Abstract
Articular cartilage is a remarkable tissue whose sophisticated composition and architecture allow it to withstand complex stresses within the joint. Once injured, cartilage lacks the capacity to self-repair, and injuries often progress to joint wide osteoarthritis (OA) resulting in debilitating pain and loss of mobility. Current palliative and surgical management provides short-term symptom relief, but almost always progresses to further deterioration in the long term. A number of bioactive factors, including drugs, corticosteroids, and growth factors, have been utilized in the clinic, in clinical trials, or in emerging research studies to stabilize the inflamed joint environment or to promote new cartilage tissue formation. However, these therapies remain limited in their duration and effectiveness. For this reason, current efforts are focused on improving the localization, retention, and activity of these bioactive factors. The purpose of this review is to highlight recent advances in drug delivery for the treatment of damaged or degenerated cartilage. First, we summarize material and modification techniques to improve the delivery of these factors to damaged tissue and enhance their retention and action within the joint environment. Second, we synthesize recent studies using novel methods to promote new cartilage formation via biofactor delivery, that have potential for improving future long-term clinical outcomes. Lastly, we review the emerging field of orthobiologics, using delivered and endogenous cells as drug-delivering “factories” to preserve and restore joint health. Enhancing drug delivery systems can improve both restorative and regenerative treatments for damaged cartilage.
Keywords: Cartilage, drug delivery, osteoarthritis, scaffolds, orthobiologics
Graphical Abstract
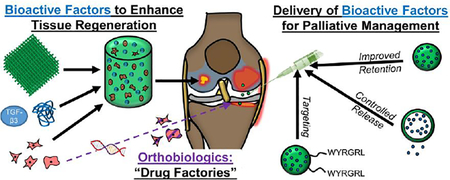
1. Introduction
Articular cartilage is a durable tissue that enables the load transmission and articulation of joints [1]. The dense extracellular matrix (ECM) of cartilage is composed of a zonally-organized network of type II collagen fibers [1], allowing the tissue to resist complex loading patterns, including compression, tension, shear, and friction [2,3]. The ECM also contains a high concentration of proteoglycans (PG), forming negatively-charged aggregates that promote fluid pressurization upon loading [4–6]. Combined, these ECM components (Fig 1A) generate a unique viscoelastic material that is optimized to bear load over a lifetime of use [7]. However, articular cartilage is also frequently injured, both traumatically and with aging and disease. Due to the relative avascular nature of the tissue and the limited ability of cells to migrate to the tissue for repair, cartilage lacks an intrinsic healing capacity [8]; once injured, the tissue loses many of its load-bearing traits, making the adjacent cartilage more vulnerable to wear [9]. Furthermore, injury can elicit an inflammatory response throughout the joint, with increased levels of synovial cytokines that elicit further degradation and damage to the tissue. Ultimately, with these accompanying chemo-mechanical insults, injuries often progress and conclude in joint-wide osteoarthritis (OA; Fig 1B), a debilitating disease that affects nearly 50 million people in the United States alone [10].
Figure 1.
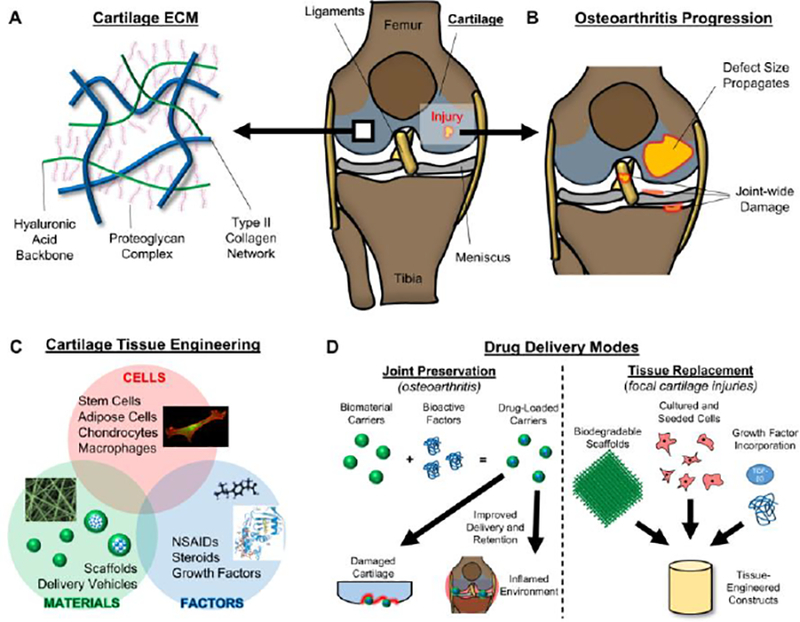
Cartilage Composition, Disease Progression, Tissue Engineering, and Treatment Modalities. [A] Cartilage is composed of a type II collagen network (blue), interwoven with long hyaluronan chains (green) and their associated proteoglycan complexes. [B] Cartilage injuries often increase in size and can result in joint-wide degenerative changes. [C] Cartilage tissue engineering combines cells, materials, and factors in order to treat damaged cartilage. [D] Two distinct modes of biofactor delivery for cartilage applications. (Left) Joint preservation via bioactive factor incorporation into biomaterial carriers to target cells within the joint. (Right) Tissue replacement via cell-based scaffolds that are supplemented with growth factors.
Common surgical interventions to treat cartilage injuries include chondroplasty [11,12] (removal of torn tissue) and microfracture [13,14] (marrow stimulation), however both provide only short-term symptomatic relief and do not prevent OA progression. More recently, chondrocyte implantation techniques [15,16] have shown superiority to microfracture for up to 5 years [17], but their ability to produce hyaline cartilage and establish long-term functionality have not yet been verified [13,14]. Due to this eventual onset of degeneration, clinicians and scientists have sought to deliver bioactive factors, consisting of proteins, nucleic acids, carbohydrates, or molecules (synthetic or biologic) that elicit a response in host tissues or cells [18–20]. Clinically speaking, most drugs used to address cartilage injury are intended for palliative OA treatment, and can provide some measure of pain and symptom relief. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as Ibuprofen and Diclofenac, have shown some benefits over placebo treatment [21–23], but both oral and topical administration results in inefficient delivery of the drug to the joint. The higher doses required to provide pain relief may also cause harmful consequences throughout the rest of the body (e.g. cardiovascular) [24,25]. Additional clinically-used bioactive factor treatments include intra-articular corticosteroid and hyaluronan injections; the former provides both pain/inflammatory relief and anti-catabolic activity [26–28], while the latter can, to some extent, restore joint lubrication and provide some chondro-protective capacity [29,30]. However, retention of these bioactive factors within the joint is limited, as these agents are readily cleared due to their relatively small molecular size [31,32], and therefore only provide short-term benefits [28]. Management of cartilage injuries and osteoarthritis would be greatly improved with localized delivery and retention of these factors.
At the other end of the spectrum, tissue engineers are developing new strategies to replace the damaged cartilage, either in small defects or after joint-wide degeneration. Unfortunately, current “replacements” do not generally result in long-term hyaline cartilage; nevertheless, there is potential in utilizing bioactive factors to induce the formation of functional tissue. For example, in vitro culture of hydrogel constructs with chemically defined medium containing the growth factor TGF-β3 promotes the chondrogenesis of mesenchymal stem cells (MSCs) [33,34], indicated by increased type II collagen and PG deposition. Furthermore, a plethora of other growth factors (BMP-2, BMP-7, IGF-1, FGF-2 [19,35]) have been used to promote neocartilage tissue formation. However, scaffolds that simply encapsulate these factors alone often release them within a matter of hours [36,37], and so cannot guide chondrogenesis and tissue formation (which occurs over the course of weeks to months). Additionally, while certainly chondrogenic, these factors can elicit negative responses, such as synovial fibrosis [38] or ossification [39], if introduced to off-target cells and tissues within the joint. Therefore, prolonged delivery without continual supplementation and precise localization to the desired cells are paramount in engineering functional cartilage tissue following injury.
With this lack of effective long-term therapeutics and treatments, novel techniques have emerged to promote repair and regeneration, via the combination of materials, cells, and bioactive factors to replace, rejuvenate, or protect the native cartilage matrix (Fig 1C). One general approach involves reprogramming the joint environment and/or wound interface with bioactive factors (Fig 1D-left), typically to restore the behavior of chondrocytes, calm the inflammatory milieu, or simply relieve pain. Another involves incorporating chondrogenic factors into scaffolds, with the intention of guiding cells to produce new cartilaginous matrix to replace the damaged or missing tissue (Fig 1D-right). Regardless of the application, drugs and growth factors can have a significant impact on these processes.
In this review, we focus on advances in drug delivery systems for cartilage treatment applications. First, we discuss innovative approaches that specifically address the inflamed joint environment and the damaged tissue interface, to improve retention and localization of palliative therapeutics. Next, we describe novel scaffold fabrication techniques that better control the release and activity of these bioactive factors in order to enhance neocartilage formation for tissue replacement. Finally, this review examines the emerging field of orthobiologics, utilizing cells as “biological producers” of bioactive factors, and discusses the strengths and current limitations of these approaches.
2. Enhanced Delivery and Retention of Bioactive Factors for Joint Preservation
2.1. Current Drug Usage for Joint Pain is Relatively Ineffective
Oral delivery of NSAIDs (Fig 2A) remains a common mode of treatment for both focally injured and degenerated cartilage, as these drugs inhibit cyclooxygenase enzymes, reducing the production of biological mediators involved in inflammation (e.g. prostaglandin E2). While pain and inflammation relief are provided to some extent, daily usage at higher doses [40] is required in order to provide a noticeable benefit. Moreover, these larger doses can lead to gastrointestinal [41–43] and cardiovascular [44,45] complications, and thus topical administration has better indications for OA management with NSAIDs [46,47]. Topical NSAIDS (Fig 2B) are applied via cream, gel, or patch 1–3 times daily, and have shown a 10- to 20-fold increase in NSAID concentration in synovial tissues over serum and plasma levels [48,49], indicating a more targeted delivery. However, the use of topical NSAIDs can be a burden on patients, resulting in skin irritations and withdrawal issues in patients [50,51]. Perhaps of greatest significance, for both oral and topical delivery, is that pharmacokinetic analyses have determined a drug half-life of 1–7 hours within the joint [52,53].
Figure 2.
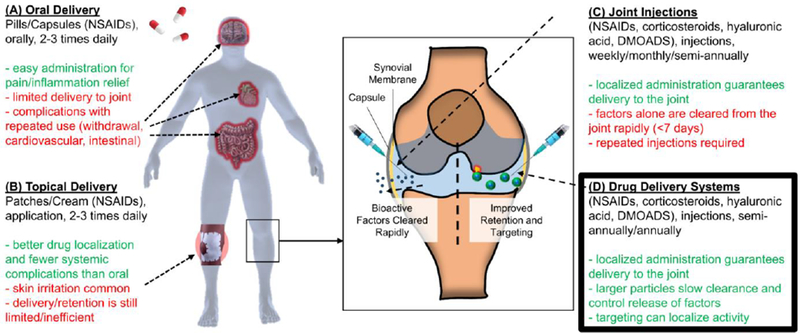
Drug Delivery Approaches. Benefits (green) and drawbacks (red) of [A] oral delivery, [B] topical delivery, and [C] joint injection of bioactive factors, highlighting the need for [D] drug delivery systems to enhance delivery and retention of these factors.
Intra-articular injections of bioactive factors (Fig 2C), as is typically done with corticosteroids (e.g. triamcinolone acetonide, dexamethasone) and hyaluronic acid (HA), can improve efficiency of delivery. For example, injected corticosteroids have shown slightly favorable outcomes to oral NSAIDs for up to 4 weeks with regards to patient-reported pain relief [54]. Similarly, HA injections show slightly improved results over NSAIDs, and significantly lower the incidence of adverse events and withdrawal [55]. However, residence time of these molecules is a concern, as corticosteroids and HA concentrations diminish rapidly post-injection [56,57], with a residence time of only 6–25 [58,59] and 22–56 days [56,60], respectively. For this reason, intra-articular delivery to maintain therapeutic levels consists of continuous monthly injections [55,61], creating a greater burden on patients with regards to comfort and finances. Moreover, even precise image-guided injection to the site of injury did not improve patient-reported pain outcomes [62], likely due to poor retention. This indicates that both localization and retention are required to improve the efficacy and longevity of bioactive corticosteroids. Lastly, while corticosteroids can provide pain and inflammatory relief for a few weeks, short-term high doses of these corticosteroids are detrimental to cartilage health (volumetric cartilage loss and tissue atrophy). The long-term release of lower concentrations of these factors could greatly improve their impact while preserving cartilage tissue structure and function. The following sections detail novel methods to improve the delivery, localization, retention, and release of these and other bioactive factors into the joint environment (Fig 2D) to provide patients with longer-term symptomatic relief.
2.2. Carriers for Enhanced Drug Delivery and Retention
The rapid clearance of NSAIDs and corticosteroids is related to their relatively low molecular weight (<1kDa), which allows the lymphatic vessels in the synovium and joint capsule to clear these small molecules. Even larger hyaluronic acid (6–100MDa) molecules are either enzymatically digested and/or cleared from the joint within weeks [60,63]. Other small molecules, including monoclonal inflammatory antibodies (e.g., TNF inhibitors), receptor antagonists (e.g., IL-1Ra), and protease inhibitors (e.g., MMP inhibitors, cathepsins), can serve as disease-modifying osteoarthritis drugs (DMOADS) and are under development, but are similarly cleared from the joint soon after injection in the absence of a delivery system [64]. To address this event, a number of new materials (microcarriers, nanocarriers, liposomes, hydrogels) have been developed to encapsulate bioactive factors and control their release from larger particles that are not as easily cleared from the joint.
Many micro-carrier – drug combinations are currently under development for OA treatment [64]. Perhaps the most advanced among these combinations (already under testing in a Phase III trial) take advantage of poly-lactic-glycolic acid (PLGA) microcarriers (FX006, Flexion Therapeutics, 35–55 microns in diameter) [65–67]. This proposed treatment consists of carriers that contain crystals (<5 microns) of the corticosteroid triamcinolone acetonide. Encapsulation results in a triphasic pharmacokinetic release profile that provided patients with pain relief lasting 12 weeks longer than placebo treatment. This release and activity profile is consistent with prior degradation profiles of the PLGA polymer [68], and can be elongated by increasing the ratio of lactide (slower degradation) to glycolide (faster degradation) groups. However, the FX0006 carrier only provided slight improvement in OA pain outcomes over intra-articular administration of the drug itself [65], leaving lots of room for clinically-relevant improvement. Other materials have been utilized for both micro- and nano-carrier development (Fig 3A), including gelatin, chitosan, polycaprolactone (PCL), polyanhydrides, and polyester amides (PEA) [69,70]. When produced at a small length scale, nanoparticles provide a greater surface-to-mass ratio than microparticles, and can be more easily taken up by the desired cells through endocytotic mechanisms in order to control cell response and fate [71,72]. For example, Kang et al [73] determined that in vitro culture of MSCs with kartogenin-loaded nano-particles (150nm) resulted in improved chondrogenesis compared to micro-particles (1.8μm) loaded similarly. However, the smaller size of nano-particles also increases the likelihood of phagocytosis and degradation by macrophages [74], as well as clearance from the joint. This phenomenon likely explains the finding that in vivo retention of kartogenin was superior when delivered from micro-particles. Many of these drug carriers can also carry multiple factors, with a differential release profile for each factor. For example, Kang et al [75] fabricated chitosan nanospheres containing diclofenac, allowing for immediate anti-inflammatory benefits, while covalently conjugating kartogenin to the particle, promoting longer-term chondrogenic enhancement [75]. This concept of polymer functionalization with bioactive factors, especially to carriers, may enhance retention and prolong release, as it creates covalent linkages via one of many chemistries (e.g. activated esters, thiol-ene processes, imine formation, Michael addition) [76,77]. Lastly, both micro- and nano-particles are typically fabricated as solid spheres with embedded bioactive factors. Alternatively, capsules can be fabricated with solid biomaterial outer shells and aqueous cores, with the release of bioactive factors regulated via permeation through the polymer shell (Fig 3A).
