Abstract
Increased use of implantable biomedical devices demonstrates their potential in treating a wide variety of ailments and disorders in bone trauma and orthopaedic, reconstructive, and craniofacial applications. However, the number of cases involving implant failure or malfunction due to bacterial infection have also increased in recent years. Implanted devices can facilitate the growth of bacteria as these micro-organisms have the potential to adhere to the implant and grow and develop to form biofilms. In an effort to better understand and mitigate these occurrences, biomaterials containing antimicrobial agents that can be released or presented within the local microenvironment have become an important area of research. In this review, we discuss critical factors that regulate antimicrobial therapy to sites of bone infection, such as key biomolecular considerations and platforms for delivery, as well as current in vivo models and current advances in the field.
Keywords: Antimicrobial, local delivery, bone defect, orthopaedic infection, implantable biomaterials
1. Introduction
Implantable medical devices have become an integral component of our lives, with over tens of millions of patients across the world and a market that is expected to reach $74 billion this year[1]. This high clinical activity has led to significant advances in the field, particularly with consideration towards the longevity and sustained performance of these devices. However, much less progress has been made towards mitigating infection and subsequent biofilm formation, and thus device-associated infection has become one of the leading causes of device failure[2].
Infections occurring from these orthopaedic devices have the potential to lead to a variety of complications. One of the most prominent diseases associated with orthopaedic device infection is osteomyelitis, which occurs in 2-5% of surgical interventions involving internal fixation devices[3]. The most common pathogens associated with these types of infections are the Staphylococcus aureus and Staphylococcus epidermidis species of bacteria, which are gram-positive bacteria with high propensity towards forming biofilms on implanted materials and are responsible for over 50% of osteomyelitis cases[4]. Biofilm formation is a problematic issue in the medical field due to the increase in tolerance and resistance of the bacteria to therapeutics and antibiotics, as well as an enhanced ability to resist clearance from the host immune system[5]. Biofilm bacterial resistance to antibiotics leads to an overall increase in the minimum inhibitory concentration (MIC) of around 10-1000 times larger than its planktonic counterpart[6].
The most common clinical solution to these kinds of infection involves debridement combined with systemic antibiotic treatment[7]. In the case of implant-associated infections, it has been shown that debridement and implant retention (DAIR) for treatment of early infection has a success rate of over 80%[8, 9]. Additionally, one-stage exchange, in which the implant is removed and a new implant is introduced within the same surgery, and two-stage exchange, in which the implant is removed and a new implant is introduced in two separate surgeries, have a success rate of over 87%[10, 11] and 91%[12, 13], respectively.
Whereas these techniques do show moderate levels of success, there are still several inherent issues which can be improved upon. It has been well established that the systemic introduction of antibiotics limits its overall performance, as there is a wide distribution of therapeutics that is collected at varying levels in different organs throughout the body, leading to poor delivery to the site of infection. This potential for off-targeting also restricts the overall amount of antibiotic that can be introduced, as high levels could lead to potential nephrotoxicity and hepatotoxicity for some antibiotics[14-16]. This toxicity limit eliminates the possibility of overcompensating dosage to make up for the loss of antibiotics to off-target effects. Additionally, nearly every type of antibiotic has been met with the development of resistance in the target bacteria, in what is sometimes referred to as the antibiotic “treadmill”[17]. The economic cost of this resistance, which can be defined as the incremental cost of treatment involving antibiotic-resistant bacteria versus susceptible bacteria, has been estimated to be around $2.8 billion in the United States alone[18].
One potential solution to this major issue is to create biomaterials that can be introduced locally to the site of infection in order to release therapeutics in close proximity. In fact, the act alone of introducing therapeutics locally has already been shown to be a more effective method of treatment for infection[19]. This result has sparked interest from many different research groups who have studied different types of antimicrobial therapeutics and utilize a multitude of different drug delivery carriers in an effort to solve this monumental issue.
2. In vivo bone infection models
Before discussing drug delivery carriers and antimicrobial agents that are introduced along with them, it is important to establish what constitutes an accurate in vivo model for bone infection. Early studies of osteomyelitis in animal models simply injected Staphylococci, either intravenously or onto the surface of the bone, in an attempt to recapitulate the physiological progression of infection[20]. Since then, it has been recognized that either introduction of a foreign substance or trauma to the bone is necessary for providing an ideal environment for bacterial seeding[21]. For cases such as modeling osteomyelitis, the location of injection can also play a role in the susceptibility of infection, as injection into the bone marrow cavity can potentially have a more substantial and relevant effect than injection onto the bone surface[22].
The choice of which animal model to use is an important consideration, and different animal models have different benefits and limitations. Rodent models make up the most extensively studied and well-understood animal models. This popularity is mostly due to the combination of low cost along with ease of handling and maintenance. However, these advantages come at the cost of small and fragile long bones that lack the Haversian-type remodeling that is typically seen in larger animal models[23]. Whereas rodents are the most well-studied animal models overall, rabbits are commonly used in musculoskeletal research, and were the first documented animal model for osteomyelitis[24]. Rabbits are a suitable model for bone infection applications due to their similar bone mineral density and fracture toughness compared to humans[25]. However, they still suffer in their small size and are known to be delicate and fragile post-surgery[26]. Larger animal models, such as pigs, sheep, dogs, and goats, make for a potentially appealing alternative model for bone infection due to their physiologically relevant size and weight of long bone. However, they are typically a much more expensive alternative as compared to small animal models, as they require larger housing, more attentive caring, and more specialized surgeons[27].
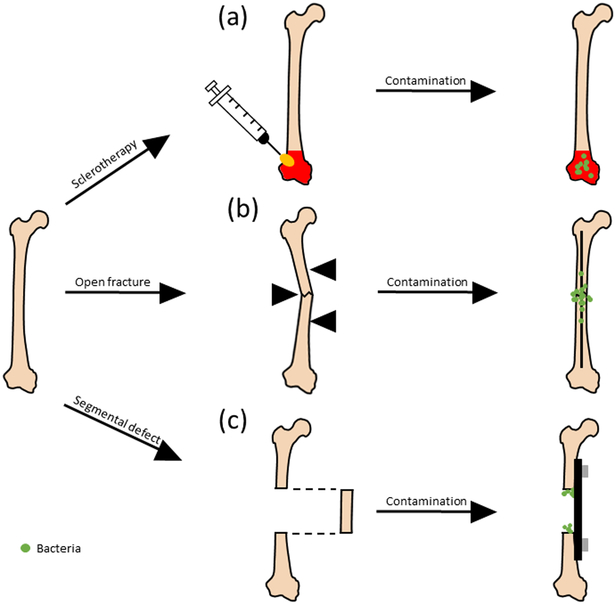
Another important consideration is the defect used to model bone infection. Most bone defect models fall into the following categories: sclerotherapic, open/closed fracture, and critical sized segmental defect (Fig. 1.).
Figure 1.
Common bone defect models for modeling infection study. (a) Sclerotherapy involves the introduction of compound such as sodium morrhuate, that causes necrosis at the surface of bone before introduction of bacteria (green). (b) Open fractures are typically induced through the use of a tool such as a 3-point bending (indicated with black triangles), after which is stabilized using either an internal or external fixation device. (c) Segmental defect occurs when a critically-sized segment of bone is removed, after which support is provided either through an internal or external fixation device (exception being when either the ulna or radius is involved).
2.1. Sclerotherapic
Sclerotherapy for bone applications is typically used as a means to induce injury at the surface of tissue without damaging the underlying vessels[28]. In the scope of bone infection, this provides a surface in which bacteria can prosper in order to more accurately model diseases such as osteomyelitis, while allowing for precise control over the area of injury and avoiding unnecessary complications and additional stabilization. Injury is typically induced through a solution, such as sodium morrhuate, that causes necrosis on the surface of the bone.
2.2. Open/closed fracture
Large trauma in the form of a fracture provide bacteria with a direct path into the body, and thus are very prone to infection. Infection rates for open fractures are typically higher than that of closed fractures, and can range from 10-50% depending on severity[29]. In animal models, fractures are typically generated transverse to the long bone, and in small animal model are typically generated through either 3-point bending (nondestructive) or 4-point bending (destructive)[30]. In this model, infection mitigation and bone healing are closely tied, as the increase of bacteria at the site of fracture is almost always correlated with a decrease in subsequent bone healing[31]. Mechanical tests can then be provided to quantify the degree of healing, with compression, torsional, tensile, and bending all being valid tests depending on the application[32].
