Abstract
HIV-1 infection continues to be a global health challenge and a vaccine is urgently needed. Broadly neutralizing antibodies (bNAbs) are considered essential as they inhibit multiple HIV-1 strains, but they are difficult to elicit by conventional immunization. In contrast, non-neutralizing antibodies that correlated with reduced risk of infection in the RV144 HIV vaccine trial are relatively easy to induce, but responses are not durable. To overcome these obstacles, adeno-associated virus (AAV) vectors were used to provide long-term expression of antibodies targeting the V2 region of the HIV-1 envelope protein, including the potent CAP256-VRC26.25 bNAb, as well as non-neutralizing CAP228 antibodies that resemble those elicited by vaccination. AAVs mediated effective antibody expression in cell culture and immunocompetent mice. Mean concentrations of human immunoglobulin G (IgG) in mouse sera increased rapidly following a single AAV injection, reaching 8–60 μg/mL for CAP256 antibodies and 44–220 μg/mL for CAP228 antibodies over 24 weeks, but antibody concentrations varied for individual mice. Secreted antibodies collected from serum retained the expected binding and neutralizing activity. The vectors generated here are, therefore, suitable for the delivery of V2-targeting HIV antibodies, and they could be used in a vectored immunoprophylaxis (VIP) approach to sustain the level of antibody expression required to prevent HIV infection.
Keywords: vectored immunoprophylaxis, AAV, gene transfer, HIV vaccine, broadly neutralizing antibodies, V2 apex
Introduction
Several studies have attempted to elicit broadly neutralizing antibodies (bNAbs) against HIV-1 by vaccination,1 but so far this goal has proved elusive. Passive infusion of bNAbs can protect against HIV-1 infection in animal models, providing a strong rationale to pursue this approach in humans.2 Numerous bNAbs have now been isolated from HIV-infected individuals,3 and they bind to one of several common epitopes on the HIV-1 envelope protein.4 These include the CD4-binding site (CD4bs), membrane-proximal external region (MPER), V2-glycan site at the apex of the envelope (Env) trimer, a gp120 V3-glycan site centered on the glycan at N332, and the interface between the MPER and gp120 protomers. The first human efficacy trial of a bNAb (https://www.clinicaltrials.gov/; antibody-mediated protection (AMP); ClinicalTrials.gov: NCT02716675) aims to determine whether VRC01, a bNAb that targets the CD4bs, can prevent the acquisition of HIV infection. However, as passively infused antibodies wane over time, participants in this trial receive infusions of VRC01 every 8 weeks for the 2-year duration of the study to sustain antibody levels.5
Antibodies targeting the V2-glycan epitope are among the most common bNAbs, which develop in a subset of HIV-infected individuals.6, 7, 8 These bNAbs have been isolated from several donors, including donor 24 (PG9 and PG16),9 donor 0219 (CH01–CH04),10 donor 256 (CAP256-VRC26.01–33),11, 12 and donor 84 (PGT141–145, PGDM1400–1412).13, 14 Antibodies targeting the V2 apex contain a characteristic heavy chain complementarity-determining region 3 (CDRH3) with an antigen-binding loop that is capable of penetrating the HIV-1 glycan shield.15, 16 The two most potent V2 bNAbs, PGDM1400 and CAP256-VRC26.25, have shown exceptional potency and protective efficacy against a clade C variant of simian HIV (SHIV) in rhesus macaques.17 Antibodies to the V2 region have also been implicated in the moderate efficacy observed in the RV144 HIV vaccine trial.18, 19 However, these antibodies are non-neutralizing, as they bind a different structural conformation of the V2 region and mediate antibody-dependent cellular cytotoxicity (ADCC).20 Unlike bNAbs, these types of V2 antibodies are readily induced by vaccination, but titers wane and re-boosting will likely be required to sustain immunity.
While the efficacy of bNAbs for passive immunity has been demonstrated,21, 22, 23 the requirement for repeat administrations may not be practical for large-scale application in resource-limited settings. Vector-mediated transfer of genes encoding antibodies has, therefore, been explored as an alternative to passive immunotherapy.24, 25, 26, 27 Adeno-associated virus (AAV) vectors are well suited for this purpose and have an excellent safety profile.28 AAVs engineered to carry antibody genes mediate effective gene transfer to muscle tissue, thus allowing for antibody expression, secretion, and systemic distribution.29 Antibodies may be subsequently detected in the sera of animals following intramuscular injection of AAV vectors, and they can achieve long-term protection against HIV-1 infection in an approach termed vectored immunoprophylaxis (VIP).30 In humanized mice, bNAbs delivered by vector-mediated gene transfer provided complete protection against HIV-1 infection following repetitive vaginal challenges with a heterosexually transmitted founder strain of the virus.31 Effective protection against simian immunodeficiency virus (SIV) or SHIV infection has also been demonstrated for modified antibodies in non-human primates.32, 33, 34, 35 Two phase I clinical trials to assess the safety and efficacy of AAV-mediated gene transfer of bNAbs as part of a VIP approach are currently underway (AAV8-VRC07; ClinicalTrials.gov: NCT03374202) or recently completed (AAV1-PG9; ClinicalTrials.gov: NCT01937455).36
In this study, we focused on AAV-mediated antibody gene transfer for the delivery of antibodies targeting the V2 region. We selected the CAP256-VRC26 bNAb lineage, including the most potent member CAP256.25, which is being developed for passive immunization, as well as non-neutralizing antibodies associated with vaccine efficacy. Sequences encoding antibodies were inserted into an AAV-based expression plasmid using a novel cloning intermediate that enables efficient assembly of antibody sequences. The resulting AAVs mediated effective antibody expression in cell culture and in mice for 6 months following intramuscular injection of the vectors. Importantly, antibodies retained the expected binding and functional activities. These newly constructed vectors thus efficiently delivered sequences encoding V2-targeting antibodies with durable expression levels. This approach reinforces the notion that VIP has clinical utility and could be used to provide protection against HIV-1 strains, including subtype C variants that are prevalent in epidemic regions of southern Africa.
Results
Selection of HIV V2-Specific Antibodies
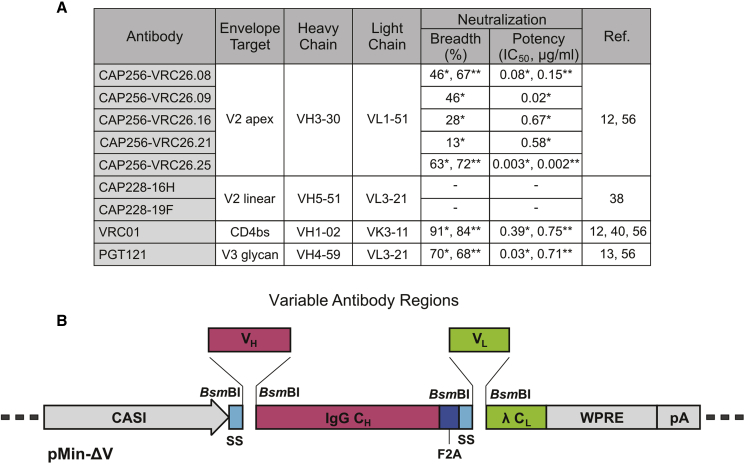
Antibodies previously isolated from HIV-infected donors in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 002 cohort37 that target the V2 region were selected for vector-mediated gene transfer. These included five from the broadly neutralizing CAP256-VRC26 lineage and two from donor CAP22811, 12, 38 (Figure 1A). The CAP256-VRC26 antibodies (CAP256.08, CAP256.09, CAP256.16, CAP256.21, and CAP256.25) are somatically related and show varying levels of neutralizing activity against HIV-1.11, 12 CAP256.25 is 10-fold more potent than previously described members of this family,12 and it is currently under clinical development. CAP228 antibodies (CAP228-16H and CAP228-19F) target the V2 linear region and are non-neutralizing. They are similar to V1V2 antibodies, which correlated with a reduced risk of HIV-1 infection and may have contributed to protection in the RV144 vaccine trial.18, 19 CAP228 antibodies have also been shown to mediate the ADCC of infected cells.39 Sequences encoding VRC0140 and PGT121,13 which target the CD4bs and V3 glycan, respectively, were used in previous VIP studies30, 31, 41 and included here as controls.
Figure 1.
