Abstract
The death of cardiomyocytes is a precursor for the cascade of hypertrophic and fibrotic remodeling that leads to cardiomyopathy. In diabetes mellitus (DM), the metabolic environment of hyperglycemia, hyperlipidemia, and oxidative stress causes cardiomyocyte cell death, leading to diabetic cardiomyopathy (DMCM), an independent cause of heart failure. Understanding the roles of the cell death signaling pathways involved in the development of cardiomyopathies is crucial to the discovery of novel targeted therapeutics and biomarkers for DMCM. Recent evidence suggests that hydrogen sulfide (H2S), an endogenous gaseous molecule, has cardioprotective effects against cell death. However, very little is known about signaling by which H2S and its downstream targets regulate myocardial cell death in the DM heart. This review focuses on H2S in the signaling of apoptotic, autophagic, necroptotic, and pyroptotic cell death in DMCM and other cardiomyopathies, abnormalities in H2S synthesis in DM, and potential H2S-based therapeutic strategies to mitigate myocardial cell death to ameliorate DMCM.
Keywords: apoptosis, autophagy, diabetes, necroptosis, pyroptosis, therapeutic strategy
INTRODUCTION
Myocardial cell death is a critical element in the pathogenesis and progression of several etiologies of cardiomyopathies, one of which is present specifically in individuals with diabetes mellitus (DM) (11, 106). The diabetic environment of hormones, cytokines, hyperglycemia, fatty acids, and adrenergic tone leads to a structural and functional cardiac muscle disorder known as diabetic cardiomyopathy (DMCM). DMCM develops independently of atherosclerosis, valvular disease, hypertension, and other common cardiovascular disease contributors (8, 10) and as such presents itself as an independent mechanism by which heart failure may occur. The cell death initiated in DMCM contributes to the loss of contractile units, arrhythmias, compensatory hypertrophy, and fibrosis (143), thus increasing the risk of DM-induced heart failure. It is well demonstrated that major cell death pathways like apoptosis, autophagy, necrosis, and pyroptosis all play a role in the development and progression of DMCM and other cardiovascular diseases (8).
The accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) have been shown to play a critical signaling role in the myocardial cell death process (153). More recently, studies have revealed a growing role of reactive sulfide species in cell death (69). Hydrogen sulfide (H2S) is an endogenous antioxidant gaseous signaling molecule that interacts with proteins present in body tissue and the circulation (112). Plasma H2S levels are decreased in T2DM patients and high-fat diet-treated mice (4, 54, 58). In addition, preclinical studies have shown that exogenous H2S treatment ameliorates DMCM, suggesting that restoring H2S levels may be a potential novel treatment option for DMCM (4). Whereas other gaseous signaling molecules such as nitric oxide (NO) have well-established therapeutic roles, comparatively little is known about signaling by H2S, which has the potential to act as a therapeutic gaseous molecule as well. Here, we review the role of H2S in cell death mechanisms in cardiomyopathies, with a focus on DMCM. We elaborate on the pathways mediating these effects and discuss the therapeutic and cardioprotective potential of H2S.
H2S PRODUCTION AND SIGNALING
H2S production is highly regulated, and it is produced on demand due to its potential toxicity at higher levels. All three enzymatic pathways that produce H2S are centered on homocysteine and cysteine metabolism. These pathways are cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and the coupling of cysteine aminotransferase (CAT) and 3-mercapto-pyruvate sulfur transferase (3-MST) (40, 69, 191). The expression of H2S-generating enzymes is subcellular and tissue specific. On a cellular level, CSE occurs strictly in the cytoplasm, whereas CAT is located in the mitochondria. Regarding tissue specificity, CSE is most abundant in the cardiovascular system (83), whereas CBS is predominant in the nervous system and liver and expressed in the heart.
A growing body of research is uncovering a role of H2S in cellular signaling similar to the other gasotransmitters. One of the mechanisms by which H2S signals cellular processes is by the sulfhydration of cysteine residues of target proteins (112). This posttranslational modification is reversible by the thioredoxin system, further lending credence to its role in signaling (6, 71). Whereas NO acts on proteins through nitrosylation, a more substantial portion of proteins is sulfhydrated by H2S, thus increasing enzyme activity. Notably, cases of inhibition of protein activity with sulfhydration also exist (105, 112). H2S also interacts with proteins to produce reactive persulfide that in turn causes structural changes in proteins (69). This modulates the function of K+ and Ca2+ ion channels, attenuates apoptosis and oxidative stress, and protects metabolic and mitochondrial functions (112). Finally, evidence suggests that H2S signaling regulates NO and CO production (112, 116). The roles of H2S in these signaling pathways in relation to cell death in cardiomyopathies, specifically DMCM, are elaborated on further in this review.
H2S SIGNALING IN CARDIOMYOPATHIES
H2S has been shown to have significant effects on the cardiovascular system. H2S reduces hypertension (2, 3, 136, 162) and improves glucose uptake and metabolism in cardiomyocytes (9, 89). Furthermore, multiple studies have shown that free H2S levels are reduced in patients with congestive heart failure (35, 117). Inhibition of H2S synthesis by knockout of CSE increases leukocyte adherence in vessels, suggesting a role for H2S in atherosclerosis development (184). Regarding DMCM specifically, plasma H2S levels are decreased in T2DM patients, DM rats, and high-fat, diet-fed mice (9, 64, 66). H2S donor administration prevents cardiomyocyte hypertrophy and increases ejection fraction in DM rats and mice (142, 194). H2S supplementation mitigates hyperglycemia-induced cardiomyocyte dysfunction, as demonstrated in multiple studies (72, 168). Furthermore, a protective effect of H2S has been shown in other cardiomyopathy models, including doxorubicin-induced cardiomyopathy, smoking-induced cardiomyopathy, and ischemia-reperfusion injury (I/R)-induced cardiomyopathy (12, 81, 90, 193). Together, these findings suggest that H2S supplementation may act as a potential therapy for DMCM and other cardiomyopathies
H2S IN CARDIOVASCULAR CELL DEATH MECHANISMS
Cell death mechanisms are a hallmark of the progression of all cardiomyopathies, including DMCM. Myocyte, endothelial, and fibroblast cell death can occur by necrosis, deregulated autophagy, or apoptosis. Each of these cell death pathways has been shown in failing human DM hearts (11, 29). In type 1 DM (T1DM) rats and STZ-induced T1DM mice, myocardial apoptosis was observed starting on day 3 after DM induction, suggesting that it occurs early in the progression of the disease (27). Recent reports have also shown a role of pyroptosis in cardiomyopathies (92). H2S signaling has been implicated in these processes within the heart, and thus it may help regulate cell death in cardiomyopathy (summarized in Table 1).
Table 1.
H2S regulation of cell death in multiple organ systems
| Type of Cell Death (Organ/Disease) | Effect of H2S | Source (Ref. Nos.) |
|---|---|---|
| Apoptosis | ||
| Heart/ischemia-reperfusion | Anti-apoptotic, ↑Bcl-2, Bcl-XL, ↓miR-1 | 47, 61, 75 |
| Heart/hyperglycemia | Anti-apoptotic, ↓p-JNK, p-cJun, NF-κB, ROS, ↑ PI3K/Akt, Nrf2 | 4, 72, 158 |
| Heart/smoke induced | Anti-apoptotic | 193 |
| Neurons | Anti-apoptotic, ↑miR-485–5p | 18 |
| Brain/traumatic injury, Parkinson’s | Anti-apoptotic, ↑Bcl-2, ↑p62 | 48, 185 |
| Gastric epithelium/ischemia-reperfusion | Anti-apoptotic, Keap1 S-sulfhydration, ↑ Nrf2 activation, ↓ NF-κB activation | 38 |
| Liver/ischemia-reperfusion | Anti-apoptotic, ↓ROS | 50, 57 |
| Aorta | ↑ Apoptosis, ↑ MAPK | 173 |
| Autophagy | ||
| Brain/traumatic injury | Anti-autophagic, ↓LC3-II, ↑p62 | 185 |
| Aorta/diabetes | Anti-autophagic, ↑ Nrf2 activation, ↓ROS | 89 |
| Heart/diabetes | Anti-autophagic, ↑PI3K/Akt1 | 166 |
| Heart/ischemia-reperfusion | Anti-autophagic, ↑ mTOR | 165 |
| Liver/NAFLD | ↑ Autophagy, AMPK activation, ↑ TG clearance | 141 |
| Pyroptosis | ||
| Bone derived macrophages | Antipyroptotic, ↓caspase 1 activity, ↓ chemokines, ↓ ROS | 14 |
| Retina | Antipyroptotic, ↓ER stress | 34 |
| Aortic endothelium | Antipyroptotic, ↓caspase 1 expression | 164 |
| Necroptosis | ||
| Heart/infarction | Antinecrotic | 33 |
| Heart/hyperglycemia | Antinecroptotic | 87 |
AMPK, AMP-activated protein kinase; ER, endoplasmic reticulum; H2S, hydrogen sulfide; Keap1, Kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NAFLD, nonalcoholic fatty liver disease; Nrf2, nuclear factor-E2-related factor; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; TG, triglyceride. ↑, Increase; ↓, decrease.
APOPTOSIS
Apoptosis is a regulated, noninflammatory cell death pathway that utilizes initiator and effector caspases to cleave cellular and nuclear components. It results in the formation of apoptotic bodies and maintenance of intact plasma membrane. Mitochondrial membrane proteins such as Bcl-2, which inhibits apoptosis, and Bax, which activates apoptosis, regulate it. Perturbations in both the intracellular and extracellular environment can induce apoptosis (30). Intrinsic apoptosis is initiated by several factors, including DNA damage, endoplasmic reticulum (ER) stress, and intracellular ROS (114). These stressors lead to irreversible permeabilization of the outer membrane of mitochondria, which leads to the release of mitochondrial proteins such as cytochrome c, which activates caspase-9 and caspase-3 (82). Alterations in the extracellular environment such as hyperglycemia and inflammation activate death receptors such as the TNF receptor that ultimately activate caspase-8, which in turn activates caspase-3 (30).