2.3. Critical sized segmental defect
Critical-sized defects, which are defects that do not fully heal by themselves, show impaired callus formation and mineralization when compared to smaller gap defects[33], which are exacerbated in the presence of bacteria[34]. An additional challenge comes along with providing reliable stabilization after the segment is removed. Researchers have overcome this barrier through using techniques such as external[35, 36] or internal[37] fixation, as well as targeting either the radius or ulna for segmental defect analysis, as the removal of one segment still provides adequate stabilization in murine model[38].
3. Antimicrobial therapeutics
3.1. Antibiotics
As one of the first and most prominent antimicrobial agents, antibiotics are widely considered one of the most successful and well-understood therapeutics for infection in the past century. Whereas the definition of antibiotic has not remained static over time, it can be loosely defined as the substance produced from a microorganism that inhibits another microorganism[39]. One of the first antibiotics used was penicillin, which was discovered by Alexander Fleming, who observed the phenomenon through an accidental encounter of the Penicillium fungus with his culture of staphylococcal bacteria[40]. From that moment on, the field of antibiotics has erupted to provide a wide range of different antibiotics suited to target different species of microorganisms with different mechanisms of action (Table 1).
Table 1.
List of clinically relevant classes of antibiotics, their main bacterial targets, and their mechanism of actions.
| Class | Main targets[50] | Mechanism of action[50] | Common antibiotics[50, 51] |
|---|---|---|---|
| Aminoglycosides | Gram-negative bacteria | Disrupt protein synthesis | Gentamicin, tobramycin, streptomycin |
| Carbapenems | Gram-positive and gram-negative bacteria | Disrupt cell wall synthesis | Ertapenem, faropenem |
| Cephalosporins | Wide range of targets: early generations target gram-positive bacteria, while later generations target gram-negative bacteria, Methicillin-resistance Staphylococcus aureus (MRSA) | Disrupt cell wall synthesis | Cefixime, cefotaxime |
| Glycopeptides | Gram-positive bacteria, MRSA | Disrupt cell wall synthesis | Teicoplanin, vancomycin |
| Lincosamides | Gram-positive and gram-negative bacteria | Disrupt protein synthesis | Clindamycin, Lincomycin |
| Lipopeptides | Gram-positive bacteria | Disrupt cell membrane potential | Daptomycin |
| Macrolides | Gram-positive bacteria, some gram-negative bacteria | Disrupt protein synthesis | Azithromycin, clarithromycin |
| Monobactams | Gram-negative bacteria | Disrupt cell wall synthesis | Aztreonam |
| Oxazolidinones | Gram-positive bacteria | Disrupt protein synthesis | Linezolid |
| Penicillins | Streptococci, Staphylococci, Neisseria species | Disrupt cell wall synthesis | Amoxicillin, ampicillin, penicillin G |
| Polymyxin | Gram-negative bacteria | Disrupt cell wall synthesis | Polymyxin B, Polymyxin E |
| Quinolones | Gram-positive and gram-negative bacteria | Disrupt DNA synthesis | Cinoxacin, nalidixic acid |
| Rifamycins | Mycobacteria | Disrupt RNA synthesis | Rifabutin, rifampin |
| Streptogramins | Gram-positive bacteria | Disrupt protein synthesis | Quinupristin, dalfopristin |
| Sulphonamides | Gram-positive and gram-negative bacteria | Disrupt folic acid synthesis | Sulfamethizole, sulfamethoxazole |
| Tetracyclines | Gram-positive and gram-negative bacteria | Disrupt protein synthesis | Tigecycline |
Researchers can take advantage of these properties of antibiotics to create novel platforms for local targeting of infection. In fact, this general idea has been put in to practice since the 1970’s, with Buchholz and Engelbrecht, who would incorporate antibiotics into a poly(methyl methacrylate) (PMMA)-based bone cement that would be introduced as a prophylactic for joint arthroplasty[41]. This idea allowed for a simple method of local release that was safe for the surrounding microenvironment, which could be loaded with multiple types of antibiotics to allow for effective results. This idea was later expanded by Klemm et al., who loaded gentamicin within PMMA beads, which would be incorporated into the open areas of the bone that were left after debridement[42]. This technique was applied to 128 patients suffering from severe chronic osteomyelitis, in which 91% experienced complete recession. This study shows the potential of this biomaterial to not only be used as a prophylactic, but also as a method for treating existing diseases.
An interesting development in recent years is the repurposing of existing therapeutics as antibiotics. Due to the significant investment of time and money that goes into drug development and subsequent FDA approval, the number of approvals has seen a drastic decrease of around 90% in recent years[43]. An alternative approach to circumvent this issue is to use current therapeutics that already have FDA approval and test them for potential antibiotic properties[44]. This method of testing FDA approved drugs has already seen success, as commercial therapeutics such as auranofin[45], celecoxib[46], and niclosamide/oxyclozanide[47] have shown promising results.
It is also important to note that one of the major limitations of antibiotics is that they can be met with resistance from bacteria. This has become a growing problem in the medical field, as each year resistant strains of bacteria are responsible for patient morbidity. For example, methicillin-resistant Staphylococcus aureus (MRSA) is responsible for the death of 19,000 people and the hospitalization of 360,000 people each year in the United States alone[48]. It is for this reason that many microbiologists have strongly advised for the controlled use and lower dosage of antibiotics in the clinical setting[49].
3.2. Antimicrobial peptides
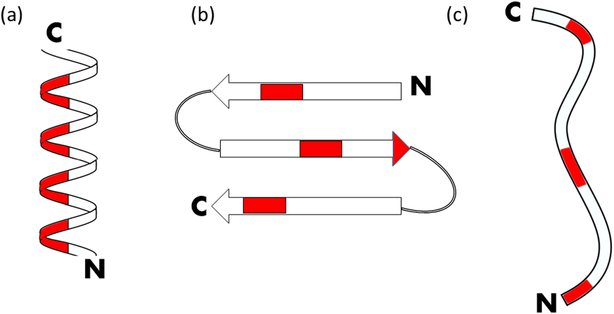
Typically considered to be a subset of modern antibiotics, antimicrobial peptides (AMPs) are oligopeptides containing a sequence of amino acids that can vary in length, which target a wide range of organisms with unique mechanisms of action that differ from conventional antibiotics[52, 53]. The use of antimicrobial peptides to treat orthopaedic infections has become an appealing alternative to antibiotics in recent years due in part because of the low levels of induced resistances against AMPs. While AMPs are inherently very diverse in structure and function, the amino acids found in AMPs tend to make them more cationic and amphipathic relative to the overall proteome[54]. The drawbacks of AMPs include their high cost for manufacturing and screening, their potential susceptibility to proteolysis[55], and their cytotoxicity tendencies towards mammalian cells[56]. AMPs can be further characterized by their structure: α helical, β sheet, and extended/flexible[57] (Fig. 2.).
Figure 2.
Antimicrobial peptides can be classified by their secondary structure: (a) α-helical, (b) β-sheet, (c) extended/flexible. Red regions indicate hydrophobic residues for the configuration of magainin, defensin 5, and indolicidin, respectively. N refers to the N-terminus, while C refers to the C-terminus.
3.2.1. Alpha helical
Alpha helical AMPs (aAMPs) are considered the most common and well-studied of the 3 subtypes. These peptides also display a wide range of efficacy in killing bacteria, as both gram-positive and gram-negative bacteria are susceptible targets[58]. These peptides have already been shown to be successfully integrated within a calcium phosphate cement carrier to assess the antimicrobial potential in an osteomyelitis model[59]. The results from this experiment showed that the local delivery of the aAMP to the site of infection showed greater efficiency in eradicating the biofilm in comparison to local delivery of antibiotics using the same conditions.
