Key Points
Question
Does the addition of pembrolizumab after locally ablative therapy in oligometastatic non–small cell lung cancer improve outcomes compared with historical data?
Findings
In this phase 2 single-arm study that included 45 patients with oligometastatic non–small cell lung cancer who received pembrolizumab after completing locally ablative therapy, the median progression-free survival was 19.1 months, which was longer than historical data.
Meaning
The use of pembrolizumab after locally ablative therapy for oligometastatic non–small cell lung cancer was associated with a clinically and statistically significant improvement in progression-free survival compared with historical data, which suggests that this treatment should be further evaluated in a randomized clinical trial.
This phase 2 single-arm study evaluates outcomes following pembrolizumab treatment after locally ablative therapy in patients with oligometastatic non–small cell lung cancer.
Abstract
Importance
Patients with oligometastatic non–small cell lung cancer (NSCLC) may benefit from locally ablative therapy (LAT) such as surgery or stereotactic radiotherapy. Prior studies were conducted before the advent of immunotherapy, and a strong biological rationale for the use of immunotherapy exists in a minimal residual disease state.
Objective
To evaluate whether the addition of pembrolizumab after LAT improves outcomes for patients with oligometastatic NSCLC.
Design, Setting, and Participants
This single-arm phase 2 trial of pembrolizumab therapy was performed from February 1, 2015, through September 30, 2017, at an academic referral cancer center. The 51 eligible patients enrolled had oligometastatic NSCLC (≤4 metastatic sites) and had completed LAT to all known sites of disease. Data were analyzed from February 1, 2015, to August 23, 2018.
Interventions
Within 4 to 12 weeks of completing LAT, patients began intravenous pembrolizumab therapy, 200 mg every 21 days, for 8 cycles, with provision to continue to 16 cycles in the absence of progressive disease or untoward toxic effects.
Main Outcomes and Measures
The 2 primary efficacy end points were progression-free survival (PFS) from the start of LAT (PFS-L), which preceded enrollment in the trial, and PFS from the start of pembrolizumab therapy (PFS-P). The study was powered for comparison with historical data on the first efficacy end point. Secondary outcomes included overall survival, safety, and quality of life as measured by the Functional Assessment of Cancer Therapy–Lung instrument.
Results
Of 51 patients enrolled, 45 (24 men [53%]; median age, 64 years [range, 46-82 years]) received pembrolizumab. At the time of analysis, 24 patients had progressive disease or had died. Median PFS-L was 19.1 months (95% CI, 9.4-28.7 months), significantly greater than the historical median of 6.6 months (P = .005). Median PFS-P was 18.7 months (95% CI, 10.1-27.1 months). Eleven patients died. Overall mean (SE) survival rate at 12 months was 90.9% (4.3%); at 24 months, 77.5% (6.7%). Neither programmed death ligand 1 expression nor CD8 T-cell tumor infiltration was associated with PFS-L. Pembrolizumab after LAT yielded no new safety signals and no reduction in quality of life.
Conclusions and Relevance
Pembrolizumab after LAT for oligometastatic NSCLC appears to improve PFS with no reduction in quality of life.
Trial Registration
ClinicalTrials.gov identifier: NCT02316002
Introduction
Approximately 7% of patients with non–small cell lung cancer (NSCLC) present with a limited number of metastatic foci.1 Weichselbaum and Hellman2 have hypothesized that cancer metastasis falls on a continuum. Although some malignant neoplasms are localized and others are systemic from diagnosis, most will fall between these 2 extremes.2 Within this revised paradigm of tumor metastasis, the concept of treating a limited number of metastatic lesions with curative intent becomes rational, because their mere presence no longer excludes the possibility of long-term disease control or even cure. Multiple retrospective studies in oligometastatic NSCLC3,4 have shown that the use of locally ablative therapy (LAT) to all sites of disease is associated with a significant improvement in overall survival (OS) and progression-free survival (PFS) when compared with historical data.
