Abstract
Histone proteins are elevated in the circulation after traumatic injury owing to cellular lysis and release from neutrophils. Elevated circulating histones in trauma contribute to coagulopathy and mortality through a mechanism suspected to involve endothelial cell (EC) dysfunction. However, the functional consequences of histone exposure on intact blood vessels are unknown. Here, we sought to understand the effects of clinically relevant concentrations of histones on the endothelium in intact, resistance-sized, mesenteric arteries (MAs). EC Ca2+ was measured with high spatial and temporal resolution in MAs from mice selectively expressing the EC-specific, genetically encoded ratiometric Ca2+ indicator, Cx40-GCaMP-GR, and vessel diameter was measured by edge detection. Application of purified histone protein directly to the endothelium of en face mouse and human MA preparations produced large Ca2+ signals that spread within and between ECs. Surprisingly, luminal application of histones had no effect on the diameter of pressurized arteries. Instead, after prolonged exposure (30 min), it reduced dilations to endothelium-dependent vasodilators and ultimately caused death of ~25% of ECs, as evidenced by markedly elevated cytosolic Ca2+ levels (793 ± 75 nM) and uptake of propidium iodide. Removal of extracellular Ca2+ but not depletion of intracellular Ca2+ stores prevented histone-induced Ca2+ signals. Histone-induced signals were not suppressed by transient receptor potential vanilloid 4 (TRPV4) channel inhibition (100 nM GSK2193874) or genetic ablation of TRPV4 channels or Toll-like receptor receptors. These data demonstrate that histones are robust activators of noncanonical EC Ca2+ signaling, which cause vascular dysfunction through loss of endothelium-dependent dilation in resistance-sized MAs.
NEW & NOTEWORTHY We describe the first use of the endothelial cell (EC)-specific, ratiometric, genetically encoded Ca2+ indicator, Cx40-GCaMP-GR, to study the effect of histone proteins on EC Ca2+ signaling. We found that histones induce an influx of Ca2+ in ECs that does not cause vasodilation but instead causes Ca2+ overload, EC death, and vascular dysfunction in the form of lost endothelium-dependent dilation.
Keywords: endothelial cell death, extracellular histone proteins, vascular endothelial cell calcium signaling, vascular dysfunction
INTRODUCTION
The surprising discovery that circulating extracellular histones play a key role in sepsis and that blocking histone proteins prevents death in rodent and primate models has led to an explosion of research over the past 10 years. Multiple groups have reported evidence for adverse clinical outcomes associated with elevated plasma levels of histones and nucleosomes—DNA-bound histone octomers—in trauma and other diseases (1, 22, 28, 42). Histones 2A (H2A), 2B (H2B), 3 (H3), and 4 (H4) form the octomer protein core of nucleosomes. Histones play a key role in the lethality of sepsis in murine and primate models, and their contribution can be abrogated by administration of antihistone antibodies (42). Histones are released into the circulation as a direct result of traumatic cell lysis and secondarily from neutrophils. During the innate immune response, neutrophils are stimulated and release neutrophil extracellular traps—fibrous networks composed largely of DNA—as part of suicidal or vital “NETosis” processes during which antimicrobial proteins, such as histones, are released into the circulation [reviewed in (23)]. Within ~4 h of injury, circulating nucleosome levels increase. DNA is degraded and nucleosome levels return to near normal within 24 h, whereas circulating histone levels remain elevated for ~3 days (1). Circulating plasma histone protein levels in healthy donors are ~2 μg/ml (1) but increase to ~25 μg/ml within 24 h of traumatic injury and can reach levels in excess of 200 μg/ml in some patients (1). High histone levels are associated with poor clinical outcome. However, the effects of histones on endothelial function are not known.
Systemic complications associated with elevated circulating histones after traumatic injury could be related to endothelial dysfunction. The vascular endothelium is a network of electrically coupled endothelial cells (ECs), which forms the barrier between blood and interstitial fluid and regulates coagulation and vascular tone. Increasing EC Ca2+ promotes vasodilation through conducted EC hyperpolarization or production of nitric oxide (NO) by endothelial nitric oxide synthase [(eNOS) reviewed in (13, 15, 37)]. Cytosolic EC Ca2+ levels are increased by release of Ca2+ from intracellular stores or influx through plasma membrane-resident ion channels. For example, endoplasmic reticulum (ER)-resident inositol trisphosphate receptors (IP3Rs) are known to mediate intracellular release, and transient receptor potential vanilloid 4 (TRPV4) Ca2+-permeable cation channels are an important, well-described EC influx pathway (24, 31). Local TRPV4-mediated Ca2+ increases at myoendothelial projections of ECs promote vasodilation though activation of small- (SK) and intermediate conductance Ca2+-activated K+ channels (IK), which in turn activate inward-rectifier K+ channels to promote hyperpolarization (18, 31–33). Hyperpolarizing signals from ECs relax smooth muscle cells (SMCs) through deactivation of smooth muscle voltage-dependent Ca2+ channels (8, 27). Elevated intracellular Ca2+ also activates eNOS and increases the production of NO, which diffuses out of ECs and activates large-conductance Ca2+-sensitive K+ channels through the guanylyl cyclase (GC)/cyclic-GMP/protein kinase G pathway in SMCs to promote hyperpolarization and vasodilation (5, 14, 19). Therefore, the a priori expectation is that a histone-induced transient increase in EC Ca2+ would promote vasodilation.
Xu et al. (42) demonstrated that histone proteins increase cytosolic Ca2+ in cultured ECs, but the source of EC Ca2+ was not demonstrated. EC Ca2+ signaling, through Ca2+ influx or release from intracellular stores, is generally localized to EC microdomains. However, regenerative Ca2+ waves in ECs can occur by stimulating release from intracellular stores through activation of IP3Rs. IP3R gating is dependent on IP3 and is also modulated by Ca2+ through a process known as Ca2+-induced Ca2+ release (6, 11, 21). Ca2+ modulation of IP3Rs is biphasic. A low cytoplasmic Ca2+ concentration ([Ca2+]) stimulates IP3R-mediated Ca2+ release, whereas a high cytoplasmic [Ca2+] inhibits IP3R-mediated Ca2+ release (7, 20, 25, 29). This sets up a condition in which Ca2+ released by IP3Rs or entering the cell via TRPV4 channels can sequentially activate adjacent IP3Rs, a process that forms the mechanistic basis for the generation of regenerative Ca2+ waves (11, 31).
A growing body of evidence indicates that circulating histone proteins are associated with poor clinical outcome after traumatic injury and suggests the possibility of an endothelium-dependent mechanism. Here, we tested the hypothesis that circulating histone proteins exert their actions through effects on EC Ca2+ signaling and endothelium-dependent vasodilation in native resistance-sized mesenteric arteries (MAs). Indeed, we found that purified histone proteins profoundly induced EC Ca2+ signals in native endothelium but contrary to expectations, failed to cause vasodilation. These histone-induced Ca2+ signals were not a result of Ca2+ release from intracellular stores but rather reflected Ca2+ influx through a noncanonical pathway. Consistent with clinical findings, prolonged histone exposure caused EC Ca2+ overload, EC death, and vascular dysfunction in the form of the loss of EC-dependent vasodilation.
MATERIALS AND METHODS
Materials.
Freshly dissected tissue was kept in 4°C HEPES-buffered physiological saline solution (HEPES-PSS; 10 mM HEPES, 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 7 mM glucose, pH 7.4) before experimentation. All experiments were performed in PSS; 118.5 mM NaCl, 24 mM NaHCO3, 7 mM glucose, 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 0.026 mM EDTA tetrasodium) or Ca2+-free PSS (118.5 mM NaCl, 24 mM NaHCO3, 7 mM glucose, 4.7 mM KCl, 1.2 mM MgCl2, 1.2 mM KH2PO4, 0.026 mM EDTA tetrasodium), bubbled with 5% CO2, 20% O2, and 75% N2 at 36°C to maintain physiological pH. All chemicals for buffer solutions and the following reagents were obtained from Sigma: digitonin, purified histone from calf thymus (cat. no. 10223565001), GSK1016790A (N-((1S)-1-[[4-((2S)-2-[[(2,4-dichlorophenyl)sulfonyl]amino]-3-hydroxypropanoyl)-1-piperazinyl]carbonyl]-3-methylbutyl)-1-benzothiophene-2-carboxamide) and GSK2193874 (3-([1,4′-bipiperidin]-1′-ylmethyl)-7-bromo-N-(1-phenylcyclopropyl)-2-[3-(trifluoromethyl)phenyl]-4-quinolinecarboxamide) (Sigma-Aldrich, St. Louis, MO). Cyclopiazonic acid (CPA) and NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) were obtained from Tocris (Bio-Techne, Minneapolis, MN). Fluo4-AM was obtained from Thermo Fisher Scientific (Waltham, MA).
Animal procedures.
