Abstract
Materials science and genetic engineering have joined forces over the last three decades in the development of so-called protein-based polymers. These are proteins, typically with repetitive amino acid sequences, that have such physical properties that they can be used as functional materials. Well-known natural examples are collagen, silk, and elastin, but also artificial sequences have been devised. These proteins can be produced in a suitable host via recombinant DNA technology, and it is this inherent control over monomer sequence and molecular size that renders this class of polymers of particular interest to the fields of nanomaterials and biomedical research. Traditionally, Escherichia coli has been the main workhorse for the production of these polymers, but the methylotrophic yeast Pichia pastoris is finding increased use in view of the often high yields and potential bioprocessing benefits. We here provide an overview of protein-based polymers produced in P. pastoris. We summarize their physicochemical properties, briefly note possible applications, and detail their biosynthesis. Some challenges that may be faced when using P. pastoris for polymer production are identified: (i) low yields and poor process control in shake flask cultures; i.e., the need for bioreactors, (ii) proteolytic degradation, and (iii) self-assembly in vivo. Strategies to overcome these challenges are discussed, which we anticipate will be of interest also to readers involved in protein expression in P. pastoris in general.
Keywords: Pichia pastoris, Protein-based polymers, Protein expression, Proteolysis, Silk, Collagen, Gelatin, Elastin, Block copolymers, Self-assembly
Abbreviations: AOX1, alcohol oxidase 1; BiP, immunoglobulin-binding protein; DPAPase A, dipeptidyl aminopeptidase A; EBP, elastin-binding protein; ECM, extracellular matrix; ELP, elastin-like polypeptide; ERAD, ER-associated degradation; FDA, food and drug administration; GAP, glyceraldehyde-3-phosphate dehydrogenase; GRAS, generally recognized as safe; his4, histidinol dehydrogenase; ITC, inverse transition cycling; LCST, lower critical solution temperature; MaSp1, major ampullate spidroin 1; MaSp2, major ampullate spidroin 2; OD600, optical density at 600 nm; P4H, peptidyl-prolyl-4-hydroxylase; PDI, protein disulfide isomerase; Pho1, acid phosphatase; ppαF, Saccharomyces cerevisiae α-factor mating pheromone prepro peptide; UPR, unfolded protein response
1. Introduction
A long-sought objective in materials science is the development of polymers with controlled monomer sequence (Van Hest and Tirrell, 2001). Although progress has been made in synthetic chemistry (Lutz et al., 2013), the level of control evident in natural sequential polymers such as DNA and proteins is unparalleled. These biological macromolecules feature a defined molecular size and a controlled sequence of the nucleotide or amino acid monomers. Proteins fold into a three-dimensional structure as defined by their primary sequence, thereby acquiring unique properties. From 20 different amino acid monomers, nature has created an awe-inspiring wealth of different proteins, including enzymes, antibodies, peptide hormones, and also proteins with a structure-forming, viscoelastic, or colloidal function. This last category includes proteins such as collagen and elastin that fulfill a major role in the structure of various tissues, and silks used in animal architecture such as silkworm cocoons and spider webs (Desai and Lee, 2015; Grunwald et al., 2009; Heslot, 1998). These proteins typically feature highly repetitive sequences with biased amino acid composition and can often reversibly self-assemble into supramolecular structures through the formation of noncovalent bonds (Freeman et al., 2015). The natural materials derived from them display remarkable toughness, elasticity, and other properties that have inspired materials scientists to mimic them using modern protein engineering (Van Hest and Tirrell, 2001). These nanomaterials are of great interest in basic research, where they provide novel insights into macromolecular structure-function relationships. Although still an emerging field, materials are also being developed for biomedical applications such as tissue engineering and drug delivery (for reviews, see Desai and Lee, 2015; Frandsen and Ghandehari, 2012; Huang et al., 2015; MacEwan and Chilkoti, 2010; Sengupta and Heilshorn, 2010).
These so-called protein-based polymers, or protein polymers for short, are produced as heterologous proteins in a suitable host, just like enzymes and other proteins. However, as will become clear in this review, their highly repetitive sequence, biased amino acid composition, and physicochemical properties do present particular difficulties. The genes encoding natural protein polymers are sometimes used, but more often genes are synthesized that encode simplified mimics, or even completely de novo-designed protein polymers (Grunwald et al., 2009; Sanford and Kumar, 2005). Multifunctional block copolymers can be prepared by combining different polymer types into one molecule. Ever since the pioneering work by Cappello and Ferrari of Protein Polymer Technologies (Cappello, 1990; Cappello et al., 1990), Escherichia coli has been the most widely used production host for protein polymers. Besides this workhorse of protein engineering, several other hosts have been used, including plants, insect cells, transgenic animals, Aspergillus nidulans, Saccharomyces cerevisiae, Hansenula polymorpha (Ogataea angusta), and Bacillus brevis (Baez et al., 2005; Girotti et al., 2011; Heidebrecht and Scheibel, 2013; Wong Po Foo and Kaplan, 2002). However, the second most used system for the production of protein polymers after E. coli is the methylotrophic yeast Pichia pastoris (Komagataella phaffii; Kurtzman, 2009). This organism may offer particular advantages as a protein production host (Section 2), and our group has almost exclusively relied on it for the production of a wide range of protein polymers during the last two decades.
The physicochemical properties and potential applications of protein polymers have been excellently reviewed (Desai and Lee, 2015; DiMarco and Heilshorn, 2012; Frandsen and Ghandehari, 2012; Grunwald et al., 2009; Heslot, 1998; Kim, 2013; Rabotyagova et al., 2011; Van Hest and Tirrell, 2001; Yang et al., 2017). However, these reviews deal mainly with polymers produced in E. coli and only occasionally mention P. pastoris. The present review is the first to specifically highlight the utility of P. pastoris in the field of protein polymer research and also takes a more biotechnological perspective. Characterization and application studies in materials science require relatively large amounts of pure protein, and, as put forward by Yang et al. (2017), a major challenge for the commercialization of many protein polymers is their eventual low cost industrial production. From this point of view, while fully acknowledging that no single expression system is a cure-all, we here aim to illustrate that P. pastoris is an efficient host for a wide variety of protein polymers.
We will first briefly describe the main features of the P. pastoris expression system and then shortly introduce the basic genetic engineering principles used in the construction of genes encoding protein polymers. Next, we will provide an overview of protein polymers that have been produced in P. pastoris, where applicable placing each polymer class in the context of previous work involving E. coli. We then proceed to draw conclusions from the work surveyed and derive strategies to meet some of the possible challenges for polymer production in P. pastoris.
2. Pichia pastoris as a protein production host
As a methylotrophic yeast, Pichia pastoris produces enzymes involved in methanol metabolism at very high levels when grown on methanol (Couderc and Baratti, 1980). It is this observation that inspired the development of the yeast into a protein expression platform, where typically the strong methanol-inducible alcohol oxidase 1 (AOX1) promoter is used to drive transcription of genes encoding heterologous proteins (Wegner, 1990). Although many more promoters have been described for use in P. pastoris (see e.g. Gasser et al., 2015; Vogl and Glieder, 2013; Vogl et al., 2016), all studies on the production of protein polymers in P. pastoris have thus far relied on the AOX1 promoter and methanol induction to drive expression (Section 4). In shake flasks this typically involves initial growth on glycerol-containing medium, transferring the cells to methanol-containing medium for induction of protein expression, and further incubation with periodic addition of doses of methanol (Clare et al., 1991). In bioreactors the process usually involves the following stages (Stratton et al., 1998): (i) a glycerol batch phase for biomass generation, (ii) a growth-limiting glycerol fed-batch phase to further increase biomass levels and simultaneously derepress the AOX1 promoter, and (iii) a methanol fed-batch phase for protein production. For details on the general bioprocessing of P. pastoris, the reader is referred to several outstanding reviews (Cos et al., 2006; Looser et al., 2015; Potvin et al., 2012; Valero, 2013; Yang and Zhang, 2018; Zhang et al., 2000).
P. pastoris has become a popular heterologous host for a number of reasons. The yeast can grow to very high cell densities (>400 g/L wet weight) in cheap chemically defined media and is highly suited for large-scale cultivation in bioreactors (Cereghino et al., 2002). It can be easily genetically manipulated, similarly to S. cerevisiae, providing mitotically stable integration of vectors at desired loci in the genome without the need for selective pressure (Cereghino and Cregg, 2000). For overviews of available vectors and basic genetic methods the reader is referred to several reviews (Ahmad et al., 2014; Cereghino and Cregg, 2000; Felber et al., 2014; Lin-Cereghino and Lin-Cereghino, 2007; Sreekrishna et al., 1997). The availability of the P. pastoris genome (De Schutter et al., 2009; Küberl et al., 2011; Mattanovich et al., 2009; Valli et al., 2016) and recent synthetic biology tools for this yeast including CRISPR-Cas9 (Kang et al., 2017; Weninger et al., 2018; Weninger et al., 2016; Zahrl et al., 2017) allow efficient strain improvement.
The P. pastoris expression system is well-known for the high protein yields that are often obtained (Cregg et al., 2000; Romanos, 1995; Vogl et al., 2013). The highest currently published intracellular expression level in P. pastoris is ~22 g/L of culture (Hasslacher et al., 1997), and the highest reported secretory expression level is ~18 g/L of cell-free broth (Mellitzer et al., 2014). In general, quite many proteins, including protein polymers, are secreted by P. pastoris at g/L levels (Cregg et al., 2000). Because P. pastoris secretes relatively small amounts of endogenous proteins, secretory production constitutes a highly efficient first purification step (Cregg et al., 2000) and obviates the need for cell disruption procedures that are cost-prohibitive at an industrial scale. The yeast's eukaryotic secretory pathway furthermore allows posttranslational modifications essential for many therapeutic proteins, including glycosylation, disulfide bond formation, folding, and proteolytic processing (Cregg et al., 2000). Overall, for many researchers the capability of P. pastoris for efficient secretory production will be the main reason for choosing this host. Accordingly, most protein polymers have been produced in secretory fashion (Section 4), and also our ‘Challenges and possible solutions’ section focuses on secretory production (Section 5).
Over the years, P. pastoris has been established as an efficient industrial host. In 2006 the FDA conferred GRAS status to a protein produced in this host (Vogl et al., 2013), and P. pastoris itself is permitted by the FDA (21CFR573.750) as an additive for broiler feed at up to 10% of the formulation. In the context of therapeutic proteins, a clear advantage of the yeast over E. coli is the absence of endotoxins (Gorbet and Sefton, 2005). A considerable number of commercial proteins have been produced in P. pastoris (Julien, 2006; Meyer et al., 2008), which already includes a few protein polymers (4.2.1, 4.2.2).
3. Genetic engineering of protein polymers
One of the distinguishing features of protein polymers is their repetitive sequence and often modular architecture. Because this is directly reflected in the encoding genes, we will briefly introduce the genetic engineering approaches used in the field.
DNA sequences that encode natural protein polymers such as spider silk or collagen have typically been isolated by hybridization-based cDNA library screening or RT-PCR (Arcidiacono et al., 1998; Werten et al., 1999). However, for spider silks, mostly partial sequences have been obtained because of the exceptionally large transcript size (~10 kb or more) and high GC-content (Ayoub et al., 2007; Hayashi and Lewis, 1998). Furthermore, PCR of repetitive sequences can be very challenging (Scheibel, 2004) in view of the high probability for false priming by the primers or by incompletely extended fragments (Hommelsheim et al., 2014). Similarly, sequencing of long repetitive genes by common primer walking methodology is not well feasible. This is problematic because minor mutations can have a detrimental impact on the folding and function of some protein polymers, e.g., collagen (Persikov et al., 2004). With the advent of next-generation sequencing these issues are starting to become less of a concern, as evident from the cloning and sequencing of the first full-length spider silk gene (Ayoub et al., 2007) and the recent determination of the genome of the golden orb-weaver spider, Nephila clavipes (Babb et al., 2017). Still, if natural homologs are not required for the intended application, synthetic biomimetic genes may be more practical than natural sequences.
In the biomimetic approach, protein polymer sequences are oligomerized, in head-to-tail orientation, from a (preferably sequence-verified) synthetic DNA fragment (hereafter referred to as ‘monomer fragment’) that encodes several repeats of a specific amino acid sequence motif. These are often idealized consensus motifs derived from relatively abundant sequence patterns in natural polymer-like proteins. Besides biomimetic sequences, also completely artificial peptide sequences can be used, selected on the basis of combinatorial approaches or by rational design (DiMarco and Heilshorn, 2012; Lakshmanan et al., 2012). In materials science, the main motivation for using simple sequence motifs instead of complex natural sequences is to gain insight into the influence of amino acid sequence on three-dimensional structure and function, and to thereby potentially allow future control. By combining different protein sequence types, multifunctional block copolymers can be created (Section 4.5).
The monomer fragment is typically codon-optimized, which may be particularly important for protein polymers because their repetitive sequences contain large fractions of certain amino acids (e.g. ~33% Gly and ~20% Pro in the collagen helical domain). To prevent rapid depletion of tRNA molecules and consequent premature translation termination, the use of several frequently used codons per amino acid is probably preferred to exclusively using the predominant codon. Making use of the degeneracy of the genetic code in this manner furthermore reduces the repetitiveness of the polymer's encoding DNA sequence, which facilitates efficient gene synthesis and likely conduces genetic stability in the heterologous host. Another means of minimizing overall repetition is to use relatively long monomer fragments for oligomerization (Cappello and Ferrari, 1994; Fahnestock and Irwin, 1997). Although these design principles have been originally established for the production of protein polymers in E. coli (Cappello and Ferrari, 1994), most studies using P. pastoris as the host followed a similar approach and none of these reported genetic instability during culturing (Section 4). Fahnestock and Bedzyk (1997) did report the occurrence of genetic rearrangements in multicopy strains of P. pastoris, but only at the time of transformation and not during growth (see discussion in Section 4.1.1).
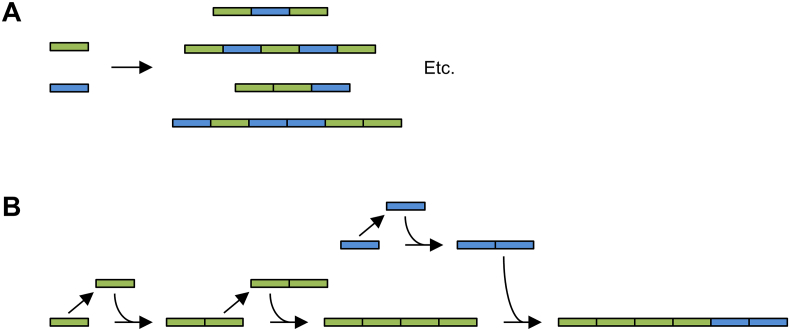
Various methods are available for the oligomerization of the monomer fragment. Although we will here shortly outline the general principles, the technical details are beyond the scope of this review and the reader is referred to the references below and a review by Mi (2006). Early oligomerization approaches involved random concatenation through self-ligation of the monomer fragment (Cappello et al., 1990; Ferrari et al., 1987; McGrath et al., 1990). Less commonly, also PCR-based random concatenation approaches have been used (Amiram et al., 2011; Chu et al., 2011; Kurihara et al., 2005). However, these methods offer no control over repeat number and block order (Fig. 1A). Such control is essential for the production of protein block copolymers with a defined size and a specific order of the functional modules (Section 4.5). Procedures that involve oligonucleotides or PCR furthermore pose a risk of introducing sequence errors, which, as discussed above, cannot readily be detected in long oligomeric genes. Therefore, techniques have been developed for the concatenation of sequence-verified monomer fragments via sequential restriction enzyme-mediated cloning steps (Fig. 1B). One such technique was developed by Kempe et al. (1985) and has been successfully used to construct protein polymers (Fahnestock and Irwin, 1997; Lewis et al., 1996; O'Brien et al., 1994). Another widely used sequential oligomerization technique was independently devised by our laboratory for the construction of synthetic gelatins (Van Heerde et al., 1998; Werten et al., 2001) and by the Chilkoti group for elastin-like sequences (Meyer and Chilkoti, 1999, Meyer and Chilkoti, 2002). The latter group aptly termed the procedure ‘Recursive Directional Ligation’ (RDL). Later implementations use class IIs restriction enzymes (Szybalski et al., 1991), which cut outside of their recognition sequence so that the oligomer can be completely free of cloning scars (McDaniel et al., 2010; Teeuwen et al., 2009b; Werten et al., 2008).
Fig. 1.
(A) Random concatenation results in a heterogeneous population. (B) Sequential concatenation methods allow the construction of block copolymers with defined repeat number and block order (vector backbones not shown). The example shows how insert size can optionally be doubled with every cloning step. Figure concept based on Chilkoti et al. (2002).
4. Protein polymers produced in P. pastoris
This section provides an overview of protein polymers produced in P. pastoris (summarized in Table 1). For each protein type, if applicable, we will first describe the properties of the natural polymer and shortly outline the historic context in terms of recombinant production in E. coli. Completely de novo-designed block copolymers produced in P. pastoris are summarized in Section 4.5.
Table 1.
Protein polymers produced in P. pastoris.a
| Protein | Molecular weight (kDa)b | Targeting | Culture | Yield (g/Lc) | Reference |
|---|---|---|---|---|---|
| Silk-like proteins | |||||
| Nephila clavipes MaSp1 mimics | 64/127d | I | S/B | 0.2–1 | Fahnestock and Bedzyk, 1997 |
| N. clavipes MaSp1 mimics | 64/127 | Ee | n/s | ≤6 μg/OD600 | Fahnestock et al., 2000 |
| N. clavipes MaSp1 mimics ± COL | ~15–30 | Ee | S | n/s | Teulé et al., 2003 |
| Nephila sp. MaSp1 and MaSp2 mimics | 94/113 | I | B | n/s | Bogush et al., 2009; Sokolova et al., 2010 |
| N. clavipes MaSp1 and MaSp2 mimics + NTD + CTD | 44 | E | B | n/s | Gaines and Marcotte Jr., 2011 |
| Euprosthenops australis MaSp1 mimic + CTD | 32 | Ee | S | n/s | Jansson et al., 2016 |
| Silk-inspired octapeptide repeats | 28–32 | E | B | 0.9–2.9f | Werten et al., 2008 |
| Gelatins (nonhydroxylated) | |||||
| Murine α1(I) and α1(III) fragments | 21–53 | E | B | 2–15f | Werten et al., 1999, Werten and de Wolf, 2005 |
| Artificial hydrophilic gelatin CP4 | 37 | E | B | 3–6f | Werten et al., 2001 |
| Human α1(I), α1(II), and α1(III) fragments | 5–90 | E | B | n/s | Olsen et al., 2003 |
| Human α1(I) fragment | 9 | E | B | 1.5f | Olsen et al., 2005 |
| Collagen (hydroxylated) | |||||
| Human type III | ~400g | Ee | S | 15 mg/L | Vuorela et al., 1997 |
| Human type I | ~400g | Ee | B | 0.2–0.5 | Nokelainen et al., 2001 |
| Human type I, II, and III | ~400g | Ee | B | 0.2–0.6 | Myllyharju et al., 2000 |
| Human type I, II, and III | ~400g | Ee | B | 0.7–1.5 | Olsen et al., 2003 |
| Human α1(I) fragments | 36–226g | Ee | S | n/s | Pakkanen et al., 2006 |
| Chondrosia reniformis nonfibrillar collagen | 210g | Ee | S | n/s | Pozzolini et al., 2015 |
| Elastin-like proteins | |||||
| V5A2G3–90, V5A2G3–40, V5L2G3–40 | 16–36 | E | B | 0.2–0.8h | Schipperus et al., 2009, Schipperus et al., 2012 |
| V4E1-105 | 47 | E | S | 2.5 mg/Lh | Sallach et al., 2009 |
| Other | |||||
| Human titin PEVK-region mimic | 54 | E | B | >1h | Tsai et al., 2012 |
| Various block copolymers | 38–115 | E | B | 0.4–6.5f | See Section 4.5 |
Abbreviations: MaSp, major ampullate spidroin; COL, amino-terminal Meloidogyne incognita collagen helical domain; NTD, amino-terminal spidroin domain; CTD, carboxy-terminal spidroin domain; XiYjZk-n, elastin-like protein with n Val-Pro-Gly-Xaa-Gly pentapeptides, where the capital letters specify the guest residue Xaa in the ELP sequence, and the subscripts indicate the number of each corresponding guest residue encoded by the DNA monomer fragment (Meyer et al., 2001); I, intracellular targeting; E, extracellular targeting; S, shake flask; B, bioreactor; n/s, not specified.
Refers to the expected molecular weight of the mature protein. For some articles this was calculated or estimated on the basis of the genetic details provided.
Except where noted, yields are given in grams of recombinant protein expressed per L of culture.
Owing to gene rearrangements during transformation, proteins in the size range of 48–261 kDa were obtained.
Retained or largely retained intracellularly despite extracellular targeting (not clearly specified for C. reniformis collagen).
Titer of secreted recombinant protein in the cell-free broth.
Molecular weight of the collagen trimer (trimer formation not demonstrated for C. reniformis collagen).
Yield of purified recombinant protein per L of cell-free broth (volume definition assumed for V4E1-105 and the titin PEVK-region mimic).
4.1. Silk-like polymers
The term “silk” is a functional description of protein fibers spun by arthropods such as silkworms, spiders, lacewings, dragonflies, crickets, and bees (Craig, 1997; Sutherland et al., 2010). Spinning refers to the extrusion of a liquid dope from a gland through a duct and spigot. This complex process involves flow, shear, and chemistry, ultimately resulting in the formation of an insoluble fiber (Heim et al., 2009). Silkworm silk has been used in the textile industry already for millennia and as a suture or wound dressing material for centuries. Spider silks display remarkable toughness (Hinman et al., 2000). The mechanical features and biocompatibility of silks (Altman et al., 2003; Hakimi et al., 2007) have sparked the interest to create recombinant silks. Besides their possible use as high-strength fibers in textiles and technical applications, these artificial silks may also be used to create high-value yarns, mats, films, microcapsules, foams, sponges, hydrogels, and implantable materials for application in regenerative medicine, coating of implants, and drug delivery (Aigner et al., 2018; Allmeling et al., 2013; Li et al., 2015; Widhe et al., 2012). Consequently, several startups are now endeavoring to mass-produce recombinant spider silks in a variety of hosts including undisclosed yeast species (DeFrancesco, 2017; Service, 2017). Below we will summarize the literature on two types of silk-like polymers that have been produced in P. pastoris, namely spider silk mimics and silk-inspired octapeptide repeats.
4.1.1. Spider silk mimics
Spider silks represent a fascinating family of fibrous proteins involved in prey capture and reproduction. At least seven types of silks are found in the golden orb-weaver spider, Nephila clavipes, each with different functions such as the orb web frame, the adhesive spiral, material for prey wrapping, etc. (Hinman et al., 2000). Dragline silk in particular is of interest, as it is thought to be nature's toughest polymer. On an equal weight basis it is tougher than steel and Kevlar, combining strength and extensibility (Tokareva et al., 2013). The mechanics of silk fiber strength have been extensively studied (Blackledge et al., 2012; Cetinkaya et al., 2011; Gosline et al., 1999).
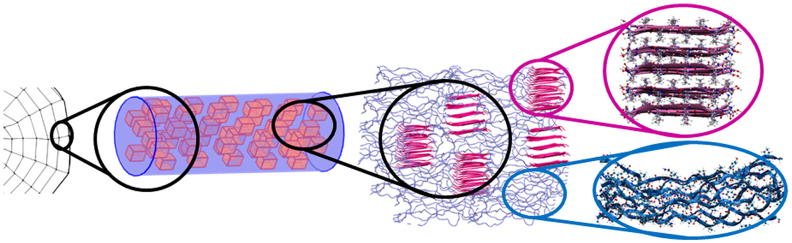
Dragline silk is secreted by specialized cells of the spider's major ampullate gland and contains two proteins, MaSp1 and MaSp2 for N. clavipes (formerly called spidroin 1 and 2; Vollrath and Knight, 2001). Both proteins have a molecular mass of >300 kDa and consist of three domains: a large repetitive middle domain responsible for the mechanical properties of the fiber and nonrepetitive N-terminal and C-terminal domains (Tokareva et al., 2013). The middle domain consists of repeats of highly similar modular units, with the following consensus sequences derived from cDNA sequences: GGAGQGGYGGLGGQGAGRGGLGGQ(GA)2A5 (MaSp1) and (GPGGYGPGQQ)2GPSGPGSA8 (MaSp2). In the actual silk fiber (Fig. 2), the poly-Ala sequences in MaSp1 and MaSp2 form ß-sheets that give rise to crystalline regions (Hinman et al., 2000). The Gly-Gly-Xaa and Gly-Pro-Gly-Xaa-Xaa of MaSp1 and MaSp2, respectively, form the fiber's elastic regions (Hinman et al., 2000). Whereas the crystalline regions provide strength, the amorphous regions likely provide extensibility (Gosline et al., 1999). How fibers are formed in the spiders' sophisticated spinning apparatus is beyond the scope of this review and has been the subject of several in-depth reviews (Heim et al., 2009; Rising, 2014; Vollrath and Knight, 2001). Replicating this process in vitro for the creation of strong fibers from artificial silk mimics remains to be one of the main challenges in the field (Doblhofer et al., 2015; Rising and Johansson, 2015).
Fig. 2.
Schematic representation of spider silk architecture at macro- to nanoscale. Spider silk fibers consist of crystalline ß-sheet domains (pink) and disordered elastic domains (blue). Reproduced from Cetinkaya et al. (2011) with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Several studies describe the production in E. coli of synthetic dragline silks, consisting of repeated spidroin consensus sequences. Prince et al. (1995) reported successful expression of N. clavipes dragline silk, in the size range of 15–41 kDa. MaSp1 oligomers with different repeat numbers were produced, as well as three block copolymers that feature both MaSp1 and MaSp2 sequences. Typical yields after purification were in the range of 2–15 mg/L of culture. Circular dichroism spectrometry showed that indeed the proteins had significant ß-sheet structure in aqueous solutions. Lewis et al. (1996) performed similar work and produced up to 32 repeats (112 kDa) of the MaSp2 consensus sequence. A 16-unit repeat was recovered at up to 10 mg/g wet cell weight, with typical yields of 1–2 mg/g. Fahnestock and Irwin (1997) of DuPont have produced 65–163 kDa analogs of MaSp1 and MaSp2 in E. coli. The authors oligomerized relatively long (>300 bps) DNA monomers, each encoding three to four variants of either consensus repeat, in an effort to minimize the repetitiveness of the overall gene (Section 3). The expression level in shake flasks was ~300 mg/L of culture, or ~15% of the total protein. The fraction of full-length products was limited to 47% for an 8-mer of the (codon-optimized) monomer and only 8.5% for a 16-mer, probably as a result of premature translation termination. Genetic instability also occurred, which produced internal gene deletions in roughly 10% of the genes in the population during a typical experiment, as well as a minor degree of gene expansions.
