Visual Abstract
Keywords: mitochondria, metabolism, Sepsis-induced AKI, biomarkers, therapeutic targets
Abstract
AKI is a common clinical condition associated with the risk of developing CKD and ESKD. Sepsis is the leading cause of AKI in the intensive care unit (ICU) and accounts for nearly half of all AKI events. Patients with AKI who require dialysis have an unacceptably high mortality rate of 60%–80%. During sepsis, endothelial activation, increased microvascular permeability, changes in regional blood flow distribution with resulting areas of hypoperfusion, and hypoxemia can lead to AKI. No effective drugs to prevent or treat human sepsis-induced AKI are currently available. Recent research has identified dysfunction in energy metabolism as a critical contributor to the pathogenesis of AKI. Mitochondria, the center of energy metabolism, are increasingly recognized to be involved in the pathophysiology of sepsis-induced AKI and mitochondria could serve as a potential therapeutic target. In this review, we summarize the potential role of mitochondria in sepsis-induced AKI and identify future therapeutic approaches that target mitochondrial function in an effort to treat sepsis-induced AKI.
Sepsis is life-threatening organ dysfunction caused by dysregulated host response to infection.1 As the leading cause of AKI in the intensive care unit, sepsis accounts for 45%–70% of all AKI cases.2 A hemodynamic hallmark of sepsis is generalized vasodilation and decreased systemic vascular resistance.3 Previous studies have suggested that hypoperfusion and intrarenal vasoconstriction, caused by activation of the sympathetic nervous system and the renin-angiotensin-aldosterone axis, and release of vasopressin may contribute to AKI in sepsis.4–7 However, recent studies have questioned old paradigms and have demonstrated that sepsis-induced AKI develops with normal or even increased renal blood flow,8–10 suggesting that blood flow redistribution, microvascular changes, and other causes may be critical in leading to injury.
During sepsis, upregulation of endothelial nitric oxide synthesis11 can affect arterial vasodilatation and decrease systemic vascular resistance.12 The vasodilatory effect of endothelial nitric oxide synthase within the kidney might be expected to lessen renal vasoconstriction induced by NE, angiotensin II, vasopressin, and endothelin during sepsis.3,13 More than likely, these vasoactive substances lead to redistribution of flow away from the renal medulla to the renal cortex, leading to medullary ischemia in sepsis-induced AKI.14–16 Furthermore, sepsis-related impairment of the endothelium may also attenuate or abolish the normal effect of endothelial nitric oxide synthase in the kidney to counteract vasoconstriction.11 Another critical pathway that has been identified in sepsis-induced AKI is alterations in primary tubular metabolism which could secondarily affect the regional circulation through decreased levels of ATP and mitochondrial dysfunction.17,18 Tran et al.19 demonstrated that mitochondrial dysfunction, cellular swelling, and a pronounced accumulation of acylglycerols developed in tubules, which led to decreased PGE2 and promoted medullary vasoconstriction in ischemia AKI. Thus, studies on mitochondria might lead to greater insights into the mechanism of AKI. Furthermore, restoration of healthy mitochondrial function and mass is likely critical to the recovery of kidney function.20
The recent progress in understanding the role of mitochondria in sepsis-induced AKI has led to an array of potential applications for mitochondria as biomarkers of kidney injury as well as targets for novel therapeutic strategies. In this review, we describe mitochondrial metabolic dysregulations in sepsis-induced AKI and discuss how this knowledge may guide the development of potential new therapies for sepsis-induced AKI.
Metabolic Regulations of Mitochondria during Sepsis-Induced AKI
A critical function of mitochondria is to provide energy (ATP) that is used by the kidney to remove waste products from the blood as well as to regulate fluid and electrolyte balance. Mitochondrial homeostasis is closely regulated by mitochondrial biogenesis. Mitochondrial biogenesis is the process by which cells increase mitochondrial mass and it is accompanied by increases in metabolic enzymes for glycolysis, oxidative phosphorylation (OXPHOS), and greater mitochondrial capacity for energy production. Under conditions such as oxidative stress and during sepsis, mitochondria can alter metabolic processes to adapt to these stressful conditions through a number of signaling pathways that maintain homeostasis.
Energy Metabolism of Mitochondria in Sepsis-Induced AKI
The provision of energy to the cell is through the electron transport chain in a process called OXPHOS. Glucose metabolism through glycolysis and β-oxidation of fatty acids (FAs) are the main energy substrates for the kidney. Energy production occurs through a series of electron transfers along the inner mitochondrial membrane leading to the eventual production of ATP. Under normal conditions, proximal tubular cells prefer FAs as the energy source and aerobic respiration is the primary mechanism of ATP production.21 However, during sepsis and hypoxic conditions, instead of feeding pyruvate into the tricarboxylic acid cycle to generate ATP through OXPHOS, proximal tubule cells convert pyruvate to lactate, a less efficient mechanism to produce ATP.22,23
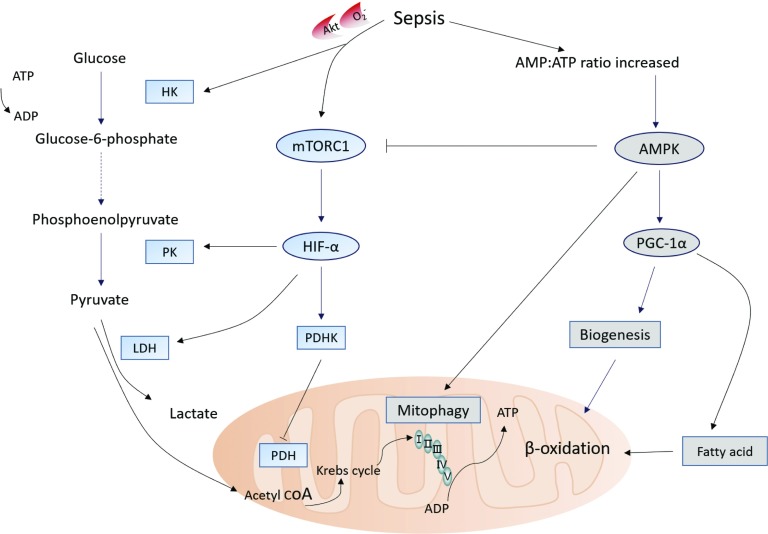
The metabolic shift during sepsis is driven by mammalian target of rapamycin complex 1 (mTORC1)–induced stabilization of hypoxia inducible factor–1α (HIF-1α) through the Akt/mTORC1/HIF-1α pathway (Figure 1).22–24 Recent studies also suggest that, during LPS-induced AKI, hexokinase activation and increased glucose-6-phosphate dehydrogenase activity are linked to increased pentose phosphate pathway activity. Despite the fact that glycolysis provides less efficient energy generation, it can provide sufficient energy for cell survival as well as for maintenance of essential structural components25 and it leads to a decrease in OXPHOS and the production of mitochondrial reactive oxygen species (ROS), at least during the early course of sepsis.
