Abstract
Tributyltin (TBT), an organotin chemical used as a catalyst and biocide, can stimulate cholesterol efflux in non-steroidogenic cells. Since cholesterol is the first limiting step for sex hormone production, we hypothesized that TBT disrupts intracellular cholesterol transport and impairs steroidogenesis in ovarian theca cells. We investigated TBT’s effect on cholesterol trafficking, luteinization, and steroidogenesis in theca cells of five species (human, sheep, cow, pig, and mice). Primary theca cells were exposed to an environmentally relevant dose of TBT (1 or 10 ng/ml) and/or retinoid X receptor (RXR) antagonist. The expression of RXRα in sheep theca cells was knocked down by using shRNA. Steroidogenic enzymes, cholesterol transport factors, and nuclear receptors were measured by RT-qPCR and western blotting, and intracellular cholesterol, progesterone, and testosterone secretion by ELISA. In ovine cells, TBT upregulated StAR, ABCA1, and SREBF1 mRNA in theca cells. TBT also reduced intracellular cholesterol and upregulated ABCA1 protein expression but did not alter testosterone or progesterone production. RXR antagonist and RXRα knockdown demonstrates that TBT’s effect is partially through RXR. TBT’s effect on ABCA1 and StAR expression was recapitulated in all five species. TBT, at an environmentally relevant dose, stimulates theca cell cholesterol extracellular efflux via the RXR pathway, triggers a compensatory upregulation of StAR that regulates cholesterol transfer into the mitochondria and SREBF for de novo cholesterol synthesis. Similar results were obtained in all five species evaluated (human, sheep, cow, pig, and mice) and are supportive of TBT’s conserved mechanism of action across mammalian species.
Keywords: theca cells, tributyltin, cholesterol, retinoid X receptor, steroidogenesis, luteinization
Introduction
Tributyltin (TBT) is an organotin compound widely used in the production of agricultural pesticides, preservatives of paper and textiles, antifouling paints, and as a stabilizer in plastic production (Antizar-Ladislao 2008). Although TBT-based paints were partially banned in 2008, its hydrophobic properties, half-life (6 weeks to 5 months) and tendency to accumulate in sediments results in a high environmental persistence of both, the parent compound, as well as its metabolites (di- and mono-butyltin) (Antizar-Ladislao 2008). Because of TBT’s use as an antifouling agent in paints for boats, aquaculture nets, and docks, environmental TBT concentrations range from 1.2 to ~160.6 μg/kg dry weight in seawater and estuaries, coastal sediments, and freshwater sediments and from 0.24 to 29.7 μg/kg w/w in aquatic organisms (Müller et al. 2013). Despite banning of TBT in some countries and applications, TBT continues to be commonly found in soil, dust, and water (Kannan et al. 2010; Shue et al. 2014). TBT is rapidly absorbed by organic materials, accumulating in aquatic species, invertebrates, birds, and mammals (Kannan et al. 1999; Shue et al. 2014; Strand and Jacobsen 2005). Humans are exposed to TBT as evidenced by the presence of TBT in liver (Nielsen and Strand 2002), placenta (Rantakokko et al. 2014), and blood (Kannan et al. 1999), with mean circulating levels between 11.6 and 85 ng/ml (Kannan et al. 1999). The most common sources of human exposures are seafood (Tsuda et al. 1995) and household dust (Fromme et al. 2005; Kannan et al. 2010). The tolerable daily intake of TBT is 250 ng/kg body weight (BW)/day (Okoro et al. 2011) and the TBT oral reference dose established by the U.S. Environmental Protection Agency is 300 ng/kg BW/day (Agency 1997). However, these were established based on immune function endpoints (Penninks 1993).
TBT can bind to the retinoid X receptor (RXR), liver X receptor (LXR), and peroxisome proliferator-activated receptor γ (PPARγ) (Baker et al. 2015; Cui et al. 2011; Harada et al. 2015; Kanayama et al. 2005; le Maire et al. 2009; Li et al. 2011). Binding to these nuclear receptors results in energy metabolism disruption supportive of its obesogenic ability (reviewed in (Veiga- Lopez et al. 2018)). TBT can also interfere with reproductive function (reviewed in (Delgado et al. 2011; Macejova et al. 2016)) and result in early embryonic loss (Harazono and Ema 2000). In aquatic organisms, TBT can act as an estradiol sulfation inhibitor and reduce steroid synthesis and specific metabolism pathways in fish (Thibaut and Porte 2004). In female mice, postnatal TBT exposure decreased ovary weight and serum 17β-estradiol, and increased serum progesterone which lengthened the metestrus and shortened the proestrus and diestrus phases (Podratz et al. 2012). Additionally, a similar ovarian weight reduction accompanied by an increase in apoptotic genes was reported after oral TBT exposure in rats (Lee et al. 2012).
Within the ovary, theca cells provide structural support to the ovarian follicle and are necessary for steroid synthesis. Before ovulation, theca cells synthesize androgens, which are then converted to estradiol by granulosa cells. Dysregulation of androgen synthesis can alter female fertility, with hyperandrogenism resulting in subfertility and reduced ovulation rates (Lebbe and Woodruff 2013) and hypoandrogenism in a diminished ovarian reserve (Gleicher et al. 2013). After ovulation, theca cells luteinize and begin to synthesize progesterone, which is essential for fertilization, embryo implantation, and pregnancy maintenance in mammalian species (Mesen and Young 2015). Low progesterone synthesis is associated with reduced conceptus elongation (Forde et al. 2011) and early pregnancy loss (Walch and Huber 2008).
Exposure to endocrine disrupting chemicals (EDCs) can impair fertility and ovarian cell function (Craig and Ziv-Gal 2018; Mlynarcikova et al. 2014), specifically altering steroidogenesis in vitro (Romani et al. 2013; Romani et al. 2014) and in vivo (Li et al. 2012; Melzer et al. 2011). TBT’s steroidogenic effects have been reported in Leydig cells and testis (Kanimozhi et al. 2018; Kariyazono et al. 2015; Mitra et al. 2014; Nakajima et al. 2005). However, TBT’s effect on ovarian steroidogenic cells has been restricted to granulosa cells. TBT reduces estradiol synthesis in human granulosa-like tumor cells and is association with aromatase activity inhibition in bovine granulosa cells (Saitoh et al. 2001; Schoenfelder et al. 2003). However, whether TBT can affect theca cells steroidogenic function remains unknown.
Cholesterol is the precursor for steroid hormone biosynthesis. In theca cells, cholesterol trafficking plays a role in progesterone synthesis. Internalized into the cytoplasm through the LDL receptor, cholesterol is transported into the endoplasmic reticulum and the mitochondrion to synthesize pregnenolone, the first intermediate of steroid hormone synthesis. Intracellular cholesterol is regulated by the cholesterol efflux regulatory protein ATP binding cassette subfamily A member 1 (ABCA1). TBT upregulates ABCA1 expression and cholesterol efflux in macrophage cells (Cui et al. 2011). TBT also upregulates ABCA1 expression in bone marrow multipotent mesenchymal stromal cells (Baker et al. 2015), which can be blocked by an RXR antagonist in a dose dependent manner (Baker et al. 2015). However, whether TBT exposure, at an environmentally relevant dose, can interfere with intracellular cholesterol homeostasis in steroidogenic ovarian cells remains unknown.
To determine if TBT can interfere with theca cell’s cholesterol trafficking and steroidogenesis, we have undertaken a multispecies approach. Mammalian species used include precocial monovulatory (human, cow, and sheep), precocial polyovulatory (pig), and altricial polyovulatory (mice). The majority of experiments were carried out in ovine theca cells. Sheep were used as the primary species in this ovarian study for the following reasons; 1) as humans, sheep are monovulatory, 2) the timeline of fetal ovarian development is similar in both species (Padmanabhan and Veiga-Lopez 2013), 3) sheep ovary size is 80% of the human premenopausal ovary and the pool of primordial follicles are located in a similar collagen-dense cortical layer (Fransolet et al. 2014), 4) ovarian steroid feedback regulatory mechanisms are similar in both species (Kasa-Vubu et al. 1992; Soules et al. 1984), and 5) sheep ovaries have been widely used as models for humans (Isachenko et al. 2014; Padmanabhan and Veiga-Lopez 2013). Additional experiments were carried out in human, bovine, porcine, and mouse theca cells. While multi- species experiments are a widely utilized in ecology (van Kleunen et al. 2014), this approach is rarely used in environmental toxicology. Multispecies approaches are a very robust approach to understand general principles governing mechanisms of action (van Kleunen et al. 2014).
The objectives of this study were to investigate if TBT, at an environmentally relevant dose 1) impairs intracellular cholesterol homeostasis and/or steroidogenesis in ovarian theca cells, 2) if the luteinization stage of the theca cells plays a role in TBT’s effect, 3) the role of RXR in mediating TBT-induced cholesterol/steroidogenic disruptions, and 4) if the TBT’s effect on intracellular cholesterol was conserved across species.