3.2.2. Beta sheet
Certain AMPs can be classified based to their β-sheet conformation, which occurs due to the cysteine residues that form disulfide bonds[60]. The largest subclass of β-sheet AMPs are the defensin family, which are endogenous peptides that were originally isolated from human skin. These peptides showed strong antimicrobial activity through targeting their cell membrane[61]. Varoga et al. has done extensive research on the human β-defensins (HBD), showing that the HBD-2 and HBD-3 are an active component of the host defense against the early onset of osteomyelitis[61-63].
3.2.3. Extended/flexible
This last subclass of AMPs is uniquely defined by its additional proline residues, which gives it a structure that strays away from both α-helix β-sheet to form a more extended coil-like structure[57]. While these peptides make up some of the least prevalent and least studied AMPs, there is ongoing research tailored towards utilizing them in orthopaedic applications. Hilpert et al. have looked at tethering indolicidin variants on a cellulose support to assess antimicrobial acitivity[64]. Optimal activity was seen when the hydrophobic residues were close to the N-terminus and the cationic residues were close to the linking site, making indolicidin an ideal choice of peptide (Fig. 2c). Results showed that by optimizing the linking chemistry and amino acid sequences of the variant, these tethered peptides were effective at eradicating gram-positive bacteria, gram-negative bacteria, and yeast.
3.3. Antimicrobial polymers
Antimicrobial polymers are polymers in which the composition, length, hydrophobicity, and cationic charge can be optimized to promote passive and active bactericidal effects, as well as biocompatibility and efficacy[65]. Polymers can passively inhibit bacteria on the surface of biomaterials through providing minimal protein adsorption[66]. Active polymers include those that are functionalized with agents directed at killing bacteria. One of the most common active components is the positively-charged quaternary ammonium groups, which provides the polymer with a positive charge without the need for pH dependence. By containing both cationic groups and hydrophobic groups, these polymers are capable of attracting the anionic components of the bacterial cell membrane, where the hydrophobic residues can then insert themselves into the membrane and disrupt it[65]. An additional strategy for active functionalization involves tethering antimicrobial peptides to the polymer structure to immobilize and direct the activity of the peptides within the surface of a biomaterial, such as catheters[67].
3.4. Antimicrobial enzymes
Enzymes play a major role in antimicrobial activity in nature. As technology has advanced, researchers have been able to extract these enzymes to play a direct role in infection mitigation and biofilm eradication. They are an appealing alternative due to their ability to not only attack the microbe directly, but also to inhibit formation and promote dismantling of biofilms[68]. While antimicrobial enzymes are a promising therapeutic, they are typically less studied than alternative antimicrobial therapeutics and their application is limited by their high cost of production[69]. Key enzymes for antimicrobial activity include lysostaphin and bacteriophage lysins.
3.4.1. Lysostaphin
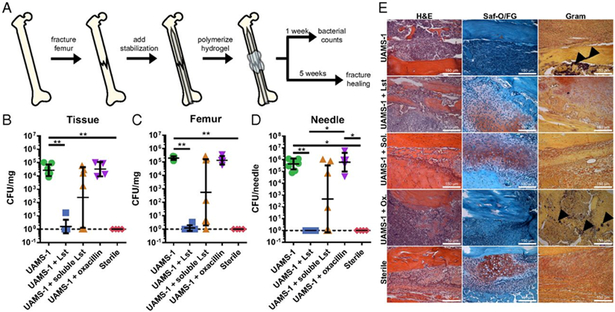
Lysostaphin is a bactericidal metalloendopeptidase derived from the gram-positive Staphylococcus simulans, which natively secretes enzymes like lysostaphin for peptidoglycan remodeling during its initial stages of growth[70]. Lysostaphin specifically targets the pentaglycine bridges on the cell membrane of staphylococcal species, making it an efficient disrupter of both planktonic bacteria and biofilm. This quality makes it an appealing agent for in vivo biofilm mitigation in orthopaedic infection. Johnson et al. utilized lysostaphin within a hydrogel carrier for controlled local delivery in a murine bone fracture model[71]. Their model tested the efficacy of their lysostaphin-encapsulated hydrogel alongside prophylactic antibiotic therapy and delivery of lysostaphin without the hydrogel carrier. Local delivery of lysostaphin via the hydrogel carrier significantly outperformed systemic delivery of oxacillin and resulted in complete healing of the fracture. This system also showed the ability to mitigate methicillin-resistant S. aureus infection, making it an overall more appealing strategy for targeting orthopaedic implant infection.
3.4.2. Bacteriophage lysins
Bacteriophages are viruses that target bacteria with high specificity. The virus infiltrates the cell membrane of the bacteria and replicates inside of the bacteria, and will then either burst out of the bacteria, or continue replicating alongside the bacteria for many generations[72]. Lysins are utilized during this process to disrupt the peptidoglycan layer and thus initiating cell lysis. This mechanism can be recreating using recombinant lysin introduced exogenously to gram-positive bacteria, which sparked interest in the scientific community to utilize this enzyme for antimicrobial applications[73]. Since then, phage lysins have been extensively studied and have seen a wide range of uses for antimicrobial studies[74-78]. Becker et al. have shown potential to utilize lysin for treatment of orthopaedic infection[79]. Their research utilized the peptidoglycan hydrolases (PGH) derived from bacteriophage endolysins (LysK) fused with lysostaphin to create a chimeric molecule containing three lytic domains. The triple-acting chimeric fusion molecules were tested in an in vivo murine femur injury model of osteomyelitis, and showed drastic reduction of bacteria.
3.5. Current and ongoing clinical trials for osteomyelitis utilizing antimicrobial agents
Recent and ongoing clinical trials that utilize antimicrobial agents for treatment of osteomyelitis can be found in Table 2.
Table 2.
List of current clinical trials utilizing antimicrobial agents for treatment of osteomyelitis, including its current status and National Clinical Trial (NCT) number.
| Responsible party | Antimicrobial agent | Status of trial | NCT number |
|---|---|---|---|
| Kaplan et al. | Ceftaroline | Active | NCT02335905 |
| Oliveira et al. | Tigecycline | Active | NCT03559530 |
| Rappo et al. | Dalbavancin | Completed | NCT02685033 |
| Ramirez et al. | Multiple antibiotics | Active | NCT02099240 |
| Cubist Pharmaceuticals LLC | Daptomycin | Completed | NCT00428844, NCT01922011 |
| Luo et al. | Vancomycin | Completed | NCT02968693 |
| VA Office of Research and Development | Rifampin | Active | NCT03012529 |
| Harrington et al. | Trimethoprim-sulfamethoxazole | Unknown | NCT00324922 |
| Borens et al. | Gentamicin | Unknown | NCT02128256 |
| Martínez et al. | Ciprofloxacin | Unknown | NCT01137903 |
| Assistance Publique – Hôpitaux de Paris | Multiple antibiotics | Completed | NCT00764114 |
| Tourcoing Hospital | Multiple antibiotics | Completed | NCT02123628 |
| University of Aarhus | Pamidronatdinatrium | Active | NCT02594878 |
| Wyeth | Tigecycline, Ertapenem | Completed | NCT00366249 |
| Infectious Diseases Physicians, Inc. | Dalbavancin | Active | NCT03426761 |
| Miller et al. | Tedizolid | Active | NCT03009045 |
| Cempra Inc. | Sodium fusidate (CEM-102) | Active | NCT02569541 |
| Merck Sharp & Dohme Corp. | Ertapenem sodium (MK-0826) | Completed | NCT01370616 |
| Microbion Corp. | MBN-101 | Active | NCT02436876 |
4. Drug Delivery Carriers
In designing a suitable delivery system for therapeutics to the site of infection, several criteria should be considered to optimize therapeutic delivery: characteristics of antimicrobial agent, invading bacterial species, anatomical location of the infection, and the therapeutic release dynamics of the biomaterial[80]. Each carrier has clear advantages and disadvantages, and while no single delivery vehicle is considered superior, selecting the right carrier is a critical step in designing the delivery system.
4.1. Bone Cement
Traditionally, bone cement is composed of a PMMA-based material, which is polymerized in the free space between the implant and bone to anchor it in place[81]. It is a useful carrier of antimicrobials and application is typically very easy to carry out, as the antimicrobial agent is mixed in with the powder component before mixing. For this reason, it is no surprise that bone cements were some of the first delivery vehicles of antimicrobial therapeutics for orthopaedic applications. In the 1970’s and 1980’s, PMMA bone cements were typically mixed with antibiotics for local treatment of infection[41, 42].
