Plant-fermenting Clostridia are anaerobic bacteria that recycle plant matter in soil and promote human health by fermenting dietary fiber in the intestine. Clostridia degrade plant biomass using extracellular enzymes and then uptake the liberated sugars for fermentation. The main sugars in plant biomass are hexoses, and here, we identify how hexoses are taken in to the cell by the model organism Clostridium phytofermentans. We show that this bacterium uptakes hexoses using a set of highly specific, nonredundant ABC transporters. Once in the cell, the hexoses are phosphorylated by intracellular hexokinases. This study provides insight into the functioning of abundant members of soil and intestinal microbiomes and identifies gene targets to engineer strains for industrial lignocellulosic fermentation.
KEYWORDS: Clostridia, biomass, fermentation
ABSTRACT
The mechanisms by which bacteria uptake solutes across the cell membrane broadly impact their cellular energetics. Here, we use functional genomic, genetic, and biophysical approaches to reveal how Clostridium (Lachnoclostridium) phytofermentans, a model bacterium that ferments lignocellulosic biomass, uptakes plant hexoses using highly specific, nonredundant ATP-binding cassette (ABC) transporters. We analyze the transcription patterns of its 173 annotated sugar transporter genes to find those upregulated on specific carbon sources. Inactivation of these genes reveals that individual ABC transporters are required for uptake of hexoses and hexo-oligosaccharides and that distinct ABC transporters are used for oligosaccharides versus their constituent monomers. The thermodynamics of sugar binding shows that substrate specificity of these transporters is encoded by the extracellular solute-binding subunit. As sugars are not phosphorylated during ABC transport, we identify intracellular hexokinases based on in vitro activities. These mechanisms used by Clostridia to uptake plant hexoses are key to understanding soil and intestinal microbiomes and to engineer strains for industrial transformation of lignocellulose.
IMPORTANCE Plant-fermenting Clostridia are anaerobic bacteria that recycle plant matter in soil and promote human health by fermenting dietary fiber in the intestine. Clostridia degrade plant biomass using extracellular enzymes and then uptake the liberated sugars for fermentation. The main sugars in plant biomass are hexoses, and here, we identify how hexoses are taken in to the cell by the model organism Clostridium phytofermentans. We show that this bacterium uptakes hexoses using a set of highly specific, nonredundant ABC transporters. Once in the cell, the hexoses are phosphorylated by intracellular hexokinases. This study provides insight into the functioning of abundant members of soil and intestinal microbiomes and identifies gene targets to engineer strains for industrial lignocellulosic fermentation.
INTRODUCTION
The mechanisms by which bacteria uptake molecules needed for growth have important consequences for cell physiology and energetics. In particular, the transport and metabolism of plant sugars by Clostridium phytofermentans (also called Lachnoclostridium phytofermentans) (1) and other bacteria that ferment lignocellulosic biomass are central to carbon flow in terrestrial and aquatic ecosystems. C. phytofermentans is an anaerobic mesophile that metabolizes plant polysaccharides, including cellulose, hemicellulose, and pectin (2–4). This bacterium expresses numerous carbohydrate-active enzymes (CAZymes) (5) to degrade plant polysaccharides into hexoses and pentoses of various chain lengths, which are taken into the cell using a panoply of transporters. C. phytofermentans is predicted to carry 572 transporter genes, including 173 genes for sugar transport (6) (see Table S1 in the supplemental material), but the transporters responsible for uptake of the specific saccharides are unknown. While in silico methods such as sequence homology and structure-based modeling (7) can make general substrate predictions, experimental approaches are generally needed to determine the substrate specificity of a transporter.
Bacteria translocate sugars using ATP-binding cassette (ABC) systems, secondary transporters, and the phosphotransferase system (PTS). ABC transporters, which power sugar translocation by ATP hydrolysis, are widely used in Clostridia; the C. phytofermentans genome contains 158 genes predicted to encode sugar ABC transporters. Secondary transporters including symporters and antiporters mediate substrate translocation independent of ATP. Symporters such as Escherichia coli LacY assimilate various molecules including hexoses by coupling their uptake with H+ or Na+ molecules to exploit energy of the proton gradient (8); C. phytofermentans is annotated as encoding 17 symporters that are primarily predicted to uptake amino acids. Antiporters couple sugar uptake with the export of inorganic phosphate or other charged molecules (9); C. phytofermentans has 8 antiporter genes predicted to exchange H+ with dicarboxylate or Na+ ions for the uptake of substrates, including citrate and gluconate. Sugar uptake by the PTS involves transfer of a phosphate from phosphoenolpyruvate (PEP) such that the sugar is both transported and phosphorylated using a single ATP equivalent. Clostridial species vary in their encoding of PTSs as follows: Clostridium acetobutylicum (10) and Clostridium beijerinckii (11) translocate sugars using many PTSs, C. phytofermentans has a single PTS predicted to transport glucose, and Clostridium cellulolyticum (12) and Clostridium thermocellum (13) appear to lack a functional PTS.
Many studies have focused on the CAZymes expressed by C. phytofermentans and other Clostridia to degrade plant biomass, but less is known about how the sugars are subsequently taken into the cell. The goal of this study is to gain insight into how plant-fermenting bacteria like C. phytofermentans metabolize biomass by identifying the mechanisms for the uptake of hexoses and hexosaccharides abundant in the three main classes of plant polysaccharides, cellulose (glucose), hemicellulose (galactose), and homogalacturonan pectin (galacturonic acid). We examine mRNA expression on a panel of plant substrates to identify transporters that are specifically upregulated on hexose substrates. While reverse genetics in Clostridia remain challenging, methods have been developed to make targeted chromosomal insertions using designed group II introns called targetrons (14) in C. phytofermentans (15) and other Clostridia (16). We inactivate the genes encoding these transporters using targetrons to identify which transporters are required for hexose uptake. We explore the basis of the substrate specificities of these transporters by measuring the thermodynamics of binding different hexoses by the solute-binding protein (SBP) subunits. As our results support the hypothesis that glucose and galactose are not phosphorylated during transport, we identify and characterize the activities of the intracellular glucokinases and galactokinases. Finally, we discuss the implications of our results for understanding carbon and energy metabolism in Clostridia.
RESULTS
mRNA expression identifies putative hexose transporters.
The C. phytofermentans genome is predicted to carry 572 transporter genes by TransportDB (6) and 485 transporter genes by KEGG (17), with 438 genes common to both databases (see Table S1 in the supplemental material). Similar to other bacteria, C. phytofermentans genes encoding a transporter are often cotranscribed as an operon to facilitate their expression at similar levels (18). Among the 173 genes annotated by TransportDB as sugar transporters, we observed that distinct and often single ABC transporter operons are transcriptionally upregulated on each carbon source (Fig. 1; see also Table S2 in the supplemental material). Single ABC transporter operons are highly expressed on mono- and disaccharides, including glucose and galactose (cphy2241-cphy2243), galacturonic acid (cphy2731-cphy2733), and cellobiose (cphy2464-cphy2466). During growth on polysaccharides, expression of cphy3588-cphy3590 is highest on galactan, and a few ABC transporters are upregulated on cellulose (cphy3858-cphy3860 and cphy2464-cphy2466), homogalacturonan (cphy2731-cphy2733 and cphy3588-cphy3590), and starch (cphy1074-cphy1076, cphy2306-cphy2308, and cphy2345-cphy2347) (Fig. 1).