Figure 3.
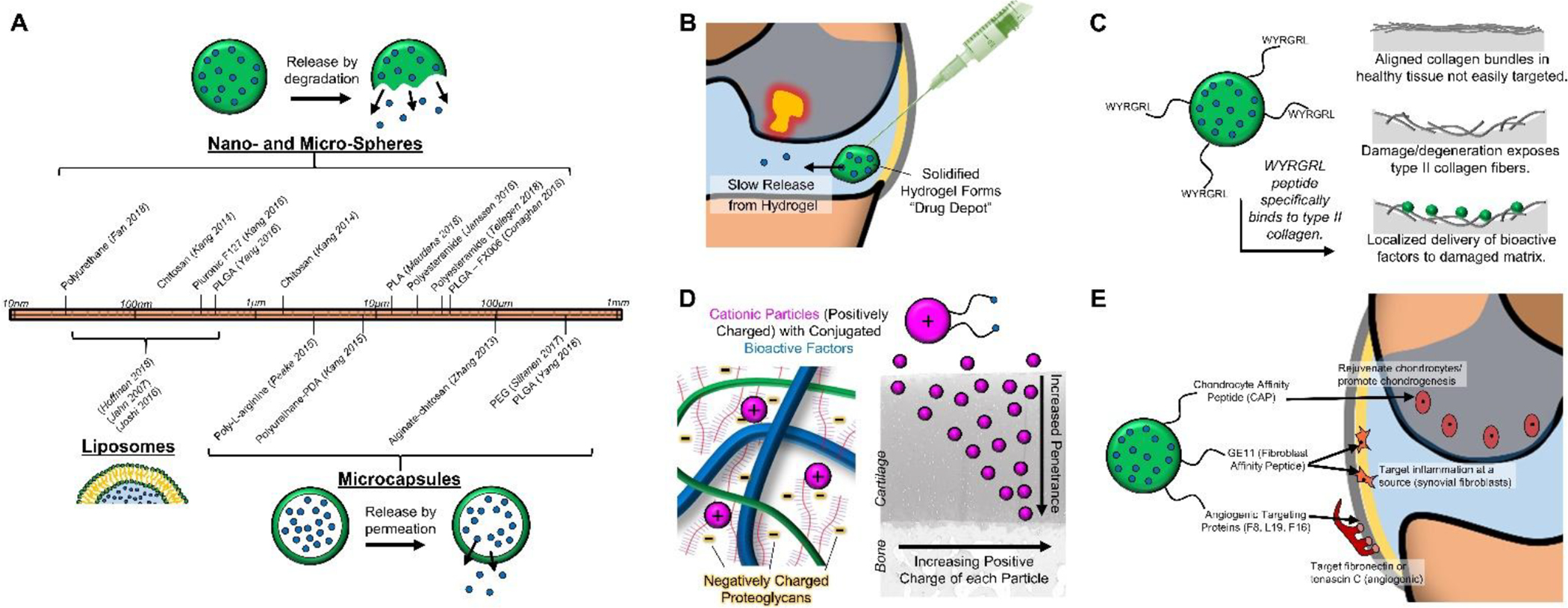
Intra-articular Drug Delivery Systems. [A] Materials used for nano- and micro-spheres, liposomes, and microcapsules span a wide range of sizes (10nm – 1mm). [B] Hydrogel “drug depots” can extend drug release within the joint. [C] Targeting molecules (e.g. WYRGRL) can focus delivery to the damaged cartilage interface. [D] Cationic particles can increase penetrance for improved drug delivery within cartilage tissue. [E] Drug delivery vehicles can target specific cells and structures (e.g. chondrocytes, synovial fibroblasts, angiogenic regions).
Drug delivery via liposomes is similar in many ways to delivery from polymeric capsules. Composed of a lipid bilayer, liposomes have high loading efficiencies for hydrophilic drugs within their cores, are well tolerated, and are tunable with respect to size (3–500nm; Fig 3A) [78,79]. Taking advantage of the fine control of flow rates afforded by microfluidic assembly, reproducible and consistent liposomes can be fabricated. Likewise loading of hydrophobic factors within the bilayer system can permit the dual delivery of multiple biofactors [79,80]. Concerns over liposomal integrity do exist, especially in the high-stress joint environment, but these are mitigated by the development of fortified liposomal fabrication, primarily through PEGylation [81]. Furthermore, liposomes can be easily functionalized for enhanced retention and targeting [82,83]. These technologies have advanced considerably for cancer and tumor targeting applications, but examples of these modifications for the treatment of cartilage injuries and osteoarthritis are limited at present [84,85]. Other drug delivery systems similar to liposomes include micelles (lipid monolayer for hydrophobic molecule delivery) and dendrimers (branched polymer system) [86,87].
The final major category of drug delivery systems is hydrogel encapsulation. These hydrogels, including hyaluronic acid or chitosan, are modified polymers that can be crosslinked to encapsulate bioactive factors and then control their delivery within the joint. Taking advantage of thermo-sensitive crosslinking behavior [88], factors can be incorporated in an aqueous solution that then crosslinks into a hydrogel when at body temperature post-injection [89,90]. Such hydrogels can encapsulate DMOADs without affecting factor bioactivity, a potential concern with synthetic carriers that require solvents or heat for fabrication. Other controlled drug release “depots” (Fig 3B) include elastin-like polypeptides (ELP), thermo-sensitive polymers that are aqueous below body temperature, but become insoluble at body temperature [91–93]. Application of this delivery system allowed for sustained release of the IL-1 receptor antagonist (IL-1Ra, anakinra), significantly reducing cartilage degeneration and synovitis in a mouse OA model [92].
Modifications can be made to natural and synthetic polymers in order to tune their responsiveness in the intended joint application, allowing bioactive factors to become available when needed. For example, enzyme cleavable particles and hydrogels are of significant interest [94], as enzyme activity is elevated in the degenerated or diseased setting. Under these conditions, bioactive factors would be released to quell inflammation and pain during periods of high cytokine activity. For instance, Joshi et al [95] developed a triglycerol monostearate hydrogel system that degrades in response to an “arthritis flare” in vivo; the incorporation of triamcinolone acetonide into these hydrogels allowed for release of the corticosteroid in the presence of elevated enzymes found in joint inflammation. Furthermore, recent studies have fabricated MMP-cleavable hydrogels that similarly degrade and release bioactive factors upon demand [96–98]. Other drug delivery systems are designed to be pH- or temperature-responsive [99–102]; a “flaring” inflamed joint is typically accompanied by a drop in pH [103] and increase in temperature [104]. Finally, an emerging arena of drug delivery relates to controlling release with external stimuli such as ultraviolet or infrared light, magnetic or electrical fields, or ultrasound [105–108]. Refining and expanding the chemical and biophysical cues that instigate biofactor delivery will increase the range of applications that can be addressed to slow or reverse joint degeneration.
2.3. Targeting the Damaged or Inflamed Cartilage Environment
Even when delivered to the joint environment using one of the carriers described above, bioactive factors are often not active at the desired site of injury or inflammation. For this reason, bioactive factors are now being targeted (Fig 3C) to inflammatory cells or the damaged cartilage interface to either quell the inflamed environment, prevent further cartilage deterioration, or even promote regeneration at the site of injury. Targeting of damaged or degenerated cartilage can be achieved via modification of drug delivery agents with a type II collagen binding peptide (WYRGRL) [109]. The peptide can be covalently linked to a multitude of biological (hyaluronan, chondroitin sulfate) and synthetic (PEG, PLGA, DOTAM) polymers (Table 1) that carry bioactive factors. Cartilage defects and degeneration expose the underlying cartilage matrix, uncovering the type II collagen-rich ECM (Fig 1A) and promote attachment of peptide-conjugated carriers. Jiang et al utilized maleimide-PEG-PLGA nanoparticles to couple WYRGRL peptides that promoted nanoparticle binding to damaged cartilage [110]. Other groups have used the WYRGRL peptide to localize pepstatin-A [111] or hyaluronic acid [112] to damaged regions of cartilage in order to promote anti-inflammatory activity or improve lubrication. Additional binding motifs on the damaged cartilage have been explored, such as hyaluronan-binding peptides [113], heparin binding peptides [114,115], β-cyclodextrin [116], aldehyde amine interactions [117–119], and even type II collagen monoclonal antibodies [120]. All of these cartilage-targeting mechanisms can enhance the delivery and retention of bioactive factors to the damaged cartilage interface.
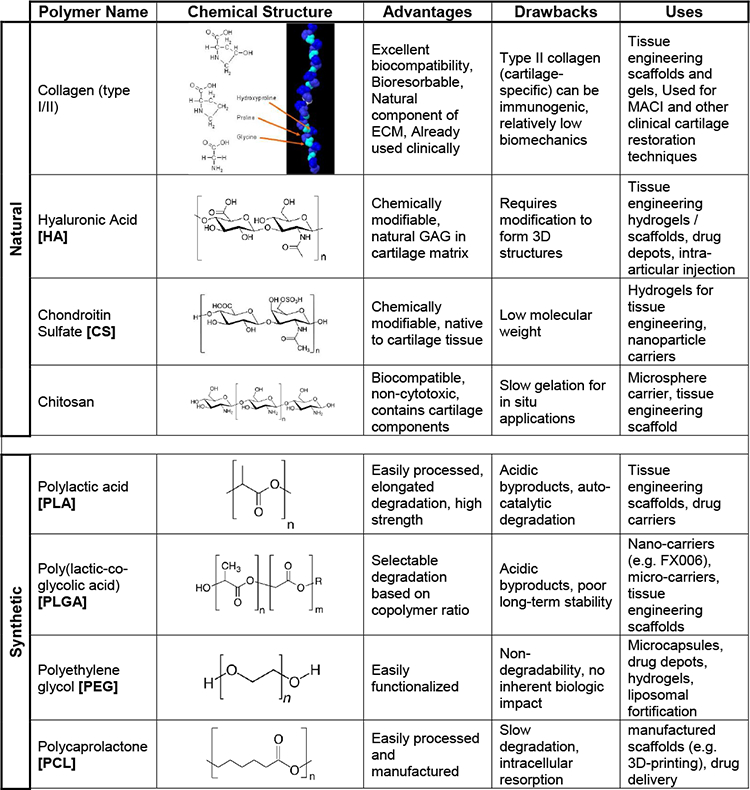
Table 1.
Common polymer types (natural and synthetic) used as drug-delivering carriers and drug-releasing scaffolds. Chemical structure, advantages, drawbacks, and cartilage applications provided.
|
Technologies have also been developed to improve penetration of delivery agents into the tissue (Fig 3D). In order to easily penetrate through the small pores of cartilaginous matrix, drug delivery agents need to be less than 5nm. However, this size restriction greatly decreases the residence time of such delivery systems within the synovial joint. In order to better promote the movement of delivered factors into the cartilage matrix, large cationic particles have shown some utility [121,122]. For example, a study by Bajpayee and colleagues determined that the charged molecule avidin (~7nm) infiltrated into cartilage explants more than 10 times further than its neutral counterpart (neutravidin). This penetration was due to electrostatic interactions [123], and this enhanced capacity for penetration was recently confirmed in vivo in a rat model of OA [124,125], and was ultimately used to deliver dexamethasone [126]. Other groups have utilized positively-charged particles in order to enhance bioactive factor delivery within the cartilage tissue [87,127,128]. For instance, Brown et al developed PLGA nanoparticles with quaternary ammonium cations that penetrated into cartilage ex vivo [129], allowing for improved delivery into the tissue prior to clearance from the joint. Improved penetration of bioactive factors can be achieved through other physical mechanisms; magnetic nanoparticles coupled with an external magnetic field can be “pushed” through the cartilage tissue at a nearly 50-fold increase over static conditions [130].
Beyond targeting moieties in the tissue itself, cells themselves offer another opportunity for biofactor localization based on surface antigens expressed on a target cell type (Fig 3E). For instance, a number of studies have utilized a chondrocyte-affinity peptide (CAP, DWRVIIPPRPSA) [131,132]. One recent study noted reductions in inflammation following injection of CAP-bound anti-Hif-2α siRNA [133], with delivery of the anti-inflammatory siRNA directly to chondrocytes in cartilage. By providing close contact between the delivery vehicle and chondrocytes, such affinity peptides likely enable efficient endocytosis of factors through clathrin and caveolin-mediated pathways [132]. Chondrocytes are not the only cells available for targeting, numerous groups have targeted both inflammatory cells and synoviocytes in the joint space. Chiesa et al [134] utilized a GE11 peptide that targets epidermal growth factor in synovial fibroblasts. This peptide was conjugated to PLGA nanocarriers containing dexamethasone, reducing expression of inflammatory markers in synovial fibroblasts. Similarly, Vanniasinghe et al [85] utilized liposomes conjugated with a Cys-HAP-1 peptide in order to target fibroblastic synoviocytes, delivering the steroid prednisolone. By specifically targeting inflammatory cells in the synovial environment, efficient drug delivery can reduce inflammatory cytokine levels and promote a return to tissue homeostasis.
A number of disease modifying OA drugs (DMOAD) under clinical investigation target TNF-α (infliximab, etanercept, adalimubab) or IL-1 (anakinra, canakinumab). While these agents can effectively abrogate signaling through these pathways, only limited short-term symptom relief has been observed in animal studies [135] and clinical trials [136,137]. To improve on these efforts, targeting of angiogenic markers, which are prevalent in arthritic regions of the joint, has been used to locally reduce inflammatory activity at these sites. Several groups have tested a fusion protein system (F8) that utilizes a human monoclonal antibody that recognizes the extra-domain A of fibronectin [138], an angiogenic marker. By conjugating recombinant IL-4 or IL-10 (anti-inflammatory cytokines) to the F8 antibody, targeted anti-inflammatory benefits were achieved [139,140]. Similar targeting antibodies for angiogenesis (L19 for extra-domain B of fibronectin [141], F16 for extra-domain A1 of tenascin C [142], and G11 for extra-domain C of tenascin C [143]) have been utilized for cancer therapeutics, and may find application for the delivery of anti-inflammatory cytokines in cartilage applications [141].
3. Scaffold Delivery Systems to Tune Growth Factor Release
Even with the improved delivery, retention, and targeting of NSAIDs, corticosteroids, hyaluronic acid, antibodies, antagonists, cytokines and siRNA, almost all outcomes have been measured relative to placebo controls. Moreover, these therapies simply act as a palliative treatment in patients, given that cartilage tissue is not replaced, but rather protected from further degenerative processes. For longer-term replacement of damaged tissue, current clinical techniques include microfracture and autologous chondrocyte implantation (ACI). Microfracture involves penetrating the subchondral bone to recruit marrow elements, most notably MSCs, in order to fill the defected tissue void space with a marrow-clot. While the short-term clinical outcomes of microfracture show moderate defect fill and pain relief [144,145], both biopsy and preclinical animal studies (Yucatan minipig) demonstrate inferior fibrocartilaginous tissue formation [146,147]. This failure in functional restoration engenders elevated stresses in the surrounding tissue, leading to long-term wear, defect expansion, and OA progression. To improve the quality of formed cartilage tissue, ACI and its subsequent iterations (matrix-induced autologous chondrocyte implantation; MACI) use chondrocytes without and with scaffold carriers to fill cartilage defects, and are governed by the principle that native cartilage cells may better reproduce cartilage matrix [148,149]. With these matrix-assisted and augmented procedures, long-term clinical pain scores show some improvement over microfracture. However, long-term quality of life measures and magnetic resonance imaging (MRI) evaluation of formed tissue showed little difference compared to microfracture [150], calling into question the durability and cost effectiveness of these procedures [16,151]. Cartilage tissue engineering aims to improve on patient-reported outcomes by utilizing scaffolds to provide a template for functional neocartilage tissue formation. Indeed, the utilization of bioactive factors within these scaffolds may better direct cells, both marrow-derived MSCs and articular chondrocytes, to regenerate cartilage tissue.
