Abstract
Vitamin B1 is an essential exogenous micronutrient for animals. Mass death and reproductive failure in top aquatic consumers caused by vitamin B1 deficiency is an emerging conservation issue in Northern hemisphere aquatic ecosystems. We present for the first time a model that identifies conditions responsible for the constrained flow of vitamin B1 from unicellular organisms to planktivorous fishes. The flow of vitamin B1 through the food web is constrained under anthropogenic pressures of increased nutrient input and, driven by climatic change, increased light attenuation by dissolved substances transported to marine coastal systems. Fishing pressure on piscivorous fish, through increased abundance of planktivorous fish that overexploit mesozooplankton, may further constrain vitamin B1 flow from producers to consumers. We also found that key ecological contributors to the constrained flow of vitamin B1 are a low mesozooplankton biomass, picoalgae prevailing among primary producers and low fluctuations of population numbers of planktonic organisms.
Subject terms: Biooceanography, Ecological modelling, Ecosystem ecology, Marine biology, Environmental impact
Introduction
Vitamin B1 (thiamin) is necessary for the proper functioning of the majority of organisms because it serves as a cofactor that associates with a number of enzymes involved in primary carbohydrate and amino acid metabolism1. Vitamin B1 deficiency compromises mitochondrial functioning2 and causes nerve signaling malfunction in animals3. On a systemic level, low vitamin B1 levels translate into impaired health, immunosuppression and reproductive failures4. Synthesis of vitamin B1 is regulated by specific biosynthetic pathways for prokaryotes, plants and fungi5. All animals, as well as many prokaryotes and unicellular eukaryotes, cannot synthesize vitamin B1 and therefore must acquire it from exogenous pools6,7. Over the recent decades, diverse taxonomic groups, including piscivorous fish and sea birds, have suffered episodic events of reproductive failure and elevated mortality associated with sub-optimal vitamin B1 contents and characteristic symptoms of paralysis8–14. These reported reproductive failures in some years create demographic generational gaps in the Baltic population of the Atlantic salmon (Salmo salar) with mass mortality of offspring in the yolk-sac fry stage15,16. Vitamin B1 deficiency has been identified as an emerging conservation issue and a potential cause of population decline in marine animals4. The problem of population viability related to vitamin B1 deficiency concerns marine and freshwater ecosystems of the Northern Hemisphere, including the North American Great Lakes, the New York Finger Lakes and the Baltic Sea10,11,15,17.
The drivers of vitamin B1 deficiency in marine organisms are currently unknown, and therefore, there is an urgent need for a general understanding of how vitamin B1 is transferred through aquatic food webs. Prokaryotes and photoautotrophs are the main producers of vitamin B1 in marine systems18, but many of these unicellular taxa, both prokaryotes and eukaryotes are vitamin B1 auxotrophs that rely on external intake of dissolved vitamin B1 or its precursor compounds6,7,18–22. Concentrations of dissolved vitamin B1, its precursors and its degradation products are generally low (picomolar range), but data from natural systems are relatively scarce18,23–26. Recent studies suggest that biotic interactions and vitamin cycling during the process of obtaining B1 for cellular processes are likely affecting aquatic microbial community dynamics and composition23,27–30. Unicellular taxa producing or taking up dissolved vitamin B1 (or its precursors) are diverse with respect to cell size and mass-specific vitamin B1 concentration7,31–33, but the mechanisms that contribute to the overall dynamics of B1 and its precursors in the microbial realm are not well known18,23. In this model, we therefore consider bacteria and photoautotrophs as sources of vitamin B1, disregarding whether they synthesize the vitamin internally or obtain it via dissolved sources and focused on the transfer of B1 up the food web to consumers. These unicellular taxa are consumed by a range of proto- and metazoans of different sizes, and in turn, vitamin B1 flows to upper level consumers such as small planktivorous fish. The mass-specific vitamin B1 levels in planktivorous fish vary significantly in a spatiotemporal manner13,34,35, but it is unclear to what extent this results from constrained vitamin B1 flow from lower trophic levels. A climate change-driven increase in freshwater discharge can reduce the primary production, negatively affecting the efficiency of biomass transfer (see, e.g.36), and potentially constrain the flow of vitamin B1. Increased nutrient load due to anthropogenic activity can trigger shifts in the size distribution of planktonic producers toward smaller cells37,38. A shift in primary producers toward picoplankton can also potentially affect the flow of vitamin B1 due to increased decay of the vitamin during subsequent trophic interactions. Our work identifies conditions responsible for the constrained flow of vitamin B1 from unicellular organisms that are a source of vitamin B1 to planktivorous fishes.
One of the marine systems in the Northern Hemisphere most affected by vitamin B1 deficiency is the Baltic Sea, one of the best-monitored ecosystems worldwide among coastal marine areas39,40. The Baltic Sea is under the pressure of both anthropogenic factors and climate change. Anthropogenic activities such as agriculture have affected this system causing very high nutrient deposition rates, while past cod overfishing has caused trophic cascades with increasing abundances of planktivorous fish41. Apart from anthropogenic pressure, climate-change-driven increased precipitation may increase river loads of particles and dissolved organic and inorganic substances42. The increased discharge of dissolved substances is forecasted to affect light and biotic conditions of the sea and in turn suppresses phytoplankton biomass production and shift the carbon flow toward microbial heterotrophy36,42. Unicellular taxa producing or taking up dissolved vitamin B1 (or its precursors) in the Baltic Sea are very diverse with respect to taxonomy, cell size and mass-specific vitamin B1 content7,31–33. In general, those with smaller cells have higher carbon-specific vitamin B1 levels than those with large cells31. High nutrient loads and changes in light attenuation due to elevated inputs of riverine soluble substances and anthropogenic pressures are expected to affect the species composition of primary producers43. In turn, this is expected to affect the size spectra of vitamin B1-containing unicellular taxa in the Baltic Sea. As a general trend, the phytoplankton size spectrum in the Baltic Sea has shifted toward smaller primary producers in recent years44, but the effect on vitamin B1 provisioning to zooplankton, fish and other consumers is unknown. The transfer of vitamin B1 from planktivorous fish to top predators and the concentration in these organisms have been extensively studied10,13,34,35. The occurrence of a vitamin B1 deficiency syndrome called M74 in salmonids has been correlated with diet quality in the case of salmonids from Great Lakes of North America10 and the high abundance of a small planktivorous fish, European sprat (Sprattus sprattus), in the case of the Baltic Sea13,35. The requirements for vitamin B1 increase with the energy density of the food source, and the vitamin requirements of cold-water species such as salmon are often higher compared to those of warm-water species45. The small sprat eaten by the salmon in the Baltic Sea are energy rich (high-lipid) and hence provide a diet which is low in vitamin B1 per an energy unit12,35. The occurrence of B1 deficiency in North American salmonids is correlated with the abundance of a planktivorous fish called the alewife (Alosa pseudoharengus)10. The mechanism suggested to induce vitamin B1 deficiency is a high activity of a vitamin B1 degrading enzyme called thiaminase I in the alewife10,46. Comparable thiaminase I activities have also been detected in Baltic Sea clupeids47. However, there are no empirical studies that have disentangled the relative effects of vitamin B1-degrading enzymes and the vitamin concentration in clupeids regulating the overall vitamin B1 transfer to Baltic salmon. There are also studies showing that the diet of Baltic salmon in fact had a lower proportion of sprat during high M74 incidence years in the 1990s as compared to a low incidence period in 1959–196248. While vitamin B1 deficiency in salmon can be related to the consumption of sprat in the Baltic Sea, other species, such as the omnivorous herring gull (Larus argentatus) and common eider ducks (Somateria mollissima), feeding on benthic organisms have also been suffering the consequences of critically low vitamin B1 levels in recent years8,9,14. To identify the drivers of the seasonal and yearly variation in vitamin B1 concentration in planktivorous fish and top consumers, we need to understand the transport pathways of this essential substance from the unicellular planktonic taxa up to the different parts of the aquatic food web.
