Visual Abstract
Keywords: prediction; osmotic demyelination syndrome; latent class analysis; Deamino Arginine Vasopressin; hyponatremia; Sodium; hypokalemia; Prevalence; Consciousness; Consensus; Tertiary Care Centers; Demyelinating Diseases; Water-Electrolyte Imbalance; Cohort Studies; Acid-Base Imbalance; Emergency Service, Hospital; Vomiting; Glucose; Neoplasms
Abstract
Background and objectives
Osmotic demyelination syndrome is the most concerning complication of severe hyponatremia, occurring with an overly rapid rate of serum sodium correction. There are limited clinical tools to aid in identifying individuals at high risk of overcorrection with severe hyponatremia.
Design, setting, participants, & measurements
We identified all patients who presented to a tertiary-care hospital emergency department in Ottawa, Canada (catchment area 1.2 million) between January 1, 2003 and December 31, 2015, with serum sodium (corrected for glucose levels) <116 mmol/L. Overcorrection was determined using 14 published criteria. Latent class analysis measured the independent association of baseline factors with a consensus overcorrection status on the basis of the 14 criteria, and was summarized as a risk score, which was validated in two cohorts.
Results
A total of 623 patients presented with severe hyponatremia (mean initial value 112 mmol/L; SD 3.2). The prevalence of no, unlikely, possible, and definite overcorrection was 72%, 4%, 10%, and 14%, respectively. Overcorrection was independently associated with decreased level of consciousness (2 points), vomiting (2 points), severe hypokalemia (1 point), hypotonic urine (4 points), volume overload (−5 points), chest tumor (−5 points), patient age (−1 point per decade, over 50 years), and initial sodium level (<110 mmol/L: 4 points; 110–111 mmol/L: 2 points; 112–113 mmol/L: 1 point). These points were summed to create the Severe Hyponatremic Overcorrection Risk (SHOR) score, which was significantly associated with overcorrection status (Spearman correlation 0.45; 95% confidence interval, 0.36 to 0.49) and was discriminating (average dichotomized c-statistic 0.77; 95% confidence interval, 0.73 to 0.81). The internal (n=119) and external (n=95) validation cohorts had significantly greater use of desmopressin, which was significantly associated with the SHOR score. The SHOR score was significantly associated with overcorrection status in the internal (P<0.001) but not external (P=0.39) validation cohort.
Conclusions
In patients presenting with severe hyponatremia, overcorrection was common and predictable using baseline information. Further external validation of the SHOR is required before generalized use.
Introduction
Hyponatremia is the most common electrolyte disorder in hospitalized patients with a prevalence of approximately 20% (1). Its rapid correction may result in osmotic demyelination syndrome (ODS). Unfortunately, no consensus exists regarding criteria for hyponatremic overcorrection with discordance among practice guidelines regarding the actual definition of overcorrection (2,3). We recently completed a systematic review of the published literature that identified nine methods to calculate sodium correction rates and 14 distinct criteria for overcorrection (4). Such variability in criteria for hyponatremic overcorrection likely reflects a lack of definitive studies correlating sodium correction rates with ODS risk.
However, latent variable models can be used in the absence of accepted definition for hyponatremic overcorrection (5). Such models could identify factors potentially influencing the risk of hyponatremic overcorrection. Knowing these factors could help clinicians stratify hyponatremic patients by their overcorrection risk to help determine the intensity of patient monitoring and treatment. In this study, we used latent class analysis (LCA) to create a risk score for hyponatremic overcorrection.
Materials and Methods
Study Setting
This study took place at a 1000-bed publicly funded teaching hospital. We used our hospital’s admission registry to identify all patients who presented to its emergency department between January 1, 2003 (the first date that data were complete) and December 31, 2015 (the last date for which long-term follow-up was complete). We identified the initial serum sodium measurement of each person’s emergency department encounter and used concurrent serum glucose measures to calculate the corrected serum sodium using a modification of the correction equation from Katz (6):
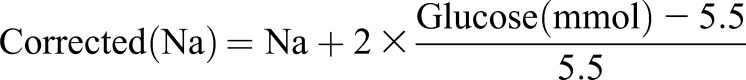 |
We increased the coefficient in the Katz (6) equation from 1.6 to 2 to ensure that patients included in our study truly had hypotonic hyponatremia. Those without a serum glucose measure (2% of patients) were imputed a normal value for this calculation.
Patients were included in our study if their initial corrected serum sodium was <116 mmol/L and their sodium was repeated at least once in the emergency department and subsequent hospitalization. We chose this threshold of 116 mmol/L because cases of ODS are exceedingly rare in patients with a higher baseline serum sodium (3), and we were primarily interested in risk stratifying patients for whom this outcome was possible. We included only the patient’s first encounter with our hospital for severe hyponatremia and excluded patients transferred to our emergency department.