All animal procedures were performed in accordance with institutional guidelines approved by the Institutional Animal Care and Use Committee of the University of Vermont. Transgenic Cx40-GCaMP-GR, TRPV4-knockout [TRPV4−/− (39)], Toll-like receptor 4 (TLR4)-knockout (TLR4−/−; Jax Laboratories, cat. no. 007227), and wild-type C57BL6 mice (Jax Laboratories, cat. no. 000664) were group-housed on a 12-h:12-h light-dark cycle with environmental enrichment and free access to food and water. Adult male mice (average age, 14 wk; weight, 28–30 g) were euthanized by intraperitoneal injection of sodium pentobarbital (150 mg/kg) followed by decapitation. Third-order branches of MAs (~100–150 μm internal diameter) were isolated, placed in 4°C HEPES-PSS, and used intact for pressurized artery studies or surgically opened longitudinally for en face Ca2+ imaging.
Construction of Cx40-GCaMP-GR mice.
Cx40-GCaMP-GR mice were developed at CHROMus (CHROMus, Cornell University) by inserting the GCaMP-GR expression cassette (30) at the translational initiation site downstream of the Cx40 promoter in bacterial artificial chromosome (BAC) clone RP24-255O4 by homologous recombination in Escherichia coli strain SW105 (38). The GCaMP-GR expression cassette encodes a ratiometric fluorescent protein created by fusion of a modified GCaMP3 Ca2+ sensor with the Ca2+-insensitive mCherry sequence, separated by an optimized linker peptide (30). The linearized Cx40BAC-GCaMP-GR DNA (Fig. 1A) was microinjected into B6SJLF/J embryos using standard pronuclear injection techniques. Founder mice were identified by polymerase chain reaction (PCR) using primers specific for both 5′ and 3′ junction sequences between Cx40 and the GCaMP-GR cassette and backcrossed to C57BL6 mice, after which offspring were genotyped for the transgene using the primers calmF (AAG GGC GAG GAG CTG TTC A) and calmR (CGA TCT GCT CTT CAG TCA GTT GGT). All procedures were approved by the Cornell Institutional Animal Care and Use Committee and adhered to the standards published in the Guide for the Care and Use of Laboratory Animals.
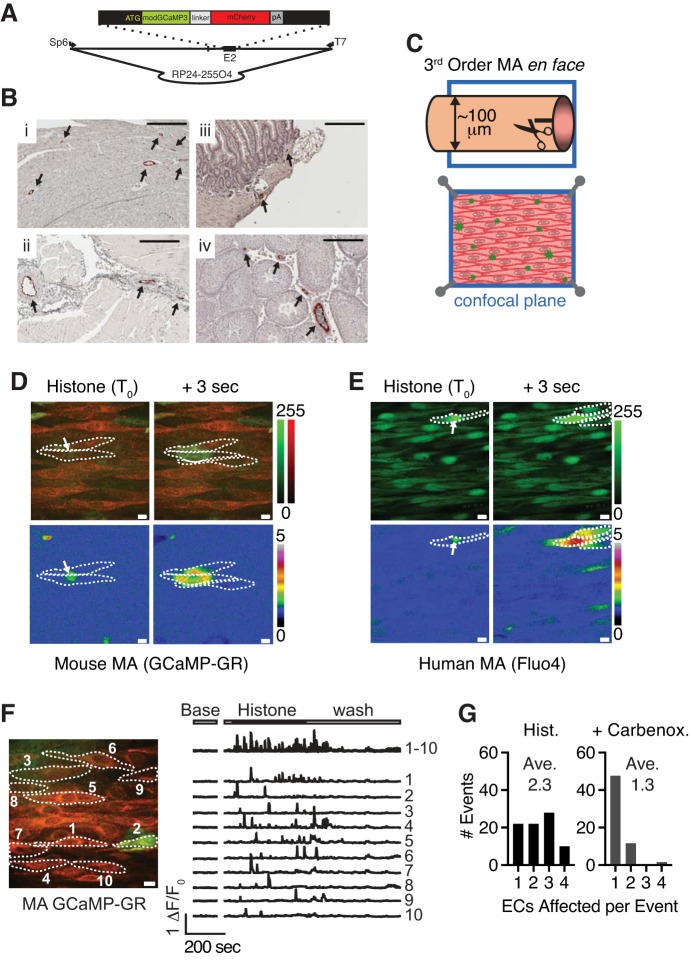
Fig. 1.
Extracellular histone proteins generate spreading Ca2+ signals in ECs of en face preparations of resistance-sized mouse MAs. A: schematic drawing of Cx40BAC-GCaMP-GR. The GCaMP-GR expression cassette with modified GCaMP3 sequence fused to mCherry was inserted at the translation initiation site of the Cx40 gene in BAC RP24-255O4 by homologous recombination, as described in materials and methods (schematic not to scale). B: distribution of GCaMP-GR expression in transgenic Cx40-GCaMP-GR mice. Paraffin-embedded tissue sections from heart (i), skeletal muscle (ii), gut (iii), and (iv) testis were immunostained with anti-green fluorescent protein antibody. Arrows mark GCaMP-GR-expressing regions (scale bar = 200 µm). C: diagram of an en face preparation of a 3rd-order mouse MA from an EC-specific Cx40-GCaMP-GR mouse. D: merged images of mCherry (red) and GCaMP (green) fluorescence showing a representative histone-induced Ca2+ signal at initiation (T0, white arrow) and 3 s after initiation. Calibration bar for each channel is on the right (top). Same field represented as ΔF/F0 (green channel) (bottom). Dotted lines indicate borders of ECs. Calibration bar for F/F0 is on the right. E: representative image of Fluo-4 fluorescence (green) in ECs in MAs from a human donor showing a representative histone-induced Ca2+ signal at initiation (T0, white arrow) and 3 s after initiation (n = 8 fields in 4 preparations from 3 donors). Calibration bar for the green channel is on the right (top). Same field represented as ΔF/F0 (bottom). Dotted lines indicate borders of ECs. Calibration bar for F/F0 is on the right. F: representative image and traces of ΔF/F0 in whole ECs from a Cx40-GCaMP-GR mouse MA treated with histones. Baseline activity was recorded for 2 min. Image acquisition was halted and resumed for 12 min to show activity from one field before, during, and after histone application. Histone proteins (10 μg/ml) were added after 30 s and remained in the circulation. Histones were washed away after 7 min. Top trace shows overlay of ΔF/F0 from 10 ECs. Each lower trace represents ΔF/F0 from a single EC indicated in the representative image on the left. G: cell-to-cell spread of a histone-induced Ca2+ signal, presented as a histogram of the number of ECs affected by histones alone (Hist; left, average = 2.32 ECs/event) or histones in presence of 100 μM carbenoxolone (Carbenox.; right, average = 1.27 ECs/event, n = 3 animals, 8–9 fields, 3–11 signals/field). BAC, bacterial artificial chromosome; EC, endothelial cell; MA, mesenteric artery.
Human tissue.
Human tissue was collected in accordance with institutional guidelines approved by The University of Vermont Committees on Human Research (protocol no. 17-00048). Small MAs (100-200-μm diameter) were dissected from discarded postoperative tissue from laparotomy patients. No extra tissue was obtained for the purpose of this study. Arteries were cleaned of perivascular adipose and connective tissue and maintained in 4°C HEPES-PSS before experimentation. MAs were prepared en face, loaded with Fluo-4 (10 μM with 0.08% pluronic acid) at 30°C for 30 min, and imaged with a spinning-disk confocal microscope, as described below.
Immunohistochemistry.
Tissues were fixed in 4% paraformaldehyde overnight at 2–8°C and embedded in paraffin for sectioning. The paraffin sections were deparaffinized before heat-induced antigen retrieval. Endogenous peroxidase activity was quenched by treating with 3% hydrogen peroxide in 1× PBS followed by avidin/biotin block, where appropriate. After blocking with normal goat serum (Vector Laboratories, Inc., Burlingame, CA) sections were incubated overnight at 2–8°C with rabbit anti-green fluorescent protein polyclonal antibody (sc-8334; Santa Cruz Biotechnology, Dallas, TX) then developed with biotinylated goat anti-rabbit secondary antibody/horseradish peroxidase-conjugated streptavidin/aminoethyl carbazole reagent. The slides were counterstained with hematoxylin, mounted with Fluoromount G, and digitally scanned on Aperio CS2 (Leica Biosystems, Buffalo Grove, IL).
En face Ca2+ imaging.
Third-order branches of MAs from Cx40-GCaMP-GR mice were dissected, cleaned of adipose and connective tissue with fine forceps, surgically opened longitudinally, and pinned en face with 100–200-μm lengths of 13-μm tungsten wire on a custom Sylguard-coated imaging chamber. Arteries were maintained at 36°C in PSS bubbled with biological air (20% O2-5% CO2-75% N2). PSS was recirculated at 10 ml/min throughout the experiment. Arteries were imaged on a dual camera Andor spinning-disk confocal microscope system equipped with a 60× water-immersion objective and 488- and 561-nm excitation lasers. For each condition, images (30-ms exposures) were captured for 2 min from both channels (power: 35 and 105 μW for 488- and 561-nm lasers, respectively, at working distance) and stored as multipage TIFF files (iQ3, Andor, Belfast, UK). After returning through the spinning disk, emission spectra were separated with a 561-nm long-pass dichromatic mirror. Reflected light (GCaMP and Fluo4 emission) was collected through a 525 ± 50-nm band pass filter on an EMCCD camera (iXon, Andor, Belfast, UK). Transmitted light [mCherry and propidium iodide (PI) emission] was collected through a 620 ± 60-nm band pass filter on a second EMCCD camera (iXon, Andor, Belfast, UK). ECs from C56BL/6J, TRPV4−/−, and TLR4−/− mice were visualized in en face MA preparations loaded with Fluo-4 (10 μM with 0.08% pluronic acid) at 30°C for 30 min and imaged as described for Cx40-GCaMP-GR mice, except total time was reduced to 1 min for each condition to minimize fluorescence decay.