Here, we present a model of nutrients and vitamin B1 flow through an aquatic food web, exemplified by the Baltic Sea, and the resulting levels of vitamin B1 in a key level of consumers, i.e., planktivorous fish. The model manipulates nutrient input, the abundance of planktivorous fish and the degree of light attenuation caused by dissolved compounds to seek conditions leading to the constrained flow of vitamin B1 from unicellular organisms to fish. Our study reveals the potential link between vitamin B1 deficiencies, the anthropogenic pressure of increased nutrient input, differences in stock size of planktivorous fish, and an increase in light attenuation by dissolved substances transported to marine coastal systems.
Materials and Methods
The model
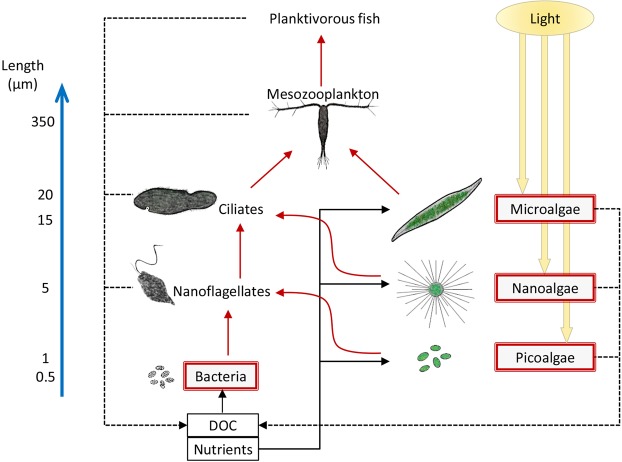
The presented model concerns a general, size-structured aquatic food web with explicit consideration of population number and nutrient and vitamin B1 flow between trophic levels with several elements parameterized to resemble the conditions of the Baltic Sea. The model considers pools of carbon, nitrogen, phosphorus and vitamin B1 to be independent and changing due to physical reactions, the physiology of organisms and population dynamics. The modeled trophic groups represent heterotrophic bacteria, primary producers (picoalgae, nanoalage and microalgae), protozoans (nanoflagellates and ciliates), mesozooplankton and small (ca. 15 cm long) planktivorous clupeid fish, with predators feeding on organisms one size level below their own (Fig. 1). The model predicts the response of the vitamin B1 concentration in planktivorous fish with constant population size to various scenarios of nutrient input and light attenuation caused by dissolved substances (see below). Due to the modeled half-year time frame (see below), we assume balanced birth and death rates in planktivorous fish, i.e., constant population size, which removes the need for consideration of complex multiyear trends in population age and size structure. The biomass of the other modeled groups in the food web (see Fig. 1) results from the balance between growth, respiration and death due to herbivory or predation, although we do not consider nonconsumptive mortality. Organisms live in a homogeneous, 10 m deep layer of water with instantaneous mixing and no stratification. Light intensity attenuates along with depth and fluctuates in the annual and day-night cycle (see Appendix 1 in Supporting Information) to represent conditions at the geographic location of Linnaeus Microbial Observatory (56.93°N and 17.06°E), which we also used to set nutrient input scenarios in our model (see below).
Figure 1.
Schematic view of the modeled food web. Primary producers grow utilizing nutrients and light, whereas heterotrophic bacteria assimilate inorganic nutrients and consume dissolved organic carbon (DOC) excreted by primary producers and consumers. Organisms in each size class are grazed by consumers positioned one size class above: nanoflagellates, ciliates, mesozooplankton or planktivorous fish. The size structure of organisms in the food web is indicated along the blue arrow. Uptake, grazing or consumption (solid lines); excretion (dot-dash lines). Unicellular taxa providing vitamin B1 to higher trophic levels (either by uptake of dissolved vitamin B1, its precursors or by internal production) are marked by fields with a red outline. Black arrows indicate the flow of macronutrients. Red arrows indicate the flow of macronutrients and vitamin B1.
Population growth of primary producers in our model depends on available light and nutrients. The way we model light attenuation follows an approach based on Lambert-Beer’s law, adopted in theoretical studies on algae primary production (see49 and SI Appendix 1). The light attenuation k [m−1] in our model is determined by self-shading and the presence of dissolved organic and inorganic substances according to
| 1 |
where ks [m2·mg C−1] sets the degree of light attenuation by algae carbon biomass WPP [mg C·m−3] (see SI Appendix 1 for parameterization) and kbg [m−1] is the background attenuation coefficient. We manipulated the background attenuation coefficient kbg to simulate scenarios with various amounts of dissolved substances. Strong light attenuation by dissolved substances reduces the overall primary production, but it also causes large primary producers to have lower rates of light absorption per unit of volume than small phytoplankton (see50). Similarly, in our model, strong light attenuation by dissolved substances constrains the growth rate of large primary producers to a higher degree than it constrains the growth rate of small primary producers. The rate of nutrient uptake and the nutrient-dependent growth rate of primary producers depend allometrically on cell volume51,52. For details of the parameterization of light- and nutrient-dependent primary production in our model, see SI Appendix 1. We modeled the aquatic food web as an enclosed system with scenarios differing by nutrient input (see below) at the start of the vegetative season. The timing of the vegetative season, between 30 March and 1 October resembles conditions in the southern part of the Baltic Sea. Apart from fish abundance, nutrient input and degree of light attenuation due to dissolved substances (see below), each scenario started from the same initial conditions. Whereas our simulations started with picomolar concentrations of vitamin B1 in the tissues of modeled organisms, the results remained qualitatively unchanged when the starting concentration of vitamin B1 was set to the maximal allowed levels (Fig. S2 in SI Appendix 1). Each type of organism was constrained by an empirically estimated maximal mass-specific concentration of vitamin B1 (given in [μmol·μmol-1 C]): bacteria 1.48e-7, picoalgae 1.48e-7, nanoalgae 1.18e-7, microalgae 1.18e-7, nanoflagellates 1.32e-7, ciliates 1.27e-7, mesozooplankton 1.28e-7 and planktivorous fish 1.04e-10 (see31,32,35 and SI Appendix 1). We excluded from the analysis two initial months (April, May) as the ca. 60-day period was long enough for planktivorous fish feeding on mesozooplankton to potentially reach realistic levels of vitamin B1 and for the biomass of phytoplankton to stabilize. The model measures the proportion of days when vitamin B1 concentration is low, i.e., drops below 6.41−11 [μmol·μmol C−1], which is an average level of vitamin B1 recorded in clupeids of the Baltic Sea see35.