The same group also described the intracellular production of MaSp1 analogs in P. pastoris (Fahnestock and Bedzyk, 1997), which represents the first example of the use of this yeast for the production of a protein polymer. Contrasting the authors' results using E. coli, genes of >3000 codons could be expressed without truncated synthesis. Multicopy strains produced various lengths of the protein, differing by an integral number of repeats of the DNA monomer. As already announced in Section 3, presumably this was due to homologous recombination during transformation, where additional vector copies inserted out of phase into the repetitive silk sequence of the first copy that had already integrated into the genome. To our knowledge, there are no other examples in the literature of such an effect in P. pastoris, and also in our laboratory we have never seen this with similarly repetitive sequences. Possibly, this discrepancy is due to differences in the transformation procedures used. Whereas Fahnestock and Bedzyk (1997) used PEG-mediated spheroplast transformation and targeted for transplacement of the AOX1 locus, we use electrotransformation and usually target for insertion into the his4 locus. The latter approach has a much lower chance of spontaneous multicopy generation (Romanos et al., 1998) and thus poses less risk of artifacts such as described. Nonetheless, once transformed, the MaSp1 analog-producing strains were genetically very stable, in that there was no alteration of the protein size distribution during growth for at least 100 doublings (Fahnestock and Bedzyk, 1997). Thus, unlike what the authors observed in E. coli, internal deletions or expansions of the encoding genes during growth did not occur. Protein purification from bead-milled cells involved precipitation of host proteins consecutively at pH 5 and at 60 °C, followed by differential ammonium sulfate precipitation. The expression level in shake flasks was in the range of 0.2–1 g/L of culture. The authors concluded that P. pastoris is a superior host for the production of large spider silks and possibly also for other long repetitive sequences.
Fahnestock et al. (2000) additionally reported secretory production of MaSp1 analogs in P. pastoris, using three different secretion signals: the acid phosphatase signal sequence (hereafter Pho1; Sreekrishna et al., 1997), the prepro region of the S. cerevisiae α-factor mating pheromone (hereafter ppαF; Brake et al., 1984), and the pre region (signal peptide) of ppαF. In all cases some level of secretion was achieved, but most of the product accumulated intracellularly. The full ppαF leader and its mere signal peptide gave similar amounts of secreted protein, which appeared higher than obtained when using the Pho1 signal peptide. At ~6 μg of secreted recombinant protein per OD600 unit or less, the yields obtained were very low (1 OD600 equals ~1 g/L wet cell weight; Zhang et al., 2007).
Teulé et al. (2003) briefly reported on the use of P. pastoris for the production of ~15 kDa proteins consisting of repeated parts of the N. clavipes MaSp1 consensus. These proteins were constructed in such a way that the content of the alanine-rich motifs was normal, reduced, or zero. Although details were not provided, the culturing of P. pastoris was probably done in shake flasks. Despite the fact that ppαF was used to drive secretion, all product was retained intracellularly. According to Western blot analysis, secretion was achieved only when the spider silk sequence was fused to an ~1 kDa natural nematode-derived collagen sequence, which suggests improved overall solubility of the fusion proteins relative to the exclusively silk-like proteins. Protein purification was by ammonium sulfate precipitation or metal ion affinity chromatography. Yields were not reported.
Bogush et al. (2009) constructed a synthetic gene monomer containing five typical repeating units selected from N. clavipes Masp1 cDNA. Nine monomers were joined, resulting in a gene encoding a 94 kDa MaSp1 analog referred to as 1F9. A 113 kDa MaSp2 analog, referred to as 2E12, was similarly constructed by concatenating 12 monomers, each consisting of 5 quasi-repeats selected from Nephila madagascariensis cDNA. P. pastoris transformants were grown in methanol-fed bioreactor cultures (Sokolova et al., 2010). The intracellularly targeted proteins were purified from disrupted cells by ion-exchange chromatography and spontaneously formed nanofibrils and micelles of ~1 μm in aqueous solutions (Bogush et al., 2009). Fibers with a tensile strength of up to 0.15 GPa could be prepared by spinning the protein, which was dissolved in lithium chloride/formic acid, into an ethanol bath, followed by stretching, annealing, plasticization, and drying. Although no yields are provided, the authors mention that the production levels of MaSp2 in P. pastoris and S. cerevisiae were comparable (Bogush et al., 2011). However, whereas in the latter host 70–80% of MaSp2 was in the water-insoluble cellular fraction, in P. pastoris 60% was in the soluble fraction. The proteins are being developed into matrices for tissue engineering and drug delivery (Agapov et al., 2009; Moisenovich et al., 2016; Moisenovich et al., 2011; Nosenko et al., 2018).
Gaines and Marcotte Jr. (2011) very briefly described the production in P. pastoris of a 44 kDa mini-silk based on N. clavipes MaSp1. The protein consists of eight MaSp1 consensus repeats, the N- and C-terminal spidroin domains, and a C-terminal His-tag. It was targeted for secretion using ppαF. The expected product, besides a minor amount of C-terminally truncated protein, was detected by Western blot analysis in the clarified broth of methanol-induced bioreactor cultures and could be purified using ion-exchange chromatography. Although not specified, yields were arguably very low. The possibility of intracellular product retention was not investigated.
Jansson et al. (2016) used P. pastoris for the production of the spider silk-like protein Z-4RepCT previously produced in E. coli (Jansson et al., 2014). The 32 kDa protein consists of an IgG-binding Z-domain, four consecutive silk-like poly-Ala/Gly-rich repeats from Euprosthenops australis MaSp1, the C-terminal CT domain from the same spidroin, and a C-terminal (His)6 tag. The nonrepetitive CT domain had previously been shown to be a prerequisite for spontaneous fiber formation by 4RepCT (Stark et al., 2007). Methanol-induced shake flask cultures were used. Although the ppαF signal was employed to achieve protein secretion, approximately half of the protein was retained intracellularly. The secreted protein was partially degraded, which could be minimized to some degree by the use of protease inhibitors during culture and purification. The CT domain was found to be N-glycosylated at the Asn-Xaa-Ser/Thr site present and O-glycosylated at one or more serines. Probably because of this, secreted Z-4RepCT did not self-assemble into fibers, yet attempts to resolve this by enzymatic deglycosylation failed. The intracellularly retained protein was mostly present in the insoluble fraction and less degraded than the secreted protein. Protein purified from the soluble intracellular fraction did self-assemble into fibers, but only after enzymatic deglycosylation. Protein purification from cell-free medium or cells disrupted by high pressure homogenization was done using metal ion affinity chromatography. The protein yield was ambiguously defined, but reportedly similar to that obtained in E. coli (Jansson et al., 2016).
4.1.2. Silk-inspired octapeptide repeats
Besides spider silk mimics, also polymers inspired by the silk of the silkworm Bombyx mori have been produced using recombinant DNA technology. Cappello and Ferrari of Protein Polymer Technologies produced repeats of the hexapeptide GAGAGS from B. mori silk fibroin in E. coli and showed that the material produced adopted crystalline structures similar to the ß-sheet structures of natural silk (Cappello et al., 1990). The Tirrell group produced repeat proteins consisting of an artificially designed silk-inspired (GA)3GE octapeptide in E. coli (Krejchi et al., 1994). The repeating Gly-Ala dipeptides were mimicked after B. mori silk and other glycylalanine-rich proteins known to adopt β-sheet structures in the solid state. The choice of three Gly-Ala repeats was based on dimensional restrictions known from the crystallization behavior of synthetic polyamides and on the fact that a fibrous cross-ß protein from lacewing egg stalks folds with a periodicity of eight amino acids. Glutamic acid was inserted because its large size sterically prevents inclusion into the lamellar interior, its polarity and charge favor strong interaction with the environment, and it is considered the weakest β-sheet former of all natural amino acids. The protein, containing 36 repeats of the octapeptide, was expressed in E. coli and purified from the soluble fraction of the cell lysate by a procedure involving sequential pH adjustments. Purity level and yield were not reported. After dissolving the protein in 70% formic acid and subsequent crystallization in methanol, a crystalline anti-parallel ß-sheet structure could be demonstrated. A lamellar structure is formed by lateral stacking of the sheets, where the polymer chain folds back at the lamellar surfaces, as dictated by the Glu residues. In a later paper (Cantor et al., 1997), the authors studied the effect of amino acid side-chain volume by substituting Glu with Asn, Phe, Ser, Val, or Tyr. They found that, although the basic structure remains the same, the average intersheet stacking distance increased linearly with the volume of the substituting residue.
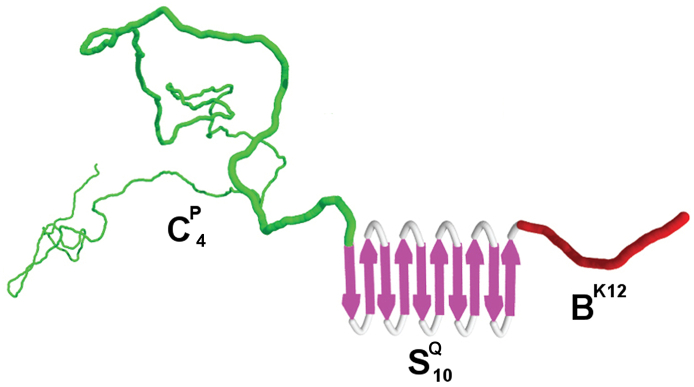
Our group chose the (GA)3GE design as a structural framework for the design of pH-responsive polymers for coating purposes (Werten et al., 2008). The silk-like nature and glutamate residues together convey self-assembling behavior at low pH. A 28 kDa protein with 48 repeats of the (GA)3GE motif, referred to hereafter as SE48, was produced in methanol fed-batch bioreactor cultures of P. pastoris. The protein was secreted using the ppαF leader. It formed a gel-like precipitate in fermentations performed at pH 3, which was resolved by using pH 5 instead. The product was fully intact according to mass spectrometry and N-terminal sequencing, and it was secreted at ~3 g/L of cell-free broth. The protein was purified essentially by a combination of isoelectric precipitation and subsequent removal of remaining host proteins by ethanol precipitation. An amphiphilic variant of this protein with 56 octapeptides was also produced, where every other glutamate residue was substituted with the highly hydrophobic amino acid leucine. The protein was monodisperse and secreted at ~1 g/L of clarified broth or more. Purification relied on the use of formic acid as a solvent to disrupt intra- and intermolecular protein interactions, followed by precipitation of host-derived proteins by dilution of the solubilized proteins with water. Upon adsorption, the purified polymer was able to render hydrophobic solid surfaces less hydrophobic and, conversely, hydrophilic substrates less hydrophilic. A more recently produced variant is SH48, which encodes 48 repeats of the octapeptide (GA)3GH. Essentially pure protein was obtained by ammonium sulfate precipitation, resulting in a recovery after dialysis of ~2 g/L of cell-free broth (unpublished). We have used several [(GA)3GX]n variants as a self-assembling module in various block copolymers capable of forming fibrils and hydrogels (Section 4.5).
4.2. Collagen-like proteins
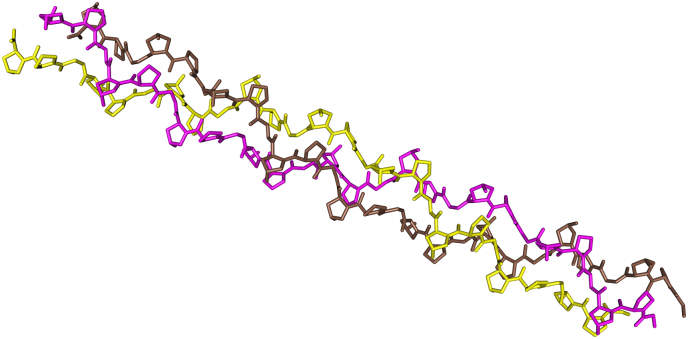
Although the family of collagens consists of multiple members, we will discuss only the most abundant, fibril-forming, collagens (types I-III, V, and XI). These extracellular matrix (ECM) proteins provide structure and strength to tissues such as skin, bones, tendons, and to internal organs (Gelse et al., 2003). Characteristic of collagen is its triple-helical structure (Fig. 3), where three polypeptide chains in extended helical conformation are coiled together. Each of the three chains consists of ~338 tandemly repeated Gly-Xaa-Yaa triplets, where Xaa and Yaa can be occupied by different amino acids. The occurrence of glycine as every third residue is a prerequisite, because only this smallest of amino acids can fit inside the triple helix interior. The Xaa and Yaa positions are frequently occupied by prolines, which limits rotation of the protein chains (Prockop and Kivirikko, 1995). Approximately half of the prolines in the Yaa position are posttranslationally modified to 4-hydroxyprolines by the enzyme peptidyl-prolyl-4-hydroxylase (P4H; Myllyharju, 2003), which allows the formation of additional hydrogen and water bridges, thereby stabilizing the triple helix at physiological temperature. Each collagen chain also contains N- and C-terminal propeptides, which allow for mutual recognition and in-register alignment of the three chains for subsequent zipper-like folding into the collagen triple helix. After secretion, the propeptides are cleaved off by specific proteases, upon which collagen self-assembles into fibrils (Prockop and Kivirikko, 1995) and higher-order fibers.
Fig. 3.
Structure of the collagen triple helix. Shown is a trimeric (Gly-Pro-Hyp)9 collagen model peptide (PDB: 3B0S; Okuyama et al., 2012), where Hyp is 4-hydroxyproline. Visualized using Cn3D (Wang et al., 2000).
Gelatin is essentially denatured collagen and is prepared by hot acid or alkaline extraction of animal tissues such as bones and hides (Asghar and Henrickson, 1982). The well-known gelling properties of heated and subsequently cooled gelatin are due to the random formation of triple-helical patches between neighboring molecules.
Collagen and gelatin are important biomaterials owing to their physical properties, excellent biocompatibility, and weak immunogenicity. Both are used in a variety of medical applications such as drug delivery, wound care, intravenous infusion, vaccine stabilization, and tissue engineering (Lee et al., 2001; Su and Wang, 2015). However, their animal origin poses risks for allergic reactions or transmission of disease-causing agents. Furthermore, since natural collagen fibrils are extensively crosslinked, the age and physiology of the animals used as source affect the physical properties of the material, leading to high batch-to-batch variation. In the case of gelatins, the extracted material consists of a variable mixture of chemically modified peptides covering a wide mass range (Asghar and Henrickson, 1982).
Several groups, therefore, endeavored to produce recombinant alternatives. Early work involved the use of E. coli. For example, Goldberg et al. (1989) produced a collagen-like sequence (Gly-Pro-Pro)32. Expression levels were not reported but appear to be very low. Similar approaches for the production in E. coli of tandemly repeated short collagen-like sequences were followed by Obrecht et al. (1991) and Gardner et al. (1992). Hori et al. (2002) produced fragments of natural bovine α2 (I) collagen up to 245 amino acids using E. coli. Yields were not reported, but were at least sufficient for use in ELISA tests. It should be noted that, because of the absence of a native P4H in prokaryotes, these fragments cannot form stable triple helices and are thus only collagen-like with respect to their sequence; they are single-chain collagen fragments. Attempts at coexpressing active P4H in E. coli proved problematic (Ramshaw et al., 2014), although Pinkas et al. (2011) were able to achieve hydroxylation of short (Pro-Pro-Gly)n peptides with n = 5, 7, and 10. In the next two sections, we will describe the use of P. pastoris for the production of gelatin and collagen.
4.2.1. Gelatin
Our group produced single-chain fragments of murine α1(I) and α1(III) collagen in P. pastoris (Werten et al., 1999), which we will hereafter refer to as Col1a1 and Col3a1, respectively. We termed these proteins ‘gelatins’ because they resemble denatured collagen, although, as desired for the intended application, they do not actually form gels owing to the absence of endogenous prolyl-4-hydroxylation in P. pastoris. The strains were grown in methanol fed-batch fermentations, and the proteins of 21–53 kDa were secreted using the ppαF leader. Although the yield of full-length Col1a1 was improved by supplementing the medium with casamino acids, the product still appeared as four major bands in SDS-PAGE, besides some minor background. The major bands were shown to result from endoproteolysis at specific sequence motifs, which was resolved by site-directed mutagenesis, or by using a protease-deficient strain (Section 5.2.1). Col1a1 was produced at several grams per liter of cell-free broth. Although Col3a1 was severely degraded in cultures grown at pH 5, this could be largely overcome by using pH 3 instead. Col3a1 expression levels were in the range of several grams per liter of clarified broth for single-copy transformants and went up with increasing copy number. A strain with an estimated 15 copies of the gene produced the polymer at a level of ~15 g/L of cell-free broth, which is among the highest heterologous protein titers reported for P. pastoris. Interestingly, no Col3a1 product could be detected when we used the Pho1 secretory signal or attempted intracellular production (unpublished). Originally conceived and developed by us for Fujifilm Corporation with a view to photography applications (De Wolf et al., 2000; Toda et al., 2002; Van Heerde et al., 1998), the company also used this technology as a basis for developing their commercial product for regenerative medicine, cellnest™ (Mumcuoglu et al., 2018; Parvizi et al., 2016; Tuin et al., 2010; Tuin et al., 2012).
In addition to gelatins with natural collagen-derived sequences we developed a fully de novo-designed gelatin CP4 (previously named P4; Werten et al., 2001). The sequence consists entirely of the Gly-Xaa-Yaa triplets typical for collagen, but was designed to be highly hydrophilic by exclusion of hydrophobic residues (other than proline) and inclusion of ~36% of polar amino acids such as asparagine, glutamine and serine. The protein consists of four repeated 99-residue long CP blocks (polar random coil). The nonhydroxylated ~37 kDa protein was secreted by P. pastoris using the ppαF leader and was completely monodisperse and expressed at 3–6 g/L of clarified broth in methanol fed-batch fermentations. The protein could be efficiently purified to >98% purity by simple ammonium sulfate precipitation. We found this to be a generally applicable strategy in P. pastoris for the purification of gelatins, including Col3a1 and Col1a1 mentioned above, or block copolymers that contain CP4-derived modules (Section 4.5). This is because, at least when using basal salts medium with PTM1 trace elements (Stratton et al., 1998), secreted P. pastoris host proteins and exopolysaccharides do not precipitate at up to 80% saturation, whereas gelatin-like proteins precipitate efficiently at around 40% saturation (Werten et al., 2001). At 100–1000L pilot-scale, we use crossflow membrane filtration instead of centrifugation to harvest the precipitate, which is then washed with ammonium sulfate solution, eluted with water, and subsequently concentrated and desalted using crossflow ultra−/diafiltration (Werten et al., 2001; unpublished). Circular dichroism spectrometry showed that the nonhydroxylated CP4 gelatin had random coil conformation over a wide temperature and pH range, as desired for our intended applications. It acts as a cytophilic protein in human cell culture (Rozkiewicz et al., 2006) and shows excellent biocompatibility as a plasma expander (Bouwstra and Toda, 2005). We have later used CP4 as a random coil constituent of many block copolymers (Section 4.5).
FibroGen Inc. followed the same approach as outlined above for murine Col3a1 and Col1a1. They produced gelatins in P. pastoris based on human α1(I) collagen chains in the range of 5–90 kDa having different pI from 4.6–10.0 and also α1(II) and α1(III) collagen fragments (Olsen et al., 2003). Most of their studies involve a small gelatin α1(I) fragment of 8.5 kDa with a pI of 9.4, for use as a stabilizer for biologics such as vaccines (Olsen et al., 2003, Olsen et al., 2005; Thyagarajapuram et al., 2007). The protein was expressed in bioreactors at ~1.5 g/L of cell-free broth and purified by ion-exchange chromatography (Olsen et al., 2005).
4.2.2. Collagen
To obtain triple-helical collagen, incorporation of 4-hydroxyprolines is required. The Myllyharju group has done extensive work on the production of triple-helical collagen in P. pastoris through coexpression of human P4H (Vuorela et al., 1997). P4H is an α2β2 tetramer, where the β subunit is the ER-resident protein disulfide isomerase (PDI). Expression of only the α subunit did not lead to P4H activity, indicating that the α subunit does not form an active enzyme with P. pastoris PDI. When both the α and ß subunits were expressed, low levels of active tetramer were obtained. However, on simultaneous coexpression with full-length prepro-α1(III) collagen chains (including the native signal peptide and N-/C-terminal propeptides), 10-fold higher P4H assembly levels were obtained. The total collagen expression level in shake flasks was ~15 mg/L of culture, of which approximately 80–90% was retained intracellularly. Trimeric collagen was purified from the soluble fraction of cell lysates by digestion with pepsin, which digests propeptides and host proteins but not triple-helical α1 chains, followed by size-exclusion chromatography. The degree of prolyl-4-hydroxylation relative to that of natural type III collagen was ~85%.
To study the observed nonsecretion of trimeric collagen in P. pastoris, immunoelectron microscopy was used (Keizer-Gunnink et al., 2000). This showed that procollagen accumulated in membranous vesicular compartments closely associated with the nuclear membrane. The authors conclude that triple-helical collagen accumulates in the ER and cannot proceed further down the secretory pathway. In an attempt to solve this, the authors replaced the native human signal peptide of type III collagen with the commonly used and often efficient ppαF secretory leader. However, this only led to a minor increase in the fraction secreted and was accompanied by a roughly 50% lower total amount of product.
Upon coexpression in P. pastoris of the proα1 and proα2 chains of type I procollagen, correctly self-assembled trimeric type I procollagen was obtained with the appropriate 2:1 ratio of the two chain types (Nokelainen et al., 2001). As expected, a fraction of the molecules also formed α1 homotrimeric molecules. Chains lacking the N-propeptide likewise correctly formed trimeric collagen but were expressed at a 1.5–3-fold higher levels. As before, the trimeric procollagen produced was intracellularly retained, at levels of 200–500 mg/L of culture in bioreactors. The use of pure oxygen supplementation in these cultures led to increased hydroxylation levels as compared to the above-mentioned earlier work with shake flasks, and the determined 4-hydroxyproline content matched that of natural type I collagen. Similarly to native collagen, mature collagen produced from the recombinant procollagen molecules by pepsin digestion formed fibrils in vitro. The group also reported the production of type II collagen (Myllyharju et al., 2000) and of shortened α1(I) chains with only the C-terminal propeptide or a C-terminal foldon domain from bacteriophage T4 fibritin for trimerization (Pakkanen et al., 2006). The shortened α1(I) variants were constructed to further investigate collagen nonsecretion (see also Section 5.3.2).
A review by FibroGen Inc. (Olsen et al., 2003), a company that partly funded the above work of the Myllyharju group, reports that the initial expression level of ~15 mg/L of culture originally reported for type III collagen in P. pastoris (Vuorela et al., 1997) was successfully increased via, unspecified, genetic means and process improvements. Collagen types I, II, and III expression levels eventually reached 1.1, 0.7, and 1.5 g/L of culture, respectively. An unusually high fermentation temperature of 32 °C was found to be beneficial for efficient hydroxylation, although this also lowered the overall collagen yield (Baez et al., 2005).
Merrett et al. (2008) conducted a phase 1 clinical study using cell-free hydrogel implants prepared from FibroGen's commercially available P. pastoris-produced collagen by carbodiimide crosslinking. The implants were intended as mimics of the largely collagenous corneal ECM, to stimulate corneal regeneration. Over four years, the implants remained stably integrated without the need for immunosuppression, and patients had improved vision from the preoperative level (Fagerholm et al., 2014). As one of few examples of protein polymers that reached the clinical testing stage, this attests to the utility of P. pastoris in this field.
P. pastoris has also been used for the production of hydroxylated nonfibrillar collagen from the marine sponge Chondrosia reniformis (Pozzolini et al., 2015). The genes encoding the α and ß subunits of P4H and collagen were all derived from this organism and were coexpressed in shake flask cultures of P. pastoris. The authors followed the example of Vuorela et al. (1997) and replaced the native secretion signal of the ß subunit of P4H with the ppαF leader. Hydroxylated collagen could be detected intracellularly, where 36% of all prolines were hydroxylated. Hydroxylation took place in both the Xaa and Yaa position of Gly-Xaa-Yaa triplets, which differs from the Yaa-specific activity of human P4H. Interestingly, the α subunit of P4H alone had an enzymatic activity of 54% relative to the tetramer, suggesting that it can either partially interact with the yeast's PDI, or does not require a β subunit. Actual formation of collagen trimers and yields were not reported.
4.3. Elastin-like polymers
Elastin is a major component of the ECM and forms the core of the elastic fibers that provide elasticity to tissues such as lungs, skin, and arteries (Van Eldijk et al., 2012). It is initially produced as tropoelastin in elastogenic cells. This is a soluble protein of 60–72 kDa, depending on the isoform, and it consists largely of alternating hydrophobic and hydrophilic regions (Gacko, 2000). The hydrophobic regions contain mainly Gly, Val, Ala, and Pro, which are frequently present as tetra-, penta-, and hexapeptides (Foster et al., 1973; Gray et al., 1973). The hydrophilic domains are Ala-rich stretches with interspersed Lys residues and function as crosslinking domains. Once synthesized and translocated into the lumen of the ER, tropoelastin is bound by an elastin-binding peripheral membrane protein (EBP) to prevent intracellular self-aggregation and degradation (Hinek and Rabinovitch, 1994). The elastin-EBP complex then moves through the Golgi to the cell surface, upon which the complex dissociates and tropoelastin is released (Mecham, 1991). Tropoelastin molecules then aggregate into globules on the cell surface through coacervation (Kozel et al., 2006). These globules are transferred to microfibrils in the extracellular space and fuse into larger fibrillar structures. In this process, tropoelastin monomers are crosslinked by the enzyme lysyl oxidase via the lysine residues in the hydrophilic regions, resulting in the insoluble elastin polymer. The elasticity of elastin, like that of rubber, is thought to be entropy-based: entropy decreases upon stretching, and recoil is driven by a return to maximal entropy (Vrhovski and Weiss, 1998).
The coacervation of tropoelastin monomers is caused by the protein's inverse temperature transition (ITT) behavior, or lower critical solution temperature (LCST) behavior. This behavior is well-known for many aqueous solutions of traditional chemically synthesized polymers such as poly(N-isopropylacrylamide) (Boutris et al., 1997). When a tropoelastin solution is heated above its transition temperature, phase separation occurs. This process is completely reversible upon cooling. At low temperature, water surrounds hydrophobic patches in a clathrate-like manner, keeping the protein unfolded. Upon a raise in temperature, the ordered water structure is disrupted and protein folding occurs through interactions between the hydrophobic domains. Although the protein structure becomes more ordered, the overall entropy increases because of the release of water (Urry and Long, 1977).
To gain more understanding of the inverse temperature transition behavior of tropoelastin, Urry et al. (1974) developed chemically synthesized model oligomers based on amino acid repeat sequences found in the hydrophobic domains of tropoelastin. Several of these synthetic polypeptides showed thermoresponsive behavior similar to that of tropoelastin (Urry, 1988, Urry, 1992), and they follow general rules of polymer (LCST) phase behavior. The most studied repeat sequence is the pentapeptide VPGXG, where X can be any amino acid except proline. A hydrophobic residue at the X position, or high molecular mass, results in a low transition temperature, whereas a hydrophilic residue at the X position, or low molecular mass, results in a high transition temperature (Urry et al., 1991a, Urry et al., 1991b, Urry et al., 1992). By using a charged amino acid at the X position, pH-dependent transition behavior is obtained (Urry et al., 1992). The transition is also dependent on protein and salt concentration, where high concentrations of either have a lowering effect on the transition temperature (Luan et al., 1991; Meyer and Chilkoti, 2004). The dependence on protein concentration is reduced in longer molecules (Meyer and Chilkoti, 2004).