Covariates
From our hospital’s data warehouse, we determined patient age and sex, ambulance status, and baseline laboratory investigations. J.D.W. used standard data abstraction methods (7) to manually abstract from each patient’s medical record all other covariates that we hypothesized might influence or indicate the cause or the severity of hyponatremia (Supplemental Table 1) as defined in the literature. Variables were defined and strict inclusion and exclusion criteria were determined a priori (Supplemental Table 1). A standardized abstraction form was developed and used for each chart. We also recorded the type and volume of intravenously administered fluids given during the first 24 hours of the encounter.
Analysis and Model Validation
We recently completed a systematic review of all peer-reviewed studies in which sodium correction rate methods were detailed and rate thresholds defining overcorrection were described (4). We identified a total of 14 distinct criteria for overcorrection (Supplemental Table 2). These criteria were applied to the cohort resulting in, for each patient, up to 14 binomial indicators for hyponatremic overcorrection status.
We used LCA to measure the prevalence of hyponatremic overcorrection and to measure the association of baseline covariates on its risk. LCA can be used when a categorical outcome cannot be measured (i.e., it is “latent”) but its status is indicated by two or more observed categorical variables. True hyponatremic overcorrection is a latent variable because it has no accepted definition. The 14 published criteria for overcorrection (Supplemental Table 2) were used in our LCA model as the observed variables. The outcome for this LCA model can be perceived as “consensus hyponatremic overcorrection status” on the basis of the 14 overcorrection criteria.
PROC LCA (SAS version 9.4) was used for all modeling (8). This procedure estimates parameters by maximum likelihood using the expectation-maximization algorithm, which assumes that absent observed variables are missing at random. LCA also assumes local independence (i.e., observed variables are independent of each other conditional upon the latent variable class) (5). This would not be the case for criteria having the same hyponatremic correction rate formula but different thresholds defining overcorrection. Of the 14 published criteria in Supplemental Table 2, these include criteria B (with two overcorrection thresholds), criteria E (two overcorrection thresholds), criteria G (three overcorrection thresholds), and criteria I (two overcorrection thresholds). To maintain local independence with these criteria, we created 1000 bootstrap datasets that varied only by a randomly selected subtype for criteria B, E, G, and I. Values for all other criteria and covariates remained the same. All analyses were conducted on these 1000 bootstrap datasets with mean results and 95% confidence intervals (95% CI; using the percentile method [9]) calculated and presented.
LCA modeling started by determining the ideal number of latent classes by fitting models (without covariates) having between one and six classes and then identifying the simplest model having the lowest mean Akaike or Bayesian information criterion values (10). We then used a variable selection strategy that was influenced by three factors: we had many potential covariates; there is a lack of consensus in the literature regarding factors influencing the likelihood of overcorrection; and we wanted to create a parsimonious and accurate method to predict overcorrection risk. Therefore, in our multinomial logistic regression model, we used forward variable selection starting with factors having the strongest univariate association with overcorrection. Variables were kept in the multivariable model if the 95% CI for its parameter estimate (determined using the mean value of 1000 bootstrap samples) excluded 0. This model was summarized as a point system using methods from Sullivan et al. (11). This point system was clustered into five similarly sized groups. The association between the point system and overcorrection status was measured with the Spearman rank correlation coefficient; discrimination was measured using the average dichotomized c-statistic (12). All percentages (except those <1%) are rounded to the nearest whole number.
We validated the final prediction model in two sites, including (1) the derivation site (“internal validation”) and (2) the University Health Network, a teaching hospital located at two sites in Toronto, Canada (“external validation”). The former included all patients presenting to the emergency room between January 1, 2016 and December 31, 2018 with a corrected serum sodium level <116 mmol/L. The latter was on the basis of a previous study (13) and included consecutive patients admitted from the emergency room with an initial uncorrected serum sodium <116 mmol/L between April 1, 2004 and March 30, 2014, excluding patients treated immediately with desmopressin to prevent hyponatremic overcorrection (n=13), with subsequent encounters after already being included in the study (n=16), with severe hyperglycemia (n=9), with non-numeric sodium values (i.e., <100 mmol/L) (n=4), transferred from another service (n=4), with diabetes insipidus (n=1), and with only one serum sodium measured (n=1). At each site, J.D.W. reviewed the hospital’s data repository (blinded to patient sodium levels or their overcorrection status) to retrieve data required to calculate the Severe Hyponatremic Overcorrection Risk (SHOR) score and record administration of desmopressin during the early hospitalization. All serum sodium measures were used to determine overcorrection status as per each criterion, which were used to classify each patient’s overcorrection status using criteria response patterns of the original LCA model. Desmopressin administration and the consensus overcorrection status was compared with the final prediction model.
Results
We identified 623 patients who were seen in the emergency department between March 1, 2003 and December 12, 2015 with an initial corrected serum sodium value <116 mmol/L and at least one repeat sodium measurement (Table 1). All patients except eight (1%) were admitted to hospital (presenting corrected sodium 110.8–115.8 mmol/L; one death).