Materials and Methods
Generation of primary sheep ovarian theca cells
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Michigan State University (MSU) and are consistent with National Research Council’s Guide for the Care and Use of Laboratory Animals and the current Animal Welfare Act. The study was conducted at Michigan State University Research Facility (East Lansing, MI; 42.7° N, 84.4° W) using an in-house flock at MSU of multiparous Polypay x Dorsett breed of sheep. Female sheep were bred using a time mated pregnancy strategy. Pregnancy was terminated at day 120 of gestation with a barbiturate overdose (pentobarbital, i.v. Fatal Plus; Henry Schein, Melville, NY) and ovaries harvested. The theca interna cell layer of antral follicles (2 – 4 mm in diameter) was isolated by microdissection as previously described (Wang et al. 2018). In brief, antral follicles were cut in half and adhering interna cells and granulosa cells microdissected by pulling the intact layer of the theca interna and granulosa cell layer from the externa theca layer. Allowing the theca interna layer to stay intact, the granulosa cell layer was gently removed using a pair of tweezers. Isolated theca interna cells were dispersed using collagenase I (1 mg/ml) supplemented with 10 μg/ml deoxyribonuclease in Ca2+/Mg2+-free buffer for 45 min at 37 °C. The cell suspension was filtered through a 35 μm nylon mesh, fractioned on a discontinuous gradient (44% and 35% Percoll), and centrifuged for 20 min at 2,000 rpm. Theca cells were collected from the Percoll interface (44% and 35%) by aspiration. Cell viability was assessed by trypan blue exclusion. Plated cells were maintained in basic medium consisting of DMEM/F12 medium supplemented with 1% fetal bovine serum, 2 mM L- glutamine, 10 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 39 °C. After 3 to 6 days of culture, primary theca cells reached ~90% confluency and were trypsin digested and store in liquid nitrogen for further study. Theca cell purity was determined using theca cell positive markers fibulin 5 (FBLN5) (Hatzirodos et al. 2015) and vimentin (VIM) (Wang et al. 2018) by immunofluorescence as described below.
Generation of primary mice, porcine, and bovine ovarian theca cells
Findings obtained in ovine theca cells were validated in 3 additional animal species (cow, pig, and mice) to determine if TBT’s effect on steroidogenic cells was conserved across species. Cow and pig ovaries were obtained from the Meat Laboratory (MSU). Mouse ovaries were obtained from 21-day old C57BL/6J mice. All ovaries were placed in transport medium (DMEM/F12 supplemented with 2% penicillin / streptomycin and 2% antibiotic-antimycotic) and processed within 1 h of sacrifice at 4 °C. The interna theca cell layer of pig and cow antral follicles was isolated and enzyme digested as described above for sheep. Mice theca-interstitial cells were isolated as previously reported (Tian et al. 2015) with minor modifications. In brief, antral follicles were punctured to release the granulosa cell layer. The residual ovarian tissue was thoroughly washed in ice-cold DPBS. The ovarian tissue was then minced, enzyme digested (4 mg/ml collagenase I, 10 mg/ml DNase, 10 mg/ml BSA) for 1 h at 37 °C in a water bath and shaken every 5 min. Tissue was then centrifuged at 1,000 rpm for 5 min and washed 3 times with growth medium. The interna theca cell layer of porcine and bovine antral follicles (2 to 5 mm in diameter), and mice (0.3 to 0.6 mm in diameter) from 2–3 animals were pooled into a single cultured cell line per species for further testing. All species’ theca cells were purified by the aforementioned discontinuous gradient centrifugation method.
Ethics, subject selection, and generation of human ovarian theca cells
Findings obtained in ovine theca cells were also validated in human theca cells. Human ovarian tissue for preparation of ovarian theca cells was obtained with written informed consent after Institutional Review Board (IRB: 17–1066M) approval of Sparrow Hospital and MSU and are consistent with relevant guidelines and regulations. Human ovaries were harvested from healthy non-pregnant women subjected to ovariectomy at Sparrow Hospital (Lansing, MI, USA). Exclusion criteria included polycystic ovarian syndrome, ovarian cancer, menopause, drug addiction, and/or diagnosed with HIV or hepatitis B or C. Age of women ranged from (31–46 years). Three human ovarian theca cultured cell lines were generated to be used in this study. Only follicles with healthy appearance that were ≥ 3 mm in size were used and pooled for the generation of a pool of theca cell line per individual. All ovaries were placed in transport medium (same as for animal cells) and processed within 1 h at 4 °C. The interna theca cell layer of human antral follicles was isolated and enzyme-digested as described for sheep. Enzyme digestion for human cells included collagenase I (3 mg/ml) and DNase I (300 IU/ml).
Cell viability assay and chemical exposure
Cell viability was determined using an MTT assay. Theca cells were seeded into 96-well plates (10,000 cells/well) and cultured overnight in basic medium. After cell attachment, cells were treated with a range of TBT (Cat# T50202, >96%, Sigma, St. Louis, MO, USA) concentrations (0, 1, 10, 20, 50, 100, 200, 500, 1,000, and 2,000 ng/ml) and/or an RXR antagonist (UVI3003;Cat# 847239-17-2, >98%, Tocris Bioscience, Bristol, UK; concentrations: 0, 0.1, 0.2, 0.5, 1, 2, 4, 8, 20 and 50 μM) in basic medium for 72 h. TBT and UVI3003 were dissolved in DMSO at 0.1% (v/v). All control groups were exposed to DMSO at 0.1% (v/v). Thereafter, medium was replaced with 100 μl of phenol red-free MTT working solution (50 μg/ml) and incubated for 4 h. MTT working solution was then discarded and 100 μl of DMSO added into each well. Plates were vortexed for 10 min at room temperature and cell viability determined by absorbance quantification at 570 nm using a microplate reader (SpectraMax M5e, Molecular Devices LLC, Sunnyvale, CA, USA).
Luteinization induction
Theca cells can be in a pre-luteinized or luteinized state and secrete testosterone or progesterone, respectively. Therefore, we tested the effect of TBT in both states. To induce theca cell luteinization, cells were cultured overnight in 6-well plates with basic medium, then serum starved for 48 h in DMEM/F12 medium supplemented with 1% penicillin/streptomycin, 2 mM L-glutamine and 0.1% BSA. Thereafter, theca cells were induced to luteinize in DMEM/F12 supplemented with 1% penicillin/streptomycin, 2 mM L-glutamine, 1% fetal bovine serum (heat inactivated), LH (250 ng/ml; ovine LH, U.S. National Hormone and Peptide Program) and 50 ng/ml IGF-I, and cultured for 72 h. Sheep theca cell luteinization was confirmed by HSD3β1 upregulation, HSD17β1 downregulation, and progesterone production in the media. The same luteinization protocol was used for all species.
Immunofluorescence
Theca cells were cultured in 24-well plastic plates with basic medium for 48 h, then washed twice with DPBS and fixed with 10% formalin for 15 min at room temperature. Cells were then washed and permeated with 0.1% Triton X-100 in DPBS for 30 min and blocked with 1% BSA in DPBS for 1 h. Cells were incubated with primary antibodies VIM and FBLN5 (details in Supplemental Table 1) overnight at 4 °C. After five DPBS washes, cells were incubated with FITC-conjugated secondary antibodies (details in Supplemental Table 1) for 1 h at 37 °C in the dark. Cells were then counterstained with DAPI nuclear stain for 10 min, and imaged under a fluorescence microscope (Eclipse Ti, Nikon, Japan). Theca cell purity was calculated by the ratio of number of FBLN5 positive or VIM positive cells to the total cells (DAPI nuclei) using Image J software (NIH 2016). Validation of primary antibody specificity was confirmed by western blotting as described below.
Western blotting
Protein was extracted from theca cells using a lysis buffer and protein concentration determined using a Pierce BCA protein assay kit (Thermo Fisher, Rockford, IL, USA). Samples were run on a 10% SDS-PAGE gel and subjected to western blotting. Protein was transferred from the gel to a nitrocellulose membrane and blocked with 5% non-fat milk and incubate with primary antibodies (Supplemental Table 1) overnight at 4 °C. After three DPBS washes, cells were incubated with HRP conjugated secondary antibodies (Supplemental Table 1) for 1 h at 37 °C in the dark. Membranes were subjected to enhanced chemiluminescence (Western Bright ECL K- 12045-D50, Advansta, Menlo Park, CA, USA) and developed using a Thermo MyECL imager. Quantification of band intensities was performed using ImageJ software (NIH 2016). Differences in loading was accounted for by normalizing target protein band to control β-actin band for each sample.
