Abstract
The incidence of pancreatic cancer has considerably increased in the past decade. Pancreatic cancer has the worst prognosis among the cancers of the digestive tract because the pancreas is located in the posterior abdominal cavity, and most patients do not show clinical symptoms for early detection. Approximately 55% of all patients are diagnosed with pancreatic cancer only after the tumors metastasize. Therefore, identifying useful biomarkers for early diagnosis and screening high-risk groups are important to improve pancreatic cancer therapy. Recent emerging evidence has suggested that genetic and epigenetic alterations play a crucial role in the molecular aspects of pancreatic tumorigenesis. Here, we summarize recent progress in our understanding of the epigenetic alterations in pancreatic cancer and propose potential synthetic lethal strategies to target these genetic defects to treat this deadly disease.
Keywords: pancreatic cancer, epigenetic regulation, SWItch/Sucrose Non-Fermentable (SWI/SNF) complex, histone methylation, synthetic lethality
1. Introduction
Pancreatic cancer is one of the leading causes of cancer-related death worldwide [1,2,3]. In addition to surgery [4,5], chemotherapy [3,5], and novel treatments [6] are required to improve therapeutic efficiency against pancreatic cancer. Biomarkers for pancreatic cancer have been widely studied [7,8] and have been clinically used for disease prediction [7] and therapeutic target identification [9], which has resulted in the development of next-generation sequencing and proteomic analysis techniques [8,9]. Genetic biomarkers in pancreatic cancer patients [9] may offer therapeutic benefits by revealing the downstream signaling pathways [10], but this benefit is also affected by the druggability of their protein products [11]. Conversely, biomarkers of epigenetic regulators may provide advantages for cancer treatment due to their versatile regulation of multiple genes [12]. Thus, researchers have focused on the biomarkers of epigenetic regulators with a high incidence rate in pancreatic cancer patients [8,9,13]. These biomarkers include the chromatin remodeler SWItch/Sucrose Non-Fermentable (SWI/SNF) members (AT-rich interaction domain 1A (ARID1A), SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 (SMARCA2), and a SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 (SMARCA4)), histone methylation regulators (lysine demethylase 6A (KDM6A), lysine methyltransferase 2C (KMT2C), and lysine methyltransferase 2D (KMT2D)), and epigenetic readers, including bromodomain and extraterminal domain (BET) proteins (bromodomain containing 2 (BRD2), bromodomain containing 3 (BRD3), bromodomain containing 4 (BRD4), and bromodomain testis associated (BRDT)). Table 1 shows the gene aliases listed in the NCBI database, and the importance of these genes in pancreatic cancer and in other gastrointestinal (GI) cancers are discussed in Table 2.
Table 1.
Aliases of ARID1A, SMARCA2, SMARCA4, KDM6A, KMT2C, KMT2D, BRD2, BRD3, BRD4, BRDT.
| Gene Symbol | Gene Name | Alias |
|---|---|---|
| ARID1A | AT-rich interaction domain 1A | B120, BAF250, BAF250a, BM029, C1orf4, CSS2, ELD, MRD14, OSA1, P270, SMARCF1, hELD, hOSA1 |
| SMARCA2 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 | BAF190, BRM, NCBRS, SNF2, SNF2L2, SNF2LA, SWI2, Sth1p, hBRM, hSNF2a |
| SMARCA4 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 | BAF190, BAF190A, BRG1, CSS4, MRD16, RTPS2, SNF2, SNF2L4, SNF2LB, SWI2, hSNF2b |
| KDM6A | lysine demethylase 6A | KABUK2, UTX, bA386N14.2 |
| KMT2C | lysine methyltransferase 2C | HALR, KLEFS2, MLL3 |
| KMT2D | lysine methyltransferase 2D | AAD10, ALR, CAGL114, KABUK1, KMS, MLL2, MLL4, TNRC21 |
| BRD2 | bromodomain containing 2 | BRD2-IT1, D6S113E, FSH, FSRG1, NAT, O27.1.1, RING3, RNF3 |
| BRD3 | bromodomain containing 3 | ORFX, RING3L |
| BRD4 | bromodomain containing 4 | CAP, HUNK1, HUNKI, MCAP |
| BRDT | bromodomain testis associated | BRD6, CT9, SPGF21 |
Table 2.
ARID1A, SMARCA2, SMARCA4, KDM6A, KMT2C, KMT2D, and BRD4 mutation status in cancers of the stomach, liver, biliary duct, pancreas, and colon.
| Cancer | Gene | Mutation | Expression | Effect | Reference |
|---|---|---|---|---|---|
| Stomach | ARID1A | Nonsense, missense, splice site | [14,15,16] | ||
| Loss | Increased proliferation | [14,17] | |||
| Loss | Increased migration and invasion | [18] | |||
| Loss | Association with tumor stage and grade | [17] | |||
| Loss | Association with lymphatic invasion and lymph node metastasis | [19] | |||
| Loss | Association with poor prognosis | [17,19,20] | |||
| SMARCA2 | Mutation | [21] | |||
| SMARCA4 | Missense | [21] | |||
| KDM6A | Nonsense, missense | [22] | |||
| KMT2C | Mutation | [14] | |||
| Liver | ARID1A | Nonsense, missense | [23,24,25] | ||
| Loss | Decreased tumorigenesis; increased metastasis | [26] | |||
| Loss | Increased steatohepatitis and tumorigenesis | [27] | |||
| Loss | Increased tumorigenesis and angiogenesis | [28] | |||
| SMARCA2 | Missense | [29] | |||
| KMT2C | Mutation | [30] | |||
| KMT2D | Mutation | [31] | |||
| BRD4 | Gain | Increased tumorigenesis | [32] | ||
| Biliary duct | ARID1A | Nonsense, missense, splice site | [33,34,35,36] | ||
| Nonsense, missense | Association with poor prognosis | [37] | |||
| SMARCA4 | Missense | [38] | |||
| KMT2C | Mutation | [39] | |||
| KMT2D | Mutation | [38] | |||
| Pancreas | ARID1A | Nonsense, missense | [40,41] | ||
| Loss | Decreased differentiation | [42] | |||
| SMARCA2 | Mutation | [43] | |||
| Gain | Decreased patient survival and drug sensitivity | [44] | |||
| SMARCA4 | Mutation | [45] | |||
| Loss | Increased IPMN; decreased PanIN | [46] | |||
| Loss | Decreased late stage tumorigenicity | [47] | |||
| KDM6A | Mutation | [48] | |||
| Loss | Increased squamous-like cancer | [48] | |||
| Loss | Decreased overall/recurrence-free survival | [49] | |||
| KMT2C | Missense | [50] | |||
| KMT2D | Missense | [50] | |||
| Loss | Increased apoptosis and drug sensitivity | [51] | |||
| Colon | ARID1A | Missense | [52,53,54] | ||
| Loss | Increased aggressive adenocarcinoma | [55] | |||
| Loss | Increased proliferation and drug resistance | [56] | |||
| Loss | Association with ageing | [57,58] | |||
| Loss | Association with poor tumor differentiation | [56,58,59] | |||
| Loss | Association with tumor size | [57] | |||
