Abstract
The meso-striatal dopamine system influences responses to rewards and the motivation to seek them out. Marked individual differences in these responses are seen in laboratory animals, related in part to input from the prefrontal cortex. Here we measured the relation between cortical morphology and drug-induced striatal dopamine release in healthy young people. Participants were 24 (17 male, 7 female; age 23.0 ± 6.2 years) stimulant drug-naive subjects who underwent PET [11C]raclopride scans with 0.3 mg/kg d-amphetamine orally and placebo, and an anatomical MRI scan for measuring cortical thickness. As expected, d-amphetamine produced significant reductions in [11C]raclopride binding potential in the striatum as a percentage of the value in the placebo condition. There was substantial individual variability in this response, which was correlated with cortical thickness in the frontal lobe as a whole. The association was strongest in the anterior part of the right lateral prefrontal cortex and bilateral supplementary motor area. A thicker cortex was correlated with a smaller dopamine response. Together, this work demonstrates in humans an association between cortical thickness and the striatal dopamine response to drugs of abuse. Although prefrontal regulation of striatal function has been well studied, it was unclear whether the thickness of the prefrontal cortex was an acceptable proxy to the function of that region. These results suggest it is.
Introduction
A common property of addictive drugs is to increase striatal dopamine neurotransmission (Di Chiara and Imperato, 1988). Striatal function is regulated in part by anatomically distinct loops connecting areas of the striatum to corresponding portions of the cortex (Selemon and Goldman-Rakic, 1985; Alexander et al., 1986; Lawrence et al., 1998; Haber and Knutson, 2010). Among the various models proposed, the tripartite model (Selemon and Goldman-Rakic, 1985; Parent and Hazrati, 1995; Nakano et al., 2000; Saint-Cyr, 2003) is particularly useful in studies of dopamine function: it divides the striatum into limbic, associative, and sensorimotor subdivisions, and it describes the striatum's connectivity with both cortical and midbrain dopamine cell body regions (Joel and Weiner, 2000; Martinez et al., 2003). Limbic striatum (LST) comprises the nucleus accumbens and the most ventral portions of the caudate and putamen, and it is connected to medial aspects of the prefrontal, orbitofrontal, and anterior cingulate cortices. Associative striatum (AST) comprises the precommissural putamen and dorsal portions of the caudate and is connected to primarily lateral aspects of prefrontal cortex. The sensorimotor striatum (SMST) comprises the postcommissural putamen and is connected to primary motor, premotor, and supplementary motor areas.
The anatomical connectivity of the striatum and frontal cortices is reflected in their functional connectivity. They are coactivated in fMRI studies (Postuma and Dagher, 2006; Choi et al., 2012; Robinson et al., 2012), and frontostriatal connections are apparent with diffusion tensor imaging (Leh et al., 2007; Kamali et al., 2010; Robinson et al., 2012). Transcranial magnetic stimulation of parts of the frontal cortex alters striatal dopamine release (Strafella et al., 2001, 2003, 2005; Ohnishi et al., 2004; Ko et al., 2008), and increased metabolic activity in prefrontal regions is associated with smaller amphetamine-induced striatal dopamine release in healthy subjects (Volkow et al., 2007). In laboratory animals, ablation of prefrontal dopamine terminals results in increased striatal dopamine function (Pycock et al., 1980).
In the present study, we set out to determine whether the anatomical and functional relationships between striatum and cortex are reflected in cortical morphology. Using relatively new automated algorithms, indices of cortical morphology, such as cortical thickness, can be reliably calculated from T1-weighted MRI information. Thicker cortex has been repeatedly associated with improved cognitive performance (Narr et al., 2007; Dickerson et al., 2008; Hartberg et al., 2010; Westlye et al., 2011; but see Hyde et al., 2007), and it may be an index of the functional integrity of the cortex. Here we assessed the relationship between cortical thickness and the magnitude of the dopaminergic response to a d-amphetamine challenge in healthy subjects. Based on the metabolic findings (Volkow et al., 2007), we specifically hypothesized that cortical thickness within the frontal lobe would be inversely related to dopamine release within the striatum as measured with PET using a [11C]raclopride tracer. Data were examined from the striatum as a whole and within its functional subdivisions.
Materials and Methods
Subjects were recruited from the community by advertisements in local newspapers and on websites. Volunteers who passed a preliminary telephone screen completed a semistructured clinical interview for DSM-IV (SCID-IV, Nonpatient edition; First et al., 1995) for assessment of present and past history of axis I disorders, and the Family Interview for Genetic Studies (1992). The exclusion criteria were as follows: (1) any personal history of axis I disorder; (2) first- or second-degree relatives with current or past axis I disorder, including substance use problems; (3) use of psychostimulant drugs; (4) use of other drug use of abuse, except modest alcohol consumption and infrequent marijuana and tobacco use; (5) cardiovascular, neurological, or other disorders that might be aggravated by participation in the study or complicate interpretation of the study's results; (6) seropositive pregnancy test; (7) adoption; or (8) being unable to provide a complete family history.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Research Ethics Board of the Montreal Neurological Institute. All participants gave written informed consent. Data from these subjects have previously appeared as control populations in two studies (Casey et al., 2010; Cherkasova et al., 2010).
Procedure.
We tested 24 healthy adults (17 male, 7 female; age 23.0 ± 6.2 years, range 18–42 years). All subjects underwent two PET scans, on separate days (subjects were scanned 21.4 ± 27.1 d apart, range 3–95 d), with the tracer [11C]raclopride after ingestion of either d-amphetamine (0.3 mg/kg, p.o., 60 min before scanning) or a lactose placebo given in a double-blind, fully randomized, and counterbalanced design. This procedure provides a reliable [11C]raclopride change signal (Leyton et al., 2002) that can be increased (Boileau et al., 2006) and decreased (Leyton et al., 2004) by experimental manipulations. On each test day, subjects arrived at the laboratory in the morning after 2 h of fasting. Before each test session, subjects abstained from tobacco for at least 12 h and from alcohol for at least 24 h; cannabis users were asked to abstain for up to 1 month, or until they could provide a negative urine drug screen. On the morning of each test day, all tested negative on a urine drug screen sensitive to cocaine, opiates, phencyclidine, barbiturates, Δ9-tetrahydrocannabinol, benzodiazepines, and amphetamines (Triage Panel for Drugs of Abuse, Biosite Diagnostics). Women were tested during the follicular phase and provided a negative urine pregnancy screen on the morning of each PET scan (Assure FastRead hCG Cassette, Conception Technologies).