Figure 1.
Energetic metabolic reprogramming in tubular epithelial cells during sepsis-induced AKI. Activated HIF-1α promotes the transformation of pyruvate into lactate, and inhibits the transformation of pyruvate into acetyl-CoA, thus blocking entry into the Krebs cycle. HIF-1α also induces the expression of PKM2, and slows down the conversion of phosphoenol pyruvate to pyruvate. HK is activated by Akt or O2− and increases glucose-6-phosphate dehydrogenase activity which is linked to increased PPP activity. As ATP levels decrease, AMPK is activated. AMPK leads to induction of mitochondrial biogenesis by PGC-1α, inhibits mTORC, and activates mitophagy by phosphorylating the serine/threonine protein kinase ULK1. Acetyl-CoA, acetyl-coenzyme A; PKM2, M2 isoform of pyruvate kinase; HK, hexokinase; PPP, pentose phosphate pathway; mTORC, mammalian target of rapamycin complex.
However, as ATP levels decrease, adenosine monophosphate–activated protein kinase (AMPK), a master sensor of energy catabolic status, is induced. Activation of AMPK can lead to the production of critical antioxidant enzymes and the induction of mitochondrial biogenesis by peroxisome proliferator–activated receptor (PPAR) γ coactivator–1α (PGC-1α). Moreover, activation of AMPK leads to an increase in glycolytic pathway flux, FA oxidation, and glucose transport. All of these events contribute to cell growth and an increase in cellular metabolism.26,27 In addition, AMPK also inhibits mTORC and activates autophagy by phosphorylation of the serine/threonine protein kinase Unc-51–like kinase 1 (ULK1).28 This AMPK-dependent response regulates metabolism, reprioritizes energy expenditure toward the functions necessary for survival, limits oxidative damage from dysfunctional mitochondria, and eventually stabilizes energy balance by mitochondrial biogenesis.
Thus, during sepsis-induced AKI, energy metabolism might first switch to glycolysis in order to decrease oxygen consumption and enhance the capacity of the cell to defend against oxidative damage through decreasing OXPHOS and mitochondrial ROS production. Later activation of AMPK may allow for cell survival and mitochondrial biogenesis.
Mitochondrial Biogenesis in Sepsis-Induced AKI
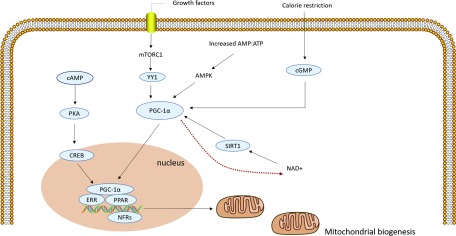
Mitochondrial biogenesis can increase ATP production in response to increasing energy demand by the generation of new and functional mitochondria. PGC-1α is a positive mitochondrial biogenesis regulator.29 During sepsis, transient local ischemia and increased cytokine levels, especially TNF-α, can reduce PGC-1α expression in tubular cells and suppress kidney recovery.30 Both mammalian target of mTORC and AMPK signaling pathways regulate mitochondrial biogenesis and help maintain healthy mitochondria during AKI (Figure 2). Activated mTORC1 triggers pathways that lead to mitochondrial biogenesis by activating the transcriptional repressor yin and yang 1 (YY1).31 AMPK can induce mitochondrial biogenesis by stimulating the transcription of the gene encoding PGC-1α (PPARGCIA) and by phosphorylating PGC-1α at Thr177 and Ser539 to increase its activity.27
Figure 2.
Mitochondrial biogenesis in sepsis-induced AKI. There are multiple pathways involved in mitochondrial biogenesis. Activated PGC-1α cooperates with NRFs and promotes the expression of multiple nuclear-encoded genes; PGC-1α activation promotes its translocation from the cytoplasm to the nucleus; activated mTOR1 triggers pathways by activating the transcriptional repressor YY1; AMPK can induce mitochondrial biogenesis; SIRT1 is activated by NAD+, and then activates PGC-1α. Stimulation of adenylyl cyclase increases cAMP, which activates PKA that in turn phosphorylates CREB. CREB can stimulate mitochondrial biogenesis; caloric restriction increases levels of cGMP and inhibits phosphodiesterases which can stimulate PGC-1α activation and mitochondrial biogenesis in vivo. mTOR, mechanistic target of rapamycin; YY1, Yin and yang 1; SIRT, Sirtuins; NAD, nicotinamide adenine dinucleotide; PKA, protein kinase A; cGMP: cyclic guanosine monophosphate.
