Abstract
Restless legs syndrome (RLS) is a common neurological disorder whose exact pathophysiological mechanism remains unclear despite the successful use of dopaminergic treatment and recent discovery of predisposing genetic factors. As iron deficiency has been associated with RLS for some patients and there is evidence for decreased spinal dopamine D3-receptor (D3R) signaling in RLS, we aimed at establishing whether D3R activity and iron deficiency share common pathways within the pathophysiology of RLS sensory and motor symptoms.
Using a combined mouse model of iron deficiency and dopamine D3-receptor deficiency (D3R−/−), circadian motor symptoms were evaluated by continuous recording of spontaneous wheel running activity. Testing the acute and persistent pain responses with the hot-plate test and formalin test, respectively, assessed sensory symptoms.
A 15 week iron-deficient (ID) diet alone increased acute and persistent pain responses as compared to control diet. As compared to C57BL/6 (WT), homozygous D3R−/− mice already exhibited elevated responses to acute and persistent pain stimuli, where the latter was further elevated by concurrent iron deficiency. ID changed the circadian activity pattern toward an increased running wheel usage before the resting period, which resembled the RLS symptom of restlessness before sleep. Interestingly, D3R−/− shifted this effect of iron deficiency to a time point 3–4 h earlier.
The results confirm the ability of iron deficiency and D3R−/− to evoke sensory and motor symptoms in mice resembling those observed in RLS patients. Furthermore this study suggests an increase of ID-related sensory symptoms and modification of ID-related motor symptoms by D3R−/−.
Introduction
Restless legs syndrome (RLS) is a common disorder with a prevalence of 2.5–15% (Satija and Ondo, 2008). It is characterized by an urge to move the legs, which is often accompanied by unpleasant sensory symptoms. These symptoms are usually worse in the evening and during rest, but they are relieved by movement. The additional symptoms of a sensory perception deficit (Schattschneider et al., 2004; Stiasny-Kolster et al., 2004) and hyperalgesia (Stiasny-Kolster et al., 2004) have been described in both primary and secondary forms of RLS. Despite the successful use of dopaminergic drugs in treating RLS, no evidence of a primary dopaminergic deficit has been identified (Earley et al., 2009). In contrast to this, a worsening of RLS symptoms may occur, observed by increased intensity of the symptoms and 2–4 h earlier onset, thus affecting patients already at daytime as opposed to an evening prevalence (García-Borreguero et al., 2007). This usually occurs after initial success using dopamine therapy and has been clinically defined as augmentation. This suggests that the underlying pathophysiological mechanisms are more complex and involve a dopaminergic dysregulation rather than a primary deficit. Clemens et al. (2006) suggested a decreased D3R-mediated inhibition of spinal sensory and reflex circuits as a pathogenetic concept in RLS (Clemens et al., 2006). This is supported by the switch from inhibitory to excitatory dopamine effects on spinal reflexes in D3R−/− mice (Clemens and Hochman, 2004) that resembles the increased excitability of the flexor reflex detected in RLS patients (Bara-Jimenez et al., 2000). Additionally, the most commonly and successfully used dopamine agonists have a relevant if not predominant D3 agonism. Along with primary RLS, several secondary forms of RLS exist. The association between RLS and iron deficiency is well established (Nordlander, 1953; Ekbom, 1960) where decreased levels of the iron storage protein ferritin have been described in patients experiencing augmentation (Trenkwalder et al., 2008). The pathogenetic importance of iron deficiency is further corroborated by the effective treatment of iron-deficient (ID) RLS patients' symptoms by using iron supplements (Earley et al., 2004; Grote et al., 2009).
The aim of this study was to investigate the behavioral effects and interactions of absent D3R signaling and iron deficiency of RLS by assessing sensory and motor symptoms, thus allowing further insight into the pathogenesis of both sensory and motor modalities present in human RLS. Experimentally, this was achieved by using a combined mouse model of iron deficiency and dopamine D3-receptor knock-out (D3R−/−).
Continuous recording of spontaneous wheel running was used to detect changes in the circadian activity pattern that resemble the increased restlessness of the legs prevailing in RLS patients predominantly during the final hours before sleep. The sensory component was determined by measuring the acute and persistent pain responses using the hot-plate and formalin tests, respectively (Dowling et al., 2009).
Materials and Methods
Animals.
Thirty male C57BL/6 mice were purchased (Charles River Laboratories) at postnatal day 28 (P28). In addition, 30 male mice homozygous deficient for the dopamine D3 receptor (D3R−/−) were bred from parents (Drd3tm1Dac) backcrossed with a C57BL/6 background. The absence of the D3R encoding region was confirmed in homozygous parents by genotyping tail DNA as originally described (Accili et al., 1996).
All mice were housed in separate cages within the same room on a 12 h light/dark cycle (lights on/off, 7:00 A.M./7:00 P.M.). Experiments were conducted under the German animal protection laws and protocols approved by the Government of Lower Saxony.
Diets.
To test the effects of iron deficiency in mice of WT and D3R−/− strains, mice were given a special diet with a reduced iron content (<8 mg/kg iron), as described earlier (Dowling et al., 2009). These formed the ID group, whereas control mice were maintained on a diet with normal iron content (179 mg/kg iron). Diets (ssniff Spezialdiäten) were started from age P28. Both food and water were available ad libitum.
Blood iron analysis.
Venous blood was extracted from the right ventricle of the anesthetized mouse. Subsequently, blood was centrifuged and the plasma isolated. Spectrophotometric analysis of plasma samples was performed on samples from both mouse strains using a Roche/Hitachi Modular Analytics P800 (Roche Diagnostics) to determine the plasma iron concentration from both ID and control groups for both WT and D3R−/− strains.
Voluntary wheel running.