Table 1.
Description of study cohort (n=623)
| Characteristic | n (%) |
|---|---|
| Demographics | |
| Mean age (SD) | 68±16 |
| Male | 245 (39%) |
| Arrived by ambulance | 389 (62%) |
| Past Medical History | |
| Stroke | 77 (12%) |
| Dementia | 70 (11%) |
| Parkinson disease | 4 (0.6%) |
| Alcoholism | 120 (19%) |
| Cancer | 114 (18%) |
| Cirrhosis | 52 (8%) |
| Hemodialysis | 3 (0.5%) |
| Adrenal insufficiency | 20 (3%) |
| Active disease | |
| Low serum albumin/total protein | 40 (6%) |
| Clinical diagnosis malnutrition | 131 (21%) |
| Drug exposure | |
| HCTZ | 215 (34%) |
| Furosemide | 107 (17%) |
| Anticonvulsant | 55 (9%) |
| SSRI | 85 (14%) |
| SNRI | 32 (5%) |
| MDMA | 1 (0.2%) |
| History | |
| Vomiting | 227 (36%) |
| Physical examination | |
| Mean Systolic BP (SD) | 137±66 |
| Baseline LOC: normal | 230 (37%) |
| Confused | 232 (37%) |
| Decreased | 129 (21%) |
| Comatose | 9 (1.4%) |
| Not documented | 23 (4%) |
| Baseline volume status: normal | 131 (21%) |
| Hypovolemic | 455 (73%) |
| Overloaded | 37 (6%) |
| Baseline investigations | |
| Mean (SD) corrected sodium (mmol/L) | 112±3 |
| Serum K+ <3mmol/L | 144 (23%) |
| Urine osmolality | |
| <150 mosm/kg | 62 (10%) |
| 150–300 mosm/kg | 173 (28%) |
| >300 mosm/kg | 261 (42%) |
| Not measured | 127 (20%) |
| Median eGFR (IQR) | 77 (50–114) |
| eGFR<30 ml/min per 1.73 m2 | 79 (13%) |
| CNS bleed | 3 (0.5%) |
| Significant chest tumor | 63 (10%) |
| Significant chest infection | 114 (18%) |
| Outcomes | |
| Median length of stay (IQR) | 6 (3–13) |
| Disposition: community | 454 (73%) |
| Rehabilitation | 17 (3%) |
| Assisted living | 101 (16%) |
| Dead | 51 (8%) |
HCTZ, hydrochlorothiazide; SSRI, sustained serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; MDMA, 3,4-methylenedioxy-methamphetamine; LOC, level of consciousness; K+, potassium; IQR, interquartile range; CNS, central nervous system.
The 14 hyponatremic overcorrection criteria identified in our systematic review (Supplemental Table 2) returned notably different results (Table 2). By these criteria, a median of 25% patients (interquartile range, 19%–45%) were classified as overcorrected, but this varied more than 11-fold, from a low of 8% (criterion D) to a maximum of 90% (criterion A). Agreement between the criteria (measured using the prevalence-adjusted bias-adjusted κ-statistic [14–30]) also varied extensively from 0.13 (for criterion D) to 0.56 (for criteria B5 and I5).
Table 2.
Prevalence of hyponatremic overcorrection by 14 different published criteria
| Criterion | Overcorrection Threshold | Patients without Missing Values | Patients Meeting Criteria for Overcorrection | % Patients with Hyponatremic Overcorrection (95% CI) | Mean (Range) Agreement with Other Criteria |
|---|---|---|---|---|---|
| A | 0.5 mmol/L per hour | 623 | 561 | 90 (87 to 92) | −0.25 (−0.74 to 0.22) |
| B3 | 8 mmol/L per day | 623 | 310 | 50 (46 to 54) | 0.44 (−0.04 to 0.87) |
| B5 | 12 mmol/L per day | 623 | 152 | 24 (21 to 28) | 0.56 (−0.31 to 0.92) |
| C | 18 mmol/L per 2 days | 623 | 102 | 16 (13 to 19) | 0.49 (−0.47 to 0.94) |
| D | 8 mmol/L per day | 520 | 44 | 8 (6 to 11) | 0.13 (−0.74 to 0.39) |
| E3 | 8 mmol/L per day | 622 | 292 | 47 (43 to 51) | 0.44 (−0.02 to 0.79) |
| E4 | 10 mmol/L per day | 622 | 193 | 31 (27 to 35) | 0.54 (−0.18 to 0.80) |
| E5 | 12 mmol/L per day | 622 | 125 | 20 (17 to 23) | 0.53 (−0.40 to 0.86) |
| F | 18 mmol/L per 2 days | 623 | 109 | 18 (14 to 20) | 0.50 (−0.45 to 0.86) |
| G3 | 8 mmol/L per day | 613 | 326 | 53 (49 to 57) | 0.39 (−0.12 to 0.87) |
| G4 | 10 mmol/L per day | 613 | 245 | 40 (36 to 44) | 0.51 (−0.02 to 0.75) |
| H | 18 mmol/L per 2 days | 552 | 110 | 20 (17 to 23) | 0.37 (−0.47 to 0.74) |
| I5 | 12 mmol/L per day | 623 | 159 | 26 (22 to 29) | 0.56 (−0.29 to 0.92) |
| I6 | 18 mmol/L per day | 623 | 111 | 18 (15 to 21) | 0.51 (−0.44 to 0.94) |
Details regarding the criteria and overcorrection thresholds are described in Supplemental Table 2. Agreement with other criteria was measured using prevalence-adjusted and bias-adjusted κ-statistic (14). 95% CI, 95% confidence interval.
