Abstract
Alternative polyadenylation (APA) is how genes choose different sites for 3′ end formation for mRNAs during transcription. APA often occurs in a tissue‐ or developmental stage‐specific manner that can significantly affect gene activity by changing the protein product generated, the stability of the transcript, its localization within the cell, or its translatability. Despite the important regulatory effects that APA has on tissue‐specific gene expression, only a few examples have been characterized mechanistically. In this 2018 update to our 2010 review, we examine mechanisms for the control of APA and update our understanding of the older mechanisms since 2010. We once postulated the existence of tissue‐specific factors in APA. However, while a few tissue‐specific polyadenylation factors are known, the emerging conclusion is that the majority of APA is accomplished by altering levels of core polyadenylation proteins. Examples of those core proteins include CSTF2, CPSF1, and subunits of mammalian cleavage factor I. But despite support for these mechanisms, no one has yet documented any of these proteins changing in either a tissue‐specific or developmental manner. Given the profound effect that APA can have on gene expression and human health, improved understanding of tissue‐specific APA could lead to numerous advances in gene activity control.
This article is categorized under:
RNA Processing > 3′ End Processing
RNA in Disease and Development > RNA in Development
Keywords: alternative polyadenylation, brain, CPSF, CstF, flowering control, immune system, polyadenylation, spermatogenesis, testis
1. INTRODUCTION
The goal of this Focus article is to describe many of the known mechanisms of regulated nuclear mRNA polyadenylation in specific metazoan tissues: tissue‐specific alternative polyadenylation. With that goal in mind, we will not address (except in passing) cytoplasmic polyadenylation, which has been reviewed elsewhere, included in this WIRES RNA series (Charlesworth, Meijer, & de Moor, 2013; De Conti, Baralle, & Buratti, 2017). Nor will we address polyadenylation in yeast and other developmentally simple organisms since they are not generally regarded as having tissues. What we will cover is alternative polyadenylation—the meaning of which has evolved since the advent of bioinformatic analyses of transcriptomes. Alternative polyadenylation has been frequently visited in the literature since 2010 when our earlier review was published, even earning its own acronym: APA. The purpose of this review is to highlight new findings on APA with a focus on tissue‐specific mechanisms, and to bring up to date our earlier review from 2010 (MacDonald & McMahon, 2010). Where possible in this article, we will identify tissue‐specific proteins that are known to be involved with the mechanisms of APA. By identifying these components in tissue‐specific APA, we will have a better opportunity to learn its principles. The advent of high‐throughput RNA‐sequencing techniques has brought new perspectives to tissue‐specific APA, so those studies will be highlighted in this review as well.
Note to the reader: we have used the mouse gene naming conventions throughout this Focus article because the majority of the genetic work, including gene knockouts, has been done in this species.
2. POLYADENYLATION AND ALTERNATIVE POLYADENYLATION
Messenger RNA polyadenylation—the cleavage of a pre‐mRNA at its 3′ end, followed by the addition of 200–250 adenosine residues—is essential for the transcriptional termination, nuclear export, stability, translation, and quality control of the mRNA. To learn more about polyadenylation, read any of the excellent reviews on its mechanisms (here are a few to get you started, Curinha, Oliveira Braz, Pereira‐Castro, Cruz, & Moreira, 2014; Di Giammartino, Nishida, & Manley, 2011; Lutz & Moreira, 2011; Tian & Manley, 2017). The choice of a transcript's cleavage/polyadenylation site occurs in the nucleus and is an important regulated process in gene expression. Changes in the site choice, APA, can lead to changes in transcript stability, localization, splicing pattern, translation, and the protein product generated from the gene. At least 70% of mammalian transcripts are alternatively polyadenylated (Derti et al., 2012; Shi, 2012; R. Wang, Zheng, Yehia, & Tian, 2018). Furthermore, a meta‐analysis of alternative transcription start sites, splicing, and cleavage/polyadenylation confirmed that the majority (65% or more) of alternative transcripts in different human cell types were due to APA or transcription start sites, not to alternative splicing (Reyes & Huber, 2018). Surprisingly, relatively few mechanisms controlling APA have been elucidated, despite its predominant role in gene expression and human health (Chang, Yeh, & Yong, 2017; Ogorodnikov, Kargapolova, & Danckwardt, 2016).
2.1. Alternative polyadenylation
The definition of APA has evolved since its discovery. In the early days, it was assumed that the first polyadenylation site identified for a given transcript was the Platonic default site, and that any subsequently identified sites were alternatives. Presumably, the thinking continued, there was also a default polyadenylation machinery (usually conceived of as the machinery in HeLa cells), and tissue‐specific auxiliary proteins resulted in the choice of alternative sites. As we will show here, this view is too simple. For example, mRNAs in individual tissues will display use of more than one polyadenylation site, although the ratios of the sites may vary from tissue to tissue or cell to cell. Therefore, for the purposes of this review, we will define tissue‐specific APA as predominant use of one or more sites in a tissue that requires augmentation or modification of the core polyadenylation machinery. We are excluding for this discussion APA in pathological states (e.g., cancer, Lin et al., 2012; Masamha et al., 2014; Xia et al., 2014). Although mechanisms in these states will likely shed light on nonpathological APA in normal tissues, their general functions in normal cellular metabolism have not yet been established.
2.2. Core polyadenylation proteins recognize signals in the pre‐mRNA
Several signals in the pre‐mRNA direct the cleavage and polyadenylation (hereinafter “polyadenylation”) machinery to a site. The sequence AAUAAA (or something similar) is called the polyadenylation signal (PAS) and is generally found 15–30 bases upstream of the site of cleavage (MacDonald & Redondo, 2002; Tian & Graber, 2012). A second signal, downstream of the cleavage site is the downstream sequence element (DSE), which can be U‐ or G/U‐rich. Other sequences upstream or downstream of the cleavage site also appear to modulate polyadenylation (Figure 1); in some cases, these other sequences may be bound by tissue‐specific factors.
Figure 1.
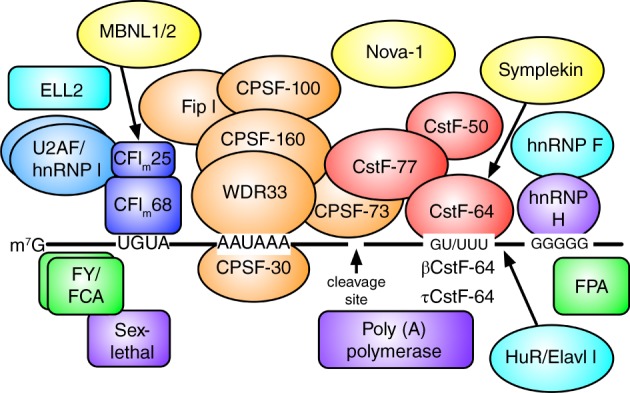
Core and auxiliary proteins involved in tissue‐specific alternative polyadenylation. The pre‐mRNA (black line) consists of upstream sequence elements (UGUA), the polyadenylation signal (AAUAAA), a cleavage site (arrow), the downstream sequence element (GU/UUU), and the downstream G‐rich element (GGGGG). The core polyadenylation proteins consist of the CPSF proteins, the CstF proteins, and CFIm (see the text for details), and the template‐independent poly(A) polymerase is indicated. Auxiliary (U2AF, hnRNP F, hnRNP H, hnRNP I) and tissue‐specific (Nova‐1, βCstF‐64, τCstF‐64, ELL2, MBNL1/2, HuR/Elavl1, sex‐lethal, FPA, FY, and FCA) proteins are indicated. It is not entirely clear whether symplekin is a core or an auxiliary polyadenylation protein, but it interacts directly with CstF‐64
Each pre‐mRNA signal is bound by a specific component of the polyadenylation machinery. The cleavage and polyadenylation specificity factor (CPSF, consisting of six different polypeptides of 160, 100, 73, and 30 kDa, Wdr33, and Fip1) and the cleavage stimulation factor (CstF, consisting of three proteins of 77, 64, and 50 kDa) bind to the AAUAAA and U‐ or G/U‐rich elements, respectively. RNA‐binding domains within CPSF‐30 and Wdr33 bind cooperatively to the PAS and CstF‐64 binds to the DSE (Grozdanov, Masoumzadeh, Latham, & MacDonald, 2018). Additional cleavage factors (CFIm and CFIIm) interact with UGUA elements upstream of the PAS. The affinity of CPSF and CstF for the pre‐mRNA determines whether a polyadenylation site is considered to be weak or strong. This affinity depends on the exact sequences of the PAS, DSE, and auxiliary elements, and responds to changes in the amounts of the core polyadenylation proteins. Stability of these complexes can also be modified by interactions with other factors, and probably by protein modification, as well. Following cleavage (by CPSF‐73), the poly(A) polymerase adds as many as 250 adenosine residues.