Apoptosis has been demonstrated extensively in hypertrophic cardiomyopathy, ischemic cardiomyopathy, myocarditis, and dilated cardiomyopathy (36, 73, 128, 161, 183). Cardiomyocyte stretching (hypertrophy) activates many pathways that in turn initiate apoptosis (43, 62, 130). High glucose concentrations induce apoptosis in cardiomyocytes, and apoptosis has been confirmed in pathological specimens of patients with DMCM (11, 181). In addition, high concentrations of ROS, circulating and local inflammatory cytokines, ER stress, and activation of the renin angiotensin system (RAS) are known activators of caspases that mediate apoptosis, and these all are present in DMCM (10). Interestingly, nitrosylation of caspases by NO have been shown to activate apoptosis in DMCM, suggesting that sulfhydration by H2S also plays an important regulatory role (120).
In fact, in a rat cigarette smoking-induced cardiomyopathy model, H2S supplementation decreased apoptosis, as measured by increased Bcl-2 expression and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. Pig and rat models of ischemia-reperfusion injury showed that H2S treatment reduced apoptosis (109). A study by Shi et al. (138) demonstrated reduced apoptosis in cells treated with H2S during I/R and was mediated by inhibiting JNK kinase, which is required for cytochrome c release into the cytosol. The role of H2S on apoptosis may be organ dependent or context dependent, as studies show that H2S prevents apoptosis in leukocytes but induces apoptosis in aorta smooth muscle cells (124, 175).
AUTOPHAGY
Autophagy is a cellular homeostatic mechanism for clearing damaged or unnecessary organelles and macronutrients by lysosomal degradation (167). Autophagy is a critical cellular process, playing both normal and pathological roles in the cardiovascular system. First described in the failing heart, it has been shown to be involved all cardiomyopathies (76). An optimal window of autophagy maintains homeostasis in response to cardiovascular events while excessive or deficient autophagy becomes maladaptive, leading to increased cell death. In ischemia-reperfusion injury, cardiomyocyte autophagy is triggered by increased afterload with both adaptive and maladaptive features (76, 79, 127). Patient biopsies from dilated cardiomyopathy showed high levels of autophagy when afterload was high (68). Increased autophagy is also observed in doxorubicin-induced cardiomyopathy (94, 95).
Autophagy is adapted to meet myocardial metabolic demand by releasing cellular components and employs a signaling network involving AMPK, which induces autophagy, and insulin pathways for regulation, making it an essential process in DM (20, 23). The nutrient-sensing mTOR complex 1 (mTORC1) integrates metabolic signals from AMPK and insulin, and when activated, mTOR inhibits the initiation of autophagy. Several studies demonstrate AMPK activation ameliorates DM-related consequences in hyperglycemic cardiomyocytes. However, in DMCM, a consensus on the role of autophagy has not been reached (20). In T1DM, some studies show increased autophagy in cardiomyocytes, whereas others show decreased autophagy (60, 169). Similarly, in T2DM mice, some studies show reduced autophagic markers, whereas others show increased autophagy (44, 60, 104). These differences may be due to differences in the model of DM used, the time of measurement, the method of measurement of autophagic flux, and glucose concentrations.
Oxidative and nitrosative mechanisms have been shown to regulate autophagy in cardiomyopathies, and recent evidence shows the importance of H2S signaling in this process (153). In smoking-induced cardiomyopathy, H2S administration decreased autophagy (193). A downregulation in autophagy markers with H2S was also seen in an ischemia-reperfusion model (165). A H2S donor has been shown to protect against cell death in high-glucose-treated cardiomyocytes via activation of AMPK. However, this study did not test whether the autophagic flux was also altered (158). Nitric oxide signaling alters autophagy in cardiomyopathy through a combination of cytokine expression, mitochondrial oxidative stress signaling, protein nitration, MMP activation, epigenetic alterations, and activation of transcription factors (153). It is likely that H2S employs similar pathways to alter autophagy. It is important to note that the effects of H2S on autophagy appear to be dependent on the pathological condition and the model system, as H2S activates AMPK in DMCM models (supposedly increasing autophagic flux) but activates mTOR to inhibit autophagy in I/R injury (165).
NECROSIS, NECROPTOSIS, AND MITOCHONDRIAL PERMEABILITY TRANSITION PORE-DRIVEN NECROSIS
Necrosis is a highly inflammatory form of cell death following severe injury, whereas necroptosis is a programmed form of necrosis that utilizes TNF receptor signaling and the caspase-independent RIPK1/RIPK3 necrosome (26, 151). Myocardial cell death by necrosis occurs after a severe injury such as ischemia. Necrosis has been observed in the autopsy of T2DM patient hearts and T1DM rat and mouse hearts (11, 29). An increase in necroptosis has been shown in the heart after ischemic insults (186). The evidence showing an effect of H2S on necrosis or necroptosis in cardiomyopathies is limited. A study by Geng et al. (33) demonstrated a decrease in necrosis markers after injection of NaHS. This decrease was measured by creatine phosphokinase and lactate dehydrogenase leakage into plasma and histology in a rat infarction model. Two more recent studies with cardiomyocytes treated with high glucose demonstrate an inhibition of necrosis and necroptosis by H2S (86, 87). Taken together, these studies suggest an anti-necroptotic effect by H2S, although the mechanism by which this occurs remains unclear.
Mitochondrial permeability transition (MPT) pore-driven necrosis is another regulated cell death pathway that ends with necrosis. It is initiated specifically by extremely high ROS and Ca2+ overload in the cytosol (30). These stressors lead to opening of the nonspecific MPT pore in the mitochondrial inner membrane, dissipate inner membrane potential, and rupture both mitochondrial membranes through osmotic swelling (30). Whereas loss of the outer membrane activates cytochrome c and caspase-3 similarly to apoptosis, the lack of ATP prevents formation of apoptotic bodies, and cell death occurs by necrosis instead (24). Hyperglycemia in DMCM induces MPT-driven necrosis through advanced glycosylation end products that elevate cytosolic ROS production (25). DMCM rodents also show impaired calcium uptake by mitochondria and elevated cytosolic calcium, which induces MPT pore opening (28). Studies show that H2S protects against MPT-driven necrosis in the heart and brain (91, 182), although its role in MPT-driven necrosis in DMCM is unclear. In I/R-induced cardiomyopathy, however, whereas H2S had no effect on MPT pore opening when applied directly to isolated mitochondria, it prevented MPT pore opening in the disease model (179).
PYROPTOSIS
Pyroptosis is a unique form of cell death in which the canonical pathway is specifically dependent on caspase-1 and the noncanonical pathway is reliant on caspase-11 (7). This cell death mechanism has several major characteristics that set it aside from apoptosis and necrosis. Originally discovered in monocytes and macrophages, pyroptosis has undergone a paradigm shift from being involved in immune response to taking part in pathological inflammation that contributes to diseases that are affiliated with inflammation and cell death (99). The rapid swelling and lysis of the cell membrane that result in the release of the inflammatory cytokines interleukin-1 β (IL-1β) and IL-18 characterize pyroptosis. This inflammatory lysis is in stark contrast to the organized membrane blebbing and chromosome packaging that occurs during apoptosis (26). Pyroptosis and its downstream inflammatory markers have been linked to pathogen defense, toxic shock, neuropathy, and nephropathy (85, 137, 176). However, we will focus on the role of pyroptosis in cardiovascular disease and DMCM.
The NOD-like receptor protein 3 (NLRP3) inflammasome is charged with activating caspase-1 in the canonical pyroptosis pathway. Blocking the inflammasome has been shown to mitigate cardiac remodeling post-infarction (96). NLRP3 also localizes to the mitochondria and contributes to increased reactive oxygen species (ROS) production in cardiomyocytes, which is linked to cardiomyopathy (190). IL-18 has been shown to induce cardiac hypertrophy through increasing the expression of atrial natriuretic peptide (ANP) (97). It is elevated in the serum and smooth muscle of infarction mouse models, and neutralizing IL-18 before ischemia significantly reduced infarct size (146). Elevated serum levels of IL-1β, a second inflammatory cytokine downstream of the pyroptosis pathway, has also been reported post-MI and in patients with cardiomyopathy (37). Although many inflammatory cytokines are elevated in cardiomyopathies, activation of IL-18 and IL-1β is unique to pyroptosis.
There is limited knowledge on the role of pyroptosis in DMCM. Pyroptosis may be triggered directly and indirectly in the DM heart. Knockout of NLRP3 that initiates pyroptosis improves glucose sensitivity in high-fat diet-fed mice (150). NLRP3-dependent pyroptosis is observed in the DM rat heart, and it contributes to DMCM (92, 93, 150). Palmate, a fatty acid upregulated in DM, also triggers the formation of the inflammasome (21). In both humans and mice, hyperglycemia and lipotoxicity upregulate cardiac NLRP3, caspase-1, and IL-1β (136, 150). Importantly, rosuvastatin treatment alleviated DMCM by inhibiting the NLRP3 inflammasome in T2DM rats (94). Targeting ELAVL1, an RNA binding protein, with miRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes (56). In a clinical trial of 551 T2DM patients, canakinumab, an IL-1β inhibitor, resulted in a small decrease in Hb A1c; however, its effect on cardiac function is unexplored (45).
Several hypotheses have been proposed for how DM induces NLRP3 inflammasome activation and its association with H2S. One hypothesis is that DM increases thioredoxin-interacting protein expression and insulin resistance, causing cellular oxidative/nitrosative stress, leading to NLRP3 inflammasome activation and subsequent cardiomyocyte pyroptosis (92, 139). Because of similarities between nitric oxide and H2S, a role for sulfide signaling is suggested in pyroptosis. Another hypothesis is that, reduced CBS and CSE in the DM heart decreases H2S generation and increases homocysteine accumulation, causing hyperhomocysteinemia (133). Hyperhomocysteinemia activates matrix metalloproteinase-9 (MMP9) in the DM heart. Notably, MMP9 ablation in the DM heart decreases pyroptosis markers in the heart (102, 171). This link suggests a vital role of CBS, CSE, H2S signaling, and MMP9 in the regulation of pyroptosis in cardiomyopathies, especially in DMCM.