Based on this principle, Gomez et al5 randomized 49 patients with oligometastatic (1-3 metastases) NSCLC who had completed first-line palliative maintenance systemic therapy or maintenance therapy and LAT. The study was stopped early owing to improved outcomes with LAT (median PFS, 11.9 vs 3.9 months; P = .005).6 Further follow-up has shown the addition of LAT was also associated with an improvement in OS.6 Concurrent with this trial, Iyengar et al7 used a similar design and randomized 29 patients with oligometastatic (1-5 metastases) NSCLC to maintenance chemotherapy alone or to maintenance LAT. The authors also performed an early interim analysis and found an improvement in PFS with LAT (median PFS, 9.7 vs 3.5 months; P = .01).7
In both trials, cytotoxic chemotherapy or targeted therapy was administered prior to LAT. At the time of trial design, such treatments were the standard of care for all patients with stage IV NSCLC.8,9 In retrospective analyses of patients with oligometastatic NSCLC treated with LAT, however, very few received chemotherapy, and its use in this setting has not been documented to improve OS or PFS to date.3 As such, the requirement for prior chemotherapy in these recent trials delays LAT to administer a treatment that has not been shown to improve clinical outcomes.
In recent years, immunotherapy has transformed our treatment approach for patients with metastatic NSCLC. The KEYNOTE-024 study10 showed that among patients with programmed death ligand 1 (PD-L1) expression greater than or equal to 50%, pembrolizumab monotherapy led to improved OS and PFS compared with platinum doublet chemotherapy. The KEYNOTE-18911 and KEYNOTE-40712 studies showed that regardless of PD-L1 expression, the addition of pembrolizumab to histology-specific chemotherapy improved OS in nonsquamous and squamous NSCLC. The best way to combine immunotherapy with ablative therapies in the curative setting is an area of active investigation. The PACIFIC trial13,14 showed that use of the PD-L1 inhibitor durvalumab after chemoradiotherapy for locally advanced NSCLC led to improved OS and PFS.
We recently completed a trial of pembrolizumab after definitive LAT for oligometastatic NSCLC. We planned to compare PFS with historical data using a median PFS of 6.6 months,4 a similar reference range to that used in the design of the trials described above. Herein we report the outcomes of our trial, including the effects of PD-L1 status and CD8 T-cell infiltration on outcomes.
Methods
Study Design and Participants
In this single-arm phase 2 trial, we enrolled patients with oligometastatic NSCLC (defined as ≤4 metastases, a number within the range of previous trials5,7) who had completed LAT to all sites of tumor. We enrolled patients with oligometastatic disease at diagnosis (synchronous disease) or who developed oligometastatic disease after initial definitive therapy (metachronous disease). Patients with polymetastatic disease (>4 metastatic sites) whose tumors regressed after initial therapy to an oligometastatic state (oligoremnant disease) were not eligible. We based TNM staging on the assigned stage at the time of initial lung cancer diagnosis. Additional key eligibility requirements included Eastern Cooperative Oncology Group performance status of 0 to 1, absence of autoimmune or immunodeficiency diseases, and adequate organ function. The complete study protocol is found in Supplement 1. We placed no limit on the number of prior therapies, although patients could not have received a prior programmed death 1 or PD-L1 inhibitor. Patients were eligible regardless of their PD-L1 or molecular target status. The trial was approved by the institutional review board at the University of Pennsylvania and was completed in accordance with international standards of good clinical practice. All patients provided written informed consent at the time of enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Procedures
Patients underwent LAT before enrollment in the trial. Any form of LAT was acceptable, including surgery, chemoradiotherapy, stereotactic radiotherapy, and/or interventional ablation. The determination that patients had completed LAT for oligometastatic disease was made by the treating investigator (J.M.B., C.C., C.A., T.E., R.B.C., or C.J.L.) with subsequent confirmation by the principal investigator (J.M.B.) to ensure eligibility. Patients began intravenous pembrolizumab therapy, 200 mg every 21 days (3 weeks), from 4 to 12 weeks after the completion of LAT. We intentionally incorporated a delay from LAT to pembrolizumab therapy because at the time of study design, the safety of early immunotherapy after LAT was not established. Treatment with pembrolizumab continued for up to 8 cycles in the absence of documented disease progression, unacceptable adverse events, intercurrent illness precluding further administration of treatment, the investigator’s decision to withdraw the participant, participant withdrawal of consent, pregnancy of the participant, or nonadherence with trial treatment. Patients without evidence of disease progression after 8 cycles could receive an additional 8 cycles of therapy with pembrolizumab, at the discretion of the treating oncologist. We chose a treatment duration of 6 to 12 months to be within the range of systemic therapy approaches used for other patients with NSCLC who have undergone therapy with curative intent.13,15,16
Patients provided a tissue sample for biomarker analysis. Per protocol, eligible patients had undergone LAT to all known tumor sites, so we were unable to obtain a recent biopsy specimen from all patients. We performed PD-L1 staining using the 22C3 assay (Dako). We also assessed samples for CD8 T-cell infiltration, as previously reported.17 Given the absence of a validated cutoff in the literature, we categorized CD8 T-cell infiltration as high or low as a function of being greater than or no greater than the median in our sample (which we calculated at 2.5% of all stained immune cells). Patients completed a Functional Assessment of Cancer Therapy–Lung (FACT-L)18 quality of life assessment at baseline and at the time of each pembrolizumab treatment (scores range from 0-136, with higher scores indicating better quality of life).