The theoretical framework we use is a modified model of an aquatic food web that explicitly considers nutrient and vitamin B1 flow created by N. Blackburn (BIORAS, Copenhagen)53,54. The model concerns interactions between organisms and their physiology based on physiochemical principals and constraints; however, processes with poorly understood proximate mechanism, are represented by empirically estimated functions (see below). The details on model parameterization not described in the main text or Supporting Information (SI Appendix 1) can be found in the work by Thelaus, et al.54. The computer program used in this study is available in SI Appendix 2.
Determinants of vitamin B1 flow rate through the food web
Vitamin B1 is synthesized or absorbed from water by primary producers and heterotrophic bacteria and is transported up the food web through predation or herbivory (Fig. 1). The pool of vitamin B1 in organism cells is subjected to constant turnover due to degradation and input processes (see below). In primary producers and heterotrophic bacteria, the input is due to synthesis or absorption from the water, whereas consumers gain vitamin B1 by feeding. Manipulation of the rate at which levels of vitamin B1 increase in cells of bacteria and phytoplankton could for example correspond to changes in the degree of auxotrophy because high microbial cycling of vitamin B1 potentially reduces the overall pool of the vitamin due to losses related to excretion and absorption. In our work, we present results for the net rate of increase in vitamin B1 levels in cells of bacteria and algae as being equal to their metabolic rate (see SI Appendix 1). However, the conclusions from our work do not change when the rate of increase of vitamin B1 levels in cells of bacteria and algae is set much faster or slower than the metabolic rate (Fig. S1 in Appendix 1). This means that conclusions from our model also apply to food webs, in which a large proportion of bacteria and phytoplankton rely on the absorption of dissolved vitamin B1 and its precursors. Similarly, the model outcomes apply to scenarios with reduced synthesis and/or uptake of vitamin B1 due to other factors. In consumers, the rate of vitamin B1 transport is dependent on the assumed volume-specific clearance rates for predators (see SI Appendix 1), prey and predator biomass and vitamin B1 bioavailability i.e., the fraction of the compound that is absorbed from the food. Below, we present mainly results for vitamin B1 bioavailability of 15%, but we also report outcomes of a sensitivity analysis with bioavailability ranging from 5 to 20% (see SI Appendix 1 for details).
Considered scenarios
The model considers scenarios differing with respect to three environmental factors: (1) abundance of planktivorous fish, (2) nutrient level at the start of the vegetative season and (3) the degree to which light is attenuated by dissolved substances. (1) The fish abundance in the model directly affects the population number of mesozooplankton and, by top-down trophic cascade, also affects lower trophic levels. We used data on the abundance of Baltic populations of herring (Clupea harengus membras) and sprat (Sprattus sprattus) in 1991–201655 to set a gradient of scenarios with planktivorous fish varying from low (0.004 [ind·m−3]) to high (0.01 [ind·m−3]) abundance (see SI Appendix 1 for details). (2) Our model also considers a set of scenarios varying with nutrient level at the start of the vegetative season. The assumed scenarios represent a broad range of nutrient concentrations found in the Baltic Sea, parameterized with the data extracted from the HELCOM database representing early spring nutrient concentration in the southern part of the Baltic Sea (from years 1998–2016) and data from the Linnaeus Microbial Observatory56. We varied the nitrogen input from a very low (2.01 μmol/l of NO3−, 0.93 μmol/l of NH4+) to a very high concentration (30 μmol/l of NO3−, 7 μmol/l of NH4+) with levels of phosphorous set relative to the nitrogen according to the Redfield ratio57 i.e., 16:1 (see SI Appendix 1 for details). (3) We also tested a gradient of the degree of light attenuation caused by substances dissolved in the water with a large amount of dissolved substances represented by a high background light attenuation coefficient kbg (see equation 1). The constrained photon flux density in our model translates to lower rates of light absorption per unit of cell volume. Thus, a high background light attenuation coefficient kbg describes a more challenging environment for large primary producers (cf. the slow growth of microalgae during the initial phase of simulations under strong light attenuation in Fig. 2c,d). We tested our model with a kbg coefficient varying from 0.04 [m−1] to 0.24 [m−1] to represent a gradient of conditions ranging from very low and very high levels of substances dissolved in the water (see SI Appendix 1 for parameterization).
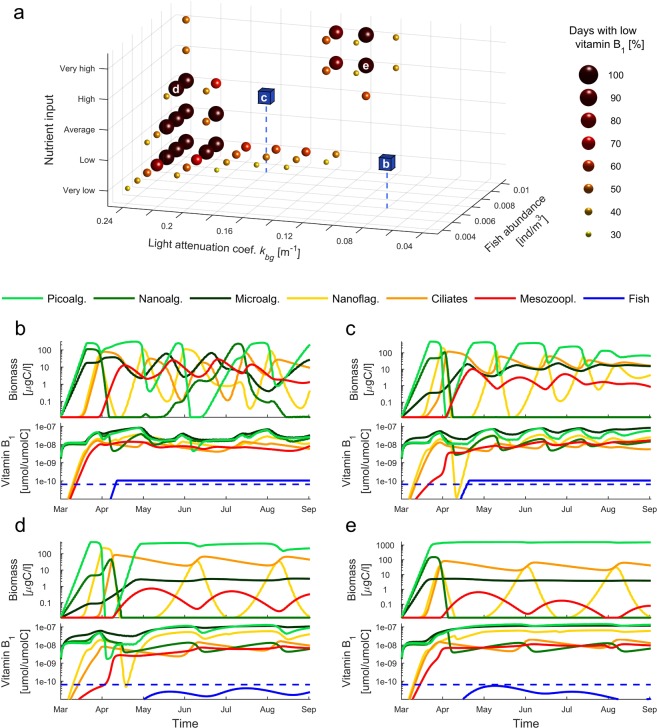
Figure 2.
Biomass and concentrations of vitamin B1 in modeled organisms. (a) Scenarios mapped on the tested parameter space of planktivorous fish abundance, background light attenuation and nutrient input. The empty spaces match the scenarios resulting in planktivorous fish rich in vitamin B1, i.e., with sustained concentrations of vitamin B1 above the levels recorded for Baltic populations of sprat and herring for more than 70% of the analyze time period (see the main text). Blue cubes represent the position of the scenarios with fish rich in vitamin B1 presented also in panels b and c (letters match the scenarios presented in b,c). Spheres represent scenarios resulting in a low level of vitamin B1 in planktivorous fish persisting for longer than 30% of the analyzed period (see the main text). Letters indicate the position of scenarios presented in (d,e). (b,c) Example scenarios with mass-specific vitamin B1 levels persisting for most of the time above average levels of vitamin B1 recorded for Baltic populations of sprat and herring (indicated by dashed line). (d,e) Example scenarios with low mass-specific vitamin B1 levels persisting for most of the time during the analyzed period. (b–e) Note the difference between scenarios, (b–e) in the dynamics of fluctuations of the modeled organisms.