It was previously believed that CPSF‐160 bound to and recognized the AAUAAA PAS. However, independent findings from two laboratories identified Wdr33 and CPSF‐30 as the actual recognition factors (Chan et al., 2014; Schonemann et al., 2014). This increases the overall importance of Wdr33, which was previously thought to be a minor player (see below). Instead, structural studies have shown a role for CPSF‐160 in coordinating the assembly of the other CPSF components to facilitate AAUAAA binding (Clerici, Faini, Aebersold, & Jinek, 2017; Clerici, Faini, Muckenfuss, Aebersold, & Jinek, 2018; Y. Sun et al., 2018).
2.3. Mechanisms of alternative polyadenylation
To our knowledge, only a small number of mechanisms of APA have been fully characterized with direct evidence leading to a change in polyadenylation site for that tissue/developmental stage (see the discussions below of CstF‐64 in B cells, FY in Arabidopsis, and Nova in brain). The remaining systems are still mysterious, although there are strong candidate proteins in each. One can imagine additional mechanisms for APA, including modification of basal polyadenylation machinery (e.g., by SUMOylation), blockade of more optimal binding sites by RNA binding proteins, and others. However, the number of validated tissue‐specific APA mechanisms remains very small.
Several studies in which key polyadenylation proteins were knocked down have revealed significant roles for each of them. For example, subunits of mammalian cleavage factor I (CFIm) have been shown to play important roles in APA, favoring use of distal polyadenylation sites (Li et al., 2015; Martin, Gruber, Keller, & Zavolan, 2012; Y. Zhu et al., 2018). As such, CFIm influences neurite growth (Fukumitsu, Soumiya, & Furukawa, 2012) and may be involved in glioblastoma (Masamha et al., 2014). Similarly, Fip1 can regulate APA by binding to elements upstream of the PAS (Lackford et al., 2014; Li et al., 2015). This upholds the belief that levels of core polyadenylation proteins can be major regulators in APA.
3. ABUNDANT ALTERNATIVE POLYADENYLATION IN THE BRAIN SUPPORTS NEURAL FUNCTION
3.1. Splicing and polyadenylation control CGRP expression
The brain and peripheral neurons express a larger proportion of the genome than any other tissue, resulting in a high level of mRNA diversity. Most of this diversity is attributed to alternative splicing, transcription start sites, and polyadenylation. In fact, the earliest described case of alternative processing of a mammalian gene was the neuronally expressed calcitonin gene‐related peptide alpha protein (CGRP). CGRP is expressed from the Calca gene, which produces calcitonin in nonneurological tissues. The earliest reports proposed that the CGRP pattern of processing was caused by APA (Amara, Evans, & Rosenfeld, 1984), but subsequent studies determined that neuronal processing of CGRP from calcitonin was a complex gallimaufry of alternative splicing and polyadenylation that still holds secrets (Zhou, Baraniak, & Lou, 2007).
Like CGRP, HuR (gene symbol Elavl1) is alternatively polyadenylated in neurons leading to predominance of an isoform with a much longer 3′ untranslated region (UTR); suppression of the shorter isoform might involve RNA binding of several other members of the Hu protein family (Mansfield & Keene, 2012). Though not always mechanistic, several high‐throughput surveys of mRNAs in neural tissues have revealed that APA in brain results in longer 3′ UTRs than other tissues (Fontes et al., 2017; Miura, Shenker, Andreu‐Agullo, Westholm, & Lai, 2013). In neurons, longer 3′ UTRs often result in selective translation of those mRNA isoforms in dendrites and axons (Ainsley, Drane, Jacobs, Kittelberger, & Reijmers, 2014; Terenzio et al., 2018) or reveal distal last exons that can localize transcripts to neurites (Taliaferro et al., 2016). We also believe that techniques like c‐Tag‐PAPERCLIP, in which epitope tags can be attached to RNA‐binding proteins of interest in specific mouse cell types (Hwang et al., 2017), will increase the understanding of APA in brain and other tissues.
3.2. Three forms of CstF‐64 are expressed in brain
Studies from our laboratory have shown that CstF‐64 (which is expressed in all tissues) and two variants, τCstF‐64 and βCstF‐64 (Figure 2), are expressed in brain. τCstF‐64 (gene name Cstf2t) is a retrotransposed paralog of CstF‐64 that is expressed at highest levels in testis, but is also detected in brain and immune cells (Wallace et al., 1999; Wallace, Denison, Attaya, & MacDonald, 2004). τCstF‐64 is found at varying levels in more tissues than testis and brain, including heart, liver (Yao et al., 2013), and embryonic stem cells (Youngblood, Grozdanov, & MacDonald, 2014; Youngblood & MacDonald, 2014). Targeted deletion of Cstf2t did not result in obvious immunological problems (Dass et al., 2007), but its loss increased performance of female but not male mice in tasks involving spatial learning and memory (Harris et al., 2016). This suggests that, while necessary for male reproduction, the presence of τCstF‐64 is deleterious to female cognition.
Figure 2.