3.1. Drug Delivery to Promote Chondrogenesis
For decades, cell-seeded hydrogel constructs have been cultured in chemically-defined media containing a variety of growth factors to promote cartilage tissue formation. While these cultured constructs have gained traction as cartilage replacements [152], and are being tested pre-clinically in animal models (e.g. rabbit, sheep) [153,154], the manufacturing variability and costs associated with their production may complicate translation and regulatory approvals. Furthermore, bioactive factors are not available in vivo at concentrations (5–100ng/mL) applied during in vitro culture, and cultured constructs often require weeks to months to mature. For this reason, numerous acellular cartilage scaffolds have incorporated bioactive factors during fabrication, with the aim of guiding endogenous cells to regenerate cartilage. A systematic literature search (Pubmed: “cartilage scaffold drug delivery”: September 2013-September 2018) determined that TGF-β1 and TGF-β3 are, by far, the most-widely used factors (18 of 44 studies), followed by BMPs (BMP-2, BMP-7), IGF-1, and FGF (Fig 4A). Other common factors, including drugs and biocompounds, include insulin [155], dexamethasone [36,156], and kartogenin [157]. When such factors are simply incorporated in a soluble form in hydrogels, the majority are released in the first week (Fig 4B – red lines), and do not substantially improve the in vivo formation of cartilage tissue that occurs over weeks to months [158]. Thus, prolonged release and activity of these factors from scaffolds is required for the long-term promotion of cartilage tissue formation.
Figure 4.
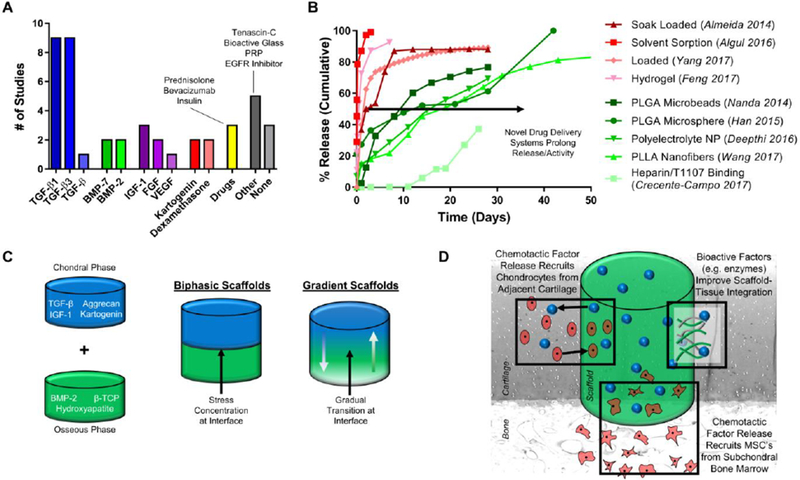
[A] Systematic literature search (Pubmed: cartilage scaffold drug delivery) revealed trends in growth factor release for promoting chondrogenesis (query window: Sep 2013-Sep 2018). [B] Characteristic drug release profiles of factors encapsulated or soak-loaded in hydrogels/scaffolds (red) or within particles/more elaborate systems (green). Values replotted from literature sources [36,37,159,163,169–173]. [C] Depiction of chondral and osseous scaffolds for spatiotemporal control, combined into biphasic and gradient scaffolds. [D] Mechanisms to improve cartilage repair by increased chondrocyte migration from adjacent cartilage, increased MSC recruitment from the subchondral bone, and improved integration between the scaffold and surrounding tissue.
Similar to drugs for the treatment of OA symptoms, micro- and nano-carriers can enable the prolonged release of bioactive factors from scaffolds to enhance chondrogenesis and tissue formation. For instance, Han et al [159] utilized PLGA microspheres to prolong the release of TGF-β1 in a chitosan-gelatin hydrogel, showing a 50% release of the growth factor by 14 days, a significant improvement from direct incorporation of TGF-β1 into HA hydrogels [37], where >90% release occurred in the first 7 days. Similarly, Deepthi et al [160] fabricated polyelectrolyte (cross-linked chondroitin sulfate) nanoparticles containing TGF-β; these delivery agents increased MSC proliferation and proteoglycan deposition in culture. Furthermore, similar to carriers discussed earlier that respond to inflammatory cues, Mohanraj et al [161] developed mechanically-activated capsules that, under loading, rupture and release bioactive factors. This technology provides on-demand release of factors to enhance matrix formation and organization in order to combat these loads. An alternative approach for promoting sustained release is the incorporation of the bioactive factors into electrospun nanofibers [162,163] or 3D-printed microfibers [164]. Compared to hydrogels, from which growth factors can diffuse, encapsulation of factors into fibrous matrices can slow or stop release until fiber degradation has occurred. Given that a variety of polymers (collagen, PLLA, PLGA, PCL; Table 1) with distinct degradation profiles can be utilized, bioactive factor release can be achieved for >50 days [163]. Additionally, functionalizing scaffolds with growth factors, similar to drug delivering carriers [76,77], can further enhance retention and activity throughout the scaffold. One such modality utilizes heparin binding domains to sequester growth factors, improving retention and prolonging release for over 28 days [165,166]. These approaches (Fig 4B – green lines), where bioavailability of factors can be achieved over longer time windows, can support the long-term maturation of cartilage-like tissue. Finally, the controlled release of growth factors from these scaffolds has also been shown to augment microfracture procedures [167,168], directing hyaline cartilage formation over fibrocartilage formation.
3.2. Spatiotemporal Control for Complex Tissues (e.g. Osteochondral Unit)
Several tissues in the body exhibit spatially varying regions of cartilaginous tissue and interfaces [174], and so require spatial control of cell phenotype and behavior that may be achieved through localized and differential biofactor release. Most notably, cartilage is part of an osteochondral unit, consisting of a gradient interface between cartilage and the underlying subchondral bone (Fig 4C). Other examples of cartilage-containing tissues include the fibro-cartilaginous meniscus, intervertebral disc (IVD), and temporomandibular joint (TMJ) tissues, as well as tendon-to-bone interfaces. Often, techniques to promote chondrogenesis must be combined with techniques to regenerate other tissues, with spatial release of factors to induce the formation of each individual tissue. Biphasic scaffolds that reconstitute the osteochondral unit typically consist of a softer hydrogel or fibrous top layer with incorporated chondrogenic factors (e.g., TGF-β), and a stiffer bottom layer that contains osteogenic factors (e.g., BMP-2) [159,175–177]. While these biphasic scaffolds have promise, stress concentrations can occur at the interface between scaffold components, rendering the construct susceptible to delamination or rupture.
For this reason, gradient scaffolds have been developed and allow for a more gradual transition between layers (Fig 4C). Di Luca et al [178] fabricated a 3D-printed PCL scaffold with a gradient of PEG-functionalized TGF-β3 and BMP-2. Brush functionalization allowed for the TGF-β3 to have its highest concentration in the top layer and gradually decline with depth, whereas the BMP-2 was concentrated in the bottom bone layer and gradually decreased over the transition to the upper layer. Similarly, Dormer et al [179] achieved gradient spatial control with TGF-β1 and BMP-2 loaded PLGA microspheres using a programmable syringe pump system to partially mix two separate dispersions carrying each growth factor. Following sintering of the microspheres, gradient scaffolds enabled spatial tissue growth in a rabbit femoral condyle model, providing the essential architecture for an anatomic osteochondral unit. Since the subchondral bone provides load support to healthy articular cartilage, the discussed techniques may improve the probability of success for cartilage constructs by also regenerating the bone underneath, while simultaneously creating a smooth transition between the two units. Ultimately, these techniques would be critical for patients with full-thickness cartilage injuries, or those with compromised subchondral bone due to prior marrow recruitment procedures (e.g. microfracture).
3.3. Improving Cell Infiltration and Integration of Cartilage Scaffolds
One additional concern for cartilage and osteochondral scaffolds, especially those that do not allow for cell encapsulation, is a limited migration into the center of scaffold. For example, electrospun nanofibrous scaffolds can be quite dense, and so cell infiltration is limited due to the small pore size [180]. Similarly, cartilage derived matrices containing chondroitin sulfate can present issues for both MSC (from bone marrow) and chondrocyte (from neighboring cartilage, possible in injury or mechanically perturbed scenarios) migration [181–183]. Aside from changing scaffold structure or porosity to better allow cell infiltration (which may compromise mechanics and other properties), the incorporation of chemotactic growth factors (e.g. PDGF [184,185], FGF [186,187], SDF-1α [188,189]) can enhance cellular recruitment and migration within scaffolds (Figure 4D). For example, Liebesny et al [184] utilized PDGF in a lysine-leucine-aspartic acid based hydrogel (AcN-KLDLKLDLKLDL-CNH2; KLD) hydrogel to improve the migration of MSCs from bone marrow, thus augmenting the microfracture procedure. Furthermore, these cell-recruitment approaches could be combined with pro-chondrogenic factors (e.g. TGF-β) to promote neo-cartilage formation by cells recruited during microfracture [168]. Similar techniques can be applied to induce chondrocyte migration from neighboring tissue [187], in the case of partial-thickness cartilage defects. Dual-delivery approaches are especially useful for these applications, in that a short burst of chemotactic factors can promote early cell migration, followed by a longer-term release of chondrogenic factors to induce the recruited cells to produce cartilage matrix [184,188].
Even if scaffolds can recruit cells and promote chondrogenesis, one potential issue that still remains is integration with the surrounding tissue. During normal load bearing, mechanical stresses at this interface can be quite significant if the scaffold is not well integrated with the surrounding healthy tissue [190]. In a recent study in meniscus, Qu et al [185] used PDGF to enhance migration from native tissue, while simultaneously providing small doses of collagenase to loosen the native matrix in order to improve integration. These findings could be directly applied to cartilage tissue engineering, using small amounts of degradation to create a more seamless border between the healthy neighboring tissue and the scaffold’s neo-tissue (Figure 4D) [191–193]. Alternatively, Allon et al [194] used a collagen adhesion protein in order to improve day zero integration mechanically, though the lack of retention of the protein may have caused a loss in these mechanics by Day 21. This retention, as noted previously, could certainly be improved; kartogenin release from scaffolds through PLGA microspheres improved integration between scaffolds and the surrounding tissue in a rabbit model [195]. Likewise, TGF-β3, with its strong matrix forming stimulus, improved the integration strength between core-ring constructs [196]. Thus both cell migration and mechanical integration can be enhanced via drug delivery approaches, increasing the likelihood of success in cartilage tissue engineering. By combining techniques to promote chondrogenesis with both spatiotemporal release and that promote integration, scaffolds are now better suited to replace injured cartilage in all shapes and sizes.
4. Orthobiologics: Delivering Cell-based Local “Drug Factories”
While many (if not most) drug delivery systems rely on a purified agent with a defined delivery profile, this need not be the case. Indeed, many factors that improve cartilage formation are produced by healthy cells, both in the joint and in other autologous cell sources. For this reason, cellular therapeutics have become increasingly popular. For instance, mesenchymal stem cell (MSC) and platelet-rich plasma (PRP) injections have become increasingly popular, as these cells produce anti-inflammatory proteins and growth factors that can quell an inflamed joint and promote tissue regeneration. Recent work has further tailored this behavior and the inherent production capabilities of cells both in vitro and in vivo, turning cells into “drug factories” that produce the desired bioactive factors or ECM components in a localized fashion after implantation. This final section will review the use of such cell based orthobiologics as potential localized drug delivery agents.
4.1. MSC and PRP Injections to Localize Cell-based “Drug Factories”
MSCs, also known as multipotent mesenchymal stromal cells, and more recently, “medicinal signaling cells” [197], represent a heterogeneous population of stromal derivatives [198,199]. Traditionally, these cells were of great interest to the musculoskeletal field given their ability to differentiate into functionalized cells resembling osteoblasts, chondrocytes, and adipocytes. MSCs have been applied in the context of regenerative medicine, where controlled differentiation of MSCs can be used to form native tissue-like constructs [200–202]. More recently, however, others have begun to consider the immuno-modulatory capacity of MSCs on their own [203,204]. In vivo, MSCs appear to migrate to sites of injury from perivascular niches and are purported to release factors that regulate tissue inflammation and recruit additional tissue reparative cells (Fig 5A). In this sense, MSCs can act as both the carrier and the producer of a potential therapeutic, “living drug producers”.
Figure 5.
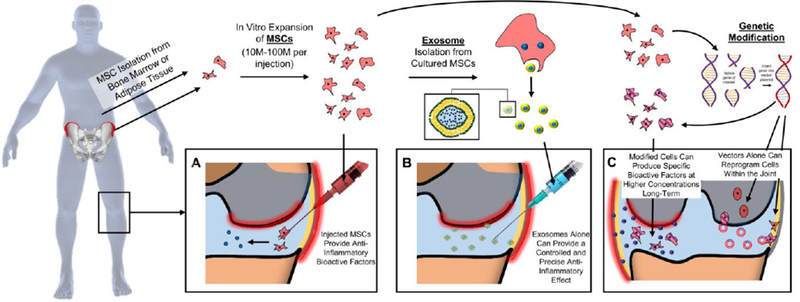
Cells and Cell-based Products as Drug-Delivery Mechanisms. MSCs are isolated from either bone marrow or adipose tissue, expanded, and [A] injected into the joint to deliver bioactive factors. Cultured MSCs can also be used to isolate the therapeutic exosomes for [B] more controlled and precise delivery of factors. [C] Genetic modification of cultured cells or direct delivery of the vector into the joint can modify cells to provide local and sustained production of therapeutic factors.
In the context of cartilage, intra-articular MSC injections have gained traction as a potential treatment for osteoarthritis (OA). These injections typically involve an aspiration (bone marrow or adipose) weeks beforehand, followed by expansion to obtain a high dose of cells, and ultimately a second “procedure” for cell application [205,206]. To avoid this second procedure, point-of-care systems have also been developed to concentrate bone marrow or adipose for same-day application [207–209], yet the long-term benefits of these therapies are not fully proven. Although there is no clear consensus on the direct chondro-regenerative effect of MSC injections, many studies have reported improved pain scores in patients with OA [210,211]. Lamo-Espinosa et al [212] showed that a high dose (100 × 106) of bone marrow-derived MSCs and hyaluronic acid delivered intra-articularly improved WOMAC scores by 16.5 points after 12 months compared to hyaluronic acid injections alone. Additionally, Soler et al [213] showed not only decreased pain and improved physical functioning, but also signs of regeneration via MRI T2-mapping, 12 months following intra-articular injection. While compelling, the exact mechanism by which MSCs are modulating the articular microenvironment has yet to be elucidated. Furthermore, the MSCs can be retained within the joint for long time periods (>10–12 weeks, [214,215]), as their size limits clearance through the lymphatic vessels. It should be noted that the majority of these cells localize to the synovial lining and fat pad [216,217], and may act to quench the activities of inflammatory cells in these tissues within the joint [218].
