Abstract
Chitin is a valuable peat substrate amendment by increasing lettuce growth and reducing the survival of the zoonotic pathogen Salmonella enterica on lettuce leaves. The production of chitin-catabolic enzymes (chitinases) play a crucial role and are mediated through the microbial community. A higher abundance of plant-growth promoting microorganisms and genera involved in N and chitin metabolism are present in a chitin-enriched substrate. In this study, we hypothesize that chitin addition to peat substrate stimulates the microbial chitinase production. The degradation of chitin leads to nutrient release and the production of small chitin oligomers that are related to plant growth promotion and activation of the plant’s defense response. First a shotgun metagenomics approach was used to decipher the potential rhizosphere microbial functions then the nutritional content of the peat substrate was measured. Our results show that chitin addition increases chitin-catabolic enzymes, bacterial ammonium oxidizing and siderophore genes. Lettuce growth promotion can be explained by a cascade degradation of chitin to N-acetylglucosamine and eventually ammonium. The occurrence of increased ammonium oxidizing bacteria, Nitrosospira, and amoA genes results in an elevated concentration of plant-available nitrate. In addition, the increase in chitinase and siderophore genes may have stimulated the plant’s systemic resistance.
Subject terms: Next-generation sequencing, Applied microbiology, Metagenomics
Introduction
Chitin is the second most abundant biopolymer in nature. It is a long-chain polymer of N-acetylglucosamine (GlcNAc), a glucose-like molecule. Major sources of chitin are the exoskeleton of arthropods (insects, crustaceans, etc.), the beaks of cephalopods and the eggs and gut linings of nematodes1.
In soil, degradation of chitin is mediated by enzymes produced by certain bacteria and several fungi, such as Streptomyces and Trichoderma2. More specifically, the biological degradation of chitin to GlcNAc monomers relies on a catalytic action of hydrolytic enzymes, called chitinases and N-acetylglucosaminidases. In addition, chitin can also be converted to a more soluble biopolymer, chitosan, through deacetylases. The enzymatic action of chitosanases further degrades chitosan into N-glucosamines3,4.
Besides its importance as structural polymer, chitin and its deacetylated form chitosan display biological activity. Beneficial effects of both compounds have been reported regarding plant growth and disease resistance. Chitin amendment of a peat substrate promotes growth of lettuce and other plants5,6. Soil amended with chitosan promotes plant growth in tomato, soybean sprouts, sweet basil and grapevine7–10. The observed increases in plant growth under chitin and chitosan supplementation can be attributed to an increase in plant-available nutrients in the growing medium. Chitin and chitosan have high N content (6.1–8.3%) and are also calcium rich11,12. The production of catabolic enzymes for chitin-degradation by micro-organisms in the rhizosphere can release Ca into the growing medium and break down N sources in forms more accessible for uptake by plants and microorganisms. For chitosan, mechanisms besides the provision of plant nutrients may also be present. In studies with orchids, chitosan affects plant growth even at concentrations under the nutritionally effective range13,14. The underlying mechanisms of this effect will need to be unraveled. Currently, they are attributed to the stimulation of plant-growth promoting rhizobacteria (PGPR)15.
Production of chitin-degradable enzymes not only affects plant growth, but also shows positive effects on plant disease resistance. The production of chitin-catabolic enzymes results in the lysis of chitin-rich cell walls of several pests and pathogens such as insects, pathogenic fungi and plant parasitic nematodes5,16–18. In addition, the application of chitin or chitosan in low concentrations in the soil or on the plant leaves can activate the plant’s own defense mechanism, also referred to as induced systemic resistance. In several crops, a number of “chitin elicitor binding proteins” (CEBiP) have been isolated, which enables the plant to respond to chitin oligomers that are either released from pests and pathogens or come from an added source of chitin19,20. The oligomers are degraded by chitinase activity5. Binding of chitin-oligomers to the CEBiP receptors will eventually activate the plant defense response, where the signal is transported throughout the plant via jasmonates5,21. Chitin and chitosan are therefore often reported as PAMPs: “pathogen-associated molecular patterns”5.
Debode et al. (2016) showed that the addition of chitin in peat substrate increases lettuce growth and reduces the survival of a zoonotic pathogen, Salmonella enterica, on lettuce leaves. The reduction in Salmonella survival can be related to chitin mediated elicitation of the plant defense response6. For both responses, the production of microbial chitin-catabolic enzymes is essential for the release of plant-available nutrients and small chitin-oligomers to the peat substrate. Sequencing of bacterial (16S rRNA gene) and fungal (ITS2 gene region) phylogenetic markers showed that chitin increased the relative abundance of PGPR and other rhizosphere microorganisms reported to be involved in the N-cycle and chitin degradation (e.g., Cellvibrio, Pedobacter, Dyadobacter, Streptomyces, Lecanicillium and Mortierella spp.). This information is based on taxonomy, however; no functional information of the microbiota on rhizosphere in peat substrate with chitin is available so far. Therefore we performed a whole genome shotgun (WGS) metagenomics analysis using the same DNA pool of a previous experiment (Debode et al.)6 to: (1) confirm taxonomic profiles; (2) analyze the functional microbial profile and focus on the presence of chitinase and chitosanase enzymes; and (3) analyze the microbial functions related with nutrient responses. To evaluate the release of N and other plant-available nutrients to the peat substrate, we also evaluated the nutrient content in unamended and chitin-amended peat substrates.
Results
Chemical properties of peat, peat amended with chitin and chitin
After eight weeks of plant growth in chitin-amended peat substrate, there was a significant increase in NO3−, total mineral N and Ca2+ levels and a significant decrease in pH and water-extractable P in the chitin-amended peat substrate.
Pure chitin contained trace elements of water-extractable SO42−, Mg, Ca, K and C. Na concentration was similar in the peat, the peat-chitin mixture and the pure chitin, while Cl content was higher in pure chitin than peat-amended with chitin and peat substrate (Table 1; Supplementary Table S1). Chitin contains a high total N content (7.1%/dry matter) but has mineral N concentrations below the detection limit (Tables 1 and S1), indicating that the N was organically bound and thus not readily available to plants.
Table 1.
Chemical properties and water-extractable nutrient concentrations of peat substrate (PS) and chitin-amended peat substrate (Chitin) samples after 8 weeks of plant growth and pure chitin.
