Introduction
Asthma is an increasing public health concern with more than 8% of children and adults now suffering from this condition (1, 2). Asthma exacerbations, or loss of symptom control that includes increased cough, wheezing, and difficulty breathing, provide the greatest morbidity to the asthmatic, and can lead to hospitalizations and, rarely, to death. Importantly, in 2016, ~54% of children (2) and ~45% of adults (1) with a diagnosis of current asthma reported having one or more asthma exacerbations. While an exact mechanism/causal relationship is not clear, rhinovirus (RV) infections have been extensively linked to asthma exacerbations. In fact, 60–80% of children with asthma exacerbations seen in the emergency department have a concomitant RV infection (3, 4).
In this article, the authors will evaluate the evidence regarding the association of RV infections to asthma exacerbations. Further, we will examine the effects of genetic polymorphisms on the generation of viral-induced asthma exacerbations. Lastly, we will discuss the role of the atopic host in the relationship between exacerbations and RV infections. Because of the overwhelming evidence linking RV infection and asthma exacerbations, our ultimate goal is to gather potential mechanisms concerning how this virus can cause such chaos in the asthmatic and speculate on potential therapeutics that may improve outcomes in the future.
The evidence that RV infection causes asthma exacerbations
Viral respiratory infections are the most common triggers for exacerbations of asthma, with human RV, particularly subtypes A and C, being the most frequent (5). In fact, hospital admission rates for asthma exacerbations in school age children correlate with the seasonal increase of RV infections in the fall and again in the spring, with similar peaks observed in adults (6). However, RV infections are ubiquitous and can occur throughout the year. Many of these infections do not cause exacerbations, and some remain completely asymptomatic (7). This lack of a consistent association has led to a debate as to whether RV infection is causal or is merely associated in asthma exacerbations.
The Con: RV is associated with asthma exacerbations
In the context of asthma exacerbations, there is ongoing debate whether RV infections are the sole cause, are synergistic with other factors, or are merely associated with exacerbations. For example, studies have detected the presence of RV in patients who are asymptomatic or have minimal symptoms. In one prospective study, 126 infants were followed for the first 2 years of life (8). Among these subjects, RV was the most predominant virus detected, regardless of the presence of symptoms. In the Early Unbiased Risk Assessment of Pediatric Asthma (EUROPA) prospective casecontrol follow-up study, 140 symptomatic and 96 asymptomatic children were selected from a birth cohort and were followed for three years (November, 2009 through December, 2012)(9). RV-A and RV-C were detected with similar prevalence between symptomatic (which included both wheezing and non-wheezing children) and control children. Of note, RV-B was detected significantly more in the control group (9). Improved detection techniques using PCR and more regular screening may lead to recognition of an even greater prevalence of indolent RV infection in the asymptomatic population.
When examining RV infections in the setting of experimental challenge models, studies have suggested that RV may not cause asthma exacerbations. In one of the first studies to examine this, 21 adult asthmatics were exposed to RV (10), and their symptoms, spirometry measurements, and histamine challenges were assessed prior to RV exposure, daily while symptomatic, and three weeks post-RV exposure. The results showed no significant changes in spirometry or histamine sensitivity, either as a whole group or when subcategorized by asthma severity.
The Pro: RV causes asthma exacerbations
In contrast, many other studies have shown a causal relationship between exposure to RV and asthma exacerbations or, alternatively, objective evidence of increased airways hyperresponsiveness (AHR). In a prospective study following subjects from the same household, one with asthma and one without, subjects had similar frequency of RV infections, though the asthmatics developed greater lower respiratory symptoms during the infection (11, 12). Other studies have shown increased susceptibility and symptoms, specifically during RV-C infections in children with asthma. For example, in one study, the frequency of RV-C infections was much higher in the asthma population than in the community as a whole, suggesting that asthmatic children are more susceptible to infection with RV-C. The same study showed that subjects with RV-C had higher asthma severity scores than children with RV-A or -B (13).