Sixty minutes before PET scanning (time 0), a baseline blood sample was drawn (collected via venipuncture directly into an EDTA vacutainer for determination of plasma amphetamine concentration). Subjects then ingested either d-amphetamine (0.3 mg/kg, p.o.) or a lactose placebo. At time 30 min, subjects were installed on the PET couch where a heparin and normal saline-primed catheter was inserted, and a transmission scan for attenuation correction was performed. At time 60 min, the [11C]raclopride tracer was injected, and a blood sample was collected. Blood samples were taken again halfway through the scan (time 90 min) and just before exiting the scanner (time 120 min).
PET and MRI testing.
All subjects were scanned on a Siemens ECAT HR+ PET scanner (CTI/Siemens) with lead septa removed (63 slice coverage, with a maximum resolution of 4.2 mm at full-width half-maximum in the center of the field of view). Immediately after the transmission scan, [11C]raclopride (range 8–10 mCi) was injected as a bolus into the antecubital vein. Emission data were collected over 60 min in 26 time frames of progressively longer duration.
For anatomical coregistration, high-resolution (1 mm) T1-weighted MRIs were obtained for all subjects on a 1.5 T Siemens scanner, using gradient echo pulse sequence (TR = 9.7 ms, TE = 4 ms, flip angle = 12°, FOV = 250, and matrix 256 × 256). These volumes were corrected for image intensity nonuniformity, and linearly and nonlinearly transformed into standardized stereotaxic Talairach-like space (Talairach and Tournoux, 1988) using an automated feature matching to the ICBM152 template (Collins et al., 1994). Each individual's MRI was also coregistered to their summed radioactivity PET images (Evans et al., 1992), and this transformation was concatenated with the MRI to Talairach transformation to move all PET data into standardize stereotaxic space. All further analysis was conducted in standardized stereotaxic space.
PET images were reconstructed using a 6 mm full-width half-maximum Hanning filter and corrected for movement (Costes et al., 2009). Parametric images were generated by computing [11C]raclopride's binding potential relative to the nondisplaceable concentration (BPND) (Innis et al., 2007) at each voxel using a simplified kinetic model that uses the cerebellum as a reference tissue nearly devoid of dopamine D2/D3 receptors to describe the kinetics of the free and specifically bound ligand (Gunn et al., 1997). BPND expresses the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable ligand in tissue using the estimated concentration of available dopamine D2/D3 receptors (Bavail), the dissociation constant of the radiotracer from D2/D3 receptors (Kd), and the free fraction of nonspecifically bound tracer in the brain (FND) (Innis et al., 2007), as follows:
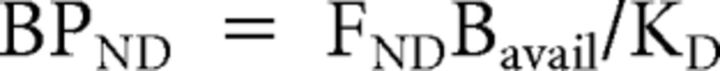 |
In a resting condition, BPND is proportional to the concentration of available D2/D3 receptors; BPND decreases when dopamine release is elicited, and the magnitude of the change in BPND has been shown to be proportional to the increase in extracellular dopamine (Laruelle et al., 1997a, b; Laruelle, 2000).
Striatal ROIs.
The striatum was divided into six ROIs as described by Martinez et al. (2003). ROIs comprising the whole striatum (caudate, putamen, and nucleus accumbens), LST, AST, and SMST were defined on both left and right sides of each individual's MRI (Martinez et al., 2003), and BPND values were extracted from those regions in both the amphetamine and placebo condition. The amphetamine-induced change in BPND (ΔBPND) was then calculated for each subject as a percentage of placebo day BPND.
Cortical thickness.
Native T1-weighted MRIs were processed through the CIVET (in-house software developed in the laboratory of Alan C. Evans, Montreal Neurological Institute) automated pipeline (version 1.1.11) (Ad-Dab'bagh et al., 2006). This pipeline includes the CLASP (in-house software developed in the laboratory of Alan C. Evans, Montreal Neurological Institute) algorithm for generating cortical thickness measurements at 40,962 vertices per hemisphere (Collins et al., 1995; MacDonald et al., 2000; Kim et al., 2005; Ad-Dab'bagh et al., 2006; Lyttelton et al., 2007). Cortical thickness is calculated as the distance between the outer CSF–gray matter and gray matter–white matter interfaces (MacDonald et al., 2000; Kim et al., 2005; Lyttelton et al., 2007).
Statistics.
Statistical analyses were implemented using SurfStat, a statistical toolbox created for MATLAB (MathWorks) by Dr. Keith Worsley (http://www.math.mcgill.ca/keith/surfstat/), and the inbuilt multiple linear regression tools in Matlab12b (linear model). Each subject's absolute native-space cortical thickness image (blurred to 20 mm) was linearly regressed against ΔBPND. A series of linear models were considered. These models included up to two continuous variables of subjects' age (age) and brain volume (total brain volume), and up to two categorical variables of sex (male/female) and the study that subjects were originally recruited for other studies (Casey et al., 2010; Cherkasova et al., 2010). The models were tested using the average thickness of the frontal lobe as the response variable. A model allowing for main effects of all factors and interactions between ΔBPND and the categorical variables sex and study was used as a starting point. This model was used as the upper limit in stepwise linear regression (linear model stepwise), terms were dropped when nested models were compared, and the more complex model was not better than the simpler model (p > 0.10, SSE method). The false discovery rate was used to correct for multiple comparisons, with a critical threshold of Q = 0.05 (interpreted similarly to a p value). We a priori predicted that ΔBPND would be positively related to the thickness of the frontal cortex (thicker cortex would correlate with smaller dopaminergic responses to amphetamine), and results within the frontal cortex were corrected only for the volume of that mask.
Cortical ROIs.
Analysis was conducted first at a cortical lobe scale of analysis, and then at each vertex within the frontal lobe and cingulate. ROIs for each lobe were defined on the average cortical surface using the AAL label set (Tzourio-Mazoyer et al., 2002), the ROIs were frontal lobe (labels 1–28), insula (labels 29 and 30), limbic (labels 31–40), occipital (labels 43–56), parietal (labels 57–70), and temporal (79–90). Average cortical thickness was calculated in each ROI and was then considered as a function of the whole striatum dopamine response using model 1 (see Results).