FIG 1.
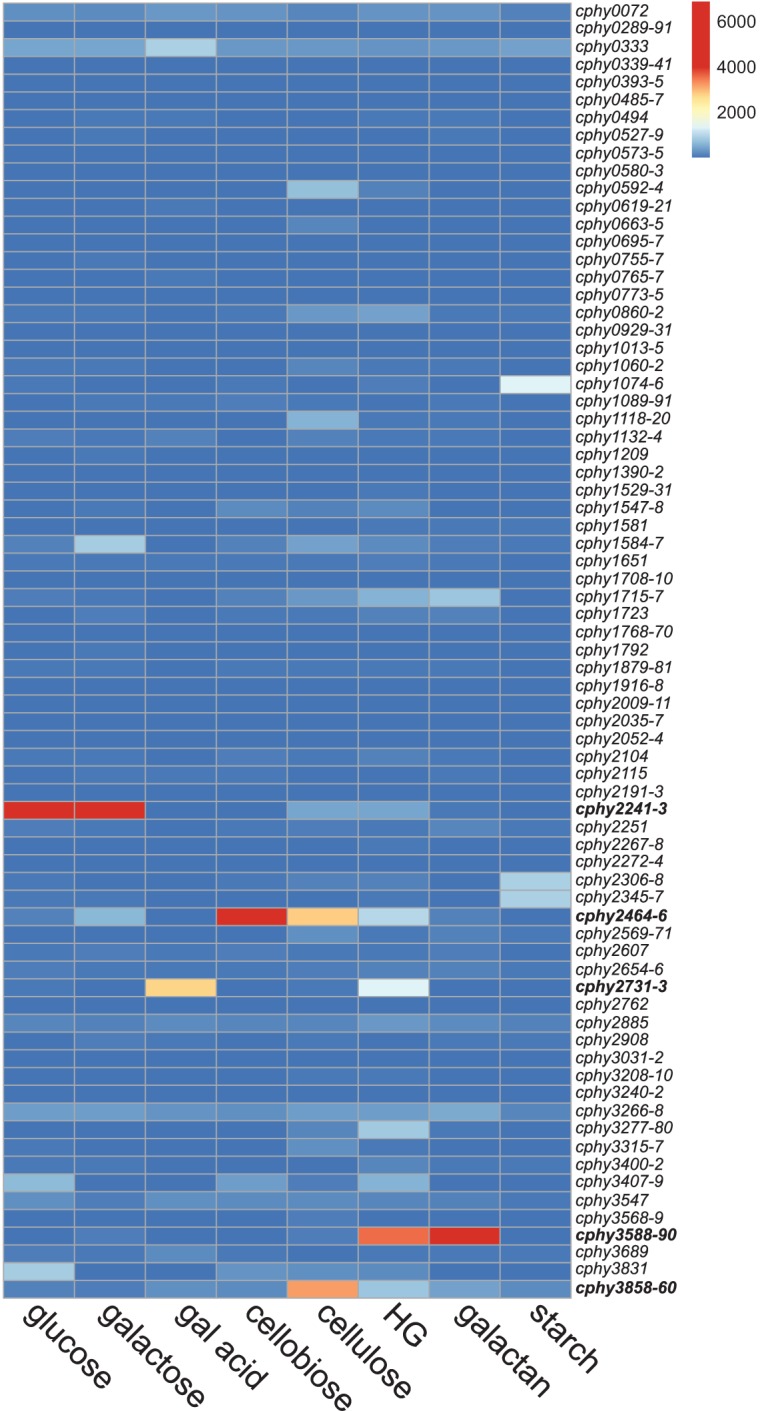
C. phytofermentans upregulates transcription of specific sugar transporters on each carbon source. The heat map is colored based on the mRNA expression measured by RNA-seq of the 173 transporter genes predicted to uptake sugars by TransportDB. Expression is reported as mean RPKM (reads per kilobase per million) mapped reads from duplicate cultures. Genes are described using NCBI GenBank gene numbers (GenBank accession number CP000885.1). Transporter genes coexpressed as an operon are shown on a single row as the average expression of all genes in the operon. Hexose-responsive ABC transporters prioritized for gene inactivation are in bold. gal acid, galacturonic acid; HG, homogalacturonan.
These mRNA expression profiles support the hypothesis that even though C. phytofermentans carries many putative sugar transporters, a small subset of the ABC transporter genes may play key roles in hexose uptake. Further, gene expression suggests that C. phytofermentans uptakes saccharides of different chain lengths, such as glucose, cellobiose, and cellodextrins, using distinct systems and that these saccharides act as intracellular inducers to upregulate expression of chain length-specific transport systems. This could have important consequences for cellular energetics because C. phytofermentans often grows faster on the polysaccharide than the constituent monomer (3), supporting the hypothesis that direct uptake of saccharides of longer chain length is energetically more efficient.
Gene inactivation shows ABC transporters required for growth on hexoses.
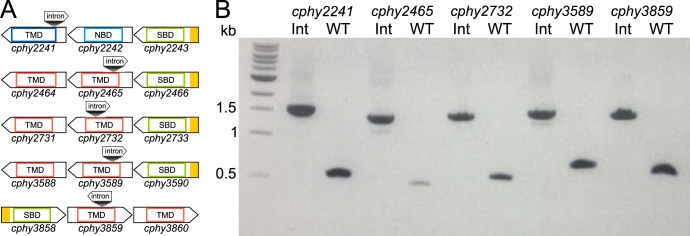
ABC transporters are comprised of three subunits as follows: the solute-binding protein (SBP) captures the substrate, the transmembrane domain (TMD) protein translocates the substrate across the membrane, and the nucleotide-binding domain (NBD) protein provides the energy by ATP hydrolysis (19). We examined the function of ABC transporters by targeted gene inactivation by inserting a designed LI.LtrB group II intron (targetron) into the coding sequence of the transmembrane domain (TMD) gene (Fig. 2A; see also Table S3 in the supplemental material), thereby disrupting translation of the mRNA into a functional protein. We targeted TMD genes associated with 5 ABC transporters whose transcriptions were elevated in response to plant hexoses as follows: cphy2241 (glucose and galactose), cphy2465 (cellobiose and cellulose), cphy2732 (galacturonic acid and homogalacturonan), cphy3589 (galactan and homogalacturonan), and cphy3859 (cellulose).
FIG 2.
Targeted inactivation of ABC transporter genes. (A) Genomic organization of ABC transporter gene operons showing intron insertion sites. Genes show secretory signal peptides predicted by SignalP (39) in yellow and protein domains defined by PFAM (40) as follows: solute-binding domains (SBD) (PFAM PF13407/PF13416), nucleotide-binding domains (NBD) (PFAM PF00005), and transmembrane domains (TMD) (PFAM PF02653 [dark blue] and PFAM PF00528 [red]). (B) PCR with primers flanking intron insertion sites of target genes in wild-type (WT) and intron insertion strains (Int) confirm insertion of the 1,040-bp intron in Int strains.