A limited number of studies have shown a role of H2S signaling in pyroptosis in other organs and in pathological conditions. In mouse bone-derived macrophages stimulated with uric acid crystals, H2S donors inhibited caspase-1 activity and IL-1β secretion usually seen after stimulation by the crystals (14). Treatment with GYY4137, a H2S donor, mitigates endoplasmic reticulum (ER) stress and pyroptosis and improves visual function in the CBS+/− mice (34). CBS−/− mice, a model of severe hyperhomocysteinemia, induces caspase-1 activation along with other pyroptosis markers determined by flow cytometry gating in isolated lung endothelial cells and in aortic endothelium (164). To date, only one study has reported a role for H2S in pyroptosis in the heart. Toldo et al. (147) demonstrated that an H2S donor suppressed several markers of pyroptosis activity (caspase-1 activity, caspase-1 recruitment via the ASC adapter protein) in an I/R cardiomyopathy model. The role of pyroptosis in cardiomyopathies and the mechanistic role of sulfide signaling in pyroptosis require further study.
Of note, whereas most studies show that H2S mediates suppression of aberrant cell death, some studies show deleterious effects of H2S, such as a study by Yang et al. (173) that demonstrated increased apoptosis after the addition of H2S gas to human aorta smooth muscle cells in culture. The study by Yang et al. (173) used a relatively high concentration of 500 µM using pure gas instead of a H2S donor. However, variability in effects of H2S is likely due to varying concentrations and active forms of H2S used. Measured physiological H2S concentrations range from nanomolar to micromolar concentrations, which is likely due to the variety of methods used to measure H2S (40, 134).
POTENTIAL MECHANISMS OF H2S-MEDIATED PROTECTION AGAINST CELL DEATH
Antioxidant Mechanisms
Oxidative damage from reactive oxygen species (ROS) is an established trigger for cell death in cardiomyopathies. Increased ROS production and decreased antioxidant capacity are observed in patients and animal models of DMCM, doxorubicin-induced cardiomyopathy, and congestive heart failure (22, 63, 160). Higher H2O2 induces apoptosis, whereas lower myocardial oxidative stress by a reduction in NADPH oxidase activity reduces apoptosis and blunts hypertrophy (129). Although there is a lack of data connecting DMCM, pyroptosis, and ROS, a transverse aortic constriction (TAC)-induced hypertrophy model showed that ROS levels were increased along with increased NLRP3 and IL-1B (157). This increase in pyroptosis and ROS was also observed in an I/R injury model (154). Taken together, there is a clear connection with ROS and apoptosis in DMCM as well as ROS and pyroptosis in other models of cardiomyopathy.
H2S appears to signal changes in ROS production and antioxidant pathways, potentially connecting H2S levels with the activation of apoptosis and pyroptosis by way of ROS signaling. The incubation of HeLa cells with H2O2, , and ONOO− resulted in the release of H2S, suggesting a homeostatic feedback mechanism (189). Incubation of cardiomyocytes with H2S led to a dose-dependent decrease in ROS and inhibition of caspase-3-dependent apoptosis in high-glucose-treated cardiomyocytes (72). Moreover, H2S treatment reversed decreased activity of superoxide dismutase and decreased autophagy in aortic endothelial cells from T2DM mice (89).
Several hypotheses have been proposed for the mechanism by which H2S reduces ROS. H2S may be a direct reducing agent, as it has been shown in endothelial cells to reduce hydrogen peroxide and oxidized lipoproteins levels (131). H2S may also impart antioxidant actions by an indirect or feedback mechanism (65). Many studies now demonstrate that H2S drives the downstream regulation of canonical transcription factors that regulate oxidative damage and antioxidant genes, including FOXO3, NF-κB, and Nrf2 (116).
Noncoding RNA
Cell death mechanisms are some of the central pathways regulated by miRNA. miRNAs are ∼22 nucleotide, noncoding RNA molecules that negatively regulate target gene by mRNA degradation or translational repression (101). Several miRNAs have been implicated in cell death during cardiomyopathies (101). miR-195 overexpression induces cardiac hypertrophy and promotes ROS generation and apoptosis and suppresses Bcl-2 in cardiomyocytes (152, 195). miR-1 and miR-144 increase apoptosis in high-glucose-treated cardiomyocytes (180, 181). Increased miR-30d was shown to increase pyroptosis in diabetic rats (84).
Cross-talk between miRNA and sulfide signaling may mediate the beneficial cardiac effects of H2S (40). Rats treated with H2S donor before I/R injury showed downregulated miR-1 and decreased apoptosis compared with the untreated I/R group in the heart (61). Several studies also show that H2S increases expression of miR-133a, which ameliorates cardiomyocyte hypertrophy and reduces apoptosis (64, 88, 123). miR-21 has anti-apoptotic effects in cardiomyocytes, and H2S donor induces cardiomyocyte expression of miR-21 both in vitro and in vivo (19, 147). In addition, miRNAs also regulate H2S production by regulating CSE, which could act as a feedback mechanism (174). Nevertheless, the list of miRNA known to be affected by H2S are small compared with the list of miRNAs known to regulate cell death, leaving ample room for further investigation.
Cytokine Signaling
Cytokines are small proteins that provide both proinflammatory and anti-inflammatory signals to the cell (119). Increased cytokine production in serum and heart muscle is a common feature of cardiovascular diseases involving cell death (119). The proinflammatory cytokine TNFα has been shown to induce both apoptosis and necrosis. Other proinflammatory cytokines such as IL-6 and IL-1 induce TNFα production. In a doxorubicin-induced cardiomyopathy cell model, treatment with NaHS, a H2S donor, reduced the expressions of IL-1β, IL-6, and TNFα, which was mediated by the p38 MAPK/NF-κB pathway (39, 168). Numerous studies have shown reduced cytokine production after H2S administration in models of sepsis and lung injury (83, 125, 126). The inflammatory and fibrotic remodeling in cardiomyopathies is also mediated by inflammatory cells such as macrophages and Treg cells in the heart, which act upon cardiomyocytes (103).
Interestingly, TNFα stimulation in the liver and macrophages leads to increased H2S production, suggesting the existence of compensatory signaling pathways (132). Alternately, two studies have shown that a decrease in cytokine production (IL-6 and TNFα) after H2S treatment is associated with increased histone acetylation, suggesting a mechanism that involves epigenetic regulation (125, 126). This effect was shown in macrophages challenged with LPS, and it may occur in cardiac macrophages in cardiomyopathies.
In cardiomyopathy following I/R injury, CD11b+Gr-1+ neutrophils are recruited to the myocardium to express IL-1β and TNFα. H2S donor treatment of mice reduced the presence of these myeloid cells, which was correlated with promoted Bcl-2 anti-apoptotic signaling, reduced cytokine release, and preserved cardiac function (187).
VEGF is a proangiogenic cytokine that promotes survival and growth of endothelial cells (78). In DMCM, reduced VEGF leads to rarefaction of microvessles in the heart and causes a redox imbalance resulting in ischemic cell death (13, 41). H2S supplementation increases cardiac VEGF in myocardial infarcted mice and improves proliferation and migration of endothelial cells (111). It suggests a cardioprotective effect of H2S on endothelial cells in cardiomyopathy via VEGF cytokine signaling (122).
TGFβ is a critical cytokine in the cardiac remodeling process during cardiomyopathies, and it induces the differentiation of fibroblasts into myofibroblasts (5). Myofibroblasts in turn promote maladaptive collagen deposition and secrete cytokines like TNFα, which induces cell death. The cardioprotective effects of H2S on cell death appear to act via cell types other than cardiomyocytes, where it reduces TGFβ expression and inhibits its signaling cascade in fibroblasts (98, 188). This limits the differentiation and proliferation of fibroblasts into myofibroblasts and prevents collagen deposition in the heart.
Renin-Angiotensin System
The role of the renin-angiotensin system (RAS) in cardiovascular diseases is well established and far-reaching. The RAS regulates oxidative stress, hypertrophy, and neuronal control of cardiovascular function (149). Studies also show that RAS partly regulates cardiomyocyte cell death. Stretching myocytes releases angiotensin II (ANG II), increases p53 binding to the ANG II promotor as well as the AT1 receptor, and results in a four to sevenfold increase in apoptosis (77). Increased apoptosis was also observed in cardiomyocytes with an adenoviral increase in p53 alone (113). Blockade of the AT1 receptor with losartan abolished stretch-induced apoptosis (77).
Laggner et al. (74) hypothesized that H2S interacts with angiotensin-converting enzyme (ACE), a zinc metalloproteinase, based on studies showing that H2S reacts with sulfide groups on metalloproteins and acts as a potent vasodilator. Their study showed a dose-dependent decrease in ACE activity in human endothelial cells after treatment with H2S. This decrease in ACE activity was without a decrease in ACE mRNA level, and it was counteracted by the addition of Zn2+. Another study showed that supplementation of H2S in DM rats reversed RAS activation and reduced ROS production (170). These studies suggest that H2S could alter RAS signaling, which may in turn reduce oxidative stress to mitigate cell death. A direct role of H2S-mediated RAS signaling in cardiomyopathy cell death, however, has not been reported.
Regulation of Epigenetic Modifications
H2S also signals through epigenetic changes. As mentioned above, H2S induces chromatin remodeling to reduce cytokine production (125). Several studies have established the role of NAD-dependent deacetylase sirtuin 1 (SIRT1) in apoptosis of cardiomyocytes. Resveratrol activation of SIRT1 in cultured cardiomyocytes after hypoxia inhibits cell death detected by TUNEL staining (15). A study by Wu et al. (163) also demonstrated a similar SIRT1-dependent protection from apoptosis in cardiomyocytes after hydrogen peroxide oxidative damage. This study also showed that treatment by H2S donor initiated this pathway by increasing the expression of SIRT1. One possible mechanism of this effect of H2S on SIRT1 is the role of miRNA. miR-34a has been shown to target SIRT1, which leads to an increase in acetylated p53 and ultimately an increase in apoptosis (172). H2S has been shown to regulate miR-34a in hepatocytes; however, this mechanism has not been shown in cardiomyocytes yet (50).
After myocardial infarction, FOXP3 transcription factor-positive CD4+ Treg cells mediate the resolution of inflammation, as opposed to maladaptive remodeling and fibrosis (159). H2S has been shown to be crucial for the differentiation of these Treg cells (177). Furthermore, the authors found that H2S drove the demethylation of Foxp3 DNA, which promotes FOXP3 expression by promoting the expression of the Ten eleven translocation (Tet) family of enzymes that reverse DNA methylation (177).