Recombinant elastin-like polypeptides (ELPs) are usually produced in E. coli (Cappello et al., 1990; McPherson et al., 1992). There is considerable biomedical interest in these materials in view of their biocompatible properties (Urry et al., 1991b) and tunable transition temperature, with possible applications mainly in controlled drug delivery and tissue engineering (for reviews, see Arias et al., 2018; MacEwan and Chilkoti, 2010; Nettles et al., 2010). The yield of ELPs in E. coli was markedly improved by relying on leaky transcription by the T7 promoter and culturing for extended periods, rather than using standard isopropyl β-D-1-thiogalactopyranoside (IPTG) induction (Guda et al., 1995). In this manner, yields in shake flasks of ~200–400 mg/L of culture have been obtained (Trabbic-Carlson et al., 2003). Purification is mostly done by exploiting the reversible phase transition (McPherson et al., 1996), in a procedure known as inverse transition cycling (ITC; Meyer and Chilkoti, 1999). This purification method involves repeated cycles of (i) aggregation of the ELPs at high temperature and/or high salt, followed by centrifugation, and (ii) resolubilization of the pellet at low temperature and low ionic strength, followed by centrifugal removal of any insoluble matter (McPherson et al., 1996; Meyer and Chilkoti, 1999). Other hosts than E. coli have been employed, including tobacco cells (Zhang et al., 1995), Aspergillus nidulans (Herzog et al., 1997), tobacco plants (Guda et al., 2000), and S. cerevisiae (Toonkool and Weiss, 2001). In all these studies the ELPs were produced (or inadvertently retained) intracellularly.
We first reported the production of an elastin-like protein in P. pastoris, which also represents the first example of secretory ELP production in any host (Schipperus et al., 2009). The ELP gene introduced into the yeast encodes 90 VPGXG pentapeptide repeats, where the X position is occupied in a quasi-random order by valine, alanine, and glycine, at 50%, 20%, and 30%, respectively. Following the nomenclature of the Chilkoti group (Meyer et al., 2001; see also legend to Table 1), we will hereafter refer to this ELP as V5A2G3–90 and use analogous notation for all other ELPs. Bioreactor cultures were run in methanol fed-batch mode, and the ~36 kDa V5A2G3–90 was purified from the cell-free broth by ITC. Within the experimental range of pH 3–7, the yields of purified protein surprisingly increased from 0 to 410 mg/L of cell-free broth with increasing culture pH. This was shown not to be related to proteolysis or differences in ITC efficiency at different pH values. Because at pH 7 the protein was partially degraded, the optimal pH for production was established at 6, with a yield of purified intact V5A2G3–90 of 255 mg/L of cell-free broth. The transition temperature of the protein as determined by turbidity measurements was in good agreement with previously reported values.
We also produced two shorter ELPs in P. pastoris (at pH 6; Schipperus et al., 2012). Variant V5A2G3-40 has the same composition as V5A2G3-90, yet features only 40 VPGXG repeats and consequently has a higher transition temperature. Variant V5L2G3-40 is identical to V5A2G3-40, except that all alanines in the X position of the pentapeptide repeats were replaced by the hydrophobic residue leucine. As expected, the transition temperature of V5A2G3-40 measured over a wide concentration range under physiological conditions was considerably higher, by 10–20 °C, than that of both V5A2G3-90 and V5L2G3-40. Whereas V5A2G3-40 was recovered by ITC at 755 mg/L of cell-free broth, the yield of the hydrophobic V5L2G3-40 was only 37 mg/L and it was partially degraded. Interestingly, at a low growth temperature of 20 °C the yield of V5L2G3-40 was roughly six times higher than at 30 °C, and the protein was fully intact. See Section 5.3.3 for a discussion on the effects of both pH and transition temperature on ELP yields.
The Conticello group produced an ELP in P. pastoris consisting of 21 repeats of the amino acid sequence (VPGVG)2VPGEG(VPGVG)2 (Sallach et al., 2009). This ~47 kDa V4E1-105 ELP includes a C-terminal c-Myc tag and a His-tag that was used for purification. Inverse transition behavior of the ELP produced was not studied, but under physiologically relevant conditions the protein is known to be present as a water-soluble flexible polymer (Sallach et al., 2009). The yield after metal ion affinity chromatography was 2.5 mg/L in shake flask cultures. Analysis of the cell lysate revealed no significant amounts of intracellularly retained ELP.
4.4. Titin
The muscle protein titin, the largest protein known (~4 MDa; Meyer and Wright, 2013), provides elasticity and structure to the sarcomere. The elastic part of the protein contains two types of extensible segments: regions containing tandem repeats of ~100 residue immunoglobulin-like domains connected by short linkers and intrinsically disordered regions including the so-called PEVK domain (Linke and Hamdani, 2014). The PEVK domain consists of tandem repeats of 28-residue sequences consisting primarily of proline, glutamate, valine, and lysine. Depending on the muscle tissue the PEVK region varies in length from <200 to 2174 residues (Meyer and Wright, 2013). According to the most accepted model of titin elasticity, low stretch forces straighten the linkers between the tandemly repeated immunoglobulin-like domains, causing the immunoglobulin-like region to elongate, whereas intermediate to high physiological stretch forces mainly unfold and extend the PEVK segment (Linke and Hamdani, 2014). At physiological force levels, unfolding of the immunoglobulin-like domains plays only a minor role. Although titin is often described as an entropic spring, where a force is needed to overcome entropic recoiling, nonentropic factors such as electrostatic and hydrophobic interactions of the PEVK region also play a role in the protein's elasticity (Linke and Hamdani, 2014).
Tsai et al. (2012) produced a PEVK analog in P. pastoris, as well as in E. coli and Sf9 insect cells (Spodoptera frugiperda). The protein consists of 15 identical repeats of a 28-residue long sequence (PEPPKEVVPEKKAPVAPPKKPEVPPVKV), derived from human titin exon 172. It is flanked at both termini by multiple tags that were included for purification, detection, and to allow single-molecule techniques such as AFM and Laser Tweezers. Random recombination of the repetitive genes occurred during all stages of cloning in E. coli. During protein production in E. coli, the protein was found to be toxic to the cells, and the use of a low growth temperature, inclusion of glucose in the media, and mid-log phase IPTG induction were required (Tsai et al., 2012). The yield of purified protein in shake flasks was only 2 mg/L of culture. Whereas SDS-PAGE of the protein produced in Sf9 insect cells showed only one band after 24h of culture, massive ladder formation was observed at 48 and 72h. These secondary species, of both lower and higher mass than expected, were attributed to recombination events (Tsai et al., 2012).
The protein was produced in P. pastoris using both secretory and intracellular expression (Tsai et al., 2012). The results of intracellular production are scarcely discussed, and the authors mention that secretory production was most successful. Indeed, in bioreactor cultures this approach resulted in a yield of purified protein of >1 g/L, and the protein appeared largely intact in SDS-PAGE. Purification involved ammonium sulfate precipitation and hydroxylapatite chromatography.
Thus far, the interest in engineered titin segments lies mainly in basic research, where it represents the foremost model for protein unfolding/refolding in response to cellular mechanical stress (Linke and Grutzner, 2008).
4.5. De novo-designed block copolymers
In a general sense, block copolymers are polymers consisting of two or more chemically distinct and covalently linked homopolymeric modules. Block copolymers have attracted much attention because the combination of different types of physical behavior in one molecule can result in interesting new properties. When the block types used are mutually poorly miscible, the block copolymer can undergo ‘microphase separation’ (Lohse and Hadjichristidis, 1997). That is, two-phase macroseparation is not possible because the blocks are covalently linked, and instead periodic nanostructures may be formed. A well-known chemically synthesized commercial polymer with such properties is Kraton™ D SBS, a triblock copolymer with polystyrene end blocks and an elastomeric polybutadiene middle block (Holden et al., 1969).
Nature has devised its own protein block copolymers, just one intriguing example of which is the mussel byssus protein preCol-P, which consists of a central collagenous domain, flanking elastic domains, and histidine-rich terminal domains (Coyne et al., 1997). Similarly, and contrasting common chemical synthesis, genetic engineering allows direct sequential ordering of the functional blocks in a protein polymer through sequential ordering of the encoding DNA fragments in the gene template (Rabotyagova et al., 2011; see also Section 3). The first example of recombinant protein block copolymers was reported by the group of Cappello and Ferrari (Cappello et al., 1990). The authors produced block copolymers in E. coli where crystalline (GAGAGS)n blocks were alternated with noncrystalline GAAGY or elastin-like (VPGVG)n blocks. Many more protein block copolymers have been produced using E. coli. Just a few examples are copolymers combining different elastin-like blocks (Meyer and Chilkoti, 2002; Wright et al., 2002), different spider silk sequences (Prince et al., 1995), and a random coil middle block with flanking associative coiled-coil domains (Petka et al., 1998; Xu et al., 2005). Besides blocks that impart particular physical properties or trigger-responsiveness, also bioactive modules can be incorporated for the creation of multifunctional materials for biomedical applications. This includes modules for cellular adhesion, growth factor activity, mineralization, proteolytic degradation, antimicrobial activity, etc. (DiMarco and Heilshorn, 2012; Liu, 2016).
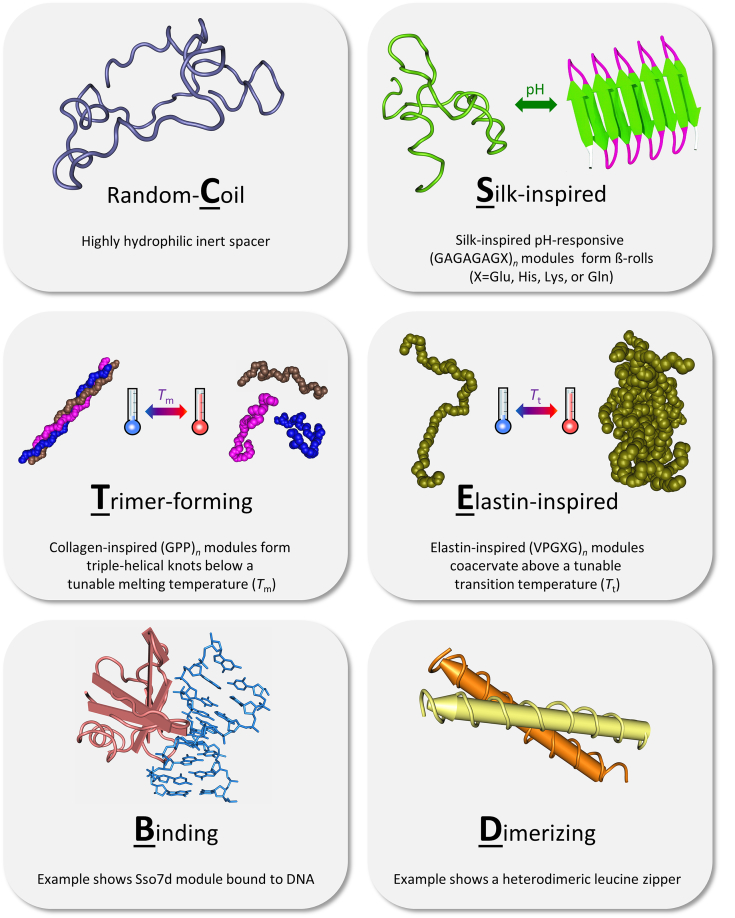
Below we will list de novo-designed block copolymers produced in P. pastoris, so far exclusively pursued by our group. Our primary interest in most of these molecules lies in basic nanomaterials research, but, where mentioned, some are being further developed towards biomedical applications. For reference, Fig. 4 provides an overview of the properties and nomenclature of the main block types used, and Table 2 lists the derived block copolymers. All polymers were produced as secreted proteins using the ppαF leader in methanol fed-batch fermentations. Unless otherwise noted, fermentations were at pH 3 and 30 °C in basal salts medium with PTM1 trace elements (Stratton et al., 1998), using P. pastoris strain GS115 and a constant ~0.2% (w/v) methanol level in the broth during the induction phase (Werten and de Wolf, 2005). Also unless otherwise noted, purification was essentially based on ammonium sulfate precipitation (Section 4.2.1).
Fig. 4.
Nomenclature and general properties of the modules used in the block copolymers described in this section. Underlined letters indicate the block codes used. Block copolymer names in Table 2 and the main text, written in bold face, reflect the block order from N- to C-terminus. Each block is written as Xsn, where X is one of the single-letter codes depicted above, s is the block subtype, and n is the number of repeats for each block (the repeating units for C, S, T, and E consist of 99, 8, 3, and 5 residues, respectively). E.g., SE12-CP4-E40 is a block copolymer consisting of an N-terminal silk-inspired 12-mer of subtype E, a random coil tetrameric spacer of subtype P, and a C-terminal elastin-inspired 40-mer. Block subtypes are described in the main text. The collagen-inspired peptide (PDB: 2CUO), Sso7d/DNA complex (PDB: 1BBX), and leucine zipper pair (PDB: 1FMH) were visualized using Cn3D (Wang et al., 2000). The image of the silk-inspired β-roll was adapted from Zhao et al. (2016) with permission from the Royal Society of Chemistry.
Table 2.
De novo-designed block copolymers produced in P. pastoris. All proteins were recovered from the extracellular medium.
| Block copolymera | Molecular weight (kDa) | Yield (g/L)b | Reference |
|---|---|---|---|
| Silk-inspired and pH-responsive | |||
| CP2-SE48-CP2/SE24-CP4-SE24 | 66 | 4.5–6.5c | Martens et al., 2009 |
| CP2-SH48-CP2/SH24-CP4-SH24 | 66 | 6.1c | Beun et al., 2012; Golinska et al., 2014; Yan et al., 2008, Yan et al., 2009 |
| CP2-SHn-CP2; n = 8, 16, 24 | 43/48/52 | >2 | Beun et al., 2014 |
| CP2-SK48-CP2/SK24-CP4-SK24 | 66 | >1 | Beun et al., 2012; unpublished |
| BRGD-CP2-SH48-CP2/BKRSR-CP2-SHn-CP2 | 68 | 3.2/3.5 | Włodarczyk-Biegun et al., 2016a |
| Collagen-inspired and thermoresponsive | |||
| T9-CP4-T9/T9-CR4-T9 | 42 | 3–6c | Werten et al., 2009 |
| T9-CPn-T9/T9-CRn-T9; n = 8, 12 | 78/115 | 1–3c | Teles et al., 2010a; unpublished |
| Tn-CP4-Tn/Tn-CR4-Tn; n = 6, 12, 16 | 40/43/45 | 3–6/0.6/0.4c | Silva et al., 2011, Silva et al., 2012 |
| Multi-responsive | |||
| SE24-E40/SE12-CP4-E40 | 30/60 | >0.5 | Golinska et al., 2013 |
| CP2-SH48-CP2-DC | 66 | 3 | Rombouts et al., 2016 |
| T9-CR4-T9-DC | 42 | 0.4 | Pham et al., 2013a |
| T9-CR4-BH6 | 40 | 0.5 | Pham et al., 2013b |
| T9-CR4-BK6 | 40 | 0.4 | Pham et al., 2016 |
| SH8-CR4-T9 | 44 | 2 | Rombouts et al., 2015 |
| DNA-binding | |||
| CP4-BK12/CP4-B(HK)6 | 38 | 1 | Hernandez-Garcia et al., 2012 |
| CP8-BSso7d | 80 | 0.7 | Hernandez-Garcia et al., 2016 |
| CP4-SQn-BK12; n = 2, 4, 10, 14 | 40–47 | 0.5d | Hernandez-Garcia et al., 2014 |
| Featuring heterodimerizing modules | |||
| CP4-DA/CP4-DB/T9-CP4-DA/T9-CP4-DB | 43–45 | 0.7–1.3e | Domeradzka et al., 2016a |
| CP2-SH48-CP2-DA/CP2-SH48-CP2-DB | 72 | 2.3/1.4 | Domeradzka et al., 2016b |
| CP4 − DWW/CP4 − DPPxY | 41/39 | 2.2/2.3 | Domeradzka et al., 2016c |
See Fig. 4 for block copolymer nomenclature. Different polymer names may have been used in the cited articles.
Except where noted, yields are given in grams of recombinant protein recovered per L of cell-free broth after purification, dialysis, and lyophilization. Note that significant (>50%) protein loss may occur particularly during dialysis owing to the linear shape of the polymers.
Titer of secreted recombinant protein in the cell-free broth, as determined by SDS-PAGE and calibrated densitometry.
Titer of secreted recombinant protein in the cell-free broth, as estimated by SDS-PAGE.
Equivalent to the yield after purification of 0.5–0.6 g/L of culture mentioned in the cited article.
4.5.1. Silk-inspired pH-responsive block copolymers
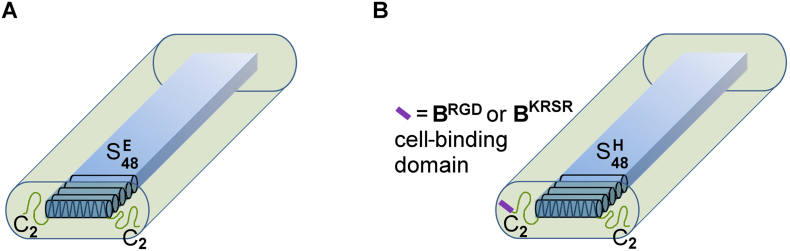
The first block copolymer we produced in P. pastoris consists of two modules mentioned above, namely the silk-like protein SE48 consisting of 48 repeats of the sequence (GA)3GE (Section 4.1.2) and two repeats of the 99-residue hydrophilic random coil block CP (Section 4.2.1). The protein, referred to as CP2-SE48-CP2, is a triblock where the silk-inspired sequence SE48 is used as the middle block, flanked by noninteracting CP2 blocks (see Fig. 5A; Martens et al., 2009). At neutral pH, the middle block is soluble owing to repulsion by the negatively charged glutamates. At low pH approaching pI, the charges are neutralized and the silk-like domain forms a β-roll or β-solenoid structure with the hydrophobic side groups facing inwards (Zhao et al., 2016, Zhao et al., 2017). These structures stack through hydrogen bonding and form the core of micrometer-long fibrils. The hydrophilic random coil end blocks surround the core and provide colloidal stability. A CP2-SH48-CP2 variant with histidines instead of glutamates was also prepared, which features charge neutralization and fibril formation at neutral pH (Golinska et al., 2014; Yan et al., 2008, Yan et al., 2009). The fibrils form gels, probably via entanglement or weak physical crosslinking. The histidine-containing triblock copolymer was also produced with middle blocks consisting of only 8, 16, or 24 octapeptides, where the first two variants formed spherical micelles rather than fibrils (Beun et al., 2014). A less studied triblock is CP2-SK48-CP2, which has lysine as the last residue of each octapeptide (unpublished). Additionally, the polymers SE24-CP4-SE24, SH24-CP4-SH24, and SK24-CP4-SK24 were produced, which feature reverse block order (Beun et al., 2012; Martens et al., 2009). All silk-inspired triblock copolymers were secreted by P. pastoris at g/L levels. To prevent possible self-assembly of the proteins in the broth, production was at pH 5 for the SEn-containing variants and at pH 3 for the SHn- and SKn-containing variants.
Fig. 5.
Tentative structure of fibrils formed by silk-inspired triblock copolymers (not to scale). By stacking, the silk-like SE48 or SH48 blocks, depicted as aligned cylinders, form a μm-long core (blue). The extremely hydrophilic C2 blocks are shown as green wires emerging from the silk-like blocks and form a hydrophilic corona surrounding the core. (A) CP2-SE48-CP2 self-assembled at low pH. (B) Functionalized variants of CP2-SH48-CP2 self-assembled at neutral pH. Adapted from Włodarczyk-Biegun et al. (2016a) under Creative Commons License BY-NC-ND 4.0. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Because CP2-SH48-CP2 forms hydrogels at physiological pH, and in view of the general biocompatibility of silk-like materials (Altman et al., 2003; Hakimi et al., 2007), this polymer is of potential interest for biomedical applications such as tissue engineering. We have shown that these hydrogels exhibit long-term stability in erosion studies and display self-healing behavior (Włodarczyk-Biegun et al., 2014). Self-healing materials are of great interest for the development of, e.g., injectable hydrogels, because they can recover from damage incurred during application to the tissue (Tseng et al., 2015), or afterwards. Rat bone marrow cells remained fully viable in direct contact with this material, although proliferation was low. Functionalized polymer variants were produced that feature either genetically incorporated general cell-adhesive RGD motifs (BRGD-CP2-SH48-CP2), or osteoblast-specific KRSR motifs (BKRSR-CP2-SH48-CP2), with purified protein yields of >3 g/L of cell-free broth (see Fig. 5B; Włodarczyk-Biegun et al., 2016c). Scaffolds were prepared by mixing polymers containing either RGD motifs, KRSR motifs, or no cell-adhesive motifs, in the desired ratio, while maintaining the same overall protein concentration. In this manner, the composition of cell-adhesive domains could be tailored independently of the material's mechanical properties. Cell adhesion, metabolic activity, spreading, and confluency of MG-63 osteoblastic cells were highly promoted on these mixed materials. Scaffolds with varying RGD content and overall protein concentration were also tested in three-dimensional culture, where the cells were embedded in the scaffold and revealed spreading (Włodarczyk-Biegun et al., 2016a). The most favorable combination featured high RGD content and low protein concentration (i.e., low stiffness). These results showcase the possibility to independently tune different properties of these hydrogels and the potential for further developing them into cell culture scaffolds.
We furthermore used the ability of KRSR domains to bind the negatively charged linear polysaccharide heparin (Cardin and Weintraub, 1989), showing that heparin could act as a binder for controlled bundling of BKRSRCP2SH48CP2 fibrils (Włodarczyk-Biegun et al., 2016b). In the natural ECM, bundling of fibrils allows for a wide variety of stiffness and organization of the network. Similarly, by modulating the protein to heparin ratio, we were able to affect fibril bundling, leading to faster formation of stiffer hydrogels. This approach for controlled fibril bundling thus has potential in the fabrication of materials that mimic the ECM.
4.5.2. Collagen-inspired thermoresponsive block copolymers
Another category of triblock copolymers uses collagen-like end blocks for self-assembly (Werten et al., 2009). As discussed in Section 4.2, natural collagen sequences require prolyl-4-hydroxylation to form stable triple helices. However, sequences with very high proline content such as (Pro-Gly-Pro)n are sufficiently stable to form triple helices also in the absence of this posttranslational modification, as has been shown using chemically synthesized peptides (Engel et al., 1977; Frank et al., 2001; Suto and Noda, 1974). In fact, the melting point of these peptides can be controlled by their length n. We used (Pro-Gly-Pro)n sequences as trimer-forming end blocks (further denoted as Tn) in the design of our triblock copolymers and used the highly hydrophilic CP4 sequence (Section 4.2.1) as a random coil middle block. These triblock copolymers are thus referred to as Tn-CP4-Tn (see Fig. 6). As mentioned in Section 4.2.1, the primary structure of CP4 is fully gelatin-like, yet it cannot form triple helices and remains a random coil down to at least 4 °C, owing to a combination of moderate proline content (22%) and absence of hydroxylation in wild-type P. pastoris. For comparison, and to formally exclude all possible triple helix-forming capacity, a variant middle block CR4 was also prepared. It has the same amino acid composition as CP4, but features a noncollagenous sequence in the sense that the glycine residues are distributed in a quasi-random fashion. As expected, both middle blocks essentially behave the same, although the hydrodynamic radius of CR4 was found to be ~8% higher than that of CP4 (Teles et al., 2010a).
Fig. 6.
Schematic representation of Tn-CP4-Tn collagen-inspired triblock copolymers. Adapted from Werten et al. (2009) with permission from American Chemical Society.
As Tn end blocks we used n = 6, 9, 12, and 16 (Silva et al., 2011, Silva et al., 2012; Werten et al., 2009). The triblock copolymers were produced in wild-type P. pastoris GS115 lacking heterologous P4H and are therefore not hydroxylated. The proteins with n = 6 or 9 were secreted at several g/L of cell-free broth and those with n = 12 or 16 at 0.6 and 0.4 g/L, respectively. Although the n = 16 variant was initially compromised by proteolysis, this problem could be solved by using a protease-deficient strain (5.2.2, 5.3.2). The tunability of the melting point known from free chemically synthesized (Pro-Gly-Pro)n peptides was retained in the triblock design (Silva et al., 2012). In fact, the melting free energy and melting points determined were similar to those of free peptides of equal length. At sufficiently high concentration the triblock copolymers can form thermoreversible hydrogels, in which exclusively the collagen-like end blocks form the trimeric knots of the network. The reversible transition of the equilibrium from the trimeric to monomeric state of the Tn blocks occurs over ~20–30 °C. The melting range shifts to increasing temperatures with increasing n. Accordingly, T16-C4-T16 gels melted at a higher temperature than T9-C4-T9 gels and also showed greater stiffness at the same temperature and molar concentration, because the equilibrium is shifted towards higher trimer content. Besides varying the length of the Tn end blocks, we also produced variants with longer random coil middle blocks, namely T9-CPn-T9 and T9-CRn-T9 with n = 8 or 12 (Teles et al., 2010a; unpublished). These large protein polymers of 78 or 115 kDa, respectively, were completely intact and efficiently secreted at 1–3 g/L of cell-free broth. By varying polymer length and concentration, elastic properties, nominal mesh size, swelling behavior, and erosion could be tuned (Teles et al., 2010a). Sustained release of encapsulated proteins was demonstrated, which offers prospects for use of these hydrogels as a drug delivery system (Teles et al., 2010b).
The demonstrated tunability of the thermoresponsive behavior of these hydrogels, as well as their expectedly biocompatible (collagen-like/gelatin-like) nature, offers promise for their use as highly defined alternatives to animal gelatins in biomedical applications. In contrast to aged, crosslinked, animal gelatins, the block copolymers showed fast self-healing behavior (Skrzeszewska et al., 2010; Fig. 7). As mentioned in Section 4.5.1, self-healing hydrogels are highly desirable in biomedical applications. We also demonstrated that a composite hydrogel network formed by T9-CR4-T9 together with the fibril-forming silk-inspired CP2-SH48-CP2 polymer (Section 4.5.1) offers synergistic enhancement of rigidity and rupture strength (Rombouts et al., 2013), thereby further expanding the potential of these polymers for biomaterial applications.
Fig. 7.

Self-healing of a T9-CP8-T9 collagen-inspired hydrogel. An orange-colored piece of broken hydrogel (top) was placed in contact with a blue-colored piece (bottom) for 15 min at RT, after which the two strongly adhered together through newly-formed triple helices. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.5.3. Multi-responsive block copolymers
In the biomaterials field, efforts are increasingly being devoted to the development of hydrogels that respond to multiple, physiological or externally applied, environmental triggers such as pH, temperature, and reducing conditions. These ‘smart’ hydrogels could be used, e.g., for site-specific drug delivery (Knipe and Peppas, 2014). In block copolymers, multi-responsiveness can be achieved by combining different trigger-sensitive modules. From a basic science point of view, these materials are very interesting because the properties of the supramolecular structures obtained often depend on the order in which the stimuli are applied (i.e., self-assembly is pathway-dependent). Next, we will describe some of the multi-responsive protein polymers we have developed based on this interest.