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using an RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. RNA quality and concentration were measured by Nanodrop (Thermo Fisher Science, Wilmington, NC, USA). RNA (1 μg; quality: A260/A280: 2.0) was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Promega, Madison, WI, USA) in a 10 μl reaction volume. Quantitative real time PCR (ABI- Quant Studio 7 Flex Real-Time PCR System, Thermo Fisher, Carlsbad, CA) was performed to examine the mRNA expression of the genes encoding sheep 3β-hydroxysteroid dehydrogenase / delta(5)-delta(4)isomerase (HSD3B1), hydroxysteroid 17β-dehydrogenase 1 (HSD17B1), ATP binding cassette subfamily A member 1 (ABCA1), steroidogenic acute regulatory protein (StAR), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), low density lipoprotein receptor (LDLR), liver X receptor α (LXRα), liver X receptor β (LXRβ), retinoid X receptor α (RXRα), retinoid X receptor β (RXRβ), hormone-sensitive lipase (HSL), scavenger receptor class B member 1 (SR-B1), translocator protein (TSPO), cAMP responsive element binding protein 1 (CREB1), and sterol regulatory element binding transcription factor 1 (SREBF1). Species- specific sequences were developed for ABCA1 and StAR. Primer sequences are provided in Supplemental Table 2. cDNA (50 ng) amplification reaction consisted of template denaturation and polymerase activation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. All RT-qPCR were run in triplicate. mRNA levels encoding the indicated genes were normalized against ov/bGAPDH (for ovine and bovine cells), h/pGAPDH (for human and porcine cells), mGAPDH (for mouse cells), (see Supplemental Table 2) and presented as relative fold change to that of the control. Melt curve analyses were performed for all genes, and the specificity as well as integrity of the PCR products were confirmed by the presence of a single peak and single PCR product band by gel electrophoresis.
Total intracellular cholesterol assay
Cells were trypsin digested, counted with hemocytometer, and cellular lipids extracted with chloroform:methanol (3:1) for 5 min at room temperature. Solvents were nitrogen-evaporated and reconstituted with isopropanol. Total cellular cholesterol was quantified using an Amplex Red cholesterol fluorometric assay following the manufacturer’s instructions (A12216, Invitrogen, Grand Island, NY, USA) and quantified using a microplate reader at 590 nm absorbance.
Hormone determination
Theca cell culture medium was collected by centrifugation at 2,500 rpm for 15 min at 4 °C, and the supernatant stored at −80 °C until assayed. Progesterone concentration in the culture medium was measured using a progesterone direct competitive ELISA assay (Ridgeway Science, Gloucestershire, United Kingdom) following manufacturer instructions as previously described (Roberts et al. 2017). The concentration of testosterone in the culture medium was measured using a solid-phase, competitive chemiluminescent enzyme immunoassay (Immulite 2000xp System Analyzer) at the American Association of Veterinary Laboratory Diagnosticians (AAVLD) accredited Diagnostic Center for Population and Animal Health (DCPAH) at MSU. Intra-and inter-coefficients of variation were < 5%.
RXRα primary theca cell knockdown
Sheep theca cells were seeded into a 6-well plate at 300,000 cells per well in growth medium. At ~60% confluency, cells were transfected with RXRα (TRCN0000330707, CAAGGACTGCCTGATTGACAA) mission shRNA plasmid DNA (SHCLNG-NM002957, Sigma, St. Louis, MO, USA) or control scramble shRNA, by using FuGENE-6 (E2692, Promega, St. Louis, MO, USA). The insert of human RXRα shRNA vector (TRCN0000330707) is a 100% match to the ovine sequence (Supplemental Figure 1). After 3 days of transfection (days 0 to 3), cells were exposed to TBT at 10 ng/ml or the vehicle (DMSO) for 48 h (days 4 to 5). qPCR and western bloting were used to assess gene and protein level of RXRα at the end of transfection (day 3), and the level of ABCA1 and StAR at the end of TBT exposure (day 5).
Statistical analysis
All data are presented as mean ± SEM. Appropriate transformations were applied, as needed, to account for the normality of data. Comparisons among the treatment groups were analyzed by ANOVA with Tukey posthoc tests and by an independent T-test using PASW Statistics. Differences were considered significant at P < 0.05.
Results
Isolation, growth, and luteinization of theca cells
Isolated sheep primary theca cells had a fibroblast-like morphology (Supplemental Figure 2A), high viability (≥ 95%) and displayed an “S” shape growth curve (Supplemental Figure 2B). Theca cell purity was identified by immunofluorescence staining using positive (FBLN5 and VIM) theca cell markers (Supplemental Figure 2C–D), with > 90% cells positive for both markers. After 72 h exposure to LH and IGF-1, sheep theca cell luteinization was confirmed by mRNA HSD3B1 upregulation, HSD17B1 downregulation (Supplemental Figure 2E) and increased progesterone secretion (Supplemental Figure 2F).
Effects of TBT on steroidogenic enzymes in theca cells
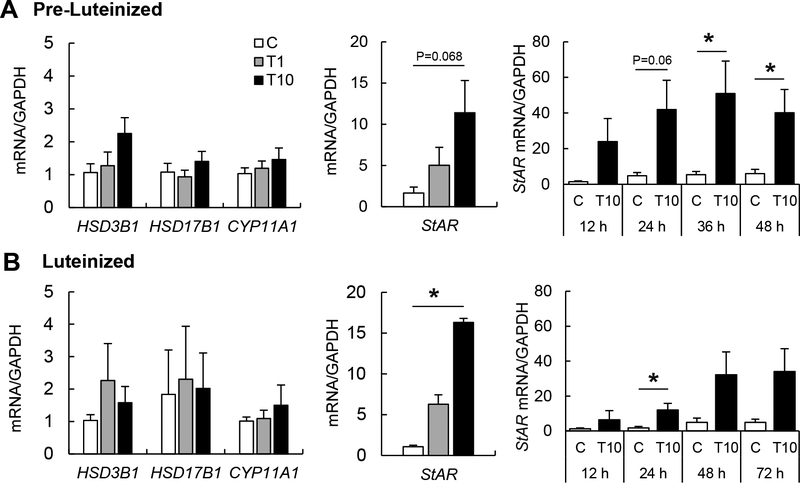
After 72 h exposure, TBT had no cytotoxicity on pre-luteinized theca cells at 1 and 10 ng/ml doses used in the current study (Supplemental Figure 3A). Cytotoxicity was only evident at the 1,000 and 2,000 ng/ml exposure doses. To evaluate the effect of TBT on steroidogenesis, pre- luteinized and luteinized theca cells were exposed to TBT (0, 1, or 10 ng/ml) for 48 h and 72 h respectively. TBT at 10 ng/ml tended to upregulate StAR mRNA expression in pre-luteinized theca cells (Figure 1A), while TBT exposure during theca cell luteinization resulted in significant upregulation of StAR mRNA at 10 ng/ml (Figure 1B). Time course exposure (12, 24, 36, and 48 h) to TBT resulted in significant StAR mRNA upregulation at 36 and 48 h in pre-luteinized cells (Figure 1A) and at 24 h in luteinized cells (Figure 1B). TBT had no effect on steroidogenic enzymes HSD3B1, HSD17B1, and CYP11A1 mRNA expression in either pre-luteinized or luteinized theca cells.
Figure 1.
