Abstract
The etiology of endemic rickets was discovered a century ago. Vitamin D is the precursor of 25-hydroxyvitamin D and other metabolites, including 1,25(OH)2D, the ligand for the vitamin D receptor (VDR). The effects of the vitamin D endocrine system on bone and its growth plate are primarily indirect and mediated by its effect on intestinal calcium transport and serum calcium and phosphate homeostasis. Rickets and osteomalacia can be prevented by daily supplements of 400 IU of vitamin D. Vitamin D deficiency (serum 25-hydroxyvitamin D <50 nmol/L) accelerates bone turnover, bone loss, and osteoporotic fractures. These risks can be reduced by 800 IU of vitamin D together with an appropriate calcium intake, given to institutionalized or vitamin D–deficient elderly subjects. VDR and vitamin D metabolic enzymes are widely expressed. Numerous genetic, molecular, cellular, and animal studies strongly suggest that vitamin D signaling has many extraskeletal effects. These include regulation of cell proliferation, immune and muscle function, skin differentiation, and reproduction, as well as vascular and metabolic properties. From observational studies in human subjects, poor vitamin D status is associated with nearly all diseases predicted by these extraskeletal actions. Results of randomized controlled trials and Mendelian randomization studies are supportive of vitamin D supplementation in reducing the incidence of some diseases, but, globally, conclusions are mixed. These findings point to a need for continued ongoing and future basic and clinical studies to better define whether vitamin D status can be optimized to improve many aspects of human health. Vitamin D deficiency enhances the risk of osteoporotic fractures and is associated with many diseases. We review what is established and what is plausible regarding the health effects of vitamin D.
Essential Points.
Vitamin D prevents and cures nutritional rickets but implementation of an adequate prevention strategy is still problematic in many countries or for subgroups of the world population
The near universal distribution of the vitamin D receptor and vitamin D metabolizing enzymes CYP24A1 and CYP27B1, along with the large number of genes under direct control of 1,25(OH)2D, all argue for a wide diversity of actions of the vitamin D endocrine system
Most animal data are in line with human data on the role of vitamin D in helping to regulate calcium and bone metabolism
Many observational studies link a poor vitamin D status to major human diseases
About 38 Mendelian randomization studies have addressed, in the past several years, a link between genetically lower 25-hydroxyvitamin D concentration and skeletal or extraskeletal health outcomes; the best documented link so far is found for multiple sclerosis
Intervention studies for extraskeletal health effects are so far inconclusive, but the results of several ongoing randomized clinical trials may help to delineate these effects more clearly
A causal role of vitamin D for bone health is well established, as vitamin D deficiency is the cause of most cases of rickets and osteomalacia. Vitamin D also plays a major role in the pathogenesis of renal osteodystrophy, and its deficiency can accelerate bone loss and osteoporosis of the elderly. Preclinical data suggest that severe vitamin D deficiency may have extraskeletal effects. Many observational studies also link a poor vitamin D status to a wide variety of extraskeletal diseases. However, cause and effect have not been confirmed, and therefore the optimal vitamin D intake or optimal levels of the major circulating metabolite 25-hydroxyvitamin D (25OHD) to achieve clinically detectable nonskeletal effects are not known.
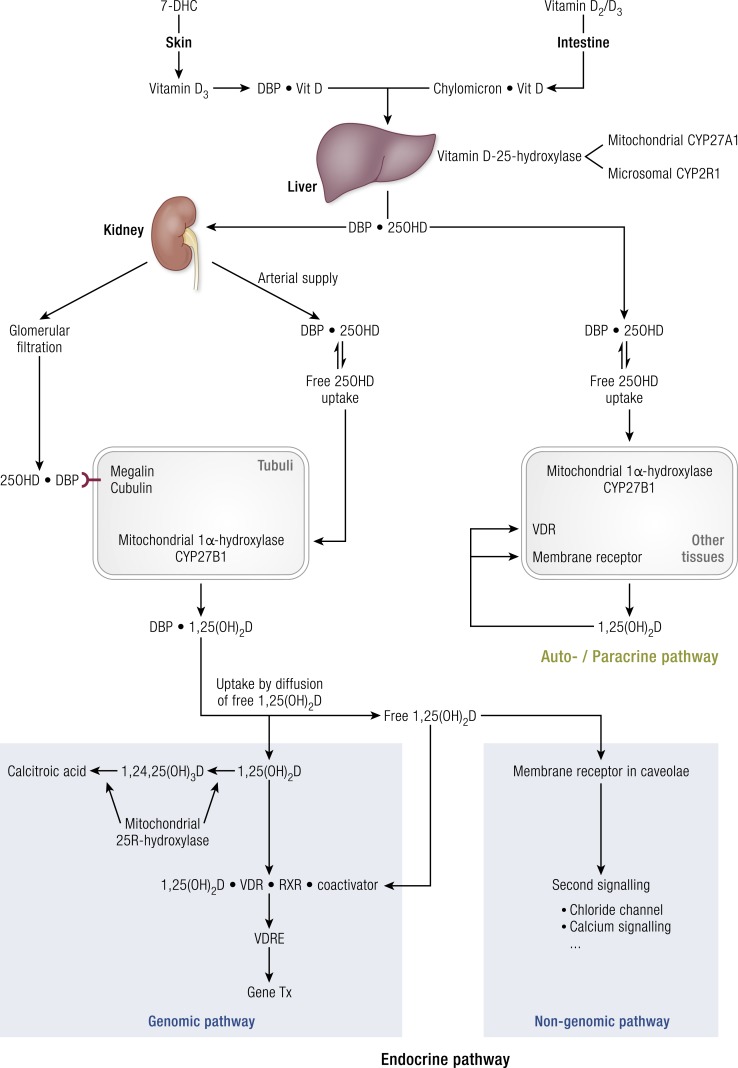
The origin, transport, metabolism, and action of vitamin D have many similarities with those of iodide/thyroid hormones. Therefore, we compare both endocrine systems in Table 1. Vitamin D is either of dietary origin or can be synthesized in the skin under the influence of UV-B light. The dietary intake of vitamin D (mostly D3 and minimal amounts of D2) is usually low. Similarly, iodide intake is variable and depends on geography and dietary habits. For both systems, a complex system allows first the storage of precursor molecules in tissues (Table 1), followed by secretion of a prohormone in the blood where it is bound to specific transport proteins. Thereafter, the hormone precursors (25OHD and T4, respectively) can be activated into the hormone [1α,25-dihydroxyvitamin D (1,25(OH)D) and T3, respectively] or inactived into other metabolites [initially mainly 24R,25(OH)2D and reverse T3, respectively, but followed later by many other metabolites]. The active hormones have a high affinity for their respective nuclear receptors [vitamin D receptor (VDR) and thyroid hormone receptor] but much lower affinity for their serum transport proteins, thereby favoring the selective nuclear uptake of the hormones whereas the precursor preferentially remains in the bloodstream. The essential aspects of vitamin D metabolism and action are presented in Fig. 1. 25OHD can enter the renal tubuli as free [not bound to the vitamin D–binding protein (DBP) or not bound to proteins in general] via the bloodstream or as a complex with DBP by uptake mediated by megalin/cubulin after filtration in the glomerulus. Most other cells only have access to free 25OHD except maybe for a few cells with low expression of megalin (placenta and parathyroid cells). Therefore, free 25OHD and the extrarenal expression of CYP2B1 define the local production of 1,25(OH)2D outside the kidney. Only kidney-produced 1,25(OH)2D can be exported to the bloodstream. Free 1,25(OH)2D then can gain access to the target tissues, activate the VDR, and thereby regulate gene transcription. A very large number of genes (∼3% of the human genome) are under the direct or indirect control of the active hormones, suggesting a broad spectrum of activities (Table 1). 1,25(OH)2D may also activate nongenomic pathways either by binding to the VDR or another receptor (1) located in lipid rafts in caveolae, thereby transiently regulating ion channel activity (especially chloride and calcium channels), kinases, and phosphatases (2). The biological consequences of VDR activation are well understood and described in greater detail in this review. The biological implications of nongenomic actions for tissue or whole-body physiology are poorly understood. We mention two examples. First, the in vivo administration of a bolus of 1,25(OH)2D can rapidly and transiently increase intestinal calcium absorption in chicks. Second, the absence of FAM57B2, a membrane receptor for 24R,25(OH)2D, results in a transient delay in fracture healing, similar to the same phenotype as found in cyp24a1-deficient mice (3). We will survey the well-documented and potential benefits of vitamin D and the risks associated with vitamin D deficiency with the goal of identifying a vitamin D status that is effective and safe in protecting health globally. First, we summarize data related to metabolic bone diseases and thereafter summarize the present state of the art regarding the possible extraskeletal actions of the vitamin D endocrine system. Throughout this review, preclinical data are briefly summarized, whereas human data are discussed in somewhat greater detail. Observational studies are briefly summarized whereas we focus more on Mendelian randomization (MR) trials and randomized controlled trials (RCTs) and their meta-analyses. Indeed, apart from an ongoing discussion about the relative value of observational vs RCTs (4, 5), most governmental authorities consider RCTs the most convincing way to demonstrate the role of vitamin D in health. There is increasing attention to the use of MR studies to evaluate the lifelong consequences of genetically predisposed lower or higher serum 25OHD concentrations and health outcomes (6).
Table 1.
Comparison Between the Vitamin D and Thyroid Endocrine Systems
| Vitamin D Endocrine System | Thyroid Endocrine System | Comment | |
|---|---|---|---|
| Substrate | Dietary vitamin D3/D2 or 7DHC and UV-B light | Iodide | • For both systems the availability of the substrate is irregular, as most food items contain little substrate (D3/D2 or iodide) |
| • UV-B light for vitamin D synthesis is also dependent on geographic, seasonal, climatic, and cultural factors | |||
| • Around the world the total supply of both substrates varies from very low to very high | |||
| Storage of inactive precursor | Vitamin D in fat, liver, muscle | Tg in thyroid colloid | • Storage of vitamin D in different tissues and its dynamics back to the plasma pool are poorly understood |
| • Active accumulation of iodide in Tg allows for storage of iodide for several weeks or months of thyroid hormone synthesis | |||
| First metabolic activation into plasma prohormone | Conversion of vitamin D into 25OHD by CYP2R1 and other CYPs | Digestion of Tg into T4 | • Synthesis of 25OHD is poorly regulated except by the dynamics of CYP2R1 and other CYPs with little or no feedback regulatory control |
| • Conversion of Tg into T4 is regulated by endogenous feedback systems (TRH–TSH–TSH receptor) | |||
| • Both mechanisms create a large plasma pool of prohormone with long half-life (2 wk for 25OHD and 1 wk for T4) | |||
| Second metabolic activation/inactivation | 25OHD can be activated by CYP27B1 into 1,25(OH)2D or inactivated into 24R,25(OH)2D by CYP24A1 | Tg or T4 activation into T3 or inactivation into reverse T3 | • Activation or inactivation is strongly controlled by hormones |
| • For vitamin D, PTH and FGF23 are the main regulators | |||
| • TSH is the main regulator for thyroid hormone metabolism | |||
| Tissues involved in activation/inactivation | Kidney is the major regulator for synthesis of 1,25(OH)2D for export to plasma; many other tissues can produce this hormone for local autocrine/paracrine action | Thyroid gland and muscle are major tissues of synthesis of T3 for export to plasma pool; most tissues have local deiodinases for activation/inactivation of T4 | |
| Control of metabolism | PTH and FGF23 are major regulators for metabolism in kidney; several cytokines regulate synthesis in other tissues | TSH is major regulator of metabolism in thyroid gland; other hormones and cytokines regulate iodinase activity in other tissues | |
| Hormone action | |||
| Binding to NR as ligand of NR | 1,25(OH)2D binding to VDR | T3 binding to TR | • Both hormones bind with high affinity to their respective receptors whereas the circulating hormone precursors have a much lower affinity for the receptors |
| • VDR and TR are both members of the large family of nuclear receptors and use the same heterodimer partner (RXR) and coactivators or repressors, and they bind to similar hexanucleotide sequences in DNA (direct repeat hormone-responsive elements) separated by three or four nucleotides, respectively | |||
| Genomic action | Gene regulation | Gene regulation | • Both hormones regulate a very large number of genes (>1% of the human genome), suggesting a broad spectrum of activities, using a complex gene transcription mechanism similar to ligands of other NRs |
| Nongenomic action | Second signaling pathways | Second signaling pathways | • As for other hormones, nongenomic actions are operational but their clinical implications are incompletely understood |
The origin of the substrate is irregular for most subjects. Therefore, evolution created a tissue storage of inactive precursors and a large circulating pool as a prohormone (25OHD and T4, respectively) with a long half-life. Thereafter, the prohormone can either be activated or inactived either for systemic transport or for local autocrine/paracrine actions. Both systems use a gene transcription regulatory mechanism based on strongly related nuclear receptors and use the same heterodimer partner (RCR) and similar hormone response elements to regulate a very large number of genes.
Abbreviations: 7DHC: 7-dehydrocholesterol; FGF23, fibroblast growth factor 23; NR, nuclear receptor; RXR, retinoic acid receptor; T3, triiodothyronine; T4, thyroxine; Tg, thyroglobulin; TR, thyroid hormone receptor.
Figure 1.
Metabolism and action of vitamin D and its metabolites, with special focus on renal and extrarenal production of 1,25(OH)2D and the genomic or nongenomic pathways of vitamin D action.
Indeed, serum 25OHD concentrations are under genetic control, and several large-scale genome-wide association studies (GWASs) have consistently identified a number of single nucleotide polymorphisms (SNPs) around genes involved in vitamin D synthesis, metabolism, and transport, which alone or in combination modify serum 25OHD concentrations (7, 8). Such data allow identifying subjects with genetically predisposed serum 25OHD significantly lower or higher than the mean of the population. Therefore, large-scale genetic studies can define whether such genetically predisposed lower serum 25OHD concentrations are linked to specific health outcomes, thereby avoiding the problem of reverse causation, residual confounding, or limited duration of observation or interventions. MR studies dealing with vitamin D status, however, are limited by the relatively low predicted differences in serum 25OHD (usually <5% of total variation). Additionally, such studies evaluate only linear correlations and cannot detect effects above or below a certain threshold (6).
Finally, we present the major outstanding research questions. For the purpose of this review, we define vitamin deficiency by serum 25OHD <50 nmol/L (or 20 ng/mL) and severe vitamin D deficiency by values <30 nmol/L (or 12 ng/mL). A more extensive analysis of the assay methodology to estimate the vitamin D status (9) and the definition of vitamin D status (10) is discussed in other recent studies (11).
Vitamin D Is Essential for Skeletal Health
Preclinical data
Growth plate
The abnormal structure of the growth plates is one of the major clinical, radiological, and histological hallmarks of rickets observed in humans and mice with vitamin D deficiency or inactivation of the VDR or 1α-hydroxylase (CYP27B1) (12–14). This phenotypic characteristic, however, does not result from direct VDR actions in growth plate chondrocytes but from the hypophosphatemia that decreases the cell death of hypertrophic chondrocytes (15, 16) and from hypocalcemia blocking chondrocyte differentiation. The growth plate structure, along with secondary hyperparathyroidism and hypophosphatemia, can be normalized by a very high oral calcium supply or by intravenous calcium administration, as demonstrated in humans and experimental animal models (17). Indeed, chondrocyte-specific deletion of Vdr or Cyp27b1 in mice does not generate a rachitic growth plate (18, 19). Alternatively, local production of hormonal 1,25(OH)2D (or calcitriol) or Vdr action in chondrocytes has temporary paracrine and endocrine actions. Mouse genetic studies show that the absence of vitamin D signaling specifically in growth plate chondrocytes generates a transient increase in bone mass related to decreased production of pro-osteoclastogenic factors by mutant chondrocytes. Additionally, serum phosphate levels are transiently increased, as FGF23 production by osteocytes and osteoblasts is decreased (18, 19).
Bone and bone cells
In conditions with defective systemic vitamin D signaling, the effects of vitamin D on bone are largely indirect and caused by a negative calcium balance resulting from reduced vitamin D action in the intestinal mucosa. This conclusion is based on several observations. First, the osteomalacia of mice with systemic inactivation of Vdr signaling can be rescued by a diet containing high calcium and lactose, similar to the restoration of the growth plate phenotype (17, 20). More precisely, the rescue in Vdr-null mice was complete, but not in Cyp27b1 mice (12, 21), and an explanation for this different response may be found in ligand-independent effects of the Vdr or differences in genetic or housing protocols. Second, mice with global Vdr inactivation and selective reintroduction of the Vdr in either the whole intestine (22) or only of the distal part of the intestine do not develop a skeletal phenotype (23). Finally, selective deletion of the Vdr in the intestine generates severe osteomalacia and decreased bone mass (24).
These data clearly indicate that the intestine is the primary target tissue for vitamin D action in calcium homeostasis and show that intestinal vitamin D signaling is necessary for adequate active transcellular and possible paracellular calcium transport (12, 20).
During a negative calcium balance, but with a normal active vitamin D system in mature osteoblasts and osteocytes, vitamin D signaling in bone cells has a role in maintaining serum calcium homeostasis by increasing bone resorption and impairing bone mineralization (24). These findings demonstrate that under these conditions, the vitamin D endocrine system primarily defends a normal serum calcium homeostasis, if needed, at the expense of bone.
During a normal calcium balance, implying normal intestinal calcium absorption, the absence of the Vdr in osteoblasts, osteocytes, or osteoclasts does not phenocopy rickets and osteomalacia, indicating a rather redundant role of the vitamin D endocrine system in bone cells (24–26). Nevertheless, mouse genetic studies have shown that both the absence and overexpression of the Vdr in osteoblasts increase bone mass modestly, in part by decreasing the expression of pro-osteoclastogenic factors (26, 27), but the exact mechanisms are incompletely understood. Moreover, the presence of the Vdr in osteoblasts mediates the bone anabolic action (in mice) of some vitamin D analogs (25). Finally, the roles of local production of 1,25(OH)2D in bone cells and its autocrine and paracrine effects have been suggested by in vitro data, but they need to be confirmed by in vivo experiments.
Human data
Pathophysiology
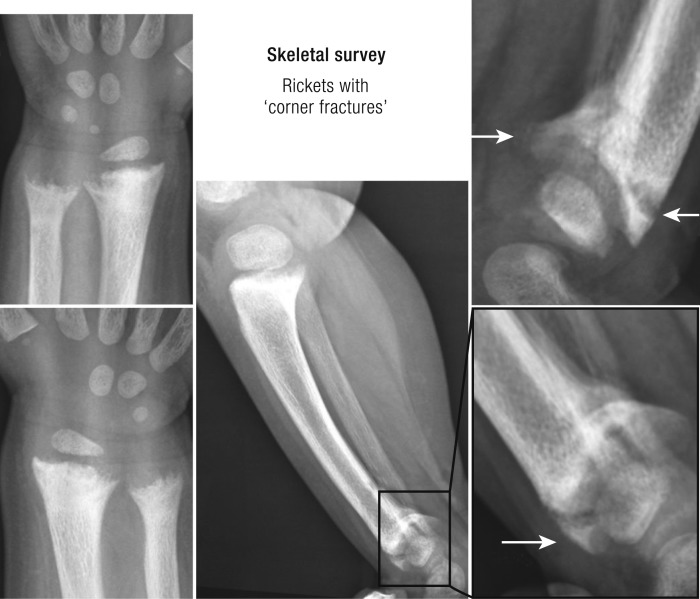
Severe vitamin D deficiency (30 nmol/L) in infants or children can cause rickets (28–30) (Fig. 2). The vitamin D endocrine system plays a limited role in prenatal life but becomes active shortly after birth. Because fetal serum 25OHD reflects that of the mother, maternal vitamin D deficiency increases the risk of nutritional rickets and hypocalcemia in the first months of life. The vitamin D content of mother milk or nonfortified cow milk is low, and exposure of infants or small children to direct sunlight may be dangerous. The incidence of rickets in Western populations is low owing to the widespread vitamin D supplementation of 400 IU/d in most Western countries for many decades. When this is not done, for example, in the case of a macrobiotic diet or in children of non-Western immigrants, the incidence of rickets increases sharply (31, 32). Low adherence rates to vitamin D supplementation in the very young in the United Kingdom have been leading to a resurgence of rickets and even casualties due to cardiomyopathy. Part of this is due to an increase of the nonwhite population, rising from 5.9% in 1991 to 14% in 2011 (33, 34). Systematic daily supply of 400 IU of vitamin D can prevent nutritional rickets, as was clearly demonstrated in Turkey where the incidence of rickets was reduced from 6% to 0.1% (35). However, rickets can still develop independent of vitamin D status with an intake of calcium of <250/300 mg daily (up to 12 months and after 12 months of age, respectively) (28, 36). Nutritional rickets is a risk factor for fractures in children and adolescents, and it can also have major consequences for tooth development (37). Despite well-established consensus guidelines on how to eliminate nutritional rickets, many infants and children are still at risk for this disease owing to the lack of implementation of simple interventions (28, 30).
Figure 2.
Radiologic image of nutritional rickets. A radiologic image of a 19-mo-old child with nutritional rickets is shown. The child was born from Indian parents, living in Australia, after a normal pregnancy of 40 wk and received exclusive breastfeeding for 18 mo without vitamin D supplementation. Height and weight were around the 50th percentile. Medical attention was asked because of genu varum and delayed walking. Serum calcium (2.01 mmol/L; 2.10 to 2.65) and phosphate were slightly decreased. Serum 25OHD was <18 nmol/L and alkaline phosphatase and PTH (126 pmol/L; 1.0 to 7.0) levels were high.
The skeletal effects of moderate vitamin D deficiency in adults or elderly subjects are mainly caused by an increase of the serum PTH concentration, leading to high bone turnover and associated cortical bone loss (38). In the MORE study, the groups with vitamin D deficiency (serum 25OHD <25 nmol/L, n = 297 and 25OHD 25 to 50 nmol/L, n = 1721) both show significantly higher serum PTH (4.8 ± 2.2 and 4.1 ± 1.8 pmol/L, respectively) compared with people with 25OHD >50 nmol/L (n = 4982, serum PTH 3.5 pmol/L). Both groups show a significant decrease of serum PTH after treatment with vitamin D (17% and 12% lower serum PTH, P < 0.001), suggesting that serum PTH was elevated on an individual level in most patients (39). Very similar observations were made in the bazedoxifene trial in >7000 participants (40). This study shows thresholds for PTH and bone mineral density (BMD) at serum 25OHD of 50 or 75 nmol/L, respectively. The Longitudinal Aging Study Amsterdam (LASA) confirms these thresholds for PTH and BMD (41). Additionally, vitamin D deficiency may increase the risk of falls (as discussed below). Severe vitamin D deficiency may cause mineralization defects in some cases. An increase of osteoid volume (>5%) was observed in 10% of hip fracture patients having a serum 25OHD <30 nmol/L (42), and in a very large postmortem series, an osteoid volume >5% was observed in 4.8% of cases (43). This study caused serious discussion because of uncertainties about the accuracy of blood 25OHD measurement in postmortem samples and the use of histological criteria for osteomalacia, which do not correspond to the standard criteria (38, 44).
Epidemiologic studies
In the National Health and Nutrition Examination Survey (NHANES), an association between BMD and serum 25OHD was observed. BMD of the hip increased 0.06 g/cm2 in whites between 20 and 50 years of age when serum 25OHD increased from 20 to 90 nmol/L (45). In older persons, the BMD increase was somewhat less. In the LASA study, BMD of the hip increased 0.06 g/cm2 when serum 25OHD increased from 20 to 50 nmol/L (41, 45). A similar relationship was found in the bazedoxifene study (41). The LASA study also found an association between vitamin D deficiency and fractures. Serum 25OHD levels below or equal to 30 nmol/L were associated with an increased fracture risk [hazard ratio (HR), 3.1] in persons aged 65 to 75 years (4). Similarly, increased fracture risk was observed in subjects with the lowest vitamin D status in several long-term follow-up studies (46). Two recent RCTs confirmed a positive effect of vitamin D supplementation on BMD in subjects with a baseline serum 25OHD <30 nmol/L (47, 48).
MR studies
Two MR studies have evaluated the impact of genetically low serum 25OHD concentrations, as predicted by polymorphisms in four genes (DHCR7 and CYP2R1 involved in synthesis of vitamin D metabolites, and GC/DBP and CYP24R1 involved in transport and metabolism of vitamin D) (7, 10). A small-scale [Table 2 (49–86)] Chinese study of 1824 postmenopausal women did not find an effect of genetically predicted lower serum 25OHD on BMD at lumbar spine or femoral neck (75). A much larger study in subjects of European descent similarly did not find an effect of the same polymorphism in four vitamin D–related genes on either BMD or ultrasound characteristics of bone (76).
Table 2.