Outcomes
This phase 2 trial had 2 efficacy end points: PFS from the start of LAT (PFS-L), which preceded enrollment in the trial, and PFS from the start of pembrolizumab therapy (PFS-P). Progression-free survival was defined from the start of LAT or pembrolizumab treatment to the first documented disease progression, death due to any cause, or last patient contact that documented progression-free status. Response status was based on investigator assessment of scans using Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). By design, no patient had measurable disease at study enrollment; RECIST 1.1 was used to assess for progressive disease. Scans of all previously involved disease sites were performed every 12 weeks, or as clinically indicated. The study was powered for comparison with historical data on the former efficacy end point. Secondary end points included safety, as defined by Common Terminology Criteria for Adverse Events toxicity profile, OS, and quality of life, as defined by FACT-L. Overall survival was defined from start of LAT to death due to any cause or last patient contact. Cause of death was recorded.
Statistical Analysis
Data were analyzed from February 1, 2015, to August 23, 2018. The null hypothesis stipulated that the median PFS-L would be 6.6 months4 compared with the alternative hypothesis that the median PFS-L would increase to 10 months. We measured survival time from a point before study enrollment to compare with available historical data in oligometastatic NSCLC, which measured survival from LAT. We recognized that such a design could introduce potential bias because any patient with oligometastatic disease who becomes ineligible during LAT would not contribute to the PFS estimate. We therefore also planned to report the PFS-P. An intention-to-treat analysis was planned, and the study was considered to have met the median PFS-L objective if the null hypothesis was rejected. Assuming 42 patients were enrolled during 2 years and followed up for an additional year, the final analysis would have 80% power to detect the stated improvement in median PFS using a 1-sided 5% significance level.
Baseline characteristics and quality-of-life measures were summarized by descriptive statistics; categorical variables were described by frequency and percentage, and continuous variables were described by mean (SE) or by median (range or interquartile range [IQR]). The median value was used to dichotomize continuous variables, when warranted. Median potential follow-up was estimated by the reverse Kaplan-Meier method. The median (95% CI) and mean (SE) rates of 12- and 24-month PFS and OS were estimated using the Kaplan-Meier method. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and generate P values for subgroup comparisons. To address the low OS event rate in the subgroup with PD-L1–positive tumors (causing nonconvergence with standard maximum likelihood estimation for Cox proportional hazards regression), we applied the Firth penalized maximum likelihood bias reduction method, with the penalized likelihood ratio test.19 Trends over time in FACT-L scores were summarized with box plots and descriptive statistics. An exploratory analysis compared baseline and end of treatment scores (at cycles 8 and 16) within patients using the t test for paired data. Normality of the distributions of FACT-L scores was established by normal probability plots. P ≤ .05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 23 (IBM Corp) or R software, version 3.0.2 (R Foundation for Statistical Computing).