Effect of TBT exposure on mRNA expression in pre-luteinized and luteinized primary ovine theca cells. mRNA expression (mean ± SEM) of steroidogenic enzymes HSD3β1, HSD17β1 and CYP11A1 and transport protein (StAR) in pre-luteinized (A) and luteinized (B) ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
Effects of TBT on intracellular cholesterol and steroid production homeostasis in theca cells
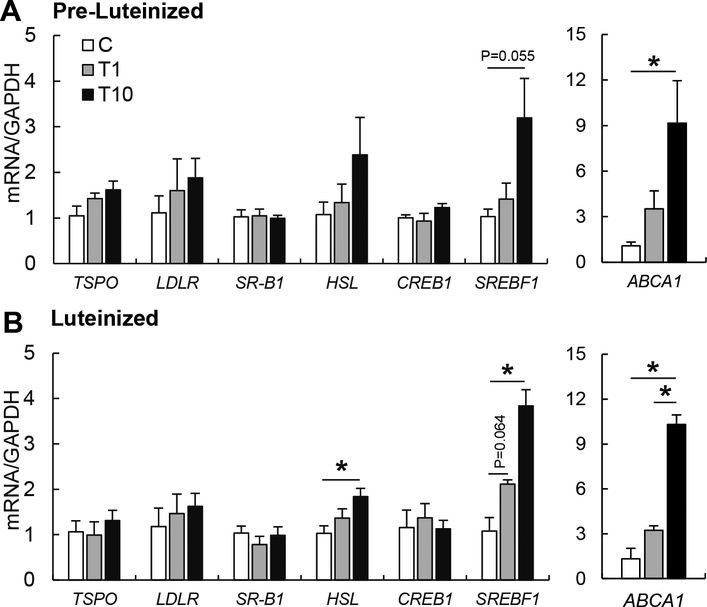
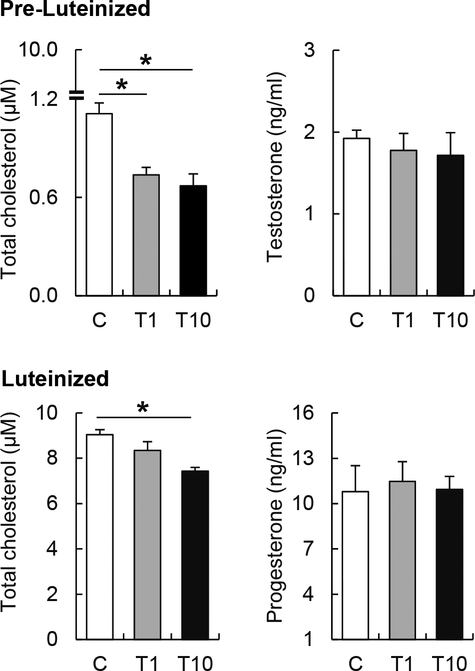
To further explore the effect of TBT on intracellular cholesterol homeostasis, pre-luteinized and luteinized theca cells were exposed to TBT for 48 h and 72 h respectively, and expression of genes involved in intracellular cholesterol influx (LDLR, SR-B1) and efflux (ABCA1), cholesteryl ester hydrolysis (HSL), cholesterol synthesis (SREBF1 and CREB1), and facilitators of mitochondrial cholesterol transport (StAR, TSPO) were assessed. TBT exposure upregulated SREBF1 and ABCA1 mRNA expression in pre- and luteinized theca cells in a dose-dependent manner (Figure 2A–B). However, the effect of TBT on cholesterol homeostasis related factors varied by dose and stage of the theca cells. In luteinized cells, TBT at 10 ng/ml significantly upregulated HSL and SREBF1 mRNA expression, but not CREB1 (Figure 2B). No significant differences were observed in TSPO, LDLR and SR-B1 expression (Figure 2A, B). Intracellular total cholesterol content was measured to evaluate if TBT-induced ABCA1 upregulation resultsin intracellular cholesterol depletion. Luteinized theca cells had 10 times higher intracellular cholesterol compared to pre-luteinized cells (Figure 3). TBT reduced intracellular cholesterol by ~40% in pre-luteinized and ~20% in luteinized theca cells (Figure 3). Given that StAR is the rate- limiting step in the synthesis of steroids in theca cells, we evaluated the effect of TBT on progesterone and testosterone synthesis. In pre-luteinized theca cells, TBT exposure for 48 h did not alter testosterone production (Figure 3). During luteinization, TBT exposure for 72 h did not alter progesterone production (Figure 3).
Figure 2.
Effect of TBT exposure on mRNA expression in pre-luteinized and luteinized ovine primary theca cells. mRNA expression (mean ± SEM) of cholesterol transport factors (TSPO, LDLR, SR-B1, ABCA1) and cholesterol biosynthesis factors (HSL, CREB1, SREBF1) in primary ovine pre-luteinized (A) and luteinized (B) ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
Figure 3.
Effect of TBT exposure on intracellular cholesterol content and steroid hormones production in pre-luteinized and luteinized ovine primary theca cells. Top Panels: Intracellular cholesterol content (left panels) and testosterone production (right panels) (mean ± SEM) in pre- luteinized ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Bottom Panels: Intracellular cholesterol content (left panels) and progesterone production (right panels) (mean ± SEM) in luteinized ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
TBT’s RXR/LXR-mediated effects
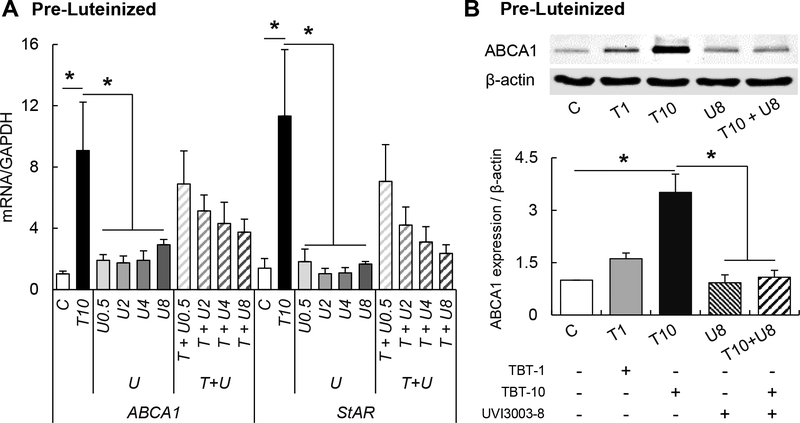
Because TBT can act through the LXR/RXR heterodimer receptor (Baker et al. 2015; Cui et al. 2011), we also evaluated the receptor expression of LXRα, LXRβ, RXRα, and RXRβ. There was no effect of TBT on either nuclear receptor at either cell stage (Supplemental Figure 4). To assess the role of RXR in TBT-mediated cholesterol efflux increase, theca cells were co- incubated with TBT and the RXR antagonist, UVI3003. First, we demonstrated that UVI3003, at the range used (0.1 – 8 μM), is not cytotoxic (Supplemental Figure 3B). Exposure to UVI3003 (0.5, 2, 4, and 8 μM) had no effect on StAR or ABCA1 mRNA expression (Figure 4A). The co- treatment with TBT and UVI3003 resulted in downregulation of StAR and ABCA1, with the 8 μM UVI3003 dose resulting in an ~80% and ~60% reduction of TBT-induced StAR and ABCA1 upregulation, respectively (Figure 4A). UVI3003 at 8 μM also blocked TBT-induced ABCA1 protein upregulation in pre-luteinized theca cells (Figure 4B). UVI3003 did not block TBT- induced mRNA upregulation of SREBF1 nor HSL (Supplemental Figure 5).
Figure 4.
Effect of TBT exposure on mRNA (ABCA1 and StAR; A) and protein (ABCA1; B) expression (mean ± SEM) in pre-luteinized ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) and / or RXR antagonist (UVI3003; 0.05, 2, 4 and 8 μM) or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
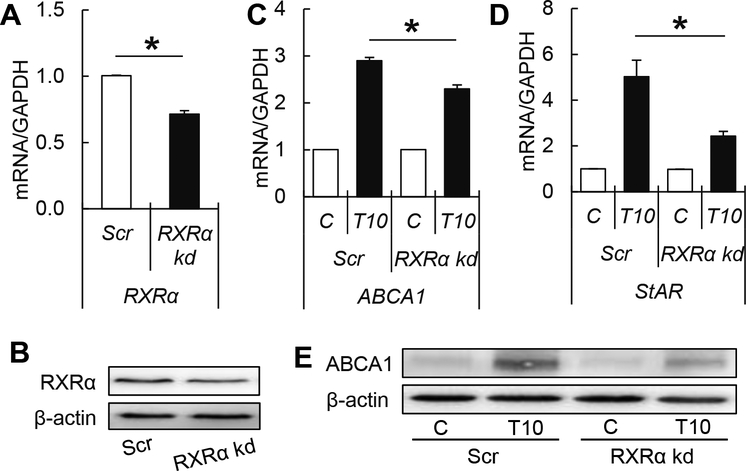
Effects of TBT on RXRα knockdown theca cells
To further support that TBT’s effect is mediated in part through RXR, we designed a loss-of- function approach by shRNA-mediated knock-down expression of RXRα in ovine theca cells. Our results demonstrated that the shRNA knock-down efficiency was ~30% (Figure 5A, B), in comparison with scramble shRNA controls. The upregulation of both ABCA1 and StAR mRNA was reduced by ~20% and ~50%, respectively upon exposure to TBT in RXRa knock-down cells (Figure 5C, D). Consistently with the previous findings, the increase in ABCA1 protein expression upon TBT exposure was decreased by ~35% in RXRα knockdown theca cells (Figure 5E).
Figure 5.
RXRα knockdown efficiency (mean ± SEM) of mRNA (A) and protein (B) in pre- luteinized ovine primary theca cells. Effect of TBT exposure on mRNA (ABCA1; C and StAR; D) and protein (ABCA1; E) expression (mean ± SEM) in RXRα knockdown or scramble controls in pre-luteinized ovine primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) and vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group. Values are normalized to scramble shRNA control cells.