Other pathways that stimulate mitochondrial biogenesis include sirtuins (SIRT), cAMP, and cyclic guanosine monophosphate (cGMP). The activity of SIRT1 is activated by NAD, leading to further activation of downstream targets such as PGC-1α.32 PGC-1α is a pivotal determinant of renal recovery from injury by regulating NAD biosynthesis in ischemia-reperfusion and in AKI secondary to nephrotoxic drugs.33 The NAD precursor niacinamide (NAM) can enhance NAD production and augment production of the fat breakdown product β-hydroxybutyrate (β-OHB), which leads to increased vasodilatory prostanoids such as PGE2, a secreted autacoid that maintains renal perfusion.34 After ischemia, mice deficient in PGC-1α (a downstream substrate of both SIRT and AMPK) develop local deficiency of the NAD+ precursor niacinamide or NAM, accumulate fat, and fail to re-establish normal renal function.19 Stimulation of adenylyl cyclase increases cAMP levels, which activates protein kinase A (PKA) that in turn phosphorylates cAMP-responsive element–binding protein (CREB).35,36 CREB is also a transcriptional activator of PGC-1α and can thus also stimulate mitochondrial biogenesis through this pathway.35 Finally, caloric restriction (food only provided on alternate days for 3 or 12 months) increases levels of cGMP and inhibits phosphodiesterases which can stimulate PGC-1α activation and mitochondrial biogenesis in vivo and folic acid–induced AKI.37,38 In summary, these findings indicate that PGC-1α may be a critical factor in the recovery of sepsis-induced AKI through mitochondrial biogenesis, and mitochondrial regeneration may be a future therapeutic target for sepsis-induced AKI.
Superoxide Anion Metabolism in Sepsis-Induced AKI
Renal mitochondrial injury occurs early in the course of sepsis and is associated with cellular damage as a result of ROS generation. Much of the data in this area of study are derived from ischemia-reperfusion models of AKI and the applicability to sepsis models has not been as well studied; the causes of mitochondrial ROS production upon reperfusion after ischemia are still unclear. It has been assumed that ROS production during reperfusion is a nonspecific consequence of oxygen interacting with dysfunctional mitochondria upon reperfusion. Recently, this view has changed, suggesting that specific metabolic pathways may be operative in which superoxide is generated through reverse electron transport at complex I of the electron transport chain. Moreover, selective accumulation of the citric acid cycle intermediate succinate is a universal metabolic signature of ischemic tissues and is responsible for mitochondrial ROS production during reperfusion.39 Importantly, pharmacologically inhibiting succinate accumulation, or slowing succinate metabolism at reperfusion, has been shown to be protective in ischemia-reperfusion models.40
ROS are toxic to the endothelium and defense mechanisms are critical to maintain organ perfusion and function.41 Mitochondria have intrinsic antioxidant mechanisms to protect against damage induced by ROS through a large array of mechanisms (e.g., SOD, glutathione, thioredoxin).42 In response to oxidative stress, NF erythroid 2–related factor 2 activates the transcription of genes encoding antioxidant enzymes such as glutathione peroxidase, SOD2, and catalase. In this manner, a mechanism to prevent ROS-induced injury is upregulated.43 Glutathione is a tripeptide (γ-glutamyl-cysteinal-glycine) nucleophile capable of preventing damage to important cellular components caused by ROS. Mitochondria contain their own pool of glutathione (mGSH), which not only helps to decrease excessive ROS levels but also prevents the release of cytochrome c from the inner mitochondrial membrane.44 mGSH directly interacts with superoxide anions and becomes oxidized to glutathione disulfide (GSSG).44,45 GSSG cannot exit the mitochondria and is converted back to mGSH by glutathione reductase, for reuse or elimination from the mitochondria.45 The conversion of GSSG to mGSH requires NADP (NADPH) that is produced by the pentose phosphate pathway. SOD2 is a mitochondrial enzyme that binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and oxygen.46
Mitochondrial uncoupling protein 2 (UCP2) also plays a role in attenuating excessive ROS production. As levels of ROS increase, UCP2 is activated and acts to dissipate the proton motive force as heat and as a result reduces ROS production.47,48 Together, these antioxidant systems can maintain optimal ATP production, and sustain mitochondrial function.
Fusion and Fission
Fusion is the combining of two mitochondria, and fission is the splitting of mitochondria into two. Mitochondria are highly dynamic organelles that exchange genetic and other information by coordinated fusion and fission. Under physiologic conditions, the processes of fusion and fission are necessary to maintain mitochondrial homeostasis and play an important role in the quality control of mitochondria.49
Mitochondria fuse together via mitofusin 1 and 2 (Mfn1 and 2) on the outer membrane and require activation of dynamin-like 120 kD (OPA1) on the inner membrane. Fusion can maintain ATP production and redistribute mitochondrial proteins. Mitochondria divide via dynamin-related protein 1 (Drp1) on the outer membrane. Fission can isolate damaged mitochondria from the mitochondrial network. Dysfunctional mitochondria, which are toxic for tubular cells, are removed by mitophagy. If the daughter mitochondria produced by fission are unbalanced and depolarized, they are targeted for mitophagy.50
In response to insults such as ischemia-reperfusion injury or sepsis, the balance between mitochondrial fusion and fission shifts to mitochondrial fission. The mitochondria become fragmented in sepsis.51,52 Changes in mitochondrial morphology, characterized by initial fragmentation of the organelles followed by ultrastructural alterations resulting in mitochondrial swelling and cristae deformation, are observed in renal tubular cells during septic AKI.51 The disruption of mitochondrial dynamics at both outer and inner membranes plays a crucial role in mitochondrial dysfunction and tubular cell injury and death in ischemia-reperfusion and cisplatin-induced AKI.53 It is likely that the same is true in sepsis.52
ROS Scavenging by Mitochondria in Sepsis-Induced AKI
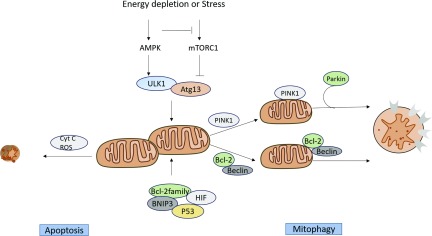
Ultrastructural changes are observed in mitochondria during sepsis-induced AKI. Mitophagy (autophagy of mitochondria) protects stressed cells from death due to mitochondrial fragmentation and can ameliorate AKI.54 Importantly, despite ATP depletion and ROS generation, the necrotic and apoptotic responses of mitochondria are limited in sepsis-induced AKI. Supporting this is that mitophagy is rapidly induced during various models of AKI and plays important roles in renal protection.54 Parikh et al.20 demonstrated that mitophagy removed damaged mitochondria in tubular epithelial cells during sepsis-induced AKI. Two main mechanisms of mitophagy have been described. In the first mechanism, mitophagy is mediated by the PTEN-induced putative kinase protein 1 (Pink1)–PARKIN mechanism. The second mechanism of mitophagy involves two Bcl-2 family proteins: BCL2/adenovirus E1B 19-kD protein–interacting protein 3 (BNIP3) and Nip3-like protein X (NIX, also known as BNIP3L).
