Abstract
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is a multifaceted transduction system that regulates cellular responses to incoming signaling ligands. STAT3 is a central member of the JAK/STAT signaling cascade and has long been recognized for its increased transcriptional activity in cancers and autoimmune disorders but has only recently been in the spotlight for its role in the progression of kidney disease. Although genetic knockout and manipulation studies have demonstrated the salutary benefits of inhibiting STAT3 activity in several kidney disease models, pharmacological inhibition has yet to make it to the clinical forefront. In recent years, significant effort has been aimed at suppressing STAT3 activation for treatment of cancers, which has led to the development of a wide variety of STAT3 inhibitors, but only a handful have been tested in kidney disease models. Here, we review the detrimental role of dysregulated STAT3 activation in a variety of kidney diseases and the current progress in the treatment of kidney diseases with pharmacological inhibition of STAT3 activity.
Keywords: glomerulosclerosis, kidney disease, podocytes, proteinuria, signal transducer and activator of transcription 3
INTRODUCTION
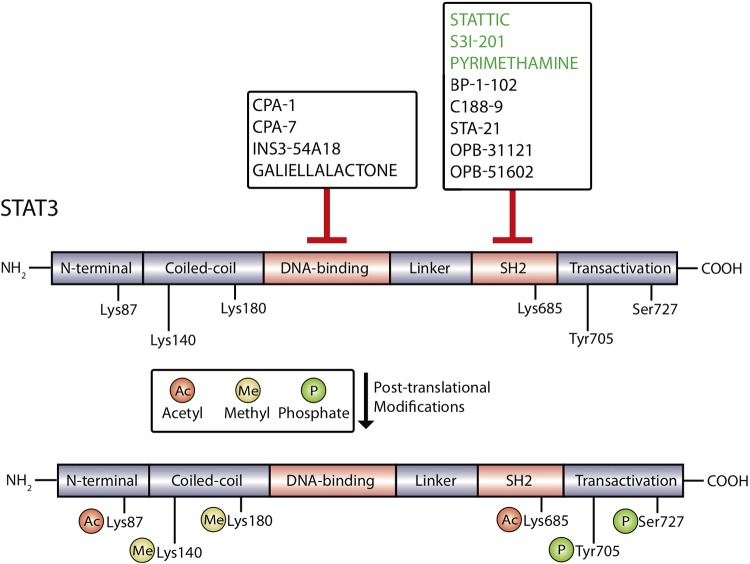
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway consists of four JAK members (JAK1–JAK3 and receptor tyrosine kinase 2) and seven STAT members (STAT1–STAT4, STAT5a, STAT5b, and STAT6). First identified through investigations focused on elucidating interferon-α- and interferon-γ-induced transcriptional pathways, the JAK/STAT signaling pathway regulates a wide variety of cellular processes, including cell growth, proliferation, angiogenesis, and immune responses (19). STAT proteins share several highly conserved domains, including the Src homology 2 (SH2) domain, DNA-binding domain (DBD), linker domain, coiled-coiled domain, and NH2-terminal domains (Fig. 1) (18). Several studies have elucidated specific roles of members of the STAT family in mediating signaling from cytokines, epidermal growth factor (EGF), hepatocyte growth factor, platelet-derived growth factor (PDGF), and colony stimulating factor 1 (47, 61).
Fig. 1.
STAT3 protein domains. The STAT3 protein contains the NH2-terminal domain, coiled-coil domain, DNA-binding domain, linker domain, Src homology 2 (SH2) domain, and transactivation domain. STAT3 protein is subject to posttranslational modifications including methylation at Lys140 and Lys180, acetylation at Lys87 and Lys685, and phosphorylation at Tyr705 and Ser727. The most commonly targeted region of STAT3 protein is the SH2 domain. Inhibitors targeting the SH2 domain that have been tested in kidney disease models are labeled in green. Inhibitors targeting the DNA-binding domain of STAT3 have also been developed but have not been investigated for potential in the treatment of kidney disease.
Of particular interest has been STAT3, which was originally identified through a series of studies on acute response factor signaling (112). Of the members of the Stat family, only Stat3 knockout in mice is embryonically lethal, demonstrating a critical role for this factor in development (83). Similarly, Stat3 knockout was detrimental for maintaining pluripotency of embryonic stem cells (70). Dysregulated STAT3 activation has been shown to play a critical role in a variety of diseased conditions, including cancers and autoimmune disorders (104), but has only recently been investigated for its role in kidney diseases (27, 67).
STAT3 SIGNALING
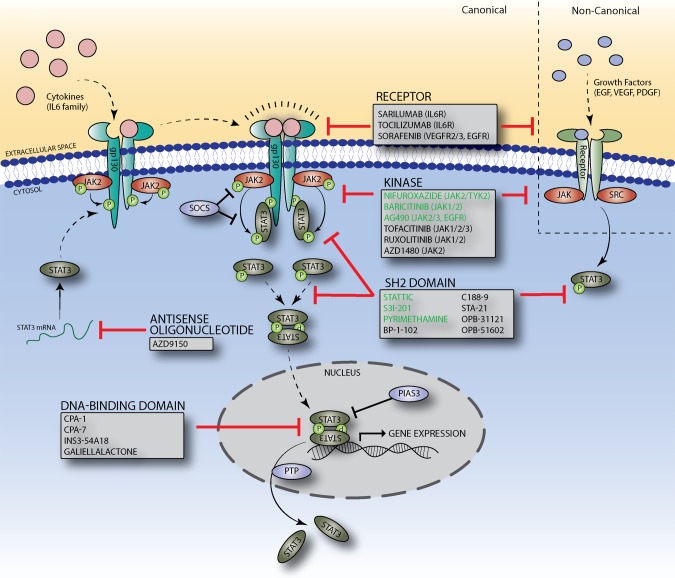
STAT3 signaling can be activated by a multitude of upstream factors, including cytokines, chemokines, and growth factors (31). Canonical STAT3 activation is mediated by JAK proteins in response to cytokine binding (Fig. 2). The most well-known activators of STAT3 are IL-6-type cytokines, which bind to cytokine receptors and induce dimerization of glycoprotein 130 receptors (57). Receptor dimerization allows for phosphorylation and activation of receptor-associated kinases, which phosphorylate tyrosine residues of the cytoplasmic receptor domain. Phosphorylated tyrosine residues serve as docking sites and recruit cytoplasmic STAT3, which, once docked, are phosphorylated at Tyr705 before dissociating from the receptor. Phosphorylated (p)STAT3 (Tyr705) proteins interact to form homodimers, which are translocated to the nucleus, where they bind to specific DNA response elements and regulate target gene expression (18).
Fig. 2.
STAT3 signaling pathway and inhibition approaches. Upon binding of a cytokine or growth factor to its appropriate receptor, JAK kinases are phosphorylated and activated, leading to phosphorylation of an intracellular receptor tyrosine residue. Free STAT3 proteins are recruited to receptor and dock to the phosphotyrosine residue of the receptor, where they are phosphorylated before dissociating from the receptor. Phosphorylated STAT3 proteins dimerize and undergo nuclear translocation, where they regulate target gene expression. Physiological regulation is achieved by suppressor of cytokine signaling (SOCS) and protein inhibitor of activated STAT3 (PIAS3), which block JAK kinase activity and DNA binding, respectively. Protein tyrosine phosphatase (PTPs) catalyze the dissociation of activated STAT3 dimers via dephosphorylation. Targets of pharmacological inhibition of STAT3 signaling at the levels of the receptor, kinase, STAT3 protein, and Stat3 mRNA are shown. Drugs labeled in green have specifically been evaluated in the treatment of kidney disease.