Two dual-responsive silk-elastin-like protein polymers, SE24-E40 and SE12-CP4-E40, were constructed (Golinska et al., 2013). Both are composed of an N-terminal silk-like block with 24 or 12 (GA)3GH octapeptide repeats (Section 4.1.2) and a C-terminal V5A2G3–40 elastin-like block (Section 4.3). These blocks impart pH-responsiveness and temperature sensitivity, respectively. The SE12-CP4-E40 polymer additionally has the flexible and inert ~37 kDa CP4 spacer (Section 4.2.1) between the silk-like and elastin-like modules, which may allow the end blocks to take part in spatially separated microphases. The triblock was purified by ammonium sulfate precipitation (Section 4.2.1) and the diblock by ITC (Section 4.3). Both proteins were found to be pure and intact. The recovery after purification was >500 mg/L of clarified broth. The combination of both pH- and temperature-responsive modules in a single polymer resulted in complex supramolecular self-assembly behavior. For SE12-CP4-E40 this behavior was studied in detail and found to be pathway-dependent (Pham et al., 2013a). Fibrils were formed upon first lowering the pH, which bundled after subsequent heating. In contrast, micelles were formed upon first heating the solution, which seemed to aggregate into large structures after subsequent lowering of the pH. Both pathways resulted in the formation of hydrogels, although with different morphologies and mechanical properties.
Redox-responsiveness was introduced into the CP2-SH48-CP2 polymer (Section 4.5.1) by addition of a cysteine residue to the C-terminus of the sequence (Rombouts et al., 2016). The polymer, referred to as CP2-SH48-CP2-DC, was produced as an intact protein with a recovery after purification of 3 g/L of cell-free broth, which is in the same range as the yield obtained for the unmodified polymer. Gels formed under oxidizing conditions had a significantly increased storage modulus as a result of increased fibril rigidity. We substantiated that the formation of intra-fibril disulfide bridges leads to a slightly reduced lateral pressure in the hydrophilic corona, which probably affects the packing of the silk-like blocks in the fibril core.
A C-terminally cysteine-extended variant of the collagen-inspired polymer T9-CR4-T9 (Section 4.5.2) was similarly constructed (Pham et al., 2013b). Unlike the unmodified monodisperse protein, the cysteine-extended variant, T9-CR4-T9-DC, was initially degraded by proteolysis. A protease-deficient strain was used to solve this problem (5.2.2, 5.3.2), resulting in intact protein with a yield after purification of ~400 mg/L of clarified broth. Under reducing conditions, the polymer behaved like unmodified T9-CR4-T9 in terms of melting point in differential scanning calorimetry and storage modulus of the hydrogel. Under oxidizing conditions, however, the thermogram showed an additional melting point shifted up by ~11 °C. This shows that a fraction of the triple helices was stabilized by disulfide formation, most likely owing to an increase in local concentration of the T9 blocks. That is, the occurrence of preexisting disulfide-bonded T9 pairs promoted the subsequent formation of triple helices. The storage modulus of hydrogels formed by T9-CR4-T9-DC under oxidizing conditions was higher than that of normal T9-CR4-T9 by a factor two. This was attributed to a shifted equilibrium towards more triple helices and to the crosslinking of different triple helices by disulfide bridges.
Two other asymmetric block copolymers based on T9-CR4-T9 were devised, where the C-terminal T9 block was replaced by a cationic binding block consisting of either six histidines (T9-CR4-BH6; Pham et al., 2013c), or six lysines (T9-CR4-BK6; Pham et al., 2016). Both proteins were intact and recovered at 500 and 400 mg/L of clarified broth, respectively. Protein T9-CR4-BH6 was used in conjunction with the polyanion sodium polystyrene sulfonate, where electrostatic interaction between the C-terminal BH6 block and the polyanion causes the formation of micelles at a temperature above the melting point of the T9 block. At low temperature, in the absence of polyanion, star polymers are formed through the formation of triple helices by the N-terminal T9 block. Under conditions where both end blocks self-assemble (low temperature, in the presence of polyanion), a thermoreversible hydrogel is formed. This network formation can start either with the charge-driven micelles (by cooling), or with the thermoresponsive star polymers (by addition of polyanion). Similarly, the T9-CR4-BK6 polymer was used in conjunction with the anionic polymer xanthan. Xanthan is a widely used water-soluble food additive with shear-thinning and pseudo-plastic properties, and its functionalization is of potential interest in biomaterial applications. The composite xanthan/T9-CR4-BK6 system formed reversible thermosensitive hydrogels, where the storage modulus was significantly increased as compared to xanthan alone.
Another dual-responsive polymer is SH8-CR4-T9, which has a short N-terminal silk-like block that self-assembles at neutral pH and a thermoresponsive collagen triple helix forming end block (Rombouts et al., 2015). The protein was intact and recovered at 2.0 g/L of clarified broth. It was used in conjunction with CP2-SE48-CP2, together forming a hybrid system. In a first step at high temperature and at a pH halfway between the pKa values of the histidine and glutamic acid side chains, coassembly occurred between the oppositely charged SH8 and SE48 silk-like domains of both proteins. Whereas the individual proteins do not appreciably self-assemble under these conditions, the mixture resulted in the formation of mixed coassembled fibrils. At high temperature, slow gel formation occurred. Upon cooling of the mixture, the storage modulus vastly increased. This can be explained by the formation of trimeric T9 nodes that crosslink CP2-SE48-CP2 fibrils. A hydrogel system with thermoreversible crosslinks was obtained that features temperature-switchable stiffness.
4.5.4. DNA-binding block copolymers and artificial viral coat proteins
DNA is a much-investigated building block for nanomaterials (Seeman, 2010). For many applications, including gene delivery, coating of DNA with suitable binder moieties is an effective approach to alter its physical properties and interactions with the environment. However, coating DNA with chemically synthesized polymers typically results in aggregates with random size and shape, such that there is a need for more controlled coatings. In its simplest form, a macromolecule for DNA coating purposes should contain a cationic module that can bind the negatively charged DNA molecule and a module that provides colloidal stability (Kabanov and Kabanov, 1998; Kakizawa and Kataoka, 2002). We have designed and produced protein polymers that meet these requirements (Hernandez-Garcia et al., 2012). They feature the hydrophilic random coil CP4 sequence (Section 4.2.1) as the module conferring colloidal stability and a short C-terminal cationic block. The latter consists of either 12 Lys residues, or 6 His-Lys dipeptides, in proteins CP4-BK12 and CP4-B(HK)6, respectively. The ~38 kDa diblock copolymers were produced in P. pastoris as monodisperse polymers and were purified with a recovery of ~1 g/L of clarified broth. The proteins were shown to complex with single DNA molecules into highly stable bottlebrushes (Hernandez-Garcia et al., 2012; Zhang et al., 2013). Inter- and intramolecular bridging of DNA molecules was prevented by virtue of the combination of a relatively small binding block and a large block for colloidal stability. The DNA bottlebrushes show liquid crystalline behavior at DNA concentrations roughly one order of magnitude below those for bare DNA (Storm et al., 2015). Besides these polymers, which feature simple electrostatic DNA binding, a variant was produced that uses the 7 kDa protein Sso7d as the nonsequence-specific DNA-binding domain (Hernandez-Garcia et al., 2016). The DNA binding properties of this protein from the thermoacidophilic archaebacterium Sulfolobus solfataricus have been well characterized, and it has been used, e.g., to improve the processivity of DNA polymerases. The length of the random coil domain of the polymer was doubled from CP4 to CP8, so as to increase the solubility and rigidity of the resulting protein-DNA complexes. The resulting protein, CP8-BSso7d, was produced in P. pastoris as an intact protein with a yield of purified protein of 0.7 g/L of cell-free medium. The protein polymer was able to coat various one-dimensional DNA templates and also two- and three-dimensional DNA origami nanostructures, without structural distortion (Estrich et al., 2017; Hernandez-Garcia et al., 2016).
The self-assembly of viruses is a highly cooperative process, where initial binding of coat proteins to the encoding viral genome triggers the binding of further coat proteins. With gene delivery applications in mind, we engineered supramolecular cooperativity into the DNA-coating protein CP4-BK12 described above. This was achieved by including the silk-like sequence SQn = [(GA)3GQ]n between the CP4 domain that provides colloidal stability and the BK12 DNA-binding domain, resulting in polymer CP4-SQn-BK12 (Fig. 8; Hernandez-Garcia et al., 2014).
Fig. 8.
Design of viral coat protein CP4-SQn-BK12, with n = 10. CP4 is an N-terminal ~400-residue hydrophilic random coil block (green), SQ10 constitutes the self-assembly block (pink) and is a tenfold repetition of the octapeptide (GA)3GQ, and BK12 is the C-terminal dodecalysine DNA-binding block (red). Adapted from Hernandez-Garcia et al. (2014) with permission from Springer Nature. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
This silk-inspired octapeptide SQ is identical to the octapeptides described in 4.1.2, 4.5.1, except that Gln replaces the charged amino acids Glu, His, or Lys, so as to rule out electrostatic behavior of this block. The number of silk-like strands n determines the propensity of the SQn block to stack and, thereby, the degree of binding cooperativity. A series of CP4-SQn-BK12 polymers with n = 0, 2, 4, 10, and 14 was efficiently produced as intact proteins in P. pastoris. Fully cooperative assembly on a DNA template was obtained for n = 10 and 14. Rod-shaped virus-like particles were formed, in which the DNA was fully protected against high concentrations of DNase I. The particles were able to transfect HeLa cells, resulting in expression of a fluorescent reporter. Similarly, CP4-SQ10-BK12 was found to coassemble with mRNA into rod-shaped virus-like particles that were able to transfect HEK-293 cells (Jekhmane et al., 2017). These rationally designed artificial virus coat proteins, produced as triblock copolymers, thus provide mechanistic insight into viral self-assembly and might be further developed as useful vectors in gene therapy.
4.5.5. Block copolymers with heterodimerizing modules
To expand the repertoire of possible topologies of nanostructured materials, a need exists to couple different protein polymers. This can be done by traditional chemical crosslinking, but increasingly also enzymatic or autocatalytic conjugation, or reversibly heterodimerizing modules are being investigated. We have reviewed these nonchemical approaches elsewhere (Domeradzka et al., 2016d). Heterodimerizing modules are of particular interest, in that they allow the linking of different protein polymers or protein domains by the mere mixing of two separately produced components. A possible medical application could be injectable hydrogels that self-assemble in situ upon mixing of two protein polymers A and B.
A first category of heterodimerizing modules that we incorporated into protein polymers are leucine zipper peptides of 47 amino acids, capable of forming high affinity α-helical heterodimeric coiled coils. These peptides were first designed and studied by Moll et al. (2001). The relatively acidic peptide RR12EE345L will be denoted here as DA, the relatively basic partner EE12RR345L as DB. We added DA and DB domains to the C-terminus of CP4 (Section 4.2.1) and T9-CP4 polymers (a diblock variant of T9-CP4-T9; Section 4.5.2). Under standard fermentation conditions, the four protein polymers were partially degraded. However, the use of a low growth temperature (20 °C) and supplementation of the medium with 1% casamino acids during the induction phase led to the production of essentially intact polymers. The yield after purification was 0.7–1.3 g/L of cell-free broth. The heterodimerizing modules were functional and mediated the coupling of the A and B counterparts. The DA and DB modules were also attached to the C-terminus of the fibril-forming silk-inspired triblock copolymer CP2-SH48-CP2 (Section 4.5.1). The ensuing CP2-SH48CP2-DA and CP2-SH48-CP2-DB polymers were purified from the cell-free broth at 2.3 and 1.4 g/L, respectively (Domeradzka et al., 2016c). In mixtures of bare CP2-SH48-CP2 and the DA/DB-containing variants, fibril bundling occurred to an extent controlled by the ratio of modified to unmodified polymer. This holds promise for the further development of biomaterials mimicking the ECM, as fibril bundling plays a key role in this environment.
Another category of heterodimerizing modules are the so-called WW-domain (~37 residues) and its complementary proline-rich ligand (~13 residues). The binding between the two occurs with high specificity and Kd values typically in the high nM to low μM range (Kay et al., 2000). WW-domains contain two highly conserved tryptophans and consist of a three-stranded antiparallel β-sheet (Macias et al., 1996). Wong Po Foo et al. (2009) successfully produced hydrogel-forming polymers in E. coli that make use of the interaction of the WW-domains CC43 or Nedd4.3 with a proline-rich sequence derived from p53-binding protein-2, referred to as PPxY. To determine if WW and PPxY domains could similarly be produced in P. pastoris, we designed variants of the CP4 polymer (Section 4.2.1) with C-terminal extensions consisting of (i) the same PPxY sequence, or (ii) a WW domain derived from human ubiquitin ligase, referred to as WWP1–1 (Domeradzka et al., 2016b). The polymers, CP4-DPPxY and CP4-DWW, respectively, were produced at a purified protein yield of 2.2–2.3 g/L of cell-free broth. The PPxY module was found to be O-glycosylated, and, interestingly, a large portion of the attached glycans was phosphorylated. Glycosylation was abolished by mutating the single serine in the PPxY module to alanine. The CP4-DWW polymer associated with both the glycosylated and nonglycosylated variants of CP4-DPPxY with a Kd of ~3–9 μM, indicating the potential of this pair of heterodimerizing modules for the generation of complex supramolecular structures.
5. Challenges and possible solutions
From the case studies in Section 4 it is clear that P. pastoris is overall an excellent host for the production of protein polymers. Repetitive genes are stably maintained, product yields are often high, and the possibility of secretory production for most of these protein polymers allows efficient downstream processing. Nonetheless, some challenges are apparent also. Several reviews on P. pastoris have already addressed general bottle necks and means of enhancing expression levels (Ahmad et al., 2014; Cereghino et al., 2002; Cregg et al., 2000; Daly and Hearn, 2005; Juturu and Wu, 2018; Sreekrishna et al., 1997; Yang and Zhang, 2018), which we will not repeat here. Instead, the purpose of this section is to provide materials scientists new to P. pastoris with some considerations drawn from our experience. The utility of bioreactors is addressed, and the nonsecretion of several self-assembling protein polymers is discussed. A major focus is also on proteolytic degradation, as this potentially threatens the hallmark feature of protein polymers: the monodispersity inherent to their genetic encoding.
5.1. Poor expression in shake flasks – the need for bioreactors
Most molecular biology and biochemistry laboratories use shake flask cultures for protein expression in E. coli, for reasons of familiarity, convenience, and the minimal infrastructure required. Some researchers similarly trying P. pastoris using shake flasks may end up being disappointed with the yields obtained and may consequently abandon the system. This especially applies to the field of protein polymer research, where the products are used as materials. Relatively large amounts of polymer are needed for physical characterization by rheometry, digital scanning calorimetry, tensile strength testing, etc., or for functional testing in, e.g., controlled release or cell culture studies.
In our opinion, meaningful use of P. pastoris for the production of protein polymers requires the use of well-controlled bioreactors. We routinely observe product levels in stirred tank fed-batch cultures that are higher than in shake flasks by a factor 20–100. A similarly large impact of the use of bioreactors is apparent from some of the protein polymers in Table 1 and heterologous proteins expressed in P. pastoris in general (Cregg et al., 2000). Unlike shake flasks, bioreactors offer strict control over feeding and dissolved oxygen levels, which enables the generation of very high biomass levels. Moreover, continuous controlled feeding minimizes cell lysis that can occur in shake flasks as a result of starvation and/or overdosing caused by intermittent manual feeding, particularly with methanol as the substrate. Whereas the pH in shake flasks is poorly controlled, typically by using buffered media, bioreactors offer continuous pH adjustment. Cell lysis and culture pH can have a dramatic impact on proteolysis (Sinha et al., 2005), and indeed we have often seen completely different degradation patterns and kinetics in shake flasks as compared to bioreactors. Although relatively uncontrolled cultures using shake flasks, multi-well plates, etc., can be useful for screening transformants, they have little bearing on eventual bioprocessing. In contrast, we find that bench-top bioreactor cultures of P. pastoris can be readily scaled-up to 1000 L pilot-scale with only limited loss of productivity and no impact on protein polymer integrity (unpublished). This is highly relevant for studies ultimately aimed at industrial production and commercial applications.
Various approaches exist for the cultivation of P. pastoris in bioreactors. Surveying these is beyond the scope of this work and the reader is referred elsewhere (Cos et al., 2006; Looser et al., 2015; Potvin et al., 2012; Valero, 2013; Yang and Zhang, 2018; Zhang et al., 2000).
5.2. Proteolysis
Like in other expression systems (see e.g. Idiris et al., 2006; Jensen et al., 2000; Jones, 1991b; Rozkov and Enfors, 2004; Van den Hombergh et al., 1997), heterologous proteins expressed in P. pastoris occasionally suffer degradation by proteases. This negatively affects not only the yield of intact product but importantly also product homogeneity. Protein polymers tend to be particularly sensitive to proteolysis owing to their often (partially) unfolded structure. In fact, it is because of such accessibility that gelatin (denatured collagen) is commonly used as a substrate in protease assays. Furthermore, owing to the repetitive sequence of protein polymers, any proteolytically sensitive motif present likely occurs multiple times.
Proteolytic degradation of secreted proteins in P. pastoris can often be minimized by optimizing culture conditions. Various strategies to this end have been reviewed elsewhere (Ahmad et al., 2014; Potvin et al., 2012). Among these approaches, the following have been successfully employed for protein polymers: pH optimization (4.2.1, 4.5.5), addition to the medium of amino acid-rich supplements such as casamino acids (4.2.1, 4.5.5), use of a low growth temperature (Section 4.5.5), and (at small scale) the use of specific protease inhibitors (Jansson et al., 2016; Section 4.1.1).
Another common strategy to counter proteolytic degradation of secreted recombinant proteins is to use mutant strains deleted in the vacuolar proteases saccharopepsin (Protease A) and/or cerevisin (Protease B). These proteases are mainly present in the extracellular medium as a result of cell lysis (Gleeson et al., 1998; Sinha et al., 2005) and can as such be involved in the degradation of secreted proteins. Although these strains (SMD1163, SMD1165, and SMD1168) have been described as the most effective protease-deficient strains (Ahmad et al., 2014), interestingly, their use has generally not reduced degradation of protein polymers in our laboratory. In view of the main specificity of vacuolar proteases for hydrophobic residues (Dreyer, 1989; Kominami et al., 1981; Remington and Breddam, 1994), this in part probably reflects the relatively hydrophilic amino acid composition of most protein polymers. In agreement with the observation by others that the vacuolar protease mutants are not as robust as wild-type strains (Cereghino and Cregg, 2000; Gleeson et al., 1998), we find that their use often results in poor growth, low polymer titers, and inefficient product purification owing to higher levels of extracellular host proteins (Domeradzka et al., 2016a; Werten et al., 1999; unpublished). In our experience, protein polymers are more often degraded by proteases of the secretory pathway than by vacuolar proteases. Because the former tend to be relatively sequence-specific (Jones, 1991a), knowledge about their sequence specificity may, to some extent, allow the design of polymers resistant to degradation. Indeed, unlike folded globular proteins with complex sequences, protein polymers typically allow considerable freedom in sequence design.
Below we will discuss proteolytic events that have been observed with protein polymers in P. pastoris, and how such degradation may be minimized through appropriate sequence design. Note that we assume the sequence specificities of P. pastoris proteases to be very similar to those of their S. cerevisiae prototypes, which agrees well with our findings so far. The utility of protease deletion mutants we have developed, for situations where the occurrence of potentially vulnerable sequences cannot be avoided, is also examined. Screening the literature, we have found that the involvement of the secretory pathway proteases in the degradation of recombinant proteins in P. pastoris is often not recognized as such and that the topic of protease specificity has so far hardly been addressed in reviews on this yeast. It is therefore our hope that the subsections below will be of value not only to those interested in protein polymers, but also to P. pastoris users in general. However, the reader is reminded that, in globular proteins, the folded structure may obscure potentially susceptible sites.
5.2.1. Endoproteolysis by Kex2 protease
We observed severe fragmentation of Col1a1 gelatins in P. pastoris by Kex2 protease (Werten et al., 1999; Section 4.2.1). In S. cerevisiae, this subtilisin-like serine protease is involved in the late-Golgi processing of the α-factor precursor, where it cleaves at the C-terminal side of dibasic Lys-Arg sites (Brake, 1990). The prepro peptide of this pheromone also constitutes ppαF, the most commonly used leader sequence for secretory protein production in P. pastoris (Damasceno et al., 2012), where Kex2 cleaves C-terminal to the Lys-Arg site present between ppαF and the fused protein of interest. Although Kex2 is mostly known for its processing of dibasic motifs (Lys-Arg and Arg-Arg), the two sites cleaved by Kex2 in Col1a1 gelatins were found to be Met-Gly-Pro-Arg. Indeed, the mono-arginyl sequence Pro-Arg is a known potential Kex2 substrate (Brenner and Fuller, 1992; Mizuno et al., 1989; Rockwell et al., 1997; Zhu et al., 1992). The Kex2-mediated degradation of Col1a1 was resolved by removal of the susceptible sites via site-directed mutagenesis.
Besides Pro-Arg, mentioned above, also other mono-arginyl motifs have been shown to be Kex2 substrates (Bevan et al., 1998; Brenner and Fuller, 1992; Riffer et al., 2002; Rourke et al., 1997). On the other hand, definitely not all arginines are substrates, even in the absence of any folded structure, as evident from noncleavage or very limited cleavage in Col1a1 of arginyl sites other than Met-Gly-Pro-Arg (Werten et al., 1999). Even a canonical dibasic Lys-Arg motif in Col1a1 was not a major site of cleavage, which led us to speculate (Werten et al., 1999) that particularly the P4 residue plays a more important role in substrate processing by Kex2 than commonly appreciated (substrate residues in the amino-terminal direction away from the scissile bond are denoted P1, P2, …, Pn, and in the carboxy-terminal direction P1’, P2’, …, Pn’; see Schechter and Berger, 1967). Our suggestion seems to be corroborated by a recent study mapping the yeast secretory ‘cleaveome’ (Li et al., 2017). Much insight into the sequence specificity of Kex2 can be gleaned from the studies by Bevan et al. (1998) and Suzuki et al. (2000), in which the effect on cleavage was investigated of substitutions at substrate positions P2 and P3/P4, respectively. Although Kex2 is thought to positively recognize only the region N-terminal to the cleaved bond (Bourbonnais et al., 1988; Rockwell et al., 2002; Rockwell et al., 1997), possibly also the P1’ residue should be taken into account, as efficient substrates tend to have small or acidic residues at this position (Bader et al., 2008; Manfredi et al., 2016; Rholam et al., 1995). Pro at P1’ appears to disturb Kex2 processing (Guisez et al., 1991; Xie et al., 2007).
Ever since the observed degradation of mono-arginyl motifs in Col1a1 gelatins (Werten et al., 1999), we have routinely screened our protein polymer sequence designs using a position weight matrix approach based on the data of Bevan et al. (1998), Suzuki et al. (2000), and others, to tentatively predict the likelihood of degradation by Kex2 (unpublished). The caveat in using position weight matrix approaches is that the relative importance of, and possible interplay between, the enzyme's subsites are not sufficiently understood to derive a truly reliable automated prediction tool. The same of course applies to attempts at deriving simple predictive sequence motifs. Nonetheless, informed use of the available specificity data, also taking into account sequence information from known Kex2 substrates, can assist the rational design of arginine-rich protein polymer sequences. For example, we incorporated repeated proteoglycan-binding Lys-Arg-Ser-Arg tetrapeptides separated by flexible poly-glycine spacers into a silk-inspired polymer in such a way that unfavorable Gly and Pro residues occupied the P4 and P3 positions, respectively. This almost entirely prevented Kex2-mediated degradation, despite the presence of the canonical dibasic Lys-Arg motif (Włodarczyk-Biegun et al., 2016c).
As an alternative to preventing undesired cleavage by Kex2 through sequence modification, we also constructed a P. pastoris kex2 disruptant (Werten and de Wolf, 2005). Indeed, endoproteolysis of the Met-Gly-Pro-Arg motifs of Col1a1 was completely abolished in this strain. Surprisingly, correct processing at the dibasic site of the ppαF leader used to drive secretion of Col1a1 still occurred (Werten and de Wolf, 2005). The protease responsible for this alternative processing of ppαF was found to be yapsin 1. Although efficient yapsin-dependent processing of ppαF may well depend on the protein fused to it, the assumption by Govindappa et al. (2014) that the use of kex2 disruptants requires leader sequences that do not rely on processing of dibasic motifs is too general. In fact, we have found complete proteolytic cleavage at the dibasic site of ppαF also for three other protein polymers in a kex2 mutant (unpublished), which shows that Kex2-independent maturation is not unique to Col1a1. Although the kex2 strain grows more slowly than the wild type (~50% increased generation time in YPD medium at 30 °C), it shows normal viability and is suitable for high-biomass fermentation (Werten and de Wolf, 2005).
5.2.2. Endoproteolysis by yapsins
We have observed partial degradation of several protein polymers by yapsin 1 protease. This includes the collagen-inspired triblock T16-CP4-T16 (Silva et al., 2011; Section 4.5.2) and the related disulfide bond-forming polymer T9-CR4-T9-Cys (Pham et al., 2013b; Section 4.5.3). The yapsin family of aspartic proteases consists of five members in S. cerevisiae (Gagnon-Arsenault et al., 2006). Their natural function appears to be in the activation and/or shedding of periplasmic proteins during cell wall stress and remodeling (Gagnon-Arsenault et al., 2008; Krysan et al., 2005). Yapsin 1 (Yps1, previously referred to as Yap3) has been studied most extensively. Although the protease is mainly active at the plasma membrane, it is also found in the extracellular medium and transiently active in the late secretory pathway (Ash et al., 1995; Cawley et al., 1998; Kang et al., 1998).
The substrate specificities of the S. cerevisiae yapsins (Egel-Mitani et al., 1990; Komano and Fuller, 1995; Olsen et al., 1999) overlap with those of Kex2 (Bourbonnais et al., 1994), in that both protease types cleave C-terminally of basic residues. This also explains the above-mentioned yapsin-mediated processing of the ppαF leader for various protein polymers in a P. pastoris kex2 strain. Nonetheless, there are notable differences between the two protease classes (Gagnon-Arsenault et al., 2006). First, Kex2 primarily recognizes residues on the N-terminal side of the scissile bond, whereas residues on both sides are important for yapsins. Second, whereas Kex2 is highly specific for Arg at P1, yapsins accept both Lys and Arg residues at this position. Indeed, in the above-mentioned examples of T16-CP4-T16 and T9-CR4-T9-Cys, cleavage occurred C-terminal of Lys residues. Third, whereas Kex2, despite its ability to process particular monobasic substrates, is primarily specific for dibasic sites, yapsins have a truly monobasic specificity. Nonetheless, additional nearby basic residues do appear to enhance catalytic efficiency. Pro at P2 is probably not tolerated by yapsin 1 (Kjeldsen et al., 1996), and also yapsin 2 appears to exclude proline from P2 (Komano et al., 1999).
In view of the limited knowledge on yapsin specificity it is difficult to design resistant protein polymers. However, degradation of a given polymer by yapsin 1 may be addressed using the yps1 strain of P. pastoris we developed (Werten and de Wolf, 2005). Overall yapsin-like activity in this strain was decreased by 95%, suggesting that Yps1 is by far the major yapsin in the yeast. The partial degradation of the T16-CP4-T16 and T9-CR4-T9-Cys polymers was completely overcome in the yps1 strain. Interestingly, in both cases the observed proteolysis was, through unknown mechanisms, triggered by the high thermal stability of the collagen trimer-forming T16 and T9-Cys modules (Section 5.3.2). However, yapsin-mediated proteolysis is not unique to self-assembling sequences, as evident from later studies that confirm the general usefulness of YPS1 disruption for protein production in P. pastoris (Sazonova et al., 2013; Wu, M. et al., 2013b; Yao et al., 2009). Disruption of YPS1 does not result in obvious phenotypic defects, and the strain is robust and suitable for high cell density fermentations at a normal growth rate (Werten and de Wolf, 2005). Note that a kex2 yps1 double disruptant does show slow growth, and that the ppαF leader is aberrantly processed in this strain (Werten and de Wolf, 2005).
