Abstract
Allogenic hematopoietic stem cell transplantation (HSCT) has been shown to be a treatment option for a selected group of patients with mucopolysaccharidoses (MPS) (MPS I, II, IVA, VI, and VII). Early diagnosis and timely referral to an expert in MPS are critical, followed by a complete examination and evaluation with a multidisciplinary team, including a transplant physician. Treatment recommendations for MPS are based on multiple factors such as biological, sociological, and financial effects. These include type of MPS, clinical severity, prognosis, present clinical signs and symptoms (disease stage), age at onset, the rate of progression, family significances and expectations, financial burdens, feasibility, availability, risks and benefits with available therapies such as HSCT, enzyme replacement therapy (ERT), surgical interventions, and other supportive care.
To evaluate therapeutic efficacy and adverse effects of HSCT for MPS, international collaboration and data review are critical. Since the first attempt of HSCT in a patient with MPS in 1981, collaborative efforts to assess HSCT for MPS have been made continuously. Accumulation of data has made it possible to identify early outcomes (transplant outcomes) and long-term disease-specific outcomes resulting from HSCT. The recent identification of predictive factors and the development of innovative regimens have significantly improved the outcomes of both engraftment failure and transplant mortality. Assessment of long-term outcomes has also been described under consideration of a variety of factors: type of MPS, graft-type, age at transplantation, stage of disease progression, etc. Studies on the long-term outcomes are considered a key achievement for the use of HSCT in MPS communities. These studies have shown the effects and limitations of HSCT to improve disease manifestations and quality of life.
In this review, we summarize the efficacy, side effects, risks, and the cost of HSCT for each type of MPS.
Keywords: allogenic hematopoietic stem cell transplantation, mucopolysaccharidoses, enzyme replacement therapy, limitations, outcomes
1. Introduction
Mucopolysaccharidoses (MPS) are a group of genetic lysosomal storage disorders (LSDs). Individuals with MPS lack a specific enzyme in the lysosome, which degrades glycosaminoglycans (GAGs) in many tissues in the body. Deficiency of the enzyme leads to an accumulation of undegraded GAGs in the body. This results in systemic clinical manifestations unique to patients with MPS. There are seven identified types of MPS, based on the specific enzyme deficiency and successive accumulation of specific GAG(s). Some of the common clinical manifestations of MPS include skeletal manifestations, cardiac and respiratory disease, and in some types of MPS, central nervous system (CNS) involvement [1–4].
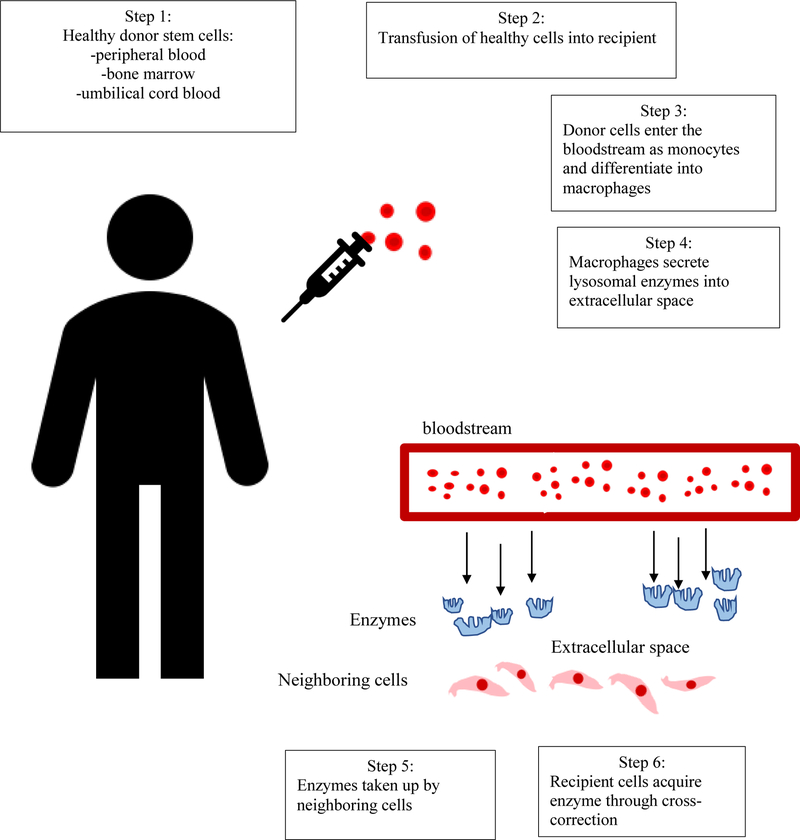
Two major treatments for MPS patients are available in practice; hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT). With HSCT, healthy donor cells are transplanted. The enzyme secreted by donor cells is then up taken by the recipient’s body through cross-correction [2, 5–8]. In contrast, intravenous ERT administers the recombinant enzyme that is deficient in the patient. These enzymes bind to the Mannose-6-phosphate (M6P) receptors on the cell surface and are delivered to the lysosome, the site of action of the lysosomal hydrolases. Figure 1 simplifies the mechanisms of HSCT treatment in MPS patients.
Figure 1.
The first stem cell transplantation on an MPS I patient was in 1981; a bone marrow transplantation (BMT) was performed on a 1-year old boy with MPS type I (Hurler syndrome) (Table 1). Initially, the boy received cells from his father who matched one haplotype, but after 2.5 months, there were no signs of successful engraftment. The boy was then transplanted using cells from his mother with three loci match. After 14 days of engraftment, 88% of his white cells had an XX chromosome. However, 8 days after engraftment, he developed graft-versus-host disease (GVHD), and manifestations of MPS I such as hepatosplenomegaly and corneal clouding regressed. A liver biopsy performed 199 days post-transplantation showed no sign of storage materials and the leukocyte and plasma enzyme levels reached the normal level of his mother [9].
Table 1.
Historical timeline of HSCT for MPS
| Year | Description | Reference |
|---|---|---|
| 1957 | First documented bone marrow transplantation in patients who suffered from a nuclear radiation accident | [196, 197]. |
| 1969 | HSCT allows cross-correction in MPS I and II patients | [21,22]. |
| 1981 | Hobbs et al. described the first HSCT on an MPS I patient. | [56, 153]. |
| 1982 – 1991 | First MPS II allo-BMT | [56, 153]. |
| 1984 | First report of a successful BMT in an MPS VI patient | [6]. |
| 1998 | First reported bone transplantation performed on a MPS VII patient | [20] |
| 2000 | First successful report of transplantation on an MPS II patient | [198] |
| 2005 | The European Group for Blood and Marrow Transplantation suggested new international guidelines for HSCT performed in MPS patients | [37] |
| 2014 | First reported case of a BMT on an MPS IVA patient. | [12] |
HSCT is considered the standard of care for those with MPS IH and an optional treatment for Hurler/Scheie syndrome (MPS IH/S) and Scheie syndrome (MPS-IS) (attenuated phenotypes of MPS I), MPS II, MPS IVA, MPS VI, and MPS VII [10–20].
One of the main differences between ERT and HSCT is that ERT only infuses the deficient enzyme, which circulates in the bloodstream with a short half-life and cannot cross the blood-brain barrier (BBB). In the case of HSCT, donor stem cells circulate into the bloodstream, which can cross the BBB and differentiate (macrophage, microglia, etc.). The microglial cells secrete the deficient enzyme to the different parts of the brain [21]. HSCT has been shown to improve CNS impairment in MPS I, II, and VII [10, 13, 18, 20, 22].
Some of the advantages of HSCT include that it is generally a one-time procedure which allows the recipient to have a continuous source of enzyme, as well as rapid clearance of GAGs [2]. Peripheral blood stem cell transplant (PBSCT) has significantly replaced the role of bone marrow transplant (BMT) in adolescent patients, due to the simplicity of donor collection associated with fewer complications and better outcomes. These outcomes include a reduced hematological and immune response, feasible process to retrieve donor stem cells, fewer antibiotics needed, shorter hospital stays, and less donor adverse effects. However, BM is still the preferred donor source for adult patients because there is less incidence of GVHD [23]. Additionally, unrelated cord blood (UCB) is a source of donor cells [3, 24–26] due to its feasible and fast retrieval, and it decreases complications with immune tolerance. However, there are many challenges associated with HSCT including the time-consuming process to find an acceptable donor for the transplantation as well as the mortality and morbidity associated with the procedure [2].
When HSCT for each type of MPS was first used as a treatment method during the 1980s and 1990s, there was a significant mortality risk associated with the procedure. However, it is important to take into account some factors that contributed to the high mortality risk. Patients, who received HSCT during this initial period, were usually in the late stages of the disease with significant clinical manifestations already present. Also, the advancements in medicine since then have allowed for a reduction in mortality risks [3, 27–33]. Specific parts of the transplant procedure have improved, reducing mortality rates. These improvements include the availability of matched cord blood, enhanced HLA-matching techniques, advanced conditioning regimens, and supportive care [28, 34–36]. Experienced HSCT centers have reported engrafted survival rates of 90% for MPS I patients, primarily due to the updated guidelines from the European Society for Blood and Marrow Transplantation (EBMT) protocol and selection of a well-suited donor [28, 31, 33, 37, 38].
ERT can cause an immune response against the infused enzyme in the recipient’s body, which can result in compromised treatment outcomes [39–42]. In 2012, Saif et al. demonstrated that HSCT can be used as a treatment to overcome immune responses caused by ERT [43]. In their study, all MPS IH patients who received ERT followed by HSCT had their antibodies reduced to an insignificant level 101 days after transplantation. The presence of normal enzyme levels did not affect the level of antibodies. Additionally, the study found that full-donor chimerism was needed for the antibody response to be reduced. Allo-HSCT corrected the immune response to ERT since it replaced the patient’s immune system with the donor’s immune system [44].
Furthermore, the age at which HSCT is performed provides a significant impact on its efficacy. In 2015, Tanjuakio et al. reported that those who receive an HSCT before the age of 5 have higher activity of daily living (ADL) scores than those who receive an HSCT after the age of 5 in MPS II patients [3, 10, 13, 18, 19, 45]. Therefore, transplantation at an early age is important for the overall outcome of the procedure. Increased awareness of MPS and introduction of newborn screening (NBS) have allowed early detection and treatment of the disease before the patient develops severe irreversible clinical manifestations.
In 2017, Kubaski et al. reported that one MPS II patient received ERT beginning at 2.3 years and that he had developed an IgG antibody response which caused the ERT to eventually become ineffective [13]. At 4.5 years, the patient received the full engraftment of HSCT. Positive results from the HSCT included decreased urinary GAG, decreased hepatosplenomegaly, and increased ADL scores. Thus, HSCT restored and even improved the outcomes of a patient who otherwise would have remained without treatment due to the immune response [13].
Over 1,000 MPS patients have received HSCT as a treatment option for their disease as of 2018[11–14, 17, 19, 20, 37, 46–50].
The common treatment for those with attenuated forms of MPS is 4–6-hour weekly intravenous ERT infusions. However, HSCT is, overall, more time efficient; also, HSCT can be a better treatment option for improving disease symptoms since it has been reported that HSCT results in better metabolic correction compared to the use of current ERT [10, 13, 18, 19, 31, 51]. In this review article, we summarize the effects and limitations, the adverse effects, predictive factors, innovative regimens, and cost/benefit of HSCT for each type of MPS.
2. General aspect of HSCT
HSCT has been shown to be effective with MPS types I, II, IVA, VI, and VII [10–14, 18–20, 45, 49, 52–58]. The extent to which the treatment is effective depends on the age of the patient and the disease stage at the time of the procedure, the type of MPS, the type of donor, and the preparative regimen [55, 59]. HSCT can improve the clinical manifestations of MPS including joint mobility, vision, hearing, cardiopulmonary function, coarse facial features, upper airway obstruction, respiratory functions, and hepatosplenomegaly [2, 5–8, 60–64]. However, HSCT has not been able to significantly correct clinical manifestations of the disease in the bone, cornea, cardiac valvular abnormalities, or any preexisting cognitive and intellectual effects of the disease [3, 65–68]. In comparison, ERT has not been able to correct skeletal dysplasia significantly. Enzymes also have a short half-life, and the use of ERT can sometimes cause an increase of antibodies which can decrease the overall efficacy of the treatment [3, 65, 66, 68, 69]. Transplantation at an early age is important for the overall outcome of the procedure.
2.1. Regimens
Preparative regimens and GVHD prophylaxis are used before the transplantation to suppress the immune system of the patient by eliminating the recipient’s immune cells and to reduce chances of rejection of the donor’s cells. These conditioning regimens include myeloablative (MA), nonmyeloablative (NMA), or reduced intensity conditioning (RIC). MA conditioning usually contains a combination of cyclophosphamide (CY) and total body irradiation (TBI) or a combination of busulfan and cyclophosphamide. NMA and RIC conditioning usually include a combination of low dose TBI with or without fludarabine or a mixture of fludarabine with alkylating agents such as thiotepa, melphalan, or busulfan [70–81]. The type of preparative conditioning used before the transplant can affect the success of the procedure. Some studies reported an increased risk of graft failure when using reduced intensity conditioning treatment [35]. Table 2 provides a summary of HSCT cases performed detailing the conditioning regimen, donor source, and outcomes of the procedure [82]. The most recent recommendation for successful engraftment was adopted in 2012, suggesting a combination of busulfan and fludarabine as a conditioning regimen. This recommendation is different from the previous recommendation of a combination of busulfan and cyclophosphamide. Fludarabine is now seen as the preferred preparative regimen since it is less toxic than cyclophosphamide when combined with busulfan and has been shown to achieve the same engraftment rate success in patients [31, 37, 83].
Table 2.
Summary of HSC depicting conditioning regimen, donor source, and graft type
| Study | MPS type | Number of Patients | Conditioning Regimen | Donor source (patients) | Graft Type | Donor chimerism | Incidence of GVHD (patients) | Survival |
|---|---|---|---|---|---|---|---|---|
| Souillet et al. 2003 [8] | MPS IH | 27 | 27 - Bu, Cy + ATG for unrelated mismatche dBMT | 13-MRD 14-MUD | 2-CB 17-BM | 70% ≥ 95% donor chimerism | 5-aGvHD | 85% |
| Boelens et al. 2007[35] | MPS IH | 146 | 68 - Bu-Cy 200mg/kg 30-Bu with high dose Cy 240–260mg/kg 15 - Bu-targeting 17 - Flu-based myeloablative 18 - RIC | 96 - HLA MD | 103 BM 20 PB 23 - CB | 71% - full donor chimerism after initial transplantation | 26-aGvHD grade I 15 - aGvHD grade II 3-aGvHD grade III 5-aGvHD grade IV 8 - cGvHD | 85% after initial transplantation |
| Tuberville et al. 2011[17] | MPS VI | 45 | 6-Cy + TBI ± other 30 - Bu + Cy ± other 1 - Flu + Mel 7 - Other | 15-HLA-MSD 3-MRD 27-MUD | 34 BM 1-PB 10-CB | N/A | 15-aGvHD grade II-IV 34-aGvHD grades III-IV 19-GvHD | 78% at 100 days 66% at 1 and 3 years |
| Aldenhoven et al. 2015 [37] | 52-MPS IH 2-MPS II 2-MPS III 2-MPS VI | 62 | 29 - BuCy 33 - FluBu | 44-HLA MD | 41 CB 21 BM or PB | 88.2% -full donor chimerism | 8-aGvHD grade II-IV 8-cGvHD | 95.20% |
| Wang et al. 2016 [18] | 12-MPS I 12-MPS II 4-MPS IVA 4-MPS VI 2-unknown | 34 | 21 -Bu, Cy, ATG 8 - Bu, Cy, Flu, ATG | 11–4/6 to 6/6 HLA-MUD 4-MSD 2-MRD 17-MUD | 11 - CB 23 - PB | 31-full donor chimerism | 14-aGvHD grade II to IV 4-aGv HD grade III to IV 2-modera te-to-severe chronic GvHD | 84.8% ± 6.3% at 3 years |
| Lum et al. 2017 [82] | MPS IH | 240 | 145 - Bu/Cy ± serotherap Y 40-Bu/Flu ± serotherap y 55 - other |
67 - MRD 151 - MUD 22 - mismatc hed donor | 132BM 16 - PB 92 - CB | 80% - full donor chimerism of 85 patients assessed | 70-aGvHD grade I -II 22 aGvHD grade III - IV 14-cGvHD | 85.20% |
| Rodgers et al. 2017 [84] | MPS IH | 134 | 35 - targeted Bu 99 - no targeted Bu | 35-MRD 97-MUD | 83 - BM 49 - CB 2 - PB | 100 - ≥ 90% 21 – 10–89% 12 - <10% 1 unknown | 70% at 1 year |
Note: BM – bone marrow; PB – peripheral blood; CB – cord blood; MRD – matched related donor; MUD – matched unrelated donor, MSD – matched sibling donor; MD – matched donor; misMUD – mismatched unrelated donor; TBI – total body irradiation; Cy – cyclophosphamide; Flu – Fludarabine; Mel – Melphalan; ATG – Thymoglobulin; RIC – reduced-intensity conditioning; aGVHD – acute graft-vs-host disease; cGvHD – chronic graft-vs-host disease; ivBU – intravenous busulfan
2.2. Best source of cells for HSCT
Recipients of HSCT can acquire donor cells from three different sources: bone marrow, peripheral blood, or umbilical cord blood (UCB) [24]. In 2005, the European Group for Blood and Marrow Transplantation released guidelines that suggested donor type hierarchy in the following order: non-carrier HLA-matched family donor, matched unrelated cord blood, matched unrelated donor [82]. In 2016, Boelens et al. reported the best way to achieve an “event-free” survival after an HSCT; the best donor sources are identically matched HLA siblings or identical antigen matched cord blood, and the next best are 5/6 HLA-matched cord blood or 10/10 HLA-matched unrelated donors [31]. In 2017, Rodger et al. reported that the 8-year survival rate for patients who receive related BMT or unrelated UCB transplantation was higher than for patients who receive unrelated BMT [84]. Additionally, the best combination of regimen and donor source was myeloablative conditioning regimen with busulfan [30, 35, 37] and a matched umbilical cord blood donor, non-carrier matched sibling, or a fully matched unrelated donor [28]. In recent years, UCB has become a popular donor source. However, in 2017, Lum et al., in a multi-center study [82] reported that all the risk of graft failure has reduced in recent years, but the pattern has changed from previous recipient autologous reconstitution to now aplastic-type graft failure. The former reflected inadequate myelosuppression and the latter is more common in UCB recipients, which was proposed to represent likely inadequate recipient immune suppression. Those who do become engrafted with UCB reported a better donor chimerism achieved compared to those engrafted with BM [28, 82, 85].