| Loss | Association with tumor grade | [57,58] | |||
| Loss | Association with metastasis | [58] |
2. Expression and Mutation of SWI/SNF Genes in Pancreatic Cancer
The epigenetic regulation of gene expression occurs through covalent modifications of DNA or histones, nucleosome positioning, and non-coding RNA, such as microRNA [60]. Therapeutics targeting the covalent modifications of DNA or histones have been approved for cancer treatment or are undergoing clinical trials in various neoplasia, including pancreatic cancer [61]. In addition, chromatin remodelers mobilize the nucleosome and regulate both chromatin accessibility/gene expression [62] and cancer progression [63]. Chromatin remodelers are categorized into the families of SWI/SNF, ISWI, Mi-2/NuRD, and INO80/SWR1, to identify histone lysine acetylation, nucleosome, histone lysine methylation, and actin-related factors, respectively [64] (Figure 1). The mutations prevailing in the SWI/SNF complex during pancreatic tumorigenesis [45,65] are discussed below:
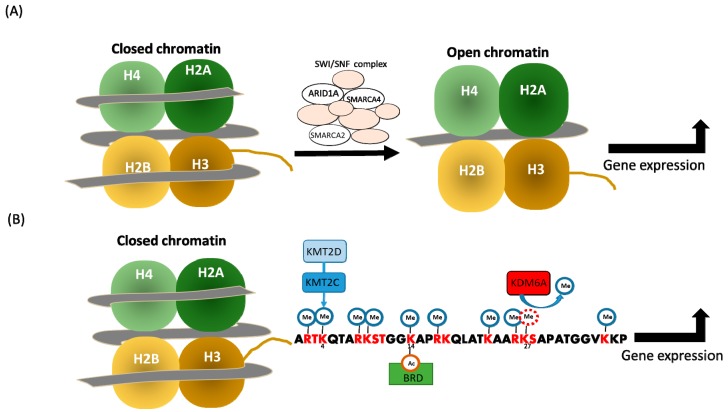
Figure 1.
Epigenetic regulation of the SWI/SNF complex, lysine methyltransferase, and demethylase. (A) The SWI/SNF complex generates an open chromatin structure to initiate transcription. (B) The activation of H3K27m2/3 demethylase KDM6A, H3K4m1 methyltransferase KMT2D/KMT2C, and bromodomain and extraterminal domain (BET) proteins leads to transcriptional activation.
2.1. ARID1A
ARID1A is a member of the SWI/SNF complex and is involved in transcriptional factor/cofactor/corepressor binding during chromatin remodeling [66]. While ARID1A is not required for pancreatic morphogenesis, its loss is associated with increased mucinous and pancreatic intra-epithelial neoplasia (PanIN) lesions [67]. ARID1A nonsense/missense mutations have been reported in tumors from patients with pancreatic cancer [40,41], and an in vitro or in vivo loss of ARID1A expression in the pancreas is associated with reduced SOX9 expression and cancer cell differentiation [42]. Furthermore, Kimura et al. used a pancreatic cancer mouse model Ptf1a-Cre; KrasG12D to show that the loss of ARID1A expression promotes tumorigenesis—from PanIN to ductal adenocarcinoma (PDAC)—and enhances intraductal papillary mucinous neoplasm (IPMN) formation from the duct cell. Following reference consultation, the authors selected SOX9 as the candidate duct cell differentiation factor. SOX9 expression was decreased in ARID1A-deficient IPMN, and this phenomenon was associated with decreased cell differentiation. However, SOX9 overexpression reversed this phenomenon. Moreover, decreased expression of ARID1A and SOX9 was observed in a subset of patients with IPMN. This result indicates that the mutational loss of ARID1A regulates pancreatic cell differentiation and cancer progression, providing clues for tumor development and progression in the pancreas.
ARID1A dysregulation plays an important role in GI cancers other than pancreatic cancer. ARID1A nonsense/missense/splice site mutations exist in patients with gastric cancer [14,15,16] and may serve to promote tumorigenesis [68]. The loss of ARID1A in vitro promotes cell proliferation [14,17] and E-cadherin-dependent migration and invasion [18]. ARID1A expression in the tumor of patients with pancreatic cancer decreased [17], and decreased ARID1A expression is associated with tumor stage/grade [17], lymphatic invasion/metastasis [19], and poor prognosis [17,19,20].
ARID1A nonsense/missense mutations are found in patients with liver cancer [23,24,25]. ARID1A increases the risk for cancer by promoting CYP450 (CYP2E1) transcriptional activation and reactive oxygen species (ROS) production in vivo, while decreasing metastasis via the transcriptional regulation of EMILIN1/MAT1A/LCN2/IL1R1 in vitro [26]. Conversely, ARID1A loss may increase the risk for steatohepatitis and cancer progression by altering immunity in vivo [27] or tumorigenesis by activating angiopoietin-2 (ANGPT2) transcription in vitro and causing angiogenesis in vivo [28]. These phenomena deserve further elucidation to clarify the context dependency of ARID1A mutation-regulated liver cancer progression, possibly using liver cancer mouse models [69,70].
ARID1A nonsense/missense/splice site mutations also exist in patients with cholangiocarcinoma [33,34,35,36] and are associated with a poor prognosis [37]. ARID1A (missense) mutations are found in patients with colon cancer [52,53,54], and ARID1A loss promotes invasive adenocarcinoma via SWI/SNF-dependent gene expression regulation in vivo [55] or proliferation/5-fluorouracil (5-FU) resistance in vitro [56]. Furthermore, ARID1A loss/decrement is associated with aging [57,58], poor tumor differentiation [56,58,59], tumor size [57], tumor grade [57,58], and metastasis [58] in human patients.
2.2. SMARCA4
SMARCA4 is an ATPase that provides energy to the SWI/SNF complex during chromatin remodeling [65] and is vital for development [71]. Similar to ARID1A, SMARCA4 is highly mutated in pancreatic cancer [45]. The in vivo loss of SMARCA4 results in the hypoplastic development of the pancreas [46] and enhances IPMN formation from the duct cell while suppressing PanIN formation from acinar cells carrying KRasG12D mutation [46]. Furthermore, in terms of the duct cell, SMARCA4 loss in the early stage increases cancer dedifferentiation from IPMN to carcinoma; however, in the late stage, SMARCA4 overexpression enhances tumorigenicity by the promoter binding/transcriptional activation of high mobility group AT-hook 2 (HMGA2) and subsequent mesenchymal transition [47]. An in vivo inhibitor against bromodomain and extra-terminal (BET; a ~110 amino acid protein domain that recognizes acetylated lysine in gene induction; please see [72] for review on BET and its inhibitor JQ-1) suppresses SMARCA4 loss-induced HMGA2 expression and affects pancreatic tumorigenicity [47]. Moreover, in clinical specimens, SMARCA4 expression is decreased in IPMN but is increased in the carcinoma [46]. Therefore, targeting dysregulated epigenetic regulators with epigenetic therapeutics may offer a therapeutic benefit. Additionally, SMARCA4 missense mutations exist in human patients with gastric cancer [21] or cholangiocarcinoma [38], indicating its broad mutation spectra across multiple GI cancers.