5.2.3. N-terminal truncation by dipeptidyl aminopeptidase A
Elastin-like protein polymers produced in P. pastoris contained 80% of species lacking the first four residues Gly-Pro-Val-Pro (Schipperus et al., 2009), which was attributed to proteolysis by the Golgi-localized dipeptidyl aminopeptidase A (DPAPase A; a serine protease encoded by the STE13 gene). In S. cerevisiae, the protease is involved in the processing of the α-factor precursor, where it stepwise removes N-terminal Xaa-Ala dipeptides. In P. pastoris, when the ppαF secretory leader is used to drive secretion of heterologous proteins, DPAPase A is responsible for the removal of the (Glu-Ala)2 spacer commonly present between the Kex2 site of this leader and the protein of interest. However, not only N-terminal Xaa-Ala dipeptides are a substrate of DPAPase A in S. cerevisiae and other yeasts, but also N-terminal Xaa-Pro dipeptides (Egel-Mitani and Hansen, 1987; Julius et al., 1983; Kreil, 1990; Matoba et al., 1988). Protein polymers containing N-terminal Xaa-Ala or Xaa-Pro dipeptides can be protected simply by adding one or more N-terminal shielding residues, such that these motifs are no longer at the N-terminus. Alternatively, P. pastoris strains disrupted in the STE13 gene homolog are likely useful (Hopkins et al., 2014; Prabha et al., 2009).
5.2.4. C-terminal truncation by Kex1 protease
Olsen et al. (2005) reported that ~30% of a 9 kDa human Col1a1 gelatin produced in P. pastoris lacked its C-terminal Arg residue, most likely as a result of cleavage by Kex1 protease. In S. cerevisiae, this membrane-bound serine carboxypeptidase is involved in the processing of the α-factor precursor (Dmochowska et al., 1987) and is localized in the late Golgi (Bryant and Boyd, 1993). Its prototypical activity is the removal of the C-terminal basic amino acids Lys and Arg. Our group has successfully produced protein polymers in P. pastoris containing C-terminal cationic DNA-binding (Lys)12 tails (Section 4.5.4), which are in principle canonical targets for Kex1-mediated proteolysis. By adding a C-terminal Gly to the sequence, as an otherwise minimally obstructive residue, we ensured that the essential cationic tails would remain intact in wild-type P. pastoris (Hernandez-Garcia et al., 2012). An alternative strategy may be to use a kex1 mutant of P. pastoris (Boehm et al., 1999), although also the vacuolar carboxypeptidase Y released into the medium through cell lysis might remove C-terminal basic amino acids (Remington and Breddam, 1994).
5.3. Self-assembly in vivo
Most protein polymers described in Section 4 are relatively hydrophilic and unfolded unless triggered to self-assemble. Self-assembly ideally occurs only in vitro after production, typically in response to an environmental trigger such as pH or temperature. In fact, because protein folding is often considered to be the major rate-limiting bottleneck in secretory production (Idiris et al., 2010), the lack of need for folding and corresponding interaction with chaperones may be why many of these polymers are secreted so efficiently, despite the demands placed on the translation machinery by their biased amino acid composition. Nonetheless, some protein polymers do self-assemble into supramolecular structures already in vivo. If the protein polymer was targeted to the cytoplasm, then the formation of such structures may not necessarily impair product yield and integrity. The protein may well sequester into stable inclusion bodies similar to those commonly seen in E. coli, as was found in P. pastoris for an aggregation-prone marker protein (Rueda et al., 2016). If, however, the protein polymer was targeted for secretion, then self-assembly during passage through the secretory pathway is likely to result in its nonsecretion, as we will discuss below.
5.3.1. (Non)secretion of silk-like polymers
It is clear from Section 4.1.1 that secretory targeting of spider silks in P. pastoris tends to result in low extracellular yields and intracellular retention of much of the product (Fahnestock et al., 2000; Jansson et al., 2016; Teulé et al., 2003). Although these studies did not investigate why retention occurred, self-assembly in vivo seems a likely cause given the propensity of poly-Ala and poly(Gly-Ala) stretches to form ß-sheets and ß-stacks. Indeed, poor solubility of recombinant spider silks in vitro is commonly noted (see e.g. Mello et al., 2004; Prince et al., 1995; Winkler et al., 2000). Also when spider silks were not targeted for secretion in P. pastoris, insect cells, or E. coli, most of the protein was found in the insoluble cellular fraction (Fahnestock and Bedzyk, 1997; Huemmerich et al., 2004; Liebmann et al., 2008). In spiders, the specialized epithelial cells of the silk glands obviously do efficiently secrete silk proteins. However, these cells' dedicated cellular machinery and the complex molecular makeup of native silk proteins differ considerably from that of yeast and simplified silk mimics, respectively.
In view of the generally poor secretion of spider silks in P. pastoris, it is surprising that, e.g., the silk-like protein SH48 and the fibril-forming triblock copolymer CP2-SH48-CP2 are produced as secreted proteins at g/L levels (4.1.2, 4.5.1). The pH of the yeast secretory pathway progressively drops from pH 7 in the ER to pH 6 in the late Golgi, through to pH 5.2 in the secretory vesicles (Orij et al., 2011). Particularly the ER and Golgi are thus in a pH range conducive to self-aggregation (SH48) or fibril formation (CP2-SH48-CP2), as the silk-like [(GA)3GH]48 block folds and stacks in vitro at pH >6 (Golinska et al., 2014). The hydrophilic end blocks and the slow kinetics of fibril formation likely play a role in the case of CP2-SH48-CP2, but this does not explain why SH48, which instantaneously aggregates at neutral pH in vitro, is secreted so efficiently. Apparently, there is a delicate balance between a protein's secretion and its inclination to aggregate, as governed by secretion kinetics, transient local concentrations in vivo, and possible effects of biological cosolutes.
5.3.2. Collagen trimers prohibit secretion
Collagen represents an example where self-assembly in vivo certainly occurs and causes nonsecretion. As described in Section 4.2.2, the Myllyharju group found that collagens produced in a strain overexpressing P4H are retained intracellularly (Vuorela et al., 1997), and that these appear to accumulate in the ER (Keizer-Gunnink et al., 2000). They devised a series of collagen fragments to study this phenomenon more closely (Pakkanen et al., 2006). Using the ppαF leader in a P. pastoris strain expressing human P4H, collagen α1(I) fragments were found to be secreted as single-chain polypeptides. In contrast, upon induction of triple helix formation by attachment of a C-terminal propeptide, the collagen molecules were fully retained intracellularly. Also when the small trimerizing foldon domain from bacteriophage T4 fibritin was used to induce trimerization, molecules accumulated inside the cells. Only a fraction of the molecules was secreted, and these were single-chain molecules. This was even the case when a small 9 kDa α1(I) fragment with foldon domain was expressed, which shows that the triple-helical conformation prohibits secretion independently of the size of the collagen fragment. The authors propose that, whereas in animal cells triple-helical collagen travels the Golgi complex by cisternal maturation, such a transport system may not be present in yeast. Indeed, also triple-helical collagen expressed in S. cerevisiae was retained intracellularly (Toman et al., 2000; Vaughan et al., 1998).
We similarly found poor secretion of the T16-CP4-T16 triblock copolymer (Section 4.5.2). The (Pro-Gly-Pro)n end blocks in this type of Tn-CP4-Tn polymer are capable of forming triple helices in the absence of prolyl-4-hydroxylation owing to their high proline content (Section 4.5.2). Whereas T9-CP4-T9 is efficiently secreted and intact, the T16-CP4-T16 variant with longer end blocks, and thus higher melting temperature, is secreted at a five times lower level and is partially degraded (Silva et al., 2011). In line with the above findings by the Myllyharju group, the formation of triple helices stable at growth temperature may prevent efficient secretion of T16-CP4-T16. Alternatively, it could be argued that the increased length of the proline-rich (Pro-Gly-Pro)n stretches may negatively impact ribosomal translation, or may, through increased hydrophobic interactions, result in protein aggregation. To discriminate between these possibilities, we produced molecules with randomized end blocks and otherwise unchanged amino acid composition. The fact that these, inherently nontrimerizing, molecules were secreted at a level similar to that of T9-CP4-T9 shows that trimer formation indeed disturbs secretion (Silva et al., 2011). Because T16-CP4-T16 triblock copolymers can form gels upon trimerization of the end blocks, we investigated whether secretion is also compromised in diblock copolymers with only a single T16 block, which are by definition unable to form gels. To this end, we produced T16-CP4 and CP4-T16 diblock copolymers (unpublished). Although these were found to be degraded similarly as the triblocks, their yields were intermediate between those of T16-CP4-T16 and the well-produced T9-CP4-T9. This suggests that, although triple helix formation as such is the main cause of poor secretion and degradation, gel formation does aggravate the condition further. The degradation of T16-CP4-T16 was resolved by using a yps1 strain (Section 5.2.2), indicating that trimer-formation in some way elicited yapsin 1-mediated proteolysis. If, like fully triple-helical hydroxylated collagen (Keizer-Gunnink et al., 2000), some of the T16-CP4-T16 molecules accumulate as trimers in the ER, this might indirectly trigger degradation of the secreted monomeric fraction by yapsin 1. A possible mechanism for this may be found in the fact that ER stress strongly induces expression of YPS1 in S. cerevisiae (Miller et al., 2010). Alternatively, if trimeric T16-CP4-T16 molecules in fact do travel beyond the ER, these might directly trigger degradation by yapsin 1 as follows. Possibly, trimeric molecules travel the secretory pathway or cross the cell wall at a relatively low rate, which would increase their exposure to yapsin 1 activity in the late secretory pathway or in the periplasmic space. Alternatively, traversal of the cell wall by trimeric T16-CP4-T16 might trigger the cell wall integrity response, which in turn could trigger increased YPS1 expression (Krysan et al., 2005). As mentioned in Section 4.5.3, we also found yapsin 1-mediated degradation of T9-CR4-T9-DC. Apparently, the demonstrated triple helix-stabilizing effect of the C-terminal disulfide bridge-forming cysteine residue in this molecule triggers degradation in the same manner as when using long T16 end blocks (Pham et al., 2013b).
5.3.3. Intracellular coacervation of elastin-like polymers
ELPs produced in P. pastoris with a low transition temperature (Schipperus et al., 2012) may represent another example of unfavorable self-assembly in vivo. As described in Section 4.3, the hydrophobic V5L2G3-40 was produced at a much lower yield at a growth temperature of 30 °C than V5A2G3-40 and was partially degraded. In cultures grown at 20 °C, the yield of V5L2G3-40 was increased by a factor of six, and the protein was no longer degraded. We propose that at a low growth temperature, V5L2G3-40 is below its transition threshold and soluble during its travel down the secretory pathway. In contrast, at 30 °C, V5L2G3-40 may undergo dehydration and transition to a more ordered ß-strand-rich structure with exposed hydrophobic residues (Li et al., 2014), which results in coacervation. This in turn could lead to retention of the protein in the secretory pathway and/or trigger the unfolded protein response (UPR), which recognizes misfolding through exposed hydrophobic regions normally buried in the core of globular proteins (Gething, 1999; Wickner et al., 1999). Degradation of the protein may then occur through ER-Associated Degradation (ERAD; Thibault and Ng, 2012), or ER-phagy (Bernales et al., 2006). For studies on UPR, ERAD, and ER-phagy in relation to aggregation-prone proteins in P. pastoris, see Kumita et al. (2006), Whyteside et al. (2011), and Vanz et al. (2012). Because the ppαF secretory signal used for V5L2G3-40 directs posttranslational ER translocation (Ng et al., 1996; Plath et al., 1998), it is also possible that the ELP coacervates at 30 °C already in the cytoplasm prior to, or during, translocation. The protein may then be targeted for proteasomal degradation (Ast and Schuldiner, 2011), or proteolytically cleared from the translocon (Ast et al., 2016), respectively. The intermediate yield and nondegradation of V5A2G3-90, which has a similar transition temperature in vitro as the more hydrophobic V5L2G3-40 owing to its length, and the fact that growth at low temperature did not increase the yield of V5A2G3-90 (Section 4.3), suggest that ELP chain length and hydrophobicity differently affect the in vivo transition temperature, intracellular retention, or stress responses.
The observed pH-dependence of ELP yield in P. pastoris (Section 4.3) may indirectly also be related to self-assembly in vivo. The yeast grows well in the pH range 3–7 (Cregg et al., 2000), and to our knowledge there is no documented major influence of the extracellular pH on the transcription and translation machinery. In support of this notion, we have obtained very similar yields at pH 3 and 5 for other types of protein polymers (Domeradzka et al., 2016a; Werten et al., 1999). Hypothetically, complete degradation in the medium of a fraction of the ELP molecules specifically at low pH might cause lower yield. However, incubation of intact ELP (produced at pH 6) with both cell-free and cell-containing medium from a non-ELP culture grown at pH 3 did not result in degradation (Schipperus et al., 2009). The effect can furthermore hardly be explained by pH-responsive behavior of the ELPs in vivo, as they do not contain any amino acids with charged side groups. Another explanation, namely reduced performance of the ITC purification procedure at low pH, was experimentally ruled out (Schipperus et al., 2009). We surmise that the pH-dependent ELP yields might indirectly result from reduced permeability of the yeast cell wall at low pH. Such a change in permeability is evident from studies with S. cerevisiae that showed reduced sensitivity at low pH to ß-1,3-glucanase or Zymolyase (Aguilar-Uscanga and Francois, 2003; Kapteyn et al., 2001), which is a measure for the porosity of the cell wall (De Nobel et al., 1990a, De Nobel et al., 1990b; Zlotnik et al., 1984). A less permeable cell wall at low pH may slow down diffusion of the ELP from the periplasm into the extracellular medium, and ensuing high local polymer concentrations might then trigger coacervation and intracellular retention. We are aware of the speculative nature of the foregoing; further study is needed.
5.3.4. Preventing self-assembly in vivo?
We do not know of any study that attempted to resolve the in vivo self-assembly and nonsecretion of some of the silks, collagen-like molecules, and ELPs in yeast. Still, it is conceivable that fine-tuning of expression levels, e.g., by using promoter libraries (see e.g. Hartner et al., 2008; Qin et al., 2011), may allow the maintenance of low transient in vivo concentrations not conducive to self-assembly. If self-assembly occurs in the periplasm or during passage through the cell wall, it might be worthwhile to evaluate the use of supersecretory cell wall mutants (Larsen et al., 2013; Marx et al., 2006).
The secretion efficiency of various globular proteins in P. pastoris has been improved by overexpression of ER chaperones such as Kar2/BiP (see e.g. Damasceno et al., 2007; Guan et al., 2016; Zhang et al., 2006). These chaperones assist folding by preventing aggregation of unfolded and misfolded species (Gething, 1999; Wickner et al., 1999). However, one should realize that nonsecretion caused by protein misfolding is very different from nonsecretion caused by polymer self-assembly. First, the ER quality control chaperones recognize the unfolded state of globular proteins by binding to exposed hydrophobic patches (Gething, 1999; Wickner et al., 1999). However, most protein polymers, except, e.g., hydrophobic ELPs (Section 5.3.3), are relatively hydrophilic and will not likely interact with these chaperones. Second, whereas common unfolded proteins undergo repeated transient binding and release by chaperones until proper folding has occurred and the protein has achieved a secretion-competent state (Gething, 1999; Wickner et al., 1999), this same dynamic process would not result in a soluble end state for self-assembling protein polymers. Rather, the polymer will (i) eventually still self-assemble and become insoluble, or (ii) be degraded by proteases (Wickner et al., 1999), or (iii) remain part of a stable complex with the chaperone that cannot leave the ER (Gething, 1999). Thus, it is unlikely that coexpression of chaperones such as Kar2/BiP is useful for resolving the nonsecretion of prematurely self-assembling protein polymers.
For short self-assembling sequences, we found that their self-assembly may be prevented by fusion to a large hydrophilic carrier domain (Moers et al., 2010). We employed this strategy for the production in P. pastoris of P11–2, an undecapeptide that strongly self-assembles at any pH (Aggeli et al., 2001). Initial attempts to secrete the free peptide using ppαF did not result in any detectable product. Subsequently, the peptide was produced as an enterokinase-cleavable fusion to the C-terminus of the highly hydrophilic random coil protein polymer CP2 (Section 4.2.1). Also here, ppαF was used to drive secretion. The fused CP2 carrier allowed convenient detection and purification. The fusion protein was recovered from the cell-free broth at 0.8–1.0 g/L, which corresponds to 60–75 mg/L of free peptide. Whereas the fusion protein did not self-assemble in vitro, the free peptide released by enterokinase digestion formed a self-supporting gel. This shows that the highly soluble CP2 domain efficiently suppressed self-assembly, which may be applicable also to other self-assembling peptides.
6. Conclusions and future prospects
Although the bacterium E. coli has been the main microbial workhorse for the production of protein polymers, P. pastoris has emerged as an attractive eukaryotic alternative. The repetitive genes encoding protein polymers are stably integrated into the yeast's genome without the need for selective pressure, and many of the protein polymers expressed in P. pastoris have been successfully produced as secreted proteins. Secretory production has the major advantage of providing a first, very effective, purification step. This is a particularly important factor in the field of protein polymer research, where physicochemical characterization or application testing of the derived supramolecular materials requires considerable amounts. With relatively little purification effort, significant amounts of protein polymers can often be obtained using bench-top bioreactors. Moreover, simple downstream processing is important in the context of eventual commercial viability.
Obviously, no single ideal host for all proteins exists. Nonetheless, P. pastoris does seem particularly efficient for silk-inspired octapeptide repeats and gelatin-like proteins, in that it routinely provides g/L levels for most of these polymers. Spider silks are also efficiently produced, but only in intracellular mode. Elastin-like proteins with high transition temperatures are expressed well, yet P. pastoris seems currently less suited for the production of hydrophobic ELPs, as these appear to self-assemble during secretion. In this respect, intracellular expression of hydrophobic ELPs in P. pastoris would be worth evaluating. The demonstrated possibility to accurately hydroxylate human collagenous sequences in appropriately engineered P. pastoris is a clear benefit over current bacterial systems, although the nonsecretion of trimeric collagen remains to be solved.
Proteolysis is one of the main challenges for the secretory production of protein polymers in P. pastoris. This is because of the typically unfolded character of these proteins, and because protease target sites may well occur multiple times in the repetitive amino acid sequences. However, as discussed, various remedies can be applied, such as optimizing fermentation conditions, applying rational sequence design, or using secretory pathway protease mutants. In relation to proteolysis, it is important to note that all protein polymers in P. pastoris have thus far been produced in methanol-grown cultures. Because cell lysis and accompanying release of proteolytic activity into the medium reportedly occur less on glycerol (Sinha et al., 2005) and glucose (Mattanovich et al., 2009) than on methanol, it would be interesting to investigate the use of methanol-free approaches in situations of persistent degradation. Strong promoters that do not require methanol induction include the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAP) gene (Waterham et al., 1997), the glucose-regulated promoter of the GTH1 gene (Prielhofer et al., 2013; Prielhofer et al., 2018), and synthetic derepression variants of the AOX1 promoter (Hartner et al., 2008; Mellitzer et al., 2014). Also recent methanol-free strategies based on overexpression or deletion of regulators of the wild-type AOX1 promoter appear promising (Chang et al., 2018; Shen et al., 2016; Vogl et al., 2018; Wang et al., 2017). Although we consider the bioprocessing requirements and potential hazards associated with the use of methanol to be well manageable, methanol-free cultivation will likely promote the accessibility of the P. pastoris system for protein polymer production.
With the availability of the P. pastoris genome and synthetic biology tools, many future improvements of this host will expectedly be realized. Systems biology and forthcoming increased understanding of the secretory pathway and the cellular stress responses evoked by protein overproduction will be invaluable (see e.g. Puxbaum et al., 2015; Zahrl et al., 2017). The incorporation of noncanonical amino acids into protein polymers is of great interest, as it allows the creation of materials with novel functionalities (Connor and Tirrell, 2007; Teeuwen et al., 2009a; Wu, I.L. et al., 2013a). Although protein polymers containing noncanonical amino acids have not yet been produced in P. pastoris, efforts towards expanding the genetic code of this host are encouraging (Young et al., 2009). Finally, also theoretical insight into the bioprocessing of the yeast is accumulating. These recent advances will undoubtedly further the utility of P. pastoris as a host for the production of protein polymers.
Acknowledgement
Financial support to M.W.T. Werten, M.A. Cohen Stuart, and F.A. de Wolf is provided by ERC Advanced Grant 267254 “BioMate”.
References
- Agapov I.I., Pustovalova O.L., Moisenovich M.M., Bogush V.G., Sokolova O.S., Sevastyanov V.I., Debabov V.G., Kirpichnikov M.P. Three-dimensional scaffold made from recombinant spider Silk protein for tissue engineering. Dokl. Biochem. Biophys. 2009;426:127–130. doi: 10.1134/s1607672909030016. [DOI] [PubMed] [Google Scholar]
- Aggeli A., Nyrkova I.A., Bell M., Harding R., Carrick L., McLeish T.C., Semenov A.N., Boden N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide ß-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11857–11862. doi: 10.1073/pnas.191250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Uscanga B., Francois J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003;37:268–274. doi: 10.1046/j.1472-765x.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Hirz M., Pichler H., Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014;98:5301–5317. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T.B., DeSimone E., Scheibel T. Biomedical applications of recombinant silk-based materials. Adv. Mater. 2018;30 doi: 10.1002/adma.201704636. [DOI] [PubMed] [Google Scholar]
- Allmeling C., Radtke C., Vogt P.M. Technical and biomedical uses of nature's strongest fiber: spider silk. In: Nentwig W., editor. Spider Ecophysiology. Springer; Berlin Heidelberg, Berlin, Heidelberg: 2013. pp. 475–490. [Google Scholar]
- Altman G.H., Diaz F., Jakuba C., Calabro T., Horan R.L., Chen J., Lu H., Richmond J., Kaplan D.L. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Amiram M., Quiroz F.G., Callahan D.J., Chilkoti A. A highly parallel method for synthesizing DNA repeats enables the discovery of 'smart' protein polymers. Nat. Mater. 2011;10:141–148. doi: 10.1038/nmat2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcidiacono S., Mello C., Kaplan D., Cheley S., Bayley H. Purification and characterization of recombinant spider silk expressed in Escherichia coli. Appl. Microbiol. Biotechnol. 1998;49:31–38. doi: 10.1007/s002530051133. [DOI] [PubMed] [Google Scholar]
- Arias F.J., Santos M., Ibanez-Fonseca A., Pina M.J., Serrano S. Elastin-like recombinamers as smart drug delivery systems. Curr. Drug Targets. 2018;19:360–379. doi: 10.2174/1389450117666160201114617. [DOI] [PubMed] [Google Scholar]
- Asghar A., Henrickson R.L. Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. Adv. Food Res. 1982;28:231–372. doi: 10.1016/s0065-2628(08)60113-5. [DOI] [PubMed] [Google Scholar]
- Ash J., Dominguez M., Bergeron J.J., Thomas D.Y., Bourbonnais Y. The yeast proprotein convertase encoded by YAP3 is a glycophosphatidylinositol-anchored protein that localizes to the plasma membrane. J. Biol. Chem. 1995;270:20847–20854. doi: 10.1074/jbc.270.35.20847. [DOI] [PubMed] [Google Scholar]
- Ast T., Schuldiner M. Protein degradation: BAGging up the trash. Curr. Biol. 2011;21 doi: 10.1016/j.cub.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Ast T., Michaelis S., Schuldiner M. The protease Ste24 clears clogged translocons. Cell. 2016;164:103–114. doi: 10.1016/j.cell.2015.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub N.A., Garb J.E., Tinghitella R.M., Collin M.A., Hayashi C.Y. Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb P.L., Lahens N.F., Correa-Garhwal S.M., Nicholson D.N., Kim E.J., Hogenesch J.B., Kuntner M., Higgins L., Hayashi C.Y., Agnarsson I., Voight B.F. The Nephila clavipes genome highlights the diversity of spider silk genes and their complex expression. Nat. Genet. 2017;49:895–903. doi: 10.1038/ng.3852. [DOI] [PubMed] [Google Scholar]
- Bader O., Krauke Y., Hube B. Processing of predicted substrates of fungal Kex2 proteinases from Candida albicans, C. glabrata, Saccharomyces cerevisiae and Pichia pastoris. BMC Microbiol. 2008;8:116. doi: 10.1186/1471-2180-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez J., Olsen D., Polarek J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 2005;69:245–252. doi: 10.1007/s00253-005-0180-x. [DOI] [PubMed] [Google Scholar]
- Bernales S., McDonald K.L., Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beun L.H., Beaudoux X.J., Kleijn J.M., de Wolf F.A., Stuart M.A. Self-assembly of silk-collagen-like triblock copolymers resembles a supramolecular living polymerization. ACS Nano. 2012;6:133–140. doi: 10.1021/nn203092u. [DOI] [PubMed] [Google Scholar]
- Beun L.H., Storm I.M., Werten M.W.T., de Wolf F.A., Cohen Stuart M.A., de Vries R. From micelles to fibers: balancing self-assembling and random coiling domains in pH-responsive silk-collagen-like protein-based polymers. Biomacromolecules. 2014;15:3349–3357. doi: 10.1021/bm500826y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan A., Brenner C., Fuller R.S. Quantitative assessment of enzyme specificity in vivo: P2 recognition by Kex2 protease defined in a genetic system. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10384–10389. doi: 10.1073/pnas.95.18.10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge T.A., Perez-Rigueiro J., Plaza G.R., Perea B., Navarro A., Guinea G.V., Elices M. Sequential origin in the high performance properties of orb spider dragline silk. Sci. Rep. 2012;2:782. doi: 10.1038/srep00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Pirie-Shepherd S., Trinh L.B., Shiloach J., Folkman J. Disruption of the KEX1 gene in Pichia pastoris allows expression of full-length murine and human endostatin. Yeast. 1999;15:563–572. doi: 10.1002/(SICI)1097-0061(199905)15:7<563::AID-YEA398>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bogush V.G., Sokolova O.S., Davydova L.I., Klinov D.V., Sidoruk K.V., Esipova N.G., Neretina T.V., Orchanskyi I.A., Makeev V.Y., Tumanyan V.G., Shaitan K.V., Debabov V.G., Kirpichnikov M.P. A novel model system for design of biomaterials based on recombinant analogs of spider silk proteins. J. Neuroimmune Pharmacol. 2009;4:17–27. doi: 10.1007/s11481-008-9129-z. [DOI] [PubMed] [Google Scholar]
- Bogush V.G., Sidoruk K.V., Davydova L.I., Zalunin I.A., Kozlov D.G., Moisenovich M.M., Agapov I.I., Kirpichnikov M.P., Debabov V.G. Recombinant analogue of spidroin 2 for biomedical materials. Dokl. Biochem. Biophys. 2011;441:276–279. doi: 10.1134/S1607672911060093. [DOI] [PubMed] [Google Scholar]
- Bourbonnais Y., Bolin D., Shields D. Secretion of somatostatin by Saccharomyces cerevisiae. Correct proteolytic processing of pro-α-factor-somatostatin hybrids requires the products of the KEX2 and STE13 genes. J. Biol. Chem. 1988;263:15342–15347. [PubMed] [Google Scholar]
- Bourbonnais Y., Germain D., Ash J., Thomas D.Y. Cleavage of prosomatostatins by the yeast Yap3 and Kex2 endoprotease. Biochimie. 1994;76:226–233. doi: 10.1016/0300-9084(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Boutris C., Chatzi E.G., Kiparissides C. Characterization of the LCST behaviour of aqueous poly(N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer. 1997;38:2567–2570. [Google Scholar]
- Bouwstra, J., Toda, Y., 2005. U.S. Pat. 20050119170.