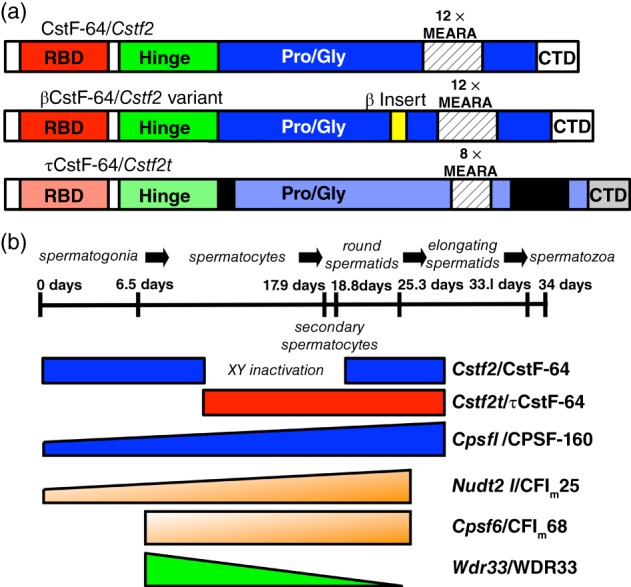
Proteins involved in testis and brain alternative polyadenylation. (a) Three forms of CstF‐64 are involved in polyadenylation. βCstF‐64 (middle) is an alternatively spliced variant of CstF‐64 (top) that is expressed in vertebrate neurons. τCstF‐64 (bottom) is an autosomal paralog of CstF‐64 that is expressed in male germ cells and other tissues in mammals. The RNA‐binding domain (RBD), CstF‐77 interaction domain (Hinge), proline‐ and glycine‐rich domain (Pro/Gly), MEARA repeats, and C‐terminal domain (CTD) are indicated. (b) Expression patterns of alternative polyadenylation proteins during spermatogenesis. Top figure shows a timeline of spermatogenesis in mice (~34 days). Cells that undergo mitotic division (spermatogonia), meiosis (spermatocytes), and postmeiotic development (round or elongating spermatids, spermatozoa) are indicated. Expression periods for CstF‐64, τCstF‐64, CPSF‐160, CFIm25, CFIm68, and Wdr33/WDR33 are shown in the bottom figure. XY inactivation indicates the period of male sex chromosome inactivation
βCstF‐64, on the other hand, seems more likely to play an essential role in brain‐specific polyadenylation. βCstF‐64 (the Greek letter β was chosen to designate its expression in brain) is a neuron‐specific splice variant that results in addition of 49 amino acids within the proline/glycine‐rich domain of CstF‐64 (Shankarling, Coates, Dass, & Macdonald, 2009). βCstF‐64 is expressed in all regions of the brain and in peripheral nerves, suggesting it has a ubiquitous function in the nervous system. Furthermore, it is present in all vertebrates, suggesting that its function is ancient and important. Finally, ectopic expression of βCstF‐64 in somatic cells stimulates expression of luciferase reporter mRNAs with neuronal polyadenylation sites (Shankarling & MacDonald, 2013), demonstrating its probable involvement in mRNA processing. However, a direct role for βCstF‐64 in control of APA has not yet been demonstrated.
Competition between CstF‐64 and hnRNP H (see Figure 1) alters both polyadenylation and splicing in acetylcholinesterase and other transcripts in human neuron‐like cells (Nazim et al., 2016). Similarly, HuR/Elavl1 competes with CstF‐64 for binding to U‐rich sequences (H. Zhu, Zhou, Hasman, & Lou, 2007), which may have consequences in neuronal cells (K. Sun et al., 2018). While HuR is ubiquitously expressed, expression of its specialized forms in brain suggests that it might be a candidate as a tissue‐specific polyadenylation factor. And revealingly, copy number variations (either duplication or deletion) of the NUDT21 gene that encodes CFIm25 results in intellectual disability due to APA of the MeCP2 mRNA (Gennarino et al., 2015).
3.3. Nova controls alternative polyadenylation as well as alternative splicing
The neuro‐oncological ventral antigen (Nova) proteins are a family of RNA‐binding proteins that were found associated with paraneoplastic opsoclonus myoclonus ataxia, a neurological disorder that is also associated with neuroblastoma. Analyses of sites of Nova‐1 (gene name: Nova1) binding in brain RNA demonstrated a strong association of Nova‐1 with intronic regions near alternatively spliced exons, as well as changes in splice patterns for key genes (Ule et al., 2003). Subsequent analyses discovered an additional association of Nova‐1 with 3′ UTRs of pre‐mRNAs near polyadenylation sites (Licatalosi et al., 2008). Inclusion of those sites in the 3′ end of reporter genes decreased polyadenylation in the presence of Nova‐1. Licatalosi et al. (op. cit.) speculate that Nova‐1 could suppress polyadenylation at some sites by binding adjacent to CPSF or CstF elements in the pre‐mRNA, or could enhance polyadenylation at other sites by antagonizing binding of unknown “auxiliary factors.” A third possibility that the authors did not enumerate was that Nova might attract auxiliary splicing factors to polyadenylation sites, and influence site selection in that way. Using a tissue‐specific crosslinking and immunoprecipitation technique (cTag‐PAPERCLIP, mentioned above), Hwang et al. (2017) better determined roles of NOVA2 and PTBP2 in APA in neurons and microglia. Though the mechanism is still undetermined, the Nova proteins have been associated with neuronal control of APA.
4. CSTF‐64 CONTROLS ALTERNATIVE POLYADENYLATION IN THE IMMUNE SYSTEM
The immune system uses myriad mechanisms to diversify gene expression, and APA is not to be left out. Immunoglobulin (Ig) is expressed in two forms: early B cells make a membrane‐bound Ig, while later stage plasma cells make a secreted form. The difference between these two protein isoforms is a short membrane‐spanning domain exhibited in B cells but not in plasma cells. This protein domain switch is decided by which of two polyadenylation sites is selected: if the upstream site is selected, then the secreted form is produced; if the downstream site is selected, then the membrane‐bound form is produced. Increasing the levels of CstF‐64 in a model B cell line is sufficient to switch Ig from the membrane‐bound to the secreted form (Takagaki, Seipelt, Peterson, & Manley, 1996). These and similar experiments have led to models in which amounts and activities of proteins of the core polyadenylation machinery (not just CstF‐64, although CstF‐64 is a common culprit) regulate poly(A) site selection. Subsequent experiments showed that there is even more complexity in Ig switching (involving splicing, hnRNP binding, RNA polymerase loading, and transcriptional elongation) than was first thought (Martincic, Alkan, Cheatle, Borghesi, & Milcarek, 2009). For example, high throughput studies demonstrated that intronic polyadenylation diversifies gene expression in immune cells (Singh et al., 2018) and reveals regulatory elements in 3′ UTRs (Yoon, Hsu, Im, & Brem, 2012). APA in other tissues likely shares this complexity, as well. In a parallel to Ig expression in B cells, the CPSF‐160 (CPSF1) polyadenylation protein can influence the ratio of soluble and membrane‐bound forms of the interleukin 7 receptor by suppressing inclusion of an exon encoding the transmembrane domain in T‐cells (Evsyukova, Bradrick, Gregory, & Garcia‐Blanco, 2013).
5. MULTIPLE TESTIS‐SPECIFIC POLYADENYLATION PROTEINS ARE EXPRESSED DURING SPERMATOGENESIS
5.1. Patterns of polyadenylation are different in male germ cells
There are many reports of mRNAs that use unique polyadenylation sites in mammalian testis (Li et al., 2016; D. Liu et al., 2007; Zhang, Lee, & Tian, 2005). In addition to APA leading to 3′ UTRs of different lengths, APA sites can lead to germ cell‐specific protein isoforms, and even control long interspersed element (LINE‐1) activity (Perepelitsa‐Belancio & Deininger, 2003). Testis‐expressed polyadenylation sites display a lower incidence of AAUAAA, contain unique upstream and DSEs, and have shorter 3′ UTRs (Ji, Lee, Pan, Jiang, & Tian, 2009; D. Liu et al., 2007). Additionally, polyadenylation regions from testis‐enriched polyadenylation sites are used inefficiently in somatic cells (McMahon, Hirsch, & MacDonald, 2006). These observations suggest that there are testis‐specific mechanisms supporting nuclear polyadenylation in male germ cells.
Alternative splicing also impacts APA and has important effects in male germ cells, especially during the transition through meiosis (Schmid et al., 2013; Zagore et al., 2015). In male germ cells, cytoplasmic chromatoid bodies are centers of multiple RNA metabolic processes. Among other things, these structures seem to be sites for nonsense‐mediated mRNA decay, especially of mRNAs bearing long 3′ UTRs (Bao et al., 2016; Fanourgakis, Lesche, Akpinar, Dahl, & Jessberger, 2016; Kashiwabara et al., 2018; Shum et al., 2016), and thus are important for catabolism of products of APA in testis (MacDonald & Grozdanov, 2017).