MR Studies: Vitamin D Status and Clinical Endpoints
| Disease | Population | Polymorphism | Results |
|---|---|---|---|
| → Cancer | |||
| Chandler et al. (49) | European women | 5 SNPs | All cancers: HR, 1.01 (NS) |
| N = 23,293 | Breast: HR, 1.02 (NS) | ||
| Subgroup with validation SNPs–25OHD | Colon: HR, 1.06 (NS) | ||
| Lung: HR, 1.00 (NS) | |||
| Cancer deaths: HR, 1.00 (NS) | |||
| 11 nmol/L difference highest/lowest NSP score | |||
| Dimitrakopoulo et al. (50) | Genetic networks | 4 SNPs | OR for predicted, 25 nmol/L |
| GAME-ON Consortium | Difference | ||
| GECCO Consortium | Colorectal cancer: 0.92 (NS) | ||
| PRACTICAL Consortium | Breast cancer: 1.05 (NS) | ||
| MR-based platform | Prostate cancer: 0.89 (NS) | ||
| N = 70,563 cancer cases | Lung cancer: 1.03 (NS) | ||
| N = 84,418 controls | |||
| Wang et al. (51) | Women of African diaspora | 2 SNPs vitamin D status SNP for pigmentation (TYRP1) | Cancer: NS |
| 1657 Cases of breast cancer | |||
| 2029 Controls | OR, 1.54 (P < 0.08) | ||
| Total of 3686 participants | |||
| Ong et al. (52) | N = 31,719 whites (10,065 cases vs 21,654 controls) | 3 SNPs | OR per 20 nmol/L |
| Ovarian cancer (all types) | Lower 25OHD: | ||
| High-grade ovarian cancer | OR, 1.27 (1.06–1.51) | ||
| OR, 1.54 (1.19–2.01) | |||
| Trummer et al. (53) | Prostate cancer | 1 SNP | NS |
| Theodoratou et al. (54) | Colorectal cancer | 1 SNP | NS |
| 2001 Cases (Scotland) | |||
| Comments: higher measured serum 25OHD associated with lower colorectal cancer incidence | |||
| Dudding et al. (55) | Oral or oropharyngeal cancer | 5 SNPs | OR, 1.01 (NS) (confirmed in validation cohort of 585 cases from UK Biobank) |
| N = 5133 | |||
| N = 5984 controls (Europe, North and South America, all with >70% European origin) | |||
| Sun et al. (56) | Lung cancer | 3 SNPs | Genes explained 3.4% of 25OHD HR, 0.96 (NS) |
| N = 676 cases | |||
| N = 54,580 controls | |||
| → Neurologic diseases | |||
| Parkinson disease | |||
| Larsson et al. (57) | 5333 Parkinson cases | 4 SNPs | OR for 10% lower predicted |
| 12,019 Controls | Serum 25OHD, 0.98 (NS) | ||
| Alzheimer’s disease | |||
| Mokry et al. (58) | Internal genomics of AD Consortium | 4 SNPs from | 1 SD lower predicted |
| N = 17,008 cases | SUNLIGHT Consortium | 25OHD: OR for AD, | |
| Versus 37,154 controls | (N = 33,996) | 1.25 (P < 0.02) | |
| CaMos; n = 2347 cases | |||
| Taylor et al. (59) | Schizophrenia | NS | |
| MS | |||
| Gianfrancesco et al. (60) | Early onset | 3 SNPs | Higher predicted 25OHD: |
| (Pediatric) MS | OR for MS, 0.72 (0.55–0.94) | ||
| N = 394 United States vs 10,875 controls | |||
| N = 175 Sweden vs 5376 controls | |||
| Mokry et al. (61) | Canada | 4 SNP | 1 SD predicted lower |
| N = 33,996 and 2347 cases? | Log 25OHD: OR for MS, 2 (1.7–2.5) (P = 10−12) | ||
| Rhead et al. (62) | N = 1056 cases vs 9015 United States controls (non-Hispanic whites) | 3 SNPs | OR for highest predicted |
| N = 6335 cases vs 5762 Sw controls | 25OHD, 0.85 (0.76–0.94) (P = 0.003) | ||
| → Diabetes and metabolic syndrome | |||
| Cooper et al. (63) | Type 1 diabetes | 5 SNPs (including CYP27B1) | OR, 1.07 cases/controls (P = 0.007) |
| n = 720 + 8517 cases | OR, 1.10 family study (P = 0.001) | ||
| n = 13,438 controls | |||
| (White Europeans) | |||
| Afzal et al. (64) | 96,423 Danish subjects with/without T2DM | SNPs in DHCR7 and CYP2R1 | 20 nmol/L genetically lower 25OHD |
| OR for T2DM, 1.51 (DHCR7) (P = 0.04) | |||
| OR for T2DM, 1.02 (CYP2R1) (NS) | |||
| Ye et al. (65) | T2DM | 4 SNPs | NS for T2DM or fasting blood glucose |
| 28,144 cases T2DM | |||
| 76,344 controls | |||
| Comments: lower measured serum 25OHD associated with risk of T2DM | |||
| Vimaleswaran et al. (66) | Obesity | 4 SNPs (vitamin D) | NS link with BMI significantly lower measured 25OHD concentrations when SNPs for higher BMI |
| 21 Cohorts of European origin | 12 SNPs (BMI) | ||
| N = 42,024 | |||
| Comment: genes for higher BMI are associated with lower measured serum 25OHD, but genes for lower 25OHD concentrations are not significantly associated with higher BMI | |||
| Husemoen et al. (67) | Adiponectin as marker of metabolic syndrome | 1 SNP (DBP/GC) | Genetically twofold higher 25OHD associated with 1.6-fold higher serum adiponectin |
| 6405 + 2656 Danish subjects | |||
| Comment: a 1.6-fold higher serum adiponectin level could explain a twofold lower risk for T2DM | |||
| Wang et al. (68) | Nonalcoholic fatty liver disease | 4 SNPs | NS |
| 9182 Subjects from East China | |||
| Comments: | |||
| • No link between eight SNPs predisposing for nonalcoholic fatty liver disease and measured serum 25OHD | |||
| • Link between four SNPs for vitamin D and serum 25OHD concentrations confirmed in Chinese subjects | |||
| → Cardiovascular events | |||
| Manousaki et al. (69) | SUNLIGHT Consortium | 4 SNPs | NS |
| N = 33,996 | |||
| Canadian artery disease | NS | ||
| N = 22,233 cases vs 64,762 controls | |||
| Brøndom-Jacobsen et al. (70) | Denmark | 4 SNPs in two genes | NS |
| N = 92,416 total participants | |||
| N = 14,455 with ischemic heart disease | Comparison: lower measured serum 25OHD Lowest/highest quartile: HR, 1.82 for ischemic heart disease (1.42–2.32) | ||
| N = 7061 with myocardial infarction | |||
| Leong et al. (71) | Cardiovascular and metabolic disease 2254 Canadian subjects Comments: no link between this SNP and fasting blood glucose, insulin, BMI, cardiovascular diseases, and stroke | 1 SNP (DBP/GC) | NS |
| Ooi et al. (72) | Nonfasting remnant cholesterol concentration | 4 SNPs | NS* |
| 85,869 Whites (Denmark) for lipoproteins | Measured nonfasting cholesterol remnants inversely associated with measured serum 25OHD | ||
| 25,862 Whites (Denmark) for 25OHD | Genes for higher cholesterol remnants associated with lower serum 25OHD | ||
| Comments: genes related to higher nonfasting cholesterol remnants are associated with lower serum 25OHD concentrations and thus may partially explain epidemiologic links between lower vitamin D status and cardiovascular risks and diseases and higher prevalence of low-grade inflammation | SNPs in vitamin D–related genes are related to serum 25OHD but only marginally with measured serum HDL | ||
| Vimaleswaran et al. (73) | Hypertension | 2 SNPs (DHCR7 and CYP2R1) | 10% Genetically higher serum 25OHD is associated with 0.3 mm Hg lower diastolic and systolic blood pressure and lower risk for hypertension |
| 142,255 Danish subjects | |||
| Skaaby et al. (74) | Cardiovascular risk factor (lipid profile) | SNPs for fillagrin | Loss of fillagrin mutations result in 10% higher measured serum 25OHD (possibly related to higher UV-B–induced efficacy in vitamin D production) and better lipid profile (higher high-density lipoprotein, lower low-density lipoprotein, and lower very-low-density lipoprotein and triglycerides) |
| 11,983 Subjects of North European origin | |||
| → Bone: BMD | |||
| Li et al. (75) | 1824 Chinese postmenopausal women | 4 SNPs | NS |
| Serum 25OHD (measured) positively associated with BMD of lumbar spine (P = 0.003), femoral neck (P = 0.006), and total hip (P = 0.005) | |||
| Larsson et al. (76) | 2 Cohorts of European descent | 5 SNPs linked to four vitamin D–related genes | NS |
| → Eye: myopic refractory disease | |||
| Cuellar-Partida et al. (77) | CREAM = 33,382 European and 8376 Asian participants | 4 SNPs | Refractory error: 0.01 to −0.02 diopters per 10 nmol/L predicted increase in serum 25OHD (NS) |
| →Immunological events | |||
| Asthma and atopic dermatitis | |||
| Manousaki et al. (78) | SUNLIGHT, GABRIEL, and EAGLE (eczema) Consortia | 4 SNPs | OR for disease per SD |
| Asthma (N = 146,761) | OR, 1.03 (NS) | ||
| Childhood onset asthma (N = 15,008) | OR, 0.95 (NS) | ||
| Atopic dermatitis (N = 40,835) | OR, 1.12 (NS) | ||
| Elevated IgE level (N = 12,835) | Effect size, 0.40 (NS) | ||
| Mao et al. (79) | Asthma GABRIEL database of 10,363 European cases vs 16,110 controls | 4 SNPs | NS |
| Inflammation (C-reactive protein) | |||
| Liefaard et al. (80) | Rotterdam study on 9649 participants Measurement of C-reactive protein as marker of inflammation | 4 SNPs | NS |
| RA | |||
| Viatte et al. (81) | RA outcome 493 + 2924 cases of RA (United Kingdom) | 4 SNPs | NS |
| Comments: study of outcome (signs and symptoms) of RA and not of prevalence or incidence of RA | |||
| → Skin aging | |||
| Noordam et al. (82) | Rotterdam and Leiden studies | ? SNPs | NS |
| N = 3831 and 661 | |||
| Facial skin aging features | |||
| Perceived age, wrinkling, pigmented spots | |||
| Serum measured 25OHD associated with skin aging: higher serum 25OHD, higher skin aging (P > 10−6) | |||
| → Mortality | |||
| Ordóñez-Mena et al. (83) | German older adults (ESTHER) | 4 SNPs | NS |
| N = 8417 | |||
| 2 SNPs associated with lower serum 25OHD | |||
| Lower serum 25OHD associated with higher mortality | |||
| Afzal et al. (84) | 3 Danish cohorts | 4 SNPs in two genes | |
| N = 95,766 | |||
| Follow-up 9–19 y | |||
| Genetically low serum 25OHD: per 20 nmol/L lower serum 25OHD | |||
| All-cause mortality: | 1.3 (1.05–1.61) | ||
| Cardiovascular mortality: | NS | ||
| Cancer mortality: | 0.43 (1.02–1.99) | ||
| Additionally measured serum 25OHD in 35,334 subjects | |||
| Per 20 nmol/L lower measured serum 25OHD | |||
| All-cause mortality: | 1.19 (1.14–1.25) | ||
| Cardiovascular mortality: | 1.18 (1.09-1.28) | ||
| Cancer mortality: | 1.12 (1.03-1.22) | ||
| Trummer et al. (85) | Mortality in 3316 German adults undergoing a coronary angiography (age 63 y) and followed up for 10 y | 4 SNPs | NS |
| → Kidney function | |||
| Teumer et al. (86) | Glomeral filtration rate 16,442 + 5123 objects of European ancestry | 3 SNPs | Negative effects of higher 25OHD on estimated glomerular filtration rate (P = 0.003) |
| → Conclusions | 1. Genetically low 25OHD associated with all-cause and cancer mortality | ||
| 2. Observational low 25OHD associated with all-cause, cardiovascular, and cancer mortality | |||
Overview of MR studies dealing with polymorphism in genes related to vitamin D synthesis, transport, or metabolism and serum 25OHD vs different biological endpoints or diseases. Unless mentioned otherwise, SNPs mentioned in this table refer to polymorphism in genes for 7-dehydrocholesterol-reductase (DHCR7), CYP2R1 or 25-hydroxylase, DBP/GC, the major serum transport protein for all vitamin D metabolites, and CYP24A1, the major catabolizing enzyme for 25OHD and 1,25(OH)2D.
Abbreviations: NS, not significant; T2DM, type 2 diabetes mellitus.
RCTs
Many RCTs have been performed with vitamin D, usually combined with calcium, on BMD and fractures as outcome criteria (87). The effects on BMD are best visible at the femoral neck, +0.8% on average (range, 0.2% to 1.4%) according to a recent meta-analysis (88). However, this meta-analysis did not include the Lyon clinical trial (89). In this trial in a very vitamin D–deficient population, the difference in total hip BMD between vitamin D and the control group was 7.3%.
In the large VIDA study of adults in New Zealand (mean age, 69 years; mean baseline serum 25OHD, 56 nmol/L) (48), a modest increase in BMD at the femoral neck (+0.5%) was observed overall in the group treated with 100,000 IU of vitamin D3 per month for 2 years. In the subgroup with a baseline serum 25OHD <30 nmol/L, BMD remained stable during 2 years in vitamin D–supplemented subjects, whereas a 2% decrease was observed in the control group. The effect of vitamin D on fracture incidence was studied in at least 19 RCTs. In these trials, vitamin D was given with different intervals, from daily to once per year. In five trials, vitamin D was given alone. In two of these, a significant decrease of fracture incidence was observed, either with annual injection or with a 4-monthly oral dose (90, 91). The three other trials with vitamin D alone were negative (92–94). In 13 RCTs, vitamin D and calcium were combined. The greatest effect was observed in the first trial in a very deficient French nursing home population (mean age, 84 years) treated with vitamin D3 (800 IU/d) and calcium (1200 mg/d) vs double placebo. In this trial, a considerable and significant decrease of hip fracture incidence (−20%) and other fracture incidence (−25%) was observed (89), as well as an increase of BMD at the hip of 6% (see above). In two other trials the combination of vitamin D and calcium showed a significant decrease of fracture incidence (95, 96). In two other trials the combined therapy showed a borderline effect, the first in a similar French nursing home population (P = 0.07 for nonvertebral fractures) (97), and the second from the Women’s Health Initiative (98) (hip fractures, intention-to-treat: HR, 0.88; 95% CI, 0.72 to 1.08; per protocol analysis in adherent subjects: HR, 0.71; 95% CI, 0.52 to 0.97]. Because of the coadministration of vitamin D and calcium, it is not possible to define the relative contribution of vitamin D and calcium supplementation. Six trials did not show a significant effect of vitamin D and calcium on fracture incidence (91, 99–101). Two RCTs, however, showed an increase in fracture incidence, both employing a very high single dose (300,000 and 500,000 IU) once per year (102, 103). One of these also showed an increased risk of falls (102). In the most recent VIDA study (104), no effect of vitamin D supplementation on fractures or falls risk was observed during a 3.4-year follow-up of New Zealand adults (mean age, 69 years) with a baseline serum 25OHD of 63 nmol/L. Whether this null result was due to the good vitamin D status at baseline, the use of monotherapy with vitamin D without calcium supplementation, or the high intermittent dose of 100,000 IU/mo is unclear. Intermittent high-dose vitamin D may paradoxically and transiently increase the fracture risk (and falls; see below) (102, 103).
Meta-analyses
Many meta-analyses have evaluated the effect of vitamin D on BMD and fracture incidence (88, 105–109). The outcomes of meta-analyses vary greatly (108). In general, the meta-analyses show that the effect of vitamin D is greater when (1) given to older (70 to 80 yrs or ≥80 years) than to younger subjects (60 to 70 years), (2) given to those in a residential care setting than to independently living elderly (109), or (3) the daily dose is at least 800 IU or when baseline serum 25OHD is low. The Cochrane systematic review and meta-analysis stated that vitamin D alone is unlikely to be effective in preventing hip fracture or any new fracture. However, it also showed that combined vitamin D and calcium supplementation induces a 16% decrease in hip fracture risk, a 14% decrease in new nonvertebral fracture risk, and a 5% decrease in risk for any fracture (105). To avoid one hip fracture, 1000 older persons must be treated for 1 year. Treatment is much more efficacious in high-risk persons, such as the institutionalized, where ∼110 persons must be treated for 1 year to save one hip fracture. This number decreases further when other nonvertebral fractures are included. Side effects of calcium and vitamin D include hypercalcemia (rare) and renal stones. Mortality decreases by 6%, but this was not significant (105). One meta-analysis concluded that the effect was trivial as the decrease in fracture incidence was not >15% (110). However, it may be argued that a 10% to 15% decrease of fracture incidence is considerable, as this therapy can be implemented in a very large number of subjects at risk, at very low cost and with limited side effects.
Research agenda
Despite major progress during the last decades in understanding the role of vitamin D and its metabolites on calcium and bone homeostasis, many important questions remain incompletely answered. There is at least one missing player in our understanding of the role and mechanism of action of the vitamin D system on transepithelial calcium transport in the intestine. The precise role of the vitamin D endocrine system in other calcium-transporting or calcium-sensing systems, such as kidney, placenta, breast, and parathyroid glands, is also still incomplete. The role of 1,25(OH)2D production in calcium-transporting or calcium-sensing tissues and its autocrine/paracrine effects should also be defined. Additionally, the precise contribution of the vitamin D system on overall phosphate homeostasis is incompletely understood. A better understanding of the risk factors for the development of nutritional rickets beyond vitamin D deficiency is required, for example, dietary calcium intake, iron deficiency, and genotype. The effect of vitamin D status on skeletal development during fetal life, childhood, and adolescence requires greater clarification. Most RCTs used daily doses of vitamin D between 400 and 1200 IU. The effect of higher doses is not well known. The effect of vitamin D metabolites or analogs on bone structure, turnover, and fracture incidence is not clear. Most trials did not select participants with a low serum 25OHD level. Forthcoming trials should select participants based on low baseline serum 25OHD. Individual participant data meta-analyses should be performed selecting vitamin D–deficient subjects only. It may be worthwhile to model the effects of vitamin D supplementation according to age, sex, residence, baseline serum 25OHD, vitamin D dose, and the addition of calcium supplements. Additionally, specific risk groups should be defined for vitamin D supplementation to prevent fractures. To improve the efficacy of vitamin D, the treatment should be targeted to the most vulnerable groups, with the institutionalized group ranking highest (89, 105, 108).
Conclusions
Vitamin D deficiency increases serum PTH, but most vitamin D–deficient subjects do not have PTH concentrations above the normal range. This results in progressive bone loss and, when severe, also mineralization defects. Epidemiologic studies show that vitamin D deficiency is associated with lower BMD and fractures. These consequences can be avoided by modest doses of vitamin D and calcium supplements (28, 35). RCTs have shown that vitamin D decreases the incidence of hip fractures and other nonvertebral fractures by ∼15%, with the effect being greater in the 80+ years of age and 70 to 80 years of age persons than in persons aged 60 to 70 years, in the institutionalized group than in community living elderly, when combined with calcium and when compliance is >80%. Vitamin D supplementation should be advised in all institutionalized and frail older persons. There is great unanimity that serum 25OHD concentrations <30 nmol/L should be corrected. Serum 25OHD levels <50 nmol/L should be avoided. For subjects with limited exposure to sunlight this requires a daily vitamin D intake of ∼800 IU/d. This advice is generally in line with most governmental guidelines (111), except for the UK Scientific Advisory Committee on Nutrition (29) who recommended serum 25OHD concentrations >30 nmol/L and a vitamin D intake of 400 IU/d for all subjects of whatever age at risk for vitamin D deficiency. This conclusion also does not contradict the conclusion of the United States Preventive Services Task Force dealing with a younger community dwelling population (mostly postmenopausal women) with a much better vitamin D status than the elderly or institutionalized subjects (112).
Extraskeletal Actions of Vitamin D
The potential extraskeletal actions of vitamin D have generated considerable excitement during the last couple of decades, with a rapidly expanding number of studies using cell-based experiments and preclinical models of diseases. These studies were spurred in part by observations that both the VDR and CYP27B1 are present in a large number of cells and tissues not related to the classical target tissues for vitamin D (113). Additionally, many observations from gene expression profiling studies indicate that 1,25(OH)2D regulates the expression of numerous genes (from zebrafish to mice and humans) unrelated to calcium homeostasis (114). It is fair to say that the position of the Institute of Medicine committee, as well as nearly all official guidelines (111), of not considering a potential role for vitamin D extraskeletal health (115) was greeted with some consternation among enthusiasts for its “nonclassical” actions. Differences in opinion have led to extensive and lifely debates in journals and handbooks (10, 116). Therefore, we critically assess the accumulating preclinical and clinical evidence for a role of vitamin D signaling in physiological systems independent of calcium homeostasis.
Skin as origin and target of vitamin D
Preclinical data
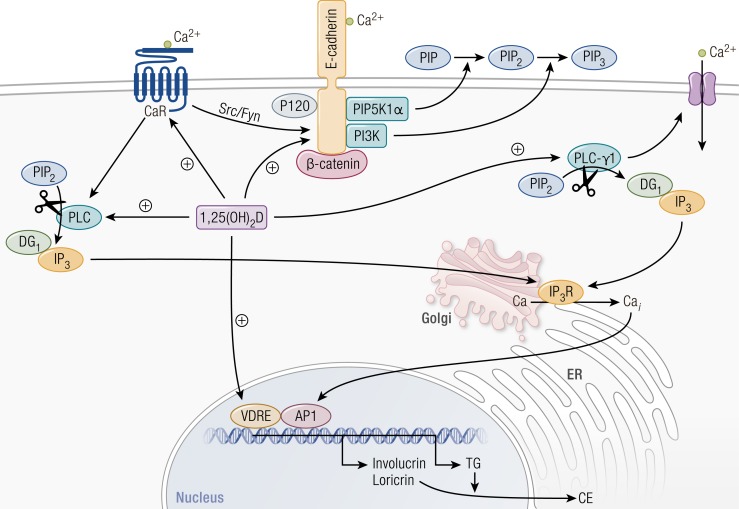
Vitamin D is produced mainly in the epidermis, where 7-dehydrocholesterol is converted to previtamin D3 under influence of UV-B and subsequently isomerized to vitamin D3. Vitamin D plays important intracrine, autocrine, and paracrine actions in the epidermis (Fig. 3) (117). Indeed, the dominant cells of the skin, the keratinocytes, are able to produce the active hormone 1,25(OH)2D, by their own 25-hydroxylase (CYP27A1) (118) and 1α-hydroxylase (CYP27B1) enzymes (119). Moreover, keratinocytes express the VDR, which is most abundant in the stratum basale and in the stem cells of the hair follicle (120). The active vitamin D locally produced does not appear to contribute to the circulating levels under normal circumstances, but it is involved in epidermal differentiation and proliferation, wound response, and tumorigenesis, acting on keratinocytes and their neighboring cells.
Figure 3.
Regulation of keratinocyte differentiation by calcium and 1,25(OH)2D. Calcium and 1,25(OH)2D interact to regulate keratinocyte differentiation at multiple steps. 1,25(OH)2D acts via its nuclear hormone receptor, VDR, to directly regulate gene transcription. Among the genes that it regulates are involucrin and loricrin, which encode major constituents of the cornified envelope (CE) as well as transglutaminase (TG) that crosslinks these proteins and others to form the CE. Although the effects of calcium on gene transcription do not appear to be direct, calcium is likely to act at least in part through protein kinase C, which phosphorylates and so activates transcription factors of the AP-1 family critical for the induction of these genes. Not shown is that 1,25(OH)2D also induces genes that encode enzymes that produce the long-chain lipids required for “waterproofing” the CE. 1,25(OH)2D also induces the calcium sensing receptor (CaR) that responds to extracellular calcium by activating phospholipase C (PLC). PLC, by cleaving phosphatidylinositol bisphosphate (PIP2), releases two important signaling molecules: diacylglycerol (DG) and inositol trisphosphate (IP3). The latter releases calcium from intracellular stores such as the endoplasmic reticulum (ER) and Golgi through the IP3 receptor (IP3R). DG works in conjunction with calcium to activate protein kinase C. 1,25(OH)2D induces both the β and γ forms of PLC, but calcium is required for their activation. The CaR also activates Src/Fyn, which phosphorylate the catenins, including β-catenin, to enable their binding to and formation of the E-cadherin complex in the membrane. Both calcium and 1,25(OH)2D are essential for the formation of this complex: 1,25(OH)2D induces E-cadherin, whereas calcium promotes its translocation to the membrane. Not shown is that α-catenin binds to β-catenin, linking the E-cadherin/catenin complex to the cytoskeleton, critical for cell migration. The E-cadherin/catenin complex also contains two enzymes, phosphatidylinositol phosphate 5 kinase 1α (PIP5K1α) and phosphatidylinositol 3 kinase (PI3K), that sequentially phosphorylate PIP to PIP2 to PIP3. PIP3 is the major activator of PLC-γ1 during keratinocyte differentiation, which in addition to promoting the cleavage of PIP2 to IP3 and DG also activates at least one of the calcium channels, TRP3. [Reproduced with permission from Bikle DD. Vitamin D, Calcium. and the Epidermis. In: Feldman D, Wesley Pike J, Bouillon R, et al., eds. Vitamin D. 4th ed. London: Academic Press; 2018:527-544.]