Analysis at each vertex was conducted within a frontal region of interest, which comprised the frontal lobe (labels 1–28) and the anterior and median portions of the cingulate (labels 31–34). Cortical thickness at each vertex was then considered as a function of the whole striatum dopamine response using model 1.
Analysis of striatal subregions.
Our goal in examining the striatal subregions was to assess the possibility that the activity in a given region would be more closely associated to thickness in cortical regions to which it is anatomically connected. To this end, we compared the t-maps resulting from fitting the selected linear model (see Results) in all six striatal subregions and assigned each vertex in the frontal cortex to the subregion with the highest t value at that vertex (we required a minimum t of 1.7, equivalent to p = 0.05 unadjusted).
We then tried to more precisely attribute cortical thickness to the striatal subregions by removing the shared variance between the striatal subregions from the model. Principal component analysis (Factor procedure in IBM SPSS Statistics v20) of ΔBPND values in the six striatal subregions yielded a one-factor solution explaining 79.34% of the variance in the ROIs. The regression scores associated with this factor were entered then entered into the selected linear model (see Results).
Plasma amphetamine determination.
Plasma amphetamine concentrations were analyzed using combined gas chromatography-mass spectrometry after extraction and derivatization of amphetamine as described by Asghar et al. (2001)
Results
BPND
Tracer dose
The data used here come from two samples with identical experimental procedures; however, because of changing standards, the ethics committees required different limits on the injected dose of [11C]raclopride. Specific activity of representative samples of [11C] labeled tracers at the Montreal Neurological Institute varies between 200 and 500 Ci/mmol. The injected dose in the Cherkasova et al. (2010) subjects was 6.42 ± 0.48 mCi (mean ± SD), the mass dose was in the range of 4.28–10.69 μg, or 0.18–0.44 nmol/kg, and receptor occupancy was in the range of 1.01–2.51%. In Casey et al. (2010), the injected dose was 9.10 ± 1.45 mCi, corresponding to a mass dose of 6.06–15.16 μg, or 0.25–0.62 nmol/kg, and receptor occupancy in the range of 1.43–3.51% (Hume et al., 1998). BPND is thought to be insensitive to mass effects of raclopride at concentrations <1–2 nmol/kg (Alexoff et al., 2003), and estimates in our work are substantially below this threshold.
The injected doses did not vary systematically by day (paired t test t(23) = 1.17, p = 0.255), and there was no association between the injected dose on a day and BPND on that day (partial correlations adjusted for study: placebo, r = −0.11, p = 0.624; amphetamine, r = −0.14, p = 0.533), nor did we find associations between an individual's mean [11C]raclopride dose and ΔBPND in the striatum (r = 0.15, p = 0.481), or between ΔBPND and Δ[11C]raclopride dose (r = −0.22, p = 0.320) (Table 1).
Table 1.
Comparison of BPND with [11C]raclopride dosea
| Placebo | Amphetamine | Mean | Δ | |
|---|---|---|---|---|
| L AST | r = −0.17, p = 0.434 | r = −0.14, p = 0.516 | r = 0.22, p = 0.303 | r = −0.17, p = 0.432 |
| L LST | r = −0.08, p = 0.724 | r = −0.22, p = 0.304 | r = −0.18, p = 0.419 | r = −0.17, p = 0.438 |
| L SMST | r = −0.03, p = 0.895 | r = −0.11, p = 0.602 | r = 0.12, p = 0.597 | r = −0.27, p = 0.211 |
| R AST | r = −0.12, p = 0.575 | r = −0.14, p = 0.533 | r = 0.17, p = 0.432 | r = −0.12, p = 0.583 |
| R LST | r = −0.14, p = 0.537 | r = −0.11, p = 0.615 | r = 0.09, p = 0.679 | r = −0.13, p = 0.554 |
| R SMST | r = 0.07, p = 0.766 | r = −0.03, p = 0.892 | r = 0.11, p = 0.629 | r = −0.11, p = 0.604 |
| Striatum | r = −0.11, p = 0.624 | r = −0.14, p = 0.533 | r = 0.15, p = 0.481 | r = −0.22, p = 0.320 |
a Partial correlations (df = 21, adjusted for study) of BPND versus [11C]raclopride dose. Placebo: BPND and dose from the placebo day; Amphetamine: BPND and dose from the amphetamine day; Mean: ΔBPND and the individual's mean dose; Δ: ΔBPND and Δdose. L, Left; R, right. No significant associations were observed.
Order effects
d-Amphetamine or placebo was given in a randomized, double-blind, and counterbalanced order. Of the 24 subjects, 13 had amphetamine followed by placebo and 11 had placebo followed by amphetamine. No order effects were observed in placebo day (BPND t(22) = −0.43, p = 0.669) or ΔBPND in the whole striatum (t(22) = −1.31, p = 0.203).
ΔBPND
d-Amphetamine administration produced significant decreases in BPND in the whole striatum (p = 0.005), the LST (p < 0.001), and SMST (p < 0.001), and AST on the right (p = 0.031) but not left (p = 0.085) sides (Table 2; Fig. 1). The magnitude of the reduction in BPND was not markedly dissimilar to previous work using an intravenous route of administration (Martinez et al., 2003) (Table 3) or longer intervals between drug and scanning (Shotbolt et al., 2012) (Table 4). Peak plasma amphetamine was 19.6 ± 13.7 ng/ml. Plasma amphetamine levels were unrelated to ΔBPND (r = −0.11, p = 0.645).
Table 2.
BPND in striatal ROIsa
| Placebo day BPND | ΔBPND (%) | t(23) | p | |
|---|---|---|---|---|
| L AST | 2.14 (0.32) | −4.14 (14.28) | −1.42 | 0.085 |
| L LST | 2.08 (0.33) | −9.45 (12.18) | −3.80 | <0.001 |
| L SMST | 2.49 (0.34) | −9.35 (11.84) | −3.87 | <0.001 |
| R AST | 2.21 (0.29) | −5.15 (12.83) | −1.97 | 0.031 |
| R LST | 2.15 (0.28) | −12.88 (11.25) | −5.61 | <0.001 |
| R SMST | 2.42 (0.33) | −9.54 (11.52) | −4.06 | <0.001 |
| Whole striatum | 2.18 (0.27) | −6.73 (11.6) | −2.84 | 0.005 |
a Placebo day BPND: related to dopamine D2/D3 receptor concentrations; ΔBPND: expresses the amphetamine-induced change in BPND as a percentage of BPND on the placebo day. One-tailed tests of the proposition that ΔBPND is <0.