2.3. Adverse effects of HSCT
Before 2000, mortality rates associated with HSCT for MPS were reported to be up to 27% [13, 18, 56]. Side effects associated with HSCT include disturbance of growth and infertility [51]. The major causes of death for MPS IH patients within the first-year post-transplantation include viral infection, pulmonary hemorrhage, and GVHD [8, 35, 84, 86, 87]. Contributors to high mortality rates include the advanced disease at the time of HSCT and the use of mismatched donors. The cause of death following HSCT includes infection, organ failure, graft rejection and disease recurrence, GVHD, and toxicity of conditioning regimens [13, 18, 56]. Total body irradiation (TBI) has not been used in MPS IH patients since 2002 because total body conditioning regimens have resulted in negative effects on neurodevelopment, growth, hypothyroidism, and cataracts in endpoints. However, the regimens used today can lead to secondary malignancies, especially in older patients [38]. The event-free survival rate for MPS IH patients rose to 91% between 2005 and 2008, which can be attributed to improved transplantation protocols.
The toxicity of the drugs needed before, during, and after the transplant can have detrimental effects on the patient. Conditioning regimens are given to achieve optimal donor chimerism and to prevent the risk of GVHD. They play an essential role in assuring the successfulness of the graft. Robust myeloablative conditioning regimen is needed; the absence of pharmacokinetic targeting of busulfan to achieve a myeloablative level is associated with a high risk (20–25%) of autologous reconstitution and late graft failure in patients with inherited metabolic disease (IMD) [63, 88, 89]. However, conditioning regimens might, alternatively, cause lysosomal storage disorder brain disease to progress further, and conditioning regimens could compromise the effectiveness of HSCT. In 2015, Aldenhoven et al. suggested that toxic conditioning regimens could be a contributor to the ongoing disease symptoms seen in MPS IH patients even after transplantation [38]. Additionally, in 2014, Ansari et al. [90] indicated that females could be more prone to busulfan toxicity, which could explain the increased mortality rate in females reported in a study by Rodgers et al. [84].
In 2018, Chen et al. [89] found that the combination of fludarabine (90 mg m−2), busulfan (9.6 mg m−2) and cyclophosphamide (200 mg kg−1) was an acceptable conditioning regimen to achieve and sustain full engraftment in haploidentical allogeneic stem cell transplantation without any increase in complications.
The 5-year survival rate for MPS I patients who have received HSCT is over 90% [7, 55, 86]. The most common time for mortality for MPS IH patients is during the first-year post-transplant [8, 28, 35, 84, 86, 87]. Rodger et al. [84, 91–93] reported that there is still a steady rate of mortality in young adult and adolescents more than a year post-transplantation, and even 10-years post-transplantation there is a higher incidence of infection and pulmonary and cardiac complications compared to a healthy population. Although untreated MPS IH patients usually die from cardiac and pulmonary causes [94], MPS IH patients who have been treated with HSCT can also die from pulmonary complications and infection during the first-year post-transplant [8, 28, 35, 84, 86, 87]. These causes are usually attributed to conditioning toxicity, GVHD, and impaired immunity. Long-term outcomes of MPS IH patients who receive HSCT show that increased rate of death from the procedure does not correlate with age at transplantation, sex, graft-recipient HLA disparity, conditioning regimen, or exposure to serotherapy [94]. However, in 2017, Rodgers et al. [84] reported a 30-year study of MPS I patients who received HSCT and found a correlation between reduced mortality of MPS I patients during the first decade after transplantation and improvements made in peri-HSCT management. The improvements in symptoms and higher survival rates make HSCT a good treatment option for those with MPS IH.
Rodgers et al. [84] also found that, regardless of the period that the transplantation took place, females had a higher rate of death than male patients. When evaluating causes of death up to 25-years post-transplantation, pulmonary-related causes accounted for 27% of the deaths, making it the highest single-organ cause of death. Infection-related cause accounted for 11.6% of the deaths, and cardiac-related causes of death accounted for 8.3% of the deaths. It is important to note that while GVHD was not reported as the main cause of death in any of the reports, it could have contributed to these single-organ causes of death. The report also found that there is no correlation between IDUA enzyme levels recorded 1-year post-transplantation and long-term survival in MPS I patients.
Along with the general risks and side effects of the procedure, there could be an increased risk for those who receive HSCT more than once. In 2017, Lum et al. [94] found that there is a correlation between MPS IH individuals who undergo two HSCT treatments and the severity of their cardiac dysfunction. Also, MPS I patients who have had serious lower respiratory tract diseases or pneumonia before transplant have higher risks of mortality [95].
The 5-year survival rate was 88.5% for those with MPS II who had HSCT in Japan between 1990 and 2003 [3]. However, there have been promising reports which suggest that survival rates are now even higher. In 2016, Wang et al. [18] reported a 100% survival rate in a 10-year follow-up study of 12 MPS II patients in China [13].
MPS IVA patients usually have a severely narrowed airway and pulmonary compromise. These abnormalities, of altered airways that have a tortuous appearance in the trachea, bronchi, and small lungs, complicate the use of anesthesia in procedures. These complications can lead to difficulty intubating and extubating patients as they age, especially above the teenage years [12, 96]. Therefore, careful selection of patients with MPS IVA is required for HSCT. Although the number of treated patients is low, no clear mortalities have been reported due to HSCT for MPS IVA [18, 19].
HSCT on MPS VI patients can result in procedure complications which include GVHD, graft failure, infection, endocrine and gonadal failure. A study reported that 36% (n=45) of MPS VI patients who had HSCT from 1982 to 2007 developed acute GVHD 100 days post-transplantation. In 2011, Tuberville et al. reported that the resulting survival rate of these MPS VI patients was 78% 100 days post-transplantation and 66% one year after transplantation [17].
2.4. Predictive factors of HSCT Cost
It is hard to accurately estimate the cost of an HSCT because of many factors which affect the cost. Factors include the country where HSCT is performed, the type of donor, preconditioning regimen, potential complications, and out-of-pocket expenses.
The cost of HSCT in the United States is often underestimated since categories such as outpatient medication, home infusions, donor search, graft procurement, and physician charges are not considered when calculating the total cost of the procedure. Most studies evaluating the cost of HSCT only focus on the short-term costs of the procedure. For this reason, there is not enough evidence to determine the financial impact of long-term care or chronic GVHD [97]. Most costs associated with transplantation occur within 5 months after the transplant [98].
Duration of hospital stay: one of the main determinants for the cost of HSCT is how long the patient stays at the hospital, as it has been reported that 80% of the total cost for an HSCT, 1-year post-transplant, is the length of stay at the hospital [23, 99, 100].
Donor: the cost of HSCT also depends on the type of donor source used. The reason for the cost difference between different donor types is the disparity in cost for cell acquisition. There is a price difference between UCB and MRD because UCB takes a longer engraftment period and has a higher graft failure rate. This disadvantage translates into longer hospital stays for the patients, which increases the total cost [101]. Cell acquisition of matched related donors includes donor evaluation, apheresis procedure, graft processing, and storage. Cell acquisition from UCB donors includes searching the cord blood bank for a suitable match, confirming HLA-typing in the donor, and shipping of cord blood. With a median cost of $69,000, the acquisition of UCB is significantly more expensive than cell acquisition from either unrelated donors or matched related donors. Cell acquisition from matched related donors is the least expensive option with a median cost of $9,500 [101].
There is also a cost difference between PBSCT and BMT, as well as between myeloablative conditioning regimens and reduced-intensity conditioning regimens [99]. Those using a non-myeloablative regimen have a shorter initial hospitalization period than those who receive a myeloablative regimen. However, over time, patients using a non-myeloablative regimen end up having to be hospitalized more than the patients using the myeloablative regimen since they have more complications such as secondary occurrences of GVHD or infections. Non-myeloablative HSCT patients usually end up spending significantly more money 6–12 months after transplantation because of the costs associated with complications and re-admissions [99].
Another factor to consider are complications that could occur as a result of the procedure; some complications include GVHD, urinary tract infection (UTI), sepsis, or pneumonia, which can incur additional costs [102].
The location where HSCT is performed; In 2006, a study in Sweden reported that HSCT costs between $145,000 and $182,000 with related and unrelated donors, respectively [103, 104]. In 2007, the 1-year median cost of HSCT in Thailand was reported to be $23,000 [103, 105]. In 2015, for the allogeneic transplant in pediatric patients, the median cost in Mexico was $13,000 and the total cost 1-year after the transplantation was $16,000, which included follow-up and out-of-pocket expenses. The median amount of inpatient days was 6 days, and the median cost was $1,400. The average for out-of-pocket expenses was $2,000 [23]. In 2016, Gale et al. studied the cost of HSCT in Latin America, suggesting the cost ranges from $25,000 to $75,000 [106]. The reported cost per HSCT in Japan is between $70,000-$205,000 [13, 47, 49, 107–109].
Costs for ERT of MPS patients are generally much higher. The annual reported cost of ERT for MPS I patients was $218,000 for a 25 kg patient in 2017 [110] (Table 8). The yearly cost for an MPS II patient was $340,000 [110]. The annual cost for an MPS IVA, MPS VI, and MPS VII patients was $578,000, $476,000, and $550,000, respectively [110].
Table 8.
| Recombinant enzyme deficient | Drug name | Dosage (mg/kg of body weight) once a week |
|---|---|---|
| Larondiase | Aldurazyme | 0.58 mg/kg |
| Idursulfase | Elaprase | 0.5 mg/kg |
| Elosulfase alfa | Vimizim | 2 mg/kg |
| Galsulfase | Naglazyme | 1 mg/kg |
| Vestronidase alfa | Mepsevii | 4 mg/kg bi-weekly |
Note; Compared with other countries, the overall annual cost for ERT differs slightly depending on the country, for example, the cost of ERT annually in Japan for a 25 kg MPS II patient is $400,000 [13].
2.5. Combination of ERT and HSCT
The combination of both ERT and HSCT is a newer, more effective approach in minimizing clinical manifestations of MPS, as well as for improving some of the patients’ clinical manifestations before transplantation [44, 111, 112]. In Australia, guidelines already suggest that patients with MPS I should receive ERT up to 12 weeks before their HSCT and up to 15–17 weeks after their transplant [2, 113]. ERT can improve some pre-transplant conditions for the recipient, which lead to better results overall, [24, 54] such as reduced respiratory and cardiac manifestations. ERT does not affect the overall engraftment of HSCT [42, 44, 54, 67, 111, 114–118]. The combination of ERT with HSCT decreases transplant-related complications for MPS I patients [119, 120] and may be able to reduce mortality caused by the transplant itself [44, 111, 112]. The use of ERT pre-transplant gives the patient time to find a donor without worrying about the worsening of the disease [120]. However, there is concern that ERT could potentially initiate an antibody response in the patients, which could, in turn, affect the outcome of the HSCT [44, 111, 112].
Since HSCT cannot entirely correct secondary musculoskeletal disorders [120], but can improve cognitive and central nervous system functions [8, 55, 64, 121–126], the combination of ERT with HSCT can result in better outcomes than either of the treatments would produce alone.
In 2010, Whitley et al. [127] reported the outcomes of a male with MPS VI who had received HSCT at 18 months of age from his HLA-identical sibling who was a heterozygous carrier. Twenty years after the transplant, the individual was still fully engrafted but had symptoms of progressive corneal opacification. The patient was then given galsulfatase via intravenous infusion at a dose of 1mg/kg. As a result of the ERT treatment, urinary GAG levels were reduced from 7.63 mg GAG/mmol creatinine to 4.4 mg GAG/mmol creatinine, just 10 days after treatment. These results are surprising for a one-time treatment of galsulfatase and could suggest the possible use of ERT with HSCT for a better outcome on MPS VI patients [127]. In 2014, Ferrara et al. reported that ERT administered pre-transplant reduced the amount of soft tissue forming around the odontoid [120]. This excess of soft tissue causes dural and cord compression in the spine. Magnetic resonance imaging (MRI) after transplantation showed a reduction of pressure in the spine. Based on this study, Ferrara et al. suggest that ERT should be given to MPS I patients pre-transplant, during marrow aplasia, and during post-transplantation until the recipient has successful engraftment [120].
Furthermore, in 2016, Ghosh et al. investigated the effect of the combination of ERT and HSCT in MPS I patients in two centers, University of Minnesota and the Royal Manchester Children’s Hospital, between September 2004 – June 2014 [112]. Patients began ERT treatment as soon as they were diagnosed with MPS I and continued until successful transplantation. All of the patients received 0.58 mg/kg of laronidase weekly. Patients at the University of Minnesota’s Division of Pediatric Blood and Marrow Transplantation received ERT an average of 8 weeks post-transplantation, while patients at the Royal Manchester Children’s Hospital ended laronidase treatment after they became engrafted. The median number of doses of laronidase given pre-transplantation was 13 doses. The overall survival rate in the study was 86%, and the overall event-free survival rate was 80%. ERT reduced urinary GAG levels, which were further reduced after HSCT. The continuous decrease of urinary GAG levels could be attributed to substrate reduction after transplantation, which has been reported by previous studies [51, 112]. Results from the study show that the combination of both HSCT and ERT did not decrease the rates of GVHD in MPS I patients.
In 2017, Lum et al. found that 52.2% of HSCT patients who reported normal/mild cardiac valvular insufficiency had received pre-transplant ERT [94]. None of 6 HSCT patients who reported moderate valvular insufficiency had pre-transplant ERT, and only 1 of 4 HSCT patients with severe valvular insufficiency/cardiomyopathy had pre-transplant ERT [94]. These data support the claim that pre-transplant ERT can help reduce cardiac manifestations of the disease when compared with HSCT alone.
Two case reports of treatment of MPS VI reported positive conclusions on the benefits of combining ERT and HSCT. One case reported positive results in respiratory function, hepatosplenomegaly, and joint range of motion by using the combination of ERT and HSCT for a 3-year old girl with MPS VI. However, the girl’s musculoskeletal manifestations and cardiac valve disease continued to worsen [128, 129]. A different case report claimed that ERT administered 10 years after an MPS VI individual received HSCT could improve joint range of motion and endurance [128, 130].
3. HSCT for each type of MPS
3.1. MPS I
MPS I is caused by the deficiency of the alpha-L-iduronidase (IDUA) enzyme leading to the accumulation of heparan sulfate (HS) and dermatan sulfate (DS) [84]. Clinical manifestations of the disease include neurocognitive, orthopedic, cardiac, and pulmonary symptoms. As a result of these manifestations, the median age of survival of untreated MPS I patients is 6.8 years of age [1, 84, 131]. HSCT has been shown to modify the course of disease in MPS I patients, as well as increase the patient’s lifespan and improve clinical parameters [21, 31, 38, 94, 120]. For this reason, HSCT is an effective treatment for MPS I [120], recommended by the EBMT for MPS I patients younger than 2.5 years [117].
Successful engraftment of donor cells improves many clinical manifestations of MPS I, such as obstructive airway disease, hepatosplenomegaly, cardiovascular functions, hearing, vision, linear growth, etc. HSCT also stabilizes or prevents hydrocephalus and prevents the deterioration of psychomotor functions [21, 31, 38, 85], as well as improves cognitive function and CNS manifestations [8, 21, 31, 36, 38, 55, 64, 85, 87, 120–126, 132].