As technology advanced, these designs branched out to include cements composed of materials such as calcium phosphate, which sets as hydroxyapatite, making it a much more biocompatible alternate that can easily be replaced with bone over time[82, 83], and glass polyalkenoate, which is used in dental applications. Although these bone cements show promise as an antimicrobial implant, they do not have the ability to degrade, and removal requires an additional surgery. As the field progressed, alternatives that provide antibiotic release through degradation became an appealing alternative. Antibiotic-loaded collagen sponges have been a popular biomaterial for this very reason, and have a popular alternative since the 1980’s. These biomaterials can either be introduced directly to the site of bone defect as a temporary scaffold, or can be manufactured as a film or membrane to be used as a coating for prosthetic or implant.[84-87]. The idea of biodegradable coating has also led to more sophisticated designs, such as the use of bioresorbable polymer films containing antibiotics, like poly(α-hydroxy acids)[88, 89], poly(ε-caprolactone)[90, 91], and poly(trimethylene carbonate)[92, 93]. The modular capabilities of these materials allow for precise control over its mechanical properties.
Bone cements have also shown the capability to be introduced in combination with other local therapeutic delivery vehicles to enhance its antimicrobial potential, such as polymer films[91], hydrogels[90, 94, 95], and nanoparticles[96, 97]. However, their main limitation is their propensity to fragmentation and creating wear debris, promoting inflammation at the site of the implant[81]. Additionally, the low surface area-to-volume ratio provides suboptimal elution characteristics that limit the amount of therapeutics that can be released from the bone cement and increasing the MIC for this vehicle[98, 99].
4.2. Collagen Sponges
Collagen is an excellent candidate for biomaterial applications due to its biodegradability, lack of toxicity, high tensile strength, and high abundancy in nature. Collagen can be prepared as a sponge by lyophilizing collagen solutions containing 0.1-5% w/v dry matter. The porosity can be modulated through altering the amount of dry matter or the rate of freezing[100]. Collagen sponges have since become commercialized, and are easy to access and affordable, making this platform an appealing candidate for antimicrobial delivery.
If one of the advantages of collagen sponges are the ease of access and affordability, it is only natural that the chosen antimicrobial to load into them is most often antibiotics. In applications involving orthopaedic infection, the collagen sponge is often paired with aminoglycosides, like gentamicin[84, 101, 102], due to the fact that aminoglycosides have been shown to be less detrimental to osteoblasts[103]. Similar to bone cements, collagen sponges can also benefit from having additional delivery vehicles introduced within the scaffold to provide stronger antimicrobial therapy. For example, Schlapp et al. showed that with gentamicin-loaded PLGA particles encapsulated within a collagen sponge matrix allowed for greater retention time and sustained antibiotic delivery for up to a week[104]. However, in comparison to bone cement, its poor mechanical strength may limit its application. Additionally, the use of animal-derived collagen may produce tissue irritation and an antigenic response from the patient.
4.3. Nanoparticles
The field of nanotechnology has seen a significant rise in popularity in recent years, as access to more sophisticated instruments has allowed more researchers to more easily manipulate, analyze, and functionalize systems in the nanoscale. Nanoparticles benefit from having unique transport qualities that can be manipulated for precise targeting. In the case of biofilms, studies have shown that not only is the small size and large surface area-to-volume ratio of the particle a useful tool in the prevention and mitigation of biofilm formation, but also the geometric shape of nanoparticles can play a role in its resulting effectiveness[105]. Nanoparticles can also combat bacteria either through the encapsulation or tethering of antimicrobial agents or through their own composition.
4.3.1. Nanoparticles with inherent antimicrobial activity
The inherent antimicrobial activity of nanoparticles typically act through one of three mechanisms: induction of oxidative stress, release of metal ions, non-oxidative stress mechanisms[106]. The use of metal ions, such as silver, as an antimicrobial agent have been an intriguing alternative therapeutic; however, they suffer from both a short lifespan of antimicrobial activity, as well as the need to be within an aqueous environment for extended periods of time in order to function properly[107]. However, groups such as Qadri et al. have synthesized metal ion nanoparticles that can provide sustained activity using a synergistic effect of silver-copper-boron composites in a bone infection model[108]. These nanoparticles showed significant reduction in bacteria within a murine osteomyelitis model, as compared to an iron oxide control nanoparticle.
4.3.2. Nanoparticles as carriers of antimicrobial agents
Nanoparticles that act as antimicrobial carriers are typically either physically entrapping or linking the antimicrobial agent to itself. Ferreira et al. looked to use a pH-sensitive liposomal carrier of radiolabeled ceftizoxime, to assess the delivery of these particles to the site of bone infection[109]. These pH-sensitive liposome carriers allow for higher affinity to sites of infection, and subsequent release of contents. Microparticles have also shown the ability to perform similar actions, such as Sofokleous et al., who looked at the release of oxidative biocides from PLGA microparticles, which showed prolonged release and activity when introduced subcutaneously in Sprague Dawley rats[110].
4.4. Hydrogels
Hydrogels are water-swollen polymeric networks that possess tunable properties, including swelling, pore size, molecular weight, and stiffness[111]. The modular nature of most synthetic hydrogels also allows for the decoration of molecules within its matrix that can direct surrounding cell function, such as cell adhesion, proliferation, and differentiation. Antimicrobial agents can be introduced into hydrogels either through physical entrapment[95, 112] or through tethering directly onto the network[113]. Johnson et al. utilized a PEG-4MAL hydrogel, which takes advantage of Michael-type addition chemistry to allow for cross-linking of a 4-arm PEG macromer at high specificity, to physically entrap lysostaphin, an antimicrobial endopeptidase, and allow for controlled release through sustained degradable of the gel[71]. The hydrogel carrier proved to be an integral part of the therapeutic delivery system, as local delivery of lysostaphin alone showed significant reduction in infection mitigation (Fig. 3.). Hydrogels have also shown prominent antimicrobial properties utilizing inorganic metals[114, 115], AMPs[116], and antibiotics[117, 118]. Similar to collagen sponges, hydrogels are limited by their low mechanical strength which make them unusable for load-bearing applications. Additionally, due to the large pore size and water-swollen nature of hydrogels, small hydrophobic drugs may require a carrier, such as liposomes or nanoparticles, in order to be utilized for long-term release applications.
Figure 3.
(a) Schematic diagram detailing the model set-up for encapsulation of lysostaphin within a hydrogel matrix for localized infection mitigation and fracture healing in a bone defect model. (b) Bacterial counts 7 days postfracture of the tissue, femur, and stabilization needle for the following conditions: bacterial strain within hydrogel (UAMS-1), bacteria and lystostaphin within hydrogel (UAMS-1 + Lst), bacteria and lysostaphin without crosslinked hydrogel (UAMS-1 + soluble Lst), bacteria and prophylactic antibiotic injections (UAMS-1 + oxacillin), and a sterile control (sterile). (c) Histological sections of femurs stained with H&E, Saf-O/FG, and Gram. Arrows indicate sites of gram-positive bacteria. Reproduced with permission [71].
4.5. Surface coatings
Surface-coated biomaterials provide a unique means of disrupting bacterial adhesion to the surface of biomaterials, and have become a well-studied and fascinating approach that can take advantage of localization of antimicrobial molecules through methods such as physical adsorption, introduction into the polymer matrix, complexation, or conjugation[119]. They can not only provide a means of antimicrobial influence by directly interacting with the bacteria, but also indirectly by promoting tissue integration at the surface site of implantation, which is in competition with bacteria in what is coined as the “race for the surface”[120]. Antimicrobial polymers work well as a surface-coating agent as they can be tethered to biomaterial surfaces without losing functionality, and can incorporate other antimicrobial agents within itself[121]. In this way, antimicrobial agents, such as antibiotics, can be administered as a surface-coating over a biomaterial, with the benefit of providing direct interaction with the site of infection. Contrary to other vehicles that can be loaded with antibiotics, surface-coatings also benefit from higher efficacy due to the control of degradation, as well as the proximity of antibiotic release being constrained to the surface[98, 99].
5. Summary
Summary of the key research contributions addressed in this paper, including the platform and therapeutic used, is summarized below (Table 3):
Table 3.