- Brake A.J. α-Factor leader-directed secretion of heterologous proteins from yeast. Methods Enzymol. 1990;185:408–421. doi: 10.1016/0076-6879(90)85036-n. [DOI] [PubMed] [Google Scholar]
- Brake A.J., Merryweather J.P., Coit D.G., Heberlein U.A., Masiarz F.R., Mullenbach G.T., Urdea M.S., Valenzuela P., Barr P.J. α-Factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 1984;81:4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C., Fuller R.S. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc. Natl. Acad. Sci. U. S. A. 1992;89:922–926. doi: 10.1073/pnas.89.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.J., Boyd A. Immunoisolation of Kex2p-containing organelles from yeast demonstrates colocalisation of three processing proteinases to a single Golgi compartment. J. Cell Sci. 1993;106:815–822. doi: 10.1242/jcs.106.3.815. [DOI] [PubMed] [Google Scholar]
- Cantor E.J., Atkins E.D., Cooper S.J., Fournier M.J., Mason T.L., Tirrell D.A. Effects of amino acid side-chain volume on chain packing in genetically engineered periodic polypeptides. J. Biochem. 1997;122:217–225. doi: 10.1093/oxfordjournals.jbchem.a021732. [DOI] [PubMed] [Google Scholar]
- Cappello J. The biological production of protein polymers and their use. Trends Biotechnol. 1990;8:309–311. doi: 10.1016/0167-7799(90)90207-e. [DOI] [PubMed] [Google Scholar]
- Cappello J., Ferrari F. Microbial production of structural protein polymers. In: Mobley D.P., editor. Plastics from microbes. Carl Hanser Verlag; Munich: 1994. pp. 35–92. [Google Scholar]
- Cappello J., Crissman J., Dorman M., Mikolajczak M., Textor G., Marquet M., Ferrari F. Genetic engineering of structural protein polymers. Biotechnol. Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- Cardin A.D., Weintraub H.J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Cawley N.X., Olsen V., Zhang C.F., Chen H.C., Tan M., Loh Y.P. Activation and processing of non-anchored yapsin 1 (Yap3p) J. Biol. Chem. 1998;273:584–591. doi: 10.1074/jbc.273.1.584. [DOI] [PubMed] [Google Scholar]
- Cereghino G.P., Cereghino J.L., Ilgen C., Cregg J.M. Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Curr. Opin. Biotechnol. 2002;13:329–332. doi: 10.1016/s0958-1669(02)00330-0. [DOI] [PubMed] [Google Scholar]
- Cereghino J.L., Cregg J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000;24:45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Cetinkaya M., Xiao S., Markert B., Stacklies W., Grater F. Silk fiber mechanics from multiscale force distribution analysis. Biophys. J. 2011;100:1298–1305. doi: 10.1016/j.bpj.2010.12.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Hsiung H.A., Hong K.L., Huang C.T. Enhancing the efficiency of the Pichia pastoris AOX1 promoter via the synthetic positive feedback circuit of transcription factor Mxr1. BMC Biotechnol. 2018;18:81. doi: 10.1186/s12896-018-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilkoti A., Dreher M.R., Meyer D.E. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv. Drug Deliv. Rev. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Chu H.S., Ryum J., Park S.Y., Kim B.G., Kim D.M., Won J.I. A new cloning strategy for generating multiple repeats of a repetitive polypeptide based on non-template PCR. Biotechnol. Lett. 2011;33:977–983. doi: 10.1007/s10529-010-0510-7. [DOI] [PubMed] [Google Scholar]
- Clare J.J., Romanos M.A., Rayment F.B., Rowedder J.E., Smith M.A., Payne M.M., Sreekrishna K., Henwood C.A. Production of mouse epidermal growth factor in yeast: high-level secretion using Pichia pastoris strains containing multiple gene copies. Gene. 1991;105:205–212. doi: 10.1016/0378-1119(91)90152-2. [DOI] [PubMed] [Google Scholar]
- Connor R.E., Tirrell D.A. Non-canonical amino acids in protein polymer design. Polym. Rev. 2007;47:9–28. [Google Scholar]
- Cos O., Ramon R., Montesinos J.L., Valero F. Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microb. Cell. Fact. 2006;5:17. doi: 10.1186/1475-2859-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc R., Baratti J. Oxidation of methanol by the yeast, Pichia pastoris. Purification and properties of the alcohol oxidase. Agric. Biol. Chem. 1980;44:2279–2289. [Google Scholar]
- Coyne K.J., Qin X.X., Waite J.H. Extensible collagen in mussel byssus: a natural block copolymer. Science. 1997;277:1830–1832. doi: 10.1126/science.277.5333.1830. [DOI] [PubMed] [Google Scholar]
- Craig C.L. Evolution of arthropod silks. Ann. Rev. Entomol. 1997;42:231–267. doi: 10.1146/annurev.ento.42.1.231. [DOI] [PubMed] [Google Scholar]
- Cregg J.M., Cereghino J.L., Shi J., Higgins D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000;16:23–52. doi: 10.1385/MB:16:1:23. [DOI] [PubMed] [Google Scholar]
- Daly R., Hearn M.T. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- Damasceno L.M., Anderson K.A., Ritter G., Cregg J.M., Old L.J., Batt C.A. Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Appl. Microbiol. Biotechnol. 2007;74:381–389. doi: 10.1007/s00253-006-0652-7. [DOI] [PubMed] [Google Scholar]
- Damasceno L.M., Huang C.J., Batt C.A. Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 2012;93:31–39. doi: 10.1007/s00253-011-3654-z. [DOI] [PubMed] [Google Scholar]
- De Nobel J.G., Klis F.M., Munnik T., Priem J., van den Ende H. An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast. 1990;6:483–490. doi: 10.1002/yea.320060605. [DOI] [PubMed] [Google Scholar]
- De Nobel J.G., Klis F.M., Priem J., Munnik T., van den Ende H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990;6:491–499. doi: 10.1002/yea.320060606. [DOI] [PubMed] [Google Scholar]
- De Schutter K., Lin Y.C., Tiels P., Van Hecke A., Glinka S., Weber-Lehmann J., Rouze P., Van de Peer Y., Callewaert N. Genome sequence of the recombinant protein production host Pichia pastoris. Nat. Biotechnol. 2009;27:561–566. doi: 10.1038/nbt.1544. [DOI] [PubMed] [Google Scholar]
- De Wolf F.A., Werten M.W.T., Wisselink W.H., Jansen-van den Bosch T.J., Toda Y., van Heerde G.V., Bouwstra J.B. Eur. Pat. Appl. 2000:1014176. [Google Scholar]
- DeFrancesco L. Hanging on a thread. Nat. Biotechnol. 2017;35:496–499. doi: 10.1038/nbt.3894. [DOI] [PubMed] [Google Scholar]
- Desai M.S., Lee S.W. Protein-based functional nanomaterial design for bioengineering applications. WIREs Nanomed. Nanobiotechnol. 2015;7:69–97. doi: 10.1002/wnan.1303. [DOI] [PubMed] [Google Scholar]
- DiMarco R.L., Heilshorn S.C. Multifunctional materials through modular protein engineering. Adv. Mater. 2012;24:3923–3940. doi: 10.1002/adma.201200051. [DOI] [PubMed] [Google Scholar]
- Dmochowska A., Dignard D., Henning D., Thomas D.Y., Bussey H. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and α-factor precursor processing. Cell. 1987;50:573–584. doi: 10.1016/0092-8674(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Doblhofer E., Heidebrecht A., Scheibel T. To spin or not to spin: spider silk fibers and more. Appl. Microbiol. Biotechnol. 2015;99:9361–9380. doi: 10.1007/s00253-015-6948-8. [DOI] [PubMed] [Google Scholar]
- Domeradzka N.E., Werten M.W.T., de Vries R., de Wolf F.A. Production in Pichia pastoris of protein-based polymers with small heterodimer-forming blocks. Biotechnol. Bioeng. 2016;113:953–960. doi: 10.1002/bit.25861. [DOI] [PubMed] [Google Scholar]
- Domeradzka N.E., Werten M.W.T., de Vries R., de Wolf F.A. Production in Pichia pastoris of complementary protein-based polymers with heterodimer-forming WW and PPxY domains. Microb. Cell. Fact. 2016;15:105. doi: 10.1186/s12934-016-0498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeradzka N.E., Werten M.W.T., de Wolf F.A., de Vries R. Cross-linking and bundling of self-assembled protein-based polymer fibrils via heterodimeric coiled coils. Biomacromolecules. 2016;17:3893–3901. doi: 10.1021/acs.biomac.6b01242. [DOI] [PubMed] [Google Scholar]
- Domeradzka N.E., Werten M.W.T., de Wolf F.A., de Vries R. Protein cross-linking tools for the construction of nanomaterials. Curr. Opin. Biotechnol. 2016;39:61–67. doi: 10.1016/j.copbio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Dreyer T. Substrate specificity of proteinase yscA from Saccharomyces cerevisiae. Carlsberg Res. Commun. 1989;54:85–97. doi: 10.1007/BF02908301. [DOI] [PubMed] [Google Scholar]
- Egel-Mitani M., Hansen M.T. Nucleotide sequence of the gene encoding the Saccharomyces kluyveri α mating pheromone. Nucleic Acids Res. 1987;15:6303. doi: 10.1093/nar/15.15.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel-Mitani M., Flygenring H.P., Hansen M.T. A novel aspartyl protease allowing KEX2-independent MFα propheromone processing in yeast. Yeast. 1990;6:127–137. doi: 10.1002/yea.320060206. [DOI] [PubMed] [Google Scholar]
- Engel J., Chen H.T., Prockop D.J., Klump H. The triple helix in equilibrium with coil conversion of collagen-like polytripeptides in aqueous and nonaqueous solvents. Comparison of the thermodynamic parameters and the binding of water to (L-Pro-L-Pro-Gly)n and (L-Pro-L-Hyp-Gly)n. Biopolymers. 1977;16:601–622. doi: 10.1002/bip.1977.360160310. [DOI] [PubMed] [Google Scholar]
- Estrich N.A., Hernandez-Garcia A., de Vries R., LaBean T.H. Engineered diblock polypeptides improve DNA and gold solubility during molecular assembly. ACS Nano. 2017;11:831–842. doi: 10.1021/acsnano.6b07291. [DOI] [PubMed] [Google Scholar]
- Fagerholm P., Lagali N.S., Ong J.A., Merrett K., Jackson W.B., Polarek J.W., Suuronen E.J., Liu Y., Brunette I., Griffith M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35:2420–2427. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]
- Fahnestock S.R., Bedzyk L.A. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 1997;47:33–39. doi: 10.1007/s002530050884. [DOI] [PubMed] [Google Scholar]
- Fahnestock S.R., Irwin S.L. Synthetic spider dragline silk proteins and their production in Escherichia coli. Appl. Microbiol. Biotechnol. 1997;47:23–32. doi: 10.1007/s002530050883. [DOI] [PubMed] [Google Scholar]
- Fahnestock S.R., Yao Z., Bedzyk L.A. Microbial production of spider silk proteins. J. Biotechnol. 2000;74:105–119. doi: 10.1016/s1389-0352(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Felber M., Pichler H., Ruth C. Strains and molecular tools for recombinant protein production in Pichia pastoris. Methods Mol. Biol. 2014;1152:87–111. doi: 10.1007/978-1-4939-0563-8_5. [DOI] [PubMed] [Google Scholar]
- Ferrari, F.A., Richardson, C., Chambers, J., Causey, S.C., Pollock, T.J., 1987. Construction of synthetic DNA and its use in large polypeptide synthesis. Pat. Appl. WO/1988/003533.
- Foster J.A., Bruenger E., Gray W.R., Sandberg L.B. Isolation and amino acid sequences of tropoelastin peptides. J. Biol. Chem. 1973;248:2876–2879. [PubMed] [Google Scholar]
- Frandsen J.L., Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chem. Soc. Rev. 2012;41:2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- Frank S., Kammerer R.A., Mechling D., Schulthess T., Landwehr R., Bann J., Guo Y., Lustig A., Bachinger H.P., Engel J. Stabilization of short collagen-like triple helices by protein engineering. J. Mol. Biol. 2001;308:1081–1089. doi: 10.1006/jmbi.2001.4644. [DOI] [PubMed] [Google Scholar]
- Freeman R., Boekhoven J., Dickerson M.B., Naik R.R., Stupp S.I. Biopolymers and supramolecular polymers as biomaterials for biomedical applications. MRS Bull. 2015;40:1089–1101. doi: 10.1557/mrs.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacko M. Elastin: structure, properties and metabolism. Cell. Mol. Biol. Lett. 2000;5:327–348. [Google Scholar]
- Gagnon-Arsenault I., Tremblay J., Bourbonnais Y. Fungal yapsins and cell wall: a unique family of aspartic peptidases for a distinctive cellular function. FEMS Yeast Res. 2006;6:966–978. doi: 10.1111/j.1567-1364.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- Gagnon-Arsenault I., Parise L., Tremblay J., Bourbonnais Y. Activation mechanism, functional role and shedding of glycosylphosphatidylinositol-anchored Yps1p at the Saccharomyces cerevisiae cell surface. Mol. Microbiol. 2008;69:982–993. doi: 10.1111/j.1365-2958.2008.06339.x. [DOI] [PubMed] [Google Scholar]
- Gaines W.A., Marcotte W.R., Jr. Recombinant dragline silk-like proteins-expression and purification. AATCC Rev. 2011;11:75–79. [PMC free article] [PubMed] [Google Scholar]
- Gardner, K., Lock, R.L., O'Brien, J.P., Salemme, F.R., 1992. Collagen-like polypeptides. Pat. Appl. PCT/US92/09655.
- Gasser B., Steiger M.G., Mattanovich D. Methanol regulated yeast promoters: production vehicles and toolbox for synthetic biology. Microb. Cell. Fact. 2015;14:196. doi: 10.1186/s12934-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelse K., Poschl E., Aigner T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gething M.J. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- Girotti A., Fernandez-Colino A., Lopez I.M., Rodriguez-Cabello J.C., Arias F.J. Elastin-like recombinamers: biosynthetic strategies and biotechnological applications. Biotechnol. J. 2011;6:1174–1186. doi: 10.1002/biot.201100116. [DOI] [PubMed] [Google Scholar]
- Gleeson M.A., White C.E., Meininger D.P., Komives E.A. Generation of protease-deficient strains and their use in heterologous protein expression. Methods Mol. Biol. 1998;103:81–94. doi: 10.1385/0-89603-421-6:81. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Salerno A.J., Patterson T., Williams J.I. Cloning and expression of a collagen-analog-encoding synthetic gene in Escherichia coli. Gene. 1989;80:305–314. doi: 10.1016/0378-1119(89)90294-1. [DOI] [PubMed] [Google Scholar]
- Golinska M.D., Pham T.T.H., Werten M.W.T., de Wolf F.A., Cohen Stuart M.A., van der Gucht J. Fibril formation by pH and temperature responsive silk-elastin block copolymers. Biomacromolecules. 2013;14:48–55. [Google Scholar]
- Golinska M.D., Włodarczyk-Biegun M.K., Werten M.W.T., Cohen Stuart M.A., de Wolf F.A., de Vries R. Dilute self-healing hydrogels of silk-collagen-like block copolypeptides at neutral pH. Biomacromolecules. 2014;15:699–706. doi: 10.1021/bm401682n. [DOI] [PubMed] [Google Scholar]
- Gorbet M.B., Sefton M.V. Endotoxin: the uninvited guest. Biomaterials. 2005;26:6811–6817. doi: 10.1016/j.biomaterials.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Gosline J.M., Guerette P.A., Ortlepp C.S., Savage K.N. The mechanical design of spider silks: from fibroin sequence to mechanical function. J. Exp. Biol. 1999;202:3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- Govindappa N., Hanumanthappa M., Venkatarangaiah K., Periyasamy S., Sreenivas S., Soni R., Sastry K. A new signal sequence for recombinant protein secretion in Pichia pastoris. J. Microbiol. Biotechnol. 2014;24:337–345. doi: 10.4014/jmb.1308.08085. [DOI] [PubMed] [Google Scholar]
- Gray W.R., Sandberg L.B., Foster J.A. Molecular model for elastin structure and function. Nature. 1973;246:461–466. doi: 10.1038/246461a0. [DOI] [PubMed] [Google Scholar]
- Grunwald I., Rischka K., Kast S.M., Scheibel T., Bargel H. Mimicking biopolymers on a molecular scale: nano(bio)technology based on engineered proteins. Philos. Trans. R. Soc. A. 2009;367:1727–1747. doi: 10.1098/rsta.2009.0012. [DOI] [PubMed] [Google Scholar]
- Guan B., Chen F., Su S., Duan Z., Chen Y., Li H., Jin J. Effects of co-overexpression of secretion helper factors on the secretion of a HSA fusion protein (IL2-HSA) in Pichia pastoris. Yeast. 2016;33:587–600. doi: 10.1002/yea.3183. [DOI] [PubMed] [Google Scholar]
- Guda C., Zhang X., McPherson D.T., Xu J., Cherry J.H., Urry D.W., Daniell H. Hyper expression of an environmentally friendly synthetic polymer gene. Biotechnol. Lett. 1995;17:745–750. [Google Scholar]
- Guda C., Lee S.-B., Daniell H. Stable expression of a biodegradable protein-based polymer in tobacco chloroplasts. Plant Cell Reports. 2000;19:257–262. doi: 10.1007/s002990050008. [DOI] [PubMed] [Google Scholar]
- Guisez Y., Tison B., Vandekerckhove J., Demolder J., Bauw G., Haegeman G., Fiers W., Contreras R. Production and purification of recombinant human interleukin-6 secreted by the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1991;198:217–222. doi: 10.1111/j.1432-1033.1991.tb16004.x. [DOI] [PubMed] [Google Scholar]
- Hakimi O., Knight D.P., Vollrath F., Vadgama P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007;38:324–337. [Google Scholar]
- Hartner F.S., Ruth C., Langenegger D., Johnson S.N., Hyka P., Lin-Cereghino G.P., Lin-Cereghino J., Kovar K., Cregg J.M., Glieder A. Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucleic Acids Res. 2008;36 doi: 10.1093/nar/gkn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasslacher M., Schall M., Hayn M., Bona R., Rumbold K., Luckl J., Griengl H., Kohlwein S.D., Schwab H. High-level intracellular expression of hydroxynitrile lyase from the tropical rubber tree Hevea brasiliensis in microbial hosts. Protein Expr. Purif. 1997;11:61–71. doi: 10.1006/prep.1997.0765. [DOI] [PubMed] [Google Scholar]
- Hayashi C.Y., Lewis R.V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 1998;275:773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- Heidebrecht A., Scheibel T. Recombinant production of spider silk proteins. Adv. Appl. Microbiol. 2013;82:115–153. doi: 10.1016/B978-0-12-407679-2.00004-1. [DOI] [PubMed] [Google Scholar]
- Heim M., Keerl D., Scheibel T. Spider silk: from soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. Engl. 2009;48:3584–3596. doi: 10.1002/anie.200803341. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia A., Werten M.W.T., Cohen Stuart M.A., de Wolf F.A., de Vries R. Coating of single DNA molecules by genetically engineered protein diblock copolymers. Small. 2012;8:3491–3501. doi: 10.1002/smll.201200939. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia A., Kraft D.J., Janssen A.F.J., Bomans P.H.H., Sommerdijk N.A.J.M., Thies-Weesie D.M.E., Favretto M.E., Brock R., de Wolf F.A., Werten M.W.T., van der Schoot P., Cohen Stuart M.A., de Vries R. Design and self-assembly of simple coat proteins for artificial viruses. Nat. Nanotechnol. 2014;9:698–702. doi: 10.1038/nnano.2014.169. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia A., Estrich N.A., Werten M.W.T., Van Der Maarel J.R.C., LaBean T.H., de Wolf F.A., Cohen Stuart M.A., de Vries R. Precise coating of a wide range of DNA templates by a protein polymer with a DNA binding domain. ACS Nano. 2016;11:144–152. doi: 10.1021/acsnano.6b05938. [DOI] [PubMed] [Google Scholar]
- Herzog R.W., Singh N.K., Urry D.W., Daniell H. Expression of a synthetic protein-based polymer (elastomer) gene in Aspergillus nidulans. Appl. Microbiol. Biotechnol. 1997;47:368–372. doi: 10.1007/s002530050942. [DOI] [PubMed] [Google Scholar]
- Heslot H. Artificial fibrous proteins: a review. Biochimie. 1998;80:19–31. doi: 10.1016/s0300-9084(98)80053-9. [DOI] [PubMed] [Google Scholar]
- Hinek A., Rabinovitch M. 67-kD elastin-binding protein is a protective "companion" of extracellular insoluble elastin and intracellular tropoelastin. J. Cell Biol. 1994;126:563–574. doi: 10.1083/jcb.126.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman M.B., Jones J.A., Lewis R.V. Synthetic spider silk: a modular fiber. Trends Biotechnol. 2000;18:374–379. doi: 10.1016/s0167-7799(00)01481-5. [DOI] [PubMed] [Google Scholar]
- Holden G., Bishop E.T., Legge N.R. Thermoplastic elastomers. J. Polym. Sci. Pol. Sym. 1969;26:37–57. [Google Scholar]
- Hommelsheim C.M., Frantzeskakis L., Huang M., Ulker B. PCR amplification of repetitive DNA: a limitation to genome editing technologies and many other applications. Sci. Rep. 2014;4:5052. doi: 10.1038/srep05052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D., Gomathinayagam S., Lynaugh H., Stadheim T.A., Hamilton S.R. Elimination of diaminopeptidase activity in Pichia pastoris for therapeutic protein production. Appl. Microbiol. Biotechnol. 2014;98:2573–2583. doi: 10.1007/s00253-013-5468-7. [DOI] [PubMed] [Google Scholar]
- Hori H., Hattori S., Inouye S., Kimura A., Irie S., Miyazawa H., Sakaguchi M. Analysis of the major epitope of the α2 chain of bovine type I collagen in children with bovine gelatin allergy. J. Allergy Clin. Immunol. 2002;110:652–657. doi: 10.1067/mai.2002.127862. [DOI] [PubMed] [Google Scholar]
- Huang W., Rollett A., Kaplan D.L. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015;12:779–791. doi: 10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huemmerich D., Scheibel T., Vollrath F., Cohen S., Gat U., Ittah S. Novel assembly properties of recombinant spider dragline silk proteins. Curr. Biol. 2004;14:2070–2074. doi: 10.1016/j.cub.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Idiris A., Bi K., Tohda H., Kumagai H., Giga-Hama Y. Construction of a protease-deficient strain set for the fission yeast Schizosaccharomyces pombe, useful for effective production of protease-sensitive heterologous proteins. Yeast. 2006;23:83–99. doi: 10.1002/yea.1342. [DOI] [PubMed] [Google Scholar]
- Idiris A., Tohda H., Kumagai H., Takegawa K. Engineering of protein secretion in yeast: strategies and impact on protein production. Appl. Microbiol. Biotechnol. 2010;86:403–417. doi: 10.1007/s00253-010-2447-0. [DOI] [PubMed] [Google Scholar]
- Jansson R., Thatikonda N., Lindberg D., Rising A., Johansson J., Nygren P.A., Hedhammar M. Recombinant spider silk genetically functionalized with affinity domains. Biomacromolecules. 2014;15:1696–1706. doi: 10.1021/bm500114e. [DOI] [PubMed] [Google Scholar]
- Jansson R., Lau C.H., Ishida T., Ramstrom M., Sandgren M., Hedhammar M. Functionalized silk assembled from a recombinant spider silk fusion protein (Z-4RepCT) produced in the methylotrophic yeast Pichia pastoris. Biotechnol. J. 2016;11:687–699. doi: 10.1002/biot.201500412. [DOI] [PubMed] [Google Scholar]
- Jekhmane S., de Haas R., Paulino Da Silva Filho O., Van Asbeck A.H., Favretto M.E., Hernandez Garcia A., Brock R., De Vries R. Virus-like particles of mRNA with artificial minimal coat proteins: particle formation, stability, and transfection efficiency. Nucleic Acid Ther. 2017;27:159–167. doi: 10.1089/nat.2016.0660. [DOI] [PubMed] [Google Scholar]
- Jensen C.L., Stephenson K., Jorgensen S.T., Harwood C. Cell-associated degradation affects the yield of secreted engineered and heterologous proteins in the Bacillus subtilis expression system. Microbiology. 2000;146(Pt 10):2583–2594. doi: 10.1099/00221287-146-10-2583. [DOI] [PubMed] [Google Scholar]
- Jones E.W. Three proteolytic systems in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1991;266:7963–7966. [PubMed] [Google Scholar]
- Jones E.W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- Julien C. Production of humanlike recombinant proteins in Pichia pastoris. Bioprocess Int. 2006;4:22–31. [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast α factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983;32:839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Juturu V., Wu J.C. Heterologous protein expression in Pichia pastoris: latest research progress and applications. ChemBioChem. 2018;19:7–21. doi: 10.1002/cbic.201700460. [DOI] [PubMed] [Google Scholar]
- Kabanov V.A., Kabanov A.V. Interpolyelectrolyte and block ionomer complexes for gene delivery: physico-chemical aspects. Adv. Drug Deliv. Rev. 1998;30:49–60. doi: 10.1016/s0169-409x(97)00106-3. [DOI] [PubMed] [Google Scholar]
- Kakizawa Y., Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv. Drug Deliv. Rev. 2002;54:203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Kang H.A., Kim S.J., Choi E.S., Rhee S.K., Chung B.H. Efficient production of intact human parathyroid hormone in a Saccharomyces cerevisiae mutant deficient in yeast aspartic protease 3 (YAP3) Appl. Microbiol. Biotechnol. 1998;50:187–192. doi: 10.1007/s002530051275. [DOI] [PubMed] [Google Scholar]
- Kang Z., Huang H., Zhang Y., Du G., Chen J. Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications. World J. Microbiol. Biotechnol. 2017;33:19. doi: 10.1007/s11274-016-2185-2. [DOI] [PubMed] [Google Scholar]
- Kapteyn J.C., ter Riet B., Vink E., Blad S., De Nobel H., Van Den Ende H., Klis F.M. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001;39:469–479. doi: 10.1046/j.1365-2958.2001.02242.x. [DOI] [PubMed] [Google Scholar]
- Kay B.K., Williamson M.P., Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- Keizer-Gunnink I., Vuorela A., Myllyharju J., Pihlajaniemi T., Kivirikko K.I., Veenhuis M. Accumulation of properly folded human type III procollagen molecules in specific intracellular membranous compartments in the yeast Pichia pastoris. Matrix Biol. 2000;19:29–36. doi: 10.1016/s0945-053x(99)00059-1. [DOI] [PubMed] [Google Scholar]
- Kempe T., Kent S.B., Chow F., Peterson S.M., Sundquist W.I., L'Italien J.J., Harbrecht D., Plunkett D., DeLorbe W.J. Multiple-copy genes: production and modification of monomeric peptides from large multimeric fusion proteins. Gene. 1985;39:239–245. doi: 10.1016/0378-1119(85)90318-x. [DOI] [PubMed] [Google Scholar]