Results
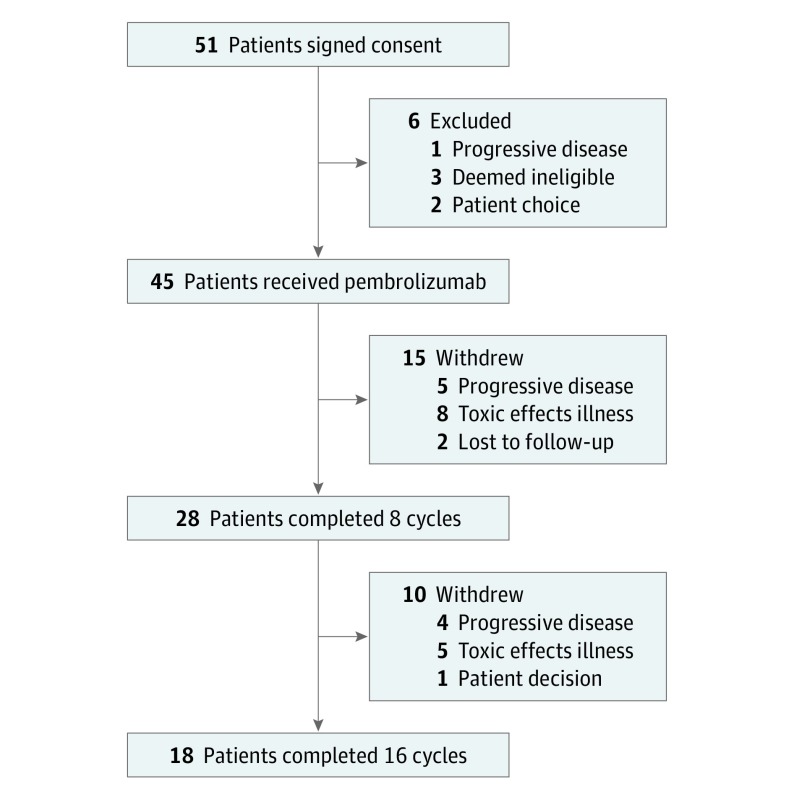
From February 1, 2015, through September 30, 2017, 51 eligible patients provided informed consent for our trial (Figure 1). Of these, 45 (24 men [53%] and 21 women [47%]; median age, 64 years [range, 46-82 years]) underwent treatment with pembrolizumab and are the focus of this analysis. Table 1 gives the baseline clinical characteristics of treated participants. Because some patients received multiple types of chemotherapy and surgery, the sum of the subgroups exceeds the total use of each modality. Thirty-two patients had adequate tissue for assessment of PD-L1, and 29 had adequate tissue for assessment of CD8 T-cell infiltration. In patients undergoing testing, 11 (34%) had results positive for PD-L1 (≥1%) and 15 (52%) had CD8 T-cell infiltration of greater than 2.5%.
Figure 1. CONSORT Diagram.
Table 1. Baseline Clinical Characteristics.
| Characteristic | Patient Data (n = 45)a |
|---|---|
| Age at enrollment, median (range), y | 64 (46-82) |
| Sex | |
| Male | 24 (53) |
| Female | 21 (47) |
| ECOG performance status | |
| 0 | 22 (49) |
| 1 | 23 (51) |
| Race/ethnicity | |
| White | 40 (89) |
| African American | 2 (4) |
| Asian American | 1 (23) |
| Unknown | 2 (4) |
| Smoking status | |
| Current | 5 (11) |
| Former | 35 (78) |
| Never | 5 (11) |
| Histologic finding | |
| Adenocarcinoma | 34 (76) |
| Squamous cell | 8 (18) |
| Other | 3 (7) |
| T stage | |
| 1 | 19 (42) |
| 2 | 12 (27) |
| 3 | 3 (7) |
| 4 | 7 (16) |
| Unknown | 4 (9) |
| N stage | |
| 0 | 16 (36) |
| 1 | 8 (18) |
| 2 | 13 (27) |
| 3 | 4 (9) |
| Unknown | 4 (9) |
| Metastatic timing | |
| Synchronous | 14 (31) |
| Metachronous | 31 (69) |
| No. of metastases | |
| 1 | 28 (62) |
| 2 | 14 (31) |
| 3 | 1 (2) |
| 4 | 2 (4) |
| Metastatic sites | |
| Lung | 14 (31) |
| Brain | 16 (36) |
| Adrenal | 6 (13) |
| Bone | 5 (11) |
| Liver | 5 (11) |
| Prior treatmentb | |
| Surgery | 30 (67) |
| Surgical resection | |
| Primary tumor | 20 (44) |
| Metastasis | 22 (49) |
| Stereotactic radiotherapy | 30 (67) |
| Chemotherapy | 24 (53) |
| Neoadjuvant or adjuvant chemotherapy | 12 (27) |
| Consolidative chemotherapy after chemoradiotherapy | 5 (11) |
| Palliative chemotherapy | 8 (18) |
| Chemoradiotherapy | 23 (51) |
| Standard fraction radiotherapy | 15 (33) |
| Radiofrequency ablation | 1 (2) |
| PD-L1 status | |
| Positive (≥1%) | 11 (24) |
| Negative | 21 (47) |
| Unknown | 13 (29) |
| CD8 T-cell infiltration, % | |
| ≤2.5 | 14 (31) |
| >2.5 | 15 (34) |
| Unknown | 16 (36) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1.
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Some patients underwent multiple types of treatment.
Patients received a median of 11 cycles of pembrolizumab (range, 1-16). Of treated patients, 28 (62%) completed 8 cycles and 18 (40%) completed 16 cycles (Figure 1). All eligible patients opted to continue pembrolizumab treatment beyond 8 cycles.