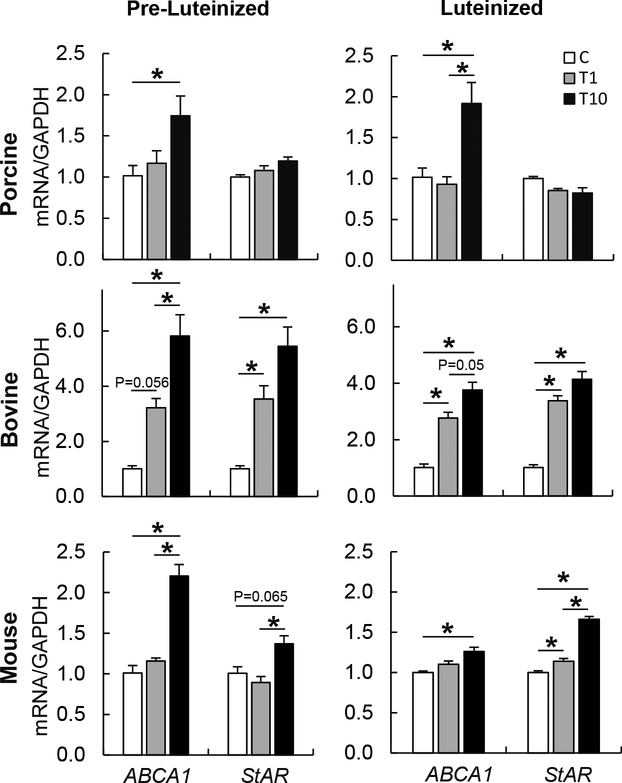
Multispecies effect of TBT on theca cells
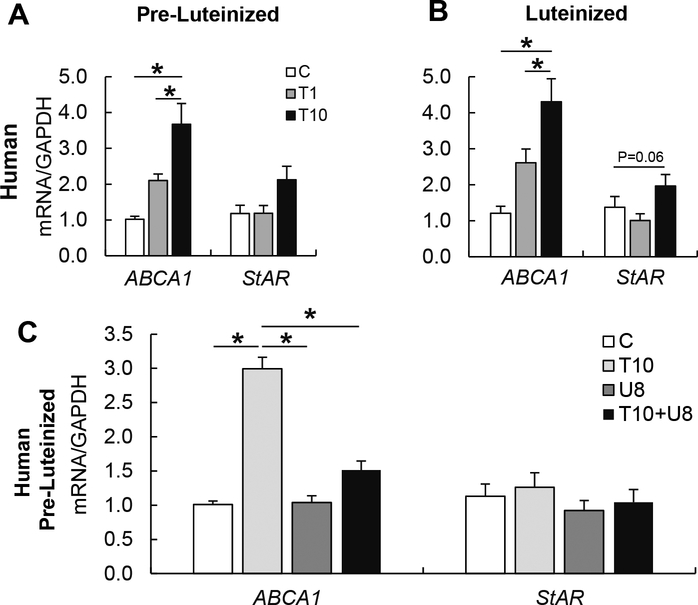
To evaluate if the findings observed in ovine theca cells are conserved in other species, human, bovine, porcine and mouse theca cells were exposed to TBT. TBT induced the upregulation of ABCA1 mRNA expression in bovine, porcine, and mouse theca cells in both, pre-luteinized and luteinized theca cells (Figure 6). StAR mRNA expression was upregulated in bovine and mouse, but not porcine theca cells (Figure 6). To address the translational relevance of the findings observed in other mammalian species, human theca cells were also exposed to TBT. Upon TBT exposure, ABCA1 mRNA expression was upregulated in a dose-dependent manner, in both pre- luteinized and luteinized human theca cells (Figure 7A–B). TBT exposure only tended to upregulate StAR mRNA expression in luteinized cells (Figure 7A, B). The RXR antagonist UVI3003 reduced the TBT-induced upregulation of ABCA1 by ~75% (Figure 7C).
Figure 6.
Effect of TBT exposure on mRNA expression (mean ± SEM) of cholesterol transporters (ABCA1 and StAR) in pre-luteinized (left) and luteinized (right) porcine (top), bovine (middle), and mouse (bottom) primary theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). One primary cultured cell line per species was used.
Figure 7.
Top Panels: Effect of TBT exposure on mRNA expression (mean ± SEM) of cholesterol transporters (ABCA1 and StAR) in pre-luteinized (A) and luteinized (B) human primary ovarian theca cells exposed to 1 ng/ml TBT (gray bars), 10 ng/ml TBT (closed bars) or vehicle (control group; open bars). Bottom Panels: Effect of TBT exposure on ABCA1 and StAR mRNA expression (mean ± SEM) in pre-luteinized human primary theca cells exposed to 10 ng/ml TBT (light gray bars), RXR antagonist (UVI3003; 8 μM; dark gray), the combination of TBT (10 ng/ml) and RXR antagonist (8 μM), or vehicle (control group; open bars). Asterisks denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
Discussion
Accumulating evidence demonstrates that TBT can modulate steroidogenesis and cholesterol trafficking. In this study, we tested whether the steroidogenesis and cholesterol trafficking could be altered in primary cultured ovarian theca cells by a short, low dose exposure to TBT. Our findings demonstrate that TBT, at environmentally relevant concentrations, stimulates theca cells intracellular cholesterol efflux by upregulating cholesterol transporter ABCA1, independent of the status of the theca cells (pre- or post-luteinization). We also demonstrate that this effect was partially driven through the nuclear receptor RXR. ABCA1 upregulation triggered a compensatory response, as shown by the upregulation of HSL, SREBF1, and StAR, that regulates cholesterol synthesis and transfer into the mitochondria. However, testosterone and progesterone production remained unchanged. Importantly, we have demonstrated that the TBT-stimulated intracellular cholesterol efflux in steroidogenic cells is a conserved effect across multiple species, including humans.
While the majority of studies have assessed TBT’s effects using doses in the range of 5.42 to 542 nM (Bertuloso et al. 2015; Chamorro-Garcia et al. 2013), doses used in this study (1 and 10 ng/ml equivalent to 3.07 nM and 30.7 nM, respectively) directly relate to human exposures. These vary from undetectable to 155 ng/ml, as reported with seafood consumption and in highly polluted areas resulting in higher exposures (Kannan et al. 1999). To note is that although TBT was banned in 1999 for certain applications, and it has a half-life of 1 to ~2 weeks in marine water, it can remain up to 30 years in the environment (Fang et al. 2017; Maguire 2000) and continues to be used worldwide for multiple industrial applications and consumer product manufacturing (Sousa et al. 2014).
TBT alters intracellular cholesterol homeostasis
Intracellular cholesterol homeostasis is essential to maintain cellular membrane structure and function. ABCA1 is the main cholesterol efflux transporter (Tsunemi et al. 2014; Wang et al. 2001), a transporter that is expressed in ovarian theca cells (Wu et al. 2003). We have demonstrated that, at an environmentally relevant dose, TBT upregulates the cholesterol efflux transporter ABCA1 mRNA expression in five mammalian species including human, sheep, cow, pig, and mouse. In sheep, TBT-stimulated ABCA1 upregulation in theca cells was evident in both mRNA and protein expression and occurred in a dose-dependent manner, with a 10-fold increase in TBT dose eliciting a ~3-fold response in ABCA1 upregulation. A similar response has been previously reported in macrophages, where TBT at 10 nM also upregulates ABCA1 expression, resulting in higher cholesterol efflux (Cui et al. 2011).
Despite TBT’s dose-dependent effect on ABCA1 upregulation, intracellular cholesterol reduction was not dose-dependent. This finding suggests that theca cells may compensate the ABCA1-mediated intracellular cholesterol loss, either by increasing intracellular cholesterol biosynthesis or internalization through the low-density lipoprotein receptor (LDLR) (Guillemot et al. 2016) and/or the scavenger receptor SR-B1 (Wu et al. 2003). While TBT did not upregulate LDLR or SR-B1 mRNA expression, we observed an upregulation of HSL, a cholesteryl ester hydrolase that stimulates cholesterol synthesis (Kraemer et al. 1997), and SREBF1, a membrane- bound transcription factor that stimulates cholesterol synthesis by upregulating cholesterol biosynthetic enzymes (Brown and Goldstein 1997). Despite HSL and SREBF1 upregulation being supportive of enhanced cholesterol biosynthesis, this did not fully counteract TBT-induced intracellular cholesterol loss.
We have also demonstrated that the effect of TBT on theca cells is independent of the phase of the reproductive cycle. This is important because theca cells have very distinct endocrine functions based on their stage (pre-luteinized vs. luteinized). Pre-luteinized theca cells produce testosterone as a precursor for estrogen synthesis while luteinized theca cells produce progesterone during the luteal phase. In our study, intracellular cholesterol content was 10-fold higher in non-exposed lutenized theca cells than pre-luteinized theca cells; supportive data for a greater cholesterol demand in luteinized vs. pre-luteinized theca cells. We predicted that the loss of intracellular cholesterol efflux may have a greater impact on pre-luteinized theca cells.
However, despite a loss of ~40% in intracellular cholesterol, testosterone production was not impaired in pre-luteinized theca cells.
TBT alters StAR, but not steroid production
Because cholesterol is the precursor for sex hormone production, we predicted that the reduction in intracellular cholesterol upon TBT exposure would reduce theca cell steroid hormone production. In support of this hypothesis, in vivo studies have demonstrated that TBT exposure inhibits steroidogenic enzymes’ transcription, and reduces serum testosterone production in mouse (Kim et al. 2008) and hamster (Kanimozhi et al. 2018) testes. In these studies, however, intracellular cholesterol was not assessed. In the current study, despite a TBT-induced reduction in intracellular cholesterol in pre-luteinized and luteinized theca cells, steroid production (testosterone or progesterone, respectively) and expression of steroid biosynthetic enzymes (HSD3B1, HSD17B1 and CYP11A1) were not altered. The lack of changes in testosterone and progesterone production, despite a reduction in cholesterol, could be due to 1) a cholesterol threshold above which steroid production remains unaffected, or 2) a compensatory mechanism in theca cell steroidogenesis within the mitochondria, such as increased activity or internalization of StAR into the outer mitochondrial membrane (Bose et al. 2007) or increased mitochondrial fusion (Duarte et al. 2012). However, these were not explored in the current study.