We found that a four-class model fit the data best (Supplemental Table 3). Overall, the latent class model showed excellent homogeneity within, and separation between, the classes (Supplemental Table 4). The prevalence of each criterion increased progressively between the four classes (that we labeled “no overcorrection,” “unlikely overcorrection,” “possible overcorrection,” and “definite overcorrection”). Within the no overcorrection class, the prevalence of each criterion was <8% (except for criterion A), whereas the prevalence was ≥90% within the definite overcorrection class for all except criterion D. The no overcorrection and unlikely overcorrection classes were statistically distinct from each other (i.e., their 95% CIs did not overlap) for all except criteria A and I. The possible overcorrection and definite overcorrection classes were statistically distinct from each other for all except criteria C and I. Overlap between unlikely overcorrection and possible overcorrection classes was common. There were 2177 distinct criteria patterns in our cohort, of which 2010 (92%) were classified into a single overcorrection class more than 95% of the time (Supplemental Table 5).
The LCA model found a prevalence for no, unlikely, possible, or definite overcorrection of 72%, 4%, 10%, and 14%, respectively. Eight baseline factors were independently associated with overcorrection (Table 3). Increased patient age, volume overload, and significant chest tumor were each associated with a decreased risk of overcorrection. Vomiting, decreased level of consciousness or coma, hypokalemia, and a low urine osmolality were independently associated with an increased risk of overcorrection. Overcorrection risk was also greater as the baseline sodium level decreased. For all variables except hypokalemia, association measures were most extreme for the definite overcorrection group; considerable overlap existed for association measures between unlikely and possible overcorrection groups.
Table 3.
Baseline factors associated with hyponatremic overcorrection and the Severe Hyponatremic Overcorrection Risk score
| Factor | Parameter Estimate (SEM) | Adjusted Odds Ratio (95% CI) | Points | ||||
|---|---|---|---|---|---|---|---|
| Unlikely | Possible | Definite | Unlikely | Possible | Definite | ||
| Intercept | 5.09 (1.31) | 7.14 (0.189) | 19.37 (1.278) | N/A | N/A | N/A | N/A |
| Patient age, yr | −0.1 (0.024) | −0.1 (0.004) | −0.39 (0.028) | 0.9 (0.86 to 0.95) | 0.9 (0.89 to 0.91) | 0.68 (0.64 to 0.72) | N/A |
| 40–50 | 0 | ||||||
| 50–60 | −1 | ||||||
| 60–70 | −2 | ||||||
| 70–80 | −3 | ||||||
| 80–90 | −4 | ||||||
| Vomiting | 0.07 (0.075) | 0.37 (0.011) | 0.63 (0.091) | 1.07 (0.92 to 1.24) | 1.45 (1.41 to 1.47) | 1.88 (1.57 to 2.25) | 2 |
| Somnolent | 0.12 (0.086) | 0.13 (−0.002) | 0.68 (0.092) | 1.13 (0.96 to 1.34) | 1.14 (1.14 to 1.14) | 1.97 (1.66 to 2.37) | 2 |
| Volume overloaded | −1.05 (0.202) | −1.23 (0.022) | −1.8 (0.409) | 0.35 (0.24 to 0.52) | 0.29 (0.28 to 0.31) | 0.17 (0.07 to 0.37) | −5 |
| Corrected sodium, mmol/L | −0.05 (0.012) | −0.07 (0.002) | −0.17 (0.011) | 0.95 (0.93 to 0.98) | 0.93 (0.93 to 0.93) | 0.84 (0.83 to 0.87) | N/A |
| <110 | 4 | ||||||
| 110 to <112 | 2 | ||||||
| 112 to <114 | 1 | ||||||
| 114–116 | 0 | ||||||
| K+ <3.0 mmol | 0.72 (0.08) | 0.77 (0.009) | 0.49 (0.106) | 2.05 (1.76 to 2.41) | 2.16 (2.12 to 2.2) | 1.63 (1.32 to 2) | 1 |
| Uosm <150 mosm/kg | 0.76 (0.119) | 0.42 (0.081) | 1.39 (0.108) | 2.14 (1.7 to 2.71) | 1.52 (1.29 to 1.78) | 4.01 (3.27 to 4.98) | 4 |
| Chest tumor | −0.8 (0.131) | −0.81 (0.029) | −1.85 (0.29) | 0.45 (0.35 to 0.58) | 0.44 (0.42 to 0.47) | 0.16 (0.09 to 0.28) | −5 |
Parameter estimates and adjusted odds ratios are compared with the no overcorrection group. Presented values are the mean of 1000 bootstrap samples. 95% CI, 95% confidence interval; N/A, not applicable; K+, potassium; Uosm, urinary osmolarity.