Following delivery of the targetron into C. phytofermentans, its chromosomal integration into the TMD gene was shown by PCR using primers flanking the programmed insertion site (Fig. 2B). We observed targetrons inserted at the expected locations in more than 75% of transconjugant colonies for all genes, and sequencing the PCR products confirmed all introns inserted at the expected genome position. Insertion strain names specify the target gene, distance of the intron insertion from the gene start, and an “a” to denote an antisense insertion. We also investigated the prevalence of additional, off-site genomic targetron insertions in the mutant strains by inverse PCR, which confirmed that cphy2241::int164a, cphy2732::int662a, and cphy3589::int332a have single genomic insertions at the expected sites (see Fig. S1 in the supplemental material). However, cphy2465::int293a and cphy3859::int586a each had an additional genomic intron insertion after curing of pQint. As cphy2465::int293a had no off-target insertions before transferring the culture to cure pQint (see Fig. S1), we used this culture for growth assays. The cphy3859::int586a strain had a second genomic insertion in cphy3242, an ABC transporter gene that is lowly expressed on all carbon sources (see Table S2). The cphy3242 insertion was present immediately after repeated conjugations, so a culture with this double insertion was used for growth experiments.
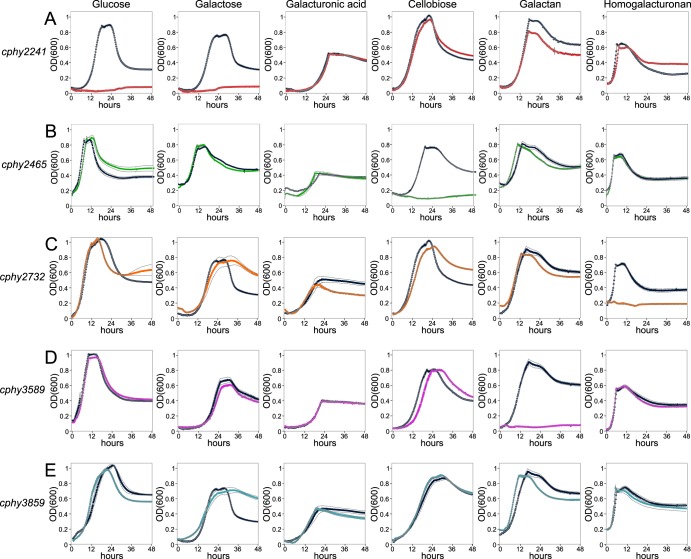
We compared the growth of C. phytofermentans transporter mutants and that of wild-type (WT) strains on monosaccharides (glucose, galactose, galacturonic acid), disaccharides (cellobiose), and polysaccharides (galactan, homogalacturonan). The cphy2241::int164a strain completely lost the ability to grow on glucose and galactose and has a small growth deficit on galactan (Fig. 3A), likely resulting from the WT consuming galactose that is residual in galactan or that is liberated by extracellular galactanase activity. The cphy2465::int293a strain specifically lost the ability to grow on cellobiose (Fig. 3B). Even though cphy2731-cphy2733 are the most highly expressed transporter genes on galacturonic acid, the cphy2732::int662a strain grows normally on galacturonic acid but has lost the ability to grow on homogalacturonan (Fig. 3C). The cphy3589::int332a strain cannot grow on galactan (Fig. 3D) but unexpectedly grows normally on homogalacturonan even though cphy3588-cphy3590 are the most highly expressed transporter genes on this substrate (Fig. 1). The high expression of cphy3588-cphy3590 on homogalacturonan suggests either the presence of galactan in the homogalacturonan preparation or that a homogalacturonan-associated metabolite triggers expression of the galactan transporter. The cphy3859::int586a strain grew normally on all 6 substrates (Fig. 3E).
FIG 3.
Transporter-inactivated strains have lost the ability to grow on distinct carbon sources. Growth (OD600) of the WT (dark blue) relative to that of cphy2241::int164a (red) (A), cphy2465::int293a (green) (B), cphy2732::int662a (orange) (C), cphy3589::int332a (magenta) (D), and cphy3859::int586a (light blue) (E) strains was measured on glucose, galactose, galacturonic acid, cellobiose, galactan, and homogalacturonan. All data are means from quadruplicate cultures and gray lines show standard deviations (SD).
We compared the cellulose degradation rates of the cphy2241, cphy2465, and cphy3859 inactivation strains relative to that of the WT. Initially, cellulose degradation was reduced by 50 to 75% in all three strains relative to that of the WT (Fig. 4). By day 14, cphy3859::int586a reestablished cellulose degradation similar to that of the WT, while the other 2 strains still had degraded 25% less cellulose than the WT. The high expression of cphy3858-cphy3860 on cellulose, but not cellobiose or glucose, suggests it uptakes cellodextrins (Fig. 1). We, thus, propose that in the initial stage of cellulose degradation, abundant cellodextrins lead to reduced growth of the cphy3859::int586a strain relative to that of the WT. As cellulases accumulate in the medium, cellobiose and glucose become the dominant products, enabling the cphy3859::int586a strain to establish growth similar to that of the WT but resulting in continued growth deficits in the cphy2465::int293a and cphy2241::int164a strains.
FIG 4.
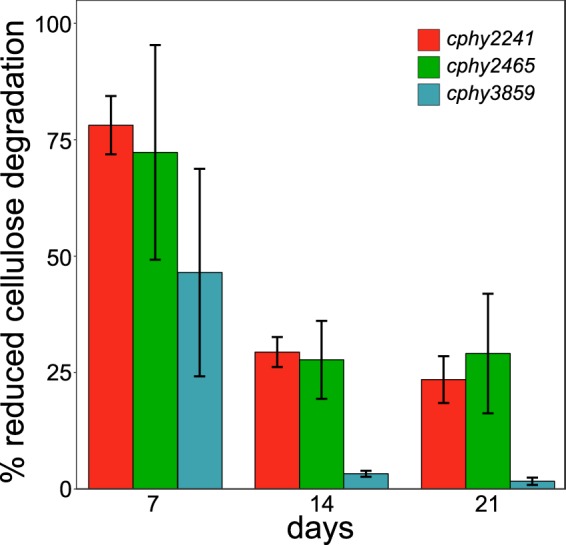
Reduced cellulose degradation of transporter-inactivated strains relative to that of the WT for cphy2241::int164a, cphy2465::int293a, and cphy3859::int586a strains. All data are means from quadruplicate cultures ± SD.
Thermodynamics of sugar binding by SBP subunits.