Because the first rate-limiting step in steroid hormone biosynthesis is the internalization of cholesterol into the mitochondria (Miller 2013), we first investigated whether cholesterol trafficking into the mitochondria may be altered upon TBT exposure. Cholesterol transport from the outer to the inner mitochondrial membrane is mediated by TSPO and StAR (Christenson and Strauss 2000; Miller 2013). We observed that TBT upregulated StAR expression in sheep theca cells. As StAR is required for steroidogenesis even in the absence of hormonal stimulation (Stocco 2001), and TBT upregulates StAR expression, it is possible that this results in an increase in internalization of cholesterol into the mitochondria to compensate TBT-induced intracellular cholesterol shortage. In addition, TBT did not alter theca cells TSPO’s expression, an outer mitochondrial membrane protein that cooperates with StAR in transporting cholesterol into the mitochondria. Since cholesterol transport is a complex process that requires the interaction of various proteins located in the inner (ATAD3A) and outer (TSPO, VDAC) mitochondrial membrane and those that form the transduceosome (StAR, ABCD3, DBI, PRKAR) (Midzak and Papadopoulos 2016), further work is required to explore the specific effect of TBT on cholesterol internalization into the mitochondria. All steroidogenic cells are capable of cholesterol de novo synthesis and thus, further work is also needed to understand if de novo cholesterol synthesis may be enhanced to compensate TBT-induced cholesterol loss.
Multispecies effect
We have demonstrated that TBT induces ABCA1 upregulation in primary cultured cells of five different species including humans, sheep, cow, pig, and mice. To note, these species have different ovarian fetal development and ovulatory rates. These findings demonstrate that TBT’s- induced ABCA1 is a conserved mechanism across five species. This provides robust evidence and reproducibility in support of TBT’s mechanism of action (49). In this study, TBT significantly stimulates the expression of cholesterol efflux transporter ABCA1, in all five species for both pre-luteinized and luteinized stages. As with sheep, the TBT-induced upregulation of ABCA1 in human theca cells was counteracted with RXR antagonist exposure. However, the expression of StAR was only significantly up-regulated in sheep, cow, and mice. The blunt response of StAR expression in human and pig theca cells may be due to the species differences, exposure time, and/or doses used. To note is that the effect observed in the current study was limited to a short exposure. Given the persistence of TBT in the environment, evaluation of chronic exposures on steroidogenesis is warranted.
TBT stimulates cholesterol efflux through the RXR signaling pathway
The expression of ABCA1 is under the modulation of multiple factors, which include cellular cholesterol content and nuclear receptors (Jiang et al. 2006). An increase in intracellular cholesterol stimulates ABCA1 expression, and inhibition of cholesterol synthesis decreases ABCA1 expression (Jiang et al. 2006). TBT exposure resulted in lower intracellular cholesterol content accompanied by ABCA1 upregulation, suggesting that TBT’s-induced upregulation of ABCA1 was likely mediated by nuclear receptors. Our findings that an RXRα antagonist counteracts TBT-induced upregulation of ABCA1 in theca cells demonstrates that TBT partially induces this effect via RXRα. This is in support of previous work demonstrating that RXR directly regulates ABCA1 expression (Cui et al. 2011; Reboulleau et al. 2012). Importantly, we demonstrated this in both, ovine and human theca cells. With a ~30% RXRα knockdown in ovine theca cells, we were able to further confirm that RXRα plays a central role in TBT’s cholesterol disruption in theca cells.
Conclusion
The current study demonstrates that TBT, at an environmentally relevant dose, results in a disruption in cholesterol trafficking homeostasis in ovarian theca cells that is partially mediated through the RXR pathway. This effect occurs independent of the stage of the theca cells (pre- or post-luteinization). Importantly, we have demonstrated that TBT acts through a conserved mechanism across five mammalian species, including humans. Further studies are required to evaluate if current TBT exposures can impair theca cell’s cholesterol metabolism in an in vivo model.
Supplementary Material
Supplemental Table 1. Antibody list.
IF: immunofluorescence, WB: western blotting.
Supplemental Table 2. Primer list.
Unless specified, all primer sequences are designed against the sheep genome sequence. b: bovine, h: human, m: mouse, p: porcine, ov: ovine.
Supplemental Figure 1. Sequence map for RXRα knockdown. Top: ovine RXRα sequence. Bottom: RXRα shRNA insert.
Supplemental Figure 2. Identification of primary cultured ovine ovarian theca cells. Morphology (A), growth curve (B) of primary cultured ovine theca cells over 8 days. Immunofluorescence stain of theca cell positive marker vimentin (VIM) (C) and fibulin 5 (FBLN5) (D). Dynamic expression pattern of steroidogenic enzymes (ovHSD3B1 and ovHSD17B1) (E) and progesterone production (F) in ovine ovarian theca cells before (growth medium) and after luteinization (luteal medium). * denote differences among treatments (P<0.05). N = 3 cultured cell lines per group. ND means not detectable.
Supplemental Figure 3. Cytotoxicity effects of TBT (A) and RXR antagonist, UVI3003 (B) on primary ovine ovarian theca cells upon three days exposure using an MTT assay. Asterisks denote differences among treatments (P<0.05). N = 3 cultured cell lines per group.
Supplemental Figure 4. Effect of TBT exposure on mRNA expression in pre-luteinized and luteinized ovine primary theca cells. mRNA expression (mean ± SEM) of nuclear receptors (LXRα, LXRβ, RXRα, RXRβ) in primary ovine pre-luteinized (A) and luteinized (B) ovine primary theca cells exposed to 1 ng/ml TBT (T1; gray bars), 10 ng/ml TBT (T10; closed bars) or vehicle (C; control group; open bars). * denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
Supplemental Figure 5. Effect of TBT (0 and 10 ng/ml) and/or RXR antagonist (UVI3003; 0.5, 2, 4 and 8 pM) exposure on mRNA (ovHSL, ovSREBFl, ovLXRα, ovLXRβand ovRXRα) expression (mean ± SEM) in pre-luteinized ovine primary theca cells. Asterisks denote differences among treatments (P<0.05). N=3 cultured cell lines per group. U: UVI3003 (μM). T: TBT.
Acknowledgements:
We thank Dr. Richard Ehrhardt and the Michigan State University (MSU) Sheep Teaching and Research Farm for help with animal husbandry. We thank Ms. Jennifer Dominguez and the Meat Laboratory in the Department of Animal Science (MSU) for help with procurement of ovarian tissue. We thank Dr. Ding and Dr. Chen (MSU) for gifting the mice ovaries. We thank Dr. Isoken Olomu and the staff at the Pathology Laboratory at Sparrow Hospital for their support during ovarian sample procurement.