Alternative upstream activators of STAT3 signaling exist, including nonreceptor tyrosine kinases, such as Src and Bcr-Abl, and receptor tyrosine kinases, such as EGF receptor, PDGF receptor, and VEGF receptor 2 (68, 91). Furthermore, research has indicated a possible synergistic and cooperative role under pathological conditions for JAK/STAT3 signaling. Sen et al. (75) showed that sustained inhibition of c-Src-mediated STAT3 signaling leads to a restoration of JAK-STAT binding and JAK activity in human head and neck squamous cell carcinoma.
A prominent mechanism by which endogenous STAT3 signaling is regulated is through the suppressor of cytokine signaling (SOCS) protein family. SOCS becomes rapidly induced after STAT activation by cytokines or growth factors and acts through a negative feedback loop to inhibit STAT activation (45). Specifically, SOCS1 downregulates STAT3 signaling through direct binding to JAK kinases, which blocks enzymatic activity of JAK via an NH2-terminal domain sequence that resembles the JAK activation loop and acts as a pseudo substrate for JAK kinases (25). SOCS3, which contains a functionally equivalent NH2-terminal domain of SOCS1, blocks JAK kinase activity by binding to cytoplasmic domains of receptors instead of JAK kinases directly (60). SOCS1 has also been shown to promote ubiquitination and subsequent degradation of activated JAK2 (42). Furthermore, in vivo studies have shown that mice lacking SOCS3 have prolonged STAT3 activation after IL-6 stimulation (14).
In addition to indirect inhibition through SOCS family proteins, STAT3 signaling can be directly inhibited by protein inhibitor of activated STAT3 (PIAS3). PIAS3 is a nuclear factor that directly interacts with activated STAT3 dimers after cytokine stimulation (13). Binding of PIAS3 inhibits the DNA-binding ability of STAT3, which negatively regulates STAT3 target gene expression. Interestingly, PIAS proteins have small ubiquitin-like modifiers (SUMO) E3 ligase activity and may also suppress target protein activity through SUMOylation, a complex process that involves conjugation of SUMOs to target proteins (74). However, the exact role of PIAS-mediated SUMOylation in regulating JAK/STAT signaling is not fully understood.
Additionally, we have recently identified Krüppel-like factor 4 (KLF4), a zinc finger transcription factor, as a key regulator of STAT3 signaling through negative feedback in podocytes (26). Specifically, podocyte-specific loss of Klf4 leads to glomerular STAT3 activation in mice on the FVB/N background. Coimmunoprecipitation experiments in immortalized human podocytes indicated that KLF4 binds to pSTAT3 (Tyr705), suggesting direct protein-protein interaction as a mechanism of inhibition. Interestingly, we also observed that induction of Klf4 suppresses STAT3 signaling in cultured human podocytes.
Because STAT3 activation is dependent on tyrosine phosphorylation, STAT3 signaling is also regulated through dephosphorylation by protein tyrosine phosphatases (PTPs). At least seven PTPs have been shown to dephosphorylate STAT3 directly, including PTP receptor type D, which is frequently deleted or mutated in human cancers, leading to aberrant STAT3 activation (65). PTP receptor type T and SH2 domain-containing phosphatase 1 have been shown to bind directly to and dephosphorylate STAT3 and JAK2, respectively (41, 110).
In addition to phosphorylation at Tyr705, STAT3 is affected by a wide range of posttranslational modifications (Fig. 1). Phosphorylation has also been observed at Ser727, which has been described as necessary for modulating STAT3 transcriptional activity (96). STAT3 can also be acetylated at two lysine residues, Lys685 and Lys87. In a prostate cancer cell line, histone acetyltransferase p300-mediated acetylation of Lys685 observed after cytokine stimulation was found to be critical for STAT3 dimer stabilization and transcriptional activation of target genes (106). Interestingly, acetylation of Lys87 has been shown to promote mitochondrial translocation of STAT3 and alter cell metabolism (100). Methylation events have also been observed at Lys140 and Lys180, leading to variable effects on STAT3 transcriptional activity in a context-dependent manner (101). Finally, oxidative stress leading to oxidation or glutathionylation of cysteine residues has been shown to diminish STAT3 transcriptional activity in HepG2 cells (97).
Although the exact mechanisms by which upregulated STAT3 signaling contributes to the pathogenesis of several kidney diseases are unknown, current evidence suggests a role for the expression of STAT3 target genes. STAT3 binding site analysis has identified several genes implicated with fibroblast activation and profibrotic signaling pathways, which are potentially regulated by direct STAT3 binding (8). Such genes include lipocalin 2, tissue inhibitor of metalloproteinase 1, and PDGF-B, which have all been investigated for specific roles in kidney fibrosis (11, 85, 90). With the use of chromatin immunoprecipitation and luciferase reporters, STAT3 has been shown to bind to the promoter of and regulate expression of kidney injury molecule-1 (KIM-1), a gene upregulated in a wide variety of acute and chronic kidney disease that has also been linked to the progression of kidney fibrosis (1, 38). Thorough investigation of STAT3 target genes in cancer has confirmed analogous involvement in pathways of inflammation, proliferation, and apoptosis (12). The number of genes responsive to STAT3 signaling is further expanded by the direct binding of STAT3 to a variety of transcription factors, including Nanog, c-Myc, and Twist, which themselves can directly bind to and regulate even more target genes (43, 52, 63). Such mechanisms provide a link between aberrant STAT3 activation and pathogenic fibrosis in progressive kidney disease.
PHARMACOLOGICAL INHIBITION OF STAT3
Pharmacological inhibition of STAT3 signaling can be achieved by direct targeting of STAT3 or upstream signaling elements (Fig. 2). Although increased awareness of the role of STAT3 activation in several cancers has been the primary driver of drug development aimed at synthesizing effective inhibitors of STAT3, their potential therapeutic benefits have extended to other pathophysiological conditions, including rheumatoid arthritis and kidney disease (6, 55).
Inhibitors targeting cytokines and cytokine receptors known to be active in diseases such as a rheumatoid arthritis and cancer have generated significant clinical interest (32). Tocilizumab, an anti-IL-6 receptor (IL-6R) monoclonal antibody, has been clinically approved for the treatment of rheumatoid arthritis and has shown promise (79, 84). Studies in human hepatocellular carcinoma stem cells have demonstrated that tocilizumab can inhibit tumor-macrophage-induced IL-6 signaling with diminished STAT3 activation and tumor growth (93). Furthermore, recent studies have demonstrated a potential role of tocilizumab in desensitization before kidney transplant (92). Sarilumab, another IL-6R monoclonal antibody, has also been approved for the treatment of rheumatoid arthritis (48). Sorafenib, a small-molecule inhibitor that binds to several targets, including VEGF receptor 2, VEGF receptor 3, and PDGF receptor, is approved for the treatment of advanced renal cell carcinoma and has been shown to induce apoptosis in prostate cancer cells, with observed decreases in STAT3 activity (36).