In addition to MSCs, other cell and cell product-based orthobiologics have become commonly used in clinical practice. For example, platelet-rich plasma (PRP) offers an unmodified biological additive that can be isolated at the time of surgery/intervention and has a much simpler regulatory pathway than other engineered delivery systems. PRP is isolated from autologous whole blood, through one or two centrifugation and isolation steps, separating plasma from red blood cells and concentrating the platelets within the plasma. Once activated by calcium chloride or other mechanisms (e.g. contact with collagen), PRP delivers a myriad of growth factors to the injection site, most notably TGF-β, PDGF, FGF, VEGF, and IGF [219,220]. The delivery of these growth factors to the joint can have a positive anti-inflammatory benefit [221,222], and may provide a cost-effective and regulatory-sensitive treatment pathway compared to delivery of specific growth factors. Furthermore, many of the factors found in PRP can increase matrix production and promote chondrogenesis. More recently, to combine the combinatorial factors of PRP with controlled delivery systems, several groups have incorporated activated-PRP into scaffolds [223–225], and have reported improved cartilage regeneration using this approach. While ease of use and low cost make PRP an intriguing option, perhaps the greatest drawback is the high variability of its components between patients, and within the same patient at different times [226,227].
4.2. Exosomes as Therapeutic Delivery Vehicles for Cartilage Repair and Regeneration
Cell-to-cell communication through soluble factors (endocrine, paracrine and autocrine signaling) is fundamental to the development of organisms and the specialization of musculoskeletal tissues. Both platelets and MSCs can release soluble factors to improve regeneration, and many have attempted to harness this soluble factor signaling between cell populations using either indirect ‘conditioned media’ [228,229] or direct ‘co-cultures’ [230,231] contexts. For instance, mixtures of chondrocytes and MSCs result in improved MSC chondrogenesis either in pellet culture [232,233] or within 3D scaffold environments [234,235]. Our understanding of the mechanisms of this cell-to-cell signaling has been further expanded by the emergence of extracellular vesicles (EVs) and other mechanisms by which cells convey information to each other [236].
Recent evidence points to a role for exosome and EV secretion as a primary mediator of the therapeutic effects exerted by MSCs [237–239]. Exosomes are naturally occurring, cell-produced nanoparticles ranging from 40–100 nm. Exosomes are formed by the folding-in of the membrane of multi-vesicular bodies, and can be released into the environment by fusion with the cell membrane (Fig 5B). MSC-generated exosomes contain a range of protein [240] and miRNA [241] products that target various cell immune and repair pathways [240,242,243]. Due to their likely role in the immunomodulatory capacity of MSCs, exosomes present an attractive therapeutic opportunity. If MSCs act as “living drug carriers”, then exosomes may represent “drug deliverables” that can be “manufactured” outside of the body and subsequently injected into damaged environments. The most common method for exosome isolation from MSCs is ultracentrifugation [244]. Once isolated, they can be delivered intra-articularly, allowing for the therapeutic effects of MSCs that can be applied in a more precise and controlled manner.
Several groups have investigated the therapeutic potential of exosome delivery. Vonk et al [245] tested the effect of bone-marrow MSC derived exosomes on OA chondrocytes cultured in the presence of TNF-α, as a model for OA cartilage inflammation. They showed that exosome delivery decreased expression of COX2 and pro-inflammatory interleukins, and reduced collagenase activity, indicating an anti-inflammatory benefit. Furthermore, increased production of proteoglycans and type II collagen was observed, implying a potential for exosomes in promoting cartilage regeneration. Similarly, Tofiño-Vian et al [246] treated OA chondrocytes with interleukin(IL)-1β to provoke an inflammatory response, and evaluated the effect of exosomes on the production of inflammatory mediators by these cells. Exosome treatment decreased production of TNF-α, IL-6, PGE2, and NO. Additionally, they noted decreased metalloproteinase activity and increased anti-inflammatory IL-10 and type II collagen expression. These studies demonstrate that exosomes may be an ideal and natural drug delivery system that can be utilized and tuned for chondro-protective and chondro-regenerative applications.
4.3. Local Biofactor Delivery via Genetic Modification
To further enhance cell-derived delivery strategies, techniques in gene therapy are being utilized as a means to reprogram cell behavior for therapeutic action [247–249]. A benefit of this approach is that one can more precisely control and tune the advantageous activity of the delivered cells. In the context of MSC injections, gene therapy can be applied to enhance their inherent immunomodulatory capacity by fine-tuning their ability to reach inflamed areas or by upregulating the expression of anti-inflammatory proteins (Fig 5C - left). For example, Shen et al [250] determined that MSCs modified to overexpress CXCR2 were more able to target inflamed mucosa in a mouse model of oral mucositis. While not intended for the knee joint environment, this approach could be adapted to target the inflamed synovium in order to protect cartilage. Furthermore, Xia et al [251] genetically engineered MSCs to express a TNFα blocker, Atsttrin, and so suppressed matrix proteases and inflammatory factors and halted the degenerative progression in a mouse model of OA.
Viral transduction is an efficient method for genetic modification of cell behavior and commonly includes the use of lenti-, retro-, and adeno-associated viral vectors [252]. Rowland et al [253] made use of scaffold-mediated lentiviral gene delivery system to induce a single MSC population towards site-specific chondrogenic and osteogenic phenotypes in osteochondral constructs. In this instance, the lentiviral vectors enabled the efficient transduction of genetic material so that MSCs overexpressed either TGF-β3 or BMP-2 in defined regions, without the antagonistic consequences of both growth factors expressed simultaneously in the same cells at the same location. Venkatesan et al [254] developed a recombinant adeno-associated vector (rAAV) genetically encoding SOX9, an early prochondrogenic transcription factor. The rAAV-SOX9 vector was applied to MSCs to induce chondrogenesis upon seeding of 3D-woven PCL scaffolds. This resulted in a prolonged period of SOX9 expression (>21 days) and protected against hypertrophic differentiation of cells in the construct. Even more sophisticated systems have been developed using CRISPR based gene editing methods [255], where the vectors reprogram host cells to produce anti-inflammatory factors when inflammation spikes. Much like the bio-response material delivery systems, these advanced gene editing and gene therapy methods can reprogram endogenous cells to act in a bio-responsive and autonomous manner to quench inflammation when it arises [256]. Ultimately, these approaches offer the potential of direct joint injection, allowing for local reprogramming of chondrocytes and synovial fibroblasts in a degenerative joint (Fig 5C – right). Such an approach could provide both long-lasting biofactor delivery, in a physiologic context, and in an on-demand manner. Large animal (equine model) and human clinical trials in this arena are now underway, with early findings showing great promise [257–260]. Several potential limitations and concerns with these approaches do however decrease enthusiasm. These relate to vector retention within the joint, the possibility of off-target transduction, and vector immunogenicity [261–263]. These attributes will certainly need to be addressed as these therapies move through clinical trials.
5. Conclusions
The use of bioactive factors in cartilage injury management clinically has progressed in recent years. Innovative biological and engineering technologies have significantly improved the efficacy and efficiency of these factors. Emerging techniques and methods in material synthesis, polymer modification and functionalization, carrier development, and scaffold fabrication, have improved: 1) delivery to the joint environment, 2) localization at damaged, inflamed, or regenerated tissue, and 3) retention and activity of therapeutic factors in the joint. Additionally, as the field of orthobiologics continues to grow, cell-based, cell-product, and cell reprogramming-based therapeutic delivery mechanisms will offer an alternative to conventional delivery approaches. Serving as endogenous “drug factories”, these cells, sometimes enhanced by gene therapy, offer the potential for durable and efficient bioactive production that might not only stabilize joints, but also enhance tissue regeneration. Each of these delivery strategies require additional evaluation, specifically with regards to safety (novel polymer systems, cell sources, gene therapy), consistency (biological variability), and clinical efficacy (significant improvement in patient-reported outcomes) over the long term. Furthermore, these clinical outcomes are often the result of numerous complex variables; for example, even structural restoration of cartilage tissue may not fully quell patient-reported pain or discomfort. Thus, new drug delivery cartilage therapies must aim to address both local functional (i.e., tissue regeneration) and joint-wide issues (i.e., inflammation, pain), and establish predictive relationships between these objective tissue-level metrics and clinical outcomes. That said, delivery of bioactive factors for both palliative OA management and cartilage regeneration has already improved preclinical outcomes and will likely see a marked increase in clinical implementation in the coming years, as the versatility and potency of these systems is increasingly validated in large animals and human clinical trials.
STATEMENT OF SIGNIFICANCE.
Articular cartilage is a remarkable and sophisticated tissue that tolerates complex stress within the joint. When injured, cartilage cannot self-repair, and these injuries often progress to joint-wide osteoarthritis, causing patients debilitating pain and loss of mobility. Current palliative and surgical treatments only provide short-term symptomatic relief and are limited with regards to efficiency and efficacy. Bioactive factors, such as drugs and growth factors, can improve outcomes to either stabilize the degenerated environment or regenerate replacement tissue. This review highlights recent advances and novel techniques to enhance the delivery, localization, retention, and activity of these factors, providing a synthesis of the cartilage drug delivery field that can guide future research in restorative and regenerative treatments for damaged cartilage.
6. Acknowledgements
This work was supported by the National Institutes of Health (R01 EB008722 and R01 AR071340) and the American Orthopaedic Society for Sports Medicine (AOSSM).
Abbreviations
- ECM
extracellular matrix
- PG
proteoglycans
- OA
osteoarthritis
- NSAID
nonsteroidal anti-inflammatory drug
- TGF
transforming growth factor
- MSC
mesenchymal stem cell
- BMP
bone morphogenetic protein
- IGF
insulin-like growth factor
- FGF
fibroblast growth factor
- HA
hyaluronic acid
- kDa
kilodaltons
- MDa
megadaltons
- TNF
tumor necrosis factor
- IL
interleukin
- MMP
matrix metalloproteinase
- DMOAD
disease-modifying osteoarthritis drug
- PLGA
poly-lactic-glycolic acid
- PCL
polycaprolactone
- PEA
polyester amides
- nm
nanometer
- PEG
polyethylene glycol
- ELP
elastin-like polypeptide
- CAP
chondrocyte-affinity peptide
- ACI
autologous chondrocyte implantation
- MACI
matrix-induced autologous chondrocyte implantation
- SDF
stromal cell-derived factor
- PRP
platelet-rich plasma
- MRI
magnetic resonance imaging
- WOMAC
Western Ontario and McMaster Universities Arthritis Index
- PDGF
platelet-derived growth factor
- VEGF
vascular endothelial growth factor
- EV
extracellular vesicle
- PGE
prostaglandin
- NO
nitric oxide
- rAAV
recombinant adeno-associated vector
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of the Drug Delivery for Musculoskeletal Applications Special Issue, edited by Robert S. Hastings and Professor Johnna S. Temenoff.
7. References
- [1].Sophia Fox AJ, Bedi A, a Rodeo S, The basic science of articular cartilage: structure, composition, and function., Sports Health. 1 (2009) 461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wong BL, Bae WC, Gratz KR, Sah RL, Shear Deformation Kinematics During Cartilage Articulation: Effect of Lubrication, Degeneration, and Stress Relaxation, Mol. Cell. Biomech 5 (2008) 197–206. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2847289/. [PMC free article] [PubMed] [Google Scholar]
- [3].Oloyede A, Broom ND, Complex nature of stress inside loaded articular cartilage, Clin. Biomech 9 (1994) 149–156. doi: 10.1016/0268-0033(94)90014-0. [DOI] [PubMed] [Google Scholar]
- [4].a Soltz M, a Ateshian G, Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage., Ann. Biomed. Eng 28 (2000) 150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- [5].Park S, Krishnan R, Nicoll SB, Ateshian GA, Cartilage interstitial fluid load support in unconfined compression, J. Biomech 36 (2003) 1785–1796. doi: 10.1016/S0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han E, Chen SS, Klisch SM, Sah RL, Contribution of Proteoglycan Osmotic Swelling Pressure to the Compressive Properties of Articular Cartilage, Biophys. J 101 (2011) 916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mak a F, The apparent viscoelastic behavior of articular cartilage--the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows., J. Biomech. Eng 108 (1986) 123–130. doi: 10.1115/1.3138591. [DOI] [PubMed] [Google Scholar]
- [8].Gomoll AH, Minas T, The quality of healing: articular cartilage, Wound Repair Regen. 22 (2014) 30–38. doi: 10.1111/wrr.12166. [DOI] [PubMed] [Google Scholar]
- [9].Venäläinen MS, Mononen ME, Salo J, Räsänen LP, Jurvelin JS, Töyräs J, Virén T, Korhonen RK, Quantitative Evaluation of the Mechanical Risks Caused by Focal Cartilage Defects in the Knee, Sci. Rep 6 (2016) 37538. doi: 10.1038/srep37538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barbour KE, Helmick CG, Boring M, Brady TJ, Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation — United States, 2013–2015, MMWR. Morb. Mortal. Wkly. Rep 66 (2017) 246–253. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C, Arthroscopic debridement for knee osteoarthritis, Cochrane Database Syst. Rev (2008) CD005118. doi: 10.1002/14651858.CD005118.pub2. [DOI] [PubMed] [Google Scholar]
- [12].Spahn G, Hofmann GO, Klinger HM, The effects of arthroscopic joint debridement in the knee osteoarthritis: Results of a meta-analysis, Knee Surgery, Sport. Traumatol. Arthrosc 21 (2013) 1553–1561. doi: 10.1007/s00167-012-2169-1. [DOI] [PubMed] [Google Scholar]
- [13].Gracitelli GC, Moraes VY, Franciozi CES, Luzo MV, Belloti JC, Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults, Cochrane Database Syst. Rev 2016 (2016). doi: 10.1002/14651858.CD010675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T, Results at 10–14 years after microfracture treatment of articular cartilage defects in the knee, Knee Surgery, Sport. Traumatol. Arthrosc 24 (2016) 1587–1593. doi: 10.1007/s00167-014-3443-1. [DOI] [PubMed] [Google Scholar]