Summary of key research, including delivery vehicle, therapeutic, and reference number.
| Group | Delivery vehicle | Therapeutic | Reference |
|---|---|---|---|
| Buchholz et al. | PMMA bone cement | Gentamicin | [33] |
| Klemm et al. | PMMA bone cement | Gentamicin | [34] |
| Luo et al. | PMMA+Ca3(PO4)2 cement | Vancomycin | [36] |
| Fang et al. | PCL+β-TCP composites | Vancomycin | [37] |
| Cometa et al. | PEG hydrogels on Ti implant | Vancomycin and ceftriaxone | [38] |
| Romanò et al. | Hydrogel + bone cement | Gentamicin, vancomycin, and cefazolin | [39] |
| Li et al. | PEG hydrogel on Ti implant | Vancomycin | [40] |
| Perni et al. | Bone cement + nanoparticles | Propylparaben | [41] |
| Shi et al. | Bone cement + nanoparticles | Chitosan, ammonium chitosan derivative, gentamicin | [42] |
| Susheel et al. | Collagen sponge | Gentamicin | [44] |
| Han et al. | Collagen sponge | Gentamicin | [45] |
| Ipsen et al. | Collagen sponge | Gentamicin | [46] |
| Schlapp et al. | Collagen sponge + PLGA particles | Gentamicin | [48] |
| Qadri et al. | Ag-Cu-B nanoparticles | Ag-Cu-B nanoparticles | [52] |
| Ferreira et al. | Liposomes | Ceftizoxime | [53] |
| Giavaresi et al. | Hydrogel | Vancomycin | [55] |
| Yeo et al. | Hydrogel | PEI star copolymer | [56] |
| Johnson et al. | PEG-4MAL hydrogel | Lysostaphin | [57] |
| Wachol-Drewek et al. | Collagen sponge | Gentamicin, cefotaxim, fusidic acid, clindamycin, vancomysin | [60] |
| Ascherl et al. | Collagen sponge | Gentamicin | [61] |
| Wernet et al. | Collagen sponge | Gentamicin | [62] |
| Aviv et al. | PLLA, PDLGA | Gentamicin | [63] |
| Price et al. | PLGA | Gentamicin | [64] |
| Neut et al. | PTMC | Gentamicin | [65] |
| Kluin et al. | PTMC | Gentamicin, vancomycin | [66] |
| Melicherčík et al. | Ca3(PO4)2 cement | HAL-1, HAL-2, HAL-2 analogues | [70] |
| Varoga et al. | Expressed in osteoblasts | HBD-2, MBD-3 | [72] |
| Varoga et al. | Expressed in healthy and osteoarthritic cartilage | HBD-2 | [73] |
| Varoga et al. | Expressed in chondrocytes | HBD-2 | [74] |
| Hilpert et al. | Cellulose support | Indolicidin variants | [75] |
| Nelson et al. | Oral and nasal introduction | C1 phage lysin | [80] |
| Schuch et al. | IP injection | PlyG lysin | [81] |
| Loeffler et al. | In vitro introduction | Pal, Cpl-1 lysin | [82] |
| Loeffler et al. | Intravenous | Cpl-1 lysin | [83] |
| Fernandes et al. | In vitro introduction | Lys168-Lys170 hybrid | [84] |
| Becker et al. | Direct introduction to femur injury | Lysk-Lysostaphin | [85] |
6. Conclusion
Local and sustained delivery of antimicrobial therapeutics for bone infections has clear advantages to the clinical standard of debridement, followed by system administration of antibiotics. As technology has become more sophisticated, the field has evolved to support many different types of carriers, as well as providing choices for antimicrobial therapeutics. Whereas this field shows exciting promise and potential, critical challenges must be addressed for this field to progress as a viable platform. Importantly, the act of implanting a biomaterial, and thus both introducing a foreign body and performing a surgery, provide a potential for bacterial introduction, as well as aggregation and colonization on the implant surface. The colony of bacteria can lie in a dormant state and remain undetectable for long periods of time, and upon growth and identification, will most likely need to be treated through a revision surgery, which can potentially exacerbate the severity of infection if the bacteria is not completely cleared[122]. Surface-coated biomaterials are one potential method of addressing this issue within the field of antimicrobial biomaterials. As mentioned previously, surface-coated biomaterials have shown the potential to be capable of both infection mitigation as well as promotion of tissue regeneration around the surface of the biomaterial, thus favoring tissue integration in the remodeled microenvironment.
It is also important to realize that the choice of animal model, bone defect, biomaterial, and therapeutic must all be compatible and designed based on the specific application being tested. While smaller animal models have had a longer history for bone infection applications, they lack some key traits that can be found in larger animal models. Additionally, the carrier plays a large role in the release kinetics of the therapeutic, as considerations such as ease of manufacturing, biodegradability, chemical tethering versus physical entrapment, and favorable transport properties can all greatly affect the overall therapeutic delivery. Lastly, the choice of antimicrobial therapeutic itself should not only be compatible with the carrier, but should also be tailored towards decisions such as cost, size, capabilities of synthesis, target bacterial strain, and ability to mitigate biofilm versus planktonic bacteria. As the field advances, these biomaterials will play a more prominent role in our daily lives, as some have already advanced to stages in which they are accepted into clinical trials.
7. Acknowledgements
The authors acknowledge funding from the U.S. National Institutes of Health (R01 AR062920).
8. References
- [1].Lind KD, Understanding the market for implantable medical devices, AARP Insight on the Issues 129 (2018). [Google Scholar]
- [2].Moriarty TF, Zaat SAJ, & Busscher HJ, Biomaterials associated infection: Immunological aspects and antimicrobial strategies, Springer; (2013). [Google Scholar]
- [3].Nair MB, Kretlow JD, Mikos AG, Kasper FK, Infection and tissue engineering in segmental bone defects--a mini review, Current opinion in biotechnology 22(5) (2011) 721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gomes D, Pereira M, Bettencourt AF, Osteomyelitis: an overview of antimicrobial therapy, Brazilian Journal of Pharmaceutical Sciences 49 (2013) 13–27. [Google Scholar]
- [5].Donlan RM, Costerton JW, Biofilms: survival mechanisms of clinically relevant microorganisms, Clinical microbiology reviews 15(2) (2002) 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J, Strategies for combating bacterial biofilm infections, International journal of oral science 7(1) (2014) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Penn-Barwell JG, Murray CK, Wenke JC, Early antibiotics and debridement independently reduce infection in an open fracture model, The Journal of bone and joint surgery. British volume 94(1) (2012) 107–12. [DOI] [PubMed] [Google Scholar]
- [8].de Vries L, van der Weegen W, Neve WC, Das H, Ridwan BU, Steens J, The Effectiveness of Debridement, Antibiotics and Irrigation for Periprosthetic Joint Infections after Primary Hip and Knee Arthroplasty. A 15 Years Retrospective Study in Two Community Hospitals in the Netherlands, Journal of bone and joint infection 1 (2016) 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weenders SGM, Nijhof MW, Schimmel JJP, Goosen JHM, Debridement, antibiotics and implant retention in early periprosthetic joint infection after primary total hip arthroplasty : 88 percent survival after two years follow-up, Acta orthopaedica Belgica 82(3) (2016) 530–538. [PubMed] [Google Scholar]
- [10].Jenny JY, Barbe B, Gaudias J, Boeri C, Argenson JN, High infection control rate and function after routine one-stage exchange for chronically infected TKA, Clinical orthopaedics and related research 471(1) (2013) 238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zahar A, Kendoff DO, Klatte TO, Gehrke TA, Can Good Infection Control Be Obtained in One-stage Exchange of the Infected TKA to a Rotating Hinge Design? 10-year Results, Clinical Orthopaedics and Related Research® 474(1) (2016) 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR, Culture-Negative Prosthetic Joint Infection, Clinical Infectious Diseases 45(9) (2007) 1113–1119. [DOI] [PubMed] [Google Scholar]