Energy-sensitive kinases mTORC1 and AMPK are known to regulate mitophagy.55–59 mTORC1 phosphorylates ULK1/2 and autophagy-related gene (Atg) 13 which inhibit ULK1/2 kinase activity,57,60 which negatively regulates autophagy. AMPK can inhibit mTORC1 and activate mitophagy by phosphorylating the serine/threonine protein kinase ULK1.56 Some studies have reported that inhibition of the mTORC pathway can impair tubular proliferation and delay recovery of kidney function during ischemia-reperfusion injury models.61–64 Additional studies are required to investigate the role of mTORC1-mediated induction of autophagy in sepsis-induced AKI. Other molecules such as antiapoptotic members of the Bcl-2 family, BNIP3, HIF, and p53 also induce autophagy in AKI (Figure 3).54
Figure 3.
ROS scavenging by mitochondria in sepsis-induced AKI. AMPK can inhibit mTORC1 and activate mitophagy by phosphorylating the serine/threonine protein kinase ULK. mTORC1 phosphorylates ULK1/2 and Atg13 which negatively regulates autophagy. Other molecules such as antiapoptotic members of the Bcl-2 family, BNIP3, HIF, and p53 also induce autophagy in AKI. The increase in the permeability of the outer mitochondrial membrane can release proapoptotic factors into the cytoplasm. These factors activate the caspase-dependent or -nondependent cascade reaction mechanism. mTORC, mechanistic target of rapamycin complex; Atg, Autophagy-related gene.
The increase in permeability of the outer mitochondrial membrane can release these proapoptotic factors into the cytoplasm. Jiang et al.65 demonstrated that mitophagy occurred before tissue damage or tubular apoptosis. In these cases, excessive mitophagy can “spill over” and digest normal components in the cell causing lethal injury, whereas, on the other hand, insufficient mitophagy can also release proapoptotic substances. The signaling pathways between autophagy and apoptosis in AKI need to be further studied.
Mitochondrial homeostasis is critical because rapid recovery of ATP levels is essential for cell survival. In this regard, the various processes of mitochondrial scavenging (mitophagy and apoptosis) and biogenesis are key steps to ensure cellular function and survival.
Therapeutic Targeting of Mitochondria in Sepsis-Induced AKI
Understanding mitochondrial metabolic regulation during sepsis-induced AKI may lead to new therapeutic targets. This section summarizes recent findings regarding the manipulation of mitochondrial metabolism, homeostasis, and recovery (Table 1).
Table 1.
Pharmacologic approaches targeting mitochondria for kidney injury
| Drugs | Pathway/Target | Process | Effect | Species | Reference |
|---|---|---|---|---|---|
| Antracyclines | Akt/mTORC/HIF-1α pathway | Energy pathway | Ameliorate injury | Animals | 67 |
| β-glucan | Akt/mTORC/HIF-1α pathway | Energy pathway | Ameliorate injury | Animals | 23 |
| AICAR | AMPK/Sirt1–6 pathway | Energy pathway | Decrease tubular damage | Animals | 68,69 |
| Formoterol | PGC1α | Biogenesis | Restore renal function | Animals | 78 |
| LY344864 | PGC1α | Biogenesis | Improve renal function | Animals | 79 |
| MA-5 | Mitofilin/Mic60 | Biogenesis | Decrease plasma BUN | Humans | 97,98 |
| Levosimendan | Mitochondrial ATP-sensitive K+ channels | Biogenesis | Restore renal function | Humans | 100 |
| Bendavia (SS-31) | Antioxidation | Reduced serum creatinine and BUN | Humans | 80–82 | |
| Mito Q | Chain-breaking antioxidant ubiquinol | Antioxidation | Improve renal function | Humans | 83 |
| Mdivi-1 | Drp1 | Fusion and fission | Ameliorate injury | Animals | 53,87 |
| Antymycin A | p53 | Mitophagy | Protect renal tubular cells | Animals | 90 |
| Ambra1 | Parkin | Mitophagy | Ameliorate injury | Animals | 92 |
| Cyclophilin-D | MPT | Protect against renal injury | Animals | 99 | |
| CsA | Drp1 | MPT | Ameliorate podocyte damage | Humans | 88,89 |
CsA, cyclosporine A; Drp1, dynamin-related protein 1; Mdivi-1, mitochondrial division inhibitor–1; MA-5, mitochonic acid 5; Mito Q, mitoquinone; MPT, mitochondrial permeability.