Figure 1.
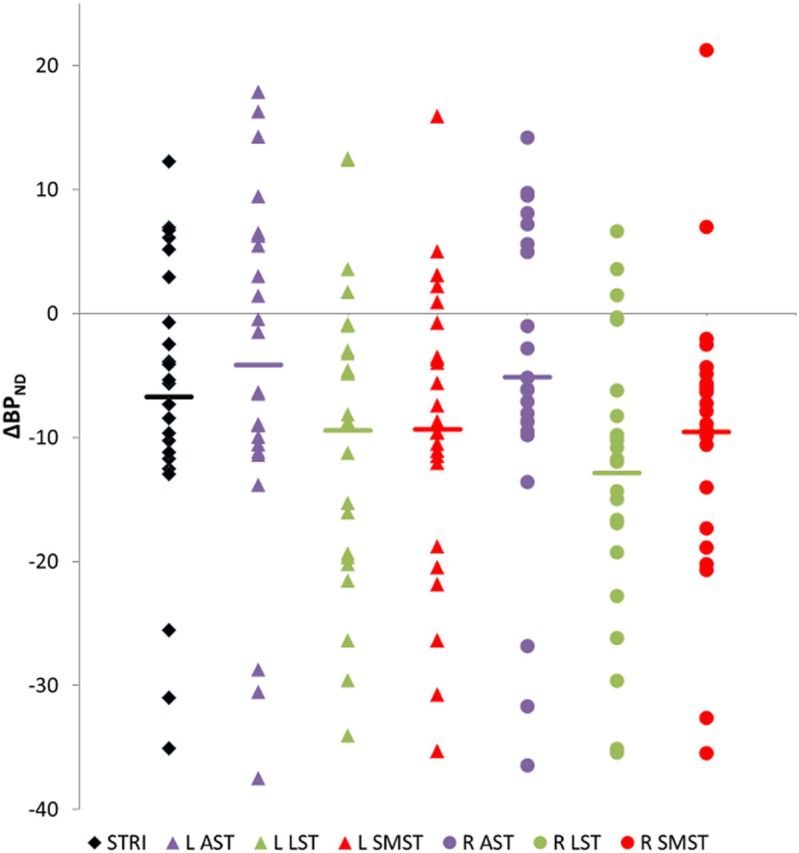
Amphetamine-induced decreases in BPND. ΔBPND in the striatal regions of interest. ΔBPND is significantly less than zero in all regions of interest, except L AST (p = 0.085). L, Left hemisphere; R, right hemisphere; STRI, whole striatum.
Table 3.
Comparison of ΔBPND across studiesa
| Current study: 0.3 mg/kg p.o., 1 h before tracer injection | Martinez et al. (2003)57: 0.3 mg/kg i.v., 2 min before tracer injection | |
|---|---|---|
| LST | −11 ± 12 | −16 ± 12 |
| AST | −5 ± 14 | −6.5 ± 8 |
| SMST | −9 ± 12 | −13 ± 8 |
a Data are mean ± SD of percent ΔBPND in LST, AST, and SMST from the present work and Martinez et al. (2003).57 ROI subregions were averaged across hemisphere for comparison.
Table 4.
Comparison of ΔBPND across studiesa
| Shotbolt et al. (2012)72: 0.3 mg/kg p.o., 3 h before tracer injection | |
|---|---|
| VST | −13 ± 7 |
| CD | −7 ± 4 |
| PU | −11 ± 6 |
a Data are mean ± SD of percent ΔBPND in ventral striatum (VST, identical to LST) and the remaining portions of the caudate (CD) and putamen (PU) from Shotbolt et al. (2012).72 ROI subregions were averaged across hemisphere for comparison.
Effects of drug use
Although all of our subjects were stimulant drug-naive, some reported use of alcohol (N = 16, 138 ± 135 uses, range 0–500 uses), cannabis (N = 14, 24 ± 29 uses, range 0–100 uses), and tobacco (N = 8, 81 ± 260 uses, range 0–1068 uses). As these data were highly right-skewed, they were log10 transformed (after adding a constant of 1 to all values) and regressed against ΔBPND. No associations were observed (alcohol: r = 0.04, p = 0.870; cannabis: r = −0.20, p = 0.437; tobacco: r = 0.06, p = 0.808). The same regressions were conducted with the reported age of first use, average uses per year, and use in past 30 d; no associations were observed. Drug use data were available for 17 of the 24 participants.
Cortical thickness
Model selection
In developing a model to assess the relationships between the average thickness of the frontal lobe and ΔBPND, we started with a model that was likely to be overfitted and then simplified it. The initial model included age, total cerebral volume, sex, and study, as well as interaction between ΔBPND and the categorical variables of sex and study. This model suggests that the ΔBPND term was the biggest contributor to the model's explanatory power (t(16) = 1.97, p = 0.067) but that overall the model did not accurately describe the variability in cortical thickness (F(1,16) = 1.82, p = 0.152). Stepwise linear regression with the preceding model as an upper bound suggested that the best-fitting model included only a constant and ΔBPND. In this simplified model (model 1), ΔBPND was a strongly associated with cortical thickness in the frontal lobe (t(22) = 3.25, p = 0.004). None of the other terms were significant contributors (age, t(16) = −0.04, p = 0.968; total cerebral volume, t(16) = 1.35, p = 0.195; sex, t(16) = −0.47, p = 0.641; study, t(16) = −0.87, p = 0.392; ΔBPND × sex, t(16) = −0.73, p = 0.478; ΔBPND × study, t(16) = 0.38, p = 0.709).
Further examination of this model at both the lobewise and voxelwise scales of analysis suggested that the data were suitable for regression with ordinary least-squares. In the frontal lobe as a whole, none of the points had leverage scores >0.33 (maximum leverage 0.30, maximum Cook's distance = 0.13). In the voxelwise analysis, only 3.7% of vertices (1026 of 27,448) had points with leverage scores >0.33, suggesting that this simplified model (model 1) was appropriate in a great majority of vertices.