- [13].Macheras GA, Kateros K, Galanakos SP, Koutsostathis SD, Kontou E, Papadakis SA, The long-term results of a two-stage protocol for revision of an infected total knee replacement, The Journal of bone and joint surgery. British volume 93(11) (2011) 1487–92. [DOI] [PubMed] [Google Scholar]
- [14].Nagai J, [Molecular mechanisms underlying renal accumulation of aminoglycoside antibiotics and mechanism-based approach for developing nonnephrotoxic aminoglycoside therapy], Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 126(5) (2006) 327–35. [DOI] [PubMed] [Google Scholar]
- [15].Garrido-Mesa N, Zarzuelo A, Gálvez J, Minocycline: far beyond an antibiotic, British Journal of Pharmacology 169(2) (2013) 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB, Increasing Vancomycin Serum Trough Concentrations and Incidence of Nephrotoxicity, The American Journal of Medicine 123(12) (2010) 1143–1149. [DOI] [PubMed] [Google Scholar]
- [17].Read AF, Huijben S, Evolutionary biology and the avoidance of antimicrobial resistance, Evolutionary Applications 2(1) (2009) 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shrestha P, Cooper BS, Coast J, Oppong R, Do Thi Thuy N, Phodha T, Celhay O, Guerin PJ, Wertheim H, Lubell Y, Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use, Antimicrobial resistance and infection control 7 (2018) 98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loc-Carrillo C, Wang C, Canden A, Burr M, Agarwal J, Local Intramedullary Delivery of Vancomycin Can Prevent the Development of Long Bone Staphylococcus aureus Infection, PLoS One 11(7) (2016) e0160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rissing JP, Animal models of osteomyelitis. Knowledge, hypothesis, and speculation, Infectious disease clinics of North America 4(3) (1990) 377–90. [PubMed] [Google Scholar]
- [21].Patel M, Rojavin Y, Jamali AA, Wasielewski SJ, Salgado CJ, Animal models for the study of osteomyelitis, Seminars in plastic surgery 23(2) (2009) 148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Norden CW, Kennedy E, Experimental Osteomyelitis. I. A Description of the Model, The Journal of Infectious Diseases 122(5) (1970) 410–418. [DOI] [PubMed] [Google Scholar]
- [23].Li Y, Chen S-K, Li L, Qin L, Wang X-L, Lai Y-X, Bone defect animal models for testing efficacy of bone substitute biomaterials, Journal of Orthopaedic Translation 3(3) (2015) 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Neyt JG, Buckwalter JA, Carroll NC, Use of animal models in musculoskeletal research, The Iowa orthopaedic journal 18 (1998) 118–123. [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Mabrey JD, Agrawal CM, An interspecies comparison of bone fracture properties, Bio-medical materials and engineering 8(1) (1998) 1–9. [PubMed] [Google Scholar]
- [26].Mapara M, Thomas BS, Bhat KM, Rabbit as an animal model for experimental research, Dental Research Journal 9(1) (2012) 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG, Animal models for implant biomaterial research in bone: a review, European cells & materials 13 (2007) 1–10. [DOI] [PubMed] [Google Scholar]
- [28].Varshney MK, Rastogi S, Khan SA, Trikha V, Is Sclerotherapy Better than Intralesional Excision for Treating Aneurysmal Bone Cysts?, Clinical Orthopaedics and Related Research® 468(6) (2010) 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lindsey BA, Clovis NB, Smith ES, Salihu S, Hubbard DF, An animal model for open femur fracture and osteomyelitis: Part I, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 28(1) (2010) 38–42. [DOI] [PubMed] [Google Scholar]
- [30].Qi B, Yu J, Zhao Y, Zhu D, Yu T, Mouse fracture models: a primer, Int J Clin Exp Med 9(7) (2016) 12418–14989. [Google Scholar]
- [31].Thomas MV, Puleo DA, Infection, Inflammation, and Bone Regeneration: a Paradoxical Relationship, Journal of Dental Research 90(9) (2011) 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Prodinger PM, Foehr P, Bürklein D, Bissinger O, Pilge H, Kreutzer K, von Eisenhart-Rothe R, Tischer T, Whole bone testing in small animals: systematic characterization of the mechanical properties of different rodent bones available for rat fracture models, European Journal of Medical Research 23(1) (2018) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mehta M, Checa S, Lienau J, Hutmacher D, Duda GN, In vivo tracking of segmental bone defect healing reveals that callus patterning is related to early mechanical stimuli, European cells & materials 24 (2012) 358–71; discussion 371. [DOI] [PubMed] [Google Scholar]
- [34].Chen X, Tsukayama DT, Kidder LS, Bourgeault CA, Schmidt AH, Lew WD, Characterization of a chronic infection in an internally-stabilized segmental defect in the rat femur, Journal of Orthopaedic Research 23(4) (2005) 816–823. [DOI] [PubMed] [Google Scholar]
- [35].DeConde AS, Lee MK, Sidell D, Defining the critical-sized defect in a rat segmental mandibulectomy model, JAMA Otolaryngology–Head & Neck Surgery 140(1) (2014) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Glatt V, Matthys R, Adjustable Stiffness, External Fixator for the Rat Femur Osteotomy and Segmental Bone Defect Models, Journal of visualized experiments : JoVE (92) (2014) 51558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu K, Li D, Huang X, Lv K, Ongodia D, Zhu L, Zhou L, Li Z, A murine femoral segmental defect model for bone tissue engineering using a novel rigid internal fixation system, The Journal of surgical research 183(2) (2013) 493–502. [DOI] [PubMed] [Google Scholar]
- [38].Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K, The effect of platelet-rich plasma on healing in critical-size long-bone defects, Biomaterials 29(29) (2008) 3983–3992. [DOI] [PubMed] [Google Scholar]
- [39].Types of Antibiotics and Synthetic Antimicrobial Agents, Hugo and Russell’s Pharmaceutical Microbiology. [Google Scholar]
- [40].Tan SY, Tatsumura Y, Alexander Fleming (1881–1955): Discoverer of penicillin, Singapore Medical Journal 56(7) (2015) 366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buchholz HW, Engelbrecht H, [Depot effects of various antibiotics mixed with Palacos resins], Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 41(11) (1970) 511–5. [PubMed] [Google Scholar]
- [42].Klemm K, [Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl)], Zentralblatt fur Chirurgie 104(14) (1979) 934–42. [PubMed] [Google Scholar]
- [43].Luepke KH, Suda KJ, Boucher H, Russo RL, Bonney MW, Hunt TD, Mohr Iii JF, Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance, Limited Antibiotic Pipeline, and Societal Implications, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 37(1) (2017) 71–84. [DOI] [PubMed] [Google Scholar]
- [44].Hernandez JJ, Pryszlak M, Smith L, Yanchus C, Kurji N, Shahani VM, Molinski SV, Giving Drugs a Second Chance: Overcoming Regulatory and Financial Hurdles in Repurposing Approved Drugs As Cancer Therapeutics, Frontiers in oncology 7 (2017) 273–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Roder C, Thomson MJ, Auranofin: repurposing an old drug for a golden new age, Drugs in R&D 15(1) (2015) 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thangamani S, Younis W, Seleem MN, Repurposing celecoxib as a topical antimicrobial agent, Frontiers in microbiology 6 (2015) 750–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, Ausubel FM, Mylonakis E, Repurposing Salicylanilide Anthelmintic Drugs to Combat Drug Resistant Staphylococcus aureus, PLOS ONE 10(4) (2015) e0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martens E, Demain AL, The antibiotic resistance crisis, with a focus on the United States, The Journal Of Antibiotics 70 (2017) 520. [DOI] [PubMed] [Google Scholar]
- [49].Ventola CL, The Antibiotic Resistance Crisis: Part 1: Causes and Threats, Pharmacy and Therapeutics 40(4) (2015) 277–283. [PMC free article] [PubMed] [Google Scholar]
- [50].Moore D, Antibiotic classification and mechanism, 2013. https://www.orthobullets.com/basic-science/9059/antibiotic-classification-and-mechanism. (Accessed December 21st, 2018.