Data accessibility
The computer program used in the work is available as Supplementary Information (see SI Appendix 2).
Results
In scenarios resulting in high concentrations of vitamin B1 in planktivorous fish, the biomass of consumers and primary producers fluctuates over time, and over the season, different size groups of primary producers contribute to the total biomass of primary producers (Fig. 2b,c). In scenarios resulting in low vitamin B1 concentrations in planktivorous fish, picoalgae biomass is high and relatively stable over time; it does not drop to the minimal levels of biomass observed in scenarios with planktivorous fish rich in vitamin B1 for most of the simulated time (cf. picoalgae biomass in Fig. 2d,e vs. b,c). The number of days with vitamin B1-poor planktivorous fish is substantial in two types of scenarios: (i.) high fish abundance, high to very high nutrient input, and intermediate light attenuation, or (ii.) intermediate to high fish abundance, very low to average nutrient input, and strong background attenuation (Fig. 2a). Below, we describe the drivers and environmental correlates restricting vitamin B1 flow through the food web in both scenarios.
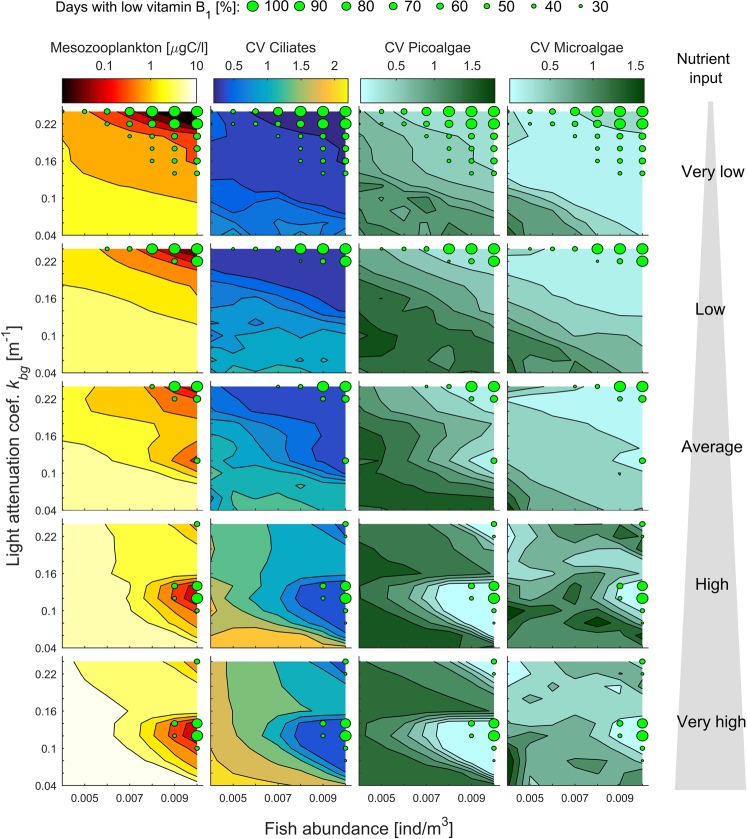
In both scenarios (see example scenario (i) illustrated in Fig. 2e and (ii) in Fig. 2d), the biomass of mesozooplankton is low for most of the time (compare Fig. 2d,e with b,c that represents conditions resulting in planktivorous fish rich in vitamin B1). Vitamin B1 flowing from mesozooplankton to planktivorous fish is diluted among the individuals when planktivorous fish are numerous. However, the low biomass of mesozooplankton in scenarios resulting in low vitamin B1 in planktivorous fish can be only partially explained by fish abundance (Fig. 3 column 1). For both regions of the parameter space with vitamin B1-poor planktivorous fish (Fig. 2a), a low biomass of mesozooplankton was associated with low fluctuations in populations of picoalgae and mesozooplankton prey (i.e., a low coefficient of variation for the biomass of ciliates and microalgae) (Fig. 3 columns 2–4). Picoalgae biomass drops in our model only if overgrazed by numerous nanoflagellates, which in turn triggers a pulse of ciliates which are in turn exploited by mesozooplankton (Fig. 2b,c). A drop in the biomass of picoalgae, the most competitive class of primary producers, also has also a positive effect on the biomass of microalgae (Fig. 2b,c) as it increases the availability of a significant amount of nutrients and improves light conditions (see below).
Figure 3.
The biomass of mesozooplankton and coefficient of variation (CV) for biomass of ciliates and key groups of primary producers presented in gradients of planktivorous fish abundance, background light attenuation and nutrient input (illustrated by the gray triangular shape). The scenarios with low vitamin B1 levels in planktivorous fish are indicated by green circles (see the legend). (Column 1) The average biomass of mesozooplankton. (Columns 2–4) The coefficient of variation calculated for the biomass of mesozooplankton food resources (ciliates and microalgae) and picoalgae.
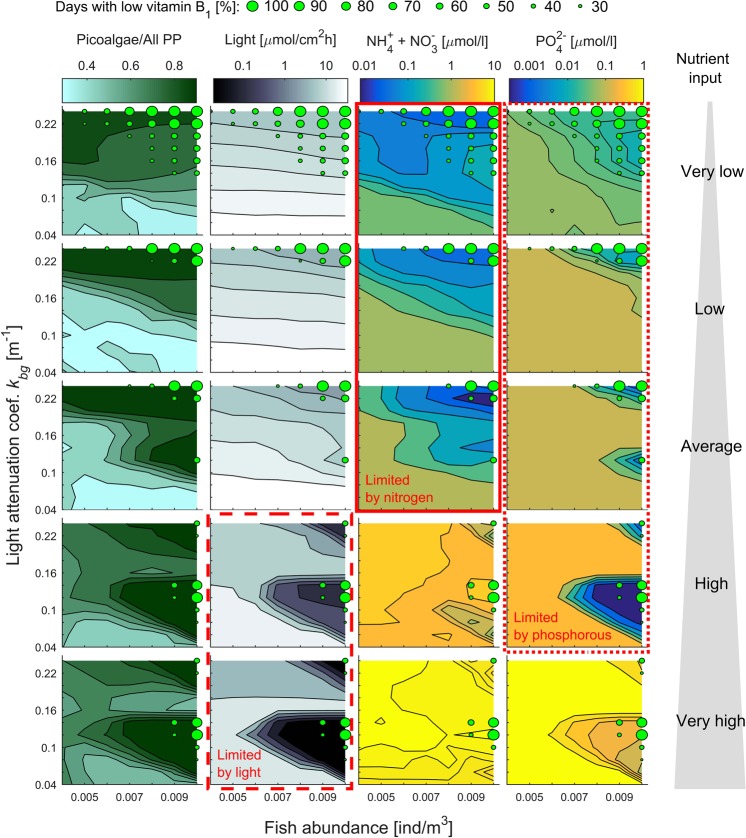
Both scenarios resulting in vitamin B1-poor planktivorous fish are associated with a skewed size spectrum of primary producers toward picoalgae (Fig. 4 column 1). However, the underlying mechanisms responsible for the skewed algae size distribution are different in the described scenarios. Under scenario (i), i.e., high fish abundance, high to very high nutrient input and intermediate light attenuation (Fig. 2a), the picoalgae bloom is long-lasting (Fig. 2e). The high biomass of picoalgae drains out phosphorous and causes severe light deprivation (Fig. 4, columns 2 and 4). Hence, under a prolonged picoalgae bloom microalgae growth is suppressed due to the low availability of phosphorus and light (high nutrient input) or of only light (very high nutrient input) (Fig. 4 columns 2 and 4). Under scenario (ii), i.e., intermediate to high fish abundance, very low to average nutrient input, and strong background attenuation (Fig. 2a,d), the growth rate of microalgae is limited by the low availability of nutrients due to the strong competitive abilities of picoalgae that drain out nitrogen and phosphorous (Figs 2d and 4 columns 3–4).