Funding: Research reported in this publication was supported by the National Institute of Environmental Health Sciences (1K22ES026208 and R01ES027863 to A.V-L.) and the National Cancer Institute (K08CA218460 to C.A.M-Z.) of the National Institute of Health, Michigan State University (MSU) General Funds, AgBioResearch and the United States Department of Agriculture (USDA) National Institute of Food and Agriculture. J.G. was supported by a Summer doctoral fellowship award through the Environmental and Integrative Toxicological Sciences (EITS) of the Institute for Integrative Toxicology at MSU, and by MSU Office of the Vice President for Research and Graduate Studies, AgBioResearch, College of Graduate Studies, and College of Human Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Agency USEP (1997) Toxicological Review Tributyltin Oxide. Washington D.C. [Google Scholar]
- Antizar-Ladislao B (2008) Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ Int 34(2):292–308 doi: 10.1016/j.envint.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Baker AH, Watt J, Huang CK, Gerstenfeld LC, Schlezinger JJ (2015) Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem Res Toxicol 28(6):1156–66 doi: 10.1021/tx500433r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuloso BD, Podratz PL, Merlo E, et al. (2015) Tributyltin chloride leads to adiposity and impairs metabolic functions in the rat liver and pancreas. Toxicology letters 235(1):45–59 doi: 10.1016/j.toxlet.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Bose M, Debnath D, Chen Y, Bose HS (2007) Folding, activity and import of steroidogenic acute regulatory protein into mitochondria changed by nicotine exposure. Journal of molecular endocrinology 39(1):67–79 doi: 10.1677/JME-07-0051 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89(3):331–40 [DOI] [PubMed] [Google Scholar]
- Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B (2013) Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Exposure to the Obesogen Tributyltin in Mice. Environ Health Persp 121(3):359–366 doi: 10.1289/ehp.1205701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LK, Strauss JF 3rd (2000) Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim Biophys Acta 1529(1–3):175–87 [DOI] [PubMed] [Google Scholar]
- Craig ZR, Ziv-Gal A (2018) Pretty Good or Pretty Bad? The Ovary and Chemicals in Personal Care Products. Toxicol Sci 162(2):349–360 doi: 10.1093/toxsci/kfx285 [DOI] [PubMed] [Google Scholar]
- Cui HY, Okuhira K, Ohoka N, et al. (2011) Tributyltin chloride induces ABCA1 expression and apolipoprotein A-I-mediated cellular cholesterol efflux by activating LXRalpha/RXR. Biochemical Pharmacology 81(6):819–824 doi: 10.1016/j.bcp.2010.12.023 [DOI] [PubMed] [Google Scholar]
- Delgado VS, Lopes PFI, Podratz PL, Graceli JB (2011) Triorganotin as a compound with potential reproductive toxicity in mammals. Braz J Med Biol Res 44(9):958–965 [DOI] [PubMed] [Google Scholar]
- Duarte A, Poderoso C, Cooke M, et al. (2012) Mitochondrial fusion is essential for steroid biosynthesis. PLoS One 7(9):e45829 doi: 10.1371/journal.pone.0045829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Xu C, Li J, Borggaard OK, Wang D (2017) The importance of environmental factors and matrices in the adsorption, desorption, and toxicity of butyltins: a review. Environ Sci Pollut Res Int 24(10):9159–9173 doi: 10.1007/s11356-017-8449-z [DOI] [PubMed] [Google Scholar]
- Forde N, Beltman ME, Duffy GB, et al. (2011) Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod 84(2):266–78 doi: 10.1095/biolreprod.110.085910 [DOI] [PubMed] [Google Scholar]
- Fransolet M, Labied S, Henry L, et al. (2014) Strategies for Using the Sheep Ovarian Cortex as a Model in Reproductive Medicine. Plos One 9(3) doi:ARTN e9107310.1371/journal.pone.0091073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Mattulat A, Lahrz T, Ruden H (2005) Occurrence of organotin compounds in house dust in Berlin (Germany). Chemosphere 58(10):1377–83 doi: 10.1016/j.chemosphere.2004.09.092 [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kim A, Weghofer A, et al. (2013) Hypoandrogenism in association with diminished functional ovarian reserve. Human reproduction 28(4):1084–91 doi: 10.1093/humrep/det033 [DOI] [PubMed] [Google Scholar]
- Guillemot J, Asselin MC, Susan-Resiga D, Essalmani R, Seidah NG (2016) Deferoxamine stimulates LDLR expression and LDL uptake in HepG2 cells. Mol Nutr Food Res 60(3):600–8 doi: 10.1002/mnfr.201500467 [DOI] [PubMed] [Google Scholar]
- Harada S, Hiromori Y, Nakamura S, et al. (2015) Structural basis for PPAR gamma transactivation by endocrine-disrupting organotin compounds. Sci Rep-Uk 5 doi:ARTN 852010.1038/srep08520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harazono A, Ema M (2000) Suppression of decidual cell response induced by tributyltin chloride in pseudopregnant rats: a cause of early embryonic loss. Arch Toxicol 74(10):632–7 [DOI] [PubMed] [Google Scholar]
- Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, Rodgers RJ (2015) Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS One 10(3):e0119800 doi: 10.1371/journal.pone.0119800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isachenko V, Rahimi G, Dattena M, et al. (2014) Whole Ovine Ovaries as a Model for Human: Perfusion with Cryoprotectants In Vivo and In Vitro . Biomed Research International doi:Artn 40901910.1155/2014/409019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Lu B, Kim P, Elias PM, Feingold KR (2006) Regulation of ABCA1 expression in human keratinocytes and murine epidermis. J Lipid Res 47(10):2248–58 doi: 10.1194/jlr.M600163-JLR200 [DOI] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J (2005) Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid x receptor pathway. Mol Pharmacol 67(3):766–774 doi: 10.1124/mol.104.008409 [DOI] [PubMed] [Google Scholar]
- Kanimozhi V, Palanivel K, Akbarsha MA, Kadalmani B (2018) Molecular mechanisms of tributyltin-induced alterations in cholesterol homeostasis and steroidogenesis in hamster testis: In vivo and in vitro studies. J Cell Biochem 119(5):4021–4037 doi: 10.1002/jcb.26564 [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP (1999) Occurrence of butyltin compounds in human blood. Environmental science & technology 33(10):1776–1779 doi:DOI 10.1021/es990011w [DOI] [Google Scholar]
- Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S (2010) Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol 58(4):901–7 doi: 10.1007/s00244-010-9513-6 [DOI] [PubMed] [Google Scholar]
- Kariyazono Y, Taura J, Hattori Y, et al. (2015) Effect of in utero exposure to endocrine disruptors on fetal steroidogenesis governed by the pituitary-gonad axis: a study in rats using different ways of administration. J Toxicol Sci 40(6):909–16 doi: 10.2131/jts.40.909 [DOI] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Dahl GE, Evans NP, et al. (1992) Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology 131(1):208–12 doi: 10.1210/endo.131.1.1611998 [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim JH, Han JH, Yoon YD (2008) Inhibitory effect of tributyltin on expression of steroidogenic enzymes in mouse testis. Int J Toxicol 27(2):175–82 doi: 10.1080/10915810801977906 [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Fong L, Patel S, Natu V, Komaromy MC (1997) Overexpression of hormone- sensitive lipase in Chinese hamster ovary cells leads to abnormalities in cholesterol homeostasis. J Lipid Res 38(8):1553–61 [PubMed] [Google Scholar]
- le Maire A, Grimaldi M, Roecklin D, et al. (2009) Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. Embo Rep 10(4):367–373 doi: 10.1038/embor.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebbe M, Woodruff TK (2013) Involvement of androgens in ovarian health and disease. Mol Hum Reprod 19(12):828–37 doi: 10.1093/molehr/gat065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lim S, Yun S, Yoon A, Park G, Yang H (2012) Tributyltin increases the expression of apoptosis- and adipogenesis-related genes in rat ovaries. Clin Exp Reprod Med 39(1):15–21 doi: 10.5653/cerm.2012.39.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu T, Zhou L, He J, Ye L (2012) Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol 34(3):869–75 doi: 10.1016/j.etap.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Li X, Ycaza J, Blumberg B (2011) The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3- L1 preadipocytes. J Steroid Biochem 127(1–2):9–15 doi: 10.1016/j.jsbmb.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macejova D, Toporova L, Brtko J (2016) The role of retinoic acid receptors and their cognate ligands in reproduction in a context of triorganotin based endocrine disrupting chemicals. Endocr Regul 50(3):154–64 doi: 10.1515/enr-2016-0018 [DOI] [PubMed] [Google Scholar]
- Maguire RJ (2000) Review of the Persistence, Bioaccumulation and Toxicity of Tributyltin in Aquatic Environments in Relation to Canada’s Toxic Substances Management Policy. Water Quality Research Journal 35(4):633–680 [Google Scholar]
- Melzer D, Harries L, Cipelli R, et al. (2011) Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect 119(12):1788–93 doi: 10.1289/ehp.1103809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesen TB, Young SL (2015) Progesterone and the luteal phase: a requisite to reproduction. Obstet Gynecol Clin North Am 42(1):135–51 doi: 10.1016/j.ogc.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzak A, Papadopoulos V (2016) Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies. Front Endocrinol (Lausanne) 7:106 doi: 10.3389/fendo.2016.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL (2013) Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 379(1–2):62–73 doi: 10.1016/j.mce.2013.04.014 [DOI] [PubMed] [Google Scholar]