- Kim W. Recombinant protein polymers in biomaterials. Front Biosci. 2013;18:289–304. doi: 10.2741/4100. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T., Brandt J., Andersen A.S., Egel-Mitani M., Hach M., Pettersson A.F., Vad K. A removable spacer peptide in an α-factor-leader/insulin precursor fusion protein improves processing and concomitant yield of the insulin precursor in Saccharomyces cerevisiae. Gene. 1996;170:107–112. doi: 10.1016/0378-1119(95)00822-5. [DOI] [PubMed] [Google Scholar]
- Knipe J.M., Peppas N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen Biomater. 2014;1:57–65. doi: 10.1093/rb/rbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano H., Fuller R.S. Shared functions in vivo of a glycosyl-phosphatidylinositol-linked aspartyl protease, Mkc7, and the proprotein processing protease Kex2 in yeast. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10752–10756. doi: 10.1073/pnas.92.23.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano H., Rockwell N., Wang G.T., Krafft G.A., Fuller R.S. Purification and characterization of the yeast glycosylphosphatidylinositol-anchored, monobasic-specific aspartyl protease yapsin 2 (Mkc7p) J. Biol. Chem. 1999;274:24431–24437. doi: 10.1074/jbc.274.34.24431. [DOI] [PubMed] [Google Scholar]
- Kominami E., Hoffschulte H., Leuschel L., Maier K., Holzer H. The substrate specificity of proteinase B from baker's yeast. Biochim. Biophys. Acta. 1981;661:136–141. doi: 10.1016/0005-2744(81)90092-9. [DOI] [PubMed] [Google Scholar]
- Kozel B.A., Rongish B.J., Czirok A., Zach J., Little C.D., Davis E.C., Knutsen R.H., Wagenseil J.E., Levy M.A., Mecham R.P. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J. Cell. Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Kreil G. Processing of precursors by dipeptidylaminopeptidases: a case of molecular ticketing. Trends Biochem. Sci. 1990;15:23–26. doi: 10.1016/0968-0004(90)90126-v. [DOI] [PubMed] [Google Scholar]
- Krejchi M.T., Atkins E.D., Waddon A.J., Fournier M.J., Mason T.L., Tirrell D.A. Chemical sequence control of ß-sheet assembly in macromolecular crystals of periodic polypeptides. Science. 1994;265:1427–1432. doi: 10.1126/science.8073284. [DOI] [PubMed] [Google Scholar]
- Krysan D.J., Ting E.L., Abeijon C., Kroos L., Fuller R.S. Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:1364–1374. doi: 10.1128/EC.4.8.1364-1374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küberl A., Schneider J., Thallinger G.G., Anderl I., Wibberg D., Hajek T., Jaenicke S., Brinkrolf K., Goesmann A., Szczepanowski R., Pühler A., Schwab H., Glieder A., Pichler H. High-quality genome sequence of Pichia pastoris CBS7435. J. Biotechnol. 2011;154:312–320. doi: 10.1016/j.jbiotec.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Kumita J.R., Johnson R.J., Alcocer M.J., Dumoulin M., Holmqvist F., McCammon M.G., Robinson C.V., Archer D.B., Dobson C.M. Impact of the native-state stability of human lysozyme variants on protein secretion by Pichia pastoris. FEBS J. 2006;273:711–720. doi: 10.1111/j.1742-4658.2005.05099.x. [DOI] [PubMed] [Google Scholar]
- Kurihara H., Morita T., Shinkai M., Nagamune T. Recombinant extracellular matrix-like proteins with repetitive elastin or collagen-like functional motifs. Biotechnol. Lett. 2005;27:665–670. doi: 10.1007/s10529-005-4477-8. [DOI] [PubMed] [Google Scholar]
- Kurtzman C.P. Biotechnological strains of Komagataella (Pichia) pastoris are Komagataella phaffii as determined from multigene sequence analysis. J. Ind. Microbiol. Biotechnol. 2009;36:1435–1438. doi: 10.1007/s10295-009-0638-4. [DOI] [PubMed] [Google Scholar]
- Lakshmanan A., Zhang S., Hauser C.A. Short self-assembling peptides as building blocks for modern nanodevices. Trends Biotechnol. 2012;30:155–165. doi: 10.1016/j.tibtech.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Larsen S., Weaver J., de Sa Campos K., Bulahan R., Nguyen J., Grove H., Huang A., Low L., Tran N., Gomez S., Yau J., Ilustrisimo T., Kawilarang J., Lau J., Tranphung M., Chen I., Tran C., Fox M., Lin-Cereghino J., Lin-Cereghino G.P. Mutant strains of Pichia pastoris with enhanced secretion of recombinant proteins. Biotechnol. Lett. 2013;35:1925–1935. doi: 10.1007/s10529-013-1290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Lewis R.V., Hinman M., Kothakota S., Fournier M.J. Expression and purification of a spider silk protein: a new strategy for producing repetitive proteins. Protein Expr. Purif. 1996;7:400–406. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- Li G., Li Y., Chen G., He J., Han Y., Wang X., Kaplan D.L. Silk-based biomaterials in biomedical textiles and fiber-based implants. Adv. Healthc. Mater. 2015;4:1134–1151. doi: 10.1002/adhm.201500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.K., Garcia Quiroz F., Hall C.K., Chilkoti A., Yingling Y.G. Molecular description of the LCST behavior of an elastin-like polypeptide. Biomacromolecules. 2014;15:3522–3530. doi: 10.1021/bm500658w. [DOI] [PubMed] [Google Scholar]
- Li Q., Yi L., Hoi K.H., Marek P., Georgiou G., Iverson B.L. Profiling protease specificity: combining yeast ER sequestration screening (YESS) with next generation sequencing. ACS Chem. Biol. 2017;12:510–518. doi: 10.1021/acschembio.6b00547. [DOI] [PubMed] [Google Scholar]
- Liebmann B., Hümmerich D., Scheibel T., Fehr M. Formulation of poorly water-soluble substances using self-assembling spider silk protein. Colloids Surf. A Physicochem. Eng. Aspects. 2008;331:126–132. [Google Scholar]
- Lin C.Y., Liu J.C. Modular protein domains: an engineering approach toward functional biomaterials. Curr. Opin. Biotechnol. 2016;40:56–63. doi: 10.1016/j.copbio.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Cereghino J., Lin-Cereghino G.P. Vectors and strains for expression. Methods Mol. Biol. 2007;389:11–25. doi: 10.1007/978-1-59745-456-8_2. [DOI] [PubMed] [Google Scholar]
- Linke W.A., Grutzner A. Pulling single molecules of titin by AFM--recent advances and physiological implications. Pflugers Arch Eur J Physiol. 2008;456:101–115. doi: 10.1007/s00424-007-0389-x. [DOI] [PubMed] [Google Scholar]
- Linke W.A., Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ. Res. 2014;114:1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- Lohse D.J., Hadjichristidis N. Microphase separation in block copolymers. Curr. Opin. Colloid In. 1997;2:171–176. [Google Scholar]
- Looser V., Bruhlmann B., Bumbak F., Stenger C., Costa M., Camattari A., Fotiadis D., Kovar K. Cultivation strategies to enhance productivity of Pichia pastoris: a review. Biotechnol. Adv. 2015;33:1177–1193. doi: 10.1016/j.biotechadv.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Luan C.H., Parker T.M., Prasad K.U., Urry D.W. Differential scanning calorimetry studies of NaCl effect on the inverse temperature transition of some elastin-based polytetra-, polypenta-, and polynonapeptides. Biopolymers. 1991;31:465–475. doi: 10.1002/bip.360310502. [DOI] [PubMed] [Google Scholar]
- Lutz J.F., Ouchi M., Liu D.R., Sawamoto M. Sequence-controlled polymers. Science. 2013;341:1238149. doi: 10.1126/science.1238149. [DOI] [PubMed] [Google Scholar]
- MacEwan S.R., Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- Macias M.J., Hyvonen M., Baraldi E., Schultz J., Sudol M., Saraste M., Oschkinat H. Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature. 1996;382:646–649. doi: 10.1038/382646a0. [DOI] [PubMed] [Google Scholar]
- Manfredi M.A., Antunes A.A., Jesus L.O., Juliano M.A., Juliano L., Judice W.A. Specificity characterization of the α-mating factor hormone by Kex2 protease. Biochimie. 2016;131:149–158. doi: 10.1016/j.biochi.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Martens A.A., Portale G., Werten M.W.T., de Vries R.J., Eggink G., Cohen Stuart M.A., de Wolf F.A. Triblock protein copolymers forming supramolecular nanotapes and pH-responsive gels. Macromolecules. 2009;42:1002–1009. [Google Scholar]
- Marx H., Sauer M., Resina D., Vai M., Porro D., Valero F., Ferrer P., Mattanovich D. Cloning, disruption and protein secretory phenotype of the GAS1 homologue of Pichia pastoris. FEMS Microbiol Lett. 2006;264:40–47. doi: 10.1111/j.1574-6968.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Matoba S., Fukayama J., Wing R.A., Ogrydziak D.M. Intracellular precursors and secretion of alkaline extracellular protease of Yarrowia lipolytica. Mol. Cell. Biol. 1988;8:4904–4916. doi: 10.1128/mcb.8.11.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattanovich D., Graf A., Stadlmann J., Dragosits M., Redl A., Maurer M., Kleinheinz M., Sauer M., Altmann F., Gasser B. Genome, secretome and glucose transport highlight unique features of the protein production host Pichia pastoris. Microb. Cell. Fact. 2009;8:29. doi: 10.1186/1475-2859-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J.R., Mackay J.A., Quiroz F.G., Chilkoti A. Recursive directional ligation by plasmid reconstruction allows rapid and seamless cloning of oligomeric genes. Biomacromolecules. 2010;11:944–952. doi: 10.1021/bm901387t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K.P., Tirrell D.A., Kawai M., Mason T.L., Fournier M.J. Chemical and biosynthetic approaches to the production of novel polypeptide materials. Biotechnol. Prog. 1990;6:188–192. doi: 10.1021/bp00003a004. [DOI] [PubMed] [Google Scholar]
- McPherson D.T., Morrow C., Minehan D.S., Wu J., Hunter E., Urry D.W. Production and purification of a recombinant elastomeric polypeptide, G-(VPGVG)19-VPGV, from Escherichia coli. Biotechnol. Prog. 1992;8:347–352. doi: 10.1021/bp00016a012. [DOI] [PubMed] [Google Scholar]
- McPherson D.T., Xu J., Urry D.W. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr. Purif. 1996;7:51–57. doi: 10.1006/prep.1996.0008. [DOI] [PubMed] [Google Scholar]
- Mecham R.P. Elastin synthesis and fiber assembly. Ann. N. Y. Acad. Sci. 1991;624:137–146. doi: 10.1111/j.1749-6632.1991.tb17013.x. [DOI] [PubMed] [Google Scholar]
- Mellitzer A., Ruth C., Gustafsson C., Welch M., Birner-Grunberger R., Weis R., Purkarthofer T., Glieder A. Synergistic modular promoter and gene optimization to push cellulase secretion by Pichia pastoris beyond existing benchmarks. J. Biotechnol. 2014;191:187–195. doi: 10.1016/j.jbiotec.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Mello C.M., Soares J.W., Arcidiacono S., Butler M.M. Acid extraction and purification of recombinant spider silk proteins. Biomacromolecules. 2004;5:1849–1852. doi: 10.1021/bm049815g. [DOI] [PubMed] [Google Scholar]
- Merrett K., Fagerholm P., McLaughlin C.R., Dravida S., Lagali N., Shinozaki N., Watsky M.A., Munger R., Kato Y., Li F., Marmo C.J., Griffith M. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: performance of type I versus type III collagen. Invest. Ophthalmol. Vis. Sci. 2008;49:3887–3894. doi: 10.1167/iovs.07-1348. [DOI] [PubMed] [Google Scholar]
- Meyer D.E., Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- Meyer D.E., Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- Meyer D.E., Chilkoti A. Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides. Biomacromolecules. 2004;5:846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- Meyer D.E., Trabbic-Carlson K., Chilkoti A. Protein purification by fusion with an environmentally responsive elastin-like polypeptide: effect of polypeptide length on the purification of thioredoxin. Biotechnol. Prog. 2001;17:720–728. doi: 10.1021/bp010049o. [DOI] [PubMed] [Google Scholar]
- Meyer H.P., Brass J., Jungo C., Klein J., Wenger J., Mommers R. An emerging star for therapeutic and catalytic protein production. Bioprocess Int. 2008;6:10–21. [Google Scholar]
- Meyer L.C., Wright N.T. Structure of giant muscle proteins. Front. Physiol. 2013;4:368. doi: 10.3389/fphys.2013.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi L. Molecular cloning of protein-based polymers. Biomacromolecules. 2006;7:2099–2107. doi: 10.1021/bm050158h. [DOI] [PubMed] [Google Scholar]
- Miller K.A., DiDone L., Krysan D.J. Extracellular secretion of overexpressed glycosylphosphatidylinositol-linked cell wall protein Utr2/Crh2p as a novel protein quality control mechanism in Saccharomyces cerevisiae. Eukaryot. Cell. 2010;9:1669–1679. doi: 10.1128/EC.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Characterization of KEX2-encoded endopeptidase from yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1989;159:305–311. doi: 10.1016/0006-291x(89)92438-8. [DOI] [PubMed] [Google Scholar]
- Moers A.P.H.A., Wolbert E.J.H., de Wolf F.A., Werten M.W.T. Secreted production of self-assembling peptides in Pichia pastoris by fusion to an artificial highly hydrophilic protein. J. Biotechnol. 2010;146:66–73. doi: 10.1016/j.jbiotec.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Moisenovich M.M., Pustovalova O.L., Arhipova A.Y., Vasiljeva T.V., Sokolova O.S., Bogush V.G., Debabov V.G., Sevastianov V.I., Kirpichnikov M.P., Agapov I.I. In vitro and in vivo biocompatibility studies of a recombinant analogue of spidroin 1 scaffolds. J. Biomed. Mater. Res. A. 2011;96:125–131. doi: 10.1002/jbm.a.32968. [DOI] [PubMed] [Google Scholar]
- Moisenovich M.M., Malyuchenko N.V., Arkhipova A.Y., Goncharenko A.V., Kotlyarova M.S., Davydova L.I., Vasil'eva T.V., Bogush V.G., Agapov I.I., Debabov V.G., Kirpichnikov M.P. Recombinant 1F9 spidroin microgels for murine full-thickness wound repairing. Dokl. Biochem. Biophys. 2016;466:9–12. doi: 10.1134/S1607672916010038. [DOI] [PubMed] [Google Scholar]
- Moll J.R., Ruvinov S.B., Pastan I., Vinson C. Designed heterodimerizing leucine zippers with a ranger of pIs and stabilities up to 10–15 M. Protein Sci. 2001;10:649–655. doi: 10.1110/ps.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumcuoglu D., de Miguel L., Jekhmane S., Siverino C., Nickel J., Mueller T.D., van Leeuwen J.P., van Osch G.J., Kluijtmans S.G. Collagen I derived recombinant protein microspheres as novel delivery vehicles for bone morphogenetic protein-2. Mater. Sci. Eng. C. 2018;84:271–280. doi: 10.1016/j.msec.2017.11.031. [DOI] [PubMed] [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- Myllyharju J., Nokelainen M., Vuorela A., Kivirikko K.I. Expression of recombinant human type I-III collagens in the yeast Pichia pastoris. Biochem. Soc. Trans. 2000;28:353–357. [PubMed] [Google Scholar]
- Nettles D.L., Chilkoti A., Setton L.A. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010;62:1479–1485. doi: 10.1016/j.addr.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D.T., Brown J.D., Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J. Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokelainen M., Tu H., Vuorela A., Notbohm H., Kivirikko K.I., Myllyharju J. High-level production of human type I collagen in the yeast Pichia pastoris. Yeast. 2001;18:797–806. doi: 10.1002/yea.730. [DOI] [PubMed] [Google Scholar]
- Nosenko M.A., Moysenovich A.M., Zvartsev R.V., Arkhipova A.Y., Zhdanova A.S., Agapov I.I., Vasilieva T.V., Bogush V.G., Debabov V.G., Nedospasov S.A., Moisenovich M.M., Drutskaya M.S. Novel biodegradable polymeric microparticles facilitate scarless wound healing by promoting re-epithelialization and inhibiting fibrosis. Front Immunol. 2018;9:2851. doi: 10.3389/fimmu.2018.02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrecht G., Lefèvre J.F., Meyrueis P. Procédure biotechnologique d'obtention et de production microbienne d'oligomères peptidiques comme substituts de la gélatine. Fr. Pat. Appl. 1991;91:16215. [Google Scholar]
- O'Brien J.P., Hoess R.H., Gardner K.H., Lock R.L., Wasserman Z.R., Weber P.C., Salemme F.R. Design, synthesis, and fabrication fabricationof a novel self-assembling fibrillar protein. In: Kaplan D., Adams W.W., Farmer B., Viney C., editors. Silk Polymers: Materials Science and Biotechnology. American Chemical Society; Washington, DC: 1994. pp. 104–117. [Google Scholar]
- Okuyama K., Miyama K., Mizuno K., Bachinger H.P. Crystal structure of (Gly-Pro-Hyp)9: implications for the collagen molecular model. Biopolymers. 2012;97:607–616. doi: 10.1002/bip.22048. [DOI] [PubMed] [Google Scholar]
- Olsen D., Yang C., Bodo M., Chang R., Leigh S., Baez J., Carmichael D., Perala M., Hamalainen E.R., Jarvinen M., Polarek J. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003;55:1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Olsen D., Jiang J., Chang R., Duffy R., Sakaguchi M., Leigh S., Lundgard R., Ju J., Buschman F., Truong-Le V., Pham B., Polarek J.W. Expression and characterization of a low molecular weight recombinant human gelatin: development of a substitute for animal-derived gelatin with superior features. Protein Expr. Purif. 2005;40:346–357. doi: 10.1016/j.pep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Olsen V., Cawley N.X., Brandt J., Egel-Mitani M., Loh Y.P. Identification and characterization of Saccharomyces cerevisiae yapsin 3, a new member of the yapsin family of aspartic proteases encoded by the YPS3 gene. Biochem J. 1999;339:407–411. [PMC free article] [PubMed] [Google Scholar]
- Orij R., Brul S., Smits G.J. Intracellular pH is a tightly controlled signal in yeast. Biochim. Biophys. Acta. 2011;1810:933–944. doi: 10.1016/j.bbagen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Pakkanen O., Pirskanen A., Myllyharju J. Selective expression of nonsecreted triple-helical and secreted single-chain recombinant collagen fragments in the yeast Pichia pastoris. J. Biotechnol. 2006;123:248–256. doi: 10.1016/j.jbiotec.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Parvizi M., Plantinga J.A., van Speuwel-Goossens C.A., van Dongen E.M., Kluijtmans S.G., Harmsen M.C. Development of recombinant collagen-peptide-based vehicles for delivery of adipose-derived stromal cells. J. Biomed. Mater. Res. A. 2016;104:503–516. doi: 10.1002/jbm.a.35588. [DOI] [PubMed] [Google Scholar]
- Persikov A.V., Pillitteri R.J., Amin P., Schwarze U., Byers P.H., Brodsky B. Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum. Mutat. 2004;24:330–337. doi: 10.1002/humu.20091. [DOI] [PubMed] [Google Scholar]
- Petka W.A., Harden J.L., McGrath K.P., Wirtz D., Tirrell D.A. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- Pham T.T.H., de Wolf F.A., Cohen Stuart M.A., van der Gucht J. Pathway-dependent properties of a multi-stimuli sensitive biosynthetic hybrid network. Soft Matter. 2013;9:8737–8744. [Google Scholar]
- Pham T.T.H., Skrzeszewska P.J., Werten M.W.T., Rombouts W.H., Cohen Stuart M.A., de Wolf F.A., van der Gucht J. Disulfide bond-stabilized physical gels of an asymmetric collagen-inspired telechelic protein polymer. Soft Matter. 2013;9:6391–6397. [Google Scholar]
- Pham T.T.H., Wang J., Werten M.W.T., Snijkers F., de Wolf F.A., Cohen Stuart M.A., van der Gucht J. Multi-responsive physical gels formed by a biosynthetic asymmetric triblock protein polymer and a polyanion. Soft Matter. 2013;9:8923–8930. [Google Scholar]
- Pham T.T.H., Snijkers F., Storm I.M., de Wolf F.A., Cohen Stuart M.A., van der Gucht J. Physical and mechanical properties of thermosensitive xanthan/collagen-inspired protein composite hydrogels. Int. J. Polym. Mater. Polym. Biomater. 2016;65:125–133. [Google Scholar]
- Pinkas D.M., Ding S., Raines R.T., Barron A.E. Tunable, post-translational hydroxylation of collagen domains in Escherichia coli. ACS Chem. Biol. 2011;6:320–324. doi: 10.1021/cb100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath K., Mothes W., Wilkinson B.M., Stirling C.J., Rapoport T.A. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- Potvin G., Ahmad A., Zhang Z. Bioprocess engineering aspects of heterologous protein production in Pichia pastoris: a review. Biochem. Eng. J. 2012;64:91–105. [Google Scholar]
- Pozzolini M., Scarfi S., Mussino F., Salis A., Damonte G., Benatti U., Giovine M. Pichia pastoris production of a prolyl 4-hydroxylase derived from Chondrosia reniformis sponge: A new biotechnological tool for the recombinant production of marine collagen. J. Biotechnol. 2015;208:28–36. doi: 10.1016/j.jbiotec.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Prabha L., Govindappa N., Adhikary L., Melarkode R., Sastry K. Identification of the dipeptidyl aminopeptidase responsible for N-terminal clipping of recombinant Exendin-4 precursor expressed in Pichia pastoris. Protein Expr. Purif. 2009;64:155–161. doi: 10.1016/j.pep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Prielhofer R., Maurer M., Klein J., Wenger J., Kiziak C., Gasser B., Mattanovich D. Induction without methanol: novel regulated promoters enable high-level expression in Pichia pastoris. Microb. Cell. Fact. 2013;12:5. doi: 10.1186/1475-2859-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prielhofer R., Reichinger M., Wagner N., Claes K., Kiziak C., Gasser B., Mattanovich D. Superior protein titers in half the fermentation time: Promoter and process engineering for the glucose-regulated GTH1 promoter of Pichia pastoris. Biotechnol. Bioeng. 2018;115:2479–2488. doi: 10.1002/bit.26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J.T., McGrath K.P., DiGirolamo C.M., Kaplan D.L. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1995;34:10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- Prockop D.J., Kivirikko K.I. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Puxbaum V., Mattanovich D., Gasser B. Quo vadis? The challenges of recombinant protein folding and secretion in Pichia pastoris. Appl. Microbiol. Biotechnol. 2015;99:2925–2938. doi: 10.1007/s00253-015-6470-z. [DOI] [PubMed] [Google Scholar]
- Qin X., Qian J., Yao G., Zhuang Y., Zhang S., Chu J. GAP promoter library for fine-tuning of gene expression in Pichia pastoris. Appl. Environ. Microbiol. 2011;77:3600–3608. doi: 10.1128/AEM.02843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabotyagova O.S., Cebe P., Kaplan D.L. Protein-based block copolymers. Biomacromolecules. 2011;12:269–289. doi: 10.1021/bm100928x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J.A., Werkmeister J.A., Dumsday G.J. Bioengineered collagens: emerging directions for biomedical materials. Bioengineered. 2014;5:227–233. doi: 10.4161/bioe.28791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington S.J., Breddam K. Carboxypeptidases C and D. Methods Enzymol. 1994;244:231–248. doi: 10.1016/0076-6879(94)44020-4. [DOI] [PubMed] [Google Scholar]
- Rholam M., Brakch N., Germain D., Thomas D.Y., Fahy C., Boussetta H., Boileau G., Cohen P. Role of amino acid sequences flanking dibasic cleavage sites in precursor proteolytic processing. The importance of the first residue C-terminal of the cleavage site. Eur. J. Biochem. 1995;227:707–714. doi: 10.1111/j.1432-1033.1995.tb20192.x. [DOI] [PubMed] [Google Scholar]
- Riffer F., Eisfeld K., Breinig F., Schmitt M.J. Mutational analysis of K28 preprotoxin processing in the yeast Saccharomyces cerevisiae. Microbiology. 2002;148:1317–1328. doi: 10.1099/00221287-148-5-1317. [DOI] [PubMed] [Google Scholar]
- Rising A. Controlled assembly: a prerequisite for the use of recombinant spider silk in regenerative medicine? Acta Biomater. 2014;10:1627–1631. doi: 10.1016/j.actbio.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Rising A., Johansson J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015;11:309. doi: 10.1038/nchembio.1789. [DOI] [PubMed] [Google Scholar]
- Rockwell N.C., Wang G.T., Krafft G.A., Fuller R.S. Internally consistent libraries of fluorogenic substrates demonstrate that Kex2 protease specificity is generated by multiple mechanisms. Biochemistry. 1997;36:1912–1917. doi: 10.1021/bi961779l. [DOI] [PubMed] [Google Scholar]
- Rockwell N.C., Krysan D.J., Komiyama T., Fuller R.S. Precursor processing by Kex2/Furin proteases. Chem. Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- Romanos M. Advances in the use of Pichia pastoris for high-level gene expression. Curr. Opin. Biotechnol. 1995;6:527–533. [Google Scholar]
- Romanos M., Scorer C., Sreekrishna K., Clare J. The generation of multicopy recombinant strains. Methods Mol. Biol. 1998;103:55–72. doi: 10.1385/0-89603-421-6:55. [DOI] [PubMed] [Google Scholar]
- Rombouts W.H., Colomb-Delsuc M., Werten M.W.T., Otto S., de Wolf F.A., van der Gucht J. Enhanced rigidity and rupture strength of composite hydrogel networks of bio-inspired block copolymers. Soft Matter. 2013;9:6936–6942. [Google Scholar]
- Rombouts W.H., de Kort D.W., Pham T.T.H., van Mierlo C.P.M., Werten M.W.T., de Wolf F.A., van der Gucht J. Reversible temperature-switching of hydrogel stiffness of coassembled, silk-collagen-like hydrogels. Biomacromolecules. 2015;16:2506–2513. doi: 10.1021/acs.biomac.5b00766. [DOI] [PubMed] [Google Scholar]
- Rombouts W.H., Domeradzka N.E., Werten M.W.T., Leermakers F.A., de Vries R.J., de Wolf F.A., van der Gucht J. Enhanced stiffness of silk-like fibers by loop formation in the corona leads to stronger gels. Biopolymers. 2016;105:795–801. doi: 10.1002/bip.22909. [DOI] [PubMed] [Google Scholar]
- Rourke I.J., Johnsen A.H., Din N., Petersen J.G., Rehfeld J.F. Heterologous expression of human cholecystokinin in Saccharomyces cerevisiae. Evidence for a lysine-specific endopeptidase in the yeast secretory pathway. J. Biol. Chem. 1997;272:9720–9727. doi: 10.1074/jbc.272.15.9720. [DOI] [PubMed] [Google Scholar]