Figure 4.
Biotic and abiotic correlates of low vitamin B1 levels in planktivorous fish presented in gradients of fish abundance, background light attenuation and nutrient input (illustrated by the gray triangular shape). Cases with vitamin B1-poor planktivorous fish are indicated by green circles (see the legend). (Column 1) The average proportion of algae biomass located in picoalgae illustrates the degree to which the primary producer cell-size spectrum shifts toward the smallest primary producers. (Column 2) Total light penetrating the water column (median) from attenuation by primary producers (self-shading) and dissolved substances (background attenuation). Note, that the light attenuation is driven by background attenuation in scenarios with very low to average nutrient input and almost entirely by self-shading in scenarios with high and very high nutrient input. (Columns 3–4) Concentration of dissolved inorganic nitrogen and phosphorous (median). (Columns 2–4) Red rectangles outline the scenarios in which low levels of nitrogen, phosphorous and/or light fit with the observed low levels of vitamin B1 in planktivorous fish.
In our model, a large fraction of vitamin B1 is degraded during consumption due to its bioavailability b = 0.15. Let us consider a hypothetic pool c of a compound synthesized by picoalgae. While flowing through the modeled food web (Fig. 1), the pool degrades at every trophic level and as a result, the amount of the compound drops in a negative exponential fashion given by cbn, where n denotes the number of consumer levels. The amount of compound c will drop 6.6 times after being absorbed by nanoflagellates and ca. 2 thousand times after being absorbed by planktivorous fish, as it needs to pass three consumer levels. Whereas only 0.5‰ of vitamin B1 produced by picoalgae can reach planktivorous fish, this value increases to ca. 2% for the pool produced by microalgae, as there are two instead of four consumer levels. Changes in bioavailability significantly affect the predicted amount of vitamin B1 that is left after passing subsequent trophic levels. For instance, by reducing bioavailability from 15% to 10%, the vitamin B1 pool synthesized by picoalgae will drop 10 thousand times, instead of 2 thousand times, when absorbed by planktivorous fish. Hence, the assumed bioavailability of vitamin B1 influenced the number of scenarios with vitamin B1-poor planktivorous fish (Fig. S3 in SI Appendix 1). However, even under relatively high vitamin B1 bioavailability scenarios, vitamin B1-deficient fish persist in two separate regions of the parameter space (see Fig. S3 in SI Appendix 1).
Discussion
Our work shows, using the Baltic Sea as a model system, that vitamin B1 flow in aquatic systems is mediated by processes that control phytoplankton diversity and abundance. Size shifts in primary producers toward picoalgae accompanied by a high abundance of small planktivorous fish lead to low vitamin B1 concentrations in the small pelagic fish and therefore to a high risk of vitamin B1 deficiencies in top consumers. A limited vitamin B1 concentration at the higher levels of the food chain is most likely to occur when there is either a high input of nutrients or a high concentration of dissolved substances that attenuates light. In addition, the sensitivity analysis revealed that one of the crucial aspects for understanding the causes of vitamin B1 deficiency in marine ecosystems, to date poorly covered in the literature, is vitamin B1 bioavailability, i.e., the proportion of the compound absorbed from food8,9,34,35,58.
Administration of vitamin B1 to animals that suffer from deficiency syndromes can reverse negative effects; fry of lake trout and Atlantic salmon as well as seabirds suffering from vitamin B1 deficiency syndrome recovered when administrated physiological concentrations of vitamin B18,14,58,59. Unicellular taxa producing or absorbing dissolved vitamin B1 (or it precursors) from water differ with respect to mass-specific vitamin B1 concentrations, but in general, they synthesize orders of magnitude more vitamin B1 than is needed to sustain physiological functions in marine top predators31,32,60,61. However, not all vitamin B1 in phytoplankton is transferred to zooplankton since grazing can be constrained. For example, large filamentous cyanobacteria contain comparably high vitamin B1 concentrations, but it is not available to zooplankton since the filaments are too large to be consumed32. Moreover, the bioavailability of vitamin B1 decreases in water environments due to the high solubility of the vitamin. Due to water-solubility, losses of vitamin B1 in fish during digestion can be very high, reaching up to 98%62. In mammals, the bioavailability of water-soluble vitamin B1 hydrochloride reaches up to 5%, with up to ca. 20% efficiency of assimilation of other vitamin B1 analogues63,64. In insects, the efficiencies of assimilation of B vitamins can range from 7 to ca. 60%65. Our work shows how differences in bioavailability on a single trophic level produce serious consequences when the full transfer through the food-web is considered (see results and Fig. S3. in SI Appendix 1). No data exist about the vitamin B1 bioavailability in lower trophic levels, such as protozoans or zooplankton, and there is a need for studies that compare the vitamin B1 bioavailability at various trophic levels of the marine food webs.
Heterotrophic prokaryotes and photoautotrophs are the main producers of vitamin B1 in marine systems18. However, a large proportion of bacteria and phytoplankton are auxotrophic, as they rely on an exogenous supply of vitamin B1 or its precursors6,7,18–22,30,66. The degree of auxotrophy in some groups, such as bacterioplankton, is very high7. Vitamin B1, due to its high water solubility, cannot be stored in other forms than those built in cellular structures, which makes auxotrophs and consumers to dependent on a continuous intake of vitamin B167,68. Concentrations of dissolved vitamin B1 and its precursors and degradation products in the water column are not well known, but when measured, they tend to be undetectable or in the picomolar range18,25. In our work, we did not consider auxotrophy or complex trophic interactions in order to keep the model general and our understanding of the results feasible. Our work focuses on the transfer of vitamin B1 from unicellular taxa up the food chain and the conclusions from our work regarding the transfer to consumers do not change after manipulation of the rates of vitamin B1 synthesis/absorption, bioavailability or initial cellular concentrations (see Figs S1–S3 in SI Appendix 1). However, the degree of auxotrophy is likely to affect the rate at which levels of dissolved vitamin B1 and microbial species composition fluctuate over time. The dynamics of dissolved and particulate-bound vitamin B1 in the microbial realm are currently receiving much attention in the literature, but the overall mechanisms regulating these dynamics are not fully understood7,18–24,26–30,66,69. Future models of microbial realm should incorporate these dynamics when the mechanisms regulating microbial cycling of vitamin B1 are understood in more detail.