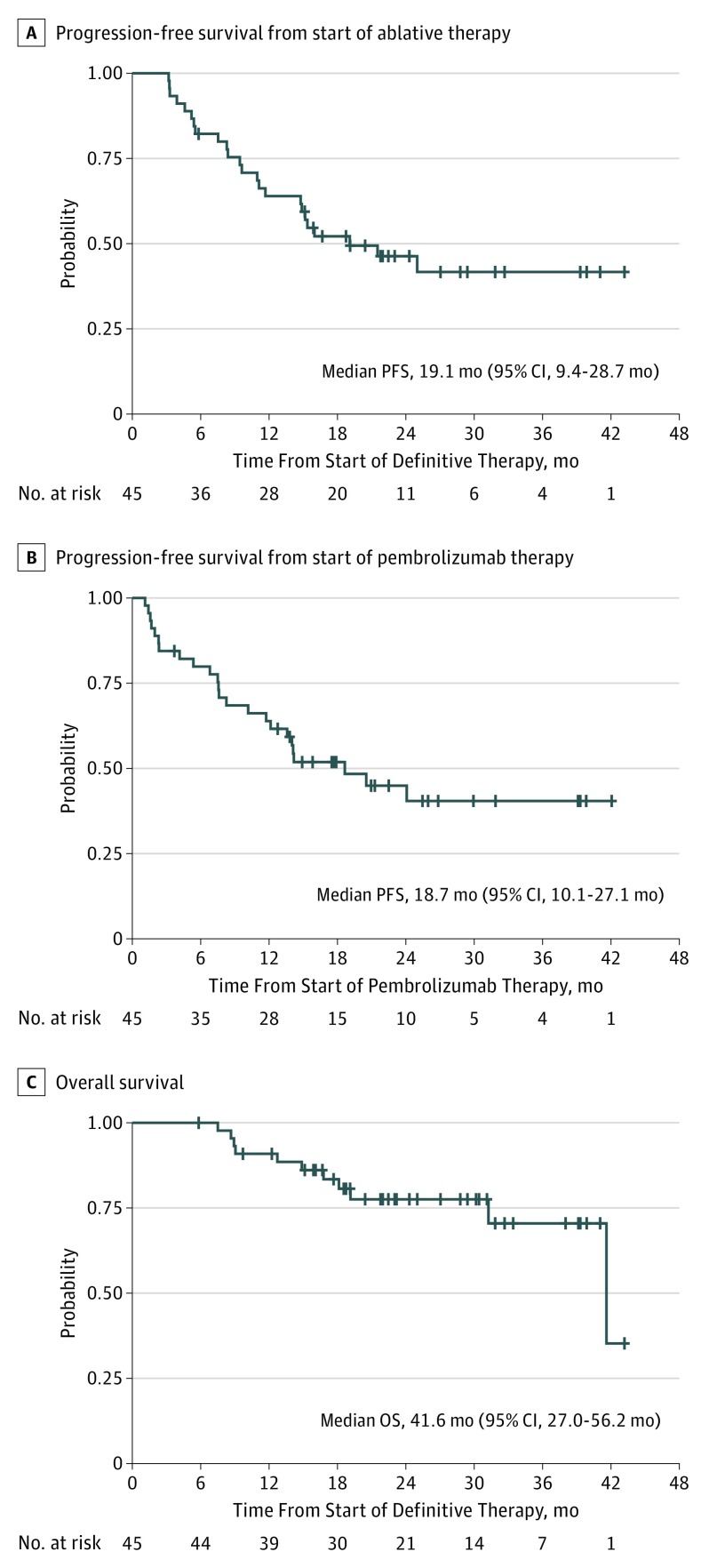
After a median potential follow-up for survival of 25.0 months (23.2 months for living patients), we observed a statistically significant improvement in median PFS-L from the historical 6.6 months to 19.1 months (95% CI, 9.4-28.7 months; P = .005) (Figure 2A). Median PFS-P was 18.7 months (95% CI, 10.1-27.1 months) (Figure 2B). Median OS was 41.6 months (95% CI, 27.0-56.2 months) (Figure 2C), although this estimate may be an artifact due to censoring and a single late event. Further follow-up is required for confirmation. The mean (SE) OS rate at 12 months was 90.9% (4.3%); at 24 months, 77.5% (6.7%). Among the patients enrolled, 23 experienced progression. Progression was local only (at a site of a prior LAT) in 2 patients, systemic only (not at a site of a prior LAT) in 15 patients, and both in 6 patients. Local progression (whether concurrent with systemic or not) occurred in 4 patients who had stereotactic radiotherapy, 2 patients who had surgery, 1 who had chemoradiotherapy, and 1 who had radiotherapy to the site in question. Treatments received after progression during the trial are summarized in eTable 1 in Supplement 2.