Quantification of Ca2+ event activity.
Analyses were performed using SparkAn software (developed by Adrian Bonev). Pixel intensity values were mapped to lookup-tables (GCaMP-GR to green, mCherry to red, and F/F0 to heat map, calibration bars shown in Fig. 1). Image brightness and contrast were adjusted to aid viewing. Nonlinear corrections were not applied (γ = 1.0). Brightness and contrast settings for each channel were held constant through all conditions in an experimental series. Quantification and analysis were performed with original pixel intensity values. Traces of GCaMP or Fluo-4 fluorescence (F/F0) were generated by monitoring fluorescence over time in selected regions of interest (ROI) from each field of view. F/F0 was calculated as fluorescence within an ROI (after subtracting camera noise) divided by the average fluorescence in the same ROI for 10 preceding images with no activity. Unless otherwise noted, ROIs were set as 1.33-μm2 (5 × 5 pixels) regions at the initiation site of a Ca2+ signal. Total Ca2+ signal activity was calculated as the area under the curve (AUC) calculated by trapezoidal numerical integration. Activity within a given ROI was calculated as the sum of the activity of each event over the course of the 2-min recording (1-min recording for Fluo-4-loaded tissue). ROIs were placed at every Ca2+ event in the field of view for each condition. The total activity of each ROI in a field was summed to determine the total activity per field represented by the summary data. Activities in the same field (1–3 fields per experiment) under multiple conditions were treated as paired experiments, as indicated by lines connecting data points from the same field under multiple conditions. Line-scan analyses of spreading Ca2+ signals were performed by extending a line from the origin of the event to the point of maximum spread along the long axis of the event. From this, we generated a spatiotemporal plot of Ca2+ signals in which the x-axis represents time and the y-axis represents distance along the line scan. These plots were used to determine signal velocity (from origin to point of maximum spread), distance (from signal origin to point of maximum spread), and propagation (Ca2+ signal origin to signal termination).
Ex vivo pressure arteriography.
MAs from C57BL/6J mice were isolated, cleaned of surrounding adipose and connective tissue, and mounted and secured with suture ties to similarly sized polished glass pipettes in an arteriograph chamber (Instrumentation and Model Facility, University of Vermont, Burlington, VT). The proximal pipette was attached to a servo-controlled pressure-regulating device (Living Systems Instrumentation, St. Albans, VT). MAs were pressurized to 80 mmHg in PSS (36°C) that was constantly bubbled with biological air (5% CO2-20% O2-75% N2) and recirculated through the arteriograph chamber. Pressurized arteries were mounted on an inverted light microscope equipped with a CCD camera and edge-detection software for continuous monitoring of internal diameter (IonOptix, Milton, MA). Only MAs that exhibited a myogenic response were used. MAs were treated with Ca2+-free PSS at the conclusion of each experiment to obtain maximal dilation. Myogenic tone was calculated as a percentage of the Ca2+-free diameter to normalize for differences in diameter between MAs. Histone or PSS (control) was delivered through the cannula into the lumen to expose the endothelium directly to histone protein. Conditions of low shear stress were used to avoid stimulating flow-mediated vasodilatory mechanisms (~2-μl/min flow, <5 dyne/cm2). Proximal and distal canula diameters were matched to vessel size to minimize pressure differential across the vessel (typically <5 mmHg). EC function was determined by measuring the dilatory response to activation of endothelial SK/IK channels with 1 μM NS309 (35).
EC Ca2+ signals in ex vivo pressurized arteries were imaged in MAs from Cx40-GCaMP-GR mice, prepared as described for pressure arteriography experiments. Vessels were pressurized to 80 mmHg and imaged on an upright spinning-disk confocal microscope (as described in En face Ca2+ imaging). Because we were not recording changes in diameter during these experiments, we used intraluminal flow rates that more closely approximate physiological flow to deliver histone proteins inside the vessel [~110 μl/min (17)]. Intraluminal PSS contained 100 ng/ml PI to allow visualization of endothelial damage in response to 10 μg/ml histone protein. Arteries were imaged before delivering histone or control solution (PSS), during exposure to histone or control solution (time 0), and after 30 min of histone or control solution exposure.
Estimation of intracellular Ca2+ concentration.
MAs were prepared as described for en face Ca2+ imaging. Tissue was placed in HEPES-PSS containing 10 mM EGTA and treated with 4 μM digitonin for 1 min to permeabilize ECs. Digitonin was washed away, and serial dilutions were performed to change free extracellular [Ca2+]. Extracellular [Ca2+] was controlled by varying the ratio of EGTA to Ca2+. The ratio of GCaMP fluorescence to mCherry fluorescence (FGCaMP/FmCherry) was recorded using the dual camera Andor spinning-disk system with the same exposure and laser power at working distance as en face imaging experiments (30 ms, 488 nm/35 μW, 561 nm/105 μW). Data were fit to a variable-slope model with free [Ca2+] of 0, 65, 193, 452, 968, 2,000, and 4,064 nM (GraphPad Prism 7, La Jolla, CA).
| (1) |
Values obtained from this fit were used to estimate intracellular [Ca2+] in nonpermeabilized ECs [best-fit values ± standard error: min = 0.16 ± 0.01, max = 0.94 ± 0.01, EC50 = 394 ± 1.0 nM, Hill slope = 2.45 ± 0.18; goodness of fit (R2) = 0.917].
Real-time cell death imaging.
MAs, prepared as for en face Ca2+ imaging, were maintained at 36°C in PSS bubbled with biological air (20% O2-5% CO2-75% N2) throughout the experiment. Identical fields from 1 artery were imaged for 30 s before histone exposure and 5, 15, and 30 min after histone exposure (Andor spinning-disk confocal microscope). For C57BL/6J, TRPV4−/−, and TLR4−/− mice, tissue was loaded with Fluo-4-AM EC cell death over time was visualized by measuring incorporation of PI (Sigma-Aldrich, St. Louis, MO), maintained at 100 ng/ml in the bath solution throughout the experiment. The percentage of ECs in a field with red, PI-labeled nuclei at each time point was calculated. ECs labeled with PI before histone exposure were excluded from the analysis.
Statistics.
Data are expressed as means ± SE. Student’s t-test was used to calculate P values for paired and unpaired data. One-way ANOVA with α = 0.05 was used for paired experiments with three or more conditions. A P value < 0.05 was considered significant. P values for each test are reported in the figure legends.
RESULTS
Extracellular histone proteins induce Ca2+ influx in ECs of resistance-sized MAs.
To test the hypothesis that histone proteins alter EC Ca2+ signaling in systemic resistance-sized arteries, we performed high-speed Ca2+ imaging on third-order branches of MAs from mice expressing GCaMP-GR fusion protein (Fig. 1A). Immunohistochemistry was used to confirm GCaMP-GR expression in the vascular endothelium of tissue from the heart, skeletal muscle, testis, and gut (Fig. 1B). Using en face MA preparations, which provide direct access to endothelium (Fig. 1C), we imaged ECs under continuous flow of PSS, 10 ml/min at 36°C. Because nucleosomes are rapidly degraded in circulation (1), we focused on the effects of unfractionated histone protein purified from calf thymus. Mass spectrometry analysis showed that this preparation consisted of 32.5% H2B, 28.9% H4, 20.8% H2A, 10.2% H3.1, and 7.5% H1.2 (data not shown). Histone H3 and H4 amino acid sequences are completely conserved between bovine, human, and mouse, whereas H2a, H2b, and H1 are 89%, 88%, and 87% homologous between bovine, human, and mouse. Addition of 10 μg/ml unfractionated purified histone protein (~0.8 μM total histone protein) to the extracellular solution caused a profound increase in transient EC Ca2+ signals in mouse and human MAs (Fig. 1, D and E; Supplemental Videos S1 and S2; all supplemental material is available at https://doi.org/10.7910/DVN/64J7MV). Because of the large size of histone-induced signals, we defined whole ECs within a field of view as ROIs and plotted ΔF/F0 versus time during histone application and removal (Fig. 1F). Ca2+ signaling activity returned to baseline after histone washout (Fig. 1F). Interestingly, histone-induced Ca2+ signals spread within and between adjacent ECs (Fig. 1D). An analysis of the number of ECs affected by a single histone Ca2+ signal, where a value of 1.0 indicates that the signal was limited to the EC of origin and did not spread to an adjacent EC, revealed that a typical histone-induced Ca2+ signal spread, on average, from the EC of origin to at least one adjacent EC (2.3 ± 0.1 cells, Fig. 1G). One possibility is that histone-induced Ca2+ signals spread to neighboring ECs through EC-EC gap junctions. To test this, we applied carbenoxolone (100 μM), an inhibitor of gap junction proteins. Carbenoxolone prevented the spread of histone-induced Ca2+ signals between cells (1.3 ± 0.1 cells, Fig. 1G), suggesting that histone-induced Ca2+ signals spread intracellularly though gap junctions.
