects had a mean (median) SMI of 35 (33) at 6 months and 36 (36) at 1 year suggesting slight increase and not statistically significant (P = 0.33). Of the 76 patients that did not have sarcopenia in the evaluation pretransplant, 20 (26%) develop sarcopenia 6 months posttransplant.
Survival outcomes.
Overall survival at 1-, 3-, and 5-year was 87.4%, 76.3%, and 66.2%, respectively. The mean OS was 38 months. Of the 106 subjects who had sarcopenia before LT, 36 had died and 70 are still alive. Of the 74 subjects who did not have sarcopenia before transplant, 19 had died and 55 are still alive. Patients with SO have a trend toward worse patient survival. Patients with SO using obesity class 1 or worse have 1-, 3-, and 5-year survival rates of 81%, 66%, and 60% compared with 88%, 79%, and 71%, respectively (P = 0.4) (Graphic 2). Perioperative mortality was higher in sarcopenic obese patients compared with their counterparts, 5% vs. 0.6% respectively.
Nonalcoholic steatohepatitis cirrhosis is significantly associated with SO in patient undergoing LT evaluation.
A multivariable logistic regression analysis found NASH as the cause of cirrhosis and MELD score as independent predictors of SO after controlling for age, gender, ALD diagnosis, and HCC (P = 0.014 and 0.038, respectively; 95% confidence interval [CI], 1.44–25.26 and 1.00–1.15, respectively; OR 6.03, 1.08, respectively). Interestingly, patients with NASH have 6-fold increase chance of having SO in this cohort (Table 4).
Table 4.
Multiple logistic regression analyses of factors associated with sarcopenic obesity
| OR | 95% CI | P value | |
|---|---|---|---|
| Age | 0.94 | 0.87–1.01 | 0.098 |
| Gender | 4.49 | 0.92–22.02 | 0.064 |
| MELD score | 1.08 | 1.00–1.15 | 0.038 |
| ALD | 0.89 | 0.21–3.77 | 0.87 |
| HCC | 1.58 | 0.35–7.15 | 0.55 |
| NASH | 6.03 | 1.44–25.27 | 0.14 |
ALD, alcoholic liver disease; HCC, hepatocellular carcinoma; MELD, model for end stage liver disease; NASH, nonalcoholic steatohepatitis.
Discussion
The two greatest epidemiological trends of our times are the aging of the population and the obesity epidemic. Each of these trends has important effects on body composition, morbidity, and mortality.
Aging causes a progressive loss of muscle mass and strength called sarcopenia. The term sarcopenia, coined by I. H. Rosenberg, originates from the Greek words sarx (flesh) and penia (loss); it can also be present as a result of chronic diseases and malignancy, and it ultimately leads to decreased functional capacity in different groups of patients.1
Sarcopenia is the most common complication of cirrhosis and adversely affects outcome before, during, and after liver transplantation. Liver transplantation reverses the biochemical abnormalities of cirrhosis and its complications but fails to reverse sarcopenia at least in the initial recovery phase. The posttransplant recovery of lean body mass reported by the majority of authors has been suboptimal and seems not to reach the premorbid levels. Some authors have proposed that sarcopenia worsens early after liver transplantation but the data on progression of the sarcopenia in the late period are scarce and conflicting.20–22 In our analysis, we found out that the prevalence of sarcopenia in pretransplant cirrhotic patients was 59% and that it was more predominant on men. Interestingly, in the majority of patients, the muscle wasting persisted at 6 months and 1 year. We also found that 26% of the patients with cirrhosis that did not have sarcopenia before liver transplantation develop new-onset sarcopenia posttransplant. These data are similar to other reports with patients failing to improve lean body mass posttransplant.20–22 Dasarathy et al. suggested that sarcopenia is an early underrecognized consequence of liver transplantation. The data regarding progression of sarcopenia in the late posttransplant period are limited. The role of interventions such as adequate nutrition and physical therapy in the setting of a well-functioning organ posttransplantion and how this can revert sarcopenia needs further investigation.20 Potential reasons for failure to reverse sarcopenia after liver transplantation include use of immunosuppressive agents that impair skeletal muscle growth and protein accretion. Also, studies have shown that repeated hospitalizations, posttransplant infections, and renal failure could play a role.20,23
Sarcopenic obesity is a critical public health problem related to two important phenomena: the rising prevalence of obesity in Western and developing countries and the increase of lifespan. Changes in body composition, occurring in ageing as well as in obesity, represent the common soil where SO develops.24 Longitudinal studies have shown that fat mass increases with age and is higher among later-birth cohorts peaking at about age 60–75 years, whereas muscle mass and strength starts to decline progressively around the age of 30 years with a more accelerated loss after the age of 60.25
Sarcopenic obesity was first defined by Baumgartner as a muscle mass index less than 2 SD below the sex-specific reference for a young, healthy population. Initial evidence indicates that when obesity and muscle impairment coexist, they act synergistically to increase the risk of developing multiple health-related illnesses. The imbalance between obesity and muscle impairment, either defined by low muscle mass or poor muscle strength, is associated with important, negative health outcomes in older individuals.