Figure 2. Progression-Free and Overall Survival.
Progression-free survival (PFS) was measured separately from the start of locally ablative therapy (LAT) (A) and from the start of pembrolizumab therapy (B). Overall survival (OS) was measured from the start of LAT (C).
We performed exploratory analyses to determine whether any clinical or pathologic features were associated with PFS-L or OS. As shown in Table 2, we were unable to identify any clinical variables that were significantly associated with clinical outcomes; therefore, we did not perform multivariable analyses. We found a suggestion of improved PFS-L (but these findings did not reach statistical significance) for patients with metachronous disease compared with those with synchronous disease (24-month PFS-L mean [SE] rate, 56.4% [9.2%] vs 16.7% [13.5%]; HR, 1.98 [95% CI, 0.87-4.52]) (eFigure 1 in Supplement 2) and for patients with tumors positive for PD-L1 compared with tumors negative for PD-L1 (24-month PFS mean [SE] rate, 69.3% [15.0%] vs 38.1% [10.6%]; HR, 3.10 [95% CI, 0.88-10.93]) (eFigure 2 in Supplement 2). These analyses should be considered preliminary and hypothesis generating.
Table 2. Univariate Analyses of Progression-Free Survival From Start of Locally Ablative Therapy and Overall Survival.
| No. of Patients | Progression-Free Survival | Overall Survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Metastases | |||||
| Metachronous | 31 | 1 [Reference] | .10 | 1 [Reference] | .59 |
| Synchronous | 14 | 1.98 (0.87-4.52) | 1.41 (0.40-4.90) | ||
| T stage | |||||
| 1 | 19 | 1 [Reference] | .08 | 1 [Reference] | .83 |
| 2 | 12 | 0.88 (0.33-2.30) | 0.92 (0.22-3.88) | ||
| 3-4 | 10 | 0.17 (0.04-0.81) | 0.60 (0.11-3.18) | ||
| N stage | |||||
| 0 | 16 | 1 [Reference] | .71 | 1 [Reference] | .62 |
| 1 | 8 | 0.63 (0.16-2.39) | 0.47 (0.05-4.62) | ||
| 2-3 | 17 | 1.08 (0.42-2.75) | 1.35 (0.32-5.74) | ||
| PD-L1 status | |||||
| Positive | 11 | 1 [Reference] | .08 | 1 [Reference] | .10a |
| Negative | 21 | 3.10 (0.88-10.93) | 6.56 (0.74-861.59)a | ||
| CD8 T-cell infiltration, % | |||||
| >2.5 | 15 | 1 [Reference] | .31 | 1 [Reference] | .82 |
| ≤2.5 | 14 | 1.72 (0.60-5.03) | 1.26 (0.18-8.95) | ||
Abbreviations: HR, hazard ratio; PD-L1, programmed death ligand 1.
Uses Firth penalized maximum likelihood bias correction, owing to only 1 failure in the PD-L1–positive group.
Pembrolizumab after LAT was safe, with no new toxicity signals among patients with oligometastatic NSCLC (Table 3). Pneumonitis occurred in 5 patients (11%), with 2 episodes each of grade 2 (4%) and grade 3 (4%), and 1 episode of grade 4 pneumonitis (2%). All 5 patients with pneumonitis had received prior thoracic radiotherapy. No other grade 4 attributable adverse events occurred, and no grade 5 attributable adverse events occurred. In addition to the more common adverse events listed in Table 3, 2 episodes of grade 3 colitis and 2 episodes of adrenal insufficiency (1 grade 2 and 1 grade 3) were deemed treatment related.
Table 3. Treatment-Related Adverse Events With at Least 10% Incidence in Study Population per Common Terminology Criteria for Adverse Events.