The final model was summarized as the SHOR score (Table 3). Chest tumor was most protective (−5 points); baseline corrected sodium <110 mmol/L and urine osmolality <150 mosm/kg were associated with the greatest risk of overcorrection (+4 points). Observed SHOR scores ranged from −10 to 11 (mean −0.06, SD 3.7). There was only a slight decrease in fit from the full model containing all covariates (log-likelihood −1507.0) with that containing SHOR only (log-likelihood −1515.2) despite 21 fewer degrees of freedom (df) in the latter model.
The SHOR score significantly predicted overcorrection risk (Table 4). As the SHOR score increased, the probability of no overcorrection decreased (< −3 points: 96%; 5+ points: 27%) and the probability of definite overcorrection increased (< −3 points: 0%; 5+ points: 55%) (chi-squared value 156.8; P<0.001; Spearman correlation 0.45; 95% CI, 0.36 to 0.49). The SHOR score was significantly discriminative (average dichotomized c-statistic 0.77; 95% CI, 0.73 to 0.81).
Table 4.
Hyponatremic overcorrection status and treatment during the first 24 hours by SHOR score
| SHOR Score | No. of Patients (N=623) | Hyponatremic Overcorrectiona | IV Fluids (L) | IV Sodium (mEq) | Desmopressin Given, | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No, n=449 (72%) | Unlikely, n=25 (4%) | Possible, n=64 (10%) | Definite, n=85 (14%) | Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | n (%) | ||
| <−3 | 98 | 94 (96%) | 3 (3%) | 1 (1%) | 0 (0%) | 0.6 (0.5 to 0.8) | 0.3 (0–1.0) | 95.4 (71.3 to 119.5) | 47.3 (0–154.0) | 0 (0%) |
| −3 to −1 | 167 | 141 (84%) | 5 (3%) | 12 (7%) | 9 (5%) | 1.1 (1.0 to 1.3) | 1.0 (0.3–1.6) | 176.3 (151.8 to 200.7) | 154 (45.7–261.8) | 0 (0%) |
| 0–2 | 191 | 138 (72%) | 10 (5%) | 25 (13%) | 18 (9%) | 1.3 (1.2 to 1.5) | 1.2 (0.5–2.0) | 206.3 (180.9 to 231.6) | 188.6 (74.4–308) | 2 (1%) |
| 3–4 | 107 | 60 (56%) | 4 (4%) | 18 (17%) | 25 (29%) | 1.5 (1.2 to 1.7) | 1.2 (0.6–2.1) | 219.0 (185.2 to 252.8) | 175.2 (100.1–326) | 6 (6%) |
| 5+ | 60 | 16 (27%) | 3 (5%) | 8 (13%) | 33 (55%) | 1.8 (1.4 to 2.1) | 1.6 (0.9–2.8) | 262.7 (216.5 to 309.0) | 232.7 (128.5–398.8) | 1 (2%) |
The total volume of fluids and amount of sodium given intravenously during the first 24 hours after the baseline sodium measurement was determined. The SHOR score was strongly associated with hyponatremic overcorrection class (chi-squared value 156.8; P<0.001; Spearman correlation 0.45; 95% CI, 0.36 to 0.49). SHOR, Severe Hyponatremia Overcorrection Risk; 95% CI, IV, intravenous; 95% confidence interval; IQR, interquartile range.
These values are on the basis the hyponatremic overcorrection category having the highest probability (Supplemental Table 5) for each patient and is the average of 1000 bootstrap samples to account for correlated hyponatremic overcorrection criteria (Supplemental Table 2).
SHOR scores were also significantly associated with both the volume of intravenously administered fluid and the amount of sodium (P<0.001, Wilcoxon test, for both). Only nine patients (1.4%) were given desmopressin but the likelihood of administration increased significantly with the SHOR score (P=0.002). Two patients were diagnosed with ODS. Both were males with alcoholism with decreased level of consciousness and initial corrected sodium levels <104 mmol/L. ODS was diagnosed approximately 2 weeks after presentation with confusion, tremor, and (in one patient) bilateral spasticity. The mean sodium correction rate (calculated using the methods presented in Supplemental Table 2) was 24.1 and 16.4 mmol/d. SHOR scores were very high in both patients (6 and 8 points).