More direct evidence of a causative role of RV in acute exacerbations of asthma has been established in the experimental RV infection model (14–16). In a study by Zambrano et al., patients with asthma and high total IgE (>371 IU/mL) infected with RV-A16 had increased methacholine sensitivity by day 4 of the study and that increase remained evident through day 21 (14). These subjects experienced significantly worse lower respiratory symptoms than their control counterparts. Another experimental infection study exposed subjects with asthma on inhaled corticosteroids (ICS) to RV-A16. In this study, subjects had increased symptoms with a mean period between peak cold and peak asthma symptoms of 2.1 (0.3–3.9) days (15). There was also increased ß-agonist use, suggesting loss of symptom control; however, these asthmatics did not experience a reduction in lung function post-inoculation. The authors suggest that long-term ICS use might inhibit this process. In a more recent study, 10 adult atopic asthmatics and 15 controls were infected with RV-A16 (16) and bronchial biopsies were taken at 14 days prior to infection, 4 days after, and 6 weeks post-infection. There were no significant differences in the number of neutrophils at baseline between asthmatics and controls. In asthmatics, the change in number of epithelial and sub-epithelial neutrophils from baseline to day 4 was significantly higher. Those asthmatics who had higher epithelial neutrophil counts had a significantly larger fall in FEV1 post-infection.
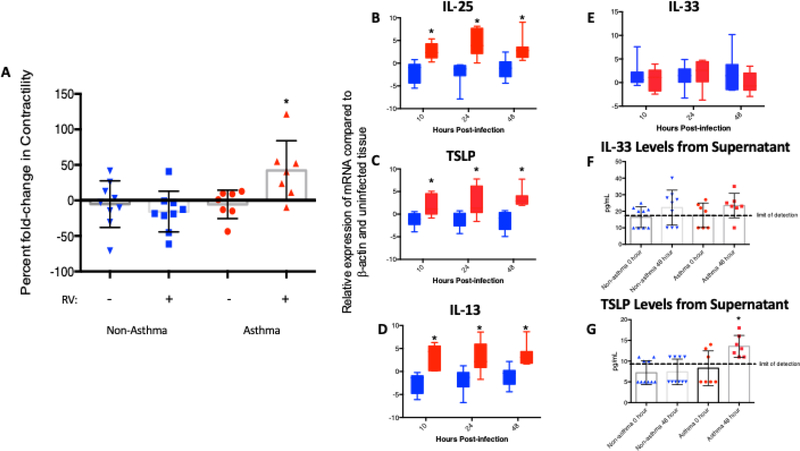
Recently, in vitro studies of human precision-cut lung slices (PCLS) from 7 asthmatics and 9 control lungs infected with RV-A39 demonstrated increased AHR (asthma mean: 35% change in AHR after infection; non-asthma mean: 17.7% change in AHR after infection; p<0.05)(17)(Figure 1A). These studies also found differences in the inflammatory responses generated by asthmatic tissue compared to controls, specifically related to type 2 (T2) inflammation in the asthmatic tissue, which will be discussed further below. To our knowledge, this study is the first to show direct evidence of AHR to RV in asthma tissue ex vivo.
Figure 1: RV39 infection of Human Precision Cut Lung Slices (PCLS) from Asthma Donors.
(A) Comparison of carbachol (CCh)-induced airway responsiveness in donors with and without asthma before and after infection with RV39. RV39 infection causes airway hyper-responsiveness to CCh only in PCLS from donors with a history of asthma. (B-E) IL25, TSLP, IL13, and IL-33 gene expression from RV39 infected PCLS. There are significant increases in the expression of IL-25, TSLP, and IL-13 in the PCLS from asthma donors compared to controls without asthma. (F, G) IL-33 and TSLP protein levels in supernatants from RV39 infected PCLS in donors with and without asthma. There are significantly increased TSLP protein levels in those donors with a history of asthma after infection with RV39.
* p<0.05
Adapted from Kennedy JL, Koziol-White CJ, Jeffus S, et al. Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J Allergy Clin Immunol 2018; 141(5): 1888; with permission.
