Visual Abstract
Keywords: chronic metabolic acidosis; kidney disease; sodium bicarbonate; Bicarbonates; Antihypertensive Agents; Confidence Intervals; Alkalies; Renal Insufficiency, Chronic; glomerular filtration rate; Kidney Failure, Chronic; acidosis; hypertension; Albumins; Bias; Risk Assessment
Abstract
Background and objectives
Metabolic acidosis is associated with progression of CKD and has significant adverse effects on muscle and bone. A systematic review and meta-analysis was conducted to evaluate the benefits and risks of metabolic acidosis treatment with oral alkali supplementation or a reduction of dietary acid intake in those with CKD.
Design, setting, participants, & measurements
MEDLINE, Embase, and Cochrane CENTRAL were searched for relevant trials in patients with stage 3–5 CKD and metabolic acidosis (<22 mEq/L) or low-normal serum bicarbonate (22–24 mEq/L). Data were pooled in a meta-analysis with results expressed as weighted mean difference for continuous outcomes and relative risk for categorical outcomes with 95% confidence intervals (95% CIs), using a random effects model. Study quality and strength of evidence were assessed using Cochrane risk of bias and the Grading of Recommendations Assessment, Development and Evaluation criteria.
Results
Fourteen clinical trials were included (n=1394 participants). Treatment of metabolic acidosis with oral alkali supplementation or a reduction of dietary acid intake increased serum bicarbonate levels (14 studies, 1378 patients, mean difference 3.33 mEq/L, 95% CI, 2.37 to 4.29) and resulted in a slower decline in eGFR (13 studies, 1329 patients, mean difference −3.28 ml/min per 1.73 m2, 95% CI, −4.42 to −2.14; moderate certainty) and a reduction in urinary albumin excretion (very-low certainty), along with a reduction in the risk of progression to ESKD (relative risk, 0.32; 95% CI, 0.18 to 0.56; low certainty). Oral alkali supplementation was associated with worsening hypertension or the requirement for increased antihypertensive therapy (very-low certainty).
Conclusions
Low-to-moderate certainty evidence suggest that oral alkali supplementation or a reduction in dietary acid intake may slow the rate of kidney function decline and potentially reduce the risk of ESKD in patients with CKD and metabolic acidosis.
Introduction
Patients with CKD often develop chronic metabolic acidosis due to a progressive reduction in kidney acid excretion with continued metabolic acid production, resulting in acid retention. The retained acid mobilizes buffer from muscle and bone and eventually depletes the principal extracellular buffer, bicarbonate, to a level below the normal lower limit of 22 mEq/L in blood. The National Kidney Foundation/Kidney Disease Outcomes Quality Initiative guidelines recommend administration of base when serum bicarbonate levels are <22 mEq/L, to maintain a level ≥22 mEq/L (1). The Kidney Disease: Improving Global Outcomes guidelines also recommend administering base when serum bicarbonate is <22 mEq/L, to maintain the value within the normal range, generally regarded as 22–29 mEq/L (2).
Observational data demonstrate an independent association between lower serum bicarbonate levels and kidney disease progression (3–6). Findings of these analyses have been supported by interventional studies with oral alkali supplementation (7,8). A previous systematic review of the effect of alkali therapy suggested a potential benefit of alkali therapy on preservation of GFR in patients with CKD (9). The small number of included trials (n=6) in this earlier report, along with a limited number of patients, precluded definitive conclusions regarding the risks and benefits of oral alkali supplementation. Subsequently, longer-term prospective trials have examined the effect of intervention with oral alkali supplementation (e.g., sodium bicarbonate or sodium citrate) (10–15) or dietary intervention (e.g., diets enriched with fruits and vegetables or very-low-protein diets supplemented with ketoanalogues, both designed to reduce the intake of dietary acids) (11–14,16) on kidney disease progression and other surrogate outcome measures. To summarize the current evidence on this topic, we performed a systematic review and meta-analysis examining the effect of oral alkali supplementation or dietary intervention compared with no treatment, usual care, or placebo, in patients with stage 3–5 CKD and metabolic acidosis and low-normal serum bicarbonate levels.
Materials and Methods
We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for reporting of systematic reviews of interventions and a prespecified registered protocol in PROSPERO (17).
Search Strategy
MEDLINE, Embase and Cochrane CENTRAL databases were searched for relevant studies (published up to October 2018) (Supplemental Appendix 1). We also searched Clinicaltrials.gov and abstracts presented in the American Society of Nephrology annual meetings (2014–2017). The references of relevant studies and review articles were manually searched.
Study Selection
All clinical trials of oral alkali supplementation, dietary intervention, or any combination used to treat nondialysis-dependent patients with CKD with chronic metabolic acidosis (defined as serum bicarbonate <22 mEq/L) or those with low-normal serum bicarbonate (22–24 mEq/L) and stage 3–5 CKD for at least 4 weeks were considered for inclusion. We planned to include studies that enrolled patients with stage 1–5 CKD and those with low serum bicarbonate (<22 mEq/L), but restricted the analyses to those with stage 3–5 CKD given its clinical relevance to this population. We also extended it to those with low-normal bicarbonate levels, on the basis of the mean serum bicarbonate levels reported in study tables, and so patients with normal serum bicarbonate levels might have been included in these studies. Studies enrolling patients with ESKD and assessing acute metabolic acidosis interventions with intravenous bicarbonate were excluded. All eligible studies had a comparison of oral alkali supplementation or dietary intervention with no treatment, usual care, placebo, or a defined control group.