| PS | Chitin | p-value | Pure Chitin | |
|---|---|---|---|---|
| pH-H2O* | 6.23 ± 0.02 | 6.03 ± 0.07 | 0.040 | 8.80 |
| EC (µS/cm) | 469.50 ± 69.92 | 634.75 ± 38.05 | 0.092 | 157.00 |
| N-NO3−*** (mg/L) | 64.58 ± 21.49 | 191.13 ± 10.80 | 0.001 | BDL |
| N-NH4+ (mg/L) | 9.45 ± 0.60 | 12.73 ± 1.65 | 0.287 | BDL |
| Total mineral N** (mg/L) | 69.30 ± 24.06 | 200.68 ± 12.53 | 0.003 | BDL |
| SO42− (mg/L) | 588.95 ± 73.01 | 571.30 ± 61.72 | 0.876 | 26.00 |
| Cl (mg/L) | 20.47 ± 1.05 | 19.97 ± 3.98 | 0.921 | 56.90 |
| P in H2O* (mg/L) | 66.43 ± 10.11 | 33.58 ± 8.41 | 0.046 | BDL |
| Fe (mg/L) | 1.1 ± 0.1 | 0.60 ± 0.02 | 0.355 | / |
| Mg (mg/L) | 75.55 ± 2.12 | 101.3 ± 1.11 | 0.081 | 1.31 |
| Ca* (mg/L) | 287.10 ± 8.04 | 393.65 ± 3.50 | 0.050 | 3.84 |
| K (mg/L) | 79.10 ± 5.40 | 56.85 ± 2.17 | 0.788 | 2.51 |
| Na (mg/L) | 35.30 ± 0.99 | 27.65 ± 0.12 | 0.202 | 34.25 |
| C (mg/L) | 433.80 ± 10.83 | 572.95 ± 12.53 | 0.166 | 86.11 |
| Si (mg/L) | 7.15 ± 0.21 | 6.95 ± 0.09 | 0.867 | / |
Values are averages ± standard errors (n = 4). Significances between treatments are indicated by an asterisk (*<0.05, **<0.01, ***< = 0.001). Elements with levels that could not be detected are indicated as BDL = below detection limit. / = not measured. Concentrations of nutrients for PS and Chitin-amended PS are measured as mg/L substrate. For the pure chitin, this is mg/L extract.
Taxonomic characterization
Four microbial peat substrate DNA samples per treatment from the study of Debode et al. (2016) were selected for WGS metagenomics6. The resulting data was initially analyzed using a new platform: Unipept Metagenomics Analysis Pipeline (UMGAP). Compared to other widely used metagenomics pipelines (e.g. MG-RAST, EBI metagenomics)22–24, UMGAP uses all sequences for taxonomic analysis instead of merely ribosomal subunits. A total of 112,765,345 reads were obtained and after quality control (QC), 14.3% on average of the sequences were removed. From these quality filtered data, an average of 14.6% of the reads were assigned to a taxon. After normalization, OTUs were clustered in 2,197 genera in the UMGAP count table. As a comparison, the MG-RAST pipeline removed 23.5% of the sequences in the QC step, and RefSeq annotation (SILVA LSU, SSU) embedded in MG-RAST allocated 0.30% of the retained reads to a taxon (Table S2). In total, 1,719 taxa were present in the MG-RAST count table.
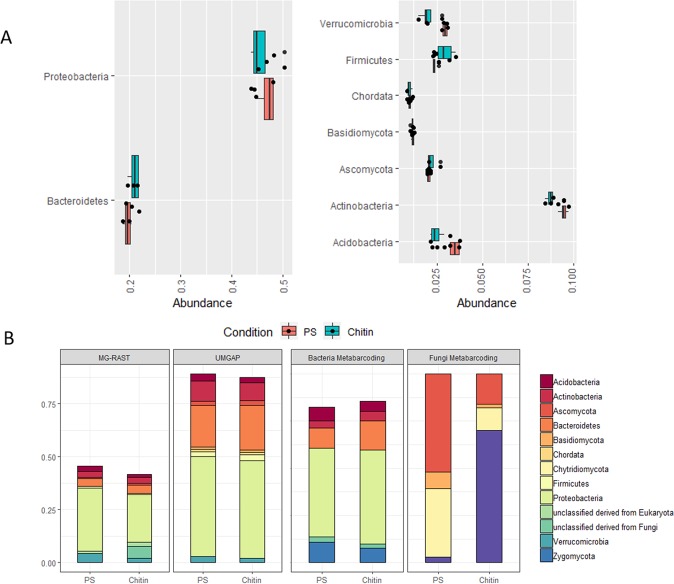
UMGAP analysis showed that independent of chitin addition, Proteobacteria, Bacteroidetes, Acidobacteria, Actinobacteria, Firmicutes and Verrucomicrobia were highly abundant in the samples. (Fig. 1A). Fungi were primarily classified as Ascomycota and Basidiomycota. Other eukaryotic phyla were highly abundant (>2%), i.e., the Chordata. Reads classified as Streptophyta (4.2% ± 2.1%), primarily consisting of OTUs mapped to the lettuce genome, were also found. In comparison, the MG-RAST analysis shares 7 of those phyla in the top 10 ranking (Fig. 1B), and consisted of a rather high amount of reads classified as Streptophyta (52.3% ± 1.9%). As a comparison, data of the previous publication6 was added.
Figure 1.
Taxonomical composition of the lettuce rhizosphere microbiome. (A) Majorly abundant phyla (relative abundance) in the lettuce rhizosphere which are present for at least 1% in at least one of the treatments (PS = peat substrate, Chitin = peat substrate + 2% chitin). Highly abundant phyla (>10%) are represented at the left, other phyla (1–10%) are represented at the right part of the figure. (B) Comparison of the top 10 phyla of the UMGAP data analysis with the conventional MG-RAST analysis. In total, 7 out of the 10 phyla are shared between the two methods. In addition, the abundance of these phyla, with the inclusion of the Zygomycota which contains the Morteriella genus in the UNITE database, from the metabarcoding data of Debode et al.6 is included.
Addition of chitin significantly influenced the rhizosphere microbial taxonomic composition (PERMANOVA, p = 0.018). In total, 96 genera were differentially abundant (gene-wise likelihood ratio tests, p-values < 0.05), of which 15 genera had an average abundance of minimum 0.10% in at least one of the two treatments (Table 2). Two fungal genera show the highest increase upon chitin addition: Hanseniaspora (18.6x) and Mortierella (18.5x), followed by a number of bacterial OTUs, e.g., Cellvibrio (7.6x), Escherichia (5.0x) and Nitrosospira (3.4x).
Table 2.
Differentially abundant genera (p < 0.05) in the lettuce rhizosphere microbiome cultivated in peat substrate (PS) and peat substrate amended with chitin (Chitin).
| Kingdom | Phylum | Genus | PS (%) | Chitin (%) | Increase/Decrease | Potential function* |
|---|---|---|---|---|---|---|
| Archaea | Thaumarchaeota | Nitrosotalea | 0.30 ± 0.03 | 0.12 ± 0.01 | 0.4 | Ammonium oxidation69 |
| Bacteria | Acidobacteria | Koribacter | 0.31 ± 0.02 | 0.17 ± 0.01 | 0.5 | Nitric oxide reduction70 |
| Bacteroidetes | Pedobacter | 1.02 ± 0.02 | 1.56 ± 0.15 | 1.5 | PGP and biocontrol71 | |
| Dyadobacter | 0.20 ± 0.01 | 0.53 ± 0.13 | 2.6 | NA | ||
| Arachidicoccus | 0.18 ± 0.02 | 0.39 ± 0.04 | 2.2 | NA | ||
| Proteobacteria | Nitrosospira | 0.59 ± 0.12 | 2.04 ± 0.33 | 3.4 | Ammonium oxidation42,72,73 | |
| Cellvibrio | 0.08 ± 0.03 | 0.58 ± 0.19 | 7.6 | PGP, chitin degradation and N-cycle25–27 | ||
| Nitrobacter | 0.19 ± 0.01 | 0.32 ± 0.02 | 1.7 | Nitrite oxidation74 | ||
| Pseudolabrys | 0.24 ± 0.01 | 0.15 ± 0.01 | 0.6 | NA | ||
| Escherichia | 0.06 ± 0.01 | 0.31 ± 0.12 | 5.0 | NA | ||
| Methylophilus | 0.23 ± 0.07 | 0.08 ± 0.02 | 0.4 | NA | ||
| Rhodoplanes | 0.18 ± 0.01 | 0.10 ± 0.01 | 0.6 | NA | ||
| Nitrosomonas | 0.06 ± 0.01 | 0.11 ± 0.02 | 1.8 | Ammonium oxidation72 | ||
| Fungi | Ascomycota | Hanseniaspora | 0.01 ± 0.01 | 0.26 ± 0.01 | 18.6 | NA |
| Zygomycota | Mortierella | 0.03 ± 0.01 | 0.57 ± 0.04 | 18.5 | Chitin degradation28 and biocontrol75 | |
| Protozoa | Ciliophora | Stylonychia | 0.11 ± 0.02 | 0.26 ± 0.04 | 2.5 | NA |
| Oxytricha | 0.11 ± 0.02 | 0.36 ± 0.06 | 3.4 | NA |
The average relative abundance of reads (±standard error, n = 4) is shown for plants grown in either in peat or chitin-amended peat. Only genera with an average relative abundance of 0.10% in at least one of the treatments are shown.