| No. (%) of Patients (n = 45) | |||||
|---|---|---|---|---|---|
| Grades 1-4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Pain | 19 (42) | 13 (29) | 5 (11) | 1 (2) | 0 |
| Fatigue | 16 (36) | 11 (24) | 5 (11) | 0 | 0 |
| Rash | 10 (22) | 10 (22) | 0 | 0 | 0 |
| Dyspnea | 8 (18) | 3 (7) | 4 (9) | 1 (2.2) | 0 |
| Cough | 7 (16) | 4 (9) | 3 (7) | 0 | 0 |
| Pruritus | 7 (16) | 7 (16) | 0 | 0 | 0 |
| Dizziness | 6 (13) | 6 (13) | 0 | 0 | 0 |
| Edema | 6 (13) | 4 (8.9) | 2 (4) | 0 | 0 |
| Nausea | 6 (13) | 5 (11) | 0 | 1 (2) | 0 |
| Pneumonitis | 5 (11) | 0 | 2 (4) | 2 (4) | 1 (2) |
| Dry eyes | 5 (11) | 4 (9) | 1 (2) | 0 | 0 |
| Headache | 5 (11) | 5 (11) | 0 | 0 | 0 |
| Insomnia | 5 (11) | 4 (9) | 1 (2) | 0 | 0 |
Median FACT-L total score was 111.3 (IQR, 95.5-120.4) at baseline (n = 38), 110.0 (IQR, 90.7-122.5) at cycle 8 (n = 25), and 115.0 (IQR, 102.5-125.0) at cycle 16 (n = 13) (eFigure 3 in Supplement 2). In patients with paired data, FACT-L scores at cycles 8 and 16 were not significantly different from FACT-L scores at baseline (eTable 2 in Supplement 2).
Discussion
Locally ablative therapy for oligometastatic NSCLC has been shown to improve clinical outcomes in 2 separate randomized clinical trials.5,7 The therapeutic approach in our trial differs from that of both studies. To stay within the standard of care for metastatic NSCLC, the published trials used palliative chemotherapy before LAT. By design in these studies, the designation of oligometastatic disease occurred after chemotherapy was completed and thus created what might be termed an oligoremnant population. Such a trial design may select for patients with an intrinsically more indolent disease trajectory or tumors more sensitive to systemic therapy because any patient whose cancer had progressed during systemic therapy would have been excluded from the trial. Indeed, in the study by Gomez et al,5 33.8% of enrolled patients were excluded from randomization, most commonly owing to disease progression during systemic therapy. By contrast, in our study, patients were required to have oligometastatic disease at the time of their diagnosis of metastatic disease. We found that the use of pembrolizumab after LAT in oligometastatic NSCLC was associated with a statistically and clinically significant improvement in PFS-L compared with historical data (P = .005). With a median follow-up of 25.0 months, patients enrolled in our trial had a median PFS-L of 19.1 months (95% CI, 9.4-28.7 months), which was nearly triple that expected from the historical median PFS of 6.6 months (P = .005). Our median using the more conservative PFS-P measurement was 18.7 months (95% CI, 10.1-27.1 months), which compares favorably with other studies using pembrolizumab in a population not preselected for PD-L1 overexpression (4 months in KEYNOTE-01020 and 7.1 months in KEYNOTE-04221). A final analysis of OS is planned after additional patient follow-up. Our results fit well with emerging data that each oligometastasis may have a different genetic profile and distinct interactions with the immune system.3
Appropriate patient selection for LAT in patients with oligometastatic NSCLC is an evolving art. Counting the number of metastases present, for example, is likely a very crude surrogate marker of the underlying biology of the oligometastatic process.2 Some patients with 3 metastases have a truly oligometastatic phenotype, while others have a clinically undetectable polymetastatic state. Prior studies have sought to identify microRNA signatures that would allow for more precise patient identification, but this assay has not been validated in a clinically available format.22,23 Although we were unable to identify clinical variables with statistically significant predictive capabilities, we must acknowledge that our biopsy-based assays (CD8 T-cell infiltration and PD-L1 staining) were relatively underpowered. Given the clear role of PD-L1 staining in the treatment of patients with stage IV NSCLC,10 we find the trend toward improved outcomes among patients with PD-L1–positive cancers particularly intriguing. We believe PD-L1 positivity should be considered as a stratification factor in future trials. Given the hypothesized effect of radiotherapy on subsequent response to immunotherapy,24,25,26 we had hoped to conduct subgroup analyses to determine the relative outcomes for patients who received and did not receive radiotherapy. Unfortunately, too few patients in our sample (5 patients) were radiotherapy naive, and thus such an analysis could not be performed.
Strengths and Limitations
Our study has numerous strengths. We focused on patients presenting with oligometastatic disease rather than an oligoremnant population in which patients with rapid progression during chemotherapy are by definition excluded. Our eligibility and enrollment strategy theoretically might have selected for patients with an inferior prognosis, and yet we observed very promising clinical outcomes. In addition, to our knowledge, this study is the first to show improved outcomes for immunotherapy after LAT in patients with oligometastatic NSCLC. Our study provides further support for the potential role of immunotherapy in the setting of minimal residual disease.