In 2015, Aldenhoven et al. reported a long-term study of 217 MPS IH patients [38]. Patients were followed for a median time of 9.2 years after treatment. The study examined the long-term effects of HSCT in more than 70% of all the MPS IH patients who were transplanted successfully. Some of the overall outcomes include significant cognitive development after transplantation for those who underwent transplantation earlier in life. Many of the individuals achieved normal enzyme levels, which suggest HSCT will greatly improve possible organ manifestations of the disease. However, there were reports that even after transplantation, some patients experienced continued manifestations of the disease [21]. Table 3 provides additional details about the report.
Table 3.
MPS I with HSCT
| Author | Details | Results |
|---|---|---|
| Yasuda et al. 2015[137] |
|
|
| Aldenhoven et al. 2015 [21, 38] |
|
|
| Rodgers et al. 2017 [84] |
|
|
| Lum et al. 2017 [82] |
|
|
| Lum et al. 2017 [94] |
|
|
Some of the most critical factors that contribute to the overall outcome and long-term effects of HSCT in MPS IH patients are the individual’s baseline status before transplantation, the age at which the recipient is transplanted, and enzyme levels achieved post-HSCT. Enzyme levels after transplantation can be a good predictor of the success of HSCT, except concerning neurodevelopmental outcomes. The most important factor that determines the level of neurocognitive outcomes after transplantation is the severity of damage in the CNS before transplantation. Factors that contribute to a normal enzyme level after transplantation are cells from non-carrier donors and full-donor chimerism [28, 38, 51].
HSCT cannot alter any pre-existing CNS symptoms of MPS I [31, 38, 85]. However, if HSCT is performed early enough, it is possible to preserve neurocognitive function in patients [10, 38, 42, 112, 117, 133]. HSCT can be a preventive treatment for the deterioration of the psychomotor system, thus, allowing for better neurodevelopment in the patient [31, 38, 85]. In 1998, a study of MPS I patients treated before the age of 2 responded more favorably to HSCT if their intellectual quotient (IQ) was more than 70 at transplantation than if it was lower than 70 [13, 36]. It is imperative that HSCT is performed at the earliest time possible because it takes considerable time, about a year, for donor-derived cells to enter the brain and replace the existing microglial cells [88]. This delivery delay might be the cause of the corresponding slow improvements or even worsening of the CNS symptoms in some cases [31].
The effectiveness of the transplantation depends on the amount of enzyme activity that results after transplantation. In 2012, one study showed that the effectiveness is poor for patients with Hurler syndrome who have low enzyme activity after HSCT [49, 134]. In 2015, another study reported better results from HSCT overall for recipients who have donor chimerism greater than 50%. It is very likely that the enzyme level achieved may be a better predictor for the outcome as shown by Pievani et al. [135, 136].
Although HSCT improves CNS manifestations of the disease, it does not seem to correct these manifestations entirely and cannot correct corneal clouding because of insufficient delivery of enzymes to the eye [136]. HSCT could not repair the preexisting or progressive bone and cartilage manifestations of the disease in MPS IH patients, even when performed between 1 and 2 years of age [136]. Since HSCT cannot correct secondary musculoskeletal disorders, surgeries are often needed to fix genu valgum, thoracolumbar kyphosis, hip dysplasia, carpal tunnel disease, as well as ribcage, finger, wrist, knee, and tibia irregularities [8, 55, 87, 121–126]. Cervical or lumbar stenosis which results in spinal cord compression is often observed during the second decade of life after transplantation [136].
In 2015, Yasuda et al. [3, 137] examined the skeletal manifestations of an MPS I patient with HSCT. Table 3 provides additional details about the report. Ten years after HSCT, characteristics of the spinal manifestations of the disease persisted and resulted in the need for surgery. His activity of daily living remained stable and after a series of surgical procedures, he became ambulant and independent in daily activities. The GAG levels in blood were normal. Later pathology revealed no vacuolization in chondrocytes with normal size [3]. Overall, skeletal abnormalities in this patient are much milder compared with untreated patients with MPS I.
In 2017, a long-term study demonstrated that even after HSCT, reports of cardiac and pulmonary manifestations of the disease are still present in patients. These manifestations range from mitral and aortic valve insufficiency, mild cardiomyopathy, and arrhythmia. Some of the individuals needed angiotensin-converting enzyme (ACE) inhibitors or cardiac surgery. A lower percentage of patients had hypoxia or needed respiratory support [94]. Although HSCT cannot fix all cardiac manifestations of MPS I, a correlation has been found between higher enzyme levels and higher donor chimerism with better cardiac outcomes after transplantation [94]. This correlation probably explains why MPS I patients who have received cells from cord blood donors have improved quality of life [138].
In 2017, Rodgers et al. investigated the long-term effects of HSCT on 134 MPS IH patients [84]. Table 3 provides additional details about the report. The survival rate of the patients was 70% one-year after transplantation. The survival rate 10-years post-transplantation was 62%, and the survival rate 25-years post-transplantation was 37%. Males had a better survival rate 25-years post-transplantation, compared to females. This higher rate of death for females could be caused by pulmonary complications or infection, which occurred more readily in females.
In 2018, Walker et al. reported that the incidence of airway complications was 14% in MPS I with HSCT, compared with 57% for those patients with MPS I treated with ERT [139]. Frawley et al. have also reported similar findings in a smaller group of post-HSCT MPS IH patients [140]. Additionally, another study reported a 0% failed intubation rate in MPS I patient who had previously received HSCT [141]. Therefore, HSCT significantly improves the ease of airway management during anesthesia and the safety of anesthesia.
It is important to note that preferred treatments changed significantly between 1983 and 2013. These changes included increased targeted busulfan dosing, reduced used of TBI/total lymphoid irradiation, increased use of UCB, and the use of peri-HSCT ERT. As a result, the percentage of patients who had undergone HSCT and reported normal IDUA enzyme levels increased after 2004. Survival rates also increased after 2004, indicating that longer-term survival rates are likely to improve. Even after the new regulations were in place, more females have died (n=5) compared to males (n=2). Promising results in pre-clinical studies using ex-vivo gene therapy on MPS I and MPS IIIA mice have led to phase I/II clinical trials of ex-vivo therapy being performed on MPS IH and MPS IIIA patients [142]. The goal of these trials is for patients to express the supranormal amount of enzymes for these enzymes to have a greater effect on the clinical manifestations of the disease.
Since MPS I is included in NBS programs in several states of the United States, some European countries, and Taiwan, patients can be treated within the first few months of life, which is expected to minimize most of the limitations seen in patients treated at later disease stages.
3.2. MPS II
Patients with MPS II have a deficiency of the enzyme iduronate-2-sulphatase (IDS), which causes the accumulation of undegraded DS and HS [2]. Major clinical manifestations of the disease are due to the accumulation of GAGs in the CNS, skeletal system, and visceral organs [13, 27, 49, 143]. During the first several years of life, MPS II patients can exhibit overgrowth in both height and body weight [144]. The most serious damage to cognitive and motor function [13, 27, 49, 143] as well as to skeletal, pulmonary, and cardiac function [145], occurs in patients with the severe phenotype. Patients with the attenuated phenotype of MPS II may also develop mild neurological symptoms and retinal deterioration, later in life [13]. ERT cannot treat the CNS symptoms of those with the severe phenotype since the infused enzyme cannot cross the BBB [10, 146, 147]. However, ERT is still the recommended treatment option for those with MPS II to improve organomegaly since it is less invasive with feasibility and has fewer risks [13, 107, 108]. In 2010, it was reported that HSCT of MPS II patients led to the appearance of donor cells in the microglia 10 months after transplantation [27, 103], while other studies have questioned the effectiveness of HSCT to alter the course of neurological decline in MPS II patients.
HSCT decreases urinary and blood GAG levels [10, 148] more than ERT, and normalizes or stabilizes the IDS enzyme activity in leukocytes [13, 149]. HSCT has been successful, not only in improving disease manifestations and slowing the overall disease progression, but also in correcting most of the disease manifestations [6, 45, 49, 53–56, 58]. It is notable that there is no question that HSCT improves somatic and skeletal symptoms of the disease [2, 52, 149, 150]. HSCT has been deemed a treatment option for patients in Japan and China with MPS II [13, 37, 45, 49, 53, 103]. More recently, Brazil has changed their guidelines for HSCT which will make the treatment accessible and efficient for MPS II patients [103, 151]. However, HSCT is not used regularly for MPS II patients in the United States at present [24, 29, 152]. In 2012, Tanaka et al. performed a study evaluating the long-term effects of HSCT [49]. Table 4 provides additional details about the report. Overall findings suggest that HSCT should be considered an effective treatment option for MPS II patients if performed before brain atrophy and heart valvular regurgitation are present. The reported 5-year survival rate after HSCT was 88.5%. Evaluated categories included ADL, IQ/DQ, FIM, Brain MRI, valvular regurgitation, and urinary GAG levels, which were measured at baseline and the most recent follow-up visit [49]. It is important to note that those patients with severe MPS II had low IQ/DQ scores at their baseline report, further suggesting that HSCT does not effectively impact brain involvements of MPS II if developmental delays are already present before transplantation. Brain MRIs demonstrated that category I and III brain lesions were positively altered by HSCT. Category I brain lesions are caused by the enlargement of perivascular spaces from GAG-loaded cells. Category III brain lesions are caused by insufficient cerebrospinal fluid (CSF) absorption or by brain atrophy. Two patients with the severe phenotype of MPS II showed deterioration in their ADL, IQ/DQ, FIM, and brain MRI questionnaires. Some results of this study question the long-term efficacy of HSCT for MPSII patients. Based upon the decline of DQ scores, HSCT might not improve brain manifestations of the disease in some patients. Category IV lesions, which are caused by neuronal cell loss, continued to progress in 6 patients. These results could be because donor cells cannot reach deep brain tissue, but they also demonstrate that HSCT does not improve brain MRI findings in more severe cases [49]. After HSCT, 32% of the patient’s heart valves had less valve regurgitation, and 56% of patients’ valves became stabilized. Valvular insufficiency is the most significant cause of death for MPS II patients [49]. MPS II patients usually experience overgrowth in height and weight during their early years, but by age 14, patients begin to report below average height compared to the age-matched control group. In 2014, Patel et al. reported that HSCT allows for similar improvement of growth in long bones compared to ERT in MPS II patients [53, 153].
Table 4.
MPS II with HSCT
| Author | Details | Results |
|---|---|---|
| Tanaka et al. 2012 [49] |
|
|
| Tanjuakio et al. 2015 [45] |
|
|
| Yasuda et al. 2015 [3, 137] |
|
|
| Kubaski et al.2017 [13, 27, 49] |
|
|
| Barth et al. 2017 [10] |
|
|
In 2015, Tanjuakio et al. compared the effectiveness of ERT and HSCT in Japanese MPS II patients, mostly comparing the ADL [45]. Table 4 provides additional details about the report. It was observed that those with the severe phenotype of MPS II had more clinical manifestations of the disease in cognitive function than those with the attenuated phenotype of MPS II. Additionally, those with the severe phenotype of MPS II reported a decrease of ADL scores over time [3, 12]. ADL scores tended to be higher in patients treated with HSCT compared with patients treated with early ERT. Results from the study also suggest that patients treated as a later age had a better ADL score if treated by HSCT than if treated by ERT. Individuals who had HSCT early in their life had the highest ADL scores. Furthermore, those who received HSCT scored substantially better on ADL score in the “movement” and “movement and cognition” categories. However, their “cognition” score remained low [45].
In 2016, Wang et al. reported improvements in speech and neurological symptoms of the disease as a result of HSCT [13, 18]. These benefits for the severe phenotype allow MPS II patients to continue to develop cognitive, adaptive, and language skills. HSCT patients still develop these skills at a slower pace; however, patients showed a significant improvement compared to untreated patients [10].
In 2017, Barth et al. reported a relatively stable IQ of a seven-year-old patient who had transplantation at 2 months of age [10]. This improvement in his cognitive function and skeletal manifestations allowed the patient to attend regular school. The patient is still behind in school compared to others at the same age, but he can gain skills and interact well with his peers. The improvements observed have also been supported through the Pediatric Evaluation of Disability Inventory, where he scored 156 out of 197 in the skills section. This score is considered very good for an individual with MPS II. The boy’s ADL score was 43 out of 60, which is higher than those with severe MPS II [10].
HSCT can improve hearing for those with MPS II if the procedure is performed before the patient is 25 months of age [10, 154]. The patient described by Barth et al. developed sensorineural hearing loss, but it is important to point out that he had cytomegalovirus (CMV) infection 75 days after transplantation, for which he received ganciclovir at 10 mg/kg/day for 6 months. He also presented an upper airway disease in the newborn period, treated with gentamicin. Possible toxicity of these drugs contributing to his hearing loss cannot be ruled out [10, 154].
In 2017, Kubaski et al. [13] reported the effects of HSCT in 146 MPS II patients (27 new cases and 119 previously published) compared to 51 patients treated only by ERT and 15 untreated cases [13]. Table 4 provides additional details about the report. There were more positive outcomes in the long-term evaluation of HSCT in MPS II patients, compared with those with ERT alone. Overall, HSCT provided an impact on brain involvements when the transplantation took place before developmental delay symptoms presented themselves.
The report suggests that HSCT could even be an effective treatment option for older MPS II patients. Some reports indicate that HSCT might even be as effective for those with MPS II asit is for those with MPS I [13, 27, 149].
It is important to mention that most studies published are limited by the age at transplantation. It is not possible to measure the precise benefits of HSCT in CNS if symptoms are already present before the procedure. Further studies in younger patients will elucidate if HSCT can improve and/or prevent CNS decline in MPS II. Taiwan is already screening for MPS II at their NBS program which will likely help to clarify the benefits of early HSCT.
3.3. MPS III
MPS IIIA and MPS IIIB are caused by a deficiency of heparan sulfate N-sulfamidase and N-acetylglucosaminidase, respectively [155]. Patients with MPS III usually exhibit neurocognitive impairment by four years of age. There have been very few reports of MPS III patients receiving HSCT, with mixed reports of effectiveness.
In 1995, Klein et al. [156] reported that HSCT was unsuccessful in preventing neurocognitive symptoms for a patient who received transplantation after these neurocognitive symptoms were present. In 1992 and 1999, HSCT was performed on two MPS IIIB patients (less than 2 years and less than one year of age) [157, 158]. These patients did not exhibit any neurocognitive symptoms before the transplantation. However, the transplantation was unsuccessful in preventing neurocognitive decline. In 2008, Prasad et al. reported that HSCT performed early in MPS III patients can have some positive effect on neurocognitive decline [25]. In 2014, it was reported that an MPS IIIB patient received HSCT at 1 year and 10months, and the treatment was deemed successful in improving clinical manifestations of MPS IIIB. At age 15, her disease symptoms were good compared to untreated MPS IIIB patients, and she had normal blood levels of HS-0S and HS-NS [148].
In 2014, Welling et al. reported a 5-year follow up study on two MPS III patients, one severe phenotype and one attenuated phenotype, after early UCBT [159]. The UCBT did not prevent the neurological effects of the disease, even though the transplantation was performed before both of the patients exhibited any neurological symptoms. Symptoms that developed included decreased cognitive skills and behavioral disturbances. Two years after HSCT, the concentration of HS in CSF in the patient with the attenuated phenotype of MPS IIIB remained very high and in the range of untreated MPS III patients. However, five years after transplantation, urinary GAG levels were normal for both patients.
Overall, HSCT for MPS III has not yet shown definitively positive results. Because of the difficulty of early detection, there are no reports of MPS III patients treated with HSCT within the first months of life.
3.4. MPS IVA
MPS IVA is caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS), leading to the accumulation of chondroitin 6-sulfate (C6S) and keratan sulfate (KS) [1, 58, 144, 160, 161]. Compared to other MPS types, no mental impairment is associated with MPS IVA. However, one of the unique manifestations of MPS IVA is hypermobility of joints [55, 161–163]. In 2014, Chinen et al. reported an MPS IVA patient with an allogeneic BMT at 15 years and 8 months of age [12]. Table 5 provides additional details about the report. Five years after the HSCT procedure, the patient’s GALNS enzyme activity in lymphocytes had reached the donor’s level, resulting in several clinical improvements and an increase in bone mineral density [3, 12].
Table 5.
MPS IVA with HSCT
| Author | Details | Results |
|---|---|---|
| Chinen et al. 2014 [3, 12, 58] |
|
|
| Yabe et al. 2016 [19] |
|
|
| Wang etal. 2016 [18] |
|
|
In 2016, Yabe et al. [19] reported successful transplantation in four MPS IVA patients (one patient from Dr. Chinen see above), who received allogeneic BMT. Table 5 provides additional details about the report. All four patients achieved full engraftment, two of whom achieved normal enzyme activity. HSCT improved the clinical course of MPS IVA. ADL scores were significantly improved after HSCT and remained high thereafter. Only one patient required bilateral osteotomies after transplantation. Additionally, HSCT reduced the bone manifestations, as well as lessened the impact on growth and laxity of joints for those with severe MPS IVA. Orthopnea and loud snoring ceased after HSCT, and overall respiratory function in the patients was improved. From the successful results of these four patients, Yabe et al. have deemed HSCT a potential treatment option for those with MPS IVA [19].