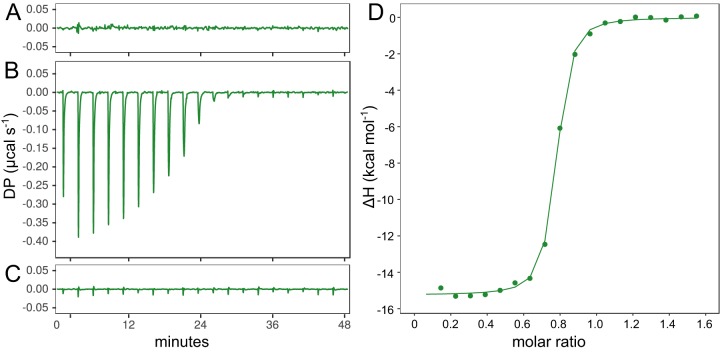
The growth phenotypes of the transporter-inactivated strains supported the hypothesis that the metabolism of hexoses generally requires a single, nonredundant ABC transporter that has high substrate specificity. We hypothesized that transporter specificity could be carried by the solute-binding protein (SBP) subunit, the transmembrane channel, or both. To examine the substrate specificity of the SBP subunits of these five ABC transporters, we cloned (see Fig. S2A and Table S3 in the supplemental material) and purified (Fig. S2B and C) the SBP subunits and performed isothermal titration calorimetry (ITC) to measure the thermodynamics of their binding to various hexoses (see Table S4 in the supplemental material). ITC is a biophysical technique that quantifies temperature changes upon ligand binding to enable calculation of thermodynamic parameters, such as binding affinity (KA), dissociation constant (KD), and enthalpy (ΔH). Using the relation ΔG = −RTln(KA), it is possible to estimate the variation in the free energy (ΔG), and the variation in entropy (ΔS) can be deduced from the relation ΔG = ΔH − TΔS.
The thermodynamics of sugar binding shows that the SBP often endows remarkable substrate specificity to the transporter. Cphy2466 did not bind glucose (Fig. 5A) or cellohexaose (Fig. 5C) but bound cellobiose with high affinity (KD = 0.03 μM) (Fig. 5B to D; see also Table S5 in the supplemental material). Thus, SBP can be specific for both the sugar subunit and chain length. Conversely, Cphy2243 bound to both glucose (KD = 2.2 μM) and galactose (KD = 4.9 μM) (see Table S5) with no binding to cellobiose. However, SBPs are not always chain length specific, as we found that Cphy3590 binds both galactose and galactan (Table S5), albeit with a much reduced affinity for galactose. Cphy3590 has no affinity for homogalacturonan even though it is the most highly expressed transporter on this substrate, which is consistent with the growth of the cphy3589::int332a strain (Fig. 3D).
FIG 5.
Thermodynamics of SBP Cphy2466 binding to sugar ligands measured by isothermal titration calorimetry. Raw binding heats of 15 μM Cphy2466 with 18- by 0.5-μl injections of either 5 mM glucose (A), 0.5 mM cellobiose (B), or 0.5 mM cellohexaose (C). Ordinate axis shows differential power (DP) between the reference and sample cells needed to maintain a zero temperature difference. (D) Integrated binding heats of Cphy2466 binding cellobiose minus the dilution control heats fitted to a single-site binding model to calculate binding enthalpy (ΔH), association binding constant (KA), and the number of binding sites per monomer (n).
While both Cphy2733 and Cphy3858 purified at yields similar to those of the other SBPs (Fig. S2B and C), we did not detect significant binding by these proteins to any of the tested substrates. These SBPs may bind other sugars or oligosaccharides derived from homogalacturonan (Cphy2733) or cellulose (Cphy3858), even though we assayed cellodextrins of 2, 4, and 6 glucose units for Cphy3858. Alternatively, this lack of binding measured by ITC may be due to incorrect folding of the SBP when expressed in E. coli or an entropic binding interaction that does not generate an enthalpy change. Challenges of measuring SBP activity were also exemplified by how while Cphy2243 bound glucose and galactose with high affinity, only 3% to 4% of the protein appeared active (Table S5). A large fraction of the Cphy2243 purified from the E. coli overproducing strain may be incorrectly folded or in an inappropriate oligomerization state.
Biochemical identification of hexose kinases.
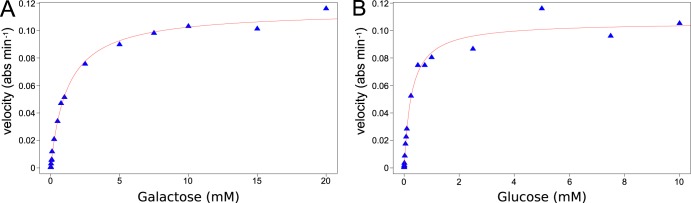
Unlike the PTSs, ABC transporters do not phosphorylate sugars during transport, requiring them to be phosphorylated by cytoplasmic kinases. Glucose is phosphorylated to glucose-6-phosphate by glucokinase. Galactose is phosphorylated to galactose-1-phosphate by galactokinase. Galacturonic acid is isomerized to 3-deoxy-d-erythro-hex-2-ulosonate and then phosphorylated by 2-keto-3-deoxy-d-gluconate kinase (20). In C. phytofermentans, the cphy2241-cphy2243 genes encoding the glucose/galactose ABC transporter are colocated in the genome with a putative hexokinase gene cphy2237, leading us to question whether this enzyme phosphorylates glucose, galactose, or both. We purified Cphy2237 (see Fig. S3 in the supplemental material) and found it phosphorylates galactose (Fig. 6A) with a kinase activity (Km and kcat) similar to galactokinases from other organisms (Table 1), but this enzyme has undetectable glucokinase activity. Among the six additional genes annotated as glucokinases in C. phytofermentans, we focused on cphy0329 based on its high expression on glucose and homology to the C. beijerinckii glucokinase (21). We purified Cphy0329 (Fig. S3) and found it phosphorylates glucose (Fig. 6B) with an activity (Km and kcat) similar to other glucokinases (Table 1) but has negligible galactokinase activity. Thus, the glucose/galactose ABC transporter genes are colocated in a gene island with galactokinase, whereas glucose phosphorylation requires an enzyme located elsewhere in the genome that was likely inherited independently.
FIG 6.
Activities of purified C. phytofermentans hexokinases Cphy2237 (A) and Cphy0329 (B). (A) Kinetics of Cphy2237 galactokinase activity at different galactose concentrations measured as NADH consumption (absorbance at 340 nm) using a coupled pyruvate kinase/lactate dehydrogenase assay. (B) Kinetics of Cphy0329 glucokinase activity at different glucose concentrations measured as NADPH formation (absorbance at 340 nm) using a coupled glucose-6-phosphate dehydrogenase assay.
TABLE 1.
Comparison of glucokinase and galactokinase activities of C. phytofermentans enzymes with those from other species
| Enzyme typea | Species | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) | Reference or source |
|---|---|---|---|---|---|
| GlcK (Cphy0329) | C. phytofermentans | 0.26 | 273.80 | 1,047.84 | This study |
| GlcK | E. coli | 0.78 | 91.26 | 117.00 | 41 |
| GlcK | Trypanosoma cruzi | 1.00 | 1,491.67 | 1,491.67 | 42 |
| GlcK | Leishmania major | 3.30 | 316.67 | 95.96 | 42 |
| GlcK | Saccharomyces cerevisiae | 0.03 | 18.93 | 631.00 | 43 |
| GlcK | Homo sapiens | 0.07 | 26.72 | 411.00 | 44 |
| GalK (Cphy2237) | C. phytofermentans | 1.19 | 176.33 | 147.63 | This study |
| GalK | E. coli | 0.70 | 9.66 | 13.80 | 45 |
| GalK | S. cerevisiae | 0.60 | 53.94 | 89.90 | 46 |
| GalK | H. sapiens | 0.12 | 68.16 | 568.00 | 47 |
GlcK, glucokinase; GalK, galactokinase.
