Abstract
Purpose
Ocular biometry varies within groups of emmetropic, hyperopic or myopic children. The aim of this study was to quantify the effect of foetal and infant growth on ocular biometry in early childhood, to determine the most important period for this association, and to examine genetic overlap with height and birth weight.
Methods
5931 children (50.1% girls) from a population‐based prospective birth cohort study underwent intra‐uterine and infant growth measurements at second and third trimester, and from birth to 72 months. An ophthalmic examination including axial length (mm) and corneal radius of curvature (mm) was performed at 6 years of age. The associations between prenatal and postnatal growth variables and axial length and corneal radius of curvature were assessed with conditional linear regression analyses. Weighted genetic risk scores for birth weight and height were calculated and causality was tested with Mendelian randomisation.
Results
Weight and length from mid‐pregnancy to 2 years of age were most important prognostic factors for axial length and corneal radius of curvature at age 4.9–9 years (mean 6.2 years S.D. 0.5). For height (Standard deviation score), the association with axial length and corneal radius of curvature was highest for the measurement at 12 months (β 0.171 p < 0.001 and 0.070 p < 0.001). The genetic height and birth weight risk scores were both significantly associated with ocular biometry.
Conclusions
Larger neonates had longer axial length and greater corneal radius of curvature. Growth during pregnancy and 2 years postnatally is the most important period underlying this association and may be partly genetically determined by genes associated with height.
Keywords: epidemiology, genetics, myopia, optics, orthokeratology
Introduction
Refractive errors are caused by a complex coordinated scaling of the eye's refractive components to place the focal plane on the retina.1, 2, 3 Two of the key biometric components in emmetropisation are axial length (AL) and corneal radius of curvature (CR). The ratio of AL/CR strongly correlates with refractive error (RE)4, 5, 6 The biometric measures show large variation even in subjects with the same refractive error;2 this calls for a better understanding of their determinants.
Growth trajectories and birth parameters such as height and weight have previously been associated with ocular biometry.7, 8 Genetic overlap between these traits has also been shown: a higher genetic risk score of height was associated with a greater CR in 15 year old children, but has not been shown for axial length.8 Approximately 75% of normal ocular growth occurs intra uterine.9 Yet, the effect of prenatal growth, growth trajectories and up to which age body growth is associated with ocular biometry is unknown.
The aim of this study was to determine the effect of intra uterine and postnatal growth on ocular biometry in schoolchildren, and to investigate potential genetic commonalities with height and birth weight.
Materials and methods
General design
This study was embedded in the Generation R Study, a population‐based prospective cohort study of pregnant women and their children in Rotterdam, the Netherlands. A total of 9778 pregnant women with children born between April 2002 and January 2006 were included in the study, and the children of 6690 women participated in a physical examination at the research centre at 6 years of age.10 The study protocol was approved by the Medical Ethical Committee of the Erasmus Medical Centre, Rotterdam (MEC 217.595/2002/20). Written informed consent was obtained from all participants.
Prenatal measurements
Foetal ultrasound examinations were carried out in early (<18 weeks), mid (18‐25 weeks), and late (≥25 weeks) pregnancy. Gestational age was determined using a questionnaire and the foetal ultrasound in the first trimester. Head circumference (HC), abdominal circumference (AC) and femur length (FL) were measured using the standardised procedures to the nearest millimetre in the second and third trimester.11 Estimated foetal weight was calculated using the Hadlock formula, an estimate based on HC, FL and AC.12 The data obtained were used to calculate gestational age adjusted standardised deviation score (SDS) for each growth outcome.11
Birth parameters and postnatal measurements, gestational age, birth weight, and HC were obtained using medical records and hospital registries. SDS for weight for gestational age were calculated according to Northern European growth standards.13 Postnatal growth characteristics were measured using standardised schedules and procedures at 6, 12, 24, 36, and 48 months in community health centres. SDS for the growth characteristics postnatal were calculated based on Dutch growth reference charts (Growth analyser 3.0, Dutch Growth Research Foundation). Prenatal growth and postnatal growth patterns, decelerated/normal/accelerated growth, were defined as weight change (in SDS) between second trimester and birth, and between birth and 6 months, with a decrease or increase of 0.67 SDS, or for normal growth within this range. Gestational age was categorised as at birth, and before and after 37 weeks of gestation, and birth weight was categorised into below and above 2500 grams according to preterm birth and low birth weight standards.