We next sought to determine the source of Ca2+ for histone-induced Ca2+ signals. Because histone-induced Ca2+ signals are visually similar to IP3R-dependent, regenerative Ca2+ waves in that they spread within and between ECs, we hypothesized that extracellular histone proteins act by inducing Ca2+ release from intracellular stores. Although small Ca2+ signals are abundant under physiological conditions in our en face preparations, spontaneous regenerative Ca2+ waves are rare (see Fig. 2F). To study the properties of regenerative Ca2+ waves, we applied 100 nm GSK1016790A (GSK101; EC50 = 18 nM), a potent TRPV4 channel agonist (39) that has been previously shown to initiate regenerative Ca2+ waves in the en face MA preparation (31). Regenerative Ca2+ signals can occur through a Ca2+-induced Ca2+ release mechanism in which Ca2+ released through IP3Rs activates nearby IP3Rs (6). TRPV4 channels are also activated by intracellular Ca2+ and can contribute to a similar regenerative Ca2+ signal if they are activated by Ca2+ release from intracellular stores or by Ca2+ influx into the cell (36). To compare properties of histone-induced Ca2+ signals and TRPV4 channel-initiated regenerative Ca2+ waves, we performed line-scan analyses (see materials and methods). The first notable difference between these two types of signals is that histone signals were detected in only a fraction (26.4 ± 5.5%) of ECs in a single field of view, whereas TRPV4 channels-initiated waves were found in nearly all ECs (98.0 ± 1.2%; Fig. 2, A and B; Supplemental Video S3). Histone-induced signals spread slower (10.8 ± 1.3 μm/s vs. 26.6 ± 2.2 μm/s) than TRPV4 channel-initiated Ca2+ waves (Fig. 2C). Moreover, histone-induced Ca2+ signals did not propagate (4.1 ± 2.2 μm), whereas TRPV4-initiated signals propagated over a considerable distance (35.9 ± 3.7 μm; Fig. 2, A and D). Histone-induced signals began (green circle) and ended (red circle) at the same location, whereas TRPV4-initated waves began and ended at different sites within the same EC or in an adjacent EC (Fig. 2, A and D). This suggests that histone-induced signals spread from a single point source and do not regenerate. If this is true, we would predict that the maximum size of a histone-induced signal—the distance from event origin to point of maximum spread—should be dependent on the duration of the event at its origin. We found a positive correlation between size and duration for histone-induced signals (R2 = 0.50) and no correlation between size and duration for TRPV4 channel-initiated regenerative Ca2+ waves (R2 = 0.03; Fig. 2E). This suggests that histone-induced signals are not generated by the same mechanism as TRPV4 channel-initiated regenerative Ca2+waves.
Fig. 2.
Histone-induced Ca2+ signals are not regenerative. A: a 50-μm line scan placed at the initiation site of a spreading Ca2+ signal induced by 100 nM GSK101 (top) or 10 μg/ml histone proteins (bottom). The green circle marks the signal origin, the white circle marks the point of maximum spread, and the red circle marks the signal termination site. B–E: comparison of the properties of histone- and GSK101-induced spreading Ca2+ signals calculated from line-scan data presented in G, including the number of ECs per field exhibiting Ca2+ signals (B); velocity of signals (μm/s) (C); propagation, calculated as the distance from signal origin to termination (A, green to red) (D); correlation between signal distance (from signal origin to point of maximum spread) and duration (E). Data are presented as means ± SE, n = 3 animals, 7–8 fields, 1–3 signals per field, *P < 0.0001, Student’s t-test. F: average total area of baseline Ca2+ signals (Base), GSK101-initiated waves, and histone-induced Ca2+ signals (Hist), with or without 30 μM CPA. Data are presented as means ± SE [n = 2–3 animals, 6–9 fields; **P < 0.0001 for groups compared with baseline (one-way ANOVA) and GSK101 compared with GSK101 + CPA (Student’s t-test), *P = 0.011 Hist compared with Hist + CPA (Student’s t-test)]. CPA, cyclopiazonic acid; GSK101, GSK1016790A; n.s., not significant.
Given the differences in physical properties between histone-induced Ca2+ signals and TRPV4 channel-initiated regenerative waves, we hypothesized that histone-induced signals are not because of Ca2+ release from intracellular stores. To test this, we measured histone-induced and TRPV channel-initiated Ca2+ waves before and after depleting intracellular Ca2+ stores with CPA. CPA blocks sarco/endoplasmic reticulum Ca2+ ATPase-dependent Ca2+ reuptake into the ER of ECs and thus depletes EC Ca2+ stores over time as Ca2+ is released by IP3Rs with no reuptake (16, 24, 26, 31). CPA treatment dramatically decreased the total area of TRPV4 channel-initiated propagating signals, reducing it from 121.0 ± 6.5 μm2 to 22.0 ± 1.2 μm2, a value comparable to the average area of baseline Ca2+ signals (25.7 ± 1.9 μm2; Fig. 2F; Supplemental Videos S4 and S5). These results confirm that TRPV4 channel-initiated Ca2+ waves are because of Ca2+ release from intracellular stores. By contrast, histones continued to cause large, spreading Ca2+ signals after CPA treatment (164.0 ± 15.6 vs. 115.0 ± 8.5 μm2). Though there was a small but significant decrease in Ca2+ signal area after CPA treatment, histone-induced Ca2+ signals were still dramatically larger than baseline Ca2+ signals (115.0 ± 8.5 vs. 25.7 ± 1.9 μm2) or TRPV4 channel Ca2+ signals after store depletion (115.0 ± 8.5 μm2 vs. 22.0 ± 1.2 μm2; Fig. 2F). This demonstrates that histone-induced Ca2+ signals spread despite depletion of intracellular stores.
To confirm that CPA effectively depletes EC Ca2+ stores in the en face preparation, we applied the muscarinic receptor agonist, carbachol (CCh; 10 μM), which acts through G-proteins of the Gq subtype to activate phospholipase C (PLC). PLC, in turn, hydrolyzes phosphoinositol 4,5-bisphosphate to generate IP3, which ultimately activates IP3Rs and increases global Ca2+ release (6, 34). If EC Ca2+ stores are depleted, CCh should not activate Ca2+ release. After incubating tissue for 30 min in PSS (control), CCh robustly increased EC Ca2+ signaling (227 ± 75 vs. 98 ± 15 events/min; Fig. 3, A and B); CCh induced a similar increase following incubation in Ca2+-free PSS for 30 min (279 ± 61 vs. 71 ± 6 events/min; Fig. 3, C and D). Notably, incubation in Ca2+-free PSS for 30 min did not significantly reduce EC Ca2+ signaling compared with baseline (untreated) conditions (Fig. 3D). In contrast, and as predicted, incubation of tissue with PSS containing 30 μM CPA for 30 min eliminated CCh-induced EC Ca2+ signals; in the presence of CCh, Ca2+ signaling frequency in CPA-pretreated tissue (6 ± 1 events/min) was comparable to that in tissues incubated with CPA-containing PSS alone (9 ± 2 events/min; Fig. 3, E and F). Thus, CPA effectively depletes EC Ca2+ stores, whereas a 30-min incubation in Ca2+-free PSS does not. Moreover, treatment with PSS containing 30 μM CPA for 30 min dramatically reduced the EC Ca2+ signaling (9 ± 2 events/min) compared with that observed under baseline conditions (46 ± 13 events/min; Fig. 3, E and F), indicating that most baseline events observed in the absence of treatment are attributable to Ca2+ release from intracellular stores.
Fig. 3.
CPA effectively depletes intracellular Ca2+ stores, whereas incubation in Ca2+-free PSS does not. A, C, and E: representative merged images of GCaMP and mCherry fluorescence. White plus signs (+) indicate the locations of each Ca2+ event during the experiment. B, D, and F: number of EC Ca2+ events per minute in an en face preparation of third-order MAs from a Cx40-GCaMP-GR mouse at baseline; after a 30-min incubation in PSS (B), Ca2+-free PSS (D), or PSS containing 30 μM CPA to deplete intracellular Ca2+ stores (F); and after treatment with 10 μM CCh. Data are presented as means ± SE [n = 4 animals, 5–10 fields (A), 3 animals, 3–6 fields (B), and 3 animals, 3–6 fields (C); *P < 0.05, Student’s t-test]. CCh, carbachol; CPA, cyclopiazonic acid; EC, endothelial cell; PSS, physiological saline solution.