2.3. SMARCA2
SMARCA2 is mutated in cancers of the stomach [21], liver [29], and pancreas [43] of human patients. SMARCA2 promotes pancreatic tumorigenesis via JAK2/STAT3 signaling in vitro and in vivo, with increased SMARCA2 correlating to advanced tumor stage and poor prognosis in human patients [44]. The diverse roles played by this ATPase family in various cancers deserve further elucidation to determine their clinical importance and therapeutic potential in different contexts.
3. Expression and Mutation of Histone Lysine Methylation Regulators in Pancreatic Cancer
Beside the positioning of the nucleosome by chromatin remodeling, covalent modification of DNA or histones plays an important role in the regulation of pancreatic cancer gene expression [61]. In addition to the modulators of histone acetylation [61] and arginine methylation [73], regulators of histone lysine methylation, such as demethylase KDM6A, methyltransferases KMT2C/KMT2D, and histone lysine methyltransferase, participate in pancreatic tumorigenesis and are frequently mutated. While the causes of mutations in these methylation regulators await elucidation, their importance and therapeutic potential [48,74,75] have been previously reported as below:
3.1. KDM6A
Lysine demethylase 6A (KDM6A) is a demethylase that targets H3K27me2 or H3K27me3 in complex proteins associated with Set1 (COMPASS), which is essential for development [76]. KDM6A also plays a vital role in embryogenesis [77], and its mutations are found in human patients with pancreatic cancer [48]. The in vivo loss of KDM6A enhances gender-specific squamous-like pancreatic cancer, as an X-chromosomal gene via super-enhancer activation can be targeted with a BET inhibitor [48] via KDM6A loss-regulated BRD4 function. Moreover, pancreatic cancer with KDM6A loss shows vulnerability to histone deacetylase (HDAC) inhibitor as KDM6A cooperates with histone acetyltransferase p300 (EP300) to regulate gene expression, and HDACi treatment reactivates p21 (CDKN1A) expression to restore cell cycle regulation in KDM6A-deficient pancreatic cancer in vitro [49]. Thus, BETi or HDACi may offer a synthetic lethality treatment strategy for pancreatic cancer upon KDM6A loss [48,49]; in this subset of cancer cells, a correlation between loss of KDM6A expression and poor prognosis was observed [49]. In addition, KDM6A nonsense/missense mutations exist in human patients with gastric cancer [22], suggesting its importance across various tumor types.
3.2. KMT2C and KMT2D
Lysine methyltransferase 2C and 2D (KMT2C and KMT2D) are monomethyltransferases in the COMPASS complex, both of which are target H3K4 for epigenetic regulation [22]. Moreover, the importance of KMT2D in development has been reported [50]. In addition to observing KMT2C (missense) mutations in patients with pancreatic cancer [50], gastric cancer [14], cholangiocarcinoma [39], or liver cancer [30], KMT2D (missense) mutations were found in patients with pancreatic cancer [50], liver cancer [31], or cholangiocarcinoma [38]. However, in vitro KMT2D depletion increases apoptosis and sensitivity toward 5-FU in pancreatic cancer [51]. This result is in accordance with RNA sequencing results that revealed downregulation in cell cycle and growth [78]. Furthermore, nonsense/missense mutations in KMT2C/D correlate with a better prognosis of patients with pancreatic cancer, as reported by Sausen et al. [50]. These results also suggest the necessity of performing a comprehensive study on the relationship between KMT2C/D mutation and its expression level and their impact on pancreatic cancer progression.
3.3. G9a
Other epigenetic modulators can also play roles in pancreatic cancer formation [78]. Regulators including euchromatic histone lysine methyltransferase 2 (EHMT2; G9a) that target H3K9 and H3K27, affect pancreatic cancer sensitivity toward gemcitabine via autocrine IL-8/CXCR1/2 stimulation in vitro [79], and further enhance mesenchymal transition by increasing polycomb repressive complex 2 (PRC2) recruitment, while decreasing the lysine demethylase 7A (KDM7A)’s expression to influence H3K27 methylation on E-cadherin promoter in vitro [80]. Furthermore, in colon cancer, G9a regulates cancer stem phenotype and chemoradioresistance via modulation of DNA damage response in vitro [81].
3.4. EZH2
Another histone lysine methyltransferase known as the enhancer of zeste homolog 2 (EZH2), also plays an important role in pancreatic cancer progression [82]. In vivo, EZH2 knockdown decreases pancreatic tumor growth and liver metastasis [83]. In clinical specimens, EZH2 expression was found to inversely correlate with E-cadherin expression and patient survival [82]. Moreover, a subset of patient-derived organoids displayed sensitivity toward EZH2 inhibition [84], suggesting the potential of EZH2 blockage for pancreatic cancer treatment, possibly in a model of personalized medicine based on an organoid platform. In addition, present synthetic lethality based on drug combinations, such as BET inhibitor plus poly (ADP-ribose) polymerase (PARP) inhibitor, has shown therapeutic efficiency in pancreatic cancer by inhibiting the BRD2/4-regulated DNA repair [85]. Hence, this study sheds light on the importance and necessity of investigating a therapeutic combination for treatment improvement with synthetic lethality strategy.
4. Therapeutics Targeting Epigenetic Regulators in Pancreatic Cancer
A synthetic lethal strategy can not only accentuate the efficacy of the cytotoxic effect, but may also decrease off-target side effects. Numerous studies have identified therapeutics causing alterations in the above epigenetic regulators in various cancer types (Table 3). For example, the loss of AIRID1A in ovarian cancer leads to increased expression of PI3K-interacting protein 1 gene (PIK3IP1), which is a negative regulator of PI3K-AKT signaling. EZH2 plays an antagonistic role in gene transcription, compared to AIRID1A. EZH2 inhibitor, GSK126, can upregulate the levels of PIK3IP1 upon the depletion of AIRID1A. Therefore, targeting EZH2 methyltransferase activity provides a personalized strategy for ARID1A-mutated cancers [86]. Moreover, HDAC2 downregulates PIK3IP1 expression. Blocking of HDAC2 induces apoptosis in ARID1A-inactivated cells. Other studies have indicated that HDAC6 inhibition could trigger cell apoptosis through p53 activation. In this way, HDAC inhibitors could be another anti-cancer agent for treating ARID1A-mutated cancers [87,88]. Furthermore, the tyrosine kinase inhibitor dasatinib mediates apoptosis by targeting YES1 inhibition in ARID1A-null tumor cells [89]. Mismatch repair (MMR) deficiency causes a molecular feature of microsatellite instability (MSI) and may contribute to response toward immune-checkpoint blockade. A prior study demonstrated that ARID1A interacts with MMR-associated protein MSH2 at the damage site. Therefore, ARID1A deficiency also contributes to providing a potential synthetic lethal strategy for targeting programmed death-ligand 1 (PD-L1; CD274) [90]. Through a high-throughput screening, targeting the BET protein with its specific inhibitor JQ1 inhibits the growth of ARID1A-mutant cells [91]. In colon cancer, ARID1A-deficient cells exhibit reduced expression of topoisomerase 2A and a decatenation defect, which render tumor cells sensitive to an ATR serine/threonine kinase inhibitor [92] or PARP inhibitor [93].