AL and CR
Ocular biometry (AL, CR) was obtained with a Zeiss IOLMaster 500 (www.zeiss.com) at the research centre during the physical examination at 6 years of age. Data were collected from right and left eyes. Five measurements of AL were taken of the right eye and the left eye and averaged. Three measurements of K1 and K2 were taken of the right eye and left eye, and were averaged. AL/CR ratio was calculated by dividing the mean AL (mm) by the mean CR (mm).
Genetics
DNA from children (cord blood or during physical examination at 6 years of age) was extracted, normalised and plated. Samples were genotyped using Illumina Infinium II HumanHap610 Quad Arrays following standard manufacturer's protocols (www.illumina.com). Intensity files were analysed using Illumina BeadStudio software Genotyping Module v.3.2.32, and genotype calling was based on default cluster files. Any sample displaying call rates below 97.5%, excess of autosomal heterozygosity (F<mean‐4SD) and mismatch between called and phenotypic gender (0.2%) were excluded. Genotypes were imputed for all polymorphic SNPs (single nucleotide polymorphisms) from phased haplotypes in autosomal chromosomes using the 1000 Genomes GIANTv3 panel (www.internationalgenome.org).
Covariates
Age, parity, smoking and alcohol use during pregnancy, pre pregnancy weight of the mother, educational level and ethnicity were obtained using questionnaires. Educational level was categorised as primary and secondary or higher education. Ethnicity was classified according to the Dutch standard classification criteria of Statistics Netherlands,14 and grouped into European and non‐European. The height of the mother was measured without shoes. Child height and weight were measured at 6 years of age, body mass index (BMI) (kg m−2) of children was calculated. Twins were excluded for analysis due to their known relation with prenatal growth.
Statistical analysis
To test the association between AL, CR and AL/CR ratio at 6 years of age with intra uterine growth parameters and postnatal growth, linear regression models were used adjusted for age, gender, and ethnicity. The association with growth trajectories were tested using restricted growth (<−0.67 SDS difference), normal growth (>−0.67 and <0.67 difference) and accelerated growth (>0.67 SDS difference) in weight during two time spans (from second trimester to birth, and from birth to 6 months postnatal). This resulted in nine groups of children in which the association with AL, CR and AL/CR ratio was tested in a linear regression model, with the normal growth children as the reference group. To identify the most important time period for the association between pre and postnatal growth with ocular biometry, conditional analyses were applied.15 Conditional analyses were performed using standardised residuals from linear regression models and consisted of two steps. Step 1 was calculating the standardised residuals in linear regression models per time point using the prior growth measurements as independent variables. This step resulted in an estimate of growth per time interval, independent of previous growth. Step 2 was the conditional analyses in which a linear regression model was performed with the ocular biometry as the dependent variable and with the standardised residuals of each time point as the independent variables, resulting in statistically independent effect per separate time interval.15 We investigated the shape of the association between non‐linear associations using ordinary least squares linear regression models with restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles. The number of splines were based on the lowest Akaike information criteria and Bayesian information criteria. Nonlinearity was tested using quadratic terms. A genetic risk score was calculated as the sum of beta * allele dosage of each top SNPs per independent locus associated with height (687/695 SNPs available) and birth weight (60/60 SNPs available).16, 17 The effect of the genetic risk scores was tested using linear regression with AL and CR as outcome. To test for causality, the genetic risk scores were used as an instrumental variable in the two‐stage least square method for the association between age, sex, and ethnicity, standardised residuals of AL and CR, and height or birth weight. Ordinary least squares linear regression models and two‐stage least square models were performed using the statistical program R v3.2.2 (www.r-project.org). All other analyses were performed in IBM SPSS Statistics v21.0.0.1 (https://www.ibm.com/uk-en/products/spss-statistics.
Results
Ocular biometry and covariates were available for 5931 children. Figure S1 shows the flow diagram for inclusion of participants. Table 1 shows the general characteristics of the participating children. The average age of the children at the eye examination was 6.2 years (S.D. ± 0.5 range 4.9–9.0 years), and 68.8% of the children were of European descent. Environmental factors or pregnancy related factors such as maternal education, season of birth, parity, alcohol or smoking during pregnancy were not associated with AL and CR (Table S1 ), and were therefore not used as covariates in the models.
Table 1.