*Species of specific genera described in literature involved in the N-cycle, chitin degradation or biocontrol.
NA = To our knowledge, these bacterial genera contain no currently known species involved in the nitrogen cycle, chitin degradation or plant growth promotion.
The abundance of Salmonella reads, the zoonotic pathogen added on the leaves, in the rhizosphere of lettuce was not significantly different between peat substrate (0.012 ± 0.001) and chitin-amended peat substrate (0.011 ± 0.001) and was generally low.
Characterization of functional categories
To study the functional potential of the rhizosphere microbiome, sequences of the shotgun metagenomics data were initially compared to the subsystem database in MG-RAST. In total 39% to 45% of the sequence reads were classified into functional categories (Table S3). At subsystem level 1, the relative abundance of 2 of the 26 functional categories was altered due to chitin addition (Table S4). An increase in category average was observed, namely for iron acquisition and metabolism (p < 0.001), and a decrease was observed in average gene abundance of the nitrogen metabolism (p = 0.002) (Table S4).
At subsystem level 3, a total of 98 functional categories were significantly different: 53 increased and 45 decreased in abundance due to chitin addition (Table S5). These categories could be classified in 22 level 1 categories, with the top 3 being the carbohydrates (n = 11), phages, prophages and plasmids (n = 9) and protein metabolism (n = 9). In most of these level 1 categories, there were both significant increases and decreases of the differentially abundant level 3 functional categories.
One of the carbohydrates categories which increases due to chitin application is named as chitin- and N-acetylglucosamine utilization. This indicates already the presence and induction of chitinase and N-acetylglucosamindase genes in the microbiome of the rhizosphere of lettuce grown in chitin-enriched peat substrate.
Five of the functional categories were clustered within the iron acquisition level. They were linked with either heme uptake, iron uptake, iron scavenging or the production of siderophores. Each of these categories that clustered within iron acquisition showed a significant increase in abundance in the chitin-amended peat substrate. In contrast, from the 98 differentially abundant functional groups, only denitrification could be linked with nitrogen metabolism.
Chitin-degradation enzyme abundance
One of the carbohydrate-related categories that increased in relative abundance due to chitin addition was chitin and N-acetylglucosaminidase utilization (Table S5). To gain deeper insight into chitin metabolism changes, we mapped the metagenome reads to chitosan- or chitin-degradation enzymes. These enzymes are classified in specific glycosyl hydrolase (GH) families. First, the enzymes capable of the deacetylation of chitin to chitosan (CE4, GH5, GH7, GH8), and chitosanases (GH46, GH75, GH80), the enzymes capable of degrading chitosan to N-glucosamines, were mapped (Table S6; Fig. 2). Independent of chitin addition in peat substrate, a high number of metagenome reads were affiliated to chitin deacetylase enzymes and chitosanases (Table S6, Fig. 2). From these chitosanases, only those grouped in GH46 (p < 0.001) and GH75 (p = 0.044) showed a significant increase in abundance due to chitin addition in peat substrate.
Figure 2.
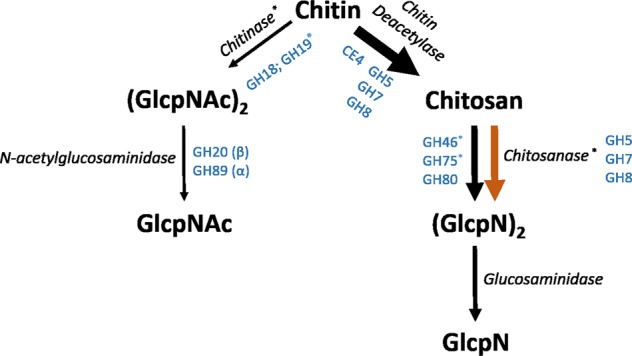
Chitin convergence and degradation pathway. First, chitin can be converted to chitosan through deacetylation and further degraded to N-glucosamines (GlcpN) through glucosaminidase enzymes. Second, chitin can be degraded by chitinases and N-acetylglucosaminidase enzymes to N-acetylglucosamines (GlcpNAc). Enzymes involved in the chitin cycle are classified in glycosyl hydrolase (GH) families and represented in blue. The metagenome reads are mapped towards these enzymes and the RPKG normalized counts representing the number of hits are illustrated by the arrow thickness in the figure. If there was a statistical effect of chitin addition, a second arrow (orange) is drawn representing the normalized count of the genes related to the chitin-added samples.
Second, the enzymes capable of the degradation of chitin to N-acetylglucosamines (chitinases: GH18, GH19; N-acetylglucosaminidases: GH20) were studied. Independent from chitin addition in peat substrate, the number of reads mapped to chitinases and N-acetylglucosaminidases was 25-times lower than those mapped to chitin-deacetylase and chitosanases. Addition of chitin did increase the potential of chitin degradation to N-acetylglucosamines, however: the number of reads mapped to GH19-related chitinases increased significantly from 0.44 ± 0.05 to 0.84 ± 0.11 (p = 0.0006) (Table S6, Fig. 2). Two genera, Cellvibrio and Mortierella, that increased in abundance due to chitin addition in peat substrate were previously described to be involved in the chitin-degradation cycle (Table 2)25–28.
Chitin affects the nitrogen cycle
The MG-RAST subsystem classification results showed a reduction in the nitrogen metabolism category in the rhizosphere of lettuce grown in chitin amended peat substrate. To analyze the changes of the nitrogen cycle in further detail, we matched the metagenome reads to profile Hidden Markov Models (HMMs) of 11 nitrification and denitrification genes: amoA, amoB, napA, nrfA, narG, nirS, nirK, norB, nosZ, nifH and ureC (Table 3). Four other genes involved in the nitrogen cycle (hao, nor, nasA, nir) were not studied as they were not available in the FunGene database.
Table 3.