The Vdr-null mouse shows signs of disrupted epidermal differentiation (namely, low levels of loricrin, involucrin, and profilaggrin), which can be partially rescued with a high calcium diet. In Cyp27b1-null mice, the disorders in epidermal differentiation were not reversed with a high calcium diet (121). In vitro and in vivo studies revealed that 1,25(OH)2D and calcium stimulate keratinocyte differentiation in a synergic and somehow redundant way at different levels by: (1) transcriptional control of cell cycle regulatory proteins; (2) stimulation of proteins crucial for cornified waterproof envelope formation; (3) induction of the E-cadherin/β-catenin complex, required for epidermal differentiation and keratinocyte cell-to-cell adhesion (122); and (4) maintenance of calcium gradient along epidermis layers, which is critical for the correct differentiation process from the basal cells to the cornified cells (90–92, 122–124). VDR functions seem to be different in each layer of epidermis owing to the recruitment of specific modulators and coactivators (123).
As discussed below, innate and acquired immune responses are modulated by ligand-dependent VDR functions, and 1,25(OH)2D is involved in the process of wound repair and host protection. This is true also in the skin. Indeed, recent studies showed that in both Vdr-null mice and in vitamin D–deficient mice normal macrophage recruitment and formation of granulation tissue after a cutaneous injury are impaired. This phenotype is due to the disruption of VDR–TGF-β interaction, which seems to be crucial for wound response (124). VDR expression within the keratinocytes is also necessary for the re-epithelialization of wounds (125). Moreover, as discussed below, active vitamin D and its analogs have been shown to stimulate keratinocyte production of cathelicidin, an antimicrobial protein that improves angiogenesis and re-epithelialization in the skin after injuries as well as protection from invading organisms (126–128).
One of the most dramatic features of Vdr-null mice is the development of alopecia 4 to 10 weeks after birth, which cannot be rescued with a high-calcium diet. This particular type of alopecia is not observed in vitamin D–deficient mice or in Cyp27b1-null mice, suggesting that the action of the VDR in the hair follicle cycle is independent from its ligand (129). The VDR plays its role in the keratinocyte stem cell population located in the bulge of hair follicles, where it is crucial for the capacity to start a new hair cycle, after the first hair coat is lost. 1,25(OH)2D seems to be not involved, but cofactors and interacting molecules that can change VDR conformation and gene transcription need to be further investigated (130, 131). The VDR is also considered as a tumor suppressor in the skin. Indeed, in the absence of the VDR there is an increased susceptibility to chemical- or UV-induced tumors in animal models (132, 133). VDR activation controls the Wnt/β-catenin and sonic hedgehog pathways, which are overexpressed in VDR-null animals, leading to uncontrolled keratinocyte proliferation and tumor formation (133, 134).
Clinical data
Despite the abundance of preclinical data regarding vitamin D and skin cell interaction, it remains speculative whether low vitamin D status has a causative role in the pathogenesis of cutaneous diseases in humans or, as suggested for other diseases, it is a marker of ill health and inflammatory status (135).
Psoriasis.
The relationship between vitamin D and psoriasis has been extensively investigated. Indeed, it is well known that psoriasis is characterized by increased proliferation and decreased final differentiation of keratinocytes, but inflammation and autoimmune reactions also contribute to its clinical expression. No association was found between vitamin D intake (dietary and/or supplemental) and psoriasis incidence in a long-term study of >70,000 US female nurses enrolled in the Nurses’ Health Study (136). Early intervention studies with oral calcitriol therapy were found to improve the clinical course of psoriasis (137, 138). These benefits have been confirmed by more recent studies in a small cohort of psoriatic patients (139, 140), as well as by a study in which 0.25 μg of calcitriol daily was combined with acitretin (141). More recently, beneficial effects have been demonstrated with the topical application to psoriasis plaques of 1,25(OH)2D or analogs such as calcipotriol, maxacalcitol, tacalcitol, and hexafluoro-1,25(OH)2D. The calcitriol analog calcipotriol is ideal for topical treatment owing to its low affinity for DBP. Its efficacy and safety were demonstrated in prospective clinical trials (142). A recent meta-analysis confirmed that topical treatment with vitamin D analogs is as efficacious as topical corticosteroid therapy alone, with the advantage of the “steroid-sparing” effects (avoiding side effects of topical glucocorticoids), and that the combined therapy further increases the beneficial effects (143). Nowadays, the use of topical vitamin D derivatives is becoming the most common treatment of psoriatic lesions (144, 145). In combination with topical steroids, it may be particularly helpful in hard-to-treat areas of the skin (146).
Skin cancer.
The role of vitamin D in skin cancer is still a matter of controversy. There is no doubt that UV-B light, responsible for the local production of vitamin D, is carcinogenic. Vitamin D metabolites, however, also display anticarcinogenic activities by activating the repair mechanisms of DNA damage (147, 148). A photoprotective effect of 1,25(OH)2D and several even nongenomic agonists was also found in mice exposed to UV-B light (149). Several studies have investigated the association between vitamin D status and nonmelanoma skin cancer (NMSC). In a sample of the Osteoporotic Fracture in Men study, an inverse association between serum 25OHD levels and the incidence of NMSC was found (47% lower odds for men in the highest quintile compared with those in the lowest quintile) (150). However, another study in a health maintenance organization cohort showed that values of serum 25OHD >37.5 nmol/L were positively associated with an increased risk, even after adjustment for additional risk factors (151, 152). In these studies, the contribution of confounding factors such as UV-B exposure is difficult to analyze, and conclusive data are still lacking. As far as the relationship between vitamin D and melanoma is concerned, there is evidence of a protective effect of vitamin D3, but UV radiation, which is a principal source of vitamin D3, is mutagenic (142). In a post hoc analysis of a large sample (n = 36,282) of postmenopausal women, enrolled into the Women’s Health Initiative, there was no difference in the incidence of melanoma between women receiving daily low-dose vitamin D (400 IU) plus calcium (1000 mg) supplementation compared with placebo during a follow-up period of 7 years. Alternatively, the incidence of melanoma was lower in a group at high risk, with a history of NMSC, receiving calcium and vitamin D supplementation (153).
Miscellanea.
Despite the experimental evidence of an established role of vitamin D in hair follicle cycling, few clinical data are available in humans. A cross-sectional study on 296 healthy men did not show any association between severity and extent of baldness and serum 25OHD levels (154). Moreover, calcipotriol failed to improve alopecia in a placebo-controlled study on patients with scalp psoriasis (155). Finally, some data suggest a possible protective role of vitamin D in acne vulgaris but an adverse effect in patients with rosacea (156).
Genetic data
Psoriasis.
Two recent meta-analyses of the most widely studied VDR gene polymorphisms and psoriasis risk have been performed, which to some extent provide contradictory results. One study showed an association between Apa1 and Taq1 polymorphisms and psoriasis in whites (157), and the other concluded that no robust and reproducible association exists between Apa1, Bsm1, Fok1, and Taq1 and psoriasis, or, at most, only a weak association present only in specific ethnic groups (158). Previous studies found no association between VDR polymorphisms and the clinical response to topical vitamin D at least in Korean patients (159). Moreover, Kontula and Mee showed no association between BsmI and response to calcipotriol therapy in psoriatic patients (160, 161). Alternatively, more recently, VDR Fok1 and Cdx2 polymorphisms were shown to influence the individual response to calcipotriol in monotherapy and when associated with steroid therapy in a Chinese population (162).
Skin cancer.
A study in a German population has shown an association between the Apa1 and Taq1 genotypes with basal cell carcinoma, but not with squamous cells carcinoma (163). Alternatively, the BSM1 genotype has been associated with both types of tumors. Several studies have looked at the association between VDR polymorphisms and melanoma. A relationship between sun exposure and VDR genotypes was evaluated in a case-control study in melanoma survivors. The authors found that the Bsm1 variant was associated with the occurrence of multiple primary melanoma (164). A meta-analysis of six studies that investigated the association between five VDR polymorphisms (TaqI, FokI, BsmI, EcoRV, and Cdx2) and the risk of melanoma also showed that the Bsm1 genotype was associated with an increased risk of melanoma development (165). Such an association has been recently confirmed by a meta-analysis, which reviewed 11 studies in European populations and analyzed the association between VDR FokI, BsmI, TaqI, ApaI, and EcoRV polymorphisms and susceptibility to melanoma (166).
So far, there is only one MR trial looking at vitamin D status and skin phenotype (Table 2). Noordam et al. (82) studied facial skin aging features in ∼4500 Dutch adults and found that higher measured serum 25OHD concentrations were associated with perceived age, skin wrinkling, and pigmented spots, but they genetically predicted that serum 25OHD was not linked with these skin characteristics. This seems to indicate that exposure to UV-B light rather than serum 25OHD concentrations are causally linked with skin aging.
Conclusions
Skin provides an excellent and well-established example of the nonskeletal actions of vitamin D signaling. The skin is indeed the only tissue capable of synthesizing all important vitamin D metabolites and is also a major target for vitamin D and its metabolites. Skin keratinocytes express all enzymes of the vitamin D metabolic pathway and can produce hormonal 1,25(OH)2D3 in the presence of sufficient UV-B irradiation. 1,25(OH)2D3 thus produced controls keratinocyte proliferation and differentiation, as well as epidermal barrier integrity (167, 168). Clinically, topical application of vitamin D analogs shows clear efficacy in alleviation of symptoms of psoriasis, which likely arises from their effects on epidermal cell proliferation and, as discussed below, their anti-inflammatory properties.
UV-B light is essential for endogenous production of vitamin D, but the same wavelengths are also oncogenic. UV-B exposure indeed causes DNA damage and p53 expression, which are associated with systemic production of endorphins, which then can produce an addictive behavior [well documented in mice (169), but likely also in humans]. Moreover, mice deficient in vitamin D signaling overexpress the oncogenic transcription factor cMYC (170) and are more susceptible to skin carcinogenesis than are their wild-type counterparts (123). There is thus a very difficult trade-off between safe exposure to sunlight as to produce sufficient vitamin D while avoiding long-term risks of skin damage and skin cancer. VDR and 1,25(OH)2D action in the skin may generate some protective mechanisms against UV-B damage.
Muscles and Falls
Preclinical data
The expression of the VDR in muscle is hotly debated, as some experts could not detect VDR protein in adult multinucleated human or rodent skeletal muscle (171), whereas others found it to be widely expressed at the mRNA and protein levels (172, 173). In immature muscle cells or its stem cells, the VDR is probably expressed at a low level in comparison with the intestine (1000-fold lower mRNA and protein level), and it is probably absent or nearly so in mature multinucleated cells (174, 175). 1,25(OH)2D has clear antiproliferative effects on cultured muscle cells and regulates several genes involved in muscle cell maturation, including a negative regulation of myostatin (172, 176). Systemic Vdr knockout mice have smaller and immature skeletal muscle cells, especially of fast-twitch (glycolytic) type II fibers (172, 173, 176), and selective Vdr deletion in cardiomyocytes generates a clear phenotype of hypertrophy and fibrosis (see “Cardiovascular System” below).
Human data
Observational data suggest that severe longstanding vitamin D deficiency is associated with muscle weakness and cardiomyopathy in infants (17). Such severe muscle weakness is also seen in patients with congenital absence of CYP27B1 or in patients with severe renal osteodystrophy. Rapid improvement of muscle function has been reported after vitamin D or 1,25(OH)2D supplementation to such patients. Vitamin D insufficiency has been associated with reduced muscle performance and loss of fast-twitch type II muscle fibers. Vitamin D may also be important for balance as measured by quantifying sway.
Several intervention studies have looked at different endpoints. Vitamin D supplementation (given daily) in deficient elderly subjects improves balance as measured by sway (99, 177). Pfeifer et al. (99) found that in vitamin D–deficient elders, with a mean age of 77 years and a mean baseline serum 25OHD level of 55 nmol/L, treatment with 800 IU of vitamin D3 per day significantly reduced body sway, when compared with the placebo group. Similarly, Cangussu et al. (177) found that supplementation with 1000 IU of vitamin D3 when compared with placebo significantly reduced body sway in 160 Brazilian women with a low mean baseline serum 25OHD level of 37.5 nmol/L. A similar conclusion was reached by Lips et al. (178) in older adults treated with a once-weekly dose of 8400 IU of vitamin D3 compared with placebo.
Based on an extensive meta-analysis, muscle (especially proximal muscle) strength may modestly improve with vitamin D supplementation of elderly subjects with serum 25OHD levels <30 nmol/L (179). Consistent with this concept, the same RCT from Brazil found that supplementation with 1000 IU of vitamin D3 per day for 9 months significantly reduced first fallers by nearly 50% and all falls by even more (177).
Several trials have examined the effect of vitamin D supplementation on incident fallers and fall rate. A meta-analysis of nine RCTs showed that daily supplementation of <IU of vitamin D was ineffective whereas 700 to 1000 IU significantly decreased the fall risk (180). A Cochrane review concluded that vitamin D supplementation reduced the risk of falls in institutionalized care patients (highly likely to be vitamin D deficient) [relative risk (RR), 0.63 (0.46 to 0.85)] (181). In ambulatory subjects, vitamin D supplementation did not decrease the risk of falls in a meta-analysis of all RCTs combined, but it decreased the risk of falls and fallers [RR, 0.57 (0.37 to 0.89) and RR, 0.70 (0.56 to 0.87), respectively] in subjects with a baseline serum 25OHD concentration <50 nmol/L (182). A more recent meta-analysis of RCTs found that supplementation with vitamin D reduced the fall rate only in subjects with a starting serum 25OHD concentration <75 nmol/L (183).
“There is at present no consensus regarding the potential beneficial effects of vitamin D supplementation on muscle function, balance, and risks of falls.”
In contrast, Bolland et al. (184) concluded from their meta-analysis that the effect estimated for vitamin D on falls lies within the futility boundary, which they defined as not altering relative risk by 15% or more. A recent post hoc analysis of falls in a large New Zealand (VIDA) study revealed that supplementation with 100,000 IU monthly for 3.4 years had no effect on risk of falling or fractures (104). The mean baseline serum 25OHD level of these subjects was 63 nmol/L, and less than one-third of all participants started with serum 25OHD levels <50 nmol/L.
High-dose vitamin D supplementation, however, may increase the risk of falling. This was first observed by Sanders et al. (102) in elderly subjects treated with a single oral dose of 500,000 IU of vitamin D3 or placebo once a year. The vitamin D–treated group had significantly more falls and fractures during the first 3 months after each loading dose during the 4-year treatment period compared with the placebo group. Smith et al. (103) found that supplementation with 300,000 IU of vitamin D2 annually by intramuscular injection had no effect on fall risk but increased fracture risk. In elderly women with baseline vitamin D deficiency, monthly doses of vitamin D greater than the equivalent of 800 IU/d for 1 year increased the risk of falls from 48% in the group treated with 24,000 IU monthly (equivalent to 800 IU/d) to 67% in the group treated with 60,000 IU/mo (equivalent to 2000 IU/d) and 66% in the group treated with 24,000 IU of vitamin D3 plus 300 μg of calcifediol per month (185). The authors concluded from a post hoc analysis that serum 25OHD concentrations higher than 112.5 nmol/L may be associated with an increased risk of falls. Ginde et al. (186) treated 107 long-term care seniors with 100,000 IU of vitamin D3 per month or placebo for 12 months. The monthly vitamin D decreased the rate of acute infections by 40% (primary endpoint) but doubled the rate of falls (secondary endpoint). Smith et al. (187) examined fall rates in 146 elderly white women (mean baseline serum 25OHD level of 38 nmol/L) treated with a full range of daily vitamin D3 doses (from 400 to 4800 IU) or placebo for 1 year. Falls were assessed by daily calendar and phone calls every three months. They found a U-shaped association with falls, the nadir of which occurred in the dose range of 1600 to 3200 IU/d. Fall rates in the higher doses, 4000 and 4800 IU/d, were significantly higher than those in the nadir. In contrast, among 91 African American women in the same study, there was no U-shaped association. Rather there was a progressive decline in percentage of fallers with increasing vitamin D dose, with the lowest rate occurring in the women taking 4000 to 4800 IU/d (187). This study had a small sample size for the number of dose groups but high-quality fall assessment.
Conclusions and perspectives
There is at present no consensus regarding the potential beneficial effects of vitamin D supplementation on muscle function, balance, and risks of falls. However, overall the data seem to indicate that modest doses and daily provision of vitamin D supplementation of elderly vitamin D–deficient subjects may modestly improve muscle function, improve balance, and decrease the risks of falling. The optimal dose and dose frequency for maximal fall reduction remain to be established, as high intermittent dosing or high serum 25OHD concentrations may increase the risk of falling in white elderly subjects. Its effect in other ethnic groups remains to be explored.
Immunity
Innate immune system
Preclinical data
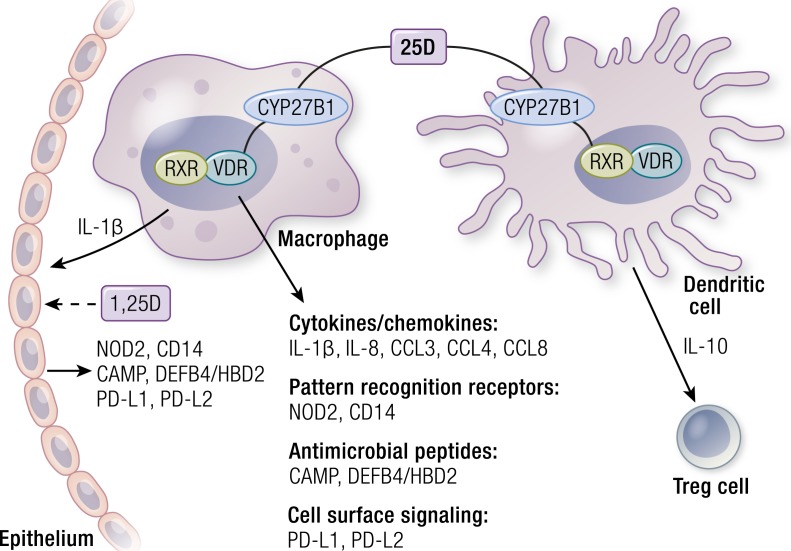
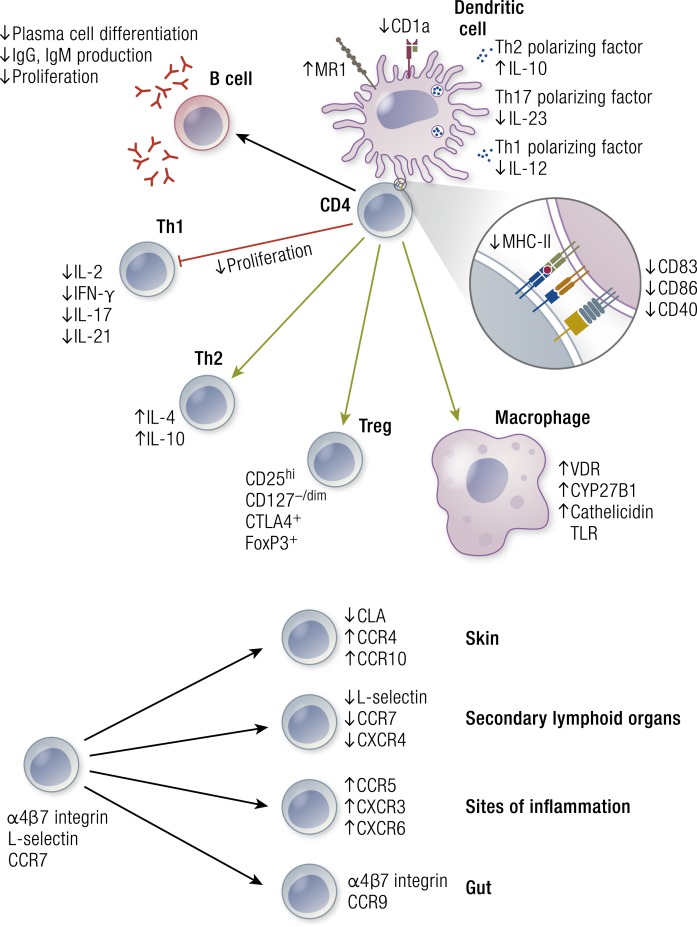
VDR and vitamin D metabolic enzymes are present in virtually all cells of the innate and adaptive arms of the immune system (188, 189). Importantly, there is compelling evidence that cells of the immune system produce 1,25(OH)2D locally and, more importantly, that expression of CYP27B1 is regulated in these cells by a network of immunoregulatory rather than calcium homeostatic inputs (189, 190). These include signaling by pattern recognition receptors, vanguards of innate immune responses to pathogen threat (190), as well as by cytokines produced by T cells of the adaptive immune system (191). In vitro studies of induced CYP27B1 expression in myeloid cells are consistent with clinical observations of excessive production of 1,25(OH)2D by macrophages in granulomatous diseases such as sarcoidosis (192). These regulatory events are important because they mirror one of the central pieces of evidence for a physiological role of vitamin D signaling in calcium homeostasis, that is, the regulation of renal CYP27B1 production by calcium regulatory hormones. Other laboratory work has provided evidence that, once activated, the VDR regulates innate immune responses upstream and downstream of pattern recognition receptor signaling by activating the transcription of several genes (summarized in Fig. 4). These encode the pattern recognition receptor nucleotide oligomerization domain protein 2 (NOD2), the Toll-like receptor cofactor CD14, antimicrobial peptides cathelicidin (CAMP, LL-37) and DEFB4/HBD2, as well as multiple cytokines, chemokines, and other signaling molecules (193–195). Notably, a combination of 1,25(OH)2D and IL-1β induced by 1,25(OH)2D in macrophages stimulated paracrine antimicrobial peptide production in epithelial cells (Fig. 3). In vitro studies analyzing induced antimicrobial peptide gene expression showed that conditioned media of 1,25(OH)2D-treated epithelial cells acquired the capacity to kill bacteria such as the lung pathogen Pseudomonas aeruginosa (196). Importantly, such findings were recently supported by results from a placebo-controlled, double-blind RCT, which provided evidence that vitamin D supplementation enhanced antimicrobial activity in pulmonary surface airway fluid (197).
Figure 4.
Vitamin D metabolism and signaling in innate immunity. The figure depicts intracrine production 1,25(OH)2D from circulating 25OHD in macrophages and DCs, as well as the effects of 1,25(OH)2D signaling on expression of several classes of proteins implicated in innate immune signaling. See text for details.
Clinical data
Vitamin D and respiratory infections.
Numerous clinical studies have revealed associations between vitamin D deficiency and increased risk of infections, particularly of the upper respiratory tract (URT) (198). Connections between vitamin D insufficiency and infections can be traced back to the 1800s with the recognition that sunlight was beneficial for patients suffering from tuberculosis (TB). Associations between vitamin D deficiency and TB susceptibility were made in the 1980s (199, 200), as was the observation that 1,25(OH)2D inhibits the growth of Mycobacterium tuberculosis in cultured human macrophages (201). Since then, many preclinical and clinical studies have investigated the potential of vitamin D supplementation to prevent or treat TB (195, 202–206). Notably, Martineau et al. (203) observed in a double-blind RCT that a single dose of 100,000 IU of vitamin D3 enhanced antimycobacterial immunity in healthy tuberculin skin test–positive donors.
Apart from TB, multiple RCTs have provided evidence for vitamin D supplementation of deficient populations in preventing infections. A highly publicized trial published in 2010 concluded that vitamin D supplementation reduced the risk of seasonal influenza infections in Japanese children, with the effect being most pronounced in children who had not been previously supplemented (207). Other studies have provided evidence for the benefit of supplementation in populations at elevated risk for URT or ear infections due to vitamin D deficiency or a history of recurrent infections (208–210). However, results of such trials are not unanimous. For example, one study in a healthy population showed no benefit of supplementation on rates of URT infections (211), perhaps because the baseline serum 25OHD level in the population studied was 73 nmol/L. Consistent with all of the above, a recently published review and meta-analysis of individual participant data from 25 RCTs concluded that vitamin D supplementation was safe and provided modest protection (adjusted OR, 0.88) against acute URTs (212). However, subgroup analysis showed that beneficial effects were observed in patients receiving daily or weekly doses (adjusted OR, 0.81) but not in those receiving bolus doses. Moreover, in groups receiving daily or weekly doses, effects were most pronounced in patients who were vitamin D deficient (<25 nmol/L; adjusted OR, 0.30).
Vitamin D and inflammatory bowel disease.
Another indication where vitamin D supplementation may be of therapeutic benefit is in the treatment of patients with inflammatory bowel disease (IBD), in particular Crohn disease (CD). Although CD is often considered an autoimmune condition, it is likely driven by defects in intestinal innate immunity (213). The genetics of CD are compelling, as they reveal the importance of variations in innate immune signaling pathways, notably those controlling autophagy, in the etiology of the disease (214, 215). Vitamin D deficiency is frequent in patients with CD owing to the combination of chronic inflammation, intestinal malabsorption of vitamin D, and lifestyle. Although vitamin D deficiency has long been associated with CD, recent evidence provides a strong mechanistic basis for a role of deficiency in the pathogenesis of CD (216). For example, the gene encoding the pattern recognition receptor NOD2 (also known as IBD1) is a direct target of 1,25(OH)2D signaling (217). GWASs revealed that NOD2 gene mutations disrupting its pattern recognition domain contribute strongly to CD development (218, 219).