Genetic susceptibilities leading to asthma exacerbations as related to RV infection
While it may be difficult to connect genetic predisposition with a future outcome like asthma exacerbation risk, researchers have recently shed light on mechanisms whereby this might be the case. Specifically, several candidate genes have been identified that provide potential associations between genetic polymorphisms and viral respiratory illness outcomes, including asthma exacerbations. Some of these genes (e.g., STAT4, JAK2, MX1, VDR, DDX58, and EIF2AK2) are important in antiviral and innate immune responses and, as such, might impart susceptibility to respiratory viruses, infection severity, and lead to virus-induced asthma exacerbations (18, 19). Studies have highlighted the importance of one particular gene in contributing to RV-induced asthma exacerbations, specifically, the genetic variant of the transmembrane protein cadherin-related family members 3 (CDHR3), which has been identified as the RV-C receptor (20). In a Swedish study, 122 wheezing subjects and 94 controls (ages 6 to 48 months) were seen both during acute episodes for wheeze and at a follow-up visit 2–3 months later (21). The genotype AA/AG at the CDHR3 gene was significantly over-represented in wheezing children; moreover, children with this genotype sought emergency care due to respiratory symptoms at a significantly higher rate. The AA/AG genetic variant results in an amino acid change (Cys529Tyr) in the CDHR3 protein, at a site that is associated with increased binding, internalization, and subsequent replication of RV-C strains. As such, these findings suggest a mechanism for greater susceptibility to and symptoms during RV-C infection that could drive exacerbations of asthma. The same outcome of this CDHR3 gene mutation was replicated in early-onset asthmatics in a different population. In this retrospective study of two cohorts (distinguished by geographical location – 967 healthy and 814 asthmatic adults in Tsukuba and 994 healthy and 591 asthmatic adults in Hokkaido), a greater number of early-onset asthmatics (≤10 years of age) were found to have the A (Cys529Tyr) allele (22). When only atopic individuals from both cohorts were examined, the association between the CDHR3 variant and early-onset asthma reached significance. These findings tie together the importance of genetic susceptibility to RV infection in combination with allergy (and by extension T2 status) in the generation of asthma exacerbations.
The role of allergy in RV-associated asthma exacerbations
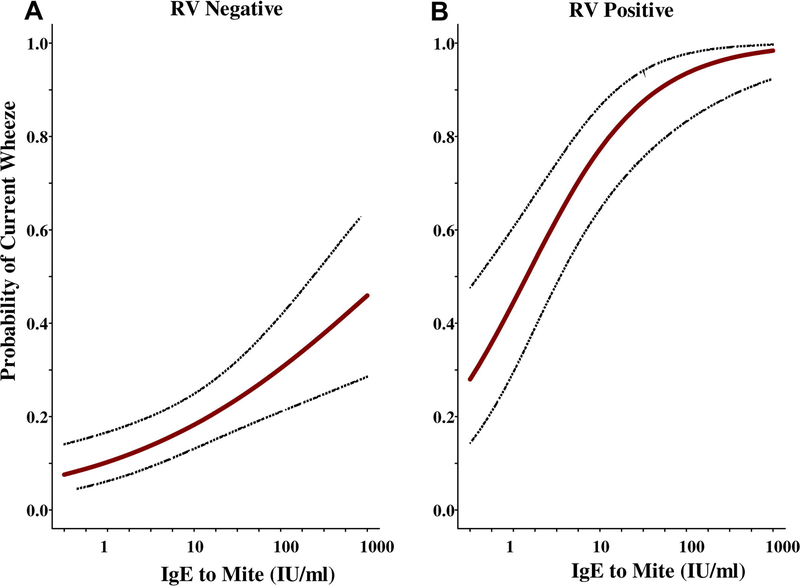
As mentioned, 60–80% of children who are seen for asthma exacerbations will have a concomitant RV infection (3, 4); however, the vast majority (i.e., ≥85%) are also allergic (23). It is reasonable to speculate that transient increases in exposure to allergen are not a convincing explanation of most attacks. However, almost all the evidence regarding the effects of RV relates primarily to allergic patients. In a study of Costa Rican children, the relationship of dust mite allergy and severe asthma exacerbations in the context of RV infection was positively correlated (24). In this study, 96 acutely wheezing children, 65 stable asthmatics, and 126 controls were enrolled through the ED. Multiple allergen-specific IgE antibodies were measured, and IgE to dust mite allergens (D. pteronyssinus, D. farinae, or Blomia tropicalis) were the most commonly detected. In this population, the probability of acute wheezing was significantly associated with increasing IgE titers to dust mite, and this probability substantially increased if the subject was RV-positive during the exacerbation (Figure 2; Table 1). These data produced a stunningly positive odds ratio of 30 (p<0.001), arguing that in the presence of sufficient allergic sensitivity to the dust mite, an RV infection virtually assured development of an asthma exacerbation sufficiently severe to require urgent care.
Figure 2:
Probability of current wheezing based on increasing specific IgE to D. pteronyssinus in children with (A) negative tests for RV and (B) positive tests for RV. The presence of high titers of specific IgE to dust mite in combination with RV leads to greatly increased risk for current wheezing.