- [15].Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RWJ, Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee, Bone Joint J. 94–B (2012) 504–509. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- [16].Peterson L, Vasiliadis HS, Brittberg M, Lindahl A, Autologous chondrocyte implantation: a long-term follow-up, Am. J. Sports Med 38 (2010) 1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- [17].Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N, Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation, Health Technol. Assess. (Rockv) 21 (2017) V–160. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ohba S, Hojo H, Chung U-I, Bioactive factors for tissue regeneration: state of the art, Muscles. Ligaments Tendons J 2 (2012) 193–203. https://www.ncbi.nlm.nih.gov/pubmed/23738297. [PMC free article] [PubMed] [Google Scholar]
- [19].Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ, The Role of Growth Factors in Cartilage Repair, Clin. Orthop. Relat. Res 469 (2011) 2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Censi R, Dubbini A, Matricardi P, Bioactive Hydrogel Scaffolds - Advances in Cartilage Regeneration Through Controlled Drug Delivery, Curr. Pharm. Des (2015). doi: 10.2174/1381612821666150115150712. [DOI] [PubMed] [Google Scholar]
- [21].da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, Trelle S, Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis, Lancet. 390 (2017) e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- [22].Simon LS, Grierson LM, Naseer Z, Bookman AAM, Zev Shainhouse J, Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis, Pain. 143 (2009) 238–245. doi: 10.1016/j.pain.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [23].Manoukian MAC, Migdal CW, Tembhekar AR, Harris JA, DeMesa C, Topical Administration of Ibuprofen for Injured Athletes: Considerations, Formulations, and Comparison to Oral Delivery, Sport. Med. - Open 3 (2017) 36. doi: 10.1186/s40798-017-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Solomon DH, Husni ME, Libby PA, Yeomans ND, Lincoff AM, Lϋscher TF, Menon V, Brennan DM, Wisniewski LM, Nissen SE, Borer JS, The Risk of Major NSAID Toxicity with Celecoxib, Ibuprofen, or Naproxen: A Secondary Analysis of the PRECISION Trial, Am. J. Med 130 (2017) 1415–1422. e4. doi: 10.1016/j.amjmed.2017.06.028. [DOI] [PubMed] [Google Scholar]
- [25].Breivik H, NSAIDs relieve osteoarthritis (OA) pain, but cardiovascular safety in question even for diclofenac, ibuprofen, naproxen, and celecoxib: what are the alternatives?, Scand. J. Pain 16 (2017) 148–149. doi: 10.1016/j.sjpain.2017.05.009. [DOI] [PubMed] [Google Scholar]
- [26].Iannitti T, McDermott MF, Laurino C, Malagoli A, Palmieri B, Corticosteroid transdermal delivery significantly improves arthritis pain and functional disability, Drug Deliv. Transl. Res 7 (2017) 156–161. doi: 10.1007/s13346-016-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Richardson DW, Dodge GR, Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes., Inflamm. Res 52 (2003) 39–49. [DOI] [PubMed] [Google Scholar]
- [28].Arroll B, Goodyear-Smith F, Corticosteroid injections for osteoarthritis of the knee: meta-analysis, BMJ Br. Med. J 328 (2004) 869. doi: 10.1136/bmj.38039.573970.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Richette P, Chevalier X, Ea HK, Eymard F, Henrotin Y, Ornetti P, Sellam J, Cucherat M, Marty M, Hyaluronan for knee osteoarthritis: An updated meta-analysis of trials with low risk of bias, RMD Open. 1 (2015). doi: 10.1136/rmdopen-2015-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bert J, Kenney J, Sgaglione NA, McClelland S, Brophy R, Toth J, Ruane J, Ali Y, Arquette S, Dasa V, Lopes M, Viscosupplementation for Osteoarthritis of the Knee: A Key Opinion Leader Panel Discussion, J. Manag. Care Spec. Pharm 24 (2018) S2–S8. doi: 10.18553/jmcp.2018.24.6-a.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Larsen NE, Dursema HD, Pollak CT, Skrabut EM, Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models, J. Biomed. Mater. Res. - Part B Appl. Biomater 100 B (2012) 457–462. doi: 10.1002/jbm.b.31971. [DOI] [PubMed] [Google Scholar]
- [32].Myers SL, Brandt KD, Effects of synovial fluid hyaluronan concentration and molecular size on clearance of protein from the canine knee, J. Rheumatol 22 (1995) 1732–1739. [PubMed] [Google Scholar]
- [33].Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL, Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels, J. Mech. Behav. Biomed. Mater 11 (2012) 92–101. doi: 10.1016/j.jmbbm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT, The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3., Osteoarthr. Cartil 15 (2007) 1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meyer MA, Urita A, Cole BJ, Chubinskaya S, Growth factors in cartilage repair, in: Cartilage, 2017: pp. 109–125. doi: 10.1007/978-3-319-53316-2_6. [DOI] [Google Scholar]
- [36].Algul D, Gokce A, Onal A, Servet E, Dogan Ekici AI, Yener FG, In vitro release and In vivo biocompatibility studies of biomimetic multilayered alginate-chitosan/β-TCP scaffold for osteochondral tissue, J. Biomater. Sci. Polym. Ed 27 (2016) 431–440. doi: 10.1080/09205063.2016.1140501. [DOI] [PubMed] [Google Scholar]
- [37].Feng Q, Lin S, Zhang K, Dong C, Wu T, Huang H, Yan X, Zhang L, Li G, Bian L, Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy, Acta Biomater. 53 (2017) 329–342. doi: 10.1016/j.actbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- [38].Blaney Davidson EN, Vitters EL, van den Berg WB, van der Kraan PM, TGF ??-induced cartilage repair is maintained but fibrosis is blocked in the presence of Smad7, Arthritis Res. Ther (2006). doi: 10.1186/ar1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Van Beuningen HM, Glansbeek HL, Van Der Kraan PM, Van Den Berg WB, Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-β injections, Osteoarthr. Cartil (2000). doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- [40].Gallelli L, Galasso O, Falcone D, Southworth S, Greco M, Ventura V, Romualdi P, Corigliano A, Terracciano R, Savino R, Gulletta E, Gasparini G, De Sarro G, The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial., Osteoarthritis Cartilage. 21 (2013) 1400–8. doi: 10.1016/j.joca.2013.06.026. [DOI] [PubMed] [Google Scholar]
- [41].Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS, Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study., Jama. 284 (2000) 1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- [42].Hunt RH, Harper S, Watson DJ, Yu C, Quan H, Lee M, Evans JK, Oxenius B, The gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events, Am. J. Gastroenterol 98 (2003) 1725–1733. doi: 10.1111/j.1572-0241.2003.07598.x. [DOI] [PubMed] [Google Scholar]
- [43].García-Rayado G, Navarro M, Lanas A, NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs, Expert Rev. Clin. Pharmacol (2018) null–null. doi: 10.1080/17512433.2018.1516143. [DOI] [PubMed] [Google Scholar]
- [44].Dubreuil M, Louie-Gao Q, Peloquin CE, Choi HK, Zhang Y, Neogi T, Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis, Ann. Rheum. Dis 77 (2018) 1137–1142. doi: 10.1136/annrheumdis-2018-213089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee T, Lu N, Felson DT, Choi HK, Dalal DS, Zhang Y, Dubreuil M, Use of non-steroidal anti-inflammatory drugs correlates with the risk of venous thromboembolism in knee osteoarthritis patients: a UK population-based case-control study., Rheumatology (Oxford). 55 (2016) 1099–105. doi: 10.1093/rheumatology/kew036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zeng C, Wei J, Persson MSM, Sarmanova A, Doherty M, Xie D, Wang Y, Li X, Li J, Long H, Lei G, Zhang W, Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies, Br. J. Sports Med (2018) bjsports-2017–098043. doi: 10.1136/bjsports-2017-098043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Roth SH, Fuller P, Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis, J. Pain Res 4 (2011) 159–167. doi: 10.2147/JPR.S20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hagen M, Baker M, Skin penetration and tissue permeation after topical administration of diclofenac, Curr. Med. Res. Opin 33 (2017) 1623–1634. doi: 10.1080/03007995.2017.1352497. [DOI] [PubMed] [Google Scholar]
- [49].Efe T, Sagnak E, Roessler PP, Getgood A, Patzer T, Fuchs-Winkelmann S, Peterlein CD, Schofer MD, Penetration of topical diclofenac sodium 4 % spray gel into the synovial tissue and synovial fluid of the knee: A randomised clinical trial, Knee Surgery, Sport. Traumatol. Arthrosc 22 (2014) 345–350. doi: 10.1007/s00167-013-2408-0. [DOI] [PubMed] [Google Scholar]
- [50].Makris UE, Kohler MJ, Fraenkel L, Adverse Effects (AEs) of Topical NSAIDs in Older Adults with Osteoarthritis (OA): a Systematic Review of the Literature, J. Rheumatol 37 (2010) 1236–1243. doi: 10.3899/jrheum.090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Derry S, Moore RA, Rabbie R, Topical NSAIDs for chronic musculoskeletal pain in adults, Cochrane Database Syst. Rev 9 (2012) CD007400–CD007400. doi: 10.1002/14651858.CD007400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Larsen C, Østergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, Andersen PH, Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders, J. Pharm. Sci 97 (2008) 4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- [53].Day RO, McLachlan a J., Graham GG, Williams KM, Pharmacokinetics of nonsteroidal anti-inflammatory drugs in synovial fluid., Clin. Pharmacokinet 36 (1999) 191–210. doi: 10.2165/00003088-199936030-00002. [DOI] [PubMed] [Google Scholar]
- [54].Sun Y, Chen J, Li H, Jiang J, Chen S, Steroid Injection and Nonsteroidal Anti-inflammatory Agents for Shoulder Pain: A PRISMA Systematic Review and Meta-Analysis of Randomized Controlled Trials, Medicine (Baltimore). 94 (2015) e2216. doi: 10.1097/MD.0000000000002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ishijima M, Nakamura T, Shimizu K, Hayashi K, Kikuchi H, Soen S, Omori G, Yamashita T, Uchio Y, Chiba J, Ideno Y, Kubota M, Kurosawa H, Kaneko K, Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial, Arthritis Res. Ther 16 (2014) R18–R18. doi: 10.1186/ar4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Merola F, Scrima M, Melito C, Iorio A, Pisano C, Giori A, Ferravante A, A novel animal model for residence time evaluation of injectable hyaluronic acid-based fillers using high-frequency ultrasound-based approach, Clin. Cosmet. Investig. Dermatol. Volume 11 (2018) 339–346. doi: 10.2147/CCID.S156740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wernecke C, Braun HJ, Dragoo JL, The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review, Orthop. J. Sport. Med 3 (2015) 2325967115581163. doi: 10.1177/2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Derendorf H, Möllmann H, Voortman G, van den Ouweland FA, VAN LBA, Putte de, Gevers G, Dequeker J, VAN Vliet‐Daskaiopoulou E, Pharmacokinetics of Rimexolone After Intra‐Articular Administration, J. Clin. Pharmacol 30 (1990) 476–479. doi: 10.1002/j.1552-4604.1990.tb03488.x. [DOI] [PubMed] [Google Scholar]
- [59].Raynauld J-P, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay J-L, Bertrand C, Pelletier J-P, Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial., Arthritis Rheum. 48 (2003) 370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- [60].Jackson DW, Simon TM, Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee, Osteoarthr. Cartil 14 (2006) 1248–1257. doi: 10.1016/j.joca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- [61].Wu B, Li Y-M, Liu Y-C, Efficacy of intra-articular hyaluronic acid injections in hip osteoarthritis: a meta-analysis of randomized controlled trials, Oncotarget. 8 (2017) 86865–86876. doi: 10.18632/oncotarget.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hirsch G, O’Neill TW, Kitas G, Sinha A, Klocke R, Accuracy of injection and short-term pain relief following intra-articular corticosteroid injection in knee osteoarthritis – an observational study, BMC Musculoskelet. Disord 18 (2017) 44. doi: 10.1186/s12891-017-1401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Edsman K, Hjelm R, Lärkner H, Nord LI, Karlsson A, Wiebensjö Å, Höglund AU, Kenne AH, Näsström J, Intra-articular Duration of Durolane™ after Single Injection into the Rabbit Knee, Cartilage. 2 (2011) 384–388. doi: 10.1177/1947603511400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Maudens P, Jordan O, Allémann E, Recent advances in intra-articular drug delivery systems for osteoarthritis therapy, Drug Discov. Today (2018). doi: 10.1016/j.drudis.2018.05.023. [DOI] [PubMed] [Google Scholar]
- [65].Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F, Lieberman JR, Jones DG, Spitzer AI, Jevsevar DS, Katz NP, Burgess DJ, Lufkin J, Johnson JR, Bodick N, on behalf of the F.−2014–008 P. Investigators, Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study, J. Bone Joint Surg. Am 100 (2018) 666–677. doi: 10.2106/JBJS.17.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kraus VB, Conaghan PG, Aazami HA, Mehra P, Kivitz AJ, Lufkin J, Hauben J, Johnson JR, Bodick N, Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA), Osteoarthr. Cartil 26 (2018) 34–42. doi: 10.1016/j.joca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- [67].Kumar A, Bendele AM, Blanks RC, Bodick N, Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis, Osteoarthr. Cartil 23 (2015) 151–160. doi: 10.1016/j.joca.2014.09.019. [DOI] [PubMed] [Google Scholar]
- [68].Park TG, Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition, Biomaterials. 16 (1995) 1123–1130. doi: 10.1016/0142-9612(95)93575-X. [DOI] [PubMed] [Google Scholar]
- [69].Rudnik-Jansen I, Colen S, Berard J, Plomp S, Que I, van Rijen M, Woike N, Egas A, van Osch G, van Maarseveen E, Messier K, Chan A, Thies J, Creemers L, Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform, J. Control. Release 253 (2017) 64–72. doi: 10.1016/j.jconrel.2017.03.014. [DOI] [PubMed] [Google Scholar]
- [70].Janssen M, Timur UT, Woike N, Welting TJM, Draaisma G, Gijbels M, van Rhijn LW, Mihov G, Thies J, Emans PJ, Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection, J. Control. Release 244 (2016) 30–40. doi: 10.1016/j.jconrel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- [71].De Jong WH, Borm PJA, Drug delivery and nanoparticles: Applications and hazards, Int. J. Nanomedicine 3 (2008) 133–149. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2527668/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dashnyam K, Lee J-H, Mandakhbayar N, Jin G-Z, Lee H-H, Kim H-W, Intra-articular biomaterials-assisted delivery to treat temporomandibular joint disorders, J. Tissue Eng 9 (2018) 2041731418776514. doi: 10.1177/2041731418776514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kang ML, Ko J-Y, Kim JE, Im G-I, Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration, Biomaterials. 35 (2014) 9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- [74].Yameen B, Il Choi W, Vilos C, Swami A, Shi J, Farokhzad OC, Insight into nanoparticle cellular uptake and intracellular targeting, J. Control. Release 190 (2014) 485–499. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kang M-L, Kim J-E, Im G-I, Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis, Acta Biomater. 39 (2016) 65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- [76].Blasco E, Sims MB, Goldmann AS, Sumerlin BS, Barner-Kowollik C, 50th Anniversary Perspective: Polymer Functionalization, Macromolecules. 50 (2017) 5215–5252. doi: 10.1021/acs.macromol.7b00465. [DOI] [Google Scholar]
- [77].Perni S, Prokopovich P, Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage, Nanomedicine Nanotechnology, Biol. Med 13 (2017) 539–548. doi: 10.1016/j.nano.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hoffman JR, Tasciotti E, Molinaro R, Microfluidic assembly of liposomes with tunable size and coloading capabilities, in: Methods Mol. Biol, 2018: pp. 205–214. doi: 10.1007/978-1-4939-7865-6_15. [DOI] [PubMed] [Google Scholar]