Potential nitrification and denitrification genes present in rhizosphere of lettuce cultivated in peat substrate (PS) and chitin-amended peat substrates (Chitin).
| Enzyme | PS | Chitin | p-value |
|---|---|---|---|
| amoA AOA | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.17 |
| amoA AOB ** | 0.21 ± 0.10 | 0.66 ± 0.21 | 0.007 |
| napA | 1.20 ± 0.05 | 1.23 ± 0.06 | 0.53 |
| narG | 3.13 ± 0.51 | 2.68 ± 0.17 | 0.14 |
| nifH | 0.04 ± 0.02 | 0.06 ± 0.02 | 0.18 |
| nirK | 2.75 ± 0.19 | 2.82 ± 0.18 | 0.61 |
| nirS | 0.05 ± 0.02 | 0.06 ± 0.04 | 0.81 |
| norB | 3.81 ± 0.45 | 3.43 ± 0.23 | 0.18 |
| nosZ ** | 1.61 ± 0.18 | 1.19 ± 0.13 | 0.0091 |
| nosZ atypical 1 ** | 1.76 ± 0.09 | 1.28 ± 0.14 | 0.0019 |
| nosZ atypical 2 *** | 2.69 ± 0.10 | 2.01 ± 0.17 | 0.0005 |
| nrfA | 0.10 ± 0.01 | 0.05 ± 0.05 | 0.08 |
| ureC | 3.16 ± 0.18 | 2.96 ± 0.29 | 0.30 |
RPKG normalized mean abundance (±standard error) of genes involved in nitrogen cycle.*p-value < 0.05; **p-value < 0.01; ***p-value < 0.001.
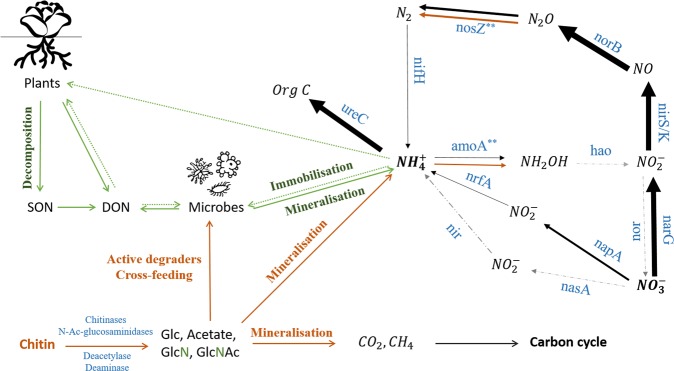
Based on the normalized counts, a high abundance of genes involved in denitrification was observed compared to the other genes related to nitrogen metabolism. The genes involved for the convergence of nitrate to dinitrogen-oxide (narG, nirS/K, norB) were most abundant in the rhizosphere of lettuce grown in peat as well as in the chitin-amended peat substrate (Fig. 3, Table 3). For the denitrification pathway, chitin only induced a significant reduction of the abundance of nitric oxide signaling genes (nosZ) which are involved in converging dinitrogen-oxide to dinitrogen. These genes are classified into three groups, all of which decreased significantly in abundance under chitin supplementation: nosZ (p = 0.0091), nosZ atypical 1 (p = 0.0019) and nosZ atypical 2 (p = 0.0005) (Table 3, Fig. 3).
Figure 3.
Microbial N-cycle and chitin convergence and degradation pathway mediated through the addition of chitin. Enzymes involved in the N-cycle are indicated in blue. The metagenome reads are mapped towards these enzymes and the RPKG normalized counts representing the number of hits are illustrated by the arrow thickness in the figure. If there was a statistical effect of chitin addition, the enzyme is indicated with an asterisk (*), and a second arrow (orange) is drawn representing the normalized count of the genes related to the chitin-added samples. In addition, the chitin-degradation and its relation with the nitrogen cycle are illustrated in orange. Part of this figure was inspired on the paper of Levy-Booth et al.76,77.
In addition, there was an increase in bacterial (AOB) ammonium-oxidizing gene (amoA, p = 0.007) in the rhizosphere of lettuce grown in chitin-amended peat (Table 3, Fig. 3). In contrast, archaeal (AOA) ammonium oxidizing gene were almost absent in the microbiome grown in peat or in chitin-enriched peat substrate. This increase in ammonium-oxidizing genes was also confirmed by taxonomic data. Within the genera that significantly increased in the rhizosphere after chitin addition, two bacterial ammonium oxidizers (Nitrosospira, Nitrosomonas) were detected in the UMGAP analysis (Table 2). Especially Nitrosospira increased significantly (p = 0.01) from 0.59% ± 0.12 to 2.04% ± 0.33.
Discussion
Lettuce is worldwide one of the favorite green leafy vegetables29. Lettuce can be considered as a high risk food due to the potential presence of human pathogens, such as Escherichia coli O157:H7 and Salmonella enterica on leaves30–32. These zoonotic bacterial pathogens enter the agricultural environment via animal feces, which can contaminate the irrigation water33. Human consumption of vegetables contaminated with zoonotic pathogens may lead to disease outbreaks. In a previous study6, we showed that chitin addition to the peat substrate promoted lettuce growth and reduced the survival of S. enterica on lettuce leaves. In this study, reads classified as Salmonella were found in low abundance in the lettuce rhizosphere microbiome. These could either be translocated from the lettuce leave to the root, or were already present in the peat substrate. Chitinases and chitosanases enzymes are necessary for chitin degradation. They have been reported to promote plant growth by releasing N and Ca due to chitin breakdown and also to promote the plant’s natural defenses5,12. The chitin-amended peat substrate increases the abundance of bacteria and fungi related to chitin degradation in the lettuce rhizosphere6. The results of the present study are based on metagenome data, and thus avoid any PCR bias due to amplification of phylogenetic markers. The present results confirm those of Debode et al.6 as related to the overrepresentation of taxonomic groups related to chitin-degradation such as the fungal genus Mortierella (increase of 18-fold) and members of the bacterial genus Cellvibrio in the lettuce rhizosphere grown in chitin-amended peat substrate. Differences are observed as well, which can be attributed partly to PCR errors and primer biases within metabarcoding, and differences in taxonomical database (SILVA versus Uniprot) between metabarcoding and metagenomics34.
Bacterial genera belonging to the phyla Proteobacteria, Bacteroidetes, Actinobacteria and Firmicutes were highly abundant in the lettuce rhizosphere and have been described to be capable of chitinase production35,36. Especially soil-borne bacteria like Bacillus, Pseudomonas and Streptomyces and fungal members are known for their chitin-degrading activity18. The fungal member Mortierella increased dramatically in abundance relative to the other fungi in the lettuce rhizosphere under chitin-added cultivation6,28. This result is reflected in the chitinase genes abundances. Chitinases are either grouped in glycosyl hydrolase (GH) families GH18 or GH1937. GH18 chitinase genes are particularly bacterial, while thus far GH19 chitinases have only been found in fungal or Streptomyces species38. Chitin addition to the lettuce growing substrate only resulted in a significant increase in GH19 family chitinase, indicating that the chitin-effect is especially fungus-related.
The addition of chitin to the peat substrate also resulted in an increase in two glycosyl hydrolase families (GH46, GH75) that contain chitosanases. Independent on the chitin addition to the peat substrate, the abundance of deacetylase enzyme genes was high. These observations indicate that the production of chitosan and further breakdown to N-glucosamines in the lettuce rhizosphere is possible.
The chitin used in this study contains a high amount of fixed total nitrogen (7.1%/dry matter), in agreement with other sources of chitin39. Although most of this N is not plant-available a significant higher amount of NO3− was measured in the chitin-amended peat substrate after eight weeks of plant growth. Chitin therefore does not provide a direct source of nutrients to plants. It has to be, at least partially, degraded by microbial enzymes, which is decomposed into compounds (e.g., NH4+, NO3− and Ca) available for the plant and finally results in increased plant biomass.