From Soto-Quiros M, Avila L, Platts-Mills TA, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 2012; 129(6): 1504; with permission.
Table 1.
Odds ratio for wheezing based on positive tests for RV and titers of IgE antibodies (IU/mL) to dust mite (D. pteronyssinus)
| Titer of IgE antibodies to mite: | <0.35 lU/ml | 0.35–17.4 lU/ml | ≥17.5 lU/ml | ||||
|---|---|---|---|---|---|---|---|
| n | PCR − | PCR + [A/C]‡ | PCR− | PCR + [A/C]‡ | PCR− | PCR + [A/C]‡ | |
| Current Wheeze | 95 | 5 | 4†[2/2] | 8 | 16 [3/10] | 20 | 42 [8/30] |
| Stable asthma | 63 | 12 | 2 [0/1] | 12 | 5 [1/2] | 31 | 1 [0/1] |
| Control | 123 | 60 | 13 [6/4] | 31 | 2[1/1] | 14 | 2[1/1] |
| Non-wheezing children# | 186 | 73 | 15 [6/5] | 43 | 7[2/3] | 45 | 3[1/2] |
| Odds Ratio* | 3.89 [0.9–16], p=0.07 | 12.3 [3.8–39], p<0.001 | 31.5 [8.3–108], p<0.001 | ||||
‡Number in brackets indicates the number of subjects positive for Group A and Group C strains of RV.
†Two of these four children had IgE antibody to Blomia tropicalis: (24.3 and 1.66 IU/ml).
Stable asthma combined with non-asthmatic controls.
Odds ratio for wheezing among RV (real-time PCR) positive compared to negative subjects.
Adapted from Soto-Quiros M, Avila L, Platts-Mills TA, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 2012; 129(6): 1503; with permission.
The findings of potential synergy between RV infection, allergic sensitization, and asthma exacerbations were further confirmed in controlled trials using omalizumab (anti-IgE) to treat severe or moderately severe asthma (25, 26). In these studies, omalizumab used to treat inner city children had a striking effect in preventing the increase in acute episodes of asthma that occurred in May and September. As previously noted, those months coincide with the season for which allergen exposure is highest and viral infections are prevalent. As also mentioned above, during experimental RV challenge, patients with higher IgE levels (>371 IU/ml) have significant changes in bronchial hyperreactivity as judged by methacholine testing and increased symptom severity. Together, these observations strongly suggest that omalizumab likely has a role in mitigating RV-induced exacerbations of asthma. However, controlled trials of anti-IgE treatment prior to RV challenge, which are ongoing (ClinicalTrials.gov: NCT02388997) will be needed to understand the exact interaction between Ig-SEmediated allergy and RV-induced exacerbations.
Mechanisms of RV-Induced Exacerbations of Asthma Related to Allergy
Current research suggests a differential response in asthmatics that consists of cytokines that could bias an allergic response, including interleukin (IL)-25, thymic stromal lymphopoietin (TSLP), and IL-33. In one study, mice that were sensitized to ovalbumin prior to experimental RV-1B infection displayed significantly elevated IL-25 mRNA levels (28-fold higher than non-infected, allergen sensitized mice at 10 hours post-infection)(27). Higher IL-25 mRNA levels were associated with increased levels of the T2 cytokines IL-4 and IL-13 in bronchoalveolar lavage fluid 10 hours post-infection and IL-5 at 24 hours post-infection. Moreover, when this group of mice was treated with a monoclonal antibody to block the IL-25 receptor, levels of IL-4, IL-5 and IL-13 dropped and were similar to controls. The researchers from this study corroborated a similar role for IL-25 in human asthmatics, demonstrating that when 28 asthmatics and 11 controls were experimentally infected with RV-A16, asthmatics displayed significantly increased levels of IL-25 compared to baseline prior to infection.
In the studies mentioned above using human PCLS, a role for IL-25 and TSLP was suggested in the increased AHR afforded to human airways after RV infection (16). In asthmatic tissue, not only was gene expression of IL-25, TSLP, and IL-13 significantly increased at 10, 24, and 48 hours post-infection, but TSLP protein release was also significantly increased at 48 hours post-infection (Figure 1B-G). In other studies, TSLP has been induced by both RV infection and dsRNA in the lungs of allergic mice (28) and in human bronchial epithelial cells (29). Further, TSLP is elevated in the nasal passages of young children with RV infection (30). These findings provide supporting evidence that epithelial generation of T2 cytokines (such as IL-13) leads to increased AHR in the setting of RV infection.