Targeting Mitochondrial Energy Pathways
Akt/mTORC/HIF-1α Pathway
The Akt/mTORC/HIF-1α pathway can regulate glucose metabolism and mitochondrial function. Activation of Akt/mTORC/HIF-1α can switch tubular epithelial cell metabolism to aerobic glycolysis in response to hypoxia and sepsis. Activation of this pathway decreases oxygen consumption and enhances the capacity of the cell to cope with oxidative damage. In addition, aerobic glycolysis supports the ability of the innate immune system to develop memory and modify the response to future insults.23,66
Treatment of rodents with antracyclines inhibits mTOR and thus activates autophagy, which limits AKI and improves survival in a model of cecal ligation and puncture (CLP)–induced sepsis.67 In monocytes, prestimulation of the Akt/mTORC1/HIF-1α pathway with β-glucan (by exposure to sublethal concentrations of Candida albicans) results in increased secretion of TNF and IL-6, and such prestimulation results in improved survival.23 The protection achieved by pretreatment with β-glucan was lost when Akt, mTOR, or HIF-1α was inhibited. This suggests that the Akt/mTORC1/HIF-1α pathway, which is involved in metabolic reprogramming and regulation during infection, is a promising target to ameliorate sepsis-induced AKI.
AMPK/Sirt1–6 Pathway
AMPK targets a number of proteins, the phosphorylation of which leads to the production of antioxidant enzymes; the induction of mitochondrial biogenesis; and an increase in glycolytic flux, FA oxidation, and glucose transport; all of these events contribute to cell growth and an increase in cellular metabolism. AMPK/SIRT1–6 pathway signaling has been implicated as a target for correcting metabolic and mitochondrial function in AKI. AMPK is a master sensor of energy status that stimulates catabolic processes. A high AMP/ATP ratio activates AMPK to stimulate cell growth and metabolism.
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) acts as an activator of AMPK and can increase the levels of PGC-1α and other mitochondrial proteins while reducing ROS production in a diabetic mouse model.68 Stimulation of AMPK with AICAR before CLP-induced sepsis was associated with significant protection from AKI and with decreased inflammatory molecules such as IL-6, IL-10, and TNF-α,69 suggesting that AMPK may play a protective role in the early response to sepsis. There is a crosstalk between AMPK and SIRT3 signaling.60,70 SIRT1 and SIRT3 are protein deacetylases that have roles in many mitochondrial processes, such as the electron transport chain, the tricarboxylic acid cycle, FA oxidation, and mitochondrial biogenesis.71 SIRT1 and SIRT3 are activated by NAD+.72
Administration of AICAR to cisplatin-treated mice attenuated the decreases in SIRT3 expression, phosphorylated AMPK level, and tubular damage.73 Compared with saline-treated control mice, cisplatin-treated mice have decreased expression and lower protein levels of SIRT3, increased tubular damage, and decreased levels of phosphorylated AMPK.73 These studies provide a therapeutic rationale for targeting AMPK to improve outcomes in AKI. The relationship between AMPK activity and SIRT in mitochondrial protection needs to be further studied.
Targeting Mitochondrial Biogenesis
Carbon monoxide upregulates mitochondrial biogenesis via activation of redox-regulated NF-E2–related factor transcription factor, nuclear respiratory factor (NRF1, NRF2), and PGC-1α. Carbon monoxide is released endogenously by activation of hemeoxygenase-1. Induction of hemeoxygenase-1 in sepsis models has shown protective effects through NRF-2 and mitochondrial biogenesis.74 PGC-1α, which is expressed highly in the kidney, is capable of driving all steps of mitochondrial biogenesis. It is an attractive pharmacologic target for kidney protection.33
After renal ischemia, PGC-1α−/− mice developed local deficiency of NAM, marked fat accumulation, and failure to re-establish normal function.19 Kidney-specific PGC-1α gene–knockout mice exhibited persistent AKI after LPS-induced sepsis.75 Remarkably, exogenous NAM improved local NAD levels, fat accumulation, and kidney function in postischemic PGC-1α−/− mice. Recent studies have demonstrated that supplementation with a NAD precursor such as nicotinamide mononucleotide or NAM can contribute to kidney protection in cisplatin- or ischemia-induced AKI.76,77 These findings imply that PGC-1α is necessary for recovery of kidney function in AKI.
In addition, two different G protein–coupled receptors, the β2 adrenergic receptor (β2AR) and the 5-hydroxytryptamine receptor 1F (5-HT1F), can restore mitochondrial function and stimulate kidney recovery by inducing mitochondrial biogenesis after IR-induced injury. Formoterol, a β2AR agonist, stimulates mitochondrial biogenesis by upregulating PGC-1α through the cAMP/PKA/CREB axis in kidney proximal tubular cells and can accelerate the recovery of mitochondrial and kidney function in mouse models of ischemia-reperfusion AKI.78 LY344864, a potent 5-HT1F agonist, induces mitochondrial biogenesis in naive mice and accelerates the recovery of mitochondrial and kidney function in the same AKI murine model.79
Antioxidant Therapy
Antioxidants that specifically target mitochondria have shown promising effects in ameliorating AKI. The Szeto-Schiller (SS) peptide selectively interacts with cardiolipin to stabilize the mitochondrial inner membrane and directly deliver antioxidants to the mitochondria.80 The peptide SS-31 (Bendavia) ameliorates mitochondrial dysfunction by reducing mitochondrial cristae disorganization in proximal tubules during ischemia and has been shown to protect mitochondrial structure and function in an in vivo study of an ischemia-reperfusion–induced AKI model in rats. Furthermore, SS-31 also ameliorated reperfusion-induced oxidative stress and accelerated the recovery of mitochondrial structure and ATP levels, which in turn preserved proximal tubular cell structure, decreased tubular cell apoptosis, and partially preserved kidney function.80 When administered immediately after CLP in mice, SS-31 normalized kidney ATP content, decreased apoptosis, reduced histologic injury score, and reduced serum creatinine and BUN levels.81 A human clinical trial is investigating the efficacy of SS-31 in ameliorating ischemia-reperfusion (NCT02436447).82 In addition, a phase 1 study is investigating the safety and pharmacokinetics of SS-31 (MTP-131) in human subjects with impaired kidney function.