Regulation of Other Gaseous Signaling Molecules by H2S
H2S is one of several biologically crucial gaseous signaling molecules. Like H2S, NO has been shown to have cardioprotective effects (59). Adding physiological levels of NO to cardiomyocytes or overexpressing endothelial NO synthase (eNOS) during I/R injury reduced oxidative stress and cell death (52). A similar study of cultured cardiomyocytes treated with doxorubicin demonstrated a reduction in apoptosis, especially after pretreatment with NO (95). In this study, caspase-3 activity decreased with NO in addition to S-nitrosylation of caspase-3.
Several studies show that H2S regulates NO expression and activity, which suggests that H2S may protect against cell death via NO. In a clinical trial of a H2S prodrug in patients with heart failure, increased H2S levels were correlated with an increase in NO (117). These effects appear to exist in cardiac cells other than cardiomyocytes, as H2S can also increase NO production in endothelial cells (118). NO regulation by H2S may be mediated by eNOS. H2S treatment increases eNOS expression and activity (1, 70). Mice lacking H2S show reduced NO and eNOS levels (66, 67).
The presence of cross-talk between H2S and NO is further supported by studies showing that H2S and NO can sulfhydrate or nitrosylate, respectively, the same cysteine residues of proteins (162). For example, nitrosylation of glyceraldehyde-3-phosphate dehydrogenase at Cys150 abolishes its activity, whereas sulfhydration at this residue by H2S increases it’s activity (42) Altaany et al. demonstrated that H2S sulfydrated eNOS and inhibited its nitrosylation at the same cysteine residue (1). This competitive cysteine modification appears to have effects on cell death since NF-κB can be nitrosylated at Cys38 to inhibit its activity and it can be sulfhydrated at the same residue to increase transcription of anti-apoptotic genes (132). These examples suggest the two gaseous molecules signal in concert and regulate each other, but the role of this interaction in cell death pathways and DMCM needs further investigation.
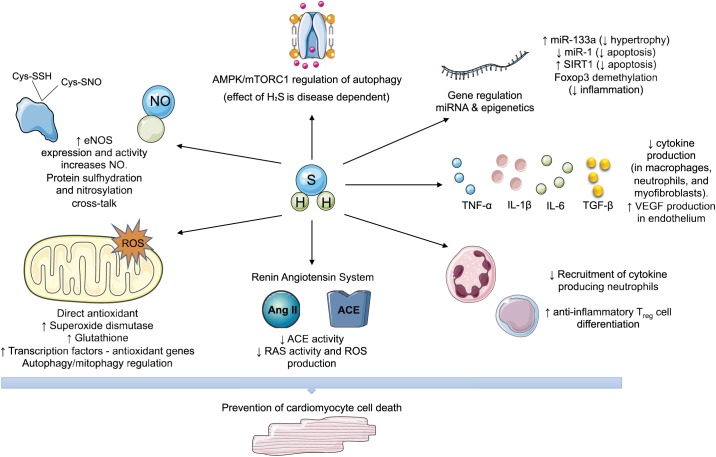
The signaling effects of H2S discussed in the preceding sections, which mediate cardioprotection of cell death in cardiomyopathies, are summarized in Fig. 1.
Fig. 1.
Hydrogen sulfide (H2S) cell death signaling by effects on oxidative stress, noncoding RNA, cytokines, and epigenetics. Proposed and experimentally demonstrated mechanisms that have been shown to be increased (↑) or decreased (↓) by H2S on cell death pathways. ACE, angiotensin-converting enzyme; AMPK, AMP-activated protein kinase; ANG II, angiotensin II; Cys-SNO, S-nitroso-cysteine; Cys-SSH, cysteine persulfide; eNOS, endothelial nitric oxide synthase; mTORC1, mammalian target of rapamycin complex 1; NO, nitric oxide; RAS, renin angiotensin system; ROS, reactive oxygen species; SIRT1, sirtuin 1.
Regulation of Transcription Factors
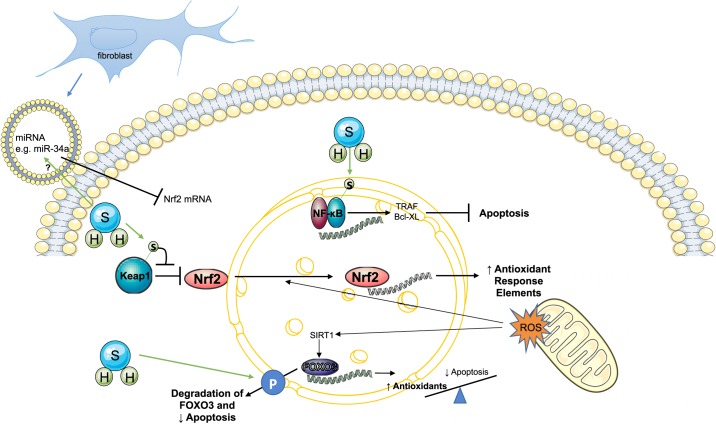
Many of the antioxidant, cytokine, and cell death mechanisms involving H2S are mediated by the effect of H2S on transcription factors. The roles of FOXO3, NF-κB, and Nrf2 in cell death mechanisms are highlighted here and are depicted in Fig. 2 with potential pathways.
Fig. 2.
Role of transcription factors in hydrogen sulfide (H2S) signaling. Proposed and experimentally demonstrated effects of H2S on the transcription factors NF-κB (top), nuclear factor-E2-related factor (Nrf2; middle), and FOXO3 (bottom). Pathways that are empirically not proven but could potentially be important in cardiomyocytes are depicted with a question mark (?). H2S can sulfhydrate the p65 subunit of NF-κB to promote its anti-apoptotic gene regulation. Sulfhydration of Kelch-like ECH-associated protein 1 (Keap1) releases its inhibition of Nrf2, allowing translocation of Nrf2 to the nucleus to increase expression of antioxidant genes. miRNA in extracellular vesicles released from fibroblasts regulate Nrf2 mRNA in cardiomyocytes, and that may interact with H2S. H2S may promote degradation of FOXO3 to limit proapoptotic effects of FOXO3. Reactive oxygen species (ROS) also induce antioxidant gene transcription via FOXO3, which may be promoted by H2S. H, hydrogen; S, sulfur.
FOXO3 belongs to the O subclass of the forkhead family of transcription factors along with FOXO1, FOXO4, and FOXO6. FOXO3 is inhibited under normal conditions and translocated out of the nucleus on phosphorylation by Akt. During cell stress, it functions as a trigger for apoptosis through upregulation of proapoptotic genes such as Bim and PUMA or downregulation of anti-apoptotic proteins such as FLIP in cardiomyocytes (3). FOXO3a can also protect against oxidative stress by upregulating antioxidants, depending on the context of its induction (155).
H2S has been shown to modulate the apoptotic or protective actions of FOXO3a. In doxorubicin-induced cardiomyopathy, NaHS treatment prevented the decrease in FOXO3 phosphorylation seen after doxorubicin treatment to maintain degradation of FOXO3 and to limit apoptosis (90). Moreover, FOXO3 has been implicated in the epigenetic effects of H2S. For example, SIRT1 deacetylation of FOXO3 increases the protective anti-apoptotic activities of FOXO3 (9). Thus, the anti-apoptotic effects of H2S in cardiomyocytes that were dependent on SIRT1 shown by Wu et al. (163) could be mediated by FOXO3. The cross-talk between H2S targets and FOXO3 is thus an important pathway for H2S-mediated cardioprotection.
The nuclear factor-E2-related factor (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) axis is a key transcriptional regulator of oxidative stress. Under oxidative stress conditions, Keap1 is degraded, and Nrf2 translocates to the nucleus to induce expression of antioxidant response elements. As highlighted above, oxidative stress plays a crucial role in cell death in cardiomyopathies, and numerous publications show that change in Nrf2 expression or function alters the progression of DMCM and other cardiomyopathies (17, 80, 157). For example, a study by Qin et al. (121) demonstrated that Nrf2-knockout increased myocardial necrosis. Nrf2-activating compounds inhibit NLRP3 inflammasome activation, and Nrf2 silencing abrogated the decrease in pyroptosis in vascular endothelial cells after antioxidant treatment (32, 49).
New evidence links H2S signaling to Nrf2-mediated cell death. Two studies have shown that incubation of mouse fibroblasts with a H2S donor directly sulfhydrated Keap1, which enhanced Nrf2 translocation and increased expression of antioxidant genes (46, 175). This protected against age-related cellular senescence and induced cytoprotective genes. Also, this H2S-mediated stabilization of Nrf2 subsequently controlled the expression of H2S-producing and -degrading enzymes. Although the effect of H2S sulfhydration of Keap1 on apoptosis or pyroptosis is yet to be explored, it is assumed that cardioprotective effects of H2S in cardiomyopathy may be mediated by this pathway.
The effects of H2S on Nrf2 may be more complex than just a direct sulfhydration effect. A recent study demonstrated that miRNA-28a, miR-27a, and miR-34a is secreted in exosomes from fibroblasts and absorbed by cardiomyocytes to decrease Nrf2 translation (145). Interestingly, in hepatic I/R injury, rats injected with NaHS attenuated miR-34a and upregulated Nrf2 to prevent I/R injury (50). These findings suggest a mechanism where H2S either directly regulates Nrf2 or indirectly regulates it through miRNA to reduce oxidative stress and cell death. Investigating these mechanisms in cardiomyopathies could reveal a promising mechanism of H2S signaling in the heart.
NF-κB is another vital transcription factor activated in response to cell stress. Typically, phosphorylation of the inhibitor-κB unmasks the p65 subunit of NF-κB, which then translocates to the nucleus to promote transcription of inflammatory and apoptotic genes (135, 148). H2S, on the other hand, has been shown to activate protective anti-apoptotic effects on NF-κB (132). In this pathway, following TNFα stimulation, CSE expression is increased to generate H2S, which sulfhydrates p65 (132). Sulfhydrated p65 translocates to the nucleus in liver macrophage cells and increases the transcription of anti-apoptotic gene targets TRAF, cIAP-2, Bcl-XL, and XIAP (132). Ablation of CSE and reduction of H2S abolished these beneficial effects. NF-κB-mediated proapoptotic effects have been shown in models of DMCM and doxorubicin-induced cardiomyopathy (81, 135, 148). The role of p65 sulfhydration by H2S in cardiomyocytes is unclear. However, a similar anti-apoptotic activation upon sulfhydration as seen in the liver may be the signaling pathway in DMCM.