Increased production of chitinases and deaminases leads to an increased degradation of chitin to GlcNAc and GlcN in the peat substrate, which can further be mineralized to CO2, CH4 and NH4+ 38. A high NH4+ concentration in soil might be toxic to plants40. High amounts of NH4+ in soils are microbially converted to NO3− by nitrification process41. NO3− is a preferred N source taken up by plants. The nitrification is carried out by a few bacterial and archaeal genera: ammonia oxidation is mediated by the ammonia-oxidizing bacteria (AOB), such as the Betaproteobacteria Nitrosomonas and Nitrosospira and by the ammonia-oxidizing archaea (AOA), such as the Thaumarchaeota, Nitrososphaera42,43; nitrite oxidation is carried out by nitrite oxidizing bacteria (NOB), including the Nitrospirae Nitrospira and the Alphaproteobacteria Nitrobacter44,45. Our results show an increase in relative abundance of the genera Nitrosospira and Nitrosomonas in the rhizosphere of lettuce cultured in chitin-amended peat substrate and an increase in amoA AOB genes, which encodes for ammonia monooygenase enzyme and transform ammonium to nitrite. The increase in ammonium oxidizing bacteria and amoA gene, can thus have resulted in the partial conversion of ammonium, produced from chitin-derived GlcNAc and GlcN.
NO3− and NO2− can also be converted by micro-organisms into N gasses that are no longer available to the plant as nutrient via denitrification. In this study, a decrease in denitrification genes was observed, which could have contributed to the increase in peat substrate nitrate level.
Besides the effect of chitin on the N cycle, there was an increase of the number of genes involved in iron acquisition, and more specifically siderophore production. Microorganisms make use of siderophores to take up iron from the environment46. Iron is necessary in many microbial and plant metabolic processes and maintains the cells in healthy state47. Iron is required for plant development and Fe deficiency disrupts many plant processes such as respiration or photosynthesis48,49. Siderophore production is also linked with an activation of the plant’s induced systemic resistance (ISR). Induced plant resistance by microorganisms, e.g. Pseudomonas aeruginosa, is often iron regulated and involves the production of ethylene and jasmonic acid50,51.
Based on the above observations, we hypothesize that the lettuce growth promotion by chitin addition is mediated through the following mechanisms. First, the addition of chitin to the peat substrate promotes and activates chitin-catabolic organisms in the lettuce rhizosphere. The production of chitinases and chitosanases leads to the degradation of chitin to plant available N, i.e. NO3− and NH4+. An increase in NH4+ concentration results in the proliferation and activation of ammonium-oxidizers, which converts ammonium to NO2− and NO3−. Thus, in the presence of chitin-degrading organisms, chitin can act as a nutrient source for plants, particularly N (Fig. 3); however an increase in plant-available Ca was also noted. Ca is important in regulating plant defense responses to pathogens52. Second, we observed a high abundance of genes that can deacetylate chitin to chitosan. Chitosan has proven to be an efficient plant growth promotor and can increase fruit yield of several plants, e.g. tomato, okra and oregano by an increase in N or polyphenol uptake53–55.
The increase in chitin-degraders and chitinase genes may result in the production of chitin oligomers. These oligomers are able to bind the plant’s CEBIP receptors5. In combination with a higher siderophore production, this may have resulted in the induction of the plant’s systemic resistance. Activation of the ISR can also affect pathogens like Botrytis cinerea and Colletotrichum coccodes, which infect the leaves of the plant51,56. The activation of the ISR might therefore have resulted in the reduction of S. enterica on the lettuce leaves through a response of chitinases and siderophores. The exact mechanism behind this reduction still needs to be unraveled further though.
To conclude, we showed that chitin addition influences the N cycle, especially nitrification by Nitrosospira bacteria; increases the number of chitin-degrading organisms; and might play a role in the chitinase and siderophore production. The increase in plant growth is probably due to an increased nutrient availability (NO3−, NH4+, and Ca) released as a result of the chitin breakdown by microbial enzymes. The high abundance of siderophore and chitinase genes might indicate an effect on the ISR of the plant.
Materials and Methods
Experimental setup
Pelletized butterhead lettuce seedlings (Lactuca sativa L. var. capitate “Alexandria”), germinated as described in Debode et al.6, were planted in a 100% peat based substrate medium pH-H2O of 5.5–6.0 (Universal Substrate LP2B, Peltracom, Belgium). Half of the plants were grown in peat substrate, the other half of the plants were grown in 2% chitin-enriched peat substrate. The chitin flakes, purified from crab shells, were obtained from BioLog Hepp Gmbh (lot: 90200705). All pots were placed in a growth chamber at 19 °C/12 °C day/night regime, 70–80% relative humidity and 14 h photoperiod. After 55 days, rhizosphere of six plants per treatment was sampled for total DNA extraction. The DNA was initially used for metabarcoding (Debode et al.)6 and for shotgun metagenomics (present study) to analyze the potential microbial functions and confirm metabarcoding results on the microbial community composition. Furthermore, samples of the peat substrate were taken for PLFA analysis (see Debode et al.)6 and chemical characterization, described in the next paragraph. In a previous study6, lettuce fresh weight was measured and the survival of S. enterica or E. coli on leaves was determined additionally.
Chemical characterization of growing medium and chitin
In addition to previous research6, we determined the chemical composition of the pure chitin flakes and evaluated differences in peat substrate chemical composition due to chitin addition. Total N (Dumas method, ISO 16634-1, Thermo Scientific flash 4000 N analyzer, Massachusetts, United States), organic matter (OM, EN 13039) and P, Ca, K, Mg, Fe, Al and Mn (ashing and digestion with 7 N HNO3− (p.a. 65%) and measured with an Agilent 5110 ICP-OES, Santa Clara, USA) concentrations were determined.
Readily available nutrients, electrical conductivity (EC) (EN 13038) and pH in H2O (EN 13037) in the pure chitin flakes and in the control and chitin amended peat were measured in a 1:5 soil to water (v/v) suspension. Water-extractable PO4-P, Cl, SO4 and NO3-N were measured with a Dionex DX-3000 IC ion chromatograph (Dionex, Sunnyvale, CA). Water soluble NH4-N was measured with a Skalar SAN++ flow analyzer (Skalar Analytical B.V, Breda, The Netherlands). Water-extractable C, Fe, Si, K, Ca, Mg and Na concentrations were measured with ICP-OES.
Rhizosphere sampling and DNA extraction
The lettuce rhizosphere was sampled according to the protocol of Lundberg et al.57. Briefly, loose soil was removed from the roots by molding and shaking. Roots were placed in a sterile 50 mL tube containing 25 mL phosphate buffer and shaken in a vortex at maximum speed for 15 s to release most of the rhizosphere soil from the roots. The solution is filtered through a 100 µm nylon mesh cell strainer into a new 50 mL tube to remove both plant parts and large sediment particles. The filtrate was then centrifuged for 15 min at 3,200 × g and 250 mg of the pellet was used for DNA extraction with the PowerSoil DNA isolation kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions.