Table 3.
Therapeutic targets in cancers with alterations in ARID1A, SMARCA2, SMARCA4, KDM6A, KMT2C, or KMT2D.
| Gene | Cancer | Therapeutic Target | Reference |
|---|---|---|---|
| ARID1A | Ovary | EZH2 | [86] |
| Pan HDAC | [87] | ||
| HDAC6 | [88] | ||
| tyrosine kinases | [89] | ||
| CD274 | [90] | ||
| BET | [91] | ||
| Colon | ATR | [92] | |
| PARP | [93] | ||
| SMARCA2 | Ovary | EZH2 | [94] |
| SMARCA4 | Lung | CDK4/6 | [95] |
| AURKA | [96] | ||
| Ovary | EZH2 | [94] | |
| KDM6A | Bladder | EZH2 | [97] |
| Multiple myeloma | EZH2 | [98] | |
| Acute myeloid leukemia | KDM1A | [99] | |
| Pancreas | BET | [48] | |
| HDAC | [49] | ||
| KMT2C | Epithelial cancer | PARP | [100] |
SMARCA2/4 deficiency leads to EZH2 dependency for cell survival. Therefore, EZH2i could be a therapeutic target in SMARCA2/4-impaired cells [94]. SMARCA4 loss results in low levels of cyclin-dependent kinase 4/6 (CDK4/6) inhibitor p16INK4a in lung cancer. This phenomenon suggests that SMARCA4-deficient cell lines are sensitized to CDK4/6 inhibitors [95]. Through RNAi-mediated depletion or chemical inhibition screening, aurora kinase A (AURKA) inhibition induces apoptosis and cell death in SMARCA4-deficient cells in vitro and in xenograft mouse models [96]. KDM6A mutation can be targeted with EZH2i in bladder cancer and multiple myeloma (MM), due to EZH2 dependency for survival [97,98].
KDM6A is most frequently mutated in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Using an epigenetic drug library screening in KDM6A-null cells, an inhibitor against lysine demethylase 1A (KDM1A; LSD1) could be a novel strategy to specifically inhibit the growth of KDM6A-deficient cells [99]. In epithelial cancers, KMT2C deficiency displays defects of homologous recombination-mediated DNA double-strand break repair that triggers PARPi sensitization of cells [100]. Moreover, in pancreatic cancer, KDM6A loss can be targeted using BETi through 78 small-molecule inhibitors [48]. Moreover, as reviewed by Xu et al. [72], the BET protein family—including BRD2, BRD3, BRD4, and BRDT—recognizes acetylated lysine on histone and transcription factors and promotes transcription with their bromodomain or extraterminal domain. The role of the BET family in tumorigenesis was implied by the promotion of liver cancer progression by BRD4 [32]. Due to the importance of bromodomain in transcriptional regulation, BET inhibitors, including diazepines JQ1 and iBET that target this domain, have been developed. Even though bromodomains from different sites on the same BET protein or from different BET proteins display diverse functions, these inhibitors still exhibit anti-tumor activity in vivo [48,72,85,86], indicating their therapeutic potential for cancer treatment. Although contextual differences exist across cancer types, the above-mentioned therapeutics identified with bioinformatics or screening strategies [48] are promising alternatives to treat tumors with synthetic lethal strategies in ARID1A, SMARCA4, KDM6A, KMT2C, or KMT2D with GI cancers as a field of future research interest (Figure 2).
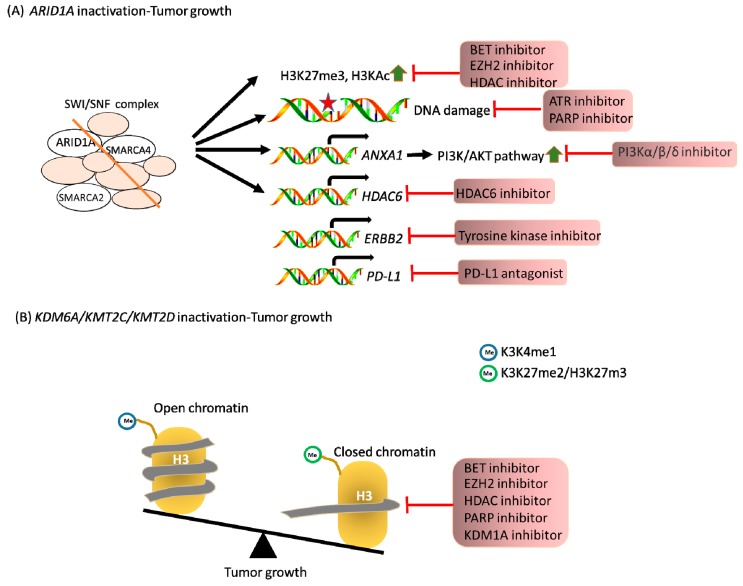
Figure 2.
Loss of ARID1A, KDM6A, KMT2C, and KMT2D triggers synthetic lethal opportunities for cancer treatment. (A) ARID1A is a member of the SWI/SNF chromatin-remodeling complex that regulates transcription and nucleosome condensation. The blockage of specific oncogenic pathways was defined as a potential strategy in ARID1A-deficient cells. (B) KDM6A and KMT2C/KMT2D represent lysine demethylase and methyltransferase, respectively. The inactivation of KDM6A, KMT2C, and KMT2D is associated with a closed chromatin structure that is specifically sensitized by the indicated inhibitors.
5. Conclusions
In the present review, we summarized the mutations of epigenetic regulators, including ARID1A, SMARCA2, SMARCA4, KDM6A, KMT2C, KMT2D, and BRD4 in GI cancers—in particular, pancreatic cancer—and found these regulators to be frequently mutated. The screening of tumor cells by utilizing chemicals, siRNAs, and shRNAs helps to identify therapeutic targets in epigenetic regulator-mutated cancers. However, the context dependency for each neoplasia needs further examination for the possible broad-spectrum application of the therapeutics mentioned in Table 3 across multiple tumor types. Accordingly, the screening strategy that targets multiple cancer types with a mutation in the same epigenetic regulator (for example, the ARID1A mutation in ovarian and colon tumors) may aid in the identification of therapeutics. This strategy could help advance the treatment of epigenetic regulator-mutated cancers.