Abiotic stress may cause variation in the vitamin B1 content of aquatic primary producers33. Our study does not consider the impacts of potential stressors on vitamin B1 concentrations, but we do show that large primary producers among phytoplankton are essential for vitamin B1 transport through aquatic food webs. Microalgae are in the optimal predator-to-prey size range for mesozooplankton70, and they are an important source of vitamin B1 for zooplankton32. The flow of vitamin B1 to the level of top consumers is expected to be more efficient when the compound is coming from microalgae rather than from picoalgae (see results). We cannot exclude the possibility that the constrained flow of vitamin B1 to higher trophic levels arises in natural conditions as an effect of abiotic stressors, e.g., pollutants that deplete vitamin B1 levels in key groups of producers. However, this hypothesis cannot be tested without a more complete picture of the spatiotemporal variation in the content of vitamin B1 in microalgae.
It has been suggested that changes in the diet composition of Atlantic salmon, with a shift from herring to sprat, which contains low levels of vitamin B1 per energy unit, is responsible for M74 syndrome in this top predatory fish13,35. However, the drivers of spatiotemporal variations in vitamin B1 levels in small planktivorous fish are not well understood, and our work indicates that they are likely constituted by both top-down and bottom-up ecosystem mechanisms of regulation. Shifts in abundance and species composition of small planktivorous fish also cannot explain the occurrence of critically low vitamin B1 levels reported for bivalves, omnivorous gulls or sea ducks8,9,14. Whereas bivalves and common eider ducks represent part of the food web that includes benthic organisms not modeled in our work, we believe that the general predictions of our study may also apply here. A shift toward smaller primary producers and less mesozooplankton, that triggers low vitamin B1 levels in planktivorous fish may affect filtrating bivalves in a similar way as the shift impacts planktivorous fish in our model.
Our results, based on the explicit analysis of vitamin B1 flow through the food web, point out two aspects that have the potential to balance the current discussion on the drivers of vitamin B1 deficiency in aquatic ecosystems. First, the factors affecting the biomass of key groups of primary producers (e.g., microalgae) may be more important for understanding the causes of vitamin B1 deficiency than the interspecific differences in mass-specific levels of vitamin B1. However, little is known about the degree to which primary producers within a size class may differ with respect to vitamin B1 content. Second, there is a great need for studies on taxa-specific differences in vitamin B1 bioavailability. Without this knowledge, it is difficult to judge the importance of different parts of the food web to the flow of vitamin B1 from producers to consumers. Knowledge of group-specific differences in the bioavailability of vitamin B1 would help us to identify key taxa among producers and consumers essential for vitamin B1 transport through aquatic food webs. To conclude, a comprehensive approach focused on the flow of vitamin B1 through an aquatic food web allows the identification of the environmental and biological factors that lead to the constrained flow of vitamin B1. Our work identifies microalgae as organisms of key importance for vitamin B1 transport from unicellular taxa to top consumers. High nutrient input, strong light attenuation by dissolved substances and a high population density of planktivorous fish promotes restricted flow of vitamin B1. Our work identifies anthropogenic pressures and climate-driven factors that may facilitate the occurrence of vitamin B1 deficiency syndrome in top consumers. Apart from the high input of nutrients and dissolved substances that attenuate light, fishing pressure on top predators may also contribute to hampered flow of vitamin B1 due to the increased abundance of planktivorous fish. Hence, models that attempt to explain the role of vitamin B1 deficiency in marine ecosystems should be an integral part of an Ecosystem-Based Management approach toward the sustainable use of living marine resources40,71.
Supplementary information
Acknowledgements
We thank A. Ejsmond for comments on earlier versions of the manuscript. The study was financially supported by the Carl Trygger Foundation, the Swedish research council FORMAS (Grant Number 215-2012-1319), the Strong Research Environment ECOCHANGE [Ecosystem dynamics in the Baltic Sea in a changing climate]), and the Linnaeus University Centre for Ecology and Evolution in Microbial Model Systems (EEMiS). Jagiellonian University (DS/WB/INoS/757/2018) also supported the work.
Author Contributions
M.E. performed simulations, analyzed results and drafted the manuscript, N.B. implemented the model into a LabView computer program, M.C. provided the data on the abundance of clupeids in the Baltic Sea, and E.F. and S.H. provided the data on vitamin B1 content in planktonic organisms. All authors took part in designing the study and contributed to the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46422-2.
References
- 1.Schowen, R. (ed. Sinnott, M.) Ch. Comprehensive biological catalysis. Vol 2, p. 217–266. (Academic Press, San Diego, Calif, 1998).
- 2.Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chemico-Biological Interactions. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Ba A. Metabolic and Structural Role of Thiamine in Nervous Tissues. Cellular and Molecular Neurobiology. 2008;28:923–931. doi: 10.1007/s10571-008-9297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland WJ, et al. A 2018 Horizon Scan of Emerging Issues for Global Conservation and Biological Diversity. Trends in Ecology & Evolution. 2018;33:47–58. doi: 10.1016/j.tree.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of thiamin biosynthesis in procaryotes - New genes and regulatory mechanisms. Journal of Biological Chemistry. 2002;277:48949–48959. doi: 10.1074/jbc.M208965200. [DOI] [PubMed] [Google Scholar]
- 6.Croft MT, Warren MJ, Smith AG. Algae need their vitamins. Eukaryotic Cell. 2006;5:1175–1183. doi: 10.1128/ec.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paerl RW, et al. Prevalent reliance of bacterioplankton on exogenous vitamin B1 and precursor availability. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:E10447–E10456. doi: 10.1073/pnas.1806425115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balk L, et al. Wild birds of declining European species are dying from a thiamine deficiency syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12001–12006. doi: 10.1073/pnas.0902903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balk, L. et al. Widespread episodic thiamine deficiency in Northern Hemisphere wildlife. Scientific Reports6, 10.1038/srep38821 (2016). [DOI] [PMC free article] [PubMed]
- 10.Fitzsimons JD, Brown SB, Honeyfield DC, Hnath JG. A Review of Early Mortality Syndrome (EMS) in Great Lakes Salmonids: Relationship with Thiamine Deficiency. AMBIO. 1999;28:9–15. [Google Scholar]
- 11.Fitzsimons, J. D., Wolgamood, M., Madenjian, C. P. & Bunnell, D. B. In Ecology and Animal Health - Ecosystem Health and Sustainable Agriculture Vol. 2 (eds Norrgren, L. & Levengood, J.) 380 (The Baltic University Programme, Uppsala University, 2013).
- 12.Keinanen M, et al. Fatty acid composition of sprat (Sprattus sprattus) and herring (Clupea harengus) in the Baltic Sea as potential prey for salmon (Salmo salar) Helgoland Marine Research. 2017;71:1–16. doi: 10.1186/s10152-017-0484-0. [DOI] [Google Scholar]
- 13.Mikkonen J, Keinanen M, Casini M, Ponni J, Vuorinen PJ. Relationships between fish stock changes in the Baltic Sea and the M74 syndrome, a reproductive disorder of Atlantic salmon (Salmo salar) Ices Journal of Marine Science. 2011;68:2134–2144. doi: 10.1093/icesjms/fsr156. [DOI] [Google Scholar]
- 14.Morner, T. et al. Thiamine deficiency impairs common eider (Somateria mollissima) reproduction in the field. Scientific Reports7, 10.1038/s41598-017-13884-1 (2017). [DOI] [PMC free article] [PubMed]
- 15.Bengtsson B, et al. Reproductive Disturbances in Baltic Fish: A Synopsis of the FiRe Project. AMBIO. 1999;28:2–8. [Google Scholar]
- 16.ICES. Report of the Baltic Salmon and Trout Assessment Working Group (WGBAST). ICES CM ACOM08, 1–347 (2014).