It has been demonstrated that inducible nitric oxide synthase inhibitors or antioxidants (l-glutathione, ebselen, or MitoQ) can specifically target mitochondria and reduce oxidative damage, preserve cytochrome c oxidase activity, prevent mitochondrial membrane potential dissipation, and improve kidney function in vitro and in LPS-83,84 and CLP85-induced sepsis models. Other compounds such as acetyl- l-carnitine, a mitochondrial antioxidant activity modulator, can ameliorate tubular injury and improve kidney function in cisplatin-induced AKI in mice.73,86
Targeting Mitochondrial Fusion and Fission
Mitochondrial division inhibitor–1 (Mdivi-1), a pharmacologic inhibitor of Drp1, blocks mitochondrial fragmentation and subsequent AKI progression in cisplatin-induced AKI53 as well as rhabdomyolysis-induced AKI87 in mice. Cho et al.88 demonstrated that cyclosporine A suppresses Drp1 dephosphorylation and prevents mitochondrial fragmentation, Bax accumulation, cytochrome c release, and apoptosis after hypoxia-induced ATP depletion in kidney proximal tubular cells in vitro. The in vivo pretreatment with Drp1 inhibitor significantly attenuated mitochondrial dysfunction and abnormal fusion-to-fission balance in a CLP-induced sepsis model.52 A phase 2 clinical trial to assess the reno-protective effect of cyclosporine A in cardiac surgery is now ongoing (NCT02397213).89
Targeting Mitophagy Activator Therapy
As described above, mitophagy can remove damaged mitochondria. Mitophagy is induced in AKI and pharmacologic enhancement of this process could minimize cell injury and accelerate recovery. In this regard, antymycin A or myxothiazol, inhibitors of the mitochondrial respiration complex, can ameliorate cisplatin-induced p53 activation and exert cytoprotective effects in cultured kidney tubular cells.90 Additionally, rapamycin protected against gentamicin-induced AKI in pigs by inducing mitophagy.91 Another compound of interest, Ambra 1, induces mitophagy by interacting with Parkin. Ambra 1 may be a therapeutic agent to induce mitophagy.92
Although the induction of mitophagy seems to ameliorate AKI, some studies have reported conflicting results, and the role of mitophagy in AKI remains a subject of debate.93 Drugs that induce mitophagy also induce mitochondrial depolarization. Mitochondrial membrane depolarization suppresses fusion, leading to fragmentation of the mitochondrial network. Fusion suppression is mostly led by the Overlapping with the m-AAA protease homolog (OMA1). After mitochondrial depolarization, PINK1 is exposed on the outer mitochondrial membrane of depolarized mitochondria in its full-length form94,95 and triggers mitophagy.95,96
Resolving these issues and conducting clinical trials on these compounds will be important to determine the ultimate role of mitophagy-targeted therapy in AKI.
Other Therapeutic Approaches Targeting Mitochondria in AKI
As presented above, there are other ways that mitochondria may be targeted for therapeutic benefit.
Mitochonic acid 5 (MA-5), a derivative of the plant hormone indole-3-acetic acid, is one of the newest class of mitochondria-targeted agents. It can reduce tubular necrosis and decrease plasma BUN after cisplatin administration.97 MA-5 is proposed to target the mitochondrial protein mitofilin/Mic60 at the cristae junction of the inner mitochondrial membrane and facilitates oligomerization of ATP synthase and super complex formation.97,98
Cyclophilin-D, a key regulator of mitochondrial permeability, protects against kidney injury in multiple experimental models of AKI.99 Levosimendan is a Ca2+ sensitizer–positive inotropic and vasodilator drug used to treat heart failure. It has renoprotective effect after cardiopulmonary bypass surgery.100 The molecular mechanism of levosimendan-evoked protection might include an interaction with mitochondrial energy conservation through mitochondrial ATP-sensitive K+ channels. A clinical study of levosimendan, investigating the safety and efficacy in intensive care patients with AKI, is now ongoing (clinicaltrials.gov identifier NCT01720030).
Mitochondrial Biomarkers for Sepsis-Induced AKI
As mitochondrial dysfunction initiates and accelerates kidney injury in sepsis, disruption of mitochondrial integrity in kidney tubular cells seems to be a hallmark in diverse forms of AKI.20 Mitochondrial DNA (mtDNA) is a circular double-stranded DNA inherited maternally which is housed in the mitochondrial matrix, encased within a double-membrane system composed of the outer and inner mitochondrial membranes.101 Increased mitochondrial ROS generation can decrease mitochondrial membrane potential, leading to impairment of membrane integrity.102,103 These changes could subsequently permit leakage of mtDNA into the cytosol. Furthermore, one of the proposed mechanisms by which mtDNA is translocated to the extracellular space is vianecroptosis.104,105 Therefore, in the kidney, disruption of mitochondrial integrity may result in release of mtDNA fragments, known as mitochondrial damage-associated molecular patterns, from the matrix into the urine, where they serve as surrogate markers of kidney mitochondrial injury.106
Therefore, recent studies evaluated the efficiency of urinary mtDNA (UmtDNA) as a marker of kidney injury in patients with AKI with various causes.107–110 Currently, because of diverse patient cohorts, and small sample sizes with type 2 statistical error, the utility of UmtDNA is unclear. Hu et al.110 reported that UmtDNA identified renal dysfunction and mitochondrial damage in sepsis-induced AKI. However, UmtDNA may serve as a valuable biomarker for the development and testing of mitochondria-targeted therapies in AKI. UmtDNA is a surrogate marker of mitochondrial integrity. Because of the role of mitochondrial function and tissue bioenergetics in tissue repair processes, UmtDNA may aid the development of new mitochondrial-targeted therapies for sepsis-induced AKI.