General and ocular characteristics of the children (n = 5931)
| All | |
|---|---|
| Child characteristics | |
| Age child at ocular measurements (years) | 6.2 (0.5) |
| Female sex (%) | 50.1 (2970) |
| Birth weight (grams) | 3427 (552) |
| Gestational age (weeks) | 39.8 (1.8) |
| Height at 6 years (m) | 1.20 (6.0) |
| Weight at 6 years (kg) | 23.3 (4.3) |
| Head circumference at 6 years (cm) | 51.4 (1.6) |
| Axial length (mm) | 22.36 (0.75) |
| Average corneal radius (mm) | 7.77 (0.26) |
| Average AL/CR ratio | 2.88 (0.08) |
| European ethnicity (%) | 66.8 (3963) |
Values are means (S.D.) or percentages (absolute numbers).
Intra‐uterine growth and ocular biometry
Table 2 shows the association of early, mid‐ and late pregnancy, birth and postnatal growth parameters with ocular biometry at age 6. At mid pregnancy, HC showed the greatest association with AL and CR, but was not associated with AL/CR. All associations were stronger in late pregnancy. Estimated foetal weight showed the strongest association with AL in this trimester. There was no evidence for non‐linearity in any of the associations with prenatal parameters. The highest effect estimates were found at 6 months (head circumference), 12 months (height) and 24 months (weight).
Table 2.
Foetal and infant growth characteristics and the association with ocular biometry at 6 years of age
| (Estimated) Weight | Axial length (mm) | Corneal radius (mm) | AL/CR ratio |
|---|---|---|---|
| Early pregnancy (n = 5093) | 0.042 (0.023–0.062) | 0.017 (0.010–0.024) | −0.001 (−0.003 to 0.002) |
| Late pregnancy (n = 5006) | 0.090 (0.072–0.109) | 0.037 (0.030–0.043) | −0.002 (−0.004 to 0.000) |
| Birth weight (kg) (n = 5923) | 0.227 (0.195–0.259) | 0.093 (0.081–0.104) | −0.005 (−0.009 to −0.002) |
| Birth weight (n = 5884) | 0.132 (0.115–0.150) | 0.050 (0.044–0.057) | −0.002 (−0.004 to 0.000) |
| 3 months (n = 3528) | 0.151 (0.129–0.172) | 0.060 (0.052–0.068) | −0.003 (−0.005 to −0.000) |
| 6 months (n = 4407) | 0.149 (0.128–0.170) | 0.063 (0.056–0.071) | −0.004 (−0.007 to −0.002) |
| 12 months (n = 4084) | 0.148 (0.126–0.170) | 0.065 (0.057–0.073) | −0.005 (−0.007 to −0.003) |
| 24 months (n = 3828) | 0.152 (0.130–0.173) | 0.061 (0.053–0.069) | −0.003 (−0.006 to −0.001) |
| 36 months (n = 3633) | 0.133 (0.111–0.155) | 0.056 (0.048–0.064) | −0.004 (−0.006 to −0.001) |
| 48 months (n = 3197) | 0.131 (0.108–0.154) | 0.056 (0.048–0.065) | −0.004 (−0.006 to −0.001) |
| 72 months (n = 5923) | 0.131 (0.115–0.148) | 0.045 (0.039–0.051) | 0.000 (−0.002 to −0.002) |
| Head circumference | |||
| Early pregnancy (n = 5103) | 0.042 (0.022–0.061) | 0.020 (0.013–0.027) | −0.002 (−0.004 to 0.000) |
| Late pregnancy (n = 5214) | 0.086 (0.066–0.105) | 0.040 (0.033–0.047) | −0.004 (−0.006 to −0.002) |
| Birth (n = 2952) | 0.071 (0.050–0.093) | 0.033 (0.025–0.040) | −0.003 (−0.005 to −0.000) |
| 6 month (n = 4323) | 0.147 (0.125–0.169) | 0.069 (0.061–0.077) | −0.007 (−0.009 to −0.004) |
| 12 months (n = 3977) | 0.146 (0.123–0.169) | 0.067 (0.059–0.076) | −0.006 (−0.009 to −0.004) |
| 72 months (n = 5778) | 0.141 (0.122–0.160) | 0.059 (0.052–0.066) | −0.004 (−0.006 to −0.002) |
| Height | |||
| Birth (cm) (n = 3700) | 0.043 (0.033–0.052) | 0.017 (0.013–0.022) | −0.001 (−0.002 to 0.000) |
| 3 months (n = 3021) | 0.153 (0.129–0.178) | 0.062 (0.052–0.071) | −0.003 (−0.006 to 0.000) |
| 6 months (n = 3956) | 0.162 (0.140–0.185) | 0.069 (0.061–0.077) | −0.005 (−0.007 to −0.002) |
| 12 months (n = 4075) | 0.171 (0.148–0.193) | 0.070 (0.062–0.078) | −0.004 (−0.006 to −0.001) |
| 24 months (n = 3774) | 0.155 (0.133–0.177) | 0.057 (0.049–0.065) | −0.001 (−0.004 to 0.001) |
| 36 months (n = 3590) | 0.146 (0.124–0.168) | 0.058 (0.050–0.066) | −0.003 (−0.005 to 0.000) |