The observation that the size (area, μm2) of histone-induced Ca2+ signals was only modestly reduced by CPA treatment, whereas GSK101-induced spreading Ca2+ events were eliminated by CPA, suggests that histone-induced Ca2+ signals are not caused by Ca2+ release from intracellular stores (Fig. 2F). To test this, we applied histone proteins to en face preparations in Ca2+-free PSS. Histone-induced Ca2+ signals were not detected in Ca2+-free PSS but were observed in the same preparation when extracellular Ca2+ was added to the bath (Fig. 4A). These data demonstrate that histone-induced Ca2+ signals are not mediated by Ca2+ release from the ER and thus must be attributable to Ca2+ influx. We depleted intracellular Ca2+ stores with CPA and measured total histone-induced Ca2+ signal activity in PSS and Ca2+ -free PSS. Figure 4, B–D shows merged fluorescence images from the same field of view and corresponding ΔF/F0 plots of all Ca2+ signals for each condition. To ensure a valid comparison between large-area histone-induced signals and local baseline Ca2+ signals (Fig. 2F), we determined total Ca2+ signal activity in each condition by placing a small ROI (5 × 5 pixel) at the initiation site of each Ca2+ signal and calculating the AUC of ΔF/F0 for every Ca2+ signal in a field of view. Many Ca2+ signals were observed at baseline (Fig. 4B). Depletion of intracellular stores with 30 μM CPA (for 30 min) dramatically reduced Ca2+ signaling (Fig. 4C). Consistent with results shown in Fig. 2F, application of histones (10 μg/ml) after CPA treatment still induced large Ca2+ signals (Fig. 4, D and E, and Table 1). Histone signals were eliminated by replacing normal PSS solution with Ca2+-free PSS (Fig. 4E and Table 1), demonstrating that histone proteins increase Ca2+ influx. We are confident that the loss of histone-induced Ca2+ signals in Ca2+-free PSS is not a result of depletion of intracellular stores, because a 30-min incubation in Ca2+-free PSS was not sufficient to deplete intracellular stores (Fig. 3, C and D). An analysis of the histone concentration-response relationship (1 to 10 μg/ml) showed that total Ca2+ signal activity per field trended higher at a histone protein concentration of 3 μg/ml and was significantly elevated compared with baseline at 10 μg/ml (Fig. 4F and Table 1). Intact histone proteins were necessary for this Ca2+ activity, as enzymatic digestion prevented histone-induced signals (Fig. 4G and Table 1). Collectively, these results indicate that purified histone proteins induce a Ca2+ signal that is attributable to Ca2+ influx.
Fig. 4.
Histone proteins induce EC Ca2+ influx. A: representative merged images and F/F0 plots generated from whole-cell ROIs. ROIs were placed around all histone-responsive cells after addition of 2 mM extracellular Ca2+ (n = 3 animals, 6 fields). B–D: representative merged images and F/F0 plots from 1.33 μm2 ROIs of Ca2+ signal activity from the same field of tissue showing baseline Ca2+ signals (B), Ca2+ signals after 20-min treatment with 30 μM CPA (C), and signals after addition of 10 μg/ml histone protein (D). E: activity in the presence of CPA and CPA + histone, with or without 2 mM extracellular Ca2+. Data are presented as means ± SE (n = 3 animals, 5 fields; *P < 0.005, paired Student’s t-test). F: activity per field in response to 1, 3, and 10 μg/ml histone protein. Data are presented as means ± SE (n = 3 animals, 8 fields; *P < 0.001, one-way ANOVA). G: activity from the same field in the presence of CPA in response to proteinase K-digested histone proteins (10 μg/ml, Pro K Histone) or intact histone proteins (10 μg/m). Data are presented as means ± SE (n = 3 animals, 7 fields; *P = 0.003, paired Student’s t-test). AUC, area under the curve; CPA, cyclopiazonic acid; His, histone; n.s., not significant; ROI, region of interest.
Table 1.
Additional parameters of histone-induced calcium signaling
| No. Cells/Field | No. ROIs/Field | No. Events/Field | % Active ECs | AUC/ROI | AUC/Event | |
|---|---|---|---|---|---|---|
| Fig. 4E | ||||||
| CPA | 12.8 ± 2.1 | 2.0 ± 1.0 | 3.6 ± 2.6 | 13.7 ± 4.2 | 3.1 ± 1.0 | 2.3 ± 0.5 |
| +Histone | 12.8 ± 2.1 | 7.4 ± 1.7* | 10.6 ± 2.7* | 58.2 ± 11.8* | 12.9 ± 2.1* | 9.1 ± 1.3* |
| CPA Ca2+-free PSS | 12.8 ± 2.1 | 0.4 ± 0.2 | 0.4 ± 0.2 | 2.3 ± 1.5 | 0.4 ± 0.3 | 0.4 ± 0.3 |
| +Histone | 12.8 ± 2.1 | 0.2 ± 0.2* | 0.2 ± 0.2* | 1.8 ± 1.8* | 0.1 ± 0.1* | 0.1 ± 0.1* |
| Fig. 4F | ||||||
| CPA | 29 ± 2.1 | 3.75 ± 0.6 | 9.75 ± 2.8 | 13.3 ± 2.4 | 6.7 ± 2.2 | 3.3 ± 1.4 |
| +1 µg/ml histone | 29 ± 2.1 | 5.0 ± 1.0 | 8.1 ± 2.1 | 16.6 ± 2.6 | 5.3 ± 0.9 | 3.6 ± 0.5 |
| +3 µg/ml histone | 29 ± 2.1 | 4.8 ± 1.1 | 7.6 ± 2.3 | 16.1 ± 3.4 | 12.3 ± 2.4 | 9.6 ± 2.6 |
| +10 µg/ml histone | 29 ± 2.1 | 9.6 ± 1.2* | 15.4 ± 2.1 | 32.8 ± 2.7* | 22.0 ± 3.0* | 14.1 ± 2.1* |
| Fig. 4G | ||||||
| CPA | 15.6 ± 1.6 | 1.6 ± 0.4 | 5.4 ± 1.6 | 9.5 ± 1.8 | 3.4 ± 1.2 | 0.9 ± 0.3 |
| +Digested histone | 15.6 ± 1.6 | 1.1 ± 0.5 | 2.6 ± 1.3* | 6.2 ± 2.5 | 1.1 ± 0.5 | 0.7 ± 0.3 |
| +Histone | 15.6 ± 1.6 | 6.0 ± 0.9* | 7.4 ± 1.4 | 39.3 ± 4.4* | 10.3 ± 3.2 | 8.8 ± 2.7* |
| Fig. 5B | ||||||
| CPA | 27.3 ± 0.4 | 2.7 ± 0.5 | 4.0 ± 0.5 | 10.0 ± 1.7 | 5.1 ± 1.5 | 2.9 ± 0.8 |
| +Histone | 27.3 ± 0.4 | 7.6 ± 0.9* | 14.4 ± 2.3* | 28.0 ± 3.7* | 19.2 ± 1.4* | 10.5 ± 1.0* |
| +10 nM GSK219 | 27.3 ± 0.4 | 6.7 ± 0.5* | 11.0 ± 1.4* | 24.7 ± 2.0* | 16.2 ± 1.0* | 10.4 ± 1.1* |
| +100 nM GSK219 | 27.3 ± 0.4 | 6.0 ± 0.5* | 9.7 ± 1.5 | 21.1 ± 2.2* | 21.1 ± 2.7* | 15.0 ± 3.0* |
| Fig. 5D | ||||||
| C57 CPA | 30.2 ± 1.2 | 1.1 ± 0.4 | 1.8 ± 0.7 | 4.0 ± 1.4 | 0.7 ± 0.4 | 0.7 ± 0.4 |
| +Histone | 30.2 ± 1.2 | 6.8 ± 0.7* | 7.8 ± 1.0* | 22.7 ± 2.5* | 5.4 ± 1.2* | 4.9 ± 1.2* |
| TRPV4 KO CPA | 32.2 ± 1.4 | 0.25 ± 0.25 | 0.25 ± 0.25 | 0.7 ± 0.7 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| +Histone | 32.2 ± 1.4 | 6.9 ± 1.0* | 7.4 ± 1.1* | 21.3 ± 3.1* | 7.5 ± 1.2* | 7.0 ± 1.3* |
| TLR4 KO CPA | 26.5 ± 1.5 | 1.0 ± 0.4 | 1.4 ± 1.5 | 3.8 ± 1.4 | 1.3 ± 0.6 | 0.8 ± 0.3 |
| +Histone | 26.5 ± 1.5 | 8.3 ± 2.1* | 9.6 ± 2.6* | 30.3 ± 7.5* | 9.0 ± 0.9* | 8.1 ± 0.7* |
| Fig. 6C | ||||||
| Baseline | 5.8 ± 1.3 | 3.3 ± 1.4 | 10.3 ± 4.8 | 45.8 ± 20.1 | 1.3 ± 0.6 | 0.4 ± 0.2 |
| Abluminal histone | 5.8 ± 1.3 | 2.0 ± 0.7 | 7.5 ± 3.9 | 30.8 ± 10.8 | 3.3 ± 1.7 | 0.9 ± 0.3 |
| Intraluminal histone | 6.8 ± 0.4 | 5.2 ± 0.4* | 7.6 ± 0.9 | 76.4 ± 3.6* | 17.1 ± 3.2* | 12.5 ± 2.8* |
Values are means ± SE; n, number of animals and fields used for analysis, the same as reported in the corresponding figure legends. AUC; area under the curve; CPA, cyclopiazonic acid; EC, endothelial cell; KO, knockout; PSS, physiological saline solution; ROI, region of interest.