Lobe scale analysis
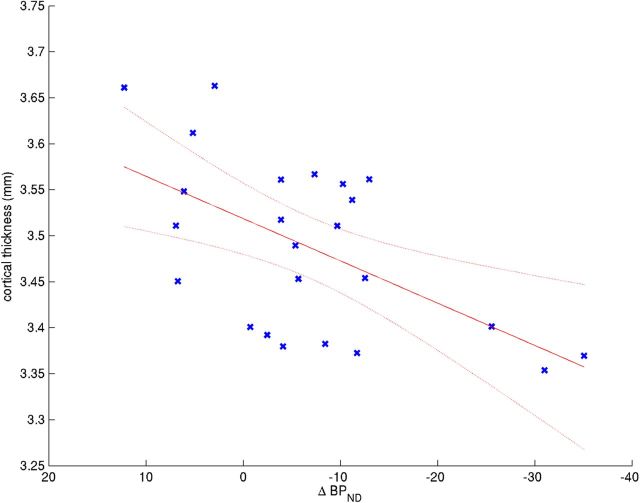
As predicted, cortical thickness was most closely associated with striatal ΔBPND in model 1 in the frontal cortex (t(22) = 3.25, Q = 0.011; Fig. 2). Significant associations were also observed in limbic cortex (t(22) = 1.93, Q = 0.050), parietal cortex (t(22) = 2.12, Q = 0.045), and temporal cortex (t(22) = 2.22, Q = 0.045). Although the effect was similar in the insula (t(22) = 1.20, Q = 0.122) and occipital cortex (t(22) = 1.74, Q = 0.058), the effect was not statistically significant in these regions.
Figure 2.
ΔBPND is correlated with the average thickness of the whole frontal lobe. ΔBPND versus average thickness of the frontal lobe in model 1. ΔBPND contributed significantly to the model (t(22) = 3.25, Q = 0.011).
Because we predicted a priori that the effect would be preferentially found in the frontal lobe, we examined this region in more detail. The frontal cortex ROI was divided into right and left hemispheres, and the effect of striatal ΔBPND in model 1 was estimated in these regions, false discovery rate correcting for only these two comparisons. We found significant associations in both left (t(22) = 2.38, Q = 0.013) and right (t(22) = 3.65, Q = 0.001) hemispheres.
Vertex scale analysis
Next, model 1 was assessed at each of the 81,924 vertices on the cortical surface, where the value at each vertex represented an individual's absolute cortical thickness at that point. We then looked within the frontal ROI (27,448 vertices) for areas where striatal ΔBPND was significantly correlated with cortical thickness, and assessed significance after adjusting for the false discovery rate. After adjustment, 1393 vertices were identified as being significantly associated with ΔBPND (Q values < 0.05). Areas of significant association were found in the left hemisphere in medial prefrontal cortex (x, y, z = −6, 58, 15), orbitofrontal cortex (x, y, z = 4, 45, −26), supplementary motor area (x, y, z = −12, −4, 76), and primary motor cortex (x, y, z = −45, −12, 56). In the right hemisphere, areas of association were found in lateral prefrontal cortex (x, y, z = 32, 52, 8; x, y, z = 39, 43, 16; and x, y, z = 45, 27, 26) and supplementary motor area, right (x, y, z = 13, 3, 70) (Figure 3).
Figure 3.
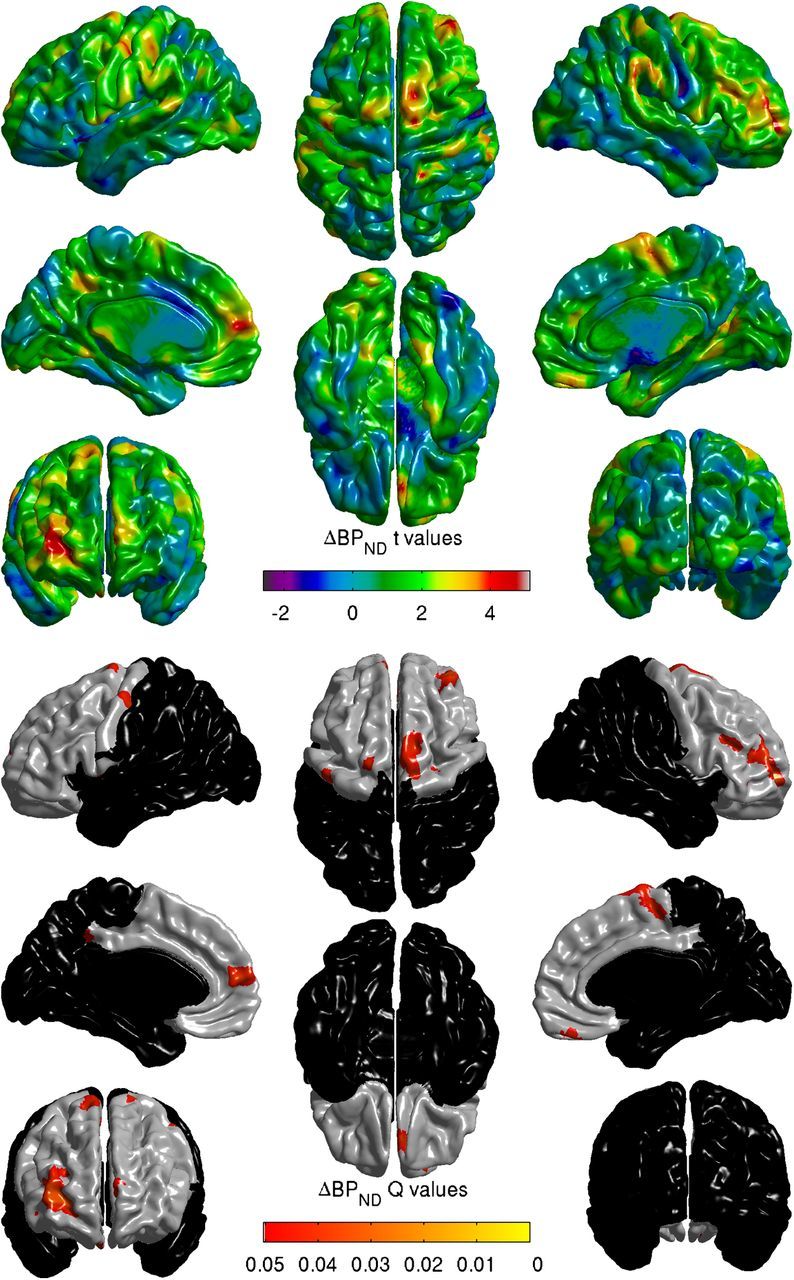
ΔBPND is correlated with cortical thickness in subregions of the frontal lobe. Top, t-map of the effect of striatal ΔBPND in model 1 fit at each vertex over the cortical mantle. Bottom, Areas of significant association between ΔBPND and cortical thickness in frontal ROI. False discovery rate-corrected Q values <0.05.