5.2. CstF‐64 and τCstF‐64 are expressed during spermatogenesis
The mRNAs encoding CstF‐64 (gene name: Cstf2) and CPSF‐160 (gene name: Cpsf1) are overexpressed in postmeiotic male germ cells (Dass, Attaya, Wallace, & MacDonald, 2001). Overexpression of CstF‐64 could influence polyadenylation site choice in some germ cell genes (Chennathukuzhi, Lefrancois, Morales, Syed, & Hecht, 2001) as it does Ig in immune cells. However, CstF‐64 is not expressed during meiosis because of male sex chromosome inactivation (Figure 2). During the critical period of meiosis in males (which lasts almost 9 days in mice and considerably longer in humans), the X and Y chromosomes are inactivated, so the X‐linked Cstf2 is not available to participate in polyadenylation. Thus, researchers in our laboratory discovered a variant of CstF‐64 in male germ cells that we named τCstF‐64 (Dass et al., 2001) (the “τ” was chosen to remind us of its expression in testis, although it is also expressed in brain, immune cells, and elsewhere). τCstF‐64 (gene name: Cstf2t) is an autosomal paralog of Cstf2 that likely resulted from retrotransposition of the CstF‐64 mRNA (P. J. Wang, 2004) around the time mammals diverged from archosaurs (B. Dass and C.C.M., unpublished). Like CstF‐64, τCstF‐64 co‐immunoprecipitates with other members of the CstF polyadenylation complex (Youngblood et al., 2014), and therefore likely participates in 3′ end formation of germ cell mRNAs (Yao et al., 2013).
Because of its pattern of expression in testis (Figure 2), it seemed certain that τCstF‐64 would be necessary for germ cell polyadenylation and thus for gene expression during spermatogenesis. Targeted deletion of Cstf2t confirmed that hypothesis: male, but not female, Cstf2t −/− mice were infertile due to aberrant meiotic and postmeiotic development, but were otherwise physiologically normal (Dass et al., 2007). Unexpectedly, spermatogenesis, while defective, did not halt, as might have been expected if polyadenylation (and thus gene expression) was blocked during meiosis. Instead, a few motile spermatozoa were seen, comparable to the human infertility known as oligoasthenoteratozoospermia. As expression of many thousands of mRNAs was altered in the Cstf2t −/− mouse testes, this suggested that cascades of genes were affected in these mice. The most likely—but by no means the only—hypothesis is that τCstF‐64 is critical for the proper polyadenylation of a few key genes, and that those key genes then affect a larger number of downstream genes.
Our laboratory has examined further roles for τCstF‐64 in global mRNA expression (Li et al., 2012), APA and splicing of the Crem mRNA (Grozdanov, Amatullah, Graber, & MacDonald, 2016), and expression of histone mRNAs (Grozdanov, Li, Yu, Yan, & MacDonald, 2018) in testis. We also found important roles for τCstF‐64 in embryonic stem cells (Youngblood et al., 2014) and during endoderm differentiation (Youngblood & MacDonald, 2014). Other laboratories have examined sites of pre‐mRNA binding for τCstF‐64 in cultured human cell lines (Hwang et al., 2016; Yao et al., 2012, 2013), and inferred functions in those cells.
5.3. Core and peripheral polyadenylation proteins in male germ cells
CFIm is a heterotetramer consisting of two identical subunits of 25 kDa (CFIm25, gene name: Nudt21) and two large subunits that are either 59, 68, or 72 kDa (the 68 and 72 kDa polypeptides are formed by alternative splicing of the Cpsf6 mRNA, while the 59 kDa polypeptide is from a different gene, Cpsf7). Together, the large and small subunits of CFIm enhance the recruitment of CPSF to polyadenylation sites that lack AAUAAA by binding to upstream UGUA motifs (Kubo, Wada, Yamaguchi, Shimizu, & Handa, 2006; Venkataraman, Brown, & Gilmartin, 2005). In male germ cells, CFIm25 and CFIm68 are expressed more highly than in other tissues and seem to contribute to their own APA (Sartini, Wang, Wang, Millette, & Kilpatrick, 2008). CFIm25 is also associated with DNA near the sterol regulatory element binding transcription factor 2 (Srebf2) polyadenylation sites (op. cit.), and probably contributes to the meiotic and postmeiotic APA of Srebf2 in germ cells (H. Wang, Sartini, Millette, & Kilpatrick, 2006).
Previously, it was proposed that WDC146 (gene symbol Wdr33), a homolog of the Pfs2 (yeast) and FY (Arabidopsis) proteins (Simpson, Dijkwel, Quesada, Henderson, & Dean, 2003), had a testis‐specific function (Ito et al., 2001). In yeast, Pfs2p bridges several core polyadenylation proteins, while in Arabidopsis, FY changes polyadenylation site choice of its own mRNA (see below). We know now (see above) that, in concert with CPSF‐30, Wdr33 binds to and recognizes the AAUAAA PAS, and is thus a core polyadenylation protein (Chan et al., 2014; Schonemann et al., 2014). The splicing factor Ptbp2 plays an important role in meiotic and postmeiotic alternative splicing (Zagore et al., 2015), and conceivably might affect APA, too.
6. OTHER TISSUES, OTHER ORGANISMS
Expansions of CTG microsatellite sequences in the myotonic dystrophy protein kinase (DMPK) gene can result in different forms of muscular dystrophy. In muscle, muscleblind‐like (MBNL) protein family members regulate RNA processing events important for prenatal‐to‐postnatal switches by binding to the CUG repeats in DMPK mRNAs. An excessively large number of CUG repeats in the DMPK mRNA can sequester MBNL proteins in muscle cells, resulting in altered polyadenylation and splicing of several other mRNAs that contribute to the dystrophic phenotype. This APA seems to be because of competition between MBNL and CPSF6 (a subunit of CFIm) for binding to pre‐mRNAs upstream of polyadenylation sites (Figure 1). In mouse models, knockouts of two or more of the MBNL genes (Mbnl1, Mbnl2, Mbnl3) resemble muscular dystrophy phenotypes due to altered polyadenylation and splicing of critical muscle RNAs (Batra et al., 2014; Thomas et al., 2017).
Mammals do not have a monopoly on tissue‐specific APA. For example, suppressor‐of‐forked is the Drosophila homolog of CstF‐77. Mutations in su(f) suppress the forked bristle phenotype of f 1 in Drosophila epidermal cells (Simonelig, Elliott, Mitchelson, & O'Hare, 1996). CstF‐77 also controls its own APA in Drosophila and possibly mammalian tissues (Hatton et al., 2000; Luo et al., 2013; Pan et al., 2006). Not to be left out, the Drosophila sex‐lethal protein, in addition to controlling gender, regulates alternative polyadenylation of enhancer of rudimentary in female germline cells (Gawande, Robida, Rahn, & Singh, 2006).
The co‐transcriptional nature of cleavage and polyadenylation seems also to influence tissue‐specific APA, at least in Drosophila. Slowed rates of transcriptional elongation led to greater use of proximal polyadenylation sites in Drosophila melanogaster in most tissues, but not in the head (X. Liu et al., 2017), suggesting that fruit fly neurons have a different mode of regulation than other cell types. A similar phenomenon occurs in mammalian cells in culture (Nagaike et al., 2011).