Another indirect approach for STAT3 inhibition has been targeting upstream kinases such as JAK and Src kinases. Tofacitinib is one such promising JAK inhibitor, which targets JAK kinases and is currently being used for the treatment of rheumatoid arthritis while also being investigated for possible use in kidney transplant rejection and psoriasis (4, 9). AG490 is a JAK2 inhibitor that was discovered for its ability to block proliferation in a human B-precursor leukemic cell line (56). Although it was found to reduce JAK2 activation in these cells, increased phosphorylation of Bcr-Abl was observed, suggesting that a compensatory mechanism may be simultaneously activated. Another JAK1/2 inhibitor, ruxolitinib, has been approved for the treatment of myelofibrosis but is associated with adverse effects, including thrombocytopenia and anemia (39, 87). Ruxolitinib has been shown to have anticancer effects in cisplatin-resistant non-small cell lung cancer and pancreatic cancer cell lines by inducing apoptosis and blocking STAT3 activation (35, 39). A randomized phase II clinical trial examining the effects of JAK1/2 inhibitor baricitinib in the progression of diabetic kidney disease (DKD) was recently completed (NCT01683409). Patients with type 2 diabetes and DKD treated with baricitinib demonstrated a reduction in albuminuria, but the exact effect on DKD progression remains to be investigated (89).
Despite promising results for some upstream inhibitors, other JAK inhibitors, such as AZD1480, have been discontinued after clinical testing because of unusual toxicity profiles and lack of clinical activity (69). Such occurrences may illuminate the risks associated with inhibition of pleiotropic JAK/STAT signaling and the need for precise downstream targets. Furthermore, some effective kinase inhibitors fail to show successful suppression of downstream STAT targets, likely because of compensatory mechanisms, which result from wide range of upstream kinases that can lead to STAT3 activation (55). This has been observed in lung cancer cell lines, which when treated with dasatinib, a potent Src family kinase inhibitor, failed to show consistent inhibition on STAT3 activation (81). Similar results have been reported in the case of EGF receptor inhibition (81). Consequently, efforts are underway for the development of inhibitors targeting STAT3 directly.
Of all the domains of STAT3, the SH2 domain has been targeted most frequently for inhibition (Fig. 1) (6). The SH2 domain mediates recruitment to JAK kinase, which allows for phosphorylation at Tyr705 and is responsible for binding to phosphorylated Tyr705 residues of other pSTAT3 molecules, leading to homodimerization and subsequent DNA binding (49). Some of the first small molecules designed to target the SH2 domain of STAT3 include Stattic, STA-21, and S3I-201, which have demonstrated success in blocking STAT3 phosphorylation (Tyr705), dimerization, and DNA-binding activity (73, 78, 80). Newer generations of these compounds have been modified to increase potency, such as BP-1-102 (a modified analog of S3I-201) and LLL12 (a modified analog of STA-21) (28, 111). BP-1-102 has been shown to inhibit STAT3 activity with high potency and exert antitumor effects in human breast and lung cancer xenografts (111). C188-9 is a compound that was identified in silico using a virtual screen of over 900,000 compounds by docking into the binding pockets of the SH2 domain of STAT3 and has been shown to induce apoptosis effectively in acute myeloid leukemia cell lines (99). This compound is currently in a phase I clinical trial for the treatment of advanced cancers (NCT03195699) but has not yet been tested in the treatment of kidney disease. OPB-31121 and OPB-51602, two SH2 domain-targeting STAT3 inhibitors, demonstrated potent inhibition of STAT3 in preclinical studies and reached phase II clinical trials before being terminated because of poor pharmacokinetic profiles and intolerability, further reinforcing the need for the continued development of more clinically efficacious compounds (62, 64)
In addition to the SH2 domain, efforts are underway to target the DBD of STAT3. In theory, targeting the protein region of STAT3 responsible for binding target DNA sequences seems to be a clear approach to blocking STAT3 transcriptional activity, but the DBD is structurally flat, making it extremely difficult to design small molecules capable of binding with sufficient specificity and potency (108). Despite the associated challenges of “drugging” the DBD, extensive structure and activity-guided hit optimization has identified InS354-A18, a compound that can specifically bind to the DBD of STAT3 and block STAT3 DNA-binding ability (37). In vivo studies have demonstrated that InS354-A18 can effectively block lung xenograft tumor growth, but testing in other disease models has not been conducted (37). Platinum-based compounds have also shown promise in targeting the DBD of STAT3 and include CPA-1, CPA-7, and platinum (IV) tetrachloride, which have all shown potent inhibition of STAT3 DNA-binding activity (88). Although treatment with CPA-7 has been shown to block growth of mouse CT26 colon tumors, experiments have indicated variable off-target effects on STAT1 and STAT5 (3). Natural products, such as galiellalactone, have also been identified as effective inhibitors of STAT3 transcriptional activity without effecting STAT3 Tyr705 or Ser727 phosphorylation (95). Interestingly, experiments have shown that galiellalactone covalently modifies multiple cysteine residues within the STAT3 linker domain and DBD, compromising STAT3 DNA-binding ability (21). Despite the increasing potential for STAT3 inhibitors targeting the DBD, therapeutic uses for kidney disease have yet to be investigated.
Another avenue of STAT3 inhibition is based on antisense oligonucleotides (ASOs), which bind Stat3 mRNA and act to silence gene expression through blocking translation or recruiting RNase H enzymes, which degrade the DNA-RNA heteroduplex (15). Next-generation ASOs have been designed to target Stat3, including AZD9150, which is a generation 2.5 ASO that has shown promise in selectively suppressing STAT3 expression and tumor growth in tumor xenografts and primary human tumor explants (34). Recent studies have also demonstrated that AZD9150 can potently induce apoptosis in leukemic cell lines and impair leukemic growth in mouse models (77). A phase I clinical trial investigating treatment of AZD9150 in patients with non-Hodgkin’s lymphoma has indicated that AZD9150 is well tolerated and shows clinical efficacy in patients (clinical trial no. NCT01563302). Current advances in ASO technology suggest potential for clinical use in kidney disease.
ROLE OF STAT3 IN KIDNEY DISEASE
DKD
In humans, DKD is characterized by excessive accumulation of the extracellular matrix, mesangial expansion, and glomerular hypertrophy, eventually leading to glomerulosclerosis and tubulointerstitial fibrosis. Although current availability of murine models representative of human DKD are limited, the extent of kidney injury observed in these diabetic models is highly dependent on the specificity of mouse strain (10). Interestingly, recent cross-species transcriptional network analysis has revealed that JAK/STAT signaling is consistently upregulated in isolated glomeruli of patients with type 2 DKD and three independent murine models of DKD (streptozotocin DBA/2, C57BLKS db/db, and endothelial nitric oxide synthase-deficient C57BLKS db/db mice) (7, 33). JAK/STAT activation has been reported in rat glomerular cells exposed to high glucose and is required for high glucose-induced transforming growth factor-β and fibronectin synthesis (5). Furthermore, angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists, which slow the progression of DKD, also block JAK/STAT signaling in isolated glomeruli of diabetic rats (94).
To investigate specifically the role of STAT3 in DKD, Lu et al. (53) used Stat3 mutant mice, which lack full STAT3, in a murine model of type I diabetes mellitus (DM). Specifically, these mice carry homozygous serine to alanine mutations at residue 727, which prevents phosphorylation of STAT3 and its downstream transcriptional activity. In the setting of low-dose streptozotocin treatment to induce type I DM, these Stat3 mutant mice exhibited significantly reduced STAT3 activity with a reduction in albuminuria, mesangial expansion, and collagen deposition compared with wild-type mice. Additionally, expression of inflammatory markers IL-6 and monocyte chemoattractant protein-1 were markedly reduced in STZ-treated Stat3 mutant mice compared with STAT-treated wild-type mice. Subsequent studies used alternative approaches to inhibit STAT3 signaling in DKD by modulating STAT3 activity indirectly. Rather than targeting STAT3 directly, Ortiz-Muñoz et al. (66) used intrarenal delivery of adenovirus expressing SOCS1 or SOCS3 to diabetic rats. As a negative regulator of JAK/STAT signaling, SOCS1 or SOCS3 expression suppressed STAT3 activation and reduced renal damage. Interstitial fibrosis, macrophage infiltration, proteinuria, and downstream STAT3 target genes were all reduced in rats given SOCS1- or SOCS3-expressing adenovirus (66). However, increased expression of SOCS1 or SOCS3 also reduced JAK2 and STAT1 phosphorylation, so the specific contributions of reduced STAT3 activation cannot be determined.