From P42, all mice had continuous and individual access to a running wheel placed inside each cage. The axis of each running wheel was connected to a rotation sensor with a resolution of 16 counts/revolution, one revolution corresponding to 35.5 cm. Using a customized recording device and software (Boenig & Kallenbach oHG), running wheel revolutions were recorded continuously at a sampling rate of 1/0.48 s. Based on these accumulative data, several parameters were calculated for time intervals of 1 h using a custom designed MatLab program (The MathWorks). We hypothesized an increased wheel usage and activity as a possible correlate for the human RLS motor symptoms, which are characterized by the recurrent urge to move the legs. The time interval corresponding best to the preponderance of human RLS symptoms during the evening would be the final stage of the dark phase before the resting phase.
This behavior was assessed by measuring the number of runs (Nrun%) and the time spent running (Trun%) each hour in relation to the cumulative value obtained during the dark phase.
To assess physical fitness and endurance of the mouse throughout the experiment, additional parameters, i.e., distance in meters (Dist), maximum velocity (Vmax), and the mean distance per run (Distmean) were measured. Distmean proved to be a suitable measure of endurance in an earlier study (Liebetanz et al., 2007). Data were averaged for each mouse across 5 consecutive days.
Hot-plate test.
An increased static hyperalgesia via Aδ-fibers has been described in human RLS patients (Stiasny-Kolster et al., 2004). The hot-plate test was used to investigate differences in acute pain response to a thermal stimulus. The Aδ high-threshold heat nociceptors activate under high thermal stimulation and mediate fast reflex responses through a greater conduction velocity compared to C-fibers (Leem et al., 1993; Yeomans et al., 1996). Moreover, action potentials via Aδ fibers reach the spinal cord before those conducted by C-fiber nociceptors, corresponding to the recorded initial reflex to the noxious heat stimulus (Leem et al., 1993; Yeomans et al., 1996). A high temperature, as used in our study, results in preferential Aδ-fiber activation, whereby temperatures below a 45°C heat stimulation tend to involve a higher C-fiber activity (Adriaensen et al., 1983). The hot-plate test was performed according to a previous study (Dowling et al., 2009). After a 15 week period of control or ID diet starting from P28, mice naive to the heat stimulus were placed directly onto a 50°C (±0.5°C) hot plate (MEDAX Nagel). The reaction time (in seconds) until the first signs of a painful response (hindpaw licking or escape) was recorded under single-blinded conditions between the hours of 7:00 A.M. and 2:00 P.M.
Formalin test.
The formalin test assesses mixed Aδ- and C-fiber function during the initial (phase I) stage of the test (Heapy et al., 1987; Ishizaki et al., 1999), where at the latter stage (phase II), formalin-induced tissue injury and inflammation causes sensitization of primary afferents, leading to the phenomenon of windup (Mendell, 1966; Li et al., 1999), which itself dominates during this phase and only occurs upon sufficient C-fiber activation (Mendell, 1966; Dickenson and Sullivan, 1987; Yamamoto and Yaksh, 1992; McCall et al., 1996). As patients suffering from secondary RLS present impaired C-fiber function (Schattschneider et al., 2004), the formalin test was used to experimentally evaluate any possible change of murine C-fiber function, moreover, to assess the differences in the persistent pain response compared to the short acute pain measurement described by the hot-plate test.
The formalin test protocol was identical to a former study (Dowling et al., 2009). Twenty-four hours after completing the hot-plate test, the same control and ID mice from WT and D3R−/− strains were subjected to this test. This time period was chosen between the two tests, as the immediate early gene c-Jun is expressed for ∼16 h (Presley et al., 1990; Herdegen et al., 1991). Therefore, hot-plate-induced c-Jun expression would not interfere with the level of formalin-induced c-Jun.
Formalin-naive mice were injected with 20 μl of 4% formalin subcutaneously into the dorsal left hindpaw. The time spent licking/lifting/biting the injected hindpaw was recorded over 45 min. Afterward, the local inflammatory response induced by the formalin injection was quantified as a control between groups, by measuring the injected (ipsilateral) and noninjected (contralateral) hindpaw thicknesses using a caliper. The value obtained was denoted the “inflammation score,” as described previously (Ko et al., 2005). The entire procedure was performed under single-blinded conditions where, similarly to the hot-plate test a predetermined order was followed.
c-Jun immunohistochemistry.
c-Jun immunoreactivity in neurons, which were labeled using a neuronal nuclei (NeuN) marker, was measured to evaluate the level of neuronal activity at dorsal laminae I/II. c-Jun immunoreactivity was measured here to determine whether any change in the formalin-induced pain response correlates with a change in neuronal activity at the level of the spinal cord. The dorsal spinal laminae I/II are known to receive primary afferent Aδ- and C-fibers from nociceptors and thermoreceptors (Shepherd, 1994). Identification of the appropriate region for quantification was achieved by general morphological analysis of the dorsal horn as performed in a previous study (Dowling et al., 2009). In addition, the use of a supplementary marker NeuN helped illustrate the segments more clearly.
After postfixation, the tissue was prepared for paraffin embedding and subsequently sectioning at 2 μm thickness. The immunohistochemistry method for investigating c-Jun expression was performed according to a previous study (Stadelmann et al., 1998). A rabbit anti-c-Jun polyclonal IgG primary antibody (Santa Cruz Biotechnology, H-79: sc-1694) was incubated at 1:1500 in 10% FCS + PBS for 24 h at 4°C. The secondary biotinylated anti-rabbit antibody (GE Healthcare) was diluted 1:200 in 10% FCS + PBS and incubated for 1 h at room temperature. A 2% 3,3′-diaminobenzidine tetra-hydrochloride (DAB) (Sigma-Aldrich) solution was applied to the tissues sections for 3 min. Identifying colocalization of c-Jun with laminae I/II neurons involved an immunofluorescence method, whereby c-Jun and mouse anti-neuronal nuclei (NeuN) monoclonal antibody (Millipore, MAB377) were incubated for 24 h at 4°C. The following secondary antibodies were used: Cy3-conjugated goat anti-rabbit IgG (Dianova) and Alexa Fluor488 rat anti-mouse (Morphosys AbD), diluted in 10% FCS + PBS to 1:200 and 1:250, respectively. This step was followed by a 1 h incubation.