- 17.Fisher JP, Spitsbergen JM, Iamonte T, Little EE, Delonay A. Pathological and Behavioral Manifestations of the “Cayuga Syndrome”, a Thiamine Deficiency in Larval Landlocked Atlantic Salmon. Journal of Aquatic Animal Health. 1995;7:269–283. doi: 10.1577/1548-8667. [DOI] [Google Scholar]
- 18.Sanudo-Wilhelmy, S. A., Gomez-Consarnau, L., Suffridge, C. & Webb, E. A. In Annual Review of Marine Science, Vol 6 Vol. 6 Annual Review of Marine Science (eds Carlson, C. A. & Giovannoni, S. J.) 339–367 (2014). [DOI] [PubMed]
- 19.Carini P, et al. Discovery of a SAR11 growth requirement for thiamin’s pyrimidine precursor and its distribution in the Sargasso Sea. Isme Journal. 2014;8:1727–1738. doi: 10.1038/ismej.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutowska, M. A. et al. Globally Important Haptophyte Algae Use Exogenous Pyrimidine Compounds More Efficiently than Thiamin. Mbio8, 10.1128/mBio.01459-17 (2017). [DOI] [PMC free article] [PubMed]
- 21.McRose D, et al. Alternatives to vitamin B-1 uptake revealed with discovery of riboswitches in multiple marine eukaryotic lineages. Isme Journal. 2014;8:2517–2529. doi: 10.1038/ismej.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paerl RW, et al. Use of plankton-derived vitamin B1 precursors, especially thiazole-related precursor, by key marine picoeukaryotic phytoplankton. Isme Journal. 2017;11:753–765. doi: 10.1038/ismej.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Consarnau L, et al. Mosaic patterns of B-vitamin synthesis and utilization in a natural marine microbial community. Environmental Microbiology. 2018;20:2809–2823. doi: 10.1111/1462-2920.14133. [DOI] [PubMed] [Google Scholar]
- 24.Heal KR, et al. Determination of four forms of vitamin B-12 and other B vitamins in seawater by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2014;28:2398–2404. doi: 10.1002/rcm.7040. [DOI] [PubMed] [Google Scholar]
- 25.Sanudo-Wilhelmy SA, et al. Multiple B-vitamin depletion in large areas of the coastal ocean. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14041–14045. doi: 10.1073/pnas.1208755109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suffridge, C., Cutter, L. & Sanudo-Wilhelmy, S. A. A New Analytical Method for Direct Measurement of Particulate and Dissolved B-vitamins and Their Congeners in Seawater. Frontiers in Marine Science4, 10.3389/fmars.2017.00011 (2017).
- 27.Gobler CJ, Norman C, Panzeca C, Taylor GT, Sanudo-Wilhelmy SA. Effect of B-vitamins (B-1, B-12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquatic Microbial Ecology. 2007;49:181–194. doi: 10.3354/ame01132. [DOI] [Google Scholar]
- 28.Koch, F. et al. Vitamin B-1 and B-12 uptake and cycling by plankton communities in coastal ecosystems. Frontiers in Microbiology3, 10.3389/fmicb.2012.00363 (2012). [DOI] [PMC free article] [PubMed]
- 29.Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F. Vitamin B1 ecophysiology of marine picoeukaryotic algae: Strain-specific differences and a new role for bacteria in vitamin cycling. Limnology and Oceanography. 2015;60:215–228. doi: 10.1002/lno.10009. [DOI] [Google Scholar]
- 30.Tang YZ, Koch F, Gobler CJ. Most harmful algal bloom species are vitamin B-1 and B-12 auxotrophs. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20756–20761. doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridolfsson, E. et al. Seasonal variation and species-specific concentrations of the essential vitamin B1 (thiamin) in zooplankton and seston. Marine Biology (in press).
- 32.Fridolfsson E, Lindehoff E, Legrand C, Hylander S. Thiamin (vitamin B1) content in phytoplankton and zooplankton in the presence of filamentous cyanobacteria. Limnology and Oceanography. 2018;63:2423–2435. doi: 10.1002/lno.10949. [DOI] [Google Scholar]
- 33.Sylvander P, Haubner N, Snoeijs P. The Thiamine Content of Phytoplankton Cells Is Affected by Abiotic Stress and Growth Rate. Microbial Ecology. 2013;65:566–577. doi: 10.1007/s00248-012-0156-1. [DOI] [PubMed] [Google Scholar]
- 34.Fitzsimons JD, et al. Thiamine content and thiaminase activity of ten freshwater stocks and one marine stock of alewives. Journal of Aquatic Animal Health. 2005;17:26–35. doi: 10.1577/h04-002.1. [DOI] [Google Scholar]
- 35.Keinanen M, et al. The thiamine deficiency syndrome M74, a reproductive disorder of Atlantic salmon (Salmo salar) feeding in the Baltic Sea, is related to the fat and thiamine content of prey fish. Ices Journal of Marine Science. 2012;69:516–528. doi: 10.1093/icesjms/fss041. [DOI] [Google Scholar]
- 36.Wikner J, Andersson A. Increased freshwater discharge shifts the trophic balance in the coastal zone of the northern Baltic Sea. Global Change Biology. 2012;18:2509–2519. doi: 10.1111/j.1365-2486.2012.02718.x. [DOI] [Google Scholar]
- 37.Jurgensone I, Carstensen J, Ikauniece A, Kalveka B. Long-term Changes and Controlling Factors of Phytoplankton Community in the Gulf of Riga (Baltic Sea) Estuaries and Coasts. 2011;34:1205–1219. doi: 10.1007/s12237-011-9402-x. [DOI] [Google Scholar]
- 38.Riemann B, et al. Recovery of Danish Coastal Ecosystems After Reductions in Nutrient Loading: A Holistic Ecosystem Approach. Estuaries and Coasts. 2016;39:82–97. doi: 10.1007/s12237-015-9980-0. [DOI] [Google Scholar]
- 39.Elmgren R, Blenckner T, Andersson A. Baltic Sea management: Successes and failures. Ambio. 2015;44:S335–S344. doi: 10.1007/s13280-015-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reusch Thorsten B. H., Dierking Jan, Andersson Helen C., Bonsdorff Erik, Carstensen Jacob, Casini Michele, Czajkowski Mikolaj, Hasler Berit, Hinsby Klaus, Hyytiäinen Kari, Johannesson Kerstin, Jomaa Seifeddine, Jormalainen Veijo, Kuosa Harri, Kurland Sara, Laikre Linda, MacKenzie Brian R., Margonski Piotr, Melzner Frank, Oesterwind Daniel, Ojaveer Henn, Refsgaard Jens Christian, Sandström Annica, Schwarz Gerald, Tonderski Karin, Winder Monika, Zandersen Marianne. The Baltic Sea as a time machine for the future coastal ocean. Science Advances. 2018;4(5):eaar8195. doi: 10.1126/sciadv.aar8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casini M, et al. Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:197–202. doi: 10.1073/pnas.0806649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson A, et al. Projected future climate change and Baltic Sea ecosystem management. Ambio. 2015;44:S345–S356. doi: 10.1007/s13280-015-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier HEM, et al. Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Climate Dynamics. 2012;39:2421–2441. doi: 10.1007/s00382-012-1339-7. [DOI] [Google Scholar]