| 48 months (n = 3185) | 0.151 (0.128–0.174) | 0.057 (0.049–0.066) | −0.002 (−0.005 to 0.001) |
| 72 months (n = 5922) | 0.146 (0.128–0.163) | 0.051 (0.044–0.057) | 0.000 (−0.002 to 0.002) |
Values are regression coefficients per standard deviation score (SDS) (except if otherwise displayed, cm, or kg) and 95% confidence intervals for the beta for increase axial length (AL; mm), corneal radius (CR; mm) or AL/CR ratio from linear regression models. “n =”represents number of total group. Models were adjusted for gender, age of anthropometry measurement, ethnicity and age of eye measurements. Bonferroni adjusted p‐values are shown in bold (0.05/75 = p < 0.0007). Estimated foetal weight was based on the Hadlock formula during pregnancy.
Gestational age (AL β 0.019, 95%CI 0.009–0.029 and CR β 0.010 95%CI 0.007–0.014), birth weight and weight for gestational age were all positively associated with AL and CR at 6 years. The effect estimate for weight increased until 3 months postnatally for AL and up to 12 months for CR (Table 2). We found evidence for non‐linear associations and between birth weight for gestational age and AL or CR (Figure 1; Table S2 ). HC and weight measurements from 2 to 6 years showed evidence for non‐linearity for AL and CR, but not for AL/CR ratio with a significant quadratic term (Table S2 ).
Figure 1.
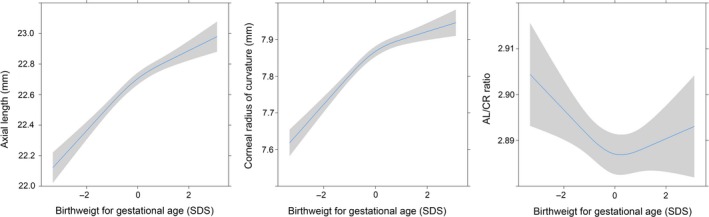
Non‐linearity in the association between axial length (left), corneal radius (middle) and AL/CR ratio (right) and birth weight for gestational age adjusted for age, gender and ethnicity.
Table 3 shows the results of the analyses for growth periods. All children with a foetal growth restriction had smaller AL and CR compared to children with normal foetal growth. Children with foetal accelerated growth had higher AL and greater CR, but no significant difference in AL/CR.
Table 3.
Foetal and infant growth patterns and correlation with ocular biometry at 6 years of age (N = 3849)
| Axial length (mm) | Corneal radius (mm) | AL/CR ratio | |
|---|---|---|---|
| Foetal restricted | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) |
| Infant restricted (n = 85) | −0.17 (−0.32 to −0.01) | −0.11 (−0.17 to −0.06) | 0.02 (0.00 to 0.04) |
| Infant normal (n = 424) | −0.19 (−0.27 to −0.11) | −0.07 (−0.10 to −0.04) | 0.00 (−0.01 to 0.01) |
| Infant accelerated (n = 490) | −0.13 (−0.21 to −0.06) | −0.07 (−0.10 to −0.04) | 0.01 (0.00 to 0.02) |
| Foetal normal | |||
| Infant restricted (n = 306) | −0.09 (−0.18 to 0.00) | −0.04 (−0.08 to −0.01) | 0.01 (−0.01 to 0.01) |
| Infant normal (n = 876) | Ref | Ref | Ref |
| Infant accelerated (n = 514) | 0.10 (0.03 to 0.18) | 0.03 (0.00 to 0.06) | 0.00 (−0.01 to 0.01) |
| Foetal accelerated | |||
| Infant restricted (n = 394) | 0.12 (0.04 to 0.20) | 0.03 (0.00 to 0.06) | 0.00 (−0.01 to 0.01) |
| Infant normal (n = 564) | 0.19 (0.12 to 0.27) | 0.07 (0.05 to 0.10) | −0.00 (−0.01 to 0.01) |
| Infant accelerated (n = 164) | 0.28 (0.17 to 0.39) | 0.11 (0.07 to 0.15) | −0.00 (−0.01 to 0.01) |
Values are regression coefficients and 95% confidence intervals for the beta for increase axial length (mm), corneal radius (mm) or AL/CR ratio from linear regression models. “n =“represents number of total group. p < 0.05 are shown in bold. Models were adjusted for gender, age at visit, ethnicity and SDS estimated foetal weight at second trimester. Restricted growth, normal growth and accelerated growth were defined as respectively <−0.67, >−0.67 and <0.67 and >0.67 standard deviation score (SDS) difference in SDS weight between second trimester and birth and birth and 6 months post‐natal. Bonferroni adjusted p‐values are shown in bold (p < 0.002).