In 2016, Wang et al. [18] reported on MPS IVA patients treated with HSCT in China. Improvements seen in one of the patients included reductions in joint hypermobility, hepatosplenomegaly, upper-airway obstruction, and recurrent otitis media. There were small improvements in height and thoracic deformity. After transplantation, the patient’s spinal cord compression was stabilized. The patient needed surgery 1-year after transplantation for genu valgus [18]. At the time of this report, HSCT had been used to treat 9 patients with MPS IVA (1.5–8 years; median age, 3 years) at Shanghai Children’s Medical Center. All patients achieved full donor chimerism with the normal enzyme activity without severe complications and were alive with significant improvement in reductions in joint hypermobility. Two patients underwent surgery for genu valgum, spinal cord compression, and hip dislocation. No long-term post-transplantation results are available yet.
3.5. MPS VI
MPS VI is caused by a deficiency of N-acteylgalacosamine-4-sulfatase (ARSB), which causes accumulation of DS and CS [1, 2]. Incorrect diagnosis of MPS VI patients has been reported, as some individuals who do not have MPS VI can have elevated total urine GAG levels similar to those of age-matched patients with MPS VI [148, 164]. The symptoms of the disease present skeletal manifestations, including dysostosis multiplex [127], but no mental impairment [1, 2]. Other symptoms of MPS VI include progressive arthropathy, hepatomegaly, pulmonary disease, cardiac abnormalities, corneal clouding, and cervical cord compression [127]. The most serious complications are cardiopulmonary complications, which most MPS VI patients die from during the second decade of their life [165–167]. Although ERT is the first recommended treatment option for those with MPS VI, ERT has had little success in reducing the cervical compression of the spinal cord [127, 168, 169]. The reported effectiveness of HSCT on those with MPS VI is varied [6, 61, 128]; therefore, HSCT is only recommended for MPS VI patients after the use of ERT has been found ineffective [7, 86, 128]. HSCT has increased survival in MPS VI patients as well as improved joint movement and cardiopulmonary function [6, 7, 61, 165].
An increase of enzyme activity and a decrease in urinary GAG levels are some of the results of HSCT for MPS VI [63, 167, 170]. HSCT can decrease the overall progression of the disease with regard to skeletal manifestations, but there have been some reports that it does not alter the progression of dysostosis multiplex [3, 11, 63, 171, 172], musculoskeletal complications, and corneal clouding [99, 128, 173, 174]. Additional positive effects of HSCT on individuals include improved endurance, joint mobility, puberty and growth, pulmonary/airway function, facial features, hepatosplenomegaly, and survival [17, 170, 175]. Although those with MPS VI do not normally have any intellectual disabilities [167, 170, 176], HSCT has been shown to reduce CNS abnormalities [6, 170, 172, 175, 177]. It remains unclear whether HSCT can substantially improve cardiac dysfunction and short stature in patients [165], although HSCT does provide some impact with regard to those symptoms. In 2013, one study suggested that haploidentical stem cell transplants are an effective donor source for MPS VI individuals. Two siblings with MPS VI had unrelated UCB transplants, which both resulted in graft failure. Later, the two individuals then received haploidentical stem cell transplants from their father’s peripheral blood. Both individuals had successful engraftment and achieved donor chimerism, although one sibling had a mixed B cell chimerism [170].
In 2017, Behfar et al. [165] reported the results of HSCT for 3 severe MPS VI patients who had non-sibling donors. Table 6 provides additional details about the report. The results conclude that the combination of a myeloablative conditioning regimen with either peripheral blood donor or cord blood is an effective pre-conditioning regimen and donor source combination for providing patients with a continuous enzyme level [6, 61, 165, 178]. Two of the patients achieved a chimerism level of over 95% after transplantation. Patient 1 received donor cells from his carrier mother through a BMT and had an increase in his enzyme levels. Patient 2 received a PBSCT from his grandmother and developed acute GVHD grade II after transplantation. Results from the transplantation included a normal size spleen and liver and an improved walk test, although mild tricuspid regurgitation and mitral regurgitation remained after transplantation. Patient 3 received HSCT from an unrelated cord blood donor that was 5/6 HLA-matched but experienced graft failure. Unfortunately, the patient died of pneumonia 11 months after transplantation. Overall, there is a need for more studies on HSCT with younger MPS VI patients to determine if HSCT is a better treatment option for MPS VI patients compared to other treatment options [165].
Table 6.
MPS VI with HSCT
| Author | Details | Results |
|---|---|---|
| Turbeville et al. 2011 [17] |
|
|
| Behfar et al. 2017 [62,165] |
|
|
3.6. MPS VII
MPS VII is caused by a deficiency of β-glucuronidase (GUSB), leading to the accumulation of DS, HS, and CS. Cognitive impairment to various extents is one of the manifestations of the disease [2, 179–182]. HSCT has been reported to be successful in patients with MPS VII regarding slowing the overall progression of the disease [20, 180, 182].
In 1998, Yamada et al. reported on a HSCT case of a 12-year-old girl who received a BMT from an HLA-identical unrelated female [20]. Table 7 provides additional details about the report. The patient presented multiple symptoms of the disease pre-transplantation and was wheelchair-bound due to the worsening of bone symptoms. [20].
Table 7.
MPS VII with HSCT
| Author | Details | Results |
|---|---|---|
| Yamada et al 1998 [20] |
|
|
| Montano et al. 2016 [180] |
|
|
| Sisinni et al. 2018 [182] |
|
|
After the BMT, uronic acid levels decreased, and the patient’s motor function, shortness of breath while moving, recurrent infections, snoring, and quality of life all improved posttransplantation. The BMT did not reverse the preexisting neurological damage, but the patient’s cardiac manifestations were stabilized.
In 2016, Montaño et al. reported the outcomes of five MPS VII individuals who received HSCT [180]. The first patient received HSCT at the age of 2 years, but the HSCT failed. At the age of 4 years, the patient had successful engraftment, with mild symptoms of the disease present. However, there is no report on the outcome of the disease. The second patient had received an HSCT at 7 years of age, with severe manifestations of the disease already present and died due to transplant-related causes. The third patient received HSCT but the age at which the transplantation occurred is unknown. The patient died a couple of years after the procedure. The fourth patient received an HSCT at the age of 3 years, with symptoms of the disease already present. At the age of 15 years, the boy still only exhibited mild symptoms of the disease. However, a more recent exam reported that manifestations of the disease had progressed severely. The fifth patient received HSCT at the age of 7 months, with some symptoms of the disease present, including fetal cardiac distress, hydrops fetalis, and tachycardia. At one year of age, the patient was meeting appropriate developmental milestones for her age. At 15 months, the patient did not have any clinical manifestations of the disease, and her hepatomegaly had digressed. Sisinni et al. reported successful HSCT results after initial graft failure on a two-year old MPS VII patient. Initially, a RIC regimen and BM from a matched unrelated donor. During the second transplantation, a myeloablative conditioning regimen was used with a matched unrelated cord blood donor. Six-years after the transplant, the patient had normal motor function, no neurological symptoms, and stabilized skeletal dysplasia [182].
These limited reports on MPS VII patients who have undergone HSCT giving variable outcomes make it difficult to make any conclusions about the suitability of this treatment for MPS VII.
4. Cost of HSCT vs. ERT
We have described the cost of HSCT in the previous sections. When compared to ERT, HSCT is a much more cost-effective treatment option. HSCT and ERT are both expensive treatments for MPS, but ERT is more expensive than HSCT because of the nature of the weekly or bi-weekly regimen that is required for the patient’s lifetime. The exact price for ERT varies for each type of MPS. Table 8 illustrates approximate costs of ERT for each type of MPS in the United States.
5. Future of HSCT
Future breakthroughs in HSCT will result in the use of the patient’s cells modified by gene therapy for HSCT. Currently, clinical trials for different types of MPS are being performed in the United States, Europe, and Australia [183–189]. For MPS patients, bone marrow, myoblast, and/or fibroblast cells would be taken from their body, altered to repair or add back the affected gene, and then those modified cells would be returned to the patient’s body. This process is called ex vivo gene therapy. After the cells are returned to the patient’s body, the promise is for the cells to then cross-correct all other cells in the body. The major benefit of using the patient’s cells is the chance for less adverse effects such as GVHD; however, there is still a need for a conditioning regimen [142]. The most promising vector used to alter patient’s cells is the lentivirus vector as reported by Visigalli et al. in MPS I mice [190]. Use of the lentivirus vector allows for transduction of the deficient enzyme (IDUA) for cells including non-diving cells, resulting in significant increases in enzyme activity [190].
In 2018, at San Raffaele Scientific Institute, Milan a Phase I/II clinical trial has been opened to evaluate the safety, tolerability, and efficacy of autologous, IDUA LV-transduced CD34+ cells in 6 patients with MPS IH undergoing myeloablative conditioning [191] (https://clinicaltrials.gov/ct2/show/NCT03488394). To accelerate hematopoietic recovery, the trial introduces G-CSF/plerixafor mobilized peripheral blood as a stem cell source, which are engineered according to a novel, shortened ex vivo manipulation protocol featuring prostaglandin E2 to boost transduction efficiency [192]. Moreover, the genetically-engineered cell product is cryopreserved, allowing its characterization before conditioning the patient and facilitating its future application in multi-center trials. The primary endpoint of efficacy is represented by IDUA activity in peripheral blood of the patients up to supraphysiologic levels at one year posttreatment. Patients included will lack a non-heterozygous (for mutated IDUA) HLA-matched sibling donor or a ≥7/8 HLA-matched cord blood donor with good cellularity after 1-month search and will have an IQ/DQ≥70. The first treated patient shows encouraging preliminary results.
6. Discussion
HSCT has been shown to provide effective results for patients with MPS. However, the risks associated with HSCT have limited the use of HSCT as a therapeutic option for MPS. Recently, increased awareness of the disease and improved medical technology have allowed for the chance of transplantation at an earlier age and have increased safety associated with the procedure.
With HSCT, the enzyme is expressed and circulated infinitely in the recipient’s body. GAGs can be rapidly cleared [2]. Current advantages of HSCT include its ability to correct certain disease symptoms which cannot be fixed with current intravenous ERT, its penetration of BBB via microglia cells, low cost compared to ERT, and that it is a one-time procedure if successful. With advancing procedural technology and new guidelines in place, HSCT has reduced mortality rates and could be considered a therapeutic option even for those with an attenuated phenotype of MPS. The use of HSCT in combination with ERT has also shown significant results in both improvements of disease and decreased mortality rates. Another advantage of HSCT is the possibility to resolve immune response such as a high-titer antibody against ERT by replacement of the recipient cells with the donor cells [2, 3, 13, 21, 27–36, 38, 44].
HSCT corrects or improves disease symptoms such as joint mobility, vision, hearing, cardiopulmonary functions, coarse facial features, upper airway and respiratory functions, and hepatosplenomegaly [2, 5–8]. Table 9 summarizes HSCT recommendations for each type of MPS.
Table 9.
Summary of clinical effect by HSCT
| Type | CNS | Bone growth | Air way | Joint mobility; rigidity | Heart | Cornea | Liver, Spleen | Overall clinical effect | Recommendation | Condition | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MPS T | ++ ~ +++ | ++ | +++ | Upper joints: ++ ~ +++ Lower joints: + ~++ | ++ ~ +++ | − ~+ | +++ | ++ ~ +++ | Yes | Patients under 2.5 years of ag. Elimination of immune response against infused enzyme with ERT | Approved and/or conducted as standard of care |
| ref | [38] | [136] | [142–144] | [5,8, 21,64,87, 122, 124, 200, 201] | [5,8, 21, 87, 202–204] | [136] | [5,8, 21, 87, 202–204] | Better than ERT | |||
| MPS II | + ~ ++ | ++ | ++ | ++ | ++ | NA | +++ | ++ | Yes | Early stage. Elimination of immune response against infused enzyme with ERT | Approved and/or conducted in Japan, China, Brazil, Europe, and USA. Reported impact on CNS although less than MPS I |
| ref | [49] | [3, 12] | [18] | [13] | [149] | [149] | Better than ERT | [13,37, 45, 49, 53, 109] | |||
| MPS III | - | NA | NA | NA | NA | NA | NA | - | No | No significant positive effect; insufficient information | |
| ref | [155] | [158] | |||||||||
| MPS IVA | NA | + | ++ | hypermobile joint; + ~ ++ | ++ | unknown | +++ | ++ | Yes | Optional treatment at an early stage. Elimination of immune response against infused enzyme with ERT | Approved and/or conducted in Japan and China |
| ref | [12, 19] | [12, 18, 19] | [12,18, 19] | [12, 18, 19] | [12, 18, 19] | Better than ERT | No criteria | ||||
| MPS VI | NA | + ~ ++ | ++ | + ~ ++ | ++ | + ~ ++ | +++ | ++ | Yes | Optional treatment. Elimination of immune response against infused enzyme with ERT | Approved and/or conducted in Japan, China, Europe, and USA. |
| ref | [172] | [17, 170,172, 175] | [6,7, 61, 164] | [164] | [97,127, 172, 173] | [3] | Similar to ERT | No criteria | |||
| MPS VII | ++ | ++ | ++ | + ~++ | ++ | unknown | +++ | ++ | Yes | Optional treatment. Elimination of immune response against infused enzyme with ERT | A few reports with significant improvements |
| ref | [20, 180, 182] | [20, 180, 182] | [20] | [20, 180, 182] | [20, 180, 182] | [20, 180, 182] | [20, 180, 182] | No criteria |
Note; The most effective+++, no improvement -; NA, not applicable
In MPS I patients, HSCT reduces obstructive airway symptoms and hepatosplenomegaly, improve cardiovascular functions, hearing, vision, and linear growth [21, 31, 38, 85]. It can also improve cognitive function and CNS manifestations [8, 21, 31, 36, 38, 55, 85, 87, 120–126, 132, 193], and either stabilize or prevent the deterioration of psychomotor functions [36, 120, 121, 132]. For this reason, HSCT has been deemed an effective treatment for MPS I [120], and the (EBMT) group recommends HSCT as a treatment method for MPS IH patients who are younger than 2.5 years of age and have an IQ higher than 70 [88, 117]. Newborn screening for MPS I has been implemented in some states of the United States, some European countries, and Taiwan will allow affected patients to be treated by HSCT within the first few months of age [136].
In 2012, a nationwide study in Japan has found that HSCT is an effective treatment if administered before brain atrophy and heart valvular regurgitation symptoms begin to manifestin MPS II patients [49]. Additionally, MPS II patients who have had HSCT early in life usually have higher ADL scores. HSCT has also shown to be more effective than ERT for those who are receiving treatment later in life [45] and to effectively diminish GAGs better than ERT [49]. HSCT has also been shown to improve speech and neurological symptoms [13, 18], improve somatic symptoms [2, 52, 149, 150], hydrocephalic changes, and perivascular enlargements [13, 49]. HSCT either diminished or stopped the progression of categories III and IV brain lesions [13] and has positively altered category I and III brain lesions in MPS II patients. Additionally, HSCT has been able to either stabilize or improve valvular regurgitation which contributes to heart failure, the most common cause of death for MPS II patients [49]. HSCT in MPS IVA improved digression of narrow airways, pulmonary function, snoring cessation, bone mineral density, and walking ability. There is a correlation between those who receive HSCT earlier in life and better ADL scores [12].
Despite the risks involved with transplantation, if HSCT is performed early in life, it will lead to better clinical improvements of the disease [55].
Due to the lack of data on the overall cost of HSCT for MPS patients, the best way to estimate the cost of HSCT for MPS patients is to find studies performed on individuals who have the same clinical background as MPS patients. For this reason, the information presented in this review focuses on the cost of HSCT for pediatric patients who have received allogeneic HSCT. The cost of ERT depends on the type of MPS the patient has, dictating what type of drug is needed for ERT. In 2017, the annual reported cost of ERT for MPS I patients was $218,000 for a 25 kg patient [110]. The early cost for an MPS II patient was $340,000 [110]. The annual cost for an MPS IVA, MPS VI, and MPS VII patients was $578,000, $476,000 and $550,000, respectively [110]. Overall, compared to the cost of ERT, HSCT is significantly cheaper.
One of the main risks of HSCT is the use of toxic preconditioning regimens. A study performed by Rodgers et al. found a positive correlation between the mortality of MPS I patients during the first decade after transplantation and improvements made in peri-HSCT managements [28, 38, 84]. Since HSCT has become much safer over the years, there is some conversation about whether HSCT should be explored as a possible treatment option for milder phenotypes of MPS [153, 194].
There are certain disadvantages associated with HSCT for MPS; the time and cost-consuming search for a donor, the urgency to perform HSCT at a younger age under good health conditions, mortality risk, and pre-existing symptoms, which cannot be reverted. An acceptable matched donor might not always be readily available to the recipient at the appropriate time of transplantation [36, 86], while early transplantation is associated with better outcomes and consequently higher ADL scores [3, 45].