Whole genome shotgun sequencing and sequence processing
In total, four samples were selected from each treatment (no chitin addition, 2% chitin addition) for whole genome shotgun metagenomics. From each sample, 1 µg DNA was sheared by ultrasonication using the covaris M20 at NxtGnt with a set length of 450 bp and a 1 min sonication (Ghent, Belgium). Further preparation of the shotgun libraries was done by Floodlight Genomics LLC (Knoxville, USA). Libraries were sequenced on an Illumina HiSeq 2500 lane (2 × 250 bp) by Macrogen, South-Korea. Demultiplexing of the shotgun data and removal of the barcodes was performed by the sequencing provider.
Data quality control, analyzed by fastqc (http://www.Bioinformatics.babraham.ac.uk/projects/fastqc), showed that TruSeq sequencing adaptors were still present in 10% of the reads. Adaptors were removed by cutadapt58. The forward and reverse reads were merged and quality filtered by the program PEAR v.0.9.859. Length cut-off values were set between 100 and 500 bp and a minimum overlap size of 100 bp and quality score threshold of 30 were used for all sequences. The raw sequence data is deposited at ENA (https://www.ebi.ac.uk/ena) under the project “ERP017180”.
Microbial taxonomy
To determine the taxonomic composition of the microbiomes, we used a new metagenomics pipeline: the Unipept Metagenomics Analysis Pipeline (UMGAP). Unipept60 is an open source web application designed for metaproteomics analysis that is now extended for the analysis of whole genome shotgun metagenome samples. UMGAP (version 2017) translated each merged sequence read into the peptide sequences of all six reading frames. Each of the amino-acid 9-mers in these peptides is taxonomically classified using a UniProt-backed index from the Uniprot Knowledgebase (version October 2017). The resulting taxa for each merged sequence read are filtered and aggregated into a single consensus taxon. All analysis of UMGAP were done making use of the reference settings. A comprehensive description of UMGAP’s workflow and a tutorial on the use of Unipept metagenomics is available at https://unipept.ugent.be/clidocs/casestudies/metagenomics.
The outcome of UMGAP OTU count tables was compared to that of MG-RAST v3.6 using RefSeq annotations, including eukaryote, bacteria, archaea and viruses22. MG-RAST analysis were done on the merged reads. First, data was preprocessed using SolexaQA to trim low-quality regions. Further on, data are dereplicated making use of a k-mer approach, followed by a removal of artificial duplicate reads by DRISEE (Duplicate Read Inferred Sequencing Error Estimation). Making use of vsearch, ribosomal RNA is identified based on a reduced RNA database (clusterd version of SILVA, Greengenes and RDP) and by a BLAT similarity search, taxonomy is annotated. More information on the pipeline can be found in the MG-RAST tutorial guideline22.
Functional analysis of metagenome data using MG-RAST
The metagenomes were compared to the subsystem database in MG-RAST to retrieve functional information. Count tables on subsystem level 1 and level 3 were created and statistically analyzed with egdeR (see statistical data-analysis).
To study the effect of chitin on the nitrogen cycle we matched the metagenome reads to the HMM profile of 11 nitrification and denitrification genes: amoA, amoB, napA, nrfA, narG, nirS, nirK, norB, nosZ, nifH, ureC. The HMM profiles of the genes were downloaded from the Functional Gene Repository database (FunGene version 8.3; http://fungene.cme.msu.edu/). The metagenome reads were first translated to protein sequences using Prodigal v2.6061. The open reading frames were queried for HMM matches using HMMsearch v3.1b262. From these matches, a count table was constructed containing the matched number per HMM per sample with an e-value of maximum 1e-10 and a coverage of 60%. The HMM matches were normalized through reads per kilobase per genome (RPKG) normalization: number of matched reads / HMM length / Number of genome equivalents62. The number of genome equivalents is calculated using MicrobeCensus v1.1.063. By applying RPKG normalization, we accounted for differences in library sizes and gene length biases to enable the comparison between samples and treatments.
In addition, we downloaded the profile HMMs of all carbohydrate enzymes listed on dbCAN64. From this set of HMM profiles, we selected glycoside hydrolase families involved in the chitin cycle: GH18, GH19 (chitinases), GH20 (N-acetylglucosaminidases), CE4, GH5, GH7, GH8 (chitin deacetylases), GH46, GH75 and GH80 (chitosanases). The translated metagenome reads obtained by prodigal are queried for HMM matches using HMMsearch. A count table was constructed from the number of matched reads having an e-value threshold of 1e-10 and at least 60% coverage. This count table was normalized by RPKG normalization.
Statistical data analysis
The differences in chemical composition of peat and chitin-amended peat substrate were checked using general linear models. First, the data was log-transformed, with the exception of pH as this is already a log-transformed variable. Second, we tested the effect of treatment through a general linear model with treatment as the main factor. Linearity, homogeneity of variances and normality were checked by plotting residuals vs fitted values, a QQ-plot of the standardized residuals and a scale-location plot.
To study the effect of treatment (peat substrate, chitin-amended peat substrate) on the taxonomic composition, we made use of the OTU tables derived from UMGAP. First, we applied a PERMANOVA analysis, implemented in the vegan package65. PERMANOVA was only applied after looking to homogeneity of variances between groups, using the betadisper function in R66. In addition, we studied shifts in the taxonomic genera. To filter out sequencing errors and technical artefacts, only taxonomic categories with a count of four per million in at least four samples were kept for analysis in the OTU table. This abundance (four per million) is based on previous research, while the four samples are chosen based on the number of replicates. In total, 30% of OTUs were removed, consisting primarily of singletons. Normalization of the filtered count table was based on the trimmed mean of m-values (TMM) in which we corrected for effective library size67. The counts were then modeled per taxonomic category using a negative binomial (NB) model with the main effect of treatment. The effective library size was used as an offset in the model for normalization purposes; hence the model parameters have an interpretation in terms of changes in relative abundance. Empirical Bayes estimation of the overdispersion parameters of the NB model were adopted using the quantile-adjusted conditional maximum likelihood method. Statistical tests were adopted on the appropriate contrasts of the model parameters. We adopted the Benjamini-Hochberg false discovery rate procedure to correct for multiple testing. After correction, only those genera with a p-value below the significance threshold of 0.05 were seen as statistical significant. All of these analyses were done using edgeR package, version 3.12.0 in R v3.4.266,68.
The effect of treatment (peat substrate, chitin-amended peat substrate) on the potential microbial functions was analyzed on the subsystem level 1 and level 3 tables retrieved from MG-RAST. Only functional categories with a count of four per million in at least four samples were kept for the analysis. Normalization, count modelling, empirical Bayes estimation and statistical tests were performed as described for the taxonomical classification.
To study differences in HMM matched number of reads after normalization for both nitrification and denitrification; and chitin-related genes, counts are modeled using a quasi-Poisson model with the main effect of treatment to correct for overdispersion. Generalized linear models (based on the quasi-poisson model) were applied on the appropriate contrasts of the model parameters, and the Benjamini-Hochberg false discovery rate procedure is used to correct for multiple testing. Genes were considered statistical significant if the p-value was below the significance thresholf of 0.05.