Conclusions
Mitochondria, given their central role in energy metabolism, play a key role in the pathophysiology of sepsis-induced AKI. Multiple targets for therapy have been identified in preclinical work but further research is needed to identify the most promising targets with the aim of improving AKI outcomes.
Disclosures
None.
Acknowledgments
This manuscript has not been published and is not under consideration elsewhere; the results presented in this paper have not been published previously. All persons listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. Consent for use of deidentified images contained in this article was given by the individuals involved.
All authors contributed to manuscript writing and editing, and read and approved the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801–810, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW: Body fluid volume regulation in health and disease: A unifying hypothesis. Ann Intern Med 113: 155–159, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW, Abraham WT: Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Benedict CR, Rose JA: Arterial norepinephrine changes in patients with septic shock. Circ Shock 38: 165–172, 1992 [PubMed] [Google Scholar]
- 7.Cumming AD, Driedger AA, McDonald JW, Lindsay RM, Solez K, Linton AL: Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis 11: 23–32, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Langenberg C, Wan L, Egi M, May CN, Bellomo R: Renal blood flow in experimental septic acute renal failure. Kidney Int 69: 1996–2002, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Di Giantomasso D, May CN, Bellomo R: Vital organ blood flow during hyperdynamic sepsis. Chest 124: 1053–1059, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Langenberg C, Bellomo R, May C, Wan L, Egi M, Morgera S: Renal blood flow in sepsis. Crit Care 9: R363–R374, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Titheradge MA: Nitric oxide in septic shock. Biochim Biophys Acta 1411: 437–455, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Landry DW, Oliver JA: The pathogenesis of vasodilatory shock. N Engl J Med 345: 588–595, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Doi K, Leelahavanichkul A, Yuen PS, Star RA: Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 119: 2868–2878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Post EH, Kellum JA, Bellomo R, Vincent JL: Renal perfusion in sepsis: From macro- to microcirculation. Kidney Int 91: 45–60, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Calzavacca P, Evans RG, Bailey M, Bellomo R, May CN: Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med 43: e431–e439, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Lankadeva YR, Kosaka J, Evans RG, Bailey SR, Bellomo R, May CN: Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int 90: 100–108, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Bartz RR, Fu P, Suliman HB, Crowley SD, MacGarvey NC, Welty-Wolf K, et al. : Staphylococcus aureus sepsis induces early renal mitochondrial DNA repair and mitochondrial biogenesis in mice. PLoS One 9: e100912, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez H, Kellum JA, Ronco C: Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13: 143–151, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al. : PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z: Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35: 108–119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houten SM, Violante S, Ventura FV, Wanders RJ: The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu Rev Physiol 78: 23–44, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, et al. : Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17: 406–413, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. : mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. : PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 5: 4436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vander Heiden MG, Cantley LC, Thompson CB: Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mihaylova MM, Shaw RJ: The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13: 1016–1023, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jäger S, Handschin C, St-Pierre J, Spiegelman BM: AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zachari M, Ganley IG: The mammalian ULK1 complex and autophagy initiation. Essays Biochem 61: 585–596, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura-Clapier R, Garnier A, Veksler V: Transcriptional control of mitochondrial biogenesis: The central role of PGC-1α. Cardiovasc Res 79: 208–217, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Stallons LJ, Funk JA, Schnellmann RG: Mitochondrial homeostasis in acute organ failure. Curr Pathobiol Rep 1: 169–177, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P: mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature 450: 736–740, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nakatani Y, Inagi R: Epigenetic regulation through SIRT1 in podocytes. Curr Hypertens Rev 12: 89–94, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Whitaker RM, Corum D, Beeson CC, Schnellmann RG: Mitochondrial biogenesis as a pharmacological target: A new approach to acute and chronic diseases. Annu Rev Pharmacol Toxicol 56: 229–249, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, et al. : Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest 120: 2910–2919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM: An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci U S A 100: 7111–7116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Marcos PJ, Auwerx J: Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93: 884S–90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. : Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG: cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther 347: 626–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. : Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pell VR, Spiroski AM, Mulvey J, Burger N, Costa ASH, Logan A, et al. : Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J Mol Cell Cardiol 123: 88–91, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath KA, Norby SM: Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Yin F, Sancheti H, Cadenas E: Mitochondrial thiols in the regulation of cell death pathways. Antioxid Redox Signal 17: 1714–1727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teruya R, Ikejiri AT, Somaio Neto F, Chaves JC, Bertoletto PR, Taha MO, et al. : Expression of oxidative stress and antioxidant defense genes in the kidney of inbred mice after intestinal ischemia and reperfusion. Acta Cir Bras 28: 848–855, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Ribas V, García-Ruiz C, Fernández-Checa JC: Glutathione and mitochondria. Front Pharmacol 5: 151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lushchak VI: Glutathione homeostasis and functions: Potential targets for medical interventions. J Amino Acids 2012: 736837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisiger RA, Fridovich I: Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem 248: 4793–4796, 1973 [PubMed] [Google Scholar]
- 47.Brand MD, Esteves TC: Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2: 85–93, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, et al. : Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37: 755–767, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Tatsuta T, Langer T: Quality control of mitochondria: Protection against neurodegeneration and ageing. EMBO J 27: 306–314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twig G, Hyde B, Shirihai OS: Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim Biophys Acta 1777: 1092–1097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Detmer SA, Chan DC: Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, et al. : Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res 48: 769–783, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livingston MJ, Dong Z: Autophagy in acute kidney injury. Semin Nephrol 34: 17–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YC, Guan KL: mTOR: A pharmacologic target for autophagy regulation. J Clin Invest 125: 25–32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J, Kundu M, Viollet B, Guan KL: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. : Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20: 1981–1991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, et al. : FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181: 