Validation.
The internal and external validation group originally included 149 and 301 patients, respectively. Eight (5%) and 100 patients (32%), respectively, were excluded because they had an overcorrection criteria pattern not seen in the derivation group (and therefore could by the LCA model into an overcorrection category; Supplemental Table 5). A total of 59 patients (20%) were excluded from the external validation because their corrected sodium exceeded 116 mmol/L. This left the internal and external validation cohorts with 141 and 142 patients, respectively (Supplemental Table 6). Overall, they were similar to the derivation cohort (Table 1) except that the external validation group was slightly older (Supplemental Table 6). Desmopressin was significantly more likely to be given in both the internal (16%) and the external validation groups (33%; chi-squared value 158; df=2, P<0.001), with the SHOR score significantly associated with desmopressin use in both the internal (Spearman correlation coefficient 0.18; P=0.03) and external groups (chi-squared value 40.3; df=4; P<0.001). In external validation patients who received no desmopressin (Table 5), hyponatremic overcorrection was significantly less likely than in the derivation group (probability of possible or definite overcorrection: derivation 24%, internal validation 25%, external validation 14%; chi-squared value 46.3; df=6; P<0.001). In the internal validation, the SHOR score (Table 3) was significantly associated with overcorrection status (Spearman correlation coefficient 0.44; P value <0.001). In the external validation, this association was smaller (Spearman correlation coefficient 0.09) and did not meet standard criteria for statistical significance (P=0.39).
Table 5.
Performance of the SHOR index in validation groups
| SHOR Points | Consensus Overcorrection Status | Total | |||
|---|---|---|---|---|---|
| No (56%) | Unlikely (18%) | Possible (19%) | Definite (9%) | ||
| Internal validation (n=119) | |||||
| < −3 | 23 | 1 | 1 | 1 | 26 |
| −3 to −1 | 17 | 6 | 3 | 2 | 28 |
| 0–2 | 17 | 6 | 6 | 2 | 31 |
| 3–4 | 6 | 5 | 6 | 2 | 19 |
| 5+ | 4 | 4 | 3 | 4 | 15 |
| No (77%) | Unlikely (8%) | Possible (5%) | Definite (9%) | ||
| External validation (n=95) | |||||
| < −3 | 20 | 3 | 1 | 1 | 25 |
| −3 to −1 | 20 | 2 | 2 | 3 | 27 |
| 0–2 | 24 | 3 | 0 | 3 | 30 |
| 3–4 | 4 | 2 | 0 | 0 | 6 |
| 5+ | 4 | 0 | 1 | 2 | 7 |
The validation patients only included patients with severe hyponatremic who did not receive desmopressin treatment. The association between the SHOR score and consensus overcorrection group was significant in the internal validation (Spearman correlation coefficient 0.41; P<0.001) but not the external validation (Spearman correlation coefficient 0.09; P=0.39). SHOR, Severe Hyponatremia Overcorrection Risk.
Discussion
Overcorrection is the most controllable risk factor for ODS but has no accepted criteria nor reliable knowledge regarding how its risk is influenced by baseline patient factors. This study used LCA to incorporate all published criteria for overcorrection and measure its association with baseline covariates. This model was summarized into the SHOR score which effectively stratified our cohort by overcorrection risk. Intravenous administration of both fluids and sodium during the first 24 hours increased significantly as SHOR score increased. The SHOR score was significantly associated overcorrection risk in an internal but not external validation cohort.
George et al. measured the association of baseline factors with hyponatremic overcorrection in a large (n=1490) multicenter study of patients with hyponatremia (31). Using criterion G3 to define overcorrection (Supplemental Table 2), they found that patient age, initial sodium, and hypokalemia were all independently associated with overcorrection risk. Results changed notably when overcorrection was defined differently (using criteria E3, G4, and H; Supplemental Table 2), highlighting that measures of association between hyponatremic overcorrection and covariates can vary with the criteria used to define this outcome. In the absence of empirical data exist to determine which overcorrection criteria best predict ODS risk, we believe that incorporating all published criteria using LCA to produce a “consensus” definition of overcorrection is the least-biased method for identifying overcorrection risk factors.
Our study identifies several important points. First, we found that the risk of hyponatremic overcorrection is heavily influenced by nonmodifiable patient factors that can be measured when severe hyponatremia is identified (Table 3). On the basis of the status of these factors, a patient’s expected risk of overcorrection could vary from as low 0% (for SHOR scores <−3) to as high as 55% (for SHOR scores of ≥5; Table 4). This information should be kept in mind when physician treatment is reviewed for cases in which overcorrection is identified. Second, knowing an individual patient’s overcorrection risk might influence the intensity of patient monitoring and their treatment. For example, desmopressin is increasingly being considered as an agent to decrease the risk of hyponatremic overcorrection. Risk stratification using the SHOR score could identify patients at a high risk of hyponatremic overcorrection and most likely to benefit from this therapy.