Supplementary information
Acknowledgements
This research was funded by the ILVO Genomics platform. Caroline De Tender and Bart Mesuere received a grant of the Research Foundation - Flanders (FWO) with application number 12S9418N and 12I5217N, respectively. Part of this work was executed within the Horti-BlueC project. This project has received funding from the Interreg 2 Seas programme 2014–2020 co-funded by the European Regional Development Fund under subsidy contract No 2S03-046. Both the Province of Antwerp and the Province of East-Flanders are co-funding ILVO for Horti-BlueC. We want to thank prof. Lieven Clement of the StatOmics group for the advice given on the statistical analysis on the paper. This work would also not have been possible without the laboratory assistance of Sarah Ommeslag, Pieter Cremelie, Cinzia Vanmalderghem and Saman Soltaninejad. We want to thank Miriam Levenson for proofreading and editing the manuscript. Publication number 6754 of the Netherlands Institute of Ecology (NIOO-KNAW).
Author Contributions
Supervision of the project: J.D. and E.E.K. Design of the project, the lettuce experiment and library preparation of the whole genome shotgun analysis were done by J.D. and C.D.T. Bio-informatical analysis of the data was done by C.D.T., B.M., F.V., A.H., P.D., E.E.K. and T.R. Chemical analysis of the peat substrate was done by BV. Statistical analysis was done by C.D.T. C.D.T. and B.M. wrote the first draft. Finalization of the draft was done by C.D.T., J.D. and E.E.K.
Data Availability
The raw sequence data of the shotgun metagenomics sequencing is deposited and freely available at ENA (https://www.ebi.ac.uk/ena) under the project “ERP017180”.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46106-x.
References
- 1.Gohel V, Singh A, Vimal M, Ashwini P, Chhatpar HS. Bioprospecting and antifungal potential of chitinolytic microorganisms. Afr. J. Biotechnol. 2006;5:54–72. [Google Scholar]
- 2.Cody RM, Davis ND, Lin J, Shaw D. Screening microorganisms for chitin hydrolysis and production of ethanol from amino sugars. Biomass. 1990;21:285–295. doi: 10.1016/0144-4565(90)90078-X. [DOI] [Google Scholar]
- 3.Zhao Y, Park RD, Muzzarelli RA. Chitin deacetylases: properties and applications. Mar. Drugs. 2010;8:24–46. doi: 10.3390/md8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragunde H, Biarnés X, Planas A. Substrate recognition and specificity of chitin deacetylases and related family 4 carbohydrate esterases. Int. J. Mol. Sci. 2017;19:412. doi: 10.3390/ijms19020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp RG. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy. 2013;3:757–793. doi: 10.3390/agronomy3040757. [DOI] [Google Scholar]
- 6.Debode J, et al. Chitin mixed in potting soil alters lettuce growth, the survival of zoonotic bacteria on the leaves and associated rhizosphere microbiology. Front. Microbiol. 2016;7:565. doi: 10.3389/fmicb.2016.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait Barka E, Eullaffroy P, Clément C, Vernet G. Chitosan improves development and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004;22:608–614. doi: 10.1007/s00299-003-0733-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Chen F, Wang X, Rajapakse NC. Effect of chitosan on the biological properties of sweet basel (Ocimum basilicum L.) J. Agric. Food Chem. 2005;53:3696–3701. doi: 10.1021/jf0480804. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Kim YH, Kim SB. Changes in the respiration, growth and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. HortScience. 2005;40:1333–1335. doi: 10.21273/HORTSCI.40.5.1333. [DOI] [Google Scholar]
- 10.Algam SAE, et al. Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J. Plant Path. 2010;92:593–600. [Google Scholar]
- 11.Yen MT, Mau JL. Selected physical properties of chitin prepared from shiitake stipes. Food Sci. Technol. 2007;40:558–563. [Google Scholar]
- 12.Boβelmann F, Romano P, Fabritius H, Raabe D, Epple M. The composition of the exoskeleton of two crustacean: the American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim. Acta. 2007;463:65–683. doi: 10.1016/j.tca.2007.07.018. [DOI] [Google Scholar]
- 13.Chandrkrachang S. The applications of chitin in agriculture in Thailand. Adv. Chitin. Sci. 2002;5:458–462. [Google Scholar]
- 14.Nahar SJ, Kazuhiko S, Haque SM. Effect of polysaccharides including elicitors on organogenesis in protocorm-like body of Cymbidium insigne in vitro. J. Agric. Sci. Technol. 2012;2:1029–1033. [Google Scholar]
- 15.Manjula K, Podile AR. Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Can. J. Microbiol. 2001;47:618–625. doi: 10.1139/w01-057. [DOI] [PubMed] [Google Scholar]
- 16.Brzezinska MS, Jankiewics U, Burkowska A, Walczak M. Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 2014;68:71–81. doi: 10.1007/s00284-013-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi N, et al. Traditional and new soil amendments reduce survival and reproduction of potato cyst nematodes, except for biochar. Appl. Soil Ecol. 2016;107:191–204. doi: 10.1016/j.apsoil.2016.06.006. [DOI] [Google Scholar]
- 18.Subbanna ARNS, Rajasekhara H, Stanley J, Mishra KK, Pattanayak A. Pesticidal prospectives of chitinolytic bacteria in agricultural pest management. Soil Biol. Bioch. 2018;116:52–66. doi: 10.1016/j.soilbio.2017.09.019. [DOI] [Google Scholar]
- 19.Kaku H, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA. 2006;103:11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinya T, et al. Functional characterization of CEBiP and CERK1 homologs in Arabidopsis and rice reveals the presence of different chitin receptor systems in plants. Plant Cell Physiol. 2012;53:1696–1706. doi: 10.1093/pcp/pcs113. [DOI] [PubMed] [Google Scholar]
- 21.Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer F, et al. The metagenomics RAST server – a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell A, et al. EBI metagenomics in 2016 – an expanding and evolving resource for the analysis and archiving of metagenomics data. Nucleic Acids Res. 2015;44:D595–603. doi: 10.1093/nar/gkv1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell A, et al. EBI metagenomics in 2017: enriching the analysis of microbial communities, from sequence reads to assemblies. Nucleic Acids Res. 2017;46:D726–D735. doi: 10.1093/nar/gkx967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolton M, et al. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011;77:4924–4930. doi: 10.1128/AEM.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson M, Habiger J. Characterization and identification of productivity-associated rhizobacteria in wheat. Appl. Environ. Microbiol. 2012;78:4434–4446. doi: 10.1128/AEM.07466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez C, Ratering S, Kramer I, Schnell S. Cellvibrio diazotrophicus sp. Nov., a nitrogen-fixing bacteria isolated from the rhizosphere of salt meadow plants and emended description of the genus Cellvirbrio. Int. J. Syst. Evol. Microbiol. 2014;64:481–486. doi: 10.1099/ijs.0.054817-0. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Zhao Y, Oh KT, Nguyen VN, Park RD. Enzymatic deacetylation of chitin by extracellular chitin deacetylase from a newly screened Mortierella sp. DY-52. Microbiol. Biotechnol. 2008;18:759–766. [PubMed] [Google Scholar]
- 29.Department agriculture and fisheries, Document “Gehele land en tuinbouw”, https://lv.vlaanderen.be/nl/voorlichting-info/feiten-cijfers/landbouwcijfers#overzichtsrapporten (2016).