6.1. Alternative polyadenylation regulates seasonal plant flowering at several levels
Plants have a complex system controlling seasonal flowering in the floral meristem. The mRNA encoding FCA (a central regulator of flowering) has several alternatively polyadenylated forms, the longer of which encodes an RNA binding protein (Simpson et al., 2003). Full length FCA binds FY, and together, the complex redirects polyadenylation from the full‐length site back upstream to the alternative site leading to the production of a truncated FCA and the loss of RNA binding. Neither FCA nor FY is a direct homolog of any member of the core mammalian polyadenylation machinery (Arabidopsis has been shown to possess the full arsenal of polyadenylation proteins observed in mammals), although the FY homolog PFS2 is a core member in Saccharomyces cerevisiae. This autoregulatory loop was therefore the first example of an auxiliary factor that redirected polyadenylation site choice from one position to another.
There is much more going on in flowering control. Functioning similarly to FY, PCFS4 (a homolog of the Pcf11 polyadenylation protein) is an auxiliary protein involved in the APA of the FCA mRNA (Xing, Zhao, Xu, & Li, 2008). Similarly, FPA (an RNA‐binding protein of the spen gene family) controls APA of antisense FCA transcripts (Hornyik, Terzi, & Simpson, 2010); CstF‐64 and CstF‐77 are probably also involved in this mechanism (F. Liu, Marquardt, Lister, Swiezewski, & Dean, 2010). Recent high‐throughput studies in Arabidopsis, red clover (Trifolium pratense L.), and rice have uncovered suggestive patterns in tissue‐specific APA in those species (Chakrabarti, Dinkins, & Hunt, 2018; de Lorenzo, Sorenson, Bailey‐Serres, & Hunt, 2017; Fu et al., 2016). Finally, we would remiss if not to mention the considerable research into polyadenylation mechanisms in Arabidopsis and other plants, which affect gene expression in embryo and gametophyte development, flowers, leaves, and other plant tissues (Hunt et al., 2008; Lorkovic, 2009; Xing, Zhao, & Li, 2008).
7. CONCLUSION—WHERE ARE ALL THE TISSUE‐SPECIFIC POLYADENYLATION FACTORS?
Tissue‐specific APA is a widespread phenomenon. Yet, in preparing this review we were struck by how much remains to be understood about its mechanisms. For example, surprisingly few tissue‐specific auxiliary proteins have been discovered that control APA. More often, changes in levels of the core polyadenylation proteins seem to be involved (CstF‐64 and its variants or CFIm) or splicing vies with polyadenylation for control (the Ig switch or Nova in brain). Authenticated mechanisms for other examples remain tantalizingly incomplete.
The overall conclusion of this review—that there are only a few tissue‐specific auxiliary factors controlling APA, and that the majority of regulation is from altered levels of core polyadenylation proteins—remains as true today as it was in 2010 when we originally surveyed the topic (MacDonald & McMahon, 2010). Knockdown studies in cell lines have implicated levels of core polyadenylation factors such as subunits of CFIm, Fip1, and CPSF. However, there have been only a few demonstrations that those levels of any core polyadenylation protein changes physiologically in metazoan or mammalian tissues (e.g., Martincic et al., 1998). Oddly, some studies conclude that highly conserved core polyadenylation proteins such as CstF‐64 do not play an important role in gene expression as was previously thought (Yao et al., 2012). However, effects of CstF‐64 might be masked in mammalian systems by the presence of τCstF‐64 (Youngblood et al., 2014), complicating interpretations. New models for APA will have to demonstrate either changes in levels of specific polyadenylation proteins or changes in overall mRNA transcription (altering the ratio of core polyadenylation proteins to polyadenylation sites). As before, we look forward to future progress in this area.
CONFLICT OF INTEREST
The author has declared no conflicts of interest for this article.
ACKNOWLEDGMENTS
I want to thank Petar Grozdanov for his careful reading of the manuscript and all the members of the MacDonald Lab for their ongoing input into this review. This review is dedicated in the memory of K. Wyatt McMahon who co‐authored its previous version and launched many of the ideas covered herein.
MacDonald CC. Tissue‐specific mechanisms of alternative polyadenylation: Testis, brain, and beyond (2018 update). WIREs RNA. 2019;10:e1526. 10.1002/wrna.1526
This article is an update of: Tissue-specific mechanisms of alternative polyadenylation: testis, brain, and beyond
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R01HD037109
REFERENCES
- Ainsley, J. A. , Drane, L. , Jacobs, J. , Kittelberger, K. A. , & Reijmers, L. G. (2014). Functionally diverse dendritic mRNAs rapidly associate with ribosomes following a novel experience. Nature Communications, 5, 4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara, S. G. , Evans, R. M. , & Rosenfeld, M. G. (1984). Calcitonin/calcitonin gene‐related peptide transcription unit: Tissue‐specific expression involves selective use of alternative polyadenylation sites. Molecular and Cellular Biology, 4, 2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, J. , Vitting‐Seerup, K. , Waage, J. , Tang, C. , Ge, Y. , Porse, B. T. , & Yan, W. (2016). UPF2‐dependent nonsense‐mediated mRNA decay pathway is essential for spermatogenesis by selectively eliminating longer 3′ UTR transcripts. PLoS Genetics, 12, e1005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra, R. , Charizanis, K. , Manchanda, M. , Mohan, A. , Li, M. , Finn, D. J. , … Swanson, M. S. (2014). Loss of MBNL leads to disruption of developmentally regulated alternative polyadenylation in RNA‐mediated disease. Molecular Cell, 56, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, M. , Dinkins, R. D. , & Hunt, A. G. (2018). Genome‐wide atlas of alternative polyadenylation in the forage legume red clover. Scientific Reports, 8, 11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. L. , Huppertz, I. , Yao, C. , Weng, L. , Moresco, J. J. , Yates, J. R., 3rd , … Shi, Y. (2014). CPSF30 and Wdr33 directly bind to AAUAAA in mammalian mRNA 3′ processing. Genes & Development, 28, 2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. W. , Yeh, H. S. , & Yong, J. (2017). Alternative polyadenylation in human diseases. Endocrinology and Metabolism, 32, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, A. , Meijer, H. A. , & de Moor, C. H. (2013). Specificity factors in cytoplasmic polyadenylation. WIREs RNA, 4, 437–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennathukuzhi, V. M. , Lefrancois, S. , Morales, C. R. , Syed, V. , & Hecht, N. B. (2001). Elevated levels of the polyadenylation factor CstF 64 enhance formation of the 1kB testis brain RNA‐binding protein (TB‐RBP) mRNA in male germ cells. Molecular Reproduction and Development, 58, 460–469. [DOI] [PubMed] [Google Scholar]
- Clerici, M. , Faini, M. , Aebersold, R. , & Jinek, M. (2017). Structural insights into the assembly and polyA signal recognition mechanism of the human CPSF complex. eLife, 6, e33111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici, M. , Faini, M. , Muckenfuss, L. M. , Aebersold, R. , & Jinek, M. (2018). Structural basis of AAUAAA polyadenylation signal recognition by the human CPSF complex. Nature Structural & Molecular Biology, 25, 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curinha, A. , Oliveira Braz, S. , Pereira‐Castro, I. , Cruz, A. , & Moreira, A. (2014). Implications of polyadenylation in health and disease. Nucleus, 5, 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass, B. , Attaya, E. N. , Wallace, A. M. , & MacDonald, C. C. (2001). Overexpression of the CstF‐64 and CPSF‐160 polyadenylation protein messenger RNAs in mouse male germ cells. Biology of Reproduction, 64, 1722–1729. [DOI] [PubMed] [Google Scholar]