Moreover, signaling downstream of NOD2 activates transcription of the gene encoding the antimicrobial peptide DEB4/HBD2, which is also a CD susceptibility locus (220) and a direct target of the VDR (196), revealing that 1,25(OH)2D signaling activates the extremities of the NOD2–DEFB4 innate immune pathway. The gene encoding programmed death ligand 1 (also known as B7-H1) is also a direct target of the VDR (158). Programmed death ligand 1 interacts with its receptor programmed death 1 on T cells to suppress inflammatory T cell responses in peripheral tissues, and its intestinal epithelial ablation in mice leads to inflammation via defects in innate immunity (221). These findings are intriguing in light of a recent GWAS study of 1812 individuals that linked VDR gene variants to alterations in the human gut microbiome (222). The study also found that VDR was upregulated in colonic biopsies of IBD patients, including those with CD. However, others found lower VDR levels in patients with CD or IBD, but confirmed that colitis is enhanced in the absence of the intestinal VDR and tapered down when the intestinal VDR is overexpressed (223). A role for vitamin D signaling in control of inflammation is also supported by clinical data. A large prospective cohort study of 72,719 women in the Nurses’ Health Study documented 122 cases of CD and found that for women with predicted serum 25OHD levels of 75 nmol/L the multivariate-adjusted HR for developing CD was 0.38 (95% CI, 0.15 to 0.97) when compared with those with predicted levels of <50 nmol/L. Retrospective cohort studies concluded that vitamin D deficiency was common among CD patients and was independently associated with greater disease activity (224, 225), as well as increased levels of markers of intestinal inflammation (226). Importantly, intervention trials have also provided positive results, with one finding a significant association between supplementation (P = 0.02), circulating serum 25OHD levels (P < 0.05), and disease quiescence in pediatric IBD patients (227). Others found that supplementation was inversely correlated with use of multiple fee-based services by IBD patients (228) and reduced rates of surgery in CD patients (229). Two double-blind, placebo-controlled RCTs in CD patients have been published to date. Ninety-four patients in remission were randomized to receive either placebo or 1200 IU/d for 12 months. The relapse rate was reduced from 14 of 48 in the placebo group to 6 of 46 in the treatment group (P < 0.06) (230). A small-scale, double-blind, placebo-controlled RCT of 27 CD patients in remission showed that 2000 IU/d of vitamin D for 3 months was sufficient to significantly increase serum 25OHD levels (231), and that supplementation enhanced circulating levels of the antimicrobial peptide LL-37 (cathelicidin, CAMP) and maintained intestinal permeability (whereas it was increased in the placebo group). Moreover, in patients whose serum 25OHD levels were at least 75 nmol/L, treatment was associated with higher quality-of-life scores (232). These findings provide strong support for conducting large-scale RCTs to examine the therapeutic efficacy of vitamin D supplementation in CD. It will be important to account for possible malabsorption in a CD patient population and to use sufficiently robust levels of supplementation.
Vitamin D, adaptive immunity, and autoimmunity
Preclinical data
We have known since the 1980s that the VDR is present in T lymphocytes and that 1,25(OH)2D is an inhibitor of T cell proliferation and activation (232, 233). T lymphocytes are composed of several subsets, including CD4+ helper T cells, cytotoxic CD8+ T cells, regulatory T (Treg) cells, natural killer cells, γδ T cells, and memory cells. The effects of vitamin D signaling on T cell subtypes have been reviewed extensively elsewhere (189, 234, 235) (Fig. 4). In general terms, vitamin D acts to suppress T cell–driven inflammation and enhance the effects of suppressive Treg cells. The communication between antigen-presenting dendritic cells (DCs) and T cells appears to be particularly important in this regard. The intracrine production of 1,25(OH)2D induces a more tolerogenic DC phenotype, characterized by the production of IL-10 (Fig. 5), which stimulates the production of Treg cells (189, 236). Recent studies have suggested that 1,25(OH)2D controls the phenotype of DCs by altering their metabolic profile (237). These anti-inflammatory effects of vitamin D signaling on DCs and T cells have stimulated extensive interest in the relationship between vitamin D status, inflammation, and autoimmunity. In autoimmune diabetes-prone NOD mice vitamin D deficiency early in life only (with normal intake later on) substantially and highly significantly increased the subsequent risk of disease in two independent studies (238, 239). In other work, dietary 1,25(OH)2D reduced arthritic lesions in two mouse models of autoimmune arthritis, murine Lyme arthritis, and collagen-induced arthritis (240). Very similar data have been made for experimental allergic encephalomyelitis [a mouse model for human multiple sclerosis (MS)] and IBDs. These data provide support for human studies exploring a role for vitamin D in the prevention or therapy of autoimmune diseases such as type 1 diabetes, IBD, MS, rheumatoid arthritis (RA), and systemic lupus erythematosus (188, 241).
Figure 5.
Vitamin D metabolism and signaling in the acquired immune system. In antigen-presenting cells (including DCs), 1,25(OH)2D3 inhibits the surface expression of major histocompatibility complex II (MHC-II)–complexed antigen and of costimulatory molecules, in addition to production of the cytokines IL-12 and IL-23, thereby indirectly shifting the polarization of T cells from a Th1 and Th17 phenotype toward a Th2 phenotype. Additionally, 1,25(OH)2D3 directly affects T cell responses by inhibiting the production of Th1 cytokines (IL-2 and IFN-γ) and Th17 cytokines (IL-17 and IL-21), as well as by stimulating Th2 cytokine production (IL-4). Moreover, 1,25(OH)2D3 favors Treg cell development via modulation of DCs and by directly targeting T cells. Finally, 1,25(OH)2D3 blocks plasma-cell differentiation, IgG and IgM production, and B cell proliferation.
Human data
Observational data have consistently confirmed an association between poor vitamin D status and all major autoimmune diseases. As detailed above, topical 1,25(OH)2D or its analogs have demonstrated efficacy against psoriasis, which is characterized by increased keratinocyte proliferation, but has inflammatory and autoimmune components as well. The relationship between vitamin D deficiency and MS has also attracted extensive interest. A large nested control study in US white army recruits demonstrated that a low vitamin D status (defined by serum 25OHD levels <50 nmol/L) at the time of recruitment conveyed a nearly twofold risk of later onset of MS compared with whites with a better vitamin D baseline status (242). The Finnish Maternity Cohort study reached similar conclusions (243). A similar study in patients with type 1 diabetes generated similar results (244). Several retrospective studies, albeit not optimally designed, showed that that vitamin D supplementation early in life reduced the risk of developing type 1 diabetes later in life by ∼30% (245). The dose of vitamin D required for this effect has not been clearly defined but a daily dose (up to 2000 IU) during the first year of life was most effective. This dose is markedly higher than the presently accepted or recommended dose for infants (200 to 400 IU/d). There are also well-established links between vitamin D deficiency and RA. In >29,000 women followed for 11 years, RA (152 cases in total) was inversely associated with intake of vitamin D (highest vs lowest tertile) (246). A small-scale MR study did not find a link between genetically predicted serum 25OHD and outcome parameters in patients with RA, but the study did not evaluate the risk of developing this disease (Table 2).
MR studies in Canadian, Swedish, and US cohorts have clearly shown an association between the presence of genes predisposing to lifelong lower serum 25OHD concentration and later onset of MS (Table 2). In the Canadian study (61), four SNPs known to be related to lower serum 25OHD were all independently associated with a significantly increased risk of MS. By extrapolation, 1 SD lower serum 25OHD concentration would imply a doubling of the risk of MS. In a US cohort and a Swedish cohort (total number of >7000 cases vs >14,000 controls), similar results were obtained using three SNPs related to serum 25OHD status. The overall OR was 0.85 for subjects without SNPs coding for higher serum 25OHD concentrations (62). Similar conclusions (OR, 0.72) were reached when only pediatric onset (<18 years of age) cases (n = 569) of MS were analyzed (60). The largest study (247) identified an additional synonymous variant in the coding region of CYP2R1, present in ∼5% of individuals of European origin (and much lower in Africans and Asians). This variant was associated with significantly lower serum 25OHD concentrations and a 2.2-fold increased risk of vitamin D deficiency, and in addition with a 1.4-fold OR of developing MS. These data thus strongly support the idea that lifelong lower vitamin D status due to genetically decreased serum 25OHD levels (albeit only about ≤12.5 nmol/L compared with subjects with genetically higher serum 25OHD levels) is a risk factor for autoimmune diseases such as MS. For type 1 diabetes, only one MR has been published (63) dealing with a very large number of patients (>9000). Using five SNPs predicting a lower lifetime serum 25OHD concentration, a significantly higher relative risk of type 1 diabetes of 1.07 was found in cases vs controls and of 1.10 in a family study.
Although there are preclinical and observational data to suggest that maintenance of vitamin D sufficiency should help prevent the onset of autoimmunity, current evidence for the therapeutic benefit of supplementation is inconclusive, and many studies are limited by group sizes. For example, in MS, a number of trials of vitamin D supplementation have been performed, generating contradictory results concerning parameters such as size of MRI lesions, rates of relapse, and effects on functionality (248). However, studies with type 1 diabetes [many of which used 1,25(OH)2D supplementation], although inconclusive, are more promising as they suggest that early intervention may preserve β-cell function (248). Moreover, 1,25(OH)2D induces a VDR-dependent transcriptional program underpinning β-cell survival (249). One trial concluded that a protective effect of 1,25(OH)2D only occurred in cases with recent (<1 year) onset of the disease (250). These findings are in line with the idea that vitamin D supplementation may contribute to prevention of type 1 diabetes (251, 252), but lose its benefit once β-cells are largely destroyed. RCTs are needed to confirm this observation and, if so, to define the optimal timing (e.g., early in life?) and safe dosage. As type 1 diabetes as well as MS have a long silent evolution before the onset of clear clinical symptoms, such studies may require a long follow-up of subjects at increased risk for such diseases.
For other autoimmune disorders, no reliable intervention trials are yet available, but the preclinical data and observational studies on IBD are promising so that such trials deserve a high-priority score. Two large-scale studies also evaluated the preventive effects of vitamin D supplementation during pregnancy on the incidence of allergy (asthma) in their offspring (up to age 3) and the combined results showed small beneficial effects (253, 254).
Vitamin D, atopy, asthma, and atopic dermatitis
Atopy is a predisposition to develop allergic diseases such as asthma and atopic dermatitis, and it is generally characterized by increased serum IgE concentrations. The vitamin D system has a number of effects on immune cells and cytokines implicated in atopy (234, 255). A poor vitamin D status has usually been associated with increased risk of wheezing or asthma and other aspects of atopy, although the opposite has also been reported. A Cochrane meta-analysis of two RCTs concluded that vitamin D supplementation reduced atopic exacerbations (256), but more recent data did not confirm this conclusion (257). High-dose vitamin D supplementation during pregnancy showed a small benefit in reducing wheezing in the offspring at age 3, at least when the results of two independent RCTs are combined (222, 223, 254, 255). A large MR study (dealing with >150,000 patients of all ages) showed that serum 25OHD, predicted based on four SNPs, did not influence the risk for (adult or pediatric onset) asthma, atopic dermatitis, or increased serum IgE (78). A similar conclusion was reached in another MR study on the same topic (258), and in another MR study of the GABRIEL asthma database (79). In a small-scale MR analysis of Dutch participants, genetically predicted serum 25OHD was not linked to an inflammation marker, C-reactive protein (80) (Table 2).
Conclusions
There is broad and growing evidence that VDR and vitamin D metabolic enzymes are present in the innate and adaptive arms of the immune system, and, more significantly, that vitamin D signaling in the immune system is physiologically important and of clinical significance in patients with deficiencies. Overall, the data suggest a role of vitamin D status in sensitivity to infections and autoimmune diseases, whereas the risk of atopic diseases is less evident. Three MR studies all showed a clear significant link between genetically predicted lower serum 25OHD concentrations and the prevalence of juvenile or adult onset MS. Intervention studies to date show particular promise in CD. However, similar to RCTs in other immune-related disorders, definitive conclusions concerning therapeutic benefit are limited by study sizes. Nonetheless, the data are sufficiently compelling to merit larger-scale RCTs for CD and other immune conditions, in particular in patients with early stage type 1 diabetes or in patients at risk for developing the disease.
Thus, a link between the vitamin D endocrine system and the immune system is highly plausible, but whether vitamin D deficiency in humans has real implications for infections or autoimmune diseases has yet to be confirmed by large-scale RCTs. It will be important to perform such trials because data accumulated to date indicate that low vitamin D status enhances the risk of upper respiratory infections and that vitamin D deficiency during early life predisposes the immune system to a higher later risk of autoimmune diseases or allergy.
Cancer
Preclinical data
In 1979, Eisman et al. (259) suggested a possible association between vitamin D and cancer, when they described for the first time the presence of the VDR in a breast cancer cell line. They later showed that these receptors were present in many, but not in all, cancer cell lines and tissues, thus concluding that the VDR was not a marker for malignancy but might play a role in the pathogenesis or evolution of cancer. Additionally, CYP27B1 is expressed in many cancers, often at higher levels than in the surrounding normal tissue. Subsequently, Abe et al. (260) and Colston et al. (261) demonstrated a clear inhibition of cell proliferation of myeloma or melanoma cells, respectively. The antiproliferative effect of 1,25(OH)2D on cancer cells has since been confirmed in most normal and cancerous cells whereby 1,25(OH)2D especially inhibits cell cycle progression at the G1 stage. This effect usually requires nanomolar concentrations of 1,25(OH)2D. Therefore, many synthetic analogs have been developed (262) aiming to find a molecule with a better ratio of anticancer vs calcemic effects (e.g., EB1089, inecalcitol) (262), and some of them have already been tested in humans (263). Some malignant cells may lose their sensitivity to 1,25(OH)2D through loss of VDR expression. However, many cancer models are wholly or partially resistant to the antiproliferative effects of 1,25(OH)2D in spite of retaining intact vitamin D signaling (264, 265). In contrast, overexpression of the catabolic enzyme CYP24A1 is fairly frequent in malignant cells and was even described as an oncogene in breast cancer screening (266). The inhibition of cell cycle progression can be explained by the effects of 1,25(OH)2D on a very large number of genes that are coherently regulated. 1,25(OH)2D regulates E2F transcription factor function, Rb phosphorylation, cyclin-dependent kinase activity, cMYC expression, and TGF-β and prostaglandin signaling. Moreover 1,25(OH)2D can inhibit angiogenesis, induce apoptosis, and decrease inflammation, invasion, and metastasis (267) (Table 3).
Table 3.
Mechanisms of Vitamin D Tumor Suppression
| Effect | Mechanism |
|---|---|
| Antiproliferative | 1. Arrest of cell cycle: G0/G1 and G1/S |
| 2. Dephosphorylation of FOXO | |
| 3. ↓ Levels of myc, fos, and jun | |
| 4. ↓ Activity of growth factors: IGF-1, IHH, and EGF | |
| 5. ↑ Activity of TGF-β | |
| 6. ↓ Activity wnt/β-catenin signaling | |
| Apoptosis | 1. ↑ Expression GOS-2 and Bax, ↓ expression Bc12 and Bc1-xL |
| 2. ↑ Expression DAP-3, CFKAR, and FADD, ↓ caspases | |
| 3. ↑ Expression PTEN | |
| 4. ↑ Autophagy | |
| DNA Repair | 1. ↑ Clearance of cyclobutane pyrimidine dimers and pyrimidine-(6,4)-pyrimidone photoproducts (in UV-B–irradiated skin) |
| 2. ↓ Oxidative DNA damage by ↑ expression antioxidant enzymes | |
| 3. ↑ Expression of DNA repair enzymes XPC and DDB2 | |
| Prostaglandin metabolism | 1. ↓ COX2 expression |
| 2. ↓ Prostaglandin receptors | |
| 3. ↑15-PDGH expression | |
| Angiogenesis | 1. ↓ Proliferation of endothelial cells |
| 2. ↓ VEGF expression | |
| Metastasis | 1. ↓ Cell migration and invasion capacity |
| 2. ↓ Expression of laminin and its receptors | |
| 3. ↑ Expression of E-cadherin | |
| 4. ↓ Expression of CEACAMI |
Animal models with knockout of the VDR have been used to understand the consequences of the lack of genomic effects of 1,25(OH)2D on cell differentiation. Although Vdr-null mice do not spontaneously develop more cancers, they are more prone to a variety of malignancies, such as breast (268), colon (269), and skin (123, 270) cancer, when exposed to carcinogenic conditions, such as oncogenes, loss of antioncogenes, or exposure to carcinogens or UV-B light (17, 271). Overexpression of Cyp24a1 in mammary cells makes such animals more sensitive to breast cancer (268). A recent study in mice with genetic absence of Cyp27b1 kept on a rescue diet to maintain normal calcium homeostasis for 1 year demonstrated a higher incidence of a variety of cancers compared with wild-type mice or null mice treated with 1,25(OH)2D (272).
Several animal studies [Table 4 (265, 273–288)] showed some beneficial effects of less hypercalcemic vitamin D analogs on the evolution of transplanted tumors, but efficacy is nonetheless limited by potential hypercalcemia (20, 289). Indeed, a large number of studies in different experimental models have demonstrated positive effects of the active form of vitamin D against tumor growth or progression of metastases, together with histological signs of decreased cell proliferation rate, inhibited angiogenesis, and induction of cell differentiation (271). Active vitamin D metabolites and analogs have been tested in clinical trials as adjuvant treatment against cancer, although, similar to preclinical models, their efficacy is limited by the development of hypercalcemia (263) (Table 4).
Table 4.
Selected List of Animal Studies on the Use of Vitamin Metabolites or Analogs on Cancer
| Cancer | Author | Study Design | Results |
|---|---|---|---|
| Colorectal | Newmark et al. (273) | Western diet | Ca + D prevents |
| Murillo et al. (274) | Chemical induced | D prevents | |
| Yang et al. (275) | APCmin + Western diet | Ca + D prevents | |
| Xu et al. (276) | APCmin + D-deficient diet | D + 1,25(OH)2D prevents | |
| Zheng et al. (277) | APCmin in VDRKO | ↑ Cancers | |
| Huerta et al. (278) | APCmin + D-deficient diet | 1,25(OH)2 D prevents | |
| Breast | Lipkin and Newmark (279) | DMBA + Western diet | Ca + D prevents |
| Zinser and Welsh (280) | DMBA + VDRKO | ↑ Cancers | |
| Zinser and Welsh (281) | MMTV-neu + VDRKO | ↑ Cancers | |
| VanWeelden et al. (282) | MCF-7 xenografts | EB1089 ↓ growth | |
| Ooi et al. (283) | Tumor inspections | D deficiency ↑ | |
| El Abdaimi et al. (284) | Xenograft breast cancer | EB1089 ↓ growth | |
| Prostate | Bhatia et al. (285) | Xenograft prostate cancer | EB1089 ↓ growth and mets |
| Zheng et al. (286) | PC3 cells in bone | Deficiency ↑ growth | |
| Mordan-McCombs et al. (287) | LPB-tag model | 1,25(OH)2D ↓ progression | |
| Krishnan et al. (288) | TRAMP model | 1,25(OH)2D ↓ growth | |
| Chung et al. (265) | TRAMP model + VDRKO | ↑ Angiogenesis |
Human data
Epidemiological data have described associations between solar radiation and cancer incidence and mortality, suggesting a possible role of vitamin D. The first hint for a link between cancer mortality and sun exposure was already mentioned in 1941 (290). The Garland brothers (291) expanded these studies in 1980 and reported that US populations with lower solar radiation had higher rates of mortality from colon cancer. Subsequently, many other types of cancer were described regarding similar associations to sun exposure (292), followed by dozens of meta-analyses looking for associations between low levels of serum 25OHD and higher incidence and mortality for different types of cancers, some with controversial results (293). This was observed in North American as well as with European whites and Japanese subjects (294–297), and it was concluded that a low vitamin D status was associated with a higher risk of bowel cancer, although the data for other cancers were inconsistent. This inconsistency was also noted in the 2011 Institute of Medicine report (298) and the UK consensus vitamin D position statement from a large number of UK scientific organizations, including Cancer UK (299). However, the association between a low vitamin D status and colon cancer is fairly consistent, as reported in several meta-analyses (5). The relationship between vitamin D status and prostate cancer incidence or aggressive types of prostate cancer is more complex, as different studies have found more prostate cancer in subjects with low as well as high vitamin D status. Several studies also suggest that the prognosis of patients with existing cancer may be better when they have a better vitamin D status (300).
In a systematic review, van der Rhee et al. (301) concluded from epidemiological data that chronic (not intermittent) sun exposure is associated with a reduced risk of colorectal, breast, and prostate cancers and non-Hodgkin’s lymphoma, but the relationship with higher levels of serum 25OHD could be demonstrated only for colorectal and breast cancer risks. Similar to data cited above, prostate cancer seems to behave differently. In Nordic men from Finland, Norway, and Sweden, the correlation between prostate cancer risk and serum 25OHD concentrations showed a U-shaped curve, increasing risk for lower as well as higher serum 25OHD concentrations (302). Although an inverse correlation between UV radiation and mortality for prostatic cancer was also described (303), a recent meta-analysis found a higher risk for prostate cancer at high levels of serum 25OHD (304), but this relationship seems to be dependent on the grade of aggressiveness of the prostatic cancer. Higher serum 25OHD levels might be associated with an increased incidence of less aggressive tumors, whereas lower levels substantially increased the risk of higher-grade (Gleason score 8 to 10) prostatic cancers (305). Overall, several meta-analyses provided a fairly consistent relationship between vitamin D status and colorectal cancer (306–309) [Table 5 (308, 310–313)]. For other tumors, such as breast, bladder, or lung tumors, the evidence is weaker or inconsistent (293). Most studies are limited to white elderly women, with scarce data for younger men and other ethnic groups such as the black population. Most of the studies concluded that high serum 25OHD levels are associated with improved cancer outcome and, less consistently, with lower cancer risk (293). Several studies also suggest that the prognosis of patients with existing cancer may be better when they have a better vitamin D status (300), and these results were confirmed using vitamin D pathway genetic variants analysis (52).
Table 5.
Meta-Analyses of Human Epidemiologic Studies Dealing With Cancer and Vitamin D Status
| Cancer |
Author | No. of Studies/Analysis | Pooled RRs | |
|---|---|---|---|---|
| Colorectal | Ma et al. (310) | 9 | 0.88 (0.8–0.96) Vitamin D intake | |
| 0.67 (0.54–0.80) 25OHD levels | ||||
| Yin et al. (311) | 10 | 0.82 (0.69–0.97) 25OHD levels | ||
| Breast | Chen et al. (312) | 11 | 0.91 (0.85–0.97) Vitamin D intake | |
| 8 | 0.55 (0.38–0.80) 25OHD levels | |||
| 0.83 (0.79–0.87) Case control (5) | ||||
| Gandini et al. (308) | 10 | 25OHD levels | ||
| 0.97 (0.92–1.03) Prospective (5) | ||||
| Prostate | Gandini et al. (308) | 11 | 0.99 (0.95–1.03) 25OHD levels | |
| Gilbert et al. (313) | 13 | 1.14 (0.99–1.31) Vitamin D intake | ||
| 14 | 1.04 (0.99–1.10) 25OHD levels | |||
There was inconsistency of the association between vitamin D status and breast or prostate cancer, but a consistent association with colorectal cancer.
In this regard, MR studies have, however, generated inconsistent results (Table 2). Ong et al. (52) found that genetically low serum 25OHD concentrations (as based on four SNPs) increased significantly the OR for ovarian carcinoma in a large (>30,000) group of whites, as well as for all types of ovarian cancer (OR, 1.27) as for high-grade cancers (OR, 1.54). Three other MR studies dealing with breast cancer or prostate cancer, however, did not find a relationship with SNPs associated with lower serum 25OHD (51, 53, 54). Additionally, two MR studies have evaluated all cancer types in relationship to four or five SNPs related to vitamin D status in a very large group of patients. Chandler et al. (49) studied 23,293 European women and found no significant link with genetically predicted serum 25OHD concentrations for all types of cancer, nor for specific (breast, colon, and lung) cancers or cancer deaths. Similarly, Dimitrakopoulo et al. (50) found no link between predicted serum 25OHD and colorectal, breast, prostate, or lung cancer in >70,000 cancer cases compared with >84,000 controls. Theodoratou et al. (54) also did not find a link between genetically predicted serum 25OHD and colorectal cancer in 2001 Scottish cases, although higher measured serum 25OHD was associated with lower risk of these cancers (Table 2). Dudding et al. (55) also did not find a link between genetically predicted serum 25OHD and oropharyngeal cancer in patients of mostly European descent living in Europe or the Americas. Finally, Sun et al. (56) did not find a link between genetically predicted serum 25OHD and lung cancer.
