Abstract
Background & Aims
Crohn’s disease (CD) is a lifelong illness with substantial morbidity, although new therapies and treatment paradigms have been developed. We provide guidance for treatment of ambulatory patients with mild to severe active luminal CD.
Methods
We performed a systematic review to identify published studies of the management of CD. The quality of evidence and strength of recommendations were rated according to the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach. Statements were developed through an iterative online platform and then finalized and voted on by a group of specialists.
Results
The consensus includes 41 statements focused on 6 main drug classes: antibiotics, 5-aminosalicylate, corticosteroids, immunosuppressants, biologic therapies, and other therapies. The group suggested against the use of antibiotics or 5-aminosalicylate as induction or maintenance therapies. Corticosteroid therapies (including budesonide) can be used as induction, but not maintenance therapies. Among immunosuppressants, thiopurines should not be used for induction, but can be used for maintenance therapy for selected low-risk patients. Parenteral methotrexate was proposed for induction and maintenance therapy in patients with corticosteroid-dependent CD. Biologic agents, including tumor necrosis factor antagonists, vedolizumab, and ustekinumab, were recommended for patients failed by conventional induction therapies and as maintenance therapy. The consensus group was unable to clearly define the role of concomitant immunosuppressant therapies in initiation of treatment with a biologic agent.
Conclusions
Optimal management of CD requires careful patient assessment, acknowledgement of patient preferences, evidence-based use of existing therapies, and thorough assessment to define treatment success.
Keywords: TNF, 5-ASA, Guidance, Mucosal Healing
Crohn’s disease (CD) is a lifelong illness with substantial morbidity and mortality. Studies have shown that up to one-third of patients require hospitalization within the first year after diagnosis and more than half within 5 years.1 In addition to increased risk of mortality from digestive conditions, CD is also associated with a significantly increased risk of all-cause mortality compared with the general population (standardized mortality ratio, 1.45; 95% confidence interval [CI], 1.34–1.58).2 Furthermore, health-related quality of life (HRQoL) is significantly lower among patients with CD compared with normal populations because of the impact of CD on physical, emotional, and social well-being.3
The cause of CD is not well-defined, which makes it challenging to develop specific targeted treatments, but a number of treatments have demonstrated efficacy in CD. In the last decade, treatment paradigms have changed, recognizing that certain clinical parameters carry an increased risk of progressive and disabling disease. In addition, as the association between mucosal healing and improved short-term and long-term outcomes has been increasingly recognized,4,5 this is becoming an important treatment goal.6 Evidence suggests that initiation of highly effective therapies can lead to symptomatic improvement and mucosal healing. For this reason the present consensus statements generally recommend that management strategies strive for complete remission, which is defined as both symptomatic and endoscopic remission. However, it is recognized that the outcome assessed in most randomized controlled trials (RCTs) has been either symptomatic remission or symptomatic response, with only more contemporary clinical trials including endoscopic outcomes. Therefore, in many cases the quality of evidence (QoE) according to Grading of Recommendation Assessment, Development and Evaluation (GRADE) methodology associated with the consensus statements had to be downgraded.
In addition, the consensus group recognized that because of the substantial impact of CD on patient daily life and HRQoL, it is imperative to consider the patient’s perspective when making treatment decisions. In many instances, factors that influence patient decisions relating to therapy choice and goals of therapy are not the same as those of the treating clinician.7,8 This is the reality of clinical practice, and it is important to keep this in mind when making therapeutic decisions.
At the time the literature searches were conducted for the present consensus (April 2016) and at the time the consensus group met (September 2016), the most recent clinical practice guideline on the treatment of CD was the second European evidence-based consensus from the European Crohn’s and Colitis Organisation (ECCO), which incorporated data published until 2008.9 Subsequently, the third European evidence-based consensus from the ECCO was published online in November 2016.10 However, there are differences between the present consensus guidelines and the ECCO consensus with respect to the methods for grading the level of evidence, the conclusions reached, the recommendations made, and the presentation of the discussions. As such, both guidelines are likely to be relevant to clinicians and their patients when managing CD.
The purpose of these consensus statements is to review the literature relating to the medical management of luminal CD and to develop specific statements regarding the various therapies available for ambulatory patients with mild to severe active disease. Furthermore, we offer practical guidance for the practicing clinician given the evidence.
METHODS
Scope and Purpose
These consensus statements focused on specific questions, identified and discussed by the participants, regarding the management of luminal CD in adults. Statements on the management of fistulizing CD were also developed and were presented in a separate publication. The development of this clinical practice guideline began in September 2015, with the full consensus group participating in a face-to-face meeting in September 2016. The entire process spanned approximately 22 months, and the final manuscript was submitted for publication in July 2017 and revised after review.
Sources and Searches
The Editorial Office of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group at McMaster University performed a systematic literature search of MEDLINE (1946 on), EMBASE (1980 on), and CENTRAL (Cochrane Central Register of Controlled Trials) for trials published through February–April 2016. Key search terms included Crohn, antibiotic, 5-aminosalicylate, corticosteroid, anti-tumor necrosis factor, thiopurine, methotrexate, vedolizumab, ustekinumab, probiotics, omega-3 fatty acid, naltrexone, and enteral nutrition. Only human studies published in English were considered; further details regarding the search strategies used for preparing the initial consensus statements can be found in Supplementary Appendix 1. Additional focused (but non-systematic) searches were also performed up to the September 2016 consensus meeting.
Review and Grading of Evidence
Two non-voting methodologists (G.L., P.M.) used the GRADE approach11 to assess the risk of bias (of individual studies and overall across studies), indirectness, inconsistency, imprecision, as well as other considerations (including publication bias) to determine the overall quality of evidence for each statement. The quality of evidence for each statement was graded as high, moderate, low, or very low, as described in GRADE11,12 and used in prior Canadian Association of Gastroenterology (CAG) consensus documents.13–16 The evidence was derived mainly from published systematic reviews and meta-analyses of RCTs. When network meta-analyses (NMAs) were available, the evidence was derived mainly from direct comparison estimates, whereas the indirect and mixed comparisons provided supportive evidence (but could not override direct evidence in case of discrepancies). When needed, we conducted our own updated analyses or subgroup analyses. When no RCT data were available, we extracted evidence from observational studies. GRADE assessments were reviewed and agreed on by voting members of the consensus group at the meeting. The finalized GRADE assessments (risk of bias assessment of included studies and evidence profiles, with revisions done at the meeting) are shown in Supplementary Appendix 2.
One statement (statement 1) was determined to meet the criteria for a “good practice statement”17; the consensus group believed the recommendation was clinically obvious, and therefore the collection and GRADE analysis of supporting evidence were deemed to be unnecessary. Although formal GRADE evaluation of the supporting evidence was not performed, information is provided in the text for this statement.
Approved product labeling from government regulatory agencies varies from country to country, and although not ignored, recommendations are based on evidence from the literature and consensus discussion and may not fully reflect the product labeling for a given country.
Consensus Process
The consensus group was composed of 20 voting participants with expertise in the management of CD, including the chairs (R.P., A.H.S.), academic and community gastroenterologists, as well as a nurse practitioner specializing in inflammatory bowel disease (IBD). Nonvoting participants included a patient representative, non-voting observers, the GRADE experts (G.L., PM), and a moderator (J.M.).
The CAG used a web-based platform (ECD Solutions, Atlanta, GA) to aid in the consensus process before the 2-day face-to-face consensus meeting held in Toronto, Ontario, Canada in September 2016. The steering committee (R.P., A.H.S., B.B., R.K., J.K.M., L.T.) and one of the nonvoting methodologists (G.L.) developed the initial statements. Using the consensus web-based platform, the steering committee reviewed the results of initial literature searches and identified relevant references that were then “tagged” (selected and linked) to each statement. All participants then used the web-based platform and a modified Delphi process18,19 to vote anonymously on their level of agreement with the statements, suggest revisions, and provide comments. The statements were revised through 2 separate iterations and finalized at the consensus meeting. All participants had access to all abstracts and electronic copies of the individual “tagged” references. The GRADE evaluations of the evidence for each statement were provided at the meeting.
At the consensus conference, participants presented data, reviewed GRADE evaluations of the evidence for the individual statements, and discussed the phrasing of specific statements before their subsequent finalization. Participants then indicated their level of agreement for each statement by voting. A statement was accepted if >75% of participants voted 4 (agree) or 5 (strongly agree) on a scale of 1–5 (with 1, 2, and 3 indicating disagree strongly, disagree, and uncertain, respectively). After acceptance of a statement, participants voted on the “strength” of the recommendation. A level of agreement of ≥75% of participants was needed to classify a statement as “strong” (we recommend); if this threshold was not met, the statement defaulted to “conditional” (we suggest). The strength of the recommendation considered risk-benefit balance, patients’ values and preferences, cost and resource allocation, and the quality of the evidence. Therefore, it was possible for a recommendation to be classified as strong despite having low-quality evidence or conditional despite the existence of high-quality evidence.20 As per the GRADE method, a strong recommendation is indicative of a more broadly applicable statement (“most patients should receive the recommended course of action”), whereas a conditional recommendation suggests that clinicians should “…recognize that different choices will be appropriate for different patients and that they must help each patient to arrive at a management decision consistent with her or his values and preferences”.20
In many cases the outcomes of clinical trials were assessed symptomatically without endoscopy; therefore, the QoE was often downgraded for indirect outcomes, resulting in a low or very low QoE, making it difficult to approve strong recommendations in many cases.
At the meeting, the group was unable to reach consensus on 5 of the initial statements (No recommendation A–E); thus, these statements were rejected. In addition, because of the absence of evidence the group decided not to vote on 2 statements (No recommendation F and G) regarding strategies in patients who had failed non–tumor necrosis factor (TNF) targeted biologic therapies, electing to discuss this issue in the “Future Directions” section.
The manuscript was initially drafted by the co-chairs (R.P., A.H.S.), after which it was then reviewed and revised by steering committee members before being disseminated to the remaining members of the consensus group for review and approval. As per CAG policy for all clinical practice guidelines, the manuscript was made available to all CAG members for commenting before submission for publication. Members were notified that the manuscript was available on the members-only section of the CAG website and open for comment for a 2-week period.
In accordance with CAG policy, written disclosures of any potential conflicts of interest for the 24 months before the consensus meeting were provided by all participants and made available to all group members.
Role of the Funding Sources
Funding for the consensus meeting was provided by unrestricted, arms-length grants to the CAG by AbbVie Corp, Janssen Inc, Pfizer Canada Inc, and Takeda Canada Inc. The CAG administered all aspects of the meeting, and the funding sources had no involvement in the process at any point, and they were not made aware of any part of the process from development of search strings and the statements to drafting and approval of these guidelines.
Crohn’s Disease Definitions
Before finalizing the individual statements for the management of CD, the consensus group first discussed and agreed on definitions of terminology that were then used throughout the consensus process. Definitions were presented by a member of the steering committee (J.K.M.), discussed and revised, and then agreed on by the group.
Disease Location and Behavior
The consensus group agreed that CD should be classified according to the Montreal classification, which considers age of onset (≤16, 17–40, >40 years), disease location (terminal ileum, colon, ileocolon, upper gastrointestinal), and disease behavior (non-stricturing/non-penetrating, stricturing, penetrating).21
Disease Activity
Although medical therapies for CD target pathways that lead to inflammation, disease activity is generally assessed in clinical trials by assessment tools that measure signs and symptoms of the disease and in clinical practice by subjective assessment of signs and symptoms.22 In the majority of clinical trials reviewed for this consensus guideline, the standard measure of severity was the Crohn’s Disease Activity Index (CDAI). Therefore, in general, descriptions of severity in this document reflect CDAI scores as described in the evidence.
The CDAI is heavily weighted toward symptoms, with a clinical response defined as a reduction from baseline of 70–100 points or more and clinical remission as a score of <150.22,23 However, the CDAI correlates poorly with scores of endoscopic disease severity and with fecal (calprotectin and lactoferrin) and serum biomarkers of inflammation (C-reactive protein [CRP]).24–26 In addition, the US Food and Drug Administration (FDA) has indicated that the CDAI will no longer be acceptable as a measure of disease activity in clinical trials because it was not created according to FDA guidance for patient-reported outcomes (PROs) (ie, index items must be generated by patients).22
The Harvey–Bradshaw Index (HBI) offers a simplified disease activity score, with a clinical response defined as a reduction from baseline of 3 points or more and remission as a score of <5.22,27,28 However, although more user-friendly, the HBI is subject to the same limitations as the CDAI in that the majority of the score is symptom-based.29
Therefore, disease activity that may be defined as mild, moderate, and severe by the tools above should not be confused with disease severity. It is acknowledged that overall disease severity encompasses many factors not captured in the CDAI or HBI as discussed below. The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) is in the midst of designing an overall disease severity index that is based on impact of the disease on the patient, objective measures of inflammatory burden, and disease course; however, this index requires validation (see statement 1).30
Disease Severity: Factors Associated With High Risk of Relapse, Surgery, or Complicated Course
Whereas the CDAI is used in clinical trials, in clinical practice, severity assessments should also take into account other factors such as overall risk profile and the disease impact on the patient. Risk factors that have been associated with a higher risk of relapse or a more aggressive or complicated disease course include clinical factors (younger age, smoking, longer disease duration, early need for corticosteroids, and fistulizing perianal CD31–33), laboratory markers (low hemoglobin, low albumin, high CRP, and high fecal calprotectin levels5,34–37), endoscopic appearance (the presence of deep ulcers), as well as overall disease burden and location (Table 1). Patients lacking these factors would generally be classified as low-risk.
Table 1.
Factors Associated With High Risk of Relapse, Surgery, or Complicated Luminal CD
| Clinical factors | Younger age Smoking Longer disease duration Early use of corticosteroids Presence of fistulizing perianal CD Previous intestinal resection |
| Disease factors | Disease location (rectal, upper GI, jejunal) Disease extent |
| Laboratory factors | Low hemoglobin Low albumin High C-reactive protein (CRP) High fecal calprotectin levels |
| Endoscopic factors | Presence of deep ulceration |
CD, Crohn’s disease; GI, gastrointestinal.
Outcomes in Luminal Crohn’s Disease
The optimal outcome in the treatment of luminal CD is control of underlying inflammation. It is well-accepted that the correlation between symptoms and the presence or absence of active disease (inflammation) can be poor. The outcomes used in this clinical practice guideline reflect a treat-to-target strategy that has been recently recommended.6 Terminology and definitions used in this guideline are shown in Table 2.
Table 2.
Definitions of Remission and Response in Patients With Luminal CD
| Complete remission | Symptomatic and endoscopic remission (defined below) OR Symptomatic and radiographic remission (defined below) |
| Endoscopic remission | Absence of ulcerations |
| Symptomatic remission | Absence of symptoms attributable to Crohn’s disease activity without the need for corticosteroids |
| Symptomatic response | Meaningful improvement in symptoms as judged by both the patient and physician in absence of remission. Response should not be considered a desirable final outcome but is useful to assess early efficacy of treatment |
| Radiographic remission | Absence of detectable disease activity on CTE, MRE, or SBUS |
CD, Crohn’s disease; CTE, computed tomography enterography; MRE, magnetic resonance enterography; SBUS, small bowel ultrasound.
Complete remission, defined as both symptomatic (corticosteroid-free) and endoscopic or radiographic remission, is the preferred outcome in keeping with the Selecting Therapeutic Targets In Inflammatory Bowel Disease recommendations.6 Assessing for complete remission requires endoscopy or cross-sectional imaging to document resolution of inflammation. Although these cannot be conducted at every assessment, the consensus group agreed that an objective measure of disease activity such as endoscopy, radiography, or suitable surrogate markers (CRP or fecal calprotectin) should be obtained when making important management decisions such as assessing efficacy at the end of induction therapy or considering a change in therapy due to inadequate response.
Many clinical trials do not incorporate endoscopic outcomes or surrogate markers, and thus there are limited data on complete remission. However, as a treatment goal, in most statements the consensus group agreed that management should strive for complete remission. The importance of the physician and patient discussing and agreeing on treatment goals was acknowledged.
Symptomatic remission was defined as the absence of symptoms specifically attributable to CD activity. Patients with CD may have symptoms that are not due to CD activity, and this needs to be ascertained by the treating clinician. Related but nonspecific symptoms, such as those associated with bile acid diarrhea, small intestinal bacterial overgrowth, superimposed irritable bowel syndrome, or complications such as intestinal stricture also need to be treated, but these would be treated by using other strategies. The symptoms that are most often attributable to CD activity are stool frequency and abdominal pain.38,39 Two PRO measures have been described, PRO2 (stool frequency and pain) and PRO3 (stool frequency, pain, and general well-being). These measures have been shown to be responsive to treatment-associated changes in disease activity. A PRO2 score of <8 corresponds to a CDAI score of <150 (clinical remission).38,39
Endoscopic remission was defined as the absence of ulcerations. This is consistent with the definition of mucosal healing used in pivotal clinical trials. Mucosal healing is an important predictor of long-term outcomes of treatment for CD. Patients who achieve mucosal healing have an almost 3-fold greater likelihood of achieving long-term clinical remission4 and a 2-fold decreased risk of relapse after treatment discontinuation.5 Mucosal healing has also been associated with higher rates of steroid-free remission40 and reduced rates of hospitalization41,42 and surgery.4 However, it is not clear whether escalation or change of therapy is warranted in patients who have achieved symptomatic remission but have evidence of residual endoscopic activity.43 The ongoing REACT-2 clinical trial will attempt to answer this question.44
Although relevant ulcerations are often defined as those >5 mm, there are few data to define the degree of endoscopic improvement that relates to improvement in long-term outcomes. There is some debate as to whether a small number of localized aphthous ulcers would be acceptable and would not warrant a change or escalation of treatment.43,45 Conversely, multiple small erosions throughout the intestine would not be considered remission and would often warrant a change or escalation of treatment. The IOIBD recently recommended the Simple Endoscopic Score for Crohn’s Disease (SES-CD) or Crohn’s Disease Endoscopic Index of Severity (CDEIS) scores to describe endoscopic response (>50% decrease) and remission (SES-CD ≤2), as well as the Rutgeerts’ score to define endoscopic remission (i0-i1) after surgery.46 Although they are used in clinical trials, the CDEIS and SES-CD remain incompletely validated.47 In addition, clinicians often do not use standardized scoring systems in clinical practice.
Radiographic remission was defined as absence of detectable active inflammation disease on computed tomography enterography (CTE), magnetic resonance enterography (MRE), or small bowel ultrasound (SBUS). Radiologic response (defined as improved lesions) to medical therapy has been associated with significant reductions in long-term risk of hospitalization, surgery, or corticosteroid usage in CD patients.48 A meta-analysis found that CTE and MRE have comparable high accuracy in grading the severity of CD, whereas data on the more operator-dependent SBUS method were inconsistent and limited. Because of the need for repeat assessments, MRE (and SBUS where available) is generally preferable to CTE because it does not involve radiation exposure.49 However, this may change as newer CTE protocols use much less radiation.