DISCUSSION
Our results show that C. phytofermentans relies on individual ABC transporters for growth on its preferred hexose substrates (glucose, galactose, cellobiose, galactan, and homogalacturonan), revealing an unexpected lack of functional overlap among its nearly 200 genes predicted to encode sugar transporters. It remains to be seen if C. phytofermentans carries nonredundant transporters for a massive diversity of saccharides, many auxiliary transporters that improve but cannot themselves support growth, or functionally redundant transporters for substrates not examined in this study. Also notable is that while ATPase activity is essential for ABC transport (19), only one of the required hexose transporters (Cphy2241-Cphy2243) has an ATPase domain (Fig. 2A). C. phytofermentans ABC transporters likely share multitasking ATPase subunits, similar to those of some other Gram-positive bacteria (22, 23).
While C. phytofermentans translocates most hexoses tested here using single, highly-expressed ABC transporters, the only highly expressed transporter on galacturonic acid (Cphy2731-Cphy2733) is required for growth on homogalacturonan but not galacturonic acid (Fig. 3C). Bacillus subtilis and other bacteria uptake galacturonate using an ExuT transporter in the major facilitator superfamily (MFS) (24, 25). C. phytofermentans has no homolog of ExuT, but it does carry 12 putative MFS transporters, 4 of which putatively transport sugars (see Table S1 in the supplemental material). We similarly did not find an individual transporter required for metabolism of cellulose. Rather, C. phytofermentans upregulated operons for multiple transporters (cphy2464-cphy2466, cphy3858-cphy3860, and cphy2241-cphy2243) that each contribute to cellulose metabolism (Fig. 4), supporting that extracellular cellulases degrade cellulose into a range of chain lengths that are simultaneously uptaken by distinct ABC transporters.
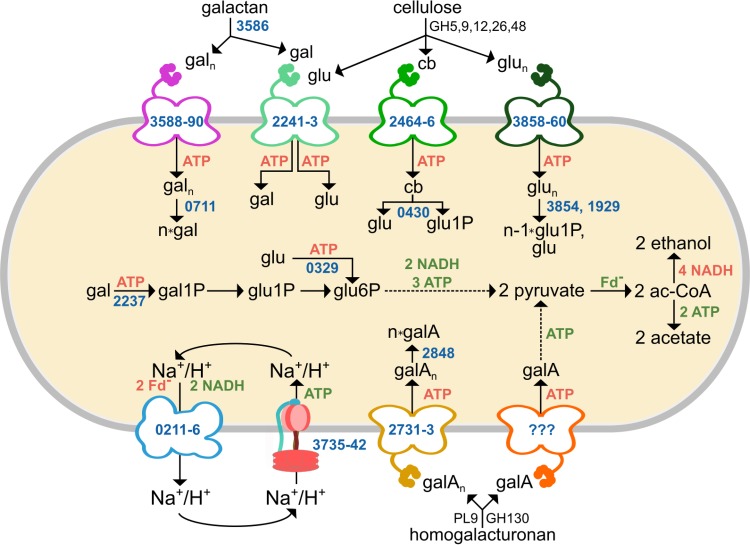
We assimilated our results into a model of how C. phytofermentans metabolizes plant-derived hexoses (Fig. 7). Extracellular glycoside hydrolases (GH) and polysaccharide lyases (PL) depolymerize cellulose, galactan, and homogalacturonan into a mix of oligosaccharides and monosaccharides (2, 3), which are translocated by substrate-specific ABC transporters. Our results show that C. phytofermentans has transport systems to uptake oligosaccharides, which conserves ATP relative to monosaccharide transport (3) and likely prevents competing microbes from filching free monosaccharides but necessitates intracellular CAZymes to split the oligosaccharides. Based on sequence homology and substrate-specific changes in mRNA expression measured by transcriptome sequencing (RNA-seq) (this study and reference 3), we propose galacto-oligosaccharides are cleaved intracellularly by the GH2 β-galactosidase Cphy0711 and galacturono-oligosaccharides by the GH4 α-galacturonase Cphy2848. Cellodextrins and cellobiose are likely split by the following three intracellular GH94 phosphorylases: Cphy1929, Cphy0430, and Cphy3854. Expression profiles show Cphy0430 responds to cellobiose and cellulose, whereas Cphy3854 is specific to cellulose. Cphy1929 is expressed at lower levels but is active on cellodextrins in vitro. Subsequently, monosaccharides are metabolized using Embden-Meyerhof-Parnas (EMP) glycolytic pathway and fermented primarily to ethanol and acetate (2).
FIG 7.
Model of C. phytofermentans hexose metabolism. Polysaccharides are degraded by CAZymes belonging to multiple glycoside hydrolase (GH) and polysaccharide lyase (PL) families. ABC transporters and metabolic enzymes are shown with NCBI GenBank numbers (blue) and associated production (green) or consumption (red) of ATP, NADH, and reduced ferredoxin. glu, glucose; gal, galactose; gala, galacturonic acid; cb, cellobiose; glun, cellodextrin; galn, galacto-oligosaccharide; galAn, galacturono-oligosaccharides; glu1P, glucose-1-phosphate; glu6P, glucose-6-phosphate; gal1P, galactose-1-phosphate; G3P, glyceraldehyde-3-phosphate; ac-CoA, acetyl coenzyme A; Fd−, reduced ferredoxin.
The use of ABC transporters for hexose transport by C. phytofermentans differs from that of E. coli, Bacillus subtilis, and solventogenic Clostridia, such as C. acetobutylicum and C. beijerinckii, which all use PTS as the primary mechanism of hexose uptake (26). C. phytofermentans has a single PTS encoded by cphy1768 (EIIABC protein), cphy1769 (Hpr protein), and cphy1770 (EI protein). While this PTS is annotated to uptake glucose, the genes are expressed at low levels on all tested carbon sources (Fig. 1; see Table S2 in the supplemental material), and the PTS cannot rescue growth of cphy2241::int164a on glucose (Fig. 3A), suggesting that the PTS is either nonfunctional or specific to another condition. Expression and in vitro evidence support that other cellulolytic Clostridia uptake hexoses with ABC transporters (27, 28), similar to C. phytofermentans. ABC transport of a sugar molecule requires 1 or 2 ATP (29) and another ATP for intracellular phosphorylation, whereas PTS uptake and phosphorylate sugars using a single ATP equivalent. In addition, the PTS indirectly facilitates further ATP savings during galactose metabolism in C. acetobutylicum, which converts galactose-6-phosphate from the PTS into glyceraldehyde-3-phosphate by the tagatose pathway (30), thereby saving another ATP relative to the Leloir pathway used by C. phytofermentans. ABC transport imposes significant cost, as transport consumes up to 60% of cellular ATP (19). This seemingly nonadaptive preference in cellulolytic Clostridia may result from ABC transporters having a higher substrate affinity to scavenge sugars in carbon-poor environments. In addition, genes for ABC transporters are often colocated with glycoside hydrolases in the C. phytofermentans genome (4), supporting that soil bacteria may swap gene cassettes for polysaccharide degradation and ABC transport by horizontal gene transfer. For example, the galactan transporter genes (cphy3588-cphy3590) are adjacent to cphy3586, encoding the main β-1,4-galactanase in this organism (3).