P < 0.05, statistical tests used are the same as reported for full field activity values in the corresponding figures.
Histone-induced Ca2+ influx is not mediated by TRPV4 channels or Toll-like receptor 4.
Extracellular histone proteins induce robust Ca2+ influx in native ECs. One possible mechanism to account for this is histone-induced activation of TRPV4 channels, which are known to play a prominent role in EC Ca2+ influx (31, 32). Additionally, histone-induced Ca2+ signals could occur through downstream effects of Toll-like receptor signaling. TLR4 has been shown to play a role in histone toxicity through activation of cytokine inflammatory response (41); however, it is not known if TLR4 receptors have an effect on EC Ca2+. To investigate the possible roles of these proteins, we used pharmacological and genetic approaches to test first whether histones cause Ca2+ influx through TRPV4 channels. In these experiments, we depleted ER Ca2+ by pretreating with CPA, then added histone proteins and applied 10–100 nM GSK2193874 (GSK219), a potent, selective TRPV4 channel antagonist [Fig. 5A (9)]. Because we are comparing large histone events to local TRPV4 influx, we placed a small (5 × 5 pixel) ROI at the initiation site of each Ca2+ signal within the field of view. We then determined the AUC for each ROI and summed all ROIs within a field to determine total activity for each condition. We found that GSK219 did not decrease histone-induced Ca2+ signals (Fig. 5B and Table 1), suggesting that TRPV4-mediated Ca2+ influx does not underlie these signals. To confirm this observation, we measured histone-induced activity in MA ECs of TRPV4−/− mice. As expected, based on previous reports by us and others (12, 31, 43), EC Ca2+ signaling was decreased in CPA-pretreated MA en face preparations from TRPV4−/− mice compared with that in C57 mice (AUC 0.22 ± 0.22 in TRPV4−/− vs. 1.30 ± 0.65 in C57; Fig. 5, C and D, and Table 1). Notably, histone-induced Ca2+ signaling in ECs was unaffected by TRPV4 knockout (Fig. 5D and Table 1). Using the same experimental paradigm, we further found that histones also induced robust EC Ca2+ signals in en face MA preparations from TLR4−/− mice (Fig. 5D and Table 1). Collectively, these results suggest that histone-induced EC Ca2+ signaling does not reflect TRPV4 channel-mediated Ca2+ influx and is independent of activation of TRL4 receptor-dependent pathways.
Fig. 5.
Histone-induced EC Ca2+ influx is not mediated by TRPV4 or TLR4. A: representative merged images of GCaMP and mCherry fluorescence from an en face preparation of third-order MAs treated with 30 μM CPA (left), 10 μg/ml histone proteins (middle), and 10 nM GSK219 (right). All images are from the same field of view. White squares indicate 1.33 μm2 ROIs placed at the initiation site of all Ca2+ signals observed during the experiment. Lower: F/F0 versus time for all signals from each treatment. B: total Ca2+ signal activity recorded over 2 min in the presence of 30 μM CPA, 30 μM CPA plus 10 μg/ml histone, or 30 μM CPA and 10 μg/ml histone plus 10 and 100 nM GSK219. Data are presented as means ± SE, n = 3 animals, 7 fields, *P < 0.001, paired one-way ANOVA). C: en face preparations of third-order MAs loaded with Fluo-4 from a C57/BL6 mouse. D: total EC Ca2+ event activity per field in en face preparations of third-order MAs from C57/Bl6 (C57), TRPV4−/−, or TLR4−/− mice recorded over 1 min in the presence of 30 μM CPA (left) or 30 μM CPA plus 10 μg/ml histone (right). Data are presented as means ± SE (n = 3–4 animals, 8–12 fields; *P = 0.012, **P < 0.001, paired Student’s t-test within groups, one-way ANOVA between groups). AUC, area under the curve; CPA, cyclopiazonic acid; EC, endothelial cell; GSK219, GSK2193874; His, histone; MA, mesenteric artery; n.s., not significant; ROI, region of interest; TLR4, Toll-like receptor 4; TRPV4, transient receptor potential vanilloid 4.
Histone proteins induce EC Ca2+ signals in pressurized ex vivo arteries but do not cause vasodilation.
Having established that histones trigger EC Ca2+ influx in the en face preparation, we sought to confirm the presence of histone-induced Ca2+ signals in intact, pressurized (80 mmHg) resistance-sized MAs ex vivo (pH 7.4, 36°C). Pressurized arteries were mounted on the stage of an upright spinning-disk confocal microscope, and PSS was continuously flowed through the vessel at an intraluminal flow rate (113 ± 34 μl/min) consistent with that reported in resistance-sized MAs in vivo (17). This allowed us to deliver histone proteins inside the lumen, where they would first interact with ECs, or outside the artery in the superfusate, where they first interact with perivascular nerves and SMCs. Using this setup, we are able to visualize 4–6 ECs in a single plane (Fig. 6, A and B). Small, local Ca2+ signals were observed at baseline (Fig. 6C and Table 1). Addition of histone proteins to the luminal solution induced large, spreading Ca2+ signals that were not seen under baseline conditions (Fig. 6, B and C, and Table 1; average intraluminal flow of PSS with 10 μg/ml histone proteins: 111 ± 22 μl/min). Exposure of the outside of the artery to histones by addition of histone proteins to the superfusate did not change Ca2+ signaling relative to baseline (Fig. 6C and Table 1). These data confirm that the histone-induced, spreading EC Ca2+ signals observed in the en face preparation are also detected in intact, pressurized arteries. Importantly, these data also demonstrate that histone-induced EC Ca2+ signals are endothelial derived and not secondary to activation of signals in perivascular nerves or SMCs.
Fig. 6.
Histone proteins induce EC Ca2+ signals in ex vivo pressurized MAs without causing dilation. A: cartoon representation of an intact third-order MA, pressurized (80 mmHg) with physiological flow (average 120 μl/min). B: representative images of baseline activity (top) and activity during a histone-induced signal (bottom) following introduction of histone proteins through the cannula directly into the lumen of the vessel. C: activity per field in intact third-order MA pressurized (80 mmHg) with intraluminal flow (112 ± 29 μl/min) under baseline conditions, following abluminal application of 10 μg/ml histone proteins or intraluminal application of 10 μg/ml histone proteins. Data are presented as means ± SE (n = 3 animals, 4–5 fields; *P = 0.0028, Student’s t-test). D: trace of diameter versus time for a pressurized (80 mmHg), ex vivo, third-order MA. Histone or control solution (PSS) was flowed through the lumen at ~2 μl/min (<5 dyne/cm2). EC SK/IK channels were activated using NS309, added to the bath, as an indicator of EC-dependent dilation. E–F: percent dilation relative to maximum passive dilation to Ca2+-free PSS before (Ctrl.) and after 30-min intralumenal histone treatment (10 μg/ml). Percent dilation to NS309 (0.1, 0.3, and 1.0 μM; E). Data are presented as means ± SE (n = 4 animals; *P < 0.001, paired Student’s t-test). Percent dilation to GSK101 (10 nM; F). Data are presented as means ± SE (10 μg/ml, n = 4 animals; *P = 0.045, paired Student’s t-test). Ab, abluminal; AUC, area under the curve; EC, endothelial cell; GSK101, GSK1016790A; Hist, histone; IK, intermediate conductance Ca2+-activated K+ channels; Intra, intraluminal; MA, mesenteric artery; PSS, physiological saline solution; SK, small conductance Ca2+-activated K+ channels.
The standard paradigm for vascular regulation by EC Ca2+ signaling is that increases in EC Ca2+ drive vasodilation through hyperpolarization caused by activation of Ca2+-dependent SK and IK channels and release of the vasodilator NO through activation of Ca2+-dependent eNOS. To test whether histone-induced increases in EC Ca2+ cause vasodilation, we measured changes in diameter of pressurized third-order branches of MAs ex vivo following exposure to histones. Arteries were pressurized to 80 mmHg and allowed to develop myogenic tone (average tone: 26.0 ± 2.0%), and histone proteins were delivered intraluminally at a low flow rate (2 μl/min) for 30 min to avoid complications of flow-mediated changes in diameter. Surprisingly, despite increasing intracellular Ca2+ event activity, intraluminal histone application did not cause vasodilation (Fig. 6D). Not only did histones fail to dilate arteries despite increasing intracellular Ca2+, prolonged exposure to histones severely blunted the dilatory effect of SK/IK channel activation with the potent SK/IK agonist, NS309 (1.0 μM) reducing it by 75% relative to the maximum dilation in Ca2+-free PSS (Fig. 6E). Exposure to the same intraluminal flow rate for 30 min without histones did not affect the dilatory response to NS309 (Fig. 6E). To confirm a histone-induced loss of endothelial dependent dilation, we measured the dilatory response to GSK101, a potent TRPV4 channel agonist, before and after a 30-min intralumenal histone application (average myogenic tone 23.8 ± 3.8%). GSK101 dilation was also eliminated after intralumenal histone treatment (75.8 ± 16.3 vs. 3.8 ± 8.2%; Fig. 6F).