Several issues should be considered when reviewing our data. First, our study was tested in only two populations distinct from the derivation cohort. In the external population, our model was significantly associated with desmopressin administration but not with overcorrection status (possibly because of the former). Therefore, it is essential that our model be evaluated in other cohorts before physicians can confidently use it. Second, although physicians make great efforts to prevent hyponatremic overcorrection, the true outcome of interest is osmotic demyelination which occurs in only a small fraction of patients who are overcorrected. Models to predict ODS risk would be much more clinically relevant. Until that model, however, we believe that the SHOR score could be helpful to clinicians (for risk stratification) and researchers (for risk adjustment when hyponatremic overcorrection is used as an outcome). Third, although we identify patient factors that predict hyponatremic overcorrection, we do not provide a mechanism explaining these associations. Some model components increasing the risk of overcorrection (e.g., vomiting) might transiently stimulate antidiuretic hormone secretion, removal of which causes a free water diuresis and rapid sodium correction. Conversely, protective factors might stem from poorly suppressed antidiuretic hormone secretion (e.g., edematous states, chest tumor, advanced age [32]). Fourth, several factors potentially influencing the likelihood of overcorrection (preadmission desmopressin use, low urine sodium or chloride without edema, high urine sodium without diuretics, serum bicarbonate concentration, hyperkalemia, high BUN-to-creatinine ratio without edema, high serum uric acid without edema, low serum uric acid) are missing from our model and could be considered in future studies. Fifth, our systematic review (4) might have missed some published criteria for overcorrection, but it is unlikely that such omissions would materially change our results. Sixth, some of the model’s associations could be due to treatment. For example, associations between overcorrection risk with lower initial sodium levels or decreased level of consciousness could stem from treatment with hypertonic saline in clinically severe patients. More detailed analysis is required to determine how such treatment relates to overcorrection. Finally, although the SHOR model was significantly associated with overcorrection risk in the internal validation cohort (Spearman correlation coefficient 0.44; P<0.001), it was not in the external validation cohort (0.09; P=0.39). The latter could be owing to a small number of patients having higher SHOR scores or a large fraction of patients (37%) whose overcorrection criteria pattern was distinct from the derivation patients (Supplemental Table 5) and therefore could not be classified into an overcorrection status. It could also be owing to a significantly more extensive use of desmopressin which itself was strongly associated with the SHOR score. If clinicians were able to identify patients having a greater likelihood of overcorrection and give them desmopressin administration, this could obscure associations between SHOR scores and overcorrection risk.
Although further validation of our model is necessary, clinicians should be aware hyponatremic overcorrection risk might be predicted by baseline patient characteristics.
Disclosures
Dr. Cavalcanti, Dr. MacMillan, Dr. Sood, Dr. van Walraven, and Dr. Woodfine have nothing to disclose.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12251018/-/DCSupplemental.
Supplemental Table 1. Variables abstracted from the patient record.
Supplemental Table 2. Published formulae for sodium correction rates and criteria for hyponatremic overcorrection.
Supplemental Table 3. Summary of LCA model fit with varying number of model classes.
Supplemental Table 4. Prevalence of indicator criteria within each overcorrection group.
Supplemental Table 5. Criteria response patterns and their overcorrection classification status in bootstrap analysis.
Supplemental Table 6. Model validation populations.
References
- 1.Upadhyay A, Jaber BL, Madias NE: Incidence and prevalence of hyponatremia. Am J Med 119[Suppl 1]: S30–S35, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ: Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med 126[Suppl 1]: S1–S42, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C, Joannidis M, Soupart A, Zietse R, Haller M, van der Veer S, Van Biesen W, Nagler E; Hyponatraemia Guideline Development Group : Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 170: G1–G47, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Woodfine J, van Walraven C: A systematic review identifying methods used to define overcorrection in patients with hyponatremia and application to a large cohort of patients with severe hyponatremia. J Gen Intern Med 2018
- 5.Collins LM, Lanza ST: The latent class model. In: Latent Class and Latent Transition Analysis, Chapter 2, Hoboken, NJ, John Wiley and Sons [Google Scholar]
- 6.Katz MA: Hyperglycemia-Induced Hyponatremia—Calculation of Expected Serum Sodium Depression. N Engl J Med. 289: 843–844, 1973 [DOI] [PubMed] [Google Scholar]