Author Contributions
Y.-H.H., M.-C.H., L.-T.C., W.-C.H. and M.-R.P. helped to complete the manuscript; all authors read and approved the final manuscript.
Funding
We acknowledge the support from the following grants: (1) KMUH107-7R32 and KMUH-107-7R31 from the Kaohsiung Medical University Hospital; (2) MOHW107-TDU-B-212-112-015 from the Ministry of Health and Welfare, Taiwan; (3) KMU-DK108011 from KMU-KMUH Co-Project of Key Research; (4) 105-2314-B-037-038-MY3 and 106-2314-B-037-049-MY3 from the Ministry of Health and Welfare, Taiwan; (5) 108CM-KMU-06 from Kaohsiung Medical University; (6) KMU-TC108A04 from Kaohsiung Medical University Research Center Grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Lai H.L., Chen Y.Y., Lu C.H., Hung C.Y., Kuo Y.C., Chen J.S., Hsu H.C., Chen P.T., Chang P.H., Hung Y.S. Effect of s-1 on survival outcomes in 838 patients with advanced pancreatic cancer: A 7-year multicenter observational cohort study in Taiwan. Cancer Med. 2019;8:2085–2094. doi: 10.1002/cam4.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Jansen L., Balavarca Y., van der Geest L., Lemmens V., Van Eycken L., De Schutter H., Johannesen T.B., Primic-Žakelj M., Zadnik V., et al. Nonsurgical therapies for resected and unresected pancreatic cancer in europe and USA in 2003–2014: A large international population-based study. Int. J. Cancer. 2018;143:3227–3239. doi: 10.1002/ijc.31628. [DOI] [PubMed] [Google Scholar]
- 4.Strobel O., Neoptolemos J., Jaeger D., Buechler M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2018;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos J.P., Kleeff J., Michl P., Costello E., Greenhalf W., Palmer D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang-Gillam A., Li C.P., Bodoky G., Dean A., Shan Y.S., Jameson G., Macarulla T., Lee K.H., Cunningham D., Blanc J.F., et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (napoli-1): A global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Shi S., Zhang B., Ni Q., Yu X., Xu J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018;8:332–353. [PMC free article] [PubMed] [Google Scholar]
- 8.Collisson E.A., Bailey P., Chang D.K., Biankin A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019;16:207–220. doi: 10.1038/s41575-019-0109-y. [DOI] [PubMed] [Google Scholar]
- 9.Witkiewicz A.K., McMillan E.A., Balaji U., Baek G., Lin W.C., Mansour J., Mollaee M., Wagner K.U., Koduru P., Yopp A., et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway J.R., Herrmann D., Evans T.J., Morton J.P., Timpson P. Combating pancreatic cancer with pi3k pathway inhibitors in the era of personalised medicine. Gut. 2019;68:742–758. doi: 10.1136/gutjnl-2018-316822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre A.J., Hahn W.C. Synthetic lethal vulnerabilities in kras-mutant cancers. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomberk G., Blum Y., Nicolle R., Nair A., Gaonkar K.S., Marisa L., Mathison A., Sun Z., Yan H., Elarouci N., et al. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat. Commun. 2018;9:1978. doi: 10.1038/s41467-018-04383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood L.D., Yurgelun M.B., Goggins M.G. Genetics of familial and sporadic pancreatic cancer. Gastroenterology. 2019;156:2041–2055. doi: 10.1053/j.gastro.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Zang Z.J., Cutcutache I., Poon S.L., Zhang S.L., McPherson J.R., Tao J., Rajasegaran V., Heng H.L., Deng N., Gan A., et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat. Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K., Yuen S.T., Xu J., Lee S.P., Yan H.H., Shi S.T., Siu H.C., Deng S., Chu K.M., Law S., et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 17.Wang D.D., Chen Y.B., Pan K., Wang W., Chen S.P., Chen J.G., Zhao J.J., Lv L., Pan Q.Z., Li Y.Q., et al. Decreased expression of the arid1a gene is associated with poor prognosis in primary gastric cancer. PLoS ONE. 2012;7:e40364. doi: 10.1371/journal.pone.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H.B., Wang X.F., Zhang Q., Tang Z.Q., Jiang Y.H., Fan H.Z., Sun Y.H., Yang P.Y., Liu F. Reduced expression of the chromatin remodeling gene arid1a enhances gastric cancer cell migration and invasion via downregulation of e-cadherin transcription. Carcinogenesis. 2013;35:867–876. doi: 10.1093/carcin/bgt398. [DOI] [PubMed] [Google Scholar]
- 19.Inada R., Sekine S., Taniguchi H., Tsuda H., Katai H., Fujiwara T., Kushima R. Arid1a expression in gastric adenocarcinoma: Clinicopathological significance and correlation with DNA mismatch repair status. World J. Gastroenterol. 2015;21:2159–2168. doi: 10.3748/wjg.v21.i7.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L., Wei S., Zhao R., Wu Y., Qiu H., Xiong H. Loss of arid1a expression predicts poor survival prognosis in gastric cancer: A systematic meta-analysis from 14 studies. Sci. Rep. 2016;6:28919. doi: 10.1038/srep28919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeshima H., Niwa T., Takahashi T., Wakabayashi M., Yamashita S., Ando T., Inagawa Y., Taniguchi H., Katai H., Sugiyama T., et al. Frequent involvement of chromatin remodeler alterations in gastric field cancerization. Cancer Lett. 2015;357:328–338. doi: 10.1016/j.canlet.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Rokutan H., Hosoda F., Hama N., Nakamura H., Totoki Y., Furukawa E., Arakawa E., Ohashi S., Urushidate T., Satoh H., et al. Comprehensive mutation profiling of mucinous gastric carcinoma. J. Pathol. 2016;240:137–148. doi: 10.1002/path.4761. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto A., Furuta M., Totoki Y., Tsunoda T., Kato M., Shiraishi Y., Tanaka H., Taniguchi H., Kawakami Y., Ueno M., et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016;48:500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- 24.Ozen C., Yildiz G., Dagcan A.T., Cevik D., Ors A., Keles U., Topel H., Ozturk M. Genetics and epigenetics of liver cancer. N. Biotechnol. 2013;30:381–384. doi: 10.1016/j.nbt.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T., Aburatani H. Exploration of liver cancer genomes. Nat. Rev. Gastroenterol. Hepatol. 2014;11:340–349. doi: 10.1038/nrgastro.2014.6. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Wang S.C., Wei Y., Luo X., Jia Y., Li L., Gopal P., Zhu M., Nassour I., Chuang J.C., et al. Arid1a has context-dependent oncogenic and tumor suppressor functions in liver cancer. Cancer Cell. 2017;32:574–589. doi: 10.1016/j.ccell.