- Dass, B. , McMahon, K. W. , Jenkins, N. A. , Gilbert, D. J. , Copeland, N. G. , & MacDonald, C. C. (2001). The gene for a variant form of the polyadenylation protein CstF‐64 is on chromosome 19 and is expressed in pachytene spermatocytes in mice. Journal of Biological Chemistry, 276, 8044–8050. [DOI] [PubMed] [Google Scholar]
- Dass, B. , Tardif, S. , Park, J. Y. , Tian, B. , Weitlauf, H. M. , Hess, R. A. , … MacDonald, C. C. (2007). Loss of polyadenylation protein τCstF‐64 causes spermatogenic defects and male infertility. Proceedings of the National Academy of Sciences of the United States of America, 104, 20374–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conti, L. , Baralle, M. , & Buratti, E. (2017). Neurodegeneration and RNA‐binding proteins. WIREs RNA, 8, e1394. [DOI] [PubMed] [Google Scholar]
- de Lorenzo, L. , Sorenson, R. , Bailey‐Serres, J. , & Hunt, A. G. (2017). Noncanonical alternative polyadenylation contributes to gene regulation in response to hypoxia. Plant Cell, 29, 1262–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derti, A. , Garrett‐Engele, P. , Macisaac, K. D. , Stevens, R. C. , Sriram, S. , Chen, R. , … Babak, T. (2012). A quantitative atlas of polyadenylation in five mammals. Genome Research, 22, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giammartino, D. C. , Nishida, K. , & Manley, J. L. (2011). Mechanisms and consequences of alternative polyadenylation. Molecular Cell, 43, 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsyukova, I. , Bradrick, S. S. , Gregory, S. G. , & Garcia‐Blanco, M. A. (2013). Cleavage and polyadenylation specificity factor 1 (CPSF1) regulates alternative splicing of interleukin 7 receptor (IL7R) exon 6. RNA, 19, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanourgakis, G. , Lesche, M. , Akpinar, M. , Dahl, A. , & Jessberger, R. (2016). Chromatoid body protein TDRD6 supports long 3′ UTR triggered nonsense mediated mRNA decay. PLoS Genetics, 12, e1005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes, M. M. , Guvenek, A. , Kawaguchi, R. , Zheng, D. , Huang, A. , Ho, V. M. , … Martin, K. C. (2017). Activity‐dependent regulation of alternative cleavage and polyadenylation during hippocampal long‐term potentiation. Scientific Reports, 7, 17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, H. , Yang, D. , Su, W. , Ma, L. , Shen, Y. , Ji, G. , … Li, Q. Q. (2016). Genome‐wide dynamics of alternative polyadenylation in rice. Genome Research, 26, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu, H. , Soumiya, H. , & Furukawa, S. (2012). Knockdown of pre‐mRNA cleavage factor Im 25 kDa promotes neurite outgrowth. Biochemical and Biophysical Research Communications, 425, 848–853. [DOI] [PubMed] [Google Scholar]
- Gawande, B. , Robida, M. D. , Rahn, A. , & Singh, R. (2006). Drosophila sex‐lethal protein mediates polyadenylation switching in the female germline. EMBO Journal, 25, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarino, V. A. , Alcott, C. E. , Chen, C. A. , Chaudhury, A. , Gillentine, M. A. , Rosenfeld, J. A. , … Zoghbi, H. Y. (2015). NUDT21‐spanning CNVs lead to neuropsychiatric disease and altered MeCP2 abundance via alternative polyadenylation. eLife, 4, e10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov, P. N. , Amatullah, A. , Graber, J. H. , & MacDonald, C. C. (2016). TauCstF‐64 mediates correct mRNA polyadenylation and splicing of activator and repressor isoforms of the cyclic AMP‐responsive element modulator (CREM) in mouse testis. Biology of Reproduction, 94, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov, P. N. , Li, J. , Yu, P. , Yan, W. , & MacDonald, C. C. (2018). Cstf2t regulates expression of histones and histone‐like proteins in male germ cells. Andrology, 6, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov, P. N. , Masoumzadeh, E. , Latham, M. P. , & MacDonald, C. C. (2018). The structural basis of CstF‐77 modulation of cleavage and polyadenylation through stimulation of CstF‐64 activity. Nucleic Acids Research, 46(22), 12022–12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. C. , Martinez, J. M. , Grozdanov, P. N. , Bergeson, S. E. , Grammas, P. , & MacDonald, C. C. (2016). The Cstf2t polyadenylation gene plays a sex‐specific role in learning behaviors in mice. PLoS One, 11, e0165976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton, L. S. , Eloranta, J. J. , Figueiredo, L. M. , Takagaki, Y. , Manley, J. L. , & O'Hare, K. (2000). The Drosophila homologue of the 64 kDa subunit of cleavage stimulation factor interacts with the 77 kDa subunit encoded by the suppressor of forked gene. Nucleic Acids Research, 28, 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornyik, C. , Terzi, L. C. , & Simpson, G. G. (2010). The Spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Developmental Cell, 18, 203–213. [DOI] [PubMed] [Google Scholar]
- Hunt, A. G. , Xu, R. , Addepalli, B. , Rao, S. , Forbes, K. P. , Meeks, L. R. , … Li, Q. Q. (2008). Arabidopsis mRNA polyadenylation machinery: Comprehensive analysis of protein–protein interactions and gene expression profiling. BMC Genomics, 9, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, H. W. , Park, C. Y. , Goodarzi, H. , Fak, J. J. , Mele, A. , Moore, M. J. , … Darnell, R. B. (2016). PAPERCLIP identifies MicroRNA targets and a role of CstF64/64tau in promoting non‐canonical poly(A) site usage. Cell Reports, 15, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, H. W. , Saito, Y. , Park, C. Y. , Blachere, N. E. , Tajima, Y. , Fak, J. J. , … Darnell, R. B. (2017). cTag‐PAPERCLIP reveals alternative polyadenylation promotes cell‐type specific protein diversity and shifts Araf isoforms with microglia activation. Neuron, 95, 1334–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, S. , Sakai, A. , Nomura, T. , Miki, Y. , Ouchida, M. , Sasaki, J. , & Shimizu, K. (2001). A novel WD40 repeat protein, WDC146, highly expressed during spermatogenesis in a stage‐specific manner. Biochemical and Biophysical Research Communications, 280, 656–663. [DOI] [PubMed] [Google Scholar]
- Ji, Z. , Lee, J. Y. , Pan, Z. , Jiang, B. , & Tian, B. (2009). Progressive lengthening of 3′ untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proceedings of the National Academy of Sciences of the United States of America, 106, 7028–7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabara, S. I. , Tsuruta, S. , Yamaoka, Y. , Oyama, K. , Iwazaki, C. , & Baba, T. (2018). PAPOLB/TPAP regulates spermiogenesis independently of chromatoid body‐associated factors. Journal of Reproduction and Development, 64, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, T. , Wada, T. , Yamaguchi, Y. , Shimizu, A. , & Handa, H. (2006). Knock‐down of 25 kDa subunit of cleavage factor Im in Hela cells alters alternative polyadenylation within 3′‐UTRs. Nucleic Acids Research, 34, 6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackford, B. , Yao, C. , Charles, G. M. , Weng, L. , Zheng, X. , Choi, E. A. , … Shi, Y. (2014). Fip1 regulates mRNA alternative polyadenylation to promote stem cell self‐renewal. EMBO Journal, 33, 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Park, J. Y. , Zheng, D. , Hoque, M. , Yehia, G. , & Tian, B. (2016). Alternative cleavage and polyadenylation in spermatogenesis connects chromatin regulation with post‐transcriptional control. BMC Biology, 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Yeh, H. J. , Shankarling, G. S. , Ji, Z. , Tian, B. , & MacDonald, C. C. (2012). The τCstF‐64 polyadenylation protein controls