Outcome Measures
We planned to analyze the following outcome measures:
Kidney disease progression end points: change in eGFR decline at the end of study period, eGFR decline per year (details outlined in the Supplemental Appendix 1), eGFR decline of >30%, >40%, and/or >50%; doubling of serum creatinine or serum cystatin C; change in urinary albumin-to-creatinine ratio (ACR); and progression to ESKD or initiation of kidney replacement therapy.
Patient-centered outcomes: mortality; hospitalization; occurrence of cardiovascular events; and measurements relating to nutritional status, muscle strength and coordination, bone density and bone fracture occurrence, and sleep quality.
Adverse effects and electrolyte changes: the incidence of electrolyte disturbances (e.g., hyperkalemia, hyperphosphatemia, hypercalcemia), new or worsening of edema or fluid status, increased or worsening calcification in tissues or vasculature, changes in antihypertensive therapy or diuretic dosing, and changes in hypertensive status.
Data Extraction
Data extraction was carried out by the authors using a standard data extraction sheet (18). The titles and abstracts identified in the initial search were screened by two authors independently (S.D.N. and J.S.), discarding studies that were not applicable before assembling a reconciled list of citations that included relevant data for the review. The authors retrieved the full-text articles of the initial studies to determine which studies satisfied the inclusion criteria. Authors of the studies (except the study by Williams et al. [19]) that tested dietary interventions were contacted to obtain baseline bicarbonate levels of whom four responded (14,16,20,21).
Assessment of Risk of Bias and Strength of Evidence
The risk of bias in the studies was assessed independently by two authors (S.D.N. and J.B.) without blinding to authorship or journal using the Cochrane risk of bias tool (18). Discrepancies were resolved by discussion. Two authors (S.D.N. and J.B.) assessed the strength of the overall evidence (as high, moderate, low, or very low) related to kidney outcome measures using the Grading of Recommendations Assessment, Development, and Evaluation approach (22), on the basis of the five domains: risk of bias, consistency, directness, precision, and publication bias (Supplemental Table 1).
Data Synthesis and Analyses
Data were pooled using the random effects model, but the fixed effects model was also used in the analysis to ensure robustness of the model chosen and susceptibility to outliers. For dichotomous outcomes (e.g., progression to ESKD, changes in antihypertensive therapy, etc.), results were expressed as relative risks (RRs) with 95% confidence intervals (95% CIs). Mean differences with 95% CIs were used where continuous scales of measurement were used to assess the effects of treatment (e.g., eGFR decline, serum electrolytes, etc.). Both separate and combined analyses were conducted for oral alkali supplementation and dietary intervention. We planned to conduct subgroup analyses according to age, stage of kidney disease, cause of kidney disease, amount of proteinuria, and severity of metabolic acidosis; however, these subgroup analyses were not conducted because of the limited number of studies. We conducted sensitivity analysis by (1) excluding studies that enrolled patients with low-normal serum bicarbonate levels, and (2) excluding studies that compared low-protein diet with usual diet group (data presented in the Supplemental Appendix 1). Publication bias was assessed by examining the funnel plot for outcomes for which more than ten studies provided data. Heterogeneity was analyzed using a chi-squared test on N-1 degrees of freedom, with an α of 0.05 used for statistical significance, to assess whether observed differences in results were from chance alone. A low P value provides evidence of heterogeneity of intervention effects. An I2 test (23) along with 95% CIs was also used to assess levels of heterogeneity in the data with 0%–30% indicating mild, 30%–60% indicating moderate, and >60% suggesting substantial heterogeneity between the included studies. We also explored the reasons for heterogeneity for eGFR decline by (1) excluding studies of low quality and (2) restricting the analysis only to those with 1-year follow-up. Meta-analyses were performed using RevMan version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and 95% CIs for the I2 values were obtained from StatsDirect software (StatsDirect Ltd., Cambridge, UK).
Results
Search Results
The combined search of MEDLINE, Embase, Cochrane CENTRAL. and other databases identified a total of 5330 citations, of which 5277 were excluded because they were review articles, duplicate publications, or studies irrelevant to this review. Full-text assessment of 53 potentially relevant articles identified 14 eligible studies involving 1394 participants (Figure 1).
Figure 1.
Flow chart showing number of citations retrieved by individual searches and number of trials included in the systematic review.
Study Characteristics
Eight of the 14 studies compared oral alkali supplementation (seven studies, sodium bicarbonate; one study, sodium citrate) with a control (no treatment, usual care, or placebo) (7,10,15,24–28); five studies compared dietary intervention (ketoanalogue-supplemented very-low-protein diet, very-low-protein diets, low-protein diet, or six-point diet) with control diets (usual diet or low-protein diets) (16,19–21,29), whereas one study compared both sodium bicarbonate and dietary intervention (fruits and vegetables) with usual care (Table 1) (14). All trials used a parallel-group design except for the study by Kendrick et al. (28), which used a crossover design. Study duration varied from 8 weeks to 5 years. Most studies included patients with metabolic acidosis, except for those in the studies by Goraya et al. (14), Gennari et al. (20), Pisani et al. (29), and Williams et al. (19), which included patients with low-normal serum bicarbonate levels (22–24 mEq/L). Inclusion and exclusion criteria of these trials are outlined in Supplemental Table 2.
Table 1.