Other work has focused on the role of upstream activators of STAT3, including JAK2. Following the observation that JAK2 is upregulated in glomerular podocytes of patients in the early stages of DKD, Zhang et al. (107) overexpressed JAK2 in podocytes to see if it could by itself exacerbate DKD. Interestingly, the level of glomerular JAK2 induction attained in these mice (two- to threefold increase) was comparable to increases observed in patients with DKD and led to significant increases in albuminuria, mesangial expansion, and glomerulosclerosis compared with wild-type mice under diabetic conditions. Mechanistically, the authors attribute the worsened injury in part to increased STAT3 activation, which was further increased in glomeruli of diabetic mice overexpressing JAK2 compared with diabetic controls (107).
In addition to activation of STAT3 by phosphorylation at Tyr705, an emerging role for acetylation is now under interrogation. Recent work has focused on investigating the role of advanced glycation end products (AGEs) in the progression of DKD, affecting STAT3 transcriptional activity indirectly through posttranslational modifications of STAT3 (105). In human podocytes, it was found that introduction of STAT3 mutants, targeting the acetylation domain, abolished AGE-induced expression of STAT3 target genes (51). In vivo experiments aimed at modulating STAT3 acetylation confirmed that STAT3 acetylation and activity correlates with extent of renal injury (51).
Nifuroxazide is a drug classically used as an antidiarrheal agent but was identified as an effective inhibitor of STAT3 activation in a STAT3-dependent luciferase reporter cell line (59). Using nifuroxazide, Said et al. (71) demonstrated that pharmacological inhibition of STAT3 in a rat model of DKD was protective against kidney injury compared with diabetic controls. Treatment with nifuroxazide reduced albuminuria, serum creatinine, and inflammation. However, it is worth noting that nifuroxazide has been shown to target other JAK/STAT members, including JAK2, STAT1, and STAT5, in addition to STAT3 (59). Additionally, treatment with nifuroxazide or vehicle was initiated 72 h after streptozotocin injection. However, treatment starting after disease establishment may be more relevant for the study of human DKD. Furthermore, studies using a genetic model or specific STAT3 inhibitor such as S3I-201 or Stattic may indicate a clearer role for STAT3 inhibition in DKD treatment.
Focal Segmental Glomerulosclerosis
Subtypes of focal segmental glomerulosclerosis (FSGS), cellular and collapsing, have been previously shown to exhibit increased STAT3 activity (86). Human immunodeficiency virus (HIV)-associated nephropathy (HIVAN) is characterized by collapsing FSGS, tubulointerstitial nephritis, and podocyte dedifferentiation and proliferation (16). It has been shown that induction of the HIV-1 accessory protein Nef specifically in the podocyte is sufficient to induce dedifferentiation and cell cycle reentry (40). Increased pSTAT3 (Tyr705) has been observed in biopsies of patients with HIVAN and kidneys of mice expressing the HIV-1 transgene, suggesting a role of STAT3 activation in HIVAN (30). Indeed, Nef-induced podocyte dedifferentiation and proliferation in immortalized podocytes is mediated by activation of Src-STAT3 and Src-MAPK1,2 pathways (30). The role of STAT3 as the critical driver of glomerular injury in HIVAN was shown using genetic knockin and deletion techniques in HIV-1 transgenic (Tg26) mice, a model of HIVAN (44). Previously, Feng et al. (27) showed that a serine to alanine manipulation at residue 727 in STAT3 was sufficient for attenuation of HIV-1-induced renal injury in Tg26 mice. Specifically, prevention of phosphorylation at Ser727, which is required for maximal activation of STAT3, resulted in reduced albuminuria and decreased glomerulosclerosis in these Tg26 mice. Furthermore, podocyte-specific deletion of Stat3 in Tg26 mice significantly attenuates the development of HIVAN, as shown by improvement of glomerular and tubulointerstitial injury with concurrent restoration of podocyte differentiation markers (29). Together, these studies demonstrated that inhibition of STAT3 activity attenuates the progression of glomerular and tubulointerstitial injury observed in HIVAN. However, the use of pharmacological agents to target STAT3 in treatment of HIVAN remains to be investigated.
We have recently shown that podocyte-specific deletion of zinc finger transcription factor Klf4 in mice leads to aberrant glomerular epithelial cell proliferation and glomerulosclerosis, with eventual FSGS in mice on the FVB/N background (26). In addition to reduced survival and proteinuria, glomerular STAT3 activation was observed in mice with podocyte-specific Klf4 knockdown. Interestingly, we observed that treatment with S3I-201, a STAT3 inhibitor at the SH2 domain, reduced albuminuria compared with vehicle-treated mice, further supporting the potential of STAT3 inhibition in proliferative glomerulopathies (Table 1) (26).
Table 1.
STAT3-specific inhibitors in models of kidney disease
| Inhibitor (Type and Animal Model | Dose, Route, and Frequency | Effects | Reference |
|---|---|---|---|
| S3I-201 (small molecule) | |||
| Interstitial fibrosis (UUO in C57BL/6 mice) | 10 mg/kg ip daily for 6 or 13 days | Reduced interstitial fibrosis and inflammation | 67 |
| Interstitial fibrosis (UUO in Sprague-Dawley rats) | 10 mg/kg ip daily for 14 days | Reduced interstitial fibrosis | 54 |
| Focal segmental glomerulosclerosis (podocin-Cre Klf4fl/fl FVB/N mice) | 10 mg/kg ip 3 times/wk for 3 wk | Reduced proteinuria | 26 |
| Lupus nephritis (MRL/lpr mice) | 10 mg/kg ip daily for 14 days | Reduced proteinuria and fibronectin expression | 22 |
| Autosomal dominant polycystic kidney disease (Mx1-Cre Pkd1fl/fl mice) | 10 mg/kg ip 5 consecutive days/wk for 5 wk | Reduced cyst formation and improved renal function | 82 |
| Stattic (small molecule) | |||
| Lupus nephritis (MRL/lpr mice) | 10 mg/kg ip 3 times/wk for 2 wk | Delayed onset of proteinuria | 24 |
| Alport syndrome (Col4a5tm1Yseg G5X mutant, C57BL/6 mice) | 10 mg/kg ip 3 times/wk for 10 wk | Reduced interstitial fibrosis and improved renal function | 103 |
The STAT3 target was the Src homology 2 domain. UUO, unilateral ureteric obstruction.
Nephrotoxic Serum-Induced Nephritis
Immunostaining studies in kidney biopsy specimens have demonstrated that STAT3 signaling is markedly upregulated in the kidneys of patients with rapidly progressive glomerulonephritis, which is marked by podocyte injury and extracapillary glomerular epithelial cell proliferation (2). Nephrotoxic serum-induced nephritis is widely used as a murine model of rapidly progressive glomerulonephritis, which is characterized by glomerular epithelial cell proliferation and accumulation in the Bowman’s space. A recent study by Dai et al. (17) showed that podocyte-specific deletion of Stat3 abrogated kidney injury subsequent to nephrotoxic serum treatment. In addition to reduced glomerular epithelial cell proliferation and crescent formation, mice with podocyte-specific Stat3 deletion showed reduced proteinuria and restored expression of podocyte markers. The marked improvement in renal function with podocyte-specific Stat3 knockdown suggests a potential role for pharmacological STAT3 inhibition in rapidly progressive glomerulonephritis.