An Olympus BX51 microscope equipped with Olympus DP71 camera was used at ×10 magnification for immunostained sections under light level for quantification and under fluorescence for photoimaging. Thirteen transverse spinal sections per mouse were selected in a random manner for quantification. The percentage number of c-Jun-immunoreactive (c-Jun-IR) nuclei within laminae I and II was quantified from both ipsilateral and corresponding contralateral dorsal horns under single-blinded conditions.
Statistics.
Separate two-factorial ANOVAs were performed to test whether the diet and/or strain had an effect upon the plasma iron concentration or the hot-plate reaction time. A three-factorial ANOVA with repeated measurements was performed to test whether strain, diet, and time point had an effect upon the formalin response score. A three-factorial ANOVA was performed to test whether strain, diet, and/or the dorsal horn side (ipsilateral vs contralateral) had an effect upon the number of c-Jun-IR neurons. Separate three-factorial ANOVAs with repeated measurements were also performed on wheel running data to test whether strain, diet, and/or time of day, had an effect on Dist, Distmean, Vmax, Nrun%, and/or Trun%. The statistics of the activity parameters were performed for the running activity of the dark phase only (7:00 P.M. to 7:00 A.M.). Post hoc tests (Student's paired t test) were performed to compare the values of different diet and strain groups for different time bins. The ANOVAs and Student's t tests were performed using SPSS 17.0.0 (SPSS Software). The level of significance for rejecting the null hypothesis was set at p < 0.05 for all statistical calculations. All data are presented as mean ± SEM.
Results
All statistical values associated with ANOVA interactions, including degrees of freedom and F and p values, can be found in supplemental Tables 1 and 2 (available at www.jneurosci.org as supplemental material).
Blood plasma iron analysis
The ID diet had an effect upon the plasma iron concentration after 15 weeks of diet starting from P28 (p < 0.001). Compared to the control diet, the ID diet decreases the plasma iron concentration in WT (29.4 μmol/L ± 2.41 vs 19.6 μmol/L ± 1.68) and D3R−/− (32.6 μmol/L ± 2.10 vs 20.4 μmol/L ± 1.70) strains (p < 0.05). Strain (WT vs D3R−/−) had no effect upon the plasma iron concentration. Also, diet and strain did not interactively change this parameter.
Iron deficiency elevates and D3R−/− shifts increased voluntary wheel running activity in mice
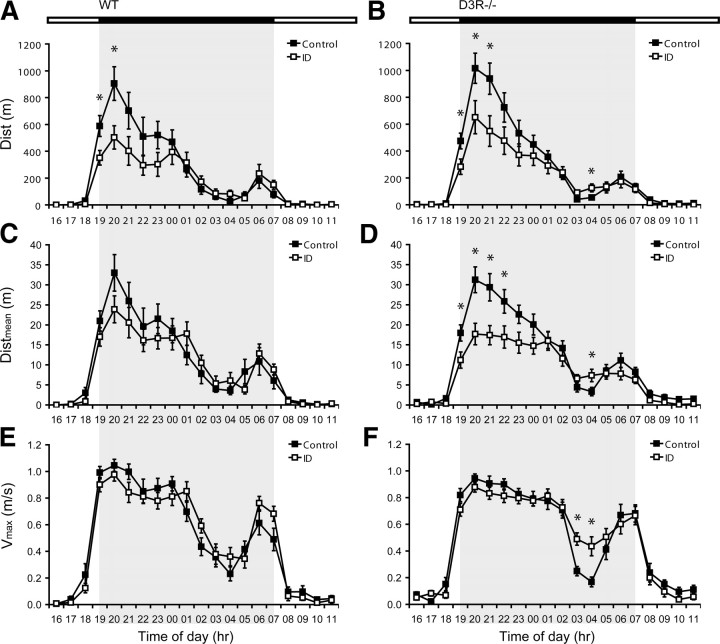
To assess the impact of the different diets and strains upon the general physical performance, the variables Dist, Distmean, and Vmax were compared (Fig. 1). Diet and also strain had no main effect upon Dist, Distmean, or Vmax. Since the running activity follows a circadian rhythm, time of day had an effect upon all three parameters (p < 0.001). In addition, diet and time of day (p < 0.001), as well as strain and time of day (p < 0.05), interactively influenced them. Moreover, the interaction of all three factors (time, diet, and strain) had an effect on Vmax (p = 0.034).
Figure 1.
Overview of voluntary wheel running performance. A–F, The parameters Dist (A, B), Distmean (C, D), and Vmax (E, F) represent the average values achieved by either the WT (A, C, E) or D3R−/− (B, D, F) groups during a 1 h time bin within the different time periods (in hours) of the circadian cycle. Both light and dark phases are shown, the latter represented by the gray boxed region. Values are means ± SEM, n = 15. *Groups differ at that time during the dark phase, p < 0.05.
According to post hoc analysis, iron deficiency decreased Dist in ID WT and ID D3R−/− groups during the early period of the activity phase (Fig. 1) and increased Dist for a single time point at 4:00 A.M. in the ID D3R−/− group as compared to the respective control groups (p < 0.05) (Fig. 1B). Iron deficiency also decreased Distmean compared to the corresponding control group during the early period of the activity phase (Fig. 1D), however, only in D3R−/− mice. In contrast, iron deficiency increased Distmean and Vmax between 3:00 and 4:00 A.M. (p < 0.05) (Fig. 1D,F) in D3R−/− mice compared to the D3R−/− group receiving a control diet.