A number of scoring systems are available to classify inflammation, including CTE0-CTE3 for computed tomography50,51 and the MaRIA and London systems for magnetic resonance imaging.51–53 However, no radiographic scoring system is currently widely accepted for use in assessing severity of CD.51 The imaging features most commonly assessed in the context of ongoing disease activity are bowel wall thickness and contrast enhancement.49
Symptomatic response was defined as meaningful improvement in symptoms as judged by both the patient and physician in the absence of symptomatic remission. This is useful to assess early improvement with therapy but should generally not be considered the goal of therapy.
Use of Corticosteroids
The consensus group defined “corticosteroid resistance” as a lack of a symptomatic response despite a course of oral prednisone of 40–60 mg/day (or equivalent) for a minimum of 14 days. “Corticosteroid dependence” was defined as the inability to withdraw oral corticosteroid therapy (within 3 months of initiation) without recurrence of symptoms, a symptomatic relapse within 3 months of discontinuing corticosteroid therapy, or the need for more than 1 course of corticosteroid therapy within 1 year.
Patient Perspectives
Although treatment recommendations help provide guidance to the clinician, treatment decisions should be made in collaboration with the individual patient. Acknowledging the need to accurately measure the patient’s experience, the FDA is encouraging the development of PROs as clinical trial endpoints for CD.6
In a patient survey, the most important treatment goals were improving quality of life and completely resolving symptoms, especially abdominal pain, and bowel movement urgency.54 However, many patients acknowledge accepting a new state of normalcy if their current treatments improved their most bothersome symptoms, even if it did not provide sustained remission.55 As might be expected, less than 15% of patients indicated having a completely normal colonoscopy as a preferred treatment objective.54 Patients often rely on their provider for treatment decisions.55 But these surveys indicate a discrepancy between patient and physician treatment goals and suggest a need for more patient education and more patient-physician collaboration and dialogue regarding treatment decisions.54,55
Recommendation Statements for Luminal Crohn’s Disease
The individual recommendation statements are provided and include the “GRADE” of supporting evidence, the voting results, and a discussion of the evidence considered for the specific statement.
Algorithms summarizing the consensus-guided approach to the medical management of mild to severe active CD are shown in Figures 1 and 2, and a summary of the individual recommendation statements is provided in Table 3. The evidence profiles, along with the results of the systematic reviews and meta-analyses conducted for this guideline, can be found in Supplementary Appendix 2.
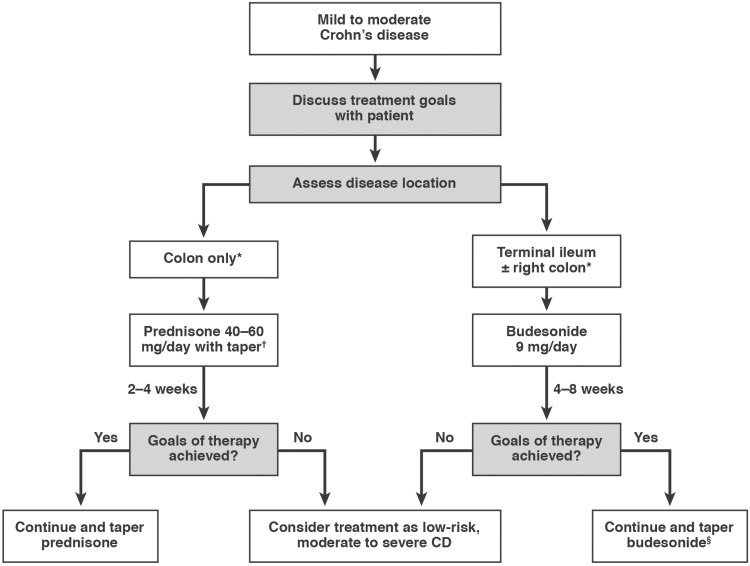
Figure 1.
Consensus guided algorithm for management of mild to moderate active CD. *If patient has multiple risk factors for poor prognosis, consider moderate to severe algorithm. †Sulfasalazine may be used in mild colonic disease (refer to text). §May consider thiopurine maintenance therapy. CD, Crohn’s disease.
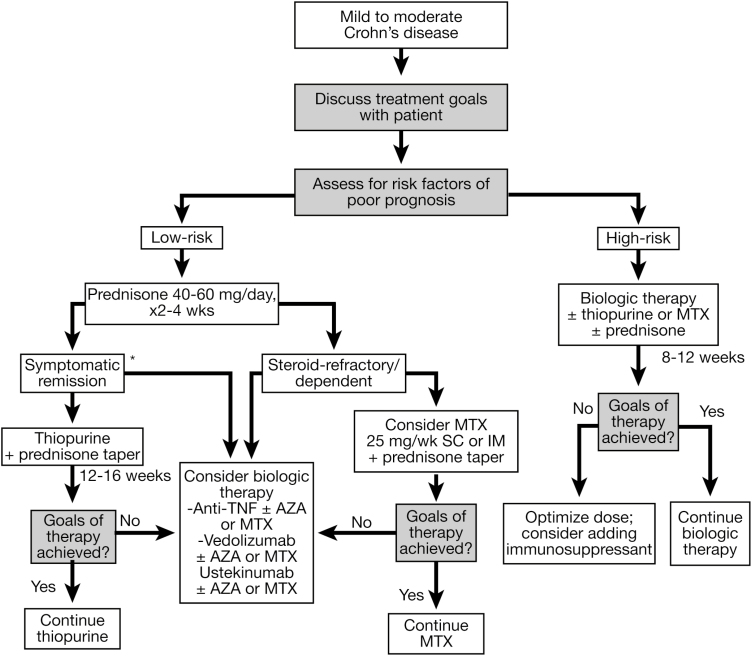
Figure 2.
Consensus guided algorithm for management of moderate to severe active CD. *Initiation of biologic therapy may be an alternative pathway to thiopurines. Despite the fact that certolizumab is FDA approved and used in the United States, it is not licensed for the treatment of CD in Canada or Europe and therefore was not included in this CPG. AZA, azathioprine; IM, intramuscular; MTX, methotrexate; SC, subcutaneous; TNF, tumor necrosis factor.
Table 3.
Summary of Consensus Recommendations for the Management of Luminal Crohn’s Diseasea
| Disease activity |
| 1. We recommend determination of disease severity be based on a combination of symptoms, objective measures of inflammation, and factors that predict an increased risk of complications. GRADE: Strong recommendation. Good practice statement, quality of evidence not assessed |
| Antibiotics |
| 2. In patients with Crohn’s disease of any severity, we suggest against the use of systemically absorbed antibiotics to induce OR maintain complete remission. GRADE: Conditional recommendation, very low-quality evidence for induction of remission, low-quality evidence for maintenance of remission |
| 5-ASA |
| 3. In patients with mild Crohn’s disease limited to the colon, we suggest the use of sulfasalazine to induce (4–6 g/day) complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 4. We suggest that patients with mild Crohn’s disease limited to the colon be evaluated for symptomatic response to sulfasalazine therapy between 2 and 4 months to determine the need to modify therapy. GRADE: Conditional recommendation, very low-quality evidence |
| 5. In patients with Crohn’s disease of any severity, we suggest against the use of oral 5-ASA to induce OR maintain complete remission. GRADE: Conditional recommendation, low-quality evidence for induction of remission, moderate-quality evidence for maintenance of remission |
| Budesonide |
| 6. In patients with mild to moderate ileal and/or right colonic Crohn’s disease, we suggest oral budesonide beginning at 9 mg/day as first-line therapy to induce complete remission. GRADE: Conditional recommendation, low-quality evidence |
| 7. We suggest that patients with mild to moderate ileal and/or right colonic Crohn’s disease be evaluated for symptomatic response to budesonide between 4 and 8 weeks to determine the need to modify therapy. GRADE: Conditional recommendation, very low-quality evidence |
| 8. In patients with mild to moderate Crohn’s disease, we suggest against the use of oral budesonide to maintain complete remission. GRADE: Conditional recommendation, low-quality evidence |
| Corticosteroids |
| 9. In patients with moderate Crohn’s disease who have failed to respond to oral budesonide 9 mg/day, we suggest the use of prednisone 40–60 mg/day to induce complete remission. GRADE: Conditional recommendation, low-quality evidence |
| 10. In patients with moderate to severe Crohn’s disease, we recommend the use of oral prednisone 40–60 mg/day to induce complete remission. GRADE: Strong recommendation, low-quality evidence |
| 11. We recommend that patients with moderate to severe Crohn’s disease be evaluated for symptomatic response to prednisone between 2 and 4 weeks to determine the need to modify therapy. GRADE: Strong recommendation, very low-quality evidence |
| 12. In patients with active Crohn’s disease of sufficient severity to require hospitalization, we suggest the use of intravenous corticosteroids (eg, methylprednisolone 40–60 mg/day) to induce symptomatic remission. GRADE: Conditional recommendation, low-quality evidence |
| 13. We recommend that patients with severe Crohn’s disease be evaluated for symptomatic response to intravenous methylprednisolone within 1 week to determine the need to modify therapy. GRADE: Strong recommendation, very low-quality evidence |
| 14. In patients with Crohn’s disease of any severity, we recommend against the use of oral corticosteroids to maintain complete remission. GRADE: Strong recommendation, low-quality evidence |
| Immunosuppressants |
| 15. In patients with Crohn’s disease of any severity, we suggest against the use of thiopurine monotherapy to induce complete remission. GRADE: Conditional recommendation, low-quality evidence |
| 16. In selected patients with Crohn’s disease who have achieved symptomatic remission on oral corticosteroids, we suggest thiopurine monotherapy to maintain complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 17. In patients with moderate to severe corticosteroid-dependent/resistant Crohn’s disease, we suggest parenteral methotrexate to induce complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 18. In patients with Crohn’s disease who have achieved symptomatic remission on oral corticosteroids and parenteral methotrexate, we suggest parenteral methotrexate to maintain complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 19. We suggest that patients with Crohn’s disease receiving thiopurine or methotrexate who do not achieve corticosteroid-free remission within 12–16 weeks should have therapy modified. GRADE: Conditional recommendation, very low-quality evidence |
| Anti-TNF biologics |
| 20. In patients with moderate to severe luminal Crohn’s disease with risk factors of poor prognosis, we recommend anti-TNF therapy (infliximab, adalimumab) as first-line therapy to induce complete remission. GRADE: Strong recommendation, moderate-quality evidence |
| 21. In patients with moderate to severe Crohn’s disease who fail to achieve complete remission with any of corticosteroids, thiopurines, or methotrexate, we recommend anti-TNF therapy (infliximab, adalimumab) to induce complete remission. GRADE: Strong recommendation, high-quality evidence |
| 22. In patients with active Crohn’s disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine over monotherapy to induce complete remission. GRADE: Conditional recommendation, low-quality evidence |
| 23. In patients with active Crohn’s disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters. GRADE: Conditional recommendation, very low-quality evidence for infliximab, very low-quality evidence for adalimumab |
| 24. We recommend that patients with Crohn’s disease be evaluated for symptomatic response to anti-TNF induction therapy between 8 and 12 weeks to determine the need to modify therapy. GRADE: Strong recommendation, very low-quality evidence |
| 25. In patients with Crohn’s disease who have achieved symptomatic response with anti-TNF induction therapy, we recommend continued anti-TNF therapy to achieve and maintain complete remission. GRADE: Strong recommendation, high-quality evidence |
| 26. In patients with Crohn’s disease who have a suboptimal response to anti-TNF induction therapy, we suggest dose intensification to achieve complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 27. In patients with Crohn’s disease who lose response to anti-TNF maintenance therapy, we suggest dose optimization to recapture complete remission. GRADE: Conditional recommendation, very low-quality evidence |
| 28. We suggest that dose optimization for patients with Crohn’s disease who lose response to anti-TNF therapy be informed by therapeutic drug monitoring. GRADE: Conditional recommendation, very low-quality evidence |
| 29. We suggest against switching between anti-TNF therapies in patients who are doing well on anti-TNF therapy. GRADE: Conditional recommendation, low-quality evidence |
| Non-anti-TNF biologics |
| 30. In patients with moderate to severe Crohn’s disease who fail to achieve complete remission with any of corticosteroids, thiopurines, methotrexate, or anti-TNF therapy, we recommend vedolizumab to induce complete remission. GRADE: Strong recommendation, moderate-quality evidence |
| 31. In patients with Crohn’s disease who fail to achieve or maintain corticosteroid-free symptomatic remission with anti-TNF therapy, we suggest vedolizumab to induce complete remission. GRADE: Conditional recommendation, low-quality evidence |
| 32. We suggest that patients with Crohn’s disease be evaluated for symptomatic response to vedolizumab therapy between 10 and 14 weeks to determine the need to modify therapy. GRADE: Conditional recommendation, very low-quality evidence |
| 33. In patients with Crohn’s disease who have achieved symptomatic response with vedolizumab induction therapy, we recommend continued vedolizumab therapy to achieve and maintain complete remission. GRADE: Strong recommendation, moderate-quality evidence |
| 34. In patients with moderate to severe Crohn’s disease who fail to achieve complete remission with any of corticosteroids, thiopurines, methotrexate, or anti-TNF therapy, we recommend ustekinumab to induce complete remission. GRADE: Strong recommendation, moderate-quality evidence |
| 35. We suggest that patients with Crohn’s disease be evaluated for symptomatic response to ustekinumab therapy between 6 and 10 weeks to determine the need to modify therapy. GRADE: Conditional recommendation, very low-quality evidence |
| 36. In patients with Crohn’s disease who have achieved symptomatic response with ustekinumab induction therapy, we recommend continued ustekinumab therapy to achieve and maintain complete remission. GRADE: Strong recommendation, moderate-quality evidence |
| Alternative treatments |
| 37. In patients with Crohn’s disease, we recommend against the use of probiotics to induce OR maintain symptomatic remission. GRADE: Strong recommendation, very low-quality evidence |
| 38. In patients with Crohn’s disease, we recommend against the use of omega-3 fatty acids to induce OR maintain symptomatic remission. GRADE: Strong recommendation, moderate-quality evidence |
| 39. In patients with Crohn’s disease, we suggest against the use of marijuana to induce OR maintain symptomatic remission. GRADE: Conditional recommendation, very low-quality evidence |
| 40. In patients with Crohn’s disease, we suggest against the use of naltrexone to induce OR maintain symptomatic remission. GRADE: Conditional recommendation, low-quality evidence for induction of remission, very low-quality evidence for maintenance of remission |
| 41. In patients with Crohn’s disease, we suggest against the use of enteral nutrition or dietary modification to induce OR maintain symptomatic remission. GRADE: Conditional recommendation, very low-quality evidence |
| Statements with no recommendations |
| A. In patients with mild Crohn’s disease limited to the colon who have failed to respond to sulfasalazine, the consensus group does not make a recommendation (neither for nor against) regarding the use of prednisone 40–60 mg/day to induce complete remission. |
| B. In patients with mild Crohn’s disease who have failed to respond to oral budesonide 9 mg/day, the consensus group does not make a recommendation (neither for nor against) regarding use of prednisone to induce complete remission. |
| C. In patients with active Crohn’s disease, when starting anti-TNF therapy, the consensus group does not make a recommendation (neither for nor against) it being combined with methotrexate over monotherapy to induce complete remission. |
| D. In patients with active Crohn’s disease starting vedolizumab, the consensus group does not make a recommendation (neither for nor against) regarding adding a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters. |
| E. In patients with active Crohn’s disease starting ustekinumab, the consensus group does not make a recommendation (neither for nor against) regarding adding a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters. |
| F. In patients with Crohn’s disease who fail to respond or lose response to vedolizumab, the consensus group agreed that it was premature, because of the lack of data and clinical experience, to recommend for or against ustekinumab to induce and maintain complete remission. |
| G. In patients with Crohn’s disease who fail to respond or lose response to ustekinumab, the consensus group agreed that it was premature, because of the lack of data and clinical experience, to recommend for or against vedolizumab to induce and maintain complete remission. |
NOTE. Despite the fact that certolizumab is FDA approved and used in the United States, it is not licensed for the treatment of CD in Canada or Europe and therefore was not included in this clinical practice guideline.
GRADE, Grading of Recommendation Assessment, Development and Evaluation; TNF, tumor necrosis factor.
aThe strength of each recommendation was assigned by the consensus group, per the GRADE system, as strong (“we recommend...”) or conditional (“we suggest...”). A recommendation could be classified as strong despite low-quality evidence to support it or conditional despite the existence of high-quality evidence because of the 4 components considered in each recommendation (risk:benefit balance, patients’ values and preferences, cost and resource allocation, and quality of evidence).
The recommendation statements are followed by a section called “Relevance, Interpretation in Clinical Practice, and Future Directions,” which discusses some of the remaining unanswered clinical questions.
Disease Activity
Statement 1. We recommend determination of disease severity be based on a combination of symptoms, objective measures of inflammation, and factors that predict an increased risk of complications.
GRADE: Strong recommendation. Good practice statement, quality of evidence not assessed.
Vote: strongly agree, 55%; agree, 40%; uncertain, 5%.
Key evidence: Good practice statement, quality of evidence not assessed.
Discussion: As discussed in the definition of “endoscopic remission”, mucosal healing is an important predictor of long-term outcomes of treatment for CD4,5,40–42 and should therefore be considered in assessment of disease severity. Conversely, the presence of deep ulcerations is considered to be a marker of more severe disease.56 Other measures of inflammation, including fecal calprotectin levels and CRP, have been shown to be useful objective, surrogate measures of inflammation.57
A number of recent reviews of the literature have highlighted the need to base the determination of disease severity and subsequent clinical decisions on multiple disease factors and not just symptoms.30,58–60 A comprehensive literature search to identify the key factors that define disease severity in IBD has been undertaken by the IOIBD. They identified 3 domains that should be considered when assessing disease severity: impact of the disease on the patient (clinical symptoms, quality of life, fatigue, and disability), measurable inflammatory burden (disease extent, endoscopic lesions, CRP, and upper gastrointestinal involvement), and disease course (including structural damage, history/length of intestinal resection, perianal disease, number of flares, and extraintestinal manifestations).59 Using these domains, the IOIBD conducted a survey of specialists to select the most important attributes related to severity of disease activity for CD. Overall, they ranked the presence of mucosal lesions, history of a fistula, history of abscess, and history of intestinal resection as the most relevant parameters. They created an overall disease severity index; however, this requires validation.30
The consensus group agreed that disease activity should not be based on symptoms alone but rather on a compilation of symptoms, endoscopic appearance, laboratory parameters, and other clinical factors that have been associated with disease progression or complications.
Antibiotics
Statement 2. In patients with CD of any severity, we suggest against the use of systemically absorbed antibiotics to induce OR maintain complete remission.
GRADE: Conditional recommendation, very low-quality evidence for induction of remission, low-quality evidence for maintenance of remission.
Vote: strongly agree, 75%; agree, 25%.