Characteristics of the population, interventions, and outcomes of included trials
| Reference | Study Design | Study Duration | Baseline eGFR or CrCl, ml/min or ml/min per 1.73 m2; Mean±SD | Baseline Serum Bicarbonate, mEq/L; Mean±SD | Intervention(s) (N) | Outcomes |
|---|---|---|---|---|---|---|
| Oral alkali supplementation | ||||||
| Bellasi et al., 2016 (24) | Randomized, open-label trial | 1 yr | I: 32±14 | I: 21.2±1.9 | I: Sodium bicarbonate (n=71) | HOMA−IR, HOMA %B |
| C: 35±15 | C: 21.6±2.0 | C: No treatment (n=74) | ||||
| de Brito-Ashurst et al., 2009 (7) | Randomized, open-label trial | 2 yr | I: 20.12±6.47 | I: 19.8±2.2 | I: Sodium bicarbonate (n=67) | Change in CrCl, ESKD, nutritional status |
| C: 20.70±5.55 | C: 19.9±1.5 | C: Standard care (n=67) | ||||
| Disthabanchong and Treeruttanawanich, 2010 (25) | Randomized, paralleled trial | 8–12 wk | I: 18.9±7.8 | I: 20.5±1.6 | I: High-dose sodium bicarbonate (n=21) | Thyroid function |
| C: 18.7±7.6 | C: 21.4±1.7 | C: No treatment or low-dose sodium bicarbonate (n=20) | ||||
| Dubey et al., 2018 (10) | Randomized, open-label trial | 6 mo | I: 29.2 (27.0 to 31.3)a | I: 18.1 (17.7 to 18.6)a | I: Sodium bicarbonate (n=94) | Change in MAMC, LBM, eGFR |
| C: 31.5 (29.3 to 33.8)a | C: 18.1 (17.6 to 18.6)a | C: Standard care (n=94) | ||||
| Jeong et al., 2014 (26) | Randomized, paralleled trial | 1 yr | I: 16.7±6.1 | I: 18.5±3.9 | I: Sodium bicarbonate (n=40) | Change in eGFR, KRT, nutritional status |
| C: 17.7±6.4 | C: 18.9±4.1 | C: Standard care (n=40) | ||||
| Kendrick et al., 2018 (28) | Open-label, crossover trial | 14 wk | I: 25±8 | I: 19.3±2.9 | n=19 | Change in GFR, PTH, FGF23 |
| C: 24±8 | C: 19.7±2.3 | I: Sodium bicarbonate | ||||
| C: No treatment | ||||||
| Mathur et al., 2006 (27) | Single-blind, paralleled trial | 3 mo | N/A | I: 19.49±5.51 | I: Sodium bicarbonate (n=20) | Serum creatinine, blood urea, PTH |
| C: 19.35±3.74 | C: Placebo (n=20) | |||||
| Phisitkul et al., 2010 (15) | Open-label, paralleled trial | 2 yr | I: 31.4±8.2 | I: 20.5±1.1 | I: Sodium citrate (n=30) | Change in eGFR, urine biomarkers of kidney injury |
| C: 31.7±7.9 | C: 20.5±0.8 | C: No treatment (n=29) | ||||
| Dietary intervention | ||||||
| Garneata et al., 2016 (16) | Randomized, open-label trial | 15 mo | I: 18.0 (15.5 to 20.1)a | I: 16.7 (15.8 to 17.6)a | I: Keto-VLPD (n=104) | Change in eGFR, KRT, nutritional status |
| C: 17.9 (14.3 to 19.3)a | C: 16.8 (15.9 to 17.8)a | C: LPD (n=103) | ||||
| Gennari et al., 2006 (20) | Randomized, open-label trial | 1 yr | Study B | Study B | Study B | Change in eGFR |
| I: 20.4±4.8 | I: 21.6±3.6 | I: VLPD (n=99) | ||||
| C: 20.2±3.9 | C: 22.3±3.7 | C: LPD (n=107) | ||||
| Mircescu et al., 2007 (21) | Randomized, open-label trial | 48 wk | I: 17.9±4.8 | I: 18.1±1.5 | I: Keto-VLPD (n=27) | Blood urea, eGFR, nutritional status |
| C: 16.1±4.8 | C: 18.3±1.3 | C: LPD (n=26) | ||||
| Pisani et al., 2016 (29) | Randomized, open-label trial | 6 mo | I: 21.2±7.4 | I: 23.4±2.4 | I: Six-point diet (n=27) | Serum urea nitrogen, eGFR, nutritional status |
| C: 21.0±8.3 | C: 24.1±3.5 | C: LPD (n=27) | ||||
| Williams et al., 1991 (19) | Randomized, paralleled trial | 1 yr | I: 23.4±15.6 | I: 23.1±4.5 | I: LPD (n=31) | Change in CrCl, KRT |
| C: 28.3±16.7 | C: 22.0±3.8 | C: Usual diet (n=29) | ||||
| Oral alkali supplementation and dietary intervention | ||||||
| Goraya et al., 2014 (14) | Randomized, open-label trial | 3 yr | I-1: 39.6±6.6 | I-1: 23.1±0.6 | I-1: Sodium bicarbonate (n=36) | Change in eGFR, urine biomarkers of kidney injury |
| I-2: 39.4±6.4 | I-2: 23.0±0.6 | I-2: Fruits/vegetables (n=36) | ||||
| C: 39.5±6.8 | C: 23.0±0.5 | C: Usual care (n=36) | ||||
CrCl, creatinine clearance; I, intervention; C, control; HOMA-IR, homeostatic model assessment– insulin resistance; HOMA %B, homeostatic model assessment–β pancreatic cell function; MAMC, midarm muscle circumference; LBM, lean body mass; KRT, kidney replacement therapy; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23; N/A, not available; Keto-VLPD, ketoanalogue-supplemented very-low-protein diet; LPD, low-protein diet; VLPD, very-low-protein diet.