- Rozkiewicz D.I., Kraan Y., Werten M.W.T., de Wolf F.A., Subramaniam V., Ravoo B.J., Reinhoudt D.N. Covalent microcontact printing of proteins for cell patterning. Chemistry. 2006;12:6290–6297. doi: 10.1002/chem.200501554. [DOI] [PubMed] [Google Scholar]
- Rozkov A., Enfors S.O. Analysis and control of proteolysis of recombinant proteins in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 2004;89:163–195. doi: 10.1007/b95567. [DOI] [PubMed] [Google Scholar]
- Rueda F., Gasser B., Sanchez-Chardi A., Roldan M., Villegas S., Puxbaum V., Ferrer-Miralles N., Unzueta U., Vazquez E., Garcia-Fruitos E., Mattanovich D., Villaverde A. Functional inclusion bodies produced in the yeast Pichia pastoris. Microb. Cell. Fact. 2016;15:166. doi: 10.1186/s12934-016-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallach R.E., Conticello V.P., Chaikof E.L. Expression of a recombinant elastin-like protein in Pichia pastoris. Biotechnol. Prog. 2009;25:1810–1818. doi: 10.1002/btpr.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford K., Kumar M. New proteins in a materials world. Curr. Opin. Biotechnol. 2005;16:416–421. doi: 10.1016/j.copbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Sazonova E.A., Zobnina A.E., Padkina M.V. Effect of disruption of Pichia pastoris YPS1 gene on viability and production of recombinant proteins. Russ. J. Genet. 2013;49:602–608. doi: 10.7868/s0016675813040127. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Scheibel T. Spider silks: recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell. Fact. 2004;3:14. doi: 10.1186/1475-2859-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipperus R., Teeuwen R.L.M., Werten M.W.T., Eggink G., de Wolf F.A. Secreted production of an elastin-like polypeptide by Pichia pastoris. Appl. Microbiol. Biotechnol. 2009;85:293–301. doi: 10.1007/s00253-009-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipperus R., Eggink G., de Wolf F.A. Secretion of elastin-like polypeptides with different transition temperatures by Pichia pastoris. Biotechnol. Prog. 2012;28:242–247. doi: 10.1002/btpr.717. [DOI] [PubMed] [Google Scholar]
- Seeman N.C. Nanomaterials based on DNA. Annu. Rev. Biochem. 2010;79:65–87. doi: 10.1146/annurev-biochem-060308-102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D., Heilshorn S.C. Protein-engineered biomaterials: highly tunable tissue engineering scaffolds. Tissue Eng. Part B. 2010;16:285–293. doi: 10.1089/ten.teb.2009.0591. [DOI] [PubMed] [Google Scholar]
- Service, R.F Silken promises. Science. 2017;358:293–294. doi: 10.1126/science.358.6361.293. [DOI] [PubMed] [Google Scholar]
- Shen W., Xue Y., Liu Y., Kong C., Wang X., Huang M., Cai M., Zhou X., Zhang Y., Zhou M. A novel methanol-free Pichia pastoris system for recombinant protein expression. Microb. Cell. Fact. 2016;15:178. doi: 10.1186/s12934-016-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.I.F., Teles H., Moers A.P.H.A., Eggink G., de Wolf F.A., Werten M.W.T. Secreted production of collagen-inspired gel-forming polymers with high thermal stability in Pichia pastoris. Biotechnol. Bioeng. 2011;108:2517–2525. doi: 10.1002/bit.23228. [DOI] [PubMed] [Google Scholar]
- Silva C.I.F., Skrzeszewska P.J., Golinska M.D., Werten M.W.T., Eggink G., de Wolf F.A. Tuning of collagen triple-helix stability in recombinant telechelic polymers. Biomacromolecules. 2012;13:1250–1258. doi: 10.1021/bm300323q. [DOI] [PubMed] [Google Scholar]
- Sinha J., Plantz B.A., Inan M., Meagher M.M. Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: case study with recombinant ovine interferon-tau. Biotechnol. Bioeng. 2005;89:102–112. doi: 10.1002/bit.20318. [DOI] [PubMed] [Google Scholar]
- Skrzeszewska P.J., Sprakel J., de Wolf F.A., Fokkink R., Cohen Stuart M.A., van der Gucht J. Fracture and self-healing in a well-defined self-assembled polymer network. Macromolecules. 2010;43:3542–3548. [Google Scholar]
- Sokolova O.S., Bogush V.G., Davydova L.I., Polevova S.V., Antonov S.A., Neretina T.V., Klinov D.V., Debabov V.G., Kirpichnikov M.P. The formation of a quaternary structure by recombinant analogs of spider silk proteins. Mol. Biol. 2010;44:150–157. [PubMed] [Google Scholar]
- Sreekrishna K., Brankamp R.G., Kropp K.E., Blankenship D.T., Tsay J.T., Smith P.L., Wierschke J.D., Subramaniam A., Birkenberger L.A. Strategies for optimal synthesis and secretion of heterologous proteins in the methylotrophic yeast Pichia pastoris. Gene. 1997;190:55–62. doi: 10.1016/s0378-1119(96)00672-5. [DOI] [PubMed] [Google Scholar]
- Stark M., Grip S., Rising A., Hedhammar M., Engstrom W., Hjalm G., Johansson J. Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules. 2007;8:1695–1701. doi: 10.1021/bm070049y. [DOI] [PubMed] [Google Scholar]
- Storm I.M., Kornreich M., Hernandez-Garcia A., Voets I.K., Roy B., Cohen Stuart M.A., Leermakers F.A.M., de Vries R. Liquid crystals of self-assembled DNA bottlebrushes. J. Phys. Chem. B. 2015;119:4084–4092. doi: 10.1021/jp511412t. [DOI] [PubMed] [Google Scholar]
- Stratton J., Chiruvolu V., Meagher M. High cell-density fermentation. Methods Mol. Biol. 1998;103:107–120. doi: 10.1385/0-89603-421-6:107. [DOI] [PubMed] [Google Scholar]
- Su K., Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015;37:2139–2145. doi: 10.1007/s10529-015-1907-0. [DOI] [PubMed] [Google Scholar]
- Sutherland T.D., Young J.H., Weisman S., Hayashi C.Y., Merritt D.J. Insect silk: one name, many materials. Annu Rev Entomol. 2010;55:171–188. doi: 10.1146/annurev-ento-112408-085401. [DOI] [PubMed] [Google Scholar]
- Suto K., Noda H. Conformational change of the triple-helical structure. II. Conformation of (Pro-Pro-Gly)n and (Pro-Pro-Gly)n (Ala-Pro-Gly)m(Pro-Pro-Pro-Gly)n in an aqueous solution. Biopolymers. 1974;13:2391–2404. doi: 10.1002/bip.1974.360131118. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ikeda N., Kataoka E., Ohsuye K. Effect of amino acid substitution at the P3 and P4 subsites of fusion proteins on kex2 protease activity. Biotechnol. Appl. Biochem. 2000;32:53–60. doi: 10.1042/ba20000012. [DOI] [PubMed] [Google Scholar]
- Szybalski W., Kim S.C., Hasan N., Podhajska A.J. Class-IIS restriction enzymes - a review. Gene. 1991;100:13–26. doi: 10.1016/0378-1119(91)90345-c. [DOI] [PubMed] [Google Scholar]
- Teeuwen R.L.M., van Berkel S.S., van Dulmen T.H., Schoffelen S., Meeuwissen S.A., Zuilhof H., de Wolf F.A., van Hest J.C. "Clickable" elastins: elastin-like polypeptides functionalized with azide or alkyne groups. Chem. Commun. 2009:4022–4024. doi: 10.1039/b903903a. [DOI] [PubMed] [Google Scholar]
- Teeuwen R.L.M., Zuilhof H., de Wolf F.A., van Hest J.C.M. Temperature-controlled positioning of fusion proteins in microreactors. Soft Matter. 2009;5:2261–2268. [Google Scholar]
- Teles H., Skrzeszewska P.J., Werten M.W.T., van der Gucht J., Eggink G., de Wolf F.A. Influence of molecular size on gel-forming properties of telechelic collagen-inspired polymers. Soft Matter. 2010;6:4681–4687. [Google Scholar]
- Teles H., Vermonden T., Eggink G., Hennink W.E., de Wolf F.A. Hydrogels of collagen-inspired telechelic triblock copolymers for the sustained release of proteins. J. Control. Release. 2010;147:298–303. doi: 10.1016/j.jconrel.2010.07.098. [DOI] [PubMed] [Google Scholar]
- Teulé F., Aubé C., Ellison M., Abbott A. Biomimetic manufacturing of customised novel fibre proteins for specialised applications. AUTEX Res. J. 2003;3:160–165. [Google Scholar]
- Thibault G., Ng D.T. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harb. Perspect. Biol. 2012;4:a013193. doi: 10.1101/cshperspect.a013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajapuram N., Olsen D., Middaugh C.R. Stabilization of proteins by recombinant human gelatins. J. Pharm. Sci. 2007;96:3304–3315. doi: 10.1002/jps.20980. [DOI] [PubMed] [Google Scholar]
- Toda Y., Mori F., Bouwstra J. Functions of gelatin in imaging materials. J. Soc. Photogr. Sci. Technol. Jpn. 2002;65:381–389. [Google Scholar]
- Tokareva O., Michalczechen-Lacerda V.A., Rech E.L., Kaplan D.L. Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 2013;6:651–663. doi: 10.1111/1751-7915.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman P.D., Chisholm G., McMullin H., Giere L.M., Olsen D.R., Kovach R.J., Leigh S.D., Fong B.E., Chang R., Daniels G.A., Berg R.A., Hitzeman R.A. Production of recombinant human type I procollagen trimers using a four-gene expression system in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:23303–23309. doi: 10.1074/jbc.M002284200. [DOI] [PubMed] [Google Scholar]
- Toonkool P., Weiss A.S. Expression of recombinant human tropoelastin in Saccharomyces cerevisiae containing a synthetic gene with a high codon adaptation index coupled to the SUC2 invertase signal sequence. Acta Biotechnol. 2001;21:189–193. [Google Scholar]
- Trabbic-Carlson K., Setton L.A., Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–580. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- Tsai W.L., Forbes J.G., Wang K. Engineering of an elastic scaffolding polyprotein based on an SH3-binding intrinsically disordered titin PEVK module. Protein Expr. Purif. 2012;85:187–199. doi: 10.1016/j.pep.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T.C., Tao L., Hsieh F.Y., Wei Y., Chiu I.M., Hsu S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015;27:3518–3524. doi: 10.1002/adma.201500762. [DOI] [PubMed] [Google Scholar]
- Tuin A., Kluijtmans S.G., Bouwstra J.B., Harmsen M.C., Van Luyn M.J. Recombinant gelatin microspheres: novel formulations for tissue repair? Tissue Eng. Part A. 2010;16:1811–1821. doi: 10.1089/ten.TEA.2009.0592. [DOI] [PubMed] [Google Scholar]
- Tuin A., Zandstra J., Kluijtmans S.G., Bouwstra J.B., Harmsen M.C., Van Luyn M.J. Hyaluronic acid-recombinant gelatin gels as a scaffold for soft tissue regeneration. Eur. Cells Mater. 2012;24:320–330. doi: 10.22203/ecm.v024a23. [DOI] [PubMed] [Google Scholar]
- Urry D.W. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J. Protein Chem. 1988;7:1–34. doi: 10.1007/BF01025411. [DOI] [PubMed] [Google Scholar]
- Urry D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992;57:23–57. doi: 10.1016/0079-6107(92)90003-o. [DOI] [PubMed] [Google Scholar]
- Urry D.W., Long M.M. On the conformation, coacervation and function of polymeric models of elastin. Adv. Exp. Med. Biol. 1977;79:685–714. doi: 10.1007/978-1-4684-9093-0_59. [DOI] [PubMed] [Google Scholar]
- Urry D.W., Long M.M., Cox B.A., Ohnishi T., Mitchell L.W., Jacobs M. The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates. Biochim. Biophys. Acta. 1974;371:597–602. doi: 10.1016/0005-2795(74)90057-9. [DOI] [PubMed] [Google Scholar]
- Urry D.W., Luan C.H., Parker T.M., Gowda D.C., Prasad K.U., Reid M.C., Safavy A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J. Am. Chem. Soc. 1991;113:4346–4348. [Google Scholar]
- Urry D.W., Parker T.M., Reid M.C., Gowda D.C. Biocompatibility of the bioelastic materials, poly(GVGVP) and its γ-irradiation cross-linked matrix: summary of generic biological test results. J. Bioact. Compat. Pol. 1991;6:263–282. [Google Scholar]
- Urry D.W., Gowda D.C., Parker T.M., Luan C.H., Reid M.C., Harris C.M., Pattanaik A., Harris R.D. Hydrophobicity scale for proteins based on inverse temperature transitions. Biopolymers. 1992;32:1243–1250. doi: 10.1002/bip.360320913. [DOI] [PubMed] [Google Scholar]
- Valero F. Bioprocess engineering of Pichia pastoris, an exciting host eukaryotic cell expression system. In: Ogawa D.T., editor. Protein Eng. InTech; 2013. [Google Scholar]
- Valli M., Tatto N.E., Peymann A., Gruber C., Landes N., Ekker H., Thallinger G.G., Mattanovich D., Gasser B., Graf A.B. Curation of the genome annotation of Pichia pastoris (Komagataella phaffii) CBS7435 from gene level to protein function. FEMS Yeast Res. 2016;16 doi: 10.1093/femsyr/fow051. [DOI] [PubMed] [Google Scholar]
- Van den Hombergh J.P., van de Vondervoort P.J., Fraissinet-Tachet L., Visser J. Aspergillus as a host for heterologous protein production: the problem of proteases. Trends Biotechnol. 1997;15:256–263. doi: 10.1016/s0167-7799(97)01020-2. [DOI] [PubMed] [Google Scholar]
- Van Eldijk M.B., McGann C.L., Kiick K.L., van Hest J.C.M. Elastomeric polypeptides. Top. Curr. Chem. 2012;310:71–116. doi: 10.1007/128_2011_205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerde, G.V., van Rijn, A.C., Bouwstra, J.B., de Wolf, F.A., Mooibroek, H., Werten, M.W.T., Wind, R.D., van den Bosch, T.J., 1998. US Pat 6,150,081.
- Van Hest J.C.M., Tirrell D.A. Protein-based materials, toward a new level of structural control. Chem. Commun. 2001:1897–1904. doi: 10.1039/b105185g. [DOI] [PubMed] [Google Scholar]
- Vanz A.L., Lunsdorf H., Adnan A., Nimtz M., Gurramkonda C., Khanna N., Rinas U. Physiological response of Pichia pastoris GS115 to methanol-induced high level production of the Hepatitis B surface antigen: catabolic adaptation, stress responses, and autophagic processes. Microb. Cell. Fact. 2012;11:103. doi: 10.1186/1475-2859-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P.R., Galanis M., Richards K.M., Tebb T.A., Ramshaw J.A., Werkmeister J.A. Production of recombinant hydroxylated human type III collagen fragment in Saccharomyces cerevisiae. DNA Cell Biol. 1998;17:511–518. doi: 10.1089/dna.1998.17.511. [DOI] [PubMed] [Google Scholar]
- Vogl T., Glieder A. Regulation of Pichia pastoris promoters and its consequences for protein production. N. Biotechnol. 2013;30:385–404. doi: 10.1016/j.nbt.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Vogl T., Hartner F.S., Glieder A. New opportunities by synthetic biology for biopharmaceutical production in Pichia pastoris. Curr. Opin. Biotechnol. 2013;24:1094–1101. doi: 10.1016/j.copbio.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T., Sturmberger L., Kickenweiz T., Wasmayer R., Schmid C., Hatzl A.M., Gerstmann M.A., Pitzer J., Wagner M., Thallinger G.G., Geier M., Glieder A. A toolbox of diverse promoters related to methanol utilization: functionally verified parts for heterologous pathway expression in Pichia pastoris. ACS Synth. Biol. 2016;5:172–186. doi: 10.1021/acssynbio.5b00199. [DOI] [PubMed] [Google Scholar]
- Vogl T., Sturmberger L., Fauland P.C., Hyden P., Fischer J.E., Schmid C., Thallinger G.G., Geier M., Glieder A. Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors. Biotechnol. Bioeng. 2018;115:1037–1050. doi: 10.1002/bit.26529. [DOI] [PubMed] [Google Scholar]
- Vollrath F., Knight D.P. Liquid crystalline spinning of spider silk. Nature. 2001;410:541–548. doi: 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- Vrhovski B., Weiss A.S. Biochemistry of tropoelastin. Eur. J. Biochem. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- Vuorela A., Myllyharju J., Nissi R., Pihlajaniemi T., Kivirikko K.I. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J. 1997;16:6702–6712. doi: 10.1093/emboj/16.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., Shi L., Qi F., Zhang P., Zhang Y., Zhou X., Song Z., Cai M. Methanol-independent protein expression by AOX1 promoter with trans-acting elements engineering and glucose-glycerol-shift induction in Pichia pastoris. Sci. Rep. 2017;7:41850. doi: 10.1038/srep41850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Geer L.Y., Chappey C., Kans J.A., Bryant S.H. Cn3D: sequence and structure views for Entrez. Trends Biochem. Sci. 2000;25:300–302. doi: 10.1016/s0968-0004(00)01561-9. [DOI] [PubMed] [Google Scholar]
- Waterham H.R., Digan M.E., Koutz P.J., Lair S.V., Cregg J.M. Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene. 1997;186:37–44. doi: 10.1016/s0378-1119(96)00675-0. [DOI] [PubMed] [Google Scholar]
- Wegner G.H. Emerging applications of the methylotrophic yeasts. FEMS Microbiol. Rev. 1990;7:279–283. doi: 10.1111/j.1574-6968.1990.tb04925.x. [DOI] [PubMed] [Google Scholar]
- Weninger A., Hatzl A.M., Schmid C., Vogl T., Glieder A. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris. J. Biotechnol. 2016;235:139–149. doi: 10.1016/j.jbiotec.2016.03.027. [DOI] [PubMed] [Google Scholar]
- Weninger A., Fischer J.E., Raschmanova H., Kniely C., Vogl T., Glieder A. Expanding the CRISPR/Cas9 toolkit for Pichia pastoris with efficient donor integration and alternative resistance markers. J. Cell Biochem. 2018;119:3183–3198. doi: 10.1002/jcb.26474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werten M.W.T., de Wolf F.A. Reduced proteolysis of secreted gelatin and Yps1-mediated α-factor leader processing in a Pichia pastoris kex2 disruptant. Appl. Environ. Microbiol. 2005;71:2310–2317. doi: 10.1128/AEM.71.5.2310-2317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werten M.W.T., van den Bosch T.J., Wind R.D., Mooibroek H., de Wolf F.A. High-yield secretion of recombinant gelatins by Pichia pastoris. Yeast. 1999;15:1087–1096. doi: 10.1002/(SICI)1097-0061(199908)15:11<1087::AID-YEA436>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Werten M.W.T., Wisselink W.H., Jansen-van den Bosch T.J., de Bruin E.C., de Wolf F.A. Secreted production of a custom-designed, highly hydrophilic gelatin in Pichia pastoris. Protein Eng. 2001;14:447–454. doi: 10.1093/protein/14.6.447. [DOI] [PubMed] [Google Scholar]
- Werten M.W.T., Moers A.P.H.A., Vong T., Zuilhof H., van Hest J.C.M., de Wolf F.A. Biosynthesis of an amphiphilic silk-like polymer. Biomacromolecules. 2008;9:1705–1711. doi: 10.1021/bm701111z. [DOI] [PubMed] [Google Scholar]
- Werten M.W.T., Teles H., Moers A.P.H.A., Wolbert E.J.H., Sprakel J., Eggink G., de Wolf F.A. Precision gels from collagen-inspired triblock copolymers. Biomacromolecules. 2009;10:1106–1113. doi: 10.1021/bm801299u. [DOI] [PubMed] [Google Scholar]
- Whyteside G., Alcocer M.J., Kumita J.R., Dobson C.M., Lazarou M., Pleass R.J., Archer D.B. Native-state stability determines the extent of degradation relative to secretion of protein variants from Pichia pastoris. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Maurizi M.R., Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- Widhe M., Johansson J., Hedhammar M., Rising A. Invited review current progress and limitations of spider silk for biomedical applications. Biopolymers. 2012;97:468–478. doi: 10.1002/bip.21715. [DOI] [PubMed] [Google Scholar]
- Winkler S., Wilson D., Kaplan D.L. Controlling ß-sheet assembly in genetically engineered silk by enzymatic phosphorylation/dephosphorylation. Biochemistry. 2000;39:12739–12746. doi: 10.1021/bi001335w. [DOI] [PubMed] [Google Scholar]
- Włodarczyk-Biegun M.K., Werten M.W.T., de Wolf F.A., van den Beucken J.J.J.P., Leeuwenburgh S.C.G., Kamperman M., Cohen Stuart M.A. Genetically engineered silk-collagen-like copolymer for biomedical applications: production, characterization and evaluation of cellular response. Acta Biomater. 2014;10:3620–3629. doi: 10.1016/j.actbio.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Włodarczyk-Biegun M.K., Farbod K., Werten M.W.T., Slingerland C.J., de Wolf F.A., van den Beucken J.J.J.P., Leeuwenburgh S.C.G., Cohen Stuart M.A., Kamperman M. Fibrous hydrogels for cell encapsulation: a modular and supramolecular approach. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Włodarczyk-Biegun M.K., Slingerland C.J., Werten M.W.T., van Hees I.A., de Wolf F.A., de Vries R., Stuart M.A., Kamperman M. Heparin as a bundler in a self-assembled fibrous network of functionalized protein-based polymers. Biomacromolecules. 2016;17:2063–2072. doi: 10.1021/acs.biomac.6b00276. [DOI] [PubMed] [Google Scholar]
- Włodarczyk-Biegun M.K., Werten M.W.T., Posadowska U., Storm I.M., de Wolf F.A., van den Beucken J.J.J.P., Leeuwenburgh S.C.G., Cohen Stuart M.A., Kamperman M. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin- and proteoglycan-binding domains. J. Biomed. Mater. Res. A. 2016;104:3082–3092. doi: 10.1002/jbm.a.35839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Po Foo C., Kaplan D.L. Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv. Drug Deliv. Rev. 2002;54:1131–1143. doi: 10.1016/s0169-409x(02)00061-3. [DOI] [PubMed] [Google Scholar]
- Wong Po Foo C.T., Lee J.S., Mulyasasmita W., Parisi-Amon A., Heilshorn S.C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E.R., McMillan R.A., Cooper A., Apkarian R.P., Conticello V.P. Thermoplastic elastomer hydrogels via self-assembly of an elastin-mimetic triblock polypeptide. Adv. Funct. Mater. 2002;12:149–154. [Google Scholar]
- Wu I.L., Patterson M.A., Carpenter Desai H.E., Mehl R.A., Giorgi G., Conticello V.P. Multiple site-selective insertions of noncanonical amino acids into sequence-repetitive polypeptides. ChemBioChem. 2013;14:968–978. doi: 10.1002/cbic.201300069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Shen Q., Yang Y., Zhang S., Qu W., Chen J., Sun H., Chen S. Disruption of YPS1 and PEP4 genes reduces proteolytic degradation of secreted HSA/PTH in Pichia pastoris GS115. J. Ind. Microbiol. Biotechnol. 2013;40:589–599. doi: 10.1007/s10295-013-1264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.F., Chen H., Huang B.R. Expression, purification and characterization of human IFN-λ1 in Pichia pastoris. J. Biotechnol. 2007;129:472–480. doi: 10.1016/j.jbiotec.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Xu C., Breedveld V., Kopecek J. Reversible hydrogels from self-assembling genetically engineered protein block copolymers. Biomacromolecules. 2005;6:1739–1749. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]
- Yan Y., Martens A.A., Besseling N.A., de Wolf F.A., de Keizer A., Drechsler M., Cohen Stuart M.A. Nanoribbons self-assembled from triblock peptide polymers and coordination polymers. Angew. Chem. Int. Ed. Engl. 2008;47:4192–4195. doi: 10.1002/anie.200705242. [DOI] [PubMed] [Google Scholar]
- Yan Y., de Keizer A., Martens A.A., Oliveira C.L., Pedersen J.S., de Wolf F.A., Drechsler M., Cohen Stuart M.A., Besseling N.A. Polypeptide nanoribbon hydrogels assembled through multiple supramolecular interactions. Langmuir. 2009;25:12899–12908. doi: 10.1021/la901834v. [DOI] [PubMed] [Google Scholar]
- Yang Y.J., Holmberg A.L., Olsen B.D. Artificially engineered protein polymers. Annu. Rev. Chem. Biomol. Eng. 2017;8:549–575. doi: 10.1146/annurev-chembioeng-060816-101620. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: a review. Biotechnol. Adv. 2018;36:182–195. doi: 10.1016/j.biotechadv.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Yao X.Q., Zhao H.L., Xue C., Zhang W., Xiong X.H., Wang Z.W., Li X.Y., Liu Z.M. Degradation of HSA-AX15(R13K) when expressed in Pichia pastoris can be reduced via the disruption of YPS1 gene in this yeast. J. Biotechnol. 2009;139:131–136. doi: 10.1016/j.jbiotec.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Young T.S., Ahmad I., Brock A., Schultz P.G. Expanding the genetic repertoire of the methylotrophic yeast Pichia pastoris. Biochemistry. 2009;48:2643–2653. doi: 10.1021/bi802178k. [DOI] [PubMed] [Google Scholar]
- Zahrl R.J., Pena D.A., Mattanovich D., Gasser B. Systems biotechnology for protein production in Pichia pastoris. FEMS Yeast Res. 2017;17 doi: 10.1093/femsyr/fox068. [DOI] [PubMed] [Google Scholar]
- Zhang C., Hernandez-Garcia A., Jiang K., Gong Z., Guttula D., Ng S.Y., Malar P.P., van Kan J.A., Dai L., Doyle P.S., Vries R., van der Maarel J.R. Amplified stretch of bottlebrush-coated DNA in nanofluidic channels. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Inan M., Meagher M.M. Fermentation strategies for recombinant protein expression in the methylotrophic yeast Pichia pastoris. Biotechnol. Bioprocess Eng. 2000;5:275–287. [Google Scholar]
- Zhang W., Zhao H.L., Xue C., Xiong X.H., Yao X.Q., Li X.Y., Chen H.P., Liu Z.M. Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnol. Prog. 2006;22:1090–1095. doi: 10.1021/bp060019r. [DOI] [PubMed] [Google Scholar]
- Zhang W., Inan M., Meagher M.M. Rational design and optimization of fed-batch and continuous fermentations. Methods Mol. Biol. 2007;389:43–64. doi: 10.1007/978-1-59745-456-8_4. [DOI] [PubMed] [Google Scholar]
- Zhang X., Guda C., Datta R., Dute R., Urry D.W., Daniell H. Nuclear expression of an environmentally friendly synthetic protein based polymer gene in tobacco cells. Biotechnol. Lett. 1995;17:1279–1284. [Google Scholar]
- Zhao B., Cohen Stuart M.A., Hall C.K. Dock 'n roll: folding of a silk-inspired polypeptide into an amyloid-like beta solenoid. Soft Matter. 2016;12:3721–3729. doi: 10.1039/c6sm00169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Cohen Stuart M.A., Hall C.K. Navigating in foldonia: Using accelerated molecular dynamics to explore stability, unfolding and self-healing of the ß-solenoid structure formed by a silk-like polypeptide. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.S., Zhang X.Y., Cartwright C.P., Tipper D.J. Kex2-dependent processing of yeast K1 killer preprotoxin includes cleavage at ProArg-44. Mol. Microbiol. 1992;6:511–520. doi: 10.1111/j.1365-2958.1992.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M.P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984;159:1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]