genome expression in testis. PLoS One, 7, e48373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , You, B. , Hoque, M. , Zheng, D. , Luo, W. , Ji, Z. , … Tian, B. (2015). Systematic profiling of poly(A)+ transcripts modulated by core 3′ end processing and splicing factors reveals regulatory rules of alternative cleavage and polyadenylation. PLoS Genetics, 11, e1005166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi, D. D. , Mele, A. , Fak, J. J. , Ule, J. , Kayikci, M. , Chi, S. W. , … Darnell, R. B. (2008). HITS‐CLIP yields genome‐wide insights into brain alternative RNA processing. Nature, 456, 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , Li, Z. , Ozsolak, F. , Kim, S. W. , Arango‐Argoty, G. , Liu, T. T. , … John, B. (2012). An in‐depth map of polyadenylation sites in cancer. Nucleic Acids Research, 40, 8460–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Brockman, J. M. , Dass, B. , Hutchins, L. N. , Singh, P. , McCarrey, J. R. , … Graber, J. H. (2007). Systematic variation in mRNA 3′‐processing signals during mouse spermatogenesis. Nucleic Acids Research, 35, 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Marquardt, S. , Lister, C. , Swiezewski, S. , & Dean, C. (2010). Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science, 327, 94–97. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Freitas, J. , Zheng, D. , Oliveira, M. S. , Hoque, M. , Martins, T. , … Moreira, A. (2017). Transcription elongation rate has a tissue‐specific impact on alternative cleavage and polyadenylation in Drosophila melanogaster . RNA, 23, 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkovic, Z. J. (2009). Role of plant RNA‐binding proteins in development, stress response and genome organization. Trends in Plant Science, 14, 229–236. [DOI] [PubMed] [Google Scholar]
- Luo, W. , Ji, Z. , Pan, Z. , You, B. , Hoque, M. , Li, W. , … Tian, B. (2013). The conserved intronic cleavage and polyadenylation site of CstF‐77 gene imparts control of 3′ end processing activity through feedback autoregulation and by U1 snRNP. PLoS Genetics, 9, e1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz, C. S. , & Moreira, A. (2011). Alternative mRNA polyadenylation in eukaryotes: An effective regulator of gene expression. WIREs RNA, 2, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, C. C. , & Grozdanov, P. N. (2017). Nonsense in the testis: Multiple roles for nonsense‐mediated decay revealed in male reproduction. Biology of Reproduction, 96, 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, C. C. , & McMahon, K. W. (2010). Tissue‐specific mechanisms of alternative polyadenylation: Testis, brain, and beyond. WIREs RNA, 1, 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, C. C. , & Redondo, J.‐L. (2002). Reexamining the polyadenylation signal: Were we wrong about AAUAAA? Molecular and Cellular Endocrinology, 190, 1–8. [DOI] [PubMed] [Google Scholar]
- Mansfield, K. D. , & Keene, J. D. (2012). Neuron‐specific ELAV/Hu proteins suppress HuR mRNA during neuronal differentiation by alternative polyadenylation. Nucleic Acids Research, 40, 2734–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, G. , Gruber, A. R. , Keller, W. , & Zavolan, M. (2012). Genome‐wide analysis of pre‐mRNA 3′ end processing reveals a decisive role of human cleavage factor I in the regulation of 3′ UTR length. Cell Reports, 1, 753–763. [DOI] [PubMed] [Google Scholar]
- Martincic, K. , Alkan, S. A. , Cheatle, A. , Borghesi, L. , & Milcarek, C. (2009). Transcription elongation factor ELL2 directs immunoglobulin secretion in plasma cells by stimulating altered RNA processing. Nature Immunology, 10, 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincic, K. , Campbell, R. , Edwalds‐Gilbert, G. , Souan, L. , Lotze, M. T. , & Milcarek, C. (1998). Increase in the 64‐kDa subunit of the polyadenylation/cleavage stimulatory factor during the G0 to S phase transition. Proceedings of the National Academy of Sciences of the United States of America, 95, 11095–11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamha, C. P. , Xia, Z. , Yang, J. , Albrecht, T. R. , Li, M. , Shyu, A. B. , … Wagner, E. J. (2014). CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature, 510, 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, K. W. , Hirsch, B. A. , & MacDonald, C. C. (2006). Differences in polyadenylation site choice between somatic and male germ cells. BMC Molecular Biology, 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, P. , Shenker, S. , Andreu‐Agullo, C. , Westholm, J. O. , & Lai, E. C. (2013). Widespread and extensive lengthening of 3′ UTRs in the mammalian brain. Genome Research, 23, 812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaike, T. , Logan, C. , Hotta, I. , Rozenblatt‐Rosen, O. , Meyerson, M. , & Manley, J. L. (2011). Transcriptional activators enhance polyadenylation of mRNA precursors. Molecular Cell, 41, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazim, M. , Masuda, A. , Rahman, M. A. , Nasrin, F. , Takeda, J. I. , Ohe, K. , … Ohno, K. (2016). Competitive regulation of alternative splicing and alternative polyadenylation by hnRNP H and CstF64 determines acetylcholinesterase isoforms. Nucleic Acids Research, 45(3), 1455–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorodnikov, A. , Kargapolova, Y. , & Danckwardt, S. (2016). Processing and transcriptome expansion at the mRNA 3′ end in health and disease: Finding the right end. Pflügers Archiv, 468, 993–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Z. , Zhang, H. , Hague, L. K. , Lee, J. Y. , Lutz, C. S. , & Tian, B. (2006). An intronic polyadenylation site in human and mouse CstF‐77 genes suggests an evolutionarily conserved regulatory mechanism. Gene, 366, 325–334. [DOI] [PubMed] [Google Scholar]
- Perepelitsa‐Belancio, V. , & Deininger, P. (2003). RNA truncation by premature polyadenylation attenuates human mobile element activity. Nature Genetics, 35, 363–366. [DOI] [PubMed] [Google Scholar]
- Reyes, A. , & Huber, W. (2018). Alternative start and termination sites of transcription drive most transcript isoform differences across human tissues. Nucleic Acids Research, 46, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartini, B. L. , Wang, H. , Wang, W. , Millette, C. F. , & Kilpatrick, D. L. (2008). Pre‐messenger RNA cleavage factor I (CFIm): Potential role in alternative polyadenylation during spermatogenesis. Biology of Reproduction, 78, 472–482. [DOI] [PubMed] [Google Scholar]
- Schmid, R. , Grellscheid, S. N. , Ehrmann, I. , Dalgliesh, C. , Danilenko, M. , Paronetto, M. P. , … Elliott, D. J. (2013). The splicing landscape is globally reprogrammed during male meiosis. Nucleic Acids Research, 41, 10170–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonemann, L. , Kuhn, U. , Martin, G. , Schafer, P. , Gruber, A. R. , Keller, W. , … Wahle, E. (2014). Reconstitution of CPSF active in polyadenylation: Recognition of the polyadenylation signal by WDR33. Genes & Development, 28, 2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankarling, G. S. , Coates, P. W. , Dass, B. , & MacDonald, C. C. (2009). A family of splice variants of CstF‐64 expressed in vertebrate nervous systems. BMC Molecular Biology, 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankarling, G. S. , & MacDonald, C. C. (2013). Polyadenylation site‐specific differences in the activity of the neuronal βCstF‐64 protein in PC‐12 cells. Gene, 529, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. (2012). Alternative polyadenylation: New insights from global analyses. RNA, 18, 2105–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum, E. Y. , Jones, S. H. , Shao, A. , Dumdie, J. , Krause, M. D. , Chan, W. K. , … Wilkinson, M. F. (2016). The antagonistic gene paralogs Upf3a and Upf3b govern nonsense‐mediated RNA decay. Cell, 165, 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelig, M. , Elliott, K. , Mitchelson, A. , & O'Hare, K. (1996). Interallelic complementation at the suppressor of forked locus of Drosophila reveals complementation between suppressor of forked proteins mutated in different regions. Genetics, 142, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, G. G. , Dijkwel, P. P. , Quesada, V. , Henderson, I. , & Dean, C. (2003). FY is an RNA 3′ end‐processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell, 113, 777–787. [DOI] [PubMed] [Google Scholar]