Key evidence: Two systematic reviews of RCTs have evaluated the efficacy of antibiotics for induction of remission in patients with CD.61,62 A meta-analysis of 10 trials found that antibiotics were superior to placebo,61 but when the 2 rifaximin trials were removed from the analysis, the efficacy was no longer significant. For maintenance of remission, 1 systematic review including 3 trials found that anti-tuberculous treatments were more effective than placebo in maintaining remission.61 A more recent systematic review (published outside the search window), which included 1 additional study, reported similar results.63
Discussion: A variety of antibiotic regimens were used in these trials, which makes interpretation difficult.61–63 Overall, antibiotics do not appear to be effective for induction, although there may be some benefit with rifamycin-derivatives (eg, rifampin, rifabutin, and rifapentine). Anti-mycobacterial agents (rifamycins or clofazamine) may be more efficacious than placebo in preventing relapse, but the available studies were small.61 The data are sparse and of poor quality; therefore, an effect of antibiotics, in general, cannot be ruled out.
Because of the low or very low quality of evidence and concerns around using antibiotics long-term, the consensus group suggested that antibiotics not be used for induction or maintenance of remission in patients with luminal disease; however, they do play a role in perianal fistulizing disease.61,64
5-ASA
Statement 3. In patients with mild CD limited to the colon, we suggest the use of sulfasalazine to induce (4–6 g/day) complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 15%; agree, 75%; uncertain, 10%.
Key evidence: Evidence for the efficacy of sulfasalazine for induction of remission is available from 2 systematic reviews of RCTs.65,66 A meta-analysis of 2 trials reports a trend toward a benefit with sulfasalazine over placebo for the failure to achieve remission (relative risk [RR], 0.83; 95% CI, 0.69–1.00).65 A Cochrane meta-analysis of the same 2 trials found a significant benefit with sulfasalazine for the induction of remission (RR, 1.38; 95% CI, 1.02–1.87) compared with placebo.66 In a recent update of the Cochrane analysis (published outside our search window), re-analysis of the 2 trials yielded a non-significant trend in favor of sulfasalazine (RR, 1.38; 95% CI, 1.00–1.89).67 The trials reported significant results with sulfasalazine only in the subgroup of patients with disease confined to the colon.68,69
A meta-analysis of 4 RCTs found that sulfasalazine was not effective in preventing relapse of CD, but there was a trend toward benefit with mesalamine.65 However, the analysis was underpowered because of the low total number of relapse events in the sulfasalazine studies.
Discussion: Meta-analyses of 2 RCTs suggest a trend to a modest benefit with sulfasalazine for induction therapy but no benefits in maintenance therapy.65–67 However, the studies assessing sulfasalazine are older and relatively small. Therefore, an effect cannot be ruled out, particularly because the dose of sulfasalazine used in the RCTs (generally 3 g/day65) may have been inadequate.
Sulfasalazine is composed of 5-ASA joined by an azo bond to sulfapyridine, which is split by colonic bacteria. This has been shown to lead to higher concentrations of 5-ASA in the sigmoid colon and rectum compared with orally administered 5-ASA.70 In addition, there is some evidence that the sulfa moiety itself has some weak immunologic effects, which may confer a therapeutic benefit in mild CD.71 The clinical studies also suggest that sulfasalazine may be more effective in colonic disease versus other sites.66–69 Therefore, on the basis of the evidence of modest effects, the consensus group recommended that sulfasalazine therapy be limited to low-risk patients with mild colonic disease. Although evidence has not shown a significant benefit with sulfasalazine for maintenance therapy, it is quite possible that a patient who responds will continue to do so.
Statement 4. We suggest that patients with mild CD limited to the colon be evaluated for symptomatic response to sulfasalazine therapy between 2 and 4 months to determine the need to modify therapy.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: agree, 95%; uncertain, 5%.
Key evidence: In the 2 RCTs included in the meta-analyses of sulfasalazine efficacy, final assessments were completed at approximately 4 months.68,69 In 1 study in which the patients were seen weekly, about 20% of patients had achieved remission after 3–4 weeks of therapy, but maximum improvements in CDAI scores were seen at 15 weeks.68 In another small study, the mean improvement in disease activity score among responders was 36.3, which was reached 4–8 weeks after initiation of therapy.72
Discussion: The limited data available suggest that symptomatic improvement should be evident by 2–4 months. In patients with mild disease, a longer therapeutic trial may be acceptable, but sulfasalazine is not without adverse events (eg, dyspeptic symptoms), and ineffective therapy should not be continued indefinitely. In addition, evidence of any worsening of symptoms during the therapeutic trial requires reevaluation of the patient.
Statement 5. In patients with CD of any severity, we suggest against the use of oral 5-ASA to induce OR maintain complete remission.
GRADE: Conditional recommendation, very low-quality evidence for induction of remission, moderate-quality evidence for maintenance of remission.
Vote: strongly agree, 50%; agree, 35%; uncertain, 15%.
Key evidence: Three systematic reviews have evaluated the efficacy of oral 5-ASA for the induction of remission in patients with active CD.65,66,73 These performed meta-analyses of various formulations and doses of non-sulfasalazine 5-ASAs (ie, mesalamine and olsalazine) and consistently reported no significant benefit with these agents over placebo for induction of remission.65,66,73 The recent update of the Cochrane analysis (published outside our search window) also reported no significant benefit of 5-ASAs over placebo for inducing response or remission.67
A meta-analysis of 11 RCTs assessing the efficacy of mesalamine for maintenance therapy found a non-significant trend toward improvement over placebo (RR, 0.94; 95% CI, 0.87–1.01).65 However, subgroup analysis of 3 RCTs that were at low risk of bias showed a significant benefit for mesalamine (RR, 0.85; 95% CI, 0.74–0.99).
Discussion: In general, oral aminosalicylates do not seem to be effective for the treatment of CD. However, the studies were small and older, and in 1 meta-analysis of 5 studies, mesalamine did offer a significant benefit over placebo for the combined endpoint of remission or improvement (RR, 0.76; 95% CI, 0.61–0.95).65 Therefore, an effect cannot be ruled out. As a result, the consensus group made a conditional recommendation against the routine use of these agents for the treatment of CD but conceded that they may have a role in selected low-risk patients (such as those with mild colonic disease, without deep ulcers or large superficial ulcers on endoscopy).
Budesonide
Statement 6. In patients with mild to moderate ileal and/or right colonic CD, we suggest oral budesonide beginning at 9 mg/day as first-line therapy to induce complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 75%; agree, 25%.
Key evidence: Evidence for the efficacy of budesonide 9 mg/day compared with placebo as first-line therapy in inducing clinical remission in patients with mild to moderate ileal and/or right colonic CD is available from 3 systematic reviews.73–75 The 2 more recent reviews meta-analyzed 3 RCTs that directly compared oral budesonide vs placebo and found that budesonide dosed at 9 mg/day or greater (15–18 mg/day) was associated with 2 to 3 times greater odds of induction of remission vs placebo.73,75 A lower dose of budesonide (3 mg/day) was not superior to placebo.73,75 In meta-analysis of 8 RCTs, budesonide was significantly less effective than conventional corticosteroids for induction of remission (RR, 0.85; 95% CI, 0.75–0.97) but was associated with fewer adverse events (RR, 0.64; 95% CI, 0.54–0.76).75 Budesonide was not significantly different from mesalamine for induction therapy.75
In an RCT, once daily and 3 times daily dosing of oral budesonide (9 mg/day) were found to be equally effective for induction of symptomatic or complete remission; however, this trial lacked a placebo control arm.76
Discussion: Although there are few trials, budesonide has demonstrated a consistent, clear benefit over placebo for induction of remission. Budesonide was inferior to conventional corticosteroids, but it was associated with significantly fewer adverse events and less suppression of adrenal function.75 Therefore, the consensus group concluded that budesonide would be a safer, better tolerated option for patients with mild to moderate disease, with conventional corticosteroids reserved for second-line use in patients who have failed budesonide or for patients with severe disease (see Statements 9 and 10).
Statement 7. We suggest that patients with mild to moderate ileal and/or right colonic CD be evaluated for symptomatic response to budesonide between 4 and 8 weeks to determine the need to modify therapy.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 85%; agree, 15%.
Key evidence: The median time to symptomatic remission in clinical trials has consistently been around 3–4 weeks, and the response rates appear to plateau around 8 weeks.75–77 Rates of symptomatic remission with budesonide were significantly better than placebo at all 3 of the time points that were assessed in the clinical trials: 2, 4, and 8 weeks.75,77
Discussion: On the basis of the evidence for significant benefits over placebo by 2 weeks and the consistent median time to symptomatic remission of 3–4 weeks, the consensus group agreed that symptomatic improvement should clearly be evident by 1–2 months. Evidence of worsening before the full 4- to 8-week trial may require intervention.
Statement 8. In patients with mild to moderate CD, we suggest against the use of oral budesonide to maintain complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 35%; agree, 50%; uncertain, 15%.
Key evidence: Most of the evidence suggests that budesonide is not more effective than placebo for maintenance of remission in patients with quiescent CD.73,74,78 Two meta-analyses of trials of at least 6-month duration suggested that budesonide was no more effective than placebo for maintenance of remission at 6 or 12 months.74,78 However, an NMA found that budesonide 6 mg/day was superior to placebo (odds ratio [OR], 1.69; credible intervals [CrI], 1.05–2.75) for maintenance of remission.73 There was no statistically significant difference at 12 months between budesonide and weaning doses of prednisolone or azathioprine, but budesonide 6 mg was better than mesalamine 3 g/day.78 All of these analyses pooled together studies using the oral controlled ileal release preparation and the pH-dependent release formulation and found no studies that used budesonide MMX for the treatment of CD.
In maintenance trials, budesonide has been associated with a significantly higher risk of corticosteroid-related adverse events compared with placebo (RR, 2.19; 95% CI, 1.08–4.46).74
Discussion: There is little evidence supporting the efficacy of budesonide for maintenance therapy. Adverse event and safety profiles are of particular concern during longer-term maintenance therapy, and budesonide has been associated with a risk of corticosteroid-related adverse events.74,79 In a pooled analysis of 5 RCTs, budesonide was associated with a higher incidence of endocrine side effects compared with placebo, particularly cutaneous corticosteroid symptoms such as acne, easy bruising, moon face, and hirsutism.79
Considering the evidence of benefit and risk evidence for its use as maintenance therapy, the consensus group made a conditional suggestion against the routine use of budesonide for maintenance therapy.
Corticosteroids
No recommendation A. In patients with mild CD limited to the colon who have failed to respond to sulfasalazine, the consensus group does not make a recommendation (neither for nor against) regarding the use of prednisone 40–60 mg/day to induce complete remission.
No recommendation B. In patients with mild CD who have failed to respond to oral budesonide 9 mg/day, the consensus group does not make a recommendation (neither for nor against) regarding use of prednisone to induce complete remission.
Key evidence: See statements 9 and 10 for evidence of the efficacy of corticosteroids. Specific data in mild disease were not evaluated.
Discussion: Two statements were voted on, but consensus could not be reached regarding whether prednisone has a role in patients with mild disease who have failed sulfasalazine or budesonide. Some members of the consensus group argued that because patients with mild disease are at low risk of complications or disease progression, a watch-and-wait strategy may be warranted, whereas others argued that if a patient had sufficient symptoms to warrant treatment with sulfasalazine or budesonide, treatment failure should not be acceptable, and the goal should still be complete remission. Regardless, patients with mild disease who have failed sulfasalazine or budesonide should be reassessed at appropriate time points to determine whether there are other causes for their symptoms and to discuss alternative treatment options.
Statement 9. In patients with moderate CD who have failed to respond to oral budesonide 9 mg/day, we suggest the use of prednisone 40–60 mg/day to induce complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 15%; agree, 80%; uncertain, 5%.
Statement 10. In patients with moderate to severe CD, we recommend the use of oral prednisone 40–60 mg/day to induce complete remission.
GRADE: Strong recommendation, low-quality evidence.
Vote: strongly agree, 50%; agree, 50%.
Key evidence: Evidence for the efficacy of oral corticosteroids over placebo is derived from 2 positive RCTs that have been included in 2 systematic reviews.74,80 In the analysis using induction of symptomatic remission as the outcome, corticosteroids were significantly more effective than placebo (RR, 1.99; 95% CI, 1.51–2.64).80 Corticosteroids were associated with higher rates of adverse events than placebo (RR, 4.89; 95% CI, 1.98–12.07).80
These studies predate the availability of budesonide, so it is unknown whether patients with previous non-response to budesonide would respond as well as budesonide-naive patients. Meta-analysis of 8 RCTs demonstrated that budesonide was significantly less effective than conventional steroids for induction of remission at 8 weeks (RR, 0.85; 95% CI, 0.75–0.97).75
The superior efficacy of conventional corticosteroids suggests that patients have a greater likelihood of responding and thus may benefit from these agents after failure of budesonide. Conversely, prednisone may be less effective in patients who have failed budesonide because these cases may be more difficult to treat, and the disease may have progressed during failure of budesonide treatment.
Discussion: Corticosteroids are an important treatment option in patients with moderate to severe CD. The use is generally limited to short-term therapy because they are associated with a high potential for serious side effects.81 On the basis of evidence for efficacy compared with placebo but because of their adverse event profile, the consensus group suggested limiting the use of prednisone to second-line use in patients with moderate disease but recommended first-line use in patients with more severe disease. In addition, the separation between moderate and severe disease is not precise, and for patients with moderate colonic disease extending beyond the right side, it is likely that prednisone would be used first-line rather than budesonide.
Corticosteroids, especially repeat courses, should be avoided in some patients such as those with poorly controlled diabetes, history of steroid-induced psychosis or depression, history of avascular necrosis, severe osteoporosis with or without pathologic fractures, or any other prior severe steroid side effect/toxicity.10 Generally all patients being started on corticosteroids should receive prophylactic therapy with adequate doses of calcium and vitamin D.82
Statement 11. We recommend that patients with moderate to severe CD be evaluated for symptomatic response to prednisone between 2 and 4 weeks to determine the need to modify therapy.
GRADE: Strong recommendation, very low-quality evidence.
Vote: strongly agree, 40%; agree, 60%.
Key evidence: The mean time to symptomatic remission reported in clinical trials with oral corticosteroids was 20 days with methylprednisone83 and 41 days with beclomethasone.75,84
Discussion: Data suggest that symptomatic improvement should be evident by 2–4 weeks. Patients with severe disease may warrant early assessment, whereas for those with more moderate symptoms, the longer time to assessment may be acceptable. Although it may not always be feasible to arrange an in-person assessment within 2 weeks, patients should be advised to report back if there is no improvement or should at least be followed up by telephone. Patients with evidence of worsening disease, unacceptable adverse events, or failure to respond during this time interval should be considered for alternate treatment strategies.
Statement 12. In patients with active CD of sufficient severity to require hospitalization, we suggest the use of intravenous corticosteroids (eg, methylprednisolone 40–60 mg/day) to induce symptomatic remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 55%; agree, 45%.
Key evidence: Evidence for the efficacy of intravenous corticosteroids is derived from 1 RCT and 1 retrospective cohort study.85,86 In the RCT, 93% of patients responded to a 10-day course of intravenous hydrocortisone. Response to therapy was not impacted by previous oral steroid use.85 In a retrospective report, 76% of patients responded to 5-day intensive intravenous corticosteroid treatment.86
Discussion: Intravenous corticosteroids appear to be effective in achieving symptomatic response and can help provide time to establish successful maintenance therapy in patients with severe CD. However, on the basis of the limited, low-quality evidence the consensus group made a conditional recommendation in favor of the use of these agents.
Statement 13. We recommend that patients with severe CD be evaluated for symptomatic response to intravenous methylprednisolone within 1 week to determine the need to modify therapy.
GRADE: Strong recommendation, very low-quality evidence.
Vote: strongly agree, 50%; agree, 50%.
Key evidence: In the RCT and retrospective cohort study mentioned above (see statement 12), symptomatic remission rates were 39% at day 3, 76%–78% at day 5, and 93% at day 10.85,86
Discussion: Generally, intravenous corticosteroid therapy requires hospitalization and serves as a short-term strategy to help stabilize the acutely ill patient while awaiting the onset of other therapies. In light of this and the potential safety issues associated with corticosteroids (statement 14), the consensus group recommended early patient assessment to determine the need for a change in therapy.
Statement 14. In patients with CD of any severity, we recommend against the use of oral corticosteroids to maintain complete remission.
GRADE: Strong recommendation, low-quality evidence.
Vote: strongly agree, 95%; agree, 5%.
Key evidence: A meta-analysis of data from 3 RCTs found no significant reduction in the odds of relapse with ongoing corticosteroid therapy compared with placebo at 6, 12, or 24 months.87 Compared with budesonide, data from 1 RCT showed no significant difference in continued remission at 12 months between budesonide and weaning doses of prednisolone (RR, 0.79; 95% CI, 0.55–1.13).78,88
Discussion: The adverse effects of long-term corticosteroid use are well-known and well-documented.10,89,90 In the TREAT registry, prednisone therapy was independently associated with serious infections (hazard ratio [HR], 1.57; 95% CI, 1.17–2.10; P = .002).90 No safe lower limit of dosing has been identified in which patients are spared from the adverse effects.
The risks of long-term corticosteroid therapy and the lack of evidence supporting efficacy over placebo in this setting led the consensus group to recommend against the use of maintenance corticosteroid therapy.
Immunosuppressants
Statement 15. In patients with CD of any severity, we suggest against the use of thiopurine monotherapy to induce complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 50%; agree, 45%; uncertain, 5%.
Key evidence: Two meta-analyses of the same 5 RCTs reported no significant difference in symptomatic remission rates between thiopurine monotherapy (azathioprine or 6-mercaptopurine) and placebo.91,92 Overall, 48% of patients receiving thiopurines (95/197) achieved remission compared with 37% of placebo patients (68/183) (RR, 1.23; 95% CI, 0.97–1.55).92 Azathioprine therapy was associated with a significant steroid-sparing effect compared with placebo (RR, 1.34; 95% CI, 1.02–1.77).92
Discussion: Thiopurine monotherapy has not been shown to be effective for induction of remission.91,92 In addition, these agents are slow-acting and therefore not desirable for use as induction therapy. Because of the safety and tolerability issues (see statement 16)93 and lack of evidence of benefit for induction therapy, the consensus group suggested against the use of these agents to induce complete remission. However, some members of the consensus group stated that they would use thiopurines in select patients in conjunction with corticosteroids during the induction period (see statement 16).
Statement 16. In selected patients with CD who have achieved symptomatic remission on oral corticosteroids, we suggest thiopurine monotherapy to maintain complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 20%; agree, 60%; uncertain, 15%; disagree, 5%.
Key evidence: Evidence for the efficacy of thiopurine monotherapy for the maintenance of remission comes from 2 systematic reviews of RCTs.91,94 A meta-analysis of 2 RCTs found a non-significant reduction in the risk of relapse with azathioprine compared with placebo (RR, 0.64; 95% CI, 0.34–1.23). However, data from 3 additional azathioprine withdrawal trials indicated that continuing medication did prevent relapse compared with switching to placebo (RR, 0.39; 95% CI, 0.21–0.74).91 A more recent meta-analysis of 6 studies found azathioprine was significantly superior to placebo in maintaining symptomatic remission during a period of 6–18 months (RR, 1.19; 95% CI, 1.05–1.34).94 Most of the maintenance studies included selected populations of patients who had achieved remission while on a thiopurine and thus are more likely to show a positive effect for thiopurines and less likely to experience adverse events. One additional RCT withdrawal trial, published after the meta-analyses, reported a significant reduction in risk of relapse with continued azathioprine therapy at 1 year but not at 2 years.95
In a meta-analysis of 10 cohort studies, thiopurine use was associated with 40% reduction in the risk of first surgical resection in patients with CD (HR, 0.59; 95% CI, 0.48–0.73).96
One meta-analysis reported that azathioprine had a significantly greater risk of adverse events (RR, 1.29; 95% CI, 1.02–1.64), withdrawal due to adverse events (RR, 3.12; 95% CI, 1.596.09), and serious adverse events (RR, 2.45; 95% CI, 1.22–4.90) compared with placebo. Common adverse events included pancreatitis, leukopenia, nausea, allergic reaction, and infection.94
Discussion: Meta-analyses suggest that among patients who achieved symptomatic remission while on a thiopurine, ongoing maintenance thiopurine therapy may be beneficial.91,94 However, the evidence is very low-quality, and there remains uncertainty as to the benefits.