Body growth measurements and emmetropisation
Conditional analysis (Figure 2a‐c) showed that the most important period for the association between body growth and ocular biometry at 6 years of age was growth up to 24 months. Up to this time, higher weight was associated with longer AL and larger CR. At the 72 months time point, significant additive associations were found only for AL and AL/CR, but not for CR (Figure 2a–c).
Figure 2.
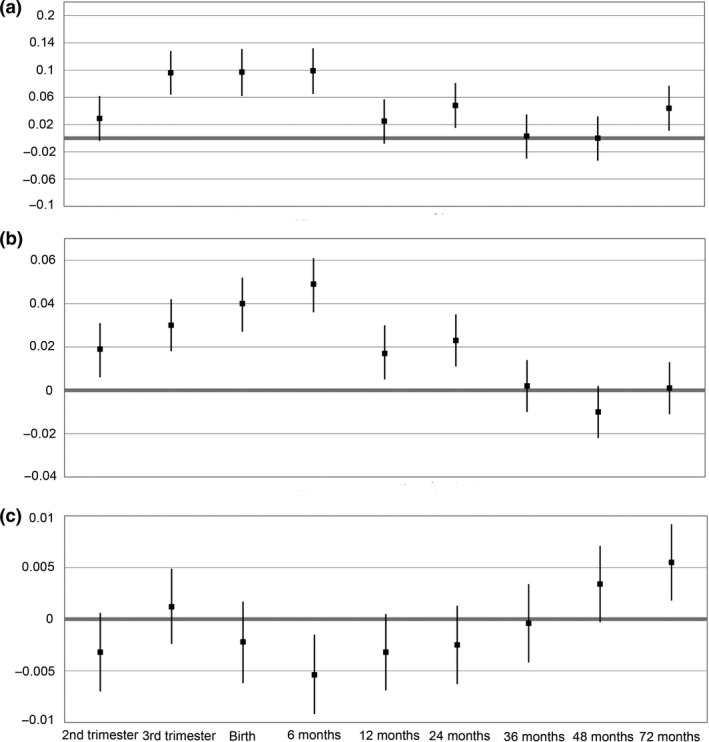
The association between foetal and infant weight SDS (standard deviation score) per time period with (a) axial length (mm), (b) corneal radius of curvature (mm) and (c) AL/CR ratio (mm mm−1) (N = 1595).
Genetics
To identify the genetic overlap in ocular biometry and growth, we created a weighted genetic risk score of 695 known SNPs associated with height and 60 SNPs associated with birth weight.16, 17 The many SNPs for height explained this trait better (6.4%) than the relatively low number of SNPs for birth weight explained birth weight (1.3%). Both genetic risk scores were significantly associated with AL as well as CR (Table 4). The genetic risk score for height explained 0.2% of the variance of AL and 0.5% of CR, and was significantly associated with AL/CR ratio (p 0.03). The genetic risk score for birth weight explained 0.23% and 0.1% for CR and AL, respectively, and was not significantly associated with AL/CR.
Table 4.