- 7.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J: Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med 27: 305–308, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Lanza ST, Dziak JJ, Huang L, Wagner A, Collins LM: PROC LCA & PROC LTA users’ guide (Version 1.3.2). University Park: The Methodology Center, Penn State. 2015. Available at: https://sites.psu.edu/pbh112/files/2019/03/proc_lca_lta_1-3-2-1_users_guide-2ggq4d3.pdf. Accessed May 31, 2019
- 9.Efron B, Tibshirani RJ: Confidence intervals based on bootstrap percentiles. In: An Introduction to the Bootstrap, New York, Chapman & Hall, 1994 [Google Scholar]
- 10.Collins LM, Lanza ST: Parameter estimation and model selection. In: Latent Class and Latent Transition Analysis, Chapter 4, Hoboken, NJ, John Wiley and Sons, 2010 [Google Scholar]
- 11.Sullivan LM, Massaro JM, D’Agostino RB Sr.: Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 23: 1631–1660, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Waegeman W, De Baets B, Boullart L: ROC analysis in ordinal regression learning. Pattern Recognit Lett 29: 1–9, 2008 [Google Scholar]
- 13.MacMillan TE, Cavalcanti RB: Outcomes in severe hyponatremia treated with and without desmopressin. Am J Med 131: 317.e1–317.e10, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Byrt T, Bishop J, Carlin JB: Bias, prevalence and kappa. J Clin Epidemiol 46: 423–429, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Velez JCQ, Dopson SJ, Sanders DS, Delay TA, Arthur JM: Intravenous conivaptan for the treatment of hyponatraemia caused by the syndrome of inappropriate secretion of antidiuretic hormone in hospitalized patients: A single-centre experience. Nephrol Dial Transplant 25: 1524–1531, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Castello LM, Baldrighi M, Panizza A, Bartoli E, Avanzi GC: Efficacy and safety of two different tolvaptan doses in the treatment of hyponatremia in the Emergency Department. Intern Emerg Med 12: 993–1001, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Morris JH, Bohm NM, Nemecek BD, Crawford R, Kelley D, Bhasin B, Nietert PJ, Velez JCQ: Rapidity of correction of hyponatremia due to syndrome of inappropriate secretion of antidiuretic hormone following tolvaptan. Am J Kidney Dis 71: 772–782, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbalis JG, Greenberg A, Burst V, Haymann JP, Johannsson G, Peri A, Poch E, Chiodo JA 3rd, Dave J: Diagnosing and treating the syndrome of inappropriate antidiuretic hormone secretion. Am J Med 129: 537.e9–537.e23, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Burst V, Grundmann F, Kubacki T, Greenberg A, Rudolf D, Salahudeen A, Verbalis J, Grohé C: Euvolemic hyponatremia in cancer patients. Report of the hyponatremia registry: An observational multicenter international study. Support Care Cancer 25: 2275–2283, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiong JR, Hauptman PJ, Dunlap ME, Chiodo JA, Chase SL: Hospital management of hyponatremia in patients with heart failure: Final report from the HN registry. J Card Fail 20: S62–S63, 2014 [Google Scholar]
- 21.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH: Hypertonic saline for hyponatremia: Risk of inadvertent overcorrection. Clin J Am Soc Nephrol 2: 1110–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Umbrello M, Mantovani ES, Formenti P, Casiraghi C, Ottolina D, Taverna M, Pezzi A, Mistraletti G, Iapichino G: Tolvaptan for hyponatremia with preserved sodium pool in critically ill patients. Ann Intensive Care 6: 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salahudeen AK, Ali N, George M, Lahoti A, Palla S: Tolvaptan in hospitalized cancer patients with hyponatremia: A double-blind, randomized, placebo-controlled clinical trial on efficacy and safety. Cancer 120: 744–751, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators : Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Shoaf SE, Bricmont P, Dandurand A: Low-dose tolvaptan PK/PD: Comparison of patients with hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion to healthy adults. Eur J Clin Pharmacol 73: 1399–1408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soupart A, Gross P, Legros J-J, Alföldi S, Annane D, Heshmati HM, Decaux G: Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin J Am Soc Nephrol 1: 1154–1160, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kim G, Heo B, Oh E, Park H, Ko J, Gwak M, Youn S, Lee S: Management of recipients with hyponatremia during and after liver transplantation; ten year experience of single center. Transplantation 98: 791, 2014 [Google Scholar]
- 28.Romanovsky A, Azevedo LCP, Meeberg G, Zibdawi R, Bigam D, Bagshaw SM: Serum sodium shift in hyponatremic patients undergoing liver transplantation: A retrospective cohort study. Ren Fail 37: 37–44, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Sood L, Sterns RH, Hix JK, Silver SM, Chen L: Hypertonic saline and desmopressin: A simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis 61: 571–578, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Winzeler B, Jeanloz N, Nigro N, Suter-Widmer I, Schuetz P, Arici B, Bally M, Blum C, Bock A, Huber A, Mueller B, Christ-Crain M: Long-term outcome of profound hyponatremia: A prospective 12 months follow-up study. Eur J Endocrinol 175: 499–507, 2016 [DOI] [PubMed] [Google Scholar]
- 31.George JC, Zafar W, Bucaloiu ID, Chang AR: Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol 13: 984–992, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowen LE, Hodak SP, Verbalis JG: Age-associated abnormalities of water homeostasis. Endocrinol Metab Clin North Am 42: 349–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