Median (95% confidence interval).
Study Outcomes
The combined effects of oral alkali supplementation and dietary intervention on kidney disease progression end points and biochemical measurements are summarized in Table 2, Figures 2 and 3, and Table 3, respectively. The effects of individual treatments are summarized in Supplemental Tables 3–6. Results using the fixed-effects model are outlined in the Supplemental Tables 7 and 8. Included studies did not report all-cause mortality and hospitalization data consistently to be pooled in a meta-analysis.
Table 2.
Effects of oral alkali supplementation or reduction of dietary acid intake on kidney disease progression end points in patients with CKD with metabolic acidosis
| Outcomes | No. of Studies/Comparisons | No. of Patients | Effect Estimate [95% CI] | P Value | I2 (95% CI), % | Certainty of Evidence, GRADE | Comments Regarding the Quality of Evidence |
|---|---|---|---|---|---|---|---|
| eGFR decline, ml/min per 1.73 m2 | 13/14 | 1329 | MD −3.3 [−4.4 to −2.1] | <0.001 | 39 (0 to 66) | 1 Moderate | 1 Lack of blinding |
| eGFR decline per year, ml/min per 1.73 m2/yr | 9/10 | 1284 | MD −2.1 [−2.8 to −1.4] | <0.001 | 0 (0 to 53) | 1 Moderate | 1 Lack of blinding |
| Progression to ESKD | 4 | 434 | RR 0.3 [0.2 to 0.6] | <0.001 | 17 (0 to 73) | 1,2 Low | 1 Lack of blinding |
| 2 Imprecision: risk estimate includes null effect | |||||||
| Urinary ACR, mg/g | 2/3 | 167 | MD −52 [−76 to −27] | <0.001 | 0 (0 to 73) | 1,2,3 Very low | 1 Lack of blinding |
| 2 Indirectness: data derived from small no. of studies | |||||||
| 3 Imprecision: data derived from small sample size |
95% CI, 95% confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; RR, risk ratio; ACR, albumin-to-creatinine ratio. For the effect estimates, “−” indicates reduction in decline in kidney function measures.
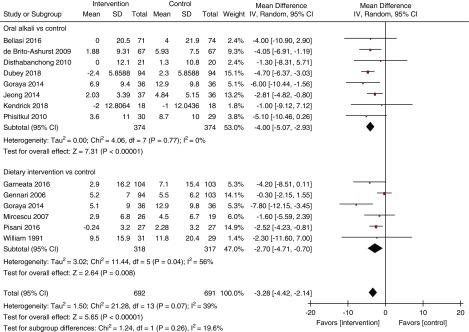
Figure 2.
Forest plot shows slower decline in eGFR at the end of study period with oral akali supplementation or reduction of dietary acid intake. I2 for the combined effect estimate: 39% (95% CI, 0% to 66%). df, degrees of freedom; IV, inverse variance.
Figure 3.
Forest plot shows slower decline in eGFR per year with oral akali supplementation or reduction of dietary acid intake. I2 for the combined effect estimate: (95% CI, 0% to 53%). df, degrees of freedom; IV, inverse variance.
Table 3.
Effects of oral alkali supplementation or reduction of dietary acid intake on change in biochemical measurements
| Outcomes | No. of Studies/Comparisons | No. of Patients | Effect Estimate MD [95% CI] | P Value | I2 (95% CI), % |
|---|---|---|---|---|---|
| Serum bicarbonate, mEq/L | 14/15 | 1378 | 3.3 [2.4 to 4.3] | <0.001 | 93 (91 to 95) |
| Serum potassium, mEq/L | 4/5 | 522 | −0.15 [−0.38 to 0.07] | 0.17 | 88 (73 to 93) |
| Serum calcium, mg/dl | 8 | 764 | 0.04 [−0.26 to 0.35] | 0.79 | 82 (64 to 89) |
| Serum phosphate, mg/dl | 9 | 818 | −0.30 [−0.62 to 0.02] | 0.06 | 59 (0 to 78) |
| Serum albumin, g/L | 7 | 741 | 0.43 [−0.54 to 1.41] | 0.39 | 68 (0 to 84) |
| Serum PTH, pg/ml | 2 | 58 | −22 [−126 to 81] | 0.67 | 42 (N/A) |
| Midarm muscle circumference, cm | 5 | 647 | 0.2 [−0.2 to 0.6] | 0.29 | 0 (0 to 64) |
MD, mean difference; 95% CI, 95% confidence interval; PTH, parathyroid hormone; N/A, not available or not applicable. For the effect estimates, “−” indicates reduction in reduction in the reported outcome measures.