C. phytofermentans fermentation products shed light on how energy-intensive ABC transport of hexoses affects cellular energetics. Fermentation of glucose and galactose produces ethanol/acetate ratios of ∼3:1 (2), supporting the hypothesis that the need for NADH oxidation outweighs that of ATP production, even though metabolism of a molecule of glucose or galactose to pyruvate yields only 1 ATP (Fig. 7). Assimilation of galacturonic acid to pyruvate yields only 1 ATP (Fig. 7), and concomitantly, the primary fermentation product shifts to acetate (18), which boosts ATP production by 2 ATPs per sugar equivalent. C. phytofermentans can likely produce additional ATP using the F1F0-ATPase (Cphy3735-Cphy3742) to harness the ion gradient generated by the Rnf complex (Cphy0211-Cphy0216), which couples ferredoxin oxidation to NAD+ reduction to pump Na+ or H+ outside the cell (Fig. 7). The high expression of the Rnf and F1F0-ATPase complexes in C. phytofermentans supports the hypothesis that their importance is similar to that in certain other Clostridia (31), potentially providing a means to maintain cellular ATP levels while transporting carbon using ATP-intensive ABC transporters.
Plant-fermenting Clostridia are critical to the functioning of soil and intestinal ecosystems and can be applied for industrial transformation of biomass. This approach combining functional genomics, targeted gene inactivation, and biophysical characterization can be generally applied to assign function to the transporters encoded by these bacteria. Further, while most efforts to engineer microbes to utilize plant polysaccharides focus on degradative enzymes, our results show that oligosaccharide-specific transporters are also required. Transfer of gene cassettes including both CAZymes and the associate transporters may help overcome the difficulties often encountered when engineering novel polysaccharide capabilities into bacteria. Understanding the mechanisms of sugar transport will facilitate future strategies for metabolic engineering to improve substrate utilization and fermentation efficiency in these organisms.
MATERIALS AND METHODS
Bacterial cultivation.
C. phytofermentans ISDg (ATCC 700394) was cultured anaerobically at 37°C in GS2 medium (32) supplemented with the appropriate carbon source. Growth curves were measured in medium containing 5 g liter−1 glucose (G5767; Sigma), galactose (G0750; Sigma), galacturonic acid (73960; Sigma), cellobiose (C7252; Sigma), galactan (P-GALLU; Megazyme), or homogalacturonan (P8471; Sigma). Log-phase cultures were resuspended in an equal volume of medium without sugars and inoculated at 1:10 volume into 100-well microtiter plates (9502550; Bioscreen) in an anaerobic chamber (2% H2, 98% N2), and plates were sealed by press-fitting adhesive pads (1018104; Qiagen) (33). Growth (optical density at 600 nm [OD600]) was measured using a Thermo Scientific Bioscreen C at 37°C with 30 s of shaking before readings each 15 min. Consumption of cellulose was measured in 10-ml cultures containing 15 g liter−1 cellulose (0.5- by 5-cm strips of Whatman filter paper 1001‐090) by collecting the remaining cellulose on 11-μm filters by vacuum filtration, drying overnight at 65°C, and weighing.
RNA sequencing.
Total RNA was extracted from log-phase C. phytofermentans galactose cultures using TRI reagent (93289; Sigma) and treated at 37°C for 30 min with 0.2 unit Turbo DNase (AM2238; Ambion) per microgram RNA. RNA was purified by Zymo Concentrator-5 (R1015; Zymo Research) (>200-bp capture) into 15 μl water. Five micrograms total RNA was rRNA depleted by Ribo-Zero (MRZMB126; Illumina) and purified by Zymo Concentrator-5 (total capture). cDNA libraries were prepared from 100 ng RNA using the TruSeq Stranded mRNA kit (15031047; Illumina) and sequenced on an Illumina MiSeq with paired-end 150-bp reads. Reads were aligned to the C. phytofermentans ISDg genome (GenBank accession number CP000885.1) using Bowtie 2 (34). RNA-seq mRNA expression was calculated as reads per kilobase per million (RPKM) mapped reads using the Bioconductor “easyRNASeq” package (35) (see Table S2 in the supplemental material). Gene expression measurements from galactose cultures were integrated with RNA-seq measurements from other carbon sources, which we previously performed using the same methods (3).
Targeted gene inactivation.
Transporter genes in the C. phytofermentans genome were inactivated by targeted insertion of group II introns. The Targetron algorithm (TA0100; Sigma-Aldrich) was used to design primers (see Table S3 in the supplemental material) to insert the intron in the antisense orientation relative to gene transcription at the site closest to the gene start. The targeted intron was PCR amplified and inserted between the NdeI and BsrGI sites of pQint (15). Targeted pQint plasmids were transferred into C. phytofermentans by conjugation with Escherichia coli under anaerobic conditions (15, 36, 37). Colonies on GS2 plates containing the 40 μg ml−1 erythromycin were picked, and the genomic intron insertion was confirmed by PCR and sequencing using primers in Table S3. pQint plasmids were cured by five successive transfers at a 1:50 volume into GS2 medium lacking antibiotics. The number of intron insertions in the C. phytofermentans genome was then examined by inverse PCR (38), whereby genomic DNA was extracted from 5-ml cultures using the Sigma GenElute bacterial genomic DNA kit (NA2110), digested with HindIII (R0104; NEB), and ligated with T4 DNA ligase (M0202; NEB). The genomic sequence flanking the intron was amplified by PCR with Q5 polymerase (M0491; NEB) with a 7-min elongation time using outward-facing primers annealing to the intron sequence (Table S3). Genomic sites of intron insertion were determined by sequencing the intron-genome junction regions in the PCR products.
Enzyme purification.
The genes encoding putative sugar kinases and solute-binding proteins (SBPs) were cloned with C-terminal His tags into pET-22B(+) (69744; Novagen) by ligation-independent cloning, as previously described (3) (primers in Table S3). SBP genes were cloned without the N-terminal signal sequence based on the cleavage site predicted by reference 39. Gene sequences were confirmed by sequencing, and plasmids were transformed into E. coli BL21(DE3) (70235; Novagen). Expression was induced by adding 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG) to cultures at an OD600 of 1.0 in 50 ml TB medium supplemented with 0.5 M sorbitol and 5 mM glycine betaine and incubating overnight at 20°C. Cells were pelleted, resuspended in 5 ml of lysis buffer (50 mM phosphate buffer, pH 8, 0.5 M NaCl, 10 mM imidazole, 15% glycerol, 1 mM Pefabloc [76307; Sigma]), and lysed by sonication (Cole-Parmer Vibracell CV33) in the presence of lysozyme (71230; Novagen).