Obesity is frequently associated with cirrhosis, and cirrhotic patients may develop simultaneous loss of skeletal muscle mass, ending in this condition called sarcopenic obesity. SO appears to be linked with the upregulation of tumor necrosis factor α, inter-leukin (IL) 6, leptin, and myostatin and the downregulation of adiponectin and IL-15.17
In our study, we found that obesity was prevalent in 48.8%, and 59% had sarcopenia pretransplant. Sarcopenia was more prevalent in men (82, 75%) than in women (26, 24%). Of 108 patients with pretransplant sarcopenia, 38 (35.2%) had obesity and 70 (64.8%) were nonobese. On multivariable regression analysis, obesity and age were found to be independently associated with pretransplant sarcopenia after controlling for gender and alcohol liver disease diagnosis (P = 0.00001, OR 0.22, and P = 0.008, OR 2.0, respectively).
The clinical profile of patients with cirrhosis is changing with the rapid increase in the number patients with nonalcoholic fatty liver disease that occurs in the setting of obesity. NASH is one of the most common forms of chronic liver disease and has become the fourth most common indication for liver transplantation in the United States. NASH describes a spectrum of liver conditions ranging from simple steatosis to severe steatosis with marked inflammation, which can be complicated by cirrhosis, end-stage liver disease (ESLD), and HCC. NASH is now the liver disease associated with the highest mortality, consequent to the increased risk of cardiovascular diseases.16 Our study reported a prevalence of NASH as liver disease etiology in this cohort of 21.7%.
Both liver and muscle are target organs for insulin action, and insulin resistance is known as a key factor in the pathophysiology for both NASH and sarcopenia. NASH and sarcopenia may share similar physiological mechanisms of systemic manifestations of chronic inflammation and damage from reactive oxygen species and other intermediates and the inability to repair the resulting injury.5,12,15,27 The association between NASH and sarcopenia was examined in 452 apparently healthy adults enrolled in a prospective observational cohort study. This study demonstrated a higher risk of NASH in individuals with sarcopenia compared with a control group. They found that patients with sarcopenia had more body fat mass, more components of metabolic syndrome, higher C-reactive protein levels, and higher arterial stiffness compared with those without sarcopenia. Moreover, high concentrations of C-reactive protein were closely correlated with both SMI and liver attenuation index, which suggests that inflammation may be an important underlying factor associated with both sarcopenia and NASH.12
Our multivariable logistic regression analysis found that NASH as cause of cirrhosis and MELD score are independent predictors of SO after controlling for age, gender, ALD diagnosis, and HCC (P = 0.014 and 0.038, respectively; 95% CI, 1.44–25.26 and 1.00–1.15, respectively; OR 6.03, 1.08, respectively). Interestingly, patients with NASH have 6-fold increase chance of having SO in our cohort.
Montano-Loza et al. published a study of 678 adult patients with cirrhosis using CT scan that indicates that skeletal muscle abnormalities are frequently present in patients with cirrhosis, with sarcopenia being present in almost half of the patients; 20% of them had SO and fatty infiltration of skeletal muscle or myosteatosis was present in more than half of the cohort. Patients with cirrhosis and sarcopenia, SO and myosteatosis tend to be older, more frequently men, and have worse liver function assessed by MELD or Child–Pugh scores compared with patients with no muscular abnormalities. Importantly, the presence of sarcopenia, SO, and myosteatosis were associated with worse survival compared with cirrhotic patients with no muscular abnormalities. The risk of mortality associated with the presence of muscular abnormalities (sarcopenia and myosteatosis) in cirrhotic patients seems to be related to a higher frequency of sepsis-related death. We found worse patient survival in patients with SO, 60% at 5 years, compared with 71% on patients without SO, although this was not found to be statistically significant. We also demonstrated a difference in perioperative mortality of 5% on obese sarcopenics versus 0.6% in nonobese sarcopenic patients.17
Baumgartner observed as well that men and women older than 60 years with SO showed, respectively, an 8- and 11-fold higher risk of having physical disabilities. More importantly, it was observed that the association with functional status impairment was stronger for SO than for either obesity or sarcopenia alone.1 Our multivariable logistic regression analysis demonstrated that obesity and age are independently associated with sarcopenia in this cohort. Obesity increases 78% the chances of having sarcopenia in our cohort.