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang J.Z., Li C., Liu X.Y., Hu T.T., Fan Z.S., Han Z.G. Hepatocyte-specific arid1a deficiency initiates mouse steatohepatitis and hepatocellular carcinoma. PLoS ONE. 2015;10:e0143042. doi: 10.1371/journal.pone.0143042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C., Li W., Tian F., Jiang K., Liu X., Cen J., He Q., Qiu Z., Kienast Y., Wang Z., et al. Arid1a regulates response to anti-angiogenic therapy in advanced hepatocellular carcinoma. J. Hepatol. 2018;68:465–475. doi: 10.1016/j.jhep.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Totoki Y., Tatsuno K., Covington K.R., Ueda H., Creighton C.J., Kato M., Tsuji S., Donehower L.A., Slagle B.L., Nakamura H., et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014;46:1267–1273. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto A., Totoki Y., Abe T., Boroevich K.A., Hosoda F., Nguyen H.H., Aoki M., Hosono N., Kubo M., Miya F., et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J., Han K.H., Gores G., Llovet J.M., Mazzaferro V. Liver cancer: Approaching a personalized care. J. Hepatol. 2015;62:144–156. doi: 10.1016/j.jhep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S.H., Eun J.W., Choi S.K., Shen Q., Choi W.S., Han J.W., Nam S.W., You J.S. Epigenetic reader brd4 inhibition as a therapeutic strategy to suppress e2f2-cell cycle regulation circuit in liver cancer. Oncotarget. 2016;7:32628–32640. doi: 10.18632/oncotarget.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao Y., Pawlik T.M., Anders R.A., Selaru F.M., Streppel M.M., Lucas D.J., Niknafs N., Guthrie V.B., Maitra A., Argani P., et al. Exome sequencing identifies frequent inactivating mutations in bap1, arid1a and pbrm1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simbolo M., Fassan M., Ruzzenente A., Mafficini A., Wood L.D., Corbo V., Melisi D., Malleo G., Vicentini C., Malpeli G., et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839–2852. doi: 10.18632/oncotarget.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruzzenente A., Fassan M., Conci S., Simbolo M., Lawlor R.T., Pedrazzani C., Capelli P., D’Onofrio M., Iacono C., Scarpa A., et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: Clinical and prognostic relevance in surgically resected patients. Ann. Surg. Oncol. 2016;23:1699–1707. doi: 10.1245/s10434-015-5046-6. [DOI] [PubMed] [Google Scholar]
- 36.Churi C.R., Shroff R., Wang Y., Rashid A., Kang H.C., Weatherly J., Zuo M., Zinner R., Hong D., Meric-Bernstam F., et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simbolo M., Vicentini C., Ruzzenente A., Brunelli M., Conci S., Fassan M., Mafficini A., Rusev B., Corbo V., Capelli P., et al. Genetic alterations analysis in prognostic stratified groups identified tp53 and arid1a as poor clinical performance markers in intrahepatic cholangiocarcinoma. Sci. Rep. 2018;8:7119. doi: 10.1038/s41598-018-25669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y.H., Hong E.K., Kong S.Y., Han S.S., Kim S.H., Rhee J.K., Hwang S.K., Park S.J., Kim T.M. Two classes of intrahepatic cholangiocarcinoma defined by relative abundance of mutations and copy number alterations. Oncotarget. 2016;7:23825–23836. doi: 10.18632/oncotarget.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito T., Sakurai-Yageta M., Goto A., Pairojkul C., Yongvanit P., Murakami Y. Genomic and transcriptional alterations of cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014;21:380–387. doi: 10.1002/jhbp.67. [DOI] [PubMed] [Google Scholar]
- 40.Birnbaum D.J., Adélaïde J., Mamessier E., Finetti P., Lagarde A., Monges G., Viret F., Gonçalvès A., Turrini O., Delpero J.R., et al. Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 2011;50:456–465. doi: 10.1002/gcc.20870. [DOI] [PubMed] [Google Scholar]
- 41.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura Y., Fukuda A., Ogawa S., Maruno T., Takada Y., Tsuda M., Hiramatsu Y., Araki O., Nagao M., Yoshikawa T., et al. Arid1a maintains differentiation of pancreatic ductal cells and inhibits development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2018;155:194–209. doi: 10.1053/j.gastro.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 43.Knudsen E.S., O’Reilly E.M., Brody J.R., Witkiewicz A.K. Genetic diversity of pancreatic ductal adenocarcinoma and opportunities for precision medicine. Gastroenterology. 2016;150:48–63. doi: 10.1053/j.gastro.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z., Wang F., Du C., Guo H., Ma L., Liu X., Kornmann M., Tian X., Yang Y. Brm/smarca2 promotes the proliferation and chemoresistance of pancreatic cancer cells by targeting jak2/stat3 signaling. Cancer Lett. 2017;402:213–224. doi: 10.1016/j.canlet.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Dal Molin M., Hong S.M., Hebbar S., Sharma R., Scrimieri F., de Wilde R.F., Mayo S.C., Goggins M., Wolfgang C.L., Schulick R.D., et al. Loss of expression of the swi/snf chromatin remodeling subunit brg1/smarca4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum. Pathol. 2012;43:585–591. doi: 10.1016/j.humpath.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Figura G., Fukuda A., Roy N., Liku M.E., Morris J.P., IV, Kim G.E., Russ H.A., Firpo M.A., Mulvihill S.J., Dawson D.W., et al. The chromatin regulator brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2014;16:255–267. doi: 10.1038/ncb2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy N., Malik S., Villanueva K.E., Urano A., Lu X., Von Figura G., Seeley E.S., Dawson D.W., Collisson E.A., Hebrok M. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015;29:658–671. doi: 10.1101/gad.256628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andricovich J., Perkail S., Kai Y., Casasanta N., Peng W., Tzatsos A. Loss of kdm6a activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to bet inhibitors. Cancer Cell. 2018;33:512–526. doi: 10.1016/j.ccell.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe S., Shimada S., Akiyama Y., Ishikawa Y., Ogura T., Ogawa K., Ono H., Mitsunori Y., Ban D., Kudo A., et al. Loss of kdm6a characterizes a poor prognostic subtype of human pancreatic cancer and potentiates hdac inhibitor lethality. Int. J. Cancer. 2019;145:192–205. doi: 10.1002/ijc.32072. [DOI] [PubMed] [Google Scholar]