- Mitra S, Srivastava A, Khanna S, Khandelwal S (2014) Consequences of tributyltin chloride induced stress in Leydig cells: an ex-vivo approach. Environ Toxicol Pharmacol 37(2):850–60 doi: 10.1016/j.etap.2014.02.018S1382-6689(14)00054-4 [pii] [DOI] [PubMed] [Google Scholar]
- Mlynarcikova A, Fickova M, Scsukova S (2014) Impact of endocrine disruptors on ovarian steroidogenesis. Endocr Regul 48(4):201–24 [DOI] [PubMed] [Google Scholar]
- Müller K, Nielse E, Ladefoged O (2013) Tributyltin compounds (TBT). Evaluation of health hazards and proposal of health based quality criteria for soil and drinking water In: Danish Ministry of the Environment EPA (ed). The Danish Environmental Protection Agency [Google Scholar]
- Nakajima Y, Sato G, Ohno S, Nakajin S (2005) Tributyltin chloride suppresses the P450cl7 transcription involved in testosterone production induced by gonadotropin stimulation in cultured pig Leydig cells. Environ Toxicol Pharmacol 20(1):11–7 doi: 10.1016/j.etap.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Strand J (2002) Butyltin compounds in human liver. Environmental research 88(2):129–33 doi: 10.1006/enrs.2001.4321 [DOI] [PubMed] [Google Scholar]
- NIH (2016) Image J. Image processing and analysis in Java. In. https://imagej.nih.gov/ij/ Accessed 10/21/2016
- Okoro HK, Fatoki OS, Adekola FA, Ximba BJ, Snyman RG, Opeolu B (2011) Human exposure, biomarkers, and fate of organotins in the environment. Rev Environ Contam Toxicol 213:27–54 doi: 10.1007/978-1-4419-9860-6_2 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A (2013) Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol 373(1–2):8–20 doi: 10.1016/j.mce.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninks AH (1993) The evaluation of data-derived safety factors for bis(tri-n-butyltin)oxide. Food Addit Contam 10(3):351–61 doi: 10.1080/02652039309374157 [DOI] [PubMed] [Google Scholar]
- Podratz PL, Delgado VS, Lopes PFI, et al. (2012) Tributyltin Impairs the Reproductive Cycle in Female Rats. J Toxicol Env Heal A 75(16–17):1035–1046 doi: 10.1080/15287394.2012.697826 [DOI] [PubMed] [Google Scholar]
- Rantakokko P, Main KM, Wohlfart-Veje C, et al. (2014) Association of placenta organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: a cohort study. Environmental health: a global access science source 13(1):45 doi: 10.1186/1476-069X-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboulleau A, Robert V, Vedie B, et al. (2012) Involvement of cholesterol efflux pathway in the control of cardiomyocytes cholesterol homeostasis. J Mol Cell Cardiol 53(2):196–205 doi: 10.1016/j.yjmcc.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Roberts JN, May KJ, Veiga-Lopez A (2017) Time-dependent changes in pregnancy-associated glycoproteins and progesterone in commercial crossbred sheep. Theriogenology 89:271–279 doi: 10.1016/j.theriogenology.2016.10.029 [DOI] [PubMed] [Google Scholar]
- Romani F, Tropea A, Scarinci E, et al. (2013) Endocrine disruptors and human corpus luteum: in vitro effects of phenols on luteal cells function. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 31(2):170–80 doi: 10.1080/10590501.2013.782180 [DOI] [PubMed] [Google Scholar]
- Romani F, Tropea A, Scarinci E, et al. (2014) Endocrine disruptors and human reproductive failure: the in vitro effect of phthalates on human luteal cells. Fertil Steril 102(3):831–7 doi: 10.1016/j.fertnstert.2014.05.041 [DOI] [PubMed] [Google Scholar]
- Saitoh M, Yanase T, Morinaga H, et al. (2001) Tributyltin or triphenyltin inhibits aromatase activity in the human granulosa–like tumor cell line KGN. Biochemical and biophysical research communications 289(1):198–204 doi: 10.1006/bbrc.2001.5952 [DOI] [PubMed] [Google Scholar]
- Schoenfelder M, Schams D, Einspanier R (2003) Steroidogenesis during in vitro maturation of bovine cumulus oocyte complexes and possible effects of tri-butyltin on granulosa cells. J Steroid Biochem Mol Biol 84(2–3):291–300 [DOI] [PubMed] [Google Scholar]
- Shue MF, Chen TC, Bellotindos LM, Lu MC (2014) Tributyltin distribution and producing androgenic activity in water, sediment, and fish muscle. J Environ Sci Health B 49(6):432–8 doi: 10.1080/03601234.2014.894780 [DOI] [PubMed] [Google Scholar]
- Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ (1984) Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. The Journal of clinical endocrinology and metabolism 58(2):378–83 doi: 10.1210/jcem-58-2-378 [DOI] [PubMed] [Google Scholar]
- Sousa ACA, Pastorinho MR, Takahashi S, Tanabe S (2014) History on organotin compounds, from snails to humans. Environ Chem Lett 12(1):117–137 doi: 10.1007/s10311-013-0449-8 [DOI] [Google Scholar]
- Stocco DM (2001) StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63:193–213 doi: 10.1146/annurev.physiol.63.1.193 [DOI] [PubMed] [Google Scholar]
- Strand J, Jacobsen JA (2005) Accumulation and trophic transfer of organotins in a marine food web from the Danish coastal waters. The Science of the total environment 350(1–3):72–85 doi: 10.1016/j.scitotenv.2005.02.039 [DOI] [PubMed] [Google Scholar]
- Thibaut R, Porte C (2004) Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J Steroid Biochem Mol Biol 92(5):485–94 doi: 10.1016/j.jsbmb.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Tian Y, Shen W, Lai Z, et al. (2015) Isolation and identification of ovarian theca-interstitial cells and granulose cells of immature female mice. Cell Biol Int 39(5):584–90 doi: 10.1002/cbin.10426 [DOI] [PubMed] [Google Scholar]
- Tsuda T, Inoue T, Kojima M, Aoki S (1995) Daily intakes of tributyltin and triphenyltin compounds from meals. J AOAC Int 78(4):941–3 [PubMed] [Google Scholar]
- Tsunemi A, Ueno T, Fukuda N, et al. (2014) A novel gene regulator, pyrrole-imidazole polyamide targeting ABCA1 gene increases cholesterol efflux from macrophages and plasma HDL concentration. J Mol Med (Berl) 92(5):509–21 doi: 10.1007/s00109-013-1118-x [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Dawson W, Bossdorf O, Fischer M (2014) The more the merrier: Multi-species experiments in ecology. Basic Appl Ecol 15(1):1–9 doi: 10.1016/j.baae.2013.10.006 [DOI] [Google Scholar]
- Veiga-Lopez A, Pu Y, Gingrich J, Padmanabhan V (2018) Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends in endocrinology and metabolism: TEM doi: 10.1016/j.tem.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch KT, Huber JC (2008) Progesterone for recurrent miscarriage: truth and deceptions. Best Pract Res Clin Obstet Gynaecol 22(2):375–89 doi: 10.1016/j.bpobgyn.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Wang H, Pu Y, Luo L, Li Y, Zhang Y, Cao Z (2018) Membrane receptor-independent inhibitory effect of melatonin on androgen production in porcine theca cells. Theriogenology 118:63–71 doi: 10.1016/j.theriogenology.2018.05.042 [DOI] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276(26):23742–7 doi: 10.1074/jbc.M102348200 [DOI] [PubMed] [Google Scholar]
- Wu Q, Sucheta S, Azhar S, Menon KM (2003) Lipoprotein enhancement of ovarian theca- interstitial cell steroidogenesis: relative contribution of scavenger receptor class B (type I) and adenosine 5’-triphosphate- binding cassette (type A1) transporter in high-density lipoprotein-cholesterol transport and androgen synthesis. Endocrinology 144(6):2437–45 doi: 10.1210/en.2002-221110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Antibody list.
IF: immunofluorescence, WB: western blotting.
Supplemental Table 2. Primer list.
Unless specified, all primer sequences are designed against the sheep genome sequence. b: bovine, h: human, m: mouse, p: porcine, ov: ovine.
Supplemental Figure 1. Sequence map for RXRα knockdown. Top: ovine RXRα sequence. Bottom: RXRα shRNA insert.
Supplemental Figure 2. Identification of primary cultured ovine ovarian theca cells. Morphology (A), growth curve (B) of primary cultured ovine theca cells over 8 days. Immunofluorescence stain of theca cell positive marker vimentin (VIM) (C) and fibulin 5 (FBLN5) (D). Dynamic expression pattern of steroidogenic enzymes (ovHSD3B1 and ovHSD17B1) (E) and progesterone production (F) in ovine ovarian theca cells before (growth medium) and after luteinization (luteal medium). * denote differences among treatments (P<0.05). N = 3 cultured cell lines per group. ND means not detectable.
Supplemental Figure 3. Cytotoxicity effects of TBT (A) and RXR antagonist, UVI3003 (B) on primary ovine ovarian theca cells upon three days exposure using an MTT assay. Asterisks denote differences among treatments (P<0.05). N = 3 cultured cell lines per group.
Supplemental Figure 4. Effect of TBT exposure on mRNA expression in pre-luteinized and luteinized ovine primary theca cells. mRNA expression (mean ± SEM) of nuclear receptors (LXRα, LXRβ, RXRα, RXRβ) in primary ovine pre-luteinized (A) and luteinized (B) ovine primary theca cells exposed to 1 ng/ml TBT (T1; gray bars), 10 ng/ml TBT (T10; closed bars) or vehicle (C; control group; open bars). * denote differences among treatments (P < 0.05). N=3 primary cultured cell lines per group.
Supplemental Figure 5. Effect of TBT (0 and 10 ng/ml) and/or RXR antagonist (UVI3003; 0.5, 2, 4 and 8 pM) exposure on mRNA (ovHSL, ovSREBFl, ovLXRα, ovLXRβand ovRXRα) expression (mean ± SEM) in pre-luteinized ovine primary theca cells. Asterisks denote differences among treatments (P<0.05). N=3 cultured cell lines per group. U: UVI3003 (μM). T: TBT.