- [79].Jahn A, Vreeland WN, Devoe DL, Locascio LE, Gaitan M, Microfluidic directed formation of liposomes of controlled size, Langmuir. 23 (2007) 6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- [80].Joshi S, Hussain MT, Roces CB, Anderluzzi G, Kastner E, Salmaso S, Kirby DJ, Perrie Y, Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs, Int. J. Pharm 514 (2016) 160–168. doi: 10.1016/j.ijpharm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- [81].Pawar VA, Manjappa AS, Murumkar PR, Gajaria TK, V Devkar R, Mishra AK, Yadav MR, Drug-fortified liposomes as carriers for sustained release of NSAIDs: The concept and its validation in the animal model for the treatment of arthritis, Eur. J. Pharm. Sci 125 (2018) 11–22. doi: 10.1016/j.ejps.2018.09.009. [DOI] [PubMed] [Google Scholar]
- [82].Allen TM, Agrawal AK, Ahmad I, Hansen CB, Zalipsky S, Antibody-mediated targeting of long-circulating (stealthr) liposomes, J. Liposome Res 4 (1994) 1–25. doi: 10.3109/08982109409037027. [DOI] [Google Scholar]
- [83].Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A, Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo, BBA - Biomembr. 1066 (1991) 29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- [84].Urbano PCM, Soccol VT, Teixeira VN, Oliveira PG, Filippin LI, Bonat WH, de Oliveira C, Rossi GR, Xavier RM, Azevedo VF, Effect of pegylated phosphatidylserine-containing liposomes in experimental chronic arthritis, BMC Pharmacol. Toxicol 16 (2015) 24. doi: 10.1186/s40360-015-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vanniasinghe AS, Manolios N, Schibeci S, Lakhiani C, Kamali-Sarvestani E, Sharma R, Kumar V, Moghaddam M, Ali M, Bender V, Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis, Clin. Immunol 151 (2014) 43–54. doi: 10.1016/j.clim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- [86].Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL, Particle Based Technologies for Osteoarthritis Detection and Therapy, Drug Deliv. Transl. Res 6 (2016) 132–147. doi: 10.1007/s13346-015-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT, Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis, Sci. Transl. Med 10 (2018) eaat8800. doi: 10.1126/scitranslmed.aat8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gallon G, Lapinte V, Robin J-J, Chopineau J, Devoisselle J-M, Aubert-Pouëssel A, Cross-Linked Castor Oil-Based Hybrid Microparticles as Drug Delivery Systems, ACS Sustain. Chem. Eng 5 (2017) 4311–4319. doi: 10.1021/acssuschemeng.7b00369. [DOI] [Google Scholar]
- [89].Wu H, Wang K, Wang H, Chen F, Huang W, Chen Y, Chen J, Tao J, Wen X, Xiong S, Novel self-assembled tacrolimus nanoparticles cross-linking thermosensitive hydrogels for local rheumatoid arthritis therapy, Colloids Surfaces B Biointerfaces. 149 (2017) 97–104. doi: 10.1016/j.colsurfb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- [90].Qi X, Qin X, Yang R, Qin J, Li W, Luan K, Wu Z, Song L, Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium–Loaded Alginate Microspheres, J. Pharm. Sci 105 (2016) 122–130. doi: 10.1016/j.xphs.2015.11.019. [DOI] [PubMed] [Google Scholar]
- [91].Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA, Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic, Arthritis Rheum. (2007). doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- [92].Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM, Huebner JL, Chilkoti A, Kraus VB, Setton LA, Guilak F, Olson SA, SUSTAINED INTRA-ARTICULAR DELIVERY OF IL-1RA FROM A THERMALLY-RESPONSIVE ELASTIN-LIKE POLYPEPTIDE AS A THERAPY FOR POST-TRAUMATIC ARTHRITIS, Eur. Cell. Mater 29 (2015) 124–140. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358781/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Luo T, David MA, Dunshee LC, Scott RA, Urello MA, Price C, Kiick KL, Thermoresponsive elastin-b-collagen-like peptide bioconjugate nanovesicles for targeted drug delivery to collagen-containing matrices, Biomacromolecules. 18 (2017) 2539–2551. doi: 10.1021/acs.biomac.7b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hampton T, Enzyme-responsive hydrogels may help treat arthritis, JAMA. 319 (2018) 2161–2162. 10.1001/jama.2017.12861. [DOI] [PubMed] [Google Scholar]
- [95].Joshi N, Yan J, Levy S, Bhagchandani S, V Slaughter K, Sherman NE, Amirault J, Wang Y, Riegel L, He X, Rui TS, Valic M, Vemula PK, Miranda OR, Levy O, Gravallese EM, Aliprantis AO, Ermann J, Karp JM, Towards an arthritis flare-responsive drug delivery system, Nat. Commun 9 (2018) 1275. doi: 10.1038/s41467-018-03691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellers KN, Doviak H, Pettaway S, Logdon CB, Shuman J, Freels PD, Gorman JH, Gorman RC, Spinale FG, Burdick JA, Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition, Nat. Mater 13 (2014) 653–661. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Purcell BP, Barlow SC, Perreault PE, Freeburg L, Doviak H, Jacobs J, Hoenes A, Zellars KN, Khakoo AY, Lee T, Burdick JA, Spinale FG, Delivery of a matrix metalloproteinase-responsive hydrogel releasing TIMP-3 after myocardial infarction: effects on left ventricular remodeling, Am. J. Physiol. Circ. Physiol 315 (2018) H814–H825. doi: 10.1152/ajpheart.00076.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kulkarni PS, Haldar MK, Nahire RR, Katti P, Ambre AH, Muhonen WW, Shabb JB, Padi SKR, Singh RK, Borowicz PP, Shrivastava DK, Katti KS, Reindl K, Guo B, Mallik S, MMP-9 Responsive PEG Cleavable Nanovesicles for Efficient Delivery of Chemotherapeutics to Pancreatic Cancer, Mol. Pharm 11 (2014) 2390–2399. doi: 10.1021/mp500108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gao C, Ren J, Zhao C, Kong W, Dai Q, Chen Q, Liu C, Sun R, Xylan-based temperature/pH sensitive hydrogels for drug controlled release, Carbohydr. Polym 151 (2016) 189–197. doi: 10.1016/j.carbpol.2016.05.075. [DOI] [PubMed] [Google Scholar]
- [100].Kim IS, Oh IJ, Drug release from the enzyme-degradable and pH-sensitive hydrogel composed of glycidyl methacrylate dextran and poly(acrylic acid), Arch. Pharm. Res 28 (2005) 983–987. doi: 10.1007/BF02973887. [DOI] [PubMed] [Google Scholar]
- [101].Baek S, Singh RK, Kim T-H, Seo J, Shin US, Chrzanowski W, Kim H-W, Triple Hit with Drug Carriers: pH- and Temperature-Responsive Theranostics for Multimodal Chemo- and Photothermal Therapy and Diagnostic Applications, ACS Appl. Mater. Interfaces 8 (2016) 8967–8979. doi: 10.1021/acsami.6b00963. [DOI] [PubMed] [Google Scholar]
- [102].Du P, Yang H, Zeng J, Liu P, Folic acid-conjugated temperature and pH dual-responsive yolk/shell microspheres as a drug delivery system, J. Mater. Chem. B 1 (2013) 5298–5308. doi: 10.1039/C3TB20975J. [DOI] [PubMed] [Google Scholar]
- [103].Cummings NA, Nordby GL, Measurement of synovial fluid pH in normal and arthritic knees, Arthritis Rheum. 9 (1966) 47–56. doi: 10.1002/art.1780090106. [DOI] [PubMed] [Google Scholar]
- [104].Tsai PF, Richards K, Tatom I, The association between knee temperature and pain in elders with osteoarthritis of the knee: A pilot study, J. Adv. Nurs 42 (2003) 373–381. doi: 10.1046/j.1365-2648.2003.02629.x. [DOI] [PubMed] [Google Scholar]
- [105].Karimi M, Ghasemi A, Zangabad PS, Rahighi R, Moosavi Basri SM, Mirshekari H, Amiri M, Pishabad ZS, Aslani A, Bozorgomid M, Ghosh D, Beyzavi A, Vaseghi A, Aref AR, Haghani L, Bahrami S, Hamblin MR, Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems, Chem. Soc. Rev 45 (2016) 1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Karimi M, Zangabad PS, Ghasemi A, Hamblin MR, Smart External Stimulus-Responsive Nanocarriers for Drug and Gene Delivery, (2015). doi: 10.1088/978-1-6817-4202-1. [DOI]
- [107].Wang H, Yi J, Mukherjee S, Banerjee P, Zhou S, Magnetic/NIR-thermally responsive hybrid nanogels for optical temperature sensing, tumor cell imaging and triggered drug release, Nanoscale. 6 (2014) 13001–13011. doi: 10.1039/C4NR03748K. [DOI] [PubMed] [Google Scholar]
- [108].Nieminen HJ, Ylitalo T, Suuronen J-P, Rahunen K, Salmi A, Saarakkala S, Serimaa R, Hæggström E, Delivering Agents Locally into Articular Cartilage by Intense MHz Ultrasound, Ultrasound Med. Biol 41 (2015) 2259–2265. doi: 10.1016/j.ultrasmedbio.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA, Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage, Nat. Mater 7 (2008) 248 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- [110].Jiang T, Kan H-M, Rajpura K, Carbone EJ, Li Y, Lo KW-H, Development of Targeted Nanoscale Drug Delivery System for Osteoarthritic Cartilage Tissue, J. Nanosci. Nanotechnol 18 (2018) 2310–2317. doi: 10.1166/jnn.2018.14311. [DOI] [PubMed] [Google Scholar]
- [111].Hu HY, Lim NH, Ding-Pfennigdorff D, Saas J, Wendt KU, Ritzeler O, Nagase H, Plettenburg O, Schultz C, Nazare M, DOTAM Derivatives as Active Cartilage-Targeting Drug Carriers for the Treatment of Osteoarthritis, Bioconjug. Chem 26 (2015) 383–388. doi: 10.1021/bc500557s. [DOI] [PubMed] [Google Scholar]
- [112].Faust HJ, Sommerfeld SD, Rathod S, Rittenbach A, Ray Banerjee S, Tsui BMW, Pomper M, Amzel ML, Singh A, Elisseeff JH, A hyaluronic acid binding peptide-polymer system for treating osteoarthritis, Biomaterials. 183 (2018) 93–101. doi: 10.1016/j.biomaterials.2018.08.045. [DOI] [PubMed] [Google Scholar]
- [113].Sharma S, Vazquez-Portalatin N, Calve S, Panitch A, Biomimetic molecules lower catabolic expression and prevent chondroitin sulfate degradation in an osteoarthritic ex vivo model, ACS Biomater. Sci. Eng 2 (2016) 241–250. doi: 10.1021/acsbiomaterials.5b00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Loffredo FS, Pancoast JR, Cai L, Vannelli T, Dong JZ, Lee RT, Patwari P, Targeted Delivery to Cartilage Is Critical for In Vivo Efficacy of Insulin-like Growth Factor 1 in a Rat Model of Osteoarthritis, Arthritis Rheumatol. 66 (2014) 1247–1255. doi: 10.1002/art.38357. [DOI] [PubMed] [Google Scholar]
- [115].Miller RE, Grodzinsky AJ, Cummings K, Plaas AHK, Cole AA, Lee RT, Patwari P, Intraarticular injection of heparin-binding insulin-like growth factor 1 sustains delivery of insulin-like growth factor 1 to cartilage through binding to chondroitin sulfate, Arthritis Rheum. 62 (2010) 3686–3694. doi: 10.1002/art.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhu M, Wei K, Lin S, Chen X, Wu CC, Li G, Bian L, Bioadhesive Polymersome for Localized and Sustained Drug Delivery at Pathological Sites with Harsh Enzymatic and Fluidic Environment via Supramolecular Host–Guest Complexation, Small. (2018). doi: 10.1002/smll.201702288. [DOI] [PubMed] [Google Scholar]
- [117].Wang D-A, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH, Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration, 6 (2007) 385 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ramaswamy S, Wang D-A, Fishbein KW, Elisseeff JH, Spencer RG, An analysis of the integration between articular cartilage and nondegradable hydrogel using magnetic resonance imaging., J. Biomed. Mater. Res. B. Appl. Biomater 77 (2006) 144–148. doi: 10.1002/jbm.b.30404. [DOI] [PubMed] [Google Scholar]
- [119].Abubacker S, Ham HO, Messersmith PB, Schmidt TA, Cartilage boundary lubricating ability of aldehyde modified proteoglycan 4 (PRG4-CHO), Osteoarthr. Cartil 21 (2013) 186–189. doi: 10.1016/j.joca.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA, Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model, Nanomedicine Nanotechnology, Biol. Med 11 (2015) 939–946. doi: 10.1016/j.nano.2015.01.011. [DOI] [PubMed] [Google Scholar]
- [121].Geiger BC, Grodzinsky AJ, Hammond P, Designing drug delivery systems for articular joints, Chem. Eng. Prog (2018). [Google Scholar]
- [122].Bajpayee AG, Grodzinsky AJ, Cartilage-targeting drug delivery: can electrostatic interactions help?, Nat. Rev. Rheumatol 13 (2017) 183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- [123].Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ, Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis, Biomaterials. 35 (2014) 538–549. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM, Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints, J. Orthop. Res 32 (2014) 1044–1051. doi: 10.1002/jor.22630. [DOI] [PubMed] [Google Scholar]
- [125].Bajpayee AG, Quadir MA, Hammond PT, Grodzinsky AJ, Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term, Osteoarthr. Cartil 24 (2016) 71–81. doi: 10.1016/j.joca.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bajpayee AG, De la Vega RE, Scheu M, Varady NH, Yannatos IA, Brown LA, Krishnan Y, Fitzsimons TJ, Bhattacharya P, Frank EH, Grodzinsky AJ, Porter RM, SUSTAINED INTRA-CARTILAGE DELIVERY OF LOW DOSE DEXAMETHASONE USING A CATIONIC CARRIER FOR TREATMENT OF POST TRAUMATIC OSTEOARTHRITIS, Eur. Cell. Mater 34 (2017) 341–364. doi: 10.22203/eCM.v034a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kar S, Smith DW, Gardiner BS, Grodzinsky AJ, Systems Based Study of the Therapeutic Potential of Small Charged Molecules for the Inhibition of IL-1 Mediated Cartilage Degradation, PLoS One. 11 (2016) e0168047 10.1371/journal.pone.0168047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Florine EM, Kopesky P, Rennard R, Kohli N, Kim J, Chubinskaya S, Bendele A, Grodzinsky AJ, Schoeberl B, A cartilage-targeted insulin-like growth factor-1 is retained in cartilage and promotes extracellular matrix maintenance in rat medial meniscus tear model, Osteoarthr. Cartil 25 (2017) S53–S54. doi: 10.1016/j.joca.2017.02.098. [DOI] [Google Scholar]
- [129].Brown S, Pistiner J, Adjei I, Sharma B, Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake, Mol. Pharm (2017). doi: 10.1021/acs.molpharmaceut.7b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jafari S, Mair LO, Chowdhury S, Nacev A, Hilaman R, Stepanov P, Baker-McKee J, Ijanaten S, Koudelka C, English B, Malik P, Weinberg IN, Magnetically targeted delivery through cartilage, AIP Adv. 8 (2017) 56717. doi: 10.1063/1.5006156. [DOI] [Google Scholar]
- [131].Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C, Gao W, Zhou C, Ao Y, Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display, Biomaterials. 32 (2011) 6324–6332. doi: 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- [132].Hu Q, Chen Q, Yan X, Ding B, Chen D, Cheng L, Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage, Nanomedicine. 13 (2018) 749–767. doi: 10.2217/nnm-2017-0335. [DOI] [PubMed] [Google Scholar]
- [133].Pi Y, Zhang X, Shao Z, Zhao F, Hu X, Ao Y, Intra-articular delivery of anti-Hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice, Gene Ther. 22 (2015) 439 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- [134].Chiesa E, Pisani S, Colzani B, Dorati R, Conti B, Modena T, Braeckmans K, Genta I, Intra-Articular Formulation of GE11-PLGA Conjugate-Based NPs for Dexamethasone Selective Targeting—In Vitro Evaluation, Int. J. Mol. Sci 19 (2018) 2304. doi: 10.3390/ijms19082304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA, Targeting pro-inflammatory cytokines following joint injury: acute intra articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis, Arthritis Res. Ther 16 (2014) R134–R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Evans CH, Kraus VB, Setton LA, Progress in intra-articular therapy, Nat. Rev. Rheumatol 10 (2014) 11–22. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE, Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study, Arthritis Care Res. (Hoboken). 61 (2009) 344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- [138].Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, Rosli C, Borsi L, Neri D, A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo, Int. J. Cancer (2008). doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- [139].Hemmerle T, Doll F, Neri D, Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 12008–12012. doi: 10.1073/pnas.1402783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, Trachsel E, Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis, Arthritis Res. Ther 11 (2009) R142–R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Trachsel E, Bootz F, Silacci M, Kaspar M, Kosmehl H, Neri D, Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis, Arthritis Res. Ther 9 (2007) R9–R9. doi: 10.1186/ar2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Mårlind J, Kaspar M, Trachsel E, Sommavilla R, Hindle S, Bacci C, Giovannon L, Neri D, Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy, Clin. Cancer Res (2008). doi: 10.1158/1078-0432.CCR-07-5041. [DOI] [PubMed] [Google Scholar]