Placebo day BPND
BPND on the placebo day, which gives an index of the number of available D2/D3 receptors, was also examined as a correlate of cortical thickness at both scales of analysis (using model 1). No associations between BPND on the placebo day and cortical thickness were observed at the lobe level (minimum Q = 0.870) or the vertex level of analysis (minimum Q = 0.998).
Associations with striatal morphology
It is conceivable that the observed association between ΔBPND and cortical thickness is secondary to changes in gray matter that affect both striatum and cortex. To address this possibility, we compared the volume of the striatum (based on the ROIs used to assess BPND) with cortical thickness. The volume of the striatum was not correlated with the thickness of the frontal cortex as a whole (t(22) = −0.67, Q = 0.745), or in left and right hemispheres (left t(20) = −0.15, Q = 0.864; right t(20) = −1.13 Q = 0.864). No associations were observed when striatal volume was regressed against cortical thickness at each vertex in the frontal lobe (minimum Q = 0.471).
We also compared thickness of the frontal lobe (left, right, and combined) to voxelwise maps of gray matter density within the striatum (Zijdenbos et al., 1998; Tohka et al., 2004) (from the same 8 mm blurred gray matter classification images that were used in the measurement of cortical thickness). Frontal thickness was not associated with gray matter density in any part of the striatal ROI (minimum Q whole frontal lobe = 0.863; left hemisphere = 0.947; right hemisphere = 0.497).
Associations with striatal subregions
ΔBPND in the functional subdivisions of the striatum were unsurprisingly highly correlated to both the whole striatum and to each other (r2 range, 0.33–0.94). We compared the t-maps resulting from fitting model 1 in all six striatal subregions and produced a plot of areas of best fit based on which subregion resulted in the highest t value at that vertex. The resulting map of best-fitting subregions is presented in Figure 4.
Figure 4.
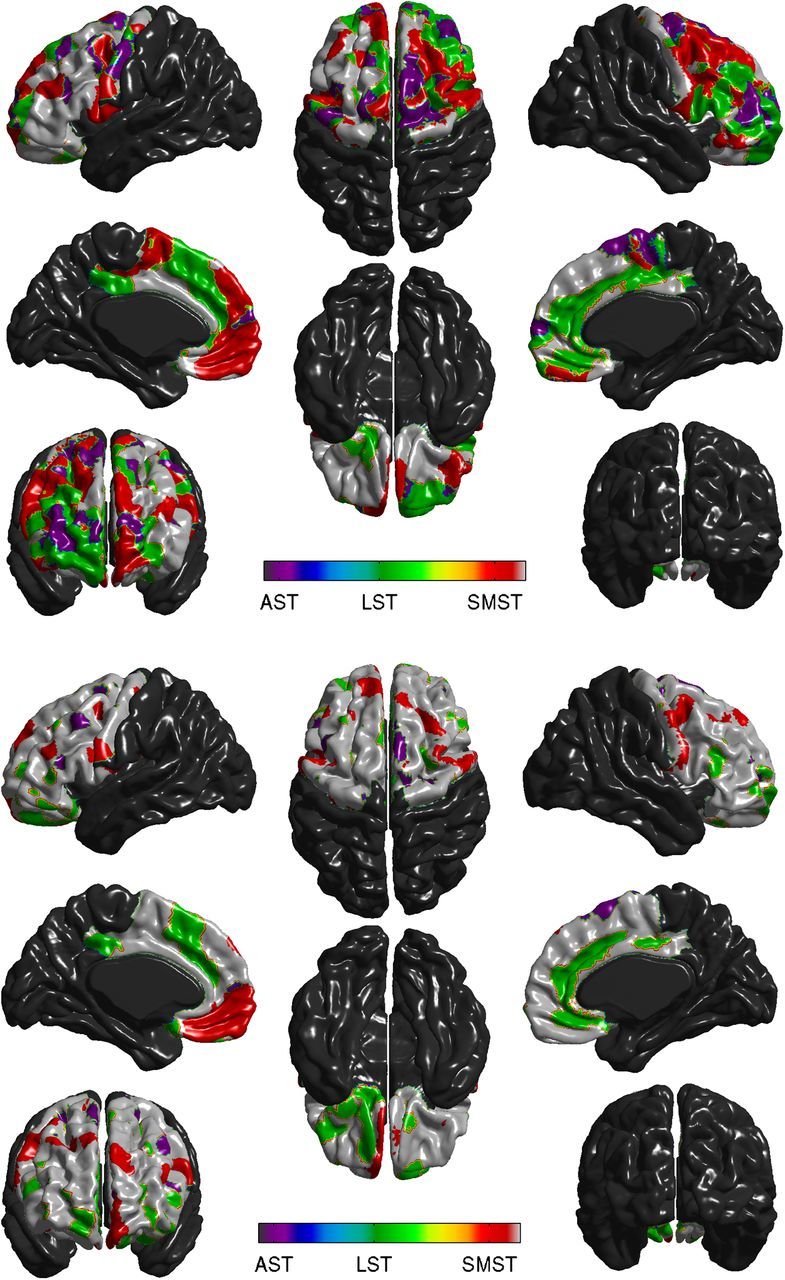
Vertexwise assignment of each frontal vertex to AST, LST, or SMST. Top, Each vertex in which ΔBPND was correlated to cortical thickness in model 1 at an unadjusted p < 0.05 was assigned to AST, LST, or SMST based on the highest t-value among the striatal subregions. Bottom, Assignment to striatal subregions as on top, but t-values were assessed in model 2, which removes the shared variance of the striatal subregions from the equation. Purple represents AST; green represents LST; red represents SMST.
We then tried to more precisely attribute cortical thickness to the striatal subregions by removing the shared variance between the striatal subregions from the model using model 2.