Selected Antibodies and the Optimized Antibody Expression Cassette
(A) Antibodies from the CAPRISA cohort used for packaging into AAVs. The epitope specificity, characteristics of the germline-encoded V gene and both the neutralization breadth and potency (median IC50) against sensitive viruses in a mixed subtype (*) or subtype C (**) panel are indicated. (B) The novel pMin-ΔV cloning vector for the facile generation of antibody constructs. Type IIs restriction sites were included in pMin-ΔV to enable fragmentation of the expression construct and incorporation of specific variable heavy- and light-chain sequences (VH and VL), using Gibson assembly. The vector contains constant regions of the IgG heavy chain (IgG CH) and kappa light chain (λ CL), as well as an enhanced promoter (CASI), human growth hormone secretion signals (SSs), self-cleaving peptide (F2A), woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and SV40 polyadenylation signal (pA).
Optimized Antibody Expression Cassettes
The level of antibody expression is important for the success of VIP. We therefore used an AAV-based plasmid (pAAV) optimized for antibody expression.30 AAVs generated using this plasmid were previously shown to provide lifelong antibody expression (∼100 μg/mL for 52 weeks) in immunocompetent mice and long-term protection against HIV-1 infection in humanized mice.30 Noteworthy features of the plasmid expression cassette include the following: (1) a CASI promoter comprising a cytomegalovirus (CMV) enhancer, chicken β-actin promoter, and ubiquitin enhancer region; (2) a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE);42 (3) an optimized F2A self-cleaving peptide43 with a furin cleavage site that separates adjacent heavy and light chains after co-expression; and (4) two unique codon-optimized human growth hormone signal sequences (SSs) for improved antibody secretion. This plasmid has been used to express several antibodies against HIV, including VRC01 and VRC07.30, 31
To re-engineer this pAAV, variable heavy-chain (VH) and variable light-chain (VL) antibody sequences of the original VRC01 expression vector (pAAV-VRC01) were replaced with those of the CAPRISA antibodies. The kappa constant region of the light chain (κ CL) was also replaced with a corresponding lambda region (λ CL) for all CAPRISA antibodies. Initially, expression vectors were generated using Gibson assembly,44 with linearized pAAV-VRC01 and six overlapping fragments to constitute the antibody-encoding expression cassette. This direct approach was successful for the construction of pAAVs encoding CAP256.08, CAP256.09, and PGT121. However, cloning efficiency was low, likely because of the number of fragments used and instability caused by AAV inverted terminal repeat (ITR) sequences.
To improve efficiency of the Gibson assembly procedure, a novel intermediate plasmid (pMin-ΔV) was generated to facilitate cloning of other pAAVs encoding antibody sequences (Figure 1B). This vector contains common elements of the antibody expression cassette in a stable backbone derived from pUC19, which could be easily fragmented for use with Gibson assembly. Linkers containing double internal type IIs restriction sites (BsmBI) were inserted in place of variable regions of the antibody expression cassette to enable the excision of only two fragments. Variable fragments (VL and VH) of novel antibodies could then be combined conveniently with these fragments in a single Gibson assembly reaction to create scarless expression cassettes. Resulting pMin antibody expression plasmids lacked ITR elements, thus eliminating problems associated with recombination. Verified antibody expression cassettes were inserted into the ITR-containing pAAVs using conventional cloning methods. This indirect approach was used successfully to construct pAAV-CAP256.16, pAAV-CAP256.21, pAAV-CAP256.25, pAAV-CAP228-16H, and pAAV-CAP228-19F.
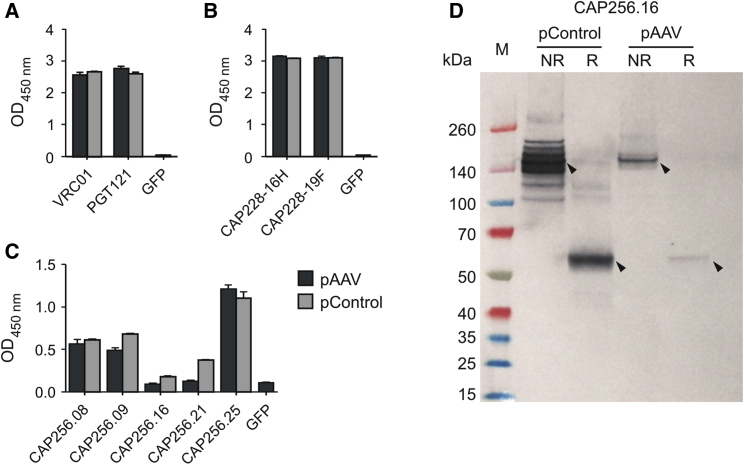
Antibody expression from the pAAVs was assessed after transient transfection of cells in culture. The expression plasmids were used to transfect HEK293T cells, and media collected 3 days later were screened for secreted immunoglobulin G (IgG) antibodies. Medium from cells transfected with a GFP expression plasmid45 was used as a negative control, while antibodies expressed and purified from non-AAV control expression plasmids (pControl) were used as positive controls. Antibody expression was successfully detected for each expression vector by ELISA using relevant antigens. VRC01 and PGT121 bound to a gp120 protein46 (Figure 2A), while the CAP228 antibodies were detected using a V1V2-scaffolded protein (Figure 2B). CAP256 antibodies were detected using a trimeric gp140 antigen (BG505 SOSIP.664), as these antibodies recognize a conformational epitope47 (Figure 2C). Binding levels varied between antibodies but were similar to the control in each case, indicating poor recognition of the gp140 antigen by some CAP256 monoclonal antibodies (mAbs). Analysis using western blot indicated that CAP256 antibodies were secreted as intact proteins (Figure 2D). A band corresponding to the full IgG molecule (∼152 kDa) was detected under non-reducing conditions, while a band corresponding to the heavy chain (∼52 kDa) was detected under reducing conditions.
Figure 2.
Antibody Expression from the AAV-Based Expression Plasmid
(A–C) Antibodies were detected using ELISA with various envelope protein derivatives as the immobilized antigen and an HRP-conjugated secondary antibody. Monoclonal antibodies expressed and purified from non-AAV expression plasmids (pControl) were used as positive controls throughout. Error bars: SEM. (A) Antibodies VRC01 and PGT121 were detected using gp120 from a subtype C consensus (ConC) env sequence as the immobilized antigen.46 (B) Antibodies CAP228-16H and CAP228-19F were detected using a V1V2 scaffolded protein as the immobilized antigen. (C) CAP256 antibodies were detected using a recombinant gp140 envelope trimer (BG505 SOSIP.664 His6)47 as the immobilized antigen captured on nickel-coated plates. (D) A representative western blot for CAP256.16 using a conjugated anti-human Fc antibody for detection. NR, non-reducing; R, reducing conditions.
AAV-Mediated Expression of V2-Targeting Antibodies
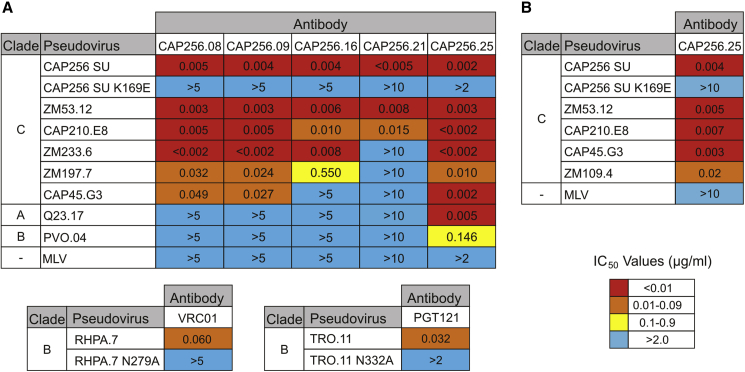
AAV-based expression plasmids were used to generate serotype 2 AAVs (AAV2) for the preliminary evaluation of antibody function in vitro. Serotype 8 AAVs (AAV8)48 were also generated for use in in vivo studies, as they were previously reported to mediate efficient gene transfer to murine muscle49 with reduced immunogenicity.50 Purified AAVs were used to infect HEK293T cells at approximately 105 viral genome copies per cell, and media were collected after 3 days. The function of secreted antibodies was assessed using a TZM-bl neutralization assay with a multi-clade reference panel of Env-pseudotyped HIV-1 viruses51, 52 with known neutralization sensitivity to CAP256 antibodies (Figure 3A).
Figure 3.