- Singh, I. , Lee, S. H. , Sperling, A. S. , Samur, M. K. , Tai, Y. T. , Fulciniti, M. , … Leslie, C. S. (2018). Widespread intronic polyadenylation diversifies immune cell transcriptomes. Nature Communications, 9, 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, K. , Li, X. , Chen, X. , Bai, Y. , Zhou, G. , Kokiko‐Cochran, O. N. , … Herjan, T. (2018). Neuron‐specific HuR‐deficient mice spontaneously develop motor neuron disease. Journal of Immunology, 201, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Zhang, Y. , Hamilton, K. , Manley, J. L. , Shi, Y. , Walz, T. , & Tong, L. (2018). Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proceedings of the National Academy of Sciences of the United States of America, 115, E1419–E1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki, Y. , Seipelt, R. L. , Peterson, M. L. , & Manley, J. L. (1996). The polyadenylation factor CstF‐64 regulates alternative processing of IgM heavy chain pre‐mRNA during B cell differentiation. Cell, 87, 941–952. [DOI] [PubMed] [Google Scholar]
- Taliaferro, J. M. , Vidaki, M. , Oliveira, R. , Olson, S. , Zhan, L. , Saxena, T. , … Burge, C. B. (2016). Distal alternative last exons localize mRNAs to neural projections. Molecular Cell, 61, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzio, M. , Koley, S. , Samra, N. , Rishal, I. , Zhao, Q. , Sahoo, P. K. , … Fainzilber, M. (2018). Locally translated mTOR controls axonal local translation in nerve injury. Science, 359, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. D. , Sznajder, L. J. , Bardhi, O. , Aslam, F. N. , Anastasiadis, Z. P. , Scotti, M. M. , … Swanson, M. S. (2017). Disrupted prenatal RNA processing and myogenesis in congenital myotonic dystrophy. Genes & Development, 31, 1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, B. , & Graber, J. H. (2012). Signals for pre‐mRNA cleavage and polyadenylation. WIREs RNA, 3, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, B. , & Manley, J. L. (2017). Alternative polyadenylation of mRNA precursors. Nature Reviews Molecular Cell Biology, 18, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule, J. , Jensen, K. B. , Ruggiu, M. , Mele, A. , Ule, A. , & Darnell, R. B. (2003). CLIP identifies Nova‐regulated RNA networks in the brain. Science, 302, 1212–1215. [DOI] [PubMed] [Google Scholar]
- Venkataraman, K. , Brown, K. M. , & Gilmartin, G. M. (2005). Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition. Genes & Development, 19, 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, A. M. , Dass, B. , Ravnik, S. E. , Tonk, V. , Jenkins, N. A. , Gilbert, D. J. , … MacDonald, C. C. (1999). Two distinct forms of the 64,000 Mr protein of the cleavage stimulation factor are expressed in mouse male germ cells. Proceedings of the National Academy of Sciences of the United States of America, 96, 6763–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, A. M. , Denison, T. , Attaya, E. N. , & MacDonald, C. C. (2004). Developmental differences in expression of two forms of the CstF‐64 polyadenylation protein in rat and mouse. Biology of Reproduction, 70, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Sartini, B. L. , Millette, C. F. , & Kilpatrick, D. L. (2006). A developmental switch in transcription factor isoforms during spermatogenesis controlled by alternative messenger RNA 3′‐end formation. Biology of Reproduction, 75, 318–323. [DOI] [PubMed] [Google Scholar]
- Wang, P. J. (2004). X chromosomes, retrogenes and their role in male reproduction. Trends in Endocrinology and Metabolism, 15, 79–83. [DOI] [PubMed] [Google Scholar]
- Wang, R. , Zheng, D. , Yehia, G. , & Tian, B. (2018). A compendium of conserved cleavage and polyadenylation events in mammalian genes. Genome Research, 28, 1427–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Z. , Donehower, L. A. , Cooper, T. A. , Neilson, J. R. , Wheeler, D. A. , Wagner, E. J. , & Li, W. (2014). Dynamic analyses of alternative polyadenylation from RNA‐seq reveal a 3′‐UTR landscape across seven tumour types. Nature Communications, 5, 5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D. , Zhao, H. , & Li, Q. Q. (2008). Arabidopsis CLP1‐SIMILAR PROTEIN3, an ortholog of human polyadenylation factor CLP1, functions in gametophyte, embryo, and postembryonic development. Plant Physiology, 148, 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, D. , Zhao, H. , Xu, R. , & Li, Q. Q. (2008). Arabidopsis PCFS4, a homologue of yeast polyadenylation factor Pcf11p, regulates FCA alternative processing and promotes flowering time. Plant Journal, 54, 899–910. [DOI] [PubMed] [Google Scholar]
- Yao, C. , Biesinger, J. , Wan, J. , Weng, L. , Xing, Y. , Xie, X. , & Shi, Y. (2012). Transcriptome‐wide analyses of CstF64‐RNA interactions in global regulation of mRNA alternative polyadenylation. Proceedings of the National Academy of Sciences of the United States of America, 109, 18773–18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, C. , Choi, E. A. , Weng, L. , Xie, X. , Wan, J. , Xing, Y. , … Shi, Y. (2013). Overlapping and distinct functions of CstF64 and CstF64τ in mammalian mRNA 3′ processing. RNA, 19, 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, O. K. , Hsu, T. Y. , Im, J. H. , & Brem, R. B. (2012). Genetics and regulatory impact of alternative polyadenylation in human B‐lymphoblastoid cells. PLoS Genetics, 8, e1002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood, B. A. , Grozdanov, P. N. , & MacDonald, C. C. (2014). CstF‐64 supports pluripotency and regulates cell cycle progression in embryonic stem cells through histone 3′ end processing. Nucleic Acids Research, 42, 8330–8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood, B. A. , & MacDonald, C. C. (2014). CstF‐64 is necessary for endoderm differentiation resulting in cardiomyocyte defects. Stem Cell Research, 13, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagore, L. L. , Grabinski, S. E. , Sweet, T. J. , Hannigan, M. M. , Sramkoski, R. M. , Li, Q. , & Licatalosi, D. D. (2015). RNA binding protein Ptbp2 is essential for male germ cell development. Molecular and Cellular Biology, 35, 4030–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Lee, J. Y. , & Tian, B. (2005). Biased alternative polyadenylation in human tissues. Genome Biology, 6, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. L. , Baraniak, A. P. , & Lou, H. (2007). Role for Fox‐1/Fox‐2 in mediating the neuronal pathway of calcitonin/calcitonin gene‐related peptide alternative RNA processing. Molecular and Cellular Biology, 27, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H. , Zhou, H. L. , Hasman, R. A. , & Lou, H. (2007). Hu proteins regulate polyadenylation by blocking sites containing U‐rich sequences. Journal of Biological Chemistry, 282, 2203–2210. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Wang, X. , Forouzmand, E. , Jeong, J. , Qiao, F. , Sowd, G. A. , … Shi, Y. (2018). Molecular mechanisms for CFIm‐mediated regulation of mRNA alternative polyadenylation. Molecular Cell, 69, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