- 50.Sausen M., Phallen J., Adleff V., Jones S., Leary R.J., Barrett M.T., Anagnostou V., Parpart-Li S., Murphy D., Kay Li Q., et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015;6:7686. doi: 10.1038/ncomms8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawkins J.B., Wang J., Maniati E., Heward J.A., Koniali L., Kocher H.M., Martin S.A., Chelala C., Balkwill F.R., Fitzgibbon J., et al. Reduced expression of histone methyltransferases kmt2c and kmt2d correlates with improved outcome in pancreatic ductal adenocarcinoma. Cancer Res. 2016;76:4861–4871. doi: 10.1158/0008-5472.CAN-16-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cajuso T., Hanninen U.A., Kondelin J., Gylfe A.E., Tanskanen T., Katainen R., Pitkanen E., Ristolainen H., Kaasinen E., Taipale M., et al. Exome sequencing reveals frequent inactivating mutations in arid1a, arid1b, arid2 and arid4a in microsatellite unstable colorectal cancer. Int. J. Cancer. 2014;135:611–623. doi: 10.1002/ijc.28705. [DOI] [PubMed] [Google Scholar]
- 53.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones S., Li M., Parsons D.W., Zhang X., Wesseling J., Kristel P., Schmidt M.K., Markowitz S., Yan H., Bigner D., et al. Somatic mutations in the chromatin remodeling gene arid1a occur in several tumor types. Hum. Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathur R., Alver B.H., San Roman A.K., Wilson B.G., Wang X., Agoston A.T., Park P.J., Shivdasani R.A., Roberts C.W. Arid1a loss impairs enhancer-mediated gene regulation and drives colon cancer in mice. Nat. Genet. 2017;49:296–302. doi: 10.1038/ng.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie C., Fu L., Han Y., Li Q., Wang E. Decreased arid1a expression facilitates cell proliferation and inhibits 5-fluorouracil-induced apoptosis in colorectal carcinoma. Tumour Biol. 2014;35:7921–7927. doi: 10.1007/s13277-014-2074-y. [DOI] [PubMed] [Google Scholar]
- 57.Chou A., Toon C.W., Clarkson A., Sioson L., Houang M., Watson N., DeSilva K., Gill A.J. Loss of arid1a expression in colorectal carcinoma is strongly associated with mismatch repair deficiency. Hum. Pathol. 2014;45:1697–1703. doi: 10.1016/j.humpath.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Ye J., Zhou Y., Weiser M.R., Gonen M., Zhang L., Samdani T., Bacares R., DeLair D., Ivelja S., Vakiani E., et al. Immunohistochemical detection of arid1a in colorectal carcinoma: Loss of staining is associated with sporadic microsatellite unstable tumors with medullary histology and high tnm stage. Hum. Pathol. 2014;45:2430–2436. doi: 10.1016/j.humpath.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Lee L.H., Sadot E., Ivelja S., Vakiani E., Hechtman J.F., Sevinsky C.J., Klimstra D.S., Ginty F., Shia J. Arid1a expression in early stage colorectal adenocarcinoma: An exploration of its prognostic significance. Hum. Pathol. 2016;53:97–104. doi: 10.1016/j.humpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paradise B., Barham W., Fernandez-Zapico M. Targeting epigenetic aberrations in pancreatic cancer, a new path to improve patient outcomes? Cancers. 2018;10:128. doi: 10.3390/cancers10050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Längst G., Manelyte L. Chromatin remodelers: From function to dysfunction. Genes. 2015;6:299–324. doi: 10.3390/genes6020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas H. Therapy: Targeting chromatin remodelling proteins to treat pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2015;12:608. doi: 10.1038/nrgastro.2015.171. [DOI] [PubMed] [Google Scholar]
- 64.Kumar R., Li D.Q., Müller S., Knapp S. Epigenomic regulation of oncogenesis by chromatin remodeling. Oncogene. 2016;35:4423–4436. doi: 10.1038/onc.2015.513. [DOI] [PubMed] [Google Scholar]
- 65.Biegel J.A., Busse T.M., Weissman B.E. Swi/snf chromatin remodeling complexes and cancer. Am. J. Med. Genet. C Semin. Med. Genet. 2014;166C:350–366. doi: 10.1002/ajmg.c.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J.N., Roberts C.W. Arid1a mutations in cancer: Another epigenetic tumor suppressor? Cancer Discov. 2013;3:35–43. doi: 10.1158/2159-8290.CD-12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Livshits G., Alonso-Curbelo D., Morris IV J.P., Koche R., Saborowski M., Wilkinson J.E., Lowe S.W. Arid1a restrains kras-dependent changes in acinar cell identity. Elife. 2018;7:35216. doi: 10.7554/eLife.35216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLean M.H., El-Omar E.M. Genetics of gastric cancer. Nat. Rev. Gastroenterol. Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 69.He L., Tian D.A., Li P.Y., He X.X. Mouse models of liver cancer: Progress and recommendations. Oncotarget. 2015;6:23306–23322. doi: 10.18632/oncotarget.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos N.P., Colaco A.A., Oliveira P.A. Animal models as a tool in hepatocellular carcinoma research: A review. Tumour Biol. 2017;39:1010428317695923. doi: 10.1177/1010428317695923. [DOI] [PubMed] [Google Scholar]
- 71.Bultman S., Gebuhr T., Yee D., La Mantia C., Nicholson J., Gilliam A., Randazzo F., Metzger D., Chambon P., Crabtree G. A brg1 null mutation in the mouse reveals functional differences among mammalian swi/snf complexes. Mol. Cell. 2000;6:1287–1295. doi: 10.1016/S1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 72.Xu Y., Vakoc C.R. Targeting cancer cells with bet bromodomain inhibitors. Cold Spring Harb. Perspect. Med. 2017;7:a026674. doi: 10.1101/cshperspect.a026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poulard C., Corbo L., Le Romancer M. Protein arginine methylation/demethylation and cancer. Oncotarget. 2016;7:67532–67550. doi: 10.18632/oncotarget.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koutsioumpa M., Hatziapostolou M., Polytarchou C., Tolosa E.J., Almada L.L., Mahurkar-Joshi S., Williams J., Tirado-Rodriguez A.B., Huerta-Yepez S., Karavias D. Lysine methyltransferase 2d regulates pancreatic carcinogenesis through metabolic reprogramming. Gut. 2019;68:1271–1286. doi: 10.1136/gutjnl-2017-315690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J., Duns G., Westers H., Sijmons R., van den Berg A., Kok K. Setd2: An epigenetic modifier with tumor suppressor functionality. Oncotarget. 2016;7:50719–50734. doi: 10.18632/oncotarget.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller T., Krogan N.J., Dover J., Erdjument-Bromage H., Tempst P., Johnston M., Greenblatt J.F., Shilatifard A. Compass: A complex of proteins associated with a trithorax-related set domain protein. Proc. Natl. Acad. Sci. USA. 2001;98:12902–12907. doi: 10.1073/pnas.231473398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van der Meulen J., Speleman F., Van Vlierberghe P. The h3k27me3 demethylase utx in normal development and disease. Epigenetics. 2014;9:658–668. doi: 10.4161/epi.28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan M.R., Hsu M.C., Chen L.T., Hung W.C. Orchestration of h3k27 methylation: Mechanisms and therapeutic implication. Cell. Mol. Life Sci. 2018;75:209–223. doi: 10.1007/s00018-017-2596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan M.R., Hsu M.C., Luo C.W., Chen L.T., Shan Y.S., Hung W.C. The histone methyltransferase g9a as a therapeutic target to override gemcitabine resistance in pancreatic cancer. Oncotarget. 