Lupus Nephritis
Systemic lupus erythematosus is an autoimmune disorder that often has direct renal manifestations (76). The pathologic changes observed in the kidney include proliferation and inflammation, affecting both glomerular and tubulointerstitial compartments. Previous studies have shown that treatment of lupus-prone (MRL/lpr) mice with Stattic, a small-molecule inhibitor of STAT3, delayed the onset of proteinuria and reduced glomerular IgG deposition and injury (Table 1) (24). Observed reductions in lymphocyte accumulation in the lymph and spleen suggest that STAT3 inhibition in the context of lupus nephritis may carry extrarenal benefits. A subsequent study (22) found similar results, in which treatment with S3I-201 in the same mouse model attenuated proteinuria and decreased interstitial fibrosis.
Alport Syndrome
Alport syndrome is a hereditary disease affecting collagen type IV, which leads to an altered composition of the glomerular basement membrane and consequential podocyte loss because of detachment (20). Glomerular dysfunction and resulting albuminuria further stimulates proximal tubule damage and eventual fibrosis, which progresses to renal failure (72). Yokota et al. (103) investigated STAT3 activation in Alport syndrome and found increased pSTAT3 (Tyr705) in both isolated glomeruli and the renal cortex of a mouse model of Alport syndrome, with accompanying increases in proinflammatory STAT3 target genes (Table 1). They tested whether or not STAT3 inhibition could ameliorate renal dysfunction in a mouse model of Alport syndrome. Treatment with Stattic improved renal function and reduced glomerular injury, proinflammatory cytokine expression, and interstitial fibrosis (Table 1).
Tubulointerstitial Fibrosis
Although small-molecule inhibitors of STAT3 have been shown to attenuate interstitial fibrosis in murine models, the role of STAT3 in specific cell types remains unclear because of the wide range of effected cell types in these models. To better understand the role of tubular STAT3 in a model of chronic kidney disease, Bienaimé et al. (8) selectively deleted Stat3 in tubular cells using the Cre-LoxP system. After 75% nephrectomy, mice with tubular Stat3 deletion showed significantly reduced interstitial fibrosis and α-smooth muscle actin (α-SMA) expression compared with controls. Using cultured mouse inner medulla collecting duct cells treated with oncostatin M, an upstream activator of STAT3, they found that STAT3 activation induces expression of genes with known roles in promoting extracellular matrix accumulation, including tissue inhibitor of metalloproteinase 1, PDBG-B, and lipocalin 2 (8). Mechanistically, the authors suggest that activated STAT3 may promote interstitial fibrosis via increased production of profibrotic paracrine factors acting on neighboring interstitial cells.
STAT3 activation has also been previously reported in other murine models of kidney fibrosis, such as unilateral ureteric obstruction (UUO) (46). Using a rat model, Kuratsune et al. (46) found that pSTAT3 (Tyr705) was elevated as early as 3 days after UUO in both tubular epithelial and interstitial cells of the obstructed kidney. A subsequent study conducted by Pang et al. (67) demonstrated the role of increased STAT3 activity as a mediator of UUO-induced interstitial fibrosis. Interestingly, they showed that mice treated with S3I-201 after UUO displayed significant improvements in interstitial fibrosis as indicated by decreased α-SMA, collagen type 1, and fibronectin expression (Table 1). Additionally, S3I-201 treatment attenuated leukocyte infiltration and proinflammatory cytokine expression (TNF-α, IL-1β, and ICAM-1) (67). These findings were corroborated by Zhang et al. (109), who demonstrated that mice treated with low-dose paclitaxel (0.3 mg/kg) after UUO exhibited a decrease in pSTAT3 (Tyr705) expression and reduced interstitial fibrosis compared with saline-treated controls. Other studies also examined interstitial fibrosis in mice treated with pifithrin-a or suramin after UUO (50, 102). Treatment with pifithrin-a, classically known as an inhibitor of p53, improved interstitial fibrosis and suppressed expression of fibronectin, collagen type I, and α-SMA in UUO mice (102). Although decreased pSTAT3 (Tyr705) was observed in human renal tubular epithelial cells after treatment with pifithrin-a, pifithrin-a is not an inhibitor specific to STAT3, so it cannot be discerned what effects are directly because of STAT3 inhibition. A single dose of suramin, a nonspecific inhibitor of several cytokine and growth factor receptor pathways, after UUO attenuated interstitial fibrosis and leukocyte infiltration, with a marked reduction in STAT3 activation (50). Together, these studies support the potential therapeutic role for pharmacological STAT3 inhibition in kidney fibrosis.
Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease is defined by severe epithelial cell proliferation and development of fluid-filled cysts, leading to loss of kidney function. Following the observation that STAT3 expression is induced in cysts of both human patients and mice with conditional polycystin-1 (Pkd1) knockout, a polycystic kidney model, Takakura et al. (82) investigated the therapeutic potential of STAT3 inhibition in polycystic kidney disease. They identified pyrimethamine, an antiparasitic compound, as an inhibitor of STAT3 activity and demonstrated that treatment with pyrimethamine blocked renal cyst formation in a cystic mouse model (Table 1). It is believed that pyrimethamine targets STAT3 specifically because inhibition of STAT3 activity is evident in the absence of effects on JAK2 and inhibition of STAT1 (82). They also showed that treatment with S3I-201, a STAT3 specific inhibitor, showed similar effects, including reduced cyst formation, reduced cyst volume, and improved serum creatinine (Table 1) (82). Although these findings highlight the potential salutary benefits of STAT3 inhibition in autosomal dominant polycystic kidney disease, additional studies are required to determine whether the benefits are targeted at a general improvement in tubulointerstitial injury or targeted at cyst development.
Acute Kidney Injury
Compared with previously described kidney injury models, the role of STAT3 signaling in acute kidney injury (AKI) models appears to be spatially and temporally dependent in the kidney; hence, the therapeutic benefit of STAT3 inhibition in AKI is not readily apparent. For instance, C57BL/6 mice harboring a genetic deletion of Stat3 restricted to the endothelium demonstrate increased serum creatinine compared with control mice after ischemia-reperfusion injury (IRI) (23). Furthermore, STAT3 activation in renal proximal tubular epithelial cells might have a protective role after IRI (98). These experiments demonstrate that overexpression of IL-22 via transgenic or adenovirus-based methods is protective against IRI. Mechanistically, the observed protection is attributed to modulation of apoptotic regulators, including Bcl-2, an antiapoptotic STAT3 target, upregulated in IL-22-overexpressing mice, which showed preserved renal function after IRI. Other studies have elucidated competing roles of IL-6 signaling in HgCl2-induced AKI, in which IL-6 promotes an injurious inflammatory response that acts in parallel to protective IL-6 signaling (58). C57BL/6 mice with IL-6 deletion are significantly protected from HgCl2-induced injury. These mice show increased survival, reduced renal neutrophil accumulation, and decreased serum urea nitrogen compared with controls, suggesting a role of IL-6 in promoting the inflammatory response that contributes to HgCl2-induced AKI. It was also found that injection of an IL-6/soluble IL-6R fusion protein before HgCl2 treatment also protects against renal injury and increases survival, whereas pretreatment with exogenous IL-6 alone does not recapitulate the same effects. Interestingly, treatment of the IL-6/soluble IL-6R fusion protein led to activated STAT3 signaling in tubular epithelial cells; however, pretreatment of IL-6 alone did not lead to similar STAT3 activation. In this context, activated STAT3 signaling in tubular epithelial cells before HgCl2-induced AKI seems to play a protective role. Studies have also investigated the role of STAT3 in aristolochic acid nephropathy, characterized by tubular epithelial cell apoptosis (113). Treatment of cultured rat tubular epithelial cells with aristolochic acid induced dephosphorylation of STAT3 in a time- and dose-dependent manner. Furthermore, overexpression of STAT3 in cultured tubular epithelial cells improved cell survival and reduced apoptosis, but corresponding in vivo studies have yet to be conducted. Together, these reports emphasize that early activation of STAT3 signaling might have a protective effect in the early phase of AKI, but studies involving the role of persistent STAT3 activation in AKI remains to be investigated.