The real answer, however, about a causal role of vitamin D status on cancer has to come from RCTs. Only a few studies have so far addressed this question. Nine of 10 such RCTs [Table 6 (91, 314–321)] did not demonstrate a clear benefit of vitamin D supplementation (400 to 2000 IU/d for up to 8 years of follow-up). The large Women’s Health Initiative trial did not demonstrate a beneficial effect of combined vitamin D (400 IU/d) and calcium supplementation on cancer in general (315) or on colorectal cancer (318). Two subsequent studies of the Women’s Health Initiativedata, either excluding women who took calcium or vitamin D supplementation at baseline (319) or excluding women who took hormone replacement therapy during the study (320), revealed a better effect of vitamin D and calcium supplementation on colorectal cancer, albeit still being a nonsignificant decrease [RR, 0.81 (0.58 to 1.13 (not significant) and RR, 0.71 (0.46 to 1.09], respectively, of the risk of colorectal cancer (Table 6)]. However, excluding women on hormone replacement therapy revealed that vitamin D and calcium supplementation modestly but significantly decreased the risk of breast cancer or all invasive cancers (320) (Table 6). Trivedi et al. (91) randomly treated 2686 elderly individuals (2037 men and 649 women, aged 65 to 85 years) with 100,000 IU of vitamin D every 4 months or placebo for 5 years and found no differences on cancer incidence or mortality. Another extensive study dealing with colon adenoma and colon cancer did not reveal a beneficial effect of daily 1000 IU of vitamin D supplementation during 3 to 5 years, even in a high-risk population (316). Two smaller studies from Omaha, Nebraska (314, 317), provided more optimistic results on overall incidence of cancer after combined treatment with vitamin D and calcium. In the first one (314), a group of women treated with 1100 IU/d of vitamin D plus calcium for 4 years showed a significant reduction on cancer incidence compared with the placebo group, with a RR of 0.402 (CI, 0.20 to 0.82; P = 0.013). However, when compared with the calcium-supplemented group or the combined placebo–calcium control groups, the significant difference was lost (Table 6). Alternatively, the results of an RTC, which included 2303 elderly women from rural counties of the United States, showed that 2000 IU of vitamin D3 plus 1500 mg of calcium per day compared with placebo had no significant effect on cancer incidence and mortality after 4 years (P = 0.06) (317). However, a post hoc analysis excluding those who withdrew from the study, died, or developed cancer within the first year showed a significant difference between groups (χ2, 3.17% vs 4.86%; P = 0.046). In proportional hazards modeling, the HR was 0.65 (95% CI, 0.42 to 0.99). A limitation of this study was that most of the women included were not vitamin D deficient at baseline (serum 25OHD 82 ± 25 nmol/L); mean ± SD), although, as expected, at the end of the study the vitamin D–calcium group reached higher concentrations of serum 25OHD than did the placebo group (109 and 79 nmol/L, respectively; P < 0.001). Besides, all participants, including those from the placebo group, were allowed to take vitamin D supplements of up to 800 IU/d of vitamin D and up to 1500 mg/d of calcium in addition to dietary intake. Moreover, even in situ cancer cases were included. In line with the data described above, a meta-analysis concluded that vitamin D or vitamin D and calcium supplementation did not decrease the risk of all types of cancer (107).
Table 6.
Vitamin D Supplementation and Prevention of Neoplasia
| Outcome | Location, Trial Reference | Population | Baseline 25OHD (ng/mL) | Intervention | Duration (y) | RR (95% CI) |
|---|---|---|---|---|---|---|
| Total cancers | Oxford, United Kingdom, Triuvedi et al. (91) | 2686 Men and women, age 65–85 y, living in the general population | Mean 21.4 | D3 100,000 IU every 4 mo vs placebo | 5 | 1.09 (0.86–1.36) |
| Total cancers | Nebraska, Lappe et al. (314) | 1179 Healthy postmenopausal women, mean age 67 y | Mean 28.8 | D3 1100 IU/d + Ca | 4 | |
| Versus placebo | 0.42 (0.21–0.83) (P = 0.013) | |||||
| Versus calcium | 0.76 (0.38–1.55) (NS) | |||||
| WHI, United States, Brunner et al. (315) | 36,282 Postmenopausal women, age 50–79 y | — | D3 400 IU/d + Ca vs Ca alone | 7 | 0.98 (0.90–1.05) | |
| Colorectal adenomas | United States, Lappe et al. (316) | 2259 Men and women, age 45–75 y, with at least one colorectal adenoma removed within 120 d before enrollment and no remaining polyps | Median 23.2 | D3 1,000 IU/d vs placebo | 3–5 | 0.99 (0.89–1.09) |
| Ca vs placebo | 0.95 (0.85–1.06) | |||||
| D3 1000 IU + Ca daily vs placebo | 0.93 (90.80–1.08) | |||||
| Total cancers (excluding skin cancers) | Nebraska, Lappe et al. (317) | 2303 Healthy postmenopausal women, mean age 65 y | Mean 32.8 | D3 2,000 IU + Ca daily vs placebo | 4 | 0.70 (0.47–1.02) |
| Colorectal cancer | WHI, United States, Wactawski-Wende et al. (318) | 36,282 Postmenopausal women, age 50–79 y | Mean 19 | D3 400 IU/d + Ca vs Ca alone | 7 | 1.08 (0.83–1.34) (NS) |
| Comment: no effect on invasive cancers | ||||||
| Colorectal breast total invasive cancers | WHI, United States, Prentice et al. (319) | WHI 23,561 women not taking calcium or vitamin D at baseline | Mean 19 | D3 400 IU/d + Ca vs Ca alone | 7 | Colorectal: |
| 0.81 (0.58–1.13) (NS) | ||||||
| Breast: | ||||||
| 0.80 (0.78–0.98) (P = 0.02) | ||||||
| All invasive cancers: | ||||||
| 0.88 (0.78–0.98) (P = 0.03) | ||||||
| Colorectal cancer | WHI, United States, Dinf et al. (320) | 8117 Postmenopausal women, age 50–79 y (excluding women on estrogen replacement therapy) | Mean 19 | D3 400 IU/d + Ca vs placebo | 7 | 0.71 (0.46–1.09) |
| All cancers | RECORD trial, United Kingdom, Avenell et al. (321) | 5292 Men and women, age >70 y | Median 15 | D3 800 IU/d vs Ca daily | 3 | Cancer incidence: |
| Versus Ca daily | +D: 12.8% | |||||
| Versus D3 800 IU + Ca daily | −D: 11.9% | |||||
| Versus placebo | HR 1.07 (NS) | |||||
| Cancer mortality: | ||||||
| + D: 5.7% | ||||||
| −D: 6.7% | ||||||
| HR 0.85 (NS) | ||||||
| Total cancers | Australia (102) | 2256 Healthy postmenopausal women, age >70 y | Mean 20 | D3 500,000 IU once a year vs placebo | 3–5 | Seven cases in D group vs 10 in placebo group (NS) |
RCTs with cancer as a safety or secondary endpoint with fewer than five cases of cancer per arm of the study are not included, such as in Komulainen et al. (389) and Prince et al. (390).
Abbreviations: Ca, supplemental calcium salt (citrate or carbonate); D3, vitamin D3; NS, not significant; WHI, Womens’ Health Initiative.
Conclusions and outcome
A wealth of preclinical data support a possible role of 1,25(OH)2D on cell cycle progression and control of tumor growth. Total loss of vitamin D signaling also predisposes mice to the development of a variety of cancers when exposed to carcinogenic agents or genes. Poor vitamin D status is strongly linked to colon cancer. MR studies suggest a link between lifelong lower vitamin D status and risks of cancer (especially colon cancer). However, the existing intervention studies so far could not demonstrate a clear benefit of vitamin D supplementation on the incidence or evolution of major human cancers. Therefore, supplementation cannot be recommended for the sole purpose of primary or secondary prevention of cancer. Serum 25OHD levels <20 ng/mL (50 nmol/L) convey the greatest association with cancer. A few studies also suggested that serum 25OHD levels >100 nmol/L might also be associated with an increased risk for some types of cancer. However, such studies might be problematic due to the use of assays overestimating the true serum 25OHD values (322). Larger ongoing studies are needed to provide more decisive conclusions, such as the VITAL study. This is a randomized, double-blind, placebo-controlled clinical trial with a 2 × 2 factorial design with four groups: (1) active vitamin D3 at 2000 IU/d and active omega-3 fatty acids; (2) active vitamin D3 and omega-3 placebo; (3) vitamin D placebo and active omega-3; and (4) double placebo. The study population includes >25,000 subjects >50 years of age and the treatment period is 5 years. The primary endpoints are cancer and cardiovascular disease (323).
Cardiovascular System
Preclinical data
Several genes playing important roles in the cardiovascular system are targets of vitamin D signaling, including those encoding renin, plasminogen activator inhibitor (PAI), and thrombomodulin. In vitro studies demonstrate that 1,25(OH)2D has beneficial effects on endothelial, vascular smooth muscle, or cardiac muscle cells (324). Vdr-null as well as Cyp27b1-null mice develop high-renin hypertension and cardiac hypertrophy. This is in line with a negative regulation of the renin gene expression in the kidney by 1,25(OH)2D (325, 326). The cardiac hypertrophy and fibrosis probably have a dual origin: an indirect one by systemic high renin hypertension and a direct effect on cardiac muscle. Indeed, cardiac muscle-specific Vdr-null mice also develop cardiac hypertrophy and fibrosis that can be accelerated by increased cardiac stress (327). In line with the in vitro studies, Vdr-null mice show increased thrombogenesis and reduced fibrinolysis (325, 328, 329). In vitro exposure of smooth muscle cells to high concentrations of 1,25(OH)2D induces transdifferentiation into bone-forming–like cells with expression of several typical osteoblast-like genes, ultimately developing matrix calcifications (330).
Observational studies in humans
Observational human studies have extensively documented an inverse relationship between vitamin D status and cardiovascular risk factors or cardiovascular events. A meta-analysis of 19 prospective studies (65,994 patients) demonstrated an inverse relationship between serum 25OHD levels (ranging from 20 to 60 nmol/L) and risk of cardiovascular disease (RR, 1.03; 95% CI, 1.00 to 1.60; per 25-nmol/L decrement in serum 25OHD) (331).
A few large studies can be summarized as follows: in the Framingham Offspring Study, vitamin D–deficient participants (serum 25OHD <37.5 nmol/L)) were more likely to have their first cardiovascular event during a mean observation period of 5.4 years in comparison with those with values ≥37.5 nmol/L (HR, 1.62; 95% CI, 1.11 to 2.36) (332). In the NHANES 2001 to 2004 study, the prevalence of coronary heart disease (angina, myocardial infarction) was more common in adults with serum 25OHD levels <50 nmol/L compared with ≥75 nmol/L (OR adjusted for age, race, and sex, 1.49; 95% CI, 1.17 to 1.91) (333, 334). Adjusting for other risk factors (body mass index (BMI), chronic kidney disease, hypertension, diabetes mellitus, smoking, and use of vitamin D supplements) attenuated the association (OR, 1.24; 95%, CI 0.95 to 1.62). The prevalence of heart failure and peripheral arterial diseases was also higher among those with serum 25OHD values <50 nmol/L (ORs, 2.10 and 1.82, respectively) with similar attenuation after adjustment for other risk factors. Several observational studies also link peripheral vascular disease with a poor vitamin D status. Indeed, an inverse dose response relationship was observed, cross-sectionally, between serum 25OHD and peripheral arterial diseases among individuals aged 40 years and older in the large US NHANES III (2001 to 2004) study (335).
MR studies
Two independent MR studies did not find a link between genetically low serum 25OHD, predicted on the basis of SNPs related to four different genes involved in vitamin D synthesis, transport, or metabolism, and cardiovascular endpoints (69, 70) (Table 2). Similarly, a Canadian MR study did not find a link between SNPs in GC/DBP and cardiovascular diseases or stroke (71). Skaaby et al. (74), however, found that filaggrin-null mutations caused a 10% increased serum 25OHD concentrations due to higher efficacy of UV-B–induced vitamin D synthesis, as well as a better lipid profile. Similarly, Ooi et al. (72) demonstrated that SNPs in genes causing higher nonfasting remnant cholesterol concentrations also caused decreased serum 25OHD concentrations. These two MR studies suggest that the observed epidemiologic link between serum 25OHD and cardiovascular risks may be more complex and involve genes that predispose to both variations in serum 25OHD and cardiovascular risk factors (Table 2).
Intervention studies in humans
The results of intervention studies are less convincing. In systematic reviews and meta-analyses, there was no effect of vitamin D supplementation on cardiovascular outcomes, including myocardial infarction and stroke (336–339). A meta-analysis did not show a significant effect of vitamin D supplementation on several cardiovascular risk factors such as lipids, glucose, and blood pressure. In one of the larger trials included in this meta-analyses, vitamin D supplementation did not generate a beneficial effect on cardiovascular or metabolic risks after increasing baseline serum 25OHD levels from 23 to well above 40 ng/mL (58 to 100 nmol/L) (339). Therefore, it is not unlikely that only subjects with a low vitamin D status at baseline (with precise threshold still to be defined) may benefit from such intervention.
There are several large-scale ongoing RCTs dealing with probably >100,000 subjects evaluating a wide variety of outcomes, including cardiovascular events. The data from the VIDA trial in New Zealand adults and elderly subjects were just published (340). A monthly dose of 100,000 IU of vitamin D3 during a mean observation period of 3.4 years did not affect cardiovascular endpoints. Whether this is due to lack of a causal relationship between vitamin D status and cardiovascular outcomes or due to other factors such as the large intermittent dose raising serum 25OHD to >125 nmol/L, to the relatively good vitamin D status at baseline (mean serum 25OHD of 63 nmol/L), is so far unknown.
Hypertension and vitamin D status
There is geographic and racial variation in blood pressure, with risk of hypertension increasing from south to north in the Northern hemisphere. One proposed explanation for the association with latitude is that exposure to sunlight may be protective, either because of an effect of UV-B radiation or of vitamin D (73). In observational studies of normotensive or hypertensive individuals, there is an inverse association between serum 25OHD concentration and blood pressure (341–345). However, this link is complicated by a strong negative association between BMI, a well-known risk factor for hypertension, and low serum 25OHD levels. One very large (n = 142,255 Danish subjects) MR study (73) found that a 10% increase in genetically higher serum 25OHD concentrations was associated with a 0.3 mm Hg lower diastolic and systolic blood pressure and a small but significantly lower risk of hypertension (Table 2). A 2015 meta-analysis of 46 intervention trials (evaluated at trial level or on individual patient level) did not show a benefit of vitamin D supplementation on systolic or diastolic blood pressure (345). This meta-analysis, however, did not include a trial in 283 black adults (∼50% with hypertension), showing that vitamin D supplementation significantly decreased systolic blood pressure (−1.4 mm Hg for each additional 1000 U/d of vitamin D3) without affecting diastolic pressure (346).
Overall, a beneficial clinical effect of vitamin D supplementation on blood pressure in unselected adults has not been demonstrated. It is unclear whether specific ethnic groups or subjects at high risk of vitamin D deficiency are more likely to benefit from vitamin D supplementation. Data on vitamin D status and supplementation (with vitamin D or active metabolites/analogs) in patients with chronic renal failure also generated no clear answer.
Excess vitamin D and cardiovascular risks and events
Excess vitamin D may also have major negative effects on the cardiovascular system, with ectopic (vascular) calcification, organ failure, and death as a consequence. Thus, it seems that too little but also frank excess of vitamin D may both be deleterious for cardiovascular events (325).
Summary
There are good preclinical (biochemical, genetic, and animal data) data linking total absence of vitamin D action and adverse events in the cardiovascular system. Human observational data also link a poor vitamin D status with several cardiovascular risk factors (including all aspects of the metabolic syndrome) and cardiovascular events and even mortality. Intervention studies, however, so far are equivocal, and this may be due to lack of causality or due to poor design of the RCTs. A minimal effect of vitamin D supplementation of vitamin D–deficient subjects on systolic blood pressure is plausible (347). The causal nature of associations between vitamin D and cardiovascular disease remains uncertain. Whether the association differs across patient populations (e.g., different sexes and racial/ethnic groups, chronic kidney disease, diabetes) also remains to be explored (348, 349).
Obesity, Diabetes, and Metabolic Syndrome and Vitamin D Status
A high BMI is linked with low serum 25OHD concentration in most of the studies globally (350). Whether this is a causal link or not or whether increased fat mass causes the low vitamin D status or the other way around is not known. One hypothesis is that the fat-soluble vitamin D is more easily stored into fat cells (sequestration) before being available for further metabolism. There is, however, also a possible closed feedback loop between fat cells and vitamin D metabolism (in mice) of leptin–FGF23–Cyp27b1–serum 1,25(OH)2D and fat mass/leptin. Indeed, Vdr-null mice as well as Cyp27b1-null mice are leaner than controls and more resistant to diet-induced obesity by actions not completely understood but essentially due to a higher metabolic rate (351). Therefore, such mice have a lower fat mass and lower serum leptin concentrations. Second, leptin-deficient or leptin-resistant mice have higher serum levels of FGF23 because of leptin’s stimulatory effects on FGF23 production, which is known to be a major renal Cyp27b1 expression (352). Finally, to close the loop, low serum 1,25(OH)2D (action) decreases fat mass in mice. These observations are in clear contrast to those in humans, as a poorer vitamin D status is linked with obesity and type 2 diabetes in humans.
Vitamin D deficiency, as well as high BMI or obesity, are frequent and the (potential) consequences of both on health are overlapping. However, the precise contribution of a poor vitamin D status to obesity, its complications, and health in general are not well established.
The contribution of a poor vitamin D status to the health consequences of a high BMI or obesity is not known, but many consequences of both situations are overlapping. Indeed, all conditions known to be part of the metabolic syndrome are associated with a poor vitamin D status (353–358) in both adults and adolescents (359). One of the most consistent associations is with type 2 diabetes. For example, low serum 25OHD levels (<52.5 nmol/L)) are associated with a nearly twofold increased risk of fasting hyperglycemia or diabetes and a 1.5-fold increased risk of hypertension or hypertriglyceridemia (360). Such low 25OHD levels are also linked to a twofold increase in the overall prevalence of the metabolic syndrome (361). Longitudinal cohort studies, summarized in recent meta-analyses, have estimated an ∼40% risk reduction for incident diabetes in the highest vs the lowest category of blood serum 25OHD level (362). However, the observational nature of these studies precludes a definitive assessment of cause and effect because reverse causation or residual confounding cannot be excluded. Confounders are especially problematic in this area because blood 25OHD level is an excellent marker of good health. In relation to type 2 diabetes mellitus, higher serum 25OHD level is monotonically associated with a lower diabetes risk, suggesting no apparent threshold for benefit (362).
MR studies may help to explore the possible lifetime consequences of a low vitamin D status. Afzal et al. (64) concluded, based on >95,000 Danish subjects, that genetically low serum 25OHD predisposed to later type 2 diabetes. Another MR trial, however, dealing with >28,000 cases of type 2 diabetes using four known SNPs related to serum 25OHD revealed a nonsignificant OR of 1.01 (65, 295, 296). Husemoen et al. (67) studied ∼9000 Danish adults and found that higher predicted serum 25OHD [based on one vitamin D related SNP (DBP/GC)] was associated with a 1.6-fold increase in serum adiponectin. Such higher adipokine levels could explain a twofold lower risk for type 2 diabetes. A large MR study of 21 cohorts of European descent with obesity used four vitamin D–related SNPs but could not find a link between BMI and predicted serum 25OHD concentrations. Twelve SNPs related to higher risk for obesity, however, were found to have a significant link with higher BMI (Table 2).
Intervention studies have not demonstrated a consistent effect of vitamin D supplementation on body weight, glycemic control, or one or more aspects of the metabolic syndrome (363). The largest trial of vitamin D supplementation for prevention of type 2 diabetes is the Tromso study from Norway (511 white adults with prediabetes received 20,000 U/wk [∼2,900 U/d] vitamin D3 or placebo; follow-up ∼3.3 years for incident diabetes) (364). The risk of diabetes was lower in the vitamin D–supplemented group compared with placebo; however, the difference was not statistically significant (HR, 0.90; 95% CI, 0.69 to 1.18). Subgroup analyses in subjects with low baseline serum 25OHD yielded similar results. Three recent meta-analyses have evaluated the effects of vitamin D supplementation on one of three aspects of glycemic control in patients with established type 2 diabetes (365, 366). Krul-Poel et al. (367) evaluated 23 RCTs with a total of 1797 patients with type 2 diabetes. No significant effects were found on HbA1c, but vitamin D supplementation decreased fasting glycemia in subjects with poor baseline HbA1c. A second meta-analysis evaluated 24 RCTs, including 1528 patients with type 2 diabetes, and found a modest reduction in HbA1c (−0.3%), fasting blood glucose (-5mg/dl), or insulin resistance (all significant) following vitamin D supplementation with a mean daily dose of ∼4000 IU (or 100 μg) of vitamin D3.
“Vitamin D may also be important for male and female reproduction.”
They found, however, marked heterogeneity, as only 10 of 23 studies showed a positive effect. Subgroup analysis revealed some strange observations, as the supplementation therapy worked only in nonobese (BMI <25) subjects (whereas most type 2 diabetic patients are either overweight or obese) and only subjects with a normal baseline serum 25OHD (>50 nmol/L) showed an improved glycemic control, whereas no beneficial effects were seen in the vitamin D–deficient (<50 nmol/L) subjects. In a third recent meta-analysis Wu et al. (365) evaluated 23 RCTs dealing with >1000 patients with type 2 diabetes. Overall, mean serum HbA1c levels decreased by 0.25% overall (just significant), but the decrease in fasting blood glucose was not significant. Subgroup analysis revealed that HbA1c and fasting blood glucose, however, decreased significantly in subjects with vitamin D deficiency at baseline (<50 nmol/L) or in patients with starting BMI <30. The daily dose of vitamin D required for an improved glycemic control was ≥1000 IU/d. Both meta-analyses thus showed a modest improvement in glycemic control, but the subgroup analysis revealed very contradictory conclusions concerning which dose of vitamin D is required and which patients respond to vitamin D supplementation. The overall conclusion or interpretation of all of these data are that poor vitamin D status is frequently linked with the metabolic syndrome and type 2 diabetes. MR studies, however, did not support this hypothesis. Less than half of the existing intervention studies showed only limited beneficial effects, so that large-scale studies are needed to define its potential benefit and, possibly, its translation into clinical recommendations. The Vitamin D and Type 2 Diabetes (368) study, an ongoing trial that tests the efficacy of 4000 IU/d of vitamin D on prevention of diabetes in people at elevated risk, is expected to answer this question. While awaiting the results from such ongoing studies, it is impossible to define a serum 25OHD threshold for the prevention of metabolic events such as type 2 diabetes (369)
Miscellaneous targets of vitamin D
As VDR is expressed in nearly all nucleated cells and its genomic activation regulates a large number of genes, it is plausible that vitamin D may have an influence on other tissues than the major target tissues described above. We herein only briefly summarize a few topics.
Neurologic disorders
Low serum 25OHD concentrations have been found in patients suffering from a variety of neurologic diseases such as Parkinson disease, Alzheimer’s disease, or schizophrenia (370, 371), apart from MS (discussed above in the section on immunity). MR studies found clear links between vitamin D status and MS (see above) and also with Alzheimer’s disease (58). In the latter study, dealing with 17,008 cases and 37,154 controls, 1 SD lower predicted that serum 25OHD caused a significant 1.25-fold higher risk of Alzheimer’s disease (Table 2). Two other MR trials did not find a link with schizophrenia (59) or Parkinson disease (57).
Reproduction
Vitamin D may also be important for male and female reproduction. Male reproduction is impaired in the Tokyo strain of VDR-null mice, and 1,25(OH)2D directly inhibits aromatase gene expression. This is in line with previous studies in rodents and humans demonstrating the essential role of the aromatase enzyme for male reproduction (372). A variety of data also suggest that the vitamin D endocrine system may be operative in male reproduction (373). A small-scale RCT, however, did not show improvement of sperm quality in men with subfertile sperm by vitamin D supplementation (374). A poor vitamin D status during pregnancy is associated with increased risk for the mother and child. A recent Cochrane analysis (375) and another review (376) found that vitamin D supplementation may reduce the risk of preeclampsia, increase mean birth weight, reduce the risk of small for gestational age, and reduce the risk for wheezing in the offspring at age 3 (376).
Liver, lung, and kidney diseases
Although the liver has a very low or undetectable level of VDR expression, hepatic stellate cells are a clear target, as selective VDR deletion causes hepatic fibrosis and a state of nonalcoholic liver disease (377).
The lung is another a possible target tissue for vitamin D action. Observational and intervention studies clearly demonstrated a possible link between poor vitamin D status and chronic obstructive lung disease [as reviewed in Ref. (378)]. Finally, vitamin D status has also been linked to a variety of other diseases such as nonalcoholic fatty liver syndrome, some eye diseases, autism, and many other diseases, as reviewed elsewhere (17, 329, 379–381).
The kidney is a major partner in vitamin D homeostasis, and renal diseases are frequently associated with lower serum 25OHD and lower 1,25(OH)2D concentrations. The complicated role of vitamin D in this context falls below the scope of the present review. One MR, however, strangely concluded that a lower predicted serum 25OHD was associated with a modestly but significantly higher estimated glomerular filtration rate (Table 2).