- [51].Fair RJ, Tor Y, Antibiotics and bacterial resistance in the 21st century, Perspectives in medicinal chemistry 6 (2014) 25–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Aoki W, Ueda M, Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics, Pharmaceuticals (Basel, Switzerland) 6(8) (2013) 1055–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bahar AA, Ren D, Antimicrobial peptides, Pharmaceuticals (Basel, Switzerland) 6(12) (2013) 1543–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wimley WC, Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model, ACS chemical biology 5(10) (2010) 905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gordon YJ, Romanowski EG, McDermott AM, A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs, Current eye research 30(7) (2005) 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bacalum M, Radu M, Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept, International Journal of Peptide Research and Therapeutics 21(1) (2015) 47–55. [Google Scholar]
- [57].Kumar P, Kizhakkedathu JN, Straus SK, Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo, Biomolecules 8(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Giangaspero A, Sandri L, Tossi A, Amphipathic alpha helical antimicrobial peptides, European journal of biochemistry 268(21) (2001) 5589–600. [DOI] [PubMed] [Google Scholar]
- [59].Melicherčík P, Nešuta O, Čeřovský V, Antimicrobial Peptides for Topical Treatment of Osteomyelitis and Implant-Related Infections: Study in the Spongy Bone, Pharmaceuticals 11(1) (2018) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ebenhan T, Gheysens O, Kruger HG, Zeevaart JR, Sathekge MM, Antimicrobial Peptides: Their Role as Infection-Selective Tracers for Molecular Imaging, BioMed Research International 2014 (2014) 867381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Varoga D, Tohidnezhad M, Paulsen F, Wruck CJ, Brandenburg L, Mentlein R, Lippross S, Hassenpflug J, Besch L, Müller M, Jürgens C, Seekamp A, Schmitt L, Pufe T, The role of human β-defensin-2 in bone, Journal of Anatomy 213(6) (2008) 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Varoga D, Paulsen F, Kohrs S, Grohmann S, Lippross S, Mentlein R, Tillmann B, Goldring M, Besch L, Pufe T, Expression and regulation of human β-defensin-2 in osteoarthritic cartilage, The Journal of Pathology 209(2) (2006) 166–173. [DOI] [PubMed] [Google Scholar]
- [63].Varoga D, Pufe T, Harder J, Meyer-Hoffert U, Mentlein R, Schroder JM, Petersen WJ, Tillmann BN, Proksch E, Goldring MB, Paulsen FP, Production of endogenous antibiotics in articular cartilage, Arthritis and rheumatism 50(11) (2004) 3526–34. [DOI] [PubMed] [Google Scholar]
- [64].Hilpert K, Elliott M, Jenssen H, Kindrachuk J, Fjell CD, Körner J, Winkler DFH, Weaver LL, Henklein P, Ulrich AS, Chiang SHY, Farmer SW, Pante N, Volkmer R, Hancock REW, Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity, Chemistry & Biology 16(1) (2009) 58–69. [DOI] [PubMed] [Google Scholar]
- [65].Ergene C, Yasuhara K, Palermo EF, Biomimetic antimicrobial polymers: recent advances in molecular design, Polymer Chemistry 9(18) (2018) 2407–2427. [Google Scholar]
- [66].Moerman FT, Antimicrobial Materials , Coatings and Biomimetic Surfaces with Modified Microtography to Control Microbial Fouling of Product Contact Surfaces within Food Processing Equipment : Legislation , Requirements , Effectiveness and Challenges, Journal of Hygienic Engineering and Design (2014). [Google Scholar]
- [67].Lim K, Chua RR, Bow H, Tambyah PA, Hadinoto K, Leong SS, Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties, Acta Biomater 15 (2015) 127–38. [DOI] [PubMed] [Google Scholar]
- [68].Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM, Antimicrobial enzymes: An emerging strategy to fight microbes and microbial biofilms, Biotechnology Journal 8(1) (2013) 97–109. [DOI] [PubMed] [Google Scholar]
- [69].Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ, Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides, Journal of Applied Microbiology 104(1) (2008) 1–13. [DOI] [PubMed] [Google Scholar]
- [70].Bastos MD, Coutinho BG, Coelho ML, Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications, Pharmaceuticals (Basel, Switzerland) 3(4) (2010) 1139–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Johnson CT, Wroe JA, Agarwal R, Martin KE, Guldberg RE, Donlan RM, Westblade LF, García AJ, Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by <em>Staphylococcus aureus</em> and supports fracture healing, Proceedings of the National Academy of Sciences (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Haq IU, Chaudhry WN, Akhtar MN, Andleeb S, Qadri I, Bacteriophages and their implications on future biotechnology: a review, Virology Journal 9(1) (2012) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fenton M, Ross P, McAuliffe O, O’Mahony J, Coffey A, Recombinant bacteriophage lysins as antibacterials, Bioengineered Bugs 1(1) (2010) 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nelson D, Loomis L, Fischetti VA, Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme, Proceedings of the National Academy of Sciences of the United States of America 98(7) (2001) 4107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schuch R, Nelson D, Fischetti VA, A bacteriolytic agent that detects and kills Bacillus anthracis, Nature 418(6900) (2002) 884–9. [DOI] [PubMed] [Google Scholar]
- [76].Loeffler JM, Fischetti VA, Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains, Antimicrobial agents and chemotherapy 47(1) (2003) 375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Loeffler JM, Djurkovic S, Fischetti VA, Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia, Infection and immunity 71(11) (2003) 6199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fernandes S, Proenca D, Cantante C, Silva FA, Leandro C, Lourenco S, Milheirico C, de Lencastre H, Cavaco-Silva P, Pimentel M, Sao-Jose C, Novel chimerical endolysins with broad antimicrobial activity against methicillin-resistant Staphylococcus aureus, Microbial drug resistance (Larchmont, N.Y.) 18(3) (2012) 333–43. [DOI] [PubMed] [Google Scholar]
- [79].Becker SC, Roach DR, Chauhan VS, Shen Y, Foster-Frey J, Powell AM, Bauchan G, Lease RA, Mohammadi H, Harty WJ, Simmons C, Schmelcher M, Camp M, Dong S, Baker JR, Sheen TR, Doran KS, Pritchard DG, Almeida RA, Nelson DC, Marriott I, Lee JC, Donovan DM, Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus, Scientific Reports 6 (2016) 25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Campoccia D, Montanaro L, Speziale P, Arciola CR, Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use, Biomaterials 31(25) (2010) 6363–77. [DOI] [PubMed] [Google Scholar]
- [81].Vaishya R, Chauhan M, Vaish A, Bone cement, Journal of Clinical Orthopaedics and Trauma 4(4) (2013) 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ambard AJ, Mueninghoff L, Calcium phosphate cement: review of mechanical and biological properties, Journal of prosthodontics : official journal of the American College of Prosthodontists 15(5) (2006) 321–8. [DOI] [PubMed] [Google Scholar]
- [83].Luo S, Jiang T, Yang Y, Yang X, Zhao J, Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis, BMC musculoskeletal disorders 17(1) (2016) 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Susheel C, Ramesh S, Chand SU, Ashwani S, Nitesh G, Daljit S, Shuang-ming S, Use of gentamicin-loaded collagen sponge in internal fixation of open fractures, Chinese Journal of Traumatology 14(4) (2011) 209–214. [PubMed] [Google Scholar]
- [85].Wachol-Drewek Z, Pfeiffer M, Scholl E, Comparative investigation of drug delivery of collagen implants saturated in antibiotic solutions and a sponge containing gentamicin, Biomaterials 17(17) (1996) 1733–1738. [DOI] [PubMed] [Google Scholar]
- [86].Ascherl R, Stemberger A, Lechner F, Plaumann L, Rupp G, Machka K, Erhardt W, Sorg KH, Blumel G, [Treatment of chronic osteomyelitis with a collagen-antibiotic compound--preliminary report], Unfallchirurgie 12(3) (1986) 125–7. [DOI] [PubMed] [Google Scholar]
- [87].Wernet E, Ekkernkamp A, Jellestad H, Muhr G, [Antibiotic-containing collagen sponge in therapy of osteitis], Der Unfallchirurg 95(5) (1992) 259–64. [PubMed] [Google Scholar]