Thiopurines are associated with a rare but important increased risk of lymphoma (including hepatosplenic T-cell lymphoma [HSTCL])97,98 and non-melanoma skin cancers.99 In 2014, Health Canada issued an alert warning of the risk of HSTCL with azathioprine/6-mercaptopurine.100 This warning led to a position statement from the CAG recommending that continuation of thiopurine therapy be considered on the basis of a balance of the evidence for risk and efficacy against an individual patient’s response to therapy, preferences, and risk tolerance.93 The risk assessment should be individualized and include factors such as underlying age-related lymphoma risk.93,101
Because of the safety and tolerability issues and weak evidence surrounding the efficacy of thiopurines, the consensus group made a conditional suggestion in favor of the use of these agents for maintenance therapy in select patients in remission. Select patients were those considered to be at low risk of disease progression or complications, for example, a patient with isolated colonic CD with superficial ulceration and no other complications. Some consensus participants were against the use of thiopurine monotherapy in patients with CD, stating that the benefits do not outweigh the risks because there are more effective therapeutic options. However, the consensus was that these agents continue to have a role, particularly in those select patients who have responded to corticosteroids and cannot access or afford biologic therapy for various reasons.
Statement 17. In patients with moderate to severe corticosteroid-dependent/resistant CD, we suggest parenteral methotrexate to induce complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 10%; agree, 65%; uncertain, 10%; disagree, 15%.
Key evidence: Evidence for the efficacy of methotrexate for the induction of symptomatic remission comes from 2 systematic reviews; 1 included 2 trials91 and the other 3 trials.102 Only 2 trials were pooled, 1 negative trial using oral methotrexate103 and 1 positive trial using intramuscular methotrexate,104 and the resulting RR expressed as the risk of having ongoing active disease was not statistically significant (RR, 0.82; 95% CI, 0.65–1.03).91 However, the trial assessing the intramuscular formulation in corticosteroid-dependent patients demonstrated a significant benefit in favor of methotrexate over placebo, with symptomatic remission being achieved by 39% of patients with methotrexate, as compared with 19% with placebo (RR, 1.95; 95% CI, 1.09–3.48; P = .025). In addition, methotrexate therapy was associated with a significant steroid-sparing effect compared with placebo (P = .026).104
A review of RCTs of methotrexate therapy versus active comparators reported that methotrexate was as effective as azathioprine or 6-mercaptopurine and more effective than 5-ASA for induction therapy.102
Most of the trials assessing the efficacy of methotrexate have included relatively small numbers of patients and may have lacked power to show a benefit of this therapy.102
Discussion: There is little evidence of the efficacy of methotrexate for induction of remission; however, the study using a parenteral formulation (intramuscular) at a higher dose (25 mg/week) did show a significant benefit in inducing symptomatic remission and reducing the need for corticosteroids.104 In contrast, the studies of oral administration generally used low doses of methotrexate, which may be subtherapeutic for induction of remission.102
The parenteral study used an intramuscular formulation of methotrexate, but subcutaneous administration is now more common in clinical practice. Pharmacokinetic studies suggest that the bioavailability of subcutaneous methotrexate is about 15%–25% greater than for the oral formulation.105,106
Primarily on the basis of the positive parenteral study demonstrating efficacy as induction therapy with steroid-sparing effects, the consensus group made a conditional suggestion in favor of the use of parenteral methotrexate in patients with corticosteroid-dependent/resistant CD.
Statement 18. In patients with CD who have achieved symptomatic remission on oral corticosteroids and parenteral methotrexate, we suggest parenteral methotrexate to maintain complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 10%; agree, 85%; uncertain, 5%.
Key evidence: Evidence for the efficacy of methotrexate for the maintenance of symptomatic remission is available from a systematic review of 5 RCTs.107 Only 1 RCT compared the efficacy of maintenance parenteral methotrexate with placebo. In this study 76 patients who had responded to intramuscular methotrexate induction therapy were randomized to continue methotrexate at a lower dose or switch to placebo.108 At week 40, 65% of patients maintained remission in the intramuscular methotrexate (15 mg/week) group compared with 39% in the placebo group (RR, 1.67; 95% CI, 1.05–2.67; P = .04).107,108 There was also a significant reduction in the use of corticosteroids for relapse among the patients in the methotrexate group.
Compared with placebo, low-dose oral methotrexate did not appear to be effective for maintenance of remission in a small study.103 In other small studies, there were no significant differences in remission rates with oral methotrexate, 6-mercaptopurine, and 5-ASA maintenance therapies.107
The most common adverse events reported in methotrexate maintenance studies were nausea and vomiting, symptoms of a cold, abdominal pain, headache, joint pain or arthralgia, and fatigue.107
Discussion: Primarily on the basis of the well-conducted, positive parenteral study demonstrating improved rates of continued corticosteroid-free symptomatic remission, the consensus group made a conditional suggestion in favor of the use of parenteral methotrexate for maintenance therapy. Regular monitoring of liver function is indicated throughout methotrexate therapy.
Statement 19. We suggest that patients with CD receiving thiopurine or methotrexate who do not achieve corticosteroid-free remission within 12–16 weeks should have therapy modified.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 40%; agree, 55%; uncertain, 5%.
Key evidence: In the meta-analysis of RCTs of thiopurines for induction therapy, patients evaluated at 17 weeks or later were significantly more likely to be in remission than those taking placebo (RR, 1.59; 95% CI, 1.05–2.41), whereas those evaluated before 17 weeks were not.92
In the methotrexate induction RCT, there were significant differences in disease activity scores between methotrexate and placebo from week 6 through the 16-week study. Corticosteroid use was significantly lower in the methotrexate group by week 4 in high-dose patients and by week 12 in those taking lower prednisone doses.104
Discussion: Thiopurine therapy has a delayed onset of action of 3–4 months,92 and methotrexate may also have a relatively slow onset of action.104 While bearing in mind that thiopurine monotherapy is not recommended for induction of remission, the consensus group concluded that improvement with these agents and methotrexate should be evident within 3–4 months. Because of the delayed onset of action it is important not to evaluate and change therapies before the completion of an adequate trial, while also considering that it is important not to delay assessment of therapeutic response and risk poor outcomes from the continuation of ineffective treatment. Failure to respond or worsening of disease within the 12- to 16-week period likely warrants modification of therapy.
Because some patients may have low or absent levels of the enzyme (thiopurine methyltransferase [TPMT]) needed to metabolize thiopurines,109 a TPMT assay should be performed before initiation of treatment to identify patients at risk for severe toxicity. It should be noted that TPMT testing does not replace the need for mandatory ongoing complete blood count monitoring. In addition, in some cases, monitoring may also include measurement of thiopurine metabolites to optimize dosing and verify adherence.
Anti–Tumor Necrosis Factor Biologics
Statement 20. In patients with moderate to severe luminal CD with risk factors of poor prognosis, we recommend anti-TNF therapy (infliximab, adalimumab) as first-line therapy to induce complete remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 60%; agree, 40%.
Statement 21. In patients with moderate to severe CD who fail to achieve complete remission with any of corticosteroids, thiopurines, or methotrexate, we recommend anti-TNF therapy (infliximab, adalimumab) to induce complete remission.
GRADE: Strong recommendation, high-quality evidence.
Vote: strongly agree, 80%; agree, 20%.
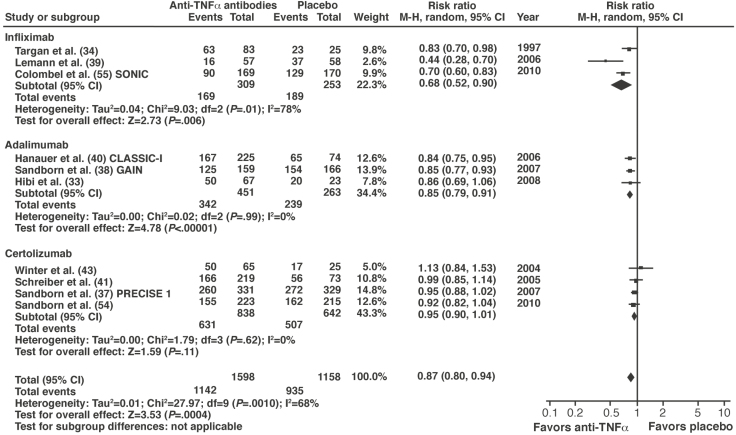
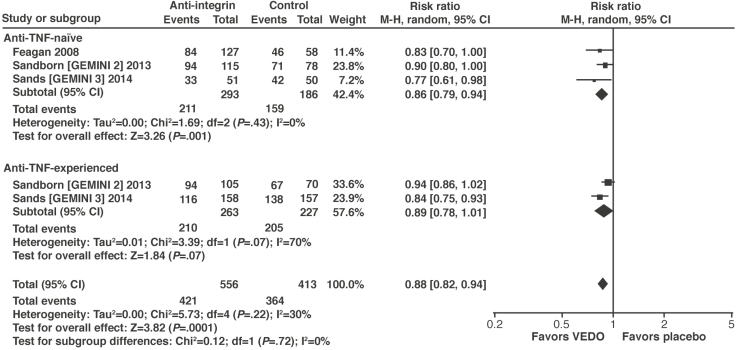
Key evidence: Anti-TNF therapies have been extensively evaluated in RCTs and systematic reviews.110–112 One meta-analysis included 10 trials evaluating the anti-TNF therapy alone or with concomitant therapies.110 Using the outcome of failure to achieve symptomatic remission, anti-TNF therapy was significantly more effective than placebo (RR, 0.87; 95% CI, 0.80–0.94; P = .0004) (Figure 3). Positive results were reported with infliximab and adalimumab but not with certolizumab pegol.110 When certolizumab pegol was removed from the analysis, the benefits of anti-TNF therapy were more robust (RR, 0.82; 95% CI, 0.73–0.91). The NMA also found significantly greater odds of induction of remission with infliximab (OR, 2.8; 95% CrI, 1.4–7.2) and adalimumab (OR, 2.9; 95% CrI, 1.6–5.5) but not certolizumab pegol (OR, 1.4; 95% CrI, 0.95–2.0) compared with placebo.111
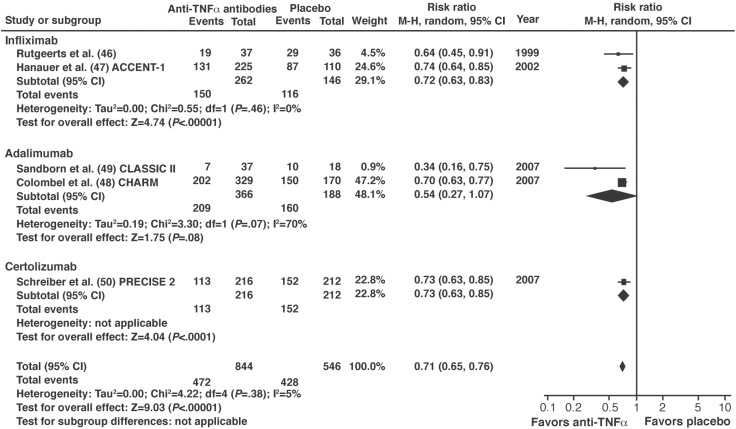
Figure 3.
Forest plot of randomized controlled trials of anti-TNF therapies versus placebo in inducing remission in active luminal CD. Reprinted by permission from Springer Nature, American Journal of Gastroenterology. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Ford AC, Sandborn WJ, Khan KJ, et al. ©2011. Reference.110 Anti-TNFα, anti-tumor necrosis factor-α; CD, Crohn’s disease; CI, confidence interval; M-H, Mantel-Haenszel.
In most of the studies, patients had previously received other treatments; therefore, the quality of evidence for statement 20 (first-line anti-TNF therapy) was downgraded for indirectness of the patient population (treatment-naive patients with risk factors for poor prognosis).
Discussion: The anti-TNF agents, adalimumab and infliximab, have demonstrated efficacy for the induction of symptomatic remission in patients with CD.110–112 Four RCTs with certolizumab pegol have all yielded negative results, failing to show a statistically significant benefit over placebo for induction of remission. Meta-analysis of these trials yielded a RR of 0.95 for failure to achieve remission (95% CI, 0.90–1.01).110 Certolizumab pegol is not licensed for the treatment of CD in Canada or most European countries. Similarly, etanercept has not demonstrated efficacy113 and is not indicated for the treatment of CD. For these reasons, the consensus group restricted the recommendations regarding anti-TNF biologics specifically to adalimumab and infliximab.
Although the primary outcome in these trials was clinical (or symptomatic) remission, anti-TNF therapy has been associated with mucosal healing, thus suggesting that the outcome of complete remission is feasible.42,114–116 In the SONIC trial, infliximab monotherapy was associated with significantly higher rates of mucosal healing at week 26 compared with azathioprine monotherapy (30% vs 17%, P = .02).115 In patients who responded to induction therapy, rates of mucosal healing at week 12 were 27% with adalimumab versus 13% with placebo (P = .056).116
The majority of clinical trials were conducted in patients who had received previous treatments (per statement 21). These data were extrapolated to the first-line recommendation described in statement 20, resulting in a lower quality of evidence. Additional support for the use of early anti-TNF therapy comes from open, prospective trials of the use of combined immunosuppressive therapy with infliximab and azathioprine in patients who had not previously received corticosteroids, immunosuppressants, or biologics.117,118 In these studies, what is being called “top-down” treatment was associated with significantly higher rates of symptomatic remission at earlier time points compared with not using early anti-TNF therapy. The study that also assessed mucosal healing demonstrated significantly higher rates of complete remission at week 30 (44.7% vs 17.9%, P = .011).118 In 1 study the higher rates of symptomatic remission remained significant at 1 year,117 whereas in the other they did not.118
In the meta-analysis, there was no statistically significant difference in the incidence of adverse events with anti-TNF therapies compared with placebo (RR, 0.99; 95% CI, 0.90–1.08).110
Biosimilar anti-TNF therapies are now available, with biosimilar infliximab being approved for CD in Canada, Europe, and the United States. At the time of the consensus meeting, no disease-specific RCTs were available on these agents, but prospective cohort studies suggested that they were effective for the treatment of CD.119–122 One study reported no change in disease activity and limited immunogenicity among patients who were switched from the originator medication.122 More recently, the 12-month NOR-SWITCH trial in patients with IBDs or arthritic diseases showed that switching from original infliximab to the biosimilar CT-P13 was not inferior to remaining on original infliximab.123 However, the study was not powered to show non-inferiority in individual diseases. In a survey of patients, the majority had concerns regarding the efficacy and safety of biosimilar agents and wished to be involved in the decision-making process.124 Currently there is insufficient evidence to support routine switching to biosimilar anti-TNF agents in patients with stable CD, and consideration of a switch should take into account patient preferences.
The consensus group concluded that anti-TNF therapy with adalimumab or infliximab is an effective and well-tolerated option in patients who have failed conventional therapy. Although sufficient evidence supports their efficacy in patients with moderate to severe CD who are treatment-naive, the consensus group agreed that these agents should likely be reserved for patients with risk factors (as described in the “definitions” section), mainly because of cost issues.
Statement 22. In patients with active CD, when starting anti-TNF therapy, we suggest it be combined with a thiopurine over monotherapy to induce complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 45%; agree, 50%; uncertain, 5%.
Statement 23. In patients with active CD, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters.
GRADE: Conditional recommendation, low-quality evidence for infliximab, very low-quality evidence for adalimumab.
Vote: strongly agree, 35%; agree, 55%; uncertain, 5%; disagree, 5%.
No recommendation C. In patients with active CD, when starting anti-TNF therapy, the consensus group does not make a recommendation (neither for nor against) it being combined with methotrexate over monotherapy to induce complete remission.
Key evidence: Evidence for the efficacy of combination therapy with an anti-TNF therapy plus a thiopurine (infliximab plus azathioprine) is available from 2 meta-analyses.111,125 In 1 analysis, the combination of infliximab plus azathioprine was more effective than either therapy alone,125 whereas in the other the combination was more effective than placebo or azathioprine alone but not more effective than infliximab alone.111 However, the SONIC trial is the only RCT directly comparing these 3 strategies.115 At 26 weeks, combination therapy was more effective in inducing corticosteroid-free symptomatic remission (56.8%) compared with either infliximab (44.4%) or azathioprine (30.0%) monotherapies (P < .001 vs azathioprine and P = .02 vs infliximab; OR vs infliximab, 1.65; 95% CI, 1.07–2.54). Significantly higher rates of mucosal healing were also seen.115 Patients who received combination therapy were less likely to develop anti-TNF antibodies (0.9% vs 14.6%) and had higher median serum infliximab trough levels (3.5 μg/mL vs 1.6 μg/mL; P < .001).115
Evidence for the efficacy of the combination of adalimumab plus azathioprine is available from a meta-analysis of observational data from RCTs and cohort studies.126 Adalimumab alone was inferior to combination therapy (OR, 0.78; 95% CI, 0.64–0.96; P = .02) for induction of symptomatic remission. However, a more recent pooled analysis of data from 4 RCTs published outside of the search window for these guidelines found no advantage with the combination of adalimumab plus an immunosuppressant over adalimumab alone.127 An open-label, randomized study in patients who had not previously received immunosuppressants or biologics found no difference in symptomatic remission rates between the combination of adalimumab plus azathioprine (68.1%) and adalimumab monotherapy (71.8%; P = .63).128 However, the rate of endoscopic improvement was significantly higher with combination therapy at 6 months (84.2% vs 63.8%; P = .019) but not 12 months (79.6% vs 69.8%; P = .36).128
One RCT, the COMMIT study, compared the efficacy of combination therapy with an anti-TNF (infliximab) plus methotrexate to infliximab alone and found no difference in rates of symptomatic remission between the 2 treatment groups (HR, 1.16; 95% CI, 0.62–2.17; P = .63).129 There appeared to be a pharmacokinetic advantage, with patients receiving combination infliximab plus methotrexate being less likely to develop antibodies to infliximab (4% vs 20%; P = .01) than those who received infliximab alone. In addition, there was a trend to higher median serum trough infliximab concentrations in patients who received combination therapy (6.35 vs 3.75 mg/mL; P = .08).129
Discussion: Evidence suggests that the addition of azathioprine to anti-TNF therapy (infliximab and possibly adalimumab) may help improve symptomatic remission rates and enhance mucosal healing.115,126,128 In addition, azathioprine, when used in combination with infliximab or adalimumab, may reduce the development of anti-TNF antibodies and improve trough drug levels. However, because of the scarce data, the consensus group made a conditional suggestion in favor of initiating azathioprine when starting anti-TNF therapy.