The main disadvantage of HSCT is its relatively high mortality rate. Before 2000, there were reports of mortality rates as high as 27% [13, 18, 56]. However, improvements of HSCT have resulted in a survival rate of over 90% for MPS IH patients in 2013 [28, 31, 37, 38]. There are several different factors which play into the survival rates for MPS patients. The type of donor cells used in transplantation could affect the overall successfulness of the procedure. In 2003, unrelated UBC blood became the favorable source for donor cells [24]. However, UCB could cause delayed engraftment in the recipient and, as a result, more frequent graft failures [101]. The toxicity of certain conditioning regimens could alter mortality rates and effectiveness inpatients, as they could cause brain disease, which could counteract the effects of HSCT [88]. The recent change of conditioning regimens to less toxic fludarabine is promising [31, 37, 83]. There is also an increased risk of degraded cardiac function in MPS IH patients who receive more than one transplantation [86, 95].
Due to the nature of the treatment, there are certain complications which decrease the safety and effectiveness of HSCT. Some of these complications include infection, organ failure, graft rejection, GVHD [13, 18, 56], resulting in growth disturbance and infertility complications [51]. The biggest cause of mortality during the first year after transplantation for MPS IH patients is viral infection, pulmonary hemorrhage, and GVHD [8, 35, 84, 86, 87]. Additionally, the drugs used to combat complications such as cytomegalovirus could also harm the effectiveness of the HSCT [10]. One of the main characteristics of MPS IVA patients are the severe bone deformities. These bone deformities in the airway and pulmonary systems pose a potential problem for MPS IVA if they need to be intubated as a result of other complications from the transplantation [12, 96]. HSCT cannot treat all MPS symptoms including manifestations in the bone, cornea, heart, and any pre-existing cognitive effects [2, 5, 7, 8, 60, 62, 64].
With the improvement of technology and research on HSCT, the use of HSCT in combination with gene therapy may be a more effective treatment option for MPS than either HSCT or ERT alone. Gene therapy takes the patient’s stem cells and genetically engineer them to produce and deliver the enzyme which their body is deficient in by gene transfer vectors [88]. The advantage of HSCT coupled with gene therapy is that the patient’s cells are being used. This decreases the risks for complications such as GVHD. Also, since gene therapy employs the patient’s cells, there will be no need for full intensity preconditioning regimens. With gene therapy, the use of the patient’s cells would lower the mortality and morbidity rates normally associated with HSCT [88]. While gene therapy as a treatment option for MPS remains in the experimental stages, the use of gene therapy with HSCT for metachromatic leukodystrophy, another lysosomal storage disorder, has rendered positive results [38, 88].
7. Conclusion
HSCT has proven to be an effective therapeutic option for various types of MPS. Previous concerns about the safety of the procedure have kept HSCT from being more widely used on different types of MPS patients. However, with the increased medical technology and awareness of the disease, the survival rates for HSCT has significantly improved. The more frequent use of HSCT in various types of MPS should be considered with careful selection of the patients since HSCT has proven to correct more clinical manifestations of the disease than only ERT. Also, one-time administration of the procedure allows for HSCT to be a more cost-effective option.
In summary:
Causes for high mortality rates include the progression of the disease at HSCT, insufficient donor selection, infection, organ failure, graft rejection, GVHD, and mortality caused by conditioning regimens [13, 18, 56].
The best pre-conditioning treatment for HSCT patients is the use of a myeloablative conditioning regimen with a combination of busulfan and fludarabine due to the reduced toxicity rates [31, 37, 83].
Umbilical cord blood is the best source of donor cells because of the higher rates of full-donor chimerism and normal enzyme level, the vast ability, and tolerance for HLA mismatch [24, 25, 28].
HSCT is the recommended treatment option for MPS IH patients who are less than 2.5 years of age [117].
HSCT is approved and administered for MPS II patients in Japan and other countries [13, 37, 45, 49, 53, 151] and should be explored as an acceptable treatment option [24, 29, 152].
Although there are limited cases, HSCT could be a potential therapeutic option for MPS IVA patients due to the positive results in recent studies [19].
There are varied results about the effects of HSCT on MPS VI patients [6, 61, 128].
The combination of HSCT and ERT has improved somatic symptoms, as well as GAG levels and enzyme levels, for both patients with MPS I and MPS VI [24, 42, 44, 54, 67, 94, 111, 114–118, 120, 127].
HSCT can eliminate immune response against ERT in patients with MPS in replacement with the donor’s cells [14, 46].
The mean health care costs for an HSCT procedure in pediatric patients receiving an allogeneic treatment transplant in the United States and Japan are around $500,000 and $100,000, respectively [195].
Overall, HSCT is a cost-effective therapeutic option for some groups of MPS patients regarding permanent affordability with careful selection and management [199]. We will also need a multidisciplinary team to take care of treatment and management of patients suffering from MPS. The guideline of treatment and management for patients with MPS should be determined under the consideration of the type of MPS, age, clinical severity, disease stage, and the socioeconomic environment by a group of experts with MPS supported by governments and non-profit organization, independent of pharmaceutical companies.
Highlights.
Several factors including type of donor, particular MPS disorder, stage of the disease, clinical severity, preconditioning regimen, and potential complications, affect the overall effect and outcome of HSCT.
HSCT can eliminate immune response against infused enzyme by ERT.
The cost-effectiveness of generally a one-time treatment makes HSCT an attractive option for MPS patients.
HSCT is currently the preferred treatment for those patients with certain MPS who have CNS manifestations.
Advancements in medicine have made HSCT a safer, more effective, and widely used treatment option for MPS patients.
8. Acknowledgments
This work was supported by grants from The Carol Ann Foundation, Angelo R. Cali & Mary V. Cali Family Foundation, Inc., The Vain and Harry Fish Foundation, Inc., The Bennett Foundation, Jacob Randall Foundation, Austrian and Japanese MPS societies, and Nemours Funds. This work was supported by the project for baby and infant in research of health and development to Adolescent and young adult from Japan Agency for Medical Research and development, AMED, under grant number JP18gk0110017. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of National Institutes of Health (NIH) under grant number P30GM114736. The content of the article has not been influenced by the sponsors.
Contributions to the project:
Madeleine Taylor is the primary author of this article and an expert in biology. She hascontributed to the concept, planning, data analysis, and reporting of the work described.
Shaukat Khan has 10 years of research experience in model mice for translational research and drug development in MPS field. He has contributed to the editing, data analysis, and reporting of the work described.
Jianmin Wang, a transplant doctor for MPS in China, has contributed to the concept andplanning of the project, interpretation of clinical data, and reporting of the work described.
Jing Chen, the most experienced transplant doctor for MPS in China, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Robert Wynn, Director of Pediatric Bone Marrow Transplant Program at Manchester University, which is one of the largest stem cell transplant centers in the work for metabolic disorders, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Marta Serafini, the well-known basic and translational researcher on HSCT, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Maria Ester Bernardo, MD, PhD, Pediatric Immunohematology and Bone Marrow Transplantation Unit, SR-TIGET, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Hiromasa Yabe, the most experienced transplant doctor for MPS in Japan, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Yasutsugu Chinen has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Jaap Jan Boelens, the most experienced transplant doctor for MPS in Europe (now moved to the United States), has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Robert W. Mason, the biochemist and the expert on GAG assay by LCMS/MS, has contributed to the concept and planning of the project, the draft of the manuscript, and reporting of the work described.
Francyne Kubaski, the biochemist and the expert on GAG assay by LCMS/MS, has contributed to the concept and planning of the project, interpretation of GAG data, and reporting of the work described.
Dafne D.G Horovitz, clinical geneticist and expert for MPS in Brazil, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Anneliese L. Barth, clinical geneticist and expert for MPS in Brazil, has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Hironori Kobayashi has contributed to the concept and planning of the project, interpretation of GAG data, and reporting of the work described.
Kenji E. Orii has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Yasuyuki Suzuki has contributed to the concept and planning of the project, interpretation of clinical data, and reporting of the work described.
Tadao Orii, the most experienced doctor in MPS field in Japan, has contributed to the concept and planning of the project, interpretation of clinical pictures, and GAG data, interpretation of published data and reporting of the work described.
Shunji Tomatsu is a Principal Investigator for this project and has over 30 years of clinical and research experience in MPS, publishing over 200 articles in MPS field. He has contributed to the concept, planning, interpretation of published data, and reporting of the work described.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Madeleine Taylor, Shaukat Khan, Molly Stapleton, Jianmin Wang, Jing Chen, Robert Wynn, Hiromasa Yabe, Yasutsugu Chinen, Jaap Jan Boelens, Robert W. Mason, Francyne Kubaski, Dafne D.G Horovitz, Anneliese L. Barth, Marta Serafini, Alessandro Aiuti, Maria Ester Bernardo, Hironori Kobayashi, Kenji E. Orii, Yasuyuki Suzuki, Tadao Orii, and Shunji Tomatsu contributed to the Review Article and had no conflict of interest with any other party. All authors declare that they have no conflict of interests.
The international investigational team of HSCT for MPS
We have established a clinical research team to evaluate HSCT for MPS, leading to accurate and feasible evaluation of prognosis, therapeutic efficacy, and cost of HSCT. This multi-disciplinary team comprises the experts in the fields of transplantation, genetics, pediatrics, metabolism, pharmacokinetics, and molecular biology in Brazil, China, Europe, Japan, and the United States. The team has over 300 publications related to MPS in both basic and clinical research for MPS, including transplantation, identification of biomarkers, diagnosis, and treatments that encompass orthopedic and tracheal surgeries, ERT, HSCT, and gene therapy. This investigational team includes basic and clinical experts on MPS, who have abundant experience in gene cloning, clinical diagnosis, ERT, and HSCT for MPS. Thus, our investigational team members are internationally recognized in this field for their expertise ranging from basic science to clinical care.
9. References
- [1].Neufeld E, Muenzer J, The Online Metabolic & Molecular Bases of Inherited Disease, in: C.B. Scriver AL; Sly WS; Valle D; Childs B; Kinzler KW; Vogelstein B. (Ed.), The mucopolysaccharidoses, McGraw-Hill;, New York, 2001, pp. 3421–3452. [Google Scholar]
- [2].Noh H, Lee JI, Current and potential therapeutic strategies for mucopolysaccharidoses Journal of clinical pharmacy and therapeutics 39 (2014) 215–224. [DOI] [PubMed] [Google Scholar]
- [3].Tomatsu S, Almeciga-Diaz CJ, Montano AM, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E, Sawamoto K, Mackenzie W, Suzuki Y, Orii KE, Barrera LA, Sly WS, Orii T, Therapies for the bone in mucopolysaccharidoses Molecular genetics and metabolism 114 (2015) 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wraith JE, The mucopolysaccharidoses: a clinical review and guide to management Archives of disease in childhood 72 (1995) 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guffon N, Souillet G, Maire I, Straczek J, Guibaud P, Follow-up of nine patients with Hurler syndrome after bone marrow transplantation The Journal of pediatrics 133 (1998) 119125. [DOI] [PubMed] [Google Scholar]
- [6].Krivit W, Pierpont ME, Ayaz K, Tsai M, Ramsay NK, Kersey JH, Weisdorf S, Sibley R, Snover D, McGovern MM, et al. , Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI). Biochemical and clinical status 24 months after transplantation The New England journal of medicine 311 (1984) 1606–1611. [DOI] [PubMed] [Google Scholar]
- [7].Prasad VK, Kurtzberg J, Transplant outcomes in mucopolysaccharidoses Seminars in hematology 47 (2010) 59–69. [DOI] [PubMed] [Google Scholar]
- [8].Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, Galambrun C, Bertrand Y, Froissart R, Dorche C, Gebuhrer L, Garin C, Berard J, Guibaud P, Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources Bone marrow transplantation 31 (2003) 1105–1117. [DOI] [PubMed] [Google Scholar]
- [9].Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC, Lucas CF, Rogers TR, Benson PF, Tansley LR, Patrick AD, Mossman J, Young EP, Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation Lancet 2 (1981) 709–712. [DOI] [PubMed] [Google Scholar]
- [10].Barth AL, de Magalhaes T, Reis ABR, de Oliveira ML, Scalco FB, Cavalcanti NC, Silva DSE, Torres DA, Costa AAP, Bonfim C, Giugliani R, Llerena JC Jr., Horovitz DDG, Early hematopoietic stem cell transplantation in a patient with severe mucopolysaccharidosis II: A 7 years follow-up Molecular genetics and metabolism reports 12 (2017) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boelens JJ, Trends in haematopoietic cell transplantation for inborn errors of metabolism Journal of inherited metabolic disease 29 (2006) 413–420. [DOI] [PubMed] [Google Scholar]
- [12].Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N, Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA Molecular genetics and metabolism reports 1 (2014) 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kubaski F, Yabe H, Suzuki Y, Seto T, Hamazaki T, Mason RW, Xie L, Onsten TGH, Leistner-Segal S, Giugliani R, Dung VC, Ngoc CTB, Yamaguchi S, Montano AM, Orii KE, Fukao T, Shintaku H, Orii T, Tomatsu S, Hematopoietic Stem Cell Transplantation for Patients with Mucopolysaccharidosis II Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 23 (2017) 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martin PL, Carter SL, Kernan NA, Sahdev I, Wall D, Pietryga D, Wagner JE, Kurtzberg J, Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 12 (2006) 184–194. [DOI] [PubMed] [Google Scholar]
- [15].Sawamoto K, Alméciga-Díaz CJ, Mackenzie W, Mason R, Orii T, S T., Mucopolysaccharidosis Type IVA: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, in: Tomatsu S, Lavery C, Giugliani R, Harmatz P, Scarpa M, Wegrzyn G, Orii T (Eds.), Mucopolysaccharidoses Update, NOVA, New York, 2018, pp. 235–271. [Google Scholar]
- [16].Tomatsu S, Kubaski F, Stapleton M, Suzuki Y, Orii K, Vairo F, Brusius-Facchin AC, Leistner-Segal S, Burin M, Moura de Souza C, R G., Mucopolysaccharidosis Type II: Clinical Features, Biochemistry, Diagnosis, Genetics, and Treatment, in: Lavery TS,C, H. P GR, Scarpa M, Wegrzyn G, Orii T (Eds.), Mucopolysaccharidoses Update, Nova, New York, 2018, pp. 165–209. [Google Scholar]
- [17].Turbeville S, Nicely H, Rizzo JD, Pedersen TL, Orchard PJ, Horwitz ME, Horwitz EM, Veys P, Bonfim C, Al-Seraihy A, Clinical outcomes following hematopoietic stem cell transplantation for the treatment of mucopolysaccharidosis VI Molecular genetics and metabolism 102 (2011) 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang J, Luan Z, Jiang H, Fang J, Qin M, Lee V, Chen J, Allogeneic Hematopoietic Stem Cell Transplantation in Thirty-Four Pediatric Cases of Mucopolysaccharidosis-A Ten-Year Report from the China Children Transplant Group. Biology of blood and marrow transplantation 22 (2016) 2104–2108. [DOI] [PubMed] [Google Scholar]