Nevertheless, our trial has several limitations. First, in a single-arm study, we cannot formally establish the role of pembrolizumab over LAT alone. Beyond this, our comparison with historical data required starting the measurement of survival time before trial enrollment. The most definitive means of characterizing the contribution of pembrolizumab to clinical outcomes after LAT would be a randomized clinical trial comparing LAT alone with LAT followed by pembrolizumab in oligometastatic NSCLC. We are currently designing such a trial. We were able to accrue 45 patients in less than 3 years at a single institution, suggesting that performing larger multicenter randomized clinical trials in the oligometastatic disease setting is feasible. Next, all but 3 patients (7%) had 1 or 2 sites of metastases, so the applicability of this approach may be limited in patients with more than 2 sites of recurrence or metastasis. In future randomized clinical trials, stratification for variables that may influence clinical outcomes for patients with oligometastatic NSCLC is imperative.
Conclusions
The use of pembrolizumab after LAT for oligometastatic NSCLC was associated with a clinically and statistically significant improvement in PFS-L compared with historical data without a decrement in quality of life. The OS outcomes in our trial are preliminary and require further follow-up but appear favorable. This treatment approach warrants further testing in a randomized clinical trial.
Trial Protocol
eTable 1. Therapy After Progression on Trial
eTable 2. FACT-L Quality-of-Life Data
eFigure 1. Progression-Free Survival From Start of Locally Ablative Therapy by Metastatic Timing
eFigure 2. Progression-Free Survival From Start of Locally Ablative Therapy by PD-L1 Status
eFigure 3. Box Plot of FACT-L Total Score at Baseline, Week 8, and Week 16, Showing Median and Interquartile Range
Data Sharing Statement
References
- 1.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? a systematic review of the literature. Lung Cancer. 2013;82(2):197-203. doi: 10.1016/j.lungcan.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 2.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378-382. doi: 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 3.Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non–small-cell lung cancer. Clin Lung Cancer. 2014;15(5):346-355. doi: 10.1016/j.cllc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Griffioen GHMJ, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non–small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer. 2013;82(1):95-102. doi: 10.1016/j.lungcan.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672-1682. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;JCO1900201. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501-e173501. doi: 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551. doi: 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 9.Socinski MA, Okamoto I, Hon JK, et al. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(9):2390-2396. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. [DOI] [PubMed] [Google Scholar]
- 12.Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators . Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. [DOI] [PubMed] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350. [DOI] [PubMed] [Google Scholar]
- 14.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. [DOI] [PubMed] [Google Scholar]
- 15.Pignon JP, Tribodet H, Scagliotti GV, et al. ; LACE Collaborative Group . Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552-3559. doi: 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 16.Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non–small-cell lung cancer. J Clin Oncol. 2019;37(2):97-104. doi: 10.1200/JCO.18.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnem T, Hald SM, Paulsen E-E, et al. Stromal CD8+ T-cell density: a promising supplement to TNM staging in non–small cell lung cancer. Clin Cancer Res. 2015;21(11):2635-2643. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy–Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199-220. doi: 10.1016/0169-5002(95)00450-F [DOI] [PubMed] [Google Scholar]
- 19.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114-119. doi: 10.1111/j.0006-341X.2001.00114.x [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 21.Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 22.Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7(12):e50141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuttig D, Baier B, Fuessel S, et al. Gene signatures of pulmonary metastases of renal cell carcinoma reflect the disease-free interval and the number of metastases per patient. Int J Cancer. 2009;125(2):474-482. doi: 10.1002/ijc.24353 [DOI] [PubMed] [Google Scholar]
- 24.Mujoo K, Hunt CR, Pandita RK, et al. Harnessing and optimizing the interplay between immunotherapy and radiotherapy to improve survival outcomes. Mol Cancer Res. 2018;16(8):1209-1214. doi: 10.1158/1541-7786.MCR-17-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016;76(20):5994-6005. doi: 10.1158/0008-5472.CAN-16-0549 [DOI] [PubMed] [Google Scholar]
- 26.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611-1618. doi: 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Therapy After Progression on Trial
eTable 2. FACT-L Quality-of-Life Data
eFigure 1. Progression-Free Survival From Start of Locally Ablative Therapy by Metastatic Timing
eFigure 2. Progression-Free Survival From Start of Locally Ablative Therapy by PD-L1 Status
eFigure 3. Box Plot of FACT-L Total Score at Baseline, Week 8, and Week 16, Showing Median and Interquartile Range
Data Sharing Statement