CONCLUSIONS
Despite the current progress in developing potent inhibitors of STAT3 activity, more effort is needed to promote the identification of novel compounds that are specific and effective at inhibiting dysregulated STAT3 activity while minimizing its systemic toxicity. Although a vast amount of STAT3 inhibitors are being developed for applications in cancer and autoimmune disorders, it is essential to investigate the potential salutary benefits of these novel compounds in kidney disease. For instance, the JAK1/2 inhibitor baricitinib has reached clinical trials for the treatment of DKD, indicating that JAK/STAT signaling is emerging as a target in kidney disease; however, agents targeting STAT3 specifically in kidney disease have yet to make it to clinical use. Existing studies suggest a therapeutic potential for STAT3 inhibition in the treatment of various kidney disease models, but studies are limited in terms of the STAT3 inhibitors used and kidney disease models tested. Future studies should include assessments of different types of STAT3 inhibitors, including DBD-targeting and ASO-based approaches, which have shown promise in other models.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-102519 and DK-112984 and the Dialysis Clinic (to S. Mallipattu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P. and S.K.M. conceived and designed research; J.P. and S.K.M. analyzed data; J.P. and S.K.M. interpreted results of experiments; J.P., B.D., and S.K.M. prepared figures; J.P., P.P., B.D., J.C.H., and S.K.M. drafted manuscript; J.P., P.P., B.D., J.C.H., and S.K.M. edited and revised manuscript; J.P., P.P., B.D., J.C.H., and S.K.M. approved final version of manuscript; S.K.M. performed experiments.
REFERENCES
- 1.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS. A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol 25: 105–118, 2014. doi: 10.1681/ASN.2013020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa T, Masaki T, Hirai T, Doi S, Kuratsune M, Arihiro K, Kohno N, Yorioka N. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant 23: 3418–3426, 2008. doi: 10.1093/ndt/gfn314. [DOI] [PubMed] [Google Scholar]
- 3.Assi HH, Paran C, VanderVeen N, Savakus J, Doherty R, Petruzzella E, Hoeschele JD, Appelman H, Raptis L, Mikkelsen T, Lowenstein PR, Castro MG. Preclinical characterization of signal transducer and activator of transcription 3 small molecule inhibitors for primary and metastatic brain cancer therapy. J Pharmacol Exp Ther 349: 458–469, 2014. doi: 10.1124/jpet.114.214619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azevedo A, Torres T. Tofacitinib: a new oral therapy for psoriasis. Clin Drug Investig 38: 101–112, 2018. doi: 10.1007/s40261-017-0596-y. [DOI] [PubMed] [Google Scholar]
- 5.Banes AK, Shaw S, Jenkins J, Redd H, Amiri F, Pollock DM, Marrero MB. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol 286: F653–F659, 2004. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 6.Beebe JD, Liu JY, Zhang JT. Two decades of research in discovery of anticancer drugs targeting STAT3, how close are we? Pharmacol Ther 191: 74–91, 2018. doi: 10.1016/j.pharmthera.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C, Argetsinger LS, Rastaldi MP, Brosius FC, Kretzler M. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienaimé F, Muorah M, Yammine L, Burtin M, Nguyen C, Baron W, Garbay S, Viau A, Broueilh M, Blanc T, Peters D, Poli V, Anglicheau D, Friedlander G, Pontoglio M, Gallazzini M, Terzi F. Stat3 controls tubulointerstitial communication during CKD. J Am Soc Nephrol 27: 3690–3705, 2016. doi: 10.1681/ASN.2015091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, Shurmur R, Singhal AK, Wei N, Rosengren S, Kaplan I, Krishnaswami S, Luo Z, Bradley J, Firestein GS. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis 74: 1311–1316, 2015. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosius FC III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium . Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai G, Zhang X, Hong Q, Shao F, Shang X, Fu B, Feng Z, Lin H, Wang J, Shi S, Yin Z, Chen X. Tissue inhibitor of metalloproteinase-1 exacerbated renal interstitial fibrosis through enhancing inflammation. Nephrol Dial Transplant 23: 1861–1875, 2008. doi: 10.1093/ndt/gfm666. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel) 6: 897–925, 2014. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science 278: 1803–1805, 1997. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 14.Croker BA, Krebs DL, Zhang J-G, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4: 540–545, 2003. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 15.Crooke ST. Molecular mechanisms of antisense oligonucleotides. Nucleic Acid Ther 27: 70–77, 2017. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agati V, Appel GB. HIV infection and the kidney. J Am Soc Nephrol 8: 138–152, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y, Gu L, Yuan W, Yu Q, Ni Z, Ross MJ, Kaufman L, Xiong H, Salant DJ, He JC, Chuang PY. Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int 84: 950–961, 2013. doi: 10.1038/ki.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darnell JE., JR STATs and gene regulation. Science 277: 1630–1635, 1997. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JE JR, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415–1421, 1994. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 20.Ding F, Wickman L, Wang SQ, Zhang Y, Wang F, Afshinnia F, Hodgin J, Ding J, Wiggins RC. Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport Syndrome. Kidney Int 92: 1515–1525, 2017. doi: 10.1016/j.kint.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Don-Doncow N, Escobar Z, Johansson M, Kjellström S, Garcia V, Munoz E, Sterner O, Bjartell A, Hellsten R. Galiellalactone is a direct inhibitor of the transcription factor STAT3 in prostate cancer cells. J Biol Chem 289: 15969–15978, 2014. doi: 10.1074/jbc.M114.564252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Y, Zhang W, Liu S, Feng X, Gao F, Liu Q. S3I-201 ameliorates tubulointerstitial lesion of the kidneys in MRL/lpr mice. Biochem Biophys Res Commun 503: 177–180, 2018. doi: 10.1016/j.bbrc.2018.05.207. [DOI] [PubMed] [Google Scholar]
- 23.Dube S, Matam T, Yen J, Mang HE, Dagher PC, Hato T, Sutton TA. Endothelial STAT3 modulates protective mechanisms in a mouse ischemia-reperfusion model of acute kidney injury. J Immunol Res 2017: 1–9, 2017. doi: 10.1155/2017/4609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards LJ, Mizui M, Kyttaris V. Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice. Clin Immunol 158: 221–230, 2015. doi: 10.1016/j.clim.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387: 921–924, 1997. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 26.Estrada CC, Paladugu P, Guo Y, Pace J, Revelo MP, Salant DJ, Shankland SJ, D’Agati VD, Mehrotra A, Cardona S, Bialkowska AB, Yang VW, He JC, Mallipattu SK. Krüppel-like factor 4 is a negative regulator of STAT3-induced glomerular epithelial cell proliferation. JCI Insight 3: e98214, 2018. doi: 10.1172/jci.insight.98214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X, Lu TC, Chuang PY, Fang W, Ratnam K, Xiong H, Ouyang X, Shen Y, Levy DE, Hyink D, Klotman M, D’Agati V, Iyengar R, Klotman PE, He JC. Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009. doi: 10.1681/ASN.2008080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuh B, Sobo M, Cen L, Josiah D, Hutzen B, Cisek K, Bhasin D, Regan N, Lin L, Chan C, Caldas H, DeAngelis S, Li C, Li PK, Lin J. LLL-3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. Br J Cancer 100: 106–112, 2009. doi: 10.1038/sj.bjc.6604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu L, Dai Y, Xu J, Mallipattu S, Kaufman L, Klotman PE, He JC, Chuang PY. Deletion of podocyte STAT3 mitigates the entire spectrum of HIV-1-associated nephropathy. AIDS 27: 1091–1098, 2013. doi: 10.1097/QAD.0b013e32835f1ea1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He JC, Husain M, Sunamoto M, D’Agati VD, Klotman ME, Iyengar R, Klotman PE. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest 114: 643–651, 2004. doi: 10.1172/JCI200421004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334: 297–314, 1998. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 4: 767–775, 2008. doi: 10.2147/TCRM.S3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgin JB, Nair V, Zhang H, Randolph A, Harris RC, Nelson RG, Weil EJ, Cavalcoli JD, Patel JM, Brosius FC III, Kretzler M. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 62: 299–308, 2013. doi: 10.2337/db11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M, Lee SJ, Yamashita M, Hughes SG, Fayad L, Piha-Paul S, Nadella MV, Mohseni M, Lawson D, Reimer C, Blakey DC, Xiao X, Hsu J, Revenko A, Monia BP, MacLeod AR. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med 7: 314ra185, 2015. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Hong Y, Xu Y, Liu P, Guo D-H, Chen Y. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC. Apoptosis 19: 1627–1636, 2014. doi: 10.1007/s10495-014-1030-z. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther 9: 742–750, 2010. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, Dong Z, Chen Y, Wang F, Wang CJ, Peng H, He Y, Hangoc G, Pollok K, Sandusky G, Fu XY, Broxmeyer HE, Zhang ZY, Liu JY, Zhang JT. Small-molecule inhibitors targeting the DNA-binding domain of STAT3 suppress tumor growth, metastasis and STAT3 target gene expression in vivo. Oncogene 35: 783–792, 2016. [Erratum in Oncogene 35: 802, 2016.] doi: 10.1038/onc.2015.215. [DOI] [PubMed] [Google Scholar]
- 38.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035, 2013. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM III, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 33: 4039–4047, 2015. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husain M, D’Agati VD, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS 19: 1975–1980, 2005. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- 41.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias LC, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol 16: 6985–6992, 1996. doi: 10.1128/MCB.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, Hattori K, Hatakeyama S, Yada M, Morita S, Kitamura T, Kato H, Nakayama K, Yoshimura A. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem 276: 12530–12538, 2001. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 43.Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med 189: 63–73, 1999. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA 89: 1577–1581, 1992. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci 113: 2813–2819, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Kuratsune M, Masaki T, Hirai T, Kiribayashi K, Yokoyama Y, Arakawa T, Yorioka N, Kohno N. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology (Carlton) 12: 565–571, 2007. doi: 10.1111/j.1440-1797.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 47.Leaman DW, Pisharody S, Flickinger TW, Commane MA, Schlessinger J, Kerr IM, Levy DE, Stark GR. Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol Cell Biol 16: 369–375, 1996. doi: 10.1128/MCB.16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee EB. A review of sarilumab for the treatment of rheumatoid arthritis. Immunotherapy 10: 57–65, 2018. doi: 10.2217/imt-2017-0075. [DOI] [PubMed] [Google Scholar]
- 49.Lim CP, Cao X. Regulation of Stat3 activation by MEK kinase 1. J Biol Chem 276: 21004–21011, 2001. doi: 10.1074/jbc.M007592200. [DOI] [PubMed] [Google Scholar]
- 50.Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 22: 1064–1075, 2011. doi: 10.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM, Chuang PY, He JC. Role of transcription factor acetylation in diabetic kidney disease. Diabetes 63: 2440–2453, 2014. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67: 9066–9076, 2007. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu TC, Wang ZH, Feng X, Chuang PY, Fang W, Shen Y, Levy DE, Xiong H, Chen N, He JC. Knockdown of Stat3 activity in vivo prevents diabetic glomerulopathy. Kidney Int 76: 63–71, 2009. doi: 10.1038/ki.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsui F, Babitz SA, Rhee A, Hile KL, Zhang H, Meldrum KK. Mesenchymal stem cells protect against obstruction-induced renal fibrosis by decreasing STAT3 activation and STAT3-dependent MMP-9 production. Am J Physiol Renal Physiol 312: F25–F32, 2017. doi: 10.1152/ajprenal.00311.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov 12: 611–629, 2013. doi: 10.1038/nrd4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyamoto N, Sugita K, Goi K, Inukai T, Iijima K, Tezuka T, Kojika S, Nakamura M, Kagami K, Nakazawa S. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia 15: 1758–1768, 2001. doi: 10.1038/sj.leu.2402260. [DOI] [PubMed] [Google Scholar]
- 57.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260: 1808–1810, 1993. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 58.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson EA, Walker SR, Kepich A, Gashin LB, Hideshima T, Ikeda H, Chauhan D, Anderson KC, Frank DA. Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood 112: 5095–5102, 2008. doi: 10.1182/blood-2007-12-129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, Metcalf D, Hilton DJ, Nicola NA, Baca M. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA 97: 6493–6498, 2000. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novak U, Harpur AG, Paradiso L, Kanagasundaram V, Jaworowski A, Wilks AF, Hamilton JA. Colony-stimulating factor 1-induced STAT1 and STAT3 activation is accompanied by phosphorylation of Tyk2 in macrophages and Tyk2 and JAK1 in fibroblasts. Blood 86: 2948–2956, 1995. [PubMed] [Google Scholar]
- 62.Ogura M, Uchida T, Terui Y, Hayakawa F, Kobayashi Y, Taniwaki M, Takamatsu Y, Naoe T, Tobinai K, Munakata W, Yamauchi T, Kageyama A, Yuasa M, Motoyama M, Tsunoda T, Hatake K. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci 106: 896–901, 2015. doi: 10.1111/cas.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okumura F, Okumura AJ, Matsumoto M, Nakayama KI, Hatakeyama S. TRIM8 regulates Nanog via Hsp90β-mediated nuclear translocation of STAT3 in embryonic stem cells. Biochim Biophys Acta 1813: 1784–1792, 2011. doi: 10.1016/j.bbamcr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Okusaka T, Ueno H, Ikeda M, Mitsunaga S, Ozaka M, Ishii H, Yokosuka O, Ooka Y, Yoshimoto R, Yanagihara Y, Okita K. Phase 1 and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol Res 45: 1283–1291, 2015. doi: 10.1111/hepr.12504. [DOI] [PubMed] [Google Scholar]
- 65.Ortiz B, Fabius AW, Wu WH, Pedraza A, Brennan CW, Schultz N, Pitter KL, Bromberg JF, Huse JT, Holland EC, Chan TA. Loss of the tyrosine phosphatase PTPRD leads to aberrant STAT3 activation and promotes gliomagenesis. Proc Natl Acad Sci USA 111: 8149–8154, 2014. doi: 10.1073/pnas.1401952111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J, Mezzano S, Ortiz A, Egido J, Gomez-Guerrero C. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 21: 763–772, 2010. doi: 10.1681/ASN.2009060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 68.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc Natl Acad Sci USA 93: 13704–13708, 1996. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plimack ER, Lorusso PM, McCoon P, Tang W, Krebs AD, Curt G, Eckhardt SG. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist 18: 819–820, 2013. doi: 10.1634/theoncologist.2013-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raz R, Lee C-K, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA 96: 2846–2851, 1999. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Said E, Zaitone SA, Eldosoky M, Elsherbiny NM. Nifuroxazide, a STAT3 inhibitor, mitigates inflammatory burden and protects against diabetes-induced nephropathy in rats. Chem Biol Interact 281: 111–120, 2018. doi: 10.1016/j.cbi.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 72.Savige J. Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol 592: 4013–4023, 2014. doi: 10.1113/jphysiol.2014.274449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 13: 1235–1242, 2006. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 74.Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 15: 23, 2017. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res 69: 1958–1965, 2009. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis: advances and implications. Arch Pathol Lab Med 133: 233–248, 2009. doi: 10.1043/1543-2165-133.2.233. [DOI] [PubMed] [Google Scholar]