Genetic risk score of height and birth weight and the correlation with ocular biometry at 6 years of age
| Genetic risk scores | ||||||
|---|---|---|---|---|---|---|
| Height (SDS) (n = 3880) | Birth weight (SDS) (n = 3880) | |||||
| Outcomes variables | β (se) | p | R 2 | β (se) | p | R 2 |
| Birth weight (kg) | 0.059 (0.012) | 3.7 × 10 −7 | 0.006 | −0.047 (0.008) | 8.7 × 10 −9 | 0.012 |
| SDS Niklasson (SDS) | 0.119 (0.022) | 4.3 × 10 −8 | 0.007 | −0.110 (0.015) | 7.2 × 10 −13 | 0.013 |
| Height (cm) | 2.134 (0.107) | 1.4 × 10 −84 | 0.064 | −0.214 (0.079) | 0.007 | 0.001 |
| Axial length (mm) | 0.046 (0.016) | 0.004 | 0.001 | −0.023 (0.011) | 0.04 | 0.001 |
| Corneal radius (mm) | 0.026 (0.006) | 4 × 10− 6 | 0.005 | −0.013 (0.004) | 0.002 | 0.002 |
| AL/CR ratio | −0.004 (0.002) | 0.03 | 0.001 | 0.002 (0.001) | 0.20 | 0.000 |
Values are regression coefficients (se) for the beta for association between genetic risk scores and birth weight, standard deviation score (SDS) Niklasson, height, axial length (mm), corneal radius (mm) or AL/CR ratio from linear regression models. “n =“represents number of total group. p < 0.05 are shown in bold. Models were adjusted for gender, age at visit and ethnicity (4 principle components).
Proportionally to the variance explained for its own trait, the genetic risk score for birth weight explained a higher variance of CR (15.4%) than the genetic risk score for body height (7.8%). To test for causality, Mendelian randomisation was performed with the two‐stage least square method. Using the genetic risk scores as instrumental variables, we found significant support for a causal association between the determinants birth weight and height, and ocular biometric outcomes. The presence of more risk alleles for a taller height or higher birth weight was associated with higher AL and greater CR (all p < 0.05).
Discussion
The aim of this paper was to learn more about ocular biometry and the association with body growth and body growth patterns pre and postnatally. Body growth patterns occurring from mid pregnancy up to 24 months after birth were highly associated with ocular biometry at 6 years of age. Restricted prenatal and postnatal growth resulted in a smaller AL and CR, and accelerated growth resulted in a longer AL and larger CR. Genetic variants associated with taller body height and higher birth weight also predisposed to longer AL and larger CR, providing evidence for genetic overlap between these traits. These results can explain variance in ocular biometry measurements in children with similar spherical equivalent.
Strengths and weaknesses
Strengths of this study were the large sample size, the unique dataset of pre‐ and postnatal growth measurements, and the prospective design. In addition, we had measurements of ocular biometry at a young age, and determined a large number of potential confounders including genetic data to perform Mendelian randomisation. Still, some limitations have to be taken into account. First, lens parameters were not available, which hampered the study of all refractive components. Second, we cannot distinguish whether height or weight at birth is the dominant factor driving the association with ocular biometry as both are highly correlated. Height is difficult to measure accurately before and at birth,18, 19 but the Mendelian randomisation and effect estimates at 1 and 2 years suggests that height is the most important factor. The effect estimates on ocular biometry are relatively small with a maximum of 0.17 mm increase in axial length per SD score for height at 1 year of age. However, this can give a difference between the highest and lowest 2 per cent of around 0.68 mm. This is a difference in spherical equivalent, without taking other refractive components into consideration, of 1.6 D.20 Compared to environmental factors, e.g. time spent outdoors, which has an effect estimate of 0.034 mm per hour spent outdoors, the influence of neonatal height on ocular biometry is relatively large.21
Larger neonates have a higher AL and greater CR in later childhood
The results of anthropometric birth parameters were comparable with cross sectional studies in Sydney,7 Singapore,6 and in the United Kingdom.8 This study adds prenatal measurements and found that the associations between weight for gestational age and ocular biometry were non‐linear; in particular, children with a below average weight have smaller AL. The effect estimates of the association between body weight, height and head circumference measurement and ocular biometry was most significant with the measurements at 3 months postnatally. The conditional analysis validated this notion, and revealed that growth in the first 2 years of life was most important period for a longer AL and larger CR. This was similar to results found in the Avon Longitudinal Study of Parents and Children (ALSPAC) Study, which also reported an association with weight up to 10–80 months of age. ALSPAC also found a higher effect of the genetic risk score on CR than on AL.8 The genetic risk score was not significant for AL in ALSPAC, as they probably incorporated fewer markers and smaller sample size. Weight change in the present study between 4 and 6 years of age was associated with AL and AL/CR, but not with CR. As CR stabilises around 18 months, this is not surprising.22
It has been demonstrated that the corneal radius of curvature stops increasing around 18 months,22, 23 whereas axial length can increase up to teenage years and adolescence.2, 24 Our observation that the highest association with CR was with weight at 1 year of age is in line with this finding. Emmetropisation is hypothesised to be an active process of ocular scaling resulting from environmental influences,25, 26, 27, 28 release of retinal neurotransmitters29, 30, 31 and feedback mechanisms.32, 33 The results of this study feed into this hypothesis, because we found a high correlation between body growth, corneal curvature, and AL without influence on AL/CR ratio. The small effect between birth weight and AL/CR ratio may be explained by lens parameters, as the lens is thinner with an increased birth weight.6 The lack of association in older ages suggests that body growth may determine refractive components up to 2 years of age, subsequently overtaken by visual input which brings the focal point on the retina by changing lens refraction and axial length.