Neutralizing Activity of Antibodies Expressed following AAV-Mediated Transfer
Neutralization potency of antibodies secreted in cell media following (A) AAV2-mediated or (B) AAV8-mediated gene transfer was measured using a TZM-bl cell assay with Env-pseudoviruses. Values indicate the antibody inhibitory concentration (IC50, μg/mL) required to inhibit 50% of viral infection. A multi-clade panel of standard reference Env-pseudoviruses was used. Clade A, Q23;75 clade B, PVO, RHPA, and TRO.11;51 clade C, CAP256 SU,53 ZM53, CAP210, ZM233, ZM197, and CAP45.52 Negative control, murine leukemia virus (MLV).
Neutralization activity, indicated by half-maximum inhibitory concentration (IC50) values, was observed for all secreted antibodies following AAV2-mediated gene transfer. CAP256 antibodies had potent neutralization activity against subtype C Env-pseudoviruses, including the autologous superinfecting virus (CAP256 SU) isolated from the CAP256 donor53 (IC50 ≤ 0.005 μg/mL). Neutralization of a mutant Env-pseudovirus lacking the K169 residue in the V2 epitope, which is essential for neutralization by antibodies of the CAP256-VRC26 lineage,54 was abrogated. CAP256.25 was the most potent antibody against subtype C Env-pseudoviruses, particularly CAP210.E8, ZM 233.6, and CAP45.G3, but it also demonstrated breadth by neutralizing both subtype A (Q23.17) and subtype B (PVO.04) Env-pseudoviruses. Potent neutralization activity against subtype C Env-pseudoviruses was also observed for CAP256.25 following AAV8-mediated gene transfer in HEK293T cells (Figure 3B). In agreement with a previous comparison of AAV infectivity,49 antibody expression in HEK293T cells was lower when using AAV8 vectors than when using AAV2 vectors (Table S1). In general, the potency of AAV2-expressed CAP256 antibodies was similar to that reported in previous studies.11, 12
Neutralization activity was also observed for the positive control antibodies. Following AAV2-mediated expression, VRC01 neutralized the subtype B RHPA.7 Env-pseudovirus with similar potency to that reported in previous studies.40 Importantly, VRC01 was unable to neutralize a corresponding mutant Env-pseudovirus with an alanine substitution in the gp120 D loop (N279A), which abolishes VRC01 binding and neutralization activity.55 Similarly, PGT121 neutralized the TRO.11 Env-pseudovirus, but it did not neutralize a mutant containing an alanine substitution that results in the removal of the binding site N332 glycan.13 There was no neutralization activity against the murine leukemia virus (MLV), which was included as a negative control. CAP228-16H and CAP228-19F are non-neutralizing antibodies38 and were, therefore, not included in these assays.
AAV-Mediated Expression of Antibody-Encoding Sequences In Vivo
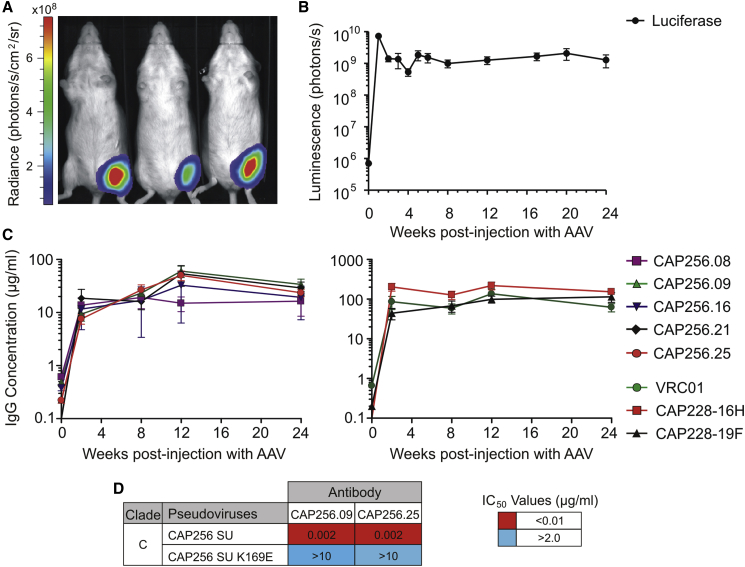
We next assessed the ability of AAVs to deliver and express antibodies in vivo. Initially, reporter gene expression after treatment with serotype 2 or 8 AAVs was compared in mice. AAV2-Fluc and AAV8-Fluc reporter vectors containing the Firefly luciferase gene were generated and administered to immunocompetent Naval Medical Research Institute (NMRI) mice by intramuscular injection at a dose of 1 × 1010 viral genome copies. Firefly luciferase expression was assessed using bioluminescence imaging (Figure S1). Luciferase transgene expression was higher in mice treated with the AAV8 vector, in accordance with previous findings.49 Mean luminescence when using the AAV8 vector was up to 250-fold higher at week 1 and stabilized at weeks 2 and 3.
Based on the analysis of reporter gene expression, AAV8 vectors were subsequently used to deliver the CAP256 and CAP228 antibodies in vivo. NMRI mice were injected with 1 × 1010 viral genome copies of the AAV8-Fluc control vector or 2.5 × 1010 viral genome copies of each antibody-encoding AAV8 vector. Bioluminescence imaging was used to monitor transgene expression in the luciferase control group (Figures 4A and 4B). Mean luminescence again peaked at week 1 following AAV administration, and it stabilized for the entire 24-week period of the study (∼1.4 × 109 photons/s).
Figure 4.
Antibody Serum Expression Levels in Mice following AAV Administration
(A) Representative bioluminescence image of mice in the AAV8-Fluc control group. This image was taken at week 1 using a 10-s exposure. (B) Mean luminescence for the AAV8-Fluc control group (n = 10) over 24 weeks. Total flux was measured using unsaturated images acquired after a 5-s exposure. Error bars, SEM. (C) Serum samples were collected over 24 weeks from mice in each AAV8-antibody treatment group. Secreted human IgG antibodies in mouse sera were detected using a sandwich ELISA and quantified using a purified IgG antibody to generate a standard curve. The mean antibody concentration (μg/mL) is shown for each group (n = 7–10). Error bars, SEM. (D) Neutralization potency of antibodies secreted in mouse sera was measured using a TZM-bl cell assay with Env-pseudoviruses following AAV8-mediated gene transfer. Values indicate the antibody inhibitory concentration (IC50, μg/mL) required to inhibit 50% of viral infection. An autologous subtype C Env-pseudovirus (CAP256 SU) and corresponding V2-epitope mutant (CAP256 SU K169E) were used in the assay.
Blood samples were collected from mice injected with the AAV8-antibody vectors, and antibodies secreted in the sera were detected and quantified using an ELISA. Mean human IgG concentrations in the sera increased for all treatments following AAV treatment (Figure 4C). Mean IgG concentrations were typically higher for CAP228 antibodies (44.1–219.8 μg/mL) than for CAP256 antibodies (7.6–60.3 μg/mL). The ranges of mean concentrations for each antibody over 24 weeks were as follows: CAP256.08, 13.8–19.2 μg/mL; CAP256.09, 9.3–60.3 μg/mL; CAP256.16, 11.6–32.7 μg/mL; CAP256.21, 16.1–53.8 μg/mL; CAP256.25, 7.6–50.2 μg/mL; CAP228-16H, 127.3–219.8 μg/mL; and CAP228-19F, 44.1–114.3 μg/mL. The mean level of expression observed for VRC01 was similar to that of a previous experiment using the same AAV dose in humanized immunodeficient mice,30 including the peak expression of 137 μg/mL at week 12.
Antibody expression varied for individual mice within each AAV8-antibody group over the course of 24 weeks (Figure S2). For example, in the group that received AAV8-CAP256.09, the antibody concentration was consistently high for five mice (56.9–106.6 μg/mL at week 12) but declined for one mouse after week 2 (1532, ≤2.1 μg/mL) and another after week 12 (1526, 1.7 μg/mL). Similarly, in the group that received AAV8-CAP256.25, the final antibody concentration at week 24 was higher for nine mice (15.3–41.4 μg/mL), but lower for one mouse (1563, 1.6 μg/mL). In the group that received AAV8-CAP256.16, the final antibody concentration was higher for only two mice (≥56.5 μg/mL), but low (≤5.0 μg/mL) for five mice. Over the course of 24 weeks, 10 of the 44 remaining mice in the AAV8-CAP256 groups consistently had antibody expression of <10 μg/mL, while only 3 mice in the CAP256.16 and CAP256.21 groups had antibody expression of <1.2 μg/mL. Levels of expression for the CAP228 mAbs were more consistent: all eight mice at week 24 that received AAV8-CAP228-16H had higher antibody concentrations (49.2–268.9 μg/mL), as did all nine mice that received AAV8-CAP228-19F (14.0–293.6 μg/mL).