In contrast, the consensus group was unable to make a recommendation for or against the use of methotrexate to improve clinical outcomes because the COMMIT study showed no significant improvement in clinical endpoints.129 However, the data suggested that methotrexate may reduce immunogenicity and improve drug levels, and thus the consensus group made a conditional suggestion in favor of the use of this agent in combination with anti-TNF therapy in an attempt to improve pharmacokinetic parameters. Methotrexate reduced the rate of development of antibodies, and there was a trend toward improved infliximab levels; importantly, 92% of the patients with detectable infliximab at trough at week 46 were treatment successes.129
Statement 24. We recommend that patients with CD be evaluated for symptomatic response to anti-TNF induction therapy between 8 and 12 weeks to determine the need to modify therapy.
GRADE: Strong recommendation, very low-quality evidence.
Vote: strongly agree, 60%; agree, 40%.
Key evidence: In clinical trials, symptomatic remission rates with adalimumab were significantly greater than placebo as early as 2–4 weeks, and significantly greater symptomatic response rates were seen by 1–2 weeks.130–132 In CHARM, the proportion of patients achieving symptomatic remission with adalimumab reached a maximum at week 8 and plateaued thereafter.130
In an induction trial with infliximab, significant improvements in symptomatic response rates were seen at week 2 and in symptomatic remission rates at week 4.133 Maintenance studies reported significantly higher rates of symptomatic remission with infliximab at weeks 12–14 (the first time points assessed).129,134
Discussion: The evidence suggests that although many patients will begin to respond to anti-TNF therapies within 2–4 weeks, response rates continue to increase up to 12–14 weeks. Therefore, the consensus group agreed that patients should complete a course of induction therapy (8–12 weeks), but that those who have failed to respond by this time are unlikely to do so. If a response occurs, subsequent assessments should include endoscopy to confirm complete remission, but the optimal timing of endoscopy is currently uncertain. Patients with more severe disease may require earlier assessments.
Statement 25. In patients with CD who have achieved symptomatic response with anti-TNF induction therapy, we recommend continued anti-TNF therapy to achieve and maintain complete remission.
GRADE: Strong recommendation, high-quality evidence.
Vote: strongly agree, 90%; agree, 10%.
Key evidence: There is high-quality evidence from 3 systematic reviews supporting the use of anti-TNF therapies for maintenance of symptomatic remission.110,111,135 In a meta-analysis of 5 RCTs, anti-TNF therapy significantly reduced the risk of relapse in patients with quiescent CD (RR, 0.71; 95% CI, 0.65–0.76; P < .00001) compared with placebo (Figure 4).110 In the NMA, infliximab (OR, 2.8; 95% CrI, 1.8–4.5), adalimumab (OR, 5.1; 95% CrI, 3.3–8.1), and certolizumab pegol (OR, 2.0; 95% CrI, 1.4–3.0) were all significantly better than placebo as maintenance therapy.111 The majority of these trials were conducted in patients who had achieved remission on anti-TNF therapy.
Figure 4.
Forest plot of randomized controlled trials of anti-TNF therapies vs placebo in preventing relapse in quiescent CD. Reprinted by permission from Springer Nature, American Journal of Gastroenterology. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Ford AC, Sandborn WJ, Khan KJ, et al. ©2011. Reference.110 Anti-TNFα, anti-tumor necrosis factor-α; CD, Crohn’s disease; CI, confidence interval; M-H, Mantel-Haenszel.
Discussion: Unlike induction therapy (statements 20 and 21), all 3 anti-TNF therapies (adalimumab, certolizumab pegol, and infliximab) have demonstrated efficacy for maintenance of remission in patients who have responded to therapy with the same agent.
However, as with induction therapy, the primary outcome in these trials was symptomatic remission, but anti-TNF therapy has been associated with mucosal healing during longer-term follow-up, suggesting the outcome of complete remission during maintenance therapy is feasible.42,116 In the endoscopic substudy of the ACCENT 1 trial, the rate of mucosal healing at week 54 was significantly higher among those who received scheduled infliximab maintenance therapy compared with those who received episodic therapy (50% vs 7%; P = .007).42 In the EXTEND trial, rates of mucosal healing were 24% with adalimumab and 0% with placebo, respectively (P < .001) at week 52.116
In the meta-analysis, there was no statistically significant difference in the incidence of any adverse event (RR, 0.93; 95% CI, 0.84–1.03) or infusion/injection site reactions (RR, 0.64; 95 % CI, 0.06–6.66) with anti-TNF therapies compared with placebo.110
On the basis of the consistently positive trials, the consensus group made a strong recommendation in favor of continuing anti-TNF maintenance therapy among patients who respond to induction therapy.
Statement 26. In patients with CD who have a suboptimal response to anti-TNF induction therapy, we suggest dose intensification to achieve complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 10%; agree, 75%; uncertain, 15%.
Statement 27. In patients with CD who lose response to anti-TNF maintenance therapy, we suggest dose optimization to recapture complete remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 35%; agree, 55%; uncertain, 10%.
Key evidence: Data on the efficacy of dose intensification in patients who did not respond to anti-TNF induction therapy (primary non-response, statement 26) and those who had an initial response (secondary loss of response, statement 27) are available from 2 systematic reviews of case series.136,137 In a meta-analysis of 23 studies, the annual rate of non-response or loss of response was about 21% in the pooled data for patients who did or did not respond to adalimumab induction therapy.136 Of those who underwent dose intensification for whom data were available, 71% achieved a symptomatic response and 40% symptomatic remission. Subgroup analysis revealed that about 20% of patients who had initially responded subsequently lost response annually, and among those for whom data were available, about 25% underwent dose intensification annually. Efficacy in this subgroup was not reported.136
A review of 16 studies calculated the annual incidence of loss of response to infliximab to be 13%.137 In the studies included in this review, rates of response to dose intensification were 54%–90%, with 1 study reporting that 31% achieved symptomatic remission.
Discussion: As stated above, statement 26 refers to primary partial responders, and statement 27 refers to patients who have initially responded and subsequently lost response. No RCT data were found for these 2 patient types; data are from subgroup analyses of patients who have a primary non-response (as opposed to partial response) and patients who have lost response, but these patients are generally pooled together. Overall, it appears that about 10%–20% of patients will lose response to anti-TNF therapy annually, and that about 54%–90% will regain symptomatic response when therapy is intensified. Although there are currently no data for dose intensification in patients who have a partial response (or have achieved symptomatic but not achieved complete remission), the ongoing cluster randomization trial, REACT-2, will address this.44
Although the quality of evidence is low, it does suggest that the likelihood of achieving a response with a dose intensification strategy is high. Therefore, the consensus group agreed that dose intensification, defined as either an increase of the anti-TNF dose or a shortening of the dosing interval, should be attempted to achieve a therapeutic goal of complete remission in patients with inadequate response or loss of response.
Statement 28. We suggest that dose optimization for patients with CD who lose response to anti-TNF therapy be informed by therapeutic drug monitoring.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 40%; agree, 50%; uncertain, 10%.
Key evidence: The evidence supporting a role for therapeutic drug monitoring (TDM) is very low-quality and largely extrapolated from observational studies that assessed the impact of trough drug levels and the development of anti-TNF antibodies on clinical responses.136,138,139 These studies do not directly assess whether TDM improves outcomes in these patients.
These data generally show that the presence of anti-TNF antibodies is associated with lower serum anti-TNF levels and a significantly higher risk of loss of clinical response to anti-TNF therapy.136,138,139 In one analysis of data from studies using infliximab, the risk of loss of clinical response among patients who had developed antibodies was 3 times greater than among those who did not develop antibodies.139 In a pooled analysis, patients with higher trough drug levels had a 2 times higher likelihood of remission compared with those with low trough levels.138
One small RCT found that using TDM to guide treatment decisions led to lower treatment costs with no significant differences in response rates versus routine dose intensification in patients who lose response to anti-TNF therapy.140
Discussion: Overall, the evidence suggests that low trough levels and the development of anti-TNF antibodies are associated with lower response rates. In addition, although the quality of evidence for TDM itself is very low, 1 study has suggested that using TDM to guide therapeutic decisions can reduce treatment costs while maintaining response rates. On the basis of these data, the consensus group made a conditional suggestion in favor of the use of TDM but agreed that more data are needed.
Statement 29. We suggest against switching between anti-TNF therapies in patients who are doing well on anti-TNF therapy.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 55%; agree, 45%.
Key evidence: The open-label, randomized SWITCH trial demonstrated that elective switching from one anti-TNF therapy to another was associated with a loss of tolerance and loss of efficacy within 1 year.141 Although the study was small and open-label, it did demonstrate a strong effect. Among patients with CD controlled on infliximab, 16% of those randomized to stay on infliximab compared with 47% switched to adalimumab required dose optimization or interruption of treatment (P = .006). Among the patients who interrupted adalimumab treatment, most were for loss of tolerance. A meta-analysis of observational studies found the rates of clinical remission were higher when the reason for switching was intolerance (61%) rather than secondary (45%) or primary failure (30%).142
Discussion: Electively switching between biologics in patients with well-controlled CD runs the risk of quickly eliminating all currently available biologic options if primary loss of response, secondary loss of response, or intolerance to therapy subsequently develops. As discussed in statement 21, there is also insufficient evidence to support routine switching to biosimilar anti-TNF agents in patients with stable CD.119–122
In light of the possible negative effects on efficacy and tolerability, the consensus group concluded that elective switching of patients controlled on anti-TNF therapy is not warranted. Any medication switch should also consider patient concerns and preferences.
Non–Anti–Tumor Necrosis Factor Biologics
Statement 30. In patients with moderate to severe CD who fail to achieve complete remission with any of corticosteroids, thiopurines, methotrexate, or anti-TNF therapy, we recommend vedolizumab to induce complete remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 60%; agree, 40%.
Key evidence: Evidence for the efficacy of vedolizumab for the induction of remission in CD is available from systematic reviews143,144 and an NMA.111 Meta-analysis of 3 RCTs (Feagan et al,145 GEMINI 2,146 and GEMINI 3147) found that vedolizumab was significantly more effective than placebo in the overall patient population (OR, 1.93; 95% CI, 1.33–2.81; P = .0006).111 Among patients who were anti-TNF-naive (see statement 31 for patients who have been previously treated with anti-TNF therapy), meta-analyses have shown that vedolizumab was significantly superior to placebo for the outcome of symptomatic remission (OR, 1.76; 95% CI, 1.11–2.78)143 or failure to achieve symptomatic remission (RR, 0.86; 95% CI, 0.79–0.94; P = .001) (Figure 5).144
Figure 5.
Forest plot of randomized controlled trials of vedolizumab in inducing remission in active luminal CD, stratified on basis of prior anti-TNF exposure. Chandar AK, Singh S, Murad MH, et al. Efficacy and safety of natalizumab and vedolizumab for the management of Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:1695–708, by permission of Oxford University Press. Reference.144 Anti-TNF, anti-tumor necrosis factor; CD, Crohn’s disease; CI, confidence interval; M-H, Mantel-Haenszel; VEDO, vedolizumab.
Discussion: Although the primary outcome in these trials was symptomatic remission, evidence from cohort studies suggests that some patients can achieve mucosal healing with vedolizumab therapy, suggesting the outcome of complete remission is feasible.148–150 In the retrospective US VICTORY study at 1 year, 63% of patients had achieved mucosal healing, and 26% had achieved deep remission defined as symptomatic remission and mucosal healing (ie, complete remission).148 In addition, compared with placebo, vedolizumab resulted in higher rates of corticosteroid-free remission at week 52 in the GEMINI 2 study.146
In the induction studies, there was no significant difference in adverse event rates (RR, 0.94; 95% CI, 0.65–1.15; P = .56) or discontinuations (RR, 0.74; 95% CI, 0.36–1.54; P = .42) with vedolizumab compared with placebo.144
The consensus group concluded that vedolizumab therapy is an effective, well-tolerated option for induction therapy in those patients who have failed conventional therapy.
No recommendation D. In patients with active CD starting vedolizumab, the consensus group does not make a recommendation (neither for nor against) regarding adding a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters.
Key evidence: No evidence was found showing that use of combination vedolizumab plus an immunosuppressant was clinically superior to monotherapy. Post hoc analysis of the GEMINI 2 trial showed no significant differences in endpoints for patients receiving vedolizumab plus a concomitant immunosuppressant at baseline compared with those receiving placebo.151 Pharmacokinetic studies report no effect of concomitant immunosuppressant therapy on the clearance of vedolizumab.152 In a post hoc analysis of pooled ulcerative colitis and CD patients in the GEMINI 1 and 2 trials, the proportion of patients developing anti-vedolizumab antibodies was similar among patients receiving an immunosuppressant compared with those receiving monotherapy (3% vs 4%).153 However, in the group that received only vedolizumab induction followed by placebo maintenance, the rates of anti-vedolizumab antibodies were 3% among those who were receiving an immunosuppressant and 18% among those who were not.153
Discussion: This statement was voted on, but consensus could not be reached regarding whether there is a role for adding a thiopurine or methotrexate when initiating vedolizumab therapy. Because vedolizumab offers “gut selectivity” and lacks systemic immunosuppression, many consensus group members would use vedolizumab monotherapy in the biologic-naive patients and reserve the addition of an immunosuppressant for patients who had previously failed anti-TNF therapy. Some members of the consensus group argued that addition of immunosuppressant therapy is likely to improve the immunogenicity of vedolizumab, an effect that may not have been detected in the studies because it appears that the presence of anti-vedolizumab antibodies were mainly detectable only after the drug was discontinued.153 However, other members of the consensus group argued that there is no evidence to suggest that an immunosuppressant will improve either the clinical or pharmacokinetic profile of vedolizumab therapy, and thus the burden of combination therapy is not warranted.
Statement 31. In patients with CD who fail to achieve or maintain corticosteroid-free symptomatic remission with anti-TNF therapy, we suggest vedolizumab to induce complete remission.
GRADE: Conditional recommendation, low-quality evidence.
Vote: strongly agree, 20%; agree, 70%; uncertain, 5%; disagree 5%.
Key evidence: Data on the use of vedolizumab in patients who have previously failed anti-TNF therapy are available from GEMINI 2146 and GEMINI 3.147 In a meta-analysis of the patients previously treated with anti-TNF therapy, the RR of failure to induce symptomatic remission was 0.89 (95% CI, 0.78–1.01), but in the study with low risk of bias (GEMINI 3) the RR was 0.84 (95% CI, 0.75–0.93) with vedolizumab compared with placebo (Figure 5).144 Among the previously treated patients in GEMINI 3 the rate of symptomatic remission with vedolizumab was not significantly greater than placebo at week 6 but was at week 10 (26.6% vs 12.1%; P = .001; RR, 2.2; 95% CI, 1.3–3.6).147
Discussion: In the RCTs, the effects of vedolizumab among patients who had previously failed anti-TNF therapy appeared to be less robust than among those who were anti-TNF-naive but did appear to be greater than placebo, particularly with longer-term follow-up.146,147 Similarly, in the VICTORY cohort study, prior anti-TNF exposure was associated with a lower likelihood of achieving symptomatic remission (HR, 0.40; 95% CI, 0.20–0.81) or mucosal healing (HR, 0.29; 95% CI, 0.12–0.73) compared with no exposure.148
In general, the consensus group recommended striving for complete remission (including both symptomatic and endoscopic remission); however, most participants argued that patients who had achieved a corticosteroid-free, symptomatic remission (but not an endoscopic remission) on anti-TNF therapy should not be switched because current evidence does not definitively show that these patients would achieve complete remission on vedolizumab. Although mucosal healing has been associated with better long-term outcomes,4,5 data suggest that patients previously exposed to anti-TNF therapies are less likely to achieve mucosal healing on vedolizumab.148 In addition, the extent of endoscopic healing required for the prevention of relapse remains unclear. Therefore, the consensus group made a conditional suggestion in favor of switching to vedolizumab only among patients who require corticosteroids when on anti-TNF therapy.
For patients who are corticosteroid-free but have failed to achieve complete remission, the clinician should use clinical judgment regarding switching therapies, taking into consideration the degree of inflammation (endoscopic or laboratory abnormalities), patient history and risk factors, as well as patient preference. Patients may be reluctant to switch their medication if they are feeling well, despite not achieving complete remission.
Statement 32. We suggest that patients with CD be evaluated for symptomatic response to vedolizumab therapy between 10 and 14 weeks to determine the need to modify therapy.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 20%; agree, 75%; uncertain, 5%.
Key evidence: In clinical trials, symptomatic remission rates with vedolizumab were significantly greater than with placebo as early as 6–8 weeks.145–147 However, in GEMINI 3, symptomatic remission rates were not significantly greater than placebo until week 10 (26.6% vs 12.1%; RR, 2.2; 95% CI, 1.3–3.6; P = .001).147
Discussion: Evidence suggests that patients will respond to vedolizumab by week 10.145–147 Vedolizumab therapy includes induction doses at 0, 2, and 6 weeks, followed by maintenance doses every 8 weeks. Therefore, the consensus group agreed that patients should complete a course of induction therapy (6 weeks), but that those who have failed to respond before the first scheduled maintenance dose should not receive the week-14 dose, and modification of therapy should be considered. If a response occurs, subsequent assessments should include endoscopy to confirm complete remission, but the optimal timing of endoscopy is currently uncertain.
Statement 33. In patients with CD who have achieved symptomatic response with vedolizumab induction therapy, we recommend continued vedolizumab therapy to achieve and maintain complete remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 80%; agree, 20%.
Key evidence: Only 1 RCT, GEMINI 2, has been conducted to assess the efficacy of vedolizumab maintenance therapy.146 At week 52, symptomatic remission rates with vedolizumab (39.0% with 8-weekly dosing and 36.4% with 4-weekly dosing) were significantly greater than with placebo (21.6%; P < .001 vs once every 8 weeks and P = .004 vs once every 4 weeks). The calculated OR for maintenance of remission with vedolizumab versus placebo was 2.20 (95% CI, 1.40–3.44).111 Among anti-TNF-naive patients, we calculated that the RR of maintenance of remission was 1.49 (95% CI, 1.19–1.86) in favor of vedolizumab over placebo. Among patients previously exposed to anti-TNF therapy, there was a statistically significant risk difference of about 15% for vedolizumab once every 4 weeks and once every 8 weeks versus placebo.146
Discussion: Cohort data from retrospective and open-label prospective studies also support the efficacy of vedolizumab for maintenance therapy.148,154,155 In the VICTORY cohort, the 12-month symptomatic remission rate was 35%, and the mucosal healing rate was 63%.148 The GEMINI long-term safety study reported that among patients who completed GEMINI 2, 74% were in symptomatic remission after 152 weeks, including 82% of anti-TNF-naive patients, and 66% of those with prior anti-TNF failure.154
During long-term follow-up, the most common adverse events were exacerbation of CD, nasopharyngitis, and arthralgia.154 In clinical trials and open-label follow-up, there has been no reported increased risk of any or serious infections associated with vedolizumab and no cases of progressive multifocal leukoencephalopathy. Infusion-related reactions were reported in ≤5% of patients and malignancy in <1%.148,154,156
On the basis of the evidence that vedolizumab was safe and effective as maintenance therapy in both anti-TNF-naive and previously treated patients, the consensus group recommended that therapy be continued in patients who respond to vedolizumab induction therapy.