Mortality
If low vitamin D status has such broad-ranging effects on so many (extraskeletal) tissues as described above, it would certainly increase the risk of a variety of diseases and thus ultimately increase mortality risks. No preclinical data exist on the relationship between longevity and vitamin D status. Many observational studies, as described above, associate a poor vitamin D status with nearly all major human diseases, and therefore it would not be surprising that a poor vitamin D status would be associated with increased mortality risk. Indeed, most observational studies found higher mortality rates in persons with the lowest vitamin D quartiles or quintiles in European (382) and US populations (383), with the highest mortality rates found in subjects with serum 25OHD <50 to 60 nmol/L. These very large studies also raised concerns about a U-shaped mortality curve, which suggests a slightly increased mortality rate in participants with the higher serum 25OHD levels (382, 383). However, subsequent recalibration of serum 25OHD determinations decreased the number of NHANES participants with high serum 25OHD [>100 nmol/L (40 ng/mL)], so that the apparent increased mortality in the two high serum 25OHD groups disappeared (384). A meta-analysis of eight prospective studies on >2600 participants (50 to 79 years of age) demonstrated a consistently higher overall mortality rate in participants with the lowest serum 25OHD concentrations (usually well below 50 nmol/L) (385). This observation included subjects living in different areas of the world (United States, Europe, and Japan). Surprisingly, the Leiden longevity study found that the offspring of nonagenarians (age >90 years) with at least one other nonagenarian in the family had lower (not higher as expected) serum 25OHD levels (386).
However, MR studies provide mixed evidence for a causal relationship. A small study (8417 German adults) confirmed an association between measured low serum 25OHD concentrations and mortality, but it did not find an effect of genetically lower serum 25OHD concentrations, based on only two SNPs, on mortality (343). The authors realized that this result may well be due to underpowering of the study. Another small-scale German study (3316 participants) also found no significant link between predicted serum 25OHD and mortality (85). However, a much larger Danish study (n = 95,766 and 9 to 19 years of follow-up) (84, 342) found an increased all-cause [RR, 1.3; confidence limit (CL), 1.05 to 1.61] and cancer (RR, 1.43; CL, 1.02 to 1.99) mortality with lower genetically predicted serum 25OHD. This effect was not much different from the excess mortality observed in a subgroup of this study using measured serum 25OHD concentrations (RR for overall mortality, 1.19; CL, 1.14 to 1.25).
Numerous RCTs designed to look at a variety of endpoints (mostly fractures or falls) also evaluated mortality risks as part of overall safety analysis. Three recent meta-analyses of such vitamin D supplementation studies generated similar conclusions (135, 306, 387): based on data of 22 RCTs on >30,000 participants (56 to 85 years of age with a median baseline serum 25OHD of 37.5 nmol/L) and 0.4 to 6.8 years of follow-up, vitamin D3 supplementation reduced all-cause mortality by 11% (387). A Cochrane analysis, including even more studies and >95,000 participants, came to a similar conclusion: vitamin D3 supplementation (most commonly used dosage of 800 IU/d) decreased the mortality rate by 6% [RR, 0.94 (0.91 to 0.98)], whereas vitamin D2 supplementation did not affect mortality, independently from calcium intake or baseline serum 25OHD (306). All-cause mortality is a hard and highly relevant endpoint and is easy to understand for the lay public. In view of the numerous large-scale ongoing intervention studies, this may well become a major endpoint to define the optimal vitamin D status and the associated recommended intake to achieve this status.
Vitamin D and Extraskeletal Health: Summary
The vitamin D endocrine system regulates a very large number of genes in many cells and tissues not related to calcium homeostasis. This effect is seen early in the evolution of vertebrates and in mammals and humans. Thus, it is plausible that vitamin D has nonskeletal effects. A large set of observational data and some MR studies support this hypothesis. Intervention studies, however, have so far been inconsistent or generated “null effects.” The strongest data for possible extraskeletal effects of vitamin D so far deal with modest effects on muscle strength and falls, acute respiratory infections, and on mortality risks. Hopefully, a large number of ongoing (large-scale) trials will generate clearer answers.
General Summary and Conclusion
The skeletal consequences of severe vitamin D deficiency are well established, as rickets was a disease that led to a search for an etiology for many centuries. An absence of vitamin D itself or of its active metabolites or of its receptor generates the same phenotype in animals as in humans. Vitamin D deficiency rickets can be prevented or cured by daily supplements of 200 to 400 IU of vitamin D per day. There is wide consensus that serum 25OHD levels <25 to 30 nmol/L (10 to 12 ng/mL) increase the risk of rickets. The pathogenesis of the disease is impaired intestinal calcium and phosphate absorption due to lack of their transporters, causing impaired mineralization and excess osteoid, whereas the typical hallmark of rachitic growth plates is due to hypophosphatemia-induced lack of apoptosis of hypertrophic growth plate chondrocytes in combination with a hypocalcemia-induced decrease in chondrocyte differentiation. Rickets is still endemic in some regions of the world or in some specific risk groups owing to a lack of implementation of systematic vitamin D supplementation during the first years of life.
“RCTs using vitamin D supplementation to prevent or improve possible nonskeletal diseases related to poor vitamin D status have generated mixed results.”
There is also general consensus that severe vitamin D deficiency or absence of 1,25(OH)2D (as in chronic renal failure) later in life can cause osteomalacia. Milder degrees of vitamin D deficiency generate secondary hyperparathyroidism in most but not all subjects of various ages and accelerates bone turnover and ultimately accelerates bone loss and the risk of fractures in the elderly.
In selected populations, RCTs with vitamin D and calcium supplementation demonstrated a decreased incidence of hip fractures and other nonvertebral fractures of ∼15%, with the effect being greater (i) in 80+ and 70 to 80 years of age persons than in persons 60 to 70 years of age, (ii) in those who are institutionalized in comparison with community living elderly, (iii) when vitamin D is combined with calcium supplementation, and (4) when compliance is >80%. Nearly all major governmental guidelines (111) confirm this policy and recommend that serum 25OHD levels <50 nmol/L should be avoided. Some expert groups (UK Scientific Advisory Committee on Nutrition) (29), however, consider this conclusion still not sufficiently validated to recommend specific supplementation beyond what is needed to prevent rickets/osteomalacia. Other experts and grassroots organizations recommend much higher intake and aim for much higher serum 25OHD concentrations based on observational data and comparison with serum 25OHD levels in individuals living in circumstances similar to those of early humans.
Because of the broad range of putative actions, there is much less consensus about the extraskeletal effects of vitamin D. Biochemical and genetic data clearly demonstrate that the vitamin D endocrine system could regulate, usually in a coherent fashion, a very large number of genes (∼3% of all genes). Evidently that includes mostly genes not related to calcium or bone homeostasis (388). Animal data largely confirm a coherent action at the cellular, tissue, or total-body level of the vitamin D endocrine system on cell proliferation, cell differentiation, the immune, muscular, cardiovascular, and other systems. These preclinical observations were made either in situations of total absence of vitamin D (action) or by exposure to supraphysiologic concentrations of 1,25(OH)2D or its analogs. A wealth of human cross-sectional and long-term prospective studies have linked a poor vitamin D status with a variety of human diseases as predicted on the basis of the preclinical data, including higher risk of cancer, infections, autoimmune diseases, cardiovascular and metabolic risk factors and events, and muscle dysfunction and falls. This has generated an intense interest and even irrational enthusiasm about the possible health effects of vitamin D supplements. Most governmental and scientific societies are more prudent and await further proof of causality before formulating optimal thresholds for serum 25OHD or optimal dosages beyond what is needed for skeletal effects. The proof of causality ultimately has to come from RCTs, but the recent introduction of MR studies has opened up a new strategy.
About 38 MR studies have so far looked at the possible health consequences of genetically lower serum 25OHD concentrations. Indeed, serum 25OHD concentrations have a strong genetic background as based on several twin studies. Large-scale GWASs have identified so far polymorphisms in at least four genes involved in vitamin D synthesis, transport, and metabolism in subjects of European or Asian descent that are associated with serum 25OHD levels. Such MR studies have intrinsic limitations, as the genes discovered so far only explain ∼5% of the variance of serum 25OHD and only allow the study of linear effects, whereas it is not unlikely that there is a threshold above which further increases in serum 25OHD would not affect the actions of the 1,25(OH)2D. Nevertheless, three MR studies (all in Europeans) so far demonstrated a clear association of genetically predicted lower serum 25OHD concentrations and the risk of MS, and one additional genetic study confirmed this association. Additionally, a large MR study in patients with type 1 diabetes confirmed a link between low serum 25OHD and the risk of this disease. As both MS and type 1 diabetes have a well-documented autoimmune origin, such data, in combination with many preclinical and observational data, make a strong case for a role of lifetime lower serum 25OHD concentrations (maybe especially early in life) and later autoimmune diseases. An MR study dealing with asthma was negative. Seven of eight MR studies dealing with a variety of cancers covering a total of >100,000 cases did not find a link between genetically lower serum 25OHD concentrations and total or organ-specific cancer incidence or mortality (Table 2). One MR study, however, found a that a lower (20 nmol/L) predicted serum 25OHD concentration (based on SNPs in the vitamin D pathway) caused a significantly increased OR of 1.27 of all types of ovarian cancer and an OR of 1.54 for high-grade ovarian cancer (52). This is unexpected, as most observational studies showed a clear link with colorectal cancer (negative in MR studies) and not with ovarian cancer. MR studies evaluating the link between vitamin D status and several aspects of the metabolic syndrome or cardiovascular events have generated complex data. Gene polymorphisms predisposing for lower serum 25OHD concentrations were not associated with higher BMI (66), higher incidence of type 2 diabetes (65), or higher incidence of cardiovascular events (71). Other MR studies, however, found a link between lower predicted serum 25OHD and adiponectin concentrations, which is a strong surrogate predictive factor for type 2 diabetes. Similarly, gene polymorphisms causing higher nonfasting cholesterol remnants are associated with lower serum 25OHD concentrations (Table 2). Hypertension was found to be associated with lower predicted serum 25OHD concentrations (73), well in line with the results of some RCTs. Mortality as studied in one large MR study was significantly linked with lower serum 25OHD concentrations predicted based on SNPs in the vitamin D pathway (84), although this was not confirmed in two smaller German studies (Table 2).
RCTs using vitamin D supplementation to prevent or improve possible nonskeletal diseases related to poor vitamin D status have generated mixed results. Null results were generated in 9 of 10 RCTs dealing with cancer (Table 6). Similarly, most RCTs dealing with aspects of the metabolic syndrome or type 2 diabetes generated “null” results. A meta-analysis of RCTs, however, demonstrated a modest effect on the risk of falls of elderly, vitamin D–deficient subjects. Vitamin D supplementation also modestly reduced the risk of upper respiratory infections or exacerbations of chronic obstructive lung disease patients. In line with an MR study mentioned above, vitamin D supplementation may have a modest blood pressure lowering effect in mildly hypertensive subjects. Based on a large number of RCTs, a modest 6% to 8% decrease in mortality risk has been observed when elderly vitamin D–deficient subjects receive modest doses of vitamin D.
Based on all of these data, the hype that vitamin D supplementation may be a cure for all major diseases of humankind is certainly not confirmed. However, several MR studies and RCTs suggest some (rather modest) beneficial effects of vitamin D supplementation for extraskeletal actions, such as muscle/falls, respiratory infections, MS, and hypertension. Local therapy with vitamin D analogs has well-documented beneficial effects on the evolution of psoriatic lesions.
According to the National Institutes of Health ClinicalTrials.gov register, ∼3000 RCTs dealing with vitamin D are still ongoing, so we may hope that within the next decade the results of these studies will further clarify the possible beneficial effects of vitamin D.
Questions for Future Research
What are the precise details of the effects of UV light on skin production of vitamin D? Indeed, the vitamin D production after full-body exposure to sunlight (below or at the minimal erythema level) has been estimated to be >10,000 IU/d, whereas others found that such exposure is equivalent to a daily intake of ∼1000 IU.
Is measured or calculated free serum 25OHD concentration a better marker than total serum 25OHD for vitamin D status and different health outcomes?
What is the role of the local production of 1,25(OH)2D for skeletal and extraskeletal effects?
Which factors regulate the local activity of 1α-hydroxylase in different tissues?
High PTH, as well as low serum 25OHD levels, is associated with cardiovascular diseases. What is the contribution of each hormone on the overall effect?
Is the optimal vitamin D status tissue-dependent, with different thresholds for different physiological systems, and does it vary by age and race?
Is the use of 25OHD supplementation advantageous over vitamin D supplementation?
How can we explain the interindividual difference in the vitamin D status and response to supplementation?
What is the best approach to improve the knowledge to completely understand the action of 1,25(OH)2D on intestinal calcium and phosphate transport?
What are the effects of vitamin D deficiency during pregnancy and early life on adolescent and adult outcomes?
What are the mechanisms underlying tissue-specific actions and can they be best explored by selective deletion of vitamin D signaling?
For most other ligands of nuclear transcription factors, such as thyroid or sex steroid hormones or glucocorticoids, low and high concentrations are found to be associated with poor health outcome. Is this also true for serum 25OHD and other vitamin D metabolites?
There are ∼50 known metabolites of vitamin D. Do metabolites other than 25OHD and 1,25(OH)2D have an independent biological role?
Acknowledgments
The authors thank Laura Macri (Pisa, Italy) for help with the references.
Financial Support: This work was supported by Research Foundation Flanders (FWO)/KU Leuven Grant G0A2416N (to R.B. and G.C.); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 AR051930 and Veterans Affairs Grant 1 I01 BX003814-01 (to D.B.); National Institutes of Health Grant DK32333, as well as grants from Genome Quebec and the Cancer Research Society (to J.H.W.); and by US Department of Agriculture Agriculture Research Service Agreement 58-1950-7-707 (to B.D.-H.).
Disclosure Summary R.B. has received lecture fees from l’Oréal and Abiogen and is co-owner of a university patent on vitamin D analogs licensed to Hybrigenix. C.M. is a consultant for Abiogen Pharma and Shire and received lecture fees from Shire. J.H.W. has received lecture fees from Abiogen. B.D.-H. received an investigator-initiated grant from Pfizer. P.L. has received lecture fees from Abiogen. M.L.-C. has received educational fees from, and is on the advisory board for, Mantecorp/Farmasa and Sanofi. A.G. has served as a consultant for Abiogen. J.B. has consultancies for Abiogen, Amgen, Radius, Ultragenyx, and Regeneron. The remaining authors have nothing to disclose.
Glossary
Abbreviations
- 1,25(OH)D
1α,25-dihydroxyvitamin D
- 25OHD
25-hydroxyvitamin D
- BMD
bone mineral density
- BMI
body mass index
- CD
Crohn disease
- CL
confidence limit
- DBP
vitamin D–binding protein
- DC
dendritic cell
- GWAS
genome-wide association study
- HR
hazard ratio
- IBD
inflammatory bowel disease
- MR
Mendelian randomization
- MS
multiple sclerosis
- NHANES
National Health and Nutrition Examination Survey
- NOD2
nucleotide oligomerization domain protein 2
- NMSC
nonmelanoma skin cancer
- RA
rheumatoid arthritis
- RCT
randomized controlled trial
- RR
relative risk
- SNP
single nucleotide polymorphism
- TB
tuberculosis
- Treg
regulatory T
- URT
upper respiratory tract
- VDR
vitamin D receptor
References
- 1. Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci USA. 2004;101(19):7392–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–559. [DOI] [PubMed] [Google Scholar]
- 3. Martineau C, Naja RP, Husseini A, Hamade B, Kaufmann M, Akhouayri O, Arabian A, Jones G, St-Arnaud R. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J Clin Invest. 2018;128(8):3546–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jorde R. RCTS are the only appropriate way to demonstrate the role of vitamin D in health. J Steroid Biochem Mol Biol. 2018;177:10–14. [DOI] [PubMed] [Google Scholar]
- 5. Scragg R. Limitations of vitamin D supplementation trials: why observational studies will continue to help determine the role of vitamin D in health. J Steroid Biochem Mol Biol. 2018;177:6–9. [DOI] [PubMed] [Google Scholar]
- 6. Harroud A, Richards JB. Mendelian randomization in multiple sclerosis: a causal role for vitamin D and obesity? Mult Scler. 2018;24(1):80–85. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouillon R. Genetic and racial differences in the vitamin D endocrine system. Endocrinol Metab Clin North Am. 2017;46(4):1119–1135. [DOI] [PubMed] [Google Scholar]
- 9. Binkley N, Dawson-Hughes B, Durazo-Arvizu R, Thamm M, Tian L, Merkel JM, Jones JC, Carter GD, Sempos CT. Vitamin D measurement standardization: the way out of the chaos. J Steroid Biochem Mol Biol. 2017;173:117–121. [DOI] [PubMed] [Google Scholar]
- 10. Bouillon R, Rosen C. The IOM–Endocrine Society controversy on recommended vitamin D targets: in support of the IOM position In: Feldman D, Wesley Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D. Vol. 1 Biochemistry, Physiology and Diagnostics. 4th ed.London, England: Academic Press; 2017:1065–1089. [Google Scholar]
- 11. Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, Giustina A, Binkley N. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br J Clin Pharmacol. 2018;84(10):2194–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279(16):16754–16766. [DOI] [PubMed] [Google Scholar]
- 13. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140(11):4982–4987. [DOI] [PubMed] [Google Scholar]
- 14. Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139(10):4391–4396. [DOI] [PubMed] [Google Scholar]
- 15. Donohue MM, Demay MB. Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology. 2002;143(9):3691–3694. [DOI] [PubMed] [Google Scholar]
- 16. Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102(27):9637–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bouillon R. Vitamin D: from photosynthesis, metabolism and action to clinical applications. In: DeGroot LJ, Jameson JL, eds. Endocrinology. Philadelphia, PA: WB Saunders; 2001:1009–1028.
- 18. Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006;116(12):3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naja RP, Dardenne O, Arabian A, St Arnaud R. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology. 2009;150(9):4024–4032. [DOI] [PubMed] [Google Scholar]
- 20. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Bone. 2003;32(4):332–340. [DOI] [PubMed] [Google Scholar]
- 22. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136(4):1317–1327.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhawan P, Veldurthy V, Yehia G, Hsaio C, Porta A, Kim KI, Patel N, Lieben L, Verlinden L, Carmeliet G, Christakos S. Transgenic expression of the vitamin D receptor restricted to the ileum, cecum, and colon of vitamin D receptor knockout mice rescues vitamin D receptor-dependent rickets. Endocrinology. 2017;158(11):3792–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P, Lafage-Proust MH, Dresselaers T, Feng JQ, Bonewald LF, Meyer MB, Pike JW, Bouillon R, Carmeliet G. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122(5):1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamichi Y, Udagawa N, Horibe K, Mizoguchi T, Yamamoto Y, Nakamura T, Hosoya A, Kato S, Suda T, Takahashi N. VDR in osteoblast-lineage cells primarily mediates vitamin D treatment-induced increase in bone mass by suppressing bone resorption. J Bone Miner Res. 2017;32(6):1297–1308. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto Y, Yoshizawa T, Fukuda T, Shirode-Fukuda Y, Yu T, Sekine K, Sato T, Kawano H, Aihara K, Nakamichi Y, Watanabe T, Shindo M, Inoue K, Inoue E, Tsuji N, Hoshino M, Karsenty G, Metzger D, Chambon P, Kato S, Imai Y. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154(3):1008–1020. [DOI] [PubMed] [Google Scholar]
- 27. Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O’Loughlin PD, Nicholson GC, Briffa KH, Eisman JA, Gardiner EM. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res. 2006;21(10):1618–1626. [DOI] [PubMed] [Google Scholar]
- 28. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scientific Advisory Committee on Nutrition Vitamin D and Health. Available at: www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition. Accessed 1 May 2018.