Sarcopenia often coexists with obesity and may have additive effects on insulin resistance and the presence of type 2 diabetes. Inflammation is a central underpinning mechanism in the pathogenesis of insulin resistance and is also seen in both obesity and sarcopenia. Inflammation may be an important mediator in restraining myogenesis and/or accelerating muscle protein degradation.28–30
There are few limitations that should be discussed. In our study, we did not have too many patients with CT scans beyond 1 year after liver transplantation and were not able to study the progression of sarcopenia after a year. Another limitation is that the real cutoff points for diagnosis of sarcopenia in cirrhosis have not been well validated. However, the cut-points we utilized in our study have been previously used and published in cirrhotic patients including the gender-specific cutoffs.
In conclusion, sarcopenia is seen in a significant amount of patients with cirrhosis undergoing LT evaluation. Sarcopenia progresses after LT initially and seems not to recover at least within the first year after surgery. Obesity is an independent predictor of pretransplant sarcopenia. SO is a relatively new concept that defines sarcopenia in the setting obesity and its incidence is increasing in patients with ESLD. NASH was associated with 6-fold increased risk of having SO in cirrhotic patients in this cohort. The mechanism for the development of this condition is not well defined and needs to be investigated. Further studies should be performed to evaluate the impact of SO on patient outcomes and resource utilization in this patient population.
Footnotes
Conflicts of interests/financial disclosures:
The authors declare no commercial or financial conflicts of interest.
References
- 1.Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J. Endocrinol 2013; 2013: 204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesari M et al. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr 2005; 82: 428–34. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R Sarcopenic obesity: the confluence of two epidemics. Obes. Res 2004; 12: 887–8. [DOI] [PubMed] [Google Scholar]
- 4.Batsis JA et al. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr 2014; 68: 1001–7. [DOI] [PubMed] [Google Scholar]
- 5.Kim TN et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int. J. Obes. (Lond) 2009; 33: 885–92. [DOI] [PubMed] [Google Scholar]
- 6.Dasarathy S Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 2012; 3: 225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda T et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014; 20: 401–7. [DOI] [PubMed] [Google Scholar]
- 8.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J. Gastroenterol 2014; 20: 8061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montano-Loza AJ et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014; 20: 640–8. [DOI] [PubMed] [Google Scholar]
- 10.Tandon P et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl 2012; 18: 1209–16. [DOI] [PubMed] [Google Scholar]
- 11.Englesbe MJ et al. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg 2010; 211: 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong HC et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014; 59: 1772–8. [DOI] [PubMed] [Google Scholar]
- 13.Kim TN et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin. Endocrinol 2013; 78: 525–32. [DOI] [PubMed] [Google Scholar]
- 14.Bertolotti M et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J. Gastroenterol 2014; 20: 14185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HY et al. , Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab J 2013; 37: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui E et al. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: role of adipokines. J. Diabetes Investig 2013; 4: 413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CMM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachex. Sarcopenia Muscle 2015, doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durand F et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J. Hepatol 2014; 60: 1151–7. [DOI] [PubMed] [Google Scholar]
- 19.Giusto M et al. Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol 2015; 27: 328–34. [DOI] [PubMed] [Google Scholar]
- 20.Dasarathy S Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig. Dis. Sci 2013; 58: 3103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergerson JT et al. Liver transplantation arrests and reverses muscle wasting. Clin. Transplant 2015; 29: 216–21. [DOI] [PubMed] [Google Scholar]
- 22.Giusto M et al. Changes in nutritional status after liver transplantation. World J. Gastroenterol 2014; 20: 10682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamaguchi Y et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014; 20: 1413–9. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo MD et al. Sarcopenic obesity: correlation with clinical, functional, and psychological status in a rehabilitation setting. J. Nutr. Food Sci 2014; 5: 2020–2031. [Google Scholar]
- 25.Stenholm S et al. Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008; 11: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schütz T et al. Weight gain in long-term survivors of kidney or liver transplantation—another paradigm of sarcopenic obesity? Nutrition 2012; 28: 378–383. [DOI] [PubMed] [Google Scholar]
- 27.Kim MK et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab 2011; 96: 3250–6. [DOI] [PubMed] [Google Scholar]
- 28.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 2010; 5: e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki A, Heying Z, Mori S. Relationship between sarcopenic obesity or sarcopenia and insulin resistance or functional disability. Nihon Ronen Igakkai Zasshi 2012; 49: 210–3. [DOI] [PubMed] [Google Scholar]
- 30.Levine ME, Crimmins EM. Sarcopenic obesity and cognitive functioning: the mediating roles of insulin resistance and inflammation? Curr. Gerontol. Geriatr. Res 2012; 2012: 826398. [DOI] [PMC free article] [PubMed] [Google Scholar]