- [19].Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, Shintaku H, Suzuki Y, Orii T, Tomatsu S, Hematopoietic stem cell transplantation for Morquio A syndrome Molecular genetics and metabolism 117 (2016) 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N, Orii T, Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation Bone marrow transplantation 21 (1998) 629–634. [DOI] [PubMed] [Google Scholar]
- [21].Aldenhoven M, Boelens JJ, de Koning TJ, The clinical outcome of Hurler syndrome after stem cell transplantation Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 14 (2008) 485–498. [DOI] [PubMed] [Google Scholar]
- [22].Fratantoni JC, Hall CW, Neufeld EF, The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation Proceedings of the National Academy of Sciences of the United States of America 64 (1969) 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jaime-Perez JC, Heredia-Salazar AC, Cantu-Rodriguez OG, Gutierrez-Aguirre H, Villarreal-Villarreal CD, Mancias-Guerra C, Herrera-Garza JL, Gomez-Almaguer D, Cost structure and clinical outcome of a stem cell transplantation program in a developing country: the experience in northeast Mexico The oncologist 20 (2015) 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prasad VK, Kurtzberg J, Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions British journal of haematology 148 (2010) 356–372. [DOI] [PubMed] [Google Scholar]
- [25].Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S, Kurtzberg J, Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes Blood 112 (2008) 2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tse W, Laughlin MJ, Umbilical cord blood transplantation: a new alternative option Hematology. American Society of Hematology. Education Program (2005) 377–383. [DOI] [PubMed] [Google Scholar]
- [27].Araya K, Sakai N, Mohri I, Kagitani-Shimono K, Okinaga T, Hashii Y, Ohta H, Nakamichi I, Aozasa K, Taniike M, Ozono K, Localized donor cells in brain of a Hunter disease patient after cord blood stem cell transplantation Molecular genetics and metabolism 98 (2009) 255–263. [DOI] [PubMed] [Google Scholar]
- [28].Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, Wraith E, Cavazzana-Calvo M, Rovelli A, Fischer A, Tolar J, Prasad VK, Escolar M, Gluckman E, O’Meara A, Orchard PJ, Veys P, Eapen M, Kurtzberg J, Rocha V, Eurocord B Inborn Errors Working Party of European, g. Marrow Transplant, B. Duke University, P. Marrow Transplantation, B. Centre for International, R. Marrow, Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning Blood 121 (2013) 3981–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C, Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders Pediatric clinics of North America 57 (2010) 123–145. [DOI] [PubMed] [Google Scholar]
- [30].Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O’Meara A, Michel G, Ionescu I, Parikh S, Prasad VK, Szabolcs P, Escolar M, Gluckman E, Cavazzana-Calvo M, Kurtzberg J, I.e.W.P.o.E. Eurocord, U. Duke, Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 15 (2009) 618–625. [DOI] [PubMed] [Google Scholar]
- [31].Boelens JJ, van Hasselt PM, Neurodevelopmental Outcome after Hematopoietic Cell Transplantation in Inborn Errors of Metabolism: Current Considerations and Future Perspectives Neuropediatrics 47 (2016) 285–292. [DOI] [PubMed] [Google Scholar]
- [32].Miller WP, Rothman SM, Nascene D, Kivisto T, DeFor TE, Ziegler RS, Eisengart J, Leiser K, Raymond G, Lund TC, Tolar J, Orchard PJ, Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report Blood 118 (2011) 1971–1978. [DOI] [PubMed] [Google Scholar]
- [33].JJ B., M. B, R. W, HSCT in inborn errors of metabolism and osteopetrosis, in: Apperley J CE, Gluckman E, Masszi T. (Ed.), The EBMT Handbook on Haemopoietic Stem Cell Transplantation, Genoa, Italy, 2012, pp. 558–571. [Google Scholar]
- [34].Boelens JJ, Orchard PJ, Wynn RF, Transplantation in inborn errors of metabolism: current considerations and future perspectives British journal of haematology 167 (2014) 293303. [DOI] [PubMed] [Google Scholar]
- [35].Boelens JJ, Wynn RF, O’Meara A, Veys P, Bertrand Y, Souillet G, Wraith JE, Fischer A, Cavazzana-Calvo M, Sykora KW, Sedlacek P, Rovelli A, Uiterwaal CS, Wulffraat N, Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure Bone marrow transplantation 40 (2007) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, Saunders EF, deAlarcon PA, Twist C, Nachman JB, Hale GA, Harris RE, Rozans MK, Kurtzberg J, Grayson GH, Williams TE, Lenarsky C, Wagner JE, Krivit W, Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group Blood 91 (1998) 2601–2608. [PubMed] [Google Scholar]
- [37].Aldenhoven M, Jones SA, Bonney D, Borrill RE, Coussons M, Mercer J, Bierings MB, Versluys B, van Hasselt PM, Wijburg FA, van der Ploeg AT, Wynn RF, Boelens JJ, Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 21 (2015) 1106–1109. [DOI] [PubMed] [Google Scholar]
- [38].Aldenhoven M, Wynn RF, Orchard PJ, O’Meara A, Veys P, Fischer A, Valayannopoulos V, Neven B, Rovelli A, Prasad VK, Tolar J, Allewelt H, Jones SA, Parini R, Renard M, Bordon V, Wulffraat NM, de Koning TJ, Shapiro EG, Kurtzberg J, Boelens JJ, Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study Blood 125 (2015) 2164–2172. [DOI] [PubMed] [Google Scholar]
- [39].de Vries JM, van der Beek NA, Kroos MA, Ozkan L, van Doorn PA, Richards SM, Sung CC, Brugma JD, Zandbergen AA, van der Ploeg AT, Reuser AJ, High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa Molecular genetics and metabolism 101 (2010) 338–345. [DOI] [PubMed] [Google Scholar]
- [40].Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT, Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants Molecular genetics and metabolism 99 (2010) 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF, Enzyme replacement in a canine model of Hurler syndrome Proceedings of the National Academy of Sciences of the United States of America 91 (1994) 12937–12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wraith JE, Beck M, Lane R, van der Ploeg A, Shapiro E, Xue Y, Kakkis ED, Guffon N, Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-L-iduronidase (laronidase) Pediatrics 120 (2007) e37–46. [DOI] [PubMed] [Google Scholar]
- [43].Saif MA, Bigger BW, Brookes KE, Mercer J, Tylee KL, Church HJ, Bonney DK, Jones S, Wraith JE, Wynn RF, Hematopoietic stem cell transplantation improves the high incidence of neutralizing allo-antibodies observed in Hurler’s syndrome after pharmacological enzyme replacement therapy Haematologica 97 (2012) 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wynn RF, Mercer J, Page J, Carr TF, Jones S, Wraith JE, Use of enzyme replacement therapy (Laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients The Journal of pediatrics 154 (2009) 135–139. [DOI] [PubMed] [Google Scholar]
- [45].Tanjuakio J, Suzuki Y, Patel P, Yasuda E, Kubaski F, Tanaka A, Yabe H, Mason RW, Montano AM, Orii KE, Orii KO, Fukao T, Orii T, Tomatsu S, Activities of daily living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation Molecular genetics and metabolism 114 (2015) 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Krivit W, Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases Springer seminars in immunopathology 26 (2004) 119–132. [DOI] [PubMed] [Google Scholar]
- [47].Prasad VK, Kurtzberg J, Emerging trends in transplantation of inherited metabolic diseases Bone marrow transplantation 41 (2008) 99–108. [DOI] [PubMed] [Google Scholar]
- [48].Rovelli AM, Steward CG, Hematopoietic cell transplantation activity in Europe for inherited metabolic diseases: open issues and future directions Bone marrow transplantation 35 Suppl 1 (2005) S23–26. [DOI] [PubMed] [Google Scholar]
- [49].Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, Tanaka T, Otomo T, Ohashi T, Ishige-Wada M, Yabe H, Ohura T, Suzuki N, Kato K, Adachi S, Kobayashi R, Mugishima H, Kato S, Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan Molecular genetics and metabolism 107 (2012) 513–520. [DOI] [PubMed] [Google Scholar]
- [50].Valayannopoulos V, Wijburg FA, Therapy for the mucopolysaccharidoses Rheumatology 50 Suppl 5 (2011) v49–59. [DOI] [PubMed] [Google Scholar]
- [51].Wynn RF, Wraith JE, Mercer J, O’Meara A, Tylee K, Thornley M, Church HJ, Bigger BW, Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy The Journal of pediatrics 154 (2009) 609–611. [DOI] [PubMed] [Google Scholar]
- [52].Coppa GV, Gabrielli O, Zampini L, Jetzequel AM, Miniero R, Busca A, De Luca T, Di Natale P, Bone marrow transplantation in Hunter syndrome Journal of inherited metabolic disease 18 (1995) 91–92. [DOI] [PubMed] [Google Scholar]
- [53].Patel P, Suzuki Y, Tanaka A, Yabe H, Kato S, Shimada T, Mason RW, Orii KE, Fukao T, Orii T, Tomatsu S, Impact of Enzyme Replacement Therapy and Hematopoietic Stem Cell Therapy on Growth in Patients with Hunter Syndrome Molecular genetics and metabolism reports 1 (2014) 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tolar J, Grewal SS, Bjoraker KJ, Whitley CB, Shapiro EG, Charnas L, Orchard PJ, Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome Bone marrow transplantation 41 (2008) 531–535. [DOI] [PubMed] [Google Scholar]
- [55].Tomatsu S, Sawamoto K, Almeciga-Diaz CJ, Shimada T, Bober MB, Chinen Y, Yabe H, Montano AM, Giugliani R, Kubaski F, Yasuda E, Rodriguez-Lopez A, Espejo-Mojica AJ, Sanchez OF, Mason RW, Barrera LA, Mackenzie WG, Orii T, Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome Drug design, development and therapy 9 (2015) 1937–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith JE, Long-term follow-up following bone marrow transplantation for Hunter disease Journal of inherited metabolic disease 22 (1999) 638–648. [DOI] [PubMed] [Google Scholar]
- [57].Tomatsu S, Patel P, Suzuki Y, Yabe H, Shimada T, Mason RW, Orii T, Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with Hunter syndrome Molecular Genetics and Metabolism, 114 (2015) S115–S116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tomatsu S, Yasuda E, Patel P, Ruhnke K, Shimada T, Mackenzie WG, Mason R, Thacker MM, Theroux M, Montano AM, Almeciga-Diaz CJ, Barrera LA, Chinen Y, Sly WS, Rowan D, Suzuki Y, Orii T, Morquio A syndrome: diagnosis and current and future therapies Pediatric endocrinology reviews : PER 12 Suppl 1 (2014) 141–151. [PMC free article] [PubMed] [Google Scholar]
- [59].Khanna G, Van Heest AE, Agel J, Bjoraker K, Grewal S, Abel S, Krivit W, Peters C, Orchard PJ, Analysis of factors affecting development of carpal tunnel syndrome in patients with Hurler syndrome after hematopoietic cell transplantation Bone marrow transplantation 39 (2007) 331–334. [DOI] [PubMed] [Google Scholar]
- [60].Braunlin EA, Stauffer NR, Peters CH, Bass JL, Berry JM, Hopwood JJ, Krivit W, Usefulness of bone marrow transplantation in the Hurler syndrome The American journal of cardiology 92 (2003) 882–886. [DOI] [PubMed] [Google Scholar]
- [61].Herskhovitz E, Young E, Rainer J, Hall CM, Lidchi V, Chong K, Vellodi A, Bone marrow transplantation for Maroteaux-Lamy syndrome (MPS VI): long-term follow-up Journal of inherited metabolic disease 22 (1999) 50–62. [DOI] [PubMed] [Google Scholar]
- [62].Muenzer J, Overview of the mucopolysaccharidoses Rheumatology 50 Suppl 5 (2011) v4–12. [DOI] [PubMed] [Google Scholar]
- [63].Rovelli AM, The controversial and changing role of haematopoietic cell transplantation for lysosomal storage disorders: an update Bone marrow transplantation 41 Suppl 2 (2008) S87–89. [DOI] [PubMed] [Google Scholar]
- [64].Weisstein JS, Delgado E, Steinbach LS, Hart K, Packman S, Musculoskeletal manifestations of Hurler syndrome: long-term follow-up after bone marrow transplantation Journal of pediatric orthopedics 24 (2004) 97–101. [DOI] [PubMed] [Google Scholar]
- [65].Chirino AJ, Mire-Sluis A, Characterizing biological products and assessing comparability following manufacturing changes Nature biotechnology 22 (2004) 1383–1391. [DOI] [PubMed] [Google Scholar]
- [66].Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED, Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I The Journal of clinical investigation 118 (2008) 2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF, Enzyme-replacement therapy in mucopolysaccharidosis I The New England journal of medicine 344 (2001) 182–188. [DOI] [PubMed] [Google Scholar]
- [68].Parveen S, Sahoo SK, Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs Clinical pharmacokinetics 45 (2006) 965–988. [DOI] [PubMed] [Google Scholar]
- [69].Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O’Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME, Enzyme replacement therapy in feline mucopolysaccharidosis I Molecular genetics and metabolism 72 (2001) 199–208. [DOI] [PubMed] [Google Scholar]
- [70].Atilla E, Ataca Atilla P, Demirer T, A Review of Myeloablative vs Reduced Intensity/Non-Myeloablative Regimens in Allogeneic Hematopoietic Stem Cell Transplantations Balkan medical journal 34 (2017) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Servais S, Baron F, Beguin Y, Allogeneic hematopoietic stem cell transplantation (HSCT) after reduced intensity conditioning Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 44 (2011) 205–210. [DOI] [PubMed] [Google Scholar]
- [72].Bensinger W, Spielberger R, Preparative Regimens and Modification of Regimen-Related Toxicities, in: Blume K, Forman S, Appelbaum F (Eds.), Thomas’ Hematopoietic Cell Transplantation, Blackwell Publishing Ltd, 2003. [Google Scholar]
- [73].Berry DA, Ueno NT, Johnson MM, Lei X, Caputo J, Smith DA, Yancey LJ, Crump M, Stadtmauer EA, Biron P, Crown JP, Schmid P, Lotz JP, Rosti G, Bregni M, Demirer T, High-dose chemotherapy with autologous hematopoietic stem-cell transplantation in metastatic breast cancer: overview of six randomized trials Journal of clinical oncology: official journal of the American Society of Clinical Oncology 29 (2011) 3224–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Brunvand MW, Bensinger WI, Soll E, Weaver CH, Rowley SD, Appelbaum FR, Lilleby K, Clift RA, Gooley TA, Press OW, Fefer A, Storb R, Sanders JE, Martin PL, Chauncey T, Maziarz RT, Zuckerman N, Montgomery P, Dorn R, Weiden PL, Demirer T, Holmberg LA, Schiffman K, McSweeney PA, Buckner CD, et al. , High-dose fractionated total-body irradiation, etoposide and cyclophosphamide for treatment of malignant lymphoma: comparison of autologous bone marrow and peripheral blood stem cells Bone marrow transplantation 18 (1996) 131–141. [PubMed] [Google Scholar]
- [75].De Giorgi U, Demirer T, Wandt H, Taverna C, Siegert W, Bornhauser M, Kozak T, Papiani G, Ballardini M, Rosti G, Solid B Tumor Working Party of the European Group for, T. Marrow, Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: the EBMT experience Annals of oncology: official journal of the European Society for Medical Oncology 16 (2005) 146–151. [DOI] [PubMed] [Google Scholar]
- [76].Demirer T, Buckner CD, Appelbaum FR, Clift R, Storb R, Myerson D, Lilleby K, Rowley S, Bensinger WI, High-dose busulfan and cyclophosphamide followed by autologous transplantation in patients with advanced breast cancer Bone marrow transplantation 17 (1996) 769–774. [PubMed] [Google Scholar]
- [77].Demirer T, Celebi H, Arat M, Ustun C, Demirer S, Dilek I, Ozcan M, Ilhan O, Akan H, Gurman G, Koc H, Autoimmune thrombocytopenia in a patient with small cell lung cancer developing after chemotherapy and resolving following autologous peripheral blood stem cell transplantation Bone marrow transplantation 24 (1999) 335–337. [DOI] [PubMed] [Google Scholar]
- [78].Demirer T, Gooley T, Buckner CD, Petersen FB, Lilleby K, Rowley S, Sanders J, Storb R, Appelbaum FR, Bensinger WI, Influence of total nucleated cell dose from marrow harvests on outcome in patients with acute myelogenous leukemia undergoing autologous transplantation Bone marrow transplantation 15 (1995) 907–913. [PubMed] [Google Scholar]
- [79].Demirer T, Petersen FB, Bensinger WI, Appelbaum FR, Fefer A, Rowley S, Sanders J, Chauncey T, Storb R, Lilleby K, Buckner CD, Autologous transplantation with peripheral blood stem cells collected after granulocyte colony-stimulating factor in patients with acute myelogenous leukemia Bone marrow transplantation 18 (1996) 29–34. [PubMed] [Google Scholar]
- [80].Kroger N, Damon L, Zander AR, Wandt H, Derigs G, Ferrante P, Demirer T, Rosti G, Solid B Tumor Working Party of the European Group for, T. Marrow, G. German Adjuvant Breast Cancer Study, S.F. University of California, Secondary acute leukemia following mitoxantrone-based high-dose chemotherapy for primary breast cancer patients Bone marrow transplantation 32 (2003) 1153–1157. [DOI] [PubMed] [Google Scholar]