One possible explanation for the loss of EC-dependent, IK/SK-mediated dilation is Ca2+ overload and subsequent EC death because of prolonged histone exposure. Although histone-induced signals are reversible, circulating histone levels are reported to remain elevated for days after a traumatic injury (1). Based on results from cell culture experiments, Xu et. al (42) postulated that elevated histone levels drive EC dysfunction through Ca2+ overload. To test this, we evaluated EC death and EC intracellular [Ca2+] in an en face MA preparation. To determine if prolonged histone exposure leads to cell death, we developed a real-time cell death assay by maintaining a low concentration of PI (100 ng/ml) in the recirculating PSS bath. Because PI does not enter intact cells but readily crosses the plasma membrane of dead or damaged cells, PI fluorescence (red) can be detected upon intercalation with DNA in the nucleus. Thus, PI uptake over time is taken as an indicator of cell death. Figure 7A shows a representative merged image of EC GCaMP (green) and mCherry (red) fluorescence before (left, baseline) and after (right) treatment with histones for 30 min; in these images, EC nuclei that became labeled during histone treatment are identifiable as ovoid regions in the center of ECs (Fig. 7A). Exposure to histone proteins caused PI labeling in 25% of EC nuclei per field (Fig. 7, A and B). PI labeling was absent in tissue treated with digested histone protein or left untreated for 30 min in PSS or Ca2+-free PSS (Fig. 7B). Application of histone protein in Ca2+-free PSS delayed, but did not prevent, EC death, suggesting that Ca2+ overload may not be the only driver of histone-induced EC death (Fig. 7B). Given that EC Ca2+ event activity in MAs was not decreased by TRPV4 or TLR4 knockout, we predicted that histone-induced EC death would not be decreased in TRPV4−/− or TLR4−/− mice. Indeed, PI uptake by ECs in en face, Fluor 4-loaded, preparations of MAs from TRPV4−/− or TLR4−/− mice was not different from that in C57 mice (Fig. 7F).
Fig. 7.
Histone proteins induce EC death and EC Ca2+ overload in en face and ex vivo pressurized MAs. A: merged image of GCaMP and mCherry fluorescence from an en face preparation of a third-order mouse MA. PI (100 ng/ml) was maintained in the circulating PSS. Dotted lines indicate borders of individual EC nuclei. The same field of view before histone exposure (baseline, left) and after 30 min of histone exposure (right). B: percentage of PI-labeled nuclei per field in tissue treated with histone proteins, histone proteins in Ca2+-free PSS, proteinase K-digested histone proteins, PSS only, or Ca2+-free PSS only. Data are presented as means ± SE (n = 3–4 animals, 4–13 fields; *P = 0.014, **P = 0.0003, ***P < 0.0001, Student’s t-test). C: FGCaMP/FmCherry in ECs in an en face MA preparation maintained in PSS containing 65, 194, 452, and 4,064 nM free Ca2+. Cells were permeabilized with digitonin (4 μM, 2 min). D: average FGCaMP/FmCherry versus free Ca2+ fit to the Hill equation (n = 3 animals, 9 fields). E: average cytosolic [Ca2+] from three cells per field treated with histone, proteinase K-digested histone, or untreated controls. Data are presented as means ± SE (n = 3 animals, 4–9 fields; *P = 0.0123, **P < 0.001, Student’s t-test). F: percentage of PI-labeled nuclei per field in Fluo-4-loaded tissue from C57 mice (histone-treated and -untreated) and histone-treated tissue from TRPV4−/− and TLR4−/− mice. Data are presented as means ± SE (n = 2–3 animals, 5–10 fields; *P < 0.01, Student’s t-test). G: representative merged image of GCaMP-GR and PI fluorescence in a pressurized third-order MA with intraluminal flow, before and after 30 min of histone exposure (10 μg/ml). H: percentage of PI-labeled ECs per field before and after 30 min of abluminal (Ab) or intraluminal (intra) histone exposure. Data are presented as means ± SE (n = 3–4 animals, 4 fields; *P = 0.01, paired Student’s t-test). [Ca2+], Ca2+ concentration; EC, endothelial cell; MA, mesenteric artery; PI, propidium iodine; PSS, physiological saline solution; TLR4, Toll-like receptor 4; TRPV4, transient receptor potential vanilloid 4.
Our ratiometric, EC-specific GCaMP-GR mouse enables us to estimate changes in intracellular EC [Ca2+] in intact native ECs. To calibrate ratiometric changes in GCaMP and mCherry fluorescence, we permeabilized the endothelium with digitonin and recorded the ratio of GCaMP fluorescence to mCherry fluorescence over a range of free [Ca2+] (see materials and methods). We fit these data to a sigmoidal variable-slope model and used the resulting standard curve to estimate intracellular Ca2+ in intact tissue (Fig. 7, C, D, and E). We examined PI-positive cells before (time 0) and after 5, 15, and 30 min of histone exposure. Cytosolic [Ca2+] increased from baseline (218 ± 12 nM Ca2+, limited by the minimum detectable GCaMP Ca2+ signal above tissue autofluorescence in our system) to 402 ± 76 nM after 5 min of histone exposure and to 793 ± 75 nM after 30 min (Fig. 7E). The GCaMP-GR fluorescence ratio plateaus at ~900 nM (Fig. 7D, see materials and methods); therefore, we may be underestimating EC [Ca2+] after 30 min of histone exposure. Cytosolic [Ca2+] did not change in untreated tissue or tissue treated with enzymatically digested histone proteins (Fig. 7E). These data demonstrate that histone proteins rapidly induce Ca2+ signals in native ECs that can lead to EC Ca2+ overload.
To confirm that histone proteins cause EC death in intact arteries, we imaged ex vivo pressurized MAs. Approximately 50% of visible EC nuclei per field were labeled with PI after 30 min of luminal histone exposure (Fig. 7, G and H; PI was included in both lumen and bath solutions). In contrast, addition of histone proteins only to the superfusate resulted in no EC PI labeling over 30 min (Fig. 7H). These findings provide a mechanistic context for endothelial dysfunction reported in the clinical literature by demonstrating that histone proteins activate a Ca2+-influx pathway that does not promote vasodilation but instead results in a decrease in EC-dependent dilation.
DISCUSSION
Histone proteins induce Ca2+ signals in ECs of native resistance arteries.
Recent evidence suggests that elevated histone protein levels in the circulation after traumatic injury correlate with markers of endothelial damage and may be responsible for systemic vascular complications (1, 22, 28). Histones mediate death in a murine sepsis model (42), but the effects of histones on native endothelium and the molecular mechanism of histone-induced increases in Ca2+ had remained unknown. Here, we demonstrate that histone proteins at a concentration of 10 μg/ml, which is at the low end of circulating histone levels reported in trauma patients, induce large, spreading Ca2+ signals in ECs of resistance-sized MAs. Interestingly, not all ECs in a field of view responded to histones. Whether Ca2+ event activity was recorded for 2 min (Fig. 2) or EC Ca2+ overload and PI labeling was measured after 30 min (Fig. 7), ~25% of ECs per field responded to histones. This indicates that the response to histones is not homogeneous throughout the endothelium, a finding consistent with literature results describing EC heterogeneity (2–4, 10, 40). However, whether this indicates heterogeneous expression of a histone receptor or reflects some other cell-to-cell variation is currently unknown.
The properties of histone-induced Ca2+ signals are distinct from those of known propagating EC Ca2+ signals. Notably, histone Ca2+ signals spread within and between ECs, even when intracellular Ca2+ stores have been depleted. How histone-induced Ca2+ signals spread is not yet clear, but unlike IP3R-mediated Ca2+ waves they are not regenerative. Histone signals initiate and terminate at the same site. Because the spread of histone-induced signals between cells is prevented by the gap junction blocker, carbenoxolone, the implication is that Ca2+ influx stimulated by histones spreads between ECs via gap junctions (Fig. 1). However, whether this spread is a result of intra- and intercellular diffusion of Ca2+ or is attributable to a small molecule activator of Ca2+ influx is unknown.
Histone-induced EC Ca2+ signals are not mediated by TRPV4 channels or TLR4 receptors.
To determine the nature of histone-induced Ca2+ signals, we used a pharmacological approach to screen potential candidate pathways, focusing on TRPV4 channel-mediated Ca2+ influx and IP3R-mediated Ca2+ release from intracellular stores—both well-documented Ca2+-elevation mechanisms. Although TRPV4 channels play a prominent role in EC Ca2+ influx (18, 31–33), we found that histone-induced EC Ca2+ signals in en face MA preparations were not blocked by the TRPV4 channel antagonist, GSK219, and were retained in MAs from TRPV4−/− mice (Figs. 5 and 6). Unlike TRPV4 channel-initiated regenerative Ca2+ waves, which were inhibited by depleting intracellular stores with CPA, histone-induced signals were still present after CPA pretreatment (Figs. 2 and 4). These observations suggest that histone-induced signals do not reflect IP3R-mediated Ca2+ release from intracellular stores and tend to rule out a role for Gq-coupled receptor signaling via PLC and activation of STIM/Orai by store depletion.