- 30.Ward LR, et al. Collaborative investigation of an outbreak of Salmonella enterica serotype Newport in England and Wales in 2001 associated with ready-to-eat salad vegetables. Commun. Dis. Public Health. 2002;5:301–304. [PubMed] [Google Scholar]
- 31.Horby PW, et al. A national outbreak of multi-resistant Salmonella enterica serovar Typhimurium definitive phage type (DT) 104 associated with consumption of lettuce. Epidemiol. Infect. 2003;130:169–178. doi: 10.1017/S0950268802008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welinder-Olsson C, et al. EHEC outbreak among staff at a children’s hospital-use of PCR for verocytotoxin detection and PFGE for epidemiological investigation. Epidemiol. Infect. 2004;132:43–49. doi: 10.1017/S0950268803001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barak JD, Schroeder BK. Interrelationships of food safety and plant pathology: the life cycle of human pathogens on plants. Annu. Rev. Phytopathol. 2012;50:241–266. doi: 10.1146/annurev-phyto-081211-172936. [DOI] [PubMed] [Google Scholar]
- 34.Zepeda Mendoza ML, Sicheritz-Pontén T, Gilbert MTP. Environmental genes and genomes: understanding the differences and challenges in the approaches and software for their analyses. Brief Bioinform. 2015;16:745–758. doi: 10.1093/bib/bbv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoushab F, Yamabhai M. Chitin research revisited. Mar. Drugs. 2010;8:1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler AJ, et al. short-chain chitin oligomers: promoters of plant growth. Mar Drugs. 2017;15:40. doi: 10.3390/md15020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beier S, Bertilsson S. Bacterial chitin degradation – mechanisms and ecophysiological strategies. Front. Microbiol. 2013;4:149. doi: 10.3389/fmicb.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramírez MG, Rodríguez AT, Alfonso L, Peniche C. Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnología Aplicada. 2010;27:270–276. [Google Scholar]
- 40.Van der Eerden LJM. Toxicity of ammonia to plants. Agric., Ecosyst. Environ. 1982;7:223–235. doi: 10.1016/0304-1131(82)90015-7. [DOI] [Google Scholar]
- 41.Prosser, J. J. Nitrification in Nitrogen in Soils, Reference module in earth systems and environmental sciences (ed. Elias, S.A.) 31–39 (Reference Module, 2005).
- 42.Lourenço, K. S., et al. Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front. Microbiol. 9, 10.3389/fmicb.2018.00674 (2018). [DOI] [PMC free article] [PubMed]
- 43.Soares, J. R. et al. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Scientific Reports6, 10.1038/srep30349 (2016). [DOI] [PMC free article] [PubMed]
- 44.Prosser JI. Autotrophic nitrification in bacteria. Adv. Microb. Physiol. 1989;30:125–181. doi: 10.1016/S0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 45.Leininger S, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed E, Holmström SJM. Siderophores in environmental research: roles and application. Microb. Biotechnol. 2014;7:196–208. doi: 10.1111/1751-7915.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crichton, R. Solution chemistry of iron in biological media. In Iron metabolism: from molecular mechanisms to clinical consequences (ed. Crichton, R.) 1–15 (John Wiley & Sons, 2002).
- 48.Guerinot, M. Iron in Cell biology of metals and nutrients. Plant cell monographs, vol 17. (eds Hell, R. & Mendel, R.-R.) 75–94 (Springer, 2010).
- 49.Zuo Y, Zhang F. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil. 2011;339:83–95. doi: 10.1007/s11104-010-0566-0. [DOI] [Google Scholar]
- 50.Aznar A, Dellagi A. New insights into the role of siderophores as triggers of plant immunity: what can we learn from animals? J. Exp. Bot. 2015;66:3001–3010. doi: 10.1093/jxb/erv155. [DOI] [PubMed] [Google Scholar]
- 51.De Meyer G, Höfte M. Salicylic acid produced by the rhizobacteria P. aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathol. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, L., Liqun, D. & Poovaiah, B. W. Plant Signal Behav 9(11) e973818 (2014). [DOI] [PMC free article] [PubMed]
- 53.Yin H, Frette XC, Christensen LP, Grevsen K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. Hirtum) J. Agric. Food Chem. 2012;60:136–143. doi: 10.1021/jf204376j. [DOI] [PubMed] [Google Scholar]
- 54.Mondal MMA, et al. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 2012;6:918–921. [Google Scholar]
- 55.Sathiyabama M, Akila G, Einstein Charles R. Chitosan-induced defence responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Prot. 2014;47:1777–1787. doi: 10.1080/03235408.2013.858423. [DOI] [Google Scholar]
- 56.Sang MK, Kim JG, Kim KD. Biocontrol activity and induction of systemic resistance in pepper by compost water extracts against Phytophthora capsici. Phytopath. 2010;100:774–783. doi: 10.1094/PHYTO-100-8-0774. [DOI] [PubMed] [Google Scholar]
- 57.Lundberg DS, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 59.Zhang L, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina paired-end read merger. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mesuere B, et al. Unipept: tryptic peptide-based biodiversity analysis of metaproteome samples. J. Proteome Res. 2012;11:5773–5780. doi: 10.1021/pr300576s. [DOI] [PubMed] [Google Scholar]
- 61.Hyatt D, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nayfach S, Pollard KS. Average genome size estimation improves comparative metagenomics and sheds light on the functional ecology of the human microbiome. Genome Biol. 2015;16:51. doi: 10.1186/s13059-015-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Y, et al. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–W451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oksanen J, et al. Vegan: community ecology pacage. R package version. 2010;2:0–10. [Google Scholar]
- 66.R Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2015).
- 67.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. PNAS. 2011;108:15892–15897. doi: 10.1073/pnas.1107196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kielak AM, Barreto CC, Kowalchuk GA, van Veen JA, Kuramae EE. The ecology of Acidobacteria: moving beyond genes and genomes. Front. Microbiol. 2016;7:744. doi: 10.3389/fmicb.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Boer W, Wagenaar AW, Gunnewiek PJAK, Van Veen JA. In vitro suppression of fungi caused by combinations of apparently non-antagonistic soil bacteria. FEMS Microbiol. Ecol. 2007;59:177–185. doi: 10.1111/j.1574-6941.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 72.Head IM, Hiorns WD, Embley TM, McCarthy AJ, Saunders JR. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J. Gen. Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 73.Utåker JB, Bakken L, Jiang QQ, Nes IF. Phylogenetic analysis of seven new isolates of ammonia-oxidizing bacteria based on 16S rRNA gene sequences. Syst. Appl. Microbiol. 1995;18:549–559. doi: 10.1016/S0723-2020(11)80415-7. [DOI] [Google Scholar]
- 74.Boon B, Laudelout H. Kinetics of nitrite oxidation by Nitrobacter winogradskyi. Biochem. J. 1962;85:440–447. doi: 10.1042/bj0850440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tagawa M, Tamaki H, Manome A, Koyama O, Kamagata Y. Isoaltion and characterization of antagonistic fungi against potato scab pathogens from potato field soils. FEMS Microbiol. Lett. 2010;305:136–142. doi: 10.1111/j.1574-6968.2010.01928.x. [DOI] [PubMed] [Google Scholar]
- 76.Donderski W, Swionetk Brzezinska M. The utilization of N-acetylglucosamine and chitin as sources of carbon and nitrogen by planktonic and benthic bacteria in Lake Jeziorak. Pl. J. Environ. Stud. 2003;6:685–692. [Google Scholar]
- 77.Levy-Booth DJ, Prescott CE, Grayston SJ. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014;75:11–25. doi: 10.1016/j.soilbio.2014.03.021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data of the shotgun metagenomics sequencing is deposited and freely available at ENA (https://www.ebi.ac.uk/ena) under the project “ERP017180”.