THERAPEUTIC POTENTIAL OF H2S IN CVD
Because of the cardioprotective benefits of H2S seen in cardiomyocytes in culture and animal models, a novel long-acting prodrug of H2S (SG1002) has been developed. SG1002 has been tested in preclinical models of cardiovascular disease and in clinical trials (2, 117). In an animal model of systolic heart failure, SG1002 protects against adverse remodeling and heart failure via upregulation of endothelial nitric oxide synthase (117). It also prevented QT interval prolongation in mice with ischemic cardiomyopathy, suggesting a decrease in cell death (2). In phase I clinical trials, SG1002 reduced BNP and increased NO in heart failure patients (115, 117). Other novel methodologies of H2S delivery are under development, such as organ-targeted delivery by nanoparticles, microbubbles, and ultrasound (16, 156). H2S encapsulated in microbubbles and released in the myocardium with ultrasound after I/R injury in rats reduced apoptosis and oxidative stress (16). The molecular and preclinical work highlighted in this review suggests that H2S-based therapies could prevent cardiomyocyte cell death; however, it remains to be seen whether these therapies can mitigate the progression of DMCM in patients. In addition, all studies highlighted in this review used prevention strategies to demonstrate that H2S protected against cell death and subsequent remodeling in cardiomyopathy. It has yet to be seen whether H2S can reverse pathological remodeling.
FUTURE DIRECTIONS
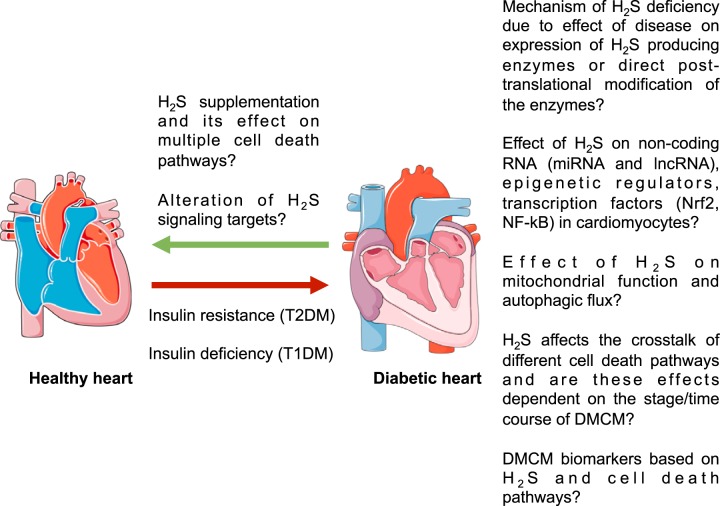
With multiple cell death mechanisms shown to be present in DMCM, the relative contribution of each mechanism is important to address (Fig. 3). It is unclear whether apoptosis, necrosis, pyroptosis, and autophagy work in concert simultaneously, if one is predominant over another, or if one pathway affects the other. The predominant cell death mechanism may be dependent on the type of stress and thus the etiology of the cardiomyopathy or on the time course of disease progression (26). Another possibility is that one cell death process may occur before another in cardiomyocytes, raising the opportunity of discovering early biomarkers for cardiomyopathy based on the earlier cell death process.
Fig. 3.
Future directions for the role of hydrogen sulfide (H2S) in protection against cell death signaling and diabetic cardiomyopathy. Both type 1 (T1DM) and type 2 diabetes mellitus (T2DM) lead to cardiomyopathy and cell death independent of other cardiovascular effects on the heart. H2S may mediate signaling that prevents cell death pathways; however, many of these effects have not been proven in cardiomyocytes. H2S and its signaling effects may also show beneficial therapeutic and preventative effects in diabetic cardiomyopathy (DMCM).
In general, suppression of one cell death pathway leads to compensation by another cell death pathway (153). For instance, inactivation of Beclin 1 protein, which is necessary for autophagy, increases apoptotic bodies in many organisms (144). Beclin 1 has a complex functional and structural interaction with anti-apoptotic protein Bcl-2, and apoptotic signals lead to caspase-mediated cleavage of Beclin 1, thereby inhibiting autophagy and vice versa (108, 192). However, there is also evidence suggesting that targeting one specific pathway may be sufficient to prevent the development of cardiomyopathy. For example, inhibiting NLRP3 gene expression in T2DM rats ameliorates DMCM (93), and inhibition of autophagy prevents hyperglycemia-induced apoptosis in cardiomyocytes (68).
H2S may also suppress or regulate multiple cell death pathways simultaneously. H2S has been shown to alter expression of both Bcl-2 and Beclin 1 (185). Similarly, Toldo et al. (147) demonstrated that H2S administration during I/R cardiomyopathy suppressed pyroptosis, apoptosis, and necrosis. However, it is unclear how H2S could regulate multiple cell death pathways together. One possibility is that H2S could regulate both apoptosis and pyroptosis simultaneously via cross-talk with autophagy. Autophagy has been shown to inhibit pyroptosis, as activation of autophagy can clear damaged mitochondria that activates pyroptosis (108) It will be essential to understand the temporal and spatial relationship of these cell death pathways in DMCM, the cross-talk between autophagy and cell death, and their relationship to an upstream signaling molecule such as H2S. If H2S can regulate both apoptosis, necroptosis, and pyroptosis simultaneously in diseases such as DMCM, future studies will be required to compare the quantitatively effects of H2S on each pathway to determine whether there is a relative gradient of cell death protection.
Although clinical trials based on H2S have already begun, many unanswered questions remain, particularly relating to its signaling mechanisms. As highlighted in this review, many gaps in understanding exist. It is unclear how H2S interacts transcription factors such as Nrf2 and NF-κB and how these pathways work in concert, especially in cardiomyocytes and cardiomyopathies. Bioinformatics such as next-generation sequencing are likely valuable unbiased approaches to study the numerous pathways that H2S affects. Because H2S is difficult to administer in optimum doses in vivo, understanding its specific signaling targets may improve existing therapies or uncover new therapies for cardiomyopathy using the H2S cardioprotection pathway.
There are also unexplored avenues regarding the role of noncoding RNAs in H2S signaling. Although studies have shown that H2S regulates miRNA expression and vice versa, the underlying mechanisms of this cross-talk are unknown. Long noncoding RNA (lncRNAs) are a class of regulatory RNAs that are >200 nucleotide in length. They can regulate miRNA expression, hypertrophy, and cell death (67). For example, lncRNA H19 has been found to be both increased and decreased in different models of DM mice hearts, and overexpression of H19 in diabetic rats decreased oxidative stress and apoptosis (59, 79). Another lncRNA, MALAT1, increases cardiomyocyte apoptosis in DM rats and is overexpressed in dilated cardiomyopathy (27, 164). lncRNA MEG3 is associated with inflammasome formation and pyroptosis, albeit in aortic endothelial cells (168). Similarly, lncRNA Kcnq1ot1 promotes pyroptosis in DCM by binding to miR-214-3p (154). To our knowledge, there is no study testing whether H2S affects lncRNA expression. However, these recent studies showing that lncRNA regulates cardiomyocyte cell death provide a rationale for determining whether H2S could modulate miRNA expression via lncRNA.
Questions also remain as to how H2S levels are reduced in disease states such as DM. One hypothesis is based on homocysteine, a precursor of H2S. Studies in some mouse models of T1DM, and T1DM and T2DM patients with renal dysfunction show elevated levels of plasma homocysteine, which is strongly associated with cardiovascular disease, including DMCM (53, 100, 107, 133). The homocysteine metabolic enzymes CBS, cystathionine γ-lyase (CSE), and methyl tetrahydrofolate reductase (MTHFR) are all downregulated in a mouse model of T1DM, leading to the buildup of homocysteine (100). Elevated homocysteine also competes with cysteine for binding to CSE, thus decreasing the production of H2S through substrate inhibition (140). Hyperhomocysteinemia also decreases expression of CSE, leading to decreased H2S generation (33, 178). Finally, homocysteine appears to directly precipitate proteins post-translationally by homocysteinylation (55), and it is possible that DM-induced hyperhomocysteinemia may precipitate CSE, resulting in decreased H2S production. This homocysteine/H2S hypothesis and/or whether DM exerts other effects on H2S metabolism requires further investigation.
H2S is also difficult to measure in vivo due to its volatility and different reactive forms. Measurement of H2S in different matrices using different methods produces highly variable results (51, 134). A consensus method for H2S measurement is necessary to reduce the variabilities in the different H2S-based experiments. Understanding the mechanisms of H2S may uncover diagnostic markers of cardiomyopathies and biomarkers of H2S-based therapies. The current gold standard for diagnosis of DMCM uses noninvasive two-dimensional and three-dimensional echocardiography and magnetic resonance imaging. However, this is problematic when early progression is asymptomatic. For example, in DMCM, diastolic dysfunction is the first clinical sign of the disease, but the heterogeneity of disease progression makes identification of diastolic dysfunction difficult, necessitating the discovery of molecular biomarkers (110). Based on the cell death mechanisms of DMCM, many molecules have been studied for potential biomarkers in DMCM when this disease is still reversible. For example, cardiotrophin-1 (CT-1) promotes fibrosis and inhibits apoptosis. Although CT-1 is elevated in plasma of patients with DMCM, it is also highly expressed in other tissues and in other cardiomyopathies (31, 110). Many of the H2S signaling pathways and its diverse effects on cell death highlighted in this review are yet to be explored, and they may reveal potential biomarkers and therapeutic targets of cardiomyopathies, including DMCM.
GRANTS
This work is supported in part by the National Heart, Lung, and Blood Institute Grants HL-113281 and HL-116205 (to P. K. Mishra).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K. and P.K.M. conceived and designed review; S.K. prepared figures; S.K., T.N.K., and P.K.M. drafted manuscript; S.K., T.N.K., and P.K.M. edited and revised manuscript; S.K., T.N.K., and P.K.M. approved final version of manuscript.