Conclusion
This study includes prenatal measurements and revealed that height and weight prenatally up to 2 years of age was related to a higher axial length and greater corneal radius of curvature at 6 years of age. Associations between weight for gestational age and ocular biometry were non‐linear; in particular children with a below average birth weight had smaller AL in later childhood. Body growth and ocular biometry at a young age may have a shared genetic background. However, with the fading effect of body growth on ocular biometry after 2 years of age, image projection on the retina may become the dominant trigger for changes in ocular biometry at later ages.
Conflicts of interest
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
Supporting information
Figure S1. Flowchart of participants.
Table S1. Pregnancy related determinants and association with axial length and corneal curvature
Table S2. Non‐linear associations of fetal and infant growth characteristics with ocular biometry (AL, CR and AL/CR ratio) in 6‐year‐old children.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Rotterdam area Municipal Health Service, Rotterdam, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR‐MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
The Generation R study is made possible by financial support from the Erasmus Medical Centre, Rotterdam; the Netherlands Organisation for Scientific Research (NWO); the Netherlands Organisation for Health Research and Development (ZonMw); the Dutch Ministry of Education, Culture and Science; the Dutch Ministry of Health, Welfare, and Sports; the European Commission (DG XII). The research was funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant 648268) and the Netherlands Organisation for Scientific Research (NWO, grant 91815655). The author was supported by the following foundations: Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, De Landelijke Stichting voor Blinden en Slechtzienden, MaculaFonds, Novartis Researchfunds, ODAS Stichting en het Oogfonds that contributed through UitZicht (Grant 2013‐24) and the Henkes Stichting. The funding organisations had no role in the design or conduct of this research. They provided unrestricted grants.
Tideman JWL, Polling JR, Jaddoe VWV, Vingerling JR & Klaver CCW. Growth in foetal life, infancy, and early childhood and the association with ocular biometry. Ophthalmic Physiol Opt 2019; 39: 245–252. 10.1111/opo.12630
Author contributions: JT, JRP and CK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JT, JRP, and CK contributed to the study concept and design, data analysis and interpretation, drafting, and revision of the manuscript. JV and VJ contributed to the study concept, data interpretation, and revision of the manuscript.