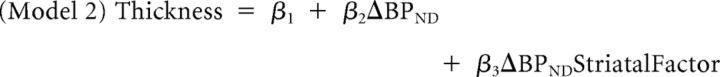 |
Although the results of model 2 are illustrative of the distinct patterns of association between striatal subregions and cortex, as expected the t-values were smaller (the shared variance of the striatum, which was associated with cortical thickness was covaried out) and they were not statistically significant after correction for multiple comparisons (all Q > 0.14).
Discussion
The present study indicates that cortical thickness, particularly within the frontal lobe, is related to drug-induced striatal dopamine release. Subjects who showed larger dopaminergic responses to amphetamine challenge, as indexed by decreases in [11C]raclopride binding potential, had thinner cortex. This effect was evident both when cortical thickness was averaged across the frontal lobe and at a higher statistical threshold in aspects of the motor cortices (primary, supplementary, premotor), the lateral PFC, medial PFC, and OFC.
Both anatomical and functional connections between the frontal cortex and striatum have been extensively reported. The novel aspect of this work is to associate a measure of striatal dopamine release to the structure of the frontal cortex in humans. This association is of particular interest because alterations in both striatal dopamine and thickness of the frontal cortex have been observed in disease states, including addiction (Volkow et al., 1997; Martinez et al., 2005, 2007; Makris et al., 2008a,b; Durazzo et al., 2011a,b), Parkinson's disease (Brooks, 2003; Lyoo et al., 2010; Sioka et al., 2010; Jubault et al., 2011; Pereira et al., 2012), and schizophrenia (Laruelle et al., 1996, 1999; Abi-Dargham et al., 1998; Bertolino et al., 2000; Kuperberg et al., 2003; Nesvåg et al., 2008; Goldman et al., 2009; Kegeles et al., 2010; Rimol et al., 2012). Evidence from these disease states suggests that the relationship between cortex and striatal dopamine function is complex. In all three diseases, patients showed cortical thinning; however, Parkinson's patients and drug-addicted populations show reduced dopamine receptor concentrations and blunted dopamine responses, whereas patients with schizophrenia show minor increases in receptor concentration and much larger enhancements in striatal responsivity. The present results, in combination with studies of glucose metabolism (Volkow et al., 2007), suggest that in healthy subjects the integrity of frontal cortex is associated with reductions in striatal dopamine responsivity. It is currently unknown whether this relationship is disturbed in disease states.
We found regions of association between ΔBPND and thickness in six aspects of the frontal cortex. Of the regions we identified, only the supplementary motor area appeared bilaterally after correction for multiple comparisons. However, there was a broad association between cortical thickness and ΔBPND in most of the frontal cortex, and the same association was present at a lower statistical threshold in the contralateral hemisphere for the groups of vertices we identified in medial PFC, OFC, and primary motor cortex. This noted, examination of the t-map (Fig. 1) and maps without adjustment for multiple comparisons (Fig. 4, left) suggests that the association tended to be right lateralized, and the aspects of lateral PFC we identified as being closely associated with ΔBPND in the right hemisphere showed no relationship on the left. It has been suggested that the right frontal lobe, particularly in (1) an area of lateral PFC overlapping that described here (Garavan et al., 1999), (2) more ventrally in the inferior frontal gyrus (Aron et al., 2003), and (3) more dorsally in the dorsolateral PFC (Knoch et al., 2006; Fecteau et al., 2007) are preferentially involved in response inhibition. This lateralization may be relevant to striatal dopamine and addiction. Dopamine synthesis capacity in AST has been associated with fMRI activation during a working memory task in an aspect of right, but not left, middle frontal gyrus, which overlaps the area described here (Fusar-Poli et al., 2010); cocaine addicts show reductions in gray matter density in similar aspects of right, but not left, middle frontal gyrus, which correlate with the duration of their cocaine use (Barrós-Loscertales et al., 2011); and activity of the right DLPFC during an emotional regulation task attenuated striatal reward encoding during reward anticipation (Staudinger et al., 2011). However, the activity of the left DLPFC has also been implicated in regulating ventral striatal activity (Kober et al., 2010). Given these observations, a unilateral association between cortical thickness in right lateral PFC and drug-induced dopamine is perhaps not unusual.
The anatomical connections between frontal cortex and the striatum appear to have a well-conserved spatial relationship, with ventral striatum receiving the bulk of its inputs from ventral and medial aspects of cortex, the associative striatum receiving inputs from lateral aspects of the frontal cortex, and the sensorimotor striatum receiving most of its inputs from motor areas (Selemon and Goldman-Rakic, 1985; Alexander et al., 1986; Parent and Hazrati, 1995; Lawrence et al., 1998; Nakano et al., 2000; Saint-Cyr, 2003; Haber and Knutson, 2010). Here we attempted to distinguish these corticostriatal networks by relating ΔBPND in the functional subdivisions of the striatum to cortical thickness. We did not find preferential associations that were able to survive statistical correction, likely because the covariance of ΔBPND between the striatal subregions was high. Using more liberal statistical thresholds and assigning each vertex of the frontal lobe to a striatal subregion produced a pattern that was only partially consistent with the predicted distribution. This issue might be better explored using tasks that preferentially induce dopamine release in different striatal subregions. Alternatively, drug administration regimens that produce larger effects in striatum might better delineate associations with cortical thickness, although this remains an empirical question.
This work should be interpreted in light of the following considerations. First, although our subject population (n = 24) is large for PET imaging, it is relatively small for studies of cortical thickness. Second, we cannot rule out the possibility that the observed association between dopamine release and cortical thickness is a consequence of some third latent factor (e.g., degradation of white matter in the corticostriatal loops) or generalized gray matter atrophy. We find this possibility unlikely as our measures of cortical thickness were only associated with the drug-induced change in dopamine release, not with a measure of dopamine receptor availability during the placebo condition, and not with either the volume or gray matter density of the striatum. Degradation of gray matter within the striatum would also be expected to result in smaller dopamine responses, which is the opposite direction of effect to that observed here. Third, cortical thickness should not be conflated with cortical function. Although thicker cortex is often associated with improved cognitive performance (Narr et al., 2007; Dickerson et al., 2008; Hartberg et al., 2010; Westlye et al., 2011), this is not always true (e.g., Hyde et al., 2007). It is tempting to speculate that thicker frontal cortex directly contributes to the inhibition of drug-induced dopamine release, but this work does not directly test that assertion.