- 77.Shastri A, Choudhary G, Teixeira M, Gordon-Mitchell S, Ramachandra N, Bernard L, Bhattacharyya S, Lopez R, Pradhan K, Giricz O, Ravipati G, Wong LF, Cole S, Bhagat TD, Feld J, Dhar Y, Bartenstein M, Thiruthuvanathan VJ, Wickrema A, Ye BH, Frank DA, Pellagatti A, Boultwood J, Zhou T, Kim Y, MacLeod AR, Epling-Burnette PK, Ye M, McCoon P, Woessner R, Steidl U, Will B, Verma A. Antisense STAT3 inhibitor decreases viability of myelodysplastic and leukemic stem cells. J Clin Invest 128: 5479–5488, 2018. doi: 10.1172/JCI120156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104: 7391–7396, 2007. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R; OPTION Investigators . Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371: 987–997, 2008. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 80.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA 102: 4700–4705, 2005. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res 66: 5542–5548, 2006. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 82.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94: 3801–3804, 1997. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 6: 843–854, 2010. doi: 10.1586/eci.10.70. [DOI] [PubMed] [Google Scholar]
- 85.Tang WW, Van GY, Qi M. Myofibroblast and alpha 1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int 51: 926–931, 1997. doi: 10.1038/ki.1997.131. [DOI] [PubMed] [Google Scholar]
- 86.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, Hartman J, Wang W, Kretzler M, Lafayette RA. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 94: 795–808, 2018. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc 86: 1188–1191, 2011. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turkson J, Zhang S, Palmer J, Kay H, Stanko J, Mora LB, Sebti S, Yu H, Jove R. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol Cancer Ther 3: 1533–1542, 2004. [PubMed] [Google Scholar]
- 89.Tuttle KR, Brosius FC III, Adler SG, Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk ME, Cardillo TE, Duffin KL, Haas JV, Macias WL, Nunes FP, Janes JM. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 33: 1950–1959, 2018. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vignais ML, Sadowski HB, Watling D, Rogers NC, Gilman M. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol 16: 1759–1769, 1996. doi: 10.1128/MCB.16.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vo AA, Sinha A, Haas M, Choi J, Mirocha J, Kahwaji J, Peng A, Villicana R, Jordan SC. Factors predicting risk for antibody-mediated rejection and graft loss in highly human leukocyte antigen sensitized patients transplanted after desensitization. Transplantation 99: 1423–1430, 2015. doi: 10.1097/TP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 93.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147: 1393–1404, 2014. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes 51: 3505–3509, 2002. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 95.Weidler M, Rether J, Anke T, Erkel G. Inhibition of interleukin-6 signaling by galiellalactone. FEBS Lett 484: 1–6, 2000. doi: 10.1016/S0014-5793(00)02115-3. [DOI] [PubMed] [Google Scholar]
- 96.Wen Z, Darnell JE Jr. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res 25: 2062–2067, 1997. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie Y, Kole S, Precht P, Pazin MJ, Bernier M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology 150: 1122–1131, 2009. doi: 10.1210/en.2008-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu MJ, Feng D, Wang H, Guan Y, Yan X, Gao B. IL-22 ameliorates renal ischemia-reperfusion injury by targeting proximal tubule epithelium. J Am Soc Nephrol 25: 967–977, 2014. doi: 10.1681/ASN.2013060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu X, Kasembeli MM, Jiang X, Tweardy BJ, Tweardy DJ. Chemical probes that competitively and selectively inhibit Stat3 activation. PLoS One 4: e4783, 2009. doi: 10.1371/journal.pone.0004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu YS, Liang JJ, Wang Y, Zhao XJ, Xu L, Xu YY, Zou QC, Zhang JM, Tu CE, Cui YG, Sun WH, Huang C, Yang JH, Chin YE. STAT3 undergoes acetylation-dependent mitochondrial translocation to regulate pyruvate metabolism. Sci Rep 6: 39517, 2016. doi: 10.1038/srep39517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, Chance MR, Chen X, Du Y, Wang Y, An L, Wang Q, Lu T, Zhang X, Wang Z, Stark GR. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci USA 107: 21499–21504, 2010. doi: 10.1073/pnas.1016147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang R, Xu X, Li H, Chen J, Xiang X, Dong Z, Zhang D. p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO. Sci Rep 7: 43409, 2017. doi: 10.1038/srep43409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yokota T, Omachi K, Suico MA, Kamura M, Kojima H, Fukuda R, Motomura K, Teramoto K, Kaseda S, Kuwazuru J, Takeo T, Nakagata N, Shuto T, Kai H. STAT3 inhibition attenuates the progressive phenotypes of Alport syndrome mouse model. Nephrol Dial Transplant 33: 214–223, 2018. doi: 10.1093/ndt/gfx246. [DOI] [PubMed] [Google Scholar]
- 104.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9: 798–809, 2009. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu J, Wu H, Liu ZY, Zhu Q, Shan C, Zhang KQ. Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med 40: 1185–1193, 2017. doi: 10.3892/ijmm.2017.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307: 269–273, 2005. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Nair V, Saha J, Atkins KB, Hodgin JB, Saunders TL, Myers MG Jr, Werner T, Kretzler M, Brosius FC. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int 92: 909–921, 2017. doi: 10.1016/j.kint.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang JT, Liu JY. Drugging the “undruggable” DNA-binding domain of STAT3. Oncotarget 7: 66324–66325, 2016. doi: 10.18632/oncotarget.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L, Xu X, Yang R, Chen J, Wang S, Yang J, Xiang X, He Z, Zhao Y, Dong Z, Zhang D. Paclitaxel attenuates renal interstitial fibroblast activation and interstitial fibrosis by inhibiting STAT3 signaling. Drug Des Devel Ther 9: 2139–2148, 2015. doi: 10.2147/DDDT.S81390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W, Polakiewicz RD, Kinzler KW, Vogelstein B, Velculescu VE, Wang ZJ. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA 104: 4060–4064, 2007. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA 109: 9623–9628, 2012. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhong Z, Wen Z, Darnell JE JR. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98, 1994. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 113.Zhou L, Fu P, Huang XR, Liu F, Lai KN, Lan HY. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J Am Soc Nephrol 21: 31–41, 2010. doi: 10.1681/ASN.2008111133. [DOI] [PMC free article] [PubMed] [Google Scholar]