Kidney Outcomes
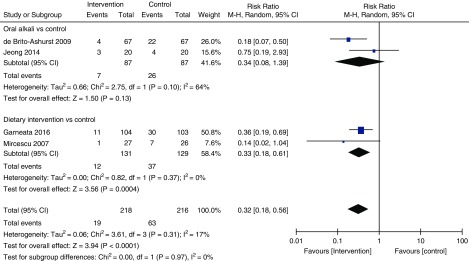
Moderate quality evidence indicated that oral alkali supplementation and dietary intervention (both individually and when pooled together) significantly slowed the decline in eGFR (Figure 2, Table 2) and eGFR decline per year (Figure 3, Table 2, moderate certainty) compared with the control groups. There was moderate heterogeneity noted (Figure 2) that attenuated substantially when the analysis was restricted to only studies with >1 year follow-up (Figure 3). Sensitivity analysis conducted by excluding studies with low-normal serum bicarbonate levels are presented in Supplemental Figures 1 and 2. There was a significant reduction in urinary ACR (two trials, 167 patients; mean difference −51.55 mg/g; 95% CI, −75.73 to −27.38; I2=0%; 95% CI, 0% to 73%; Table 2) with treatment (very low certainty). In addition, on the basis of low certainty, oral alkali supplementation or dietary intervention significantly reduced the risk of progression to ESKD (four trials, 434 patients; RR, 0.32; 95% CI, 0.18 to 0.56; I2=17%; 95% CI, 0% to 73%; Figure 4) with no heterogeneity between these two types of interventions.
Figure 4.
Forest plot shows potentially reduced risk of end stage kidney disease with oral alkali supplementation or reduction of dietary acid intake. I2 for the combined effect estimate: (95% CI, 0% to 73%). df, degrees of freedom; M-H, Mantel-Haenszel.
Biochemical Measurements
Oral alkali supplementation or dietary intervention significantly increased the serum bicarbonate level compared with the control groups (Table 3). There were no significant differences in serum potassium, calcium, phosphate, albumin, and parathyroid hormone levels, and midarm muscle circumference between the groups (Table 3).
Adverse Effects
Oral Alkali Supplementation.
One trial reported that oral alkali supplementation significantly increased 24-hour urinary sodium excretion (n=134) (7), and urinary sodium-to-creatinine ratio (n=59) (15). Pooled analyses also showed worsening edema requiring increased diuretic therapy and worsening hypertension or the requirement for increased antihypertensive therapy in the treatment (sodium bicarbonate or sodium citrate) group (Table 4, very-low certainty). Table 4 outlines other adverse events.
Table 4.
Adverse effects of oral alkali supplementation or reduction of dietary acid intake in patients with CKD with metabolic acidosis
| Outcomes | No. of Comparisons | No. of Participants | Effect Estimate [95% CI] | P Value | I2 (95% CI) |
|---|---|---|---|---|---|
| Oral alkali supplementation | |||||
| Body weight, kg | 5 | 518 | MD 0.2 [−0.6 to 0.9] | 0.67 | 0 (0 to 64) |
| Systolic BP, mm Hg | 7 | 711 | MD −0.1 [−1.9 to 1.7] | 0.93 | 23 (0 to 67) |
| Diastolic BP, mm Hg | 5 | 580 | MD 1.6 [−0.4 to 3.5] | 0.12 | 49 (0 to 79) |
| Worsening hypertension or requiring increase in antihypertensive therapy | 3 | 362 | RR 1.38 [1.07 to 1.79] | 0.01 | 0 (0 to 73) |
| Worsening edema or requiring increase in loop diuretics | 5 | 420 | RR 1.39 [1.02 to 1.89] | 0.04 | 25 (0 to 73) |
| Achieving goal of systolic BP <130 mm Hg | 1 | 72 | RR 0.60 [0.24 to 1.48] | 0.27 | N/A |
| 24 h urinary sodium excretion, mEq/24 h | 1 | 134 | MD 24.6 [19.8 to 29.4] | <0.001 | N/A |
| Urinary sodium-to-creatinine ratio, mEq/g | 1 | 59 | MD 13 [7.3 to 18.7] | <0.001 | N/A |
| Dietary intervention | |||||
| Body weight, kg | 2 | 126 | MD −1.9 [−5.5 to 1.8] | 0.31 | 0 (N/A) |
| Systolic BP, mm Hg | 2 | 117 | MD −11.3 [−16.8 to −5.9] | <0.001 | 0 (N/A) |
| Diastolic BP, mm Hg | 1 | 45 | MD −3.4 [−14.3 to 7.5] | 0.54 | N/A |
95% CI, 95% confidence interval; MD, mean difference; RR, risk ratio; N/A, not applicable or available. For the effect estimates,“−” indicates reduction in outcome measures.
Dietary Intervention.
In contrast to oral alkali supplementation, treatment of metabolic acidosis with dietary intervention significantly reduced systolic BP. There was also a trend toward decreased body weight and diastolic BP, although these were not statistically significant (Table 4).
Study Quality and Publication Bias.
Supplemental Figure 3 outlines the risk of bias of the included studies. Publication bias was not tested for outcomes other than eGFR decline and serum bicarbonate because of the smaller number of studies (fewer than ten studies) (Supplemental Figures 4 and 5). There was no evidence of publication bias for these outcome measures.
Discussion
This meta-analysis of clinical trials using oral alkali supplementation or reduction in dietary acid intake, compared with no treatment, usual care, or placebo, for the treatment of metabolic acidosis in patients with stage 3–5 CKD found that these treatments significantly increased serum bicarbonate and resulted in a slower decline in eGFR and a reduction in ACR, along with a reduction in the risk of progression to ESKD. Oral alkali supplementation, however, was associated with worsening edema requiring increased diuretic therapy and worsening hypertension or the requirement for increased antihypertensive therapy. Dietary intervention was associated with a significant reduction in systolic BP. In general, the strength of evidence for reported outcomes varied from very low to moderate certainty evidence using the Grading of Recommendations, Assessment, Development and Evaluations criteria (22).