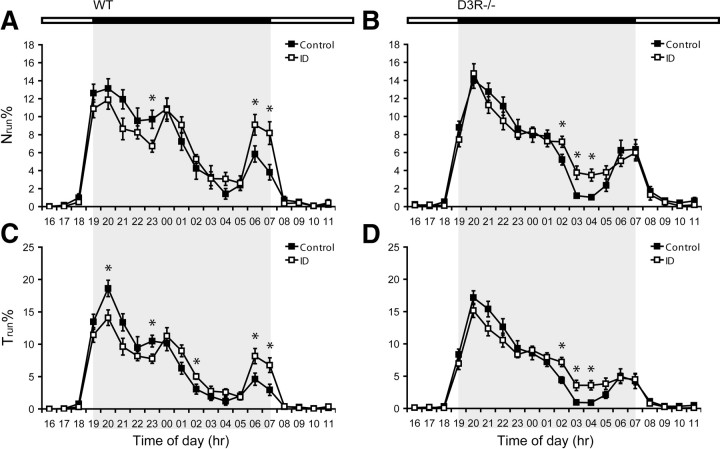
To investigate for possible changes in motor behavior that may correspond to the restlessness of human RLS patients, an increased use of the running wheel was assessed by the variables number of runs (Nrun%) and time spent running (Trun%), expressed in percentage of the cumulative value obtained during the dark phase. The results of the three-factorial ANOVA showed that strain had an effect upon Nrun% (p = 0.038) and time of day had an effect upon both Nrun% and Trun% (p < 0.001). Moreover, the interaction of diet and time of day (p < 0.05) as well as strain and time of day (p < 0.001) had an effect on both activity parameters. In contrast, the interaction of diet and strain as well as of all three factors (time, diet, and strain) had no effect.
As affirmed by the post hoc analysis, iron deficiency increased Nrun% and Trun% in WT mice during the final hours before the start of the rest phase (Fig. 2A,C). Iron deficiency also increased these activity parameters in D3R−/− mice. However, as compared to the WT mice, this heightened activity was shifted ∼2–4 h earlier in the ID D3R−/− group (p < 0.05) (Fig. 2B,D). In the groups fed control diet, D3R−/− led to a slightly increased activity; however, this effect was not significant (p = 0.085). Additionally, the ID WT groups decreased in activity at single time points (8:00 and 11:00 P.M.; p < 0.05).
Figure 2.
Differences in voluntary wheel running activity. A–D, The parameters Nrun% (A, B) and Trun% (C, D) represent the average values obtained by either the WT (A, C) or D3R−/− (B, D) groups during a 1 h time bin in relation to the cumulative value achieved within the different time periods (in hours) of the dark phase as represented by the gray boxed region. Values are means ± SEM, n = 15. *Groups differ at that time during the dark phase, p < 0.05.
Shortened hot-plate reaction time in ID WT and both ID and control D3R−/− groups
The hot-plate test helped determine whether the iron deficiency-increased sensitivity to the thermal stimulus depends on the presence of D3R (Fig. 3A). According to the two-factorial ANOVA, diet (p = 0.006) and strain of mouse (p < 0.001) had main effects on the hot-plate reaction time, where the interaction was not significant. As compared to the corresponding WT groups, D3R−/− led to a faster reaction time to the thermal stimulus (p < 0.05) in both ID and control diet groups. Confirming our previous study (Dowling et al., 2009), iron deficiency shortened the response time in WT mice when compared to the control diet group (p < 0.05). The reaction time was not further decreased in D3R−/− on ID diet.
Figure 3.
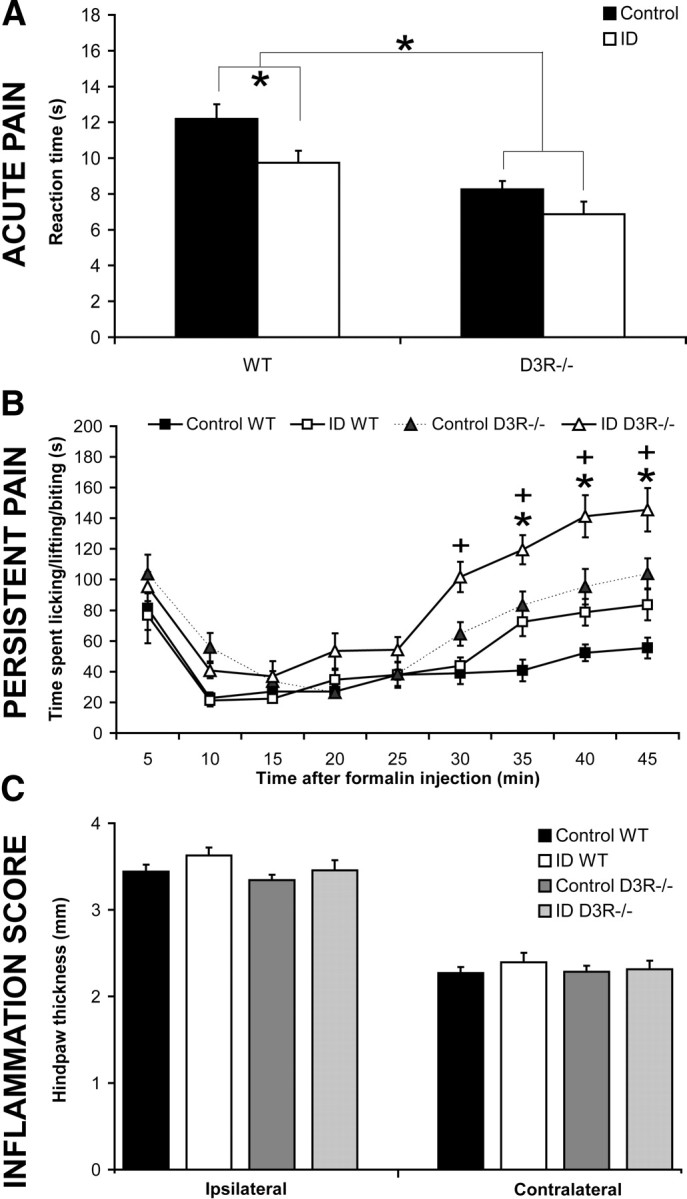
Response differences to acute and persistent pain. A, The acute pain response scores (in seconds) until the initial hindpaw licking or escape were recorded by the hot-plate test. Values are means ± SEM, n = 7. *WT and D3R−/− groups differ at a diet duration of 15 weeks beginning at age P28. B, The persistent pain response to the formalin injection over time (in minutes) in WT and D3R−/− mice fed control or ID diets for 15 weeks starting from age P28. Values are means ± SEM, n = 7. *WT and +D3R−/− groups differ at certain time points, p < 0.05. C, After the formalin injection, the injected (ipsilateral) and noninjected (contralateral) hindpaw thicknesses were measured (in millimeters), and the value denoted the level of inflammation. Values are means ± SEM, n = 7.