IL-33 has a vital role in the induction and effector phases of type 2 immune responses, and, as such, it is important in many allergic diseases including asthma, atopic dermatitis, and allergic rhinitis (31). In fact, genetic polymorphisms of both the IL-33 gene and its receptor are strongly linked to asthma, suggesting atopic individuals may be genetically predisposed to secrete more and respond better to IL-33, especially during allergen challenge (32). In a recent study by Jackson and colleagues, experimental RV inoculation and BAL sampling of human subjects revealed induction of IL-4, IL-5, IL-13, and IL-33 in the airways of asthmatics (33). Peripheral blood T and ILC2 cells from the same subjects cultured with the supernatants of RV-infected bronchial epithelial cells also induced these cytokines, and this process was entirely dependent upon IL-33 (34, 35).
Future therapeutic options for patients with viral-induced exacerbations of asthma
Given the studies which demonstrate a muted allergic response in asthmatic subjects treated with biologics directed against IgE, it is reasonable to expect lower severity of viral-induced exacerbations due to less of a T2-biasing response. One such biologic, omalizumab, effectively blocks the activity of much of the circulating IgE by targeting its binding to the high-affinity receptor-binding site on IgE. As a result, omalizumab therapy improves asthma impairment, risk and degree of control, as illustrated by reduction in days with asthma symptoms, reduction in exacerbations and hospitalizations, and reduced need for inhaled corticosteroids to maintain control (26). More importantly, as previously noted, the seasonal peaks of asthma exacerbations seen in spring and fall were nearly eliminated in the omalizumab treatment group compared to the control. This association remained in spite of similar detection of viruses throughout the year in both the treatment and control groups (26). However, since the treatment group reported a significantly decreased number of exacerbations, this further supports the previously discussed synergistic association between viral infections and allergen responses during asthma exacerbations.
In addition to biologics directed against IgE, additional pharmacological agents have also been developed. Given the central importance of TSLP to RV-mediated asthma exacerbations, it is reasonable to speculate that this agent would also attenuate the presence and severity of exacerbations. A monoclonal antibody to TSLP (tezepelumab) has been evaluated in both an allergen challenge model (35) and in severe uncontrolled asthmatics and shown to significantly improve asthma control and prevent exacerbations while decreasing eosinophils and FeNO (36). Human monoclonal antibodies to IL-25 and IL-33 are currently also being developed as asthma therapeutics and the central importance of these cytokines to RV-mediated exacerbations in pre-clinical studies support their potential to improve symptoms during RV infections. For example, as previously discussed, blockade of the IL-25 receptor in murine models correlated with decreased T2 biasing response during RV infections (27). Similarly, in an in vitro study utilizing human asthma bronchial epithelial cells experimentally infected with RV-A16, antibodies to IL-33 resulted in attenuated T2 cytokine production (33). In summary, these studies support the premise that biologics directed against TSLP, IL-25 and IL-33 could be additional therapeutic targets for prevention of viral-induced asthma exacerbations.
Conclusions
In recent years, we have made significant strides towards improving asthma control; however, despite our best efforts, we continue to have significant morbidity and, tragically, mortality from asthma. RV is ubiquitous and typically causes only minor upper respiratory symptoms in the general population. However, RV causes asthma exacerbations, especially in the presence of another impetus, especially allergy. Because exacerbations do not happen in every asthmatic with an RV infection, it is possible that exacerbations may be related to the genetic background of the host, occur in the context of a concomitant allergen exposure, or require altered immune responses to the virus that leads to greater inflammation and loss of asthma control. In the allergic subject, mechanisms of the host immune response appear to bias toward increased allergic inflammation and worsening AHR. Given these responses, there are a number of possible treatments available and even more on the horizon that should improve our ability to control exacerbations related to RV infection.
Key Points:
Most asthma exacerbations in children and adolescents are associated with development of a rhinovirus (RV) infection.
These RV-mediated exacerbations appear to require concomitant exposure to a bystander allergen to which the asthmatic is highly sensitized.
Asthmatics can be genetically prone to RV-mediated exacerbations.
Targeting IgE and type 2 inflammation-promoting cytokines can mitigate likelihood of developing RV-induced asthma exacerbations.