References
- 1. Mutti DO, Mitchell GL, Jones LA et al Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci 2005; 46: 3074–3080. [DOI] [PubMed] [Google Scholar]
- 2. Tideman JWL, Polling JR, Vingerling JR et al Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol 2018; 96: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown NP, Koretz JF & Bron AJ. The development and maintenance of emmetropia. Eye (Lond) 1999; 13: 83–92. [DOI] [PubMed] [Google Scholar]
- 4. Hashemi H, Khabazkhoob M, Miraftab M et al Axial length to corneal radius of curvature ratio and refractive errors. J Ophthalmic Vis Res 2013; 8: 220–226. [PMC free article] [PubMed] [Google Scholar]
- 5. Ojaimi E, Rose KA, Morgan IG et al Distribution of ocular biometric parameters and refraction in a population‐based study of Australian children. Invest Ophthalmol Vis Sci 2005; 46: 2748–2754. [DOI] [PubMed] [Google Scholar]
- 6. Saw SM, Tong L, Chia KS et al The relation between birth size and the results of refractive error and biometry measurements in children. Br J Ophthalmol 2004; 88: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ojaimi E, Robaei D, Rochtchina E, Rose KA, Morgan IG & Mitchell P. Impact of birth parameters on eye size in a population‐based study of 6‐year‐old Australian children. Am J Ophthalmol 2005; 140: 535–537. [DOI] [PubMed] [Google Scholar]
- 8. Northstone K, Guggenheim JA, Howe LD et al Body stature growth trajectories during childhood and the development of myopia. Ophthalmology 2013; 120: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim LS, Chua S, Tan PT et al Eye size and shape in newborn children and their relation to axial length and refraction at 3 years. Ophthalmic Physiol Opt 2015; 35: 414–423. [DOI] [PubMed] [Google Scholar]
- 10. Jaddoe VW, van Duijn CM, Franco OH et al The Generation R Study: design and cohort update 2012. Eur J Epidemiol 2012; 27: 739–756. [DOI] [PubMed] [Google Scholar]
- 11. Verburg BO, Steegers EA, De Ridder M et al New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population‐based cohort study. Ultrasound Obstet Gynecol 2008; 31: 388–396. [DOI] [PubMed] [Google Scholar]
- 12. Hadlock FP, Harrist RB, Carpenter RJ, Deter RL & Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984; 150: 535–540. [DOI] [PubMed] [Google Scholar]
- 13. Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C & Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977‐1981). Acta Paediatr Scand 1991; 80: 756–762. [DOI] [PubMed] [Google Scholar]
- 14. Allochtonen in Nederland 2004. Voorburg/Heerlen: Statistics Netherlands, 2004.
- 15. Keijzer‐Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP & Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol 2005; 58: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 16. Wood AR, Esko T, Yang J et al Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 2014; 46: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horikoshi M, Beaumont RN, Day FR et al Genome‐wide associations for birth weight and correlations with adult disease. Nature 2016; 538: 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson TS, Engstrom JL & Gelhar DK. Intra‐ and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr 1997; 24: 497–505. [DOI] [PubMed] [Google Scholar]
- 19. Wood AJ, Raynes‐Greenow CH, Carberry AE & Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length‐board. J Paediatr Child Health 2013; 49: 199–203. [DOI] [PubMed] [Google Scholar]
- 20. Ophthalmology AAO . Clinical Optics Basic and Clinical Science Course 2017 ‐ 2018:200.
- 21. Tideman JW, Polling JR, Voortman T et al Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol 2016; 31: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Augusteyn RC, Nankivil D, Mohamed A, Maceo B, Pierre F & Parel JM. Human ocular biometry. Exp Eye Res 2012; 102: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inagaki Y. The rapid change of corneal curvature in the neonatal period and infancy. Arch Ophthalmol 1986; 104: 1026–1027. [DOI] [PubMed] [Google Scholar]
- 24. Zadnik K, Manny RE, Yu JA et al Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci 2003; 80: 226–236. [DOI] [PubMed] [Google Scholar]
- 25. He M, Xiang F, Zeng Y et al Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 26. Rose KA, Morgan IG, Ip J et al Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 27. Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA & Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta‐analysis. Ophthalmology 2012; 119: 2141–2151. [DOI] [PubMed] [Google Scholar]
- 28. Tideman JWL, Polling JR, Hofman A, Jaddoe VW, Mackenbach JP & Klaver CC. Environmental factors explain socioeconomic prevalence differences in myopia in 6‐year‐old children. Br J Ophthalmol 2018; 102: 243–247. [DOI] [PubMed] [Google Scholar]
- 29. Verhoeven VJ, Hysi PG, Wojciechowski R et al Genome‐wide meta‐analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet 2013; 45: 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feldkaemper M & Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 31. Tideman JW, Fan Q, Polling JR et al When do myopia genes have their effect? Comparison of genetic risks between children and adults. Genet Epidemiol 2016; 40: 756–766. [DOI] [PubMed] [Google Scholar]
- 32. Zhou X, Pardue MT, Iuvone PM & Qu J. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wildsoet CF & Schmid KL. Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res 2000; 40: 3273–3282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of participants.
Table S1. Pregnancy related determinants and association with axial length and corneal curvature
Table S2. Non‐linear associations of fetal and infant growth characteristics with ocular biometry (AL, CR and AL/CR ratio) in 6‐year‐old children.