His-tagged, putative kinases were purified from 50-ml cultures on Ni-nitrilotriacetic acid (Ni-NTA) spin columns (31014; Qiagen). To attain sufficient yield for isothermal titration calorimetry, the 5 putative SBP were purified from 1,000 ml cell culture (IPTG induction at an OD600 of 1.9) using an Äkta Pure chromatographic system (GE Healthcare Life Sciences). Cell pellets were lysed using BugBuster 10X (VWR) supplemented with 1 mM dithiothreitol (DTT), 1 mM Pefabloc SC (Sigma-Aldrich), and Lysonase (Novagen). The lysate was incubated for 30 min at room temperature with stirring and centrifuged (20,000 × g, 4°C, 30 min). Lysates were filtered on a 0.22-μm membrane (Millipore), assayed by the Bradford method, and diluted in lysis buffer to 3 mg ml−1. Protein (1,000 mg) was loaded onto a His Trap FF 5-ml column (GE Healthcare Life Sciences) and eluted with 250 mM imidazole, and fractions were collected. The collected fractions were desalted on a HiPrep 26/10 desalting column equilibrated with 25 mM HEPES-KOH buffer, pH 7.0, and 150 mM NaCl. Both the putative kinases and SBPs were quantified by Bradford assay and visualized on a 12% SDS-PAGE gel (see Fig. S2B and S3A in the supplemental material).
Substrate affinities of solute-binding proteins.
Thermodynamics of sugar binding by SBP was measured by isothermal titration calorimetry (ITC) using a MicroCal iTC200 microcalorimeter (Malvern-Microcal). The binding of each protein was measured for 3 potential sugar ligands at different protein concentrations and injection regimes (see Table S4 in the supplemental material). Product numbers for the sugar ligands were the same as those for growth experiments, plus cellotetraose (O-CTE; Megazyme) and cellohexaose (C8286; Megazyme). All experiments were carried out at 25°C in 150 mM NaCl and 25 mM HEPES, pH 7.0. Control runs were performed for all experiments in which buffer was injected instead of sugar to ensure the lack of a binding signal. A theoretical titration curve was fit to the experimental data using Origin software (Malvern-Microcal). The relationship between the heat generated by each injection was used to calculate ΔH (enthalpy change), KD (binding constant), and n (number of binding sites per monomer). The variation in the entropy (ΔS in calories per mole per degree) of each binding reaction was inferred from the variation in the free energy (ΔG), which was calculated based on the following formula: ΔG = −RTln(KA). All experiments were repeated in duplicate.
Activities of purified kinases.
Glucokinase and galactokinase activities were quantified in PMMA cuvettes (Z330388; Sigma-Aldrich) using a SAFAS UVmc1 spectrophotometer at room temperature. Glucokinase activities were calculated based on glucose-6-phosphate formation using a glucose-6-phosphate dehydrogenase assay. Specifically, NADPH formation was measured as absorbance at 340 nm in 50 mM Tris-HCl (pH 7.5) containing glucose concentrations from 2.5 μM to 10 mM, 5 mM MgCl2, 1 mM ATP, 0.25 mM NADP+, 5 U ml−1 glucose-6-phosphate dehydrogenase (G7877; Sigma), and 0.035 μg ml−1 Cphy0329 or 0.0345 mg ml−1 Cphy2237. Galactokinase activities were measured based on galactose-1-phosphate formation using a coupled assay with pyruvate kinase and lactate dehydrogenase. In this assay, NADH oxidation was measured as absorbance at 340 nm in 50 mM Tris-HCl (pH 7.5) containing galactose concentrations from 2.5 μM to 25 mM, 0.3 mM NADH, 5 mM MgCl2, 1 mM ATP, 5 mM phosphoenolpyruvate, 10 U ml−1 pyruvate kinase (P1506; Sigma), 23 U ml−1 lactate dehydrogenase (L2500; Sigma), and 0.085 μg ml−1 Cphy2237 or 0.034 mg ml−1 Cphy0329.
Data availability.
We confirm that all data underlying the findings are fully available without restriction. RNA sequencing files in FASTQ format have been deposited in the European Nucleotide Archive under accession numbers ERP019898 and ERP019903.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. Cruaud and K. Labadie for DNA/RNA sequencing and J. L. Petit for biochemistry advice.
This work was funded by the Genoscope-CEA, the Université d’Évry, and the Agence Nationale de la Recherche grant ANR-16-CE05-0020.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00241-19.
REFERENCES
- 1.Warnick TA, Methé BA, Leschine SB. 2002. Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int J Syst Evol Microbiol 52:1155–1160. doi: 10.1099/00207713-52-4-1155. [DOI] [PubMed] [Google Scholar]
- 2.Tolonen AC, Haas W, Chilaka AC, Aach J, Gygi SP, Church GM. 2011. Proteome-wide systems analysis of a cellulosic biofuel-producing microbe. Mol Syst Biol 7:461. doi: 10.1038/msb.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutard M, Cerisy T, Nogue P-Y, Alberti A, Weissenbach J, Salanoubat M, Tolonen AC. 2014. Functional diversity of carbohydrate-active enzymes enabling a bacterium to ferment plant biomass. PLoS Genet 10:e1004773. doi: 10.1371/journal.pgen.1004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petit E, Coppi M, Hayes J, Tolonen A, Warnick T, Latouf G, Amisano D, Biddle A, Mukherjee S, Ivanova N, Lykidis A, Land M, Hauser L, Kyrpides N, Henrissat B, Lau J, Schnell D, Church G, Leschine S, Blanchard J. 2015. Genome and transcriptome of Clostridium phytofermentans, catalyst for the direct conversion of plant feedstocks to fuels. PLoS One 10:e0118285. doi: 10.1371/journal.pone.0118285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. 2014. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Q, Chen K, Paulsen IT. 2007. TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res 35:D274–D279. doi: 10.1093/nar/gkl925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ecker GF, Stockner T, Chiba P. 2008. Computational models for prediction of interactions with ABC-transporters. Drug Discov Today 13:311–317. doi: 10.1016/j.drudis.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Iancu CV, Zamoon J, Woo SB, Aleshin A, Choe J. 2013. Crystal structure of a glucose/H+ symporter and its mechanism of action. Proc Natl Acad Sci U S A 110:17862–17867. doi: 10.1073/pnas.1311485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Island MD, Wei BY, Kadner RJ. 1992. Structure and function of the uhp genes for the sugar phosphate transport system in Escherichia coli and Salmonella typhimurium. J Bacteriol 174:2754–2762. doi: 10.1128/jb.174.9.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voigt C, Bahl H, Fischer R-J. 2014. Identification of PTS(Fru) as the major fructose uptake system of Clostridium acetobutylicum. Appl Microbiol Biotechnol 98:7161–7172. doi: 10.1007/s00253-014-5809-1. [DOI] [PubMed] [Google Scholar]
- 11.Essalem MEE, Mitchell WJ. 2016. Identification of a glucose-mannose phosphotransferase system in Clostridium beijerinckii. FEMS Microbiol Lett 363:fnw053. doi: 10.1093/femsle/fnw053. [DOI] [PubMed] [Google Scholar]
- 12.Abdou L, Boileau C, de Philip P, Pagès S, Fiérobe H-P, Tardif C. 2008. Transcriptional regulation of the Clostridium cellulolyticum cip-cel operon: a complex mechanism involving a catabolite-responsive element. J Bacteriol 190:1499–1506. doi: 10.1128/JB.01160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nochur SV, Jacobson GR, Roberts MF, Demain AL. 1992. Mode of sugar phosphorylation inClostridium thermocellum. Appl Biochem Biotechnol 33:33–41. doi: 10.1007/BF02922182. [DOI] [Google Scholar]