- [81].Pedrazzoli P, Ferrante P, Kulekci A, Schiavo R, De Giorgi U, Carminati O, Marangolo M, Demirer T, Siena S, Rosti G, European Group for B, Marrow Transplantation STWP, Autologous hematopoietic stem cell transplantation for breast cancer in Europe: critical evaluation of data from the European Group for Blood and Marrow Transplantation (EBMT) Registry 1990–1999 Bone marrow transplantation 32 (2003) 489–494. [DOI] [PubMed] [Google Scholar]
- [82].Lum SH, Miller WP, Jones S, Poulton K, Ogden W, Lee H, Logan A, Bonney D, Lund TC, Orchard PJ, Wynn RF, Changes in the incidence, patterns and outcomes of graft failure following hematopoietic stem cell transplantation for Hurler syndrome Bone marrow transplantation 52 (2017) 846–853. [DOI] [PubMed] [Google Scholar]
- [83].Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, Lindemans CA, Bierings MB, Boelens JJ, Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 20 (2014) 345–353. [DOI] [PubMed] [Google Scholar]
- [84].Rodgers NJ, Kaizer AM, Miller WP, Rudser KD, Orchard PJ, Braunlin EA, Mortality after hematopoietic stem cell transplantation for severe mucopolysaccharidosis type I: the 30-year University of Minnesota experience Journal of inherited metabolic disease 40 (2017) 271–280. [DOI] [PubMed] [Google Scholar]
- [85].Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J, Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome The New England journal of medicine 350 (2004) 1960–1969. [DOI] [PubMed] [Google Scholar]
- [86].Mitchell R, Nivison-Smith I, Anazodo A, Tiedemann K, Shaw PJ, Teague L, Fraser CJ, Carter TL, Tapp H, Alvaro F, O’Brien TA, Outcomes of haematopoietic stem cell transplantation for inherited metabolic disorders: a report from the Australian and New Zealand Children’s Haematology Oncology Group and the Australasian Bone Marrow Transplant Recipient Registry Pediatric transplantation 17 (2013) 582–588. [DOI] [PubMed] [Google Scholar]
- [87].Vellodi A, Young EP, Cooper A, Wraith JE, Winchester B, Meaney C, Ramaswami U, Will A, Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres Archives of disease in childhood 76 (1997) 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Biffi A, Hematopoietic Stem Cell Gene Therapy for Storage Disease: Current and New Indications Molecular therapy : the journal of the American Society of Gene Therapy 25 (2017) 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen Y, Xu L, Zhang X, Chen H, Wang F, Liu1 K, Qin J, Yang Y, Huang X, Busulfan, Fludarabine, and Cyclophosphamide (BFC) conditioning allowed stable engraftment after haplo-identical allogeneic stem cell transplantation in children with adrenoleukodystrophy and mucopolysaccharidosis Bone marrow transplantation 53 (2018) 770–773. [DOI] [PubMed] [Google Scholar]
- [90].Ansari M, Theoret Y, Rezgui MA, Peters C, Mezziani S, Desjean C, Vachon MF, Champagne MA, Duval M, Krajinovic M, Bittencourt H, Pediatric B Disease Working Parties of the European, G. Marrow Transplant, Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematopoietic stem cell transplantation Therapeutic drug monitoring 36 (2014) 93–99. [DOI] [PubMed] [Google Scholar]
- [91].Arias E, United States Life Tables, 2000, National vital statistics reports, in: CDC (Ed.), Hyattsville, Maryland:, 2002, pp. 1–40. [PubMed] [Google Scholar]
- [92].FEINLEIB M, Vita Statistics of the United States, 1980, Life tables, in: U.S.D.O.H.A.H. SERVICES (Ed.), DEPARTMENT OF HEALTH AND HUMAN SERVICES Hyattsville, Maryland, 1984, pp. 1–21. [Google Scholar]
- [93].FEINLEIB M, Vital Statistics of the United States, 1990 LIFE “TABLES:, in: U.S.D.O.H.A.H. SERVICES (Ed.), DEPARTMENT OF HEALTH AND HUMAN SERVICES, Hyattsville, Maryland, 1994, pp. 1–27. [Google Scholar]
- [94].Lum SH, Stepien KM, Ghosh A, Broomfield A, Church H, Mercer J, Jones S, Wynn R, Long term survival and cardiopulmonary outcome in children with Hurler syndrome after haematopoietic stem cell transplantation Journal of inherited metabolic disease 40 (2017) 455460. [DOI] [PubMed] [Google Scholar]
- [95].Orchard PJ, Milla C, Braunlin E, DeFor T, Bjoraker K, Blazar BR, Peters C, Wagner J, Tolar J, Pre-transplant risk factors affecting outcome in Hurler syndrome Bone marrow transplantation 45 (2010) 1239–1246. [DOI] [PubMed] [Google Scholar]
- [96].Theroux MC, Nerker T, Ditro C, Mackenzie WG, Anesthetic care and perioperative complications of children with Morquio syndrome Paediatric anaesthesia 22 (2012) 901–907. [DOI] [PubMed] [Google Scholar]
- [97].Preussler JM, Denzen EM, Majhail NS, Costs and cost-effectiveness of hematopoietic cell transplantation Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 18 (2012) 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uyl-de Groot CA, Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study Annals of hematology 91 (2012) 1945–1952. [DOI] [PubMed] [Google Scholar]
- [99].Cordonnier C, Maury S, Esperou H, Pautas C, Beaune J, Rodet M, Lagrange JL, Rouard H, Beaumont JL, Bassompierre F, Gluckman E, Kuentz M, Durand-Zaleski I, Do minitransplants have minicosts? A cost comparison between myeloablative and nonmyeloablative allogeneic stem cell transplant in patients with acute myeloid leukemia Bone marrow transplantation 36 (2005) 649–654. [DOI] [PubMed] [Google Scholar]
- [100].Majhail NS, Rizzo JD, Hahn T, Lee SJ, McCarthy PL, Ammi M, Denzen E, Drexler R, Flesch S, James H, Omondi N, Pedersen TL, Murphy E, Pederson K, Pilot study of patient and caregiver out-of-pocket costs of allogeneic hematopoietic cell transplantation Bone marrow transplantation 48 (2013) 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ, Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 15 (2009) 564–573. [DOI] [PubMed] [Google Scholar]
- [102].Kitazawa T, Matsumoto K, Fujita S, Seto K, Hasegawa T, Cost Analysis of Transplantation in Japan, Performed With the Use of the National Database Transplantation proceedings 49 (2017) 4–9. [DOI] [PubMed] [Google Scholar]
- [103].Barth A, Horovitz D, Hematopoietic Stem Cell Transplantation in Mucopolysaccharidosis Type II: A Literature Review and Critical Analysis Journal of Inborn Errors of Metabolism & Screening 6 (2018) 1–11. [Google Scholar]
- [104].Svahn BM, Alvin O, Ringden O, Gardulf A, Remberger M, Costs of allogeneic hematopoietic stem cell transplantation Transplantation 82 (2006) 147–153. [DOI] [PubMed] [Google Scholar]
- [105].Ngamkiatphaisan S, Sriratanaban J, Kamolratanakul P, Intragumtornchai T, Noppakun N, Jongudomsuk P, Cost analysis of hematopoietic stem cell transplantation in adult patients with acute myeloid leukemia at King Chulalongkorn Memorial Hospital Journal of the Medical Association of Thailand = Chotmaihet thangphaet 90 (2007) 2565–2573. [PubMed] [Google Scholar]
- [106].Gale RP, Seber A, Bonfim C, Pasquini M, Haematopoietic cell transplants in Latin America Bone marrow transplantation 51 (2016) 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Muenzer J, Bodamer O, Burton B, Clarke L, Frenking GS, Giugliani R, Jones S, Rojas MV, Scarpa M, Beck M, Harmatz P, The role of enzyme replacement therapy in severe Hunter syndrome-an expert panel consensus European journal of pediatrics 171 (2012) 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J, Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy European journal of pediatrics 167 (2008) 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Okuyama T, Tanaka A, Suzuki Y, Ida H, Tanaka T, Cox GF, Eto Y, Orii T, Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Molecular genetics and metabolism 99 (2010) 18–25. [DOI] [PubMed] [Google Scholar]
- [110].Li V, Making of Mepsevii, 2017. [Google Scholar]
- [111].Cox-Brinkman J, Boelens JJ, Wraith JE, O’Meara A, Veys P, Wijburg FA, Wulffraat N, Wynn RF, Haematopoietic cell transplantation (HCT) in combination with enzyme replacement therapy (ERT) in patients with Hurler syndrome Bone marrow transplantation 38 (2006) 17–21. [DOI] [PubMed] [Google Scholar]
- [112].Ghosh A, Miller W, Orchard PJ, Jones SA, Mercer J, Church HJ, Tylee K, Lund T, Bigger BW, Tolar J, Wynn RF, Enzyme replacement therapy prior to haematopoietic stem cell transplantation in Mucopolysaccharidosis Type I: 10 year combined experience of 2 centres Molecular genetics and metabolism 117 (2016) 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Guidelines for the treatment of Mucopolysaccharidosis Type I (MPS I) disease through the Life Saving Drugs Program, 2013, in: A.G.D.o.H.a. Ageing (Ed.), 2018.
- [114].Clarke LA, Wraith JE, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Sidman M, Kakkis ED, Cox GF, Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I Pediatrics 123 (2009) 229–240. [DOI] [PubMed] [Google Scholar]
- [115].Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED, A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years Molecular genetics and metabolism 90 (2007) 171–180. [DOI] [PubMed] [Google Scholar]
- [116].Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF, Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) The Journal of pediatrics 144 (2004) 581–588. [DOI] [PubMed] [Google Scholar]
- [117].de Ru MH, Boelens JJ, Das AM, Jones SA, van der Lee JH, Mahlaoui N, Mengel E, Offringa M, O’Meara A, Parini R, Rovelli A, Sykora KW, Valayannopoulos V, Vellodi A, Wynn RF, Wijburg FA, Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure Orphanet journal of rare diseases 6 (2011) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Grewal SS, Wynn R, Abdenur JE, Burton BK, Gharib M, Haase C, Hayashi RJ, Shenoy S, Sillence D, Tiller GE, Dudek ME, van Royen-Kerkhof A, Wraith JE, Woodard P, Young GA, Wulffraat N, Whitley CB, Peters C, Safety and efficacy of enzyme replacement therapy in combination with hematopoietic stem cell transplantation in Hurler syndrome Genetics in medicine : official journal of the American College of Medical Genetics 7 (2005) 143–146. [DOI] [PubMed] [Google Scholar]
- [119].Baxter MA, Wynn RF, Schyma L, Holmes DK, Wraith JE, Fairbairn LJ, Bellantuono I, Marrow stromal cells from patients affected by MPS I differentially support haematopoietic progenitor cell development Journal of inherited metabolic disease 28 (2005) 1045–1053. [DOI] [PubMed] [Google Scholar]
- [120].Ferrara G, Maximova N, Zennaro F, Gregori M, Tamaro P, Hematopoietic stem cell transplantation effects on spinal cord compression in Hurler Pediatric transplantation 18 (2014) E96–99. [DOI] [PubMed] [Google Scholar]
- [121].Aldenhoven M, Sakkers RJ, Boelens J, de Koning TJ, Wulffraat NM, Musculoskeletal manifestations of lysosomal storage disorders Annals of the rheumatic diseases 68 (2009) 1659–1665. [DOI] [PubMed] [Google Scholar]
- [122].Field RE, Buchanan JA, Copplemans MG, Aichroth PM, Bone-marrow transplantation in Hurler’s syndrome. Effect on skeletal development The Journal of bone and joint surgery. British volume 76 (1994) 975–981. [PubMed] [Google Scholar]
- [123].Langereis EJ, Borgo A, Crushell E, Harmatz PR, van Hasselt PM, Jones SA, Kelly PM, Lampe C, van der Lee JH, Odent T, Sakkers R, Scarpa M, Schafroth MU, Struijs PA, Valayannopoulos V, White KK, Wijburg FA, Treatment of hip dysplasia in patients with mucopolysaccharidosis type I after hematopoietic stem cell transplantation: results of an international consensus procedure Orphanet journal of rare diseases 8 (2013) 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Masterson EL, Murphy PG, O’Meara A, Moore DP, Dowling FE, Fogarty EE, Hip dysplasia in Hurler’s syndrome: orthopaedic management after bone marrow transplantation Journal of pediatric orthopedics 16 (1996) 731–733. [DOI] [PubMed] [Google Scholar]
- [125].Stoop FJ, Kruyt MC, van der Linden MH, Sakkers RJB, van Hasselt PM, Castelein RMC , Prevalence and development of orthopaedic symptoms in the dutch hurler patient population after haematopoietic stem cell transplantation JIMD reports 9 (2013) 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Taylor C, Brady P, O’Meara A, Moore D, Dowling F, Fogarty E, Mobility in Hurler syndrome Journal of pediatric orthopedics 28 (2008) 163–168. [DOI] [PubMed] [Google Scholar]
- [127].Whitley CB, Utz JR, Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI): a single dose of galsulfase further reduces urine glycosaminoglycans after hematopoietic stem cell transplantation Molecular genetics and metabolism 101 (2010) 346–348. [DOI] [PubMed] [Google Scholar]
- [128].Harmatz P, Shediac R, Mucopolysaccharidosis VI: pathophysiology, diagnosis and treatment Frontiers in bioscience 22 (2017) 385–406. [DOI] [PubMed] [Google Scholar]
- [129].Sillence D, Waters K, Donaldson S, Shaw PJ, Ellaway C, Combined Enzyme Replacement Therapy and Hematopoietic Stem Cell Transplantation in Mucopolysacharidosis Type VI JIMD reports 2 (2012) 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sohn YB, Park SW, Kim SH, Cho SY, Ji ST, Kwon EK, Han SJ, Oh SJ, Park YJ, Ko AR, Paik KH, Lee J, Lee DH, Jin DK, Enzyme replacement therapy improves joint motion and outcome of the 12-min walk test in a mucopolysaccharidosis type VI patient previously treated with bone marrow transplantation American journal of medical genetics. Part A 158A (2012) 1158–1163. [DOI] [PubMed] [Google Scholar]
- [131].Moore D, Connock MJ, Wraith E, Lavery C, The prevalence of and survival in Mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK Orphanet journal of rare diseases 3 (2008) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, Henslee-Downey J, Trigg ME, Cowan MJ, Sanders J, Bunin N, Weinstein H, Lenarsky C, Falk P, Harris R, Bowen T, Williams TE, Grayson GH, Warkentin P, Sender L, Cool VA, Crittenden M, Packman S, Kaplan P, Lockman LA, Anderson J, Krivit W, Dusenbery K, Wagner J, Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome Blood 87 (1996) 4894–4902. [PubMed] [Google Scholar]
- [133].Poe MD, Chagnon SL, Escolar ML, Early treatment is associated with improved cognition in Hurler syndrome Annals of neurology 76 (2014) 747–753. [DOI] [PubMed] [Google Scholar]
- [134].Boelens J, Aldenhoven M, Escolar M, Poe M, Wynn R, Maera A, Rovel A, Veys P, Orchard P, Kurtzberg J, Boelens J, International multicenter study to identify predictors of long term outcome of Hurler syndrome patients after successful hematopoietic stem cell transplantation, 12th International Symposium on MPS and Related Diseases, Noordwijkerhout, The Netherlands, 2012, pp. S08.07 [Google Scholar]
- [135].Pievani A, Azario I, Antolini L, Shimada T, Patel P, Remoli C, Rambaldi B, Valsecchi MG, Riminucci M, Biondi A, Tomatsu S, Serafini M, Neonatal bone marrow transplantation prevents bone pathology in a mouse model of mucopolysaccharidosis type I Blood 125 (2015) 1662–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kurtzberg J, Early HSCT corrects the skeleton in MPS Blood 125 (2015) 1518–1519. [DOI] [PubMed] [Google Scholar]
- [137].Yasuda E, Mackenzie W, Ruhnke K, Shimada T, Mason RW, Zustin J, Martin PL, Thacker M, Orii T, Sai Y, Tomatsu S, Molecular Genetics and Metabolism Report Long-term follow-up of post hematopoietic stem cell transplantation for Hurler syndrome: clinical, biochemical, and pathological improvements Molecular genetics and metabolism reports 2 (2015) 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Church H, Tylee K, Cooper A, Thornley M, Mercer J, Wraith E, Carr T, O’Meara A, Wynn RF, Biochemical monitoring after haemopoietic stem cell transplant for Hurler syndrome (MPSIH): implications for functional outcome after transplant in metabolic disease Bone marrow transplantation 39 (2007) 207–210. [DOI] [PubMed] [Google Scholar]
- [139].Walker RWM, Garbarino J, Anesthesia Risk and the Mucopolysaccharidoses: A Challenging and Changing Landscape Journal of Child Science (JCS) 8 (2018) e116–e123. [Google Scholar]
- [140].Frawley G, Fuenzalida D, Donath S, Yaplito-Lee J, Peters H, A retrospective audit of anesthetic techniques and complications in children with mucopolysaccharidoses Paediatric anaesthesia 22 (2012) 737–744. [DOI] [PubMed] [Google Scholar]
- [141].Kirkpatrick K, Ellwood J, Walker RW, Mucopolysaccharidosis type I (Hurler syndrome) and anesthesia: the impact of bone marrow transplantation, enzyme replacement therapy, and fiberoptic intubation on airway management Paediatric anaesthesia 22 (2012) 745751. [DOI] [PubMed] [Google Scholar]
- [142].Fraldi A, Serafini M, Sorrentino NC, Gentner B, Aiuti A, Bernardo ME, Gene therapy for mucopolysaccharidoses: in vivo and ex vivo approaches Italian journal of pediatrics 44 (2018) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nelson J, Incidence of the mucopolysaccharidoses in Northern Ireland Human genetics 101 (1997) 355–358. [DOI] [PubMed] [Google Scholar]