REFERENCES
- 1.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 2.Balan B, Mauro AG, Cain C, Polhemus DJ, Lefer DJ, Salloum FN. Abstract 16815: Novel orally active hydrogen sulfide-releasing compound, SG1002, attenuates QT interval prolongation and improves LV function and survival in a murine model of ischemic cardiomyopathy. Circulation 136: A16815 LP-A16815, 2017. http://circ.ahajournals.org/content/136/Suppl_1/A16815. [Google Scholar]
- 3.Bao W, Pan F, Chen L, Su G, Gao X, Li Y, Sun Q, Sun J, He K, Song H. The PI3K/AKT Pathway and FOXO3a Transcription Factor Mediate High Glucose-Induced Apoptosis in Neonatal Rat Ventricular Myocytes. Iran Red Crescent Med J 16: e14914, 2014. doi: 10.5812/ircmj.14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr LA, Shimizu Y, Lambert JP, Nicholson CK, Calvert JW. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide 46: 145–156, 2015. doi: 10.1016/j.niox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57: 376–379, 2011. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10: 721–732, 2009. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 7.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11: 31–39, 2010. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 10.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57: 660–671, 2014. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 3: 219–228, 2003. doi: 10.1385/CT:3:3:219. [DOI] [PubMed] [Google Scholar]
- 12.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Ng Y-S, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med 5: 495–502, 1999. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 14.Castelblanco M, Lugrin J, Ehirchiou D, Nasi S, Ishii I, So A, Martinon F, Busso N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem 293: 2546–2557, 2018. doi: 10.1074/jbc.M117.806869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun 378: 389–393, 2009. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Yang L, Zhong L, Kutty S, Wang Y, Cui K, Xiu J, Cao S, Huang Q, Liao W, Liao Y, Wu J, Zhang W, Bin J. Delivery of hydrogen sulfide by ultrasound targeted microbubble destruction attenuates myocardial ischemia-reperfusion injury. Sci Rep 6: 30643, 2016. doi: 10.1038/srep30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Zhang Z, Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status [Online]. Diabetes Metab J 38: 337–345, 2014. doi: 10.4093/dmj.2014.38.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Zhang Z, Zhang D, Li H, Sun Z. Hydrogen sulfide protects against TNF-α induced neuronal cell apoptosis through miR-485-5p/TRADD signaling. Biochem Biophys Res Commun 478: 1304–1309, 2016. doi: 10.1016/j.bbrc.2016.08.116. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 87: 431–439, 2010. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delbridge LMD, Mellor KM, Taylor DJ, Gottlieb RA. Myocardial stress and autophagy: mechanisms and potential therapies. Nat Rev Cardiol 14: 412–425, 2017. doi: 10.1038/nrcardio.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol 32: 373–379, 2011. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 18: 655–673, 2000. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 23.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19: 349–364, 2018. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 24.Dorn GW 2nd. Molecular mechanisms that differentiate apoptosis from programmed necrosis. Toxicol Pathol 41: 227–234, 2013. doi: 10.1177/0192623312466961. [DOI] [PubMed] [Google Scholar]
- 25.Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813: 1351–1359, 2011. doi: 10.1016/j.bbamcr.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916, 2005. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II- dependent [Online]. Lab Invest 80: 513–527, 2000. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- 28.Flarsheim CE, Grupp IL, Matlib MA. Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol Heart Circ Physiol 271: H192–H202, 1996. doi: 10.1152/ajpheart.1996.271.1.H192. [DOI] [PubMed] [Google Scholar]
- 29.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000. doi: 10.1161/01.RES.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 30.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JH, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541, 2018. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamella-Pozuelo L, Fuentes-Calvo I, Gómez-Marcos MA, Recio-Rodriguez JI, Agudo-Conde C, Fernández-Martín JL, Cannata-Andía JB, López-Novoa JM, García-Ortiz L, Martínez-Salgado C. Plasma cardiotrophin-1 as a marker of hypertension and diabetes-induced target organ damage and cardiovascular risk. Medicine (Baltimore) 94: e1218, 2015. doi: 10.1097/MD.0000000000001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garstkiewicz M, Strittmatter GE, Grossi S, Sand J, Fenini G, Werner S, French LE, Beer HD. Opposing effects of Nrf2 and Nrf2-activating compounds on the NLRP3 inflammasome independent of Nrf2-mediated gene expression. Eur J Immunol 47: 806–817, 2017. doi: 10.1002/eji.201646665. [DOI] [PubMed] [Google Scholar]
- 33.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318: 756–763, 2004. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 34.George AK, Singh M, Homme RP, Majumder A, Tyagi S. Role of Hydrogen Sulfide (H2S) on Homocysteine Mediated Glutamate Excitotoxicity, Endoplasmic Reticulum Stress and Pyroptosis in Retina. FASEB J 32: 748.5, 2018. [Google Scholar]
- 35.Kovačić D, Glavnik N, Marinšek M, Zagožen P, Rovan K, Goslar T, Marš T, Podbregar M. Total plasma sulfide in congestive heart failure. J Card Fail 18: 541–548, 2012. doi: 10.1016/j.cardfail.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci 874: 412–426, 1999. doi: 10.1111/j.1749-6632.1999.tb09255.x. [DOI] [PubMed] [Google Scholar]
- 37.Guillén I, Blanes M, Gómez-Lechón MJ, Castell JV. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol Regul Integr Comp Physiol 269: R229–R235, 1995. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Liang F, Shah Masood W, Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-κB dependent anti-inflammation pathway. Eur J Pharmacol 725: 70–78, 2014. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D, Chen P, Chen G, Xu W, Feng J. Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell Physiol Biochem 32: 1668–1680, 2013. doi: 10.1159/000356602. [DOI] [PubMed] [Google Scholar]
- 40.Hackfort BT, Mishra PK. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am J Physiol Heart Circ Physiol 310: H802–H812, 2016. doi: 10.1152/ajpheart.00660.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han B, Baliga R, Huang H, Giannone PJ, Bauer JA. Decreased cardiac expression of vascular endothelial growth factor and redox imbalance in murine diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 297: H829–H835, 2009. doi: 10.1152/ajpheart.00222.2009. [DOI] [PubMed] [Google Scholar]
- 42.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 43.Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol 194: 355–365, 2011. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 62: 1270–1281, 2013. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hensen J, Howard CP, Walter V, Thuren T. Impact of interleukin-1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab 39: 524–531, 2013. doi: 10.1016/j.diabet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid Redox Signal 19: 465–481, 2013. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 47.Hu LF, Li Y, Neo KL, Yong QC, Lee SW, Tan BK, Bian JS. Hydrogen sulfide regulates Na+/H+ exchanger activity via stimulation of phosphoinositide 3-kinase/Akt and protein kinase G pathways. J Pharmacol Exp Ther 339: 726–735, 2011. doi: 10.1124/jpet.111.184754. [DOI] [PubMed] [Google Scholar]
- 48.Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 75: 27–34, 2009. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 49.Hu Q, Zhang T, Yi L, Zhou X, Mi M. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors 44: 123–136, 2018. doi: 10.1002/biof.1395. [DOI] [PubMed] [Google Scholar]
- 50.Huang X, Gao Y, Qin J, Lu S. The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS One 9: e113305, 2014. doi: 10.1371/journal.pone.0113305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47: 1346–1353, 2009. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Iwase H, Robin E, Guzy RD, Mungai PT, Vanden Hoek TL, Chandel NS, Levraut J, Schumacker PT. Nitric oxide during ischemia attenuates oxidant stress and cell death during ischemia and reperfusion in cardiomyocytes. Free Radic Biol Med 43: 590–599, 2007. doi: 10.1016/j.freeradbiomed.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes 47: 1967–1970, 1998. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- 54.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA Jr. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 12: 1333–1337, 2010. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J 13: 2277–2283, 1999. doi: 10.1096/fasebj.13.15.2277. [DOI] [PubMed] [Google Scholar]
- 56.Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471: 423–429, 2016. doi: 10.1016/j.bbrc.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol 295: H801–H806, 2008. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin S, Pu SX, Hou CL, Ma FF, Li N, Li XH, Tan B, Tao BB, Wang MJ, Zhu YC. Cardiac H2S generation is reduced in ageing diabetic mice. Oxid Med Cell Longev 2015: 1–14, 2015. doi: 10.1155/2015/758358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, Minatoguchi S. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 11: 1146–1160, 2015. doi: 10.1080/15548627.2015.1051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang B, Hong J, Xiao J, Zhu X, Ni X, Zhang Y, He B, Wang Z. Involvement of miR-1 in the protective effect of hydrogen sulfide against cardiomyocyte apoptosis induced by ischemia/reperfusion. Mol Biol Rep 41: 6845–6853, 2014. doi: 10.1007/s11033-014-3570-2. [DOI] [PubMed] [Google Scholar]
- 62.Kawai C. From Myocarditis to Cardiomyopathy : Mechanisms of Host Factors That Influence Susceptibility to. Heart 99: 1091–1100, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 31: 1352–1356, 1998. doi: 10.1016/S0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 64.Kesherwani V, Shahshahan HR, Mishra PK. Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS One 12: e0182828, 2017. doi: 10.1371/journal.pone.0182828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Neurosci Res 71: e88, 2011. doi: 10.1016/j.neures.2011.07.375. [DOI] [PubMed] [Google Scholar]
- 66.King AL, Lefer DJ. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp Physiol 96: 840–846, 2011. doi: 10.1113/expphysiol.2011.059725. [DOI] [PubMed] [Google Scholar]