To confirm antibody function in mouse sera, two of the more potent antibodies were selected for use in a TZM-bl neutralization assay with subtype C reference Env-pseudoviruses. Neutralizing activity was retained for CAP256.09 and CAP256.25 against the autologous subtype C virus (CAP256 SU),53 but not the corresponding V2 epitope mutant virus (K169E), confirming antibody specificity (Figure 4D). Importantly, the impressive antibody potency of these antibodies (IC50 = 0.002 μg/mL) was maintained following AAV-mediated delivery and secretion in vivo.
Anti-antibody Response In Vivo
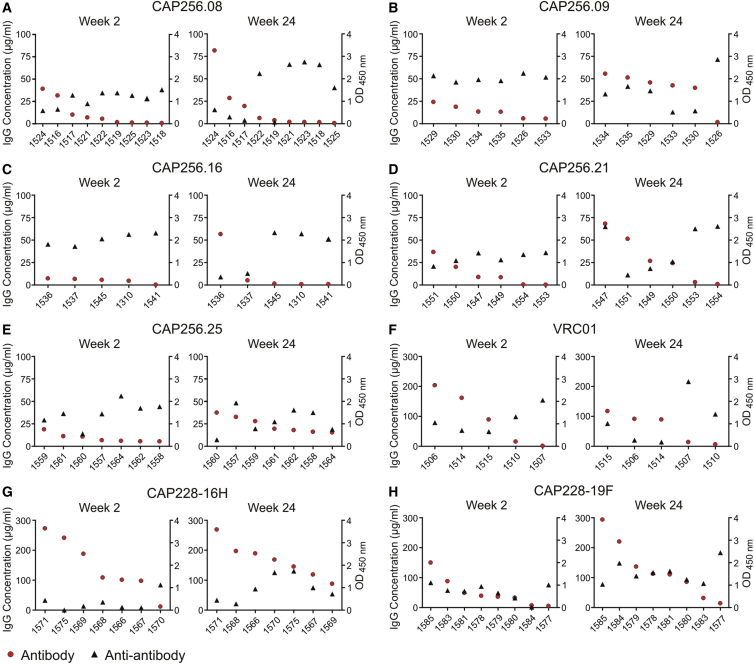
To assess the humoral anti-antibody response, we performed ELISAs to detect mouse antibodies against the human CAP256 and CAP228 antibodies at weeks 2 and 24 post-injection with AAV8-antibody vectors. Mouse anti-antibodies were detected in all treatment groups, but levels varied for individual mice within each group (Figure 5). Higher anti-antibody expression was often observed for mice with lower antibody levels at each time point, and vice versa. Higher anti-antibody levels were also observed for mice with a consistently low antibody expression of <10 μg/mL (1518, 1523, 1525, 1310, 1545, and 1553) (Figures 5A, 5C, and 5D) or <1.2 μg/mL (1541 and 1554) (Figures 5C and 5D). Anti-antibody levels increased at week 24 for mouse 1526, which showed a loss of CAP256.09 expression (Figure 5B). Anti-antibody levels were typically lower for mice expressing CAP228-16H, which showed the highest antibody concentrations (Figure 5G). An inverse correlation was noted between antibody and anti-antibody expression when assessing all mice at week 2 (r2 = 0.33, p < 0.0001) and week 24 (r2 = 0.08, p = 0.04) or mice expressing CAP256 antibodies at weeks 2 and 24 (r2 = 0.19, p = 0.01 for both), but not for mice in each antibody group (Table S2).
Figure 5.
Anti-antibodies Detected in Individual Mice at Weeks 2 and 24 after AAV Administration
Anti-antibodies were measured at weeks 2 and 24 post-injection with AAV8 using an ELISA with a goat anti-mouse IgG. Both antibody (IgG concentration) and anti-antibody (O.D. 450 nm) results are shown for individual mice in each of the eight treatment groups (A–H). Data is ordered according to the level of antibody expression. Anti-antibody data were not available for all mice at each time point because of limited serum volumes.
However, this trend in antibody expression was not observed for all mice. Comparable antibody expression was observed despite different anti-antibody levels in some mice. Examples include mice expressing CAP256.09 (week 24, 1529 and 1533) (Figure 5B) and mice expressing CAP256.25 (1557 and 1560) (Figure 5E). The inverse was also observed, where different levels of antibody expression were noted despite comparable anti-antibody levels. Examples include mice expressing CAP256.16 (week 24, 1536 and 1537) (Figure 5C), CAP228-19F (week 2, 1585 and 1577) (Figure 5H), and 6 of 7 mice expressing the CAP228-16H antibody at week 2 (Figure 5G). Therefore, anti-antibody expression may influence the levels of CAP256 and CAP228 expression, but alone it cannot explain the observed variation in antibody expression.
Discussion
We have successfully generated AAVs that may be used in vivo for intramuscular delivery and expression of antibodies targeting the V2 region of the HIV-1 envelope protein. An optimized AAV-based expression plasmid30 was easily adapted for expression of CAPRISA antibodies that are relevant for both passive and active immunization. Serotype 8 AAVs provided long-term antibody expression in immunocompetent mice, and the expressed antibodies retained binding specificity and potent neutralizing activity. We have, therefore, expanded the repertoire of antibodies that may be encoded by AAVs and translated for use in VIP and HIV prevention in southern Africa.
Previous VIP studies have shown that the minimum protective serum concentration against HIV infection in NOD/SCID/γc (NSG) humanized mice was 34 μg/mL for the b12 antibody and 8.3 μg/mL for the second-generation VRC01 antibody.30 This protection was achieved using intramuscular injection of AAVs at a dose of 1.3 × 1010 viral genome copies per mouse. More potent antibodies, such as 3BNC117 and VRC07, expressed after the administration of AAVs at doses of 2.5 × 1010 viral genome copies or less, provided protection at serum concentrations of 2–6 μg/mL.31 For VRC-PG04, a protective serum concentration as low as 0.35 μg/mL was reported.31 The AAV dose used here (2.5 × 1010 viral genome copies) achieved substantially higher mean serum antibody concentrations of 8–60 μg/mL for the CAP256 antibodies. Based on previously published studies, this level of antibody expression would be expected to provide protection against HIV infection in mouse models. Protection may be particularly good against clade C isolates, which were responsible for eliciting the antibody response in the CAP256 donor. Greater neutralization potency (IC50) against a panel of clade C viruses was observed in vitro for CAPRISA antibodies used here (CAP256.25, 0.002 μg/mL; CAP256.08, 0.151 μg/mL) than for other potent bNAbs (3BNC117, 0.304 μg/mL; VRC07, 0.245 μg/mL).56 In a passive infusion study, CAP256.25 was more effective than PDGM1400 against a clade C variant of SHIV in rhesus macaques at the lowest dose administered (0.08 mg/kg).17 The AAVs developed here for intramuscular delivery and expression of potent V2-targeting antibodies may, therefore, provide effective prophylaxis against HIV-1 infection.
The in vivo serum concentrations of CAP256 antibodies were generally lower than those of CAP228 antibodies by approximately 3-fold. While the reasons for different serum concentrations of antibodies expressed using the same vector are unclear, it may be related to the structural features of the paratopes of individual antibodies. CAP256 antibodies have an unusually long, anionic CDRH3, which penetrates the glycan shield to affect neutralization. This complex structure also requires sulfation, which may further impact expression. Low levels of expression were also noted in the clinical trial of AAV1-PG9,36 another V2 antibody with an unusually long CDRH3. Whether there are inherent difficulties expressing antibodies with long CDRH3 regions in vivo will require further investigation, particularly as antibodies such as CAP256.25 and PDGM1400 are being developed for human clinical trials. CAP228 antibodies were expressed at similar levels to VRC01, and both have CDRH3 lengths in the normal range. The CAP228 antibodies recognize a different conformation of V2 not present on the trimeric envelope, and they are, therefore, not broadly neutralizing.57 However, these antibodies mediate ADCC39 and are similar to antibodies associated with moderate efficacy in the RV144 trial. A major limitation of this vaccine regimen is that antibody responses are not durable and wane after 12 months, requiring re-boosting. Thus, alternative strategies, such as VIP, could be useful to provide sustained antibody concentrations for long-lasting immunity.