- [144].Patel P, Suzuki Y, Maeda M, Yasuda E, Shimada T, Orii KE, Orii T, Tomatsu S, Growth charts for patients with Hunter syndrome Molecular genetics and metabolism reports 1 (2014) 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Selvanathan A, Ellaway C, Wilson C, Owens P, Shaw PJ, Bhattacharya K, Effectiveness of Early Hematopoietic Stem Cell Transplantation in Preventing Neurocognitive Decline in Mucopolysaccharidosis Type II: A Case Series JIMD reports 41 (2018) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Boado RJ, Hui EK, Lu JZ, Sumbria RK, Pardridge WM, Blood-brain barrier molecular trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey Bioconjugate chemistry 24 (2013) 1741–1749. [DOI] [PubMed] [Google Scholar]
- [147].Lampe C, Bosserhoff AK, Burton BK, Giugliani R, de Souza CF, Bittar C, Muschol N, Olson R, Mendelsohn NJ, Long-term experience with enzyme replacement therapy (ERT) in MPS II patients with a severe phenotype: an international case series Journal of inherited metabolic disease 37 (2014) 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Shimada T, Kelly J, LaMarr WA, van Vlies N, Yasuda E, Mason RW, Mackenzie W, Kubaski F, Giugliani R, Chinen Y, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T, Tomatsu S, Novel heparan sulfate assay by using automated high-throughput mass spectrometry: Application to monitoring and screening for mucopolysaccharidoses Molecular genetics and metabolism 113 (2014) 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R, Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years The Journal of pediatrics 154 (2009) 733–737. [DOI] [PubMed] [Google Scholar]
- [150].McKinnis EJ, Sulzbacher S, Rutledge JC, Sanders J, Scott CR, Bone marrow transplantation in Hunter syndrome The Journal of pediatrics 129 (1996) 145–148. [DOI] [PubMed] [Google Scholar]
- [151].CONITECH CLINICAL PROTOCOL AND THERAPEUTIC GUIDELINES MUCOPOLISSACARIDOSE TYPE II, in: Health M.o.. (Ed.), 2018, pp. 1–40. [Google Scholar]
- [152].Bradley LA, Haddow HRM, Palomaki GE, Treatment of mucopolysaccharidosis type II (Hunter syndrome): results from a systematic evidence review Genetics in medicine: official journal of the American College of Medical Genetics 19 (2017) 1187–1201. [DOI] [PubMed] [Google Scholar]
- [153].Stapleton M, Kubaski F, Mason RW, Yabe H, Suzuki Y, Orii KE, Orii T, Tomatsu S, Presentation and Treatments for Mucopolysaccharidosis Type II (MPS II; Hunter Syndrome) Expert opinion on orphan drugs 5 (2017) 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Da Costa V, O’Grady G, Jackson L, Kaylie D, Raynor E, Improvements in sensorineural hearing loss after cord blood transplant in patients with mucopolysaccharidosis Archives of otolaryngology--head & neck surgery 138 (2012) 1071–1076. [DOI] [PubMed] [Google Scholar]
- [155].Valstar MJ, Ruijter GJ, van Diggelen OP, Poorthuis BJ, Wijburg FA, Sanfilippo syndrome: a mini-review Journal of inherited metabolic disease 31 (2008) 240–252. [DOI] [PubMed] [Google Scholar]
- [156].Klein K, Krivit W, CB W, Peters C, Cool V, Fuhrman M, De Alarcon P, Klemperer M, Miller L, Nelson R, Henslee-Downey J, Chang P, Wraith J, Lockman L, Shapiro E, Poor cognitive outcome of eleven children with Sanfilippo syndrome after bone marrow transplantation and successful engraftment Bone marrow transplantation 15 (1995). [Google Scholar]
- [157].Vellodi A, Young E, New M, Pot-Mees C, Hugh-Jones K, Bone marrow transplantation for Sanfilippo disease type B Journal of inherited metabolic disease 15 (1992) 911–918. [DOI] [PubMed] [Google Scholar]
- [158].Sivakumur P, Wraith JE , Bone marrow transplantation in mucopolysaccharidosis type IIIA: a comparison of an early treated patient with his untreated sibling Journal of inherited metabolic disease 22 (1999) 849–850. [DOI] [PubMed] [Google Scholar]
- [159].Welling L, Marchal JP, van Hasselt P, van der Ploeg AT, Wijburg FA, Boelens JJ, Early Umbilical Cord Blood-Derived Stem Cell Transplantation Does Not Prevent Neurological Deterioration in Mucopolysaccharidosis Type III JIMD reports 18 (2015) 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tomatsu S, Montaño A, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T, Impairment of Body Growth in Mucopolysaccharidoses,Impairment of Body Growth in Mucopolysaccharidoses, Springer science+Businessmedia, LLC, Springer New York Dordrecht Heidelberg London, 2012. [Google Scholar]
- [161].Tomatsu S, Montano AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T, Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment Current pharmaceutical biotechnology 12 (2011) 931–945. [DOI] [PubMed] [Google Scholar]
- [162].Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T, International Morquio A Registry: clinical manifestation and natural course of Morquio A disease Journal of inherited metabolic disease 30 (2007) 165–174. [DOI] [PubMed] [Google Scholar]
- [163].Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montano AM, Rowan D, Sly W, Almeciga-Diaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T, Current and emerging treatments and surgical interventions for Morquio A syndrome: a review Research and reports in endocrine disorders 2012 (2012) 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Whitley CB, Spielmann RC, Herro G, Teragawa SS, Urinary glycosaminoglycan excretion quantified by an automated semimicro method in specimens conveniently transported from around the globe Molecular genetics and metabolism 75 (2002) 56–64. [DOI] [PubMed] [Google Scholar]
- [165].Behfar M, Dehghani SS, Rostami T, Ghavamzadeh A, Hamidieh AA, Non-sibling hematopoietic stem cell transplantation using myeloablative conditioning regimen in children with Maroteaux-Lamy syndrome: A brief report Pediatric transplantation 21 (2017). [DOI] [PubMed] [Google Scholar]
- [166].Giugliani R, Harmatz P, Wraith JE, Management guidelines for mucopolysaccharidosis VI Pediatrics 120 (2007) 405–418. [DOI] [PubMed] [Google Scholar]
- [167].Valayannopoulos V, Nicely H, Harmatz P, Turbeville S, Mucopolysaccharidosis VI Orphanet journal of rare diseases 5 (2010) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ, Group MVPS, Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study The Journal of pediatrics 148 (2006) 533–539. [DOI] [PubMed] [Google Scholar]
- [169].Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Ketteridge D, Hopwood JJ, Plecko B, Steiner R, Whitley CB, Kaplan P, Yu ZF, Swiedler SJ, Decker C, Group MVS, Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase Molecular genetics and metabolism 94 (2008) 469–475. [DOI] [PubMed] [Google Scholar]
- [170].Jester S, Larsson J, Eklund EA, Papadopoulou D, Mansson JE, Bekassy AN, Turkiewicz D, Toporski J, Ora I, Haploidentical stem cell transplantation in two children with mucopolysaccharidosis VI: clinical and biochemical outcome Orphanet journal of rare diseases 8 (2013) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Orchard PJ, Blazar BR, Wagner J, Charnas L, Krivit W, Tolar J, Hematopoietic cell therapy for metabolic disease The Journal of pediatrics 151 (2007) 340–346. [DOI] [PubMed] [Google Scholar]
- [172].Wang CC, Hwu WL, Lin KH, Long-term follow-up of a girl with Maroteaux-Lamy syndrome after bone marrow transplantation World journal of pediatrics : WJP 4 (2008) 152154. [DOI] [PubMed] [Google Scholar]
- [173].Armitage J BMRO, Bone marrow transplantation, in: C.W L Lanza RP. (Ed.), Yearbook of Cell and Tissue Transplantation 1996–1997, Springer, Dordrecht, Dordrecht, The Netherlands, 1996, pp. 3–12. [Google Scholar]
- [174].McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA, Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients Annals of internal medicine 118 (1993) 255–267. [DOI] [PubMed] [Google Scholar]
- [175].Giugliani R, Federhen A, Rojas MV, Vieira T, Artigalas O, Pinto LL, Azevedo AC, Acosta A, Bonfim C, Lourenco CM, Kim CA, Horovitz D, Bonfim D, Norato D, Marinho D, Palhares D, Santos ES, Ribeiro E, Valadares E, Guarany F, de Lucca GR, Pimentel H, de Souza IN, Correa J Sr., Fraga JC, Goes JE, Cabral JM, Simionato J, Llerena J Jr., Jardim L, Giuliani L, da Silva LC, Santos ML, Moreira MA, Kerstenetzky M, Ribeiro M, Ruas N, Barrios P, Aranda P, Honjo R, Boy R, Costa R, Souza C, Alcantara FF, Avilla SG, Fagondes S, Martins AM, Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment Genetics and molecular biology 33 (2010) 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Coutinho MF, Lacerda L, Alves S, Glycosaminoglycan storage disorders: a review Biochemistry research international 2012 (2012) 471325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hendriksz CJ, Giugliani R, Harmatz P, Lampe C, Martins AM, Pastores GM, Steiner RD, Leao Teles E, Valayannopoulos V, Group CSPS, Design, baseline characteristics, and early findings of the MPS VI (mucopolysaccharidosis VI) Clinical Surveillance Program (CSP) Journal of inherited metabolic disease 36 (2013) 373–384. [DOI] [PubMed] [Google Scholar]
- [178].Lee V, Li CK, Shing MM, Chik KW, Lam CW, Tsang KS, Pong H, Huen KF, Yuen PM, Umbilical cord blood transplantation for Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI) Bone marrow transplantation 26 (2000) 455–458. [DOI] [PubMed] [Google Scholar]
- [179].Tomatsu S, Sukegawa K, Ikedo Y, Fukuda S, Yamada Y, Sasaki T, Okamoto H, Kuwabara T, Orii T, Molecular basis of mucopolysaccharidosis type VII: replacement of Ala619 in beta-glucuronidase with Val. Gene. 1990. May 14;89(2):283–7. [DOI] [PubMed] [Google Scholar]
- [180].Montano AM, Lock-Hock N, Steiner RD, Graham BH, Szlago M, Greenstein R, Pineda M, Gonzalez-Meneses A, Coker M, Bartholomew D, Sands MS, Wang R, Giugliani R, Macaya A, Pastores G, Ketko AK, Ezgu F, Tanaka A, Arash L, Beck M, Falk RE, Bhattacharya K, Franco J, White KK, Mitchell GA, Cimbalistiene L, Holtz M, Sly WS, Clinical course of sly syndrome (mucopolysaccharidosis type VII) Journal of medical genetics 53 (2016) 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Tomatsu S, Fukuda S, Sukegawa K, Ikedo Y, Yamada S, Yamada Y, Sasaki T, Okamoto H, Kuwahara T, Yamaguchi S, et al. , Mucopolysaccharidosis type VII: characterization of mutations and molecular heterogeneity Am J Hum Genet. 1991. January;48(1):89–96.. [PMC free article] [PubMed] [Google Scholar]
- [182].Sisinni L, Pineda M, Coll MJ, Gort L, Turon E, Torrent M, Ey A, Tobajas E, Badell I, Birkenmeier, Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates Laboratory investigation; a journal of technical methods and pathology 68 (1993) 676–686. [PubMed] [Google Scholar]
- [183].I. Abeona Therapeutics, Phase I/II Gene Transfer Clinical Trial of scAAV9.U1a.hSGSH, Phase I/II Gene Transfer Clinical Trial of scAAV9.U1a.hSGSH for Mucopolysaccharidosis (MPS) IIIA, 2016. [Google Scholar]
- [184].LYSOGENE, Intracerebral Gene Therapy for Sanfilippo Type A Syndrome, An Open-label, Single Arm, Monocentric, Phase I/II Clinical Study of Intracerebral Administration of Adeno-associated Viral Vector Serotype 10 Carrying the Human SGSH and SUMF1 cDNAs for the Treatment of Sanfilippo Type A Syndrome., France, 2011. [Google Scholar]
- [185].LYSOGENE, Long-term Follow-up of Sanfilippo Type A Patients Treated by Intracerebral SAF-301 Gene Therapy, Long-term Follow-up of Patients With Sanfilippo Type A Syndrome Who Have Previously Been Treated in the P1-SAF-301 Clinical Study Evaluating the Tolerability and Safety of the Intracerebral Administration of SAF-301., France, 2013. [Google Scholar]
- [186].Nicola Brunetti-Pierri FT, Gene Therapy in Patients With Mucopolysaccharidosis Disease, A Phase I/II Open Label, Dose Escalation, Safety Study in Subjects With Mucopolysaccharidosis Type VI (MPS VI) Using Adeno-Associated Viral Vector 8 to Deliver the Human ARSB Gene to Liver, 2017.
- [187].Sawamoto K, Chen HH, Almeciga-Diaz CJ, Mason RW, Tomatsu S, Gene therapy for Mucopolysaccharidoses Molecular genetics and metabolism 123 (2018) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Therapeutics S, Ascending Dose Study of Genome Editing by the Zinc Finger Nuclease (ZFN) Therapeutic SB-318 in Subjects With MPS I, A Phase I, Multicenter, Open-label, Singledose, Dose-ranging Study to Assess the Safety and Tolerability of SB-318, a rAAV2/6-based Gene Transfer in Subjects With Mucopolysaccharidosis I (MPS I), 2017.
- [189].Therapeutics S , Ascending Dose Study of Genome Editing by the Zinc Finger Nuclease (ZFN) Therapeutic SB-913 in Subjects With MPS II, A Phase I, Multicenter, Open-label, Singledose, Dose-ranging Study to Assess the Safety and Tolerability of SB-913, a rAAV2/6-based Gene Transfer in Subjects With Mucopolysaccharidosis II (MPS II), 2017.
- [190].Visigalli I, Delai S, Politi LS, Di Domenico C, Cerri F, Mrak E, D’Isa R, Ungaro D, Stok M, Sanvito F, Mariani E, Staszewsky L, Godi C, Russo I, Cecere F, Del Carro U, Rubinacci A, Brambilla R, Quattrini A, Di Natale P, Ponder K, Naldini L, Biffi A, Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model Blood 116 (2010) 5130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Aiuti A, Gene Therapy With Modified Autologous Hematopoietic Stem Cells for the Treatment of Patients With Mucopolysaccharidosis Type I, Hurler Variant (TigetT10_MPSIH), Italy, 2018. [Google Scholar]
- [192].Zonari E, Desantis G, Petrillo C, Boccalatte FE, Lidonnici MR, Kajaste-Rudnitski A, Aiuti A, Ferrari G, Naldini L, Gentner B, Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy Stem cell reports 8 (2017) 977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, Sandmaier BM, Storb R, Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors Blood 104 (2004) 1550–1558. [DOI] [PubMed] [Google Scholar]
- [194].Yokoi T, Yokoi K, Akiyama K, Higuchi T, Shimada Y, Kobayashi H, Sato T, Ohteki T, Otsu M, Nakauchi H, Ida H, Ohashi T, Non-myeloablative preconditioning with ACK2 (anti-c-kit antibody) is efficient in bone marrow transplantation for murine models of mucopolysaccharidosis type II Molecular genetics and metabolism 119 (2016) 232–238. [DOI] [PubMed] [Google Scholar]
- [195].Broder MS, Quock TP, Chang E, Reddy SR, Agarwal-Hashmi R, Arai S, Villa KF, The Cost of Hematopoietic Stem-Cell Transplantation in the United States American health & drug benefits 10 (2017) 366–374. [PMC free article] [PubMed] [Google Scholar]
- [196].Ezzone SA, History of hematopoietic stem cell transplantation Seminars in oncology nursing 25 (2009) 95–99. [DOI] [PubMed] [Google Scholar]
- [197].Thomas ED, Lochte HL Jr., Lu WC, Ferrebee JW, Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy The New England journal of medicine 257 (1957) 491–496. [DOI] [PubMed] [Google Scholar]
- [198].Mullen CA, Thompson JN, Richard LA, Chan KW, Unrelated umbilical cord blood transplantation in infancy for mucopolysaccharidosis type IIB (Hunter syndrome) complicated by autoimmune hemolytic anemia Bone marrow transplantation 25 (2000) 1093–1097. [DOI] [PubMed] [Google Scholar]
- [199].Parenti G, Pignata C, Vajro P, Salerno M, New strategies for the treatment of lysosomal storage diseases (review) International journal of molecular medicine 31 (2013) 1120. [DOI] [PubMed] [Google Scholar]
- [200].Bjoraker KJ, Delaney K, Peters C, Krivit W, Shapiro EG, Long-term outcomes of adaptive functions for children with mucopolysaccharidosis I (Hurler syndrome) treated with hematopoietic stem cell transplantation Journal of developmental and behavioral pediatrics: JDBP 27 (2006) 290–296. [DOI] [PubMed] [Google Scholar]
- [201].Hopwood JJ, Vellodi A, Scott HS, Morris CP, Litjens T, Clements PR, Brooks DA, Cooper A, Wraith JE, Long-term clinical progress in bone marrow transplanted mucopolysaccharidosis type I patients with a defined genotype Journal of inherited metabolic disease 16 (1993) 1024–1033. [DOI] [PubMed] [Google Scholar]
- [202].Hoogerbrugge PM, Brouwer OF, Bordigoni P, Ringden O, Kapaun P, Ortega JJ, O’Meara A, Cornu G, Souillet G, Frappaz D, et al. , Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation Lancet 345 (1995) 1398–1402. [DOI] [PubMed] [Google Scholar]
- [203].Whitley CB, Belani KG, Chang PN, Summers CG, Blazar BR, Tsai MY, Latchaw RE, Ramsay NK, Kersey JH, Long-term outcome of Hurler syndrome following bone marrow transplantation American journal of medical genetics 46 (1993) 209–218. [DOI] [PubMed] [Google Scholar]
- [204].Fleming DR, Henslee-Downey PJ, Ciocci G, Romond EH, Marciniak E, Munn RK, Thompson JS, The use of partially HLA-mismatched donors for allogeneic transplantation in patients with mucopolysaccharidosis-I Pediatric transplantation 2 (1998) 299–304. [PubMed] [Google Scholar]