- [143].Silacci M, Brack SS, Späth N, Buck A, Hillinger S, Arni S, Weder W, Zardi L, Neri D, Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo, Protein Eng. Des. Sel (2006). doi: 10.1093/protein/gzl033. [DOI] [PubMed] [Google Scholar]
- [144].Gill TJ, Asnis PD, Berkson EM, The Treatment of Articular Cartilage Defects Using the Microfracture Technique, J. Orthop. Sport. Phys. Ther 36 (2006) 728–738. doi: 10.2519/jospt.2006.2444. [DOI] [PubMed] [Google Scholar]
- [145].Steadman JR, Rodkey WG, Briggs KK, Microfracture to Treat Full-Thickness Chondral Defects: Surgical Technique, Rehabilitation, and Outcomes, J. Knee Surg 15 (2002) 170–176. [PubMed] [Google Scholar]
- [146].Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, Pfeifer C, Meloni G, Dodge GR, Burdick JA, Schaer TP, Steinberg DR, Mauck RL, Cartilage Repair and Subchondral Bone Remodeling in Response to Focal Lesions in a Mini-Pig Model: Implications for Tissue Engineering, Tissue Eng. Part A 21 (2015) 850–860. doi: 10.1089/ten.tea.2014.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Pfeifer CG, Fisher MB, Saxena V, Kim M, Henning EA, Steinberg DA, Dodge GR, Mauck RL, Age-Dependent Subchondral Bone Remodeling and Cartilage Repair in a Minipig Defect Model, Tissue Eng. Part C Methods 23 (2017) 745–753. doi: 10.1089/ten.tec.2017.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Crawford DC, DeBerardino TM, Williams RJ, NeoCart, an Autologous Cartilage Tissue Implant, Compared with Microfracture for Treatment of Distal Femoral Cartilage Lesions, J. Bone Jt. Surgery-American Vol 94 (2012) 979–989. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- [149].Basad E, Ishaque B, Bachmann G, Stü H, Jü @bullet, Steinmeyer R, Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study, Knee Surgery, Sport. Traumatol. Arthrosc (2010). doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- [150].Brittberg M, Recker D, Ilgenfritz J, Saris DBF, Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial, Am. J. Sports Med 46 (2018) 1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]
- [151].Frøseth Aae T, Randsborg P-H, Lurås H, Årøen A, Øystein, Lian B, Aae TF, Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up, Knee Surgery, Sport. Traumatol. Arthrosc 0 (123AD). doi: 10.1007/s00167-017-4802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Cheng N-C, Estes BT, Young T-H, Guilak F, Engineered cartilage using primary chondrocytes cultured in a porous cartilage-derived matrix, Regen. Med 6 (2011) 81–93. doi: 10.2217/rme.10.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kim YS, Park D-Y, Cho YH, Chang JW, Choi JW, Park JK, Min BH, Shin YS, Kim CH, Cultured chondrocyte and porcine cartilage-derived substance (PCS) construct as a possible dorsal augmentation material in rhinoplasty: A preliminary animal study, J. Plast. Reconstr. Aesthetic Surg 68 (2015) 659–666. doi: 10.1016/j.bjps.2014.12.017. [DOI] [PubMed] [Google Scholar]
- [154].Bichara DA, Pomerantseva I, Zhao X, Zhou L, Kulig KM, Tseng A, Kimura AM, Johnson MA, Vacanti JP, Randolph MA, Sundback CA, Successful Creation of Tissue-Engineered Autologous Auricular Cartilage in an Immunocompetent Large Animal Model, Tissue Eng. Part A (2014). doi: 10.1103/PhysRevE.54.R50. [DOI] [PubMed] [Google Scholar]
- [155].Nanda HS, Chen S, Zhang Q, Kawazoe N, Chen G, Collagen Scaffolds with Controlled Insulin Release and Controlled Pore Structure for Cartilage Tissue Engineering, Biomed Res. Int 2014 (2014) 623805. doi: 10.1155/2014/623805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Jung H, Park JS, Yeom J, Selvapalam N, Park KM, Oh K, Yang JA, Park KH, Hahn SK, Kim K, 3D tissue engineered supramolecular hydrogels for controlled chondrogenesis of human mesenchymal stem cells, Biomacromolecules. (2014). doi: 10.1021/bm401123m. [DOI] [PubMed] [Google Scholar]
- [157].Shi D, Xu X, Ye Y, Song K, Cheng Y, Di J, Hu Q, Li J, Ju H, Jiang Q, Gu Z, Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration, ACS Nano. (2016). doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- [158].Fisher MB, Belkin NS, Milby AH, Henning EA, Söegaard N, Kim M, Pfeifer C, Saxena V, Dodge GR, Burdick JA, Schaer TP, Steinberg DR, Mauck RL, Effects of Mesenchymal Stem Cell and Growth Factor Delivery on Cartilage Repair in a Mini-Pig Model, Cartilage. (2016). doi: 10.1177/1947603515623030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Han F, Zhou F, Yang X, Zhao J, Zhao Y, Yuan X, A pilot study of conically graded chitosan–gelatin hydrogel/PLGA scaffold with dual-delivery of TGF-β1 and BMP-2 for regeneration of cartilage–bone interface, J. Biomed. Mater. Res. Part B Appl. Biomater 103 (2014) 1344–1353. doi: 10.1002/jbm.b.33314. [DOI] [PubMed] [Google Scholar]
- [160].Deepthi S, Jayakumar R, Prolonged release of TGF-β from polyelectrolyte nanoparticle loaded macroporous chitin-poly(caprolactone) scaffold for chondrogenesis, Int. J. Biol. Macromol 93 (2016) 1402–1409. doi: 10.1016/j.ijbiomac.2016.03.068. [DOI] [PubMed] [Google Scholar]
- [161].Mohanraj B, Duan G, Peredo A, Kim M, Tu F, Lee D, Dodge GR, Mauck R, Mechanically-Activated Microcapsules for “On-Demand” Drug Delivery in Dynamically Loaded Musculoskeletal Tissues, Adv. Funct. Mater (2019)In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kim IL, Pfeifer CG, Fisher MB, Saxena V, Meloni GR, Kwon MY, Kim M, Steinberg DR, Mauck RL, Burdick JA, Fibrous Scaffolds with Varied Fiber Chemistry and Growth Factor Delivery Promote Repair in a Porcine Cartilage Defect Model, Tissue Eng. Part A 21 (2015) 2680–2690. doi: 10.1089/ten.tea.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wang J, Sun B, Tian L, He X, Gao Q, Wu T, Ramakrishna S, Zheng J, Mo X, Evaluation of the potential of rhTGF- β3 encapsulated P(LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton’s jelly of human umbilical cord, Mater. Sci. Eng. C 70 (2017) 637–645. doi: 10.1016/j.msec.2016.09.044. [DOI] [PubMed] [Google Scholar]
- [164].Hung KC, Tseng CS, Dai LG, hui Hsu S, Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering, Biomaterials. (2016). doi: 10.1016/j.biomaterials.2016.01.019. [DOI] [PubMed] [Google Scholar]
- [165].Florine EM, Miller RE, Liebesny PH, Mroszczyk KA, Lee RT, Patwari P, Grodzinsky AJ, Delivering Heparin-Binding Insulin-Like Growth Factor 1 with Self-Assembling Peptide Hydrogels, Tissue Eng. Part A (2015). doi: 10.1089/ten.tea.2013.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Fernández-Muiños T, Recha-Sancho L, López-Chicón P, Castells-Sala C, Mata A, Semino CE, Bimolecular based heparin and self-assembling hydrogel for tissue engineering applications, Acta Biomater. (2015). doi: 10.1016/j.actbio.2015.01.008. [DOI] [PubMed] [Google Scholar]
- [167].Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M, Transforming Growth Factor Beta-Releasing Scaffolds for Cartilage Tissue Engineering, Tissue Eng. Part B Rev (2014). doi: 10.1089/ten.teb.2013.0271. [DOI] [PubMed] [Google Scholar]
- [168].Kim IL, Pfeifer CG, Fisher MB, Saxena V, Meloni GR, Kwon MY, Kim M, Steinberg DR, Mauck RL, Burdick JA, Fibrous Scaffolds with Varied Fiber Chemistry and Growth Factor Delivery Promote Repair in a Porcine Cartilage Defect Model, Tissue Eng. Part A (2015). doi: 10.1089/ten.tea.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Almeida HV, Liu Y, Cunniffe GM, Mulhall KJ, Matsiko A, Buckley CT, O’Brien FJ, Kelly DJ, Controlled release of transforming growth factor-β3 from cartilage-extra-cellular-matrix-derived scaffolds to promote chondrogenesis of human-joint-tissue-derived stem cells, Acta Biomater. (2014). doi: 10.1016/j.actbio.2014.05.030. [DOI] [PubMed] [Google Scholar]
- [170].Yang Q, Teng B-H, Wang L-N, Li K, Xu C, Ma X-L, Zhang Y, Kong D-L, Wang L-Y, Zhao Y-H, Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells, Int. J. Nanomedicine 12 (2017) 6721–6733. doi: 10.2147/IJN.S141888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Nanda HS, Chen S, Zhang Q, Kawazoe N, Chen G, Collagen scaffolds with controlled insulin release and controlled pore structure for cartilage tissue engineering, Biomed Res. Int (2014). doi: 10.1155/2014/623805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Deepthi S, Jayakumar R, Prolonged release of TGF-β from polyelectrolyte nanoparticle loaded macroporous chitin-poly(caprolactone) scaffold for chondrogenesis, Int. J. Biol. Macromol (2016). doi: 10.1016/j.ijbiomac.2016.03.068. [DOI] [PubMed] [Google Scholar]
- [173].Crecente-Campo J, Borrajo E, Vidal A, Garcia-Fuentes M, New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation, Eur. J. Pharm. Biopharm 114 (2017) 69–78. doi: 10.1016/j.ejpb.2016.12.021. [DOI] [PubMed] [Google Scholar]
- [174].Bonnevie ED, Mauck RL, Physiology and Engineering of the Graded Interfaces of Musculoskeletal Junctions, Annu. Rev. Biomed. Eng (2018). doi: 10.1146/annurev-bioeng-062117-121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, Tabata Y, Wong ME, Jansen JA, Mikos AG, Kasper FK, Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair, Biomaterials. (2014). doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Reyes R, Delgado A, Sánchez E, Fernández A, Hernández A, Evora C, Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold, J. Tissue Eng. Regen. Med (2014). doi: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- [177].Reyes R, Delgado A, Solis R, Sanchez E, Hernandez A, Roman JS, Evora C, Cartilage repair by local delivery of transforming growth factor-β1 or bone morphogenetic protein-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold, J. Biomed. Mater. Res. Part A 102 (2013) 1110–1120. doi: 10.1002/jbm.a.34769. [DOI] [PubMed] [Google Scholar]
- [178].Di Luca A, Klein-Gunnewiek M, Vancso JG, van Blitterswijk CA, Benetti EM, Moroni L, Covalent Binding of Bone Morphogenetic Protein-2 and Transforming Growth Factor-β3 to 3D Plotted Scaffolds for Osteochondral Tissue Regeneration, Biotechnol. J 12 (2017) 1700072. doi: 10.1002/biot.201700072. [DOI] [PubMed] [Google Scholar]
- [179].Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS, OSTEOCHONDRAL INTERFACE REGENERATION OF THE RABBIT KNEE WITH MACROSCOPIC GRADIENTS OF BIOACTIVE SIGNALS, J. Biomed. Mater. Res. A 100 (2012) 162–170. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Ahmed M, Ramos T, Wieringa P, van Blitterswijk C, de Boer J, Moroni L, Geometric constraints of endothelial cell migration on electrospun fibres, Sci. Rep 8 (2018) 6386. doi: 10.1038/s41598-018-24667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Davies LC, Blain EJ, Caterson B, Duance VC, Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes, Osteoarthr. Cartil 16 (2008) 855–864. doi: 10.1016/j.joca.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [182].Lyman JR, Chappell JD, Morales TI, Kelley SS, Lee GM, Response of Chondrocytes to Local Mechanical Injury in an Ex Vivo Model, Cartilage. 3 (2012) 58–69. doi: 10.1177/1947603511421155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kouri JB, Jimenez SA, Quintero M, Chico A, Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage, Osteoarthr. Cartil (1996). doi: 10.1016/S1063-4584(05)80320-6. [DOI] [PubMed] [Google Scholar]
- [184].Liebesny PH, Byun S, Hung H-H, Pancoast JR, Mroszczyk KA, Young WT, Lee RT, Frisbie DD, Kisiday JD, Grodzinsky AJ, Growth Factor-Mediated Migration of Bone Marrow Progenitor Cells for Accelerated Scaffold Recruitment, Tissue Eng. Part A 22 (2016) 917–927. doi: 10.1089/ten.tea.2015.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Qu F, Holloway JL, Esterhai JL, Burdick JA, Mauck RL, Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair, Nat. Commun 8 (2017) 1780. doi: 10.1038/s41467-017-01955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Filová E, Rampichová M, Litvinec A, Držík M, Míčková A, Buzgo M, Košťáková E, Martinová L, Usvald D, Prosecká E, Uhlík J, Motlík J, Vajner L, Amler E, A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs, Int. J. Pharm 447 (2013) 139–149. doi: 10.1016/j.ijpharm.2013.02.056. [DOI] [PubMed] [Google Scholar]
- [187].Pabbruwe MB, Esfandiari E, Kafienah W, Tarlton JF, Hollander AP, Induction of cartilage integration by a chondrocyte/collagen-scaffold implant, Biomaterials. 30 (2009) 4277–4286. doi: 10.1016/j.biomaterials.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Chim H, Miller E, Gliniak C, Alsberg E, Stromal-cell-derived factor (SDF) 1-alpha in combination with BMP-2 and TGF-β1 induces site -directed cell homing and osteogenic and chondrogenic differentiation for tissue engineering without the requirement for cell seeding, Cell Tissue Res. 350 (2012) 89–94. doi: 10.1007/s00441-012-1449-x. [DOI] [PubMed] [Google Scholar]
- [189].Zhang W, Chen J, Tao J, Jiang Y, Hu C, Huang L, Ji J, Ouyang HW, The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair, Biomaterials. 34 (2013) 713–723. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- [190].Chen T, McCarthy MM, Guo H, Warren R, Maher SA, The Scaffold–Articular Cartilage Interface: A Combined In Vitro and In Silico Analysis Under Controlled Loading Conditions, J. Biomech. Eng 140 (2018) 91002–91007. 10.1115/1.4040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ng KW, Wanivenhaus F, Chen T, Hsu H-C, Allon AA, Abrams VD, Torzilli PA, Warren RF, Maher SA, A Novel Macroporous Polyvinyl Alcohol Scaffold Promotes Chondrocyte Migration and Interface Formation in an In Vitro Cartilage Defect Model, Tissue Eng. Part A 18 (2012) 1273–1281. doi: 10.1089/ten.tea.2011.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Hunziker EB, Kapfinger DE, Müller ME, Hunziker EB, Kapfinger E, Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells, J Bone Jt. Surg [Br]. 80 (1998) 144–50. doi: 10.1302/0301-620X.80B1.7531. [DOI] [PubMed] [Google Scholar]
- [193].Janssen LM, In Der Maur CD, Koen Bos P, Hardillo JA, Van Osch GJVM, Short-duration enzymatic treatment promotes integration of a cartilage graft in a defect, Ann. Otol. Rhinol. Laryngol (2006). doi: 10.1177/000348940611500611. [DOI] [PubMed] [Google Scholar]
- [194].Allon AA, Ng KW, Hammoud S, Russell BH, Jones CM, Rivera JJ, Schwartz J, Hook M, Maher SA, Augmenting the articular cartilage-implant interface: functionalizing with a collagen adhesion protein, J. Biomed. Mater. Res. A 100 (2012) 2168–2175. doi: 10.1002/jbm.a.34144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sun X, Wang J, Wang Y, Zhang Q, Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering, Artif. Cells, Nanomedicine, Biotechnol (2017). doi: 10.1080/21691401.2017.1397000. [DOI] [PubMed] [Google Scholar]