VIP is a promising approach in the field of HIV prophylaxis, but several factors may limit its clinical application in humans.58 First, the AAV transduction efficiency relative to muscle mass in mice, as used in this study, may not be achievable in humans. Second, a humoral response against the encoded antibody could reduce or abrogate antibody expression, rendering VIP ineffective. In our study, effective antibody expression was achieved in the majority of mice from 2 weeks after AAV administration, but expression varied among individual mice. Over the course of 24 weeks, expression of CAP256 antibodies was lower (<10 μg/mL) in 10 of the 44 remaining mice, while there was virtually no expression (<1.2 μg/mL) of CAP256.16 and CAP256.21 antibodies in 3 mice. It is tempting to speculate that the anti-antibody response may limit CAP256 antibody expression in these mice. However, higher anti-antibody expression did not correspond to lower antibody expression in all mice. Further investigation is, therefore, required to elucidate the timing and level of humoral response that may limit CAP256 and CAP228 antibody expression and prophylactic efficacy, as well as other potential contributions of the immune response.
Anti-antibodies against b12 were detected previously using the same AAV delivery system in immunocompetent mouse models (C57BL/6 and BALB/c), but the response did not appear to impact human IgG levels or the efficacy of prophylaxis.30 However, limiting anti-antibody responses have been reported in non-human primates32, 34, 35, 59, 60 and humans.36 The nature of the anti-antibody response depends on the identity of the transferred antibody, but variable heavy and light chain sequences of rhesus or human origin appear to be predominant targets in rhesus macaques, including the rhesus CDRH3.61 Other elements of the expressed transgene may also prove immunogenic in humans, such as the expressed F2A peptide, which elicited varied humoral and cellular immune responses in individual macaques.34 Effective prophylaxis has, nonetheless, been achieved with long-term antibody expression of up to a year in mice30, 31 and for several years in non-human primates,32, 62 with sustained viral suppression in one SHIV-infected macaque.35 A humoral response to transferred bNAbs occurred at a lower frequency when using autologous, naturally arising antibodies,63 and it was circumvented in non-human primates by transient immunosuppressive therapy.34 Anti-antibody responses therefore remain a challenge to the clinical application of VIP. However, it is still unclear whether a prohibitive anti-antibody response to CAP256 or CAP228 antibodies delivered by AAV8 will be mounted in humans or if such a response could be circumvented by immunosuppressive therapy.
A third major concern in the clinical application of VIP and AAV-mediated gene therapy is that of pre-existing immunity to the AAV vector.64 Seroprevalence of antibodies to AAV8 was significantly lower than that observed for antibodies to AAV1 and AAV2, both worldwide and in South Africa.65 Therefore, while the efficacy of AAV8 demonstrated in mice may not be mirrored in humans, AAV8 remains a promising candidate for use in VIP to prevent HIV-1 infection, and it was selected for use in a clinical trial (AAV8-VRC07; ClinicalTrials.gov: NCT03374202). Serotype 8 also appears to be well suited for long-term gene expression, and it was shown to avoid capsid-specific activation of T cells50 and immune activation in murine muscle.66, 67 Reduced immunogenicity of the AAV8 serotype may also explain the similar level of antibody expression observed in immunocompetent and immunodeficient mouse models, both here and in previous studies.30 However, additional strategies may be required in the future clinical application of VIP in humans, including the use of novel AAV serotypes, capsid engineering, and transient immunosuppressive therapy.68
The use of potent antibodies is crucial to achieving effective VIP in humans. Potent antibodies enable effective prophylaxis at a lower level of antibody expression, while a lower vector dose can reduce injection volumes for improved intramuscular delivery regimens. Moreover, durable expression of the exogenous antibodies is important to obviate a requirement for repeat administrations of vectors, as an immune response to a particular AAV serotype may render subsequent gene transduction ineffective. The recombinant vectors described here have properties that are potentially useful to achieve these goals and advance VIP. Importantly similar AAVs have previously been shown to provide mucosal protection to HIV challenge of humanized mice.31 Our approach is also well suited for generating other AAV-based expression plasmids and AAVs for new potentially useful antibodies as they are characterized. A combinatorial VIP approach, where several potent antibodies are administered in a single cocktail of AAVs, is likely to have benefit by providing broad coverage of circulating strains. Moreover, vector-mediated gene transfer may prove useful as an immunotherapy for existing HIV-1 infection, as conventional passive infusion of bNAbs may control viral replication and even affect the viral reservoir.24 Although several limitations must still be overcome in its clinical application, VIP has exciting potential for the prevention and treatment of HIV infection.
Materials and Methods
Direct Assembly of AAV-Based Expression Plasmids
The sequences encoding CAP256.08, CAP256.09, and PGT121 were inserted into the AAV vector as six fragments with ∼20-bp overlaps using Gibson assembly. The fragments covering the SS, constant heavy chain (CH), and F2A/SS regions were generated by PCR amplification (HiFi, KAPA Biosystems, Wilmington, MA, USA) of pAAV-VRC01,30 while the fragments covering the λ CL, VH, and VL regions were synthesized as gBlock Gene Fragments (Integrated DNA Technologies, Coralville, IA, USA) or amplified from non-AAV expression plasmids. The λ CL DNA sequence was assessed in silico for possible splice sites using NNSPLICE 0.9,69 as previously described,30 and silent mutations were introduced to prevent aberrant splicing. The linearized AAV backbone was prepared from pAAV-VRC01 using a BamHI and NotI (Thermo Fisher Scientific, Waltham, MA, USA) restriction digestion and then purified following agarose gel electrophoresis (QIAquick Gel Extraction Kit, QIAGEN, Hilden, Germany). Equimolar amounts of each fragment and the linearized AAV backbone were added to Gibson Assembly Master Mix (New England Biolabs, Ipswich, MA, USA) and incubated at 50°C for 1 h. The reaction was used to transform the recombination-deficient XL-10 Gold strain of E. coli (Stratagene, San Diego, CA, USA), followed by plating and overnight incubation at 30°C. pAAV expression plasmids were purified from transformed colonies and screened for correct construct assembly by restriction digestion and DNA sequencing (Inqaba Biotech, Pretoria, South Africa).
pMin Expression Vectors
The region spanning the origin of replication (Ori) and ampicillin resistance gene (AmpR) of pUC19 (New England Biolabs) was amplified using primers with 5′ restriction sites (pMinF, SpeI: 5′-ACT AGT GAT ATC AAG CTT TGA CTC GCT GCG CTC GGT CGT TCG-3′; pMinR, HindIII: 5′-AAG CTT GAT ATC ACT AGT GAC GAA AGG GCC TCG TGA TAC GCC-3′). This product was 5′ phosphorylated with T4 polynucleotide kinase (New England Biolabs) and circularized using T4 DNA ligase (New England Biolabs) to create pMin. The antibody-encoding sequence of pAAV-CAP256.09, excluding flanking ITRs, was removed using SpeI and HindIII and then ligated to corresponding sites of linearized pMin (pMin-CAP256.09). This ligation reaction was used to transform competent NEB 5-alpha E. coli, followed by plating and overnight incubation at 37°C. pMin expression plasmids were identified using restriction digestion.
pMin-ΔV Cloning Vector
pMin-ΔV was generated using Gibson assembly of common antibody fragments and the linearized pMin-CAP256.09 backbone containing the promoter, enhancer, and transcription termination signal. Common elements of the antibody expression cassette were amplified from pMin-CAP256.09 as three overlapping fragments (SS, CH/F2A/SS, λ CL), using the primers listed in Table S3. To replace variable regions of the pMin-CAP256.09 cassette with double BsmBI restriction sites, short 5′ linkers were included on PCR primers of the CH/F2A/SS fragment. The linearized pMin backbone was prepared from pMin-CAP256.09 using a BamHI and NotI restriction digestion and purified following agarose gel electrophoresis. Equimolar amounts of each fragment and the linearized backbone were subjected to Gibson assembly, bacterial transformation, and colony screening, as described above. To create novel pMin expression plasmids, variable antibody sequences were generated as gBlock Gene Fragments and combined with purified, BsmBI-treated pMin-ΔV fragments using Gibson assembly. Antibody constructs were verified by DNA sequencing.