Retrospective studies in humans have found that serum bicarbonate levels below the normal range were associated with more rapid decline in kidney function (3–6). Experimental studies in animals with reduced nephron mass also have shown that acid-inducing diets cause a progressive decline in GFR that is mediated by metabolic acidosis (30,31). Proposed mechanisms through which acidosis accelerates the progression of CKD include increased production of hormones (e.g., endothelin, angiotensin II, and aldosterone) and proinflammatory cytokines, and activation of complement induced by increased ammonia production per nephron, each of which promotes acute acid excretion but chronically results in kidney inflammation and fibrosis through enhanced complement and renin-angiotensin system activation (32). Studies using oral alkali therapy in animals and humans with reduced kidney function have demonstrated a slower decline in eGFR (7,33,34). A previous meta-analysis included six studies (two short-term, <7 days; four long-term, >2 months) on the effects of alkali therapy. In aggregate, they demonstrated improvement in kidney function but differences in study protocol and small sample size precluded the authors from reaching definitive conclusions (9). Dietary interventions were not included in this earlier review.
Our analyses of pooled data from the existing clinical trials note that treatment of metabolic acidosis, using either oral alkali or dietary interventions, significantly increased serum bicarbonate levels, and offered kidney benefits in patients with stage 3–5 CKD and metabolic acidosis. These data also indicate that there may be a potentially higher effect eGFR decline at the end of study period with oral alkali (mean difference −4.00 [95% CI, −5.07 to −2.93] ml/min slower decline) than dietary interventions (mean difference −2.70 [95% CI, −4.71 to −0.70] ml/min slower decline), but the test of subgroup difference was not significant (P=0.26) (Figure 2). As expected, interventions testing oral alkali or dietary interventions had several differences in their characteristics (study population, interventions, follow-up, etc.). Effects of oral alkali on albuminuria were assessed only in two trials, limiting the reliability of ascertaining the benefits of treatment of metabolic acidosis on this parameter. Although it is encouraging to see potential benefits, it is important to note that data for kidney disease progression to ESKD were derived from only four trials, two each for oral alkali supplementation and dietary intervention, with significant reduction for ESKD using dietary intervention but not with oral alkali supplementation (Figure 4). Given the lack of trials comparing oral alkali and dietary interventions head to head, superiority of one over the other is unclear. Additional adequately powered trials are needed to derive definitive conclusions regarding the effect of treatment of metabolic acidosis on the risk of kidney disease progression to ESKD with these different types of interventions.
Higher consumption of acid-producing animal protein contributes to metabolic acidosis in patients with CKD. Dietary interventions have been shown to provide an effective means of raising serum bicarbonate in patients with CKD who have metabolic acidosis or low-normal bicarbonate levels. Our summary data from the dietary intervention studies showed smaller but significantly slower reduction in kidney function, although with significant heterogeneity between the included studies that could be attributed to the differences among the dietary intervention protocols. These data should be interpreted with caution since these studies were not primarily designed to address the metabolic effect of these diets and included different levels of protein restriction. Base-providing diets tend to have a high potassium content and hence participants in these studies were carefully selected to be at very low risk to develop hyperkalemia (11,13,14). Further, willingness to adhere to a restrictive diet is a challenging factor in dietary intervention studies in patients with CKD. In one study, only 14% of screened patients who met all eligibility criteria agreed to adhere to the dietary requirements and were randomized (16). Other dietary interventions that could alter acid-base status in patients with CKD were not included in this analysis because of a lack of bicarbonate data. Further, some patients with normal serum bicarbonate levels could have been included these studies; future individual patient-level (rather than study-level) meta-analysis could provide additional insights. Further, it is possible that the observed benefits with dietary interventions could be ascribed to effects apart from the noted improvement in metabolic acidosis. Despite these limitations, the potential benefits of dietary intervention merit carefully designed larger studies.
Potential benefits of oral alkali supplementation on nutritional assessments such as serum albumin and potassium, midarm muscle circumference, and handgrip strength were suggested in a few single-center studies with a limited number of patients (7,35), but no significant differences were noted in our analysis. Ongoing trials could provide additional details about the effects of bicarbonate supplementation on physical function and quality of life (36). We also did not find outcome data for several prespecified outcomes (outlined in the Materials and Methods), and thus, a meta-analysis could not be conducted for these outcomes. In this analysis, there was a significant increase in worsening edema requiring increased diuretic therapy and worsening hypertension or the requirement for increased antihypertensive therapy associated with oral alkali supplementation in patients with CKD. Further, two trials reported a significantly increased urinary sodium excretion associated with oral alkali supplementation in those with CKD. Observational data associate higher urinary sodium excretion with kidney disease progression and cardiovascular events (37,38). It is important to note that the prospective studies included in this analysis were designed to exclude patients with CKD and comorbidities including uncontrolled hypertension, decompensated congestive heart failure, morbid obesity, volume overload, or hyperkalemia (39). Hence, the generalizability of these data to patients with CKD and multiple comorbidities is unclear.