- [196].Maher SA, Mauck RL, Rackwitz L, Tuan RS, A Nano-Fibrous Cell Seeded Hydrogel Promotes Integration in a Cartilage Gap Model, J. Tissue Eng. Regen. Med 4 (2010) 25–29. doi: 10.1002/term.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Caplan AI, Mesenchymal stem cells: Time to change the name!, Stem Cells Transl. Med (2017). doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Majore I, Moretti P, Hass R, Kasper C, Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord, Cell Commun. Signal (2009). doi: 10.1186/1478-811X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Hass R, Kasper C, Böhm S, Jacobs R, Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC., Cell Commun. Signal (2011). doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Ren L, Ma D, Liu B, Li J, Chen J, Yang D, Gao P, Preparation of three-dimensional vascularized MSC cell sheet constructs for tissue regeneration, Biomed Res. Int (2014). doi: 10.1155/2014/301279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sasaki JI, Hashimoto M, Yamaguchi S, Itoh Y, Yoshimoto I, Matsumoto T, Imazato S, Fabrication of biomimetic bone tissue using mesenchymal stem cell-derived three-dimensional constructs incorporating endothelial cells, PLoS One. (2015). doi: 10.1371/journal.pone.0129266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Saxena V, Kim M, Keah NM, Neuwirth AL, Stoeckl BD, Bickard K, Restle DJ, Salowe R, Wang MY, Steinberg DR, Mauck RL, Anatomic Mesenchymal Stem Cell-Based Engineered Cartilage Constructs for Biologic Total Joint Replacement, Tissue Eng. Part A 22 (2016) 386–395. doi: 10.1089/ten.tea.2015.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wang Y, Chen X, Cao W, Shi Y, Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications, Nat. Immunol (2014). doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- [204].van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM, Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture, Osteoarthr. Cartil (2012). doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [205].Mardones R, Jofré CM, Tobar L, Minguell JJ, Mesenchymal stem cell therapy in the treatment of hip osteoarthritis, J. Hip Preserv. Surg 4 (2017) 159–163. doi: 10.1093/jhps/hnx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS, Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial, Stem Cells. 32 (2014) 1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- [207].Coughlin RP, Oldweiler A, Mickelson DT, Moorman CT, Adipose-Derived Stem Cell Transplant Technique for Degenerative Joint Disease, Arthrosc. Tech 6 (2017) e1761–e1766. doi: 10.1016/j.eats.2017.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Yokota N, Yamakawa M, Shirata T, Kimura T, Kaneshima H, Clinical results following intra articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee, Regen. Ther 6 (2017) 108–112. doi: 10.1016/j.reth.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Darrow M, Shaw B, Darrow B, Wisz S, Short-Term Outcomes of Treatment of Hip Osteoarthritis With 4 Bone Marrow Concentrate Injections: A Case Series, Clin. Med. Insights. Case Rep 11 (2018) 1179547618791574–1179547618791574. doi: 10.1177/1179547618791574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J, Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial, Transplantation. (2015). doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- [211].Davatchi et al. , Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients., Int. J. Rheum. Dis (2011). doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- [212].Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Cañizo MC, Valentí-Nin JR, Prósper F, Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II), J. Transl. Med (2016). doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, Coll R, Codinach M, Garcia-Lopez J, Final results of a phase I–II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration, Knee. (2016). doi: 10.1016/j.knee.2015.08.013. [DOI] [PubMed] [Google Scholar]
- [214].Delling U, Brehm W, Metzger M, Ludewig E, Winter K, Jülke H, In Vivo Tracking and Fate of Intra-Articularly Injected Superparamagnetic Iron Oxide Particle-Labeled Multipotent Stromal Cells in an Ovine Model of Osteoarthritis, Cell Transplant. 24 (2015) 2379–2390. doi: 10.3727/096368914X685654. [DOI] [PubMed] [Google Scholar]
- [215].Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, Cao W, Wang R, Wang W, In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model, Stem Cell Res. Ther 7 (2016) 160. doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, Saito T, Sekiya I, Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats, Osteoarthr. Cartil 24 (2016) 1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- [217].Murphy JM, Fink DJ, Hunziker EB, Barry FP, Stem cell therapy in a caprine model of osteoarthritis, Arthritis Rheum. 48 (2003) 3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- [218].Kehoe O, Cartwright A, Askari A, El Haj AJ, Middleton J, Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis, J. Transl. Med 12 (2014) 157. doi: 10.1186/1479-5876-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lubkowska A, Dolegowska B, Banfi G, Growth factor content in PRP and their applicability in medicine., J. Biol. Regul. Homeost. Agents (2012). [PubMed] [Google Scholar]
- [220].Wen Y-H, Lin W-Y, Lin C-J, Sun Y-C, Chang P-Y, Wang H-Y, Lu J-J, Yeh W-L, Chiueh T-S, Sustained or higher levels of growth factors in platelet-rich plasma during 7-day storage, Clin. Chim. Acta 483 (2018) 89–93. doi: 10.1016/j.cca.2018.04.027. [DOI] [PubMed] [Google Scholar]
- [221].Osterman C, Mccarthy MBR, Cote MP, Beitzel K, Bradley J, Polkowski G, Mazzocca AD, Platelet-Rich Plasma Increases Anti-inflammatory Markers in a Human Coculture Model for Osteoarthritis, Am. J. Sports Med (2015). doi: 10.1177/0363546515570463. [DOI] [PubMed] [Google Scholar]
- [222].Martini LI, Via AG, Fossati C, Randelli F, Randelli P, Cucchi D, Single Platelet-Rich Plasma Injection for Early Stage of Osteoarthritis of the Knee, Joints. 5 (2017) 2–6. doi: 10.1055/s-0037-1601405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Beigi M-H, Atefi A, Ghanaei H-R, Labbaf S, Ejeian F, Nasr-Esfahani M-H, Activated platelet-rich plasma improves cartilage regeneration using adipose stem cells encapsulated in a 3D alginate scaffold, J. Tissue Eng. Regen. Med 12 (2018) 1327–1338. doi: 10.1002/term.2663. [DOI] [PubMed] [Google Scholar]
- [224].Carneiro M. de O., Barbieri CH, Barbieri J, Platelet-rich plasma gel promotes regeneration of articular cartilage in knees of sheeps, Acta Ortop. Bras 21 (2013) 80–86. doi: 10.1590/S1413-78522013000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sermer C, Devitt B, Chahal J, Kandel R, Theodoropoulos J, The Addition of Platelet-Rich Plasma to Scaffolds Used for Cartilage Repair: A Review of Human and??Animal Studies, Arthroscopy. (2015). doi: 10.1016/j.arthro.2015.01.027. [DOI] [PubMed] [Google Scholar]
- [226].Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD, Variability of platelet-rich plasma preparations, Sports Med. Arthrosc (2013). doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- [227].Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, Arciero RA, Beitzel K, Platelet-Rich Plasma Differs According to Preparation Method and Human Variability, J. Bone Jt. Surgery-American Vol (2012). doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- [228].Liu J, Liu X, Zhou G, Xiao R, Cao Y, Conditioned Medium from Chondrocyte/Scaffold Constructs Induced Chondrogenic Differentiation of Bone Marrow Stromal Cells, Anat. Rec (2012). doi: 10.1002/ar.22500. [DOI] [PubMed] [Google Scholar]
- [229].Platas J, Guillén MI, Del Caz MDP, Gomar F, Mirabet V, Alcaraz MJ, Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by interleukin-1 β in osteoarthritic chondrocytes, Mediators Inflamm. (2013). doi: 10.1155/2013/357014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Kulesza A, Burdzinska A, Szczepanska I, Zarychta-Wisniewska W, Pajak B, Bojarczuk K, Dybowski B, Paczek L, The mutual interactions between mesenchymal stem cells and myoblasts in an autologous co-culture model, PLoS One. (2016). doi: 10.1371/journal.pone.0161693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Qu C, Puttonen KA, Lindeberg H, Ruponen M, Hovatta O, Koistinaho J, Lammi MJ, Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture, Int. J. Biochem. Cell Biol (2013). doi: 10.1016/j.biocel.2013.05.029. [DOI] [PubMed] [Google Scholar]
- [232].Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, Marcucio RS, Schneider RA, Lotz JC, Alliston T, Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy, Osteoarthritis Cartilage. 19 (2011) 1210–1218. doi: 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lopa S, Colombini A, Sansone V, Preis FWB, Moretti M, Influence on Chondrogenesis of Human Osteoarthritic Chondrocytes in Co-Culture with Donor-Matched Mesenchymal Stem Cells from Infrapatellar Fat Pad and Subcutaneous Adipose Tissue, Int. J. Immunopathol. Pharmacol 26 (2013) 23–31. doi: 10.1177/03946320130260S104. [DOI] [PubMed] [Google Scholar]
- [234].V Meretoja V, Dahlin RL, Kasper FK, Mikos AG, Enhanced Chondrogenesis in Co-Cultures with Articular Chondrocytes and Mesenchymal Stem Cells, Biomaterials. 33 (2012) 6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kim M, Steinberg DR, Burdick JA, Mauck RL, Extracellular Vesicles Mediate Improved Functional Outcomes in Engineered Cartilage Produced from MSC/Chondrocyte Co-Cultures, Proc. Natl. Acad. Sci (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Malda J, Boere J, Van De Lest CHA, Van Weeren PR, Wauben MHM, Extracellular vesicles - New tool for joint repair and regeneration, Nat. Rev. Rheumatol (2016). doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, V de Kleijn DP, Lim SK, Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury, Stem Cell Res. (2010). doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- [238].Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W, HucMSc-exosome mediated-Wnt4 signaling is required for cutaneous wound healing, Stem Cells. (2015). doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- [239].Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK, Mesenchymal Stem Cells Secrete Immunologically Active Exosomes, Stem Cells Dev. (2014). doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- [240].Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK, Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome, Int. J. Proteomics (2012). doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK, Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs, Nucleic Acids Res. (2009). doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP, Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury, Stem Cell Res. (2013). doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- [243].Lai RC, Yeo RWY, Lim SK, Mesenchymal stem cell exosomes., Semin. Cell Dev. Biol (2015). doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- [244].Willis GR, Kourembanas S, Mitsialis SA, Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency, Front. Cardiovasc. Med (2017). doi: 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ, Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro, Theranostics. (2018). doi: 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ, Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes., Cell. Physiol. Biochem (2018). doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- [247].Nixon AJ, Goodrich LR, Scimeca MS, Witte TH, Schnabel LV, Watts AE, Robbins PD, Gene therapy in musculoskeletal repair, in: Ann. N. Y. Acad. Sci, 2007. doi: 10.1196/annals.1402.065. [DOI] [PubMed] [Google Scholar]
- [248].Evans CH, Ghivizzani SC, Robbins PD, Arthritis gene therapy is becoming a reality, Nat. Rev. Rheumatol (2018). doi: 10.1038/s41584-018-0009-5. [DOI] [PubMed] [Google Scholar]
- [249].Evans CH, Huard J, Gene therapy approaches to regenerating the musculoskeletal system, Nat. Rev. Rheumatol (2015). doi: 10.1038/nrrheum.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Zhang X, Shen Z, Wang J, Huang Q, Shi Y, Wei Z, Zhang X, Qiu Y, Zhang M, Wang Y, Qin W, Huang S, Huang Y, Liu X, Xia K, Lin Z, Genetic modification to induce CXCR2 overexpression in mesenchymal stem cells enhances treatment benefits in radiation-induced oral mucositis article, Cell Death Dis. (2018). doi: 10.1038/s41419-018-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Xia Q, Zhu S, Wu Y, Wang J, Cai Y, Chen P, Li J, Heng BC, Ouyang HW, Lu P, Intra-Articular Transplantation of Atsttrin-Transduced Mesenchymal Stem Cells Ameliorate Osteoarthritis Development, Stem Cells Transl. Med (2015). doi: 10.5966/sctm.2014-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Park JS, Suryaprakash S, Lao Y-H, Leong KW, Engineering mesenchymal stem cells for regenerative medicine and drug delivery, Methods. (2015). doi: 10.1016/j.ymeth.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Rowland CR, Glass KA, Ettyreddy AR, Gloss CC, Matthews JRL, Huynh NPT, Guilak F, Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs, Biomaterials. 177 (2018) 161–175. doi: 10.1016/j.biomaterials.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Venkatesan JK, Moutos FT, Rey-Rico A, Estes BT, Frisch J, Schmitt G, Madry H, Guilak F, Cucchiarini M, Chondrogenic Differentiation Processes in Human Bone-Marrow Aspirates Seeded in Three-Dimensional-Woven Poly(ɛ-Caprolactone) Scaffolds Enhanced by Recombinant Adeno-Associated Virus–Mediated SOX9 Gene Transfer, Hum. Gene Ther (2018). doi: 10.1089/hum.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Adkar SS, Brunger JM, Willard VP, Wu C-L, Gersbach CA, Guilak F, Genome Engineering for Personalized Arthritis Therapeutics, Trends Mol. Med 23 (2017) 917–931. doi: 10.1016/j.molmed.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F, Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs, Stem Cell Reports. (2017). doi: 10.1016/j.stemcr.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Watson Levings RS, Smith AD, Broome TA, Rice BL, Gibbs EP, Myara DA, Hyddmark EV, Nasri E, Zarezadeh A, Levings PP, Lu Y, White ME, Dacanay EA, Foremny GB, Evans CH, Morton AJ, Winter M, Dark MJ, Nickerson DM, Colahan PT, Ghivizzani SC, Self-Complementary Adeno-Associated Virus–Mediated Interleukin-1 Receptor Antagonist Gene Delivery for the Treatment of Osteoarthritis: Test of Efficacy in an Equine Model, Hum. Gene Ther. Clin. Dev (2018). doi: 10.1089/humc.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Watson Levings RS, Broome TA, Smith AD, Rice BL, Gibbs EP, Myara DA, Hyddmark EV, Nasri E, Zarezadeh A, Levings PP, Lu Y, White ME, Dacanay EA, Foremny GB, Evans CH, Morton AJ, Winter M, Dark MJ, Nickerson DM, Colahan PT, Ghivizzani SC, Gene Therapy for Osteoarthritis: Pharmacokinetics of Intra-Articular Self-Complementary Adeno-Associated Virus Interleukin-1 Receptor Antagonist Delivery in an Equine Model, Hum. Gene Ther. Clin. Dev 29 (2018) 90–100. doi: 10.1089/humc.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Evans CH, Ghivizzani SC, Robbins PD, Arthritis Gene Therapy Approved in Korea, J. Am. Acad. Orthop. Surg (2018). doi: 10.5435/JAAOS-D-17-00695. [DOI] [PubMed] [Google Scholar]
- [260].Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW, Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene, Gene Ther. (2002). doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- [261].Kaufmann KB, Büning H, Galy A, Schambach A, Grez M, Gene therapy on the move, EMBO Mol. Med 5 (2013) 1642–1661. doi: 10.1002/emmm.201202287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, Hobbs KF, Greenwald M, Hou A, Bookbinder SA, Graham GE, Wiesenhutter CW, Willis L, Ruderman EM, Forstot JZ, Maricic MJ, Dao KH, Pritchard CH, Fiske DN, Burch FX, Prupas HM, Anklesaria P, Heald AE, Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: Results of a phase 1/2 study, J. Rheumatol (2010). doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- [263].Kyostio-Moore S, Bangari DS, Ewing P, Nambiar B, Berthelette P, Sookdeo C, Hutto E, Moran N, Sullivan J, Matthews GL, Scaria A, Armentano D, Local gene delivery of heme oxygenase-1 by adeno-associated virus into osteoarthritic mouse joints exhibiting synovial oxidative stress, Osteoarthr. Cartil 21 (2013) 358–367. doi: 10.1016/j.joca.2012.11.002. [DOI] [PubMed] [Google Scholar]