Synopsis:
Rhinovirus (RV) is a ubiquitous pathogen and typically causes only minor upper respiratory symptoms in the general population. However, especially in children and adolescent asthmatics, RV is responsible for most exacerbations. This ability of RV to drive exacerbations typically requires the concomitant presence of exposure to a bystander allergen to which the asthmatic is sensitized. Susceptibility to RV-mediated exacerbations is also related to the genetic background of the host, which will variously contribute to greater infectivity, more severe infections, altered immune responses and ultimately to greater inflammation and loss of asthma control. Given these responses, there are a number of treatments available or on the horizon that should improve our ability to control exacerbations related to RV infection. These includes biologics that target IgE and the cytokines that either promote type 2 immune deviation (TSLP, interleukin (IL)-25, and IL-33) or are components of type 2 inflammation (IL-4, IL-5, and IL-13).
Acknowledgments
Joshua L. Kennedy and his laboratory are supported by the NIH (K08AI121345, UL1TR000039, KL2TR000063, P20GM121293), the Centers for Translational Science Award Western Consortium Grant, Arkansas Children’s Research Institute Marion B. Lyon New Scientist Career Development Award, and the Arkansas Biosciences Institute.
Larry Borish does consulting work for Novartis, Teva, Astra Zeneca, and Regeneron. His laboratory at the University of Virginia receives research grants from the NIH and from Astra Zeneca.
Footnotes
Disclosures:
Sarah Pham reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mazurek JM, Syamlal G. Prevalence of Asthma, Asthma Attacks, and Emergency Department Visits for Asthma Among Working Adults - National Health Interview Survey, 2011–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in Children - United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng SY, Wang LL, Ren L, Luo J, Liao W, Liu EM. Epidemiological analysis and follow-up of human rhinovirus infection in children with asthma exacerbation. J Med Virol 2018; 90: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, Erwin EA, Shaker MS, Hellems M, Peerzada J, Hayden FG, Hatley TK, Chamberlain R. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004; 114: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WM, Lemanske RF Jr., Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 2012; 186: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo JR, Peters SP, Busse WW. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J Allergy Clin Immunol Pract 2017; 5: 918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinke JW, Borish L. Immune Responses in Rhinovirus-Induced Asthma Exacerbations. Curr Allergy Asthma Rep 2016; 16: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Benten I, Koopman L, Niesters B, Hop W, van Middelkoop B, de Waal L, van Drunen K, Osterhaus A, Neijens H, Fokkens W. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol 2003; 14: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildenbeest JG, van der Schee MP, Hashimoto S, Benschop KS, Minnaar RP, Sprikkelman AB, Haarman EG, van Aalderen WM, Sterk PJ, Pajkrt D, Wolthers KC. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin Microbiol Infect 2016; 22: 736 e739–736 e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halperin SA, Eggleston PA, Beasley P, Suratt P, Hendley JO, Groschel DH, Gwaltney JM Jr. Exacerbations of asthma in adults during experimental rhinovirus infection. Am Rev Respir Dis 1985; 132: 976–980. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie AI, Farne HA, Singanayagam A, Jackson DJ, Mallia P, Johnston SL. Pathogenesis of Viral Infection in Exacerbations of Airway Disease. Ann Am Thorac Soc 2015; 12 Suppl 2: S115–132. [DOI] [PubMed] [Google Scholar]
- 12.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 2002; 359: 831–834. [DOI] [PubMed] [Google Scholar]
- 13.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, McMinn PC, Goldblatt J, Gern JE, Le Souef PN. Association between human rhinovirus C and severity of acute asthma in children. The European respiratory journal 2011; 37: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambrano JC, Carper HT, Rakes GP, Patrie J, Murphy DD, Platts-Mills TA, Hayden FG, Gwaltney JM Jr., Hatley TK, Owens AM, Heymann PW. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol 2003; 111: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 15.Adura PT, Reed E, Macintyre J, Del Rosario A, Roberts J, Pestridge R, Beegan R, Boxall CB, Xiao C, Kebadze T, Aniscenko J, Cornelius V, Gern JE, Monk PD, Johnston SL, Djukanovic R. Experimental rhinovirus 16 infection in moderate asthmatics on inhaled corticosteroids. Eur Respir J 2014; 43: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Message SD, Qiu Y, Mallia P, Kebadze T, Contoli M, Ward CK, Barnathan ES, Mascelli MA, Kon OM, Papi A, Stanciu LA, Jeffery PK, Johnston SL. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest 2014; 145: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy JL, Koziol-White CJ, Jeffus S, Rettiganti MR, Fisher P, Kurten M, Eze A, House S, Sikes JD, Askew E, Putt C, Panettieri RA, Jones SM, Kurten RC. Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J Allergy Clin Immunol 2018; 141: 1887–1890 e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loisel DA, Du G, Ahluwalia TS, Tisler CJ, Evans MD, Myers RA, Gangnon RE, Kreiner-Moller E, Bonnelykke K, Bisgaard H, Jackson DJ, Lemanske RF Jr., Nicolae DL, Gern JE, Ober C. Genetic associations with viral respiratory illnesses and asthma control in children. Clin Exp Allergy 2016; 46: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchinson K, Kerley CP, Faul J, Greally P, Coghlan D, Louw M, Elnazir B, Rochev Y. Vitamin D receptor variants and uncontrolled asthma. Eur Ann Allergy Clin Immunol 2018; 50: 108–116. [DOI] [PubMed] [Google Scholar]
- 20.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, Palmenberg AC, Gern JE. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A 2015; 112: 5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenberg Hammar K, Niespodziana K, van Hage M, Kere J, Valenta R, Hedlin G, Soderhall C. Reduced CDHR3 expression in children wheezing with rhinovirus. Pediatr Allergy Immunol 2018; 29: 200–206. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa J, Masuko H, Yatagai Y, Sakamoto T, Yamada H, Kaneko Y, Kitazawa H, Iijima H, Naito T, Saito T, Noguchi E, Konno S, Nishimura M, Hirota T, Tamari M, Hizawa N. Genetic association of the functional CDHR3 genotype with earlyonset adult asthma in Japanese populations. Allergol Int 2017; 66: 563–567. [DOI] [PubMed] [Google Scholar]
- 23.Ayres JG, Mansur AH. Vocal cord dysfunction and severe asthma: considering the total airway. Am J Respir Crit Care Med 2011; 184: 2–3. [DOI] [PubMed] [Google Scholar]
- 24.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, Murphy DD, Odio S, James HR, Patrie JT, Hunt W, O’Rourke AK, Davis MD, Steinke JW, Lu X, Kennedy J, Heymann PW. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. The Journal of allergy and clinical immunology 2012; 129: 1499–1505 e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001; 108: E36. [DOI] [PubMed] [Google Scholar]
- 26.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine 2011; 364: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, Zhu J, Ching YM, Shamji B, Edwards M, Westwick J, Cousins DJ, Hwang YY, McKenzie A, Johnston SL, Bartlett NW. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 2014; 6: 256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmutovic-Persson I, Akbarshahi H, Bartlett NW, Glanville N, Johnston SL, Brandelius A, Uller L. Inhaled dsRNA and rhinovirus evoke neutrophilic exacerbation and lung expression of thymic stromal lymphopoietin in allergic mice with established experimental asthma. Allergy 2014; 69: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato A, Favoreto S Jr., Avila PC, Schleimer RP. TLR3- and Th2 cytokinedependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez GF, Pancham K, Huseni S, Preciado D, Freishtat RJ, Colberg-Poley AM, Hoffman EP, Rose MC, Nino G. Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur Respir J 2014; 44: 1075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohno T, Morita H, Arae K, Matsumoto K, Nakae S. Interleukin-33 in allergy. Allergy 2012; 67: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 32.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson W, Consortium G. A large-scale, consortiumbased genomewide association study of asthma. N Engl J Med 2010; 363: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, Jerico D-R, Telcian AG, Nikonova A, Zhu J, Aniscenko J, Gogsadze L, Bakhsoliani E, Traub S, Dhariwal J, Porter J, Hunt D, Hunt T, Hunt T, Stanciu LA, Khaitov M, Bartlett NW, Edwards MR, Kon OM, Mallia P, Papadopoulos NG, Akdis CA, Westwick J, Edwards MJ, Cousins DJ, Walton RP, Johnston SL. IL33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014; 190: 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond C, Kurten M, Kennedy JL. Rhinovirus and asthma: a storied history of incompatibility. Curr Allergy Asthma Rep 2015; 15: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, FitzGerald JM, Boedigheimer M, Davis BE, Dias C, Gorski KS, Smith L, Bautista E, Comeau MR, Leigh R, Parnes JR. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. [DOI] [PubMed] [Google Scholar]
- 36.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, van der Merwe R. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377: 936–946. [DOI] [PubMed] [Google Scholar]