Iron deficiency further elevates the inflammatory pain response in D3R−/− mice
The formalin test was implemented to determine whether the iron deficiency-enhanced persistent pain response in WT mice depends upon the presence of D3R (Fig. 3B), or whether a further, “additive” increase in pain response occurs. According to the three-factorial repeated-measures ANOVA, diet (p = 0.014), strain (p < 0.001), and time point (p < 0.001) had individual main effects upon the formalin-induced response score. Diet and time point, as well as strain and time point, interactively influenced the pain response (p < 0.001). Diet and strain, as well as all three factors (time, diet, and strain), did not interactively change the pain response score.
During phase II of the formalin test, iron deficiency increased the pain response in ID WT and ID D3R−/− groups when compared to controls (30–45 min, p < 0.05). Although D3R−/− mice had a higher pain response compared to WT mice, iron deficiency increased this further in the ID D3R−/− group (Fig. 3B). As a control, the diet or strain did not change the local tissue inflammation score after the formalin injection (Fig. 3C).
Heightened central neuronal activity detected by ipsilateral c-Jun expression in ID WT and ID D3R−/− groups
After completion of the formalin test, the number of c-Jun-IR neurons was quantified at superficial laminae I/II of the ipsilateral and contralateral dorsal horns (Fig. 4A). This evaluated whether the iron deficiency-increased neuronal activity after the formalin test depends on the presence of D3R. According to the three-factorial ANOVA, diet (p < 0.001), strain (p = 0.004), and dorsal horn side (ipsilateral vs contralateral, p < 0.001) had individual main effects upon the number of c-Jun-IR neurons. The interactions between diet and strain, diet and dorsal horn side, strain and dorsal horn side, or all three factors (diet, strain, and dorsal horn side) were not significant. Post hoc tests revealed that the number of c-Jun-IR neurons at the ipsilateral horn was increased in all four groups (ID WT, control WT, ID D3R−/−, and control D3R−/−), compared to the level at the contralateral horn (p < 0.05) (Fig. 4A). Furthermore, as compared to control groups, iron deficiency increased the expression of c-Jun-IR neurons at the ipsilateral horn in ID WT and ID D3R−/− mice (p < 0.05) (Fig. 4A). Neuronal c-Jun expression was confirmed by double immunofluorescence labeling (Fig. 4B,C).
Figure 4.
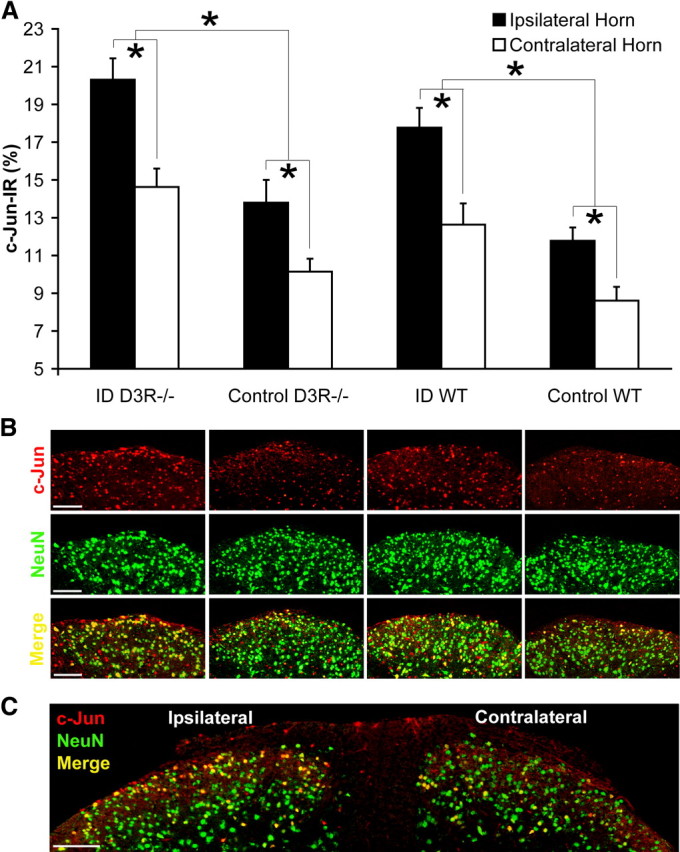
Altered c-Jun expression at the ipsilateral dorsal horn after formalin-induced pain. A, Percentages of c-Jun-IR neurons were quantified in relation to total neuron count in laminae I/II at either the ipsilateral or contralateral dorsal horns from WT and D3R−/− mouse strains fed control or ID diets for 15 weeks starting from age P28. Values are means ± SEM, n = 7. *WT and D3R−/− groups differ in the percentage level of c-Jun-IR expression, p < 0.05. B, Colocalization (yellow) of c-Jun immunoreactivity (red) in relation to the neuronal marker NeuN (green), at laminae I/II of the ipsilateral dorsal horn (scale bar, 75 μm). C, c-Jun expression is increased at the ipsilateral dorsal horn of an ID D3R−/− mouse (scale bar, 100 μm).
Discussion
In the current study, we investigated the changes in the circadian pattern of locomotor activity and of acute and persistent pain responses in a combined model of iron deficiency and D3R−/−. The aim was to acquire further insight into the interaction of these two influences that are thought to be associated with human RLS.
The increased running wheel usage before the resting period evoked by iron deficiency resembled RLS motor symptoms. Furthermore, iron deficiency resulted in a sensitization to acute and persistent pain resembling RLS sensory symptoms. Additional D3R deficiency modified the time course of the iron deficiency-evoked symptoms and resulted in an additive increase of the persistent pain response.