- 44.Suikkanen S, et al. Climate Change and Eutrophication Induced Shifts in Northern Summer Plankton Communities. PLOS ONE. 2013;8:e66475. doi: 10.1371/journal.pone.0066475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodward B. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture. 1994;124:133–168. doi: 10.1016/0044-8486(94)90375-1. [DOI] [Google Scholar]
- 46.Brown SB, Fitzsimons JD, Honeyfield DC, Tillitt DE. Implications of thiamine deficiency in Great Lakes Salmonines. Journal of Aquatic Animal Health. 2005;17:113–124. doi: 10.1577/h04-015.1. [DOI] [Google Scholar]
- 47.Wistbacka S, Bylund G. Thiaminase activity of Baltic salmon prey species: a comparision of net- and predator-caught samples. Journal of Fish Biology. 2008;72:787–802. doi: 10.1111/j.1095-8649.2007.01722.x. [DOI] [Google Scholar]
- 48.Hansson S, et al. Stomach analyses of Baltic salmon from 1959–1962 and 1994–1997: possible relations between diet and yolk-sac-fry mortality (M74) Journal of Fish Biology. 2001;58:1730–1745. doi: 10.1006/jfbi.2001.1585. [DOI] [Google Scholar]
- 49.Huisman J, Weissing FJ. Competition for Nutrients and Light in a Mixed Water Column: A Theoretical Analysis. American Naturalist. 1995;146:536–564. doi: 10.1086/285814. [DOI] [Google Scholar]
- 50.Edwards KF, Thomas MK, Klausmeier CA, Litchman E. Light and growth in marine phytoplankton: allometric, taxonomic, and environmental variation. Limnology and Oceanography. 2015;60:540–552. doi: 10.1002/lno.10033. [DOI] [Google Scholar]
- 51.Edwards KF, Thomas MK, Klausmeier CA, Litchman E. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnology and Oceanography. 2012;57:554–566. doi: 10.4319/lo.2012.57.2.0554. [DOI] [Google Scholar]
- 52.Lindemann, C., Fiksen, Ø., Andersen, K. H. & Aksnes, D. L. Scaling Laws in Phytoplankton Nutrient Uptake Affinity. Frontiers in Marine Science3, 10.3389/fmars.2016.00026 (2016).
- 53.Blackburn N, Azam F, Hagstrom A. Spatially explicit simulations of a microbial food web. Limnology and Oceanography. 1997;42:613–622. doi: 10.4319/lo.1997.42.4.0613. [DOI] [Google Scholar]
- 54.Thelaus J, Haecky P, Forsman M, Andersson A. Predation pressure on bacteria increases along aquatic productivity gradients. Aquatic Microbial Ecology. 2008;52:45–55. doi: 10.3354/ame01200. [DOI] [Google Scholar]
- 55.Casini M, et al. Spatial and temporal density dependence regulates the condition of central Baltic Sea clupeids: compelling evidence using an extensive international acoustic survey. Population Ecology. 2011;53:511–523. doi: 10.1007/s10144-011-0269-2. [DOI] [Google Scholar]
- 56.Legrand C, et al. Interannual variability of phyto-bacterioplankton biomass and production in coastal and offshore waters of the Baltic Sea. AMBIO. 2015;44:427–438. doi: 10.1007/s13280-015-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geider RJ, La Roche J. Redfield revisited: variability of C: N: P in marine microalgae and its biochemical basis. European Journal of Phycology. 2002;37:1–17. doi: 10.1017/s0967026201003456. [DOI] [Google Scholar]
- 58.Fitzsimons JD. The effect of B-vitamins on a swim-up syndrome in Lake Ontario lake trout. Journal of Great Lakes Research. 1995;21:286–289. doi: 10.1016/s0380-1330(95)71102-9. [DOI] [Google Scholar]
- 59.Borjeson H, Amcoff P, Ragnarsson B, Norrgren L. Reconditioning of sea-run Baltic salmon (Salmo salar) that have produced progeny with the M74 syndrome. Ambio. 1999;28:30–36. [Google Scholar]
- 60.Brown MR, Mular M, Miller I, Farmer C, Trenerry C. The vitamin content of microalgae used in aquaculture. Journal of Applied Phycology. 1999;11:247–255. doi: 10.1023/a:1008075903578. [DOI] [Google Scholar]
- 61.Ladago BJ, Marsden JE, Evans AN. Early Feeding by Lake Trout Fry. Transactions of the American Fisheries Society. 2016;145:1–6. doi: 10.1080/00028487.2015.1073622. [DOI] [Google Scholar]
- 62.Tacon, A. G. J. Nutritional fish pathology. Morphological signs of nutrient deficiency and toxicity in farmed fish., Vol. 330 (FAO, 1992).
- 63.Loew D. Pharmacokinetics of thiamine derivatives especially of benfotiamine. International Journal of Clinical Pharmacology and Therapeutics. 1996;34:47–50. [PubMed] [Google Scholar]
- 64.Tallaksen CME, Sande A, Bohmer T, Bell H, Karlsen J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. European Journal of Clinical Pharmacology. 1993;44:73–78. doi: 10.1007/bf00315284. [DOI] [PubMed] [Google Scholar]
- 65.Barbehenn, R. V., Reese, J. C. & Hagen, K. S. In Ecological Entomology (eds Huffaker, C. B. & Gutierrez, A. P.) (John Wiley & Sons, 1999).
- 66.Paerl, R. W. et al. Carboxythiazole is a key microbial nutrient currency and critical component of thiamin biosynthesis. Scientific Reports8, 10.1038/s41598-018-24321-2 (2018). [DOI] [PMC free article] [PubMed]
- 67.Combs, G. F. & Combs, G. F. The Vitamins. (Elsevier Science, 2012).
- 68.Jurgenson CT, Begley TP, Ealick SE. The Structural and Biochemical Foundations of Thiamin Biosynthesis. Annual Review of Biochemistry. 2009;78:569–603. doi: 10.1146/annurev.biochem.78.072407.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sardans J, Rivas-Ubach A, Penuelas J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry. 2012;111:1–39. doi: 10.1007/s10533-011-9640-9. [DOI] [Google Scholar]
- 70.Hansen B, Bjornsen PK, Hansen PJ. The size ratio between planktonic predators and their prey. Limnology and Oceanography. 1994;39:395–403. doi: 10.4319/lo.1994.39.2.0395. [DOI] [Google Scholar]
- 71.Belgrano A, Fowler CW. How Fisheries Affect Evolution. Science. 2013;342:1176–1177. doi: 10.1126/science.1245490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The computer program used in the work is available as Supplementary Information (see SI Appendix 2).