Statement 34. In patients with moderate to severe CD who fail to achieve complete remission with any of corticosteroids, thiopurines, methotrexate, or anti-TNF therapy, we recommend ustekinumab to induce complete remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 70%; agree, 30%.
Key evidence: Evidence for the efficacy of ustekinumab for the induction of symptomatic remission of CD is available from 4 RCTs.157–159 A Cochrane systematic review conducted in 2015160 included 2 of the RCTs,157,158 and we added the 2 more recently published UNITI trials, UNITI-1 and UNITI-2,159 to the meta-analysis. Ustekinumab was significantly superior to placebo for the outcome of failure to achieve symptomatic remission at week 6 (RR, 0.88; 95% CI, 0.85–0.92) (Figure 6). Ustekinumab was effective in patients who had previously responded to anti-TNF therapy and anti-TNF-naive patients.
Figure 6.
Forest plot of randomized controlled trials of ustekinumab in inducing remission in active luminal CD. Meta-analysis conducted for the consensus. Note the placebo groups in the UNITI trials have been split to avoid double-counting. CD, Crohn’s disease; CI, confidence interval; M-H, Mantel-Haenszel.
Discussion: As with other treatments, the primary outcome in these trials was symptomatic remission. However, evidence from retrospective cohort studies suggests that some patients can achieve mucosal healing with ustekinumab therapy, suggesting the outcome of complete remission is feasible.161–163 In addition, ustekinumab resulted in higher rates of corticosteroid-free remission at week 52 in the IM-UNITI study compared with placebo.159
Data suggest that ustekinumab may be more effective in patients who are anti-TNF-naive compared with those who have been previously treated with anti-TNF therapy. Symptomatic response rates in UNITI-2 among patients who were anti-TNF-naive were 54%–56%, and in UNITI-1 among patients who had received previous anti-TNF therapy they were 34%.159 In addition, in UNITI-1 symptomatic response rates with ustekinumab were significantly greater than placebo among patients who had previously responded to anti-TNF therapy and lost response (secondary non-responder), but not among patients with an initial non-response to anti-TNF therapy (primary non-responder), although there were small patient numbers in the latter group.159
The consensus group concluded that ustekinumab therapy is an effective, well-tolerated option for induction therapy. Because early use of ustekinumab in CD patients who are treatment-naive has not yet been reported, the consensus group agreed that this agent should likely be reserved for patients who have failed conventional therapy or anti-TNF therapy.
In general, the consensus group recommended striving for complete remission (including both symptomatic and endoscopic remission); however, among patients who have achieved a corticosteroid-free, symptomatic remission (but not endoscopic remission) on anti-TNF therapy, evidence is not available to demonstrate whether these patients would achieve complete remission with mucosal healing on ustekinumab. Therefore, in patients who have achieved corticosteroid-free, symptomatic remission on anti-TNF therapy, the decision to switch should be made only after consideration of patient preference.
No recommendation E. In patients with active CD starting ustekinumab, the consensus group does not make a recommendation (neither for nor against) regarding adding a thiopurine or methotrexate over monotherapy to improve pharmacokinetic parameters.
Key evidence: The combination ustekinumab plus an immunosuppressant has not been adequately studied. In the UNITI trials among those receiving concomitant immunosuppressants, symptomatic response rates were higher in most of the ustekinumab dosing groups compared with placebo. These rates were generally numerically higher than those in patients not receiving immunosuppressants; however, this comparison was not statistically assessed. In IM-UNITI, the incidence of antidrug antibodies was low (27/1154 patients, 2.3%), and no data on the use of immunosuppressants in these patients were provided. In addition, the presence of antidrug antibodies did not impact efficacy.159
Discussion: This statement was voted on, but consensus could not be reached regarding whether there is a role for adding a thiopurine or methotrexate when initiating ustekinumab therapy because of the lack of evidence.
Statement 35. We suggest that patients with CD be evaluated for symptomatic response to ustekinumab therapy between 6 and 10 weeks to determine the need to modify therapy.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 20%; agree, 75%; uncertain, 5%.
Key evidence: In RCTs, symptomatic response rates with ustekinumab were significantly greater than with placebo at 6–8 weeks.157–160 In the UNITI trials, significant improvements in symptomatic response rates were reported as early as week 3, which increased through the week 8 visit, and were maintained through the 1-year maintenance trial.159
Discussion: Evidence suggests that most patients who are going to respond will respond after a single intravenous dose of ustekinumab (~6 mg/kg) by week 6, and indeed this is the approved induction dose globally.157–160 Other dosing regimens should not be used in clinical practice. However, there appears to be a delayed-responder population that improves after the first subcutaneous dose at week 8. Maintenance consists of additional subcutaneous doses every 8 or 12 weeks. Therefore, the consensus group agreed that although there is some uncertainty about timing, patients who have not demonstrated a response before the second subcutaneous dose warrant modification of therapy. If a response occurs, subsequent assessments should include endoscopy to confirm complete remission, but the optimal timing of endoscopy is currently uncertain.
Statement 36. In patients with CD who have achieved symptomatic response with ustekinumab induction therapy, we recommend continued ustekinumab therapy to achieve and maintain complete remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 85%; agree, 15%.
Key evidence: Evidence for the efficacy of ustekinumab as maintenance therapy is available from the CERTIFI and IM-UNITI trials.158,159 In the CERTIFI trial, symptomatic remission rates at week 22 were statistically significantly greater in the ustekinumab group than in the placebo group (41.7% vs 27.4%; absolute difference, 14.3%; 95% CI, 2.0–27.1; P = .03).158 In the IM-UNITI trial, symptomatic remission rates after 1 year of treatment were significantly greater among patients treated with ustekinumab compared with placebo (51.0% vs 35.9%; absolute difference, 15.1%; 95% CI, 4.86–25.33; P = .005).159 Rates of corticosteroid-free remission were significantly greater with ustekinumab compared with placebo (44.7% vs 29.8%, P = .004).159
Discussion: Ustekinumab has demonstrated efficacy as maintenance therapy; however, data suggest greater efficacy in patients who are anti-TNF-naive, compared with those who have been previously treated with anti-TNF therapy. The symptomatic remission rates at week 44 among patients from UNITI-2 who were anti-TNF-naive were 56.9%–62.5%, and in UNITI-1 among patients who had received previously received anti-TNF therapy, they were 38.6%–41.1%. Among patients who had previously received anti-TNF therapy, the symptomatic remission rates in the combined ustekinumab group were not significantly greater than placebo (39.8% vs 26.2%; absolute difference, 13.6%; 95% CI, –0.67 to 27.85; P = .07).159
In the UNITI trials, the rates of overall adverse events, serious adverse events, serious infections, and infusion-related reactions occurred at similar rates across groups.159 During long-term follow-up, the most common adverse events were arthralgia, headache, nasopharyngitis, and CD events.159
On the basis of the evidence that ustekinumab was safe and effective as maintenance therapy, the consensus group recommended that therapy be continued in patients who respond to ustekinumab induction therapy.
No recommendation F. In patients with CD who fail to respond or lose response to vedolizumab, the consensus group agreed that it was premature, because of the lack of data and clinical experience, to recommend for or against ustekinumab to induce and maintain complete remission.
No recommendation G. In patients with CD who fail to respond or lose response to ustekinumab, the consensus group agreed that it was premature, because of the lack of data and clinical experience, to recommend for or against vedolizumab to induce and maintain complete remission.
Discussion: The intent of these statements was to make recommendations on strategies for patients who have failed therapy with a non-anti-TNF biologic. However, this was deemed premature because the issue of switching from 1 non-anti-TNF biologic to another and the proper ordering of these agents have not yet been studied. Therefore, the consensus group agreed not to vote on formal statements but rather to discuss this issue in the section called “Future Directions”.
Alternative Treatments
Statement 37. In patients with CD, we recommend against the use of probiotics to induce OR maintain symptomatic remission.
GRADE: Strong recommendation, very low-quality evidence.
Vote: strongly agree, 85%; agree, 15%.
Key evidence: Systematic reviews of RCTs (n = 1–14) showed no significant benefits of probiotic treatments on clinical outcomes when used for either induction or maintenance therapy in patients with CD.164–167 The majority of studies were small, evaluated maintenance therapy with a variety of different probiotics, and the probiotics were generally used as adjunct to conventional CD treatments.
Discussion: The available RCTs have assessed a variety of probiotic strains and regimens, making it challenging or even inappropriate to pool studies. The majority of trials used Lactobacillus GG, Lactobacillus johnsonii, Escherichia coli strain Nissle 1917, or Saccharomyces boulardii with varying results.164,165 Current data do not support a significant effect with the use of probiotics as a whole for either induction therapy or maintenance therapy. As a result, the consensus group recommended against the use of these agents for the treatment of CD. However, because data are scarce, individual probiotics may prove useful, and further study is warranted.
Statement 38. In patients with CD, we recommend against the use of omega-3 fatty acids to induce OR maintain symptomatic remission.
GRADE: Strong recommendation, moderate-quality evidence.
Vote: strongly agree, 90%; agree, 10%.
Key evidence: Two systematic reviews including 6 RCTs concluded that omega-3 fatty acids (primarily monotherapy) were likely not more effective than placebo for maintenance therapy in CD.168,169 The Cochrane meta-analysis (n = 1039) found marginally significant lower 12-month relapse rates with omega-3 fatty acids over placebo (39% vs 47%; RR, 0.77; 95% CI, 0.61–0.98; P = .031). This was primarily driven by 2 of the smaller studies that had a higher risk of bias. Analysis of the 2 largest and highest quality studies resulted in no significant benefit with omega-3 fatty acids over placebo (n = 738; RR, 0.88; 95% CI, 0.74–1.05).169 The earlier systematic review also included 2 small RCTs assessing induction therapy; both trials were negative, and the authors concluded that there was insufficient evidence for use of these agents in this context.168
No serious adverse events were reported in any of the 6 RCTs, but the pooled analysis showed significantly higher risks of diarrhea (RR, 1.36; 95% CI, 1.01–1.84; P = .045) and upper gastrointestinal tract symptoms (eg, nausea, vomiting, halitosis, heartburn, dyspepsia, dysgeusia, bloating) (RR, 1.65; 95% CI, 1.25–2.18; P = .00043) with active treatment.
Discussion: Because the majority of RCTs assessing the use of omega-3 fatty acids for the induction or maintenance of remission in patients with CD have been negative, the consensus group made a recommendation against the use of these agents in patients with CD.
Statement 39. In patients with CD, we suggest against the use of marijuana to induce OR maintain symptomatic remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 45%; agree, 40%, uncertain, 5%.
Key evidence: The role of cannabis for the treatment of CD has been inadequately studied. In a small RCT in 21 patients with active CD who had previously failed corticosteroids, immunosuppressants, or anti-TNF agents, cannabis was not more effective than placebo for inducing symptomatic remission (45% vs 10%; P = .43).170 However, the symptomatic response rate was greater than with placebo (90% vs 40%; P = .028).170 The blinding of patients in this trial failed largely because of the psychotropic effects of active treatment, which may account for the perceived symptomatic improvement.
Discussion: Low-quality data suggest there may be some symptomatic improvement. In addition to the trial cited above, a large survey of IBD outpatients reported improvements in abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%), and diarrhea (28.6%) in patients using vs not using cannabis for IBD.171 Side effects were frequent, and a duration of cannabis use for IBD symptoms of more than 6 months was found to be a significant risk factor for surgery in patients with CD (OR, 5.03; 95% CI, 1.45–17.46) after adjusting for other risk factors.171
Because the quality of evidence is very low and suggests possible symptomatic improvements, cannabis use may warrant further study. Therefore, the consensus group made a conditional suggestion against its use for the induction or maintenance of remission in CD at the present time.
Statement 40. In patients with CD, we suggest against the use of naltrexone to induce OR maintain symptomatic remission.
GRADE: Conditional recommendation, low-quality evidence for induction of remission, very low-quality evidence for maintenance of remission.
Vote: strongly agree, 65%; agree, 35%.
Key evidence: A Cochrane meta-analysis included 2 small studies that assessed the use of naltrexone for inducing symptomatic remission, 1 in 34 adults and 1 in 12 pediatric patients.172 In the study in adult patients, there was no significant difference in rates of symptomatic remission between naltrexone and placebo (RR, 1.48; 95% CI, 0.42–5.24; P = NS). There were significant improvements in rates of symptomatic and endoscopic response but not endoscopic remission. No studies were found assessing naltrexone use as maintenance therapy in CD. Pooled analysis of patients from both trials showed no serious adverse events and no significant differences in the frequency of or discontinuations due to adverse events between naltrexone and placebo therapy.172
Discussion: Interest in naltrexone in the treatment of CD arises from the fact that opioids affect secretion and motility by interacting with opioid receptors in the gut.172 Naltrexone is a long-acting opioid antagonist that acts at the mu-opioid receptor. This receptor is present in the gut and has been found to be overexpressed by CD4+ and CD8+ T-lymphocytes in inflamed bowel.173
The positive trends in response rates and the favorable tolerability profile suggest that further study of naltrexone may be warranted. However, in light of the lack of sufficient evidence, the consensus group conditionally suggested that naltrexone should not currently be used to manage CD.
Statement 41. In patients with CD, we suggest against the use of enteral nutrition or dietary modification to induce OR maintain symptomatic remission.
GRADE: Conditional recommendation, very low-quality evidence.
Vote: strongly agree, 25%; agree, 60%; uncertain, 15%.
Key evidence: A Cochrane review found only 2 studies (n = 42) assessing the utility of glutamine supplementation for induction of remission, 1 in adults and 1 in pediatric patients.174 The authors concluded that there was insufficient evidence to make a determination as to its efficacy and safety in patients with CD. A second Cochrane review (2 studies, n = 84) assessed supplemental enteral nutrition in the maintenance of remission in CD and found conflicting results between the 2 included studies.175 In both systematic reviews, pooling of the included trials was not possible because of differences in study populations, interventions, and outcome assessment methods.174,175
One systematic review (4 studies, n = 342) assessed the efficacy of adjunctive enteral nutrition (elemental or polymeric diet with low-fat or regular diet) in patients receiving infliximab as maintenance therapy in CD.176 The combination was associated with a significantly higher rate of symptomatic remission at 1 year compared with infliximab monotherapy (74.5% vs 49.2%; OR, 2.93; 95% CI, 1.66–5.17; P < .01).176
No RCTs were found that assessed specific dietary modifications to induce or maintain remission in patients with CD.
Discussion: There are few data assessing enteral nutrition as monotherapy for induction or maintenance of remission in patients with CD, with the Cochrane reviews including only 2 trials each.174,175 These trials assessed a variety of interventions, controls, and outcomes; in addition, it is unlikely that such trials can be adequately blinded. Although data suggested that enteral nutrition may have a role as adjunct to anti-TNF therapy, all 4 studies included in the meta-analysis were conducted in Japanese patients,176 and it is unknown whether the results would be generalizable to other ethnicities. Adverse event data are sparse; however, 1 study reported 3 central catheter infections among patients receiving a glutamine-enriched diet vs none among those who received the standard diet (RR, 7.00; 95% CI, 0.40–122.44).174
On the basis of current data it does not appear that enteral nutrition is an effective or practical strategy for use in adults with CD. The consensus group did not assess the evidence for use of this strategy in children. Although there are few data on enteral nutrition or dietary modifications, there are also few data to definitively state that these strategies would not be useful; therefore, the consensus group conditionally suggested these strategies not be used routinely for the management of CD.
Relevance, Interpretation in Clinical Practice, and Future Directions
Antibiotics and Altering the Microbiome
Because of the hypothesis that IBD may result in part from alterations in the intestinal microbiome, there remains substantial interest in trying to manipulate the microbiome for therapeutic benefit. Although the antibiotic regimens that have been studied to date have not consistently demonstrated efficacy, non-absorbable antibiotics such as rifaximin warrant further study in CD. Several studies are underway, and the results of these studies are eagerly awaited. In addition, manipulating the microbiome through diet or other means, including fecal transfer, may prove to be beneficial and is also under investigation.
Sulfasalazine for Mild Colonic Crohn’s Disease
Although the evidence suggests a minimal benefit for sulfasalazine in a subgroup of patients with mild colonic CD, it was acknowledged that most consensus members rarely if ever use this agent in their clinical practice as a stand-alone therapy. The evidence is based on older studies with poor methodology and lack of robust outcomes. The recent update of the Cochrane analysis (published outside our search window) also reported no significant benefit of 5-ASAs over placebo for inducing response or remission.67
Corticosteroids in Crohn’s Disease
Corticosteroids such as budesonide and prednisone have been the cornerstone of the management of CD for many decades. These agents are recommended for the treatment of mild to moderate and severe ileal, ileocolonic, and colonic CD. The choice between budesonide and prednisone depends not only on location but also on severity of disease. In patients with mild disease who have failed budesonide, there was no consensus on whether these patients would transition to prednisone, but more than half of the participants would try at least 1 course of prednisone in this patient population.
No formal dose-response trials have been performed with systemic corticosteroids in CD. The usual starting dose for induction of remission in active CD is 40–60 mg prednisone or equivalent. A higher starting dose of 1 mg/kg seems to increase the short-term remission rate,177,178 but no comparative studies have been performed.
Thiopurines
The use of thiopurines as monotherapy has slowly fallen out of favor because of evidence suggesting lack of efficacy179,180 and issues of drug tolerability and toxicity.181–183 In general, biologic therapies are favored over thiopurines in clinical practice in high-risk patients (Table 1). Specifically, anti-TNF therapy has been shown to be superior to azathioprine in the SONIC study.115 Thiopurines should be restricted to select low-risk patients who are steroid dependent184 or as part of combination therapy with biologics. It was acknowledged that sometimes physician choice is limited because payers may require the use of immunosuppressants before prescribing biologic therapy. This represents a knowledge translation gap between the medical literature and these payers whose decisions are often driven by cost containment.
Sequencing or Combining Biologic Therapies
As new biologic agents are introduced, one of the biggest remaining questions in the pharmacologic treatment of CD surrounds the order of placement of the different classes of biologics. In the absence of head-to-head studies or companion diagnostic testing to predict response or non-response, this becomes difficult. In most instances, this is left to the discretion of the physician and the patient. Physicians often make these decisions on the basis of personal experience with a particular class of drug while considering efficacy, safety, and patient comorbidities. Patients often choose therapies partly on the basis of efficacy but more so on safety concerns and routes of administration.185
In this consensus, we do not differentiate between biologics in moderate to severe CD because they can all be used as first-line agents. If mucosal healing is desired, anti-TNF agents are positioned first in high-risk patients primarily because of the experience with these agents and the lack of robust mucosal healing data with vedolizumab and ustekinumab. If rapid onset is desired, both anti-TNF therapy and ustekinumab would be favored over vedolizumab because of the slower onset of action of vedolizumab. In patients with multiple comorbidities or safety concerns, vedolizumab is often the agent of choice because of the gut selective mechanism of action. In patients with significant extraintestinal manifestations such as uveitis, ankylosing spondylitis, or pyoderma gangrenosum, anti-TNF therapies once again would be preferred.