D3R−/− and iron deficiency alter circadian motor activity
In the current study, we were able to assess changes in the circadian motor activity induced by D3R−/− in combination with iron deficiency in mice by an increased wheel usage, as demonstrated by the parameters Nrun% and Trun%. These parameters proved more sensitive in displaying circadian changes than did absolute running distance or time, both of which depend more on the overall running performance.
Iron deficiency increased Nrun% and Trun% in WT mice during the final hours of the dark phase (6:00–7:00 A.M.). This is in line with the findings of Dean et al. (2006), who, using polysomnographic recordings, described an increased awake time during the late active period in ID WT mice, probably due to the increased dopaminergic activation of neurons in the periaqueductal gray matter. Interestingly, in D3R−/− mice the iron deficiency-induced phase of increased running wheel usage was shifted to a time point 3 h earlier, whereas no change was seen at the period from 6:00 to 7:00 A.M. in these mice, suggesting a modulating effect of the D3R on the timing of the iron deficiency-induced activity. A comparable increase of physical activity has been seen after lesioning the diencephalic-spinal A11 region, although that study did not investigate circadian patterns (Qu et al., 2007). Therefore in light of this study, spinal dopaminergic circuits may be involved in the observed changes.
The performance parameters Dist, Distmean, and Vmax represent physical fitness and running motivation. Iron deficiency led to a decrease in Dist, especially during the early night period in both strains, this being consistent with previous reports (Youdim et al., 1981; Hunt et al., 1994). Youdim et al. (1981) suggested that this is caused by an iron deficiency-mediated reduction of dopamine D2 receptors (D2Rs). In contrast to our performance parameters, Youdim et al. (1981) reported an increase of spontaneous motor activity of ID animals in the morning. This divergence is probably due to the different methods used, and might correspond to the increase in activity parameters (Nrun%, Trun%) in the morning phase of our study. Since no anemia was seen in our ID animals (unpublished data) and iron deficiency reduced Distmean only in D3R−/− mice, reduced physical endurance or fitness is an unlikely cause for a change in the performance parameters. This is also supported by the fact that Vmax remained unaffected by iron deficiency.
On the other hand, D3R−/− mice did show an increased running performance (Dist and Distmean) during the active period as described earlier (Accili et al., 1996). Contrary to the congruent effects on circadian activity, Dist is influenced contrarily by iron deficiency and D3R−/−. D3R activation decreases overall locomotor activity (Menalled et al., 1999), possibly due to dopaminergic inhibition in the Calleja islands (Guitart-Masip et al., 2008), which suggests that the lack of D3R in D3R−/− might lead to the increase in Dist.
ID mice in our study had increased wheel usage in the phase before the resting period. Both the induction of symptoms by iron deficiency and the time point of restlessness as represented by increased wheel usage resemble the symptoms of RLS in humans, which are also characterized by the urge to move during the last hours before the resting phase (Nordlander, 1953; Ekbom, 1960). The increased urge to move and restlessness that RLS patients suffer from is believed to involve the dopaminergic system, because dopamine agonists alleviate these symptoms (Akpinar, 1982; Earley and Allen, 1996). Iron deficiency influences the dopaminergic system on different levels. D2R and dopamine transporter (DAT) are downregulated in ID rats (Ashkenazi et al., 1982; Erikson et al., 2000). A decrease of inhibitory D2R activation leads to a preponderance of excitatory dopamine D1 receptor (D1R) activation, which is associated with increased locomotor activity in rats (Shieh and Walters, 1996; Heijtz et al., 2002). This effect may further be enhanced by an iron deficiency-induced decrease of DAT expression as described in mice (Bianco et al., 2008, 2009) that would increase dopamine levels in the synaptic cleft. Paulus and Trenkwalder (2006) proposed that the misbalance between D1-like and D2-like activation might further be increased by treatment-related downregulation of D2-like receptors leading to the phenomenon of augmentation. A disproportionately extensive downregulation of D2-like receptors compared to D1-like receptors due to specific stimulation has been previously reported (Chen et al., 1993). This implicates D2-like receptor (especially D2R and D3R) dysfunction as one pathogenetic agent leading to augmentation. Interestingly, combining iron deficiency—which is associated with decreased D2R-expression—and D3R deficiency led to a time shift of the iron deficiency-induced increased motor activity in our study. Since augmentation in human RLS is associated with a comparable time shift of circadian symptoms (Allen et al., 2003; García-Borreguero et al., 2007), a similar mechanism of decreased D2-like dopaminergic signaling might be speculated.
Partially additive alteration of pain responses by D3R−/− and iron deficiency
Confirming our previous findings (Dowling et al., 2009), iron deficiency increased the response to acute and persistent pain in the WT mouse group. Moreover, we also demonstrated that the D3R−/− status alone is associated with an elevated response to acute and persistent pain. Interestingly, only the persistent pain response was additively increased by iron deficiency.
The increased acute and persistent pain responses in D3R−/− compared to WT mice are presumably caused by the lack of D3R-mediated regulation of pronociceptive postsynaptic D1R activity (Fleetwood-Walker et al., 1988; Xu et al., 1997), which increases in activity upon deletion of D3R (Mizuo et al., 2004). The increase of D1R mRNA expression in the spinal cord of ID WT mice (Zhao et al., 2007) suggests a similar mechanism for the effects of ID diet in WT mice.
Due to the lack of any measurable difference in local inflammatory response (ID vs control and WT vs D3R−/−), our behavioral data suggest sensitization of primary afferents and windup occurs more rapidly in ID and D3R−/− mice during phase II of the formalin test (Mendell, 1966; Dickenson and Sullivan, 1987; Yamamoto and Yaksh, 1992; McCall et al., 1996). Similarly to the Aδ-fiber acute pain response, ID and D3R−/− may also reduce the primary afferent C-fiber pain threshold. This likely occurs through a series of biochemical changes, most notably a dysfunction of the descending dopaminergic system through ID-induced effects and a change of antinociceptive D3R expression, as described above. Moreover, other processes are likely to play a role, including synaptic long-term potentiation, which after C-fiber stimulation during the formalin test is dependent on group I metabotropic glutamate receptors 1 and 5 (Azkue et al., 2003) and NMDA receptors (Liu and Sandkühler, 1995). Iron deficiency- or D3R−/−-mediated changes in the activity of these receptors might be the reason for the additive increase of the persistent pain response we observed in our study.