- 14.Karberg M, Guo H, Zhong J, Coon R, Perutka J, Lambowitz AM. 2001. Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat Biotechnol 19:1162–1167. doi: 10.1038/nbt1201-1162. [DOI] [PubMed] [Google Scholar]
- 15.Tolonen AC, Chilaka AC, Church GM. 2009. Targeted gene inactivation in Clostridium phytofermentans shows that cellulose degradation requires the family 9 hydrolase Cphy3367. Mol Microbiol 74:1300–1313. doi: 10.1111/j.1365-2958.2009.06890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. 2016. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutard M, Ettwiller L, Cerisy T, Alberti A, Labadie K, Salanoubat M, Schildkraut I, Tolonen AC. 2016. Global repositioning of transcription start sites in a plant-fermenting bacterium. Nat Commun 7:13783. doi: 10.1038/ncomms13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees DC, Johnson E, Lewinson O. 2009. ABC transporters: the power to change. Nat Rev Mol Cell Biol 10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard P, Hilditch S. 2009. d-Galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl Microbiol Biotechnol 82:597–604. doi: 10.1007/s00253-009-1870-6. [DOI] [PubMed] [Google Scholar]
- 21.Seo S-O, Janssen H, Magis A, Wang Y, Lu T, Price ND, Jin Y-S, Blaschek HP. 2017. Genomic, transcriptional, and phenotypic analysis of the glucose derepressed Clostridium beijerinckii mutant exhibiting acid crash phenotype. Biotechnol J 12:1700182. doi: 10.1002/biot.201700182. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira MJ, Sá-Nogueira I. 2010. A multitask ATPase serving different ABC-type sugar importers in Bacillus subtilis. J Bacteriol 192:5312–5318. doi: 10.1128/JB.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fosses A, Maté M, Franche N, Liu N, Denis Y, Borne R, de Philip P, Fierobe H-P, Perret S. 2017. A seven-gene cluster in Ruminiclostridium cellulolyticum is essential for signalization, uptake and catabolism of the degradation products of cellulose hydrolysis. Biotechnol Biofuels 10:250. doi: 10.1186/s13068-017-0933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Binns AN. 2016. Involvement of Agrobacterium tumefaciens galacturonate tripartite ATP-independent periplasmic (TRAP) transporter GaaPQM in virulence gene expression. Appl Environ Microbiol 82:1136–1146. doi: 10.1128/AEM.02891-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekjian KR, Bryan EM, Beall BW, Moran CP. 1999. Regulation of hexuronate utilization in Bacillus subtilis. J Bacteriol 181:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell WJ. 2016. Sugar uptake by the solventogenic clostridia. World J Microbiol Biotechnol 32:32. doi: 10.1007/s11274-015-1981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataf Y, Yaron S, Stahl F, Lamed R, Bayer EA, Scheper T-H, Sonenshein AL, Shoham Y. 2009. Cellodextrin and laminaribiose ABC transporters in Clostridium thermocellum. J Bacteriol 191:203–209. doi: 10.1128/JB.01190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravachol J, de Philip P, Borne R, Mansuelle P, Maté MJ, Perret S, Fierobe H-P. 2016. Mechanisms involved in xyloglucan catabolism by the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Sci Rep 6:22770. doi: 10.1038/srep22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poolman B, Doeven MK, Geertsma ER, Biemans-Oldehinkel E, Konings WN, Rees DC. 2005. Functional analysis of detergent-solubilized and membrane-reconstituted ATP-binding cassette transporters. Methods Enzymol 400:429–459. doi: 10.1016/S0076-6879(05)00025-X. [DOI] [PubMed] [Google Scholar]
- 30.Sund CJ, Servinsky MD, Gerlach ES. 2013. Differing roles for Clostridium acetobutylicum’s galactose utilization pathways. AiM 03:490. doi: 10.4236/aim.2013.36065. [DOI] [Google Scholar]
- 31.Tremblay P-L, Zhang T, Dar SA, Leang C, Lovley DR. 2012. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4:e00406-12. doi: 10.1128/mBio.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavedon K, Leschine SB, Canale-Parola E. 1990. Cellulase system of a free-living, mesophilic clostridium (strain C7). J Bacteriol 172:4222–4230. doi: 10.1128/jb.172.8.4222-4230.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerisy T, Souterre T, Torres-Romero I, Boutard M, Dubois I, Patrouix J, Labadie K, Berrabah W, Salanoubat M, Doring V, Tolonen A. 2017. Evolution of a biomass-fermenting bacterium to resist lignin phenolics. Appl Environ Microbiol 83:e00289-17. doi: 10.1128/AEM.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delhomme N, Padioleau I, Furlong EE, Steinmetz LM. 2012. easyRNASeq: a bioconductor package for processing RNA-seq data. Bioinformatics 28:2532–2533. doi: 10.1093/bioinformatics/bts477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolonen AC, Zuroff TR, Ramya M, Boutard M, Cerisy T, Curtis WR. 2015. Physiology, genomics, and pathway engineering of an ethanol-tolerant strain of Clostridium phytofermentans. Appl Environ Microbiol 81:5440–5448. doi: 10.1128/AEM.00619-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolonen AC, Cerisy T, El-Sayyed H, Boutard M, Salanoubat M, Church GM. 2015. Fungal lysis by a soil bacterium fermenting cellulose. Environ Microbiol 17:2618–2627. doi: 10.1111/1462-2920.12495. [DOI] [PubMed] [Google Scholar]
- 38.Ochman H, Gerber AS, Hartl DL. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 40.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer D, Schneider-Fresenius C, Horlacher R, Peist R, Boos W. 1997. Molecular characterization of glucokinase from Escherichia coli K-12. J Bacteriol 179:1298–1306. doi: 10.1128/jb.179.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cáceres AJ, Quiñones W, Gualdrón M, Cordeiro A, Avilán L, Michels PAM, Concepción JL. 2007. Molecular and biochemical characterization of novel glucokinases from Trypanosoma cruzi and Leishmania spp. Mol Biochem Parasitol 156:235–245. doi: 10.1016/j.molbiopara.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Maitra PK. 1970. A glucokinase from Saccharomyces cerevisiae. J Biol Chem 245:2423–2431. [PubMed] [Google Scholar]
- 44.Zeng C, Aleshin AE, Chen G, Honzatko RB, Fromm HJ. 1998. The roles of glycine residues in the ATP binding site of human brain hexokinase. J Biol Chem 273:700–704. doi: 10.1074/jbc.273.2.700. [DOI] [PubMed] [Google Scholar]
- 45.Verhees CH, Koot DGM, Ettema TJG, Dijkema C, de Vos WM, van der Oost J. 2002. Biochemical adaptations of two sugar kinases from the hyperthermophilic archaeon Pyrococcus furiosus. Biochem J 366:121–127. doi: 10.1042/bj20011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schell MA, Wilson DB. 1977. Purification and properties of galactokinase from Saccharomyces cerevisiae. J Biol Chem 252:1162–1166. [PubMed] [Google Scholar]
- 47.Blume KG, Beutler E. 1971. Purification and properties of galactokinase from human red blood cells. J Biol Chem 246:6507–6510. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We confirm that all data underlying the findings are fully available without restriction. RNA sequencing files in FASTQ format have been deposited in the European Nucleotide Archive under accession numbers ERP019898 and ERP019903.