- 30. Schoenmakers I, Pettifor JM, Peña-Rosas JP, Lamberg-Allardt C, Shaw N, Jones KS, Lips P, Glorieux FH, Bouillon R. Prevention and consequences of vitamin D deficiency in pregnant and lactating women and children: a symposium to prioritise vitamin D on the global agenda. J Steroid Biochem Mol Biol. 2016;164:156–160. [DOI] [PubMed] [Google Scholar]
- 31. Beck-Nielsen SS, Jensen TK, Gram J, Brixen K, Brock-Jacobsen B. Nutritional rickets in Denmark: a retrospective review of children’s medical records from 1985 to 2005. Eur J Pediatr. 2009;168(8):941–949. [DOI] [PubMed] [Google Scholar]
- 32. Dagnelie PC, Vergote FJ, van Staveren WA, van den Berg H, Dingjan PG, Hautvast JG. High prevalence of rickets in infants on macrobiotic diets. Am J Clin Nutr. 1990;51(2):202–208. [DOI] [PubMed] [Google Scholar]
- 33. Uday S, Gorman S, Feltbower RG, Mathai M. Ethnic variation in the correlation between waist to height ratio and total daily insulin requirement in children with type 1 diabetes: a cross-sectional study. Pediatr Diabetes. 2017;18(2):128–135. [DOI] [PubMed] [Google Scholar]
- 34. Uday S, Högler W. Prevention of rickets and osteomalacia in the UK: political action overdue. Arch Dis Child. 2018;103(9):901–906. [DOI] [PubMed] [Google Scholar]
- 35. Hatun Ş, Ozkan B, Bereket A. Vitamin D deficiency and prevention: Turkish experience. Acta Paediatr. 2011;100(9):1195–1199. [DOI] [PubMed] [Google Scholar]
- 36. Aggarwal V, Seth A, Aneja S, Sharma B, Sonkar P, Singh S, Marwaha RK. Role of calcium deficiency in development of nutritional rickets in Indian children: a case control study. J Clin Endocrinol Metab. 2012;97(10):3461–3466. [DOI] [PubMed] [Google Scholar]
- 37. Foster BL, Nociti FH Jr, Somerman MJ. The rachitic tooth. Endocr Rev. 2014;35(1):1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. [DOI] [PubMed] [Google Scholar]
- 39. Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–1221. [DOI] [PubMed] [Google Scholar]
- 40. Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24(4):693–701. [DOI] [PubMed] [Google Scholar]
- 41. Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–1250. [DOI] [PubMed] [Google Scholar]
- 42. Lips P, Netelenbos JC, Jongen MJ, van Ginkel FC, Althuis AL, van Schaik CL, van der Vijgh WJ, Vermeiden JP, van der Meer C. Histomorphometric profile and vitamin D status in patients with femoral neck fracture. Metab Bone Dis Relat Res. 1982;4(2):85–93. [DOI] [PubMed] [Google Scholar]
- 43. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25(2):305–312. [DOI] [PubMed] [Google Scholar]
- 44. Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–639. [DOI] [PubMed] [Google Scholar]
- 46. Bouillon R, Van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, Lips P. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98(8):E1283–E1304. [DOI] [PubMed] [Google Scholar]
- 47. Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25-Hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J Bone Miner Res. 2018;33(8):1464–1469. [DOI] [PubMed] [Google Scholar]
- 48. Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, Taylor L, Fenwick S, Camargo CA, Stewart AW, Scragg R. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017;282(5):452–460. [DOI] [PubMed] [Google Scholar]
- 49. Chandler PD, Tobias DK, Wang L, Smith-Warner SA, Chasman DI, Rose L, Giovannucci EL, Buring JE, Ridker PM, Cook NR, Manson JE, Sesso HD. Association between vitamin D genetic risk score and cancer risk in a large cohort of U.S. women. Nutrients. 2018;10(1):E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, Lewis SJ, Gunter MJ, Mondul A, Shui IM, Theodoratou E, Nimptsch K, Lindström S, Albanes D, Kühn T, Key TJ, Travis RC, Vimaleswaran KS, Kraft P, Pierce BL, Schildkraut JM; GECCO Consortium; PRACTICAL Consortium; GAME-ON Network (CORECT, DRIVE, ELLIPSE, FOCI-OCAC, TRICL-ILCCO) . Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359:j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Huo D, Kupfer S, Alleyne D, Ogundiran TO, Ojengbede O, Zheng W, Nathanson KL, Nemesure B, Ambs S, Olopade OI, Zheng Y. Genetic variation in the vitamin D related pathway and breast cancer risk in women of African ancestry in the root consortium. Int J Cancer. 2018;142(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ong JS, Cuellar-Partida G, Lu Y, Fasching PA, Hein A, Burghaus S, Beckmann MW, Lambrechts D, Van Nieuwenhuysen E, Vergote I, Vanderstichele A, Anne Doherty J, Anne Rossing M, Chang-Claude J, Eilber U, Rudolph A, Wang-Gohrke S, Goodman MT, Bogdanova N, Dörk T, Dürst M, Hillemanns P, Runnebaum IB, Antonenkova N, Butzow R, Leminen A, Nevanlinna H, Pelttari LM, Edwards RP, Kelley JL, Modugno F, Moysich KB, Ness RB, Cannioto R, Høgdall E, Høgdall CK, Jensen A, Giles GG, Bruinsma F, Kjaer SK, Hildebrandt MA, Liang D, Lu KH, Wu X, Bisogna M, Dao F, Levine DA, Cramer DW, Terry KL, Tworoger SS, Stampfer M, Missmer S, Bjorge L, Salvesen HB, Kopperud RK, Bischof K, Aben KK, Kiemeney LA, Massuger LF, Brooks-Wilson A, Olson SH, McGuire V, Rothstein JH, Sieh W, Whittemore AS, Cook LS, Le ND, Gilks CB, Gronwald J, Jakubowska A, Lubiński J, Kluz T, Song H, Tyrer JP, Wentzensen N, Brinton L, Trabert B, Lissowska J, McLaughlin JR, Narod SA, Phelan C, Anton-Culver H, Ziogas A, Eccles D, Campbell I, Gayther SA, Gentry-Maharaj A, Menon U, Ramus SJ, Wu AH, Dansonka-Mieszkowska A, Kupryjanczyk J, Timorek A, Szafron L, Cunningham JM, Fridley BL, Winham SJ, Bandera EV, Poole EM, Morgan TK, Risch HA, Goode EL, Schildkraut JM, Pearce CL, Berchuck A, Pharoah PD, Chenevix-Trench G, Gharahkhani P, Neale RE, Webb PM, MacGregor S; Australian Ovarian Cancer Study . Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2016;45(5):1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trummer O, Langsenlehner U, Krenn-Pilko S, Pieber TR, Obermayer-Pietsch B, Gerger A, Renner W, Langsenlehner T. Vitamin D and prostate cancer prognosis: a Mendelian randomization study. World J Urol. 2016;34(4):607–611. [DOI] [PubMed] [Google Scholar]
- 54. Theodoratou E, Palmer T, Zgaga L, Farrington SM, McKeigue P, Din FV, Tenesa A, Davey-Smith G, Dunlop MG, Campbell H. Instrumental variable estimation of the causal effect of plasma 25-hydroxy-vitamin D on colorectal cancer risk: a Mendelian randomization analysis. PLoS One. 2012;7(6):e37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dudding T, Johansson M, Thomas SJ, Brennan P, Martin RM, Timpson NJ. Assessing the causal association between 25-hydroxyvitamin D and the risk of oral and oropharyngeal cancer using Mendelian randomization. Int J Cancer. 2018;143(5):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun YQ, Brumpton BM, Bonilla C, Lewis SJ, Burgess S, Skorpen F, Chen Y, Nilsen TIL, Romundstad PR, Mai XM. Serum 25-hydroxyvitamin D levels and risk of lung cancer and histologic types: a Mendelian randomisation analysis of the HUNT study. Eur Respir J. 2018;51(6):1800329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Larsson SC, Singleton AB, Nalls MA, Richards JB; International Parkinson’s Disease Genomics Consortium (IPDGC) . No clear support for a role for vitamin D in Parkinson’s disease: a Mendelian randomization study. Mov Disord. 2017;32(8):1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, Richards JB. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology. 2016;87(24):2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Davey Smith G, Munafò MR. Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep. 2016;6(1):26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, Chitnis T, Waldman A, Lotze T, Schreiner T, Belman A, Greenberg B, Weinstock-Guttman B, Aaen G, Tillema JM, Hart J, Caillier S, Ness J, Harris Y, Rubin J, Candee M, Krupp L, Gorman M, Benson L, Rodriguez M, Mar S, Kahn I, Rose J, Roalstad S, Casper TC, Shen L, Quach H, Quach D, Hillert J, Bäärnhielm M, Hedstrom A, Olsson T, Kockum I, Alfredsson L, Metayer C, Schaefer C, Barcellos LF, Waubant E; Network of Pediatric Multiple Sclerosis Centers . Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88(17):1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, Leong A, Greenwood CM, Thanassoulis G, Richards JB. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study [published correct appears in PLoS Med. 2016;13(3):e1001981]. PLoS Med. 2015;12(8):e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, Shen L, Schaefer C, Link J, Gyllenberg A, Hedström AK, Olsson T, Hillert J, Kockum I, Glymour MM, Alfredsson L, Barcellos LF. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, Greissl C, Ramos-Lopez E, Hyppönen E, Dunger DB, Spector TD, Ouwehand WH, Wang TJ, Badenhoop K, Todd JA. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(4):298–306. [DOI] [PubMed] [Google Scholar]
- 65. Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, Langenberg C, Wareham NJ, Forouhi NG; InterAct Consortium . Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, Wood AR, Michaëlsson K, Vandenput L, Zgaga L, Yerges-Armstrong LM, McCarthy MI, Dupuis J, Kaakinen M, Kleber ME, Jameson K, Arden N, Raitakari O, Viikari J, Lohman KK, Ferrucci L, Melhus H, Ingelsson E, Byberg L, Lind L, Lorentzon M, Salomaa V, Campbell H, Dunlop M, Mitchell BD, Herzig KH, Pouta A, Hartikainen AL, Streeten EA, Theodoratou E, Jula A, Wareham NJ, Ohlsson C, Frayling TM, Kritchevsky SB, Spector TD, Richards JB, Lehtimäki T, Ouwehand WH, Kraft P, Cooper C, März W, Power C, Loos RJ, Wang TJ, Järvelin MR, Whittaker JC, Hingorani AD, Hyppönen E; Genetic Investigation of Anthropometric Traits (GIANT) Consortium . Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Husemoen LL, Skaaby T, Martinussen T, Jørgensen T, Thuesen BH, Kistorp C, Jeppesen J, Thyssen JP, Meldgaard M, Szecsi PB, Fenger M, Linneberg A. Investigating the causal effect of vitamin D on serum adiponectin using a Mendelian randomization approach. Eur J Clin Nutr. 2014;68(2):189–195. [DOI] [PubMed] [Google Scholar]
- 68. Wang N, Chen C, Zhao L, Chen Y, Han B, Xia F, Cheng J, Li Q, Lu Y. Vitamin D and nonalcoholic fatty liver disease: bi-directional Mendelian randomization analysis. EBioMedicine. 2018;28:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian Randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet. 2016;9(4):349–356. [DOI] [PubMed] [Google Scholar]
- 70. Brøndum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):651–661. [DOI] [PubMed] [Google Scholar]
- 71. Leong A, Rehman W, Dastani Z, Greenwood C, Timpson N, Langsetmo L, Berger C, Fu L, Wong BY, Malik S, Malik R, Hanley DA, Cole DE, Goltzman D, Richards JB; METASTROKE . The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. PLoS Med. 2014;11(10):e1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ooi EM, Afzal S, Nordestgaard BG. Elevated remnant cholesterol in 25-hydroxyvitamin D deficiency in the general population: Mendelian randomization study. Circ Cardiovasc Genet. 2014;7(5):650–658. [DOI] [PubMed] [Google Scholar]
- 73. Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M, Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schöttker B, Saum KU, McCarthy MI, Dupuis J, Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G, Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP, Hernandez DG, Byberg L, Hagström E, Melhus H, Ingelsson E, Mellström D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O, Hayward C, Wilson JF, Rudan I, Salomaa V, Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaëlsson K, Bandinelli S, Frayling TM, Hartman CA, Sørensen TI, Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimäki T, Kuh D, Humphries SE, Cooper C, Ohlsson C, März W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C, Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD, Pilz S, Whittaker JC, Järvelin MR, Hyppönen E; LifeLines Cohort Study Investigators; International Consortium for Blood Pressure (ICBP); Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; Global Blood Pressure Genetics (Global BPGen) consortium . Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Skaaby T, Husemoen LL, Martinussen T, Thyssen JP, Melgaard M, Thuesen BH, Pisinger C, Jørgensen T, Johansen JD, Menné T, Carlsen B, Szecsi PB, Stender S, Fenger RV, Fenger M, Linneberg A. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach. PLoS One. 2013;8(2):e57647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li SS, Gao LH, Zhang XY, He JW, Fu WZ, Liu YJ, Hu YQ, Zhang ZL. Genetically low vitamin D levels, bone mineral density, and bone metabolism markers: a Mendelian randomisation study. Sci Rep. 2016;6(1):33202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Larsson SC, Melhus H, Michaëlsson K. Circulating serum 25-hydroxyvitamin D levels and bone mineral density: Mendelian randomization study. J Bone Miner Res. 2018;33(5):840–844. [DOI] [PubMed] [Google Scholar]
- 77. Cuellar-Partida G, Williams KM, Yazar S, Guggenheim JA, Hewitt AW, Williams C, Wang JJ, Kho PF, Saw SM, Cheng CY, Wong TY, Aung T, Young TL, Tideman JWL, Jonas JB, Mitchell P, Wojciechowski R, Stambolian D, Hysi P, Hammond CJ, Mackey DA, Lucas RM, MacGregor S; Consortium for Refractive Error and Myopia (CREAM) . Genetically low vitamin D concentrations and myopic refractive error: a Mendelian randomization study. Int J Epidemiol. 2017;46(6):1882–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Manousaki D, Paternoster L, Standl M, Moffatt MF, Farrall M, Bouzigon E, Strachan DP, Demenais F, Lathrop M, Cookson WOCM, Richards JB. Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: a Mendelian randomization study. PLoS Med. 2017;14(5):e1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mao Y, Zhan Y, Huang Y.. Vitamin D and asthma: a Mendelian randomization study. Ann Allergy Asthma Immunol. 2017;119(1):95–97.e1. [DOI] [PubMed] [Google Scholar]
- 80. Liefaard MC, Ligthart S, Vitezova A, Hofman A, Uitterlinden AG, Kiefte-de Jong JC, Franco OH, Zillikens MC, Dehghan A. Vitamin D and C-reactive protein:a Mendelian randomization study. PLoS One. 2015;10(7):e0131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Viatte S, Yarwood A, McAllister K, Al-Mudhaffer S, Fu B, Flynn E, Symmons DP, Young A, Barton A. The role of genetic polymorphisms regulating vitamin D levels in rheumatoid arthritis outcome: a Mendelian randomisation approach. Ann Rheum Dis. 2014;73(7):1430–1433. [DOI] [PubMed] [Google Scholar]
- 82. Noordam R, Hamer MA, Pardo LM, van der Nat T, Kiefte-de Jong JC, Kayser M, Slagboom PE, Uitterlinden A, Zillikens MC, Beekman M, Nijsten T, van Heemst D, Gunn DA. No causal association between 25-hydroxyvitamin D and features of skin aging: evidence from a bidirectional Mendelian randomization study. J Invest Dermatol. 2017;137(11):2291–2297. [DOI] [PubMed] [Google Scholar]
- 83. Ordóñez-Mena JM, Maalmi H, Schöttker B, Saum KU, Holleczek B, Wang TJ, Burwinkel B, Brenner H. Genetic variants in the vitamin D pathway, 25(OH)D levels, and mortality in a large population-based cohort study. J Clin Endocrinol Metab. 2017;102(2):470–477. [DOI] [PubMed] [Google Scholar]
- 84. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, März W, Pieber TR, Obermayer-Pietsch B, Renner W. Vitamin D and mortality: a Mendelian randomization study. Clin Chem. 2013;59(5):793–797. [DOI] [PubMed] [Google Scholar]
- 86. Teumer A, Gambaro G, Corre T, Bochud M, Vollenweider P, Guessous I, Kleber ME, Delgado GE, Pilz S, März W, Barnes CLK, Joshi PK, Wilson JF, de Borst MH, Navis G, van der Harst P, Heerspink HJL, Homuth G, Endlich K, Nauck M, Köttgen A, Pattaro C, Ferraro PM. Negative effect of vitamin D on kidney function: a Mendelian randomization study. Nephrol Dial Transplant. 2018;33(12):2139–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585–591. [DOI] [PubMed] [Google Scholar]
- 88. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383(9912):146–155. [DOI] [PubMed] [Google Scholar]
- 89. Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637–1642. [DOI] [PubMed] [Google Scholar]
- 90. Heikinheimo RJ, Inkovaara JA, Harju EJ, Haavisto MV, Kaarela RH, Kataja JM, Kokko AM, Kolho LA, Rajala SA. Annual injection of vitamin D and fractures of aged bones. Calcif Tissue Int. 1992;51(2):105–110. [DOI] [PubMed] [Google Scholar]
- 91. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Komulainen MH, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, Saarikoski S. HRT and Vit D in prevention of non-vertebral fractures in postmenopausal women; a 5 year randomized trial. Maturitas. 1998;31(1):45–54. [DOI] [PubMed] [Google Scholar]
- 93. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400–406. [DOI] [PubMed] [Google Scholar]
- 94. Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. 2002;17(4):709–715. [DOI] [PubMed] [Google Scholar]
- 95. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. [DOI] [PubMed] [Google Scholar]
- 96. Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19(3):370–378. [DOI] [PubMed] [Google Scholar]
- 97. Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, Garnero P, Meunier PJ. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–264. [DOI] [PubMed] [Google Scholar]
- 98. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. [DOI] [PubMed] [Google Scholar]
- 99. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2):315–322. [DOI] [PubMed] [Google Scholar]
- 100. Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, Baverstock M, Birks Y, Dumville J, Francis R, Iglesias C, Puffer S, Sutcliffe A, Watt I, Torgerson DJ. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Salovaara K, Tuppurainen M, Kärkkäinen M, Rikkonen T, Sandini L, Sirola J, Honkanen R, Alhava E, Kröger H. Effect of vitamin D3 and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial—the OSTPRE-FPS. J Bone Miner Res. 2010;25(7):1487–1495. [DOI] [PubMed] [Google Scholar]
- 102. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822. [DOI] [PubMed] [Google Scholar]
- 103. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46(12):1852–1857. [DOI] [PubMed] [Google Scholar]
- 104. Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA Jr, Scragg R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5(6):438–447. [DOI] [PubMed] [Google Scholar]
- 105. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014; (4):CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention [published correction appear in N Engl J Med. 2012;367(5):481]. N Engl J Med. 2012;367(1):40–49. [DOI] [PubMed] [Google Scholar]
- 107. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes—authors’ reply. Lancet Diabetes Endocrinol. 2014;2(5):364–365. [DOI] [PubMed] [Google Scholar]
- 108. Lips P, Gielen E, van Schoor NM. Vitamin D supplements with or without calcium to prevent fractures. Bonekey Rep. 2014;3:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–666. [DOI] [PubMed] [Google Scholar]
- 110. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–320. [DOI] [PubMed] [Google Scholar]
- 111. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13(8):466–479. [DOI] [PubMed] [Google Scholar]
- 112. Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kubik M, Landefeld S, Mangione CM, Silverstein M, Simon MA, Tseng CW; US Preventive Services Task Force . Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(15):1592–1599. [DOI] [PubMed] [Google Scholar]
- 113. Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–321. [DOI] [PubMed] [Google Scholar]
- 114. Wang T-T, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19(11):2685–2695. [DOI] [PubMed] [Google Scholar]
- 115. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vieth R, Holick M. The IOM–Endocrine Society controversy on recommended vitamin D targets: in support of the IOM position In: Feldman D, Wesley Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D. Vol. 1 Biochemistry, Physiology and Diagnostics. 4th ed.London, England: Academic Press; 2017:1091–1107. [Google Scholar]
- 117. Bikle DD. Vitamin D, calcium. and the epidermis In: Feldman D, Wesley Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D. Vol. 1 Biochemistry, Physiology and Diagnostics. 4th ed.London, England: Academic Press; 2017:527–544. [Google Scholar]
- 118. Lehmann B, Meurer M. Extrarenal sites of calcitriol synthesis: the particular role of the skin. Recent Results Cancer Res. 2003;164:135–145. [DOI] [PubMed] [Google Scholar]
- 119. Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25(7):1545–1548. [DOI] [PubMed] [Google Scholar]
- 120. Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol. 2004;89–90(1–5):355–360. [DOI] [PubMed] [Google Scholar]
- 122. Oh JE, Kook JK, Park KH, Lee G, Seo BM, Min BM. Phospholipase C-γ1 is required for subculture-induced terminal differentiation of normal human oral keratinocytes. Int J Mol Med. 2003;11(4):491–498. [PubMed] [Google Scholar]
- 123. Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of vitamin D and calcium signaling in keratinocytes predisposes to skin cancer. Front Physiol. 2016;7:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Oda Y, Hu L, Nguyen T, Fong C, Tu CL, Bikle DD. Combined deletion of the vitamin d receptor and calcium-sensing receptor delays wound re-epithelialization. Endocrinology. 2017;158(6):1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. [DOI] [PubMed] [Google Scholar]
- 127. Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, Deftos LJ, Gallo RL. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci Transl Med. 2012;4(135):135ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Luderer HF, Demay MB. The vitamin D receptor, the skin and stem cells. J Steroid Biochem Mol Biol. 2010;121(1–2):314–316. [DOI] [PubMed] [Google Scholar]
- 130. Demay MB. The hair cycle and Vitamin D receptor. Arch Biochem Biophys. 2012;523(1):19–21. [DOI] [PubMed] [Google Scholar]
- 131. Oda Y, Hu L, Bul V, Elalieh H, Reddy JK, Bikle DD. Coactivator MED1 ablation in keratinocytes results in hair-cycling defects and epidermal alterations. J Invest Dermatol. 2012;132(4):1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128(10):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131(11):2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jiang YJ, Bikle DD. LncRNA profiling reveals new mechanism for VDR protection against skin cancer formation. J Steroid Biochem Mol Biol. 2014;144(Pt A):87–90. [DOI] [PubMed]
- 135. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. [DOI] [PubMed] [Google Scholar]
- 136. Merola JF, Han J, Li T, Qureshi AA. No association between vitamin D intake and incident psoriasis among US women. Arch Dermatol Res. 2014;306(3):305–307. [DOI] [PubMed] [Google Scholar]
- 137. Morimoto S, Yoshikawa K, Kozuka T, Kitano Y, Imanaka S, Fukuo K, Koh E, Kumahara Y. An open study of vitamin D3 treatment in psoriasis vulgaris. Br J Dermatol. 1986;115(4):421–429. [DOI] [PubMed] [Google Scholar]
- 138. Perez A, Raab R, Chen TC, Turner A, Holick MF. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134(6):1070–1078. [PubMed] [Google Scholar]
- 139. Abramovits W. Calcitriol 3 μg/g ointment: an effective and safe addition to the armamentarium in topical psoriasis therapy. J Drugs Dermatol. 2009;8(8, Suppl):s17–s22. [PubMed] [Google Scholar]
- 140. Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079. [DOI] [PMC free article] [PubMed]
- 141. Ezquerra GM, Regaña MS, Millet PU. Combination of acitretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Derm Venereol. 2007;87(5):449–450. [DOI] [PubMed] [Google Scholar]
- 142. Reichrath J, Zouboulis CC, Vogt T, Holick MF. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: an historical view and outlook. Rev Endocr Metab Disord. 2016;17(3):405–417. [DOI] [PubMed] [Google Scholar]
- 143. Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54(4):383–392. [DOI] [PubMed] [Google Scholar]
- 144. Ahn CS, Awadalla F, Huang KE, Yentzer B, Dabade TS, Feldman SR. Patterns of vitamin D analog use for the treatment of psoriasis. J Drugs Dermatol. 2013;12(8):906–910. [PubMed] [Google Scholar]
- 145. Mason A, Mason J, Cork M, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69(5):799–807. [DOI] [PubMed] [Google Scholar]
- 146. Trémezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: where are we standing and where will we be going? Dermatoendocrinol. 2011;3(3):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78(2):141–148. [DOI] [PubMed] [Google Scholar]
- 148. Tongkao-on W, Gordon-Thomson C, Dixon KM, Song EJ, Luu T, Carter SE, Sequeira VB, Reeve VE, Mason RS. Novel vitamin D compounds and skin cancer prevention. Dermatoendocrinol. 2013;5(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, Halliday GM, Mason RS. 1α,25(OH)2-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila). 2011;4(9):1485–1494. [DOI] [PubMed] [Google Scholar]
- 150. Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, Cummings SR, Epstein EH Jr, Bauer DC; Osteoporotic Fractures in Men (MrOS) Study Group . Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21(3):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Asgari MM, Tang J, Warton ME, Chren MM, Quesenberry CP Jr, Bikle D, Horst RL, Orentreich N, Vogelman JH, Friedman GD. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130(5):1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Eide MJ, Johnson DA, Jacobsen GR, Krajenta RJ, Rao DS, Lim HW, Johnson CC. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147(12):1379–1384. [DOI] [PubMed] [Google Scholar]
- 153. Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, Vitolins MZ, Zeitouni NC, Larson J, Stefanick ML. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29(22):3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bolland MJ, Ames RW, Grey AB, Horne AM, Mason BH, Gamble GD, Reid IR. Does degree of baldness influence vitamin D status? Med J Aust. 2008;189(11–12):674–675. [DOI] [PubMed] [Google Scholar]
- 155. van der Vleuten CJ, van de Kerkhof PC. Management of scalp psoriasis: guidelines for corticosteroid use in combination treatment. Drugs. 2001;61(11):1593–1598. [DOI] [PubMed] [Google Scholar]
- 156. Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6(6):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Liu JL, Zhang SQ, Zeng HM. ApaI, BsmI, FokI and TaqI polymorphisms in the vitamin D receptor (VDR) gene and the risk of psoriasis: a meta-analysis. J Eur Acad Dermatol Venereol. 2013;27(6):739–746. [DOI] [PubMed] [Google Scholar]
- 158. Stefanic M, Rucevic I, Barisic-Drusko V. Meta-analysis of vitamin D receptor polymorphisms and psoriasis risk. Int J Dermatol. 2013;52(6):705–710. [DOI] [PubMed] [Google Scholar]
- 159. Lee DY, Park BS, Choi KH, Jeon JH, Cho KH, Song KY, Kim IG, Youn JI. Vitamin D receptor genotypes are not associated with clinical response to calcipotriol in Korean psoriasis patients. Arch Dermatol Res. 2002;294(1–2):1–5. [DOI] [PubMed] [Google Scholar]
- 160. Kontula K, Välimäki S, Kainulainen K, Viitanen AM, Keski-Oja J. Vitamin D receptor polymorphism and treatment of psoriasis with calcipotriol. Br J Dermatol. 1997;136(6):977–978. [DOI] [PubMed] [Google Scholar]
- 161. Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. J Invest Dermatol. 1998;110(3):301–302. [DOI] [PubMed] [Google Scholar]
- 162. Zhao Y, Chen X, Li J, He Y, Su J, Chen M, Zhang W, Chen W, Zhu W. VDR gene polymorphisms are associated with the clinical response to calcipotriol in psoriatic patients. J Dermatol Sci. 2015;79(3):305–307. [DOI] [PubMed] [Google Scholar]
- 163. Köstner K, Denzer N, Koreng M, Reichrath S, Gräber S, Klein R, Tilgen W, Vogt T, Reichrath J. Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): a pilot study in a German population. Anticancer Res. 2012;32(1):327–333. [PubMed] [Google Scholar]
- 164. Mandelcorn-Monson R, Marrett L, Kricker A, Armstrong BK, Orlow I, Goumas C, Paine S, Rosso S, Thomas N, Millikan RC, Pole JD, Cotignola J, Rosen C, Kanetsky PA, Lee-Taylor J, Begg CB, Berwick M. Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol. 2011;35(6):e105–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Mocellin S, Nitti D. Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer. 2008;113(9):2398–2407. [DOI] [PubMed] [Google Scholar]
- 166. Lee YH, Gyu Song G. Vitamin D receptor FokI, BsmI, TaqI, ApaI, and EcoRV polymorphisms and susceptibility to melanoma: a meta-analysis. J BUON. 2015;20(1):235–243. [PubMed] [Google Scholar]
- 167. Barrea L, Savastano S, Di Somma C, Savanelli MC, Nappi F, Albanese L, Orio F, Colao A. Low serum vitamin D-status, air pollution and obesity: a dangerous liaison. Rev Endocr Metab Disord. 2017;18(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1–2):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin β-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Salehi-Tabar R, Nguyen-Yamamoto L, Tavera-Mendoza LE, Quail T, Dimitrov V, An BS, Glass L, Goltzman D, White JH. Vitamin D receptor as a master regulator of the c-MYC/MXD1 network. Proc Natl Acad Sci USA. 2012;109(46):18827–18832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152(2):354–363. [DOI] [PubMed] [Google Scholar]
- 172. Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. [DOI] [PubMed] [Google Scholar]
- 173. Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Gunton JE. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology. 2014;155(2):347–357. [DOI] [PubMed] [Google Scholar]
- 174. Bouillon R, Gielen E, Vanderschueren D. Vitamin D receptor and vitamin D action in muscle. Endocrinology. 2014;155(9):3210–3213. [DOI] [PubMed] [Google Scholar]
- 175. Pike JW. Closing in on vitamin D action in skeletal muscle: early activity in muscle stem cells? Endocrinology. 2016;157(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155(9):3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Cangussu LM, Nahas-Neto J, Orsatti CL, Poloni PF, Schmitt EB, Almeida-Filho B, Nahas EA. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause. 2016;23(3):267–274. [DOI] [PubMed] [Google Scholar]
- 178. Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, Chandler J, Rosenberg E, Papanicolaou DA. Once-weekly dose of 8400 IU vitamin D3 compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010;91(4):985–991. [DOI] [PubMed] [Google Scholar]
- 179. Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–4345. [DOI] [PubMed] [Google Scholar]
- 180. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2012;12:CD005465. [DOI] [PubMed] [Google Scholar]