Paralleling the iron deficiency-induced increase in the persistent pain response, the spinal expression of the neuronal activity marker c-Jun following chronic pain stimulation was increased by iron deficiency. A similar correlation has been reported recently for ipsilateral c-Fos expression in the dorsal horn of ID WT mice (Dowling et al., 2009).
Surprisingly, the additive increase in pain response by iron deficiency and D3R−/− did not go along with a further increase in spinal c-Jun-IR expression despite the expression of these receptors on primary afferent fibers at laminae I/II of the spinal cord (Benarroch, 2008). A reason for this finding might be a ceiling effect, since c-Jun is expressed at a generally higher basal level than c-Fos in WT mice (Li et al., 2004) and as contralateral c-Jun expression in the present study was already relatively high.
Previous clinical studies in RLS patients have identified a change in Aδ- and C-fiber function (Schattschneider et al., 2004; Stiasny-Kolster et al., 2004). The hot-plate test as performed in the current study mainly assesses pain transmission through Aδ-fibers (Frölich et al., 2005), whereas phase II of the formalin test mostly describes C-fiber-mediated pain transmission (Dubuisson and Dennis, 1977; Yaksh et al., 1999). The fact that the sensory dorsal horn receives inputs from both Aδ- and C-fibers (Shepherd, 1994), together with the finding that c-Jun expression was elevated at this region in ID WT and ID D3R−/− mice suggests that this sensory region is involved in the generation of the sensory symptoms observed in these animals. Furthermore, the high D3R population at the sensory dorsal horn region (Levant and McCarson, 2001), in addition to the existence of D3R expression upon nociceptor afferent fibers (Benarroch, 2008), implies an involvement of D3Rs in modulating central pain transmission. The demonstrated increase in acute and persistent pain responses in D3R−/− mice further supports the hypothesis that D3Rs play a critical role in the pathophysiology of RLS sensory symptoms.
Conclusions
In this study, we present detailed circadian activity patterns as well as acute and persistent pain reaction data in a novel model of combined iron and D3R deficiency.
The increase of locomotor activity before rest and the sensitization toward pain stimuli observed in the ID state resemble motor and sensory symptoms of human RLS. This underlines the value of the presented model as a potential animal model of RLS. A major advantage of our continuous running wheel recording is having the possibility of obtaining a detailed assessment of circadian patterns lacking in many other approaches (Qu et al., 2007), where to date only punctual activity levels were recorded. In addition, our model requires a minimum of animal handling, since neither surgery nor pharmacologic interventions were implemented [unlike, for example, the A11-lesion model (Zhao et al., 2007) or the pharmacologic nafadotride model (Sautel et al., 1995)], thus preventing a disturbance of the very sensitive day–night rhythm. Our model is, however, limited by the use of a nonconditional knock-out, since ontogenetic compensatory mechanisms are probable. Furthermore, in human RLS a dysfunction of D3 receptors is more likely than the complete absence of D3 activity in our model.
In furtherance to this, pharmacologic studies assessing the reaction toward dopamine agonists would be suited when further validating the presented model.
Our results also affirm the important role of iron deficiency as a pathogenetic factor in RLS. The increased pain response in D3R−/− indicates an influence of D3R upon pain regulation possibly at the spinal level. Our results suggest that a dysfunction of either D2R—as present in ID animals—or D3R—as in D3R−/− animals—evoke symptoms comparable to those in RLS patients. Furthermore, our results implicate different sensitivity of motor and sensory symptoms toward these influences: the circadian profile of running activity was not affected by D3R−/− alone and ID-induced motor changes were modified, but not increased by D3R−/−. On the other hand, D3R−/− as well as ID increased acute and persistent pain responses, where D3R−/− combined with ID had additive effects upon the persistent pain response. In light of these findings different pathogenetic pathways could be considered as a reason for individual differences in the preponderance of sensory or motor symptoms in human RLS patients.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (GK 632) and in part by the DFG Center for Molecular Physiology of the Brain. We thank M. Schedensack and Angela Dettmar for excellent technical assistance.