- 67.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 111: 3182–3187, 2014. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi S, Xu X, Chen K, Liang Q. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 8: 577–592, 2012. doi: 10.4161/auto.18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35: 5–20, 2013. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo WW, Wang WJ, Tsai CY, Way CL, Hsu HH, Chen LM. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int J Cardiol 168: 270–280, 2013. doi: 10.1016/j.ijcard.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 73.Kytö V, Saraste A, Saukko P, Henn V, Pulkki K, Vuorinen T, Voipio-Pulkki LM. Apoptotic cardiomyocyte death in fatal myocarditis. Am J Cardiol 94: 746–750, 2004. doi: 10.1016/j.amjcard.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 74.Laggner H, Hermann M, Esterbauer H, Muellner MK, Exner M, Gmeiner BM, Kapiotis S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens 25: 2100–2104, 2007. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- 75.Lambert JP, Nicholson CK, Amin H, Amin S, Calvert JW. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res 4: 20, 2014. doi: 10.1186/s13618-014-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest 125: 55–64, 2015. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 101: 1326–1342, 1998. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 79.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477, 2004. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 80.Li B, Liu S, Miao L, Cai L. Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res 2012: 1–7, 2012. doi: 10.1155/2012/216512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li D, Li J, An Y, Yang Y, Zhang SQ. Doxorubicin-induced apoptosis in H9c2 cardiomyocytes by NF-κB dependent PUMA upregulation. Eur Rev Med Pharmacol Sci 17: 2323–2329, 2013. [PubMed] [Google Scholar]
- 82.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 83.Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 233: 1081–1087, 2008. doi: 10.3181/0712-RM-354. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C, Fang Z, Wei Y, Wang R, Du Z, Zhang Y, Lu Y. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5: e1479, 2014. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Zeng L, Cao C, Lu C, Lian W, Han J, Zhang X, Zhang J, Tang T, Li M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350: 327–335, 2017. doi: 10.1016/j.yexcr.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Liang W, Chen M, Jianhua HE, Zhang W, Cheng F, Lan J, Feng J, Huang H. Role of ATP-sensitive potassium channels in inhibitory effect of hydrogen sulfide on high glucose-induced inflammation mediated by necroptosis in H9c2 cardiac cells. Chinese J Pathophysiol 32: 1364–1369, 2016. [Google Scholar]
- 87.Liang W, Jieyi HE, Zhang W, Shenglong YU, Chen J, Song M, Chen J, Zheng D, Liao X. Hydrogen sulfide protects H9c2 cardiomyocytes against high glucose-in-duced injury by inhibiting necroptosis. Chinese J Pathophysiol 32: 385–391, 2016. [Google Scholar]
- 88.Liu J, Hao DD, Zhang JS, Zhu YC. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem Biophys Res Commun 413: 342–347, 2011. doi: 10.1016/j.bbrc.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Wu J, Sun A, Sun Y, Yu X, Liu N, Dong S, Yang F, Zhang L, Zhong X, Xu C, Lu F, Zhang W. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell Biosci 6: 33, 2016. doi: 10.1186/s13578-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu MH, Zhang Y, He J, Tan TP, Wu SJ, Guo DM, He H, Peng J, Tang ZH, Jiang ZS. Hydrogen sulfide protects H9c2 cardiac cells against doxorubicin-induced cytotoxicity through the PI3K/Akt/FoxO3a pathway. Int J Mol Med 37: 1661–1668, 2016. doi: 10.3892/ijmm.2016.2563. [DOI] [PubMed] [Google Scholar]
- 91.Lu M, Zhao FF, Tang JJ, Su CJ, Fan Y, Ding JH, Bian JS, Hu G. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal 17: 849–859, 2012. doi: 10.1089/ars.2011.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke H, Zeng Z, Huang W, He Y. NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol 8: 519, 2017. doi: 10.3389/fphys.2017.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, An F. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 9: e104771, 2014. doi: 10.1371/journal.pone.0104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo B, Li B, Wang W, Liu X, Liu X, Xia Y, Zhang C, Zhang Y, Zhang M, An F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther 28: 33–43, 2014. doi: 10.1007/s10557-013-6498-1. [DOI] [PubMed] [Google Scholar]
- 95.Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol 38: 163–174, 2005. doi: 10.1016/j.yjmcc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 96.Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, Federici M, Van Tassell BW, Zhang S, Abbate A. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol 63: 316–322, 2014. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsushita N, Ishida N, Ibi M, Saito M, Sanbe A, Shimojo H, Suzuki S, Koepsell H, Takeishi Y, Morino Y, Taira E, Sawa Y, Hirose M. Chronic Pressure Overload Induces Cardiac Hypertrophy and Fibrosis via Increases in SGLT1 and IL-18 Gene Expression in Mice. Int Heart J 59: 1123–1133, 2018. doi: 10.1536/ihj.17-565. [DOI] [PubMed] [Google Scholar]
- 98.Meng G, Zhu J, Xiao Y, Huang Z, Zhang Y, Tang X, Xie L, Chen Y, Shao Y, Ferro A, Wang R, Moore PK, Ji Y. Hydrogen sulfide donor GYY4137 protects against myocardial fibrosis. Oxid Med Cell Longev 2015: 1–14, 2015. doi: 10.1155/2015/691070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142, 2010. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mishra PK, Givvimani S, Metreveli N, Tyagi SC. Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem Biophys Res Commun 401: 175–181, 2010. doi: 10.1016/j.bbrc.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med 13: 778–789, 2009. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys 55: 153–162, 2009. doi: 10.1007/s12013-009-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra PK, Ying W, Nandi SS, Bandyopadhyay GK, Patel KK, Mahata SK. Diabetic cardiomyopathy: An immunometabolic perspective. Front Endocrinol (Lausanne) 8: 72, 2017. doi: 10.3389/fendo.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munasinghe PE, Riu F, Dixit P, Edamatsu M, Saxena P, Hamer NSJ, Galvin IF, Bunton RW, Lequeux S, Jones G, Lamberts RR, Emanueli C, Madeddu P, Katare R. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol 202: 13–20, 2016. doi: 10.1016/j.ijcard.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 105.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-Sulfhydration. Sci Signal 2: ra72, 2009. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw B-A. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189, 1996. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 107.Ndrepepa G, Kastrati A, Braun S, Koch W, Kölling K, Mehilli J, Schömig A. Circulating homocysteine levels in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 18: 66–73, 2008. doi: 10.1016/j.numecd.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 108.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833: 3448–3459, 2013. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Osipov RM, Robich MP, Feng J, Chan V, Clements RT, Deyo RJ, Szabo C, Sellke FW. Effect of hydrogen sulfide on myocardial protection in the setting of cardioplegia and cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 10: 506–512, 2010. doi: 10.1510/icvts.2009.219535. [DOI] [PubMed] [Google Scholar]
- 110.Palomer X, Pizarro-Delgado J, Vázquez-Carrera M. Emerging actors in diabetic cardiomyopathy: heartbreaker biomarkers or therapeutic targets? Trends Pharmacol Sci 39: 452–467, 2018. doi: 10.1016/j.tips.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 111.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 113.Pierzchalski P, Reiss K, Cheng W, Cirielli C, Kajstura J, Nitahara JA, Rizk M, Capogrossi MC, Anversa P. p53 Induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res 234: 57–65, 1997. doi: 10.1006/excr.1997.3604. [DOI] [PubMed] [Google Scholar]
- 114.Pihán P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ 24: 1478–1487, 2017. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polhemus DJ, Giordano T, Lefer DJ. Abstract 16779: A Novel Hydrogen Sulfide Donor Promotes Nitric Oxide Bioavailability in a Phase I Clinical Trial. Circulation 130: A16779 LP-A16779, 2014. http://circ.ahajournals.org/content/130/Suppl_2/A16779. [Google Scholar]
- 116.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Polhemus DJ, Li Z, Pattillo CB, Gojon G Sr, Gojon G Jr, Giordano T, Krum H. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther 33: 216–226, 2015. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Predmore BL, Julian D, Cardounel AJ. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front Physiol 2: 104, 2011. doi: 10.3389/fphys.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pulkki KJ. Cytokines and cardiomyocyte death. Ann Med 29: 339–343, 1997. doi: 10.3109/07853899708999358. [DOI] [PubMed] [Google Scholar]
- 120.Puthanveetil P, Zhang D, Wang Y, Wang F, Wan A, Abrahani A, Rodrigues B. Diabetes triggers a PARP1 mediated death pathway in the heart through participation of FoxO1. J Mol Cell Cardiol 53: 677–686, 2012. doi: 10.1016/j.yjmcc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 121.Qin Q, Qu C, Niu T, Zang H, Qi L, Lyu L, Wang X, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. Nrf2-mediated cardiac maladaptive remodeling and dysfunction in a setting of autophagy insufficiency. Hypertension 67: 107–117, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci 8: 430–441, 2012. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ren L, Wang Q, Chen Y, Ma Y, Wang D. Involvement of MicroRNA-133a in the Protective Effect of Hydrogen Sulfide against Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress and Cardiomyocyte Apoptosis. Pharmacology 103: 1–9, 2019. doi: 10.1159/000492969. [DOI] [PubMed] [Google Scholar]
- 124.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 86: 391–397, 2006. doi: 10.1038/labinvest.3700391. [DOI] [PubMed] [Google Scholar]
- 125.Rios ECS, Soriano FG, Olah G, Gerö D, Szczesny B, Szabo C. Hydrogen sulfide modulates chromatin remodeling and inflammatory mediator production in response to endotoxin, but does not play a role in the development of endotoxin tolerance. J Inflamm (Lond) 13: 10, 2016. doi: 10.1186/s12950-016-0119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rios ECS, Szczesny B, Soriano FG, Olah G, Szabo C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int J Mol Med 35: 1741–1746, 2015. doi: 10.3892/ijmm.2015.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res 103: 1363–1369, 2008. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saraste A, Arola A, Vuorinen T, Kytö V, Kallajoki M, Pulkki K, Voipio-Pulkki L-M, Hyypiä T. Cardiomyocyte apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc Pathol 12: 255–262, 2003. doi: 10.1016/S1054-8807(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 129.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA 103: 7432–7437, 2006. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schaper J, Elsässer A, Kostin S. The role of cell death in heart failure. Circ Res 85: 867–869, 1999. doi: 10.1161/01.RES.85.9.867. [DOI] [PubMed] [Google Scholar]