- 182. Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. LeBlanc ES, Chou R. Vitamin D and falls-fitting new data with current guidelines. JAMA Intern Med. 2015;175(5):712–713. [DOI] [PubMed] [Google Scholar]
- 184. Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(7):573–580. [DOI] [PubMed] [Google Scholar]
- 185. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–183. [DOI] [PubMed] [Google Scholar]
- 186. Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, Schwartz RS. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46(4):1061–1094. [DOI] [PubMed] [Google Scholar]
- 189. Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. [DOI] [PubMed] [Google Scholar]
- 191. Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA. 2010;107(52):22593–22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. [DOI] [PubMed] [Google Scholar]
- 193. White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2012;13(1):21–29. [DOI] [PubMed] [Google Scholar]
- 194. Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Verway M, Bouttier M, Wang T-T, Carrier M, Calderon M, An B-S, Devemy E, McIntosh F, Divangahi M, Behr MA, White JH. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9(6):e1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression [published correction appears in J Immunol. 2004;173(10):following 6489]. J Immunol. 2004;173(5):2909–2912. [DOI] [PubMed] [Google Scholar]
- 197. Vargas Buonfiglio LG, Cano M, Pezzulo AA, Vanegas Calderon OG, Zabner J, Gerke AK, Comellas AP. Effect of vitamin D3 on the antimicrobial activity of human airway surface liquid: preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Respir Res. 2017;4(1):e000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40(3):187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985;66(3):187–191. [DOI] [PubMed] [Google Scholar]
- 201. Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 202. Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13(3):117–124. [DOI] [PubMed] [Google Scholar]
- 203. Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Woodward NJ, Venton TR, Barnes KE, Mullett CJ, Coussens AK, Rutterford CM, Mein CA, Davies GR, Wilkinson RJ, Nikolayevskyy V, Drobniewski FA, Eldridge SM, Griffiths CJ. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J, Rodriguez R, Cabrera L, Gilman RH. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004;190(5):920–927. [DOI] [PubMed] [Google Scholar]
- 205. Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103(3–5):793–798. [DOI] [PubMed] [Google Scholar]
- 206. Mily A, Rekha RS, Kamal SMM, Arifuzzaman AS, Rahim Z, Khan L, Haq MA, Zaman K, Bergman P, Brighenti S, Gudmundsson GH, Agerberth B, Raqib R. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS One. 2015;10(9):e0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–1260. [DOI] [PubMed] [Google Scholar]
- 208. Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N, Rich-Edwards JW. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561–e567. [DOI] [PubMed] [Google Scholar]
- 209. Bergman P, Norlin A-C, Hansen S, Rekha RS, Agerberth B, Björkhem-Bergman L, Ekström L, Lindh JD, Andersson J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(6):e001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, Tirelli S, Esposito S, Principi N. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J. 2013;32(10):1055–1060. [DOI] [PubMed] [Google Scholar]
- 211. Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, Florkowski CM, Livesey JH, Camargo CA, Scragg R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–1339. [DOI] [PubMed] [Google Scholar]
- 212. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Kumar GT, Urashima M, Camargo CA. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017:356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Coulombe F, Behr MA. Crohn’s disease as an immune deficiency? Lancet. 2009;374(9692):769–770. [DOI] [PubMed] [Google Scholar]
- 214. Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8(6):458–466. [DOI] [PubMed] [Google Scholar]
- 215. Deretic V. Autophagy in leukocytes and other cells: mechanisms, subsystem organization, selectivity, and links to innate immunity. J Leukoc Biol. 2016;100(5):969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. White JH. Vitamin D deficiency and the pathogenesis of Crohn’s disease. J Steroid Biochem Mol Biol. 2018;175:23–28. [DOI] [PubMed] [Google Scholar]
- 217. Wang T-T, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cézard J-P, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel J-F, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. [DOI] [PubMed] [Google Scholar]
- 219. Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Núñez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276(7):4812–4818. [DOI] [PubMed] [Google Scholar]
- 220. Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, Reinisch W, Teml A, Schwab M, Lichter P, Radlwimmer B, Stange EF. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79(3):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Scandiuzzi L, Ghosh K, Hofmeyer KA, Abadi YM, Lázár-Molnár E, Lin EY, Liu Q, Jeon H, Almo SC, Chen L, Nathenson SG, Zang X. Tissue-expressed B7-H1 critically controls intestinal inflammation. Cell Reports. 2014;6(4):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, Hübenthal M, Koch M, Schwarz K, Rimbach G, Hübbe P, Pan WH, Sheibani-Tezerji R, Häsler R, Rosenstiel P, D’Amato M, Cloppenborg-Schmidt K, Künzel S, Laudes M, Marschall HU, Lieb W, Nöthlings U, Karlsen TH, Baines JF, Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J, Zheng C, Bissonnette M, Hanauer SB, Li YC. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123(9):3983–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35(3):308–316. [DOI] [PubMed] [Google Scholar]
- 225. Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56(3):830–836. [DOI] [PubMed] [Google Scholar]
- 226. Raftery T, Merrick M, Healy M, Mahmud N, O’Morain C, Smith S, McNamara D, O’Sullivan M. Vitamin D status is associated with intestinal inflammation as measured by fecal calprotectin in Crohn’s disease in clinical remission. Dig Dis Sci. 2015;60(8):2427–2435. [DOI] [PubMed] [Google Scholar]
- 227. Samson CM, Morgan P, Williams E, Beck L, Addie-Carson R, McIntire S, Booth A, Mendez E, Luzader C, Tomer G, Saeed S, Donovan E, Bucuvalas J, Denson LA. Improved outcomes with quality improvement interventions in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2012;55(6):679–688. [DOI] [PubMed] [Google Scholar]
- 228. Youssef D, Bailey B, Atia A, El-Abbassi A, Manning T, Peiris AN. Differences in outcomes between cholecalciferol and ergocalciferol supplementation in veterans with inflammatory bowel disease. Geriatr Gerontol Int. 2012;12(3):475–480. [DOI] [PubMed] [Google Scholar]
- 229. Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng S-C, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, Shaw SY, Churchill S, Karlson EW, Kohane I, Plenge RM, Murphy SN, Liao KP. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis. 2013;19(9):1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32(3):377–383. [DOI] [PubMed] [Google Scholar]
- 231. Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3(3):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–1310. [DOI] [PubMed] [Google Scholar]
- 233. Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133(4):1748–1754. [PubMed] [Google Scholar]
- 234. Vanherwegen AS, Gysemans C, Mathieu C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol Cell Endocrinol. 2017;453:52–67. [DOI] [PubMed] [Google Scholar]
- 235. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–496. [DOI] [PubMed] [Google Scholar]
- 236. Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70(5):345–352. [DOI] [PubMed] [Google Scholar]
- 237. Ferreira GB, Vanherwegen A-S, Eelen G, Gutiérrez ACF, Van Lommel L, Marchal K, Verlinden L, Verstuyf A, Nogueira T, Georgiadou M, Schuit F, Eizirik DL, Gysemans C, Carmeliet P, Overbergh L, Mathieu C. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Reports. 2015;10(5):711–725. [DOI] [PubMed] [Google Scholar]
- 238. Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92(1):60–64. [DOI] [PubMed] [Google Scholar]
- 239. Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47(3):451–462. [DOI] [PubMed] [Google Scholar]
- 240. Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72. [DOI] [PubMed] [Google Scholar]
- 241. Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4(8):404–412. [DOI] [PubMed] [Google Scholar]
- 242. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. [DOI] [PubMed] [Google Scholar]
- 243. Munger KL, Åivo J, Hongell K, Soilu-Hänninen M, Surcel HM, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol. 2016;73(5):515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Munger KL, Levin LI, Massa J, Horst R, Orban T, Ascherio A. Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am J Epidemiol. 2013;177(5):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–1257. [DOI] [PubMed] [Google Scholar]
- 246. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG; Iowa Women’s Health Study . Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–77. [DOI] [PubMed] [Google Scholar]
- 247. Manousaki D, Dudding T, Haworth S, Hsu YH, Liu CT, Medina-Gómez C, Voortman T, van der Velde N, Melhus H, Robinson-Cohen C, Cousminer DL, Nethander M, Vandenput L, Noordam R, Forgetta V, Greenwood CMT, Biggs ML, Psaty BM, Rotter JI, Zemel BS, Mitchell JA, Taylor B, Lorentzon M, Karlsson M, Jaddoe VVW, Tiemeier H, Campos-Obando N, Franco OH, Utterlinden AG, Broer L, van Schoor NM, Ham AC, Ikram MA, Karasik D, de Mutsert R, Rosendaal FR, den Heijer M, Wang TJ, Lind L, Orwoll ES, Mook-Kanamori DO, Michaëlsson K, Kestenbaum B, Ohlsson C, Mellström D, de Groot LCPGM, Grant SFA, Kiel DP, Zillikens MC, Rivadeneira F, Sawcer S, Timpson NJ, Richards JB. Low-frequency synonymous coding variation in CYP2R1 has large effects on vitamin D levels and risk of multiple sclerosis. Am J Hum Genet. 2017;101(2):227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2017;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wei Z, Yoshihara E, He N, Hah N, Fan W, Pinto AFM, Huddy T, Wang Y, Ross B, Estepa G, Dai Y, Ding N, Sherman MH, Fang S, Zhao X, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM. Vitamin D switches BAF complexes to protect β cells. Cell. 2018;173(5):1135–1149.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Li X, Liao L, Yan X, Huang G, Lin J, Lei M, Wang X, Zhou Z. Protective effects of 1-α-hydroxyvitamin D3 on residual β-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2009;25(5):411–416. [DOI] [PubMed] [Google Scholar]
- 251. Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–517. [DOI] [PubMed] [Google Scholar]
- 252. Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5(9):3551–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, Sandel M, Iverson RE Jr, Lee-Paritz A, Strunk RC, Bacharier LB, Macones GA, Zeiger RS, Schatz M, Hollis BW, Hornsby E, Hawrylowicz C, Wu AC, Weiss ST. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, Arianto L, Hallas HW, Heickendorff L, Brix S, Rasmussen MA, Bisgaard H. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353–361. [DOI] [PubMed] [Google Scholar]
- 255. Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30(3):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, Sheikh A, Griffiths CJ. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Luo J, Liu D, Liu CT. Can vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma?: a meta-analysis. Medicine (Baltimore). 2015;94(50):e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Hysinger EB, Roizen JD, Mentch FD, Vazquez L, Connolly JJ, Bradfield JP, Almoguera B, Sleiman PM, Allen JL, Levine MA, Hakonarson H. Mendelian randomization analysis demonstrates that low vitamin D is unlikely causative for pediatric asthma. J Allergy Clin Immunol. 2016;138(6):1747–1749.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-Dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2(8156–8157):1335–1336. [DOI] [PubMed] [Google Scholar]
- 260. Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78(8):4990–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108(3):1083–1086. [DOI] [PubMed] [Google Scholar]
- 262. Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16(2):200–257. [DOI] [PubMed] [Google Scholar]
- 263. Duffy MJ, Murray A, Synnott NC, O’Donovan N, Crown J. Vitamin D analogues: potential use in cancer treatment. Crit Rev Oncol Hematol. 2017;112:190–197. [DOI] [PubMed] [Google Scholar]
- 264. Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11(56):7–17. [PMC free article] [PubMed] [Google Scholar]
- 265. Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69(3):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000;25(2):144–146. [DOI] [PubMed] [Google Scholar]
- 267. Bandera Merchan B, Morcillo S, Martin-Nuñez G, Tinahones FJ, Macías-González M. The role of vitamin D and VDR in carcinogenesis: through epidemiology and basic sciences. J Steroid Biochem Mol Biol. 2017;167:203–218. [DOI] [PubMed] [Google Scholar]
- 268. Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol Cell Endocrinol. 2017;453:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Tong WM, Kállay E, Hofer H, Hulla W, Manhardt T, Peterlik M, Cross HS. Growth regulation of human colon cancer cells by epidermal growth factor and 1,25-dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur J Cancer. 1998;34(13):2119–2125. [DOI] [PubMed] [Google Scholar]
- 270. Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1α,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J Steroid Biochem Mol Biol. 2013;136:229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. [DOI] [PubMed] [Google Scholar]
- 272. Chen L, Yang R, Qiao W, Yuan X, Wang S, Goltzman D, Miao D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int J Cancer. 2018;143(2):368–382. [DOI] [PubMed] [Google Scholar]
- 273. Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30(1):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol. 2010;121(1–2):403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Yang K, Lamprecht SA, Shinozaki H, Fan K, Yang W, Newmark HL, Kopelovich L, Edelmann W, Jin B, Gravaghi C, Augenlicht L, Kucherlapati R, Lipkin M. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. J Nutr. 2008;138(9):1658–1663. [DOI] [PubMed] [Google Scholar]
- 276. Xu H, Posner GH, Stevenson M, Campbell FC. ApcMIN modulation of vitamin D secosteroid growth control. Carcinogenesis. 2010;31(8):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Zheng W, Wong KE, Zhang Z, Dougherty U, Mustafi R, Kong J, Deb DK, Zheng H, Bissonnette M, Li YC. Inactivation of the vitamin D receptor in APCmin/+ mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer. 2012;130(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 1α,25-(OH)2-D3 and its synthetic analogue decrease tumor load in the Apcmin mouse. Cancer Res. 2002;62(3):741–746. [PubMed] [Google Scholar]
- 279. Lipkin M, Newmark HL. Vitamin D, calcium and prevention of breast cancer: a review. J Am Coll Nutr. 1999;18(5, Suppl):392S–397S. [DOI] [PubMed] [Google Scholar]
- 280. Zinser GM, Welsh J. Effect of vitamin D3 receptor ablation on murine mammary gland development and tumorigenesis. J Steroid Biochem Mol Biol. 2004;89-90(1–5):433–436. [DOI] [PubMed] [Google Scholar]
- 281. Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25(12):2361–2372. [DOI] [PubMed] [Google Scholar]
- 282. VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139(4):2102–2110. [DOI] [PubMed] [Google Scholar]
- 283. Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ, Dunstan CR. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70(5):1835–1844. [DOI] [PubMed] [Google Scholar]
- 284. El Abdaimi K, Dion N, Papavasiliou V, Cardinal PE, Binderup L, Goltzman D, Ste-Marie LG, Kremer R. The vitamin D analogue EB 1089 prevents skeletal metastasis and prolongs survival time in nude mice transplanted with human breast cancer cells. Cancer Res. 2000;60(16):4412–4418. [PubMed] [Google Scholar]
- 285. Bhatia V, Saini MK, Shen X, Bi LX, Qiu S, Weigel NL, Falzon M. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther. 2009;8(7):1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zheng Y, Zhou H, Ooi LL, Snir AD, Dunstan CR, Seibel MJ. Vitamin D deficiency promotes prostate cancer growth in bone. Prostate. 2011;71(9):1012–1021. [DOI] [PubMed] [Google Scholar]
- 287. Mordan-McCombs S, Brown T, Wang WL, Gaupel AC, Welsh J, Tenniswood M. Tumor progression in the LPB-Tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. J Steroid Biochem Mol Biol. 2010;121(1–2):368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39(2):401–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol. 2014;5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Apperly F. The relation of solar radiation to cancer mortality in North America. Cancer Res. 1941;1:191–195. [DOI] [PubMed] [Google Scholar]
- 291. Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):227–231. [DOI] [PubMed] [Google Scholar]
- 292. Egan KM. Commentary: sunlight, vitamin D, and the cancer connection revisited. Int J Epidemiol. 2006;35(2):227–230. [DOI] [PubMed] [Google Scholar]
- 293. Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39(1):28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. International Agency for Research on Cancer. Vitamin D and cancer. Lyon, France: World Health Organization Press; 2008.
- 295. Dou R, Ng K, Giovannucci EL, Manson JE, Qian ZR, Ogino S. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br J Nutr. 2016;115(9):1643–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Jacobs ET, Kohler LN, Kunihiro AG, Jurutka PW. Vitamin D and colorectal, breast, and prostate cancers: a review of the epidemiological evidence. J Cancer. 2016;7(3):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Lee DH, Keum N, Giovannucci EL. Colorectal cancer epidemiology in the Nurses’ Health Study. Am J Public Health. 2016;106(9):1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. Available at: www.ncbi.nlm.nih.gov/books/NBK56070/. [PubMed]
- 299. UK National Health Service. Consensus vitamin D position statement. Available at: www.nhs.uk/livewell/summerhealth/documents/concensus_statement%20_vitd_dec_2010.pdf.
- 300. Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, Brouckaert O, Peuteman G, Laenen A, Verlinden L, Kriebitzsch C, Dieudonné AS, Paridaens R, Neven P, Christiaens MR, Bouillon R, Wildiers H. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis. 2012;33(7):1319–1326. [DOI] [PubMed] [Google Scholar]
- 301. van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer. 2013;49(6):1422–1436. [DOI] [PubMed] [Google Scholar]
- 302. Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–108. [DOI] [PubMed] [Google Scholar]
- 303. Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70(12):2861–2869. [DOI] [PubMed] [Google Scholar]
- 304. Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, Kristal AR, Peters U, Neuhouser ML. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–838. [DOI] [PubMed] [Google Scholar]
- 308. Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414–1424. [DOI] [PubMed] [Google Scholar]
- 309. Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29(28):3775–3782. [DOI] [PubMed] [Google Scholar]
- 311. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: Serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53(1–2):10–16. [DOI] [PubMed] [Google Scholar]
- 312. Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–477. [DOI] [PubMed] [Google Scholar]
- 313. Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE, Fraser WD, Sterne JA, Metcalfe C. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22(3):319–340. [DOI] [PubMed] [Google Scholar]
- 314. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. [DOI] [PubMed] [Google Scholar]
- 315. Brunner RL, Wactawski-Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML, Jacobs ET, LaCroix AZ, Lane D, Larson J, Margolis KL, Millen AE, Sarto GE, Vitolins MZ, Wallace RB. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63(6):827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, Beck GJ, Bresalier RS, Burke CA, Church TR, Cruz-Correa M, Figueiredo JC, Goodman M, Kim AS, Robertson DJ, Rothstein R, Shaukat A, Seabrook ME, Summers RW. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373(16):1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Lappe J, Watson P, Travers-Gustafson D, Recker R, Garland C, Gorham E, Baggerly K, McDonnell SL. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234–1243. [DOI] [PubMed] [Google Scholar]
- 318. Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. [DOI] [PubMed] [Google Scholar]
- 319. Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M, LeBlanc ES, Cauley JA, Rossouw JE. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women’s Health Initiative randomized trial. Int J Cancer. 2008;122(8):1690–1694. [DOI] [PubMed] [Google Scholar]
- 321. Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA; RECORD Trial Group . Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97(2):614–622. [DOI] [PubMed] [Google Scholar]
- 322. Binkley N, Sempos CT; Vitamin D Standardization Program (VDSP) . Standardizing vitamin D assays: the way forward. J Bone Miner Res. 2014;29(8):1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Bassuk SS, Manson JE, Lee IM, Cook NR, Christen WG, Bubes VY, Gordon DS, Copeland T, Friedenberg G, D’Agostino DM, Ridge CY, MacFadyen JG, Kalan K, Buring JE. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. [DOI] [PubMed] [Google Scholar]
- 325. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124(17):1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Bouillon R. Vitamin D: from photosynthesis, metabolism and action to clinical applications In: Jameson JL, De Groot LJ, eds. Endocrinology. Vol 1 6th ed.Philadelphia, PA: Saunders Elsevier; 2010:1089–1110. [Google Scholar]
- 329. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 330. Han MS, Che X, Cho GH, Park HR, Lim KE, Park NR, Jin JS, Jung YK, Jeong JH, Lee IK, Kato S, Choi JY. Functional cooperation between vitamin D receptor and Runx2 in vitamin D-induced vascular calcification. PLoS One. 2013;8(12):e83584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008;102(11):1540–1544. [DOI] [PubMed] [Google Scholar]
- 334. Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205(1):255–260. [DOI] [PubMed] [Google Scholar]
- 335. Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152(5):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–1942. [DOI] [PubMed] [Google Scholar]
- 338. Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group . Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–755. [DOI] [PubMed] [Google Scholar]
- 339. Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267(5):462–472. [DOI] [PubMed] [Google Scholar]
- 340. Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol. 2017;2(6):608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Bouillon R. Vitamin D as potential baseline therapy for blood pressure control. Am J Hypertens. 2009;22(8):816. [DOI] [PubMed] [Google Scholar]
- 342. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. [DOI] [PubMed] [Google Scholar]
- 343. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713–719. [DOI] [PubMed] [Google Scholar]
- 344. Schmitz KJ, Skinner HG, Bautista LE, Fingerlin TE, Langefeld CD, Hicks PJ, Haffner SM, Bryer-Ash M, Wagenknecht LE, Bowden DW, Norris JM, Engelman CD. Association of 25-hydroxyvitamin D with blood pressure in predominantly 25-hydroxyvitamin D deficient Hispanic and African Americans. Am J Hypertens. 2009;22(8):867–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD; D-PRESSURE Collaboration . Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, Giovannucci EL, Fuchs CS, Chan AT. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61(4):779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014;29(1):1–14. [DOI] [PubMed] [Google Scholar]
- 348. Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Norris KC, Williams SF. Race/ethnicity, serum 25-hydroxyvitamin D, and heart disease. JAMA. 2013;310(2):153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Ozfirat Z, Chowdhury TA. Vitamin D deficiency and type 2 diabetes. Postgrad Med J. 2010;86(1011):18–25. [DOI] [PubMed] [Google Scholar]
- 351. Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, Schoonjans K, Verstuyf A. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10(2):79–87. [DOI] [PubMed] [Google Scholar]
- 352. Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3-1α-hydroxylase and -24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145(3):1367–1375. [DOI] [PubMed] [Google Scholar]
- 353. Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, Jacques PF. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am J Clin Nutr. 2010;91(6):1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Pittas AG, Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol. 2010;121(1–2):425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Zhao G, Ford ES, Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003–2006. Diabetes Care. 2010;33(2):344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, Perkins BA, Harris SB, Zinman B, Hanley AJ. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes [published correction appears in Diabetes Care. 2011;34(1):247]. Diabetes Care. 2010;33(6):1379–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Kositsawat J, Freeman VL, Gerber BS, Geraci S. Association of A1C levels with vitamin D status in U.S. adults: data from the National Health and Nutrition Examination Survey. Diabetes Care. 2010;33(6):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O’Donnell CJ, Hoffmann U, Jacques PF, Booth SL, Vasan RS, Wolf M, Wang TJ. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59(1):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Reis JP, von Mühlen D, Miller ER III, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159–1165. [DOI] [PubMed] [Google Scholar]
- 361. Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28(5):1228–1230. [DOI] [PubMed] [Google Scholar]
- 362. Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. 2016;101(4):1647–1655. [DOI] [PubMed] [Google Scholar]
- 365. Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism. 2017;73:67–76. [DOI] [PubMed] [Google Scholar]
- 366. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab. 2017;102(9):3097–3110. [DOI] [PubMed] [Google Scholar]
- 367. Krul-Poel YH, Ter Wee MM, Lips P, Simsek S. Management of endocrine disease: the effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 368. Pittas AG, Dawson-Hughes B, Sheehan PR, Rosen CJ, Ware JH, Knowler WC, Staten MA; D2d Research Group . Rationale and design of the vitamin D and type 2 diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37(12):3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Angellotti E, Pittas AG. The role of vitamin D in the prevention of type 2 diabetes: to D or not to D? Endocrinology. 2017;158(7):2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Landel V, Annweiler C, Millet P, Morello M, Féron F. Vitamin D, cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis. 2016;53(2):419–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Shen L, Ji HF. Associations between vitamin D status, supplementation, outdoor work and risk of Parkinson’s disease: a meta-analysis assessment. Nutrients. 2015;7(6):4817–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Blomberg Jensen M. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175–186. [DOI] [PubMed] [Google Scholar]
- 374. Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jorgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103(3):870–881. [DOI] [PubMed] [Google Scholar]
- 375. De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016; (1):CD008873. [DOI] [PubMed] [Google Scholar]
- 376. Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ. 2017;359:j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, Atkins AR, Barish GD, Gunton JE, Liddle C, Downes M, Evans RM. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Mathyssen C, Gayan-Ramirez G, Bouillon R, Janssens W. Vitamin D supplementation in respiratory diseases: evidence from randomized controlled trials. Pol Arch Intern Med. 2017;127(11):775–784. [DOI] [PubMed] [Google Scholar]
- 379. Bikle DD. Extraskeletal actions of vitamin D. Ann N Y Acad Sci. 2016;1376(1):29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Rosen CJ, Taylor CL. Common misconceptions about vitamin D—implications for clinicians. Nat Rev Endocrinol. 2013;9(7):434–438. [DOI] [PubMed] [Google Scholar]
- 382. Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97(8):2644–2652. [DOI] [PubMed] [Google Scholar]
- 383. Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, Cao G, Burt V, Kramer H, Bailey RL, Dwyer JT, Zhang X, Gahche J, Coates PM, Picciano MF. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013;98(7):3001–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL, Pfeiffer CM, Johnson CL. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, Streppel M, Gardiner J, Ordóñez-Mena JM, Perna L, Wilsgaard T, Rathmann W, Feskens E, Kampman E, Siganos G, Njølstad I, Mathiesen EB, Kubínová R, Pająk A, Topor-Madry R, Tamosiunas A, Hughes M, Kee F, Bobak M, Trichopoulou A, Boffetta P, Brenner H; Consortium on Health and Ageing: Network of Cohorts in Europe and the United States . Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Noordam R, de Craen AJ, Pedram P, Maier AB, Mooijaart SP, van Pelt J, Feskens EJ, Streppel MT, Slagboom PE, Westendorp RG, Beekman M, van Heemst D. Levels of 25-hydroxyvitamin D in familial longevity: the Leiden Longevity Study. CMAJ. 2012;184(18):E963–E968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9(12):941–955. [DOI] [PubMed] [Google Scholar]
- 389. Komulainen M, Kröger H, Tuppurainen MT, Heikkinen AM, Alhava E, Honkanen R, Jurvelin J, Saarikoski S. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population-based 5-year randomized trial. J Clin Endocrinol Metab. 1999;84(2):546–552. [DOI] [PubMed] [Google Scholar]
- 390. Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168(1):103–108. [DOI] [PubMed] [Google Scholar]