497–510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunlop EA, Tee AR: mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol 36: 121–129, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, et al. : Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285: 3133–3144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakagawa S, Nishihara K, Inui K, Masuda S: Involvement of autophagy in the pharmacological effects of the mTOR inhibitor everolimus in acute kidney injury. Eur J Pharmacol 696: 143–154, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, et al. : Rapamycin impairs recovery from acute renal failure: Role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 281: F693–F706, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Lieberthal W, Fuhro R, Andry C, Patel V, Levine JS: Rapamycin delays but does not prevent recovery from acute renal failure: Role of acquired tubular resistance. Transplantation 82: 17–22, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Esposito C, Grosjean F, Torreggiani M, Esposito V, Mangione F, Villa L, et al. : Sirolimus prevents short-term renal changes induced by ischemia-reperfusion injury in rats. Am J Nephrol 33: 239–249, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Jiang M, Liu K, Luo J, Dong Z: Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176: 1181–1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zager RA: ‘Biologic memory’ in response to acute kidney injury: Cytoresistance, toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrol Dial Transplant 28: 1985–1993, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figueiredo N, Chora A, Raquel H, Pejanovic N, Pereira P, Hartleben B, et al. : Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 39: 874–884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. : AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, et al. : Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res 194: 262–272, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL 3rd, et al. : Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging (Albany NY) 1: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. : A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, et al. : Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol Rev 92: 1479–1514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, et al. : Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715–726, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, et al. : Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286: 16374–16385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. : PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, et al. : Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J Am Soc Nephrol 28: 2337–2352, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, et al. : De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, et al. : Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG: Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther 350: 257–264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, et al. : The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G, Wu J, Li R, Yuan D, Fan Y, Yang J, et al. : Protective effects of antioxidant peptide SS-31 against multiple organ dysfunctions during endotoxemia. Inflammation 39: 54–64, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Wijermars LG, Schaapherder AF, de Vries DK, Verschuren L, Wüst RC, Kostidis S, et al. : Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int 90: 181–191, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Lowes DA, Thottakam BMV, Webster NR, Murphy MP, Galley HF: The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45: 1559–1565, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Quoilin C, Mouithys-Mickalad A, Lécart S, Fontaine-Aupart M-P, Hoebeke M: Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta 1837: 1790–1800, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR: Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: Mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol 306: F734–F743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukhopadhyay P, Horváth B, Zsengellér Z, Zielonka J, Tanchian G, Holovac E, et al. : Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med 52: 497–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang WX, Wu WH, Qiu HY, Bo H, Huang SM: Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol 26: 1073–1082, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Cho SG, Du Q, Huang S, Dong Z: Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol 299: F199–F206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ederoth P, Grins E, Dardashti A, Brondén B, Metzsch C, Erdling A, et al. : Ciclosporin to Protect Renal function In Cardiac Surgery (CiPRICS): A study protocol for a double-blind, randomised, placebo-controlled, proof-of-concept study. BMJ Open 6: e012299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J, Biju MP, Wang MH, Haase VH, Dong Z: Cytoprotective effects of hypoxia against cisplatin-induced tubular cell apoptosis: Involvement of mitochondrial inhibition and p53 suppression. J Am Soc Nephrol 17: 1875–1885, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Cui J, Bai XY, Sun X, Cai G, Hong Q, Ding R, et al. : Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep 5: 11256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, et al. : AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ 22: 517, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Decuypere JP, Ceulemans LJ, Agostinis P, Monbaliu D, Naesens M, Pirenne J, et al. : Autophagy and the kidney: Implications for ischemia-reperfusion injury and therapy. Am J Kidney Dis 66: 699–709, 2015 [DOI] [PubMed] [Google Scholar]
- 94.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ: Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin SM, Youle RJ: PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci 125: 795–799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. : PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki T, Yamaguchi H, Kikusato M, Hashizume O, Nagatoishi S, Matsuo A, et al. : Mitochonic acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J Am Soc Nephrol 27: 1925–1932, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsuhashi T, Sato T, Kanno SI, Suzuki T, Matsuo A, Oba Y, et al. : Mitochonic Acid 5 (MA-5) facilitates ATP synthase oligomerization and cell survival in various mitochondrial diseases. EBioMedicine 20: 27–38, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh D, Chander V, Chopra K: Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207: 339–347, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Gamilla-Crudo AK, Kadambi PV, Prough DS: Test driving levosimendan as the new “kidney protector”: First impressions... Crit Care Med 41: 2445–2446, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N: Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23: 147, 1999 [DOI] [PubMed] [Google Scholar]
- 102.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. : Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrington JS, Choi AMK, Nakahira K: Mitochondrial DNA in sepsis. Curr Opin Crit Care 23: 284–290, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaczmarek A, Vandenabeele P, Krysko DV: Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Linkermann A, Green DR: Necroptosis. N Engl J Med 370: 455–465, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC; Working Group on DAMPs in Cardiovascular Disease: Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 35: 1172–1177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eirin A, Saad A, Tang H, Herrmann SM, Woollard JR, Lerman A, et al. : Urinary mitochondrial DNA copy number identifies chronic renal injury in hypertensive patients. Hypertension 68: 401–410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ho PW, Pang WF, Luk CC, Ng JK, Chow KM, Kwan BC, et al. : Urinary mitochondrial DNA level as a biomarker of acute kidney injury severity. Kidney Dis (Basel) 3: 78–83, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whitaker RM, Stallons LJ, Kneff JE, Alge JL, Harmon JL, Rahn JJ, et al. : Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney Int 88: 1336–1344, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu Q, Ren J, Ren H, Wu J, Wu X, Liu S, et al. : Urinary mitochondrial DNA identifies renal dysfunction and mitochondrial damage in sepsis-induced acute kidney injury. Oxid Med Cell Longev 2018: 8074936, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]