2016;7:61136–61151. doi: 10.18632/oncotarget.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan M.R., Hsu M.C., Chen L.T., Hung W.C. G9a orchestrates pcl3 and kdm7a to promote histone h3k27 methylation. Sci. Rep. 2015;5:18709. doi: 10.1038/srep18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo C.W., Wang J.Y., Hung W.C., Peng G., Tsai Y.L., Chang T.M., Chai C.Y., Lin C.H., Pan M.R. G9a governs colon cancer stem cell phenotype and chemoradioresistance through pp2a-rpa axis-mediated DNA damage response. Radiother. Oncol. 2017;124:395–402. doi: 10.1016/j.radonc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Toll A.D., Dasgupta A., Potoczek M., Yeo C.J., Kleer C.G., Brody J.R., Witkiewicz A.K. Implications of enhancer of zeste homologue 2 expression in pancreatic ductal adenocarcinoma. Hum. Pathol. 2010;41:1205–1209. doi: 10.1016/j.humpath.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Xie D., Yin Li W., Man Cheung C., Yao H., Chan C.Y., Chan C.Y., Xu F.P., Liu Y.H., Sung J.J., et al. Rnai targeting ezh2 inhibits tumor growth and liver metastasis of pancreatic cancer in vivo. Cancer Lett. 2010;297:109–116. doi: 10.1016/j.canlet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Huang L., Holtzinger A., Jagan I., BeGora M., Lohse I., Ngai N., Nostro C., Wang R., Muthuswamy L.B., Crawford H.C., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller A.L., Fehling S.C., Garcia P.L., Gamblin T.L., Council L.N., van Waardenburg R., Yang E.S., Bradner J.E., Yoon K.J. The bet inhibitor jq1 attenuates double-strand break repair and sensitizes models of pancreatic ductal adenocarcinoma to parp inhibitors. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bitler B.G., Aird K.M., Garipov A., Li H., Amatangelo M., Kossenkov A.V., Schultz D.C., Liu Q., Shih Ie M., Conejo-Garcia J.R., et al. Synthetic lethality by targeting ezh2 methyltransferase activity in arid1a-mutated cancers. Nat. Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukumoto T., Park P.H., Wu S., Fatkhutdinov N., Karakashev S., Nacarelli T., Kossenkov A.V., Speicher D.W., Jean S., Zhang L., et al. Repurposing pan-hdac inhibitors for arid1a-mutated ovarian cancer. Cell Rep. 2018;22:3393–3400. doi: 10.1016/j.celrep.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bitler B.G., Wu S., Park P.H., Hai Y., Aird K.M., Wang Y., Zhai Y., Kossenkov A.V., Vara-Ailor A., Rauscher F.J., III, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat. Cell Biol. 2017;19:962–973. doi: 10.1038/ncb3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller R.E., Brough R., Bajrami I., Williamson C.T., McDade S., Campbell J., Kigozi A., Rafiq R., Pemberton H., Natrajan R., et al. Synthetic lethal targeting of arid1a-mutant ovarian clear cell tumors with dasatinib. Mol. Cancer Ther. 2016;15:1472–1484. doi: 10.1158/1535-7163.MCT-15-0554. [DOI] [PubMed] [Google Scholar]
- 90.Shen J., Ju Z., Zhao W., Wang L., Peng Y., Ge Z., Nagel Z.D., Zou J., Wang C., Kapoor P., et al. Arid1a deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 2018;24:556–562. doi: 10.1038/s41591-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berns K., Caumanns J.J., Hijmans E.M., Gennissen A.M.C., Severson T.M., Evers B., Wisman G.B.A., Jan Meersma G., Lieftink C., Beijersbergen R.L., et al. Arid1a mutation sensitizes most ovarian clear cell carcinomas to bet inhibitors. Oncogene. 2018;37:4611–4625. doi: 10.1038/s41388-018-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williamson C.T., Miller R., Pemberton H.N., Jones S.E., Campbell J., Konde A., Badham N., Rafiq R., Brough R., Gulati A., et al. Atr inhibitors as a synthetic lethal therapy for tumours deficient in arid1a. Nat. Commun. 2016;7:13837. doi: 10.1038/ncomms13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen J., Peng Y., Wei L., Zhang W., Yang L., Lan L., Kapoor P., Ju Z., Mo Q., Shih Ie M., et al. Arid1a deficiency impairs the DNA damage checkpoint and sensitizes cells to parp inhibitors. Cancer Discov. 2015;5:752–767. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan-Penebre E., Armstrong K., Drew A., Grassian A.R., Feldman I., Knutson S.K., Kuplast-Barr K., Roche M., Campbell J., Ho P., et al. Selective killing of smarca2- and smarca4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of ezh2: In vitro and in vivo preclinical models. Mol. Cancer Ther. 2017;16:850–860. doi: 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 95.Xue Y., Meehan B., Fu Z., Wang X.Q.D., Fiset P.O., Rieker R., Levins C., Kong T., Zhu X., Morin G., et al. Smarca4 loss is synthetic lethal with cdk4/6 inhibition in non-small cell lung cancer. Nat. Commun. 2019;10:557. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tagal V., Wei S., Zhang W., Brekken R.A., Posner B.A., Peyton M., Girard L., Hwang T., Wheeler D.A., Minna J.D., et al. Smarca4-inactivating mutations increase sensitivity to aurora kinase a inhibitor vx-680 in non-small cell lung cancers. Nat. Commun. 2017;8:14098. doi: 10.1038/ncomms14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ler L.D., Ghosh S., Chai X., Thike A.A., Heng H.L., Siew E.Y., Dey S., Koh L.K., Lim J.Q., Lim W.K., et al. Loss of tumor suppressor kdm6a amplifies prc2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of ezh2. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai8312. [DOI] [PubMed] [Google Scholar]
- 98.Ezponda T., Dupere-Richer D., Will C.M., Small E.C., Varghese N., Patel T., Nabet B., Popovic R., Oyer J., Bulic M., et al. UTX/KDM6A loss enhances the malignant phenotype of multiple myeloma and sensitizes cells to EZH2 inhibition. Cell Rep. 2017;21:628–640. doi: 10.1016/j.celrep.2017.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu B., Pan X., Chen X., Chen M., Shi K., Xu J., Zheng J., Niu T., Chen C., Shuai X., et al. Epigenetic drug library screening identified an lsd1 inhibitor to target utx-deficient cells for differentiation therapy. Signal Transduct. Target. Ther. 2019;4:11. doi: 10.1038/s41392-019-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rampias T., Karagiannis D., Avgeris M., Polyzos A., Kokkalis A., Kanaki Z., Kousidou E., Tzetis M., Kanavakis E., Stravodimos K., et al. The lysine-specific methyltransferase kmt2c/mll3 regulates DNA repair components in cancer. EMBO Rep. 2019;20:e46821. doi: 10.15252/embr.201846821. [DOI] [PMC free article] [PubMed] [Google Scholar]