Therefore, questions remain around the issue of patients who fail to respond or lose response to a biologic therapy; there are few or no data to guide strategies for these patients. Potential strategies include switching between the non-anti-TNF biologics or switching to another anti-TNF biologic, provided there are no immediate indications for surgery or other contraindications to medical therapy. In patients who are primary non-responders to one mechanism of action despite optimization, it seems logical to switch to a different mechanism of action. However, more data are needed to better define optimized induction with the different agents, as well as the optimal sequencing of anti-TNF and non-anti-TNF biologics.
Combination Therapy
Especially in light of the potential adverse effects, it is important to have a more definitive clarification of whether the addition of immunosuppressive therapy, with methotrexate or a thiopurine, during initiation of a biologic provides any real efficacy benefits, with acceptable side effects. All currently available biologics are immunogenic; however, the rates of immunogenicity seem to be lower with both vedolizumab and ustekinumab. Therefore, in the absence of clear studies demonstrating superior efficacy of combination therapy with these agents and an immunosuppressant, proper guidance is difficult.
“Combination therapy” may also take on a new meaning. With more than 1 class of biologic therapy available, there is appeal in combining agents with different mechanisms of action. Whether during the induction period only or during both induction and maintenance is yet to be determined. Overall safety needs to be taken into consideration as well as the potential health economic impact.
Treat-to-Target Approach
The role of complete remission with demonstrated endoscopic healing requires further study. It is currently unclear whether escalation of therapy is warranted in patients who have achieved clinical remission but have evidence of residual endoscopic activity. The ongoing REACT-2 clinical trial should help to answer this question.44 Even if this trial demonstrates that treating to a target of mucosal healing improves hard outcomes such as hospitalization and surgery, the next question becomes one of feasibility. Adequate biomarkers or predictive indices of mucosal healing would likely be needed to allow for a treat-to-target approach in clinical practice.
Therapeutic Drug Monitoring
TDM is valuable in patients who lose response to anti-TNF therapy, and there is an association between drug concentrations and clinical outcomes.136,138,139 However, there is a need for more accurate descriptions of the optimal therapeutic drug ranges to help patients with CD on biologic therapies achieve complete remission. These ranges may also depend on the desired outcome or disease phenotype. Prospective testing remains controversial. The studies evaluating proactive TDM have been negative but have several limitations; further studies are necessary to clarify the utility of TDM in this context. Extending beyond anti-TNF therapy, the utility of TDM with vedolizumab or ustekinumab remains poorly understood but will likely evolve.
Canadian Association of Gastroenterology Statement
This clinical practice guideline (CPG) on the management of luminal Crohn’s disease was developed under the direction of Drs Remo Panaccione and A. Hillary Steinhart, in accordance with the policies and procedures of the Canadian Association of Gastroenterology (CAG) and under the direction of CAG Clinical Affairs. It has been reviewed by the CAG Practice Affairs and Clinical Affairs Committees and the CAG Board of Directors. The CPG was developed following a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian and International panel composed of experts on this topic. The CPG aims to provide a reasonable and practical approach to care for specialists and allied health professionals who are charged with the duty of providing optimal care to patients and families and can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The CPG is not intended to be a substitute for physicians using their individual judgment in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic and treatment options available, and available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
Conflicts of interest
These authors disclose the following:
Advisory board: AbbVie (AB, AHS, BB, BH, CB, CS, CW, GR, LT, MB, RK, RP, SM, SP, UC, WA), Abbott (RP), Actavis (AHS, BB, CS), Allergan (AB), Amgen (RP), Aptalis (RP), AstraZeneca (RP), Baxter (RP), Bristol-Myers Squibb (RP), Celgene (RP), Celltrion (BB), Centocor (RP), Cubist (CB, RP), Eisai (RP), Elan (RP), Ferring (AB, AHS, BB, CW, RP, WA), Genentech (BB, RP), Glaxo-Smith Kline (RP), Janssen (AB, AHS, BH, CS, GR, LT, RK, RP, SP, UC, WA), Merck (AB, AHS, RP), Pendopharm (AHS, BB, GR), Pfizer (AB, AHS, RP), Salix (RP), Schering-Plough (RP), Shire (AB, AHS, BB, CB, CS, CW, GR, MB, RP, SM, WA), Takeda (AB, AHS, BB, CB, CS, GR, JJ, LT, RK, RP, SM, SP, UC, WA), UCB (RP), Warner Chilcott (RP).
Consulting: AbbVie (DS, GR, JJ, EL, JKM, RP), Abbott (RP), Actavis (JKM), Amgen (EL, RP), Aptalis (RP), AstraZeneca (JKM, RP), Baxter (RP), Bristol-Myers Squibb (EL, RP), Celgene (EL, RP), Celltrion (JKM), Centocor (RP), Cubist (JKM, RP), CVS Caremark (EL), Eisai (RP), Elan (RP), Eli Lilly (EL), Ferring (JKM, RP), Genentech (EL), Glaxo-Smith Kline (RP), Hospira (JKM), Janssen (DS, JJ, EL, JKM, RP), Merck (RP), Mesoblast (EL), Pfizer (AHS, EL, RP), Salix (EL), Schering-Plough (RP), Seres Therapeutics (EL), Shire (JKM, RP), Sun Pharmaceuticals (EL), Takeda (DS, JJ, EL, JKM, RP), Theradig (CB, EL), Tigenix (DS), UCB (EL, RP, DS), Warner Chilcott (RP).
Educational support: AbbVie (AB, RP, SP, UC), Abbott (RP), Allergan (AB), Aptalis (UC), Bristol-Myers Squibb (RP), Centocor (RP), Elan (RP), Ferring (RP), Janssen (AB, AHS, GR, RP), Millennium (RP), Pfizer (LT), Proctor and Gamble (RP), Schering-Plough (RP), Shire (LT), Takeda (AB, LT). Research grants/clinical trial funding: AbbVie (AB, AHS, BB, EL, DS, LT, RP), Abbott (RP), Alvine (BB), Amgen (AHS, BB, EL), Boehringer Ingelheim (BB), Bristol-Myers Squibb (BB, RP), Celgene (AHS, BB, EL), Centocor (RP), Elan (RP), Ferring (RP), Genentech (AHS, BB, EL), Gilead (EL), Glaxo-Smith Kline (BB), Janssen (BB, EL, RP), MedImmune (EL), Merck (BB), Millennium (AHS, RP), Pfizer (EL, LT), Proctor and Gamble (RP), Prometheus (WA), Qu Biologic (BB), Receptos (EL), RedHill Biopharm (AHS, BB), Robarts Clinical Trials (EL), Schering-Plough (RP), Seres Therapeutics (EL), Takeda (EL), UCB (EL, DS).
Speaker’s bureau: AbbVie (AB, AHS, BB, BH, CB, GR, JKM, MB, RK, RP, SM, SP, UC, WA), Abbott (RP), Actavis (BB, JKM), Allergan/Forest (MB), Aptalis (UC), AstraZeneca (RP), Centocor (RP), Elan (RP), Ferring (AHS, BB, JJ, JKM), Hospira (AHS, JKM), Janssen (AHS, BH, CB, CW, GR, JJ, JKM, MB, RK, RP, SP, UC, WA), Pendopharm (BH, MB), Prometheus (RP), Schering-Plough (RP), Shire (AB, AHS, BB, BH, CB, GR, JJ, JKM, MB, RP, SP), Takeda (AB, AHS, BB, CB, CW, GR, JKM, MB, RK, RP, SM, WA), Warner Chilcott (RP).
Other: Qu Biologic (BB-stock options).
The remaining authors disclose no conflicts.
Funding
Supported through unrestricted grants to the Canadian Association of Gastroenterology by AbbVie Corp, Janssen Inc, Pfizer Canada Inc, and Takeda Canada Inc, who had no involvement in any aspect of the guideline development.
Reprint requests
Address requests for reprints to: Remo Panaccione, MD, FRCPC, Inflammatory Bowel Disease Unit, Division of Gastroenterology, University of Calgary, Room 6D30, TRW Building, 3280 Hospital Drive NW, Calgary, Alberta, Canada T2N 4N1. e-mail: rpanacci@ucalgary.ca; fax: (403) 270–7287.
Supplementary Material
Acknowledgments
The Canadian Association of Gastroenterology thanks AbbVie Corp, Janssen Inc, Pfizer Canada Inc, and Takeda Canada Inc for their generous support of the guideline process. The consensus group would like to thank the following people for their contributions: Paul Sinclair and Lesley Marshall (CAG representatives, administrative and technical support, and logistics assistance), Pauline Lavigne and Steven Portelance (unaffiliated, editorial assistance). Finally, they thank their patient advocate, Jenna Rines, for invaluable insights.
Glossary
Abbreviations used in this paper
- CAG
Canadian Association of Gastroenterology
- CD
Crohn’s disease
- CDAI
Crohn’s Disease Activity Index
- CDEIS
Crohn’s Disease Endoscopic Index of Severity
- CI
confidence interval
- CPG
clinical practice guideline
- CrI
credible intervals
- CRP
C-reactive protein
- CTE
computed tomography enterography
- ECCO
European Crohn’s and Colitis Organisation
- FDA
Food and Drug Administration
- GRADE
Grading of Recommendation Assessment, Development and Evaluation
- HBI
Harvey–Bradshaw Index
- HR
hazard ratio
- HRQoL
health-related quality of life
- HSTCL
hepatosplenic T-cell lymphoma
- IBD
inflammatory bowel disease
- IOIBD
International Organization for the Study of Inflammatory Bowel Diseases
- MRE
magnetic resonance enterography
- NMA
network meta-analysis
- OR
odds ratio
- PRO
patient-reported outcome
- QoE
quality of evidence
- RCT
randomized controlled trial
- RR
relative risk
- SBUS
small bowel ultrasound
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
- TDM
therapeutic drug monitoring
- TNF
tumor necrosis factor
- TPMT
thiopurine methyltransferase
References
- 1. Golovics PA, Lakatos L, Mandel MD, et al. . Prevalence and predictors of hospitalization in Crohn’s disease in a prospective population-based inception cohort from 2000–2012. World J Gastroenterol 2015;21:7272–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bitton A, Vutcovici M, Sewitch M, et al. . Mortality trends in Crohn’s disease and ulcerative colitis: a population-based study in Quebec, Canada. Inflamm Bowel Dis 2016;22: 416–423. [DOI] [PubMed] [Google Scholar]
- 3. Floyd DN, Langham S, Severac HC, et al. . The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci 2015;60:299–312. [DOI] [PubMed] [Google Scholar]
- 4. Shah SC, Colombel JF, Sands BE, et al. . Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016;43:317–333. [DOI] [PubMed] [Google Scholar]
- 5. Gisbert JP, Marin AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther 2015;42:391–405. [DOI] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 7. van Deen WK, Nguyen D, Duran NE, et al. . Value redefined for inflammatory bowel disease patients: a choice-based conjoint analysis of patients’ preferences. Qual Life Res 2017;26:455–465. [DOI] [PubMed] [Google Scholar]
- 8. Bewtra M, Fairchild AO, Gilroy E, et al. . Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am J Gastroenterol 2015;110:1675–1681. [DOI] [PubMed] [Google Scholar]
- 9. Dignass A, Van Assche G, Lindsay JO, et al. . The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 10. Gomollon F, Dignass A, Annese V, et al. . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1—diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 11. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sultan S, Falck-Ytter Y, Inadomi JM. The AGA institute process for developing clinical practice guidelines part one: grading the evidence. Clin Gastroenterol Hepatol 2013;11:329–332. [DOI] [PubMed] [Google Scholar]
- 13. Bressler B, Marshall JK, Bernstein CN, et al. . Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148:1035–1058 e3. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen GC, Bernstein CN, Bitton A, et al. . Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014;146:835–848 e6. [DOI] [PubMed] [Google Scholar]
- 15. Fallone CA, Chiba N, van Zanten SV, et al. . The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69 e14. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen GC, Seow CH, Maxwell C, et al. . The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016;150:734–757 e1. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Schunemann HJ, Djulbegovic B, et al. . Guideline panels should not GRADE good practice statements. J Clin Epidemiol 2015;68:597–600. [DOI] [PubMed] [Google Scholar]
- 18. Dalkey N. An experimental study of group opinion: the Delphi method. Futures 1969;1:408–426. [Google Scholar]
- 19. Cook DJ, Greengold NL, Ellrodt AG, et al. . The relation between systematic reviews and practice guidelines. Ann Intern Med 1997;127:210–216. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Kunz R, et al. . Going from evidence to recommendations. BMJ 2008;336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 22. Levesque BG, Sandborn WJ, Ruel J, et al. . Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51 e1. [DOI] [PubMed] [Google Scholar]
- 23. Best WR, Becktel JM, Singleton JW, et al. . Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–444. [PubMed] [Google Scholar]
- 24. Cellier C, Sahmoud T, Froguel E, et al. . Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease: a prospective multicentre study of 121 cases—The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut 1994;35:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sipponen T, Savilahti E, Kolho KL, et al. . Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2008;14:40–46. [DOI] [PubMed] [Google Scholar]
- 26. Jones J, Loftus EV Jr, Panaccione R, et al. . Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008;6:1218–1224. [DOI] [PubMed] [Google Scholar]
- 27. Vermeire S, Schreiber S, Sandborn WJ, et al. . Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010;8:357–363. [DOI] [PubMed] [Google Scholar]
- 28. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 29. Zittan E, Kabakchiev B, Kelly OB, et al. . Development of the Harvey-Bradshaw Index-pro (HBI-PRO) score to assess endoscopic disease activity in Crohn’s disease. J Crohns Colitis 2017;5:543–548. [DOI] [PubMed] [Google Scholar]
- 30. Siegel CA, Whitman CB, Spiegel BM, et al. . Development of an index to define overall disease severity in IBD. Gut 2018;67:244–254. [DOI] [PubMed] [Google Scholar]
- 31. Beaugerie L, Seksik P, Nion-Larmurier I, et al. . Predictors of Crohn’s disease. Gastroenterology 2006;130:650–656. [DOI] [PubMed] [Google Scholar]
- 32. Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol 2008;43:948–954. [DOI] [PubMed] [Google Scholar]
- 33. Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol 2012;18:3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiss LS, Papp M, Lovasz BD, et al. . High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification? Inflamm Bowel Dis 2012;18:1647–1654. [DOI] [PubMed] [Google Scholar]
- 35. Mao R, Xiao YL, Gao X, et al. . Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18:1894–1899. [DOI] [PubMed] [Google Scholar]
- 36. Ghaly S, Murray K, Baird A, et al. . High vitamin D-binding protein concentration, low albumin, and mode of remission predict relapse in Crohn’s disease. Inflamm Bowel Dis 2016;22:2456–2464. [DOI] [PubMed] [Google Scholar]
- 37. Qin G, Tu J, Liu L, et al. . Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s disease activity. Med Sci Monit 2016;22:4393–4400. [DOI] [PubMed] [Google Scholar]
- 38. Khanna R, Zou G, D’Haens G, et al. . A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther 2015;41:77–86. [DOI] [PubMed] [Google Scholar]
- 39. Khanna R, D’Haens G, Feagan BG, et al. . Patient reported outcome measures derived from the Crohn’s Disease Activity Index: correlation between PRO2 and PRO3 scores and CDAI-defined clinical thresholds [abstract P176]. J Crohns Colitis 2014;8:S135. [Google Scholar]
- 40. Baert F, Moortgat L, Van Assche G, et al. . Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010;138:463–468; quiz e10–e11. [DOI] [PubMed] [Google Scholar]
- 41. Schnitzler F, Fidder H, Ferrante M, et al. . Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–1301. [DOI] [PubMed] [Google Scholar]
- 42. Rutgeerts P, Diamond RH, Bala M, et al. . Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc 2006;63:433–442, quiz 464. [DOI] [PubMed] [Google Scholar]
- 43. Bouguen G, Levesque BG, Feagan BG, et al. . Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:1042–1050 e2. [DOI] [PubMed] [Google Scholar]
- 44. Enhanced agorithm for Crohn’s treatment incorporating early combination therapy (REACT-2). Last update 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT01698307. Accessed May 9, 2018. [Google Scholar]
- 45. Panaccione R, Colombel JF, Louis E, et al. . Evolving definitions of remission in Crohn’s disease. Inflamm Bowel Dis 2013;19:1645–1653. [DOI] [PubMed] [Google Scholar]
- 46. Vuitton L, Marteau P, Sandborn WJ, et al. . IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut 2016;65:1447–1455. [DOI] [PubMed] [Google Scholar]
- 47. Khanna R, Nelson SA, Feagan BG, et al. . Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev 2016;CD010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deepak P, Fletcher JG, Fidler JL, et al. . Radiological response is associated with better long-term outcomes and is a potential treatment target in patients with small bowel Crohn’s disease. Am J Gastroenterol 2016;111:997–1006. [DOI] [PubMed] [Google Scholar]
- 49. Puylaert CA, Tielbeek JA, Bipat S, et al. . Grading of Crohn’s disease activity using CT, MRI, US and scintigraphy: a meta-analysis. Eur Radiol 2015;25:3295–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Minordi LM, Vecchioli A, Poloni G, et al. . Enteroclysis CT and PEG-CT in patients with previous small-bowel surgical resection for Crohn’s disease: CT findings and correlation with endoscopy. Eur Radiol 2009;19:2432–2440. [DOI] [PubMed] [Google Scholar]
- 51. Bruining DH, Bhatnagar G, Rimola J, et al. . CT and MR enterography in Crohn’s disease: current and future applications. Abdom Imaging 2015;40:965–974. [DOI] [PubMed] [Google Scholar]
- 52. Steward MJ, Punwani S, Proctor I, et al. . Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 2012;81:2080–2088. [DOI] [PubMed] [Google Scholar]
- 53. Rimola J, Rodriguez S, Garcia-Bosch O, et al. . Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009;58:1113–1120. [DOI] [PubMed] [Google Scholar]
- 54. Casellas F, Herrera-de Guise C, Robles V, et al. . Patient preferences for inflammatory bowel disease treatment objectives. Dig Liver Dis 2017;49:152–156. [DOI] [PubMed] [Google Scholar]
- 55. Norton BA, Thomas R, Lomax KG, et al. . Patient perspectives on the impact of Crohn’s disease: results from group interviews. Patient Prefer Adherence 2012;6:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allez M, Lemann M, Bonnet J, et al. . Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol 2002;97:947–953. [DOI] [PubMed] [Google Scholar]
- 57. Benitez JM, Meuwis MA, Reenaers C, et al. . Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut 2013;62:1806–1816. [DOI] [PubMed] [Google Scholar]
- 58. Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13:567–579. [DOI] [PubMed] [Google Scholar]
- 59. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. . Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016;14:348–354 e17. [DOI] [PubMed] [Google Scholar]
- 60. Panes J, Jairath V, Levesque BG. Advances in use of endoscopy, radiology, and biomarkers to monitor inflammatory bowel diseases. Gastroenterology 2017;152:362–373 e3. [DOI] [PubMed] [Google Scholar]
- 61. Khan KJ, Ullman TA, Ford AC, et al. . Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–673. [DOI] [PubMed] [Google Scholar]
- 62. Su JW, Ma JJ, Zhang HJ. Use of antibiotics in patients with Crohn’s disease: a systematic review and meta-analysis. J Dig Dis 2015;16:58–66. [DOI] [PubMed] [Google Scholar]
- 63. Patton PH, Parker CE, MacDonald JK, et al. . Anti-tuberculous therapy for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2016;CD000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dewint P, Hansen BE, Verhey E, et al. . Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut 2014;63:292–299. [DOI] [PubMed] [Google Scholar]
- 65. Ford AC, Kane SV, Khan KJ, et al. . Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:617–629. [DOI] [PubMed] [Google Scholar]
- 66. Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2010;CD008870. [DOI] [PubMed] [Google Scholar]