Together, these findings suggest that the structure of the frontal cortex is related to the dopamine release produced by a test dose of amphetamine in a healthy, stimulant drug-naive population. The T1-weighted MRI scans used to calculate cortical thickness are routinely obtained in PET imaging studies for anatomical coregistration, which suggests that many of the datasets required to explore potential differences in these relationships in disease states have already been collected and that these questions can be rapidly addressed.
Footnotes
This work was supported by Canadian Institutes of Health Research Grant MOP-64426. We thank K. Auclaire and F. Durand for nursing support; R. Fukasawa, G. Sauchuck, and S. Mattei for technical assistance at the PET unit; D. Jolly and M. Kovacevic for preparation of radiotracers; G. Rauw for technical assistance in plasma amphetamine determinations; S.M. Cox, K. Welfeld, and I. Boileau for assistance in PET image analysis; and C. Lepage for assistance in calculating cortical thickness.
The authors declare no competing financial interests.
References
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Ad-Dab'bagh Y, Lyttelton O, Muehlboeck J, Lepage C, Einarson D, Mok K. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. Proceedings of the 12th annual meeting of the organization for human brain mapping; Florence, Italy. 2006. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alexoff DL, Vaska P, Marsteller D, Gerasimov T, Li J, Logan J, Fowler JS, Taintor NB, Thanos PK, Volkow ND. Reproducibility of [11C]-raclopride binding in the rat brain measured with the microPET R4: effects of scatter correction and tracer specific activity. J Nucl Med. 2003;44:815–822. [PubMed] [Google Scholar]
- Anon. NIMH Genetics Initiative: Family Interview for Genetic Studies. Rockville, MD: National Institute of Mental Health; 1992. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Asghar SJ, Baker GB, Rauw GA, Silverstone PH. A rapid method of determining amphetamine in plasma samples using pentafluorobenzenesulfonyl chloride and electron-capture gas chromatography. J Pharmacol Toxicol Methods. 2001;46:111–115. doi: 10.1016/S1056-8719(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Frank JA, Pickar D, Weinberger DR. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22:125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. PET studies on the function of dopamine in health and Parkinson's disease. Ann N Y Acad Sci. 2003;991:22–35. doi: 10.1111/j.1749-6632.2003.tb07460.x. [DOI] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M. Attenuated amphetamine-induced dopamine release in subjects at high familial risk for substance dependence. Neuropsychopharmacology; Abstracts of the ACNP (American College of Neuropsychopharmacology) 49th Annual Conference; December 5–9, 2010; Miami. 2010. pp. S1–S447. [Google Scholar]
- Cherkasova MV, Faridi N, Casey KF, O'Driscoll GA, Hechtman L, Baker GB, Dagher A, Leyton M, Benkelfat C. Attenuated amphetamine-induced dopamine release in subjects at high familial risk for substance dependence. Neuropsychopharmacology; Abstracts of the ACNP (American College of Neuropsychopharmacology) 49th Annual Conference; December 5–9, 2010; Miami. 2010. pp. S1–S447. [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. doi: 10.1097/00004728-199403000-00005. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes C, Peters TM, Evans AC. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. doi: 10.1002/hbm.460030304. [DOI] [Google Scholar]
- Costes N, Dagher A, Larcher K, Evans AC, Collins DL, Reilhac A. Motion correction of multi-frame PET data in neuroreceptor mapping: simulation based validation. Neuroimage. 2009;47:1496–1505. doi: 10.1016/j.neuroimage.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol. 2011a;18:379–391. doi: 10.1111/j.1369-1600.2011.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011b;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M. Axis 1 disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Grasby PM, McGuire PK. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 2010;182:123–133. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hume SP, Gunn RN, Jones T. Pharmacological constraints associated with positron emission tomographic scanning of small laboratory animals. Eur J Nucl Med. 1998;25:173–176. doi: 10.1007/s002590050211. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: when less is better than more. J Neurosci. 2007;27:13028–13032. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/S0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O. Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage. 2011;55:462–467. doi: 10.1016/j.neuroimage.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Kamali A, Kramer LA, Hasan KM. Feasibility of prefronto-caudate pathway tractography using high resolution diffusion tensor tractography data at 3T. J Neurosci Methods. 2010;191:249–254. doi: 10.1016/j.jneumeth.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[11C]raclopride PET study. Eur J Neurosci. 2008;28:2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997a;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997b;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/S0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington's disease. Trends Cogn Sci. 1998;2:379–388. doi: 10.1016/S1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, Gunn R, Young SN, Benkelfat C. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29:427–432. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Ryu YH, Lee MS. Topographical distribution of cerebral cortical thinning in patients with mild Parkinson's disease without dementia. Mov Disord. 2010;25:496–499. doi: 10.1002/mds.22975. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC. Cortical thickness abnormalities in cocaine addiction: a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008a;60:174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008b;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: II. Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/appi.ajp.164.4.622. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(Suppl 5):V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Nesvåg R, Lawyer G, Varnäs K, Fjell AM, Walhovd KB, Frigessi A, Jönsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Hayashi T, Okabe S, Nonaka I, Matsuda H, Iida H, Imabayashi E, Watabe H, Miyake Y, Ogawa M, Teramoto N, Ohta Y, Ejima N, Sawada T, Ugawa Y. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol Psychiatry. 2004;55:484–489. doi: 10.1016/j.biopsych.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia: I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-C. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junqué C, Bargallo N, Tolosa E. Assessment of cortical degeneration in patients with parkinson's disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp. 2012;33:2521–2534. doi: 10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvåg R, Hagler DJ, Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioka C, Fotopoulos A, Kyritsis AP. Recent advances in PET imaging for evaluation of Parkinson's disease. Eur J Nucl Med Mol Imaging. 2010;37:1594–1603. doi: 10.1007/s00259-009-1357-9. [DOI] [PubMed] [Google Scholar]
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–2588. doi: 10.1093/cercor/bhr041. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson's disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Georg Thieme Verlag; 1988. [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A, Forghani R, Evans A. Automatic quantification of MS lesions in 3D MRI brain datasets: validation of INSECT. In: Wells WM, Colchester A, Delp S, editors. Medical image computing and computer-assisted intervention — MICCAI'98. Berlin: Springer; 1998. pp. 439–448. [Google Scholar]