- 131.Sen N. Functional and Molecular insights of hydrogen sulfide signaling and protein sulfhydration. J Mol Biol 429: 543–561, 2017. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sen U, Mishra PK, Tyagi N, Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem Biophys 57: 49–58, 2010. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheu ML, Chiang CK, Tsai KS, Ho FM, Weng TI, Wu HY, Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med 44: 2043–2050, 2008. doi: 10.1016/j.freeradbiomed.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 136.Shi H, Zhang Z, Wang X, Li R, Hou W, Bi W, Zhang X. Inhibition of autophagy induces IL-1β release from ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem Biophys Res Commun 463: 1071–1076, 2015. doi: 10.1016/j.bbrc.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 137.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42: 245–254, 2017. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Shi S, Li QS, Li H, Zhang L, Xu M, Cheng JL, Peng CH, Xu CQ, Tian Y. Anti-apoptotic action of hydrogen sulfide is associated with early JNK inhibition. Cell Biol Int 33: 1095–1101, 2009. doi: 10.1016/j.cellbi.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 139.Singh LP. The NLRP3 inflammasome and diabetic cardiomyopathy: editorial to: “Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model” by Beibei Luo et al. Cardiovasc Drugs Ther 28: 5–6, 2014. doi: 10.1007/s10557-013-6501-x. [DOI] [PubMed] [Google Scholar]
- 140.Stabler SP, Steegborn C, Wahl MC, Oliveriusova J, Kraus JP, Allen RH, Wagner C, Mudd SH. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism 51: 981–988, 2002. doi: 10.1053/meta.2002.34017. [DOI] [PubMed] [Google Scholar]
- 141.Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao J, Wang X, Yun F, Zhao H, Yan S, Yuan Y, Wang D, Ding X, Liu G, Li W, Zhao X, Liu Z, Li Y. Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am J Physiol Endocrinol Metab 309: E925–E935, 2015. doi: 10.1152/ajpendo.00294.2015. [DOI] [PubMed] [Google Scholar]
- 142.Sun X, Zhao D, Lu F, Peng S, Yu M, Liu N, Sun Y, Du H, Wang B, Chen J, Dong S, Lu F, Zhang W. Hydrogen sulfide regulates muscle RING finger-1 protein S-sulfhydration at Cys44 to prevent cardiac structural damage in diabetic cardiomyopathy. Br J Pharmacol, 2019. doi: 10.1111/bph.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev 79: 215–262, 1999. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 144.Takacs-Vellai K, Vellai T, Puoti A, Passannante M, Wicky C, Streit A, Kovacs AL, Müller F. Inactivation of the autophagy gene bec-1 triggers apoptotic cell death in C. elegans. Curr Biol 15: 1513–1517, 2005. doi: 10.1016/j.cub.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 145.Tian C, Gao L, Zimmerman MC, Zucker IH. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol 314: H928–H939, 2018. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 15: 203–214, 2018. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 147.Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ Cardiovasc Genet 7: 311–320, 2014. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsai KH, Wang WJ, Lin CW, Pai P, Lai TY, Tsai CY, Kuo WW. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. J Cell Physiol 227: 1347–1357, 2012. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 149.Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol 89: 3–9, 2002. doi: 10.1016/S0002-9149(01)02321-9. [DOI] [PubMed] [Google Scholar]
- 150.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714, 2010. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 152.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Varga ZV, Giricz Z, Liaudet L, Haskó G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852: 232–242, 2015. doi: 10.1016/j.bbadis.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem 284: 7853–7865, 2009. doi: 10.1074/jbc.M808824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal 14: 579–592, 2011. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- 156.Wang W, Liu H, Lu Y, Wang X, Zhang B, Cong S, Zhao Y, Ji M, Tao H, Wei L. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia-reperfusion injury. Int J Nanomedicine 14: 875–888, 2019. doi: 10.2147/IJN.S186225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y, Wu Y, Chen J, Zhao S, Li H. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology 126: 1–11, 2013. doi: 10.1159/000351179. [DOI] [PubMed] [Google Scholar]
- 158.Wei WB, Hu X, Zhuang XD, Liao LZ, Li WD. GYY4137, a novel hydrogen sulfide-releasing molecule, likely protects against high glucose-induced cytotoxicity by activation of the AMPK/mTOR signal pathway in H9c2 cells. Mol Cell Biochem 389: 249–256, 2014. doi: 10.1007/s11010-013-1946-6. [DOI] [PubMed] [Google Scholar]
- 159.Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 160.Wold LE, Ceylan-Isik AF, Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin 26: 908–917, 2005. doi: 10.1111/j.1745-7254.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 161.Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, See V, Stewart CL, Conner DA, Berul CI, Seidman CE, Seidman JG. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol 44: 293–303, 2008. doi: 10.1016/j.yjmcc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT, Li Q, Oka S, Sadoshima J, Beuve A, Simmons WJ, Li H. Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 15: 2565–2604, 2011. doi: 10.1089/ars.2010.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu D, Hu Q, Liu X, Pan L, Xiong Q, Zhu YZ. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide 46: 204–212, 2015. doi: 10.1016/j.niox.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 164.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ Res 118: 1525–1539, 2016. doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao J, Zhu X, Kang B, Xu J, Wu L, Hong J, Zhang Y, Ni X, Wang Z. Hydrogen sulfide attenuates myocardial hypoxia-reoxygenation injury by inhibiting autophagy via mTOR activation. Cell Physiol Biochem 37: 2444–2453, 2015. doi: 10.1159/000438597. [DOI] [PubMed] [Google Scholar]
- 166.Xiao T, Luo J, Wu Z, Li F, Zeng O, Yang J. Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats. Mol Med Rep 13: 1765–1773, 2016. doi: 10.3892/mmr.2015.4689. [DOI] [PubMed] [Google Scholar]
- 167.Xie M, Morales CR, Lavandero S, Hill JA. Tuning flux: autophagy as a target of heart disease therapy. Curr Opin Cardiol 26: 216–222, 2011. doi: 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu W, Wu W, Chen J, Guo R, Lin J, Liao X, Feng J. Exogenous hydrogen sulfide protects H9c2 cardiac cells against high glucose-induced injury by inhibiting the activities of the p38 MAPK and ERK1/2 pathways. Int J Mol Med 32: 917–925, 2013. doi: 10.3892/ijmm.2013.1462. [DOI] [PubMed] [Google Scholar]
- 169.Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, Gulick J, Yue Z, Robbins J, Epstein PN, Liang Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 288: 18077–18092, 2013. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xue H, Yuan P, Ni J, Li C, Shao D, Liu J, Shen Y, Wang Z, Zhou L, Zhang W, Huang Y, Yu C, Wang R, Lu L. H2S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS One 8: e74366, 2013. doi: 10.1371/journal.pone.0074366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yadav SK, Mishra PK. Abstract 20116: Ablation of Mmp9 Mitigates High Glucose-induced Cardiac Stem Cell Death. Circulation 136: A20116 LP-A20116, 2017. http://circ.ahajournals.org/content/136/Suppl_1/A20116. [Google Scholar]
- 172.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105: 13421–13426, 2008. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18: 1782–1784, 2004. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 174.Yang G, Pei Y, Cao Q, Wang R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J Cell Physiol 227: 3192–3200, 2012. doi: 10.1002/jcp.24006. [DOI] [PubMed] [Google Scholar]
- 175.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 176.Yang J, Zhao Y, Zhang P, Li Y, Yang Y, Yang Y, Zhu J, Song X, Jiang G, Fan J. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: role in pulmonary inflammation following LPS. Cell Death Dis 7: e2363, 2016. doi: 10.1038/cddis.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, Chen C, Liu S, Liu D, Chen Y, Zandi E, Chen W, Zhou Y, Shi S. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 43: 251–263, 2015. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yao K. Effects of several unusual sulfur-containing amino acids on rat liver cystathionine-gamma-lyase. Physiol Chem Phys 7: 401–408, 1975. [PubMed] [Google Scholar]
- 179.Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3β-dependent opening of mPTP. Am J Physiol Heart Circ Physiol 298: H1310–H1319, 2010. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 180.Yu M, Liu Y, Zhang B, Shi Y, Cui L, Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc Pathol 24: 375–381, 2015. doi: 10.1016/j.carpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 181.Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun 376: 548–552, 2008. doi: 10.1016/j.bbrc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 182.Yuan YQ, Wang YL, Yuan BS, Yuan X, Hou XO, Bian JS, Liu CF, Hu LF. Impaired CBS-H2S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun 67: 77–90, 2018. doi: 10.1016/j.bbi.2017.07.159. [DOI] [PubMed] [Google Scholar]
- 183.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW II. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med 8: 725–730, 2002. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 184.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 185.Zhang M, Shan H, Chang P, Wang T, Dong W, Chen X, Tao L. Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice. PLoS One 9: e87241, 2014. doi: 10.1371/journal.pone.0087241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao C-M, Xiao RP. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis [Online]. Nat Med 22: 175–182, 2016. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li Z, Zhu H, Zou Y, Yang X, Ge J. Hydrogen sulfide attenuates the recruitment of CD11b+Gr-1+ myeloid cells and regulates Bax/Bcl-2 signaling in myocardial ischemia injury. Sci Rep 4: 4774, 2014. doi: 10.1038/srep04774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y, Wang J, Li H, Yuan L, Wang L, Wu B, Ge J. Hydrogen sulfide suppresses transforming growth factor-β1-induced differentiation of human cardiac fibroblasts into myofibroblasts. Sci China Life Sci 58: 1126–1134, 2015. doi: 10.1007/s11427-015-4904-6. [DOI] [PubMed] [Google Scholar]
- 189.Zhao Y, Pluth MD. Hydrogen sulfide donors activated by reactive oxygen species. Angew Chem Int Ed Engl 55: 14638–14642, 2016. doi: 10.1002/anie.201608052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhao Y, Shi J, Shao F. Inflammatory caspases: activation and cleavage of gasdermin-D in vitro and during pyroptosis. Methods Mol Biol 1714: 131–148, 2018. doi: 10.1007/978-1-4939-7519-8_9. [DOI] [PubMed] [Google Scholar]
- 191.Zheng Y, Ji X, Ji K, Wang B. Hydrogen sulfide prodrugs-a review. Acta Pharm Sin B 5: 367–377, 2015. doi: 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J 278: 403–413, 2011. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 193.Zhou X, An G, Chen J. Hydrogen sulfide improves left ventricular function in smoking rats via regulation of apoptosis and autophagy. Apoptosis 19: 998–1005, 2014. doi: 10.1007/s10495-014-0978-z. [DOI] [PubMed] [Google Scholar]
- 194.Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin Sci (Lond) 128: 325–335, 2015. doi: 10.1042/CS20140460. [DOI] [PubMed] [Google Scholar]
- 195.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res 92: 75–84, 2011. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]