- 67. Lim WC, Wang Y, MacDonald JK, et al. . Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2016;CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Summers RW, Switz DM, Sessions JT Jr, et al. . National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology 1979;77:847–869. [PubMed] [Google Scholar]
- 69. Malchow H, Ewe K, Brandes JW, et al. . European Cooperative Crohn’s Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984;86:249–266. [PubMed] [Google Scholar]
- 70. Naganuma M, Iwao Y, Ogata H, et al. . Measurement of colonic mucosal concentrations of 5-aminosalicylic acid is useful for estimating its therapeutic efficacy in distal ulcerative colitis: comparison of orally administered mesalamine and sulfasalazine. Inflamm Bowel Dis 2001;7:221–225. [DOI] [PubMed] [Google Scholar]
- 71. Lodowska J, Gruchlik A, Wolny D, et al. . The effect of sulfasalazine and 5-aminosalicylic acid on the secretion of interleukin 8 by human colon myofibroblasts. Acta Pol Pharm 2015;72:917–921. [PubMed] [Google Scholar]
- 72. Van Hees PA, Van Lier HJ, Van Elteren PH, et al. . Effect of sulphasalazine in patients with active Crohn’s disease: a controlled double-blind study. Gut 1981;22:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moja L, Danese S, Fiorino G, et al. . Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Aliment Pharmacol Ther 2015;41:1055–1065. [DOI] [PubMed] [Google Scholar]
- 74. Ford AC, Bernstein CN, Khan KJ, et al. . Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:590–599; quiz 600. [DOI] [PubMed] [Google Scholar]
- 75. Rezaie A, Kuenzig ME, Benchimol EI, et al. . Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2015;CD000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dignass A, Stoynov S, Dorofeyev AE, et al. . Once versus three times daily dosing of oral budesonide for active Crohn’s disease: a double-blind, double-dummy, randomised trial. J Crohns Colitis 2014;8:970–980. [DOI] [PubMed] [Google Scholar]
- 77. Suzuki Y, Motoya S, Takazoe M, et al. . Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn’s disease: a multicentre, double-blind, randomized, parallel-group Phase II study. J Crohns Colitis 2013;7:239–247. [DOI] [PubMed] [Google Scholar]
- 78. Kuenzig ME, Rezaie A, Seow CH, et al. . Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2014;CD002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lichtenstein GR, Bengtsson B, Hapten-White L, et al. . Oral budesonide for maintenance of remission of Crohn’s disease: a pooled safety analysis. Aliment Pharmacol Ther 2009;29:643–653. [DOI] [PubMed] [Google Scholar]
- 80. Benchimol EI, Seow CH, Steinhart AH, et al. . Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2008;CD006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang YX, Lichtenstein GR. Corticosteroids in Crohn’s disease. Am J Gastroenterol 2002;97:803–823. [DOI] [PubMed] [Google Scholar]
- 82. Homik J, Suarez-Almazor ME, Shea B, et al. . Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database Syst Rev 2000;CD000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gross V, Andus T, Caesar I, et al. . Oral pH-modified release budesonide versus 6-methylprednisolone in active Crohn’s disease: German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol 1996;8:905–909. [PubMed] [Google Scholar]
- 84. Tursi A, Giorgetti GM, Brandimarte G, et al. . Beclomethasone dipropionate for the treatment of mild-to-moderate Crohn’s disease: an open-label, budesonide-controlled, randomized study. Med Sci Monit 2006;12:PI29–PI32. [PubMed] [Google Scholar]
- 85. Chun A, Chadi RM, Korelitz BI, et al. . Intravenous corticotrophin vs hydrocortisone in the treatment of hospitalized patients with Crohn’s disease: a randomized double-blind study and follow-up. Inflamm Bowel Dis 1998;4:177–181. [DOI] [PubMed] [Google Scholar]
- 86. Shepherd HA, Barr GD, Jewell DP. Use of an intravenous steroid regimen in the treatment of acute Crohn’s disease. J Clin Gastroenterol 1986;8:154–159. [DOI] [PubMed] [Google Scholar]
- 87. Steinhart AH, Ewe K, Griffiths AM, et al. . Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2003;CD000301. [DOI] [PubMed] [Google Scholar]
- 88. Schoon EJ, Bollani S, Mills PR, et al. . Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clin Gastroenterol Hepatol 2005;3:113–121. [DOI] [PubMed] [Google Scholar]
- 89. Lichtenstein GR, Abreu MT, Cohen R, et al. . American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130:940–987. [DOI] [PubMed] [Google Scholar]
- 90. Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khan KJ, Dubinsky MC, Ford AC, et al. . Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:630–642. [DOI] [PubMed] [Google Scholar]
- 92. Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2013;CD000545. [DOI] [PubMed] [Google Scholar]
- 93. Marshall JK, Otley AR, Afif W, et al. . Canadian Association of Gastroenterology position statement regarding the use of thiopurines for the treatment of inflammatory bowel disease. Can J Gastroenterol Hepatol 2014;28:371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chande N, Patton PH, Tsoulis DJ, et al. . Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2015;CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wenzl HH, Primas C, Novacek G, et al. . Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig Dis Sci 2015;60:1414–1423. [DOI] [PubMed] [Google Scholar]
- 96. Chatu S, Subramanian V, Saxena S, et al. . The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:23–34, quiz 35. [DOI] [PubMed] [Google Scholar]
- 97. Smith MA, Irving PM, Marinaki AM, et al. . Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther 2010;32:119–130. [DOI] [PubMed] [Google Scholar]
- 98. Kotlyar DS, Osterman MT, Diamond RH, et al. . A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:36–41 e1. [DOI] [PubMed] [Google Scholar]
- 99. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2014;109:163–169. [DOI] [PubMed] [Google Scholar]
- 100. Health Canada. Imuran (azathioprine) or Purinethol (mercaptopurine): association with a type of blood cancer - hepatosplenic T-cell lymphoma - for health professionals. Last update 2014. Available at: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/38691a-eng.php. Accessed January 17, 2015. [Google Scholar]
- 101. Beaugerie L, Brousse N, Bouvier AM, et al. . Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–1625. [DOI] [PubMed] [Google Scholar]
- 102. McDonald JW, Wang Y, Tsoulis DJ, et al. . Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev 2014;CD003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oren R, Moshkowitz M, Odes S, et al. . Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol 1997;92:2203–2209. [PubMed] [Google Scholar]
- 104. Feagan BG, Rochon J, Fedorak RN, et al. . Methotrexate for the treatment of Crohn’s disease: the North American Crohn’s Study Group Investigators. N Engl J Med 1995;332:292–297. [DOI] [PubMed] [Google Scholar]
- 105. Wilson A, Patel V, Chande N, et al. . Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2013;37:340–345. [DOI] [PubMed] [Google Scholar]
- 106. Kurnik D, Loebstein R, Fishbein E, et al. . Bioavailability of oral vs subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther 2003;18:57–63. [DOI] [PubMed] [Google Scholar]
- 107. Patel V, Wang Y, MacDonald JK, et al. . Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2014;CD006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Feagan BG, Fedorak RN, Irvine EJ, et al. . A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease: North American Crohn’s Study Group Investigators. N Engl J Med 2000;342:1627–1632. [DOI] [PubMed] [Google Scholar]
- 109. Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 110. Ford AC, Sandborn WJ, Khan KJ, et al. . Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011;106:644–659; quiz 660. [DOI] [PubMed] [Google Scholar]
- 111. Hazlewood GS, Rezaie A, Borman M, et al. . Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015;148:344–354 e5; quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 112. Singh S, Garg SK, Pardi DS, et al. . Comparative efficacy of biologic therapy in biologic-naive patients with Crohn disease: a systematic review and network meta-analysis. Mayo Clin Proc 2014;89:1621–1635. [DOI] [PubMed] [Google Scholar]
- 113. Sandborn WJ, Hanauer SB, Katz S, et al. . Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:1088–1094. [DOI] [PubMed] [Google Scholar]
- 114. D’Haens G, Van Deventer S, Van Hogezand R, et al. . Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: a European multicenter trial. Gastroenterology 1999;116:1029–1034. [DOI] [PubMed] [Google Scholar]
- 115. Colombel JF, Sandborn WJ, Reinisch W, et al. . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 116. Rutgeerts P, Van Assche G, Sandborn WJ, et al. . Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–1111 e2. [DOI] [PubMed] [Google Scholar]
- 117. D’Haens G, Baert F, van Assche G, et al. . Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–667. [DOI] [PubMed] [Google Scholar]
- 118. Fan R, Zhong J, Wang ZT, et al. . Evaluation of “top-down” treatment of early Crohn’s disease by double balloon enteroscopy. World J Gastroenterol 2014;20:14479–14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gecse KB, Lovasz BD, Farkas K, et al. . Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis 2016;10:133–140. [DOI] [PubMed] [Google Scholar]
- 120. Jahnsen J, Detlie TE, Vatn S, et al. . Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol 2015;9(Suppl 1):45–52. [DOI] [PubMed] [Google Scholar]
- 121. Keil R, Wasserbauer M, Zadorova Z, et al. . Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn’s disease and ulcerative colitis. Scand J Gastroenterol 2016;51:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Buer LC, Moum BA, Cvancarova M, et al. . Switching from Remicade(R) to Remsima(R) is well tolerated and feasible: a prospective, open-label study. J Crohns Colitis 2017;11:297–304. [DOI] [PubMed] [Google Scholar]
- 123. Jorgensen KK, Olsen IC, Goll GL, et al. . Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389:2304–2316. [DOI] [PubMed] [Google Scholar]
- 124. Peyrin-Biroulet L, Lonnfors S, Roblin X, et al. . Patient perspectives on biosimilars: a survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohns Colitis 2017;11:128–133. [DOI] [PubMed] [Google Scholar]
- 125. Dassopoulos T, Sultan S, Falck-Ytter YT, et al. . American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology 2013;145:1464–1478, e1–e5. [DOI] [PubMed] [Google Scholar]
- 126. Kopylov U, Al-Taweel T, Yaghoobi M, et al. . Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 2014;8:1632–1641. [DOI] [PubMed] [Google Scholar]
- 127. Colombel JF, Jharap B, Sandborn WJ, et al. . Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn’s disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther 2017;45:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Matsumoto T, Motoya S, Watanabe K, et al. . Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis 2016;10:1259–1266. [DOI] [PubMed] [Google Scholar]
- 129. Feagan BG, McDonald JW, Panaccione R, et al. . Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–688 e1. [DOI] [PubMed] [Google Scholar]
- 130. Colombel JF, Sandborn WJ, Rutgeerts P, et al. . Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 131. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. . Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006;130:323–333, quiz 591. [DOI] [PubMed] [Google Scholar]
- 132. Sandborn WJ, Rutgeerts P, Enns R, et al. . Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 133. Targan SR, Hanauer SB, van Deventer SJ, et al. . A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease: Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–1035. [DOI] [PubMed] [Google Scholar]
- 134. Lemann M, Mary JY, Duclos B, et al. . Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology 2006;130:1054–1061. [DOI] [PubMed] [Google Scholar]
- 135. Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2008;CD006893. [DOI] [PubMed] [Google Scholar]
- 136. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 2011;106:674–684. [DOI] [PubMed] [Google Scholar]
- 137. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009;104:760–767. [DOI] [PubMed] [Google Scholar]
- 138. Vande Casteele N, Khanna R, Levesque BG, et al. . The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015;64:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40–47; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Steenholdt C, Brynskov J, Thomsen OO, et al. . Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–927. [DOI] [PubMed] [Google Scholar]
- 141. Van Assche G, Vermeire S, Ballet V, et al. . Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012;61:229–234. [DOI] [PubMed] [Google Scholar]
- 142. Gisbert JP, Marin AC, McNicholl AG, et al. . Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–623. [DOI] [PubMed] [Google Scholar]
- 143. Singh S, Heien HC, Sangaralingham LR, et al. . Comparative effectiveness and safety of anti-tumor necrosis factor agents in biologic-naive patients with Crohn’s disease. Clin Gastroenterol Hepatol 2016;14:1120–1129 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chandar AK, Singh S, Murad MH, et al. . Efficacy and safety of natalizumab and vedolizumab for the management of Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:1695–1708. [DOI] [PubMed] [Google Scholar]
- 145. Feagan BG, Greenberg GR, Wild G, et al. . Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol 2008;6:1370–1377. [DOI] [PubMed] [Google Scholar]
- 146. Sandborn WJ, Feagan BG, Rutgeerts P, et al. . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 147. Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–627 e3. [DOI] [PubMed] [Google Scholar]
- 148. Dulai PS, Singh S, Jiang X, et al. . The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY Consortium. Am J Gastroenterol 2016;111:1147–1155. [DOI] [PubMed] [Google Scholar]
- 149. Shafran I, Laughlin R, Burgunder P. Evidence of mucosal healing in patients with Crohn’s disease treated with open-label vedolizumab: a case series [abstract P-006]. Inflamm Bowel Dis 2016;22:S11. [Google Scholar]
- 150. Noman M, Ferrante M, Bisschops R, et al. . Mucosal healing and dysplasia surveillance in a large referral center cohort of patients with Crohn’s disease and ulcerative colitis treated with vedolizumab [abstract Mo1880]. Gastroenterology 2016;150:S804. [Google Scholar]
- 151. Colombel J-F, Loftus EV, Siegel CA, et al. . Efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with Crohn’s disease from GEMINI 2 [abstract Sa1270]. Gastroenterology 2015;148:S277. [Google Scholar]
- 152. Rosario M, Dirks NL, Gastonguay MR, et al. . Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015;42:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rosario M, Wyant T, Milch C, et al. . Pharmacokinetic and pharmacodynamic relationship and immunogenicity of vedolizumab in adults with inflammatory bowel disease: additional results from the GEMINI 1 and 2 studies [abstract DOP058]. J Crohns Colitis 2014;8:S42–S43. [Google Scholar]
- 154. Vermeire S, Loftus EV Jr, Colombel JF, et al. . Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017;11:412–424. [DOI] [PubMed] [Google Scholar]
- 155. Sands BE, Sandborn WJ, Van Assche G, et al. . Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 2017;23:97–106. [DOI] [PubMed] [Google Scholar]
- 156. Colombel JF, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sandborn WJ, Feagan BG, Fedorak RN, et al. . A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008;135:1130–1141. [DOI] [PubMed] [Google Scholar]
- 158. Sandborn WJ, Gasink C, Gao LL, et al. . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 159. Feagan BG, Sandborn WJ, Gasink C, et al. . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 160. Khanna R, Preiss JC, MacDonald JK, et al. . Anti-IL-12/23p40 antibodies for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2015;CD007572. [DOI] [PubMed] [Google Scholar]
- 161. Wils P, Bouhnik Y, Michetti P, et al. . Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016;14:242–250 e1–e2. [DOI] [PubMed] [Google Scholar]
- 162. Khorrami S, Ginard D, Marin-Jimenez I, et al. . Ustekinumab for the treatment of refractory Crohn’s disease: the spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis 2016;22:1662–1669. [DOI] [PubMed] [Google Scholar]
- 163. Harris KA, Horst S, Gadani A, et al. . Patients with refractory Crohn’s disease successfully treated with ustekinumab. Inflamm Bowel Dis 2016;22:397–401. [DOI] [PubMed] [Google Scholar]
- 164. Ghouri YA, Richards DM, Rahimi EF, et al. . Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 2014;7:473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol 2014;7:1–13. [DOI] [PubMed] [Google Scholar]
- 166. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2008;CD006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rahimi R, Nikfar S, Rahimi F, et al. . A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci 2008;53:2524–2531. [DOI] [PubMed] [Google Scholar]
- 168. Cabre E, Manosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases: a systematic review. Br J Nutr 2012;107(Suppl 2):S240–S252. [DOI] [PubMed] [Google Scholar]
- 169. Lev-Tzion R, Griffiths AM, Leder O, et al. . Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2014;CD006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Naftali T, Bar-Lev Schleider L, Dotan I, et al. . Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013;11:1276–1280 e1. [DOI] [PubMed] [Google Scholar]
- 171. Storr M, Devlin S, Kaplan GG, et al. . Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:472–480. [DOI] [PubMed] [Google Scholar]
- 172. Segal D, Macdonald JK, Chande N. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2014;CD010410. [DOI] [PubMed] [Google Scholar]
- 173. Philippe D, Chakass D, Thuru X, et al. . Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut 2006;55:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Akobeng AK, Elawad M, Gordon M. Glutamine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2016;CD007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Akobeng AK, Thomas AG. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2007;CD005984. [DOI] [PubMed] [Google Scholar]
- 176. Nguyen DL, Palmer LB, Nguyen ET, et al. . Specialized enteral nutrition therapy in Crohn’s disease patients on maintenance infliximab therapy: a meta-analysis. Therap Adv Gastroenterol 2015;8:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Munkholm P, Langholz E, Davidsen M, et al. . Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 1994;35:360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Modigliani R, Mary JY, Simon JF, et al. . Clinical, biological, and endoscopic picture of attacks of Crohn’s disease: evolution on prednisolone—Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology 1990;98:811–818. [DOI] [PubMed] [Google Scholar]
- 179. Panes J, Lopez-Sanroman A, Bermejo F, et al. . Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 2013;145:766–774 e1. [DOI] [PubMed] [Google Scholar]
- 180. Cosnes J, Bourrier A, Laharie D, et al. . Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 2013;145:758–765 e2, quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 181. Macaluso FS, Renna S, Maida M, et al. . Tolerability profile of thiopurines in inflammatory bowel disease: a prospective experience. Scand J Gastroenterol 2017;52:981–987. [DOI] [PubMed] [Google Scholar]
- 182. Lopez A, Mounier M, Bouvier AM, et al. . Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol 2014;12:1324–1329. [DOI] [PubMed] [Google Scholar]
- 183. Beaugerie L, Carrat F, Colombel JF, et al. . Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 2014;63:1416–1423. [DOI] [PubMed] [Google Scholar]
- 184. Mottet C, Schoepfer AM, Juillerat P, et al. . Experts opinion on the practical use of azathioprine and 6-mercaptopurine in inflammatory bowel disease. Inflamm Bowel Dis 2016; 22:2733–2747. [DOI] [PubMed] [Google Scholar]
- 185. Kariburyo MF, Xie L, Teeple A, et al. . Predicting pre-emptive discussions of biologic treatment: results from an openness and preference survey of inflammatory bowel disease patients and their prescribers. Adv Ther 2017;34:1398–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.