- [88].Aviv M, Berdicevsky I, Zilberman M, Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants, Journal of biomedical materials research. Part A 83(1) (2007) 10–9. [DOI] [PubMed] [Google Scholar]
- [89].Price JS, Tencer AF, Arm DM, Bohach GA, Controlled release of antibiotics from coated orthopedic implants, Journal of biomedical materials research 30(3) (1996) 281–6. [DOI] [PubMed] [Google Scholar]
- [90].Li D, Lv P, Fan L, Huang Y, Yang F, Mei X, Wu D, The immobilization of antibiotic-loaded polymeric coatings on osteoarticular Ti implants for the prevention of bone infections, Biomaterials science 5(11) (2017) 2337–2346. [DOI] [PubMed] [Google Scholar]
- [91].Fang T, Wen J, Zhou J, Shao Z, Dong J, Poly (ε-caprolactone) coating delays vancomycin delivery from porous chitosan/β-tricalcium phosphate composites, Journal of Biomedical Materials Research Part B: Applied Biomaterials 100B(7) (2012) 1803–1811. [DOI] [PubMed] [Google Scholar]
- [92].Neut D, Kluin OS, Crielaard BJ, van der Mei HC, Busscher HJ, Grijpma DW, A biodegradable antibiotic delivery system based on poly-(trimethylene carbonate) for the treatment of osteomyelitis, Acta Orthopaedica 80(5) (2009) 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kluin OS, van der Mei HC, Busscher HJ, Neut D, A surface-eroding antibiotic delivery system based on poly-(trimethylene carbonate), Biomaterials 30(27) (2009) 4738–42. [DOI] [PubMed] [Google Scholar]
- [94].Cometa S, Mattioli-Belmonte M, Cafagna D, Iatta R, Ceci E, De Giglio E, Antibiotic-modified hydrogel coatings on titanium dental implants, Journal of biological regulators and homeostatic agents 26(2 Suppl) (2012) 65–71. [PubMed] [Google Scholar]
- [95].Romanò CL, Malizos K, Capuano N, Mezzoprete R, D’Arienzo M, Van Der Straeten C, Scarponi S, Drago L, Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty?, Journal of bone and joint infection 1 (2016) 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Perni S, Thenault V, Abdo P, Margulis K, Magdassi S, Prokopovich P, Antimicrobial activity of bone cements embedded with organic nanoparticles, International journal of nanomedicine 10 (2015) 6317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shi Z, Neoh KG, Kang ET, Wang W, Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles, Biomaterials 27(11) (2006) 2440–2449. [DOI] [PubMed] [Google Scholar]
- [98].Duey RE, Chong ACM, McQueen DA, Womack JL, Song Z, Steinberger TA, Wooley PH, Mechanical properties and elution characteristics of polymethylmethacrylate bone cement impregnated with antibiotics for various surface area and volume constructs, The Iowa orthopaedic journal 32 (2012) 104–115. [PMC free article] [PubMed] [Google Scholar]
- [99].Swearingen MC, Granger JF, Sullivan A, Stoodley P, Elution of antibiotics from poly(methyl methacrylate) bone cement after extended implantation does not necessarily clear the infection despite susceptibility of the clinical isolates, Pathogens and disease 74(1) (2016) ftv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chattopadhyay S, Raines RT, Review collagen-based biomaterials for wound healing, Biopolymers 101(8) (2014) 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Han J-S, Kim S-H, Jin S-W, Lee S-H, Kim B-J, Kim S-D, Lim D-J, The Use of Gentamicin-Impregnated Collagen Sponge for Reducing Surgical Site Infection after Spine Surgery, Korean Journal of Spine 13(3) (2016) 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ipsen T, Jorgensen PS, Damholt V, Torholm C, Gentamicin-collagen sponge for local applications. 10 cases of chronic osteomyelitis followed for 1 year, Acta orthopaedica Scandinavica 62(6) (1991) 592–4. [DOI] [PubMed] [Google Scholar]
- [103].Duewelhenke N, Krut O, Eysel P, Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines, Antimicrobial agents and chemotherapy 51(1) (2007) 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Schlapp M, Friess W, Collagen/PLGA microparticle composites for local controlled delivery of gentamicin, Journal of pharmaceutical sciences 92(11) (2003) 2145–51. [DOI] [PubMed] [Google Scholar]
- [105].Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH, Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles, ACS Applied Materials & Interfaces 5(19) (2013) 9322–9329. [DOI] [PubMed] [Google Scholar]
- [106].Wang L, Hu C, Shao L, The antimicrobial activity of nanoparticles: present situation and prospects for the future, International journal of nanomedicine 12 (2017) 1227–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO, A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus, Journal of biomedical materials research 52(4) (2000) 662–668. [DOI] [PubMed] [Google Scholar]
- [108].Qadri S, Haik Y, Mensah-Brown E, Bashir G, Fernandez-Cabezudo MJ, al-Ramadi BK, Metallic nanoparticles to eradicate bacterial bone infection, Nanomedicine: Nanotechnology, Biology and Medicine 13(7) (2017) 2241–2250. [DOI] [PubMed] [Google Scholar]
- [109].Ferreira D.d.S., Boratto FA, Cardoso VN, Serakides R, Fernandes SO, Ferreira LAM, Oliveira MC, Alendronate-coated long-circulating liposomes containing (99m)technetium-ceftizoxime used to identify osteomyelitis, International journal of nanomedicine 10 (2015) 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sofokleous P, Ali S, Wilson P, Buanz A, Gaisford S, Mistry D, Fellows A, Day RM, Sustained antimicrobial activity and reduced toxicity of oxidative biocides through biodegradable microparticles, Acta Biomaterialia 64 (2017) 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, García AJ, Maleimide Cross-Linked Bioactive PEG Hydrogel Exhibits Improved Reaction Kinetics and Cross-Linking for Cell Encapsulation and In Situ Delivery, Advanced Materials 24(1) (2012) 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Giavaresi G, Meani E, Sartori M, Ferrari A, Bellini D, Sacchetta AC, Meraner J, Sambri A, Vocale C, Sambri V, Fini M, Romano CL, Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant, International orthopaedics 38(7) (2014) 1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yeo CK, Vikhe YS, Li P, Guo Z, Greenberg P, Duan H, Tan NS, Chan-Park MB, Hydrogel Effects Rapid Biofilm Debridement with ex situ Contact-Kill to Eliminate Multidrug Resistant Bacteria in vivo, ACS Applied Materials & Interfaces 10(24) (2018) 20356–20367. [DOI] [PubMed] [Google Scholar]
- [114].Murthy PS, Murali Mohan Y, Varaprasad K, Sreedhar B, Mohana Raju K, First successful design of semi-IPN hydrogel-silver nanocomposites: a facile approach for antibacterial application, J Colloid Interface Sci 318(2) (2008) 217–24. [DOI] [PubMed] [Google Scholar]
- [115].Li S, Dong S, Xu W, Tu S, Yan L, Zhao C, Ding J, Chen X, Antibacterial Hydrogels, Advanced science (Weinheim, Baden-Wurttemberg, Germany) 5(5) (2018) 1700527–1700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Salick DA, Kretsinger JK, Pochan DJ, Schneider JP, Inherent antibacterial activity of a peptide-based beta-hairpin hydrogel, Journal of the American Chemical Society 129(47) (2007) 14793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Marchesan S, Qu Y, Waddington LJ, Easton CD, Glattauer V, Lithgow TJ, McLean KM, Forsythe JS, Hartley PG, Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel, Biomaterials 34(14) (2013) 3678–87. [DOI] [PubMed] [Google Scholar]
- [118].Das D, Das R, Ghosh P, Dhara S, Panda AB, Pal S, Dextrin cross linked with poly(HEMA): a novel hydrogel for colon specific delivery of ornidazole, RSC Advances 3(47) (2013) 25340–25350. [Google Scholar]
- [119].Francolini I, Vuotto C, Piozzi A, Donelli G, Antifouling and antimicrobial biomaterials: an overview, APMIS 125(4) (2017) 392–417. [DOI] [PubMed] [Google Scholar]
- [120].Gristina AG, Biomaterial-centered infection: microbial adhesion versus tissue integration, Science (New York, N.Y.) 237(4822) (1987) 1588–95. [DOI] [PubMed] [Google Scholar]
- [121].Siedenbiedel F, Tiller JC, Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles, Polymers 4(1) (2012) 46. [Google Scholar]
- [122].Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJAM, Zaat SAJ, Schultz MJ, Grainger DW, Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface, Science Translational Medicine 4(153) (2012) 153rv10. [DOI] [PubMed] [Google Scholar]