Generating pAAVs Using Conventional Cloning
Antibody expression constructs were excised from corresponding pMin vectors following digestion with SpeI and HindIII and then purified by gel extraction. The pAAV backbone was prepared in the same manner. Purified fragments were joined using T4 DNA ligase (New England Biolabs), then used to transform SURE 2 cells (Agilent Technologies, Santa Clara, CA, USA), and cultures were grown at 30°C for 20–24 h in 2 × YT broth with carbenicillin (100 μg/mL) to avoid the occurrence of recombination events. The integrity of pAAVs was confirmed after every round of plasmid purification using restriction digestion.
Preparation of AAVs
AAVs were generated as described previously.70, 71 HEK293T cells were cultured at 37°C and 5% CO2 in DMEM supplemented with 10% v/v heat-inactivated fetal bovine serum, 2 mM L-alanyl-L-glutamine (GlutaMAX), penicillin (100 units/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). To generate a single AAV, HEK293T cells were transfected with the pAAV antibody expression vector (6 μg), adenohelper plasmid (8 μg), and Rep-Cap plasmid from AAV serotype 2 or 8 (10 μg) using PEI MAX (Polysciences, Warrington, PA, USA), at a w/w ratio of 5:1 to DNA. To generate AAV-GFP, the antibody expression vector was replaced by pAAV-CAG-GFP (a gift from Karel Svoboda, Addgene 28014).45 To generate AAV-Fluc, pAAV-Fluc30 was used. AAVs were purified from both cells and media. Briefly, cells were resuspended in 5 mL lysis buffer, followed by five freeze-thaw cycles, sonication, benzonase digestion (50 U/mL, Sigma-Aldrich, St. Louis, MO, USA), and centrifugation (2,950 × g, 30 min) to give AAV-containing cell lysate. Media collected on days 1, 3, 5, and 7 were filtered using a Steritop 0.2-μm unit (Millipore, Billerica, MA, USA), and AAVs were precipitated overnight at 4°C using polyethylene glycol (8% w/v PEG-8000, 0.5 M NaCl). Precipitate was collected by centrifugation at 4,400 × g for 30 min at 4°C in a JA-10 rotor (Beckman Coulter, Brea, CA, USA) and resuspended in the cell lysate described above. The AAV sample was separated using an iodixanol density gradient (Optiprep, Sigma-Aldrich). The 40% fraction containing purified AAVs was collected following ultracentrifugation at 229,600 × g for 2 h at 4°C in a 70.1 Ti rotor (Beckman Coulter). AAV aliquots (50 μL) were stored at −80°C.
AAV Quantification
The number of viral genomes per milliliter (vg/mL) was measured using droplet digital PCR (ddPCR)72 (QuantaLife, Bio-Rad, Hercules, CA, USA) and a reported protocol.73 Briefly, a 20-μL PCR reaction was assembled in duplicate with 5 μL diluted AAV sample, 1 × ddPCR Supermix for probes (Bio-Rad), a forward and reverse primer (0.9 μM each), and fluorescein-labeled probe (0.25 μM). Primer and probe sequences for AAV-GFP were reported previously.71 Primers for AAV-Fluc were reported previously,30 while the probe sequence was (5′(6-FAM)-GAT AGC AAG ACC GAC TAC CAG GGC T-(BHQ1)3′). Primer and probe sequences for AAV-antibody samples (IgG CH) were as follows: forward primer 5′-CAG CCG GAG AAC AAC TAC AA-3′, reverse primer 5′-CTC TTG TCC ACG GTG AGT TT-3′; and probe 5′(6-FAM)-CTC CGA CGG CTC CTT CTT CCT CTA-(BHQ1)3′. Droplets containing the PCR template were generated according to the manufacturer’s protocol using a QX200 Droplet Generator, and PCR was performed using a thermocycler (Bio-Rad C1000 Touch). The PCR products were analyzed using a QX200 Droplet Reader and QuantaSoft Analysis Pro (version 1.0).
Antibody Expression
To assess antibody expression from the pAAV expression vector, HEK293T cells were seeded in a 15-cm diameter plate, followed by transfection with the pAAV antibody expression vector (6 μg) as described above. Media were collected for testing at 72 and 120 h after transfection, and then stored at 4°C. To assess antibody expression following AAV treatment of cultured cells, HEK293T cells were seeded at ∼2.0 × 105 cells/well of a 24-well plate and infected with AAVs at a dose of approximately 1 × 105 vg/cell. Untreated cells and cells treated with an AAV carrying GFP (AAV-GFP) were included as controls. Media were collected at 72, 120, and 168 h post-infection.
Antibody Detection by ELISA
High protein-binding plates (Thermo Fisher Scientific) were coated with a V1V2-scaffolded protein to detect CAP228 antibodies, ConC gp12046 to detect VRC01 and PGT121, and BG505.664 SOSIP47 to detect CAP256 mAbs, and they were incubated overnight at 4°C. Plates were washed using PBS-Tween 20 and blocked with 5% non-fat dry milk in PBS (Bio-Rad). Control mAbs were expressed by co-transfecting 293F cells with non-AAV expression plasmids encoding separate heavy or light antibody chains. Purified control mAbs (10 μM) or harvested media were added to the plate and incubated for 1 h at room temperature. Plates were washed, and 50 μL horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Sigma-Aldrich) was added (1:2,000 dilution) for detection. The reaction was stopped after incubating at room temperature for 1 h and plates were washed. TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Thermo Fisher Scientific) was added, followed by a brief incubation. The reaction was stopped with 1 M H2SO4, and absorbance was measured at 450 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Quantification of Antibody Levels by ELISA
A quantitative ELISA was used to measure the IgG antibody concentration in cell culture media and mouse sera. Briefly, high protein-binding plates were coated with 50 μL goat anti-human fragment crystallizable (Fc) antibody (Sigma-Aldrich) diluted 1:1,000 and incubated overnight at 4°C. The next day, plates were washed three times with 0.05% Tween 20 in PBS and blocked with 5% w/v skimmed milk powder reconstituted in PBS. Diluted cell culture media or mouse serum samples (50 μL) were added to each well and incubated at room temperature for 1 h. Purified VRC01 IgG was diluted (50 μg/mL–0.8 ng/mL) and used as the standard for quantification. After incubation, plates were washed, and 50 μL HRP-conjugated goat anti-human Fc (1:2,000 dilution) was added to each well. TMB substrate was added and the absorbance was measured as described above. To detect mouse anti-human antibodies, high binding plates were coated with monoclonal CAP256 or CAP228 antibodies (4 μg/mL). Sera were diluted 1:100, and mouse anti-human antibodies were detected with 1:3,000 dilution of goat anti-mouse IgG-HRP (EMD Millipore, Burlington, MA, USA). A standard sample was included as a positive control on every plate.
Western Blot
Cell culture medium was collected following transfection of HEK293T cells with pAAV-CAP256.16, and the expressed antibody was purified using protein A affinity chromatography. Control antibodies were expressed and purified from non-AAV expression vectors. Equivalent amounts of purified protein were loaded in duplicate under reducing and non-reducing conditions on a 4%–15% gradient SDS-polyacrylamide gel (Bio-Rad). Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using the Transblot Turbo transfer system (Bio-Rad). Membranes were blocked (1× Tris-buffered saline [TBS] and 5% w/v milk powder) for 1 h and washed twice (1 × TBS and 0.1% Tween 20) (Sigma-Aldrich) before a 1-h incubation in 1× TBS containing a 1:5,000 dilution of anti-human Fc Alkaline Phosphatase (Sigma-Aldrich). The membrane was washed three times, developed using the nitro blue tetrazolium chloride (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Sigma-Aldrich), and then washed with 1× TBS and photographed.
Neutralization Assays
In vitro neutralization was assessed using the TZM-bl assay as previously described.74 This assay measures a reduction in luminescence following a single round of infection in TZM-bl cells. Briefly, culture media or sera were serially diluted using 3-fold titration in a 96-well plate. Various HIV-1 pseudotyped viruses, generated as previously described,51 were added to each well and incubated for 1 h. TZM-bl cells were then added and incubated for a further 48 h. Luminescence was measured using the Bright-Glo Luciferase Assay System (Promega, Madison, WI, USA). Viral titers were calculated as the IC50 causing a 50% reduction of relative light units (RLU) compared to the control virus or untreated wells.