Previous research has focused on histones as mediators of death through activation of the immune system (41, 42). In the current work, we focused on the acute vascular response to histones. Although TLR4 has been shown to mediate histone-evoked cytokine release and lethality in murine models (41), it was not known if TLR4 receptors contribute to histone-induced EC Ca2+ signals or histone-induced EC death. We found that genetic ablation of TRL4 did not decrease histone-induced EC Ca2+ signals or EC death (Fig. 7). This suggests that acute histone-induced EC Ca2+ signaling occurs through a TLR4-independent pathway.
What is the physiological significance of histone-induced Ca2+ influx?
In the current study, we demonstrated that histone proteins induce Ca2+ influx in ECs of native resistance-sized arteries. Published work clearly demonstrates that circulating histone proteins are elevated after traumatic injury (1, 22, 28, 42). Surprisingly, although histone proteins caused a profound increase in EC Ca2+, they exerted no vasodilatory effect. How do we reconcile this with the known vasodilatory role of elevated EC Ca2+? There are two possible explanations. First, histone proteins may activate a nonselective cation pathway with a low Ca2+/Na+ bi-ionic permeability ratio, thereby overriding Ca2+-dependent, SK/IK channel-mediated hyperpolarization and subsequent vasodilation by allowing sufficient Na+ influx to depolarize ECs (Fig. 8). This is supported by the observation that removal of extracellular Ca2+ did not prevent PI labeling in response to histone treatment, demonstrating that Ca2+ is not necessary for histone-induced EC death (Fig. 7E). Second, prolonged histone exposure causes Ca2+ overload and EC death, which ultimately causes vascular dysfunction through loss of endothelial dependent hyperpolarization. This is supported by the loss of EC SK/IK and TRPV4 dependent dilation after prolonged histone exposure (Fig. 6, E and F). We focused on the SK/IK dilatory mechanism for two reasons. First, because SK/IK-mediated dilation is downstream of a cytosolic Ca2+ increase, if it were impaired, one would not expect mechanisms that increase Ca2+ through any other pathway—be it influx through TRPV4 channels or release from the ER via stimulation of IP3 generation through muscarinic receptor agonism—to have an effect. We were able to confirm a loss of a TRPV4 channel-mediated dilation after histone treatment (Fig. 6F). Second, SK/IK dilations are rapidly reversible and highly reproducible over multiple applications in the same preparation (Fig. 6, D and E). Both possibilities imply the involvement of a noncanonical EC Ca2+ influx pathway that is still under investigation.
Fig. 8.
Model of histone-induced loss of EC function. Circulating histone proteins are elevated after traumatic injury through cellular lysis and production of neutrophil extracellular traps (NETs). Histones activate a nonselective cation channel (NS+) with a low Ca2+/Na+ bi-ionic permeability ratio that allows rapid global increases in EC Ca2+ that do not engage vasodilatory pathways through myoendothelial projections (MEPs) that are normally activated by Ca2+ released from intracellular stores or local Ca2+ influx mediated by TRPV4 channels. [Ca2+], Ca2+ concentration; EC, endothelial cell; IK, intermediate conductance Ca2+-activated K+ channels; SK, small conductance Ca2+-activated K+ channels; TRPV4, transient receptor potential vanilloid 4.
Our data demonstrate that circulating histone proteins cause a robust influx in Ca2+ that is not a result of TRPV4 channels, TLR4-dependent signaling, or Ca2+ release from intracellular stores. Prolonged exposure to histone proteins causes EC Ca2+ overload and EC death in native tissue. Furthermore, we demonstrated that histone proteins are detrimental to endothelium-dependent dilation in native tissue. We speculate that EC death and loss of endothelium-dependent function may contribute to trauma-associated vascular dysfunction reported in the clinical literature.
GRANTS
D. M. Collier is supported by NIH (grant no. 5K99HL133451); K. Freeman by NIH (grant no. 1R01GM123010); and M. T. Nelson by NIH (grant nos. 5R01HL131181, 5R01HL121706, 5R37DK053832, and 7UM1HL120877), The Fondation Leducq, European Union's Horizon 2020 research and innovation programme, grant agreement no. 666881, and the Totman Medical Research Trust. The mouse strain Cx40-GCaMP-GR was developed by CHROMus which is supported by the National Heart Lung Blood Institute of the National Institute of Health under award number R24HL120847 to M. I. Kotlikoff.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M.C., M.T.N., and K.F. conceived and designed research; D.M.C., N.V., A.S., Z.D.M., B.S., J.C.L., F.K.L., and S.R. performed experiments; D.M.C., N.V., A.S., and A.D.B. analyzed data; D.M.C., N.V., A.S., A.D.B., M.T.N., and K.F. interpreted results of experiments; D.M.C., N.V., B.S., J.C.L., F.K.L., and M.I.K. prepared figures; D.M.C. drafted manuscript; D.M.C., J.S.M., F.K.L., M.I.K., M.T.N., and K.F. edited and revised manuscript; D.M.C., N.V., A.S., A.D.B., Z.D.M., J.S.M., B.S., J.C.L., F.K.L., S.R., M.I.K., M.T.N., and K.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mike Previs (University of Vermont) for mass spectrometry analysis of unfractionated histone proteins and University of California Irvine (UCI) Transgenic Mouse Facility (TMF) for production of BAC transgenic mice. The UCI TMF is a shared resource funded in part by the Chao Family Comprehensive Cancer Center Support Grant P30CA062203) from the National Cancer Institute.
REFERENCES
- 1.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, Wang G, Toh CH. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187: 160–169, 2013. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100: 174–190, 2007. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 74: 3203–3207, 1977. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 7.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754, 1991. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 8.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 9.Cheung M, Bao W, Behm DJ, Brooks CA, Bury MJ, Dowdell SE, Eidam HS, Fox RM, Goodman KB, Holt DA, Lee D, Roethke TJ, Willette RN, Xu X, Ye G, Thorneloe KS. Discovery of GSK2193874: An orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med Chem Lett 8: 549–554, 2017. doi: 10.1021/acsmedchemlett.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 100: 10623–10628, 2003. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 110: 6157–6162, 2013. doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459: 863–879, 2010. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. doi: 10.1016/S0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 16.Goeger DE, Riley RT, Dorner JW, Cole RJ. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol 37: 978–981, 1988. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 17.Henrion D, Terzi F, Matrougui K, Duriez M, Boulanger CM, Colucci-Guyon E, Babinet C, Briand P, Friedlander G, Poitevin P, Lévy BI. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. J Clin Invest 100: 2909–2914, 1997. doi: 10.1172/JCI119840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122, 1990. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe LF. Classes and mechanisms of calcium waves. Cell Calcium 14: 736–745, 1993. doi: 10.1016/0143-4160(93)90099-R. [DOI] [PubMed] [Google Scholar]
- 22.Johansson PI, Windeløv NA, Rasmussen LS, Sørensen AM, Ostrowski SR. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock 6: 171–175, 2013. doi: 10.4103/0974-2700.115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 23: 279–287, 2017. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 24.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci USA 95: 15821–15825, 1998. [Erratum in: Proc Natl Acad Sci USA 96: 3330, 1999] doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moncoq K, Trieber CA, Young HS. The molecular basis for cyclopiazonic acid inhibition of the sarcoplasmic reticulum calcium pump. J Biol Chem 282: 9748–9757, 2007. doi: 10.1074/jbc.M611653200. [DOI] [PubMed] [Google Scholar]
- 27.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 28.Russell RT, Christiaans SC, Nice TR, Banks M, Mortellaro VE, Morgan C, Duhachek-Stapelman A, Lisco SJ, Kerby JD, Wagener BM, Chen MK, Pittet JF. Histone-complexed DNA fragments levels are associated with coagulopathy, endothelial cell damage, and increased mortality after severe pediatric trauma. Shock 49: 44–52, 2018. doi: 10.1097/SHK.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 29.Shinohara T, Michikawa T, Enomoto M, Goto J, Iwai M, Matsu-ura T, Yamazaki H, Miyamoto A, Suzuki A, Mikoshiba K. Mechanistic basis of bell-shaped dependence of inositol 1,4,5-trisphosphate receptor gating on cytosolic calcium. Proc Natl Acad Sci USA 108: 15486–15491, 2011. doi: 10.1073/pnas.1101677108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shui B, Lee JC, Reining S, Lee FK, Kotlikoff MI. Optogenetic sensors and effectors: CHROMus-the Cornell Heart Lung Blood Institute Resource for Optogenetic Mouse Signaling. Front Physiol 5: 428, 2014. doi: 10.3389/fphys.2014.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol 594: 3271–3285, 2016. doi: 10.1113/JP271652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69, 1983. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 35.Strøbæk D, Teuber L, Jørgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta 1665: 1–5, 2004. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278: 26541–26549, 2003. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MN, Earley S. TRP channel Ca(2+) sparklets: fundamental signals underlying endothelium-dependent hyperpolarization. Am J Physiol Cell Physiol 305: C999–C1008, 2013. doi: 10.1152/ajpcell.00273.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 39.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 326: 432–442, 2008. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 40.Wilson C, Saunter CD, Girkin JM, McCarron JG. Clusters of specialized detector cells provide sensitive and high fidelity receptor signaling in the intact endothelium. FASEB J 30: 2000–2013, 2016. doi: 10.1096/fj.201500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 187: 2626–2631, 2011. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med 15: 1318–1321, 2009. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]