This review has several strengths and limitations. Strengths include a systematic search of all major medical databases, data extraction and analysis, and trial quality assessment according to a prespecified protocol (17). Most included studies enrolled patients with stage 3–5 CKD and metabolic acidosis (serum bicarbonate <22 mEq/L) or low-normal serum bicarbonate (22–24 mEq/L), but few likely included patients with normal serum bicarbonate levels. A sensitivity analysis that excluded studies that enrolled patients with low-normal serum bicarbonate showed findings similar to those of the main analysis. The major limitation of our analysis is the lack of long-term outcome studies analyzing the effect of oral alkali supplementation or dietary intervention on patient-centered end points, including mortality. Most of the included studies were single-center, open-label trials that enrolled a small population of patients, excluded patients with multiple comorbidities, and were not powered to analyze patient-centered end points. In addition, for some outcomes, significant clinical heterogeneity existed among included trials, including differences in the types and doses of intervention, strategies of the control group, baseline eGFR and serum bicarbonate levels, and treatment duration. We were not able to explore the acute versus long-term effect of bicarbonate supplementation and the potential dose response on the basis of available trial evidence. Finally, most included studies with oral alkali supplementation did not report data on changes in BP status or antihypertensive and loop diuretic therapy, precluding definitive conclusions on the potential adverse effects associated with treatment with oral alkali therapy. We were not able to conduct several analyses planned a priori (such as 30% or 40% decline in eGFR, doubling of serum creatinine, hospitalization, and mortality) as these data were not reported in the included studies. Ongoing trials (Clincialtrials.gov identifiers: NCT01452412, NCT02915601) (40,41) could provide additional evidence on the effects of sodium bicarbonate supplementation in CKD populations.
In summary, current clinical trial evidence suggests that oral alkali supplementation or a reduction of dietary acid load improved serum bicarbonate levels and may slow the progression of kidney disease, on the basis of very-low- to moderate-certainty clinical evidence. Further larger, long-term studies of better quality are warranted to establish the benefits (such as delaying initiation of kidney replacement therapy or slowing progression to ESKD) and risks of treatment with oral alkali and/or a reduction in dietary acid load in patients with CKD.
Disclosures
Dr. Navaneethan served as a consultant for Tricida for the work under consideration. Outside the submitted work, Dr. Navaneethan has served on an independent event adjudication committee for clinical trials sponsored by Bayer and Boehringer Ingelheim and has received investigator-initiated research support from Keryx Pharmaceuticals. Dr. Bushinsky is a consultant and member of the Scientific Advisory Board for Tricida and reports consulting fees and owns stock and stock options in Tricida. Dr. Bushinsky reports additional consulting fees from Amgen, Sanofi/Genzyme, Fresenius/Relypsa/Vifor, Novo Nordisk/Covance/Quintiles, speaker fees from Sanofi/Genzyme and stock ownership in Amgen and past stock ownership in Relypsa, all outside of the submitted work. Dr. Bushinsky also receives grant support from the National Institutes of Health and Renal Research Institute, both outside of the submitted work. Dr. Buysse and Dr. Shao are employees of Tricida and have a stock ownership interest in Tricida. In addition, Dr. Buysse reports personal fees and other from Tricida, Inc., during the conduct of the study and personal fees and other from Tricida,Inc., outside the submitted work. Dr. Buysse has a patent US9,205,107 issued, a patent US9,925,214 issued, a patent US9,993,500 issued, a patent EP3003327 issued, a patent WO2014/197725 pending, a patent WO2017193050 pending, a patent WO2017193064 pending, a patent WO2017193024 pending, a patent (Treatment of Eubicarbonatemic Metabolic Acidosis) pending, and a patent (Treatment of Metabolic Acidosis) pending. Dr. Shao reports personal fees and other from Tricida, Inc., during the conduct of the study and personal fees and other from Tricida, Inc., outside the submitted work.
Supplementary Material
Acknowledgments
The authors would like to thank Dawn Parsell for editorial comments and review of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13091118/-/DCSupplemental.
Supplemental Appendix 1. Search strategy.
Supplemental Table 1. GRADE quality of evidence.
Supplemental Table 2. Key inclusion and exclusion criteria of the included studies.
Supplemental Table 3. Effects of oral alkali supplementation on kidney disease progression end points in patients with CKD.
Supplemental Table 4. Effects of dietary intervention on kidney disease progression end points in patients with CKD.
Supplemental Table 5. Effects of oral alkali supplementation on change in biochemical measurements.
Supplemental Table 6. Effects of dietary intervention on change in biochemical measurements and midarm muscle circumference.
Supplemental Table 7. Effects of oral alkali supplementation/dietary intervention on kidney disease progression end points in patients with CKD using fixed effects method.
Supplemental Table 8. Effects of oral alkali supplementation/dietary intervention on change in biochemical measurements using fixed effects method.
Supplemental Figure 1. Effects of oral alkali supplementation or dietary intervention on kidney disease progression at the end of study period in patients with CKD.
Supplemental Figure 2. Effects of oral alkali supplementation or dietary intervention on eGFR slope (eGFR decline per year).
Supplemental Figure 3. Assessment of risk of bias using Cochrane Collaboration tool.
Supplemental Figure 4. Funnel plot for publication bias on end of-study eGFR decline.
Supplemental Figure 5. Funnel plot for publication bias on change in serum bicarbonate.