Antibody Expression in Mice
Animal studies were approved by the University of the Witwatersrand (Wits) Animal Ethics Screening Committee (AESC 2017-05-34C) and conducted at Wits Central Animal Services (CAS) facilities. Female NMRI mice were 3–5 weeks of age and weighed 19.5–29.0 g at the start of the study. Mice receiving each treatment (n = 10) were housed together in groups of five. AAVs were administered as a single intramuscular injection into the gastrocnemius muscle using a 27G tuberculin syringe (Becton Dickinson, Thermo Fisher Scientific). AAV aliquots were thawed on ice and diluted in saline to give the appropriate dose in 50 μL (AAV-Fluc, 1 × 1010 vg/mouse; AAV-Ab, 2.5 × 1010 vg/mouse).
After AAV administration, mice in the AAV-Fluc group were imaged using whole-body bioluminescence imaging (IVIS Kinetic imaging system, Caliper Life Sciences, Hopkinton, MA, USA) weekly for 6 weeks, then every 4 weeks thereafter. Mice receiving the antibody-expressing AAVs were subjected to retroorbital puncture under anesthetic, and serum samples were collected and stored at −20°C. The bleeding procedure was carried out at weeks 2, 4, 8, 12, and 24 after AAV administration. For imaging, mice were anesthetized using isofluorane inhalation, and 15 mg/mL D-luciferin (PerkinElmer, Waltham, MA, USA) was administered by intraperitoneal injection at a dose of approximately 150 mg/kg. Whole-body bioluminescence imaging was performed within 10 min of D-luciferin administration, and images were acquired after exposure for 1–25 s. Images were analyzed (Living Image Software version 4.2, Caliper Life Sciences), and Firefly luciferase signal was quantified using an automatic contour region of interest.
Author Contributions
Conceptualization, M.S.W. and L.M.; Methodology, F.T.v.d.B., N.A.M., S.A.A., T.A.S., N.N.M., M.S.W., A.E., P.B.A., and L.M.; Investigation, F.T.v.d.B., N.A.M., S.A.A., T.A.S., R.E.M., L.Z.M., N.N.M., B.E.L., P.D.K., C.C., A.E., and P.B.A.; Resources, S.S.A.K., A.B.B., M.S.W., P.B.A., and L.M.; Writing – Original Draft, F.T.v.d.B. and N.A.M.; Writing – Review & Editing, F.T.v.d.B., N.A.M., S.A.A., A.E., P.B.A., and L.M.; Visualization, F.T.v.d.B. and N.A.M.; Supervision, L.M.; Funding Acquisition, M.S.W. and L.M.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We gratefully acknowledge support from the South African Medical Research Council provided through the FLAGSHIP program and Antiviral Gene Therapy Extramural Unit. Funding for the bioluminescence imager was also provided from the South African National Research Foundation (GUN 78565). We also wish to thank Dirk Grimm of the University of Heidelberg for AAV-packaging plasmids, Mohube Betty Maepa at the AGTRU for advice on AAV transduction, and Penny Moore at the NICD for constructive comments on serum antibody quantification.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.06.002.
Supplemental Information
References
- 1.Stephenson K.E., D’Couto H.T., Barouch D.H. New concepts in HIV-1 vaccine development. Curr. Opin. Immunol. 2016;41:39–46. doi: 10.1016/j.coi.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris L., Mkhize N.N. Prospects for passive immunity to prevent HIV infection. PLoS Med. 2017;14:e1002436. doi: 10.1371/journal.pmed.1002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCoy L.E., Burton D.R. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol. Rev. 2017;275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton D.R., Mascola J.R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert P.B., Juraska M., deCamp A.C., Karuna S., Edupuganti S., Mgodi N., Donnell D.J., Bentley C., Sista N., Andrew P. Basis and Statistical Design of the Passive HIV-1 Antibody Mediated Prevention (AMP) Test-of-Concept Efficacy Trials. Stat. Commun. Infect. Dis. 2017;9:20160001. doi: 10.1515/scid-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker L.M., Simek M.D., Priddy F., Gach J.S., Wagner D., Zwick M.B., Phogat S.K., Poignard P., Burton D.R. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 2010;6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgiev I.S., Doria-Rose N.A., Zhou T., Kwon Y.D., Staupe R.P., Moquin S., Chuang G.Y., Louder M.K., Schmidt S.D., Altae-Tran H.R. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013;340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 8.Landais E., Huang X., Havenar-Daughton C., Murrell B., Price M.A., Wickramasinghe L., Ramos A., Bian C.B., Simek M., Allen S. Broadly Neutralizing Antibody Responses in a Large Longitudinal Sub-Saharan HIV Primary Infection Cohort. PLoS Pathog. 2016;12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker L.M., Phogat S.K., Chan-Hui P.Y., Wagner D., Phung P., Goss J.L., Wrin T., Simek M.D., Fling S., Mitcham J.L., Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M., Hwang K.K., Chen X., Tsao C.Y., Morris L., Gray E., Marshall D.J., Crump J.A., Kapiga S.H., Sam N.E. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose N.A., Schramm C.A., Gorman J., Moore P.L., Bhiman J.N., DeKosky B.J., Ernandes M.J., Georgiev I.S., Kim H.J., Pancera M., NISC Comparative Sequencing Program Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria-Rose N.A., Bhiman J.N., Roark R.S., Schramm C.A., Gorman J., Chuang G.Y., Pancera M., Cale E.M., Ernandes M.J., Louder M.K. New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. J. Virol. 2015;90:76–91. doi: 10.1128/JVI.01791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker L.M., Huber M., Doores K.J., Falkowska E., Pejchal R., Julien J.P., Wang S.K., Ramos A., Chan-Hui P.Y., Moyle M., Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sok D., van Gils M.J., Pauthner M., Julien J.P., Saye-Francisco K.L., Hsueh J., Briney B., Lee J.H., Le K.M., Lee P.S. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc. Natl. Acad. Sci. USA. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan J.S., Pancera M., Carrico C., Gorman J., Julien J.P., Khayat R., Louder R., Pejchal R., Sastry M., Dai K. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pancera M., Shahzad-Ul-Hussan S., Doria-Rose N.A., McLellan J.S., Bailer R.T., Dai K., Loesgen S., Louder M.K., Staupe R.P., Yang Y. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat. Struct. Mol. Biol. 2013;20:804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julg B., Tartaglia L.J., Keele B.F., Wagh K., Pegu A., Sok D., Abbink P., Schmidt S.D., Wang K., Chen X. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 2017;9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R., Premsri N., Namwat C., de Souza M., Adams E., MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 19.Haynes B.F., Gilbert P.B., McElrath M.J., Zolla-Pazner S., Tomaras G.D., Alam S.M., Evans D.T., Montefiori D.C., Karnasuta C., Sutthent R. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao H.X., Bonsignori M., Alam S.M., McLellan J.S., Tomaras G.D., Moody M.A., Kozink D.M., Hwang K.K., Chen X., Tsao C.Y. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch D.H., Whitney J.B., Moldt B., Klein F., Oliveira T.Y., Liu J., Stephenson K.E., Chang H.W., Shekhar K., Gupta S. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caskey M., Klein F., Lorenzi J.C., Seaman M.S., West A.P., Jr., Buckley N., Kremer G., Nogueira L., Braunschweig M., Scheid J.F. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood J.E., Coates E.E., Yamshchikov G., Saunders J.G., Holman L., Enama M.E., DeZure A., Lynch R.M., Gordon I., Plummer S., VRC 602 Study Team Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deal C.E., Balazs A.B. Vectored antibody gene delivery for the prevention or treatment of HIV infection. Curr. Opin. HIV AIDS. 2015;10:190–197. doi: 10.1097/COH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs S.P., Desrosiers R.C. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol. Ther. Methods Clin. Dev. 2016;3:16068. doi: 10.1038/mtm.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady J.M., Baltimore D., Balazs A.B. Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. Immunol. Rev. 2017;275:324–333. doi: 10.1111/imr.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin A., Balazs A.B. Adeno-associated virus gene delivery of broadly neutralizing antibodies as prevention and therapy against HIV-1. Retrovirology. 2018;15:66. doi: 10.1186/s12977-018-0449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 29.Lewis A.D., Chen R., Montefiori D.C., Johnson P.R., Clark K.R. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J. Virol. 2002;76:8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balazs A.B., Ouyang Y., Hong C.M., Chen J., Nguyen S.M., Rao D.S., An D.S., Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson P.R., Schnepp B.C., Zhang J., Connell M.J., Greene S.M., Yuste E., Desrosiers R.C., Clark K.R. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner M.R., Kattenhorn L.M., Kondur H.R., von Schaewen M., Dorfman T., Chiang J.J., Haworth K.G., Decker J.M., Alpert M.D., Bailey C.C. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders K.O., Wang L., Joyce M.G., Yang Z.Y., Balazs A.B., Cheng C., Ko S.Y., Kong W.P., Rudicell R.S., Georgiev I.S. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection. J. Virol. 2015;89:8334–8345. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Navio J.M., Fuchs S.P., Pantry S.N., Lauer W.A., Duggan N.N., Keele B.F., Rakasz E.G., Gao G., Lifson J.D., Desrosiers R.C. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity. 2019;50:567–575.e5. doi: 10.1016/j.immuni.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priddy F.H., Lewis D.J.M., Gelderblom H.C., Hassanin H., Streatfield C., LaBranche C., Hare J., Cox J.H., Dally L., Bendel D. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV. 2019;6:e230–e239. doi: 10.1016/S2352-3018(19)30003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Loggerenberg F., Mlisana K., Williamson C., Auld S.C., Morris L., Gray C.M., Abdool Karim Q., Grobler A., Barnabas N., Iriogbe I., Abdool Karim S.S., CAPRISA 002 Acute Infection Study Team Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS ONE. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray E.S., Madiga M.C., Hermanus T., Moore P.L., Wibmer C.K., Tumba N.L., Werner L., Mlisana K., Sibeko S., Williamson C., CAPRISA002 Study Team The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Eeden C., Wibmer C.K., Scheepers C., Richardson S.I., Nonyane M., Lambson B., Mkhize N.N., Vijayakumar B., Sheng Z., Stanfield-Oakley S. V2-Directed Vaccine-like Antibodies from HIV-1 Infection Identify an Additional K169-Binding Light Chain Motif with Broad ADCC Activity. Cell Rep. 2018;25:3123–3135.e6. doi: 10.1016/j.celrep.2018.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X., Yang Z.Y., Li Y., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badamchi-Zadeh A., Tartaglia L.J., Abbink P., Bricault C.A., Liu P.T., Boyd M., Kirilova M., Mercado N.B., Nanayakkara O.S., Vrbanac V.D. Therapeutic efficacy of vectored PGT121 gene delivery in HIV-1-infected humanized mice. J. Virol. 2018;92 doi: 10.1128/JVI.01925-17. e01925-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zufferey R., Donello J.E., Trono D., Hope T.J. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szymczak A.L., Workman C.J., Wang Y., Vignali K.M., Dilioglou S., Vanin E.F., Vignali D.A. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 44.Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., 3rd, Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 45.Mao T., Kusefoglu D., Hooks B.M., Huber D., Petreanu L., Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kothe D.L., Li Y., Decker J.M., Bibollet-Ruche F., Zammit K.P., Salazar M.G., Chen Y., Weng Z., Weaver E.A., Gao F. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology. 2006;352:438–449. doi: 10.1016/j.virol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Sanders R.W., Derking R., Cupo A., Julien J.P., Yasmeen A., de Val N., Kim H.J., Blattner C., de la Peña A.T., Korzun J. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimm D., Lee J.S., Wang L., Desai T., Akache B., Storm T.A., Kay M.A. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandenberghe L.H., Wang L., Somanathan S., Zhi Y., Figueredo J., Calcedo R., Sanmiguel J., Desai R.A., Chen C.S., Johnston J. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 51.Li M., Gao F., Mascola J.R., Stamatatos L., Polonis V.R., Koutsoukos M., Voss G., Goepfert P., Gilbert P., Greene K.M. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li M., Salazar-Gonzalez J.F., Derdeyn C.A., Morris L., Williamson C., Robinson J.E., Decker J.M., Li Y., Salazar M.G., Polonis V.R. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 2006;80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhiman J.N., Anthony C., Doria-Rose N.A., Karimanzira O., Schramm C.A., Khoza T., Kitchin D., Botha G., Gorman J., Garrett N.J. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat. Med. 2015;21:1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore P.L., Gray E.S., Sheward D., Madiga M., Ranchobe N., Lai Z., Honnen W.J., Nonyane M., Tumba N., Hermanus T., CAPRISA 002 Study Potent and broad neutralization of HIV-1 subtype C by plasma antibodies targeting a quaternary epitope including residues in the V2 loop. J. Virol. 2011;85:3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., O’Dell S., Walker L.M., Wu X., Guenaga J., Feng Y., Schmidt S.D., McKee K., Louder M.K., Ledgerwood J.E. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J. Virol. 2011;85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagh K., Bhattacharya T., Williamson C., Robles A., Bayne M., Garrity J., Rist M., Rademeyer C., Yoon H., Lapedes A. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wibmer C.K., Richardson S.I., Yolitz J., Cicala C., Arthos J., Moore P.L., Morris L. Common helical V1V2 conformations of HIV-1 Envelope expose the α4β7 binding site on intact virions. Nat. Commun. 2018;9:4489. doi: 10.1038/s41467-018-06794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colella P., Ronzitti G., Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuchs S.P., Martinez-Navio J.M., Piatak M., Jr., Lifson J.D., Gao G., Desrosiers R.C. AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity. PLoS Pathog. 2015;11:e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner M.R., Fetzer I., Kattenhorn L.M., Davis-Gardner M.E., Zhou A.S., Alfant B., Weber J.A., Kondur H.R., Martinez-Navio J.M., Fuchs S.P. Anti-drug Antibody Responses Impair Prophylaxis Mediated by AAV-Delivered HIV-1 Broadly Neutralizing Antibodies. Mol. Ther. 2019;27:650–660. doi: 10.1016/j.ymthe.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Navio J.M., Fuchs S.P., Pedreño-López S., Rakasz E.G., Gao G., Desrosiers R.C. Host Anti-antibody Responses Following Adeno-associated Virus-mediated Delivery of Antibodies Against HIV and SIV in Rhesus Monkeys. Mol. Ther. 2016;24:76–86. doi: 10.1038/mt.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balazs A.B., West A.P., Jr. Antibody gene transfer for HIV immunoprophylaxis. Nat. Immunol. 2013;14:1–5. doi: 10.1038/ni.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welles H.C., Jennewein M.F., Mason R.D., Narpala S., Wang L., Cheng C., Zhang Y., Todd J.P., Lifson J.D., Balazs A.B. Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog. 2018;14:e1007395. doi: 10.1371/journal.ppat.1007395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mays L.E., Wang L., Lin J., Bell P., Crawford A., Wherry E.J., Wilson J.M. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol. Ther. 2014;22:28–41. doi: 10.1038/mt.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mays L.E., Vandenberghe L.H., Xiao R., Bell P., Nam H.J., Agbandje-McKenna M., Wilson J.M. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J. Immunol. 2009;182:6051–6060. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tse L.V., Moller-Tank S., Asokan A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin. Biol. Ther. 2015;15:845–855. doi: 10.1517/14712598.2015.1035645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reese M.G., Eeckman F.H., Kulp D., Haussler D. Improved splice site detection in Genie. J. Comput. Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 70.Börner K., Niopek D., Cotugno G., Kaldenbach M., Pankert T., Willemsen J., Zhang X., Schürmann N., Mockenhaupt S., Serva A. Robust RNAi enhancement via human Argonaute-2 overexpression from plasmids, viral vectors and cell lines. Nucleic Acids Res. 2013;41:e199. doi: 10.1093/nar/gkt836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott T., Moyo B., Nicholson S., Maepa M.B., Watashi K., Ely A., Weinberg M.S., Arbuthnot P. ssAAVs containing cassettes encoding SaCas9 and guides targeting hepatitis B virus inactivate replication of the virus in cultured cells. Sci. Rep. 2017;7:7401. doi: 10.1038/s41598-017-07642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hiddessen A.L., Legler T.C. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lock M., Alvira M.R., Chen S.J., Wilson J.M. Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Hum. Gene Ther. Methods. 2014;25:115–125. doi: 10.1089/hgtb.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montefiori D.C. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. 2005;Chapter 12 doi: 10.1002/0471142735.im1211s64. Unit 12.11. [DOI] [PubMed] [Google Scholar]
- 75.Poss M., Overbaugh J. Variants from the diverse virus population identified at seroconversion of a clade A human immunodeficiency virus type 1-infected woman have distinct biological properties. J. Virol. 1999;73:5255–5264. doi: 10.1128/jvi.73.7.5255-5264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.