References
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol. 1983;49:111–122. doi: 10.1152/jn.1983.49.1.111. [DOI] [PubMed] [Google Scholar]
- Akpinar S. Treatment of restless legs syndrome with levodopa plus benserazide. Arch Neurol. 1982;39:739. doi: 10.1001/archneur.1982.00510230065027. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Ashkenazi R, Ben-Shachar D, Youdim MB. Nutritional iron and dopamine binding sites in the rat brain. Pharmacol Biochem Behav. 1982;17(Suppl 1):43–47. doi: 10.1016/0091-3057(82)90509-3. [DOI] [PubMed] [Google Scholar]
- Azkue JJ, Liu XG, Zimmermann M, Sandkühler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Aksu M, Graham B, Sato S, Hallett M. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54:1609–1616. doi: 10.1212/wnl.54.8.1609. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008;71:217–221. doi: 10.1212/01.wnl.0000318225.51122.63. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Unger EL, Earley CJ, Beard JL. Iron deficiency alters the day-night variation in monoamine levels in mice. Chronobiol Int. 2009;26:447–463. doi: 10.1080/07420520902820905. [DOI] [PubMed] [Google Scholar]
- Chen JF, Aloyo VJ, Weiss B. Continuous treatment with the D2 dopamine receptor agonist quinpirole decreases D2 dopamine receptors, D2 dopamine receptor messenger RNA and proenkephalin messenger RNA, and increases mu opioid receptors in mouse striatum. Neuroscience. 1993;54:669–680. doi: 10.1016/0306-4522(93)90238-b. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci. 2004;24:11337–11345. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- Dean T, Jr, Allen RP, O'Donnell CP, Earley CJ. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med. 2006;7:634–640. doi: 10.1016/j.sleep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dowling P, Klinker F, Amaya F, Paulus W, Liebetanz D. Iron-deficiency sensitizes mice to acute pain stimuli and formalin-induced nociception. J Nutr. 2009;139:2087–2092. doi: 10.3945/jn.109.112557. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP. Pergolide and carbidopa/levodopa treatment of the restless legs syndrome and periodic leg movements in sleep in a consecutive series of patients. Sleep. 1996;19:801–810. doi: 10.1093/sleep/19.10.801. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–235. doi: 10.1016/j.sleep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP, Connor JR, Ferrucci L, Troncoso J. The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 2009;10:1155–1157. doi: 10.1016/j.sleep.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom KA. Restless legs syndrome. Neurology. 1960;10:868–873. doi: 10.1212/wnl.10.9.868. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Hope PJ, Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J Physiol. 1988;399:335–348. doi: 10.1113/jphysiol.1988.sp017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölich MA, Price DD, Robinson ME, Shuster JJ, Theriaque DW, Heft MW. The effect of propofol on thermal pain perception. Anesth Analg. 2005;100:481–486. doi: 10.1213/01.ANE.0000142125.61206.7A. [DOI] [PubMed] [Google Scholar]
- García-Borreguero D, Allen RP, Kohnen R, Högl B, Trenkwalder C, Oertel W, Hening WA, Paulus W, Rye D, Walters A, Winkelmann J, Earley CJ. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med. 2007;8:520–530. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Grote L, Leissner L, Hedner J, Ulfberg J. A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24:1445–1452. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Johansson B, Fernández-Teruel A, Tobeña A, Giménez-Llort L. Divergent effect of the selective D3 receptor agonist pd-128,907 on locomotor activity in Roman high- and low-avoidance rats: relationship to NGFI-A gene expression in the Calleja islands. Psychopharmacology (Berl) 2008;196:39–49. doi: 10.1007/s00213-007-0925-6. [DOI] [PubMed] [Google Scholar]
- Heapy CG, Jamieson A, Russel NJW. Afferent C-fiber and A-delta activity in models of inflammation. Br J Pharmacol. 1987;90:164p. [Google Scholar]
- Heijtz RD, Beraki S, Scott L, Aperia A, Forssberg H. Sex differences in the motor inhibitory and stimulatory role of dopamine D1 receptors in rats. Eur J Pharmacol. 2002;445:97–104. doi: 10.1016/s0014-2999(02)01716-8. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Kovary K, Leah J, Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991;313:178–191. doi: 10.1002/cne.903130113. [DOI] [PubMed] [Google Scholar]
- Hunt JR, Zito CA, Erjavec J, Johnson LK. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr. 1994;59:413–418. doi: 10.1093/ajcn/59.2.413. [DOI] [PubMed] [Google Scholar]
- Ishizaki K, Sasaki M, Karasawa S, Obata H, Nara T, Goto F. The effect of intrathecal magnesium sulphate on nociception in rat acute pain models. Anaesthesia. 1999;54:241–246. doi: 10.1046/j.1365-2044.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci. 2005;25:977–984. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Levant B, McCarson KE. D(3) dopamine receptors in rat spinal cord: implications for sensory and motor function. Neurosci Lett. 2001;303:9–12. doi: 10.1016/s0304-3940(01)01692-5. [DOI] [PubMed] [Google Scholar]
- Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Li X, Lighthall G, Liang DY, Clark JD. Alterations in spinal cord gene expression after hindpaw formalin injection. J Neurosci Res. 2004;78:533–541. doi: 10.1002/jnr.20274. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Baier PC, Paulus W, Meuer K, Bähr M, Weishaupt JH. A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson's disease. Exp Neurol. 2007;205:207–213. doi: 10.1016/j.expneurol.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Dziewczapolski G, Garcia MC, Rubinstein M, Gershanik OS. D3 receptor knockdown through antisense oligonucleotide administration supports its inhibitory role in locomotion. Neuroreport. 1999;10:3131–3136. doi: 10.1097/00001756-199910190-00002. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mizuo K, Narita M, Miyatake M, Suzuki T. Enhancement of dopamine-induced signaling responses in the forebrain of mice lacking dopamine D3 receptor. Neurosci Lett. 2004;358:13–16. doi: 10.1016/j.neulet.2003.12.119. [DOI] [PubMed] [Google Scholar]
- Nordlander NB. Therapy in restless legs. Acta Med Scand. 1953;145:453–457. [PubMed] [Google Scholar]
- Paulus W, Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 2006;5:878–886. doi: 10.1016/S1474-4422(06)70576-2. [DOI] [PubMed] [Google Scholar]
- Presley RW, Menétrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383–388. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- Satija P, Ondo WG. Restless legs syndrome: pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22:497–518. doi: 10.2165/00023210-200822060-00004. [DOI] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A, Wermuth CG. Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther. 1995;275:1239–1246. [PubMed] [Google Scholar]
- Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251:977–982. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Neurobiology. New York: Oxford UP; 1994. [Google Scholar]
- Shieh GJ, Walters DE. Stimulating dopamine D1 receptors increases the locomotor activity of developing rats. Eur J Pharmacol. 1996;311:103–107. doi: 10.1016/0014-2999(96)00417-7. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Brück W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol. 1998;57:456–464. doi: 10.1097/00005072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Magerl W, Oertel WH, Möller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127:773–782. doi: 10.1093/brain/awh079. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Högl B, Benes H, Kohnen R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med. 2008;9:572–574. doi: 10.1016/j.sleep.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci U S A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre- and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992;77:757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Yehuda S, Ben-Uriah Y. Iron deficiency-induced circadian rhythm reversal of dopaminergic-mediated behaviours and thermoregulation in rats. Eur J Pharmacol. 1981;74:295–301. doi: 10.1016/0014-2999(81)90048-0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Pan T, Xie W, Zhang A, Ondo WG, Le W. Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J Neurosci Res. 2007;85:1065–1076. doi: 10.1002/jnr.21207. [DOI] [PubMed] [Google Scholar]