References
- 1.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 2.Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 3.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M; CRIC Investigators : Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 62: 670–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ: Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56: 907–914, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE: Short- and long-term effects of alkali therapy in chronic kidney disease: A systematic review. Am J Nephrol 35: 540–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubey AK, Sahoo J, Vairappan B, Haridasan S, Parameswaran S, Priyamvada PS: Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial [published online ahead of print July 24, 2018]. Nephrol Dial Transplant 10.1093/ndt/gfy214 [DOI] [PubMed] [Google Scholar]
- 11.Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE: Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol 49: 438–448, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Goraya N, Maldonado YM, Simoni J, Wesson DE: Treatment of metabolic acidosis in chronic kidney disease with fruits and vegetables but not sodium bicarbonate yields fewer adverse cardiovascular events after five-year follow up. Circulation 136: A16628, 2017 [Google Scholar]
- 13.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G: Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol 27: 2164–2176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan S, Buysse J, Shao J, Parsell D, Li E, Bushinsky D: Treatment of chronic metabolic acidosis in CKD: A systematic review and meta-analysis. PROSPERO: CRD42017079464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S (editors): Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: https://urldefense.proofpoint.com/v2/url?u=http-3A__www.handbook.cochrane.org&d=DwMF-g&c=ZQs-KZ8oxEw0p81sqgiaRA&r=IArnAw3VCzk5UPvCZuFXCfOUlGoUVMNFkhzyx2dyGjo&m=o4jeLazyGg5D8QaW3_iByeQlluW9Y2344XA9votrcQ8&s=qrDG549VOo78JiTLUAuJHy67_wsiT8Y2Mm4LPIEp40w&e=. Accessed May 27, 2019
- 19.Williams PS, Stevens ME, Fass G, Irons L, Bone JM: Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: A prospective, randomized, controlled trial. Q J Med 81: 837–855, 1991 [PubMed] [Google Scholar]
- 20.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS: Effect of dietary protein intake on serum total CO2 concentration in chronic kidney disease: Modification of diet in renal disease study findings. Clin J Am Soc Nephrol 1: 52–57, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Mircescu G, Gârneaţă L, Stancu SH, Căpuşă C: Effects of a supplemented hypoproteic diet in chronic kidney disease. J Ren Nutr 17: 179–188, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group : GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellasi A, Di Micco L, Santoro D, Marzocco S, De Simone E, Cozzolino M, Di Lullo L, Guastaferro P, Di Iorio B; UBI Study Investigators : Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol 17: 158, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Disthabanchong S, Treeruttanawanich A: Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol 32: 549–556, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Jeong J, Kwon SK, Kim HY: Effect of bicarbonate supplementation on renal function and nutritional indices in predialysis advanced chronic kidney disease. Electrolyte Blood Press 12: 80–87, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D: Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: A prospective randomized single blind controlled trial. Ren Fail 28: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kendrick J, Shah P, Andrews E, You Z, Nowak K, Pasch A, Chonchol M: Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: A pilot randomized cross-over study. Clin J Am Soc Nephrol 13: 1463–1470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisani A, Riccio E, Bellizzi V, Caputo DL, Mozzillo G, Amato M, Andreucci M, Cianciaruso B, Sabbatini M: 6-tips diet: A simplified dietary approach in patients with chronic renal disease. A clinical randomized trial. Clin Exp Nephrol 20: 433–442, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE: Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int 73: 192–199, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Wesson DE, Simoni J: Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Kraut JA, Madias NE: Adverse effects of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24: 289–297, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Wesson DE, Jo CH, Simoni J: Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30: 762–770, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Wesson DE, Simoni J: Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH: Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8: 714–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witham MD, Band MM, Littleford RC, Avenell A, Soiza RL, McMurdo ME, Sumukadas D, Ogston SA, Lamb EJ, Hampson G, McNamee P; BiCARB Study Group : Does oral sodium bicarbonate therapy improve function and quality of life in older patients with chronic kidney disease and low-grade acidosis (the BiCARB trial)? Study protocol for a randomized controlled trial. Trials 16: 326, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators : Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, Delafontaine P, Keane MG, Mohler E, Ojo A, Rahman M, Ricardo AC, Soliman EZ, Steigerwalt S, Townsend R, He J; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 315: 2200–2210, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bushinsky DA: Tolerance to sodium in patients with CKD-induced metabolic acidosis: Does the accompanying anion matter? [published online ahead of print December 3, 2018]. Am J Kidney Dis 10.1053/j.ajkd.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 40.Albert Einstein College of Medicine: Alkali therapy in Chronic Kidney Disease. Available at: https://urldefense.proofpoint.com/v2/url?u=https-3A__clinicaltrials.gov_ct2_show_NCT01452412&d=DwMF-g&c=ZQs-KZ8oxEw0p81sqgiaRA&r=IArnAw3VCzk5UPvCZuFXCfOUlGoUVMNFkhzyx2dyGjo&m=o4jeLazyGg5D8QaW3_iByeQlluW9Y2344XA9votrcQ8&s=3hzllq3cUreKzA6_zfhD6bedIpxNgCbJkQ6-mI5aYIE&e=. Accessed May 14, 2019
- 41.University of Colorado, Denver: Bicarbonate administration in CKD. Available at: https://urldefense.proofpoint.com/v2/url?u=https-3A__clinicaltrials.gov_ct2_show_NCT02915601&d=DwMF-g&c=ZQs-KZ8oxEw0p81sqgiaRA&r=IArnAw3VCzk5UPvCZuFXCfOUlGoUVMNFkhzyx2dyGjo&m=o4jeLazyGg5D8QaW3_iByeQlluW9Y2344XA9votrcQ8&s=DEn2qq0r89B67IVbWCo7hrApMuccyD9FvX-y5VpTZUc&e=. Accessed May 14, 2019
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.